Abstract
There are great interest and demand for the development of vaccines to prevent and treat diverse microbial infections. Mucosal vaccines elicit immune protection by stimulating the production of antibodies at mucosal surfaces and systemic districts. Being positioned in close proximity to a large community of commensal microbes, the mucosal immune system deploys a heterogeneous population of cells and a complex regulatory network to maintain the balance between surveillance and tolerance. A successful mucosal vaccine relies on leveraging the functions of these immune cells and regulatory components. We review the important cellular interactions and molecular pathways underlying the induction and regulation of mucosal antibody responses and discuss their implications on mucosal vaccination.
Main Text
Introduction
Infections caused by many old, emerging, and re-emerging pathogens, such as Mycobacterium tuberculosis, Vibrio cholerae, Aspergillus fumigatus, helminths, coxsackie virus, influenza viruses, rhinovirus, severe acute respiratory syndrome (SARS) coronavirus, and human immunodeficiency virus (HIV), pose severe health problems to the world's population and great challenges to the realization of Millennium Development Goals outlined in the United Nations Millennium Declaration. These health problems are particularly serious to people living in developing countries and areas of poor hygiene and people with conditions causing compromised immunity. Although effective antibiotics are available against most of those bacterial, fungal, and parasitic pathogens, these drugs are not applicable for disease prevention and give rise to an insidious trend of drug resistance after prolonged use. In addition, there are no approved antiviral drugs highly effective against many viral pathogens, such as coxsackie virus, rhinovirus, and SARS coronavirus. Vaccination, which works by stimulating our immune system to combat infections, appears a promising and much-needed approach for the treatment and prevention of these diseases. A common feature of infections caused by these diverse types of pathogens is that they usually occur or initiate at a mucosal surface. While ample evidence exists that systemic vaccination is adequate to offer protection against selected pathogens, such as polio and influenza viruses (Haan et al., 2001, Herremans et al., 1999), an increasing number of studies have shown that induction of mucosal immunity is required for effective protection against other important pathogens, such as HIV, human papillomavirus, herpes viruses, Vibrio cholera, and Mycobacterium species (Belyakov et al., 2001, Chen et al., 2004, Gallichan and Rosenthal, 1996, Neutra and Kozlowski, 2006, Wang et al., 2004). In addition to the superior ability of mucosal vaccination to induce local mucosal immune responses over systemic vaccination (Neutra and Kozlowski, 2006), mucosal vaccination also offers many logistic and additional immunological advantages over systemic vaccination. By simply ingesting or inhaling the vaccine, mucosal vaccination does not require injection and causes less pain and, thus, has a high compliance among patients of all ages. Simplified manufacturing and storage methods, as well as independence on trained medical personnel for delivery, make mucosal vaccines suitable for mass vaccination programs, especially in developing countries and during emergency. The mucosal immune system is more accessible for the induction of an immune response because all mucosal surfaces can, in principle, act as sites of antigen entry. More importantly, mucosal vaccination targets specific mucosal districts and induces “frontline immunity” at the site of pathogen entry that can prevent the establishment and dissemination of an infection. In addition, immunization at one mucosal site can result in antibody secretion systemically, as well as at other selected mucosal sites (Holmgren and Czerkinsky, 2005).
However, no more than a dozen of mucosal vaccines are currently approved for human use (Holmgren and Czerkinsky, 2005). This constitutes an embarrassing contrast to the severe health problem posed by mucosal pathogens and the many advantages of mucosal vaccination. Such a situation results largely from the numerous immunological and technological challenges confronting the quest of successful mucosal vaccines. A successful mucosal vaccine should be able to penetrate the mucosal barrier at the right mucosal district in a controlled manner and induce both innate and adaptive immune responses, such as the activation of dendritic cells (DCs), macrophages, epithelial cells (ECs) of the innate immune system, as well as antigen-specific effector and memory T and B cells of the adaptive immune system, which cooperate with one another to achieve optimal potency and duration of protection (Holmgren and Czerkinsky, 2005). To achieve this goal, adjuvants are frequently required (see Coffman et al., 2010). Improper mucosal vaccine formulations can cause poor absorption and limited bioavailability because of high rates of mucosal enzyme-mediated inactivation and mucosal clearance or may lead to the delivery of vaccines to improper mucosal districts and targeting to the wrong mucosal cell types (Holmgren and Czerkinsky, 2005). In addition, because of the delicate and dynamic immunological balance maintained at mucosal surfaces resulting from the presence of large numbers of commensal flora, a successful mucosal vaccine must simultaneously evoke a mucosal response and avoid mucosal inflammation or tolerance. Systemic vaccination can deliver a known dose of an antigen into the body and generate readily measurable humoral and cell-mediated responses, but the dose of mucosal vaccines that enters the body is difficult to be measured accurately. The efficacy of mucosal vaccination is also difficult to be determined because of the difficulties in capturing and quantitating antibodies in mucosal secretions and the technical challenges in determining the function of diverse mucosal T cell subsets (Neutra and Kozlowski, 2006).
As mentioned earlier, mucosal immunity involves an intimate interplay between the innate and adaptive immune systems through a complex web of cellular and signaling networks. Thus, a vaccine is expected to achieve optimal responses if it leverages the interaction between innate and adaptive components of the mucosal immune system. This article reviews our current knowledge of the cellular and molecular pathways underlying mucosal antibody responses and mucosal homeostasis and discusses the implications of such knowledge on the design of effective mucosal vaccines.
General Architecture of the Mucosal Immune System
The MALT (mucosa-associated lymphoid tissue) includes the nasopharynx-associated lymphoid tissue (NALT), the bronchus-associated lymphoid tissue (BALT), and the gut-associated lymphoid tissue (GALT), which comprises Peyer's patches (PPs) and isolated lymphoid follicles (ILFs) (Kunisawa et al., 2008). Humans generally do not have an anatomically well-defined NALT, except at an early age, but possess oropharyngeal lymphoid tissues such as pharyngeal, tubal, palatine, and lingual tonsils (Kunisawa et al., 2008). This set of lymphatic tissues is called Waldeyer's ring and may constitute the human equivalent of mouse NALT (Kiyono and Fukuyama, 2004, Kunisawa et al., 2008). Conjunctiva-associated lymphoid tissue (CALT), lacrimal duct-associated lymphoid tissue (LDALT), larynx-associated lymphoid tissue (LALT), and salivary duct-associated lymphoid tissue (SDALT) have also been described in humans (Gebert and Pabst, 1999). All these segments of the MALT comprise anatomically and functionally distinct inductive and effector sites. Inductive sites include mucosa-associated follicles, such as intestinal PPs, IFLs, and mesenteric lymph nodes, where antigen-specific T and B cells undergo activation, clonal expansion, and differentiation into T and B effector cells. These cells migrate from inductive sites to effector sites to carry out their effector functions. Effector sites are present in all mucosal districts as a nonorganized lymphoid tissue diffusely distributed throughout the lamina propria (LP) (Kiyono and Fukuyama, 2004, Kunisawa et al., 2008). Here, cytotoxic T lymphocytes (CTLs) lyse infected cells, and B cells differentiate into plasma cells that secrete large amounts of immunoglobulin A (IgA), the predominant antibody isotype in intestinal secretions together with IgM. Respiratory and urogenital secretions also contain IgG and IgD, two antibody isotypes with less defined mucosal functions. IgA and IgM are transported across ECs by polymeric Ig receptor (pIgR), whereas IgG is transported across ECs by neonatal Fc receptor (Rojas and Apodaca, 2002). The antibody transporter utilized by IgD is unknown (Chen and Cerutti, 2010).
Cells of the Mucosal Immune System
MALT has a general immune architecture resembling that of systemic lymphoid tissues, including presence of B cell-rich follicles and T cell-rich interfollicular areas. However, MALT also has several unique anatomic and cellular features. Indeed, GALT and NALT are provided with efferent, but not afferent, lymphatics and, therefore, sample exogenous antigens directly from the mucosal surface (Brandtzaeg et al., 1999). In addition, GALT and NALT harbor highly heterogeneous populations of immune cells that establish an intimate interaction with ECs. In this regard, PPs are capped by a follicle-associated epithelium equipped with microfold or membranous cells (M cells) specialized in antigen sampling. Similar M cells are present in the crypts of tonsils and adenoids. Owing to their short microvilli, thin mucus layer, abundant cytoplasmic vesicles, and efficient transcytosis activity, M cells effectively take up lumenal antigens and then transfer them to neighboring antigen-presenting cells (APCs) (Neutra et al., 2001). In general, the MALT contains a complex network of APCs that differentially modulate mucosal immune responses. These APCs include multiple DC subsets that populate specific regions of the MALT and perform distinct functions (Helft et al., 2010, Iwasaki, 2007a, Iwasaki, 2007b, Pulendran, 2006, Varol et al., 2010; for review, see Palucka et al., 2010). DCs expressing the C-X3-C chemokine receptor 1 (CX3CR1) are involved in the processing and presentation of antigens to initiate adaptive T cell responses (Varol et al., 2009), whereas DCs expressing the αEβ7 integrin CD103 condition local CD4+ T cells to generate T regulatory (Treg) cells, a CD4+ T cell subset that is pivotal to maintain mucosal immune tolerance and homeostasis (Coombes et al., 2007, Jaensson et al., 2008). The phenotype of NALT DCs has not been clearly determined. Human tonsillar DCs comprise a heterogeneous group of immature DCs in the reticular epithelium of the crypts and mature DCs in interfollicular regions (Dieu et al., 1998, Xu et al., 2007, Xu et al., 2008), but the functional differences among these DC subsets remain to be investigated. In addition to DCs, the MALT contains many other cell types with potential antigen-presenting and regulatory functions, such as macrophages (Denning et al., 2007, Smythies et al., 2005). The roles of these cells in the regulation of mucosal antibody production and immune homeostasis are just beginning to be understood.
