Abstract
Although perturbation of organic anion transport protein (oatp) cell surface expression can result in drug toxicity, little is known regarding mechanisms regulating its subcellular distribution. Many members of the oatp family, including oatp1a1, have a COOH-terminal PDZ consensus binding motif that interacts with PDZK1, while serines upstream of this site (S634 and S635) can be phosphorylated. Using oatp1a1 as a prototypical member of the oatp family, we prepared plasmids in which these serines were mutated to glutamic acid [E634E635 (oatp1a1EE), phosphomimetic] or alanine [A634A635 (oatp1a1AA), nonphosphorylatable]. Distribution of oatp1a1AA and oatp1a1EE was largely intracellular in transfected human embryonic kidney (HEK) 293T cells. Cotransfection with a plasmid encoding PDZK1 revealed that oatp1a1AA was now expressed largely on the cell surface, while oatp1a1EE remained intracellular. To quantify these changes, studies were performed in HuH7 cells stably transfected with these oatp1a1 plasmids. These cells endogenously express PDZK1. Surface biotinylation at 4°C followed by shift to 37°C showed that oatp1a1EE internalizes quickly compared with oatp1a1AA. To examine a physiological role for phosphorylation in oatp1a1 subcellular distribution, studies were performed in rat hepatocytes exposed to extracellular ATP, a condition that stimulates serine phosphorylation of oatp1a1 via activity of a purinergic receptor. Internalization of oatp1a1 under these conditions was rapid. Thus, although PDZK1 binding is required for optimal cell surface expression of oatp1a1, phosphorylation provides a mechanism for fast regulation of the distribution of oatp1a1 between the cell surface and intracellular vesicular pools. Identification of the proteins and motor molecules that mediate these trafficking events represents an important area for future study.
Keywords: transporter, endocytosis, PDZ, plasma membrane, biotinylation
a major function of the hepatocyte is removal of organic anionic compounds from the circulation (2, 33, 44). These include drugs as well as endogenous substances, such as hormones, bile acids, and bilirubin (2, 33, 42, 44). For the most part, these compounds circulate bound tightly to albumin, but they are cleared rapidly by interaction with specific transporters on the hepatocyte surface (37, 45). A number of hepatocyte plasma membrane transporters have been identified. These include several members of the organic anion transport protein (oatp) family. The initial member of this family was identified and cloned in a Xenopus laevis oocyte expression system on the basis of its ability to mediate transport of the organic anion sulfobromophthalein (20, 21). Subsequently, >20 additional members of the oatp family have been described (17, 18); 4 of these are expressed in the liver (7). The originally identified oatp is now termed oatp1a1. This integral membrane protein has 12 transmembrane domains and is localized to the basolateral (sinusoidal) plasma membrane of hepatocytes (1, 18, 40). It has been used as a prototypical member of the oatp family in studies of mechanism and regulation. Oatp1a1-mediated transport is an electroneutral process in which uptake of an organic anion is accompanied by efflux of HCO3− (25, 32). Previous studies suggested that transport activity of oatp1a1 was markedly reduced in rat hepatocytes following 10 min of incubation in extracellular ATP (5, 15). This was accompanied by serine phosphorylation of the transporter (15). Subsequent study showed that serines at amino acid positions 634 and 635 in the oatp1a1 protein sequence were phosphorylated (46). One or both of these serine residues are conserved in many of the oatp family members.
It was also recognized that members of the oatp family have a highly conserved PDZ consensus binding site at the COOH terminus (7, 41). In most of these cases, the terminal four amino acids that define this binding domain are KTKL, and previous studies with oatp1a1 showed that this sequence mediates binding to PDZK1 (41). Binding to PDZK1 is required for trafficking of oatp1a1 to the plasma membrane. In PDZK1 knockout mice, oatp1a1 accumulates within the cell, but little is found on the hepatocyte plasma membrane (41). Members of the oatp family can mediate uptake of ligands only when they are expressed on the cell surface. Little is known regarding this potential point of functional regulation. The present study examines the role of interaction with PDZK1 in subcellular trafficking of oatp1a1 and the influence of serine phosphorylation of the transporter on this process.
MATERIALS AND METHODS
Antibodies and Reagents
A rabbit antibody recognizing a 13-amino acid sequence near the COOH terminus of oatp1a1 was prepared in rabbits by Covance Research Products (Denver, PA), as previously described (1). A rabbit polyclonal antibody that recognizes the full-length recombinant murine PDZK1 was provided by Dr. David Silver (24). Horseradish peroxidase-conjugated affinity-purified goat anti-rabbit IgG was obtained from Jackson ImmunoResearch (West Grove, PA), and goat anti-mouse IgG was obtained from GE Healthcare Biosciences (Piscataway, NJ). Enhanced chemiluminescence (ECL) substrate (ECL reagent) for Western blotting was obtained from PerkinElmer Life Sciences (Waltham, MA). All other chemicals, unless noted otherwise, were purchased from Sigma-Aldrich (St. Louis, MO).
Plasmids
A monomeric enhanced green fluorescence protein (mEGFP) expression plasmid was kindly provided by Dr. Erik Snapp (Albert Einstein College of Medicine) (35). cDNA encoding rat oatp1a1 or its phosphomimetic (oatp1a1EE) or nonphosphorylatable (oatp1a1AA) derivatives (see below) was inserted into the Bgl II (5′) and Xba I (3′) restriction sites of this plasmid after PCR amplification from a pcDNA3.1-oatp1a1 expression plasmid (41), with 5′-GAAGATCTATGGAAGAAACAGAGAAAAAGATTGCA-3′ used as sense primer and 5′-GCTCTAGATTACAGCTTCGTTTTCAGTTCTCCGTC-3′ used as antisense primer. A mEGFP expression plasmid containing cDNA encoding oatp1a1 lacking its terminal four amino acids (KTKL) was prepared by PCR of the pcDNA 3.1-oatp1a1 plasmid using a similar strategy, in which an early stop codon was introduced into the oatp1a1 sequence by amplification of the template with 5′-GAAGATCTATGGAAGAAACAGAGAAAAAGATTGCA-3′ as sense primer and 5′-GCTCTAGATTACAGCTTCGTTTACAGTTCTCCGTC-3′ as antisense primer. The resulting PCR product was inserted into the Bgl II and Xba I restriction sites of the mEGFP plasmid. In previous studies, we showed that oatp1a1 can be phosphorylated at serines at amino acid positions 634 and 635 (15, 46). Expression plasmids were prepared; in these plasmids, serine residues 634 and 635 were mutagenized to glutamic acid (E634E635), resulting in oatp1a1EE, or alanine (A634A635), resulting in oatp1a1AA. This mutagenesis of the pcDNA3.1-oatp1a1 plasmid was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's directions. All constructs were confirmed by full-length sequencing in the Albert Einstein College of Medicine DNA sequencing facility using appropriate primers. FLAG-linked murine PDZK1 was prepared in the pFLAG-CMV-5c plasmid, as described previously (41).
