Abstract
MicroRNAs are small, highly conserved non-coding RNA molecules involved in the regulation of gene expression. MicroRNAs are transcribed by RNA polymerases II and III, generating precursors that undergo a series of cleavage events to form mature microRNA. The conventional biogenesis pathway consists of two cleavage events, one nuclear and one cytoplasmic. However, alternative biogenesis pathways exist that differ in the number of cleavage events and enzymes responsible. How microRNA precursors are sorted to the different pathways is unclear but appears to be determined by the site of origin of the microRNA, its sequence and thermodynamic stability. The regulatory functions of microRNAs are accomplished through the RNA-induced silencing complex (RISC). MicroRNA assembles into RISC, activating the complex to target messenger RNA (mRNA) specified by the microRNA. Various RISC assembly models have been proposed and research continues to explore the mechanism(s) of RISC loading and activation. The degree and nature of the complementarity between the microRNA and target determine the gene silencing mechanism, slicer-dependent mRNA degradation or slicer-independent translation inhibition. Recent evidence indicates that P-bodies are essential for microRNA-mediated gene silencing and that RISC assembly and silencing occurs primarily within P-bodies. The P-body model outlines microRNA sorting and shuttling between specialized P-body compartments that house enzymes required for slicer –dependent and –independent silencing, addressing the reversibility of these silencing mechanisms. Detailed knowledge of the microRNA pathways is essential for understanding their physiological role and the implications associated with dysfunction and dysregulation.
Keywords: MicroRNA, RNA interference (RNAi), Post-transcriptional gene regulation, Cancer.
INTRODUCTION
MicroRNA (miRNA), originally discovered in Caenorhabditis elegans, is found in most eukaryotes, including humans [1-3]. It is predicted that miRNA account for 1-5% of the human genome and regulate at least 30% of protein-coding genes [4-8]. To date, 940 distinct miRNAs molecules have been identified within the human genome [9-12] (http://microrna.sanger.ac.uk accessed July 20, 2010). Although little is currently known about the specific targets and biological functions of miRNA molecules thus far, it is evident that miRNA plays a crucial role in the regulation of gene expression controlling diverse cellular and metabolic pathways [13-19].
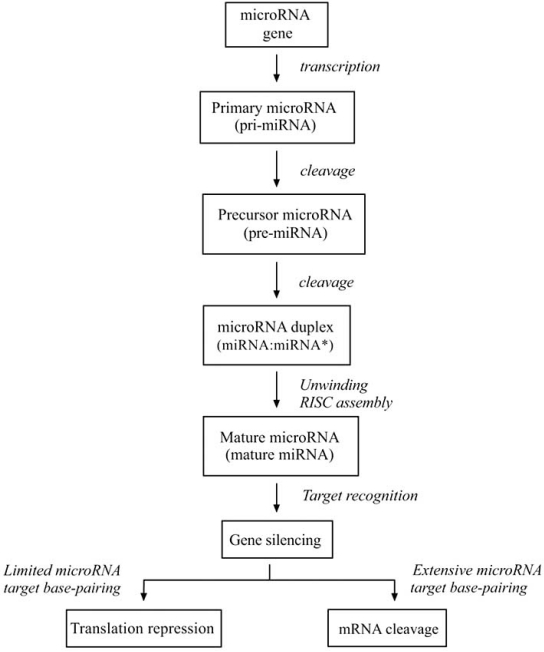
MiRNA are small, evolutionary conserved, single-stranded, non-coding RNA molecules that bind target mRNA to prevent protein production by one of two distinct mechanisms. Mature miRNA is generated through two-step cleavage of primary miRNA (pri-miRNA), which incorporates into the effector complex RNA-induced silencing complex (RISC). The miRNA functions as a guide by base-pairing with target mRNA to negatively regulate its expression. The level of complementarity between the guide and mRNA target determines which silencing mechanism will be employed; cleavage of target messenger RNA (mRNA) with subsequent degradation or translation inhibition Fig. (1) [1, 20].
Fig. (1). MicroRNA maturation and function.
The miRNA gene is transcribed to generate a primary microRNA (pri-miRNA) precursor molecule that undergoes nuclear cleavage to form a precursor microRNA (pre-miRNA). The pre-miRNA is cleaved in the cytoplasm to create a microRNA duplex (miRNA:miRNA*, passenger strand designated with asterisk) containing the mature miRNA. The duplex unwinds and the mature miRNA assembles into RISC. The miRNA base-pairs with target mRNA to direct gene silencing via mRNA cleavage or translation repression based on the level of complementarity between the miRNA and the mRNA target.
There is a general understanding of miRNA function but the mechanistic details of miRNA biogenesis and gene silencing are still unclear. This new and exciting field of molecular biology continues to advance, having profound implications in medicine. Although the biological function of identified miRNAs may be unknown, examination of the expression profiles of these molecules provides information on their regulation and function. Such observations have indicated that miRNA expression profiles are altered in specific tumors, implying that miRNA may be involved in development of cancer and other diseases [21-27]. Despite the limited knowledge of these molecules, basic expression profiling is proving to be clinically relevant to cancer diagnosis, progressions and outcome [24, 28-31].
This review will explore recent advances in human miRNA biogenesis and function to propose a working model of miRNA gene silencing. Secondly, it will examine miRNA involvement in cancer development and the medical implications.
MICRORNA – IN THE GENOME
There are many classes of small endogenous RNA molecules, such as small transfer RNA (tRNA), ribosomal RNA (rRNA), small nucleolar RNA (snoRNA), small interfering RNA (siRNA) and microRNA (miRNA). MiRNA and siRNA are biochemically and functionally indistinguishable. Both are 19-20 nucleotides (nt) in length with 5’-phosphate and 3’-hydroxyl ends, and assemble into RISC to silence specific gene expression [32-34]. Therefore, these molecules are distinguished based on their respective origins. MicroRNA is derived from the double-stranded region of a 60-70nt RNA hairpin precursor while siRNA is generated from long double-stranded RNA (dsRNA) [20, 32, 34]. Originally all small RNA that mediated post-transcriptional gene silencing via RISC was referred to as siRNA regardless of origin, however now it is common procedure to distinguish between miRNA and siRNA.
MiRNA precursors are commonly found in clusters through many different regions of the genome, most frequently within intergenic regions and introns of protein-coding genes. Historically these regions were referred to as “junk DNA” because their function was unknown. The discovery of miRNA genes in this vast component of our genome implies ‘junk DNA’ is not useless as originally thought. MiRNA precursors are less commonly found within exons of transcripts and in antisense transcripts [35, 36].
MiRNA transcriptional units and their regulation vary with gene loci. Intronic miRNAs located within a host gene, in the same orientation are transcribed along with primary transcript by the same promoter [36, 37]. In contrast, intergenic miRNA presumably rely on their own promoters [35, 36, 38]. The nature of intergenic miRNA transcriptional units is just beginning to unfold. Recent genomic analysis indicates that primary miRNA precursors are long polycistronic transcripts that are similar to mRNA in that they have distinct 5’ and 3’ boundaries, 7-methyl guanylate (m7G) caps and poly(A) tails [12, 38-41].
The discovery of intergenic miRNA and protein-coding intronic miRNA coupled with work of Lee et al. (2004) indicates that the majority of miRNA are transcribed by RNA polymerase II (pol II) [38, 42-44]. Pol II is the catalytic component of the complex of protein responsible for transcribing DNA into mRNA [45-47]. However, miRNA can also be transcribed by RNA polymerase III (pol III) which specifically synthesizes small non-protein coding RNAs that are linked to regulating cell cycle and growth [48-57]. Investigation of the miRNA cluster C19MC on the human chromosome 19 transcribed by pol III revealed that it was interspersed with Alu repeats, a characteristic of pol III transcription [57, 58]. Extrapolating this observation Borchert et al. suggest that pol III transcription of miRNA might be more prevalent than originally thought, noting that at least 50 additional human miRNA loci are among repetitive elements associated with pol III transcript [57]. A recent study by Gu et al. (2010) identified 68 new miRNAs that appear to be transcribed by an Alu-dependent pol III mechanism [59].
The synthesis of miRNA by pol II and pol III implies that miRNA is a fundamental regulatory element generated from diverse loci within the human genome, which are involved in controlling gene expression essential for normal cellular function.
Regulation of miRNA expression remains largely unknown. MiRNA may be expressed in a tissue- or developmental- specific manner [19, 20, 60-62]. Primary miRNA transcripts generated by pol II are presumably regulated similar to protein coding transcripts. MiRNA expression can be controlled by transcription factors and possibly other miRNA in response to a variety of endogenous and exogenous stimuli [63-69]. Regulation of the multiple processing steps in miRNA biogenesis can also influence expression. Proteins such as HnRNPA1, SMAD1 and SMAD5 have been shown to interact with miRNA precursors and regulate their subsequent processing to mature miRNA [70, 71]. Regulatory proteins can also bind mature miRNA to direct their degradation, preventing their expression. Lin28 is an example of such a regulatory protein, it binds let-7 miRNA and targets its degradation [72].
It is estimated that 10% of miRNA expression is controlled through DNA methylation [73]. Additional evidence supports miRNA regulation in response to hypoxia, hormonal and dietary changes [63-65]. MiRNA regulation becomes even more complicated with the discovery of intricate feedback loops, which will be discussed in detail later [67-69, 74].
MICRORNA BIOGENESIS – NUCLEAR FIG. (2)
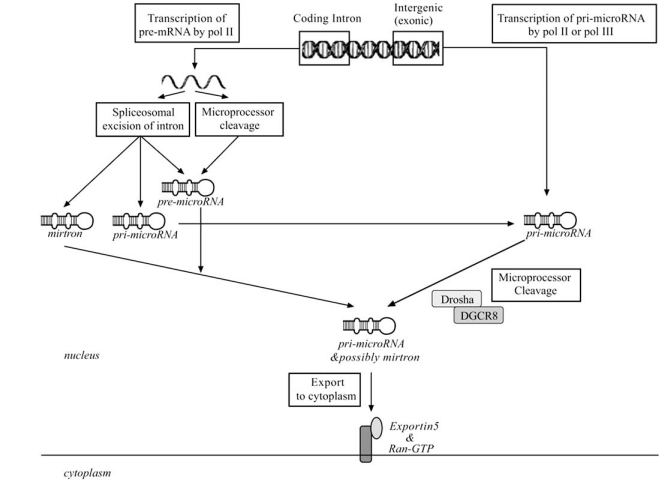
Fig. (2). Nuclear component of microRNA biogenesis.
Intergenic miRNAs are transcribed by RNA polymerase II or III generating a primary miRNA (pri-miRNA) molecule, which is processed into a precursor miRNA (pre-miRNA) by the microprocessor complex comprised of DGCR8 and Drosha. Pre-miRNAs are exported to the cytoplasm in a nucleocytoplasmic transporter containing Exportin 5 and Ran-GTP. MiRNA within introns are transcribed as part of precursor mRNA (pre-mRNA) by RNA polymerase II. The miRNA sequence is excised from the pre-mRNA by spliceosomal components or the microprocessor to liberate a mirtron or a pre-miRNA that is exported. Alternatively, a primary miRNA (pri-miRNA) is released which undergoes microprocessor cleavage to generate pre-miRNA.
Human miRNA biogenesis is a two-step process, with both nuclear and subsequent cytoplasmic cleavage events performed by two ribonuclease III endonucleases, Drosha and Dicer [75-81]. The miRNA gene is transcribed to produce a primary miRNA (pri-miRNA) that is processed into a precursor miRNA (pre-miRNA) and subsequently miRNA duplex (miRNA:miRNA*, passenger strand designated with asterisk) which ultimately releases mature miRNA [44]. MiRNA origin and size appear to determine what nuclear pathway the miRNA will proceed through.
The first determinant is the origin of the miRNA, being either intergenic or coding-intronic [36, 82].
Intergenic microRNA
Intergenic miRNA are transcribed by pol II or pol III producing a pri-miRNA which is a large stem-loop structure with single-stranded RNA extensions at both ends [42, 57, 83]. Only those pri-miRNA with the appropriate stem length, a large flexible terminal loop (≥ 10bp) and the capability of producing 5’ and 3’ single-stranded RNA overhangs will be efficiently processed and mature into functional miRNA [80, 84-88]. The maturation process begins with the nuclear cleavage of pri-miRNA by a protein complex known as “the microprocessor”, which is comprised of the RNase III endonuclease Drosha and the double-stranded RNA-binding protein DiGeorge syndrome critical region gene 8 (DGCR8) [75, 77, 78, 80, 81]. Recent evidence indicates unique cross-regulation between Drosha and DGCR8 is important for control of miRNA biogenesis. In this cross-regulation DGCR8 stabilizes Drosha via protein-protein interaction and assists in controlling Drosha protein levels [89]. Conversely, Drosha in the context of the microprocessor negatively regulates DGCR8 mRNA post-transcriptionally by cleaving an 88 nt hairpin located in the 5’ untranslated region (UTR) which destabilizes the transcript [89, 90]. Additional microprocessor-associated proteins are required for processing of particular pre-miRNAs, such an example is miR-18a which requires the protein factor hnRNPA1 [71, 91]. The most current processing model suggest that DGCR8 recognizes the pri-miRNA at the ssRNA-dsRNA junction and directs Drosha to a specific cleavage site ~11 base pairs (bp) from the junction on the stem where Drosha cuts to liberate a ~ 60-70 bp miRNA hairpin precursor (pre-miRNA) [78, 80, 81, 83, 86, 92, 93]. The pre-miRNA has one end of the mature miRNA defined by the cut from Drosha and contains the mature miRNA in either the 5’ or 3’ arm [76].
Coding-Intronic microRNA
In contrast, miRNA located within an intron of a protein coding gene is transcribed by pol II as part of the pre-mRNA [36]. Evidence supports two possible miRNA excision processes which may be occurring simultaneously or independently [94]. Initial investigation determined that introns are excised out of the pre-mRNA and debranched by spiceosomal components [95, 96]. It is unclear whether the splicing process releases pre-miRNA that proceeds to nuclear export for maturation within the cytoplasm or if a pri-miRNA is released acquiring a secondary stem-loop structure and proceeding like intergenic pri-miRNA to be processed by the microprocessor complex [95, 96]. An additional hypothesized nuclear pathway might be possible for those small (~50-200nt) excised debranched introns containing miRNA, which have the structure to support hairpin formation. Recently such introns, referred to as mirtrons, have been discovered in invertebrates and mammals [97-99]. In invertebrates mirtrons bypass microprocessor cleavage, entering the miRNA maturation pathway during nuclear export to proceed through cytosolic processing. Although mammalian mirtrons have been identified it is unclear whether they actually proceed via the same miRNA maturation/functional pathway suggested by the evidence from invertebrates.
The alternate miRNA excision hypothesis is that pre-mRNA splicing is not a requirement for pri-miRNA processing. In this hypothesis miRNA are excised after the splicing commitment has been made but prior to intron excision. Splice sites in the pre-mRNA are assumably marked and tethered by a splicing commitment complex permitting the microprocessor to selectively excises and liberate pre-miRNA, while splicing continues as normal to produce mature mRNA. This proposes that the microprocessor has an alternate recognition method from that described with intergenic pre-miRNA processing which is yet to be investigated [94, 99, 100].
MicroRNA Nuclear Export
Subsequent pre-miRNA (and possibly mirtron) processing occurs in the cytoplasm. Pre-miRNA assembles into a complex with the nucleocytoplasmic transporter factor Exportin-5 and RanGTP, which prevents nuclear degradation and facilitates translocation into the cytoplasm [101-105]. The Exportin-5/RanGTP nuclear export pathway can support mirtron transfer to the cytoplasm in some species including Xenopus laevis oocytes and Drosophila but it remains unclear whether this occurs in mammals [97, 102, 106]. It is possible that unidentified factors participate in mirtron nuclear export.
