Abstract
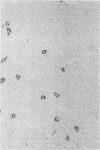
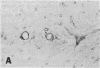
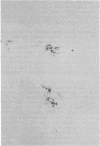
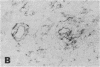
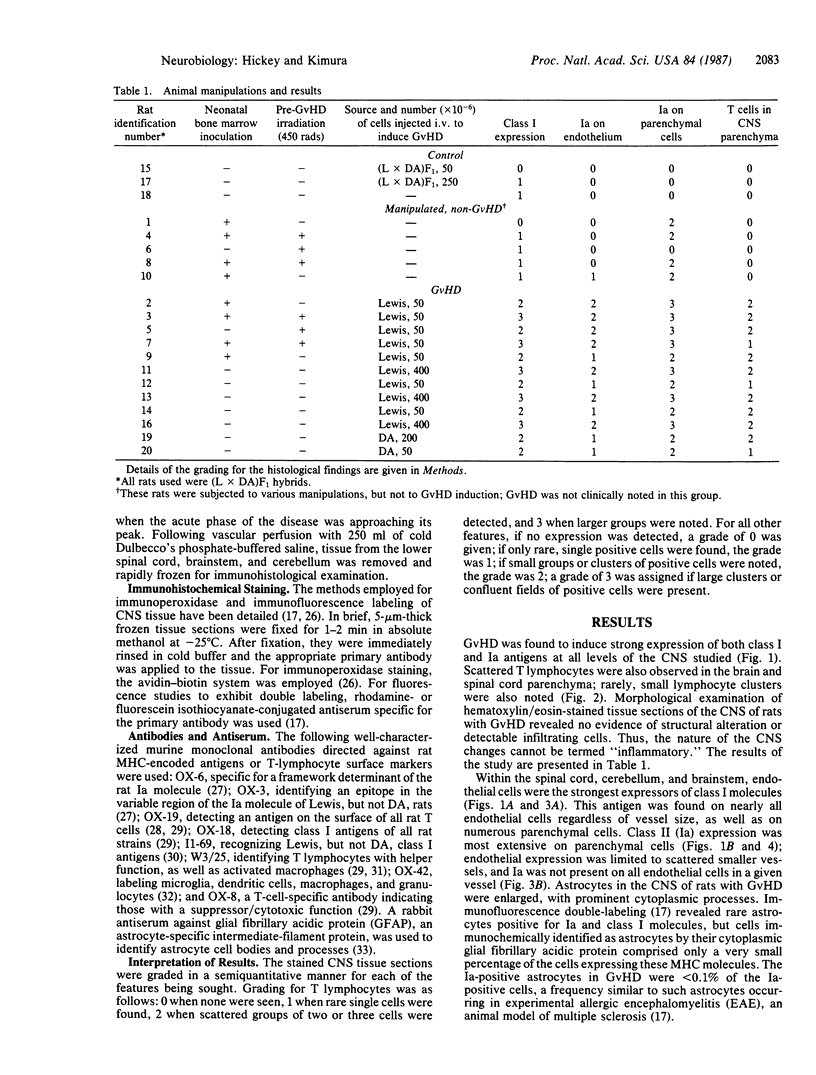
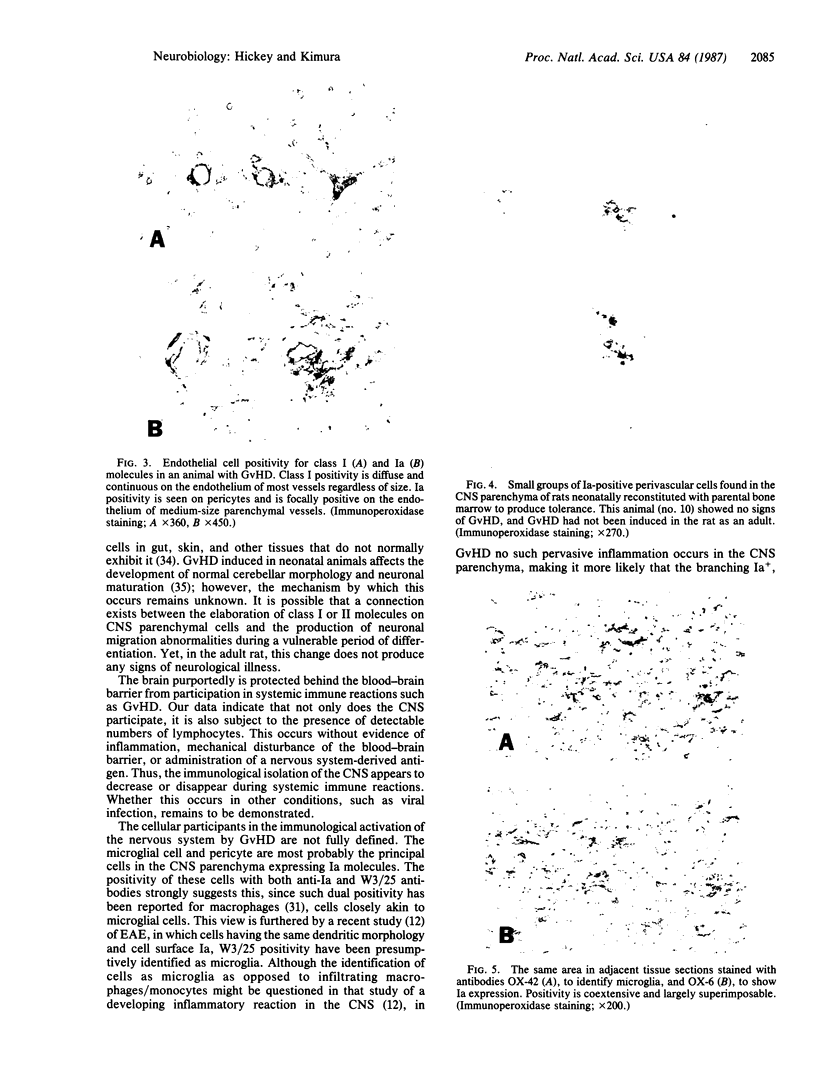
In the central nervous system (CNS) of healthy animals, T lymphocytes and cellular expression of major histocompatibility complex (MHC) gene products are virtually undetectable. Yet, in CNS immunological diseases, such as multiple sclerosis in humans, these constituents of the immune response must appear by some mechanism. Immunohistochemical examination of the CNS of F1 hybrid rats following induction of graft-vs.-host disease by parental lymphocytes revealed extensive parenchymal and vascular expression of host class I and II (Ia) MHC-encoded cell surface molecules. In addition, occasional scattered T lymphocytes were detected in the CNS of these animals. F1 hybrid rats reconstituted during the neonatal period with bone marrow cells from one parental strain also expressed increased levels of MHC antigens in the CNS. Thus, evidence is presented that the "immunological privilege" of the CNS seems to decrease or disappear during a strong systemic immune response such as graft-vs.-host disease. These findings may have important implications concerning the mechanism of induction of human CNS immunological diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker C. F., Billingham R. E. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Bellgrau D., Smilek D., Wilson D. B. Induced tolerance in F1 rats to anti-major histocompatibility complex receptors on parental T cells. Implications for self tolerance. J Exp Med. 1981 Jun 1;153(6):1660–1665. doi: 10.1084/jem.153.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Craggs R. I., Webster H. D. Ia antigens in the normal rat nervous system and in lesions of experimental allergic encephalomyelitis. Acta Neuropathol. 1985;68(4):263–272. doi: 10.1007/BF00690828. [DOI] [PubMed] [Google Scholar]