Functional Connectivity of the Mucosal Immune System
The mucosal immune system can act independently of the systemic immune system (Kiyono and Fukuyama, 2004). Although anatomically separated, different regions of the MALT are functionally connected in what has been termed the “common mucosal immune system,” which permits T cells and B cells activated by antigen at one specific mucosal site to appear as effector T cells and B cells in distant mucosal sites (Kiyono and Fukuyama, 2004). This functional connectivity is achieved through the induction of specific sets of mucosal homing receptors during the interaction on T and B cells with mucosal DCs. For example, oral and intranasal immunization can stimulate effector T and B cell responses in distant mucosal tissues such as the intestinal and urogenital tracts (Kiyono et al., 2008). In spite of this functional connectivity, NALT-targeted immunization preferentially induces antigen-specific immunity in respiratory and reproductive tissues, whereas GALT-targeted immunization predominantly elicits protective responses in gastrointestinal tissues. Further support for a compartmentalized common mucosal immune system derives from evidence indicating that nasal immunization induces IgA-producing B cells to express C-C chemokine receptor 10 (CCR10) and α4β1 integrin. Binding of CCR10 and α4β1 to C-C chemokine ligand 28 (CCL28) and vascular cell adhesion molecule-1 (VCAM-1), respectively, mediates trafficking of IgA-producing B cells to the respiratory and genitourinary tracts (Kunkel et al., 2003, Lazarus et al., 2003). By contrast, oral immunization stimulates IgA-producing B cells to express CCR9 and CCR10 chemokine receptors, as well as α4β7 and α4β1 integrins (Kunkel et al., 2003, Lazarus et al., 2003). Binding of CCR9, CCR10, α4β7, and α4β1 to CCL25, CCL28, mucosal vascular addressin cell adhesion molecule-1 (MAdCAM-1), and VCAM-1, respectively, permits IgA-producing B cells to home to the small intestine (Kunkel and Butcher, 2003, Mora et al., 2006, Mora et al., 2008). These examples highlight the existence of subtle differences in the “common mucosal immune system” that may result from the exposure of anatomically distinct mucosal sites to different populations of microorganisms.
Complexity of Mucosal Antibody Responses
It is important for vaccine developers to realize that regions of the mucosal immune system as different as PPs and LP have enormous potential to complement the function of one another in the development of mucosal antibody responses and that although IgA constitutes the predominant class of mucosal antibodies, multiple layers of antibody responses comprising several antibody isotypes are in place to ensure frontline surveillance at mucosal surfaces. This is reflected by the marked increase of IgM- and IgG-secreting B cells in the GALT and IgD-, IgG-, and IgM-secreting B cells in the NALT from subjects with selective IgA deficiency (Brandtzaeg et al., 1999), which may explain the frequently asymptomatic nature of this primary immunodeficiency in both humans (Cunningham-Rundles, 2001) and mice (Mbawuike et al., 1999). It must be also noted that humans have two IgA1 and IgA2 isotypes with distinct regulation, function, and anatomic distribution, while mice have only one IgA isotype (Cerutti, 2008). Underlying these multiple layers of antibody responses are the collaborative mechanisms of follicular and extrafollicular machineries involving T cell-dependent and T cell-independent pathways of mucosal B cell activation (Chen et al., 2009, Cong et al., 2009, Fagarasan et al., 2010, He et al., 2007, Tsuji et al., 2008, Tsuji et al., 2009). This implies that a mucosal vaccine will be more efficient if a response can be initiated by synergistically targeting multiple components of these complex B cell-activating pathways.
Mechanisms Underlying Mucosal IgA Responses
Mature B cells acquire IgA expression by undergoing a deletional DNA recombination event called class switch recombination (CSR) that takes place on the chromosome containing the recombined Ig heavy (H) chain locus (Cerutti, 2008). In mature B cells, the IgH locus includes a recombined VHDJH exon, which encodes the antigen-binding variable region of an antibody, and multiple sets of constant H chain (CH) exons, which encode the Cμ, Cδ, Cγ, Cα, or Cɛ region of an antibody (Stavnezer et al., 2008). Each of these CH regions accounts for the effector functions of an antibody, and the Cα region is critical for the transportation of antibodies across ECs (Cerutti and Rescigno, 2008). CSR from IgM to IgA involves an exchange of the upstream donor Cμ gene with the downstream acceptor Cα gene and the deletion of the intervening CH genes (Cerutti, 2008). CSR is guided by a promoter positioned upstream of a genetic unit comprising a short intronic (I) exon, a switch (S) region, and a CH gene (Stavnezer et al., 2008). The selectivity of IgA CSR is achieved by specific cytokine signals that initiate transcription at the Iα promoter. Germline transcription allows the CSR machinery (Stavnezer et al., 2008), which includes the DNA-editing enzyme activation-induced cytidine deaminase (AID), to target the Sμ and Sα regions to introduce double-strand DNA breaks (Chaudhuri and Alt, 2004, Muramatsu et al., 2000). Subsequent joining of these breaks by DNA repair machineries results in intrachromosomal deletion of the intervening Sμ-Sα sequence and juxtaposition of the downstream Cα gene with the upstream VHDJH exon encoding the antigen-binding V region to allow the expression of a full-length IgA. AID also mediates affinity maturation by introducing high-rate somatic point mutations into the VDJ exons to produce a high-affinity IgA antibody (Honjo et al., 2002, Muramatsu et al., 2000). IgA class-switched B cells can further differentiate into plasmablasts or plasma cells and secrete IgA in the appropriate cytokine environment (Cerutti and Rescigno, 2008, Macpherson et al., 2008). Understanding the signals that regulate IgA CSR and production is critical for the design of effective vaccines to boost mucosal IgA responses.
