Abstract
Antigen presenting cells, specifically dendritic cells (DCs) are a focal point in the delicate balance between T cell tolerance and immune responses contributing to the onset of type I diabetes (T1D). Weak adjuvant proteins like the cholera toxin B subunit when linked to autoantigens may sufficiently alter the balance of this initial immune response to suppress the development of autoimmunity. To assess adjuvant enhancement of autoantigen mediated immune suppression of Type 1 diabetes, we examined the cholera toxin B subunit (CTB)-proinsulin fusion protein (CTB-INS) activation of immature dendritic cells (iDC) at the earliest detectable stage of the human immune response. In this study, Incubation of human umbilical cord blood monocyte-derived immature DCs with CTB-INS autoantigen fusion protein increased the surface membrane expression of DC toll-like receptor (TLR-2) while no significant upregulation in TLR-4 expression was detected. Inoculation of iDCs with CTB stimulated the biosynthesis of both CD86 and CD83 co-stimulatory factors demonstrating an immunostimulatory role for CTB in both DC activation and maturation. In contrast, incubation of iDCs with proinsulin partially suppressed CD86 co-stimulatory factor mediated DC activation, while incubation of iDCs with CTB-INS fusion protein completely suppressed iDC biosynthesis of both CD86 and CD83 costimulatory factors. The incubation of iDCs with increasing amounts of insulin did not increase the level of immune suppression but rather activated DC maturation by stimulating increased biosynthesis of both CD86 and CD83 costimulatory factors. Inoculation of iDCs with CTB-INS fusion protein dramatically increased secretion of the immunosuppressive cytokine IL-10 and suppressed synthesis of the pro-inflammatory cytokine IL12/23 p40 subunit protein suggesting that linkage of CTB to insulin (INS) may play an important role in mediating DC guidance of cognate naïve Th0 cell development into immunosuppressive T lymphocytes. Taken together, the experimental data suggests Toll like receptor 2 (TLR-2) plays a dominant role in CTB mediated INS inhibition of DC induced type 1 diabetes onset in human Type 1 diabetes autoimmunity. Further, fusion of CTB to the autoantigen was found to be essential for enhancement of immune suppression as co-delivery of CTB and insulin did not significantly inhibit DC costimulatory factor biosynthesis. The experimental data presented supports the hypotheses that adjuvant enhancement of autoantigen mediated suppression of islet beta cell inflammation is dependent on CTB stimulation of dendritic cell TLR2 receptor activation and co-processing of both CTB and the autoantigen in the same dendritic cell.
Keywords: adjuvant, autoimmunity, cholera toxin-B (CTB), dendritic cells, IDDM, juvenile diabetes
Introduction
Insulin dependent diabetes mellitus, or Type 1 diabetes (T1D), is the most destructive metabolic disease of children. T1D is caused by autoreactive lymphocyte destruction of insulin-producing islet beta cells of the pancreas (Eisenbarth, 1986, Tisch and McDevitt, 1996). The progressive loss of islet β-cell function results in an increasing deficiency of insulin production resulting in elevated blood sugar levels (hyperglycemia). The inability to transfer glucose from the blood into the cells of the body results in increased levels of cellular oxidative stress which leads to chronic inflammation throughout the body resulting in an increased and premature risk for secondary neural and circulatory health problems, including amputation of extremities, blindness, heart attack and stroke (Libby, et al., 2005). Young T1D patients must inject insulin several times a day for the rest of their lives or risk diabetic shock and death (Lodinova-Zadnikova, et al., 2004).
The first step in diabetes mediated breakdown of immunological homeostasis leading to islet β-cell mortality is initiated by autoantigen stimulated maturation of antigen-presenting cells (APCs), largely dendritic cells (DC). Maturation of DCs induces the development of autoreactive CD8+, and CD4+ T helper (Th1) cells as well as B cell production of auto-antigen specific antibodies (Atkinson and Maclaren, 1994, Han, et al., 2005, Tang, et al., 2006, Tisch and McDevitt, 1996). Following autoreactive CD4+ Th1 cell infiltration of pancreatic islets in NOD mice, autoreactive Th1 lymphocytes were shown to secrete the inflammatory cytokines IFN-gamma and IL-2. These diabetes autoantibodies are known to stimulate macrophage and CTL secretion of oxidative compounds NO, O2, H202) in addition to inflammatory cytokines (IL-1 beta, TNF-alpha, TNF-beta, IFN-gamma) [5–7]. The persistence of these immune responses induces chronic pancreatic inflammation (insulitis), which results in the induction of apoptosis of approximately 90% of the islet insulin-producing β-cells leading to insufficient levels of insulin production (Piccinni, et al., 1998). A variety of immune cell types including B cells, dendritic cells, macrophages and natural killer (NK) cells were shown to be involved in the onset of diabetes pathogenesis (Cardell, 2006, Kent, et al., 2005, Silveira and Grey, 2006, Tian, et al., 2009, Tian, et al., 2006, Wang, et al., 2005, Yoon and Jun, 2005). Specifically, dendritic cells (DCs) were shown to play a primary role in antigen priming of naïve T helper cells (Th0) and in the modulation of their development into autoreactive Th1 lymphocytes or immunosuppressive Th2 cells critical for maintenance of immunological homeostasis (Itano, et al., 2003, Pulendran, et al., 2001, Pulendran, et al., 1999). Immuno-cytochemical analyses showed that oral inoculation results in auto-antigen uptake through M cells of the intestinal epithelium into peripheral DCs via several routes that may aid in the establishment of immune suppression (Yoon and Jun, 2005). Autoantigens (AutoAg) are taken up and processed by immature DC subsets(Figdor, et al., 2004). Following DC activation by autoantigens, the DCs migrate to adjacent lymph nodes, where they present antigen peptides on MHCII receptors, synthesize co-stimulatory molecules and secrete IL-12 which guides the development of naïve cognate Th0 cells into Ag-specific inflammatory Th1 lymphocytes. In contrast, oral inoculation with small amounts of autoantigen was shown to induce DC production of the anti-inflammatory cytokine IL-10 which stimulates the development of naïve Th0 lymphocytes into anti-inflammatory Th2 lymphocytes, or alternatively into IL-10 or TGF-beta producing CD4+CD25+ Tr1 or Th3 regulatory T cells (D’Ambrosio, et al., 2008, Kapsenberg, 2003). Thus, interactions between autoantigens, DCs and T cells in the gut associated lymphoid tissues, may dictate the onset of inflammatory or tolerogenic outcomes following initial autoAg presentation. In addition, DCs residing in lymphoid follicles and the Peyer’s patches shown to synthesize IL-10 were found to down-regulate Th1 cell mediated autoimmunity (Steinbrink, et al., 1997). Further, immature or peripheral DCs (iDCs), that displayed low levels of co-stimulatory molecule expression and that secreted cytokine IL-10, were shown to remain in the periphery and were found to induce Th2 lymphocyte mediated immunological tolerance (Holmgren, et al., 2005, Li, et al., 2006, Liu, et al., 2001, Rissoan, et al., 1999). In an alternative set of experiments, the addition of IL-12 to immature dendritic cells (iDCs), induced autoreactive Th1 cell morphogenesis and accelerated type 1 diabetes in NOD mice (Trembleau, et al., 1995). In composite, the available experimental data suggests that initial stages of type 1 diabetes progression may be largely under DC control and may set the stage for anti-inflammatory or inflammatory disease outcomes (Shinomiya, et al., 1999).
Oral administration of auto-antigens has shown promise for prevention of spontaneous autoimmune diabetes (Trentham, et al., 1993, Zhang, et al., 1991). However, the need for repeated autoantigen administration over an extended period of time poses a limitation to such therapy. Further, a low efficiency of immune suppression was reported in previously sensitized hosts (Arakawa, et al., 1998). These limitations were largely overcome through application of the non toxic B subunit of the cholera enterotoxin (CTB) from Vibrio cholera. The CTB molecule was shown to be a strong immunomodulator for induction of oral tolerance when used as a carrier molecule for conjugated autoantigens (Sun, et al., 1994, Sun, et al., 2000, Sun, et al., 1996, Sun, et al., 2000). The bacterial AB enterotoxin from Vibrio cholerae, cholera toxin (CTX) contains a toxic ADP-ribosyltransferase subunit A1 (CTA1), linked through a small helical (A2) peptide to a pentamer of non-toxic B carrier subunits (CTB). The CTB subunits were shown to be required for binding the toxin to monosialoganglioside receptor molecules embedded in gut epithelial cell membranes facilitating entry of the holotoxin into the cell (Eriksson and Holmgren, 2002). The CTB subunit was shown to bind specifically to GM1-ganglioside, a receptor molecule found in common on the membrane of most types of epidermal cells. Thus, CTB can provide an efficient trans-mucosal carrier molecule for autoantigen induction of peripheral tolerance (Shreedhar, et al., 2003, Sun, et al., 1994).