MICRORNA BIOGENESIS – CYTOPLASMIC FIG. (3)
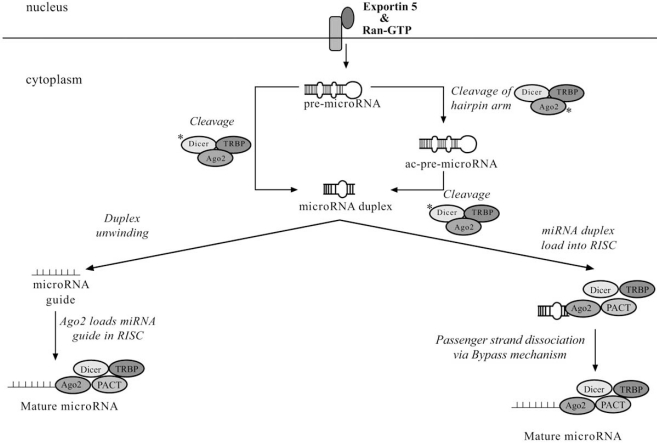
Fig. (3). Cytoplasmic component of microRNA biogenesis.
Pre-miRNA is cleaved by Dicer to generate miRNA duplex or by Ago2 to generate an Ago2-cleaved precursor miRNA” (ac-pre-miRNA) that subsequently acts as a substrate for Dicer. The asterisk (*) near a protein symbolizes it is responsible for the cleavage event. The miRNA duplex liberates the mature miRNA to assemble into RISC loading complex comprised of Ago2, TRBP, PACT and Dicer. The mechanism of mature miRNA release is unclear. Possible mechanisms involving RNA Helicase A (RHA), Dicer cleavage, Ago2 cleavage and uncharacterized proteins have been illustrated.
The cytosolic component of miRNA maturation revolves around the RNase III endonuclease Dicer, which has been found in all eukaryotes examined to date except budding yeast [107]. Humans have a single isoform of Dicer that functions in both the siRNA and miRNA pathways [108, 109]. In contrast, Drosophila have separate isoforms of Dicer for the siRNA pathway and miRNA pathways [110, 111]. It is unknown how human Dicer distinguishes between siRNA and miRNA pathways. Dicer is a multi-domain protein located within the cytoplasm and/or the rough endoplasmic reticulum which is comprised of a N-terminal ATPase/Helicase domain, DUF283 (domain of unknown function), PAZ (Piwi/Argonaute/Zwilli) domain, and two tandem RNaseIII nuclease domains (RNase IIIa and RNase IIIb) located at the C-terminal followed by a dsRNA-binding domain (dsRBD) [112]. The PAZ domain, RNase III nuclease domains and the dsRBD are involved in the binding and cleavage of dsRNA [107, 113-116]. However, the function of the ATPase/Helicase domain and the DUF283 domain are unknown. Dicer characteristically cleaves dsRNA in an ATP independent manner but it has been suggested that ATP might be required for enzyme turnover assisting in product release and/or protein conformational changes [107, 117].
Single Cleavage Pre-miRNA Processing
In one proposed model pre-miRNA translocates to the cytoplasm and is incorporated into the performed pre-miRNA processing complex composed for Dicer, the human immunodeficiency virus transactivating response RNA-binding protein (TRBP) and Protein Kinase R-activating protein (PACT) [1, 109, 118, 119]. This complex is the core of the RISC-loading complex [120]. The PAZ domain of Dicer recognizes the 2 nt 3’-overhang of the pre-miRNA and initiates binding [107, 113, 114]. The distance between Dicer’s PAZ and RnaseIII domains is used as a ruler to determine the exact cleavage site on the pre-miRNA [1, 113]. Intermolecular dimerization of Dicer’s two RNaseIII domains forms a single processing center with two catalytic sites that cleaves the single strand opposite that previously cleaved by Drosha in the pre-miRNA. The resulting product is a double stranded miRNA duplex with 3’ overhanging ends [113].
A recent model proposed by Cifuentes et al. (2010) centers around the Argonaute 2 protein (Ago2), also known as eukaryotic translation initiation factor 2C2 (eIF2C2) [121]. Ago2 contains a Piwi domain and a PAZ domain that possesses endonuclease activity [122-124]. This novel step-wise model describes a pre-miRNA processing pathway that is Ago2-dependent and Dicer-independent. Upon Ago2 pre-miRNA binding the endonuclease cleaves the passenger strand of the precursor hairpin 10 nucleotides upstream of the guide strand 5’end which facilitates unwinding. Those remaining nucleotides around the cleavage site that are not protected by Ago2 binding subsequently undergo polyuridylation and nuclease-mediated trimming to generate mature miRNA.
Double Cleavage Pre-miRNA Processing
An additional Ago2-dependent pre-miRNA processing model proposed by Diedrichs and Haber (2007) suggests Dicer, TRBP and Ago2 form a protein complex that recognizes and binds the pre-miRNA through the PAZ domain of Dicer and/or Ago2. Ago2 then cleaves a single-strand of the pre-miRNA 11-12 nucleotides from the end of the on the 3’arm to generate a nicked hairpin structure referred to as “Ago2-cleaved precursor miRNA” or “ac-pre-miRNA”. The ac-pre-miRNA is the Dicer substrate cleaved to generate the double stranded miRNA duplex.
There is evidence that Dicer, TRBP, PACT and Ago2 can all interact but no group has yet to describe a processing complex composed of all proteins. It appears the key players have been identified but the exact protein interactions remain unclear. Throughout the stages of miRNA biogenesis, proteins dissociate and re-associate with the active complexes, making it difficult to determine where in the miRNA pathway they are active. There is a consensus within the literature that TRBP associates with Ago2 and Dicer to play an integral role in the miRNA pathway [109, 118, 125, 126]. However, the exact function remains debated. Two studies performed by Haase et al. 2005 and Chendrimada et al. 2005 are in conflict [127]. Hasse et al. proposes that TRBP is essential for Dicer cleavage of pre-miRNA and the subsequent transfer to Ago2 for assembly into RISC. In contrast, Chendrimada et al. believe TRBP is only crucial for miRNA assembly into RISC. Additionally, an independent study has shown that TRBP can regulate Dicer protein levels via direct protein-protein interaction [128]. Further investigation is required to clearly define TRBP’s function.
Interestingly, TRBP inhibits the interferon-induced dsRNA-activated protein kinase R (PKR) pathway and its associated protein PACT, which activates the PKR pathway [1, 129-132]. Activation of the PKR pathway inhibits general protein synthesis throughout the cell and induces the production of interferons, which mediate anti-proliferative and pro-apoptotic activity [133-135]. It appears that TRBP and PACT function to prevent cytoplasmic pre-miRNA activation of the PKR pathway and/or regulate PKR phosphorylation which controls components of the miRNA functional pathway [1, 119, 127, 136].
RISC ASSEMBLY
Double stranded microRNA is generally a transient imperfect duplex molecule consisting of a passenger strand and a mature microRNA strand (also referred to as guide strand) commonly denoted miRNA:miRNA*, where the passenger strand is designated miRNA* [20, 33, 137]. However, some miRNA duplexes are near-perfect (extensive base-pairing in RNA duplex) [138]. Ultimately, the RNA duplex is unwound and the single strand mature miRNA is incorporated into the protein complex RISC to function as a guide, directing the silencing of target mRNA [20]. RISC assembly and activation has mainly been studied in Drosophila with relation to near-perfect base pair matches between the guide and passenger strands of the miRNA duplex. The RISC assembly model from Drosophila proposed ATP-dependent unwinding of the duplexes which enables the mature single-stranded guide to load into Ago2 of the RISC complex resulting in its activation [139, 140].
Drosophila RISC Loading
In the Drosophila model of RISC loading, the passenger strand of the miRNA:miRNA* duplex is selected based on the thermodynamic stability of the 5’end. The strand with the most unstable 5’end will become the miRNA guide [141, 142]. In rare instances mature miRNA can form from both strands of a miRNA duplex [126, 143-145]. The process of miRNA duplex unwinding and subsequent degradation is similar to siRNA, where Ago2 cleavage of the passenger strand releases single strand mature miRNA to function in RISC [19, 20, 33, 60-62, 76, 126, 139-142, 146, 147]. Whether passenger strand cleavage occurs prior to RISC loading is unclear. The model proposed by Matranga et al. (2005) suggests that RNA duplexes (siRNA and miRNA) are loaded into Ago2 of RISC, which then cleaves the passenger strand, leaving the guide strand bound to Ago2. Single strand mature miRNA bound to Ago2 facilitates the RISC activation. However, the typical mismatch pairs within a miRNA:miRNA* duplex are thought to prevent the Ago2 cleavage-assisted mechanism and ultimately propel a “bypass mechanism”. The bypass mechanism is a slower process that ultimately results in the passenger strand dissociating from the guide strand although the mechanism remains unclear.
Human RISC Loading
In humans there are eight classes of RISC complexes, which are based on protein composition centered around the four argonaute proteins, Ago 1-4 [148]. Genomic analysis indicates that argonaute proteins have evolved from translation initiation protein, hence the synonym eukaryotic translation initiation factor [149]. Ago2 is the only argonaute protein with “slicer” activity capable of catalyzing cleavage of mRNA. However, all four Ago proteins associate with miRNA and appear to function in gene silencing. It is unclear whether specific Ago proteins are associated with a particular silencing mechanism. The core components of the RISC loading complex are Dicer, Ago2, PACT and TRBP [119, 126, 148]. RISCs that load miRNA are designated microRNA containing ribonucleoprotein complex (miRISC or miRNP).
The mechanism of human miRNA RISC assembly is unclear. Hypotheses are mainly based on the Drosophila model. The mechanism of duplex unwinding is unknown, however evidence indicates the process is ATP-independent in humans [125, 126]. There are many hypotheses of duplex unwinding: Dicer could cleave the passenger strand, initiating unwinding and releasing the mature single strand miRNA that could be captured by Ago2. Alternatively, Ago2 could cleave the passenger strand of a loaded duplex, keeping the miRNA guide. Duplex unwinding also could occur simultaneously with conformational changes in RISC during assembly or by an unidentified helicase [126]. A recent study suggests that RNA Helicase A (RHA), also referred to as DHX9 or NDHll, is responsible for RNA duplex unwinding associated with RISC activation in the human miRNA pathway [150]. RHA is a DEAH-box protein with helicase activity capable of binding to ssRNA, dsRNA and dsDNA [150-152]. RHA is capable of unwinding RNA:RNA duplexes with 3’overhangs but the efficiency of RHA is unclear and is believed to be dependent on RHA’s interactions with associated proteins [150, 152, 153]. RHA interacts with Dicer, Ago2 and TRBP in a spatial arrangement optimal for unwinding RNA:RNA duplexes [150]. Additionally, the putative helicase MOV10 (Moloney leukemia virus 10 homolog) has been found to associate with some Ago2 RISC complexes but it remains unclear whether MOV10 is capable of unwinding RNA duplex structures [150, 154]. Interestingly, a very recent study by Yoda et al. (2010) suggests that Dicer cleavage and RISC assembly is uncoupled and ATP-dependent, which is in contrast to earlier studies [155]. Future studies are needed to confirm this finding.
TARGET RECOGNITION
Activated RISC binds target mRNA through Watson-Crick base-pairing between the guide strand and the 3’UTR of the target [2, 3]. Target recognition relies heavily on base-pairing between the seed (residues 2-8 at the 5’end) of the miRNA guide [156-159]. The degree and nature of complementary sites between the guide and target appear to determine the gene silencing mechanism [20, 138]. Ago2 endonuclease cleavage is generally favored by near-perfect (extensive) base-pairing whereas the more common translation inhibition seems to require multiple complementary sites with only moderate (limited) base-pairing in each site [20, 138]. In animals, miRNA are generally 100% complementary in the seed but not over the whole miRNA which results in imperfect RNA hybrids with characteristic bulges [20, 93, 160, 161]. Binding at the seed is important for the thermal stability of the interaction, which contributes to miRNA specificity and activity [157, 162]. The phenomenon of G:U wobble (mismatch) base pairing in the seed was initially thought to be detrimental to miRNA function. miRNA:mRNA interaction can still occur in the presence of a G:U wobble but it effects specificity and activity of miRNA [156, 157, 163]. Subsequent investigation revealed this is not the case for all miRNAs. Mammalian miR-196 perfectly base pairs with its target HOXB8, with the exception of a G:U wobble involving U5 within the seed region and still efficiently down-regulates HOXB8 through mRNA cleavage [138].
SILENCING
It is widely accepted that miRNA bind to their target mRNA and negatively regulate its expression. A single miRNA guide can regulate several mRNA targets and conversely multiple miRNAs can cooperatively regulate a single mRNA target [20]. However, the means of silencing remains unclear. Evidence supports two distinct silencing mechanisms; mRNA cleavage and translation repression, which can be defined as slicer-dependent and slicer-independent silencing [164-171]. Slicer activity refers to the endonuclease cleavage of target mRNA by Ago2 which requires extensive base-pairing between miRNA and mRNA target [124, 172-174]. Slicer-dependent and slicer-independent silencing mechanisms have one of two downstream effects, mRNA degradation or translation inhibition, both ultimately leading to down-regulation of gene expression. One significant difference between the downstream effects is reversibility, mRNA decay is an irreversible process. In contrast, translation inhibition is reversible because stable mRNA can be translated following elimination of translation repression [165, 174-177]. The following sections discuss the slicer-dependent and -independent silencing mechanisms.
Slicer-Dependent Silencing Fig. (4)
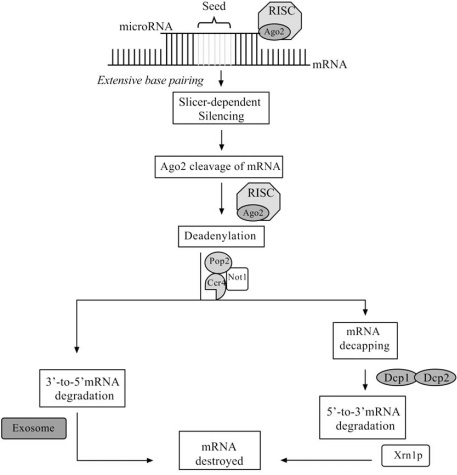
Fig. (4). Slicer-dependent microRNA mediated gene silencing.
Extensive base-pairing between the miRNA guide and mRNA target permit Ago2 mRNA cleavage. Subsequently, mRNA is deadenylated by protein complexes comprised of Pop2, Ccr4 and Not1. mRNA is then degraded from 3’ to 5’ by the exosome complex or from 5’ to 3’ by the Xrn1p exonuclease following decapping by Dcp enzymes.
MicroRNA-directed mRNA cleavage is catalyzed by Ago2, when the target and miRNA are extensively base-paired over regions including the seed and bases 10-11 of the guide [124, 138, 172, 174, 178, 179]. A single extensive complementary region is usually sufficient for cleavage. However, exceptions have been noted which suggest unidentified additional requirements might be necessary for slicer-dependent mRNA cleavage [86, 93, 157, 160, 174, 180]. Cleavage products are degraded by one of two processes responsible for bulk cellular mRNA degradation, both beginning with the deadenylation of the mRNA to remove the poly (A) tail [174, 181]. Subsequent degradation can occur via the exosome, which is a multi-protein complex with 3’-to-5’ exonuclease activity. Alternatively, the mRNA can undergo decapping by the enzymes Dcp1 and Dcp2 which facilitates 5’-to-3’ degradation by the exoribonuclease Xrn1p [174, 182].
