Abstract
Background and Purpose
Osteopontin (OPN) is neuroprotective in ischemic brain injuries in adult experimental models, therefore, we hypothesized that OPN would provide neuroprotection and improve long term neurological function in the immature brain after hypoxic-ischemic (HI) injury.
Methods
HI was induced by unilateral ligation of the right carotid artery followed by hypoxia (8% O2 for 2h) in postnatal day 7 rats. OPN (0.03 µg or 0.1 µg) was injected intracerebroventricularly at 1h post HI. Temporal expression of endogenous OPN was evaluated in the normal rat brain at the age of day 0, 4, 7, 11, 14, and 21, and in the ipsilateral hemisphere following HI. The effects of OPN were evaluated using TTC staining, apoptotic cell death assay, and cleaved caspase-3 expression. Neurological function was assessed by Morris water maze test.
Results
Endogenous OPN expression in the brain was the highest at the age of day 0, with continuous reduction till the age of day 21 during development. After HI injury, endogenous OPN expression was increased and peaked at 48h. Exogenous OPN decreased infarct volume and improved neurological outcomes 7 weeks after HI injury. OPN-induced neuroprotection was blocked by an integrin antagonist.
Conclusions
OPN-induced neuroprotection was associated with cleaved-caspase-3 inhibition and antiapoptotic cell death. OPN treatment improved long-term neurological function against neonatal HI brain injury.
Keywords: Osteopontin, Neonatal, Hypoxic/Ischemic, Neuroprotection
Hypoxia-ischemia (HI) brain injury in the preterm infant impairs normal development and results in long term neurological deficits.1 Previous studies suggest that apoptotic cell death is prominent in the neonatal brain after HI insults, 2 and it is more common in the immature brain than adult brain.3 Caspase-3 cleavage and activation has been shown as a major cause of brain injury following neonatal stroke.4 To date, however, there is no effective pharmacological strategies available for neonatal brain neuroprotection following injury.
Osteopontin (OPN) is a secreted glycosylated phosphoprotein which exists in all the body fluids and involves in multiple biological functions, including inflammation, cell migration, and anti-apoptotic processes.5 OPN is widely overexpressed in various cancer diseases5,6 in accordance with the increased cell survival.7 Other than in vitro study, a protective role of OPN in ischemia has also been suggested in the kidney and brain in the adult animal.8,9 The present study was designed to investigate the effect of OPN in the neonatal brain after hypoxic-ischemic insult.
Materials and Methods
Animal model
The Institutional Animal Care and Use Committee (IUCAC) at Loma Linda University approved all protocols. A modified Rice-Vannucci model10 was used as previously described.11 Briefly, 7-day-old rat pups were anesthetized with 3% isoflurane. The right common carotid artery of each pup was identified, exposed, and permanently ligated. After recovering with their dam for 2h, the pups were then placed in a jar perfused with 8% oxygen (balanced with nitrogen) at 4L/min for 2h. A constant temperature of 37°C was maintained throughout all the procedures. After hypoxia, the animals returned to their dams and the ambient temperature was maintained at 37°C for 24h. Sham animals underwent anesthesia and the common carotid artery was exposed without ligation and hypoxia.
Drug Administration
Pups were randomly assigned to one of the following groups: sham+PBS, sham+OPN-0.1 (0.1µg OPN injection), HI+PBS, HI+OPN-0.03 (0.03µg OPN treatment), HI+OPN-0.1 (0.1µg OPN treatment). OPN (Calbiochem, CA) was prepared followed as others described with modification.12 OPN was dissolved in PBS (0.03µg/µl or 0.1 µg/µl) and total volume of 1.0µl was administered intra-cerebroventricularly at 0.03µg or 0.1µg per animal 1h post HI. Briefly, 7-day-old rat pups were fixed on a stereotaxic apparatus (Stoelting, Wood Dale, IL) under isoflurane inhalation (2%). A scalp incision was made on the skull surface and the bregma was exposed. OPN was injected with a 10-µl syringe (Hamilton, NV) at the location of 1.0mm posterior and 1.0mm lateral to the bregma, and 2.0mm deep to the skull surface at the contralateral hemisphere. The control rats were injected with sterile PBS. The injection was completed in 5 min and the needle was kept in the injection position for an additional 2 min. Then the needle was removed slowly out of the brain and the wound was sutured. After recovery from the anesthesia, the pups were returned to their dams.13 To investigate whether integrin receptor is involved, additional group was administered 1 µl GRGDSP (5µmol/L, Sigma-Aldrich, MO) intracerebroventricularly 15 min before OPN treatment (0.1µg).
Infarct volume evaluation
2,3,5-triphenyltetrazolium chloride monohydrate (TTC, Sigma-Aldrich, MO) staining was used to measure infarct volume as previously described.11
Immunohistochemistry
At 48h after HI, animals were anesthetized and ten-micron-thick coronal brain sections were cut using the cryostat (CM3050S, Leica Microsystems) as previous described.11 Brain sections were incubated with primary antibody GFAP (Dako, CA), MAP-2 (Santa cruz biotechnology, CA), or Iba-1 (Dako, CA) overnight at 4°C. Fluorescein isothiocyanate (FITC)- or Texas red-conjugated secondary antibodies (Jackson Immunoresearch, PA) were used. The sections were then visualized using a fluorescent microscope (Olympus BX51, Olympus Optical Co. Ltd, Japan) and pictures were recorded and analyzed (MagnaFire SP 2.1B software).