In previous studies, oral delivery of CTB conjugated to specific autoantigens was shown to enhance autoantigen mediated protection of mice against several organ-specific autoimmune diseases including autoimmune encephalomyelitis (Sun, et al., 2000) autoimmune chondritis (Kim, et al., 2001) and uveitis (Phipps, et al., 2003). In addition, CTB-INS conjugates were shown to substantially suppress diabetes in NOD mice (Arakawa, et al., 1998, Bergerot, et al., 1997). The observed suppression of diabetes onset was associated with a reduction in Th1 cell IFN-γ production and the migration of Tr1 regulatory T cells into pancreatic islets (Aspord and Thivolet, 2002, Roncarolo, et al., 2001). Further, the fusion of CTB to insulin was shown to provide up to a 10,000 fold reduction in autoantigen amounts required for immuno-tolerization (Arakawa, et al., 1998, George-Chandy, et al., 2001). Mechanisms underlying CTB modulated immune suppression of T1D may include the inhibition of DC maturation, inhibition of autoreactive T cell development and/or induction of Th2 and regulatory T cell (iTreg) proliferation and activation (Lavelle, et al., 2004, Lavelle, et al., 2003, Marinaro, et al., 1995).
Recent immunotherapy and vaccination strategies strongly target receptors that mediate immune cell activation. Pathogen recognition receptors, especially APC Toll-like receptors (TLRs) have received increasing attention because activation of innate immunity through pathogen protein, nucleic acid and lipopolysaccharide pattern recognition has been increasingly identified as an essential first line of immunological defense (Hemmi, et al., 2000, Schnare, et al., 2001, Takeda, et al., 2003). In general, TLRs interact with a variety of microbial structures widely expressed by fungi, bacteria, protozoa and viruses conferring a high degree of specificity to the immune response (Takeda, et al., 2003). Recent developments support the concept that activation of immunity by microbial molecules may involve cooperative interaction with multiple host receptors within the membrane lipid raft (Beutler, et al., 2006, Hoebe, et al., 2006, Triantafilou, et al., 2002). That is, TLRs are usually present as preformed homodimers with the exception of TLR2 which preferentially forms heterodimers with either TLR1 or TLR6 (Akira and Takeda, 2004). Toll like receptors TLR2/TLR1 and TLR2/TLR6 were shown to be activated in response to agonists such as lipoteichoic acid and lipoproteins, while other APC surface and internal TLRs respond to a variety of bacterial and virus DNA and lipopolysaccharide immunostimulatory molecules (Akira and Hemmi, 2003, Kanzler, et al., 2007, Roger, et al., 2005, Takeda, et al., 2003). Recently, the type II heat-labile enterotoxin from E. coli which has an AB5 subunit structure similar to cholera toxin was found to stimulate cytokine release in mouse and human cells through interactions with TLR2. However, up to the present, the mechanism of CTB mediated TLR activation is only poorly understood. An increased understanding of the initial interactions among CTB and its fusion proteins with TLRs is predicted to improve our understanding of mechanisms by which these molecules exert their immunomodulatory activities.
The objectives of this study are to clarify mechanisms underlying early events in CTB-INS vaccine induction of immunological tolerance leading to the suppression of Type 1 diabetes through analysis of CTB-proinsulin autoantigen induced inhibition of human immature DC activation and maturation leading to induction of Th0 lymphocyte development into inflammatory Th cells resulting in chronic pancreatic islet inflammation and the death of insulin producing islet beta cells. Examination of CTB and CTB-INS fusion protein interactions with DC membrane Toll-like receptors (TLR) will provide information on whether they or other membrane receptors may play a role in the initiation of adjuvant-autoantigen mediated DC activation and maturation. Through analysis of CTB-autoantigen fusion protein interactions with immature DCs we may be able to gain a clearer understanding of how CTB-INS fusion proteins interact with DCs to suppress the onset of Type 1 diabetes.
Materials and Methods
Construction of Bacterial Plasmids Containing CTB-INS
A gene encoding approximately 258bp of human proinsulin (INS) was physically linked by gene splicing to the carboxyl-terminus (309bp) of CTB to generate the fusion gene CTB-INS. The cholera toxin B subunit–autoantigen fusion gene CTB-INS was cloned into the (A) configuration of the E. coli expression vector pRSET (Invitrogen™, Carlsbad, CA), under control of the bacteriophage T7 promoter in order to achieve high levels of transgene expression. The pRSET vector also contained an oligonucleotide encoding 6 histidines immediately upstream of the CTB permitting isolation of the transgene product. The CTB-INS fusion gene (567bp) was cloned between the BamH1/Bgl1 and EcoR1 sites flanked by a termination sequence and a poly (A) adenylation sequence in the 3′ region of the transgene. Selective clones were assessed by DNA sequence analysis to confirm the in-frame linkage of CTB and Insulin DNA fragments. The expression vector PRSET-CTB-INS containing the gene encoding the CTB-INS fusion protein was introduced into E. coli producer strain BL21 (DE3)pLysS (Invitrogen, Carlsbad, CA), for nickel affinity column isolation of the recombinant protein (Carter, et al., 2006).
Synthesis and Isolation of CTB-INS Fusion Proteins
The CTB-INS transformed E. coli strain BL-21, was grown in 250 ml Luria Broth (LB) medium containing ampicillin (100mg/ml). While still in log phase of growth, CTB-INS protein synthesis was stimulated by addition of 90 mg isopropyl β-D-1thiogalacto-pyranoside (IPTG), (Sigma Chemical Co. St. Louis, MO), to the bacterial culture. After 6 hr continued growth at 37°C, the bacterial culture was transferred into 50 ml polystyrene Oakridge tubes and the cells harvested by centrifugation in a SA-600 rotor for 10 min, at 5,000 rpm and 4°C, in a Sorvall RC5B centrifuge. The cell pellet was resuspended in 1.0 ml / tube of 10 mM HEPES buffer (pH 7.5), containing 100 mM imidazole. The cells were disrupted by sonication at 3 × 10 sec bursts at 10 W, with a Sonic 60 Dismembrator, (Fisher Sci. Sunnyvale, CA). The CTB-INS protein was isolated and purified from the bacterial homogenate using a Maxwell Model 16 robotic protein purification system (Promega Inc.™), according to the protein isolation protocol provided by the manufacturer (Promega Inc., Madison, WI). In order to obtain a pure protein product, the robot employs electromagnetically charged Magne-His Nickel-Iron alloy particles with an affinity for the 6-HIS tag linked to the N terminus of the recombinant CTB-INS fusion protein. Imidazole was removed from the protein mixture by dialysis of the preparation against 2 × 1.0 Liter, 10 mM HEPES buffer (pH 7.5), for 4 hr at 4°C. The purity of the isolated CTB-INS protein (23.4kDa) was determined by electrophoretic mobility analysis in a 12% polyacrylamide gel in comparison with protein molecular weight standards (Figure 1b). The purified CTB-INS protein was stored at −20°C until further use.
Figure 1. Construction of the CTB-INS Fusion Gene and Isolation of the Gene Product.
