Summary
The genome of Aspergillus fumigatus encodes two isoforms of the catalytic subunit of the cAMP-dependent Protein Kinase (PKA). Although deletion of the class I isoform, pkaC1, leads to an attenuation of virulence, the function of the class II subunit, PkaC2, was previously uninvestigated. In this report, we demonstrate that both isoforms act in concert to support various physiologic processes that promote the virulence of this pathogen. Whereas pkaC1 and pkaC2 single-deletion mutants display wild-type conidial germination, a double-deletion mutant is delayed in germination in response to environmental nutrients. Furthermore, PkaC1 and PkaC2 interact to positively regulate flux through the carbohydrate catabolic pathway and, consequently, the ΔpkaC1ΔpkaC2 mutant is unable to grow on low glucose concentrations. Importantly, the reduced germinative capacity and inability to utilize glucose observed for the ΔpkaC1ΔpkaC2 strain correlated with an inability of the mutant to establish infection in a murine model. Conversely, overexpression of pkaC2 both promotes the in vitro growth on glucose, and restores the fungal burden and mortality associated with the ΔpkaC1 to that of the wild-type organism. Taken together, these data demonstrate the functional capacity of pkaC2 and emphasize the importance of PKA-mediated metabolic control in the pathogenic potential of A. fumigatus.
Introduction
Aspergillus fumigatus has emerged as the predominant mould pathogen among severely immunocompromised patients (Latge, 1999; Upton et al., 2007). Current evidence suggests that the clinical prevalence of A. fumigatus is related to attributes that have evolved in the fungus that promote its survival in its ex vivo niche of the compost. This environment confronts the fungus with a variety of stresses: elevated temperatures, changes in pH, nutritional limitations and fluctuations in oxygen and carbon dioxide levels, as examples (Beffa et al., 1998; van Heerden et al., 2002; Askew, 2008). As these stresses are also present within the host, the adaptability of A. fumigatus translates into its success as an opportunistic pathogen. Accordingly, understanding the signalling pathways that detect these environmental conditions and mediate appropriate physiological responses will provide significant insights into the virulence of this organism.
The cAMP-dependent Protein Kinase A (PKA) pathway is a key regulator of fungal biology, and in many cases, virulence (Adachi and Hamer, 1998; Dürrenberger et al., 1998; Yang and Dickman, 1999; D’Souza and Heitman, 2001; D’Souza et al., 2001; Chen et al., 2007). The central core of the pathway is well conserved among all eukaryotes, with the holoenzyme consisting of two catalytic subunits that are bound and inactivated by a dimer of regulatory subunits. Activation occurs after the second messenger, cAMP, binds to the regulatory subunits, thereby inducing a conformational change that releases the active kinases. In the model yeast Saccharomyces cerevisiae, nutrients, particularly fermentable sugars, are important stimulators of the pathway (Thevelein et al., 2005). Downstream targets of PKA include proteins that fall within a variety of functional groups, including enzymes, transcriptional factors, and other signalling effectors that co-ordinately regulate fungal metabolism, morphogenesis and stress response. In this way, PKA is strategically positioned to drastically alter cellular physiology in response to diverse extracellular cues.
In an early effort to identify A. fumigatus genes involved in virulence, we analysed patterns of fungal gene expression following co-culture with human endothelial and epithelial cells (Rhodes et al., 2001; Oliver et al., 2002a). These studies revealed an upregulation of core components of the PKA pathway, including the catalytic subunit gene, pkaC1. Deletion of pkaC1 resulted in attenuation of virulence in a murine model of invasive aspergillosis (IA), demonstrating an essential role for PKA signalling in pathogenesis (Liebmann et al., 2004).