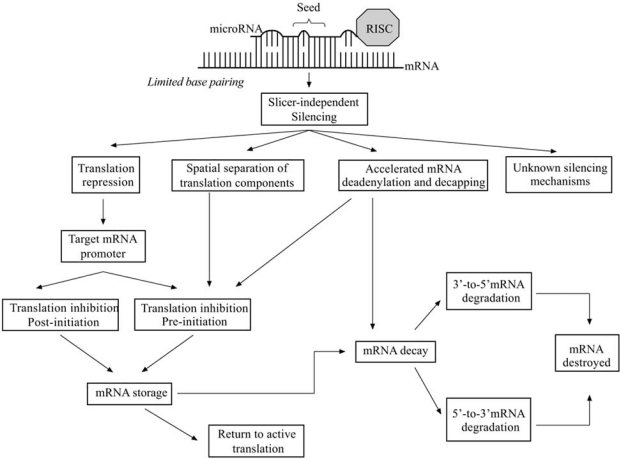
Slicer-Independent Silencing Fig. (5)
Fig. (5). Slicer-independent microRNA mediated gene silencing.
Limited base-pairing between the miRNA guide and mRNA target create bulges that inhibit Ago2 mRNA cleavage and propels slicer-independent gene silencing mechanisms. MiRNA can repress translation directly pre- or post- translation initiation which is determined by the target mRNA promoter. Alternatively, miRNA can repress translation indirectly by segregating mRNA away from ribosomes to cytoplasmic foci and accelerating deadenylation and decapping processes. Ultimately, mRNA can be isolated for storage or be targeted for decay via Xrn1p or exosome degradation. Stored mRNA can return to active translation or be targeted for degradation.
Multiple complementary sites with imperfect base-pairing create bulges in the RNA duplex inhibit the slicer activity of Ago2 [2, 3, 20, 174, 183, 184]. This does not affect the argonaute proteins ability to repress translation of the target mRNA [124, 172, 173, 185]. There is no distinct model of how miRNAs inhibit translation but it is clear that this can occur in various ways. Translation occurs in three stages: initiation, elongation and termination, which require coordination of multiple protein factors [186]. Experiments performed with mammalian cells provided evidence supporting miRNA inhibition of translation at both the initiation and elongation stages [183, 187]. However the selection mechanism is unclear. Recent data suggests the promoter used to transcribe the target mRNA determines which translation repression mechanisms will be employed [188]. Translation can also be regulated indirectly by spatial separation of components, such that miRNA-targeted mRNA is sequestered away from translational machinery into cytoplasmic foci known as P-bodies (also known as processing bodies, GW bodies and Dcp bodies) [165, 189-194]. Additionally, miRNA can accelerate target mRNA deadenylation and decapping independently of slicer activity consequently effecting translation initiation efficiency and/or transcript stability [169, 195-197]. The latter effect will lead to target mRNA decay via the same exosome and Xrn1p enzymatic degradation as slicer-dependent silencing [169, 182]. Unresolved issues with slicer-independent mRNA degradation also suggest involvement of unknown alternative mRNA decay pathways.
P-Bodies
P-bodies are dynamic small cytoplasmic protein spheroid domains found in cells ranging from yeast to humans [164, 166]. A large number of studies investigating the formation and function of P-bodies have been conducted in yeast. Although these structures are similar in humans there are mammalian specific proteins that might relate to functional diversity. There are a number of similar cytoplasmic domains found in cells, including stress granules, exosomes and multivesicular bodies that contain some of the same protein components [198, 199]. However, there are a few protein markers that appear to be specific to P-bodies such as Dcp1, 4E-T, GE-1/hedls, p54/RCK and Xrn1 [199]. There is some controversy in the field with regards to the classification of these small cytoplasmic domains that relates to varying function, localization, size and contained proteins. This is partly due to the dynamic nature of these structures. P-bodies are affected by a variety of cellular factors including glucose level, osmotic pressure and cell proliferation [194]. Similarly, stress granules respond in number and size to environmental stresses such as temperature, infection, hypoxia and ultraviolet light [200].
Collective research indicates P-bodies are the functional site of reversible mRNA repression and mRNA decay which are mediated by a variety of mechanisms including miRNA-mediated gene silencing [189, 190, 201, 202]. P-bodies contain mRNA along with a variety of enzymes and factors required for processes such as mRNA decapping, deadenylation, RNA degradation and translational repression [164, 182]. Further investigation of the link between miRNA silencing and P-bodies revealed that the RISC effector proteins Ago 1-4 localize in P-bodies with passenger and guide strands miRNA duplexes [183, 184, 189, 190, 203-206]. This suggests that in addition to being the functional site for general cellular mRNA turnover, P-bodies are also the functional site of miRNA-mediated gene silencing. The role of P-bodies in miRNA silencing is actively being investigated. Recent studies have compiled data to develop a P-body model of miRNA-mediated gene silencing.
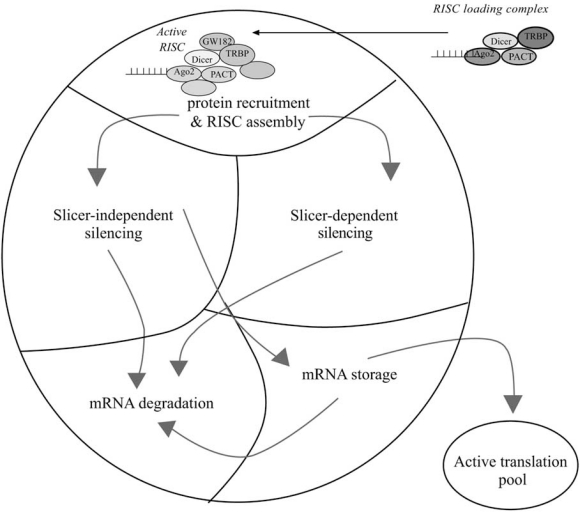
The P-Body Model Fig. (6)
Fig. (6). The P-body model.
The RISC loading complex containing mature miRNA translocates to a P-body compartment specific for RISC assembly and activation. Upon target recognition the silencing mechanism is determine as slicer -depepndent or –independent, both having distinct functional compartments. Target mRNA will then be redirected for degradation or mRNA storage, which can be later returned to active translation or degraded.
P-bodies and miRNA function are crucial to each other. Evidence clearly indicates that P-bodies are essential for miRNA function as inhibition of P-body formation by depletion of GW182 (the major protein component of P-bodies) significantly impairs miRNA function [190, 203, 207]. Conversely, removal of the microprocessor complex inhibits P-bodies formation [164, 194, 205, 207, 208].
RISC assembly and activation occurs within the P-bodies, which is supported the co-localization of miRNA duplexes and Ago 1-4 proteins to GW182 [183, 189, 190, 203-206]. GW182 localization and protein associations suggest it is a major component of RISC, having functional involvement in slicer-dependent and slicer-independent silencing mechanisms [154, 189, 190, 203, 204, 209-212]. This model hypothesizes specialized compartments within P-bodies for RISC recruitment of silencing effector proteins, slicer-dependent silencing, slicer-independent silencing.
Slicer directed mRNA cleavage conceivably occurs in a specialized P-body compartment responsible for mRNA degradation [183, 184]. Enzymes involved in the mRNA degradation process such as deadenylation enzymes Ccr4, Not1, Pop2, decapping enzymes Dcp1, Dcp2 and nucleases Xrn1p and the exosome, reside within P-bodies [182, 191, 192, 202, 213]. Alternatively, slicer-independent mechanisms inhibit translation by various means.
P-bodies house translational control enzymes and factors, notably p54, FMRP, Gemin5 and RAP55, required for miRNA mediated translational repression and/or directed mRNA storage [192, 204, 213-218]. Storage compartments must be isolated from degradation enzymes and have a mechanism to manage mRNA status, which would determine if transcripts get degraded or return to the cytoplasm for active translation [171, 219, 220]. On the other hand, mRNAs being actively translated by polyribosomes are targeted by miRISC in the cytoplasm to inhibit translation initiation and/or elongation [165, 175-177]. These mRNAs could then be directed to P-bodies for storage or degradation. MiRNA repression of actively translated mRNAs may explain why miRNAs and argonaute proteins are also found within the cytoplasm.
MicroRNA Silencing in the Nucleus?
The majority of data indicates that miRNA mediated post-transcriptional gene silencing occurs in the cytoplasm and P-bodies. However, the recently miRNA and argonaute proteins have been discovered in the nucleus of human cells and shown to repress gene expression of nuclear target RNA [221-225]. MiRISC could conceivably assemble and/or be imported into nuclease. A study in Caenorhabditis elegans identified an argonaute protein, NRDE-3 (nuclear RNAi defective-3), that imports siRNA to the nucleus [226, 227]. It is possible that humans have transporter proteins with similar function.
The function of nuclear miRISC is unclear. However, the discovery designates nuclear non-coding RNA as a new class of miRNA targets. MiRNA may function in the nucleus to regulation gene transcription, prevent RNA export or affect target mRNA splicing. Perhaps nuclear miRNA, in conjunction with argonaute proteins, directs DNA methylation. Alternatively, miRNA could localize in the nucleus for modification. Nuclear A-to-I editing of miRNA by adenosine deaminase that act on RNA (ADAR) has been implicated in the regulation of miRNA processing [217, 228-231]. The scope of miRNA silencing remains unclear, as does a relationship between different silencing mechanisms.
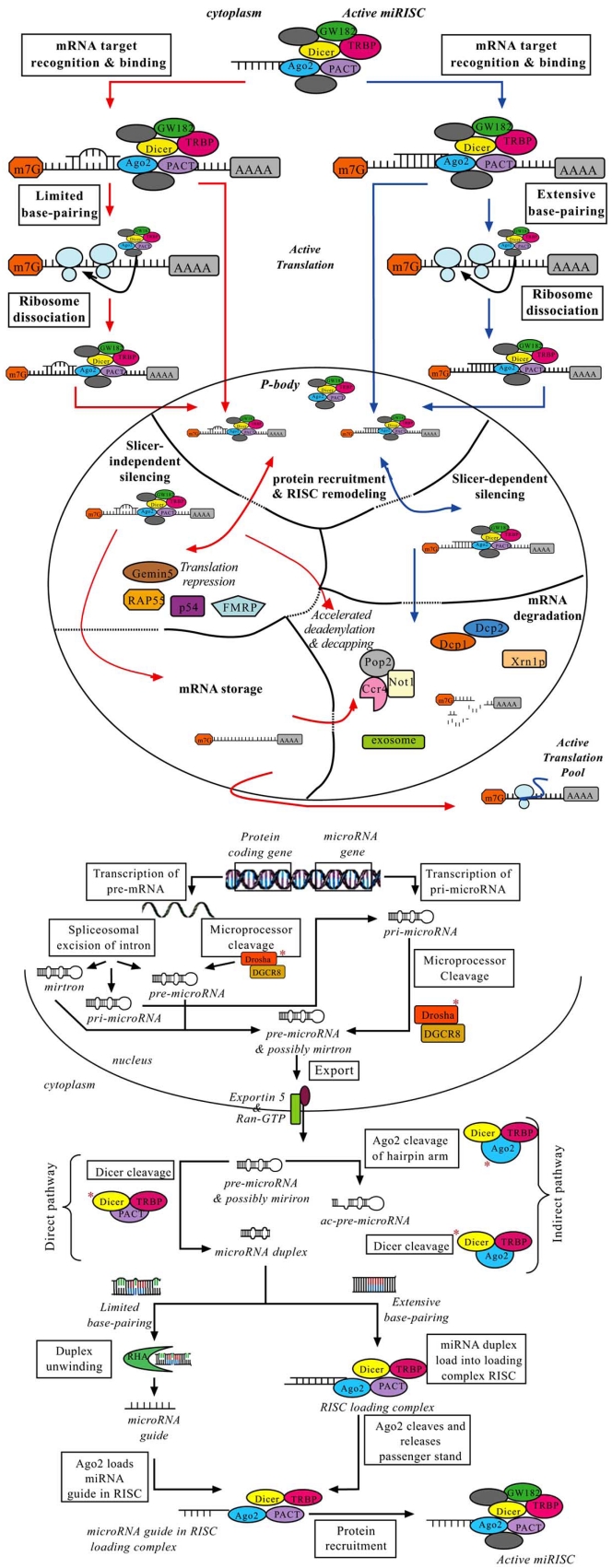
PROPOSED MICRORNA WORKING MODEL FIG. (7)
Fig. (7). Proposed microRNA working model.
duplexes have mismatched pairs an Ago2 independent bypass mechanism will be employed. I hypothesize that this mechanism uses RNA helicase A to unwind the miRNA duplex which permits Ago2 of the RISC loading complex to load the arm with the least stable 5’end, the miRNA guide. Once the mature miRNA guide is bound to Ago2 additional RISC associated proteins, such as GW182, assemble to form the active miRISC which will seek out and bind its target mRNA.
Upon stimulation miRNA is transcribed from the genome either as a component of pre-mRNA or a polycistronic primary miRNA transcript. Cleavage of the pre-mRNA by the spliceosome or microprocessor releases one of three possible miRNA precursor molecules, a mirtron, pri-miRNA or pre-miRNA. Pri-miRNA are processed into pre-miRNA by the microprocessor complex which cleaves one arm 11 bp from the ssRNA-dsRNA junction. Pre-miRNA (and possibly mirtrons) are exported to the cytoplasm via the Exportin-5/RanGTP pathway. In the cytoplasm pre-miRNA can mature into a miRNA duplex through two alternative pathways. The direct pathway is a single cleavage event performed by Dicer, which is associated with TRBP and PACT. In contrast, the indirect pathway is a two-step cleavage process carried out by a complex comprised of Dicer, Ago2 and TRBP. An initial Ago2 cleavage generates a nicked intermediated, ac-pre-miRNA, which acts as a substrate for Dicer. The miRNA duplex loads into Ago2 of the RISC loading complex that contains Dicer, TRBP, and PACT. If the miRNA duplex is extensively base-paired Ago2 will cleave the arm with the most stable 5’end, designated the passenger strand, triggering its degradation and leaving the guide strand bound to Ago2. However, in the instance that miRNA.
Active miRISC will direct mRNA awaiting translation or being translated to the recruitment compartment of a P-body, where the level of guide-target complementarity will regulate the recruitment of proteins required for slicer-dependent or slicer-independent silencing. Extensive guide-target base-pairing will relocate miRISC, with the bound mRNA target and recruited proteins, to the P-body compartment designated for slicer-dependent silencing where Ago2 will cleave the mRNA target. Subsequent mRNA decay will occur in the specialized mRNA degradation compartment at which time miRISC will return to the recruitment unit for ATP-dependent remodeling necessary for additional silencing. Alternatively, limited guide-target base-pairing will direct relocation to a compartment specific for slicer-independent silencing. In this unit mRNA will either associate with translation repression factors or be relocated for long-term mRNA storage or accelerated mRNA decay which releases miRISC for remodeling.
MicroRNA IN CANCER
Cancer is a multistep process in which normal cells experience genetic changes that progress them through a series of pre-malignant states (initiation) into invasive cancer (progression) that can spread throughout the body (metastasis). The resulting transformed cellular phenotype has several distinct characteristics that enables cells to proliferate excessively in an autonomous manner; Cancer cells are able to proliferate independent of growth signals, unresponsive to inhibitory growth signals, evade programmed cell death (apoptosis) pathways, overcome intrinsic cell replication limits, induce and sustain angiogenesis, and form new colonies discontinuous with the primary tumor [232].