Cells
Rat hepatocytes.
Male Roman high-avoidance (RHA) rats (200–250 g body wt) bred in the Albert Einstein College of Medicine small animal facility were kindly provided by Dr. Namita RoyChowdhury. Experiments were approved by the institutional animal use committee. Livers were perfused with collagenase type I, and isolated hepatocytes were prepared as described previously (28, 30, 45). Viability as judged by trypan blue exclusion was >90%. After isolation, cells were suspended in Waymouth's 752/1 medium (Sigma) supplemented with 5% heat-inactivated fetal bovine serum (Gemini Bioproducts, Calabasas, CA), 1.7 mM additional CaCl2, 5 μg/ml bovine insulin (Sigma), 50 U/ml penicillin and 50 μg/ml streptomycin (Fisher), and 25 mM HEPES, pH 7.2. Hepatocytes (1.5 × 106) were plated in 60-mm Primaria culture plates (BD Biosciences, Franklin Lakes, NJ). Cells were incubated at 37°C in 5% CO2 atmosphere for 2 h; then the medium was changed, and incubation was continued overnight for ∼18 h.
Cell lines.
HuH7 cells, obtained from the Cell Culture and Genetic Engineering Core of the Marion Bessin Liver Research Center were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma), 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were plated in 60-mm plastic dishes (Falcon, BD Biosciences) and grown to confluence before each experiment unless otherwise indicated. HuH7 cell lines stably expressing wild-type (WT) oatp1a1, oatp1a1EE, or oatp1a1AA with green fluorescence protein (GFP) at the NH2 terminus were prepared. Briefly, HuH7 cells were transfected with GFP-oatp1a1 plasmids using LipofectAMINE PLUS reagent (Invitrogen) according to the manufacturer's protocol and selected for plasmid expression in neomycin (200 μg/ml).
Studies requiring transient expression of oatp1a1 constructs were performed in HEK 293T cells (41) cultured in DMEM (Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Transient transfection of these cells with GFP-oatp1a1 and FLAG-PDZK1 plasmids was performed using FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions.
Assay of oatp1a1 Cell Surface Expression
HEK 293T cells on 60-mm plates were cotransfected with GFP-oatp1a1 expression plasmids and FLAG plasmid or FLAG-PDZK1 plasmid. At 48 h after transfection, cells were washed with ice-cold PBS and then treated with 0.5 mg/ml sulfo-N-hydroxy-succinimide-SS-biotin, a membrane-impermeant biotinylation agent (Pierce, Thermo Fisher Scientific, Rockford, IL) at 4°C for 30 min according to the manufacturer's instructions. Subsequently, the cells were washed three times with ice-cold PBS containing 50 mM NH4Cl and then incubated in the same buffer for 15 min at 4°C to quench unreacted reagent. Cells were harvested, frozen on dry ice, and disrupted with 100 μl of lysis buffer (PBS containing 1% Triton X-100, pH 7.4) containing protease inhibitors (catalog no. P-8340, Sigma) at 4°C for 1 h. Lysate was centrifuged for 20 min at 14,000 rpm in a fixed-angle rotor (model F45-30-11, Eppendorf, Hamburg, Germany) in a desktop centrifuge (model 5810R, Eppendorf) at 4°C. The protein concentration in the supernatant was determined using the bicinchoninic acid protein assay reagent kit (Thermo Scientific) according to the manufacturer's instructions, with BSA used as standard. Streptavidin-agarose beads (50 μl; Pierce) were added to 200 μg of cell lysate protein in 1 ml of lysis buffer with protease inhibitors and incubated for 1 h at room temperature. Beads were washed four times with ice-cold lysis buffer, and biotinylated proteins were released by addition of 2× SDS sample buffer containing 0.2 M DTT and heating for 15 min at 50°C. The eluates were subjected to Western blot analysis for detection of oatp1a1.
Transporter Internalization Assay
For transporter internalization assay, we used a cell surface biotinylation strategy that has been employed to quantify trafficking of occludin to and from the surface of cells in culture (29, 31). HuH7 cells (2 × 106) stably expressing GFP-oatp1a1 were washed three times with ice-cold PBS containing 0.9 mM CaCl2 and 0.33 mM MgCl2 (PBS/CM), pH 7.2. Cells were biotinylated at 4°C for 30 min with 0.5 mg/ml sulfo-NHS-SS-biotin (Pierce) in PBS/CM, washed three times with ice-cold 50 mM NH4Cl in PBS/CM, and incubated again for 15 min at 4°C to quench unreacted reagent. Cells were then incubated at 37°C for 0–120 min in fresh medium to allow internalization of labeled transporter. After this internalization period, biotin on the cell surface was cleaved from protein by incubation in 50 mM 2-mercaptoethanesulfonic acid (MESNA) in 100 mM Tris·HCl (pH 8.6) containing 100 mM NaCl and 2.5 mM CaCl2 at 4°C for 30 min. Unreacted MESNA was quenched with 5 mg/ml iodoacetamide in PBS/CM at 4°C for 15 min. Biotin-linked protein within the cell was recovered following lysis of cells in 1% Triton X-100/PBS containing protease inhibitors (catalog no. P-8340, Sigma) and incubation for 1 h with 60 μl of streptavidin-agarose beads. Recovered oatp1a1 was analyzed by Western blotting, as described above.
Experiments were also performed using rat hepatocytes. A similar protocol was followed, except after biotin was quenched with 50 mM NH4Cl in PBS/CM, cells were washed with buffer containing 135 mM NaCl, 27.8 mM glucose, and 25 mM HEPES, pH 7.2. They were then incubated in this buffer in the presence or absence of 1 mM ATP at 37°C for 0, 10, or 30 min and washed with PBS/CM, and surface biotin was removed with MESNA, as described above.
Coimmunoprecipitation of GFP-oatp1a1 Constructs and FLAG-PDZK1
At 2 days after transfection, HEK 293T cells were washed with ice-cold PBS and lysed with Tris-buffered saline containing 1% CHAPS and protease inhibitors (catalog no. P-8340, Sigma) on ice for 40 min. The lysate was centrifuged for 20 min at 4°C, and the supernatant was incubated overnight at 4°C with anti-FLAG M2 affinity gel (Sigma; 25 μl gel/1 × 106 cells). The gel was washed with lysis buffer, incubated with SDS sample buffer at 95°C, and centrifuged; the supernatant was subjected to Western blot analysis for oatp1a1 and FLAG; and densitometric quantification was performed using ImageJ (National Institutes of Health public domain; http://rsb.info.nih.gov/ij/).
GST pull-down.
