Abstract
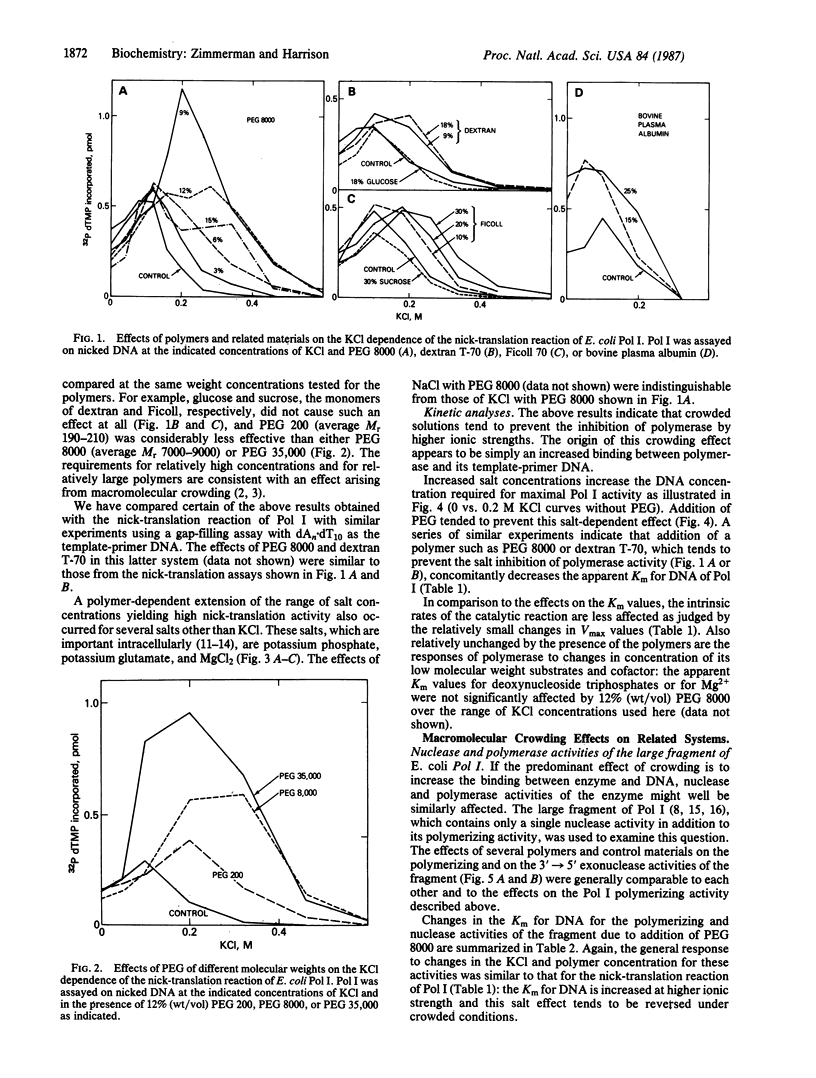
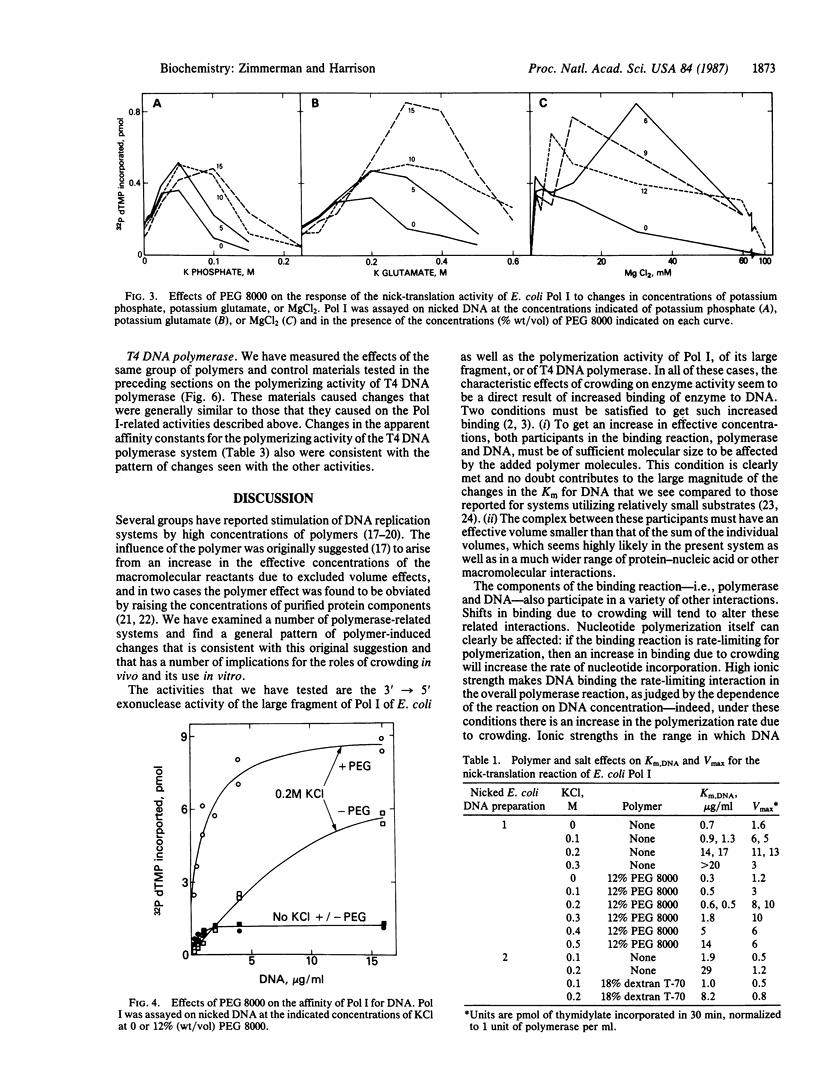
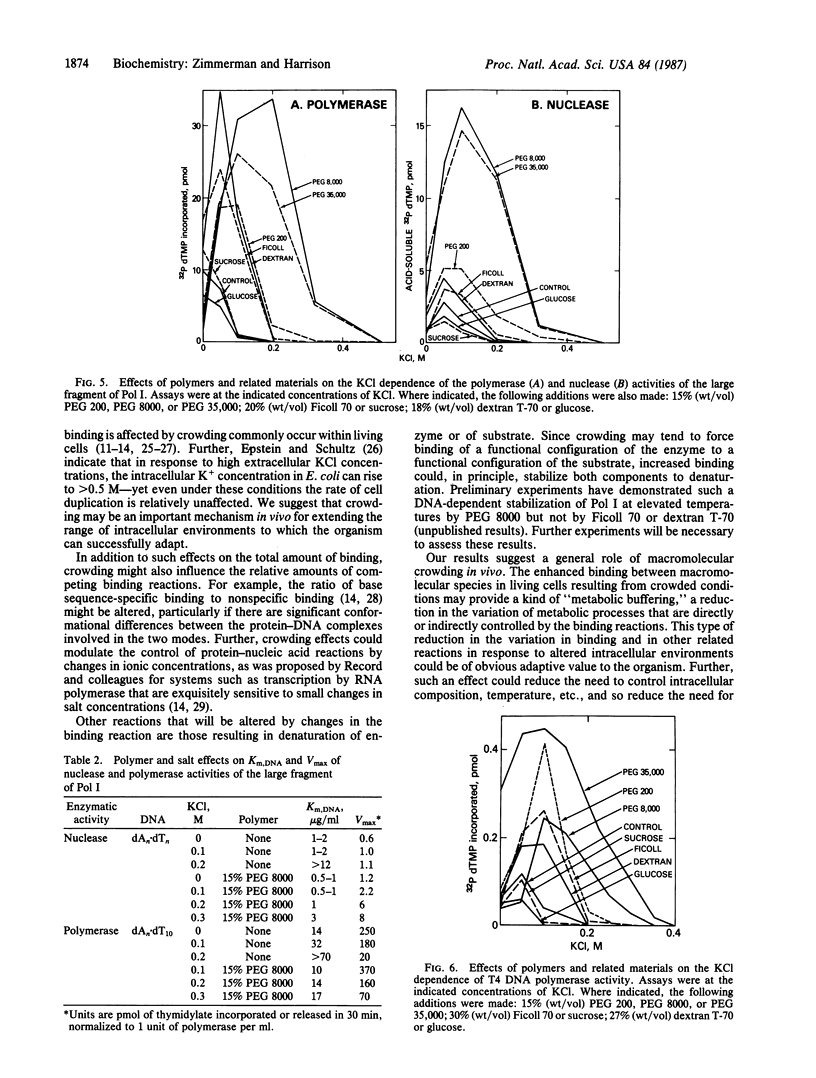
Macromolecular crowding extends the range of ionic conditions supporting high DNA polymerase reaction rates. Reactions tested were nick-translation and gap-filling by DNA polymerase I of Escherichia coli, nuclease and polymerase activities of the large fragment of that polymerase, and polymerization by the T4 DNA polymerase. For all of these reactions, high concentrations of nonspecific polymers increased enzymatic activity under otherwise inhibitory conditions resulting from relatively high ionic strength. The primary mechanism of the polymer effect seems to be to increase the binding of polymerase to DNA. We suggest that this effect on protein-DNA complexes is only one example of a general "metabolic buffering" action of crowded solutions on a variety of macromolecular interactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutlag D., Atkinson M. R., Setlow P., Kornberg A. An active fragment of DNA polymerase produced by proteolytic cleavage. Biochem Biophys Res Commun. 1969 Dec 4;37(6):982–989. doi: 10.1016/0006-291x(69)90228-9. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. Solute concentrations within cells of halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1962 Dec 17;65:506–508. doi: 10.1016/0006-3002(62)90453-5. [DOI] [PubMed] [Google Scholar]
- Fairfield F. R., Newport J. W., Dolejsi M. K., von Hippel P. H. On the processivity of DNA replication. J Biomol Struct Dyn. 1983 Dec;1(3):715–727. doi: 10.1080/07391102.1983.10507477. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Harrison B., Zimmerman S. B. Polymer-stimulated ligation: enhanced ligation of oligo- and polynucleotides by T4 RNA ligase in polymer solutions. Nucleic Acids Res. 1984 Nov 12;12(21):8235–8251. doi: 10.1093/nar/12.21.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B., Zimmerman S. B. Stabilization of T4 polynucleotide kinase by macromolecular crowding. Nucleic Acids Res. 1986 Feb 25;14(4):1863–1870. doi: 10.1093/nar/14.4.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell. 1984 Aug;38(1):183–190. doi: 10.1016/0092-8674(84)90539-7. [DOI] [PubMed] [Google Scholar]