The dysregulation of genes involved in cell proliferation, differentiation and/or apoptosis is associated with cancer initiation and progression. Genes linked with cancer development are characterized as oncogenes and tumor suppressors. Oncogene products can be categorized into six groups based on their function; they can be transcription factors, growth factors, growth factor receptors, chromatin remodelers, apoptosis regulators or signal transducers [233]. Over expression of these gene products provide selective growth advantages that can drive tumor development. Oncogenes can be activated by genetic alterations that amplify the gene, alter promoters/enhancers to increase gene expression or alter protein structure to a permanent active state [233-235]. Conversely, tumor suppressor gene products have regulatory roles in biological processes. Loss or reduction of function of tumor suppressors results in dysregulation associated with cancer [236]. Recently, the definition of oncogenes and tumor suppressors has been expanded from the classical protein coding genes to include miRNA [24, 237]. MiRNAs play a vital role in regulating numerous metabolic and cellular pathways, notably those controlling cell proliferation, differentiation and survival [13-18, 238-240].
Dysregulation of miRNA expression profiles has been demonstrated in most tumors examined [24, 241]. However, the specific classification of miRNA as oncogenes or tumor suppressors can be difficult because of the intricate expression patterns of miRNAs. MiRNAs expression patterns differ for specific tissues and differentiation states, which poses two difficulties in classification [21, 22, 31, 242, 243]. It is not always clear if altered miRNA patterns are the direct cause of the cancer or rather an indirect effect of changes in cellular phenotype. Additionally, a single miRNA can regulate multiple targets [158]. This coupled with tissue specific expression could implicate a single miRNA as a tumor suppressor in one context and an oncogene in another. This is the topic of the next section.
MicroRNAs AS TUMOR SUPPRESSORS & ONCOGENES
Let-7
Let-7 miRNAs (let-7s) were one of the first miRNAs discovered in Caenorhabditis elegans [244]. Let-7s are highly conserved among invertebrates and vertebrates, including humans who have twelve let-7 genes encoding nine miRNAs [245]. Several let-7 genes have been mapped to regions within the human genome that are frequently altered or deleted in various cancers [245]. This discovery along with many other studies have implicated let-7 as a tumor suppressor. Two well-defined let-7 targets are the known oncogenes Ras and high mobility group AT-hook 2 (HMGA2).
Ras is a signal transducing GTPase that delivers signals from cell surface receptors to functional intracellular pathway effecting cell proliferation, growth, cytoskeleton organization, cell movements and survival [246-249]. Constitutively active Ras mutants (H-Ras, K-Ras and N-Ras) are found within a variety of human cancers including pancreas, colon, thyroid and lung [250]. Let-7s regulate the expression of Ras, K-Ras and N-Ras via 3’UTR binding that inhibits translation [251]. Functioning as a tumor suppressor, let-7 mediates the suppression of Ras and the cellular processes it modulates [251-254].
HMG2A is non-histone architectural transcription factor that alters DNA conformation to direct transcriptional activation of a variety of genes that influence cell growth, differentiation, proliferation and survival [255]. HMG2A is undetectable in normal adult tissue but highly expressed in embryonic tissues, lung cancer and uterine leiomyomas [256-258]. Studies reveal that let-7s regulate HMG2A by destabilizing its mRNA through 3’UTR binding [21-23]. This regulation can explain the HMG2A expression pattern as let-7 exhibits a reciprocal expression pattern compared to HMG2A in embryonic and mature tissue [22]. Loss of let-7 HMG2A control promotes cell growth and proliferation [21, 22].
The let-7 family of miRNAs regulate numerous genes not all of which are as well defined as HMG2A and Ras. Recent data indicates that let-7s play a much larger role in controlling cell proliferation than initially thought as they have been shown to functionally inhibit numerous cell cycle regulators including c-myc, CDC25A, CDK6 and cyclin D2 [253, 259, 260].
miR15 and miR16
The miR15/16 family of miRNAs has four members that are putative tumor suppressors. These members are organized in two distinct clusters, miR15a/miR16-1 located at 13q14.3 and miR15b/miR16-2 at 3q26 [144, 261, 262]. All four miRNAs share a 9-nucleotide seed region that targets the 3’UTR of the anti-apoptotic protein BCL2 for post-transcriptional repression [261, 263]. The oncogenic BCL2 protein, commonly over expressed in various tumors and hematopoetic malignancies, promotes cell survival by evading apoptosis [264-266].
The miR15a/16-1 miRNA cluster was found to be a frequent deletion site in B cell chronic lymphocytic leukemia (CLL) patients [262]. Evidence suggests that in this instance the absence of miR15a/16-1 is associated with a loss of regulation resulting in elevated BCL2 protein levels [261-263, 267]. BCL2 functions to block apoptosis by inhibiting mitochondrial cytochrome C release necessary to activate the caspase pathway of enzymes responsible for directing programmed cell death [268, 269]. Overall, the consensus is that normal miR15a/16-1 expression induces intrinsic apoptosis pathways by mediating BCL2 repression.
A recent study focusing on miR15b/miR16-2 expression found similar results as those observed with miR15a/16-1 [263]. Additionally, evidence was provided that suggested miR15b/miR16-2 plays a role in increasing chemosensitivity. It is hypothesized that BCL2 repression via miR15b/miR16-2 reduces a cell’s sensitivity to drug-induced apoptosis [263, 270-272].
The miR15/16 family of miRNAs has numerous predicted targets in addition to BCL2. Computer algorithms and proteomic analysis predict a variety of targets linked to cell growth, cell cycle and apoptosis [267, 273]. However, it is unclear whether these are direct or indirect miRNA targets.
miR-21
MiR-21 is a relatively new member of the oncogenic miRNA group. Initially found to be over expressed in human glioblastoma tumors, miR-21 was described as an anti-apoptotic factor predicted to down regulate genes associated with advancing apoptosis [274]. Subsequently, miR-21 over-expression was observed in a variety of human cancer, including those derived from breast, colon, liver, brain, pancreas and prostate [241, 274-279].
Recent studies have revealed that miR-21 down-regulate four tumor suppressors genes: mapsin, programmed cell death 4 (PDCD4), tropomyosin1 (TPM1) and phosphatase and tensin homolog (PTEN). Evidence suggests that miR-21 directly binds to the 3’UTR of the gene transcripts preventing their translation. MiR-21 repression of these tumor suppressor genes promotes cell transformation, tumor growth, invasion, and metastasis [275, 277, 280-282].
PTEN is a phosphatidylinositol phosphate (PIP) phosphatase that functions in conjunction with phosphoinositide 3-kinase (PI3K) to balance PIP3 levels that regulate the Akt pathway [283]. PTEN dephosphorylates PIP3 and PI3K phosphorylates PIP2 to maintain the crucial balance of PIP3 levels [Li, 2007 #8933, 284]. Translational repression of PTEN by miR-21 leads to an accumulation of PIP3 that over stimulates the Akt pathway, activating multiple downstream pathways that stimulate cell growth and survival [275, 280, 285]. Additionally, loss of PTEN is associated with an increase in focal adhesion kinase (FAK) activity that promotes cell migration and metastasis by increasing the expression of several matrix metalloproteases [275, 280, 286].
TPM1 is an actin binding protein involved in the control of anchorage-independent growth and cellular microfilament organization [287, 288]. It is hypothesized that miR-21 repression of TMP1 leads to cytoskeleton changes that promote neoplastic transformation, cell invasion and metastasis [282]. Similarly, repression of PDCD4 and mapsin are thought to promote cancer progression by promoting cell invasion and metastasis [277, 280, 281, 289]. There are multiple proposed mechanisms of PDCD4 and mapsin action, all of which are still under investigation. Both molecules are associated with apoptosis and regulation of the urokinase receptor (uPAR), which is involved in degradation of the extracellular matrix [280, 290-292]. Thus implicating a role in tumor progression and metastasis.
miR-17-92
The miR-17-92 miRNA cluster is the most complex. The sequence and organization of the miR-17-92 cluster is highly conserved among all vertebrates examined [293]. In humans, the cluster produces six mature miRNAs (miR-17, miR-18a, miR-19a, miR-19b-1, miR-20a and miR-92-1) from a polycistronic transcript generated from the third exon of the open reading frame C13orf25 at loci 13q31.3 [293, 294]. MiR-17-92 can be directly regulated by c-myc and E2F transcription factors (E2Fs) 1-3 but, E2F3 is thought to be the predominant regulator [67-69, 295]. Evidence supports a dual role for miR-17-92 as both an oncogene and a tumor suppressor. However, the miR-17-92 functional network is extremely complex and remains under extensive investigation.
MiR-17-92 is most commonly thought of as an oncogene [296]. The 13q31.3 gene locus is a frequent site for gene amplification, which explains highly elevated levels of miR-17-92 miRNAs observed within a variety of lymphomas, lung cancer, colon, pancreas and prostate cancers [241, 294, 297-299]. Additionally, over expression of c-myc is commonly observed in human cancers, which would result in an increase in miR-17-92 expression [300-303]. MiR-17-5p and miR-20a of the miR-17-92 cluster bind to target sequences in the 3’UTR of E2F transcription factors to inhibit their translation [67-69].
E2Fs are critical regulatory components for apoptosis and cell proliferation [304-312]. E2F 1-3 are associated with the activation of genes required for G1/S phase progression in the cell cycle [305, 306]. However, isoform specificity is apparent. E2F1 is primarily involved in promoting apoptosis and E2F3 signals more specifically for proliferation [68, 69, 313]. The crucial balance of E2F isoform expression modulates apoptosis and proliferation in normal cells. E2Fs stimulate the transcription of their own genes and of their regulators, c-myc and the miR-17-92 cluster. This creates an E2F positive auto-regulatory feedback loop which is controlled by miR-17-92 in a negative feedback loop [67-69, 314, 315]. Additionally, c-myc regulates E2Fs and miR-17-92, establishing a double feed-forward loop [68, 316]. The oncogenic activity of miR-17-92 is hypothesized to promote tumorigenesis by selectively repressing E2F1. Over expression of miR17-92 would decrease E2F1 levels needed to induce apoptosis. This does not trigger the negative feedback loop because E2F3 is the predominant miR-17-92 regulator instead there is an increase in E2F3 levels that will stimulate proliferation.
Although the majority of data supports miR-17-92 as an oncogene there is some evidence that it also acts as a tumor suppressor. Coincidently, loss of heterozygosity and deletions at 13q31.3 have been observed in various cancers, including breast cancer, hepatocellular carcinoma, retinoblastoma and nasopharyngeal carcinomas [317-320]. Recent data from breast cancer cell lines shows that miR-17-5p of the miR17-92 cluster down regulates the proto-oncogenic transcriptional activator AIB1 (amplified in breast cancer 1), also known as SRC-3, TRAM1, NCOA3 and RAC3 [321, 322]. AIB1 is a coactivator that increases the activity of transcription factors and various steroid receptors, which are involved in breast cell proliferation, growth and hormone signaling [323-329]. In this cellular context miR-17-5p is a tumor suppressor that regulates proliferation, growth, survival, differentiation and anchorage-independent growth by inhibiting AIB1 translation [321-324].
CONCLUSION
Since their discovery, miRNAs have provided a new perspective on regulation of gene expression. Extensive research over the last decade revealed that miRNA are more prevalent that originally thought, with 940 miRNA identified to date in humans and over 1000 predicted [9-11, 330]. Although, much more investigation is required to determine all miRNA targets, silencing mechanisms and networks it is accepted that miRNAs play an integral role in regulating an array of fundamental biological processes. Evidence indicates that miRNA dysregulation is associated with disease, notably cancer as miRNA can function as oncogenes and tumor suppressors. MiRNAs are currently being exploited to advance cancer diagnosis, classification, prognosis and treatment [30].
MiRNA profiling was originally done with glass slide microarrays [331-334]. New advances have lead to the development of bead-based technology, specifically bead-based flow cytometric expression and bead-array profiling which is highly accurate, specific and feasible to implement within a clinical setting [31, 335]. This technology, and other techniques including quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR), can identify distinct miRNA expression patterns that can characterize specific tissue and disease states, distinguishing between subtypes of normal and malignant tissues [278, 336, 337]. MiRNA signature profiles can be used to determine prognosis [338-340], response to drug therapy [341], predict treatment efficiency [342, 343], race susceptibility [23] and patient susceptibility to cancer and metastasis [344-346]. This has been demonstrated with lung cancer where unique miRNA profiles can distinguish between normal lung and tumor tissue and correlate to patient prognosis [340]. Additionally, miRNA profiles can discriminate between normal and malignant B-cells in chronic lymphatic leukemia and could hypothetically be utilized to classify undifferentiated tumors to their organ of origin [344].
Initially miRNA profiling was conducted on samples extracted from tissues. However recent studies have found stable miRNAs in readily available body fluids including, serum [347, 348], plasma [349-351], urine [352] and saliva [353]. The source of the endogenous circulating miRNAs is unclear. The predominant hypothesis suggests that miRNAs (more likely pre-miRNAs) are circulating in exosomes that are shed from normal or tumor-derived cells [354]. Containment of miRNAs (pre-miRNA) within exosomes would protect the molecules from degradation and explain the molecules stability, even in harsh experimental conditions [355, 356]. However, a combination of other theories collectively suggest it is possible that the circulating miRNAs are from lysed cells and their stability is due to binding of DNA, proteins and or lipids that protect them from degradation [355, 357, 358].
Although the use of miRNAs as biomarkers in body fluids is exciting there are limitations that need to be overcome before there is wide-spread clinical application. More studies need to examine the ‘normal’ levels and diversity of miRNAs present with particular body fluids. It would appear classification of miRNAs would be necessary to account for different miRNA profiles that may arise depending on race, gender and age. The effects of cancer treatment, whether it be chemotherapy, surgery, radiation or a combination of these methods, should be investigated as it will presumably change miRNA profiles. One particular study showed specific reduction of miR-184 plasma levels following surgical resection of squamous cell carcinoma of the tongue [359]. Additionally, the mechanism of miRNA released into these body fluids needs to be characterized. Within the clinical setting the technological approach needs be standardized as a variety of handling and processing factors can results in dramatics changes in miRNA profiles [360]. Establishment of universal profiling approaches for specific samples (i.e. tissue, serum, plasma, urine, saliva) that addresses collection, extraction (sample purity), storage (frozen vs. fresh), profiling technique (noting RNA amount), normalization/standardization and statistical comparison.
Further research exploring the outcome of therapeutic treatment associated with different miRNA profiles could provide valuable information to advance drug regime selection. A recent study of patients with colon adenocarcinoma receiving fluorouracil-based chemotherapy revealed that high miR-21 expression is associated with a poor therapeutic outcome [361]. It is possible other chemotherapy drugs might have a better outcome in comparison to fluorouracil. As we gain understanding of specific miRNA mechanisms and function it could become evident that certain chemotherapeutic drugs might be favorable based on their mode of action in relation to a particular miRNA profile.
MiRNA modulation is emerging as a novel therapeutic target with the use of antagomirs. Antagomirs are chemically engineered oligonucleotides that inhibit miRNA target binding through competitive miRNA binding [14]. A variety of chemical modifications can be made to increase the stability and potency of antagomirs, including 2’-O-methyl modified ribose sugars [14], 2’-O-methoxyethyl-modified ribose sugars [13] and addition of an extra 2’-O, 4’-C methylene bridge in the ribose sugar [362]. A study conducted in vivo with mice utilized this novel therapeutic approach to inhibit miR-122 expression. Intraperitoneal injections administered the antagomirs to the liver successfully inhibited miR-122 liver expression, which decreased plasma cholesterol levels in normal and obese mice [13]. Thus miR-122 may be a good therapeutic target for hepatic metabolic diseases. This study suggests that antagomirs could be employed as a new treatment because they are long lasting, specific and have no significant associated toxicity.
