Abstract
Zinc deficiency is associated with high incidences of esophageal and other cancers in humans and leads to a highly proliferative hyperplastic condition in the upper gastrointestinal tract in laboratory rodents. Zn replenishment reduces the incidence of lingual, esophageal and forestomach tumors in Zn-deficient rats and mice. While previous animal studies focused on Zn deficiency, we have investigated the effect of Zn supplementation on carcinogenesis in Zn-sufficient mice of wild-type and tumor suppressor-deficient mouse strains. All mice received N-nitrosomethylbenzylamine and half the mice of each strain then received Zn supplementation. At killing, mice without Zn supplementation had developed more tumors than Zn-supplemented mice: wild-type C57BL/6 mice developed an average of 7.0 versus 5.0 tumors for Zn supplemented (P < 0.05); Zn-supplemented Fhit−/− mice averaged 5.7 versus 8.0 for control mice (P < 0.01); Zn-supplemented Fhit−/−Nit1−/− mice averaged 5.4 versus 9.2 for control mice (P < 0.01) and Zn-supplemented Fhit−/−Rassf1a−/− (the murine gene) mice averaged 5.9 versus 9.1 for control mice (P < 0.01). Zn supplementation reduced tumor burdens by 28% (wild-type) to 42% (Fhit−/−Nit1−/−). Histological analysis of forestomach tissues also showed significant decreases in severity of preneoplastic and neoplastic lesions in Zn-supplemented cohorts of each mouse strain. Thus, Zn supplementation significantly reduced tumor burdens in mice with multiple tumor suppressor deficiencies. When Zn supplementation was begun at 7 weeks after the final carcinogen dose, the reduction in tumor burden was the same as observed when supplementation began immediately after carcinogen dosing, suggesting that Zn supplementation may affect tumor progression rather than tumor initiation.
Introduction
Analysis of esophageal cancer rates in high- and low-incidence areas of China has shown an inverse correlation between esophageal cancer mortality rate and the contents of magnesium, molybdenum and Zn in the hair samples (1); and in Africa, Zn deficiency was found to be associated with a high incidence of esophageal cancer (2). Also, Doerr et al. (3,4) in the USA reported that Zn status in plasma and white blood cells is a better indicator of tumor burden and stage of the disease in head and neck cancer patients than overall nutritional status.
Nearly three decades ago, Fong et al. began using rodent models to investigate the effect of Zn deficiency on tumor development. Rats and mice were made Zn-deficient through maintenance on a Zn-deficient diet and treated with carcinogens, such as 4-nitroquinoline 1-oxide and nitrosomethylbenzylamine (NMBA) (5–10). The Zn-deficient rodents developed significantly more tumors and severe histopathological lesions than Zn-sufficient control rodents and Fong et al. have elucidated mechanisms underlying the effect of Zn deficiency on the epithelial tissues of the upper gastrointestinal tract. Gene expression profiling studies of Zn-deficient versus Zn-sufficient esophageal tissue identified Zn-responsive genes, including metallothionein-1 (Mt1), keratin 14 (Krt14), carbonic anhydrase II, cyclin B, Cox2 and nuclear factor-kappaB, that were upregulated in Zn-deficient rat esophagus (11,12). In addition, Zn replenishment reversed overexpression of S100A8 (S100 Ca-binding protein A8) and modulated the link between S100A8–RAGE interaction and downstream nuclear factor-kappaB signaling (13).
While previous animal studies focused on the effect of Zn deficiency and replenishment on mechanisms of cancer development, where the effect of Zn replenishment on reversal of the Zn-induced precancerous state was dramatic, we wished to determine if Zn supplementation in mice receiving a Zn-sufficient diet could prevent tumor development or progression. That is, we were interested in a preclinical model to determine if Zn supplementation, an inexpensive regimen, might be an effective cancer prevention treatment. We began these studies using Fhit−/− mice because we had already defined conditions in which 100% of these mice develop NMBA-induced forestomach tumors (14,15), with the forestomach a preclinical model for the human lower esophagus. We were also interested in examining mouse models with complex genetic deficiencies because it is often suggested that mouse tumors are less complex genetically than human tumors, making them less genuine preclinical models; thus we chose to study several mouse strains with double tumor suppressor deficiency. In addition, the majority of esophageal and other upper gastrointestinal tract tumors of humans are deficient for Fhit (human or mouse protein) and other tumor suppressor proteins, including Rassf1a (the protein), so we wished to examine the effect of Zn in tumor models with multiple deficiencies of relevant tumor suppressor proteins, Fhit and Rassf1a. Absence of expression of Nit1 (the murine protein) in doubly deficient Fhit−/−Nit1−/− mice led to significantly increased NMBA-induced forestomach tumor burdens in previous studies (16,17). Rassf1a is a tumor suppressor whose inactivation has been implicated in a wide variety of sporadic human cancers and is frequently lost together with Fhit in tumors. It regulates several biologic processes, including cell cycle progression, apoptosis and microtubule stability (18,19), and Rassf1a-mutated mice develop more spontaneous tumors than wild-type mice (20,21). Fhit-deficient mice are exquisitely sensitive to induction of forestomach and other cancers (14,15,22) and Fhit expression is reduced in >50 of most types of human cancer, including esophageal cancers, both squamous cell and adenocarcinoma (23).