Cell death assay
Apoptosis induced by HI at 24h in the ipsilateral hemisphere was evaluated by quantitation of DNA fragmentation using Cell Death Detection ELISA kit (Roche Applied Science, IN) in accordance with the manufacture’s specification, as we previously described.14
Western Blotting
Normal animals were sacrificed at the age of day 0, 4, 7, 11, 14, and 21 (n = 4 for each group) for endogenous OPN measurement. To evaluate endogenous OPN expression following HI injury, rat pups were sacrificed at 3, 24, 48hs, 4, 7, and 14 days post HI. The level of cleaved caspase-3 in the ipsilateral hemisphere was measured at 24h post HI, with n=6 for each group. Western blot analysis was performed as described previously.11 Primary antibodies (osteopontin, Santa cruz biotechnology, CA; cleaved caspase-3, Chemicon, CA) and secondary antibody were used.
Morris water maze test
Water maze test is used to evaluate the ability to learn spatial locations and memory. It was performed at 7 weeks after HI injury as previous described.11 Briefly, the rats need to find a visualized (cued test) or submerged (special test) platform in a pool of water with visual cues in the room. The water maze consisted of a pool (118cm diameter) filled with water and a platform (22cm diameter) that rats could step on to escape the water. All trials last a maximum of 60s, at which point the rats were manually guided to be placed on the platform. All the activities were recorded and the animals' swimming paths were measured for quantification of distance, latency, and swimming speed by the Video Tracking System SMART-2000 (San Diego Instruments Inc., CA).
Statistical Analysis
Data are presented as mean ± SEM. Statistical differences between more than two groups were analyzed by using one-way ANOVA followed by Turkey multiple comparison procedure. Water maze data were analyzed using the general linear models repeated measures ANOVA (Statistica 6.0). P value below 0.05 was considered as statistically significant.
Results
Endogenous OPN Expression in the Developing Brain
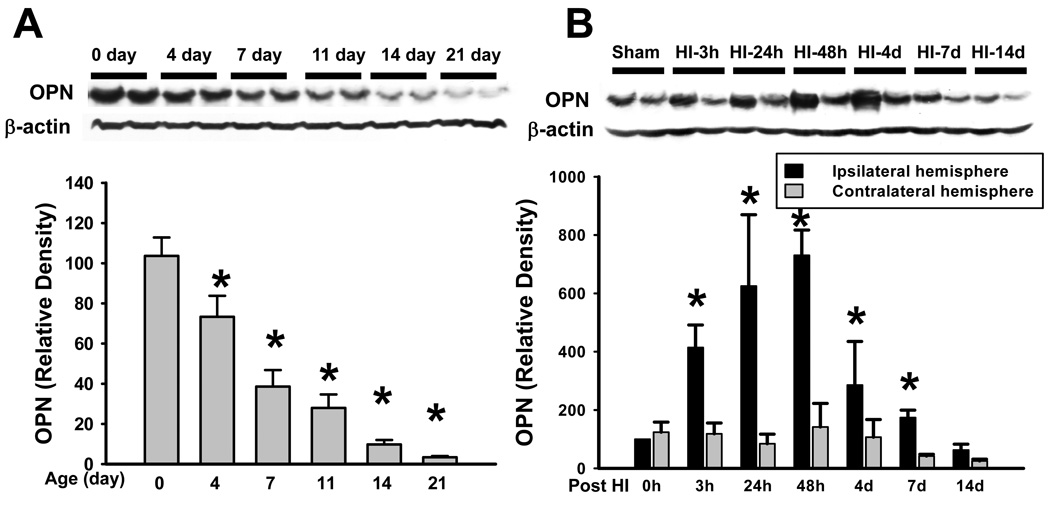
First, the temporal profile of endogenous OPN expression in the normal rat brain was measured by Western blot at the age of day 0, 4, 7, 11, 14, and 21 (Figure 1A). The level of OPN in the brain was the highest at the age of day 0, and then significantly decreased by 29.1% since the age of day 4, with continuous reduction till the age of day 21 during development, which was 96.7% less than the level at day 0. Next, we evaluated the changes of endogenous OPN expression in the brain following HI injury in the 7-day-old rat pups (Figure 1B). Endogenous OPN expression in the ipsilateral hemisphere started to significantly increase from 3h post HI, and gradually peaked at 48h after HI injury (730.5 ± 86.4, P=0.009 vs. 0h-ipsilater hemisphere, ANOVA). OPN expression started to decrease 4 days post-HI compared with 48h post-HI, however, the level of OPN in the ipsilateral hemisphere is still significantly higher than in the contralateral hemisphere 7 days after HI injury.
Figure 1.
Endogenous OPN Expression. (A) Endogenous OPN expression in the normal rat brain at age of day 0, 4, 7, 11, 14, and 21. The level of OPN was significantly decreased during development (*P<0.05, vs. day 0). N=4 in each time point. (B) Endogenous OPN expression in the brain was significantly increased and peaked at 48h following HI injury (*P<0.001, vs. contralateral hemisphere). N= 4 in each time point.
