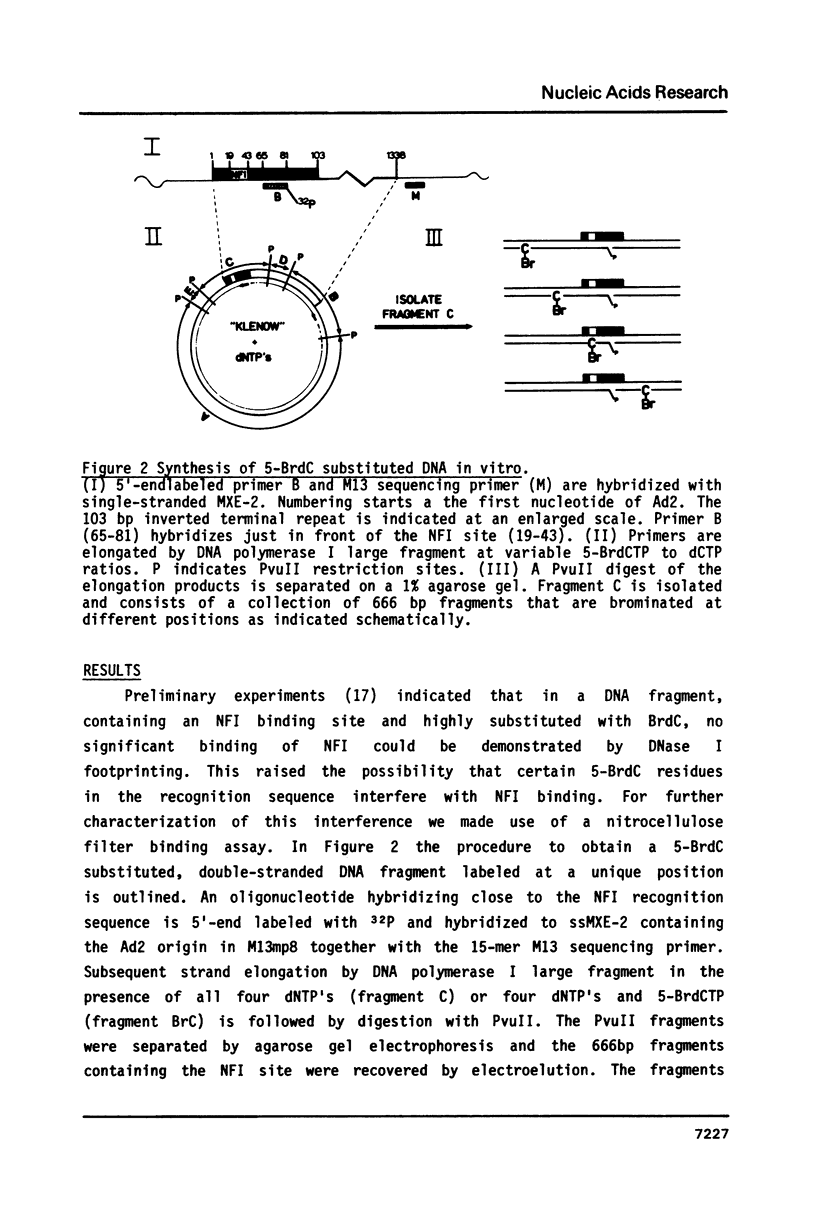
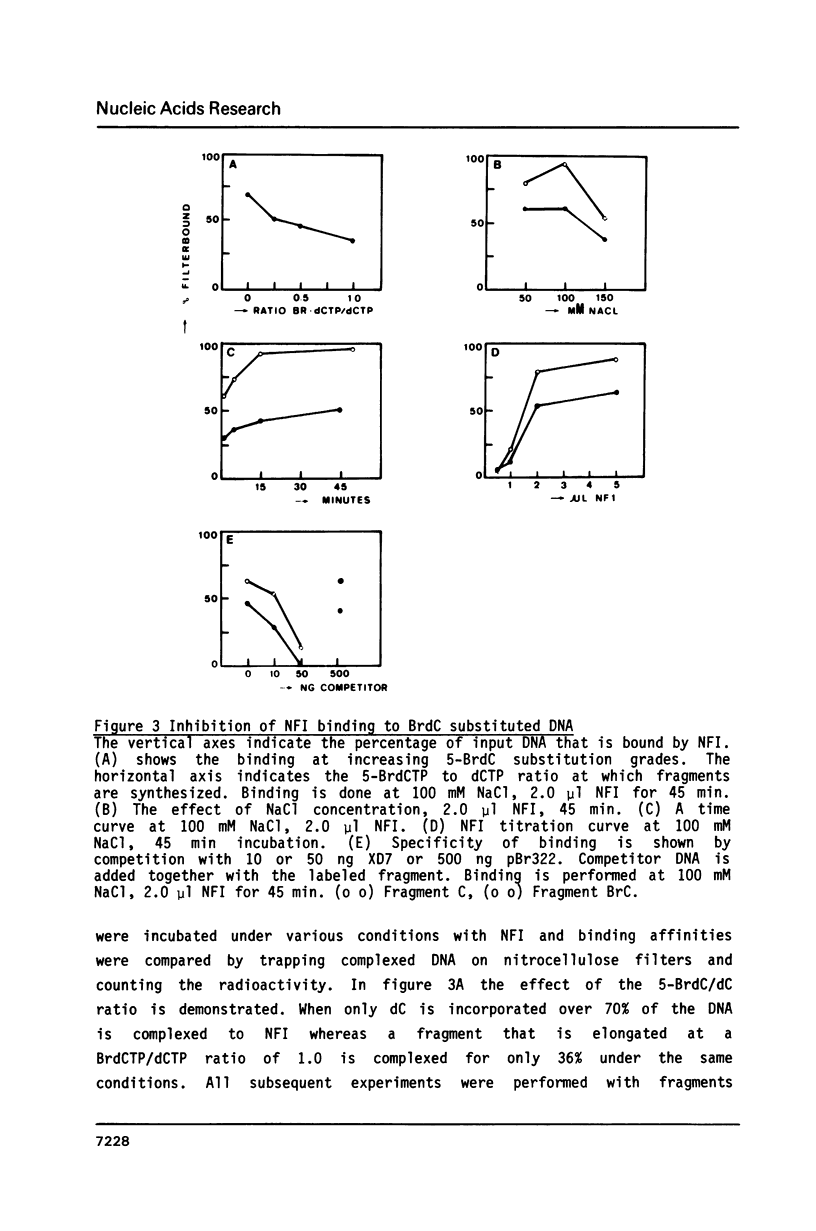
Abstract
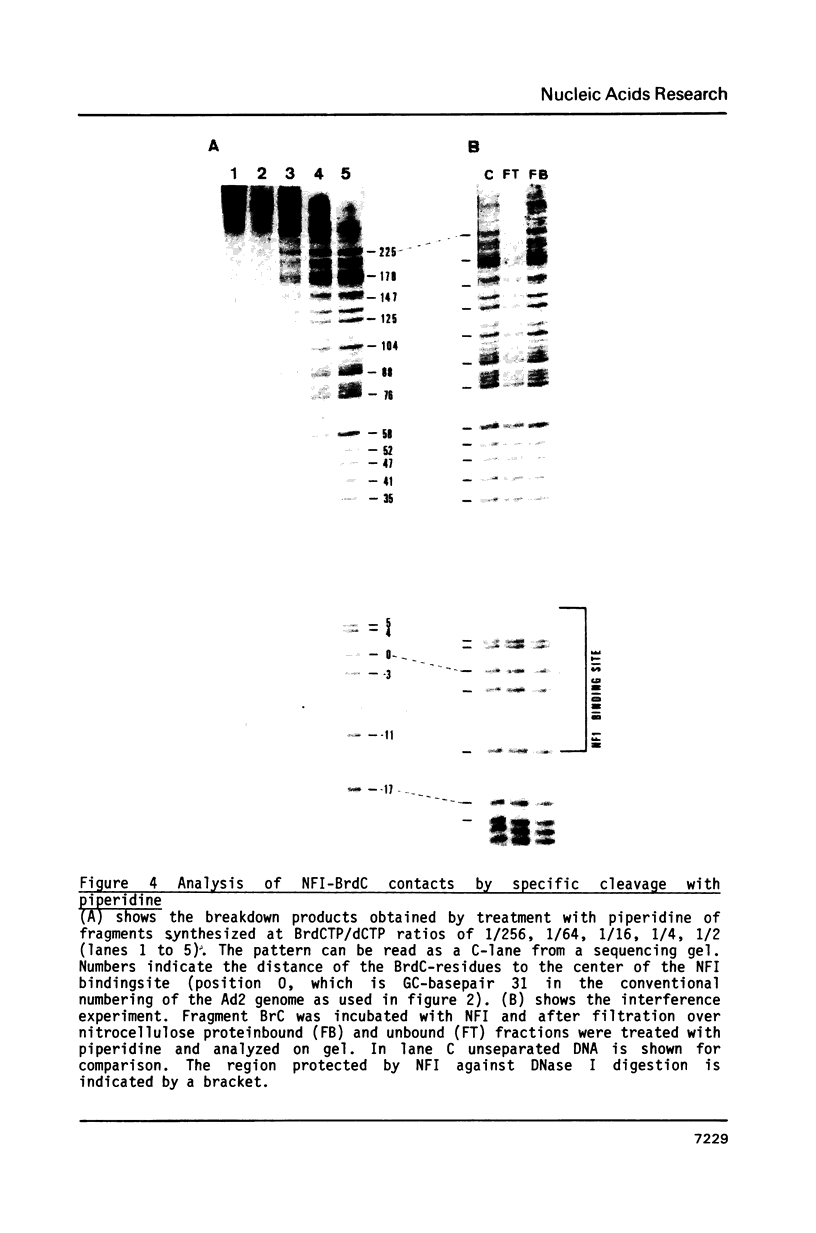
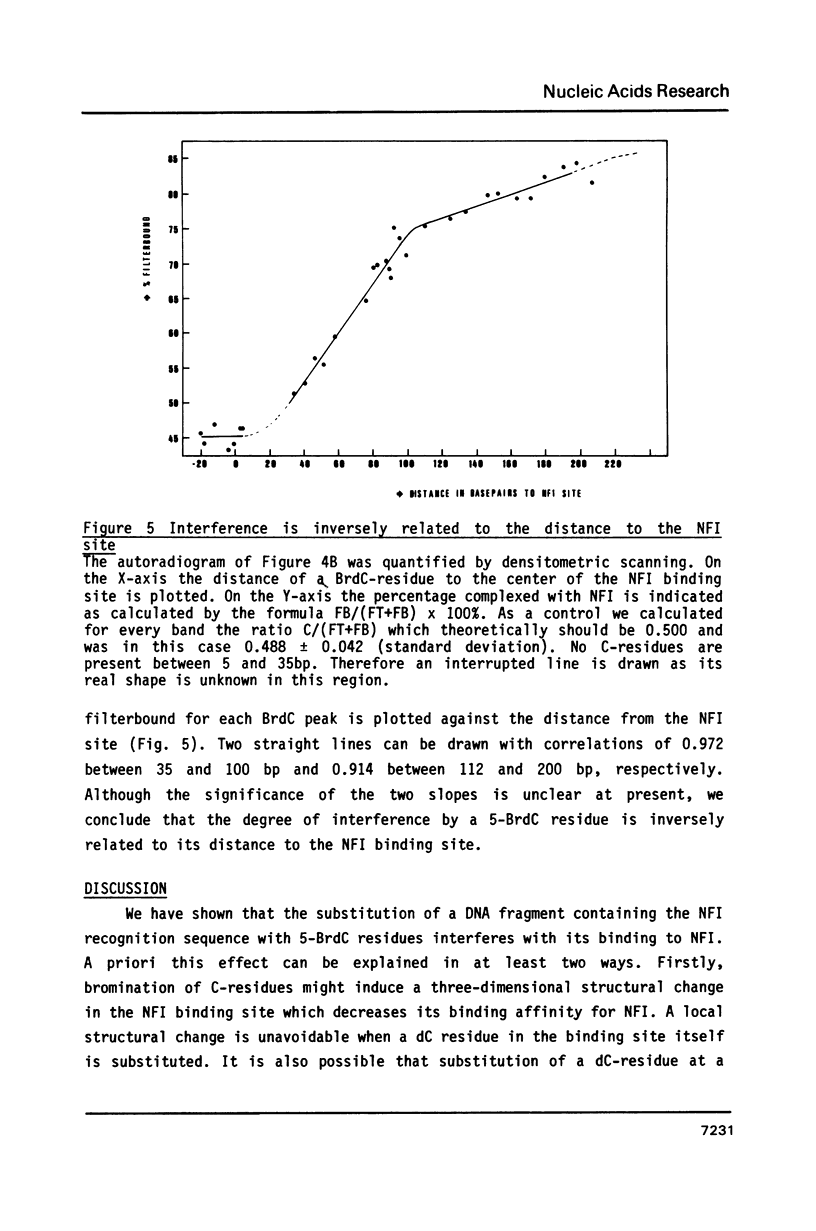
We have studied the binding of nuclear factor 1 (NFI), a human sequence-specific DNA-binding protein, to a DNA fragment substituted in vitro with 5-bromodeoxycytidine (5-BrdC). Even at low substitution grades binding of NFI to its recognition sequence was considerably lower than with the unsubstituted control fragment. We developed a procedure to cleave substituted DNA specifically at a BrdC residue and searched for contacts between NFI and 5-BrdC residues by an interference assay. Surprisingly, no specific contacts were found in or near the recognition sequence. It appeared instead that interference was inversely related to the distance of a 5-BrdC residue from the NFI binding site. Models to explain these results, including a possible sliding mechanism, are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgmeyer U., Nowock J., Sippel A. E. The TGGCA-binding protein: a eukaryotic nuclear protein recognizing a symmetrical sequence on double-stranded linear DNA. Nucleic Acids Res. 1984 May 25;12(10):4295–4311. doi: 10.1093/nar/12.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Purification of a cellular, double-stranded DNA-binding protein required for initiation of adenovirus DNA replication by using a rapid filter-binding assay. Mol Cell Biol. 1986 May;6(5):1363–1373. doi: 10.1128/mcb.6.5.