However, there are still many limitations associated with antagomirs as a therapeutic treatment for humans. Firstly, miRNA sequences and targets need to be further characterized and cataloged. MiRNAs can have multiple targets and a full understanding of their functional mechanisms would be required to design appropriate antagomirs. Although computational bioinformatics programs have made great improvements they still do not have a high enough accuracy to predict miRNA targets [363, 364]. Secondly, an effective method of delivery has yet to be developed for humans. High-pressure injection and electroporation can effectively delivery small RNAs, but they cause unwanted tissue damage [365-367]. A variety of viral vectors can efficiently introduce small RNA into cells [367-371]. While some vectors have been useful in animal studies they are not ideal for human because they can elicit an immune response that deflates their effectiveness [371-373]. Vectors that integrate into the genome can cause further problems with mutations associated with genome insertion [374, 375]. Strategies exploring liposome/cationic encapsulation delivery methods are advancing efficiency however, they may also elicit an immune response [365, 376]. Progress is also being made in the expanding field of nanobiotechnology to develop a means to package and deliver RNA to specific sites [377-380]. Viral vectors are being modified to improve expression specificity and make them safer for human trials [374, 381].
The young field of miRNA research is continually expanding. Current and future investigation will hopefully provide the much need information to sufficiently characterize the targets and functional mechanisms of miRNAs. A deeper understanding of miRNA molecular pathways would provide great insight in the initiation and progression of cancer, among other diseases and is essential to advance therapeutic treatments.
REFERENCES
- 1.Perron MP, Provost P. Protein interactions and complexes in human microRNA biogenesis and function. Front. Biosci. 2008;13:2537–2547. doi: 10.2741/2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wightman BHI, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediated temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee RCFR, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 4.Rajewsky N. L(ou)sy miRNA targets? Nat. Struct. Mol. Biol. 2006;13:754–755. doi: 10.1038/nsmb0906-754. [DOI] [PubMed] [Google Scholar]
- 5.Rajewsky N. microRNA target predictions in animals. Nat. Genet. 2006;38(Suppl):S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Mao SY, Zhu WY. Impact of tiny miRNAs on cancers. World J. Gastroenterol. 2007;13:497–502. doi: 10.3748/wjg.v13.i4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120:21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol. Biol. 2006;342:129–138. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32:D109–111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen BR. Transcription and processing of human microRNA precursors. Mol. Cell. 2004;16:861–865. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 16.Naguibneva I, Ameyar-Zazoua M, Polesskaya A, Ait-Si-Ali S, Groisman R, Souidi M, Cuvellier S, Harel-Bellan A. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat. Cell Biol. 2006;8:278–284. doi: 10.1038/ncb1373. [DOI] [PubMed] [Google Scholar]
- 17.Garzon R, Pichiorri F, Palumbo T, Iuliano R, Cimmino A, Aqeilan R, Volinia S, Bhatt D, Alder H, Marcucci G, Calin GA, Liu CG, Bloomfield CD, Andreeff M, Croce CM. MicroRNA fingerprints during human mega-karyocytopoiesis. Proc. Natl. Acad. Sci. USA. 2006;103:5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monticelli S, Ansel KM, Xiao C, Socci ND, Krichevsky AM, Thai TH, Rajewsky N, Marks DS, Sander C, Rajewsky K, Rao A, Kosik KS. MicroRNA profiling of the murine hematopoietic system. Genome Biol. 2005;6:R71. doi: 10.1186/gb-2005-6-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 21.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315(5818):1576–9. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. A micro-RNA signature associated with race tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 24.Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol. Med. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Nelson PT, Wang WX, Rajeev BW. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008;18:130–138. doi: 10.1111/j.1750-3639.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, Rigoutsos I, Nelson PT. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, Andreassen OA, Djurovic S, Melle I, Agartz I, Hall H, Timm S, Wang AG, Werge T. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS ONE. 2007;2:e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jay C, Nemunaitis J, Chen P, Fulgham P, Tong AW. miRNA profiling for diagnosis and prognosis of human cancer. DNA Cell Biol. 2007;26:293–300. doi: 10.1089/dna.2006.0554. [DOI] [PubMed] [Google Scholar]
- 29.Hernando E. microRNAs and cancer: role in tumorigenesis, patient classification and therapy. Clin. Transl. Oncol. 2007;9:155–160. doi: 10.1007/s12094-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 30.Barbarotto E, Schmittgen TD, Calin GA. MicroRNAs and cancer: profile, profile, profile. Int. J. Cancer. 2008;122:969–977. doi: 10.1002/ijc.23343. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 32.Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. Rna. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell. Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 34.Kim VN. Small RNAs: classification, biogenesis, and function. Mol. Cells. 2005;19:1–15. [PubMed] [Google Scholar]
- 35.Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. 2003;9:175–179. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying SY, Chang CP, Lin SL. Intron-mediated RNA interference intronic microRNAs and applications. Methods Mol. Biol. 2010;629:205–237. doi: 10.1007/978-1-60761-657-3_14. [DOI] [PubMed] [Google Scholar]
- 38.Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. USA. 2007;104:17719–17724. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–2706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Wang F, Yang GH, Wang FL, Ma YN, Du ZW, Zhang JW. Human microRNA clusters: genomic organization and expression profile in leukemia cell lines. Biochem. Biophys. Res. Commun. 2006;349:59–68. doi: 10.1016/j.bbrc.2006.07.207. [DOI] [PubMed] [Google Scholar]
- 41.Cullen BR. Derivation and function of small interfering RNAs and microRNAs. Virus Res. 2004;102:3–9. doi: 10.1016/j.virusres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 45.Cramer P. Structure and function of RNA polymerase II. Adv. Protein Chem. 2004;67:1–42. doi: 10.1016/S0065-3233(04)67001-X. [DOI] [PubMed] [Google Scholar]
- 46.Ohkuma Y. [Function and structural biology of general transcription factors and RNA polymerase II in eukaryotes] Tanpakushitsu Kakusan Koso. 1999;44:438–456. [PubMed] [Google Scholar]
- 47.Woychik NA, Young RA. RNA polymerase II: subunit structure and function. Trends Biochem. Sci. 1990;15:347–351. doi: 10.1016/0968-0004(90)90074-l. [DOI] [PubMed] [Google Scholar]
- 48.Goodfellow SJ, White RJ. Regulation of RNA polymerase III transcription during mammalian cell growth. Cell Cycle. 2007;6:2323–2326. doi: 10.4161/cc.6.19.4767. [DOI] [PubMed] [Google Scholar]
- 49.Felton-Edkins ZA, Kenneth NS, Brown TR, Daly NL, Gomez-Roman N, Grandori C, Eisenman RN, White RJ. Direct regulation of RNA polymerase III transcription by RB p53 and c-Myc. Cell Cycle. 2003;2:181–184. [PubMed] [Google Scholar]
- 50.Scott PH, Cairns CA, Sutcliffe JE, Alzuherri HM, McLees A, Winter AG, White RJ. Regulation of RNA polymerase III transcription during cell cycle entry. J. Biol. Chem. 2001;276:1005–1014. doi: 10.1074/jbc.M005417200. [DOI] [PubMed] [Google Scholar]
- 51.White RJ, Gottlieb TM, Downes CS, Jackson SP. Cell cycle regulation of RNA polymerase III transcription. Mol. Cell Biol. 1995;15:6653–6662. doi: 10.1128/mcb.15.12.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costanzo G, Camier S, Carlucci P, Burderi L, Negri R. RNA polymerase III transcription complexes on chromosomal 5S rRNA genes in vivo: TFIIIB occupancy and promoter opening. Mol. Cell Biol. 2001;21:3166–3178. doi: 10.1128/MCB.21.9.3166-3178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young LS, Rivier DH, Sprague KU. Sequences far downstream from the classical tRNA promoter elements bind RNA polymerase III transcription factors. Mol. Cell Biol. 1991;11:1382–1392. doi: 10.1128/mcb.11.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Besser D, Gotz F, Schulze-Forster K, Wagner H, Kroger H, Simon D. DNA methylation inhibits transcription by RNA polymerase III of a tRNA gene, but not of a 5S rRNA gene. FEBS Lett. 1990;269:358–362. doi: 10.1016/0014-5793(90)81193-r. [DOI] [PubMed] [Google Scholar]
- 55.Kassavetis GA, Riggs DL, Negri R, Nguyen LH, Geiduschek EP. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol. Cell Biol. 1989;9:2551–2566. doi: 10.1128/mcb.9.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 57.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat. Struct. Mol. Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 58.Hess J, Perez-Stable C, Wu GJ, Weir B, Tinoco I, Jr. Shen CK. End-to-end transcription of an Alu family repeat. A new type of polymerase-III-dependent terminator and its evolutionary implication. J. Mol. Biol. 1985;184:7–21. doi: 10.1016/0022-2836(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 59.Gu TJ, Yi X, Zhao XW, Zhao Y, Yin JQ. Alu-directed transcriptional regulation of some novel miRNAs. BMC Genomics. 2009;10:563. doi: 10.1186/1471-2164-10-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ambros V, Lee RC. Identification of microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods Mol. Biol. 2004;265:131–158. doi: 10.1385/1-59259-775-0:131. [DOI] [PubMed] [Google Scholar]
- 61.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 63.Davis CD, Ross SA. Evidence for dietary regulation of microRNA expression in cancer cells. Nutr. Rev. 2008;66:477–482. doi: 10.1111/j.1753-4887.2008.00080.x. [DOI] [PubMed] [Google Scholar]
- 64.Fiedler SD, Carletti MZ, Hong X, Christenson LK. Hormonal regulation of microRNA expression in periovulatory mouse mural granulosa cells. Biol. Reprod. 2008;79(6):1030–7. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulshreshtha R, Ferracin M, Negrini M, Calin GA, Davuluri RV, Ivan M. Regulation of microRNA expression: the hypoxic component. Cell Cycle. 2007;6:1426–1431. [PubMed] [Google Scholar]
- 66.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol. Cell. Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 68.Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G, Chartrand P. An E2F/miR-20a autoregulatory feedback loop. J. Biol. Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 69.Woods K, Thomson JM, Hammond SM. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J. Biol. Chem. 2007;282:2130–2134. doi: 10.1074/jbc.C600252200. [DOI] [PubMed] [Google Scholar]
- 70.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol. Cell. 2010;39:373–384. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michlewski G, Guil S, Semple CA, Caceres JF. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol. Cell. 2008;32:383–393. doi: 10.1016/j.molcel.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Han L, Witmer PD, Casey E, Valle D, Sukumar S. DNA Methylation Regulates MicroRNA Expression. Cancer Biol. Ther. 2007;6:1284–8. doi: 10.4161/cbt.6.8.4486. [DOI] [PubMed] [Google Scholar]
- 74.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T, McCue P, Pestell RG. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 76.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 77.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 78.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 80.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 81.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin SL, Kim H, Ying SY. Intron-mediated RNA interference and microRNA (miRNA) Front. Biosci. 2008;13:2216–2230. doi: 10.2741/2836. [DOI] [PubMed] [Google Scholar]
- 83.Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, Sohn SY, Cho Y, Zhang BT, Kim VN. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 84.Abbott AL, Alvarez-Saavedra E, Miska EA, Lau NC, Bartel DP, Horvitz HR, Ambros V. The let-7 MicroRNA family members mir-48, mir-84 and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev. Cell. 2005;9:403–414. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng Y, Cai X, Cullen BR. Use of RNA polymerase II to transcribe artificial microRNAs. Methods Enzymol. 2005;392:371–380. doi: 10.1016/S0076-6879(04)92022-8. [DOI] [PubMed] [Google Scholar]
- 86.Zeng Y, Cullen BR. Sequence requirements for micro RNA processing and function in human cells. RNA. 2003;9:112–123. doi: 10.1261/rna.2780503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng Y, Cullen BR. Efficient processing of primary microRNA hairpins by Drosha requires flanking nonstructured RNA sequences. J. Biol. Chem. 2005;280:27595–27603. doi: 10.1074/jbc.M504714200. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Zeng Y. The terminal loop region controls microRNA processing by Drosha and Dicer. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq645. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han J, Pedersen JS, Kwon SC, Belair CD, Kim YK, Yeom KH, Yang WY, Haussler D, Blelloch R, Kim VN. Posttranscriptional crossregulation between Drosha and DGCR8. Cell. 2009;136:75–84. doi: 10.1016/j.cell.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Triboulet R, Chang HM, Lapierre RJ, Gregory RI. Post-transcriptional control of DGCR8 expression by the Microprocessor. RNA. 2009;15:1005–1011. doi: 10.1261/rna.1591709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guil S, Caceres JF. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat. Struct. Mol. Biol. 2007;14:591–596. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- 92.Gregory RI, Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 2005;65:3509–3512. doi: 10.1158/0008-5472.CAN-05-0298. [DOI] [PubMed] [Google Scholar]
- 93.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. USA. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim YK, Kim VN. Processing of intronic microRNAs. EMBO J. 2007;26:775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ying SY, Lin SL. Intron-derived microRNAs--fine tuning of gene functions. Gene. 2004;342:25–28. doi: 10.1016/j.gene.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 96.Lin SL, Chang D, Wu DY, Ying SY. A novel RNA splicing-mediated gene silencing mechanism potential for genome evolution. Biochem. Biophys. Res. Commun. 2003;310:754–760. doi: 10.1016/j.bbrc.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 97.Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol. Cell. 2007;28:328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rainer J, Ploner C, Jesacher S, Ploner A, Eduardoff M, Mansha M, Wasim M, Panzer-Grumayer R, Trajanoski Z, Niederegger H, Kofler R. Glucocorticoid-regulated microRNAs and mirtrons in acute lymphoblastic leukemia. Leukemia. 2009;23:746–752. doi: 10.1038/leu.2008.370. [DOI] [PubMed] [Google Scholar]
- 99.Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448:83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin SL, Miller JD, Ying SY. Intronic MicroRNA (miRNA) J. Biomed. Biotechnol. 2006;2006:26818. doi: 10.1155/JBB/2006/26818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lund E, Dahlberg JE. Substrate Selectivity of Exportin 5 and Dicer in the Biogenesis of MicroRNAs. Cold Spring Harb. Symp. Quant. Biol. 2006;71:59–66. doi: 10.1101/sqb.2006.71.050. [DOI] [PubMed] [Google Scholar]
- 103.Okada C, Yamashita E, Lee SJ, Shibata S, Katahira J, Nakagawa A, Yoneda Y, Tsukihara T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326:1275–1279. doi: 10.1126/science.1178705. [DOI] [PubMed] [Google Scholar]