Despite the advances, the contribution of PKA to the virulence of A. fumigatus remains incompletely understood. The ΔpkaC1 mutant is growth impaired in vitro (Liebmann et al., 2004), which may partially explain the attenuated virulence of the strain. But other studies in A. fumigatus suggest that a decreased in vitro growth rate cannot fully explain the virulence of other mutants in the PKA pathway. For example, GpaB is the Gα subunit of a heterotrimeric G–protein complex that stimulates adenylate cyclase (AcyA) to produce cAMP. As expected, deletion of either gpaB or acyA leads to a loss of detectable PKA activity in A. fumigatus (Liebmann et al., 2003). However, although both mutants were severely attenuated for virulence, only the ΔacyA strain showed a reduction in growth rate in vitro (Liebmann et al., 2003; Liebmann et al., 2004). This implies that PKA signalling governs specific physiologic requirements for growth within the host environment.
Analysis of PKA signalling in fungi is complicated by the fact that most organisms contain two or more genes encoding a PKA catalytic subunit. For instance, S. cerevisiae encodes three PKA catalytic isoforms, Tpk1p, 2p and 3p. Signalling through any one of the isoforms is sufficient to support normal growth of the organism, whereas deletion of all three is synthetically lethal (Toda et al., 1987). In addition to their functional redundancy, phenotypic and transcriptional analyses have revealed non-overlapping roles for each of these genes. Tpk2p positively regulates pseudohyphal filamentation, whereas Tpk1p and Tpk3p are repressive in this respect (Robertson and Fink, 1998; Pan and Heitman, 1999). Moreover, Tpk2p uniquely downregulates a variety of genes involved in high-affinity iron uptake, and Tpk1p is the sole isoform involved in the biosynthesis of branched chain amino acids that function in mitochondrial homeostasis. In this regard, Tpk1p and Tpk2p play opposite roles in regulating processes involved in the maintenance of respiratory growth (Robertson et al., 2000).
Despite the independent functionalities associated with the PKA catalytic isoforms of yeast, the three proteins share a high degree of sequence identity (Robertson and Fink, 1998). This is in contrast with the filamentous fungi, in which the PKA isoforms are more evolutionarily divergent from one another. For example, Aspergillus nidulans encodes two catalytic subunits, PkaA and PkaB (Ni et al., 2005). PkaA demonstrates high homology to the TPK proteins of S. cerevisiae, and together, they form a phylogenetic group called the class I PKAs. However, pkaB and its homologues form a separate phylogenetic group of PKA-related kinases, the class II PKAs, which are uniquely found in filamentous fungi (Ni et al., 2005). The lack of appreciable phenotypes associated with class II deletion mutants, as exemplified by ΔpkaB in A. nidulans, is in strong contrast to the significant growth and developmental defects imparted by the deletion of class I isoforms (Dürrenberger et al., 1998; Ni et al., 2005). However, deletion of both pkaA and pkaB in A. nidulans is synthetically lethal, confirming that the class II gene retains some important function. Moreover, differential roles between the two catalytic isoforms with regard to germination, asexual development and oxidative stress response have been described (Ni et al., 2005). These findings underscore the importance of studying both PKA isoforms in order to obtain a comprehensive understanding of PKA signalling within filamentous fungi.
In this study, we report the first functional characterization of the class II PKA, PkaC2, in A. fumigatus. Our data demonstrate that, although PkaC2 activity alone is largely redundant to that of PkaC1, both isoforms act in concert to regulate germination, cell wall homeostasis and growth on fermentable carbon substrates. Importantly, these PkaC2-controlled processes contribute to the pathogenic potential of A. fumigatus, as deletion of pkaC2 eliminates any residual virulence associated with ΔpkaC1, and overexpression of pkaC2 fully restores virulence to the ΔpkaC1 mutant. Taken together, these data underscore the importance of PKA signalling to the expression of virulence-related attributes of A. fumigatus that facilitate its adaptation to, and growth within the host.