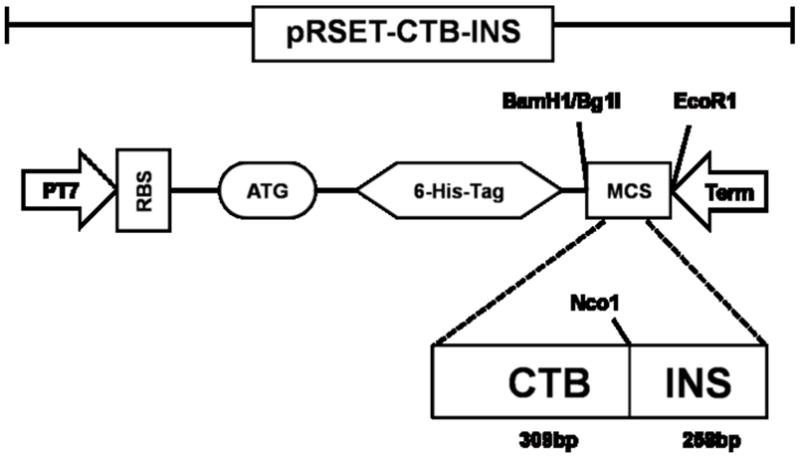
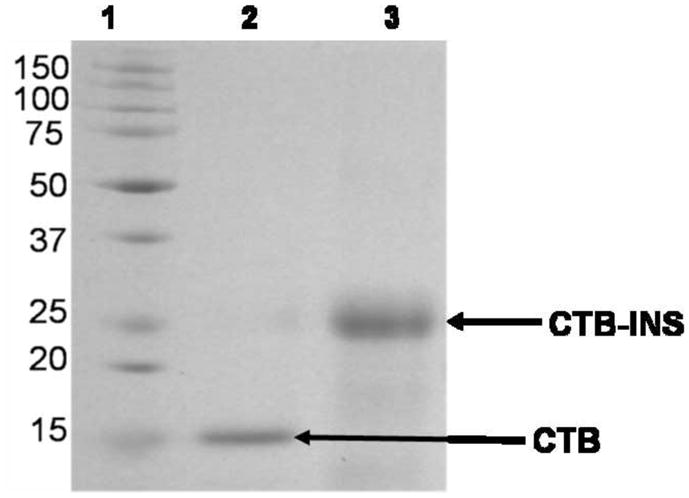
(Panel A) is a plasmid map of the E. coli expression vector pRSET A (Invitrogen™, Carlsbad, CA), carrying the CTB-INS fusion gene. The cholera toxin B subunit– proinsulin autoantigen gene fusion CTB-INS (567bp) was cloned into pRSET (A) between the BamH1/Bgl 1 and EcoR1 sites. The expression vector PRSET-CTB-INS with the CTB-INS coding sequence under control of the bacteriophage T7 promoter also contains an oligonucleotide region encoding 6 histidine amino acid residues immediately 5′ upstream of the CTB DNA sequence. The recombinant plasmid was introduced into the E. coli recipient strain BL21 (DE3) pLysS, for CTB-INS fusion protein expression and for nickel binding isolation of the recombinant protein using a Maxwell 16 ™ protein isolation robot (Promega Inc, Madison, WI, USA). (Panel B) is an SDS polyacrylamide gel stained with Commassie blue, Lane 1: Molecular Weight Marker Proteins (BIO-RAD, Hercules, CA); Lane 2: pRSET-CTB protein (16.1kDa); Lane 3: the CTB-INS fusion protein isolated and purified from BL21 E. coli cells containing pRSET-CTB-INS (23.4kDa).
Isolation and Culture of Monocyte- derived DC from Human Cord Blood
Monocyte-derived dendritic cells (MDDC) were prepared from freshly collected human umbilical cord blood. The leukocyte fraction of approximately 50 ml of cord blood obtained from normal healthy placenta donors (by LLU IRB approved protocols), was separated from red blood cell and platelet fractions by Ficoll-paque density gradient centrifugation for 30 minutes at 2,000 RPM @ 4°C, in a Beckman Coulter Allegra X-15R centrifuge, equipped with a SX4750 rotor. The CD14+ monocytes were obtained from the total lymphocyte fraction by incubation with anti-CD14 PE (Phycoerythrin) for 10 minutes @ 4°C followed by incubation with anti-PE magnetic microbeads for 15 minutes at 4°C. The cells were separated magnetically by passing them through the MACS column as described by the manufacturer (Miltenyi Biotech, Auburn, CA) (Devaraj, et al., 2008, Devaraj, et al., 2006). The purity of the monocyte fraction was determined flow cytometry in a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
The isolated monocytes were cultured in 6 cm non-pyrogenic polystyrene culture plates in RPMI 1640 culture medium (Mediatech Inc. Manassas, VA, USA) in a humidified atmosphere of 5% CO2 at 37 °C (Preprotech, Rocky Hill, NJ). The medium was supplemented with 10% FBS, 1 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 ng/ml human recombinant GMCSF, and 10 ng/ml human recombinant IL-4 (ProSpec-Tany TechnoGene, Rehovot Science Park, Israel). The monocyte cell culture was fed every 2 days by gentle replacement of 50% of the medium with fresh medium. The cell cultures were kept in their original plates until harvested to avoid induction of DC maturation caused by mechanical stress associated with replating the cells. The percentage of monocyte-derived immature dendritic cells (M-iDC) was determined directly by flow cytometry based on expression of DC specific cell surface markers (CD14−HLA-DR+CD11c+) after 6 days further incubation.
DC Maturation Assay and Phenotyping
The immature dendritic cells (iDCs) were stimulated by the addition of CTB (10ug/ml), insulin (10ug/ml), CTB-INS fusion protein (20ug/ml) and Phorbol myristate acetate (PMA) and Ionomycin at 10ng/ml (PMA+ionomycin) for 48hrs at 37 degrees, 5% CO2. After incubation, the expression of DC surface markers indicating their state of activation and maturation (CD14, CD11c, HLA-DR), was determined by flow cytometry. Briefly, the gated CD14− HLA-DR+ CD11c+ cell population was analyzed for the expression of CD86, and CD83 activation markers (BD Pharmingen, San Jose, CA USA).
Flow Cytometry Analysis
For flow cytometry examination of DC surface markers, the DCs were surface stained with antibodies conjugated to Phycoerythrin (PE), Fluorescein isothiocyanate (FITC), Allophycocyanin (APC), and Peridinin Chlorophyll Protein Complex (PerCP) (Parrish, et al., 2009) and resuspended in 1.0% of freshly prepared formaldehyde for analysis using the FACSCalibur flow cytometer. The following antibodies were used to identify the different lymphocyte types: anti- CD86-FITC, anti- CD83-PE, anti- HLA-DR PerCP anti- CD14-APC anti- Mouse IgG1 FITC, anti- Mouse IgG1 PE, anti- Mouse IgG1 HLA-DR, anti- Mouse IgG1 APC (BD Pharmingen); 7-Amino-actinomycin D (7-AAD) was used to assess viability of the collected cells. Briefly, a pooled sample of harvested cells was incubated with 7-Amino-actinomycin D (7-AAD) and prepared for flow cytometry according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA). Fluorescence intensity on all flow histograms is shown on a log scale. Forward scatter (FSC) and Side Scatter (SSC) and percentage of cell population on histograms are shown in a linear scale.
Secreted Cytokine Analysis by Cytometric Bead Assay
Immature DCs were stimulated with PMA and Ionomycin and proteins for 48 hours as described above. The supernatant was collected and stored at −20°C until analyzed for concentrations of IL-10 and IL-12 using the cytometric Bead Array (CBA) kit (Becton Dickinson Biosciences, San Jose, CA, USA). Briefly, 50 μl of premixed beads coated with capture antibodies was incubated with 50μl of cytokine standards or test samples in the dark at room temperature for 1 hour. Following the incubation, 50μl of a mixture of Phycoerythrin-conjugated antibodies prepared against the specific cytokines was added to each preparation, and the samples incubated for 2 hours in the dark at room temperature. The beads were washed once with the wash buffer supplied with the CBA kit and analyzed immediately in a BD FACSCalibur flow cytometer (Becton, Dickinson Inc.). Data analysis was performed using the FCAP Array software packages supplied by BD Biosciences. Three thousand counting events were acquired from each sample. Cytokine calibration curves were generated using cytokine standards supplied by BD Biosciences (LaFrance, et al., 2008, Potapova, et al., 2007).
Results
CTB-INS mediated suppression of DC maturation involves upregulation of TLR2
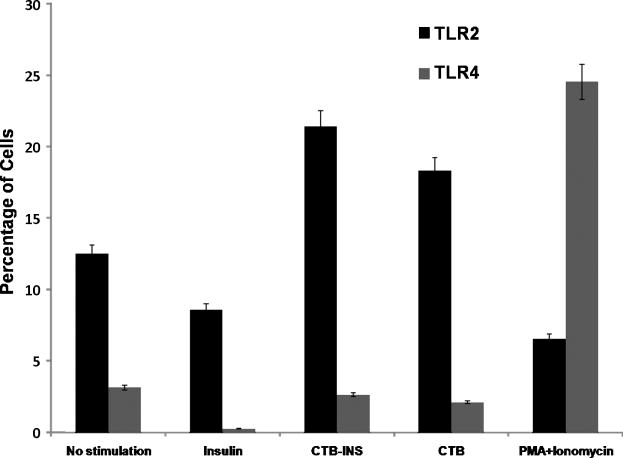
To determine whether Toll-like receptor 2 (TLR2) or TLR4 was involved in CTB-INS mediated DC activation, flow cytometric analysis of TLR2 and TLR4 markers was carried out on vaccine inoculated activated DCs. The results of flow cytometry analysis indicated that CTB and CTB-INS inoculated DCs significantly upregulated the presence of TLR2 proteins on the surface of the vaccine inoculated DC membrane in comparison with unstimulated DCs, insulin treated DCs and PMA Ionomycin treated DCs (Figure 2). No significant up-regulation of TLR4 receptor protein was detected on the surface of CTB, CTB-INS and insulin treated DCs. However, TLR4 was significantly up-regulated in PMA and Ionomycin treated cells a result consistent with those obtained earlier by Thierry et al. (2005) (Roger, et al., 2005). The finding that TLR2 is upregulated by CTB-INS treated cells and not on insulin treated cells suggests that CTB-INS mediated suppression of DC maturation, similar to that found for the E. coli heat sensitive enterotoxin B subunit is attributable to CTB activation of immature DCs through induction of TLR2 rather than TLR4.