Materials and methods
Mouse genotypes, tumor induction and Zn supplementation
We used four strains of mice of the following genotypes in this study: wild-type C57BL/6 (B6), Fhit−/− (murine fragile histidine triad gene) on B6 background, Fhit−/−Nit1−/− (murine nitrilase 1-related gene) and Fhit−/−Rassf1a−/− on mixed backgrounds. B6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Fhit−/− mice were generated as described (14,15). Fhit−/−Nit1−/− were generated and maintained on a mixed background of 129/SvJ × B6, as previously reported (16,17). Fhit−/− and Fhit−/−Nit1−/− mice were genotyped to confirm correct gene deficiency before tumor induction (14,16). B6129 Rassf1a−/− mice (20) were mated with B6 Fhit−/− animals to obtain double heterozygous mice which were intercrossed. Fhit−/−Rassf1a−/− double-knockout mice were genotyped using polymerase chain reaction amplification methods described previously (14,20).
All procedures involving mice were performed following protocols approved by the Ohio State University Institutional Animal Care and Use Committee. We first performed a pilot study with ∼10 Fhit−/− mice in each of three groups: a group with Zn supplementation begun after the last NMBA dose (Zn1); a group with Zn supplementation begun 7 weeks after the last NMBA dose (Zn7) and the control group receiving no Zn. The main goal of this pilot experiment was to determine if the two different Zn supplement regimens would reduce the tumor burden relative to the control group and if there was any difference when the Zn supplement treatment was delayed. In subsequent experiments, we included the mouse strains with multiple tumor suppressor gene deficiencies to study the effect of Zn supplementation in the absence of these genes. All four genotypes of mice were NMBA treated and then half of the mice (∼25) for each strain were Zn supplemented and half received no Zn.
All mice used in the experiments were 8–10 weeks of age at the start of the protocols and the experiments always began with NMBA treatment on day 1 of the protocols; the three tumor suppressor-deficient cohorts were treated with NMBA (Midwest Research Institute, Kansas City, MO) twice a week at a dosage of 2 mg/kg for 3 weeks. Because wild-type B6 mice are relatively resistant to tumor induction by NMBA (9), B6 mice received eight doses over 4 weeks; Fhit-deficient and FhitNit1-deficient mice on B6 or mixed backgrounds are highly susceptible to NMBA induction of forestomach tumors (14,16), and FhitRassf1a-deficient mice were expected to be highly susceptible; thus, these mouse strains received six NMBA doses over 3 weeks. Immediately after the last NMBA treatment, mice of each strain were randomly divided into control and Zn supplement groups. Control mice received regular water, whereas supplemented mice received Zn-gluconate (Life Extension, Ft Lauderdale, FL) supplemented water (0.086 mg Zn per day per mouse, assuming 7 ml water consumption per day per mouse; the equivalent of ∼200 mg elemental Zn/day for a human, depending on the volume of water consumed by individual mice) continuously for the duration of the experiment. The minimum ‘recommended daily allowance’ for human Zn intake is approximately10–15 mg/day, so the dose for these mice was in large excess. Mice were observed weekly and were killed 16 weeks after the last administration of NMBA. Upon dissection, esophagus, forestomach, glandular stomach, spleen, liver, kidney and intestine were examined for gross changes; changes were observed only in forestomach. Forestomach tumors were measured, enumerated and tissues were fixed and processed for histological and immunohistochemical (IHC) analyses.
To examine protein expression changes early after Zn treatment, six Fhit−/− mice were treated with NMBA as described above and supplied with regular water. Seven weeks after the last NMBA administration, halfway through the tumor development period, three of these mice were switched to Zn-gluconate water, whereas the others continued to receive unsupplemented water. One unsupplemented and one Zn-supplemented mouse were killed on days 1, 3 and 5 after beginning Zn supplementation. Esophagus, forestomach and glandular stomach were examined for tumor burden and fixed for IHC analysis.
Histopathological analysis
Tissues were fixed in buffered formalin, processed and embedded in paraffin. Forestomach and esophagus were cut widthwise and stood on cut ends in paraffin. Slides were stained with hematoxylin and eosin for pathological assessment or with specific antisera for IHC analyses. Squamous hyperplasia and squamous cell carcinoma (in situ and invasive) were enumerated in forestomach by a pathologist (J.L.) who was blinded to treatments of all mice.
Forestomach tissue is designated normal for stratified squamous epithelium with no increased thickness (two to three layers). Squamous hyperplasia shows mildly or severely thickened squamous epithelium covering fibrovascular stalks which may be papillary (or papilloma-like) endophytic or with stromal spikes; in squamous cell carcinoma, the epithelium is totally replaced by dysplastic cells with an intact basal lamina [carcinoma in situ (CIS)] or with stromal invasion through basal membrane (invasive squamous cell carcinoma).
IHC analysis
Specific proteins were chosen for assessment of expression in the forestomach tissues based on previous mechanistic studies showing alteration of expression after Zn replenishment (7,11,13,24). The antisera used in IHC detection of expression in the mouse tissues are listed in Table I, with source and detection method. IHC of mouse forestomachs was performed as described previously (16). Paraffin embedded tissue was cut at 4 μm and placed on positively charged slides. Slides were deparaffinized and rehydrated through xylene and graded ethanol solutions to water. All slides were quenched for 5 min in a 3% hydrogen peroxide solution in water to block endogenous peroxidase.
Table I.
