Abstract
Pyrethroid insecticide resistance due to reduced nerve sensitivity, known as knockdown resistance (kdr or kdr-type), is linked to multiple point mutations in the para-homologous sodium channel genes. Previously we demonstrated that two mutations (E434K and C764R) in the German cockroach sodium channel greatly enhanced the ability of the L993F mutation (a known kdr -type mutation) to reduce sodium channel sensitivity to deltamethrin, a pyrethroid insecticide. Neither E434K nor C764R alone, however, altered sodium channel sensitivity. To examine whether E434K and C764R also enhance the effect of pyrethroid resistance-associated sodium channel mutations identified in other insects, we introduced a V to M mutation (V409M) into the cockroach sodium channel protein at the position that corresponds to the V421M mutation in the Heliothis virescens sodium channel protein. We found that the V409M mutation alone modified the gating properties of the sodium channel and reduced channel sensitivity to deltamethrin by 10-fold. Combining the V409M mutation with either the E434K or C764K alone did not reduce the V409M channel sensitivity to deltamethrin further. However, the triple mutation combination (V409M, E434K and C764R) dramatically reduced channel sensitivity by 100-fold compared with the wild-type channel. These results suggest that the E434K and C764R mutations are important modifiers of sodium channel sensitivity to pyrethroid insecticides.
Keywords: Knockdown resistance, Pyrethroids, Insecticide resistance, Sodium channel, Xenopus oocyte expression system
1. Introduction
Pyrethroid insecticides modify sodium channel gating properties by inhibiting deactivation and inactivation, leading to repetitive discharge and membrane depolarization in the nervous system (Narahashi, 1988). For decades, pyrethroids have been widely and intensively used against many insect pests, as a result, many have developed resistance to these compounds. One type of resistance mechanism is knockdown resistance (kdr), which results from reduced nerve sensitivity to pyrethroids and also to DDT (Soderlund and Bloomquist, 1990). Although first reported in 1951 in the house fly (Busvine, 1951), our understanding of the molecular basis of kdr and kdr-type resistance was limited until the recent cloning of sodium channel genes (homologous to the Drosophila para gene) from several kdr and kdr-type insects (Park and Taylor, 1997; Miyazaki et al., 1996; Williamson et al., 1996; Dong, 1997).
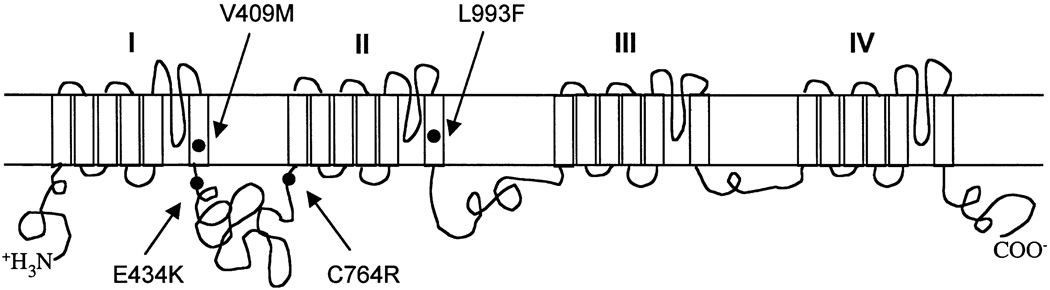
Like their vertebrate counterparts, insect sodium channel proteins are large transmembrane proteins of approximately 260 kDa, comprised of four homologous domains (I–IV), each domain consisting of six transmembrane segments (S1–S6) (Fig. 1). A single leucine (L) to phenylalanine (F) substitution (L1014F in the house fly, and L993F in the German cockroach, see Fig. 1) in segment 6 of domain II (IIS6) is associated with kdr and kdr-type resistance (Miyazaki et al., 1996; Williamson et al., 1996; Dong, 1997). Subsequently, the same mutation was found in other important insect pests, including diamondback moth, Plutella xylostella (Martinez-Torres et al., 1997), horn fly, Haematobia irritans (Guerrero et al., 1997), mosquitoes, Anopheles gambiae and Culex pipiens (Martinez-Torres et al., 1998), aphids, Myzus persicae (Martinez-Torres et al., 1999), and Colorado potato beetle, Leptinotarsa decemlineata (Lee et al., 1999a). Conversely, in pyrethroid-resistant tobacco budworm (Heliothis virescens), an L1029H mutation in IIS6 is associated with pyrethroid resistance (Park and Taylor, 1997).