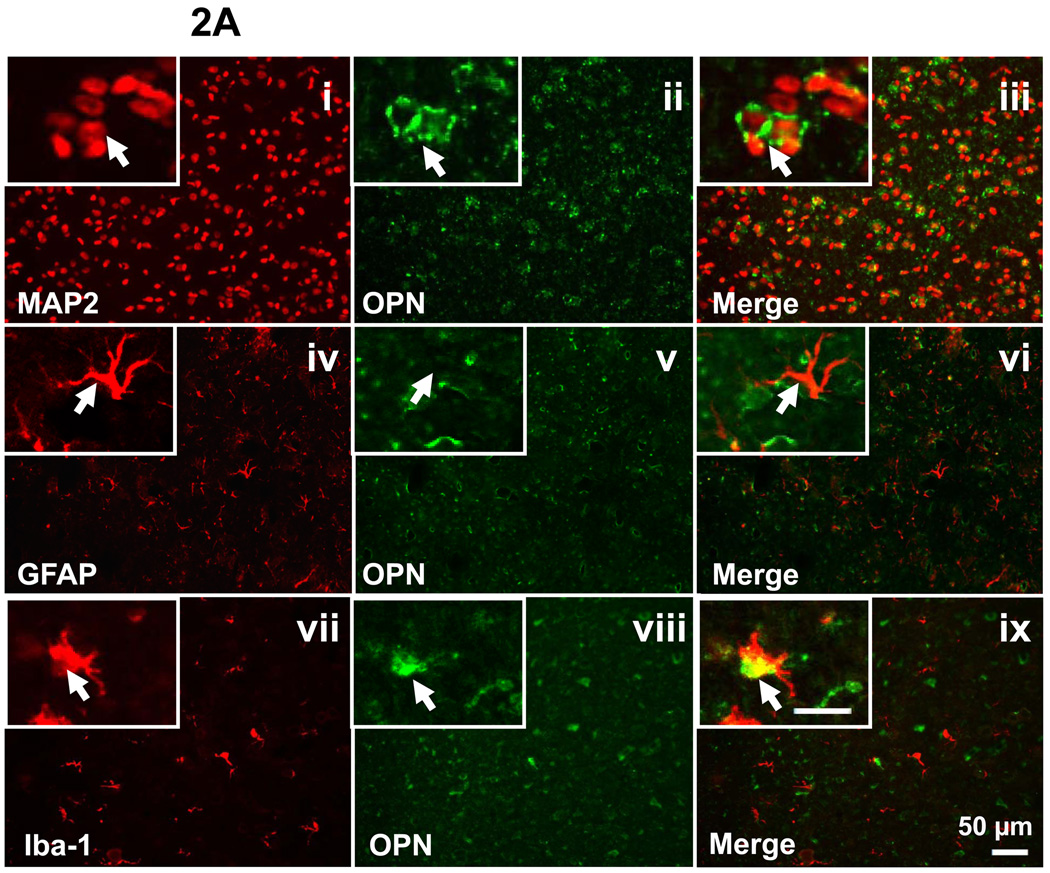
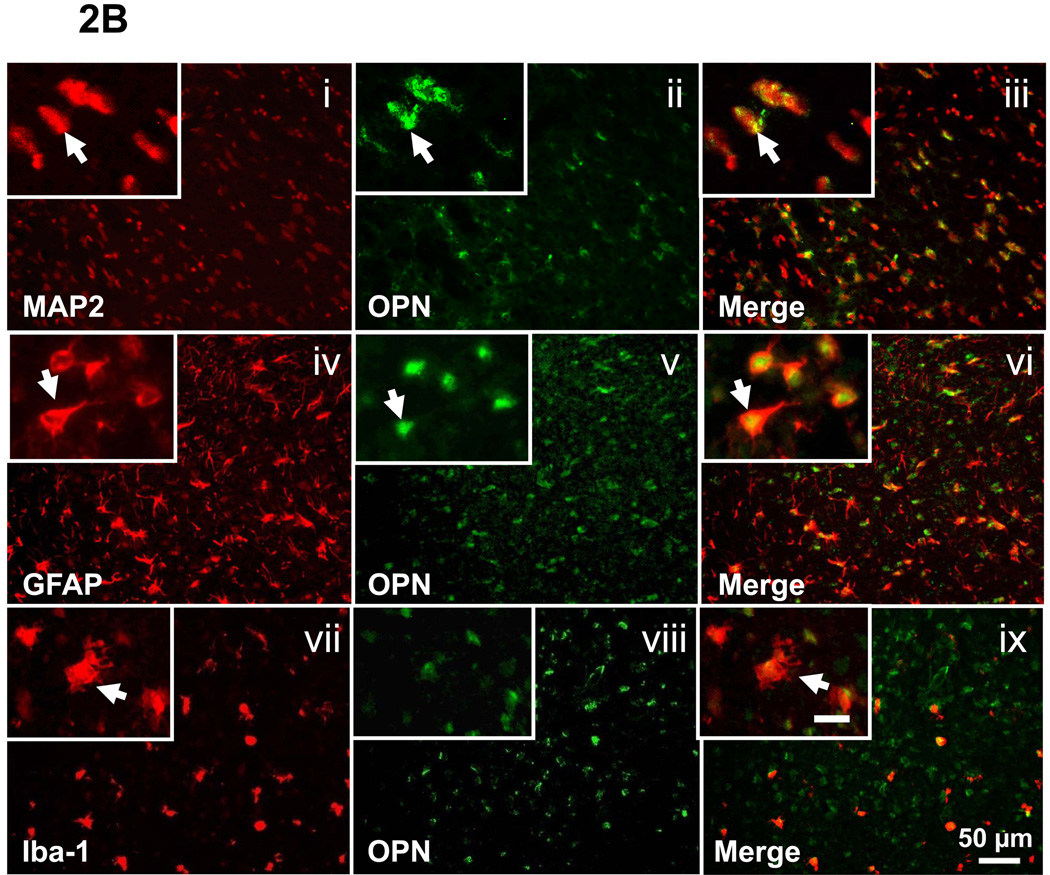
To determine the cell-type and sources of OPN expression, double staining for GFAP/OPN, MAP2/OPN, and Iba-1/OPN was performed on the brain section of P7 sham-operated animals and the ipsilateral brain section at 48h after HI injury. Sham group showed positive expression of OPN in the neurons and macrophage (Figure 2A, iii and ix), but negative expression in the astrocytes (Figure 2A, vi). However, in the frontal cortex region of the ipsilateral hemisphere at 48h after HI injury, the OPN expression was co-localized with neurons, astrocytes, and macrophage (Figure 2B, iii, vi, and ix).