Follicular Pathways for Mucosal IgA Production
In the GALT, antibody responses are strongly biased toward IgA and involve activation of follicular B cells by antigen and CD4+ T cells expressing CD40 ligand (CD40L) (Macpherson et al., 2008). Most of IgA-producing B cells emerge from the germinal center (GC) of PPs and mesenteric lymph nodes, because these organized lymphoid structures contain a cellular composition and cytokine environment conducive to IgA CSR and production (Figure 1 ). In particular, PPs have a B:T cell ratio four to six times higher than that of peripheral lymph nodes (Stevens et al., 1982). Engagement of CD40 on B cells by CD40L on CD4+ T cells recruits tumor necrosis factor receptor-associated factor (TRAF) proteins that activate the nuclear factor-κB (NF-κB) pathway and induce expression of AID, an essential requirement for IgA CSR (Cerutti, 2008). The second seemingly essential requirement for IgA CSR in PPs is transforming growth factor-β1 (TGF-β1), a cytokine produced by Foxp3+ Treg cells, CXCR5+ T follicular helper (Tfh) cells, interleukin-10 (IL-10)-producing T regulatory-1 (Tr1) cells, DCs, stromal cells, B cells, and perhaps also ECs (Barnes and Powrie, 2009, Cong et al., 2009, Fagarasan et al., 2001, Fillatreau et al., 2008, Maynard et al., 2007, Strober, 2009, Tsuji et al., 2009). TGF-β1 plays a pivotal role in promoting IgA CSR by inducing nuclear translocation of mothers against decapentaplegic (SMAD) proteins, runt-related transcription factor 3 (RUNX3), and cyclic AMP response element binding protein (CREB), which cooperatively initiate germline Cα gene transcription (Cerutti, 2008). Mice with a B cell-specific deficiency of TGF-β receptor type II and its downstream signaling proteins show profound deficiency of steady-state and antigen-induced IgA both systemically and in the GALT (Cazac and Roes, 2000). PPs also contain other cytokines, such as IL-2, IL-4, IL-5, IL-6, and IL-10, which promote the expansion of IgA class-switched B cells and their differentiation into IgA-secreting plasma cells (Cerutti, 2008). In addition to PPs, ILFs constitute another inductive site for IgA CSR and production (Fagarasan and Honjo, 2003, Fagarasan et al., 2010). The contribution of ILFs to the generation of IgA was demonstrated using retinoic acid-related orphan receptor-γt (RORγt, encoded by the Rora gene)-deficient mice, which do not have PPs and ILFs because of the instrumental role of RORγt+ lymphoid tissue inducer (LTi) cells in the induction of these structures (Tsuji et al., 2008). Adoptive transfer to RORγt+ LTi cells into Rora −/− mice resulted in the formation of ILFs, but not PPs, and the appearance of many IgA+ B cells in these ILFs (Tsuji et al., 2008). Interestingly, activation and IgA CSR in ILFs does not require help to B cells from CD4+ T cells (Tsuji et al., 2008). However, ILFs probably contribute only to steady-state IgA production to commensal flora but have a marginal role in antigen-specific IgA production in response to mucosal immunization, as mice treated in utero with soluble TNF decoy receptor and soluble lymphotoxin β (LTβ) decoy receptor, two compounds that abrogate the formation of PPs and mesenteric lymph nodes, but not that of ILFs, failed to develop antigen-specific IgA responses after oral immunization despite having unaltered intestinal IgA antibodies (Yamamoto et al., 2004). Interestingly, B cells from PPs can undergo IgA CSR and antigen-specific IgA production without requiring the expression of a somatically recombined antigen Ig receptor (Casola et al., 2004), which is usually needed to internalize and present antigen to CD4+ T cells in the context of a cognate B-T cell interaction. Instead, B cells from PPs heavily rely on germline gene-encoded innate antigen receptors known as Toll-like receptors (TLRs) to mount IgA responses, at least in mice (Casola et al., 2004). TLRs initiate innate and adaptive immune responses after recognize highly conserved microbial molecular signatures and provide protection against mucosal infections by enhancing both IgA CSR and V(D)J somatic hypermutation (Casola and Rajewsky, 2006, Delgado et al., 2009). These findings highlight the pivotal role of PPs in oral immunization and indicate the importance of orally administered vaccines to target M cells and PPs in order to generate efficient antigen-specific IgA responses (Neutra and Kozlowski, 2006).
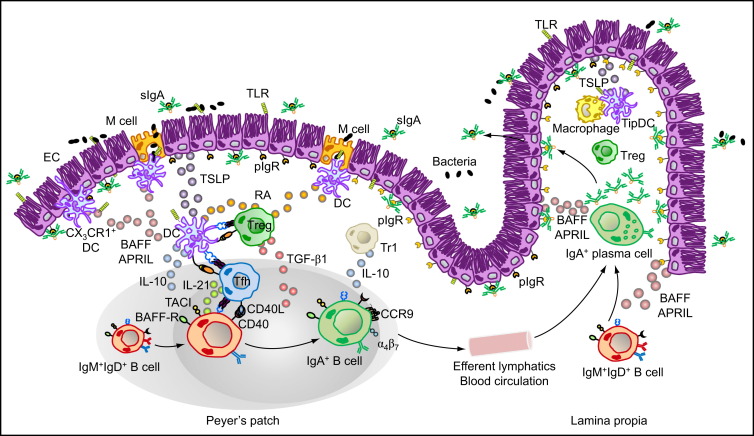
Figure 1.
Major Cellular Interactions and Regulatory Pathways Involved in IgA Responses to Intestinal Antigens
M cells from PPs sample commensal and vaccine antigens from the intestinal lumen and deliver them to subepithelial DCs. Antigen sampling is also carried out by CX3CR1+ DCs that project dendrites into the intestinal lumen across ECs. These cells release immunoregulatory (TSLP) and IgA-inducing (BAFF and APRIL) molecules upon sensing microbial signatures through TLRs and NLRs. TSLP stimulates the formation of tolerogenic DCs that suppress proinflammatory Th1 responses and induce noninflammatory Treg and Th2 responses by releasing IL-6, IL-10, TGF-β1, and RA. Treg cells may further differentiate into TFH cells, which together with Treg and perhaps Th2 cells stimulate IgA CSR and production by stimulating naive IgM+IgD+ B cells through CD40L, TGF-β1, IL-6, IL-10, and IL-21. In the presence of RA, IgA-expressing B cells emerging from mucosal germinal centers acquire expression of gut-homing receptors such as CCR9 and α4β7, which direct subsequent B cell migration to the intestinal LP through efferent lymphatics, regional mesenteric lymph nodes, and blood circulation. In the LP, IgA-expressing B cells differentiate into IgA-secreting plasma cells that secrete IgA dimers. Interaction of IgA dimers with the pIgR results into IgA transcytosis and formation of a secretory IgA (SIgA) complex that binds antigen in the intestinal lumen. The LP also contains IL-10-producing macrophages and BAFF-APRIL-nitric oxide-producing TipDCs whose development is promoted by microbial and epithelial factors such as TSLP. TipDCs and macrophages would enhance local IgA production by triggering CSR and stimulating plasma cell survival.
Following activation, CSR and upregulation of gut-homing receptors such as CCR9, α4β7 integrin, and type 1 sphingosine-1-phosphate (S1P) receptor and downregulation of follicular localization receptors such as CXCR5, IgA-producing B cells migrate from the inductive site of PPs to the effector site of the gut LP through efferent lymphatics, regional mesenteric lymph nodes, and general circulation (Mora and von Andrian, 2008, Suzuki and Fagarasan, 2009). In the LP, IgA-producing B cells further differentiate into IgA-secreting plasma cells (Figure 1).
Extrafollicular Pathways for Mucosal IgA Production
Studies in mice and humans have shown that the LP also has all the necessary cellular and molecular machineries to induce IgA CSR independently of T cells (Mora and von Andrian, 2008, Suzuki and Fagarasan, 2009). In the absence of PPs, ILFs, and mesenteric lymph nodes, intestinal IgA responses can be generated in the LP. Indeed, mice deficient in inhibitor of DNA binding 2 (Id2) or LTα, which do not have PPs, ILFs, and mesenteric lymph nodes, retain some antigen-specific IgA plasma cells in the LP (Eberl and Littman, 2004, Kang et al., 2002). In addition, protective mucosal antibody responses can be generated by intranasal immunization with an inactive influenza virus in mice deficient in CD4+ T cells (Sha et al., 2005). Furthermore, humans deficient in CD40 or advanced AIDS patients deficient in CD4+ T cells retain intestinal IgA responses in the LP (He et al., 2007, Xu et al., 2009). These findings have important implications for the development of mucosal vaccines, especially for people with a pre-existing condition of immunosuppression, such as transplant or diabetic patients and HIV-1-infected subjects.
Indeed, the LP contains IgM+ B cells that have migrated from either the bone marrow or PPs (Suzuki et al., 2005). These B cells can undergo differentiation into IgM-secreting plasma cells or serve as local precursors of IgA- and IgG-producing B cells in a CD4+ T cell- and CD40L-independent manner (Fagarasan et al., 2001). T cell-independent antigens initiate IgA CSR by activating multiple innate immune pathways. Some T cell-independent antigens, such as microbial lipopolysaccharide (LPS) and nucleic acids, activate B cells through TLRs (He et al., 2007, Pasare and Medzhitov, 2005, Peng, 2005, Xu et al., 2007, Xu et al., 2008), whereas other T cell-independent antigens, such as bacterial capsular polysaccharides (CPS), activate B cells through surface Ig receptors (Mond et al., 1995). DCs, monocytes, neutrophils, basophils, ECs, and stromal cells can release powerful IgA CSR-inducing factors, such as B cell activating factor of TNF family (BAFF) and a proliferation-inducing ligand (APRIL), in response to microbial TLR ligands (Charles et al., 2010, Chen et al., 2009, Gorelik et al., 2003, He et al., 2007, Litinskiy et al., 2002, Scapini et al., 2008, Xu et al., 2007). This places innate immune cells, epithelial cells, and stromal cells as prominent targets for mucosal vaccination. Some human IgA1 class-switched B cells can undergo sequential CSR from IgA1 to IgA2 in the LP under the influence of APRIL expressed by DCs and ECs. This process is particularly evident in the distal portions of the intestine, which may account for the greater abundance of IgA2 found there (He et al., 2007). BAFF and APRIL induce IgA and IgG CSR by activating germline Iα and Iγ transcription and AID expression through NF-κB signaling via receptor transmembrane activator and calcium modulator and cyclophylin ligand interactor (TACI) and the adaptor protein myeloid differentiation primary response gene 88 (MyD88) (He et al., 2010). When combined with microbial TLR ligands, BAFF and APRIL are sufficient to induce IgA production in both mouse and human B cells (He et al., 2007, Tsuji et al., 2008, Xu et al., 2007), suggesting the existence of an intimate interplay between TACI and TLRs. Our recent finding that TACI triggers CSR via MyD88 (He et al., 2010) and the fact that MyD88 is usually associated with TLRs raises the possibility of signaling integration between TACI and TLR pathways through MyD88 and suggests that a vaccine formulation simultaneously activating both pathways could be superior in boosting mucosal IgA responses in humans.