Primary antisera and detection methods used in IHC studies
| Primary antiserum | Source | Description | Dilution | Secondary Ab/Detection Kit |
| Ki67 | Dako | M7249, mouse monoclonal | 1:500 | Biotinylated Rabbit anti-rat/Vectastain Elite |
| Cyclin D1 | Labvision | RM-9104-S, rabbit monoclonal | 1:100 | Biotinylated Goat anti-rabbit/Vectastain Elite |
| Krt14 | Leica Microsystems | NCL-L-LL002, mouse monoclonal | 1:80 | Animal Research Kit (Dako) |
| S100A8 | R&D Systems | MAB3059, rabbit monoclonal | 1:200 | Biotinylated Rabbit anti-rat/Vectastain Elite |
| Mt1 | Dako | M0639, mouse monoclonal | 1:400 | Animal Research Kit (Dako) |
| Bax | Abcam | Rabbit Ab32503 | 1:2500 | Biotinylated Goat anti-rabbit/Vectastain Elite |
Antigen retrieval was in Dako target retrieval solution in a vegetable steamer. The primary antisera, at dilutions indicated in Table I, were placed on slides for 1 h at room temperature. Secondary antiserum was added and incubated for 30 min. The detection system was added and incubated for 30 min and substrate chromogen was added. Slides were counterstained with Richard Allen hematoxylin, dehydrated through graded ethanol solutions and coverslipped. For Bax staining to detect cells expressing this proapoptotic protein, a serum-free protein block (Serum-Free Protein Block, catalog X0909; Dako, Carpinteria, CA) was used to decrease background after the primary antiserum incubation.
Statistical analyses
The aim of the pilot study was to investigate the effect of Zn supplementation and the difference between two Zn treatment groups. A general linear model with fixed treatment effects was used to test the primary hypothesis about the differences in the number of tumors per mouse between Zn and the control cohorts. The secondary hypothesis test compared the Zn treated cohorts; a general linear model with fixed effects of treatment, genotype and their interaction was used to test the hypothesis concerning differences between the Zn treatment and no Zn treatment across all four different genotypes. If Zn treatment works through any of these genes, the Zn supplementation effect may be suppressed in mice with specific tumor suppressor deficiencies, and interaction between the treatment and the genotype would be significant. To account for the multiple testing between the B6 mice and the three gene deficiency genotypes, all P values were adjusted using Holmes procedure. In addition, to support our results, the proportion of the tumors with different diameters and the incidence of normal or hyperplasia without dysplasia versus dysplasia or CIS were compared between the Zn supplemented and control cohorts using chi-square tests.
Results
NMBA-induced tumor incidence in Zn-sufficient Fhit−/− mice was reduced by Zn supplementation
Small cohorts of Fhit−/−mice were treated with six NMBA doses and 14 mice received Zn gluconate in drinking water after the final NMBA dose (Zn1), 7 received Zn supplementation at 7 weeks after the final NMBA dose (Zn7) and 7 received no Zn supplement (no Zn). At 14 weeks after NMBA treatment, mice were killed and gross appearance of esophagi and forestomachs were recorded, tumors enumerated, as summarized in Table II. The epithelial lining of the forestomachs of the no Zn group was thicker and tumors of the squamocolumnar junctions fused, whereas in the Zn-supplemented groups, forestomachs were thin and squamocolumnar junctions clear of tumor or hyperplasia.
Table II.
Effect of Zn supplementation on the incidence of tumors in Fhit−/− mice
| Treatment | Totalmice | Mice bearing tumors | Tumors per mouse (mean ± SE) |
aTumors of specific diameter per mouse |
||
| ≤0.5 mm | 0.5–2 mm | ≥2 mm | ||||
| No Zn | 7 | 7 (100%) | 8.00 ± 0.98 | 6.00 | 1.00 | 1.00 |
| Zn1: Zn supplement starting after last NMBA dose | 14 | 13 (92.6%) | 3.14 ± 0.49b | 2.43b | 0.21 | 0.50 |
| Zn7: Zn supplement starting 7 weeks after last NMBA dose | 9 | 9 (100%) | 3.67 ± 0.96b | 2.89b | 0.67 | 0.11a |
Mice were separated into three treatment groups: no Zn supplementation, Zn supplementation starting immediately (Zn1) or Zn supplementation begun 7 weeks after NMBA administration (Zn7). Mice were killed and esophagus, stomach, liver, kidney, spleen and intestine grossly examined at 14 weeks after final dose of NMBA. Tumors were found only in forestomach and were measured and enumerated. Statistical analyses showed significant differences between control and the two Zn-supplemented groups (P < 0.01). There was no significant difference between two Zn treatment groups.
Significantly lower than no Zn supplement group with P value ≤ 0.05.
Significantly lower than no Zn supplement group with P value ≤ 0.01.
The two Zn-supplemented cohorts, Zn1 and Zn7, had 3.14 ± 0.49 and 3.67 ± 0.96 (mean ± standard error) tumors per mouse, respectively, whereas the no Zn group showed an average of 8.0 ± 0.98 tumors per mouse (Table II). For the primary hypothesis, Zn1 cohorts had statistically significant fewer tumors per mouse than the no Zn cohort (mean and standard error of 4.86 ± 0.92, P < 0.001). The mean difference between the Zn1 and Zn7 cohorts was not significant (0.52 ± 0.85, P = 0.81). In addition, the tumor burden distributions, as measured by the proportion of tumors with specific diameters, were similar among all three cohorts, with P values of 0.60, 0.08 and 0.27 between Zn1 and control, between Zn1 and Zn7 and between Zn7 and control, respectively. Because we know from previous experiments (25) that at 7 weeks after NMBA dosing, the mice have multiple forestomach tumors, these results suggested that Zn supplementation effects progression rather than initiation of the tumors.