Figure 2.
Endogenous OPN expression in P7 sham-operated group (A) and at 48h following HI (B). Double fluorescent labeling for OPN with MAP2 (i-iii), GFAP (iv-vi) and Iba-1 (vii-ix) in the cortex of the ipsilateral hemisphere is shown (scale bar represents 50 µm). In the brain of sham-operated P7 rats, OPN expression was co-localized with the neurons and macrophage (2A, iii and ix, as indicated by arrowhead), but not with the astrocytes (2A, vi, as indicated by arrowhead). At 48h after HI, OPN expression is co-localized with neurons, astrocytes, and macrophage (2B, iii, vi, and ix, as indicated by arrowhead). Insets in the left corner of i-ix are higher magnification (scale bar represents 20µm).
Effect of OPN on Body Weight and Infarction Volume
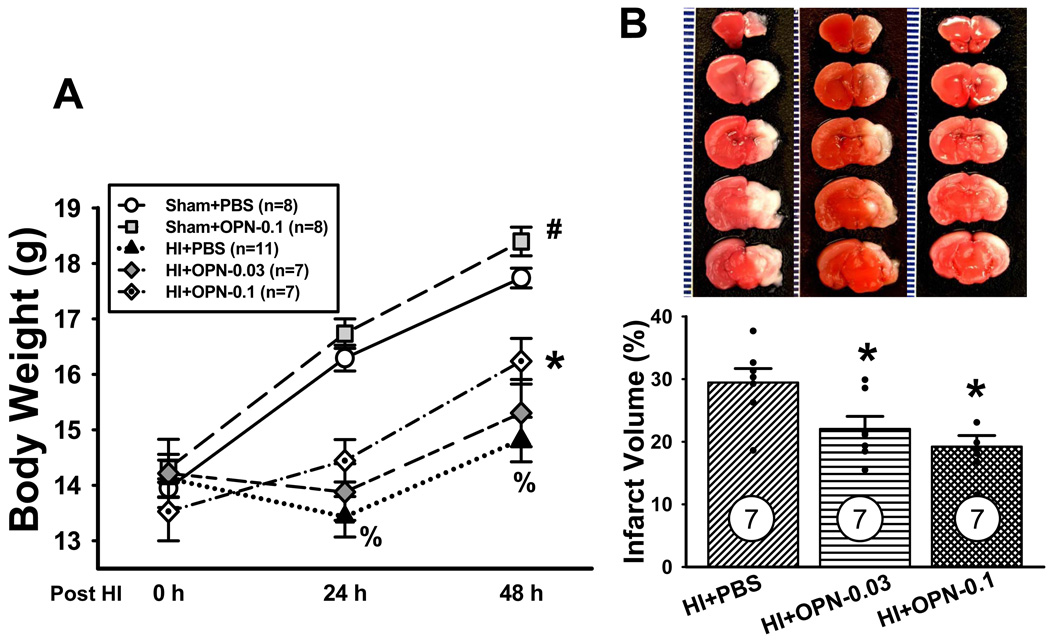
Body weight of rat pups was monitored after HI injury as an indicator of their general health. Pups body weight was significantly reduced at both 24 and 48h post HI compared with sham+PBS group (%P<0.001, ANOVA, Figure 3A). OPN treatment with high dosage (0.1µg) significantly improved the rat pups body weight at 48h after HI injury (*P=0.01 vs. OPN+PBS, ANOVA, Figure 3A). Moreover, there is a significantly increase of body weight in OPN-treated sham group (0.1µg) compared with the sham+PBS group at 48h post HI (#P<0.001 vs. OPN+PBS, ANOVA, Figure 3A).
Figure 3.