IgD Production in the Upper Respiratory Mucosa
In humans, one of the most prominent differences between the respiratory and intestinal mucosal surfaces lies in their immunoglobulin composition. Unlike mice, humans have a substantial percentage of IgD-producing B cells in the MALT associated with the nasal cavities, tonsils, pharynx, salivary glands, and lacrimal glands, but not in the GALT (Brandtzaeg et al., 1999, Chen et al., 2009, Chen and Cerutti, 2010). The possibility that IgD may have an important value in mucosal vaccination is supported by the observation that the percentage of IgD-producing B cells in the upper respiratory tract increases to as much as 60% of all plasmacytes in individuals with selective IgA deficiency (Brandtzaeg et al., 1999). Similar to humans, some teleosts have a subset of B cells expressing IgD, but not IgM, suggesting some evolutionarily conserved immunological advantages of IgD over IgM (Chen and Cerutti, 2010). Remarkably, IgD is the most hypermutated human antibody isotype and has a long heavy-chain complementarity-determining region 3 (CDR3) (Chen and Cerutti, 2010, Koelsch et al., 2007) that may form a protruding finger-like structure capable of targeting recessed neutralizing viral epitopes (Burton et al., 2005, Saphire et al., 2001). Moreover, IgD can bind to many bacterial virulence factors and induce B cell-stimulating and antimicrobial programs in basophils, thereby contributing to mucosal and systemic antibody production and antimicrobial defense (Chen and Cerutti, 2010, Chen et al., 2009). Similar to IgA, multiple follicular and extrafollicular pathways, as well as T cell-dependent and T cell-independent signals, including CD40L, BAFF, APRIL, as well as IL-2, IL-15, and IL-21, participate in promoting IgD CSR and production in mucosal B cells (Chen and Cerutti, 2010, Chen et al., 2009). The requirement for CD40L, BAFF, APRIL, and IL-21 in the induction of IgD highlights the importance of targeting upper aerodigestive mucosal Tfh cells, DCs, and ECs should mucosal vaccination be aimed at boosting IgD responses. The dual ability of IgD to bind antigen and amplify immune activation both systemically and at mucosal sites of entry may explain why, in some studies, the nasal route is more advantageous than other routes of vaccine administration (Holmgren and Czerkinsky, 2005, Chen and Cerutti, 2010).
Mucosal Immunity and Homeostasis: A Tale of Many Cells
It is important to understand that the mucosal immune system operates at the forefront of our body's immune battlefield in close proximity to an enormous amount of commensal flora. It deploys multiple types of immune cells organized in phenotypically and functionally distinct subsets and utilizes an intricate regulatory signaling network to reinforce mucosal antibody responses and maintain a harmonious coexistence with the local microbiota. Understanding this complex regulatory network is pivotal to the development of effective mucosal vaccines.
Tfh cells play an important role in promoting mucosal IgA CSR, IgA production, and homing of IgA-committed B cells to mucosal sites. Tfh cells in tonsils and PPs produce IL-21 that synergizes with TGF-β1 to skew CSR toward IgA (Dullaers et al., 2009, Tsuji et al., 2009). IL-21 also downregulates CXCR5, the chemokine receptor that promotes follicular localization of B cells, and upregulates CCR10, the chemokine receptor that facilitates the migration of IgA class-switched B cells to local mucosal effector sites (Hieshima et al., 2004, Kunkel et al., 2003). Tfh cells may derive from Treg cells in PPs upon receiving signals from IL-6, IL-21, and activated PP B cells (Tsuji et al., 2009). Therefore, one prediction is that mucosal vaccines should robustly promote the generation of Tfh cells from Treg cells to induce protective IgA production and concurrently avoid mucosal tolerance.
Besides Tfh cells, important regulation of mucosal follicular antibody responses is also carried out by ECs, DCs, macrophages, Treg cells, Tr1-like cells, granulocytes, NK cells, as well as B cells themselves. ECs as gatekeepers of the mucosa release myriad factors in response to the commensal microbes present in the lumen, probably by sensing these microbes through innate immune receptors, such as TLRs and intracellular Nod-like receptors (NLRs) (Abreu, 2010, Hooper and Macpherson, 2010). Among such important factors are BAFF, APRIL, secretory leukocyte protease inhibitor (SLPI), and thymic stromal lymphopoietin (TSLP). Epithelial BAFF and APRIL promote T cell-independent IgA CSR and plasma cell survival, while SLPI serves as a homeostatic regulator to attenuate BAFF signaling in B cells (He et al., 2007, Xu et al., 2007). TSLP stimulates DCs to produce more BAFF and APRIL (He et al., 2007, Xu et al., 2007) and to skew T cell differentiation toward the Th2 cell direction through repression of IL-12 (Rimoldi et al., 2005, Zaph et al., 2007) and the induction of OX40 ligand (OX40L) (Ito et al., 2005), engendering a Th2 cell-dominated environment in PPs helpful for the production of IgA. A subset of mucosal DCs express CD103 (also called αE integrin), which may bind the epithelial adhesion molecule E-cadherin to facilitate DC conditioning by ECs (Strober, 2009). TSLP-conditioned CD103+ DCs also produce TGF-β1 and IL-10 (Li and Flavell, 2008). In addition to facilitating IgA CSR, TGF-β1 and IL-10 cooperate with other DC-derived factors, such as retinoic acid (RA) and indoleamine-2,3-dioxygenase (IDO), to induce the development of Treg cells and suppress the development of proinflammatory Th17 cells (Coombes et al., 2007, Matteoli et al., 2010, Mucida et al., 2007). Furthermore, TGF-β1 and IL-10 cooperate with IL-27 to induce the development of Tr1-like cells (Barnes and Powrie, 2009). Human intestinal macrophages do not produce proinflammatory cytokines in response to inflammatory stimuli (Smythies et al., 2005), and mouse LP macrophages promote the development of Treg cells (Denning et al., 2007). All these mucosal cell types are important to maintain a noninflammatory environment and prevent harmful immune responses against commensals under physiological conditions. As mentioned earlier, Treg cells, Treg-derived Tfh cells, and Tr1 cells are also important sources of IgA-inducing signals such as TGF-β1, IL-10, and IL-21 (Cong et al., 2009, Tsuji et al., 2009). Conversely, IgA-producing B cells and their precursors may enhance the generation of Treg cells and Tr1 (or Tr1-like) cells by releasing IL-10 and TGF-β1 (Asseman et al., 1999, Cerutti and Rescigno, 2008, Fillatreau et al., 2008, Mizoguchi et al., 2002). Of note, TGF-β1 is produced in a latent form and requires either proteolytic cleavage of a proregion by matrix metalloproteinases (MMPs) or a conformational change by binding to certain cell-surface receptors in order to convert an inactive TGF-β1 precursor into active TGF-β1 (Taylor, 2009). Unlike DCs from systemic lymph nodes, mucosal DCs uniquely express high levels of MMP9 and MMP13 and can activate TGF-β1 with high efficiency (Tsuji et al., 2008). In addition to TGF-β1, mucosal DCs release RA, which cooperates with IL-5 and IL-6 to enhance IgA secretion (Iwata et al., 2004, Mora et al., 2006). Mucosal DCs would further augment IgA production through nitric oxide. In mice, the intestinal mucosa contains a subset of DCs called TipDCs that express TNF and inducible nitric oxide synthase (iNOS) and release large amounts of nitric oxide after recognizing commensal bacteria through TLRs (Tezuka et al., 2007). Nitric oxide enhances IgA CSR and production by upregulating TGF-βRII expression on PP B cells and by inducing DC expression of BAFF and APRIL through unknown mechanisms (Tezuka et al., 2007). As for mucosal NK and NKT cells, the role of these innate cell types in mucosal immunity is not well understood, but several studies show that they contribute to mucosal homeostasis by releasing IL-22, a cytokine that enhances the survival, proliferation, and antimicrobial activity of ECs (Colonna, 2009, Satoh-Takayama et al., 2008, Vivier et al., 2009, Zenewicz et al., 2008). Given the broad role of IL-22 in mucosal homeostasis, it is conceivable that this cytokine also augments the production of IgA-inducing factors by ECs.