- 104.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeng Y, Cullen BR. Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res. 2004;32:4776–4785. doi: 10.1093/nar/gkh824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H, Kolb FA, Brondani V, Billy E, Filipowicz W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002;21:5875–5885. doi: 10.1093/emboj/cdf582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr. Opin. Struct. Biol. 2005;15:331–341. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 109.Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W. TRBP a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang F, Ye X, Liu X, Fincher L, McKearin D, Liu Q. Dicer-1 and R3D1-L catalyze microRNA maturation in Drosophila. Genes Dev. 2005;19:1674–1679. doi: 10.1101/gad.1334005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 112.Provost P, Dishart D, Doucet J, Frendewey D, Samuelsson B, Radmark O. Ribonuclease activity and RNA binding of recombinant human Dicer. EMBO J. 2002;21:5864–5874. doi: 10.1093/emboj/cdf578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 114.Song JJ, Liu J, Tolia NH, Schneiderman J, Smith SK, Martienssen RA, Hannon GJ, Joshua-Tor L. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat. Struct. Biol. 2003;10:1026–1032. doi: 10.1038/nsb1016. [DOI] [PubMed] [Google Scholar]
- 115.Yan KS, Yan S, Farooq A, Han A, Zeng L, Zhou MM. Structure and conserved RNA binding of the PAZ domain. Nature. 2003;426:468–474. doi: 10.1038/nature02129. [DOI] [PubMed] [Google Scholar]
- 116.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 117.Billy E, Brondani V, Zhang H, Muller U, Filipowicz W. Specific interference with gene expression induced by long double-stranded RNA in mouse embryonal teratocarcinoma cell lines. Proc. Natl. Acad. Sci. USA. 2001;98:14428–14433. doi: 10.1073/pnas.261562698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 121.Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, Ma E, Mane S, Hannon GJ, Lawson ND, Wolfe SA, Giraldez AJ. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328:1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 123.O'Carroll D, Mecklenbrauker I, Das PP, Santana A, Koenig U, Enright AJ, Miska EA, Tarakhovsky A. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21:1999–2004. doi: 10.1101/gad.1565607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 125.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 126.Maniataki E, Mourelatos Z. A human ATP-independent RISC assembly machine fueled by pre-miRNA. Genes Dev. 2005;19:2979–2990. doi: 10.1101/gad.1384005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rossi JJ. Mammalian Dicer finds a partner. EMBO Rep. 2005;6:927–929. doi: 10.1038/sj.embor.7400531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melo SA, Ropero S, Moutinho C, Aaltonen LA, Yamamoto H, Calin GA, Rossi S, Fernandez AF, Carneiro F, Oliveira C, Ferreira B, Liu CG, Villanueva A, Capella G, Schwartz S Jr, Shiekhattar R, Esteller M. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nat. Genet. 2009;41:365–370. doi: 10.1038/ng.317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Daher A, Longuet M, Dorin D, Bois F, Segeral E, Bannwarth S, Battisti PL, Purcell DF, Benarous R, Vaquero C, Meurs EF, Gatignol A. Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression. J. Biol. Chem. 2001;276:33899–33905. doi: 10.1074/jbc.M103584200. [DOI] [PubMed] [Google Scholar]
- 130.Gupta V, Huang X, Patel RC. The carboxy-terminal M3 motifs of PACT and TRBP have opposite effects on PKR activity. Virology. 2003;315:283–291. doi: 10.1016/s0042-6822(03)00589-0. [DOI] [PubMed] [Google Scholar]
- 131.Peters GA, Hartmann R, Qin J, Sen GC. Modular structure of PACT: distinct domains for binding and activating PKR. Mol. Cell. Biol. 2001;21:1908–1920. doi: 10.1128/MCB.21.6.1908-1920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patel RC, Sen GC. PACT a protein activator of the interferon-induced protein kinase PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 134.Kalai M, Suin V, Festjens N, Meeus A, Bernis A, Wang XM, Saelens X, Vandenabeele P. The caspase-generated fragments of PKR cooperate to activate full-length PKR and inhibit translation. Cell Death Differ. 2007;14:1050–1059. doi: 10.1038/sj.cdd.4402110. [DOI] [PubMed] [Google Scholar]
- 135.Vattem KM, Staschke KA, Wek RC. Mechanism of activation of the double-stranded-RNA-dependent protein kinase, PKR: role of dimerization and cellular localization in the stimulation of PKR phosphorylation of eukaryotic initiation factor-2 (eIF2) Eur. J. Biochem. 2001;268:3674–3684. doi: 10.1046/j.1432-1327.2001.02273.x. [DOI] [PubMed] [Google Scholar]
- 136.Aaltonen T, Abulencia A, Adelman J, Affolder T, Akimoto T, Albrow MG, Ambrose D, Amerio S, Amidei D, Anastassov A, Anikeev K, Annovi A, Antos J, Aoki M, Apollinari G, Arguin JF, Arisawa T, Artikov A, Ashmanskas W, Attal A, Azfar F, Azzi-Bacchetta P, Azzurri P, Bacchetta N, Badgett W, Barbaro-Galtieri A, Barnes VE, Barnett BA, Baroiant S, Bartsch V, Bauer G, Bedeschi F, Behari S, Belforte S, Bellettini G, Bel-linger J, Belloni A, Benjamin D, Beretvas A, Beringer J, Berry T, Bhatti A, Binkley M, Bisello D, Blair RE, Blocker C, Blumenfeld B, Bocci A, Bodek A, Boisvert V, Bolla G, Bolshov A, Bortoletto D, Boudreau J, Boveia A, Brau B, Brigliadori L, Bromberg C, Brubaker E, Budagov J, Budd HS, Budd S, Budroni S, Burkett K, Busetto G, Bussey P, Byrum KL, Cabrera S, Campanelli M, Campbell M, Canelli F, Canepa A, Carillo S, Carlsmith D, Carosi R, Casarsa M, Castro A, Catastini P, Cauz D, Cavalli-Sforza M, Cerri A, Cerrito L, Chang SH, Chen YC, Chertok M, Chiarelli G, Chlachidze G, Chlebana F, Cho I, Cho K, Chokheli D, Chou JP, Choudalakis G, Chuang SH, Chung K, Chung WH, Chung YS, Ciljak M, Ciobanu CI, Ciocci MA, Clark A, Clark D, Coca M, Compostella G, Convery ME, Conway J, Cooper B, Copic K, Cordelli M, Cortiana G, Crescioli F, Almenar CC, Cuevas J, Culbertson R, Cully JC, Cyr D, Daronco S, Datta M, D'Auria S, Davies T, D'Onofrio M, Dagenhart D, de Barbaro P, De Cecco S, Deisher A, De Lentdecker G, Dell'orso M, Delli Paoli F, Demortier L, Deng J, Deninno M, De Pedis D, Derwent PF, Di Giovanni GP, Dionisi C, Di Ruzza B, Dittmann JR, Dituro P, Dorr C, Donati S, Donega M, Dong P, Donini J, Dorigo T, Dube S, Efron J, Erbacher R, Errede D, Errede S, Eusebi R, Fang HC, Farrington S, Fedorko I, Fedorko WT, Feild RG, Feindt M, Fernandez JP, Field R, Flanagan G, Foland A, Forrester S, Foster GW, Franklin M, Freeman JC, Furic I, Gallinaro M, Galyardt J, Garcia JE, Garberson F, Garfinkel AF, Gay C, Gerberich H, Gerdes D, Giagu S, Giannetti P, Gibson A, Gibson K, Gimmell JL, Ginsburg C, Giokaris N, Giordani M, Giromini P, Giunta M, Giurgiu G, Glagolev V, Glenzinski D, Gold M, Goldschmidt N, Goldstein J, Golossanov A, Gomez G, Gomez-Ceballos G, Goncharov M, Gonzalez O, Gorelov I, Goshaw AT, Goulianos K, Gresele A, Griffiths M, Grinstein S, Grosso-Pilcher C, Grundler U, da Costa JG, Gunay-Unalan Z, Haber C, Hahn K, Hahn SR, Halkiadakis E, Hamilton A, Han BY, Han JY, Handler R, Happacher F, Hara K, Hare M, Harper S, Harr RF, Harris RM, Hartz M, Hatakeyama K, Hauser J, Heijboer A, Heinemann B, Heinrich J, Henderson C, Herndon M, Heuser J, Hidas D, Hill CS, Hirschbuehl D, Hocker A, Holloway A, Hou S, Houlden M, Hsu SC, Huffman BT, Hughes RE, Husemann U, Huston J, Incandela J, Introzzi G, Iori M, Ishizawa Y, Ivanov A, Iyutin B, James E, Jang D, Jayatilaka B, Jeans D, Jensen H, Jeon EJ, Jindariani S, Jones M, Joo KK, Jun SY, Jung JE, Junk TR, Kamon T, Karchin PE, Kato Y, Kemp Y, Kephart R, Kerzel U, Khotilovich V, Kilminster B, Kim DH, Kim HS, Kim JE, Kim MJ, Kim SB, Kim SH, Kim YK, Kimura N, Kirsch L, Klimenko S, Klute M, Knuteson B, Ko BR, Kondo K, Kong DJ, Konigsberg J, Korytov A, Kotwal AV, Kovalev A, Kraan AC, Kraus J, Kravchenko I, Kreps M, Kroll J, Krumnack N, Kruse M, Krutelyov V, Kubo T, Kuhlmann SE, Kuhr T, Kusakabe Y, Kwang S, Laasanen AT, Lai S, Lami S, Lammel S, Lancaster M, Lander RL, Lannon K, Lath A, Latino G, Lazzizzera I, Lecompte T, Lee J, Lee YJ, Lee SW, Lefevre R, Leonardo N, Leone S, Levy S, Lewis JD, Lin C, Lin CS, Lindgren M, Lipeles E, Lister A, Litvintsev DO, Liu T, Lockyer NS, Loginov A, Loreti M, Loverre P, Lu RS, Lucchesi D, Lujan P, Lukens P, Lungu G, Lyons L, Lys J, Lysak R, Lytken E, Mack P, MacQueen D, Madrak R, Maeshima K, Makhoul K, Maki T, Maksi-movic P, Malde S, Manca G, Margaroli F, Marginean R, Marino C, Marino CP, Martin A, Martin M, Martin V, Martinez M, Maruyama T, Mastrandrea P, Masubuchi T, Matsunaga H, Mattson ME, Mazini R, Mazzanti P, McFarland KS, McIntyre P, McNulty R, Mehta A, Mehtala P, Menzemer S, Menzione A, Merkel P, Mesropian C, Messina A, Miao T, Miladinovic N, Miles J, Miller R, Mills C, Milnik M, Mitra A, Mitselmakher G, Miyamoto A, Moed S, Moggi N, Mohr B, Moore R, Morello M, Fernandez PM, Mulmenstadt J, Mukherjee A, Muller T, Mumford R, Murat P, Nachtman J, Nagano A, Naganoma J, Nakano I, Napier A, Necula V, Neu C, Neubauer MS, Nielsen J, Nigmanov T, Nodulman L, Norniella O, Nurse E, Oh SH, Oh YD, Oksuzian I, Okusawa T, Oldeman R, Orava R, Osterberg K, Pagliarone C, Palencia E, Papadimitriou V, Paramonov AA, Parks B, Pashapour S, Patrick J, Pauletta G, Paulini M, Paus C, Pellett DE, Penzo A, Phillips TJ, Piacentino G, Piedra J, Pinera L, Pitts K, Plager C, Pondrom L, Portell X, Poukhov O, Pounder N, Prakoshyn F, Pronko A, Proudfoot J, Ptohos F, Punzi G, Pursley J, Rademacker J, Rahaman A, Ranjan N, Rappoccio S, Reisert B, Rekovic V, Renton P, Rescigno M, Richter S, Rimondi F, Ristori L, Robson A, Rodrigo T, Rogers E, Rolli S, Roser R, Rossi M, Rossin R, Ruiz A, Russ J, Rusu V, Saarikko H, Sabik S, Safonov A, Sakumoto WK, Salamanna G, Salto O, Saltzberg D, Sanchez C, Santi L, Sarkar S, Sartori L, Sato K, Savard P, Savoy-Navarro A, Scheidle T, Schlabach P, Schmidt EE, Schmidt MP, Schmitt M, Schwarz T, Scodellaro L, Scott AL, Scribano A, Scuri F, Sedov A, Seidel S, Seiya Y, Semenov A, Sexton-Kennedy L, Sfyrla A, Shapiro MD, Shears T, Shepard PF, Sherman D, Shimojima M, Shochet M, Shon Y, Shreyber I, Sidoti A, Sinervo P, Sisakyan A, Sjolin J, Slaughter AJ, Slaunwhite J, Sliwa K, Smith JR, Snider FD, Snihur R, Soderberg M, Soha A, Somalwar S, Sorin V, Spalding J, Spinella F, Spreitzer T, Squillacioti P, Stanitzki M, Staveris-Polykalas A, St Denis R, Stelzer B, Stelzer-Chilton O, Stentz D, Strologas J, Stuart D, Suh JS, Sukhanov A, Sun H, Suzuki T, Taffard A, Takashima R, Takeuchi Y, Takikawa K, Tanaka M, Tanaka R, Tecchio M, Teng PK, Terashi K, Thom J, Thompson AS, Thomson E, Tipton P, Tiwari V, Tkaczyk S, Toback D, Tokar S, Tollefson K, Tomura T, Tonelli D, Torre S, Torretta D, Tourneur S, Trischuk W, Tsuchiya R, Tsuno S, Turini N, Ukegawa F, Unverhau T, Uozumi S, Usynin D, Vallecorsa S, van Remortel N, Varganov A, Vataga E, Vazquez F, Velev G, Veramendi G, Veszpremi V, Vidal R, Vila I, Vilar R, Vine T, Vollrath I, Volobouev I, Volpi G, Wurthwein F, Wagner P, Wagner RG, Wagner RL, Wagner J, Wagner W, Wallny R, Wang SM, Warburton A, Waschke S, Waters D, Wester W. C 3rd, Whitehouse B, Whiteson D, Wicklund A B, Wicklund E, Williams G, Williams HH, Wilson P, Winer BL, Wittich P, Wolbers S, Wolfe C, Wright T, Wu X, Wynne SM, Yagil A, Yamamoto K, Yamaoka J, Yamashita T, Yang C, Yang UK, Yang YC, Yao WM, Yeh GP, Yoh J, Yorita K, Yoshida T, Yu GB, Yu I, Yu SS, Yun JC, Zanello L, Zanetti A, Zaw I, Zhang X, Zhou J, Zucchelli S. Measurement of the top-quark mass in all-hadronic decays in pp collisions at CDF II. Phys. Rev. Lett. 2007;98:142001. doi: 10.1103/PhysRevLett.98.142001. [DOI] [PubMed] [Google Scholar]
- 137.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 138.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 139.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 140.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 141.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 142.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 143.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 144.Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zeng Y, Cullen BR. RNA interference in human cells is restricted to the cytoplasm. RNA. 2002;8:855–860. doi: 10.1017/s1355838202020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tomari Y, Zamore PD. MicroRNA biogenesis: drosha can't cut it without a partner. Curr. Biol. 2005;15:R61–64. doi: 10.1016/j.cub.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 147.Leuschner PJ, Ameres SL, Kueng S, Martinez J. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO Rep. 2006;7:314–320. doi: 10.1038/sj.embor.7400637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Macrae IJ, Ma E, Zhou M, Robinson CV, Doudna JA. In vitro reconstitution of the human RISC-loading complex. Proc. Natl. Acad. Sci. USA. 2008;105(2):512–7. doi: 10.1073/pnas.0710869105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anantharaman V, Koonin EV, Aravind L. Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Res. 2002;30:1427–1464. doi: 10.1093/nar/30.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Robb GB, Rana TM. RNA helicase A interacts with RISC in human cells and functions in RISC loading. Mol. Cell. 2007;26:523–537. doi: 10.1016/j.molcel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 151.Abdelhaleem M, Maltais L, Wain H. The human DDX and DHX gene families of putative RNA helicases. Genomics. 2003;81:618–622. doi: 10.1016/s0888-7543(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 152.Lee CG, Hurwitz J. A new RNA helicase isolated from HeLa cells that catalytically translocates in the 3 to 5 direction. J. Biol. Chem. 1992;267:4398–4407. [PubMed] [Google Scholar]
- 153.Friedemann J, Grosse F, Zhang S. Nuclear DNA helicase II (RNA helicase A) interacts with Werner syndrome helicase and stimulates its exonuclease activity. J. Biol. Chem. 2005;280:31303–31313. doi: 10.1074/jbc.M503882200. [DOI] [PubMed] [Google Scholar]
- 154.Meister G, Landthaler M, Peters L, Chen PY, Urlaub H, Luhrmann R, Tuschl T. Identification of novel argonaute-associated proteins. Curr. Biol. 2005;15:2149–2155. doi: 10.1016/j.cub.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 155.Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki S, Liu Q, Tomari Y. ATP-dependent human RISC assembly pathways. Nat. Struct. Mol. Biol. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat. Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 159.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 160.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saxena S, Jonsson ZO, Dutta A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J. Biol. Chem. 2003;278:44312–44319. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- 162.Stark A, Brennecke J, Russell RB, Cohen SM. Identification of Drosophila MicroRNA targets. PLoS Biol. 2003;1:E60. doi: 10.1371/journal.pbio.0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cougot N, Babajko S, Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 2004;165:31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brengues M, Teixeira D, Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486–489. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eystathioy T, Chan EK, Mahler M, Luft LM, Fritzler ML, Fritzler MJ. A panel of monoclonal antibodies to cytoplasmic GW bodies and the mRNA binding protein GW182. Hybrid Hybridomics. 2003;22:79–86. doi: 10.1089/153685903321947996. [DOI] [PubMed] [Google Scholar]
- 168.Eystathioy T, Jakymiw A, Chan EK, Seraphin B, Cougot N, Fritzler MJ. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9:1171–1173. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Dijk E, Cougot N, Meyer S, Babajko S, Wahle E, Seraphin B. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002;21:6915–6924. doi: 10.1093/emboj/cdf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–886. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 173.Meister G, Landthaler M, Dorsett Y, Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–550. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 175.Maroney PA, Yu Y, Fisher J, Nilsen TW. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat. Struct. Mol. Biol. 2006;13:1102–1107. doi: 10.1038/nsmb1174. [DOI] [PubMed] [Google Scholar]