- Kao-Huang Y., Revzin A., Butler A. P., O'Conner P., Noble D. W., von Hippel P. H. Nonspecific DNA binding of genome-regulating proteins as a biological control mechanism: measurement of DNA-bound Escherichia coli lac repressor in vivo. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4228–4232. doi: 10.1073/pnas.74.10.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenow H., Henningsen I. Effect of monovalent cations on the activity of the DNA polymerase of Escherichia coli B. Eur J Biochem. 1969 May 1;9(1):133–141. doi: 10.1111/j.1432-1033.1969.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Klenow H., Henningsen I. Selective elimination of the exonuclease activity of the deoxyribonucleic acid polymerase from Escherichia coli B by limited proteolysis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):168–175. doi: 10.1073/pnas.65.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C. Enzyme reactions in polymer media. Eur J Biochem. 1971 Aug 25;21(4):498–506. doi: 10.1111/j.1432-1033.1971.tb01495.x. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The bacteriophage lambda O and P protein initiators promote the replication of single-stranded DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3069–3088. doi: 10.1093/nar/12.7.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Zylicz M., Georgopoulos C., McMacken R. Initiation of DNA replication on single-stranded DNA templates catalyzed by purified replication proteins of bacteriophage lambda and Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3988–3992. doi: 10.1073/pnas.82.12.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman T. M. Kinetics of protein-nucleic acid interactions: use of salt effects to probe mechanisms of interaction. CRC Crit Rev Biochem. 1986;19(3):191–245. doi: 10.3109/10409238609084656. [DOI] [PubMed] [Google Scholar]
- Medina R., Aragón J. J., Sols A. Effect of polyethylene glycol on the kinetic behaviour of pyruvate kinase and other potentially regulatory liver enzymes. FEBS Lett. 1985 Jan 21;180(1):77–80. doi: 10.1016/0014-5793(85)80235-0. [DOI] [PubMed] [Google Scholar]
- Minton A. P. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55(2):119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K. In vitro transposition of bacteriophage Mu: a biochemical approach to a novel replication reaction. Cell. 1983 Dec;35(3 Pt 2):785–794. doi: 10.1016/0092-8674(83)90111-3. [DOI] [PubMed] [Google Scholar]