Food for Thought: Mucosal Immune Regulation by Vitamin A
As discussed above, many regulatory DC subsets in the GALT produce RA, a metabolic derivative of vitamin A found in food. Indeed, the ability to synthesize RA is not limited to GALT DCs, but seems to be a broad phenomenon found in many mucosal immune cells, such as LP CD11b+ macrophages (Denning et al., 2007), mesenteric lymph node CD103+ DCs (Coombes et al., 2007), PP DCs (Iwata et al., 2004), LP CD103+ DCs (Uematsu et al., 2008), LP TipDCs (Tezuka et al., 2007), ECs (Iwata et al., 2004), mast cells and basophils (Spiegl et al., 2008). By promoting IgA CSR, plasma cell differentiation and Treg cell development, RA appears to be a central regulator of intestinal immunity and homeostasis. Moreover, RA is a central regulator of T cell and B cell trafficking toward the effector site of the intestinal LP. RA induces the expression of gut homing receptors such as α4β7 integrin and CCR9 on CD4+ T cells (Iwata et al., 2004), CD8+ T cells (Iwata et al., 2004), CD8α+ intraepithelial lymphocytes (IELs) (Iwata et al., 2004), Treg cells (Benson et al., 2007), and B cells (Mora et al., 2006, Tezuka et al., 2007). Gut homing is achieved by α4β7 integrin binding to MAdCAM-1 expressed on endothelial cells at the post-capillary venules in intestinal tissues, and CCR9 binding to the chemokine CCL25 released by ECs in the small intestine (Iwata et al., 2004, Mora et al., 2006). RA originates from vitamin A by a series of enzymatic reactions that culminate in a final irreversible step catalyzed by retinal dehydrogenase 1 (RALDH1) and RALDH2. Peroxisome proliferator-activated receptor γ (PPARγ) agonist, IL-4 as well as bacterial flagellin, a ligand of TLR5, have been shown to activate RALDH2 expression in DCs (Elgueta et al., 2008, Szatmari et al., 2006, Uematsu et al., 2008). Low amounts of these signals might be involved in the maintenance of basal RALDH expression and RA production in GALT. As RA is a diet derivative, mucosal vaccination strategies that encompass stimulation of RA production through dietary supplementation of vitamin A as well as induction of RALDH expression seem appealing and should be explored, particularly for vaccination trials in developing countries where vitamin A deficiency is common.
Toll-Like Receptors: Swords with a Toll
A great number of vaccination studies in animal models and humans have demonstrated the benefits of taking advantage of TLR signaling system to boost the efficacy of mucosal vaccines (Mbow et al., 2010). As discussed earlier, TLRs are involved in the recognition of microbial molecular structures and are expressed by multiple cells types in the mucosal immune system, such as ECs, DCs, T cells, and B cells. By sensing products of commensal and pathogenic microorganisms, they induce the expression of critical regulatory factors to contribute to mucosal defense and homeostasis. In the presence of microbial TLR ligands, intestinal ECs express antimicrobial factors such as RegIIIγ (Cash et al., 2006), B cell-activating factors such as BAFF and APRIL (He et al., 2007, Xu et al., 2007), and immunoregulatory factors such as TSLP (Liu et al., 2007), whereas MALT DCs express iNOS and produce nitric oxide (Tezuka et al., 2007). LP TLR5+ DCs express RALDH2 and produce RA in response to flagellin stimulation (Uematsu et al., 2008). B cells express TLRs and stimulation with a variety of TLR ligands triggers B cell proliferation, differentiation into antibody secreting cells, antigen presentation, cytokine secretion, and CSR (Avalos et al., 2010, Lanzavecchia and Sallusto, 2007). The importance of this pathway in vaccination is exemplified by a recent study showing the critical requirement of providing TLR stimulation directly to B cells in order for a formalin-inactivated respiratory syncytial virus vaccine to induce affinity maturation and confer protection (Delgado et al., 2009).
However, the benefits of targeting TLRs in mucosal vaccination come at a price. Administration of TLR ligands may cause inflammation and autoimmune diseases, especially after long-term stimulation (Avalos et al., 2010, Israeli et al., 2009, Mullick et al., 2005). Certain TLR ligands may play a pathogenic role in tumorigenesis. Existing neoplastic processes may divert TLR signaling in tumors and facilitate their survival or immune evasion (Huang et al., 2005, Rakoff-Nahoum and Medzhitov, 2007, Wang et al., 2003). Imprudent therapeutic targeting of TLRs in mucosal vaccination should be cautioned, and circumvention of these potential adverse effects will rely on a thorough understanding of the responses of different mucosal immune cell types to various TLR ligand stimulations.
Advantages to Target Mucosal Antibody Responses
Antibody responses initiated at mucosal inductive sites exhibit their protective function by myriad mechanisms, some of which are distinct from their systemically initiated counterparts. Mucosal antibody responses offer a critical step for the protection of the host by inhibiting the very first step of microbial pathogenesis, i.e., microbial adherence to mucosal ECs (Cerutti and Rescigno, 2008). Mucosal antibodies can also inhibit the activity of many detrimental microbial enzymes and toxins in the lumen and neutralize mucosal viruses inside ECs (Cerutti and Rescigno, 2008). For invasive pathogens that have breached the epithelial barrier, mucosal IgG can mediate opsonization and internalization of the pathogens by phagocytes, which in turn leads to the activation of phagocytes and killing of the pathogen or presentation of pathogen-derived antigens that activates multiple types of mucosal effector T cells, such as Th17 cells, γδ T cells, and CD8+ CTL cells, to promote pathogen containment or clearance (Cerutti and Rescigno, 2008). Mucosal antibodies, such as IgG and IgA, can promote antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells, neutrophils, and eosinophils to lyse infected cells (Nimmerjahn and Ravetch, 2006). IgD can recruit basophils to the aerodigestive mucosa and activate their antimicrobial and B cell-stimulating responses to directly participate in frontline antimicrobial defense (Chen et al., 2009).
There are some added advantages for vaccines to boost mucosal IgA responses. IgA can mediate apical-to-basolateral retrotranscytosis of antigens across M cells via an unknown receptor or across duodenal epithelial cells via the transferring receptor (also known as CD71), thereby enabling controlled entry of specific antigens to initiate mucosal immune responses (Cerutti and Rescigno, 2008). This retrotranscytosis process can deliver intact antigens to the basolateral side of ECs (Phalipon and Corthésy, 2003). Conversely, IgA can transport invasive pathogens that have breached the epithelial barrier back to the lumen for elimination through pIgR-mediated basolateral-to-apical transcytosis (Kaetzel et al., 1991). This process would limit the amount of immune complexes available in the LP, thereby avoiding unnecessary inflammatory mucosal responses in situations where there is no severe infection. Vaccinations targeting mucosal IgA responses have the additional advantage of triggering systemic production of monomeric IgA (Macpherson et al., 2008). This IgA promotes immune protection without causing detrimental systemic inflammation by removing invading pathogens through a noninflammatory FcαRI receptor expressed on the surface of phagocytes, including neutrophils (Cerutti and Rescigno, 2008, Pasquier et al., 2005). Finally, recent evidence indicates that secretory IgA “appeases” the mucosal immune system that is in close contact with heavy indigenous microbial loads by restraining commensal flora-induced activation of the host oxidative system (Peterson et al., 2007).
Mucosal Immune Dynamics and Implications for Vaccine Design
Recent studies on intestinal immune responses to commensal flora have yielded important insights into the dynamics of mucosal antibody responses and revealed novel differences from systemic antibody responses. Commensal flora is known to orchestrate the postnatal maturation of mucosal immune systems (Artis, 2008, Macpherson and Uhr, 2004). Using a controlled, reversible intestine colonization system, intestinal IgA responses were shown to be directed against only a few major commensal microbial species at a given time (Cerutti, 2010, Hapfelmeier et al., 2010, Jiang et al., 2001, Suzuki et al., 2004), similar to intestinal T cell responses, which are mainly directed against major segmented filamentous bacteria species (Gaboriau-Routhiau et al., 2009, Ivanov et al., 2008). Furthermore, intestinal IgA responses have a high threshold of 108–109 bacteria, below which there is no elicitation of IgA production. It lacks classical memory prime-boost features and displays constant attrition, where subsequent challenges with different antigens diminish the response to previous antigenic challenges. This deviates substantially from classical systemic immune memory paradigms. Therefore, intestinal IgA responses have a built-in “algorithm” for total strength control that constantly adapts intestinal IgA to the current microbiota. Such “mucosal immunological logic” is conceivable considering the fact that the most abundant microbial species have a higher chance of breaching the epithelial barrier. Therefore, steady-state IgA should predominantly target those species to avoid potentially wasteful over-diversified responses. Because of the great abundance of commensal bacteria, intestinal IgA responses also involve a high antigenic threshold. All these features highlight the importance of the integrity of the epithelial barrier. Indeed, mucosal inflammation, clonal exhaustion, and immunological paralysis could ensue should these abundant commensal species breach the epithelia in overwhelming amounts (Halliday, 1971, Mitchison, 1964). IgA limits these potentially lethal outcomes by tightly controlling the amount of antigen input across ECs. If we assume that mucosal immune responses to antigens from pathogens and vaccines follow a similar set of principles, induction of long-lasting protective IgA responses would require the use of vaccination strategies that ensure prolonged supply of antigens and sustained stimulation of intestinal B cells. These strategies could include embedding appropriate immunogens in stable components of the microbiota, edible probiotic bacteria, or genetically modified food, such as transgenic plants (Tokuhara et al., 2010). However, caution must be exercised in vaccine design and evaluation to avoid the induction of mucosal tolerance as a result of prolonged antigenic stimulation.