Zn supplementation lowers tumor burden in mice with multiple tumor suppressor deficiencies
Because human esophageal cancers sustain numerous genetic alterations during development, including loss of expression of Fhit, Rassf1a, other tumor suppressors, as well as activation of oncogenes (18,26), we were interested in testing the effects of Zn supplementation in mice with similarly complex genetic aberrations. Thus, we next examined the effects of Zn supplementation on NMBA induction of forestomach tumors in larger cohorts of 8-week old Fhit deficient, Fhit−/−Nit1−/− and Fhit−/−Rassf1a−/− and B6 mice. All the genetically modified mice received six doses of NMBA by oral gavage starting at day 1 of the experiments. For comparison, wild-type B6 mice, also beginning on day 1 of the experiment, were treated with eight doses due to relative resistance to NMBA induction of forestomach tumors, and mice were observed frequently during the 16-week-tumor development period. Half the mice of each strain received Zn supplementation in drinking water immediately after the final NMBA dose. Mice appeared healthy throughout the observation period and all were killed at 16 weeks; esophagi and forestomachs were opened longitudinally, photographed, tumors enumerated and tissues fixed for histological and IHC analyses.
Table III shows a summary of tumor burdens of Zn supplemented (Zn) and non-supplemented (no Zn or control) mice and Figure 1 shows representative photographs illustrating appearance of 16 week forestomachs of mice of two mouse strains with and without Zn supplementation. Zn had a beneficial effect on all four mouse genotypes. The mean reductions in tumor numbers per mouse for B6, Fhit−/−, Fhit−/−Nit1−/− and Fhit−/−Rassf1a−/− mice were 2.00 ± 0.75, 2.28 ± 0.69, 3.82 ± 0.77, 3.19 ± 0.75 (mean ± standard error), respectively, which were all statistically significant (P values < 0.01). When compared with the B6 (wild-type) mice, the effect of Zn supplementation was not significantly different for Fhit−/−, Fhit−/−Nit1−/− and Fhit−/−Rassf1a−/− mice with P values of 0.79, 0.09 and 0.27, respectively. Also, the distributions of the numbers of tumors with specific diameters were similar between the Zn and the no Zn cohorts for each genotype of mice (all P > 0.05 based on chi-square tests).
Table III.
Effect of Zn supplementation on NMBA-induced tumor development in four mouse strains
| Genotype | Treatment | Total mice | Tumors per mouse (mean ± SE) | # Tumors of specific diameter per mouse |
||
| ≤0.5 mm | ∼1 mm | ≥2 mm | ||||
| B6 | No Zn | 24 | 7.00 ± 1.21 | 4.08 | 2.04 | 0.88 |
| Zn | 29 | 5.00 ± 0.74a | 3.21 | 1 | 0.79 | |
| Fhit−/− | No Zn | 34 | 8.00 ± 0.69 | 5.41 | 1.62 | 0.97 |
| Zn | 29 | 5.72 ± 1.01a | 3.93 | 1.24 | 0.55 | |
| Fhit−/−Nit1−/− | No Zn | 24 | 9.21 ± 1.63 | 4.46 | 3.21 | 1.54 |
| Zn | 26 | 5.39 ± 0.82a | 3.23 | 1.31 | 0.85 | |
| Fhit−/−Rassf1a−/− | No Zn | 26 | 9.12 ± 1.54 | 5.35 | 2.58 | 1.19 |
| Zn | 27 | 5.93 ± 0.59a | 3.48 | 1.52 | 0.93 | |
Immediately after the final NMBA dose, mice were supplied with regular or Zn-supplemented water. Mice were killed and esophagus, stomach, liver, kidney, spleen and intestine grossly examined at 16 weeks after final NMBA treatment. All mice developed tumors. Statistical analyses showed significant differences between control and Zn-supplemented groups (P < 0.0001), and the reduction of number of total tumors per mouse due to Zn treatment was similar for all genotypes (P = 0.30). In addition, the numbers of tumors with specific diameter were all distributed similarly between control and Zn-supplemented groups based on chi-square test (P = 0.07).
Significantly lower tumor numbers per mouse than no Zn supplement group, P value ≤ 0.01.
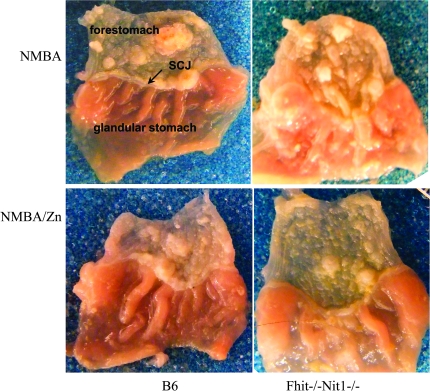
Fig. 1.
Representative photographs of forestomachs of NMBA treated and NMBA-treated Zn-supplemented mice. Gross appearance of forestomachs of B6 and Fhit−/−Nit1−/− mouse pairs, killed at 16 weeks after NMBA treatment and Zn supplemented or unsupplemented water. Note the thickened squamocolumnar junction of forestomach with glandular stomach tissue in photo at upper right, and the differences in tumor numbers in the unsupplemented versus Zn-supplemented tissues.
The results also showed that the B6 mice developed smaller numbers of tumors per mouse (7.00 ± 1.21) than tumor suppressor-deficient mice (8.00 ± 0.69, 9.21 ± 1.63 and 9.12 ± 1.54 for Fhit−/−, Fhit−/−Nit1−/− and Fhit−/−Rassf1a−/− mice, respectively) even though they received two additional NMBA doses; they were not as well protected by Zn supplementation in terms of the reduction of the number of tumors per mouse due to the Zn treatment. The cumulative results for B6 mice were influenced by differences in tumor burdens and Zn response of B6 males and females. B6 males showed fewer tumors (average 5.33 versus 8.1 for females) and showed very little protection by Zn (4.9 tumors per mouse for males and females); on the other hand, the Fhit−/− mice are also on B6 background and did not show a significant disparity in tumor burden by sex (7.8 for females versus 8.2 for males). Clarifying the basis for male/female difference for this strain of wild-type B6 mice will require further investigation.