Figure 2. TLR2 upregulation by CTB-INS.
The bar graph represents the percentages of dendritic cells expressing Toll like Receptors 2 (TLR2) and Toll like Receptor 4 (TLR4). Umbilical cord blood monocyte derived Immature DC were stimulated for 48 hours with CTB, CTB-INS, Insulin and PMA Ionomycin and the induction of TLR2(CD282) and TLR4(CD284) determined by flow cytometric TLR specific antibody surface staining methods. The mean and standard error of the mean (SE) were calculated for three separate experiments (p<0.05).
CTB-INS Treatment Suppresses Maturation of Dendritic Cells
The influence of CTB, Insulin, CTB-INS and PMA + Ionomycin on immature DC activation and maturation was examined by measuring the expression of DC synthesized co-stimulatory molecules CD86 and CD83. Immature dendritic cells (iDC) were differentiated from monocytes as described in methods and materials section and assessed for the expression of CD14-HLA-DR+CD11C+ surface markers indicating DC differentiation from monocytes (Figure 3A). The Immature dendritic cells (CD14-HLA-DR+CD11C+) were inoculated with CTB, INS and CTB-INS fusion proteins and assessed for stimulation of DC membrane surface markers indicating activation and maturation CD86 and CD83 respectively (Figure 3B). Flow cytometric data obtained for CTB-INS treated cells revealed that immature DC exposure to CTB-INS resulted in a significant level of suppression of CD86 and CD83 costimulatory factors as compared with CTB, INS, and PMA and Ionomycin. These results suggest that the physical linkage of CTB to the Insulin autoantigen is responsible for suppression of dendritic cell activation and maturation by CTB-INS.
Figure 3. Dendritic Cell Activation and Maturation is Suppressed by CTB-INS Fusion Protein.
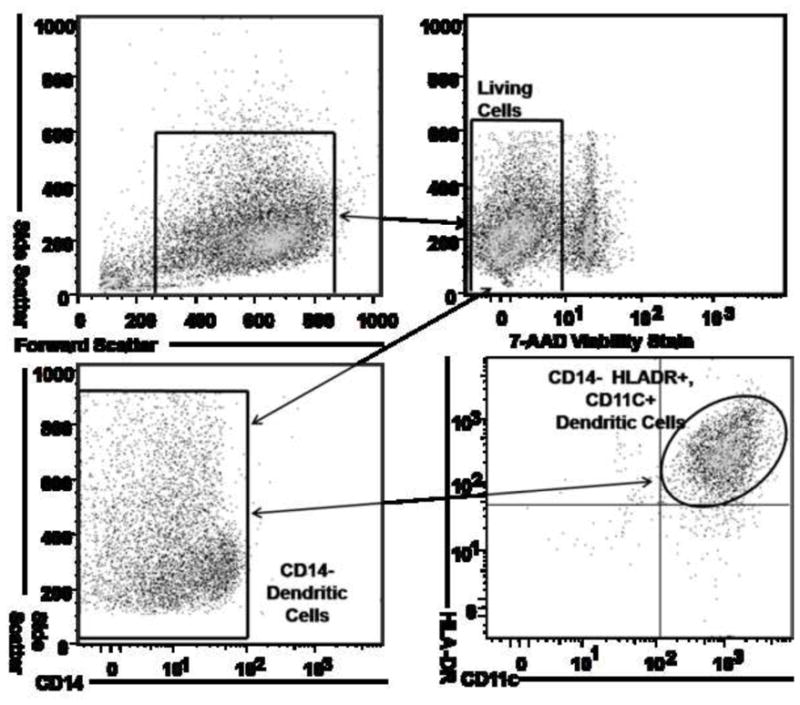
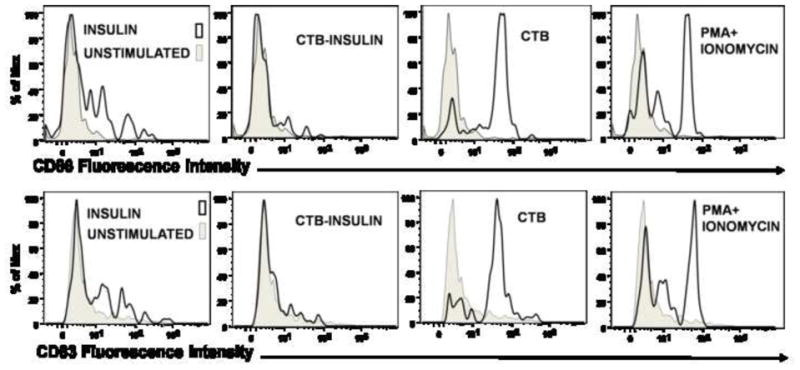
(Panel A) Flow cytometry dot plot showing dendritic cells differentiated from monocytes isolated by ficoll gradient centrifugation from human umbilical cord blood. The CD14+ monocytes were supplemented with granulocyte macrophage colony stimulating factor (GMCSF) and IL-4 and were cultured for 6 days to obtain CD14-HLA-DR+CD11c+ immature human DCs. To determine the viability of DCs isolated from the entire population of collected cells (Upper left panel), 7-AAD negative cells were gated (Upper right panel) and analyzed for expression of the surface marker CD14 (Lower left panel). The CD14- cells were gated and analyzed for co-expression of HLA-DR and CD11C differentiation markers by flow cytometry. (Panel B) The histograms depict the expression of dendritic cell CD83 and CD86 after stimulation of DCs for 48 hours with CTB, CTB-INS, Insulin and PMA Ionomycin. Maturation state of the DCs was determined by flow cytometric analysis. The data are representative of three independent experiments with comparable results.
Linkage of CTB to Autoantigen is Necessary for Suppression of DC Activation
Based on the results obtained from the insulin stimulation studies described above, it was important to examine the role of CTB in the observed suppression of DC activation. Therefore immature DCs were stimulated with 10ug insulin alone, co-delivered with 10ug CTB and finally stimulated with equivalent amounts of CTB-INS fusion protein. Co-delivery of insulin + CTB resulted in significant dendritic cell CD83 and CD86 co-stimulatory factor upregulation. However, cells treated with CTB-INS fusion proteins showed almost no CD83 and CD86 upregulation as measured by mean fluorescent intensity analysis for each treatment group (Figure 4A). Samples treated with CTB, CTB-INS and PMA+Ionomycin showed a significant increase in cell populations expressing CD86 and CD83 in comparison with the control (unstimulated) DC sample. Interestingly, the population of cells expressing CD83 was significantly reduced in comparison with the population of cells expressing the CD86 costimulatory factor. However the ratios of costimulatory expression for each treatment group remained unchanged (data not shown). Taken together, the data indicate that the physical linkage of CTB to the autoantigen is essential for achieving high levels of immunological suppression of DC activation.
Figure 4. Effects of CTB on Insulin Mediated DC Activation.
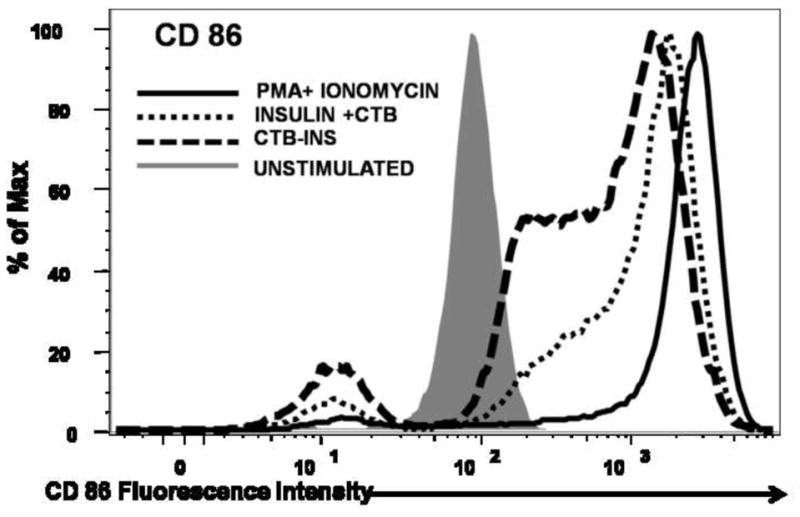
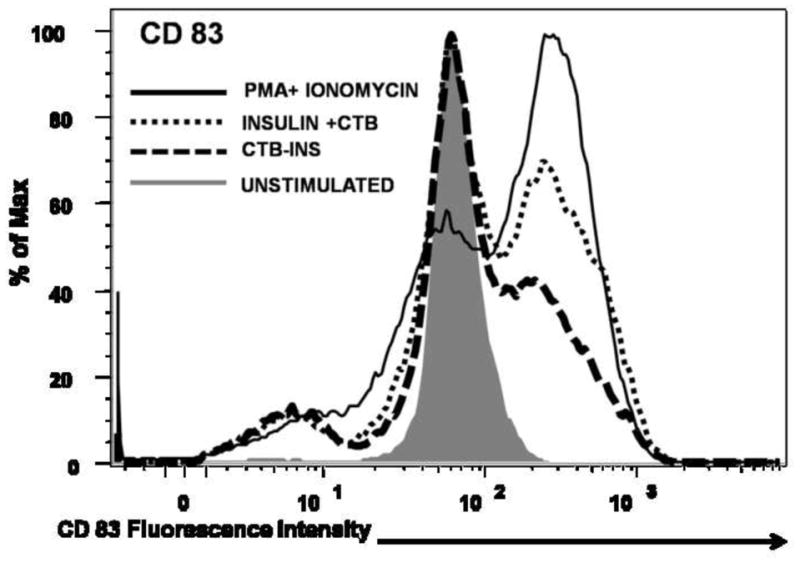
Immature monocyte derived DCs were stimulated for 48 hours with 10ug insulin + 10ug CTB, 20ug CTB-INS and PMA + Ionomycin as a positive control for DC activation. The activation and maturation of DCs measured by expression of CD86 and CD83 cell surface markers was determined by flow cytometric methods. The mean fluorescence intensity (MFI) and percentage population data is representative of four repeated independent experiments with comparable results. (Panel A) The overlapping histograms depict the expression of CD83 and CD86 costimulatory factors in immature dendritic cells after stimulation with PMA + Ionomycin, Insulin + CTB and CTB-INS. The statistical significance was calculated based on P<0.005.