Yin and Yang of Mucosal Vaccination: Immunity versus Tolerance
As discussed earlier, the induction of mucosal IgA responses involves multiple regulatory mechanisms that also function to promote mucosal and systemic T cell hyporesponsiveness by inducing Treg cell development. Indeed, administration of antigens to human gastrointestinal or respiratory tracts can induce a profound reduction in cellular immunity, evidenced by diminished delayed type hypersensitivity reactions, reduced T cell proliferation, and increased T cell secretion of immunosuppressive cytokines, without altering humoral immunity (Mayer and Shao, 2004, Mowat, 2003). This phenomenon, termed oral tolerance, remains a challenge for mucosal vaccine development. In the past, mucosal immunization protocols eliciting concomitant induction of B cell and Treg cell responses were exploited to develop antibody-mediated protection against poliovirus and influenza virus (Bergerot et al., 1997, Phipps et al., 2003, Tamura et al., 1997, Tarkowski et al., 1999). Similar approaches could be harnessed to dampen inflammatory systemic and mucosal responses present in disorders such as asthma, uveitis, diabetes, and rheumatoid arthritis (Mayer and Shao, 2004), but may be a hurdle for mucosal vaccination against pathogens requiring the development of humoral and cellular immune responses, both systemically and at mucosal sites of entry (Figure 2 ). In such cases, an approach that combines mucosal and systemic vaccination or one that targets additional immune cell types with less regulatory functions but TH1- or CTL-activating abilities, such as neutrophils (Appelberg, 2007) and CD14+CD33+ LP mononuclear phagocytes (Kamada et al., 2008), may be effective.
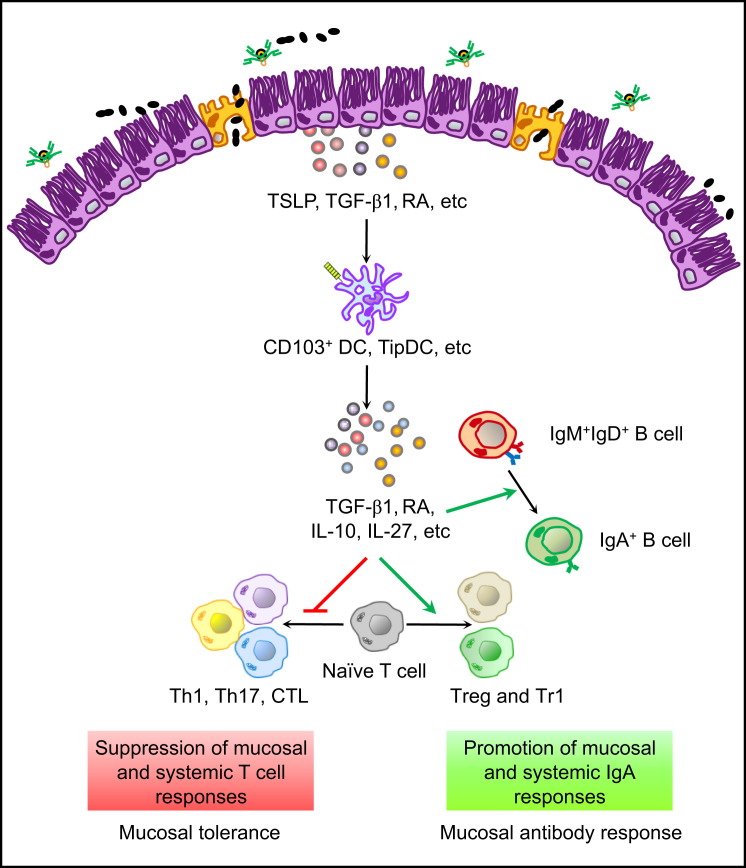
Figure 2.
Parallel Regulatory Mechanisms for Induction of Mucosal Immunity and Tolerance
Intestinal ECs release immune regulatory factors, such as TSLP, RA, and TGF-β1, upon sensing antigens from the lumen. These immune mediators condition DCs to generate CD103+ DCs and TipDCs. Such regulatory DCs produce more immune regulatory factors, such as TGF-β1, RA, IL-10, and IL-27, which reinforce mucosal homeostasis and tolerance by promoting the development of IgA responses and regulatory T cell responses, including Treg and Tr1 responses. Treg and Tr1 cells suppress mucosal and systemic responses by Th1 cells, Th17 cells, and CTLs. Such a situation may represent a challenge for mucosal vaccines aimed at eliciting mucosal and systemic T cell-mediated immunity.
Summary and Future Challenges
Studies based on various mouse models, human diseases, and successful examples of mucosal vaccines have clearly demonstrated the pivotal role of mucosal antibodies in frontline immune protection and their instructive functions in systemic immunity. In spite of recent advances in structural biology and nanotechnology improving vaccine delivery (Chadwick et al., 2010) and additional advances in genome sequencing technology fostering reverse vaccinology and antigen discovery (see review by Sette and Rappuoli, 2010), the development of effective mucosal vaccines and adjuvants is still hampered by our inability to fully understand the intricate regulatory networks governing mucosal immunity. A better understanding of these networks would permit the elicitation of sustained protective immune responses in both mucosal and systemic districts without causing excessive immune activation or inappropriate immune tolerance. Our current knowledge of the intricacies characterizing the mucosal immune system may only allow us to have a glimpse of the tip of the iceberg. The lineage and functional heterogeneity and plasticity of the mucosal immune cell types reviewed here may already seem exceedingly confusing (Geissmann et al., 2010), yet more mucosal cell types with key regulatory functions are being described (Buonocore et al., 2010, Dardalhon et al., 2008, Neill et al., 2010, Saenz et al., 2010, Siddiqui et al., 2010, Veldhoen et al., 2008). In addition, the functions of old cell types and molecules abundant in mucosal districts, such as eosinophils, mast cells, mucins, secretory component, antimicrobial peptides, as well as diet-derived molecules, remain poorly understood. While trying to take advantage of these regulatory mechanisms for vaccination, it is also important to bear in mind that some sophisticated pathogens hitch a ride on certain regulatory mechanisms to establish or reinforce infection (Fontenot et al., 2009). In addition, mucosal IgA responses induced by harnessing these regulatory mechanisms can result in a self-limiting situation because of the shielding of inductive lymphoid structures such as PPs (Brandtzaeg and Johansen, 2007).
There are several more points for immunologists and vaccine developers to ponder. Although mucosal immunization elicits mucosal and systemic antibody responses, its impact on cell-mediated immunity, particularly the induction of systemic and mucosal CTL responses, has not been adequately investigated. Such studies are particularly relevant to the design of prophylactic vaccines against pathogens such as HIV, which might take advantage of the undesirable effect of diminishing beneficial CTL responses to spread with higher speed throughout the body. Furthermore, the mucosal immune system declines as the subject becomes older, a phenomenon called immunosenescence, with GALT declining at an earlier age than NALT, as evidenced by a reduction in GALT mass, intestinal antigen-specific IgA responses, and defective oral tolerance induction (Fujihashi and Kiyono, 2009). This may necessitate that vaccination strategies be different for older people compared to younger people and suggests that the nasal route may be more desirable for effective mucosal vaccination in the elderly. The mucosal immune system also changes under certain physiological and pathological conditions, such as female menstrual cycles and metabolic syndromes (Black et al., 2000, Vijay-Kumar et al., 2010), which can affect the outcome of mucosal vaccination. Solving these various issues are necessary prerequisites before we can reach the milestone of “vaccine for all” and truly realize the aspirations set forth in the Millennium Development Goals.
Acknowledgments
Supported by U.S. National Institutes of Health grants R01 AI-05753, R01 AI-074378, ARRA AI-61093; funds from The Hemsley Foundation for IBD research; Ministerio de Ciencia e Innovación grant SAF 2008-02725; and funds from Fundacio' IMIM (to A.C.).