- 176.Maroney PA, Yu Y, Nilsen TW. MicroRNAs mRNAs and Translation. Cold Spring Harb. Symp. Quant. Biol. 2006;71:531–535. doi: 10.1101/sqb.2006.71.043. [DOI] [PubMed] [Google Scholar]
- 177.Nottrott S, Simard MJ, Richter JD. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat. Struct. Mol. Biol. 2006;13:1108–1114. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- 178.Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, Barton MK, Bartel DP. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5' region. EMBO J. 2004;23:3356–3364. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mallory AC, Vaucheret H. MicroRNAs: something important between the genes. Curr. Opin. Plant. Biol. 2004;7:120–125. doi: 10.1016/j.pbi.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 180.Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, Mourelatos Z, Hatzigeorgiou A. A combined computational-experimental approach predicts human microRNA targets. Genes Dev. 2004;18:1165–1178. doi: 10.1101/gad.1184704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- 182.Coller J, Parker R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 2004;73:861–890. doi: 10.1146/annurev.biochem.73.011303.074032. [DOI] [PubMed] [Google Scholar]
- 183.Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 185.Pillai RS, Artus CG, Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Richter D, Isono K. The mechanism of protein synthesis-initiation elongation and termination in translation of genetic messeges. Curr. Top. Microbiol. Immunol. 1977;76:83–125. doi: 10.1007/978-3-642-66653-7_3. [DOI] [PubMed] [Google Scholar]
- 187.Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol. Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 188.Kong YW, Cannell IG, de Moor CH, Hill K, Garside PG, Hamilton TL, Meijer HA, Dobbyn HC, Stoneley M, Spriggs KA, Willis AE, Bushell M. The mechanism of micro-RNA-mediated translation repression is determined by the promoter of the target gene. Proc. Natl. Acad. Sci. USA. 2008;105:8866–8871. doi: 10.1073/pnas.0800650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu J, Rivas FV, Wohlschlegel J, Yates J. R 3rd, Parker R, Hannon GJ. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005;7:1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Andrei MA, Ingelfinger D, Heintzmann R, Achsel T, Rivera-Pomar R, Luhrmann R. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA. 2005;11:717–727. doi: 10.1261/rna.2340405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anderson P, Kedersha N. RNA granules. J. Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 196.Fischer N, Weis K. The DEAD box protein Dhh1 stimulates the decapping enzyme Dcp1. EMBO J. 2002;21:2788–2797. doi: 10.1093/emboj/21.11.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Coller JM, Tucker M, Sheth U, Valencia-Sanchez MA, Parker R. The DEAD box helicase Dhh1p functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA. 2001;7:1717–1727. doi: 10.1017/s135583820101994x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 199.Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- 200.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphory-lation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chan SP, Slack FJ. microRNA-Mediated Silencing Inside P-Bodies. RNA Biol. 2006;3:97–100. doi: 10.4161/rna.3.3.3499. [DOI] [PubMed] [Google Scholar]
- 202.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol. Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 203.Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, Fritzler MJ, Chan EK. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 2005;7:1267–1274. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- 204.Jakymiw A, Pauley KM, Li S, Ikeda K, Lian S, Eystathioy T, Satoh M, Fritzler MJ, Chan EK. The role of GW/P-bodies in RNA processing and silencing. J. Cell Sci. 2007;120:1317–1323. doi: 10.1242/jcs.03429. [DOI] [PubMed] [Google Scholar]
- 205.Pauley KM, Eystathioy T, Jakymiw A, Hamel JC, Fritzler MJ, Chan EK. Formation of GW bodies is a consequence of microRNA genesis. EMBO Rep. 2006;7:904–910. doi: 10.1038/sj.embor.7400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sen GL, Blau HM. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 2005;7:633–636. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- 207.Yang Z, Jakymiw A, Wood MR, Eystathioy T, Rubin RL, Fritzler MJ, Chan EK. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J. Cell Sci. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- 208.Eystathioy T, Chan EK, Tenenbaum SA, Keene JD, Griffith K, Fritzler MJ. A phosphorylated cytoplasmic autoantigen GW182 associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell. 2002;13:1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4: NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Behm-Ansmant I, Rehwinkel J, Izaurralde E. MicroRNAs Silence Gene Expression by Repressing Protein Expression and/or by Promoting mRNA Decay. Cold Spring Harb. Symp. Quant. Biol. 2006;71:523–530. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- 211.Ding L, Han M. GW182 family proteins are crucial for microRNA-mediated gene silencing. Trends Cell Biol. 2007;17:411–416. doi: 10.1016/j.tcb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 212.Ikeda K, Satoh M, Pauley KM, Fritzler MJ, Reeves WH, Chan EK. Detection of the argonaute protein Ago2 and microRNAs in the RNA induced silencing complex (RISC) using a monoclonal antibody. J. Immunol. Methods. 2006;317:38–44. doi: 10.1016/j.jim.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4:e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ferraiuolo MA, Basak S, Dostie J, Murray EL, Schoenberg DR, Sonenberg N. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J. Cell Biol. 2005;170:913–924. doi: 10.1083/jcb.200504039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Caudy AA, Myers M, Hannon GJ, Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tanaka Y, Kozu T. [MiRNA in disease and therapeutic development] Tanpakushitsu Kakusan Koso. 2006;51:2509–2514. [PubMed] [Google Scholar]
- 217.Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, Shiekhattar R, Nishikura K. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat. Struct. Mol. Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kahvejian A, Svitkin YV, Sukarieh R, M'Boutchou MN, Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 2005;19:104–113. doi: 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 220.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Stress-induced Reversal of MicroRNA Repression and mRNA P-body Localization in Human Cells. Cold Spring Harb. Symp. Quant. Biol. 2006;71:513–521. doi: 10.1101/sqb.2006.71.038. [DOI] [PubMed] [Google Scholar]
- 221.Jakymiw A, Ikeda K, Fritzler MJ, Reeves WH, Satoh M, Chan EK. Autoimmune targeting of key components of RNA interference. Arthritis Res. Ther. 2006;8:R87. doi: 10.1186/ar1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liao JY, Ma LM, Guo YH, Zhang YC, Zhou H, Shao P, Chen YQ, Qu LH. Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3' trailers. PLoS One. 2010;5:e10563. doi: 10.1371/journal.pone.0010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Marcon E, Babak T, Chua G, Hughes T, Moens PB. miRNA and piRNA localization in the male mammalian meiotic nucleus. Chromosome Res. 2008;16:243–260. doi: 10.1007/s10577-007-1190-6. [DOI] [PubMed] [Google Scholar]
- 224.Politz JC, Zhang F, Pederson T. MicroRNA-206 colocalizes with ribosome-rich regions in both the nucleolus and cytoplasm of rat myogenic cells. Proc. Natl. Acad. Sci. USA. 2006;103:18957–18962. doi: 10.1073/pnas.0609466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat. Struct. Mol. Biol. 2005;12:133–137. doi: 10.1038/nsmb886. [DOI] [PubMed] [Google Scholar]
- 226.Guang S, Bochner AF, Pavelec DM, Burkhart KB, Harding S, Lachowiec J, Kennedy S. An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science. 2008;321:537–541. doi: 10.1126/science.1157647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Meister G. Molecular biology. RNA interference in the nucleus. Science. 2008;321:496–497. doi: 10.1126/science.1161854. [DOI] [PubMed] [Google Scholar]
- 228.Blow MJ, Grocock RJ, van Dongen S, Enright AJ, Dicks E, Futreal PA, Wooster R, Stratton MR. RNA editing of human microRNAs. Genome Biol. 2006;7:R27. doi: 10.1186/gb-2006-7-4-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Desterro JM, Keegan LP, Lafarga M, Berciano MT, O'Connell M, Carmo-Fonseca M. Dynamic association of RNA-editing enzymes with the nucleolus. J. Cell Sci. 2003;116:1805–1818. doi: 10.1242/jcs.00371. [DOI] [PubMed] [Google Scholar]
- 230.Luciano DJ, Mirsky H, Vendetti NJ, Maas S. RNA editing of a miRNA precursor. RNA. 2004;10:1174–1177. doi: 10.1261/rna.7350304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 233.Croce CM. Oncogenes and cancer. N. Engl. J. Med. 2008;358:502–511. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 234.Konopka JB, Watanabe SM, Singer JW, Collins SJ, Witte ON. Cell lines and clinical isolates derived from Ph1-positive chronic myelogenous leukemia patients express c-abl proteins with a common structural alteration. Proc. Natl. Acad. Sci. USA. 1985;82:1810–1814. doi: 10.1073/pnas.82.6.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tsujimoto Y, Jaffe E, Cossman J, Gorham J, Nowell PC, Croce CM. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11 14) chromosome translocation. Nature. 1985;315:340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- 236.Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 237.Wu W, Sun M, Zou GM, Chen J. MicroRNA and cancer: Current status and prospective. Int. J. Cancer. 2007;120:953–960. doi: 10.1002/ijc.22454. [DOI] [PubMed] [Google Scholar]
- 238.Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25:6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- 239.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 240.Hwang HW, Mendell JT. MicroRNAs in cell proliferation cell death and tumorigenesis. Br. J. Cancer. 2006;94:776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, Dell'Aquila ML, Alder H, Rassenti L, Kipps TJ, Bullrich F, Negrini M, Croce CM. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc. Natl. Acad. Sci. USA. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 244.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 245.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nobes CD, Hall A. Rho GTPases control polarity protrusion and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Holzer M, Rodel L, Seeger G, Gartner U, Narz F, Janke C, Heumann R, Arendt T. Activation of mitogen-activated protein kinase cascade and phosphorylation of cytoskeletal proteins after neurone-specific activation of p21ras II Cytoskeletal proteins and dendritic morphology. Neuroscience. 2001;105:1041–1054. doi: 10.1016/s0306-4522(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 248.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 249.Walsh AB, Bar-Sagi D. Differential activation of the Rac pathway by Ha-Ras and K-Ras. J. Biol. Chem. 2001;276:15609–15615. doi: 10.1074/jbc.M010573200. [DOI] [PubMed] [Google Scholar]
- 250.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 251.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 252.Lee CT, Risom T, Strauss WM. Evolutionary conservation of microRNA regulatory circuits: an examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny. DNA Cell Biol. 2007;26:209–218. doi: 10.1089/dna.2006.0545. [DOI] [PubMed] [Google Scholar]
- 253.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 254.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol. Pharm. Bull. 2006;29:903–906. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 255.Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- 256.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ. Health Perspect. 2003;111:1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sarhadi VK, Wikman H, Salmenkivi K, Kuosma E, Sioris T, Salo J, Karjalainen A, Knuutila S, Anttila S. Increased expression of high mobility group A proteins in lung cancer. J. Pathol. 2006;209:206–212. doi: 10.1002/path.1960. [DOI] [PubMed] [Google Scholar]
- 258.Ligon AH, Morton CC. Leiomyomata: heritability and cytogenetic studies. Hum. Reprod. Update. 2001;7:8–14. doi: 10.1093/humupd/7.1.8. [DOI] [PubMed] [Google Scholar]
- 259.Koscianska E, Baev V, Skreka K, Oikonomaki K, Rusinov V, Tabler M, Kalantidis K. Prediction and preliminary validation of oncogene regulation by miRNAs. BMC Mol. Biol. 2007;8:79. doi: 10.1186/1471-2199-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 261.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 264.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 265.Sanchez-Beato M, Sanchez-Aguilera A, Piris MA. Cell cycle deregulation in B-cell lymphomas. Blood. 2003;101:1220–1235. doi: 10.1182/blood-2002-07-2009. [DOI] [PubMed] [Google Scholar]
- 266.Kitada S, Krajewska M, Zhang X, Scudiero D, Zapata JM, Wang HG, Shabaik A, Tudor G, Krajewski S, Myers TG, Johnson GS, Sausville EA, Reed JC. Expression and location of pro-apoptotic Bcl-2 family protein BAD in normal human tissues and tumor cell lines. Am. J. Pathol. 1998;152:51–61. [PMC free article] [PubMed] [Google Scholar]
- 267.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI, Alder H, Volinia S, Rassenti L, Liu X, Liu CG, Kipps TJ, Negrini M, Croce CM. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. USA. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 269.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 270.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 271.Kaufmann SH, Gores GJ. Apoptosis in cancer: cause and cure. Bioessays. 2000;22:1007–1017. doi: 10.1002/1521-1878(200011)22:11<1007::AID-BIES7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 272.Cao X, Rodarte C, Zhang L, Morgan CD, Littlejohn J, Smythe WR. Bcl2/bcl-xL inhibitor engenders apoptosis and increases chemosensitivity in mesothelioma. Cancer Biol. Ther. 2007;6:246–252. doi: 10.4161/cbt.6.2.3626. [DOI] [PubMed] [Google Scholar]
- 273.Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, Bartz SR, Johnson JM, Cummins JM, Raymond CK, Dai H, Chau N, Cleary M, Jackson AL, Carleton M, Lim L. Transcripts Targeted by the MicroRNA-16 Family Cooperatively Regulate Cell Cycle Progression. Mol. Cell Biol. 2007;27:2240–2252. doi: 10.1128/MCB.02005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 275.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J. Gastrointest. Surg. 2008;12(12):2171–6. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 278.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 279.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 280.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 281.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J. Biol. Chem. 2007;282(19):14328–36. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 283.Kim RH, Mak TW. Tumours and tremors: how PTEN regulation underlies both. Br. J. Cancer. 2006;94:620–624. doi: 10.1038/sj.bjc.6602994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 285.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J. Clin. Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 286.Park MJ, Kim MS, Park IC, Kang HS, Yoo H, Park SH, Rhee CH, Hong SI, Lee SH. PTEN suppresses hyaluronic acid-induced matrix metalloproteinase-9 expression in U87MG glioblastoma cells through focal adhesion kinase dephosphorylation. Cancer Res. 2002;62:6318–6322. [PubMed] [Google Scholar]
- 287.Perry SV. Vertebrate tropomyosin: distribution, properties and function. J. Muscle Res. Cell Motil. 2001;22:5–49. doi: 10.1023/a:1010303732441. [DOI] [PubMed] [Google Scholar]
- 288.Boyd J, Risinger JI, Wiseman RW, Merrick BA, Selkirk JK, Barrett JC. Regulation of microfilament organization and anchorage-independent growth by tropomyosin 1. Proc. Natl. Acad. Sci. USA. 1995;92:11534–11538. doi: 10.1073/pnas.92.25.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J. Biol. Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 290.Yin S, Lockett J, Meng Y, Biliran H, Jr., Blouse G E, Li X, Reddy N, Zhao Z, Lin X, Anagli J, Cher ML, Sheng S. Maspin retards cell detachment via a novel interaction with the urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor system. Cancer Res. 2006;66:4173–4181. doi: 10.1158/0008-5472.CAN-05-3514. [DOI] [PubMed] [Google Scholar]