Exorgenous OPN is neuroprotective against HI. (A) The body weight of rat pups was significantly improved after OPN (0.1µg) treatment 48h post HI (*P<0.05, vs. HI+PBS). Meanwhile, OPN (0.1µg) treatment significantly increased the body weight in the sham animal (#P<0.05, vs. sham+PBS). Vertical bars indicate SEM. (B) Reduction of infarct volume in OPN treated group at 48h post HI. Representative TTC stained coronal brain sections from HI and treatment groups with different dosages of OPN are shown. The scale is shown on the left side of each TTC-stained brain with 1mm being the shortest interval. Quantitative analysis revealed that OPN treatment significantly reduced infarct volume (*P<0.05, vs. HI+PBS). N=7 in each group. Vertical bars indicate SEM.
OPN treatment resulted in a significantly decrease in brain infarct volume 48h after HI injury. Quantitative assessment of TTC-stained sections indicated that using both low and high dosage of OPN: 0.03µg (22±2.0%) and 1µg (19.2±1.8%) significantly reduced infarct volume as compared with the vehicle-treated group (29.4±2.2%) (*P<0.05, mean±SEM, Figure 3B). There was no difference between low and high dose OPN treatment.
Long term effects of OPN treatment
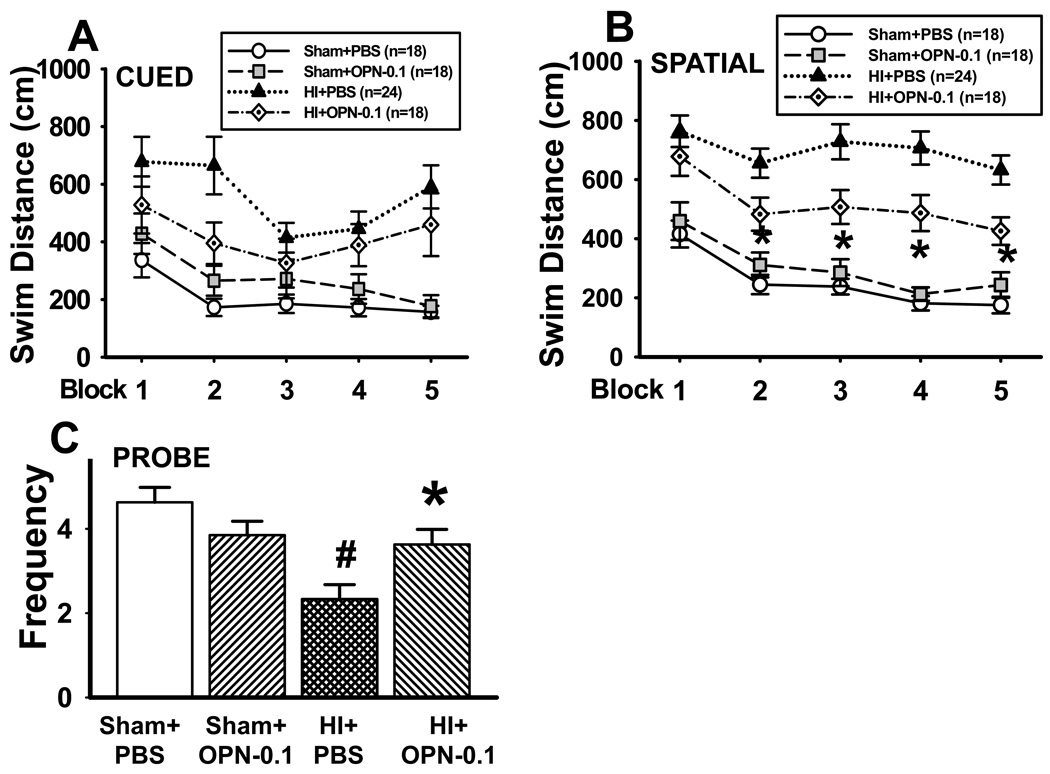
First, OPN improves functional recovery 7 weeks after neonatal HI injury. Morris water maze test was performed at 7 weeks post HI. The swimming distance (from releasing point to reach the platform on the cued and spatial maze) is shown in Figure 4A and 4B. There is no significant difference in swimming distance among all the groups on the cued maze test (Figure 4A, P>0.05, repeated measures ANOVA). However, evaluated by spatial maze test, the swimming distance in the HI+PBS group was significantly increased, whereas OPN treatment (0.1µg) significantly reduced the swimming distance (Figure 4B, *P<0.001, ANOVA). There is no significant difference of the swimming distance between the sham+PBS and sham+OPN group (Figure 4B, P>0.05, repeated measures ANOVA).
Figure 4.
Morris water maze test at 7 weeks after HI. (A) Cued maze test. The swimming distance was not significantly different in any of the groups. (B) Hidden maze test. The swimming distance in the treatment group was significantly shorter than the HI group (*P<0.05). (C) Probe trials for platform crossing frequency. There was a significantly improved performance in OPN-treated group compared with HI group (*P<0.05, vs. HI+PBS). Vertical bars indicate SEM.
The probe trial was carried following the spatial maze training. The frequency of crossing target quadrant was recorded. Animals in sham+PBS and sham+OPN group had about 4 times crossing frequency, and there is no significant difference between the two groups (Figure 4C). The crossing frequency was reduced to about 2 times in the HI group (HI+PBS vs. sham+PBS = 2.3 ± 0.3 vs. 4.6 ± 0.4, #P<0.05, ANOVA), and OPN treatment significantly recovered the crossing frequency (3.6 ± 0.4 *P<0.05, ANOVA).