References
- Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 2007;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- Asseman C., Mauze S., Leach M.W., Coffman R.L., Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos A.M., Busconi L., Marshak-Rothstein A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity. 2010;43:76–83. doi: 10.3109/08916930903374618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M.J., Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Belyakov I.M., Hel Z., Kelsall B., Kuznetsov V.A., Ahlers J.D., Nacsa J., Watkins D.I., Allen T.M., Sette A., Altman J., et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- Benson M.J., Pino-Lagos K., Rosemblatt M., Noelle R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerot I., Ploix C., Petersen J., Moulin V., Rask C., Fabien N., Lindblad M., Mayer A., Czerkinsky C., Holmgren J., Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 1997;94:4610–4614. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C.A., Rohan L.C., Cost M., Watkins S.C., Draviam R., Alber S., Edwards R.P. Vaginal mucosa serves as an inductive site for tolerance. J. Immunol. 2000;165:5077–5083. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Johansen F.E. In: Mucosal immune defense. Kaetzel C.S., editor. Springer; New York: 2007. IgA and intestinal homeostasis; pp. 221–268. [Google Scholar]
- Brandtzaeg P., Farstad I.N., Johansen F.E., Morton H.C., Norderhaug I.N., Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol. Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D.R., Stanfield R.L., Wilson I.A. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA. 2005;102:14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash H.L., Whitham C.V., Behrendt C.L., Hooper L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola S., Rajewsky K. B cell recruitment and selection in mouse GALT germinal centers. Curr. Top. Microbiol. Immunol. 2006;308:155–171. doi: 10.1007/3-540-30657-9_7. [DOI] [PubMed] [Google Scholar]
- Casola S., Otipoby K.L., Alimzhanov M., Humme S., Uyttersprot N., Kutok J.L., Carroll M.C., Rajewsky K. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Cazac B.B., Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Cerutti A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A. IgA changes the rules of memory. Science. 2010;329:1646–1647. doi: 10.1126/science.1192488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A., Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick S., Kriegel C., Amiji M. Nanotechnology solutions for mucosal immunization. Adv. Drug Deliv. Rev. 2010;62:394–407. doi: 10.1016/j.addr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Charles N., Hardwick D., Daugas E., Illei G.G., Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat. Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri J., Alt F.W. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- Chen K., Cerutti A. New insights into the enigma of immunoglobulin D. Immunol. Rev. 2010;237:160–179. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang J., Zganiacz A., Xing Z. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect. Immun. 2004;72:238–246. doi: 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Xu W., Wilson M., He B., Miller N.W., Bengtén E., Edholm E.S., Santini P.A., Rath P., Chiu A., et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Cong Y., Feng T., Fujihashi K., Schoeb T.R., Elson C.O. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J. Clin. Immunol. 2001;21:303–309. doi: 10.1023/a:1012241117984. [DOI] [PubMed] [Google Scholar]
- Dardalhon V., Awasthi A., Kwon H., Galileos G., Gao W., Sobel R.A., Mitsdoerffer M., Strom T.B., Elyaman W., Ho I.C., et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.F., Coviello S., Monsalvo A.C., Melendi G.A., Hernandez J.Z., Batalle J.P., Diaz L., Trento A., Chang H.Y., Mitzner W., et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Dieu M.C., Vanbervliet B., Vicari A., Bridon J.M., Oldham E., Aït-Yahia S., Brière F., Zlotnik A., Lebecque S., Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullaers M., Li D., Xue Y., Ni L., Gayet I., Morita R., Ueno H., Palucka K.A., Banchereau J., Oh S. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G., Littman D.R. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Elgueta R., Sepulveda F.E., Vilches F., Vargas L., Mora J.R., Bono M.R., Rosemblatt M. Imprinting of CCR9 on CD4 T cells requires IL-4 signaling on mesenteric lymph node dendritic cells. J. Immunol. 2008;180:6501–6507. doi: 10.4049/jimmunol.180.10.6501. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Kinoshita K., Muramatsu M., Ikuta K., Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Kawamoto S., Kanagawa O., Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Fillatreau S., Gray D., Anderton S.M. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat. Rev. Immunol. 2008;8:391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- Fontenot D., He H., Hanabuchi S., Nehete P.N., Zhang M., Chang M., Nehete B., Wang Y.H., Wang Y.H., Ma Z.M., et al. TSLP production by epithelial cells exposed to immunodeficiency virus triggers DC-mediated mucosal infection of CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2009;106:16776–16781. doi: 10.1073/pnas.0907347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K., Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 2009;30:334–343. doi: 10.1016/j.it.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V., Rakotobe S., Lécuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G., et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Gallichan W.S., Rosenthal K.L. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert A., Pabst R. M cells at locations outside the gut. Semin. Immunol. 1999;11:165–170. doi: 10.1006/smim.1999.0172. [DOI] [PubMed] [Google Scholar]
- Geissmann F., Gordon S., Hume D.A., Mowat A.M., Randolph G.J. Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L., Gilbride K., Dobles M., Kalled S.L., Zandman D., Scott M.L. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J. Exp. Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan L., Verweij W.R., Holtrop M., Brands R., van Scharrenburg G.J., Palache A.M., Agsteribbe E., Wilschut J. Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine. 2001;19:2898–2907. doi: 10.1016/s0264-410x(00)00556-9. [DOI] [PubMed] [Google Scholar]
- Halliday W.J. Immunological paralysis of mice with pneumococcal polysaccharide antigens. Bacteriol. Rev. 1971;35:267–289. doi: 10.1128/br.35.3.267-289.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S., Lawson M.A.E., Slack E., Kirundi J.K., Maaike S., Heikenwalder M., Cahenzli J., Velykoredko Y., Balmer M.L., Endt K., et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;329:1646–1647. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Xu W., Santini P.A., Polydorides A.D., Chiu A., Estrella J., Shan M., Chadburn A., Villanacci V., Plebani A., et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- He B., Santamaria R., Xu W., Cols M., Chen K., Puga I., Shan M., Xiong H., Bussel J.B., Chiu A., et al. TACI triggers immunoglobulin class switching by activating B cells through the adaptor protein MyD88. Nat. Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J., Ginhoux F., Bogunovic M., Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol. Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Herremans T.M., Reimerink J.H., Buisman A.M., Kimman T.G., Koopmans M.P. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 1999;162:5011–5018. [PubMed] [Google Scholar]
- Hieshima K., Kawasaki Y., Hanamoto H., Nakayama T., Nagakubo D., Kanamaru A., Yoshie O. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J. Immunol. 2004;173:3668–3675. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11(Suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- Honjo T., Kinoshita K., Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu. Rev. Immunol. 2002;20:165–196. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Macpherson A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Huang B., Zhao J., Li H., He K.L., Chen Y., Chen S.H., Mayer L., Unkeless J.C., Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- Israeli E., Agmon-Levin N., Blank M., Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18:1217–1225. doi: 10.1177/0961203309345724. [DOI] [PubMed] [Google Scholar]
- Ito T., Wang Y.H., Duramad O., Hori T., Delespesse G.J., Watanabe N., Qin F.X., Yao Z., Cao W., Liu Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Frutos Rde.L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. Division of labor by dendritic cells. Cell. 2007;128:435–436. doi: 10.1016/j.cell.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Annu. Rev. Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J.L., Berg P.L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W.W. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.Q., Bos N.A., Cebra J.J. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect. Immun. 2001;69:3611–3617. doi: 10.1128/IAI.69.6.3611-3617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel C.S., Robinson J.K., Chintalacharuvu K.R., Vaerman J.P., Lamm M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc. Natl. Acad. Sci. USA. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K., et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.S., Chin R.K., Wang Y., Yu P., Wang J., Newell K.A., Fu Y.X. Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production. Nat. Immunol. 2002;3:576–582. doi: 10.1038/ni795. [DOI] [PubMed] [Google Scholar]
- Kiyono H., Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H., Kunisawa J., McGhee J.R., Mestecky J. In: Fundamental Immunology. Paul W.E., editor. Academic Press; San Diego, CA: 2008. The mucosal immune system; pp. 983–1030. [Google Scholar]