- Munro G. F., Hercules K., Morgan J., Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972 Feb 25;247(4):1272–1280. [PubMed] [Google Scholar]
- Ollis D. L., Brick P., Hamlin R., Xuong N. G., Steitz T. A. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. 1985 Feb 28-Mar 6Nature. 313(6005):762–766. doi: 10.1038/313762a0. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Mills P., Mossing M., Roe J. H. Ions as regulators of protein-nucleic acid interactions in vitro and in vivo. Adv Biophys. 1985;20:109–135. doi: 10.1016/0065-227x(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Roe J. H., Record M. T., Jr Regulation of the kinetics of the interaction of Escherichia coli RNA polymerase with the lambda PR promoter by salt concentration. Biochemistry. 1985 Aug 27;24(18):4721–4726. doi: 10.1021/bi00339a002. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., WILSON N. L., EPSTEIN W. Cation transport in Escherichia coli. II. Intracellular chloride concentration. J Gen Physiol. 1962 Sep;46:159–166. doi: 10.1085/jgp.46.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Replication of lambda dv plasmid in vitro promoted by purified lambda O and P proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7639–7643. doi: 10.1073/pnas.79.24.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwicker J., Ollis D., Richards F. M., Steitz T. A. Electrostatic field of the large fragment of Escherichia coli DNA polymerase I. J Mol Biol. 1985 Dec 5;186(3):645–649. doi: 10.1016/0022-2836(85)90136-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Harrison B. Macromolecular crowding accelerates the cohesion of DNA fragments with complementary termini. Nucleic Acids Res. 1985 Apr 11;13(7):2241–2249. doi: 10.1093/nar/13.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Macromolecular crowding allows blunt-end ligation by DNA ligases from rat liver or Escherichia coli. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5852–5856. doi: 10.1073/pnas.80.19.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. H., Berg O. G. On the specificity of DNA-protein interactions. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1608–1612. doi: 10.1073/pnas.83.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]