Histopathological analysis of forestomach lesions
Fixed forestomach tissues of all mice of each strain were processed, embedded and mounted for histopathological analyses (by J.L., blinded to strain and treatment). Several 4 μm sections from each forestomach were scored for frequency of mild hyperplasia, severe hyperplasia and carcinoma; results are summarized in Table IV and sections illustrating tissues with normal histology, hyperplasia and squamous cell CIS in an Fhit−/−Nit1−/− forestomach are shown in Figure 2. CIS were found in multiple non-supplemented B6, Fhit−/−Nit1−/− and Fhit−/−Rassf1a−/− mice but in only one Zn-supplemented Fhit−/−Rassf1a−/− mouse (Table III). Examples of forestomach tissues with normal and mildly hyperplastic histology were more frequent among the Zn-supplemented forestomachs of each mouse strain, except for mild hyperplasia in Fhit−/− tissue. Tissues with severe hyperplasia were also greatly reduced in B6 and Fhit−/−Rassf1a−/− forestomachs.
Table IV.
Histopathological analysis of forestomach lesions
| Genotype | Treatment | Total mice | Incidence |
|||||
| Missing sample | Normal | Mild hyperplasia | Severe hyperplasia | Hyperplasia with dysplasiaa | Carcinoma | |||
| B6 | No Zn | 24 | 0 | 0 | 1 (4%) | 19 (79%) | 11 (46%) | 4 (17%) |
| Zn | 29 | 1 | 3 (11%) | 17 (61%) | 8 (29%) | 6 (21%) | 0 | |
| Fhit−/− | No Zn | 34 | 1 | 4 (12%) | 25 (76%) | 4 (12%) | 7 (21%) | 0 |
| Zn | 29 | 0 | 6 (21%) | 18 (62%) | 5 (17%) | 2 (7%) | 0 | |
| Fhit−/−Nit1−/− | No Zn | 24 | 2 | 0 | 2 (9%) | 16 (73%) | 8 (36%) | 4 (18%) |
| Zn | 26 | 1 | 2 (8%) | 6 (24%) | 17 (68%) | 3 (12%) | 0 | |
| Fhit−/−Rassf1a−/− | No Zn | 26 | 0 | 0 | 7 (27%) | 17 (65%) | 7 (27%) | 2 (8%) |
| Zn | 27 | 0 | 1 (4%) | 13 (48%) | 12 (44%) | 6 (22%) | 1 (4%) | |
The hyperplasia with dysplasia column lists the number of mice with mild or severe hyperplasia, that also exhibited dysplasia. chi-square test showed significant differences between Zn and no Zn treatment for B6 (P = 0.003) and Fhit−/−Nit1−/− (P = 0.002) mice in terms of the number of mice with carcinoma and dysplasia (hyperplasia w dysplasia + carcinoma) versus those without dysplasia (normal + mild + severe hyperplasia—hyperplasia w dysplasia), and the P values for Fhit−/− and Fhit−/−Rassf1a−/− mice were 0.11 and 0.491, respectively.
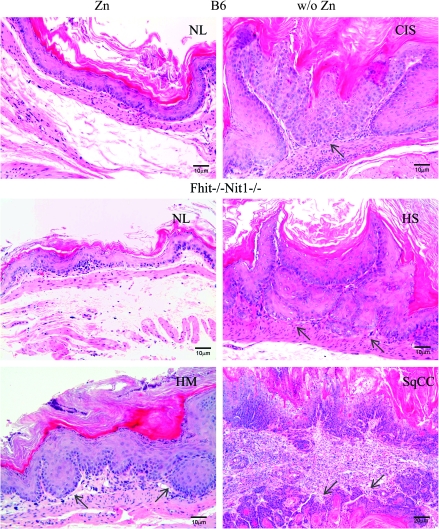
Fig. 2.
Histopathology of representative forestomach tissues of unsupplemented and Zn-supplemented mice. In the left panels from mice that received Zn supplementation immediately after final carcinogen treatment are photomicrographs of hematoxylin- and eosin-stained forestomach tissues: normal mouse forestomach (NL) tissue sections with two to three layers keratinizing stratified squamous epithelium without cytological and nuclear atypia or architectural dysplasia (left, top two panels) and mild hyperplasia (HM, lower left panel) in which the squamous epithelium is up to 10 layers thick and focal nuclear atypia may be present, indicated by arrow. In the right panels from mice that did not receive Zn supplementation are photomicrographs of hematoxylin- and eosin-stained forestomach tissues: CIS (carcinoma confined to epithelium with intact basement membrane, top right panel), indicated by arrow; severe hyperplasia (HS, squamous epithelium >10 layers thick with or without nuclear atypia and papillomatous change, middle right panel), indicated by arrows; squamous cell carcinoma (SqCC, right bottom panel) with invasion through the basement membrane; arrows in SqCC denote well-differentiated invasive SqCC with keratin pearls. The tumor cells show loss of maturation and loss of nuclear polarity. Nuclei are enlarged with high nuclear/cytoplasmic ratio and denser hyperchromasia. Mitotic activity is frequently present and atypical keratinizing squamous epithelium is also seen.