- 291.Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007;26:4550–4562. doi: 10.1038/sj.onc.1210234. [DOI] [PubMed] [Google Scholar]
- 292.Stephens RW, Nielsen HJ, Christensen IJ, Thorlacius-Ussing O, Sorensen S, Dano K, Brunner N. Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis. J. Natl. Cancer Inst. 1999;91:869–874. doi: 10.1093/jnci/91.10.869. [DOI] [PubMed] [Google Scholar]
- 293.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, Yoshida Y, Seto M. Identification and characterization of a novel gene C13orf25 as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 295.Giangrande PH, Zhu W, Rempel RE, Laakso N, Nevins JR. Combinatorial gene control involving E2F and E Box family members. EMBO J. 2004;23:1336–1347. doi: 10.1038/sj.emboj.7600134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hammond SM. MicroRNAs as oncogenes. Curr. Opin. Genet. Dev. 2006;16:4–9. doi: 10.1016/j.gde.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 297.Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco L P, Baldassarre G, Croce CM, Vecchione A. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 298.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster miR-17-92 is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 299.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Dang CV, Resar LM, Emison E, Kim S, Li Q, Prescott JE, Wonsey D, Zeller K. Function of the c-Myc oncogenic transcription factor. Exp. Cell Res. 1999;253:63–77. doi: 10.1006/excr.1999.4686. [DOI] [PubMed] [Google Scholar]
- 301.Cole MD, McMahon SB. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene. 1999;18:2916–2924. doi: 10.1038/sj.onc.1202748. [DOI] [PubMed] [Google Scholar]
- 302.Eisenman RN. Deconstructing myc. Genes Dev. 2001;15:2023–2030. doi: 10.1101/gad928101. [DOI] [PubMed] [Google Scholar]
- 303.Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, Cocito A, Amati B. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, Greenberg ME, Orkin S, Nevins JR, Robinson ML, Leone G. The E2F1-3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 305.DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Johnson DG, Schwarz JK, Cress WD, Nevins JR. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 307.Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M, Zhang MQ, Lazebnik Y, Bar-Sagi D, Lowe SW. Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 2002;4:859–864. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 308.Furukawa Y, Nishimura N, Furukawa Y, Satoh M, Endo H, Iwase S, Yamada H, Matsuda M, Kano Y, Nakamura M. Apaf-1 is a mediator of E2F-1-induced apoptosis. J. Biol. Chem. 2002;277:39760–39768. doi: 10.1074/jbc.M200805200. [DOI] [PubMed] [Google Scholar]
- 309.Urist M, Tanaka T, Poyurovsky MV, Prives C. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 2004;18:3041–3054. doi: 10.1101/gad.1221004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lin WC, Lin FT, Nevins JR. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 2001;15:1833–1844. [PMC free article] [PubMed] [Google Scholar]
- 311.Kowalik TF, DeGregori J, Schwarz JK, Nevins JR. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J. Virol. 1995;69:2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ginsberg D. E2F1 pathways to apoptosis. FEBS Lett. 2002;529:122–125. doi: 10.1016/s0014-5793(02)03270-2. [DOI] [PubMed] [Google Scholar]
- 313.Coller HA, Forman JJ, Legesse-Miller A. “Myc'ed messages”: myc induces transcription of E2F1 while inhibiting its translation via a microRNA polycistron. PLoS Genet. 2007;3:e146. doi: 10.1371/journal.pgen.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Johnson DG, Cress WD, Jakoi L, Nevins JR. Oncogenic capacity of the E2F1 gene. Proc. Natl. Acad. Sci. USA. 1994;91:12823–12827. doi: 10.1073/pnas.91.26.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Thalmeier K, Synovzik H, Mertz R, Winnacker EL, Lipp M. Nuclear factor E2F mediates basic transcription and trans-activation by E1a of the human MYC promoter. Genes Dev. 1989;3:527–536. doi: 10.1101/gad.3.4.527. [DOI] [PubMed] [Google Scholar]
- 316.Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. USA. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhang XL, Fu WL, Zhao HX, Zhou LX, Huang JF, Wang JH. Molecular studies of loss of heterozygosity in Chinese sporadic retinoblastoma patients. Clin. Chim. Acta. 2005;358:75–80. doi: 10.1016/j.cccn.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 318.Lin YW, Sheu JC, Liu LY, Chen CH, Lee HS, Huang GT, Wang JT, Lee PH, Lu FJ. Loss of heterozygosity at chromosome 13q in hepatocellular carcinoma: identification of three independent regions. Eur. J. Cancer. 1999;35:1730–1734. doi: 10.1016/s0959-8049(99)00205-1. [DOI] [PubMed] [Google Scholar]
- 319.Eiriksdottir G, Johannesdottir G, Ingvarsson S, Bjornsdottir IB, Jonasson JG, Agnarsson BA, Hallgrimsson J, Gudmundsson J, Egilsson V, Sigurdsson H, Barkardottir RB. Mapping loss of heterozygosity at chromosome 13q: loss at 13q12-q13 is associated with breast tumour progression and poor prognosis. Eur. J. Cancer. 1998;34:2076–2081. doi: 10.1016/s0959-8049(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 320.Shao J, Li Y, Wu Q, Liang X, Yu X, Huang L, Hou J, Huang X, Ernberg I, Hu LF, Zeng Y. High frequency loss of heterozygosity on the long arms of chromosomes 13 and 14 in nasopharyngeal carcinoma in Southern China. Chin. Med. J. (Engl) 2002;115:571–575. [PubMed] [Google Scholar]
- 321.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol. Cell. Biol. 2006;26:8191–8201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yan J, Tsai SY, Tsai MJ. SRC-3/AIB1: transcriptional coactivator in oncogenesis. Acta Pharmacol. Sin. 2006;27:387–394. doi: 10.1111/j.1745-7254.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- 323.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1 a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 324.Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol. Cell. Biol. 2004;24:5157–5171. doi: 10.1128/MCB.24.12.5157-5171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Oh A, List HJ, Reiter R, Mani A, Zhang Y, Gehan E, Wellstein A, Riegel AT. The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res. 2004;64:8299–8308. doi: 10.1158/0008-5472.CAN-04-0354. [DOI] [PubMed] [Google Scholar]
- 326.Wang Z, Rose DW, Hermanson O, Liu F, Herman T, Wu W, Szeto D, Gleiberman A, Krones A, Pratt K, Rosenfeld R, Glass CK, Rosenfeld MG. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA. 2000;97:13549–13554. doi: 10.1073/pnas.260463097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai MJ. Role of the steroid receptor coactivator SRC-3 in cell growth. Mol. Cell Biol. 2003;23:7742–7755. doi: 10.1128/MCB.23.21.7742-7755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Han SJ, DeMayo FJ, Xu J, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol. Endocrinol. 2006;20:45–55. doi: 10.1210/me.2005-0310. [DOI] [PubMed] [Google Scholar]
- 329.Werbajh S, Nojek I, Lanz R, Costas MA. RAC-3 is a NF-kappa B coactivator. FEBS Lett. 2000;485:195–199. doi: 10.1016/s0014-5793(00)02223-7. [DOI] [PubMed] [Google Scholar]
- 330.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Babak T, Zhang W, Morris Q, Blencowe BJ, Hughes TR. Probing microRNAs with microarrays: tissue specificity and functional inference. RNA. 2004;10:1813–1819. doi: 10.1261/rna.7119904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Nelson PT, Baldwin DA, Scearce LM, Oberholtzer JC, Tobias JW, Mourelatos Z. Microarray-based, high-throughput gene expression profiling of microRNAs. Nat. Methods. 2004;1:155–161. doi: 10.1038/nmeth717. [DOI] [PubMed] [Google Scholar]
- 333.Barad O, Meiri E, Avniel A, Aharonov R, Barzilai A, Bentwich I, Einav U, Gilad S, Hurban P, Karov Y, Lobenhofer EK, Sharon E, Shiboleth YM, Shtutman M, Bentwich Z, Einat P. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res. 2004;14:2486–2494. doi: 10.1101/gr.2845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, Perera RJ. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32:e188. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Chen J, Lozach J, Garcia EW, Barnes B, Luo S, Mikoulitch I, Zhou L, Schroth G, Fan JB. Highly sensitive and specific microRNA expression profiling using BeadArray technology. Nucleic Acids Res. 2008;36:e87. doi: 10.1093/nar/gkn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chen YT, Kitabayashi N, Zhou XK, Fahey T.J 3rd, Scognamiglio T. MicroRNA analysis as a potential diagnostic tool for papillary thyroid carcinoma. Mod. Pathol. 2008;21:1139–1146. doi: 10.1038/modpathol.2008.105. [DOI] [PubMed] [Google Scholar]
- 337.Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol. Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis and patient survival. Clin. Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin. Chem. 2008;54:1696–1704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 340.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 341.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 342.Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Svoboda M, Izakovicova Holla L, Sefr R, Vrtkova I, Kocakova I, Tichy B, Dvorak J. Micro-RNAs miR125b and miR137 are frequently upregulated in response to capecitabine chemoradiotherapy of rectal cancer. Int. J. Oncol. 2008;33:541–547. [PubMed] [Google Scholar]
- 344.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 345.Chin LJ, Ratner E, Leng S, Zhai R, Nallur S, Babar I, Muller RU, Straka E, Su L, Burki EA, Crowell RE, Patel R, Kulkarni T, Homer R, Zelterman D, Kidd KK, Zhu Y, Christiani DC, Belinsky SA, Slack FJ, Weidhaas JB. A SNP in a let-7 microRNA complementary site in the KRAS 3 untranslated region increases non-small cell lung cancer risk. Cancer Res. 2008;68:8535–8540. doi: 10.1158/0008-5472.CAN-08-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 347.Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, Chen Y, Xu L, Zen K, Zhang C, Shen H. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J. Clin. Oncol. 2010;28:1721–1726. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 348.Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS One. 2009;4:e6229. doi: 10.1371/journal.pone.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hausler SF, Keller A, Chandran PA, Ziegler K, Zipp K, Heuer S, Krockenberger M, Engel JB, Honig A, Scheffler M, Dietl J, Wischhusen J. Whole blood-derived miRNA profiles as potential new tools for ovarian cancer screening. Br. J. Cancer. 2010;103:693–700. doi: 10.1038/sj.bjc.6605833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Keller A, Leidinger P, Borries A, Wendschlag A, Wucherpfennig F, Scheffler M, Huwer H, Lenhof HP, Meese E. miRNAs in lung cancer - studying complex fingerprints in patient's blood cells by microarray experiments. BMC Cancer. 2009;9:353. doi: 10.1186/1471-2407-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Leidinger P, Keller A, Borries A, Reichrath J, Rass K, Jager SU, Lenhof HP, Meese E. High-throughput miRNA profiling of human melanoma blood samples. BMC Cancer. 2010;10:262. doi: 10.1186/1471-2407-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, Warnecke JM, Sczakiel G. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol. Oncol. 2009 doi: 10.1016/j.urolonc.2009.01.027. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 353.Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq601. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 356.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.El-Hefnawy T, Raja S, Kelly L, Bigbee WL, Kirkwood JM, Luketich JD, Godfrey TE. Characterization of amplifiable, circulating RNA in plasma and its potential as a tool for cancer diagnostics. Clin. Chem. 2004;50:564–573. doi: 10.1373/clinchem.2003.028506. [DOI] [PubMed] [Google Scholar]
- 358.Sisco KL. Is RNA in serum bound to nucleoprotein complexes? Clin. Chem. 2001;47:1744–1745. [PubMed] [Google Scholar]
- 359.Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue. Clin. Cancer Res. 2008;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 360.Gaarz A, Debey-Pascher S, Classen S, Eggle D, Gathof B, Chen J, Fan JB, Voss T, Schultze JL, Staratschek-Jox A. Bead array-based microrna expression profiling of peripheral blood and the impact of different RNA isolation approaches. J. Mol. Diagn. 2010;12:335–344. doi: 10.2353/jmoldx.2010.090116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, Liu CG, Calin GA, Croce CM, Harris CC. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 363.Zhang B, Pan X, Anderson TA. MicroRNA: a new player in stem cells. J Cell Physiol. 2006;209:266–269. doi: 10.1002/jcp.20713. [DOI] [PubMed] [Google Scholar]
- 364.Zhang B, Pan X, Wang Q, Cobb GP, Anderson TA. Computational identification of microRNAs and their targets. Comput. Biol. Chem. 2006;30:395–407. doi: 10.1016/j.compbiolchem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 365.Yang M, Mattes J. Discovery biology and therapeutic potential of RNA interference microRNA and antagomirs. Pharmacol. Ther. 2008;117:94–104. doi: 10.1016/j.pharmthera.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 366.Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat. Genet. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]
- 367.Lefesvre P, Attema J, van Bekkum D. A comparison of efficacy and toxicity between electroporation and adenoviral gene transfer. BMC Mol. Biol. 2002;3:12. doi: 10.1186/1471-2199-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Boden D, Pusch O, Lee F, Tucker L, Ramratnam B. Efficient gene transfer of HIV-1-specific short hairpin RNA into human lymphocytic cells using recombinant adeno-associated virus vectors. Mol. Ther. 2004;9:396–402. doi: 10.1016/j.ymthe.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 369.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells stem cells and transgenic mice by RNA interference. Nat. Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 370.Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat. Methods. 2006;3:109–116. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- 371.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 372.Harvey BG, Hackett NR, El-Sawy T, Rosengart TK, Hirschowitz EA, Lieberman MD, Lesser ML, Crystal RG. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J. Virol. 1999;73:6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Sinn PL, Sauter SL, McCray P.B Jr. Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors--design, biosafety, and production. Gene Ther. 2005;12:1089–1098. doi: 10.1038/sj.gt.3302570. [DOI] [PubMed] [Google Scholar]
- 375.Hackett CS, Geurts AM, Hackett PB. Predicting preferential DNA vector insertion sites: implications for functional genomics and gene therapy. Genome Biol. 2007;8(Suppl 1):S12. doi: 10.1186/gb-2007-8-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Miller AD. The problem with cationic liposome/micelle-based non-viral vector systems for gene therapy. Curr. Med. Chem. 2003;10:1195–1211. doi: 10.2174/0929867033457485. [DOI] [PubMed] [Google Scholar]
- 377.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 378.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 379.Tan W, Wang K, He X, Zhao XJ, Drake T, Wang L, Bagwe RP. Bionanotechnology based on silica nanoparticles. Med. Res. Rev. 2004;24:621–638. doi: 10.1002/med.20003. [DOI] [PubMed] [Google Scholar]
- 380.He XX, Wang K, Tan W, Liu B, Lin X, He C, Li D, Huang S, Li J. Bioconjugated nanoparticles for DNA protection from cleavage. J. Am. Chem. Soc. 2003;125:7168–7169. doi: 10.1021/ja034450d. [DOI] [PubMed] [Google Scholar]
- 381.Apolonia L, Waddington SN, Fernandes C, Ward NJ, Bouma G, Blundell MP, Thrasher AJ, Collins MK, Philpott NJ. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 2007;15:1947–1954. doi: 10.1038/sj.mt.6300281. [DOI] [PubMed] [Google Scholar]