As described previously (41), each of the four PDZ binding domains of PDZK1 was amplified by PCR, digested, and cloned into pGEX6p-1 GST expression plasmids using a pcDNA3.1/hygro-PDZK1 plasmid as the PCR template. Each plasmid was transformed into BL21 Escherichia coli (Invitrogen). LB ampicillin (5 ml) was inoculated with GST-PDZ domain-expressing bacteria and grown at 37°C overnight. The culture was then diluted in 50 ml of LB ampicillin and grown to optical density at 600 nm of 0.6–0.8, and 0.1 mM isopropyl 1-thio-β-d-galactopyranoside (Sigma) was added for 4 h to induce protein expression. Bacteria were centrifuged at 6,000 rpm for 15 min at 4°C in a fixed-angle rotor (model F45-30-11). The pellet was resuspended in 10 ml of ice-cold PBS and divided into 1.5-ml aliquots. Each aliquot was centrifuged at 13,000 rpm for 20 min at 4°C, and the pellet was resuspended in 1 ml of lysis buffer (1% Triton X-100 in PBS with 1 mg/ml final concentration lysozyme, 5 mg/ml aprotinin, and 5 mg/ml leupeptin) and incubated on ice for 30 min. The suspension was sonicated four times for 10 s each with 30-s intervals on ice and centrifuged at 12,000 rpm for 15 min, and GSH-linked protein in the supernatant was captured with 100 μl of GSH-agarose gel (GE Healthcare). The gel was washed with lysis buffer and incubated by slow rotation at 4°C for 90 min with cell extract in 1% Triton X-100/PBS containing protease inhibitors. The gel was washed five times with lysis buffer, and proteins were eluted by boiling the beads in 2× SDS sample buffer containing 3% DTT (wt/vol). The eluate was analyzed for oatp1a1 by Western blotting.
Fluorescence Microscopy
HEK 293T cells (5 × 105) were cultured on collagen-coated 35-mm glass-bottom dishes (MatTek, Ashland, MA). At 2 days after transfection with appropriate plasmids, cells were washed twice with PBS and fixed in PBS containing 4% formaldehyde for 10 min at room temperature. After permeabilization with 0.1% Triton X-100 in PBS for 10 min, the cells were washed three times with PBS and then blocked in PBS containing 4% donkey serum and 0.1% Tween for 1 h. Cells that were cotransfected with FLAG-PDZK1 and GFP-oatp1a1 vectors were immunolabeled with rabbit PDZK1 antibody for 1 h. Cells were washed with PBS and exposed to Cy3-labeled donkey antibody to rabbit IgG at a dilution of 1:400 (Jackson ImmunoResearch). After immunolabeling of cells, subcellular localization of each protein was assessed by confocal microscopy in the Analytical Imaging Facility of the Albert Einstein College of Medicine. Immunofluorescence images were captured with a ×60 objective (1.4 numerical aperture) on a confocal microscope (Radiance 2000, Bio-Rad). Fixed cells were excited with a Kr/Ar ion laser source and red diode laser source with excitation at 488, 568, or 638 nm. Fluorescent images were analyzed using ImageJ and Adobe Photoshop version 9.0 (Adobe Systems, San Jose, CA).
Fluorescence Recovery After Photobleaching
HuH7 cells stably expressing GFP-oatp1a1EE or GFP-oatp1a1AA were cultured on glass-bottom dishes (MatTek), washed into serum-free medium (135 mM NaCl, 1.2 mM MgCl2, 0.8 mM MgSO4, 2.5 mM CaCl2, 28 mM d-glucose, and 25 mM HEPES, pH 7.4), and examined using a confocal microscope mounted with a CARV II spinning-disk confocal imager (Crisel Instruments, Rome, Italy) containing a digital fluorescence recovery after photobleaching (FRAP) iris, a iXion back-illuminated 897 electron-multiplying charge-coupled device camera (Andor Technologies, Belfast Ireland), a PhotoFluor metal halide white light source (Chroma Technologies, Bellows Falls, VT), and a ×60 (1.4 numerical aperture) oil immersion lens. The FRAP iris was set to 490 to allow bleaching of an 8-μm-wide vertical band under GFP illumination for exactly 2 min. Images of the plasma membrane ventral surface were acquired under low-light confocal microscopy from 10 to 195 s after photobleaching at 1 frame per 5 s. Mean intensity of ≥10 cells total from ≥3 plates was quantified within a 3 × 7 μm box at the center of the bleached region. Background and maximum for each experiment were measured at an empty region and unbleached neighboring cell region, respectively. Normalized fluorescence intensity was expressed as (fluorescence − background)/(maximum − background) and was fit to the exponential y = y0 + (plateau − y0) * [1 − exp(−k * x)], where plateau is 1, x is time, and k is rate of fluorescence recovery, with use of GraphPad Prism 5 software (La Jolla, CA). Values for background and maximum fluorescence did not differ significantly between the oatp1a1 derivatives.
RESULTS
Influence of Phosphorylation on oatp1a1 Subcellular Distribution
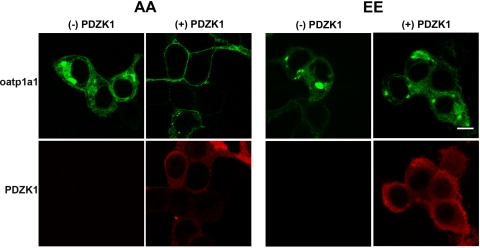
Our previous studies in WT and PDZK1 knockout mice indicated that plasma membrane expression of oatp1a1 in hepatocytes requires the presence of PDZK1 (41). In these studies, total cell expression was normal, but the transporter accumulated within the cell. In other studies, we showed that oatp1a1 can be phosphorylated at serine residues at positions 634 and 635 of the amino acid sequence (46) and that this correlated with reduced transport activity (5, 15). To examine the mechanism by which serine phosphorylation could modulate transport activity, HEK 293T cells were transiently transfected with plasmids encoding GFP-oatp1a1EE and GFP-oatp1a1AA. In these constructs, the GFP group was contiguous with the NH2 terminus of the transporter, so that the PDZ-interacting domain on the COOH terminus was free. As seen in Fig. 1, in the absence of PDZK1, distribution of oatp1a1AA or oatp1a1EE was mainly intracellular. When coexpressed with PDZK1, oatp1a1AA was primarily distributed on the plasma membrane. In contrast, even when coexpressed with PDZK1, oatp1a1EE remained largely intracellular. These results suggest that, in the presence of PDZK1, the phosphorylation state of oatp1a1 is a major determinant of its subcellular distribution.
Fig. 1.
Influence of phosphorylation and interaction with PDZK1 on subcellular distribution of organic anionic transport protein 1a1 (oatp1a1). Human embryonic kidney (HEK) 293T cells were transfected with plasmids encoding nonphosphorylatable (AA) or phosphomimetic (EE) oatp1a1 (oatp1a1AA and oatp1a1EE, respectively) linked to green fluorescence protein (GFP) at the NH2 terminus. Experiments were performed without or with coexpression of PDZK1. After transfection, cells were cultured for 2 days, fixed and permeabilized with 0.1% Triton X-100 in PBS, and incubated with primary antibody to PDZK1 and Cy3-labeled secondary antibody. Distribution of fluorescence was examined by confocal microscopy. Scale bar, 8 μm.