Increased Amounts of Insulin Induce Dendritic Cell Activation
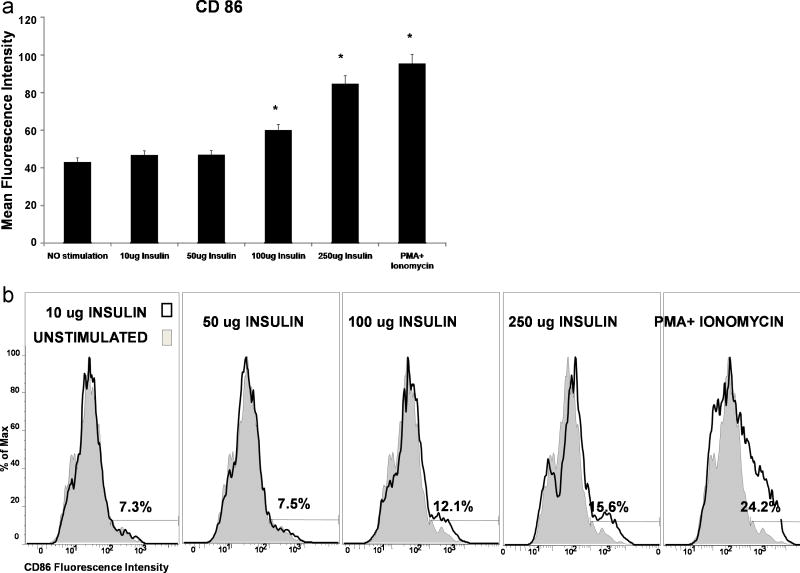
The effect of increasing concentrations of Insulin protein on DC activation was investigated by stimulating immature DCs with increasing amounts of insulin from 10ug/ml up to 250ug/ml of insulin. There was no significant difference in the upregulation of CD86 co-stimulatory molecules on the DC cell surface for concentration 10ug/ml up to 50ug insulin/ml. However, mean fluorescence intensity measurements suggest a progressive increase in the expression of CD86, when the concentration of insulin was increased to 100ug/ml and 250ug/ml respectively (Figure 5A). We also observed an increase in the percentage of the DC cell population activated by increasing the amount of insulin P<0.05 (Figure 5B). Based on the increasing levels of CD86 fluorescence intensity detected, increases in the percentage of activated DCs were shown to correlate with increasing amounts of insulin. Our experimental data did not reveal a significantly different level of CD83 protein synthesized in cells incubated with 100ug/ml and 250ug/ml insulin. Predictably, PMA+ Ionomycin induced significantly increased levels of DC synthesis of CD86 and CD83 costimulatory factors.
Figure 5. Increasing Insulin Dosage Results in DC Activation.
Immature monocyte derived DCs were stimulated for 48 hours with different amounts of insulin and PMA Ionomycin. Activation of DCs was determined by flow cytometric methods. The data presented are representative of more than three independent experiments with comparable results. (Panel A) Graph indicating increasing mean fluorescence intensity of CD86 expression on DC cell surfaces with increasing amounts of Insulin. (Panel B) is a histogram showing the percentages of cells activated by different concentrations of insulin as measured by CD86 costimulatory factor expression on the DC cell surface.
CTB-INS Stimulates Increased Synthesis of IL-10
To determine whether CTB-INS suppression of DC maturation (suppression of co-stimulatory factor synthesis), resulted in increased secretion of immunosuppressive cytokine IL-10 and decreased synthesis of IL-12, the supernatant medium was collected from DCs exposed to 48hrs incubation with CTB-INS fusion protein and analyzed by cytometric bead assay (CBA) based flow cytometry for the presence of secreted cytokines. The results of this experiment showed that there was a significant increase in IL-10 production by DCs incubated with CTB-INS (P<0.05) with a concurrent decrease in proinflammatory cytokine IL-12/23p40 oligomer. Incubation of immature DCs with CTB-INS resulted in a 2.5 fold pg/ml increase in synthesis of IL-10 in comparison with DC incubation with CTB, insulin or PMA + Ionomycin treated cells. Although CTB alone appeared to stimulate DC IL-10 synthesis, the cytokine levels were significantly lower than CTB-INS induced DC IL-10 production (Figure 6). In summary, CTB-INS induced suppression of DC activation and maturation appears to be followed by an increased secretion of the anti-inflammatory cytokine IL-10 and a decrease in secreted IL-12/23p40 suggesting the possibility of immune suppression of inflammatory cytokine synthesis.
Figure 6. CTB-INS Fusion Protein Stimulates IL-10 Synthesis.
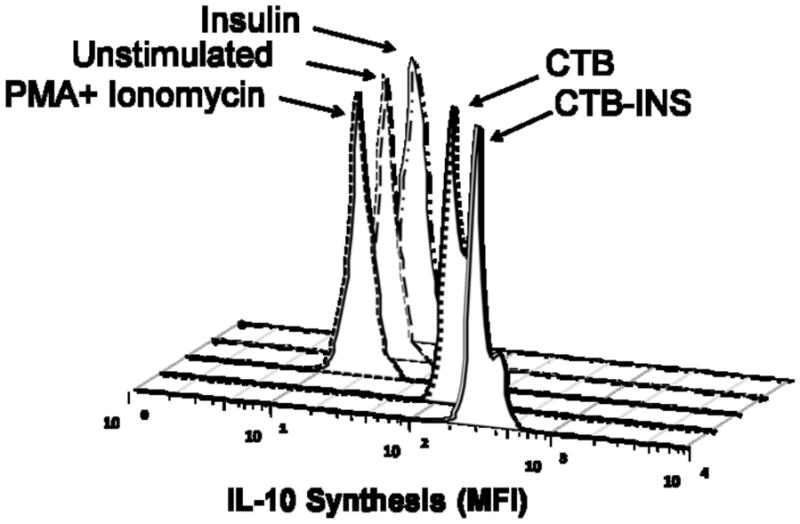
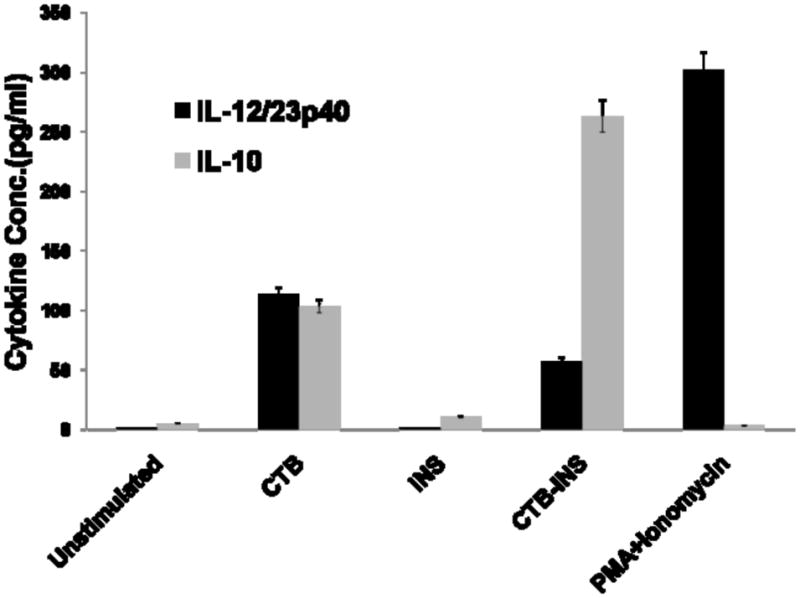
(Panel A) The histograms show the mean fluorescence intensity of IL-10. Premixed plastic beads coated with capture antibodies (BD Biosciences, San Jose, CA, USA).) and a mixture of Phycoerythrin-conjugated antibodies against IL-10 were incubated for 2 hours with the supernatant removed from different treatment conditions of proteins with DCs as described in the Materials and methods section. The beads were washed and analyzed by flow cytometry for determination of the fluorescence intensity of bound IL-10. (Panel B) shows Cytometric Bead Array-defined concentrations of IL-10 and IL-12/23p40 subunit measured from cell supernatants taken from DCs receiving different treatment conditions. For each treatment the concentration of IL-10 and IL-12/23p40 subunit was normalized to standard IL-10 and IL-12/23p40 cytokine curve and given in pg/ml. The data are the Mean and SE (*P<0.001) of repeated independent experiments as compared to the control sample.