The brain atrophy was measured after water maze test as previously described.11 There is a trend of the recovery of brain tissue loss after OPN treatment; however, it is not significantly different between HI group and OPN-treated group (data not shown).
Caspase-3 cleavage inhibition is involved in OPN-Induced neuroprotection
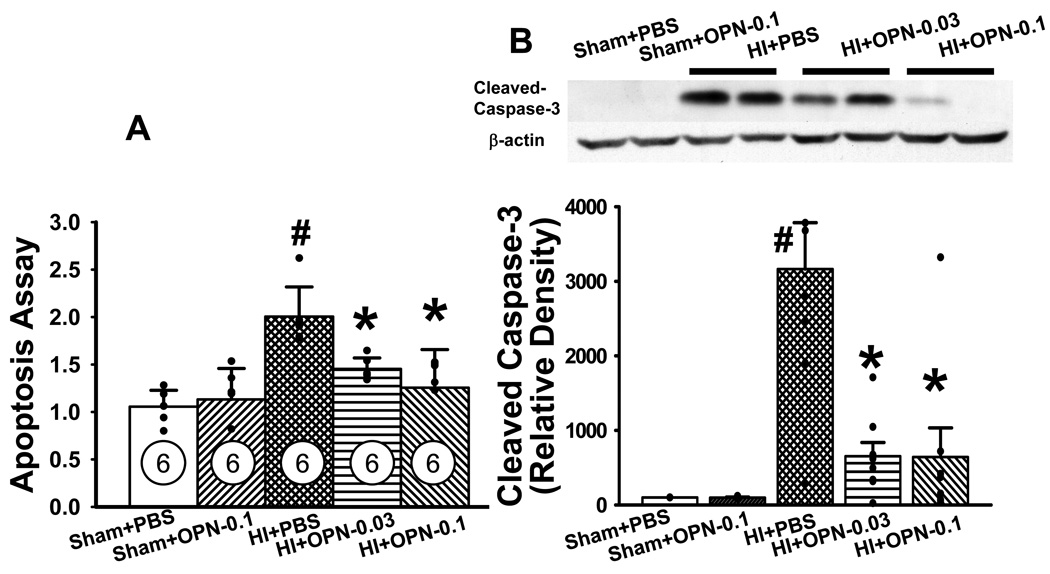
Significantly higher apoptotic cell death was detected at 24h in the HI+PBS group compared to both sham and sham+OPN (0.1µg) group (#P<0.05 vs. sham+PBS and sham+OPN, respectively, ANOVA, Figure 5A). It was significantly attenuated by both low-dose and high-dose OPN treatment (*P<0.01, vs. HI+PBS). Apoptotic marker, cleaved caspase-3 expression was evaluated by Western blotting analyses. The level of pro-caspase-3 was not significantly different between all the groups (data not shown). Cleaved caspase-3 (17kD) expression was dramatically increased in the vehicle-treated group compared with both sham and sham+OPN (0.1µg) group, respectively (#P<0.05, ANOVA, Figure 5B). It was significantly reversed by both low-dose and high-dose OPN treatment (*P<0.01, vs. HI+PBS, Figure 5B). OPN treatment didn’t change the level of 29 kD caspase-3 expression compared with the HI group (data not shown).
Figure 5.
OPN inhibits apoptotic cell death via caspase-3 dependent pathway. Significantly increased apoptotic cell death (A) and caspase-3 activation (B) was observed in HI+PBS group compared to both sham and sham+OPN-0.1µg group at 24h after HI. Both high dose (0.1µg) and low dose (0.03µg) OPN significantly decreased apoptotic cell death (A) and reduced caspase-3 activation (B). #P<0.05 vs. Sham+PBS and Sham+OPN-0.1 group, respectively; *P<0.05 vs. HI+PBS group; n=6 in each group.