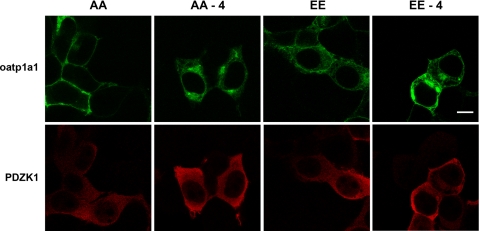
To examine directly the role of interaction with PDZK1 in oatp1a1 subcellular distribution, the plasmids encoding the GFP-linked constructs were mutagenized, so that a stop codon was inserted into the sequence, resulting in protein expressed without the terminal four amino acids. Previous studies showed that, without its terminal four amino acids, oatp1a1 no longer binds to PDZK1 (41). As seen in Fig. 2, expression of these modified oatp1a1 constructs results in intracellular accumulation of the transporter in the presence of PDZK1, irrespective of whether oatp1a1 is in the nonphosphorylatable or phosphomimetic form. These results indicate that optimal expression of oatp1a1 on the plasma membrane requires interaction of its dephosphorylated form with PDZK1.
Fig. 2.
An intact PDZ binding motif is required for cell surface expression of oatp1a1 independent of its phosphorylation state. HEK 293T cells were transfected with plasmids encoding FLAG-PDZK1 as well as full-length or truncated oatp1a1AA or oatp1a1EE linked to GFP at the NH2 terminus. Truncated plasmids, indicated as −4, encoded oatp1a1 without its last 4 amino acids (KTKL), which define its PDZ binding motif. After transfection, cells were cultured for 2 days, fixed and permeabilized with 0.1% Triton X-100 in PBS, and incubated with primary antibody to PDZK1 and Cy3-labeled secondary antibody. Distribution of fluorescence was determined by confocal microscopy. Scale bar, 8 μm.
Influence of Phosphorylation on Binding of oatp1a1 to PDZK1
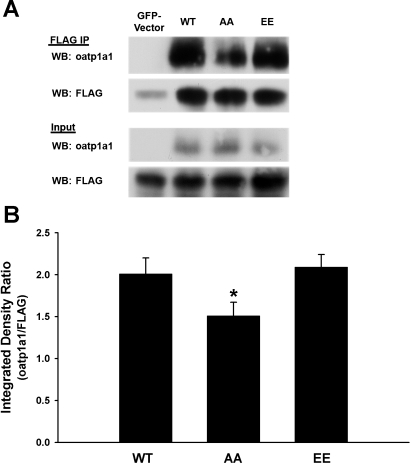
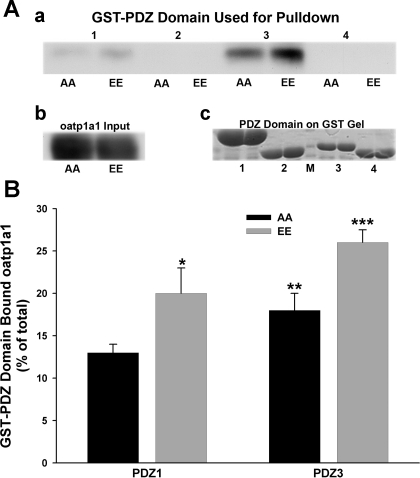
We next asked whether differential interaction with PDZK1 could explain the differences in subcellular distribution that were seen following expression of oatp1a1AA and oatp1a1EE. HEK 293T cells were cotransfected with a plasmid encoding FLAG-PDZK1 and a plasmid encoding WT, nonphosphorylatable, or phosphomimetic forms of GFP-oatp1a1. Oatp1a1 in the FLAG immunoprecipitates was quantified by Western blotting (Fig. 3A). There was no reduction in binding of oatp1a1EE to PDZK1. Rather, there was significantly more oatp1a1EE than oatp1a1AA in the FLAG immunoprecipitate, suggesting greater accessibility or affinity of oatp1a1EE for PDZK1 (Fig. 3B). PDZK1 has four independent PDZ binding domains (22), and we next determined whether they bound oatp1a1 differentially depending on its phosphorylation state. Our previous studies showed that oatp1a1 binds to the first and third PDZ domains of PDZK1 (41). Expression plasmids encoding each of the PDZK1 binding domains were expressed as GST fusion proteins, which were then bound to GSH-agarose gel. Each preparation was incubated with a Triton X-100 extract of transfected HEK 293T cells expressing oatp1a1AA or oatp1a1EE and, after washing, oatp1a1 bound to the gel was quantified by Western blot densitometry. As seen in the representative study in Fig. 4A and in the quantification of three experiments shown in Fig. 4B, only domains 1 and 3 bound oatp1a1, with domain 3 being predominant, consistent with our previous results (41). Each of these two domains bound significantly more oatp1a1EE than oatp1a1AA (Fig. 4B).
Fig. 3.
Influence of phosphorylation on interaction of oatp1a1 with PDZK1. HEK 293T cells were cotransfected with a plasmid encoding FLAG-PDZK1 and a plasmid encoding GFP alone or wild-type (WT), nonphosphorylatable (AA), or phosphomimetic (EE) GFP-oatp1a1 constructs. After transfection, cells were cultured for 2 days and then lysed with 1% CHAPS. Lysates were subjected to immunoprecipitation (IP) using anti-FLAG coupled to agarose beads. Immunoprecipitates were subjected to SDS-PAGE and Western blot analysis for oatp1a1 and FLAG. A: representative Western blot (WB). B: densitometric quantitation of 3 experiments and normalization to immunoprecipitated FLAG-PDZK1. Values are means ± SE. Significantly more oatp1a1EE than oatp1a1AA was in the FLAG immunoprecipitate (*P < 0.04).
Fig. 4.
Influence of phosphorylation on interaction of oatp1a1 with each of the 4 binding domains of PDZK1. A: representative study in which each of the 4 binding domains of PDZK1 was expressed as a GST-fusion protein, bound to GSH-agarose, and incubated with a 1% Triton X-100 extract of HEK 293T cells expressing oatp1a1AA or oatp1a1EE. After multiple washes, bound proteins were eluted into SDS-PAGE sample buffer, and Western blot analysis was performed for oatp1a1 (a). A Western blot representing total oatp1a1 was incubated with agarose beads (b). Equal aliquots of beads used in this experiment were heated in sample buffer, subjected to SDS-PAGE, and stained with Coomassie blue (c). Lanes in this gel correspond to those in the pull-down experiment, with the exception of protein markers run in lane M. B: densitometric quantitation of 3 experiments and normalization to protein input. Values are means ± SE. Significantly more binding of oatp1a1EE than oatp1a1AA to domains 1 and 3: *P < 0.03, PDZ1AA vs. EE; **P < 0.02, PDZ3AA vs. EE; ***P < 0.03 vs. PDZ1EE vs. PDZ3EE.