Fig. 1.
Schematic drawing of the ParaCSMA sodium channel protein indicating kdr-associated mutations. Four homologous domains (I–IV) and six transmembrane segments in each domain are shown. The V409M mutation corresponding to the tobacco budworm V421M mutation and the three cockroach kdr-associated mutations, E434K, C764R and L993F, are indicated.
Evidence shows that the L to F mutation in IIS6 acts in house fly, Drosophila, and cockroach (Smith et al., 1997; Vais et al., 2000; Tan et al., 2001) by reducing sodium channel sensitivity to pyrethroids. The level of resistance conferred by this mutation, however, is relatively low based on results from both toxicological bioassays and channel sensitivity assays in Xenopus oocytes. For example, the L993F mutation reduces cockroach sodium channel sensitivity to deltamethrin by only five-fold (Tan et al., 2001). Interestingly, higher levels of pyrethroid resistance are found if the L to F mutation is combined with additional sodium channel mutations. In the house fly, for example, a methionine to threonine mutation (M918T) in the linker region between IIS4 and IIS5 together with L1014F confers a much higher level of kdr (super-kdr) and sodium channel insensitivity (Williamson et al., 1996; Lee et al., 1999c; Vais et al., 2000). The German cockroach has additional four mutations, D57G, E434K, C764R and P1880L that are present in several highly pyrethroid-resistant German cockroach populations (Liu et al., 2000). The cockroach sodium channel carrying the E434K or C764R mutation alone is as sensitive to deltamethrin as the wild-type channel. However, coexistence of either E434K or C764R with L993F reduces channel sensitivity to deltamethrin by 100-fold (Tan et al., 2001).
The ability of cockroach E434K and C764R mutations to drastically enhance the effect of the L993F mutation suggests an interesting interaction of pyrethroids with residues E434, C764, and L993, possibly as part of a pyrethroid-binding site or a pyrethroid-response domain. To further define amino acid residues involved in such a putative pyrethroid-binding site or a pyrethroid-response domain, we sought to characterize a possible interaction between the cockroach E434K or C764R mutations and other kdr-associated mutations. V421M in IS6 is a unique sodium channel gene mutation of the tobacco budworm associated with pyrethroid resistance (Park et al., 1997). Neurons isolated from the resistant population carrying the V421M mutation are more resistant to permethrin than those from a pyrethroid susceptible population (Lee et al., 1999b). Curiously, the predicted topological location of the V421M mutation in IS6 is similar to that for L to F/H mutations in IIS6 in the German cockroach and other insects. We introduced the V421M into the cockroach sodium channel protein to determine whether this mutation alone reduced cockroach sodium channel sensitivity to deltamethrin, and if so, whether the effect could be enhanced by the presence of the cockroach E434K and C764R mutations.
2. Materials and methods
The V409M mutation, which corresponds to V421M in the Heliothis virescens sodium channel was introduced into a full-length cDNA clone, KD1 (Tan et al., 2001) using site-directed mutagenesis. Briefly, a 689-bp SphI/AccI fragment containing V409 and also E434 was cloned into the pUC19 vector. The fragment plus an additional 11 bp vector sequence was then excised from pUC19 using SphI and BamHI and cloned into pAlter-Ex1 (Promega Corp., Madison, WI). The E to K and/or V to M substitutions were introduced using the Altered Sites II in vitro Mutagensis System (Promega Corp., Madison, WI). The mutated SphI/AccI fragment was excised from pAlter-Ex1 and cloned back into the wild-type cDNA clone to generate single or double mutations, i.e. V409M or V409M+E434K. An EcoRV fragment containing C764R (Tan et al., 2001) was introduced into the constructs containing V409M or V409M+E434K to produce double and triple mutations.
Methods for oocyte preparation, cRNA synthesis and injection for expression of cockroach sodium channels in Xenopus oocytes were identical to those previously reported by Tan et al. (2001). Briefly, oocytes were obtained surgically from oocyte-positive female Xenopus laevis (Nasco, Ft. Atkinson, WI) and incubated with 1 mg/ml Type IA collagenase (Sigma Co., St. Louis, MO) in Ca2+-free ND 96 medium, which contains 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, pH 7.5. Follicle cells remaining on the oocytes were removed with forceps. Isolated oocytes were incubated in ND-96 medium containing 1.8 mM CaCl2 supplemented with 50 µg/ml gentamicin, 5 mM pyruvate, and 0.5 mM theophylline (Goldin, 1992). To prepare cRNA for oocyte injection, plasmid DNA of KD1 or Drosophila tipE construct was linearized with NotI, which does not cut the insert, followed by in vitro transcription with T7 polymerase using the mMESSAGE mMACHINE kit (Ambion Inc., Austin, TX). According to Feng et al. (1995) and Warmke et al. (1997), a robust expression in Xenopus oocyte of sodium current is achieved by co-injection of Drosophila tipE and para cRNAs. Therefore, we co-injected healthy stage VI Xenopus oocytes with 1 ng of both Drosophila tipE and KD1 cRNAs. Oocytes were incubated at 19°C for 3–10 days before recording.