IHC analyses of forestomach tissues early and late after Zn supplementation
Fong et al. have examined mechanisms involved in effects of Zn replenishment on reversal of hyperplastic esophageal tissues early after replenishment by Zn-gluconate bolus. Zn replenishment of NMBA-treated Zn-deficient rats rapidly induced apoptosis in esophageal epithelial cells and reduced development of esophageal cancer (10). To identify Zn-responsive genes, gene expression profiles were assessed in precancerous Zn-deficient and Zn-replenished esophageal epithelial layers. The Zn-sensitive gene metallothionein-1 (Mt1) was upregulated 7-fold, and the keratin 14 (Krt14) gene, a biomarker in esophageal tumorigenesis, was upregulated >4-fold in the Zn-deficient condition. IHC showed that Mt1 and Krt14 proteins were overexpressed in Zn-deficient esophagus, as well as in lingual and esophageal squamous cell carcinoma (ESCC) from carcinogen-treated rats, emphasizing their roles in carcinogenesis (11,13).
It seemed probably that similar signal pathways would participate in the tumor reduction effects of Zn supplementation in these NMBA-treated mouse strains. To investigate this possibility, we examined expression of several proteins, Krt14, Mt1 and Ki67, in forestomach tissues of NMBA-treated Fhit−/− mice at 7 weeks after final NMBA dose and 1, 3 and 5 days after receiving Zn-supplemented water or continuing on non-supplemented water. We examined several sections from forestomachs of each mouse, stained for each protein listed in Table I, for all mice killed at 16 weeks after Zn supplementation. IHC results, with or without Zn supplementation, did not show differences in expression levels of Ki67, Cyclin D1 or S100A8, though due to overall lower level lesions, there was an impression of fewer strongly staining cell layers in the Zn-supplemented tissues (data not shown). For Mt1 and Krt14 staining, there were fewer areas of thick epithelial layers with strong staining (see Figure 3A, without Zn panels versus Zn panels for representative photos); also there were areas without staining (see Figure 3A, Zn, Mt1, far right) and areas with one or two cell layers stained versus >10 cell layers stained for without Zn tissues. This difference between without Zn and Zn supplementation is no doubt driven by the overall lower level of lesions in the tissues of Zn-supplemented mice of each strain.
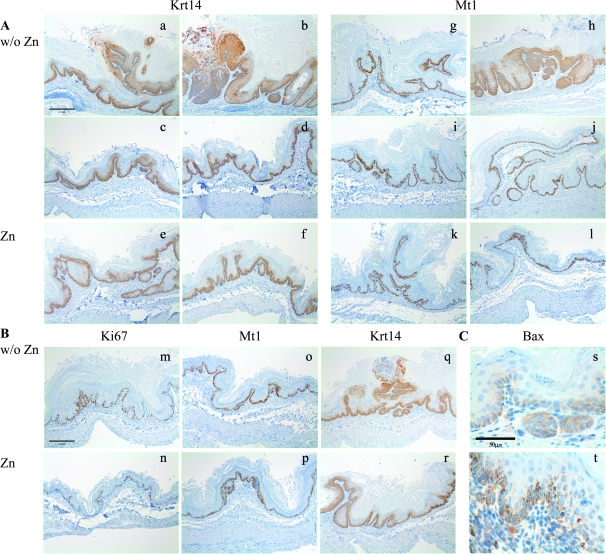
Fig. 3.
IHC detection of Krt-14, Mt-1, Ki67 and Bax in forestomach tissues of with and without Zn supplementation. (A) IHC analysis late after Zn supplementation: (a–f) Krt14 staining and (g–l) Mt-1 staining. Zn supplementation led to a reduction in the number of epithelial layers expressing of Krt14 and Mt-1 in lesions of approximately equal thickness, as shown in the photographs (magnification: ×100, bar denotes 200 μm). (B) IHC analysis early after Zn supplementation: (m and n) Ki67, (o and p) Mt1 and (q and r) Krt14. Ki67, Mt1 and Krt14 were expressed in fewer epithelial layers within days after Zn supplementation, as shown in the representative photographs (magnification: ×100, bar denotes 200 μm) (C) IHC showed increased mitochondrial Bax staining in 16-week-Zn-supplemented mouse forestomach as compared with non-supplemented forestomach tissue (magnification: ×600, bar denotes 50 μm). In complete forestomach sections, the fraction of cells expressing proapoptotic mitochondrial Bax protein in Zn-supplemented forestomach was 21.5 ± 6.3% versus 6.6 ± 2.4% in forestomach sections of mice not receiving Zn supplementation.
The analysis of IHC results for assessment of protein expression early after Zn supplementation, at time points, we expected to reveal changes similar to that reported for Zn replenishment of Zn-deficient rodents; we did observe an overall lower level expression of Ki67, Mt1 and Krt14, as illustrated in Figure 3B photos of tissues without Zn and with Zn supplementation, differences that should not be due to lesion differences so soon after supplementation. The photos show comparisons of areas of similar hyperplasia level. We did not see a dramatic difference, as reported previously for Zn-deficient mice, replenished with an oral bolus of Zn gluconate (10,13).
Apoptosis in tissues of Zn-supplemented mice
We also examined frequency of expression of the proapoptotic Bax protein in the forestomach tissues of 16-week-Zn-supplemented mice versus the tissues of the non-supplemented control mice, by IHC and observed a larger fraction (21.5 ± 6.3% versus ∼6.6 ± 2.4%, see Figure 3C legend) of Bax-positive cells in forestomach tissues of the Zn-supplemented tissues. Representative photos of Bax staining in forestomach tissue sections of non-supplemented and supplemented mice are shown in Figure 3C.