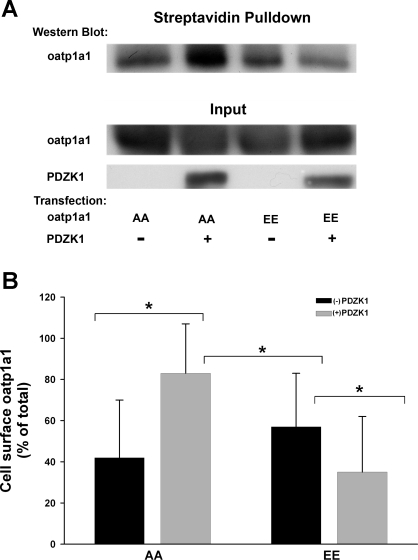
Quantification of oatp1a1 on the Cell Surface
The immunofluorescence studies presented above (Figs. 1 and 2) indicate that optimal plasma membrane distribution of oatp1a1 requires interaction of its unphosphorylated form with PDZK1. In further studies, cell surface localization of oatp1a1 was quantified following labeling of cell surface proteins with a membrane-impermeant biotinylation reagent. These studies were performed using HEK 293T cells that were cotransfected with a plasmid encoding oatp1a1AA or oatp1a1EE and a plasmid encoding FLAG or FLAG-PDZK1. Oatp1a1 on the cell surface was recovered on streptavidin-agarose beads and quantified by densitometry following Western blot analysis for oatp1a1. As seen in Fig. 5, cell surface expression of oatp1a1 was significantly higher in cells coexpressing PDZK1 and oatp1a1AA than in the absence of PDZK1 or in cells expressing oatp1a1EE. Interestingly, cell surface expression of oatp1a1 was significantly lower in cells coexpressing oatp1a1EE and PDZK1 than in cells expressing oatp1a1EE alone (Fig. 5B).
Fig. 5.
Biochemical quantification of cell surface oatp1a1: influence of phosphorylation and interaction with PDZK1. HEK 293T cells were cotransfected with plasmids encoding GFP-oatp1a1AA or GFP-oatp1a1EE and FLAG plasmid or FLAG-PDZK1 plasmid. At 2 days after transfection, cells were biotinylated with membrane-impermeant sulfo-NHS-SS-biotin for 30 min at 4°C. Biotinylated proteins were collected on streptavidin-agarose beads. Proteins bound to the beads were eluted with SDS sample buffer containing 3% DTT, resolved on 10% SDS-PAGE, and subjected to Western blot analysis for oatp1a1. A: representative study. B: densitometric quantitation of 3 experiments and normalization to protein input. Values are means ± SE. These studies are consistent with results found by confocal microscopy (Figs. 1 and 2) but also reveal significantly less expression of oatp1a1 on the cell surface with coexpression of GFP-oatp1a1EE with PDZK1 than in the absence of PDZK1 (*P < 0.03).
Quantification of Internalization of Cell Surface oatp1a1
The preceding experiments show that oatp1a1EE is largely located within the cell compared with oatp1a1AA. This could be due to reduced rate of trafficking to the cell surface or increased rate of internalization of transporter that is on the cell surface. To study this latter possibility, cell surface proteins were biotinylated with a membrane-impermeant biotinylation reagent at 4°C, as in the preceding experiments. In this reagent, biotin is attached via a disulfide bond that can be cleaved by reduction with MESNA (see materials and methods). In these studies, HuH7 cells stably expressing GFP-oatp1a1 constructs were used. These cells express endogenous PDZK1 (11) but do not express oatp1a1 (data not shown). After washout of unbound reagent, cells were warmed to 37°C for up to 120 min. They were then returned to 4°C, and biotin remaining on the cell surface was removed by reduction, leaving only biotinylated protein that had been internalized and inaccessible to reducing agent. Internalized biotinylated proteins were recovered on streptavidin-agarose beads, and oatp1a1 was quantified from Western blot. As seen in the representative study in Fig. 6A and in the densitometric results of four experiments in Fig. 6B, internalization of oatp1a1EE was substantially greater than internalization of oatp1a1AA.
Fig. 6.
Influence of phosphorylation on internalization of cell surface GFP-oatp1a1 in stably transfected HuH7 cells. HuH7-derived cell lines stably expressing GFP-oatp1a1AA or GFP-oatp1a1EE were prepared. These cells constitutively express PDZK1. Cells were surface biotinylated with membrane-impermeant sulfo-NHS-SS-biotin for 30 min at 4°C and then incubated at 37°C for up to 120 min to allow internalization. After removal of residual biotin from the cell surface by reduction, internalized biotinylated GFP-oatp1a1 was collected on streptavidin-agarose beads and subjected to immunoblot for oatp1a1. A: representative experiment. B: results of densitometric quantitation of 4 individual experiments. Data were normalized to total starting cell surface biotinylated oatp1a1. Lines are drawn through means at each time. Open symbols, oatp1a1AA; filled symbols, oatp1a1EE.
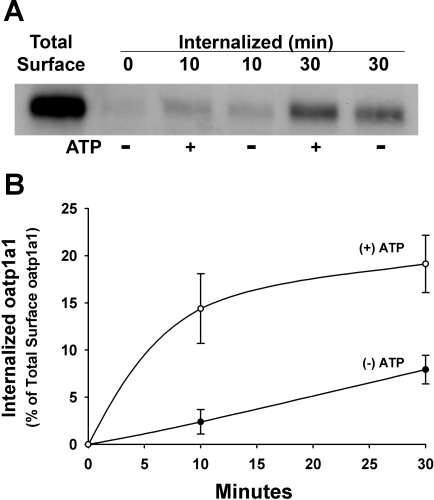
Phosphorylation-Dependent Trafficking of oatp1a1 in Rat Hepatocytes
The studies presented above were performed in cell lines transfected with mutagenized oatp1a1 constructs. To see if these results are applicable to events that occur in vivo, we took advantage of our previously described model in which a short incubation of rat hepatocytes in extracellular ATP results in serine phosphorylation of oatp1a1 and reversible downregulation of its transport activity (5, 15, 46). We determined in these studies whether there was increased internalization of phosphorylated oatp1a1, as was observed in the studies presented above. Overnight-cultured rat hepatocytes were surface-labeled with biotin, as described above. They were then incubated at 37°C with or without 1 mM ATP for 10 or 30 min, and internalized oatp1a1 was assayed as described above. As seen in Fig. 7, although a small baseline of internalization of oatp1a1 occurred under control conditions, there was a rapid stimulation of internalization in ATP-treated cells.
Fig. 7.