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M., Nagata K., Hurwitz J. Isolation of human DNA sequences that bind to nuclear factor I, a host protein involved in adenovirus DNA replication. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4013–4017. doi: 10.1073/pnas.81.13.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheimer R. A., Stillman B. W., Nagata K., Tamanoi F., Hurwitz J. DNA sequences required for the in vitro replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3069–3073. doi: 10.1073/pnas.81.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T. The origin of adenovirus DNA replication: minimal DNA sequence requirement in vivo. EMBO J. 1985 Feb;4(2):421–426. doi: 10.1002/j.1460-2075.1985.tb03645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Fleckenstein B. Nuclear factor 1 interacts with five DNA elements in the promoter region of the human cytomegalovirus major immediate early gene. EMBO J. 1986 Jun;5(6):1367–1371. doi: 10.1002/j.1460-2075.1986.tb04368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Siebenlist U., Danner D., Leder P., Rawlins D., Rosenfeld P., Kelly T., Jr High-affinity binding site for a specific nuclear protein in the human IgM gene. Nature. 1985 Mar 21;314(6008):289–292. doi: 10.1038/314289a0. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kim J. G., Takeda Y., Matthews B. W., Anderson W. F. Kinetic studies on Cro repressor-operator DNA interaction. J Mol Biol. 1987 Jul 5;196(1):149–158. doi: 10.1016/0022-2836(87)90517-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng M. Left-handed Z-DNA. Biochim Biophys Acta. 1985 Aug 21;825(4):339–344. doi: 10.1016/0167-4781(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Borgmeyer U., Nowock J. Interaction of the TGGCA-binding protein with upstream sequences is required for efficient transcription of mouse mammary tumor virus. EMBO J. 1987 May;6(5):1355–1360. doi: 10.1002/j.1460-2075.1987.tb02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Rosenfeld P. J., Wides R. J., Challberg M. D., Kelly T. J., Jr Structure and function of the adenovirus origin of replication. Cell. 1984 May;37(1):309–319. doi: 10.1016/0092-8674(84)90327-1. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., Kelly T. J. Purification of nuclear factor I by DNA recognition site affinity chromatography. J Biol Chem. 1986 Jan 25;261(3):1398–1408. [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Gessner R. V., van der Marel G. A., van Boom J. H., Rich A. Crystal structure of Z-DNA without an alternating purine-pyrimidine sequence. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3611–3615. doi: 10.1073/pnas.82.11.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wides R. J., Challberg M. D., Rawlins D. R., Kelly T. J. Adenovirus origin of DNA replication: sequence requirements for replication in vitro. Mol Cell Biol. 1987 Feb;7(2):864–874. doi: 10.1128/mcb.7.2.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter R. B., Berg O. G., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 3. The Escherichia coli lac repressor--operator interaction: kinetic measurements and conclusions. Biochemistry. 1981 Nov 24;20(24):6961–6977. doi: 10.1021/bi00527a030. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- de Vries E., van Driel W., Tromp M., van Boom J., van der Vliet P. C. Adenovirus DNA replication in vitro: site-directed mutagenesis of the nuclear factor I binding site of the Ad2 origin. Nucleic Acids Res. 1985 Jul 11;13(13):4935–4952. doi: 10.1093/nar/13.13.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., van Driel W., van den Heuvel S. J., van der Vliet P. C. Contactpoint analysis of the HeLa nuclear factor I recognition site reveals symmetrical binding at one side of the DNA helix. EMBO J. 1987 Jan;6(1):161–168. doi: 10.1002/j.1460-2075.1987.tb04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. H., Berg O. G. On the specificity of DNA-protein interactions. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1608–1612. doi: 10.1073/pnas.83.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]