Discussion
Our laboratory and others previously demonstrated the phenomenon of suppression of insulitis and autoimmune diabetes in NOD mice inoculated with CTB-INS (Arakawa, et al., 1998, Bergerot, et al., 1997, Sobel, et al., 1998). However, the cellular and molecular mechanisms underlying CTB-proinsulin – immune cell interactions involved in the suppression of disease pathology are incompletely understood. Because dendritic cells are the dominant population of antigen presenting cells involved in the induction of inflammatory and autoreactive T cell morphogenesis, we investigated early effects of CTB-INS on human immature dendritic cell activation and maturation in vitro and attempted to correlate these results with findings from NOD mice diabetes onset studies.
Our experiments suggest that CTB when conjugated to insulin (CTB-INS) results in the suppression of dendritic cell activation and maturation as determined by a failure of CTB-INS and to a limited extent proinsulin alone to stimulate the upregulation of immature dendritic cell CD86 and CD83 costimulatory factors. This observation was further confirmed when CTB-INS stimulated DCs resulted in an increase in DC secretion of the anti-inflammatory cytokine IL-10. Further, our experimental data appears to confirm previous studies with the heat sensitive enterotoxin from E. coli (LTB), that CTB, a molecule similar in structure to LTB like LTB selectively stimulates TLR2 activation in response to DC incubation with CTB or CTB-INS proteins. Previous observations show that CTB can greatly enhance the immunogenicity of linked antigens (D’Ambrosio, et al., 2008, George-Chandy, et al., 2001) by inducing DC upregulation of CD86 and CD83 (George-Chandy, et al., 2001, Isomura, et al., 2005). Morphological changes which included enlargement of immature dendritic cells, elongation of DC dendrites and increased migration of DCs into draining lymph nodes observed in those studies was not monitored in the present study. D’Ambrosio et al. suggested that CTB could partially prevent LPS-induced maturation of monocyte derived DCs while Morita et, al., reported that CTB induced DC maturation. Careful consideration of these findings appears to suggest that CTB may play a dual role as both an immunostimulatory and immunosuppressive modulator molecule. We report that CTB activation of DCs appears to be less than PMA-Ionomycin induced activation of DC maturation. However when compared to insulin and CTB-INS, CTB shows a significantly increased level of DC activation. In essence, the question of the duality of CTB activity either as a pro-inflammatory or anti-inflammatory immune response modulator may be resolved by understanding the activity of CTB in relation to another molecule (antigen or autoantigen).
Earlier NOD mouse insulin inoculation experiments conducted by Bergerot et. al., demonstrated sustained prevention of diabetes onset, even when oral insulin treatment was initiated as late as 15 weeks after birth of the mice. In addition, the protective effect against continued insulitis development was transferable to untreated NOD mice through transfer of CD4+ T cells from CTB-INS inoculated animals (Bergerot, et al., 1997). Our recent finding that CTB-INS is active in the suppression of DC maturation provides a possible explanation for CTB enhanced insulin mediated diabetes suppression observed in NOD mice studies employing CTB-INS fusion protein produced in viruses, plants, and silkworm larvae (Denes, et al., 2005, Gong, et al., 2007, Gong, et al., 2009).
Previous studies demonstrated that insulin can function as a prime target for autoantigen directed type 1 diabetes therapies (Jaeckel, et al., 2003, Jaeckel, et al., 2004, Kent, et al., 2005, Palmer, et al., 1983). These studies showed that administration of insulin analogs such as altered B: 9-23 insulin induced T cell mechanisms involved in the prevention of diabetes development in NOD mice (Atkinson and Leiter, 1999, Elliott, et al., 1994, Kobayashi, et al., 2007, Weiner, 2001, Weiner, et al., 1991, Zhang, et al., 1991). However, a major concern is that increasing amounts of insulin can act as a double edged sword with the potential to protect against the development of autoimmunity at low insulin concentrations while stimulating pathogenic immunity at higher insulin concentrations (Kobayashi, et al., 2007, Romagnani, 1998). Qui-Liang Lui et al (2009) recently demonstrated that increased insulin concentration (2.5–25mg/L) significantly increased DC synthesis of co-stimulatory factors, increasing DC ability to activate autologous lymphocytes.(Liu, et al., 2009) Our experimental data showed that exposure of immature DCs to increasing amounts of insulin resulted in an increase rather than a further suppression of DC activation. This result provides a plausible answer to the earlier counter intuitive observation that increased insulin levels might be expected to stimulate rather than suppresses diabetes onset in NOD mice.
The observed increase in suppression of DC maturation by CTB-INS fusion protein in comparison with insulin alone raises the question of whether CTB-INS suppression of DC maturation is mediated through the activation of Toll like receptor signaling processes. The type II heat-labile enterotoxin (LT-IIB) of Escherichia coli, which has an AB5 type structure similar to CTB was shown to induce DC activation through TLR2 resulting in downstream activation of NF-κB (Connell, et al., 1995, Hajishengallis, et al., 2005, Lencer, et al., 1995, Merritt, et al., 1995). Examination of CTB mediated suppression of DC activation and maturation similarly suggests that TLR2 may be selectively upregulated as well in CTB stimulated DCs. The cholera toxin B subunit may be selective for TLR2 since TLR2 depends specifically on hydrophobic interactions for ligand binding (Okusawa, et al., 2004, Seong and Matzinger, 2004) as observed for LTBIIb. While the specific mechanism by which CTB may interact with TLR2 is largely inferred from LTB studies, further insights may be provided from the binding properties of CTB and other pentameric enterotoxin B subunits that have the capacity to participate in both hydrophobic and hydrophilic interactions (Tinker, et al., 2003, van den Akker, et al., 1996).
DC activation correlates with expression of the major immunosuppressive cytokine-IL-10 identified in APCs including dendritic cell subsets (de Waal Malefyt, et al., 1991, Sica, et al., 2000). Autocrine production of IL-10 by immature DCs (iDCs) was shown to inhibit synthesis and release of pro-inflammatory cytokines and other molecules (IL-12, TNF-α, IL-6, LTB4, NO, PGE2). Further, IL-10 secretion by DCs was shown to suppress Th1 lymphocyte activity by enhancing down-regulation of costimulatory molecule expression on the DC surface (Grutz, 2005, Harizi and Gualde, 2006). In the case of experimental encephalitis, oral administration of CTB linked to myelin basic protein (MBP) increased suppression of encephalitis induced by TGF-β CD4 T cells in the animal spinal cord (Sun, et al., 2000). In the same way, immunological analyses of patients with Behcet’s disease treated with CTB conjugated to HSP60 p336–351 in a pilot clinic trial showed the presence of increased synthesis of IL-10 by CD4+ cells as well as a reduction in T cell synthesis of pro-inflammatory cytokines IL-2 and IFN-γ (Stanford, et al., 2004). Through inhibition of the above endogenous pro-inflammatory mediators, IL-10 was shown to be central to maintenance of DCs in an immature state and in the down-regulation of DC mediated inflammatory responses (Harizi and Gualde, 2006). In paracrine fashion, IL-10 synthesized by DCs can regulate immunity by altering the function of different adjacent cell types. In several studies, IL-10 secreted by iDCs stimulated the development of cognate naïve Th0 cells into either Th2 lymphocytes, or suppressor regulatory T cells (Romagnani, 1998, Romagnani, et al., 1998, Stassen, et al., 2004, Stassen, et al., 2004). Studies in knockout mice showed that IL-10 can down-regulate autoimmunity in the intestine and can be active in the suppression of Crohn’s disease (Grimbaldeston, et al., 2007, Minderhoud, et al., 2007).