Discussion
ESCC is among the most common cancers in the world (1). ESCC is the predominant histological subtype and is more common in Asia, with esophageal adenocarcinoma increasing in the west. ESCC patients are frequently diagnosed at an advanced stage of the disease and have a poor prognosis, with an overall 5 years survival rate at ∼10% worldwide. Epidemiologic studies have implicated dietary Zn deficiency in the etiology of ESCC (2,3). Abnet et al. (27) provided the strongest evidence of an association between dietary Zn deficiency and esophageal cancer in humans by establishing an inverse relationship between zinc concentration in biopsy samples from a high ESCC incidence area in China and subsequent risk of developing cancer.
Because of the epidemiological link between Zn deficiency and ESCC, we were interested in determining, in a Zn-sufficient model system, if supplementation with a dose of Zn attainable with Zn supplemental capsules meant for human use could affect esophageal tumor development after massive carcinogen doses that induce tumors in mice.
This study demonstrated that Zn supplementation significantly reduced forestomach/lower esophagus tumor burdens in wild-type and tumor suppressor-deficient mice, even after multiple massive carcinogen exposures. There was no indication that expression of these tumor suppressor genes was associated with pathways through which Zn supplementation suppresses tumor development.
As mentioned above, we did not see dramatic differences in expression of the Zn-responsive proteins discovered by replenishing Zn-deficient mice (10,13), possibly because the mice are not Zn-deficient so supplementation does not provide a stark change in protein expression. It is also possible that the large bolus of Zn gluconate used in Zn-deficient rodent studies contributed to a dramatic change not duplicated by the gradual Zn supplementation provided in the drinking water in this study. The 3-fold increased fraction of mitochondrial Bax-expressing cells in forestomach tissue of Zn-supplemented mice relative to non-supplemented mice suggests that apoptosis induced by Zn supplementation contributes to the suppression and/or regression of neoplastic and preneoplastic lesions in these initially Zn-sufficient supplemented animals. These preclinical studies suggest that Zn supplementation might be an effective adjunct to treatment of upper gastrointestinal tract cancers and might contribute to preventive measures. We have not yet determined an optimum dose for the mice and are aware of contraindications for long-term high-dose Zn supplementation in humans.
Zn is an essential micronutrient for human metabolism needed for catalysis of >200 enzymes, protein folding and regulation of gene expression and malnourished individuals are at an increased risk of Zn deficiency. Symptoms of Zn deficiency include growth retardation, diarrhea, alopecia, decreased immunity and hypogonadism in males and Zn supplementation may be effective for the prevention of upper respiratory infection and diarrhea. Zn is well tolerated at recommended dosages. Adverse effects of long-term extreme high-dose zinc use include suppressed immunity, decreased high-density lipoprotein cholesterol levels, anemia, copper deficiency, possible genitourinary complications and neuropathy (for review, see refs. 28,29). Thus, before considering Zn supplementation for use in cancer prevention or treatment, safe doses for long-term usage must be determined.
Lin et al. (30) examined the effect of Zn supplementation on survival of patients with advanced nasopharyngeal carcinoma receiving concomitant chemotherapy and radiotherapy and concluded that Zn supplementation attenuated local tumor recurrence and improved overall survival of patients with advanced nasopharyngeal carcinoma but failed to reduce distant metastasis survival. And Lin et al. (31) evaluated the impact of zinc supplementation on the survival of patients after receiving radiotherapy for head and neck cancers. One impact observed was that Zn supplementation improved local recurrence-free survival at 3 years after beginning treatment for patients with Stages III–IV disease. In both of the above studies, the advanced stage disease of the patients may have contributed to the modest but significant effect of short-term Zn supplementation.
Several other animal models examining the role of Zn in prevention or treatment of colon cancer or prostate tumors, respectively, have been reported (32,33). We believe the cumulative evidence suggests that Zn supplementation at carefully determined dosages and lengths of treatment could have an important role in prevention and perhaps treatment of specific human cancers and should be more thoroughly studied in preclinical models.
Funding
National Cancer Institute (CA115965, CA132453, CA118560); Ohio State University Comprehensive Cancer Center.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CIS
carcinoma in situ
- ESCC
esophageal squamous cell carcinoma
- NMBA
N-nitrosomethylbenzylamine
- IHC
immunohistochemical
References
- 1.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 2.Joint Iran-International Agency for Research on Cancer Study Group. Esophageal cancer studies in the Caspian littoral of Iran: results of population studies—a prodrome. J. Natl Cancer Inst. 1977;59:1127–1138. [PubMed] [Google Scholar]
- 3.Doerr TD, et al. Zinc deficiency in head and neck cancer patients. J. Am. Coll. Nutr. 1997;16:418–422. doi: 10.1080/07315724.1997.10718707. [DOI] [PubMed] [Google Scholar]
- 4.Doerr TD, et al. Effects of zinc and nutritional status on clinical outcomes in head and neck cancer. Nutrition. 1998;14:489–495. doi: 10.1016/s0899-9007(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 5.Fong LY, et al. Zinc deficiency and methylbenzylnitrosamine-induced esophageal cancer in rats. J. Natl Cancer Inst. 1978;61:145–150. doi: 10.1093/jnci/61.1.145. [DOI] [PubMed] [Google Scholar]