Methods for electrophysiological recording and data analysis were similar to those described previously (Kontis and Goldin, 1993). Sodium currents were recorded using standard two-electrode voltage clamping. Resistance of the borosilicate glass electrodes filled with filtered 3 M KCl was less than 0.5 MΩ. Currents were measured using the oocyte clamp instrument OC725C (Warner Instrument Corp., Hamden, CT), Digidata 1200 interface (Axon Instrument, Foster City, CA) and pCLAMP 6 software (Axon Instrument Foster City, CA). All experiments were performed at room temperature (20–22°C). Capacitive transient and linear leak currents were corrected using P/N subtraction or by subtraction of records obtained in the presence of 20 nM tetrodotoxin (TTX), which completely blocks Para sodium channels.
For application of deltamethrin, the disposable perfusion system developed by Tatebayashi and Narahashi (1994) was used. Briefly, the test solution was transferred into a Petri dish placed on a support stand. Two glass capillary tubes (10 cm in length) joined together with a short length of Tygon tubing connected the Petri dish to the recording chamber. The solution flow was controlled by hydrostatic force created by adjusting the level of the Petri dish relative to the recording chamber. Disposable recording chambers (1–1.5 ml volume) were made with glue dams in Petri dishes. Because deltamethrin is extremely lipophilic, recording chambers, perfusion systems, and the glass agarose bridges connecting the oocyte chamber with the ground electrode chamber were all discarded after a single use. The deltamethrin stock solution (100 mM) was prepared in dimethylsulfoxide (DMSO). The working solutions were made in ND-96 medium immediately before use. Effects of deltamethrin on sodium channel tail currents reached a steady-state level within 5 min after perfusion.
To examine the voltage-dependence of activation of the sodium channel, sodium currents were measured by applying a series of depolarizing voltage steps from a holding potential of −120 mV. Inward sodium currents were recorded during a 14-ms depolarization from −120 to 60 mV in 5 mV increments. The voltage-dependence of sodium channel conductance (G) was calculated by measuring the peak current at test potentials ranging from −120 to +60 mV in 5 mV increments and dividing by (Vt−Vrev), where Vt is the test potential and Vrev is the reversal potential for sodium. Reversal potentials were determined from the I–V curves. The average relative sodium conductance was plotted as a function of depolarizing test potentials (Fig. 2A). Peak conductance values were fit with a two-state Boltzmann equation of the form G=1−[1+exp(V−V1/2)/k]−1, where V is the potential of the voltage pulse, V1/2 the half-maximal voltage for activation, and k is the slope factor.
Fig. 2.
Voltage-dependent activation (A) and steady state inactivation (B) of ParaCSMA and five mutant channels. (A) Normalized conductance–voltage curve. The sodium currents were recorded upon depolarization to various membrane potentials (Vt) from a holding potential of −120 mV for a duration of 14 ms. The peak current was converted to conductance as described in Section 2 and plotted against Vt. (B) The voltage-dependence of inactivation was determined using 200 ms inactivating pre-pulses from a holding potential of −120 to 0 mV in 5 mV increments, followed by test pulses (Vt) to −5 mV for 14 ms. The peak current amplitude during the test depolarization was normalized to the maximum current amplitude, and plotted as a function of the pre-pulse potential (Vp). The smooth curves represent the best fit using Boltzmann equations, as described in Section 2. Symbols represent means and error bars indicate the SEM for three oocytes.
The voltage-dependence of sodium channel inactivation was determined using 200 ms inactivating pre-pulses from a holding potential of −120 to +40 mV in 5 mV increments, followed by test pulses to −5 mV for 12 ms. The peak current amplitude during the test depolarization was normalized to the maximum current amplitude, and plotted as a function of the pre-pulse potential (Fig. 2B). The data were fit with a two-state Boltzmann equation of the form I=Imax*[1+(exp(V−V1/2)/k)]−1, where Imax is the maximal current evoked, V the potential of the voltage pulse, V1/2 the voltage at which 50% of the current is inactivated (the midpoint of the inactivation curve), and k is the slope factor.
