Abstract
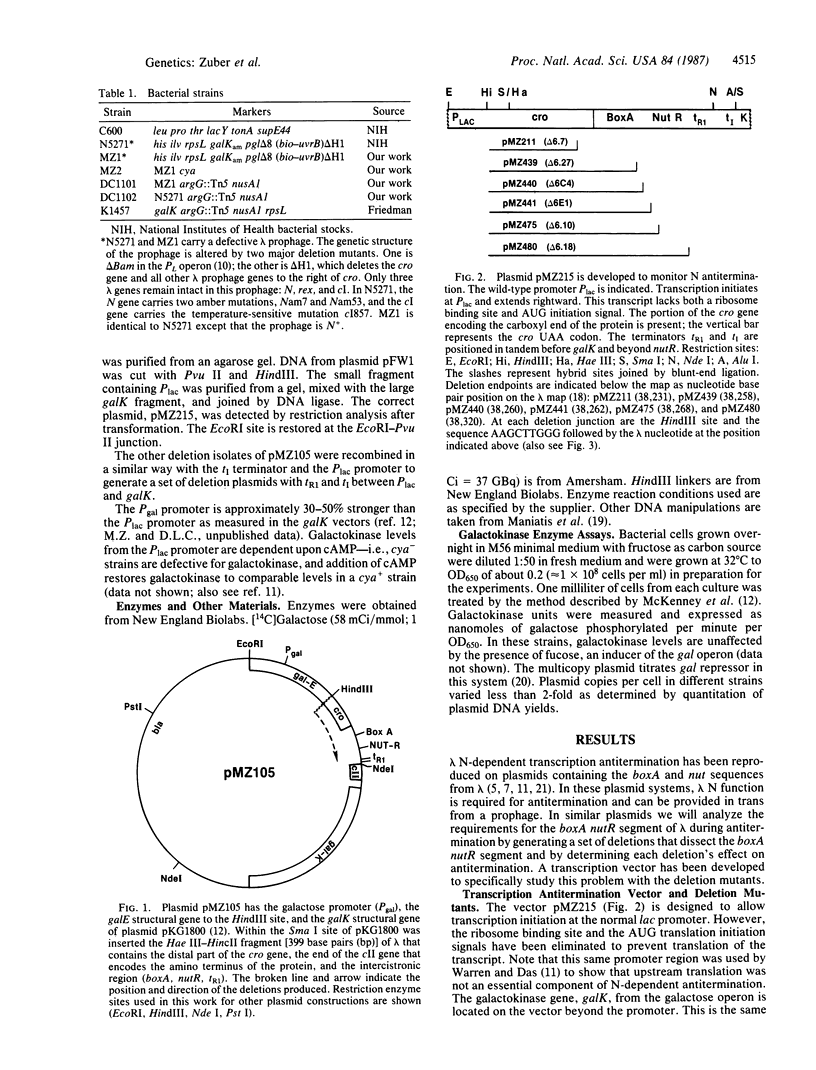
Deletions extending from the cro gene into boxA and nutR of the Rho-dependent tR1 terminator of bacteriophage lambda have been generated and cloned between promoters and the galK gene of Escherichia coli on a multicopy plasmid. Terminators placed between the promoters and galK restrict transcription and expression of galK on these plasmids. However, when lambda N protein is provided, and if a functional N interaction site, nutR, is intact, transcription antitermination occurs and galK expression increases. Deletions into the nutR region affect the ability to antiterminate. From the results obtained we conclude that: boxA, a site believed to bind host factors (Nus), is not required for transcription antitermination in this system; the host NusA function is required even in the absence of boxA; nutR is required for N antitermination; translation across the nutR sequence prevents N-dependent antitermination.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. L., Szybalski W. Transcriptional antitermination activity of the synthetic nut elements of coliphage lambda. I. Assembly of the nutR recognition site from boxA and nut core elements. Gene. 1986;42(1):E125–E132. doi: 10.1016/0378-1119(86)90159-9. [DOI] [PubMed] [Google Scholar]
- Dambly-Chaudière C., Gottesman M., Debouck C., Adhya S. Regulation of the pR operon of bacteriophage lambda. J Mol Appl Genet. 1983;2(1):45–56. [PubMed] [Google Scholar]
- Das A., Wolska K. Transcription antitermination in vitro by lambda N gene product: requirement for a phage nut site and the products of host nusA, nusB, and nusE genes. Cell. 1984 Aug;38(1):165–173. doi: 10.1016/0092-8674(84)90537-3. [DOI] [PubMed] [Google Scholar]
- Drahos D., Galluppi G. R., Caruthers M., Szybalski W. Synthesis of the nutL DNA segments and analysis of antitermination and termination functions in coliphage lambda. Gene. 1982 Jun;18(3):343–354. doi: 10.1016/0378-1119(82)90173-1. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R. Evidence that a nucleotide sequence, "boxA," is involved in the action of the NusA protein. Cell. 1983 Aug;34(1):143–149. doi: 10.1016/0092-8674(83)90144-7. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Georgopoulos C., Tilly K., Herskowitz I., Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984 Dec;48(4):299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980 Jun 15;140(1):57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- Grayhack E. J., Yang X. J., Lau L. F., Roberts J. W. Phage lambda gene Q antiterminator recognizes RNA polymerase near the promoter and accelerates it through a pause site. Cell. 1985 Aug;42(1):259–269. doi: 10.1016/s0092-8674(85)80121-5. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Wu R. Exonuclease III: use for DNA sequence analysis and in specific deletions of nucleotides. Methods Enzymol. 1983;100:60–96. doi: 10.1016/0076-6879(83)00046-4. [DOI] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Montañez C., Bueno J., Schmeissner U., Court D. L., Guarneros G. Mutations of bacteriophage lambda that define independent but overlapping RNA processing and transcription termination sites. J Mol Biol. 1986 Sep 5;191(1):29–37. doi: 10.1016/0022-2836(86)90420-1. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Mizusawa S., Court D. L., Tsugawa A. Regulatory defects of a conditionally lethal nusAts mutant of Escherichia coli. Positive and negative modulator roles of NusA protein in vivo. J Mol Biol. 1986 May 5;189(1):103–111. doi: 10.1016/0022-2836(86)90384-0. [DOI] [PubMed] [Google Scholar]
- Olson E. R., Flamm E. L., Friedman D. I. Analysis of nutR: a region of phage lambda required for antitermination of transcription. Cell. 1982 Nov;31(1):61–70. doi: 10.1016/0092-8674(82)90405-6. [DOI] [PubMed] [Google Scholar]
- Olson E. R., Tomich C. S., Friedman D. I. The nusA recognition site. Alteration in its sequence or position relative to upstream translation interferes with the action of the N antitermination function of phage lambda. J Mol Biol. 1984 Dec 25;180(4):1053–1063. doi: 10.1016/0022-2836(84)90270-5. [DOI] [PubMed] [Google Scholar]
- Peltz S. W., Brown A. L., Hasan N., Podhajska A. J., Szybalski W. Thermosensitivity of a DNA recognition site: activity of a truncated nutL antiterminator of coliphage lambda. Science. 1985 Apr 5;228(4695):91–93. doi: 10.1126/science.3156406. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Szybalski W. Coliphage lambdanutL-: a unique class of mutants defective in the site of gene N product utilization for antitermination of leftward transcription. J Mol Biol. 1978 Sep 5;124(1):195–221. doi: 10.1016/0022-2836(78)90156-0. [DOI] [PubMed] [Google Scholar]
- Tsugawa A., Kurihara T., Zuber M., Court D. L., Nakamura Y. E. coli NusA protein binds in vitro to an RNA sequence immediately upstream of the boxA signal of bacteriophage lambda. EMBO J. 1985 Sep;4(9):2337–2342. doi: 10.1002/j.1460-2075.1985.tb03935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren F., Das A. Formation of termination-resistant transcription complex at phage lambda nut locus: effects of altered translation and a ribosomal mutation. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3612–3616. doi: 10.1073/pnas.81.12.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Mudryj M., DiLauro R., Gottesman M. Specificity of the bacteriophage lambda N gene product (pN): nut sequences are necessary and sufficient for antitermination by pN. Cell. 1979 Dec;18(4):1145–1151. doi: 10.1016/0092-8674(79)90227-7. [DOI] [PubMed] [Google Scholar]