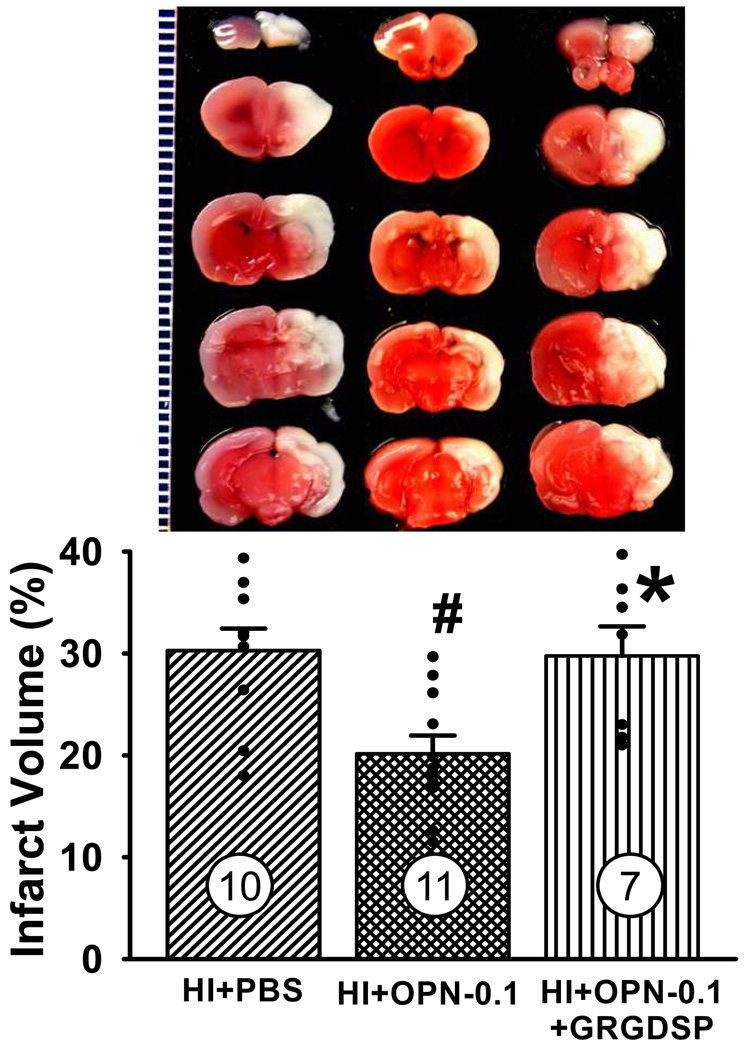
OPN-induced neuroprotection is blocked by an integrin antagonist
To determine whether OPN-induced neuroprotection following neonatal HI brain injury involves an interaction with an integrin receptor, a RGD-containing peptide which binds to various integrin receptors was used. Quantitative analysis of TTC-stained sections indicated that GRGDSP peptide blocked OPN-induced neuroprotection at 48h post HI (*P<0.01, vs. HI+OPN-0.1, Figure 6). The infarct volume in the GRGDSP treated group has no significant difference compared with HI+PBS group (29.8±2.9 vs. 30.3±2.2, P>0.05, ANOVA). It suggests that integrin receptor is involved in OPN-induced neuroprotection.
Figure 6.
OPN-induced neuroprotection is blocked by integrin receptor analog. Rat pups were treated with GRGDSP (5 µmol/L, 1µl) 15 minutes before OPN treatment. TTC staining at 48h post HI revealed that OPN-induced neuroprotection is blocked by integrin receptor analog treatment (*P<0.05, vs. HI+OPN-0.1). Vertical bars indicate SEM. N=10 in HI+PBS group, n=11 in HI+OPN-0.1 group, n=7 in HI+OPN-0.1+GRGDSP group.
Discussion
Neonatal brain injury is an important cause of mortality and long-term morbidity. To date, no pharmacologic or therapeutic interventions have been proven effective in improving neurologic outcome of infants.15 In this study, we have investigated the potential neuroprotection of OPN in the developing brain following HI injury. Intracerebroventricular administration of OPN significantly reduced infarct volume, ameliorated body weight loss, and improved long term neurological impairment. OPN reduced apoptotic cell death and cleaved caspase-3 activity, possibly by activation of integrin receptors.
We firstly investigated the level of endogenous OPN in the brain at different developmental stage, and its expression in response to HI. The level of OPN was highest at day 0 and decreased dramatically in the normal brain during maturation. After HI, an up-regulation of OPN started from 3h post HI and persists till 7 days after HI injury. Our data are consistent with published results in adult animals that elevated endogenous OPN expression has been observed in various cell types, in response to inflammation, growth factor, and ischemia etc.16 In acute ischemia, OPN is neuroprotective8,9, and OPN has also been implicated in tissue remodeling processes such as angiogenesis, endothelial cell migration, and VEGF up-regulation.17,18 The fast and prolonged OPN upregulation in response to HI injury as observed in the present study in neurons, astrocytes, and macroglial cells may indicate that OPN plays a role at both acute and delayed stage following HI brain injury. More interestingly, the role of endogenous OPN in the astrocytes post HI needs to be examined in the future, since there is no OPN expression in the astrocytes in the normal neonatal brain.
The neuroprotection of OPN administration has been suggested in the adult rat after focal ischemic brain injury.8,19 Compared with the adult, the primary mechanisms of neuronal cell death after injury are different in the immature brain. The adult brain usually demonstrates necrotic cell death with early cytoplasmic and organelle swelling when responds to most acute insults such as HI and excitoxicity.20 However, the normal developing brain retains a part of the programmed cell death for natural neuronal elimination, for instance, during the development of the vertebrate nervous system, up to 50% of many types of developing neurons are eliminated by apoptosis.21 The apoptotic machinery is more easily engaged and activated in response to injury in the immature brain is one of the reasons why the immature brain is more sensitive to HI injury.3 Previous studies demonstrated that OPN promotes cell survival through Akt activation,6,8 which might be associated with integrin-linked kinase and P13K pathway. The effects of OPN silence were accompanied by the activation of mitochondria-related apoptosis pathway involving cytochrome c, cleaved caspase-3 and Bcl-2/Bax.23 In accordance with previous data, our results suggest that apoptotic cell death was significantly reduced after OPN administration following neonatal HI injury. Moreover, caspase-3 cleavage was significantly blocked in the OPN treated group. Other than anti-apoptotic effects, OPN was shown to prevent cytotoxicity via inhibition of MMP-9 and NF-kB activity, or nitric oxide synthesis.5,12 OPN is also involved in cell migration via CD44 signaling and FAK/Src kinase induction.22 The other biological function of OPN against neonatal HI needs to be tested in the future.