- Koelsch K., Zheng N.Y., Zhang Q., Duty A., Helms C., Mathias M.D., Jared M., Smith K., Capra J.D., Wilson P.C. Mature B cells class switched to IgD are autoreactive in healthy individuals. J. Clin. Invest. 2007;117:1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisawa J., Nochi T., Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008;29:505–513. doi: 10.1016/j.it.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Kunkel E.J., Butcher E.C. Plasma-cell homing. Nat. Rev. Immunol. 2003;3:822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- Kunkel E.J., Kim C.H., Lazarus N.H., Vierra M.A., Soler D., Bowman E.P., Butcher E.C. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J. Clin. Invest. 2003;111:1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr. Opin. Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Lazarus N.H., Kunkel E.J., Johnston B., Wilson E., Youngman K.R., Butcher E.C. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 2003;170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- Li M.O., Flavell R.A. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Litinskiy M.B., Nardelli B., Hilbert D.M., He B., Schaffer A., Casali P., Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., Malefyt Rde.W., Omori M., Zhou B., Ziegler S.F. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., McCoy K.D., Johansen F.E., Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Matteoli G., Mazzini E., Iliev I.D., Mileti E., Fallarino F., Puccetti P., Chieppa M., Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- Mayer L., Shao L. Therapeutic potential of oral tolerance. Nat. Rev. Immunol. 2004;4:407–419. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- Maynard C.L., Harrington L.E., Janowski K.M., Oliver J.R., Zindl C.L., Rudensky A.Y., Weaver C.T. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat. Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- Mbawuike I.N., Pacheco S., Acuna C.L., Switzer K.C., Zhang Y., Harriman G.R. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J. Immunol. 1999;162:2530–2537. [PubMed] [Google Scholar]
- Mbow M.L., De Gregorio E., Valiante N.M., Rappuoli R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Mitchison N.A. Induction of Immunological Paralysis in Two Zones of Dosage. Proc. R. Soc. Lond. B Biol. Sci. 1964;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E., Takedatsu H., Blumberg R.S., Bhan A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Mond J.J., Vos Q., Lees A., Snapper C.M. T cell independent antigens. Curr. Opin. Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Mora J.R., von Andrian U.H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., von Andrian U.H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Mullick A.E., Tobias P.S., Curtiss L.K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K., Bucks C., Kane C.M., Fallon P.G., Pannell R., et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutra M.R., Kozlowski P.A. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- Neutra M.R., Mantis N.J., Kraehenbuhl J.P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Palucka K., Banchereau J., Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–478. doi: 10.1016/j.immuni.2010.10.007. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C., Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Pasquier B., Launay P., Kanamaru Y., Moura I.C., Pfirsch S., Ruffié C., Hénin D., Benhamou M., Pretolani M., Blank U., Monteiro R.C. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Peng S.L. Signaling in B cells via Toll-like receptors. Curr. Opin. Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Peterson D.A., McNulty N.P., Guruge J.L., Gordon J.I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Phalipon A., Corthésy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55–58. doi: 10.1016/s1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- Phipps P.A., Stanford M.R., Sun J.B., Xiao B.G., Holmgren J., Shinnick T., Hasan A., Mizushima Y., Lehner T. Prevention of mucosally induced uveitis with a HSP60-derived peptide linked to cholera toxin B subunit. Eur. J. Immunol. 2003;33:224–232. doi: 10.1002/immu.200390025. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Division of labor and cooperation between dendritic cells. Nat. Immunol. 2006;7:699–700. doi: 10.1038/ni0706-699. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G.M., Nespoli A., Viale G., Allavena P., Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- Rojas R., Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat. Rev. Mol. Cell Biol. 2002;3:944–955. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- Saenz S.A., Siracusa M.C., Perrigoue J.G., Spencer S.P., Urban J.F., Jr., Tocker J.E., Budelsky A.L., Kleinschek M.A., Kastelein R.A., Kambayashi T., et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire E.O., Parren P.W., Pantophlet R., Zwick M.B., Morris G.M., Rudd P.M., Dwek R.A., Stanfield R.L., Burton D.R., Wilson I.A. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N., Vosshenrich C.A., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., Mention J.J., Thiam K., Cerf-Bensussan N., Mandelboim O., et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Scapini P., Bazzoni F., Cassatella M.A. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol. Lett. 2008;116:1–6. doi: 10.1016/j.imlet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Sette A., Rappuoli R. Reverse vaccinology: Developing vaccines in the era of genomics. Immunity. 2010;33:530–541. doi: 10.1016/j.immuni.2010.09.017. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z., Kang S.M., Compans R.W. Mucosal immunization of CD4+ T cell-deficient mice with an inactivated virus induces IgG and IgA responses in serum and mucosal secretions. Virology. 2005;331:387–395. doi: 10.1016/j.virol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Siddiqui K.R., Laffont S., Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies L.E., Sellers M., Clements R.H., Mosteller-Barnum M., Meng G., Benjamin W.H., Orenstein J.M., Smith P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegl N., Didichenko S., McCaffery P., Langen H., Dahinden C.A. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood. 2008;112:3762–3771. doi: 10.1182/blood-2008-01-135251. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Guikema J.E., Schrader C.E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S.K., Weissman I.L., Butcher E.C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J. Immunol. 1982;128:844–851. [PubMed] [Google Scholar]
- Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2:468–471. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Meek B., Doi Y., Muramatsu M., Chiba T., Honjo T., Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. USA. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Meek B., Doi Y., Honjo T., Fagarasan S. Two distinctive pathways for recruitment of naive and primed IgM+ B cells to the gut lamina propria. Proc. Natl. Acad. Sci. USA. 2005;102:2482–2486. doi: 10.1073/pnas.0409539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I., Pap A., Rühl R., Ma J.X., Illarionov P.A., Besra G.S., Rajnavolgyi E., Dezso B., Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Hatori E., Tsuruhara T., Aizawa C., Kurata T. Suppression of delayed-type hypersensitivity and IgE antibody responses to ovalbumin by intranasal administration of Escherichia coli heat-labile enterotoxin B subunit-conjugated ovalbumin. Vaccine. 1997;15:225–229. doi: 10.1016/s0264-410x(96)00135-1. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Sun J.B., Holmdahl R., Holmgren J., Czerkinsky C. Treatment of experimental autoimmune arthritis by nasal administration of a type II collagen-cholera toxoid conjugate vaccine. Arthritis Rheum. 1999;42:1628–1634. doi: 10.1002/1529-0131(199908)42:8<1628::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Taylor A.W. Review of the activation of TGF-beta in immunity. J. Leukoc. Biol. 2009;85:29–33. doi: 10.1189/jlb.0708415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka H., Abe Y., Iwata M., Takeuchi H., Ishikawa H., Matsushita M., Shiohara T., Akira S., Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- Tokuhara D., Yuki Y., Nochi T., Kodama T., Mejima M., Kurokawa S., Takahashi Y., Nanno M., Nakanishi U., Takaiwa F., et al. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc. Natl. Acad. Sci. USA. 2010;107:8794–8799. doi: 10.1073/pnas.0914121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M., Suzuki K., Kitamura H., Maruya M., Kinoshita K., Ivanov I.I., Itoh K., Littman D.R., Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Komatsu N., Kawamoto S., Suzuki K., Kanagawa O., Honjo T., Hori S., Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- Uematsu S., Fujimoto K., Jang M.H., Yang B.G., Jung Y.J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.D., Shakhar G., Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Varol C., Zigmond E., Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol. 2010;10:415–426. doi: 10.1038/nri2778. [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Uyttenhove C., van Snick J., Helmby H., Westendorf A., Buer J., Martin B., Wilhelm C., Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M., Aitken J.D., Carvalho F.A., Cullender T.C., Mwangi S., Srinivasan S., Sitaraman S.V., Knight R., Ley R.E., Gewirtz A.T. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Spits H., Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat. Rev. Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- Wang J.H., Manning B.J., Wu Q.D., Blankson S., Bouchier-Hayes D., Redmond H.P. Endotoxin/lipopolysaccharide activates NF-kappa B and enhances tumor cell adhesion and invasion through a beta 1 integrin-dependent mechanism. J. Immunol. 2003;170:795–804. doi: 10.4049/jimmunol.170.2.795. [DOI] [PubMed] [Google Scholar]
- Wang J., Thorson L., Stokes R.W., Santosuosso M., Huygen K., Zganiacz A., Hitt M., Xing Z. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 2004;173:6357–6365. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- Xu W., He B., Chiu A., Chadburn A., Shan M., Buldys M., Ding A., Knowles D.M., Santini P.A., Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- Xu W., Santini P.A., Matthews A.J., Chiu A., Plebani A., He B., Chen K., Cerutti A. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J. Immunol. 2008;181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Santini P.A., Sullivan J.S., He B., Shan M., Ball S.C., Dyer W.B., Ketas T.J., Chadburn A., Cohen-Gould L., et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 2009;9:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Kweon M.N., Rennert P.D., Hiroi T., Fujihashi K., McGhee J.R., Kiyono H. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J. Immunol. 2004;173:762–769. doi: 10.4049/jimmunol.173.2.762. [DOI] [PubMed] [Google Scholar]
- Zaph C., Troy A.E., Taylor B.C., Berman-Booty L.D., Guild K.J., Du Y., Yost E.A., Gruber A.D., May M.J., Greten F.R., et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., Flavell R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]