In our experiments, CTB-INS stimulated iDCs produced significantly higher levels of IL-10 than in CTB or proinsulin stimulated iDCs. However of interest, the biosynthesis of IL-12/23p40 subunit was significantly inhibited. This result is critical because induction of cognate naïve T helper cell (Th0) morphogenesis into Th1 effector cells that secrete IFN-γ and IL-2 responsible for islet inflammation and beta cell death is dependent on DC synthesis of IL-12 in addition to expression of surface costimulatory molecules (Itano, et al., 2003, Kang and Kim, 2006, Pulendran, et al., 1999, Zorena, et al., 2008). Secretion of IL-10 was shown to be essential for the inhibition of DC maturation through its effect of blocking IL-12 synthesis. As a result of increased DC biosynthesis of IL-10, inflammatory autoreactive effector Th1 cell proliferation and secretion of downstream inflammatory cytokines IFN-γ and IL-2 is inhibited. This finding in combination with our experiments demonstrating CTB-INS inhibition of DC co-stimulatory factor upregulation and IL-12/23p40 synthesis may be critical to determine the probable cellular mechanisms underlying CTB-INS mediated immune suppression of T1D. Future experiments incubating CTB linked autoantigens with naïve DCs will reveal the capacity of CTB-INS to arrest the progression of type 1 diabetes once hyperglycemia has become established. Establishment of CTB-INS mediated immunological suppression of type 1 diabetes progression will help to determine whether this form of interventional therapy in combination with anti-inflammatory cytokines can prevent both the onset and the progression of diabetes once hyperglycemia has developed. Once durable immunological suppression of diabetes progression has been achieved, interventional therapy with insulin producing mesenchymal stem cells may provide an effective, safe and durable cure for the present ravages of type 1 diabetes.
Acknowledgments
We would like to thank the Loma Linda University Medical Center, Division of Labor and Delivery, for assistance with acquisition of umbilical cord blood. We also thank Dr. Daila Gridley and Mr. Gordon Harding for providing LPS for the dendritic cell stimulation studies. Further, We would also like to thank Dequina Nicholas, Terry-ann Milford, Abigail Benitez and Abby Weldon for their technical assistance. This project was supported in part by funding from grant 1-2000-812 to W.H.R.L from the Juvenile Diabetes Foundation, and R21 grant DK-99-013 awarded to W.H.R.L. and I. F. from the National Institutes of Health.
List of Abbreviations
- DC
dendritic cell
- TLR
Toll Like receptor
- T1D
type 1 diabetes
- PMA
Phorbol myristate acetate
- LPS
lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Chong DK, Langridge WH. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat Biotechnol. 1998;16:292. doi: 10.1038/nbt0398-292. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- Aspord C, Thivolet C. Nasal administration of CTB-insulin induces active tolerance against autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol. 2002;130:204. doi: 10.1046/j.1365-2249.2002.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:4610. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Cardell SL. The natural killer T lymphocyte: a player in the complex regulation of autoimmune diabetes in non-obese diabetic mice. Clin Exp Immunol. 2006;143:194. doi: 10.1111/j.1365-2249.2005.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JE, 3rd, Yu J, Choi NW, Hough J, Henderson D, He D, Langridge WH. Bacterial and plant enterotoxin B subunit-autoantigen fusion proteins suppress diabetes insulitis. Mol Biotechnol. 2006;32:1. doi: 10.1385/MB:32:1:001. [DOI] [PubMed] [Google Scholar]
- Connell TD, Metzger DJ, Wang M, Jobling MG, Holmes RK. Initial studies of the structural signal for extracellular transport of cholera toxin and other proteins recognized by Vibrio cholerae. Infect Immun. 1995;63:4091. doi: 10.1128/iai.63.10.4091-4098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio A, Colucci M, Pugliese O, Quintieri F, Boirivant M. Cholera toxin B subunit promotes the induction of regulatory T cells by preventing human dendritic cell maturation. J Leukoc Biol. 2008;84:661. doi: 10.1189/jlb.1207850. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes B, Krausova V, Fodor N, Timiryasova T, Henderson D, Hough J, Yu J, Fodor I, Langridge WH. Protection of NOD mice from type 1 diabetes after oral inoculation with vaccinia viruses expressing adjuvanted islet autoantigens. J Immunother. 2005;28:438. doi: 10.1097/01.cji.0000171315.82997.9a. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- Elliott JF, Qin HY, Bhatti S, Smith DK, Singh RK, Dillon T, Lauzon J, Singh B. Immunization with the larger isoform of mouse glutamic acid decarboxylase (GAD67) prevents autoimmune diabetes in NOD mice. Diabetes. 1994;43:1494. doi: 10.2337/diab.43.12.1494. [DOI] [PubMed] [Google Scholar]
- Eriksson K, Holmgren J. Recent advances in mucosal vaccines and adjuvants. Curr Opin Immunol. 2002;14:666. doi: 10.1016/s0952-7915(02)00384-9. [DOI] [PubMed] [Google Scholar]
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- George-Chandy A, Eriksson K, Lebens M, Nordstrom I, Schon E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001;69:5716. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Jin Y, Zhang Y. Suppression of diabetes in non-obese diabetic (NOD) mice by oral administration of a cholera toxin B subunit-insulin B chain fusion protein vaccine produced in silkworm. Vaccine. 2007;25:1444. doi: 10.1016/j.vaccine.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Gong Z, Long X, Pan L, Le Y, Liu Q, Wang S, Guo J, Xiao B, Zhou M, Mei D. Cloning, expression, purification and characterization of the cholera toxin B subunit and triple glutamic acid decarboxylase epitopes fusion protein in Escherichia coli. Protein Expr Purif. 2009;66:191. doi: 10.1016/j.pep.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Martin MH, Nawar H, Lyle EA, Russell MW, Connell TD. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect Immun. 2005;73:1343. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, Dilorenzo TP, Santamaria P. Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J Clin Invest. 2005;115:1879. doi: 10.1172/JCI24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harizi H, Gualde N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell Mol Immunol. 2006;3:271. [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Jiang Z, Georgel P, Tabeta K, Janssen E, Du X, Beutler B. TLR signaling pathways: opportunities for activation and blockade in pursuit of therapy. Curr Pharm Des. 2006;12:4123. doi: 10.2174/138161206778743466. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Adamsson J, Anjuere F, Clemens J, Czerkinsky C, Eriksson K, Flach CF, George-Chandy A, Harandi AM, Lebens M, Lehner T, Lindblad M, Nygren E, Raghavan S, Sanchez J, Stanford M, Sun JB, Svennerholm AM, Tengvall S. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol Lett. 2005;97:181. doi: 10.1016/j.imlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Isomura I, Yasuda Y, Tsujimura K, Takahashi T, Tochikubo K, Morita A. Recombinant cholera toxin B subunit activates dendritic cells and enhances antitumor immunity. Microbiol Immunol. 2005;49:79. doi: 10.1111/j.1348-0421.2005.tb03632.x. [DOI] [PubMed] [Google Scholar]
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Jaeckel E, Klein L, Martin-Orozco N, von Boehmer H. Normal incidence of diabetes in NOD mice tolerant to glutamic acid decarboxylase. J Exp Med. 2003;197:1635. doi: 10.1084/jem.20030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol. 2004;5:1028. doi: 10.1038/ni1120. [DOI] [PubMed] [Google Scholar]
- Kang BY, Kim TS. Targeting cytokines of the interleukin-12 family in autoimmunity. Curr Med Chem. 2006;13:1149. doi: 10.2174/092986706776360879. [DOI] [PubMed] [Google Scholar]
- Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, Hering BJ, Hafler DA. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- Kim N, Cheng KC, Kwon SS, Mora R, Barbieri M, Yoo TJ. Oral administration of collagen conjugated with cholera toxin induces tolerance to type II collagen and suppresses chondritis in an animal model of autoimmune ear disease. Ann Otol Rhinol Laryngol. 2001;110:646. doi: 10.1177/000348940111000710. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Abiru N, Arakawa T, Fukushima K, Zhou H, Kawasaki E, Yamasaki H, Liu E, Miao D, Wong FS, Eisenbarth GS, Eguchi K. Altered B:9-23 insulin, when administered intranasally with cholera toxin adjuvant, suppresses the expression of insulin autoantibodies and prevents diabetes. J Immunol. 2007;179:2082. doi: 10.4049/jimmunol.179.4.2082. [DOI] [PubMed] [Google Scholar]
- LaFrance MW, Kehinde LE, Fullard RJ. Multiple cytokine analysis in human tears: an optimized procedure for cytometric bead-based assay. Curr Eye Res. 2008;33:525. doi: 10.1080/02713680802190085. [DOI] [PubMed] [Google Scholar]