- 6.Fong LY, et al. Nitrosobenzylmethylamine, zinc deficiency and oesophageal cancer. IARC Sci. Publ. 1978;19:503–513. [PubMed] [Google Scholar]
- 7.Fong LY, et al. Cell proliferation and esophageal carcinogenesis in the zinc-deficient rat. Carcinogenesis. 1996;17:1841–1848. doi: 10.1093/carcin/17.9.1841. [DOI] [PubMed] [Google Scholar]
- 8.Fong LY, et al. Zinc replenishment reduces esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in zinc-deficient rats. Carcinogenesis. 1998;19:1591–1596. doi: 10.1093/carcin/19.9.1591. [DOI] [PubMed] [Google Scholar]
- 9.Fong LY, et al. Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Lett. 1999;143:63–69. doi: 10.1016/s0304-3835(99)00191-3. [DOI] [PubMed] [Google Scholar]
- 10.Fong LY, et al. Esophageal cancer prevention in zinc-deficient rats: rapid induction of apoptosis by replenishing zinc. J. Natl Cancer Inst. 2001;93:1525–1533. doi: 10.1093/jnci/93.20.1525. [DOI] [PubMed] [Google Scholar]
- 11.Liu CG, et al. Modulation of gene expression in precancerous rat esophagus by dietary zinc deficit and replenishment. Cancer Res. 2005;65:7790–7799. doi: 10.1158/0008-5472.CAN-05-1345. [DOI] [PubMed] [Google Scholar]
- 12.Fong L, et al. Prevention of upper aerodigestive tract cancer in zinc-deficient rodents: inefficacy of genetic or pharmacological disruption of COX-2. Int. J. Cancer. 2008;122:978. doi: 10.1002/ijc.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taccioli C, et al. Zinc replenishment reverses overexpression of the proinflammatory mediator S100A8 and esophageal preneoplasia in the rat. Gastroenterology. 2009;136:953. doi: 10.1053/j.gastro.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong L, et al. Muir-Torre-like syndrome in Fhit-deficient mice. Proc. Natl Acad. Sci. USA. 2000;97:4742–4747. doi: 10.1073/pnas.080063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanesi N, et al. The tumor spectrum in FHIT-deficient mice. Proc. Natl Acad. Sci. USA. 2001;98:10250–10255. doi: 10.1073/pnas.191345898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, et al. Nit1 and Fhit tumor suppressor activities are additive. J. Cell. Biochem. 2009;107:1097–1106. doi: 10.1002/jcb.22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semba S, et al. Biological functions of mammalian Nit1, the counterpart of the invertebrate NitFhit Rosetta stone protein, a possible tumor suppressor. J. Biol. Chem. 2006;281:28244–28253. doi: 10.1074/jbc.M603590200. [DOI] [PubMed] [Google Scholar]
- 18.Donninger H, et al. The RASSF1A tumor suppressor. J. Cell Sci. 2007;120:3163–3172. doi: 10.1242/jcs.010389. [DOI] [PubMed] [Google Scholar]
- 19.Palakurthy RK, et al. Epigenetic silencing of the RASSF1A tumor suppressor gene through HOXB3-mediated induction of DNMT3B expression. Mol. Cell. 2009;36:219–230. doi: 10.1016/j.molcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tommasi S, et al. Tumor susceptibility of Rassf1a knockout mice. Cancer Res. 2005;65:92–98. [PubMed] [Google Scholar]
- 21.van der Weyden L, et al. Megaoesophagus in Rassf1a-null mice. Int. J. Exp. Pathol. 2009;90:101–108. doi: 10.1111/j.1365-2613.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanesi N, et al. Lung cancer susceptibility in Fhit-deficient mice is increased by Vhl haploinsufficiency. Cancer Res. 2005;65:6576–6582. doi: 10.1158/0008-5472.CAN-05-1128. [DOI] [PubMed] [Google Scholar]
- 23.Pichiorri F, et al. Fhit tumor suppressor: guardian of the preneoplastic genome. Future Oncol. 2008;4:815–824. doi: 10.2217/14796694.4.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fong LY, et al. Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats. J. Natl Cancer Inst. 2005;97:40–50. doi: 10.1093/jnci/dji006. [DOI] [PubMed] [Google Scholar]
- 25.Ishii H, et al. Regression of upper gastric cancer in mice by FHIT gene delivery. FASEB J. 2003;17:1768–1770. doi: 10.1096/fj.03-0241fje. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Ordonez B, et al. Molecular biology of squamous cell carcinoma of the head and neck. J. Clin. Pathol. 2006;59:445–453. doi: 10.1136/jcp.2003.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abnet CC, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J. Natl Cancer Inst. 2005;97:301–306. doi: 10.1093/jnci/dji042. [DOI] [PubMed] [Google Scholar]
- 28.Saper RB, et al. Zinc: an essential micronutrient. Am. Fam. Physician. 2009;79:768–772. [PMC free article] [PubMed] [Google Scholar]
- 29.Nations SP, et al. Denture cream: an unusual source of excess zinc, leading to hypocupremia and neurologic disease. Neurology. 2008;71:639–643. doi: 10.1212/01.wnl.0000312375.79881.94. [DOI] [PubMed] [Google Scholar]
- 30.Lin YS, et al. Effects of zinc supplementation on the survival of patients who received concomitant chemotherapy and radiotherapy for advanced nasopharyngeal carcinoma: follow-up of a double-blind randomized study with subgroup analysis. Laryngoscope. 2009;119:1348–1352. doi: 10.1002/lary.20524. [DOI] [PubMed] [Google Scholar]
- 31.Lin LC, et al. Effects of zinc supplementation on clinical outcomes in patients receiving radiotherapy for head and neck cancers: a double-blinded randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:368–373. doi: 10.1016/j.ijrobp.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 32.Dani V, et al. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicol. Lett. 2007;171:10–18. doi: 10.1016/j.toxlet.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Shah MR, et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J. Exp. Clin. Cancer Res. 2009;28:84. doi: 10.1186/1756-9966-28-84. [DOI] [PMC free article] [PubMed] [Google Scholar]