The sensitivity of the mutant sodium channels to deltamethrin was determined by measuring the amplitudes of deltamethrin-induced tail currents. The tail current recording protocol by Vais et al. (2000) was used: a 100-pulse train of a 5-ms depolarization from −120 to 0 mV with a 2-ms interval between each depolarization. Traces of tail current were recorded 5 min after the application of each deltamethrin concentration. The percentage of channels modified by deltamethrin was calculated using the equation M={[Itail/(Eh−ENa)]/[INa/(Et−ENa)]}×100 (Tatebayashi and Narahashi, 1994), where Itail is the maximal tail current amplitude, Eh the potential to which the membrane is repolarized, ENa the reversal potential for sodium current determined from the I–V curve, INa the amplitude of the peak current during depolarization before deltamethrin exposure, and Et the potential of step depolarization.
The concentration-response data were fitted to the Hill equation: M=Mmax/{1+(EC50/[deltamethrin])n}, where [deltamethrin] and EC50 represent the concentration of deltamethrin and the concentration to produce the half-maximal effect, respectively, n represents the Hill coefficient, and the Mmax is the maximal percentage of sodium channels modified.
3. Results
Four cockroach sodium channel mutants with single (V409M), double (V409M+E434K or V409M+C764R) and triple (V409M+E434K+C764R) mutations were constructed using site-directed mutagenesis. The mutant channel carrying the previously tested L993F mutation (Tan et al., 2001) was also included in this study for direct comparison. For simplicity, the mutants will be referred to by the single letter code for the new amino acid that is present, with multiple letters indicating the presence of multiple mutations, as listed in Table 1. All four mutant channels, co-expressed with Drosophila tipE, produced sodium currents in Xenopus oocytes. The amplitude of the peak current for all five mutant channels was 1.5 to 2.0-fold smaller than that of the wild-type channel ParaCSMA (data not shown).
Table 1.
Voltage-dependence of activation and inactivation of wild-type and mutant ParaCSMA sodium channelsb
| Channel type | Activation | Inactivation | ||
|---|---|---|---|---|
| V1/2 (mV) | Slope (mV) | V1/2 (mV) | Slope (mV) | |
| ParaCSMA | −25.9±0.5 | 5.1±0.2 | −45.2±1.7 | 4.8±0.2 |
| ParaCSMA-F | −24.4±1.2 | 5.8±0.4 | −44.2±0.9 | 4.8±0.7 |
| ParaCSMA-M | −20.6±0.3a | 5.2±0.2 | −40.0±1.3a | 4.4±0.6 |
| ParaCSMA-MR | −17.9±1.3a | 5.6±0.3 | −41.3±0.8a | 5.0±0.5 |
| ParaCSMA-MK | −17.2±1.8a | 5.6±0.3 | −41.8±1.7a | 5.0±0.6 |
| ParaCSMA-MKR | −18.9±0.5a | 5.6±0.2 | −41.5±1.2a | 4.9±0.2 |
Indicates a statistically significant difference compared to the wild-type channel at p<0.05.
The voltage-dependence of activation and inactivation data were fit with two-state Boltzmann equations, as described in Section 2, to determine V1/2, the voltage for half-maximal conductance or inactivation and k, the slope factor for conductance or inactivation. Each value represents the mean±SEM for three oocytes
For ParaCSMA, the voltage for half-maximal activation was −25.9±0.5 mV and the slope factor was 5.1±0.2 (Table 1). All four mutant channels exhibited similar gating properties (Table 1 and Fig. 2A). Compared with ParaCSMA, the voltage-dependence of activation was shifted about 4–5 mV in the depolarizing direction for all four mutant channels. The voltage for half-maximal inactivation was −45.2±1.7 mV, and the slope factor was 4.8±0.2 (Table 1). The voltage-dependence of steady-state inactivation was shifted about 4–5 mV in the depolarizing direction for all four mutant channels. Because ParaCSMA channels carrying the E434K, C764R, or E434K+C764R mutation do not exhibit significant changes in gating properties (Tan et al., 2001), we believe that the shifts in voltage-dependence of activation and inactivation in ParaCSMA-M, ParaCSMA-MK, ParaCSMA-MR and ParaCSMA-MKR channels resulted from the introduction of the V409M mutation.