Influence of phosphorylation on internalization of cell surface oatp1a1 in overnight-cultured rat hepatocytes. Hepatocytes were isolated from rat liver and cultured overnight, surface biotinylated with membrane-impermeant sulfo-NHS-SS-biotin for 30 min at 4°C, and then incubated at 37°C for 10 or 30 min in the absence (−) or presence (+) of 1 mM ATP. Previous studies showed that this short incubation of rat hepatocytes in extracellular ATP stimulates serine phosphorylation of oatp1a1 via activity of a purinergic receptor. After removal of residual biotin from the cell surface by reduction, internalized biotinylated oatp1a1 was collected on streptavidin-agarose beads and subjected to immunoblot for oatp1a1. A: representative study. B: densitometric quantitation of 3 experiments. Data were normalized to total starting cell surface biotinylated oatp1a1. Values are means ± SE.
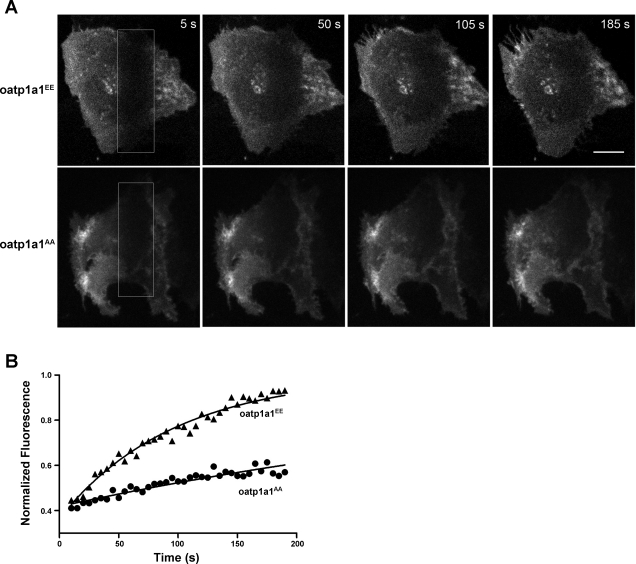
Effect of Phosphorylation on Mobility of oatp1a1 in Cells
The preceding studies indicate that phosphorylation of oatp1a1 results in more rapid retrieval of oatp1a1 from the plasma membrane (Figs. 6 and 7). Additional confocal microscopy-based FRAP experiments were performed to determine whether phosphorylation of oatp1a1 results in increased mobility within cells (Fig. 8). In these studies, the plasma membrane ventral surface of GFP-oatp1a1-expressing cells was photobleached for 2 min, and time-lapse images were acquired for an additional 195 s under low-light spinning-disk confocal microscopy. The rate of fluorescence recovery within the bleached region was used to indicate the mobility of oatp1a1 within the cell. Representative experiments are presented in Fig. 8A. At the start of imaging, the photobleached region is visible as a dark, vertical stripe on the right side of the image field (boxed region). Over time, fluorescence increases within the region due to the movement of unbleached GFP-oatp1a1 from elsewhere in the cell. Individual FRAP experiments were fit by least squares to a single-exponential curve (see materials and methods). Quantitative data for the experiments in Fig. 8A (Fig. 8B) show a higher rate of recovery after photobleaching for oatp1a1EE- than oatp1a1AA-expressing cells, with mean rate constants (k) of 0.48 ± 0.07 and 0.30 ± 0.04 min−1 for oatp1a1EE and oatp1a1AA, respectively (P < 0.05).
Fig. 8.
Mobility of GFP-oatp1a1 in stably transfected HuH7 cells as determined by fluorescence recovery after photobleaching. A: confocal images showing living cells undergoing recovery from photobleaching. Enclosed areas (white boxes) were photobleached, and oatp1a1AA- and oatp1a1EE-expressing cells were imaged for 195 s. Scale bar, 8 μm. B: normalized fluorescence within the recovering region from experiments in A as a function of time. Exponential curves were fit to data for studies in cells expressing oatp1a1AA (●) and oatp1a1EE (▴).
DISCUSSION
Perturbation of drug transporters in the liver can produce significant variation in drug clearance and metabolism among individuals (9). In particular, a number of recent studies have shown that members of the oatp family play important roles in xenobiotic transport and metabolism and that alteration of their function can have substantial consequences to health (6, 27, 47). Although transporters such as members of the oatp family can be seen within the cell, the fraction that resides on the plasma membrane is required to mediate cellular ligand uptake. Mechanisms that influence the net distribution of transporter between intracellular and plasma membrane locations can be important regulators of their transport activity. In the case of members of the oatp family, this has been shown in a genome-wide association study that examined genetic variants in patients who developed myopathy while taking simvastatin. In this study, a strong association with toxicity was obtained only with a polymorphism in the gene encoding OATP1B1 (26). This polymorphism (Val174Ala) encodes a protein variant that traffics poorly to the plasma membrane and accumulates within cells (39). Patients with this polymorphism have reduced liver uptake of the drug with correspondingly increased levels in the circulation, which can lead to muscle toxicity.
Little is known regarding mechanisms by which members of the oatp family traffic through the cell, and this was the focus of the present study, in which oatp1a1 was used as a prototypical member of the oatp family of transporters. Since the initial description of this first oatp, >20 additional members of the oatp family have been described (17, 18). These proteins have a high degree of amino acid homology (18) as well as overlap of transported substrates. Several members of the oatp family, including oatp1a1, are highly expressed in hepatocytes from rats, mice, and humans, where they are localized primarily to the basolateral plasma membrane (18, 47). All members of the oatp family are hydrophobic glycoproteins with 12 transmembrane domains (43), and most have PDZ consensus binding sites at their COOH termini (41). In previous studies, we showed that the last four amino acids (KTKL) of oatp1a1 represent a PDZ consensus binding site and that PDZK1 is the PDZ binding protein with which they interact (41). In the PDZK1 knockout mouse, oatp1a1 accumulates in intracellular vesicles, with little expression on the cell surface (41). In addition, we previously showed that oatp1a1 transport activity is rapidly downregulated by phosphorylation (5, 15) of serines at positions 634 and 635 (46). These serine residues are conserved throughout the oatp family. Although it might be speculated that phosphorylation of the transporter could alter its binding to PDZK1, leading to altered trafficking, this was not found in the present study. Phosphorylation of other PDZ domain-containing proteins has been described as altering their interaction with their binding proteins (36, 38). However, unlike oatp1a1, where the site of phosphorylation is 31 amino acids upstream of the PDZ binding consensus site, these other proteins are phosphorylated within the consensus site.