- Lavelle EC, Jarnicki A, McNeela E, Armstrong ME, Higgins SC, Leavy O, Mills KH. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J Leukoc Biol. 2004;75:756. doi: 10.1189/jlb.1103534. [DOI] [PubMed] [Google Scholar]
- Lavelle EC, McNeela E, Armstrong ME, Leavy O, Higgins SC, Mills KH. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J Immunol. 2003;171:2384. doi: 10.4049/jimmunol.171.5.2384. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Constable C, Moe S, Jobling MG, Webb HM, Ruston S, Madara JL, Hirst TR, Holmes RK. Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995;131:951. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhong J, Chen Y, Qiu X, Zhang T, Ma D, Han W. Expression of chemokine-like factor 1 is upregulated during T lymphocyte activation. Life Sci. 2006;79:519. doi: 10.1016/j.lfs.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- Liu H, MacKenzie-Graham AJ, Kim S, Voskuhl RR. Mice resistant to experimental autoimmune encephalomyelitis have increased thymic expression of myelin basic protein and increased MBP specific T cell tolerance. J Neuroimmunol. 2001;115:118. doi: 10.1016/s0165-5728(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Liu QL, Wang YS, Wang JX. Effect of insulin on functional status of cord blood-derived dendritic cells and on dendritic cell-induced CTL cytotoxicity against pancreatic cancer cell lines. Hepatobiliary Pancreat Dis Int. 2009;8:529. [PubMed] [Google Scholar]
- Lodinova-Zadnikova R, Prokesova L, Tlaskalova H, Kocourkova I, Zizka J, Stranak Z. Influence of oral colonization with probiotic E. coli strain after birth on frequency of recurrent infections, allergy and development of some immunologic parameters. Long-term studies. Ceska Gynekol. 2004;69(Suppl 1):91. [PubMed] [Google Scholar]
- Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee JR. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621. [PubMed] [Google Scholar]
- Merritt EA, Sarfaty S, Chang TT, Palmer LM, Jobling MG, Holmes RK, Hol WG. Surprising leads for a cholera toxin receptor-binding antagonist: crystallographic studies of CTB mutants. Structure. 1995;3:561. doi: 10.1016/s0969-2126(01)00190-3. [DOI] [PubMed] [Google Scholar]
- Minderhoud IM, Samsom M, Oldenburg B. What predicts mucosal inflammation in Crohn’s disease patients? Inflamm Bowel Dis. 2007;13:1567. doi: 10.1002/ibd.20233. [DOI] [PubMed] [Google Scholar]
- Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infect Immun. 2004;72:1657. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW, Sahakian E, Kagoda M, Huang G, Hao QL, Sevilla Y, Barsky LW, Zielinska E, Price MA, Wall NR, Dovat S, Payne KJ. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps PA, Stanford MR, Sun JB, Xiao BG, Holmgren J, Shinnick T, Hasan A, Mizushima Y, Lehner T. Prevention of mucosally induced uveitis with a HSP60-derived peptide linked to cholera toxin B subunit. Eur J Immunol. 2003;33:224. doi: 10.1002/immu.200390025. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A. 1999;96:1036. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Roger T, Miconnet I, Schiesser AL, Kai H, Miyake K, Calandra T. Critical role for Ets, AP-1 and GATA-like transcription factors in regulating mouse Toll-like receptor 4 (Tlr4) gene expression. Biochem J. 2005;387:355. doi: 10.1042/BJ20041243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. T-cell subsets (Th1, Th2) and cytokines in autoimmunity. 3. Academic Press; 1998. p. 163. [Google Scholar]
- Romagnani S. The Th1/Th2 paradigm and allergic disorders. Allergy. 1998;53:12. doi: 10.1111/j.1398-9995.1998.tb04951.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S, Kapsenberg M, Radbruch A, Adorini L. Th1 and Th2 cells. Res Immunol. 1998;149:871. doi: 10.1016/s0923-2494(99)80016-9. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Shinomiya M, Fazle Akbar SM, Shinomiya H, Onji M. Transfer of dendritic cells (DC) ex vivo stimulated with interferon-gamma (IFN-gamma) down-modulates autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol. 1999;117:38. doi: 10.1046/j.1365-2249.1999.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreedhar VK, Kelsall BL, Neutra MR. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-cell areas of Peyer’s patches. Infect Immun. 2003;71:504. doi: 10.1128/IAI.71.1.504-509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- Silveira PA, Grey ST. B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab. 2006;17:128. doi: 10.1016/j.tem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Sobel DO, Yankelevich B, Goyal D, Nelson D, Mazumder A. The B-subunit of cholera toxin induces immunoregulatory cells and prevents diabetes in the NOD mouse. Diabetes. 1998;47:186. doi: 10.2337/diab.47.2.186. [DOI] [PubMed] [Google Scholar]
- Stanford M, Whittall T, Bergmeier LA, Lindblad M, Lundin S, Shinnick T, Mizushima Y, Holmgren J, Lehner T. Oral tolerization with peptide 336–351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet’s disease. Clin Exp Immunol. 2004;137:201. doi: 10.1111/j.1365-2249.2004.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen M, Fondel S, Bopp T, Richter C, Muller C, Kubach J, Becker C, Knop J, Enk AH, Schmitt S, Schmitt E, Jonuleit H. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303. doi: 10.1002/eji.200324656. [DOI] [PubMed] [Google Scholar]
- Stassen M, Schmitt E, Jonuleit H. Human CD(4+)CD(25+) regulatory T cells and infectious tolerance. Transplantation. 2004;77:S23. doi: 10.1097/00007890-200401151-00009. [DOI] [PubMed] [Google Scholar]
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772. [PubMed] [Google Scholar]
- Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci U S A. 1994;91:10795. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JB, Li BL, Czerkinsky C, Holmgren J. Enhanced immunological tolerance against allograft rejection by oral administration of allogeneic antigen linked to cholera toxin B subunit. Clin Immunol. 2000;97:130. doi: 10.1006/clim.2000.4927. [DOI] [PubMed] [Google Scholar]
- Sun JB, Rask C, Olsson T, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc Natl Acad Sci U S A. 1996;93:7196. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JB, Xiao BG, Lindblad M, Li BL, Link H, Czerkinsky C, Holmgren J. Oral administration of cholera toxin B subunit conjugated to myelin basic protein protects against experimental autoimmune encephalomyelitis by inducing transforming growth factor-beta-secreting cells and suppressing chemokine expression. Int Immunol. 2000;12:1449. doi: 10.1093/intimm/12.10.1449. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Hao J, Zhang Y, Tian L, Yi H, O’Brien TD, Sutherland DE, Hering BJ, Guo Z. Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation. 2009;87:198. doi: 10.1097/TP.0b013e3181933261. [DOI] [PubMed] [Google Scholar]
- Tian J, Zekzer D, Lu Y, Dang H, Kaufman DL. B cells are crucial for determinant spreading of T cell autoimmunity among beta cell antigens in diabetes-prone nonobese diabetic mice. J Immunol. 2006;176:2654. doi: 10.4049/jimmunol.176.4.2654. [DOI] [PubMed] [Google Scholar]
- Tinker JK, Erbe JL, Hol WG, Holmes RK. Cholera holotoxin assembly requires a hydrophobic domain at the A-B5 interface: mutational analysis and development of an in vitro assembly system. Infect Immun. 2003;71:4093. doi: 10.1128/IAI.71.7.4093-4101.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- Trembleau S, Germann T, Gately MK, Adorini L. The role of IL-12 in the induction of organ-specific autoimmune diseases. Immunol Today. 1995;16:383. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- van den Akker F, Sarfaty S, Twiddy EM, Connell TD, Holmes RK, Hol WG. Crystal structure of a new heat-labile enterotoxin, LT-IIb. Structure. 1996;4:665. doi: 10.1016/s0969-2126(96)00073-1. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Zhang H, Chen XL, Jin WR, Cong YQ, Han B, Gu Y. Detection of IFN-gamma level in single CD8+ T cell. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2005;21:72. [PubMed] [Google Scholar]
- Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Zhang ZJ, Khoury SJ, Miller A, al-Sabbagh A, Brod SA, Lider O, Higgins P, Sobel R, Nussenblatt RB, et al. Antigen-driven peripheral immune tolerance. Suppression of organ-specific autoimmune diseases by oral administration of autoantigens. Ann N Y Acad Sci. 1991;636:227. doi: 10.1111/j.1749-6632.1991.tb33454.x. [DOI] [PubMed] [Google Scholar]
- Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A. 1991;88:10252. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorena K, Mysliwska J, Mysliwiec M, Balcerska A, Lipowski P, Raczynska K. Interleukin-12 and tumour necrosis factor-alpha equilibrium is a prerequisite for clinical course free from late complications in children with type 1 diabetes mellitus. Scand J Immunol. 2008;67:204. doi: 10.1111/j.1365-3083.2007.02054.x. [DOI] [PubMed] [Google Scholar]