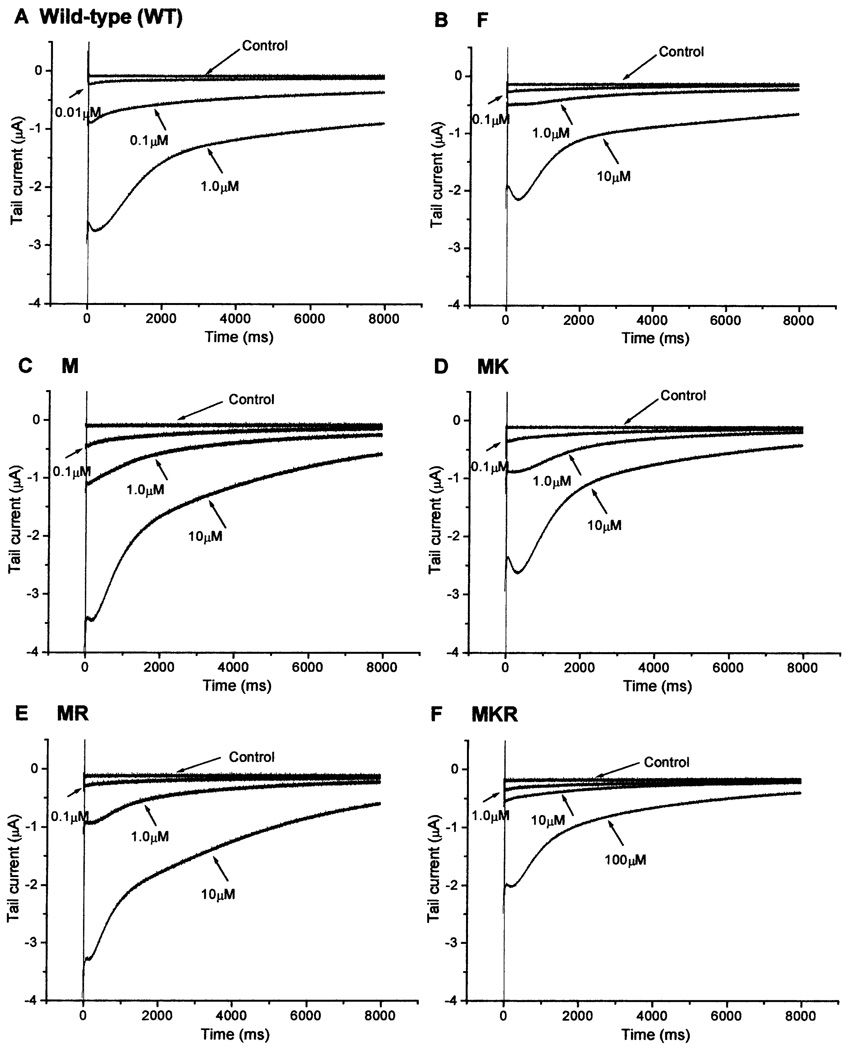
A large tail current was elicited in oocytes expressing the wild-type channel, at 1 µM concentration of deltamethrin (Fig. 3A). Tail currents at the higher concentrations could not be obtained because high concentrations (>10 µM) of deltamethrin resulted in huge leakage currents that made it impossible to clamp the oocyte. However, at 1 µM concentration of deltamethrin, oocytes expressing ParaCSMA-F or ParaCSMA-M channels produced much smaller tail currents (Fig. 3B and C). A higher concentration (10 µM) was required to induce a large tail current. These results indicated that the ParaCSMA-M channel, like the ParaCSMA-F channel, was less sensitive to deltamethrin than ParaCSMA. The deltamethrin sensitivity of ParaCSMA-MK and ParaCSMA-MR channels, however, was similar to that of the ParaCSMA-M channel (Fig. 3D and E). Oocytes expressing the ParaCSMA-MKR channel required 100 µM to produce a large tail current (Fig. 3F), indicating that the sensitivity of the triple mutation channel was greatly reduced. The percentage of channel modification quantified from two experiments (Fig. 4) showed that modification of 20% of sodium channels required 0.3 µM deltamethrin for the ParaCSMA channel, 1.5 µM for the ParaCSMA-F channel, 4.1 µM for the ParaCSMA-MR channel, 5.0 µM for both ParaCSMA-M and ParaCSMA-MK channels, and 30.6 µM for the ParaCSMA-MKR channel (Fig. 4 inset). Clearly, the sensitivity of ParaCSMA-M, ParaCSMA-MK or ParaCSMA-MR channels was reduced by about 15-fold when compared to the wild-type channel ParaCSMA whereas the triple mutation channel ParaCSMA-MKR exhibited 100-fold reduction in deltamethrin sensitivity (Fig. 4). Interestingly, the tobacco budworm derived V409M mutation provided the cockroach sodium channel three-fold more resistance to deltamethrin than the naturally occurring L993F mutation.