- DAMESHEK W., SCHWARTZ R. S. Leukemia and auto-immunization- some possible relationships. Blood. 1959 Oct;14:1151–1158. [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M., Ramshaw I. A., Grant C. K. Rejection of allogeneic tumor cells growing in mouse cerebrospinal fluid. Functional analysis of the inflammatory process. J Neuroimmunol. 1981 Mar;1(1):93–99. doi: 10.1016/0165-5728(81)90011-4. [DOI] [PubMed] [Google Scholar]
- Elkins W. L. Cellular immunology and the pathogenesis of graft versus host reactions. Prog Allergy. 1971;15:78–187. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fontana A., Weber E., Grob P. J., Lim R., Miller J. F. Dual effect of glia maturation factor on astrocytes. Differentiation and release of interleukin-1 like factors. J Neuroimmunol. 1983 Dec;5(3):261–269. doi: 10.1016/0165-5728(83)90046-2. [DOI] [PubMed] [Google Scholar]
- Gasser D. L., Palm J., Gonatas N. K. Genetic control of susceptibility to experimental allergic encephalomyelitis and the Ag-B locus of rats. J Immunol. 1975 Aug;115(2):431–433. [PubMed] [Google Scholar]
- Griffin W. S., Head J. R., Morrison M. R. Graft-versus-host disease (GVHD) and immunotherapy: effects on basic cell functions in the developing brain. Transplant Proc. 1981 Mar;13(1 Pt 2):1237–1240. [PubMed] [Google Scholar]
- Hickey W. F., Gonatas N. K., Kimura H., Wilson D. B. Identification and quantitation of T lymphocyte subsets found in the spinal cord of the Lewis rat during acute experimental allergic encephalomyelitis. J Immunol. 1983 Dec;131(6):2805–2809. [PubMed] [Google Scholar]
- Hickey W. F., Osborn J. P., Kirby W. M. Expression of Ia molecules by astrocytes during acute experimental allergic encephalomyelitis in the Lewis rat. Cell Immunol. 1985 Apr 1;91(2):528–535. doi: 10.1016/0008-8749(85)90251-5. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., von Hanwehr R. I., Dinarello C. A., Mizel S. B., Hinton D., Merrill J. E. Immunoregulatory molecules and IL 2 receptors identified in multiple sclerosis brain. J Immunol. 1986 May 1;136(9):3239–3245. [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Wilson D. B. Anti-idiotypic cytotoxic T cells in rats with graft-versus-host disease. 1984 Mar 29-Apr 4Nature. 