We also showed that OPN treatment in the developing brain improved long-term neurological function at 7 weeks after HI injury, although we didn’t see the improvement of the brain atrophy. The performance of the Water maze test was determined by both motor and cognitive function. The swimming distance in cued maze test didn’t show any differences among all the groups. Analysis of the swimming distance on hidden maze test showed that the performance by rats in HI group was significantly worse compare to sham+PBS and sham+OPN groups, which could be associated with the motor deficit after HI injury. OPN treatment significantly reduced the swimming distance on hidden maze test. However, repeated measures analysis showed no difference among all the groups, which suggested that the learning curve is not different among all the groups. In addition, all the behavioral tests have no difference between sham+PBS and sham+OPN group, which implies that the OPN treatment in the developing brain had no significant effects on the normal developmental process. However, it is interesting to notice that the body weight of OPN-treated sham group was significantly higher than vehicle-treated sham group at 48h post-HI. The related mechanism is not clear and will need to explore in the future.
The OPN signaling pathway might be depended on specific receptors. OPN can act on neurons, astrocytes, endothelial cells, and macrophages via integrin and CD44 receptors and trigger various signal transduction pathways.22 Our data suggested that the effect of OPN in the developing brain might be through integrin receptors because an integrin antagonist attenuated the neuroprotective effect of OPN. However, whether CD44 receptors are involved in OPN-induced neuroprotection following neonatal HI needs to be further investigated.
Supplementary Material
Acknowledgments
This study was partially supported by grants from the NIH HD43120, NS43338, and NS54685 to J. H. Z, and NIH 5R01NS60936-2 to J.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: Stroke [44] Acute Cerebral Infarction Stroke Treatment - Medical [72] Neuroprotectors
Conflicts of interest disclosures: none
References
- 1.Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.Pulera MR, Adams LM, Liu H, Santos DG, Nishimura RN, Yang F, Cole GM, Wasterlain CG. Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke. 1998;29:2622–2630. doi: 10.1161/01.str.29.12.2622. [DOI] [PubMed] [Google Scholar]
- 3.Sidhu RS, Tuor UI, Del Bigio MR. Nuclear condensation and fragmentation following cerebral hypoxia-ischemia occurs more frequently in immature than older rats. Neurosci Lett. 1997;223:129–132. doi: 10.1016/s0304-3940(97)13426-7. [DOI] [PubMed] [Google Scholar]
- 4.Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 2003;34:207–213. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin--a molecule for all seasons. QJM. 2002;95:3–13. doi: 10.1093/qjmed/95.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Song G, Cai QF, Mao YB, Ming YL, Bao SD, Ouyang GL. Osteopontin promotes ovarian cancer progression and cell survival and increases HIF-1alpha expression through the PI3-K/Akt pathway. Cancer Sci. 2008;99:1901–1907. doi: 10.1111/j.1349-7006.2008.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Zhang XF, Zhou HJ, Xue YH, Dong QZ, Ye QH, Qin LX. Expression and prognostic significance of osteopontin and caspase-3 in hepatocellular carcinoma patients after curative resection. Cancer Sci. 2010;101:1314–1319. doi: 10.1111/j.1349-7006.2010.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meller R, Stevens SL, Minami M, Cameron JA, King S, Rosenzweig H, Doyle K, Lessov NS, Simon RP, Stenzel-Poore MP. Neuroprotection by osteopontin in stroke. J Cereb Blood Flow Metab. 2005;25:217–225. doi: 10.1038/sj.jcbfm.9600022. [DOI] [PubMed] [Google Scholar]
- 9.Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int. 2003;63:543–553. doi: 10.1046/j.1523-1755.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- 10.Rice JE, III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Hartman R, Ayer R, Marcantonio S, Kamper J, Tang J, Zhang JH. Matrix metalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in the developing brain. J Neurochem. 2009;111:726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Ayer R, Sugawara T, Chen W, Sozen T, Hasegawa Y, Kanamaru K, Zhang JH. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2010;38:612–618. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- 14.Sugawara T, Jadhav V, Ayer R, Chen W, Suzuki H, Zhang JH. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 2009;40:1530–1532. doi: 10.1161/STROKEAHA.108.531699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez FF, Ferriero DM. Therapeutics for neonatal brain injury. Pharmacol Ther. 2008;120:43–53. doi: 10.1016/j.pharmthera.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23:123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- 17.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 18.Takano S, Tsuboi K, Tomono Y, Mitsui Y, Nose T. Tissue factor, osteopontin, alphavbeta3 integrin expression in microvasculature of gliomas associated with vascular endothelial growth factor expression. Br J Cancer. 2000;82:1967–1973. doi: 10.1054/bjoc.2000.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vosler PS, Chen J. Potential molecular targets for translational stroke research. Stroke. 2009;40:S119–S120. doi: 10.1161/STROKEAHA.108.533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 21.Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 22.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang A, Liu Y, Shen Y, Xu Y, Li X. Osteopontin silencing by small interfering RNA induces apoptosis and suppresses invasion in human renal carcinoma Caki-1 cells. Med Oncol. 2009 doi: 10.1007/s12032-009-9356-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.