Fig. 3.
ParaCSMA and mutant channel sensitivity to deltamethrin. (A)–(F) Tail currents induced by deltamethrin in oocytes expressing the ParaCSMA and mutant channels. Tail currents were recorded in response to a 100-pulse train of 5 ms depolarizations from −120 to 0 mV with a 2-ms interval between each depolarization. Note the differences in the scales of tail current and also the concentrations of deltamethrin. At 100 µM, the recording solution showed a slight cloudiness, indicating that deltamethrin was not completely soluble at this concentration.
Fig. 4.
Quantification of ParaCSMA and mutant channel sensitivity to deltamethrin. Percentage of channel modification is plotted as a function of deltamethrin concentration. The percentage of channels modified by deltamethrin was determined using the equation M={[Itail/(Eh−ENa)]/[INa/(Et−ENa)]}×100 (Tatebayashi and Narahashi, 1994) (see Section 2). Percentage of modification of sodium channels is plotted as a function of deltamethrin concentration. The data were fitted with the Hill equation (see Section 2). EC20 values (inset) are derived from the fitted curves. The values of the concentration that modifies 20% of channels (EC20) are presented in the inset. Each point represents mean±SEM (pooled data from three oocytes).
4. Discussion
Sodium channels are the targets of a variety of neurotoxins including DDT and the widely used pyrethroids. Pharmacological approaches are of limited utility in revealing the pyrethroid-binding site on the sodium channel because of the highly lipophilic nature of pyrethroids. Recently, the successful identification of multiple pyrethroid resistance-associated mutations that affect sodium channel sensitivity to pyrethroids provides us with a powerful and alternative approach for understanding the molecular basis of sodium channel interactions with pyrethroids. In this study, we showed that the V409M mutation, equivalent to the V421M mutation identified in the tobacco budworm sodium channel, altered the voltage-dependence of activation and inactivation of a cockroach sodium channel, ParaCSMA, and reduced channel sensitivity to deltamethrin. Our results are consistent with two very recent independent reports of a similar effect of the V421M mutation on the sensitivity of Drosophila and house fly sodium channels expressed in Xenopus (Zhao et al., 2000; Lee and Soderlund, 2001). These studies provide direct evidence that sodium channel mutations from one insect species can effectively confer pyrethroid insensitivity to heterologous sodium channels.
A major finding of this study is that two cockroach kdr-type mutations, E434K and C764R, which by themselves do not alter sodium channel sensitivity, significantly, enhanced the ability of the V409M mutation to reduce channel sensitivity to deltamethrin. These mutations naturally co-exist with the L993F mutation only in highly pyrethroid-resistant cockroach populations (Liu et al., 2000). Concomitant expression of either E434K or C764R with L993F reduced the ParaCSMA sodium channel sensitivity to deltamethrin by 100-fold (Tan et al., 2001). Moreover, co-expression of all three mutations further reduced cockroach sodium channel sensitivity by 500-fold compared with the wild-type channel. The current study showed that E434K and C764R also enhance the effect of the heterologous V409M mutation on the ParaCSMA sodium channel sensitivity to pyrethroids. Therefore, E434K and C764R appear to represent ‘modifiers’ of kdr or kdr-type resistance, that are capable of increasing the effects of different kdr-type mutations.
The molecular mechanism by which the E434K and C764R mutations enhance kdr mutations is not clear. E434K and C764R are located in the intracellular linker connecting domains I and II. The fact that E434K is only 19 amino acid residues downstream of IS6, and C764R is only 12 amino acid residues upstream of IIS1 indicates that V409, E434 and C764 residues could be in close proximity in the sodium channel protein assembled in the membrane. We hypothesized that L993F, E434K and C764R may affect different pyrethroid-binding sites. The E434K and C764R mutations may be part of a low-affinity binding site and the L993F mutation part of a high-affinity binding site. The existence of two pyrethoid-binding sites was originally proposed by Vais et al. (2000), and is based on their detailed analysis of the effect of deltamethrin on Drosophila wild-type and kdr Para channels. Our results support this model. We believe that V409 is part of a high-affinity binding site altered by V409M, similar to the modification of L993F, which results in a moderate decrease in channel sensitivity to pyrethroids. E434K and C764R would not be ‘visible’ by themselves if they effected the low-affinity site due to the presence of the high-affinity site. The triple mutations, however, would dramatically alter both high- and low-affinity binding sites, resulting in the most drastic decrease in channel sensitivity. The high-affinity-pyrethroid-binding site is located likely at the interface between domains I and II where the amino acid residues V409 and L993 reside, which is also suggested in the house fly sodium channel (Lee and Soderlund, 2001). Such domain interface models have been proposed for several classes of lipophilic neurotoxins acting on calcium and sodium channels (Hockerman et al., 1997; Linford et al., 1998). For example, sodium channel site 2 neurotoxin BTX activates sodium channels by binding to a site formed by unique amino acid determinants in IS6 and IVS6 (Linford et al., 1998).