To examine the role of oatp1a1 phosphorylation and interaction with PDZK1 in its subcellular distribution, oatp1a1 expression plasmids encoding oatp1a1EE and oatp1a1AA were used. As seen in Figs. 1 and 2, coexpression with PDZK1 enhances surface localization of oatp1a1AA in transfected HEK 293T cells. In contrast, there is substantially less plasma membrane localization of oatp1a1EE, even in the presence of PDZK1 (Fig. 5). This is not due to reduced interaction with PDZK1; if anything, oatp1a1EE binds more readily than oatp1a1AA to PDZK1 (Figs. 3 and 4). Of interest is the fact that phosphorylated oatp1a1 on the cell surface is more mobile than unphosphorylated oatp1a1. This is reflected in a much higher internalization rate of oatp1a1EE than oatp1a1AA (Fig. 6), as well as more rapid recovery following fluorescence bleaching of the cell surface (Fig. 8). These results indicate that interaction with PDZK1 is essential for optimal cell surface expression of oatp1a1. Phosphorylation of the transporter is sufficient to obviate this pathway, resulting in substantial intracellular accumulation of oatp1a1, even in the presence of PDZK1. The mechanism mediating this effect of phosphorylation on trafficking of oatp1a1 is not known.
The fact that oatp1a1EE binds more readily to PDZK1 but that its retrieval rate from the cell surface is greater than that of oatp1a1AA (Fig. 6) suggests involvement of other interacting proteins, as has been described in regulation of the trafficking of the CFTR (14). It is possible that oatp1a1 phosphorylation alters this interaction, resulting in reduced plasma membrane localization. Several studies have described complexes of proteins, including syntaxin 16, small GTPases such as Sar1 and Arf1, and PDZ domain proteins such as NHERF-1 and PDZK1, that regulate trafficking and subcellular localization of CFTR (3, 16). The high-density lipoprotein receptor scavenger receptor class B, type I (SR-BI), also binds to PDZK1 through its COOH terminus (19, 34), and this interaction is required for trafficking of SR-BI to the cell surface (34). In contrast to results for oatp1a1, expression of SR-BI is reduced by 95% in PDZK1 knockout mice (23). Interestingly, although the COOH terminus of SR-BI binds to only the first PDZ domain of PDZK1 (19), overexpression in PDZK1 knockout mice of a truncated mutant transgene of PDZK1, primarily encoding the PDZ1 domain, did not restore SR-BI cell surface localization or function (13). All four PDZ domains were required to fully restore the abundance, localization, and function of SR-BI (12). Moreover, hepatic overexpression of the PDZ1 domain in WT mice resulted in the mislocalization of SR-BI to cytoplasm (13), suggesting that as-yet-unidentified proteins bind to the three other PDZ domains of PDZK1, stabilizing SR-BI at the cell surface. Aside from these functions, PDZK1 also plays a role in normal expression and subcellular localization of MRP2 (ABCC2) (10), the glutamate transporter (EEAC1) (8), and the Na+/H+ exchanger 3 (NHE3) (4).
In a previous study (15), we incubated rat hepatocytes in ATP for 10 min and then subjected them to surface biotinylation with a membrane-impermeant reagent to quantify cell surface oatp1a1 after ATP exposure. A cell lysate was prepared from which oatp1a1 was immunoprecipitated, and biotinylated oatp1a1 in the immunoprecipitate was quantified by blotting with horseradish peroxidase-conjugated avidin. We did not find a difference in recovered biotinylated oatp1a1 in these experiments. However, we know from the present study that there is an intracellular pool of oatp1a1 that could traffic to the plasma membrane to replace phosphorylated protein that has been internalized. In addition, there could be a fraction of internalized phosphorylated transporter that becomes dephosphorylated and returns to the cell surface. Consistent with this latter point is the finding in our previous study of a small (12%) reduction in cell surface oatp1a1 following incubation of hepatocytes in the phosphatase inhibitor okadaic acid. In contrast, the experiment represented in Fig. 7 of the present study was designed to quantify directly the internalization of cell surface oatp1a1 following exposure of rat hepatocytes to ATP. In this study, oatp1a1 on the cell surface was biotinylated prior to exposure to ATP. At 10 min after removal of cell surface biotin, ∼10–15% of biotinylated oatp1a1 was found to have been internalized. Unlike the previous study, recovery of biotinylated oatp1a1 in the present study represents only oatp1a1 within the cell; biotin bound to oatp1a1 that trafficked to the cell surface during the 10-min ATP exposure would be removed during the reduction with MESNA. In addition, if there were some recycling of internalized biotinylated oatp1a1 back to the cell surface, this would only serve to reduce the recovery of that which was within the cell.
The present study shows that although PDZK1 binding is required for optimal cell surface expression of oatp1a1, this effect can be modulated by the phosphorylation state of the transporter. As phosphorylation of oatp1a1 can occur rapidly (15), this provides a mechanism for fast regulation of the distribution of oatp1a1 between the cell surface and intracellular vesicular pools. This rapid movement of oatp1a1 to and from the plasma membrane has been documented in the experiments presented above. The proteins and motor molecules that mediate these trafficking events are unknown, but they represent an important area for future study.
GRANTS
This work was supported by National Institutes of Health Research Grants DK-23026 and DK-41296 and Medical Scientist Training Program Grant T32 GM-007288.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Pijun Wang for providing oatp1a1 plasmids used for PCR.