308(5958):463–464. doi: 10.1038/308463a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A. Weak HLA and beta 2-microglobulin expression of neuronal cell lines can be modulated by interferon. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6476–6480. doi: 10.1073/pnas.81.20.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Hickey W. F. Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from "histologically normal" to that showing different levels of glial tumor involvement. J Immunol. 1986 Jun 1;136(11):4054–4062. [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Tabira T., Endoh M., Steinman L. Ia expression in chronic relapsing experimental allergic encephalomyelitis induced by long-term cultured T cell lines in mice. Lab Invest. 1986 Mar;54(3):345–352. [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M., Kondo N., Itoh T., Yoshiki T. Autoimmune disease and malignant lymphoma associated with host-versus-graft disease in mice. Clin Exp Immunol. 1985 Dec;62(3):535–544. [PMC free article] [PubMed] [Google Scholar]
- Traugott U., Scheinberg L. C., Raine C. S. On the presence of Ia-positive endothelial cells and astrocytes in multiple sclerosis lesions and its relevance to antigen presentation. J Neuroimmunol. 1985 Apr;8(1):1–14. doi: 10.1016/s0165-5728(85)80043-6. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Rolinson A., Danta G. Reactivation of allergic encephalomyelitis by means of allogeneic confrontation. Cell Immunol. 1985 Feb;90(2):614–619. doi: 10.1016/0008-8749(85)90226-6. [DOI] [PubMed] [Google Scholar]
- Williams K. A., Hart D. N., Fabre J. W., Morris P. J. Distribution and quantitation of HLA-ABC and DR (Ia) antigens on human kidney and other tissues. Transplantation. 1980 Apr;29(4):274–279. doi: 10.1097/00007890-198004000-00002. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., Harris A. W., Schrader J. W. Effect of cloned interferon-gamma on expression of H-2 and Ia antigens on cell lines of hemopoietic, lymphoid, epithelial, fibroblastic and neuronal origin. Eur J Immunol. 1984 Jan;14(1):52–56. doi: 10.1002/eji.1830140110. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]