The evolutionary mechanism for German cockroach adaptation to pyrethroid selection pressure through sodium channel gene mutations can be explained by a ‘successive mutation’ mechanism. In nature, the E434K and C764R mutations always co-exist with the L993F mutation. No individual carries the E434K or C764R mutation alone without the L993F mutation (Liu et al., 2000). Recombinant ParaCSMA channels carrying the E434K or C764R mutation alone generated sodium currents poorly in Xenopus oocytes, suggesting a possible fitness disadvantage associated with these single mutations. We therefore suggest that the L993F is the primary kdr mutation because this mutation alone confers pyrethroid resistance, albeit at a low level. Because the effects of the kdr modifier mutations E434K and C764R are visible only in the presence of the L993F mutation, the L993F mutation must play an essential role in the development of high levels of kdr in the German cockroach. E434K and C764R likely evolved after the initial selection of L993F (Tan et al., 2001). The positive interaction of V409M with E434K and C764R revealed in this study raises an interesting question: Are E434K and C764R or other kdr-modifier mutations also present naturally in other insects with notably high levels of pyrethroid resistance? The current sequence analysis of sodium channel genes is often limited to particular regions, especially IIS6 where the L993F mutation is located. A more extensive sequence survey in pyrethroid-resistant insects may shed light on how widespread the E434K and C764R mutations or other kdr-modifier mutations are in insects.
Acknowledgements
We thank Dr N. Koller for critical review of the manuscript. This work was supported by National Institutes of Health Grant 08-1GM57440A (to K.D.) and the Michigan State University Rackham Endowment Fund (to K.D.).
References
- Busvine JR. Mechanism of resistance to insecticide in houseflies. Nature (London) 1951;168:193–195. doi: 10.1038/168193a0. [DOI] [PubMed] [Google Scholar]
- Dong K. A single amino acid change in the Para sodium channel protein is associated with knockdown-resistance (kdr) to pyrethroid insecticides in German cockroach. Insect Biochem. Mol. Biol. 1997;27:93–100. doi: 10.1016/s0965-1748(96)00082-3. [DOI] [PubMed] [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila Para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–279. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]
- Guerrero FD, Jamroz RC, Kammlah D, Kunz SE. Toxicological and molecular characterization of pyrethroid-resistant horn flies, Haematobia irritans: identification of kdr and super-kdr point mutations. Insect Biochem. Mol. Biol. 1997;27:745–755. doi: 10.1016/s0965-1748(97)00057-x. [DOI] [PubMed] [Google Scholar]
- Hockerman GH, Perterson BZ, Johnson BD, Catterall WA. Molecular determinants of drug binding and action of L-type calcium channels. Annu. Rev. Pharmacol. Toxicol. 1997;37:361–396. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- Kontis KJ, Goldin AL. Site-directed mutagenesis of the putative pore region of the rat IIA sodium channel. Mol. Pharmacol. 1993;43:635–644. [PubMed] [Google Scholar]
- Lee SH, Dunn JB, Clark JM, Soderlund DM. Molecular analysis of kdr-like resistance in a permethrin-resistant strain of Colorado potato beetle. Pestic. Biochem. Physiol. 1999a;63:63–75. [Google Scholar]
- Lee D, Park Y, Brown TM, Adams ME. Altered properties of neuronal sodium channels associated with genetic resistance to pyrethroids. Mol. Pharmacol. 1999b;55:584–593. [PubMed] [Google Scholar]
- Lee SH, Smith TJ, Knipple DC, Soderlund DM. Mutations in the house fly Vssc1 sodium channel gene associated with super-kdr resistance abolish the pyrethroid sensitivity of Vssc1/tipE sodium channels expressed in Xenopus oocytes. Insect Biochem. Mol. Biol. 1999c;29:185–194. doi: 10.1016/s0965-1748(98)00122-2. [DOI] [PubMed] [Google Scholar]