REFERENCES
- 1. Bergwerk AJ, Shi X, Ford AC, Kanai N, Jacquemin E, Burk RD, Bai S, Novikoff PM, Stieger B, Meier PJ, Schuster VL, Wolkoff AW. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am J Physiol Gastrointest Liver Physiol 271: G231–G238, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Bernstein LH, Ezzer JB, Gartner L, Arias IM. Hepatic intracellular distribution of tritium-labeled unconjugated and conjugated bilirubin in normal and Gunn rats. J Clin Invest 45: 1194–1201, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilan F, Nacfer M, Fresquet F, Norez C, Melin P, Martin-Berge A, Costa de Beauregard MA, Becq F, Kitzis A, Thoreau V. Endosomal SNARE proteins regulate CFTR activity and trafficking in epithelial cells. Exp Cell Res 314: 2199–2211, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Broere N, Chen M, Cinar A, Singh AK, Hillesheim J, Riederer B, Lunnemann M, Rottinghaus I, Krabbenhoft A, Engelhardt R, Rausch B, Weinman EJ, Donowitz M, Hubbard A, Kocher O, de Jonge HR, Hogema BM, Seidler U. Defective jejunal and colonic salt absorption and altered Na+/H+ exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflügers Arch 457: 1079–1091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell CG, Spray DC, Wolkoff AW. Extracellular ATP4− modulates organic anion transport by rat hepatocytes. J Biol Chem 268: 15399–15404, 1993 [PubMed] [Google Scholar]
- 6. Chen C, Stock JL, Liu X, Shi J, Van Deusen JW, DiMattia DA, Dullea RG, de Morais SM. Utility of a novel Oatp1b2 knockout mouse model for evaluating the role of Oatp1b2 in the hepatic uptake of model compounds. Drug Metab Dispos 36: 1840–1845, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Choi JH, Murray JM, Wolkoff AW. Hepatocyte basolateral membrane organic anion transporters. In: The Liver Biology and Pathobiology, edited by Arias IM, Alter HJ, Boyer JL, Cohen DE, Fausto N, Shafritz DA, Wolkoff AW. Chichester, UK: Wiley-Blackwell, 2009, p. 305–321 [Google Scholar]
- 8. D'Amico A, Soragna A, Di Cairano E, Panzeri N, Anzai N, Vellea Sacchi F, Perego C. The surface density of the glutamate transporter EAAC1 is controlled by interactions with PDZK1 and AP2 adaptor complexes. Traffic 11: 1455–1470, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Degorter MK, Kim RB. Hepatic drug transporters, old and new: pharmacogenomics, drug response, and clinical relevance. Hepatology 50: 1014–1016, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Emi Y, Nomura S, Yokota H, Sakaguchi M. ATP-binding cassette transporter isoform C2 (ABCC2) localizes to the apical plasma membrane via interactions with PDZK1 scaffolding protein. J Biochem In press [DOI] [PubMed] [Google Scholar]
- 11. Eyre NS, Drummer HE, Beard MR. The SR-BI partner PDZK1 facilitates hepatitis C virus entry. PLoS Pathog 10: e1001130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenske SA, Yesilaltay A, Pal R, Daniels K, Barker C, Quinones V, Rigotti A, Krieger M, Kocher O. Normal hepatic cell surface localization of the high density lipoprotein receptor, scavenger receptor class B, type I, depends on all four PDZ domains of PDZK1. J Biol Chem 284: 5797–5806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fenske SA, Yesilaltay A, Pal R, Daniels K, Rigotti A, Krieger M, Kocher O. Overexpression of the PDZ1 domain of PDZK1 blocks the activity of hepatic scavenger receptor, class B, type I by altering its abundance and cellular localization. J Biol Chem 283: 22097–22104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gee HY, Tang BL, Kim KH, Lee MG. Syntaxin 16 binds to CFTR and regulates its membrane trafficking in epithelial cells. J Biol Chem 285: 35519–35527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glavy JS, Wu SM, Wang PJ, Orr GA, Wolkoff AW. Down-regulation by extracellular ATP of rat hepatocyte organic anion transport is mediated by serine phosphorylation of oatp1. J Biol Chem 275: 1479–1484, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Arch 447: 653–665, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta 1609: 1–18, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Ikemoto M, Arai H, Feng D, Tanaka K, Aoki J, Dohmae N, Takio K, Adachi H, Tsujimoto M, Inoue K. Identification of a PDZ-domain-containing protein that interacts with the scavenger receptor class B type I. Proc Natl Acad Sci USA 97: 6538–6543, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na+-independent organic anion transporter. Proc Natl Acad Sci USA 91: 133–137, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression of the hepatocellular chloride-dependent sulfobromophthalein uptake system in Xenopus laevis oocytes. J Clin Invest 88: 2146–2149, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kocher O, Comella N, Tognazzi K, Brown LF. Identification and partial characterization of PDZK1: a novel protein containing PDZ interaction domains. Lab Invest 78: 117–125, 1998 [PubMed] [Google Scholar]
- 23. Kocher O, Yesilaltay A, Cirovic C, Pal R, Rigotti A, Krieger M. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J Biol Chem 278: 52820–52825, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Lan D, Silver DL. Fenofibrate induces a novel degradation pathway for scavenger receptor B-I independent of PDZK1. J Biol Chem 280: 23390–23396, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol 296: C570–C582, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 359: 789–799, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Lu H, Choudhuri S, Ogura K, Csanaky IL, Lei X, Cheng X, Song PZ, Klaassen CD. Characterization of organic anion transporting polypeptide 1b2-null mice: essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol Sci 103: 35–45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Min AD, Johansen KL, Campbell CG, Wolkoff AW. Role of chloride and intracellular pH on the activity of the rat hepatocyte organic anion transporter. J Clin Invest 87: 1496–1502, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem 280: 2220–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Neufeld DS. Isolation of rat liver hepatocytes. Methods Mol Biol 75: 145–151, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Nishimura N, Sasaki T. Cell-surface biotinylation to study endocytosis and recycling of occludin. Methods Mol Biol 440: 89–96, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Satlin LM, Amin V, Wolkoff AW. Organic anion transporting polypeptide mediates organic anion/HCO3− exchange. J Biol Chem 272: 26340–26345, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Scharschmidt BF, Waggoner JG, Berk PD. Hepatic organic anion uptake in the rat. J Clin Invest 56: 1280–1292, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silver DL. A carboxyl-terminal PDZ-interacting domain of scavenger receptor B, type I is essential for cell surface expression in liver. J Biol Chem 277: 34042–34047, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Snapp EL, Hegde RS, Francolini M, Lombardo F, Colombo S, Pedrazzini E, Borgese N, Lippincott-Schwartz J. Formation of stacked ER cisternae by low affinity protein interactions. J Cell Biol 163: 257–269, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein EL, Chetkovich DM. Regulation of stargazin synaptic trafficking by C-terminal PDZ ligand phosphorylation in bidirectional synaptic plasticity. J Neurochem 113: 42–53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stollman YR, Gartner U, Theilmann L, Ohmi N, Wolkoff AW. Hepatic bilirubin uptake in the isolated perfused rat liver is not facilitated by albumin binding. J Clin Invest 72: 718–723, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tandon C, De Lisle RC, Boulatnikov I, Naik PK. Interaction of carboxyl-terminal peptides of cytosolic-tail of apactin with PDZ domains of NHERF/EBP50 and PDZK-1/CAP70. Mol Cell Biochem 302: 157–167, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 276: 35669–35675, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol 294: G1052–G1059, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Wang P, Wang JJ, Xiao Y, Murray JW, Novikoff PM, Angeletti RH, Orr GA, Lan D, Silver DL, Wolkoff AW. Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J Biol Chem 280: 30143–30149, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Wolkoff AW. Mechanisms of hepatocyte organic anion transport. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD. San Diego, CA: Elsevier Academic, 2006, p. 1463–1481 [Google Scholar]
- 43. Wolkoff AW. Hepatocellular sinusoidal membrane organic anion transport and transporters. Semin Liver Dis 16: 121–127, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Wolkoff AW, Ketley JN, Waggoner JG, Berk PD, Jakoby WB. Hepatic accumulation and intracellular binding of conjugated bilirubin. J Clin Invest 61: 142–149, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolkoff AW, Samuelson AC, Johansen KL, Nakata R, Withers DM, Sosiak A. Influence of Cl− on organic anion transport in short-term cultured rat hepatocytes and isolated perfused rat liver. J Clin Invest 79: 1259–1268, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao Y, Nieves E, Angeletti RH, Orr GA, Wolkoff AW. Rat organic anion transporting protein 1A1 (Oatp1a1): purification and phosphopeptide assignment. Biochemistry 45: 3357–3369, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zaher H, zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, Palandra J, Stock JL, Kim RB, Ware JA. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol 74: 320–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]