- Lee SH, Soderlund DM. The V410M mutation associated with pyrethroid resistance in Heliothis virescens reduces the pyrethroid sensitivity of house fly sodium channels expressed in Xenopus oocytes. Insect Biochem. Mol. Biol. 2001;31(1):19–29. doi: 10.1016/s0965-1748(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Cantrell AR, Qu Y, Scheuer T, Catterall WA. Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc. Natl. Acad. Sci. USA. 1998;95:13947–13952. doi: 10.1073/pnas.95.23.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Valles SM, Dong K. Novel point mutations in the German cockroach para sodium channel gene are associated with knockdown resistance (kdr) to pyrethroid insecticides. Insect Biochem. Mol. Biol. 2000;30:991–997. doi: 10.1016/s0965-1748(00)00074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae. Insect Mol. Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Torres D, Devonshire AL, Williamson MS. Molecular studies of knockdown resistance to pyrethroids: cloning of domain II sodium channel sequences from insects. Pestic. Sci. 1997;51:265–270. [Google Scholar]
- Martinez-Torres D, Foster SP, Field LM, Devonshire AL, Williamson MS. A sodium channel point mutation is associated with resistance to DDT and pyrethroid insecticides in the peach-potato aphid, myzus persicae. Insect Mol. Biol. 1999;8:339–346. doi: 10.1046/j.1365-2583.1999.83121.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Ohyama K, Dunlap DY, Matsumura F. Cloning and sequencing of the para-type sodium channel gene from susceptible and kdr-resistant German cockroaches (Blattella germanica) and house fly (Musca domestica) Mol. Gen. Genet. 1996;252:61–68. [PubMed] [Google Scholar]
- Narahashi T. Molecular and cellular approaches to neurotoxicology: past, present and future. In: Lunt GG, editor. Neurotox ’88: Molecular Basis of Drug and Pesticide Action. New York: Elsevier; 1988. pp. 563–582. [Google Scholar]
- Park Y, Taylor MF. A novel mutation L1029H in sodium channel hscp associated with pyrethroid resistance for Heliothis virescens (Lepidoptera: Noctuidae) Insect Biochem. Mol. Biol. 1997;27:9–13. doi: 10.1016/s0965-1748(96)00077-x. [DOI] [PubMed] [Google Scholar]
- Park Y, Taylor MF, Feyereisen R. A valine421 to methionine mutation in IS6 of the hscp voltage-gated sodium channel associated with pyrethroid resistance in Heliothis virescens. Biochem. Biophys. Res. Commun. 1997;239:688–691. doi: 10.1006/bbrc.1997.7511. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Lee SH, Ingles PJ, Knipple DC, Soderlund DM. The L1014F point mutation in the House Fly Vssc1 sodium channel confers knockdown resistance to pyrethroids. Insect Biochem. Mol. Biol. 1997;27:807–812. doi: 10.1016/s0965-1748(97)00065-9. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Bloomquist JR. Molecular mechanisms of insecticide resistance. In: Roush RT, Tabashnik BE, editors. Pesticide Resistance in Arthropods. New York: Chapman and Hall; 1990. pp. 58–96. [Google Scholar]
- Tan J, Liu Z, Tsai T-D, Valles SM, Goldin AL, Dong K. Novel para mutations abolish sodium channel sensitivity to pyrethroids. Insect Biochem. Molec. Biol. 2001 doi: 10.1016/s0965-1748(01)00122-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Vais H, Williamson MS, Goodson SJ, Devonshire AL, Warmke JW, Usherwood PNR, Cohen C. Activation of Drosophila sodium channels promotes modification by deltamethrin: reductions in affinity caused by knock-down resistance mutations. J. Gen. Physiol. 2000;115:305–318. doi: 10.1085/jgp.115.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke JW, Warmke JW, Reenan RAG, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van Der Ploeg LHT, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels: modulation by the membrane protein tipE and toxin Pharmacology. J. Gen. Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol. Gen. Genet. 1996;252:51–60. doi: 10.1007/BF02173204. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Park Y, Adams ME. Functional and evolutionary consequences of pyrethroid resistance mutations in S6 transmembrane segments of a voltage-gated sodium channel. Biochem. Biophys. Res. Commun. 2000;278:516–521. doi: 10.1006/bbrc.2000.3832. [DOI] [PubMed] [Google Scholar]