Results
A. fumigatus genome encodes two PKA catalytic subunits that are not essential for viability
Aspergillus fumigatus, like other filamentous fungi, contains two PKA catalytic genes within its genome, pkaC1 and pkaC2. PkaC1 is the canonical class I PKA, the homologues of which have been studied in multiple species and shown to play a role in A. fumigatus growth and virulence (Liebmann et al., 2004). In comparison, pkaC2 encodes a member of the class II PKA subunits found uniquely in filamentous fungi, the function of which is not yet known in this fungal pathogen.
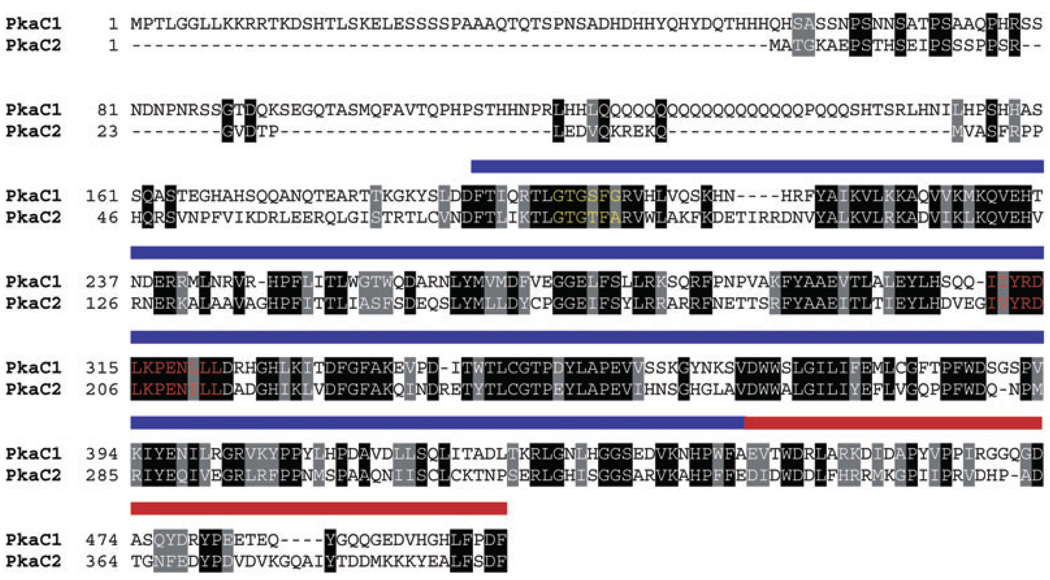
Sequence analysis of the pkaC2 cDNA identified three exons spanning 1188 base pairs, confirming the genomic organization predicted in the GenBank database (data not shown, Accession No. AJ575357.1 and Q70KQ0). The PkaC2 protein contains a typical serine/threonine kinase domain as well as a C-terminal AGC domain, which is representative of a large family of kinases that includes PKA (Pearce et al., 2010) (Fig. 1). The PkaC1 and PkaC2 proteins share approximately 32% sequence identity overall, with the highest degree of divergence existing at the N-terminal region. PkaC1 and its class I homologues in filamentous fungi contain N-terminal extensions that are absent in the class II PKAs. As would be expected, higher degrees of conservation between the two isoforms exist across the two kinase domains, with approximately 48% identity and 65% similarity.
Fig. 1. Sequence analysis of PkaC1 versus PkaC2.
The protein sequences of PkaC1 and PkaC2 were aligned using DNAMAN software (Lynnon Corp. Canada) using default parameters and shaded using BOXSHADE 3.21. The blue and red bars span the conserved serine/threonine kinase domain and the conserved AGC domain respectively. The catalytic active site is highlighted in red font and the ATP-binding loop is highlighted in yellow font.
To determine the relative contributions of PkaC1 and PkaC2 to the biology and virulence of A. fumigatus, deletion mutants of each gene were constructed by homologous replacement with the hygromycin and phleomycin resistance genes respectively (Fig. S1). In addition, a strain deficient in both pkaC1 and pkaC2 was isolated (Fig. S1), demonstrating that PKA signalling is not essential for the viability of A. fumigatus. This is in striking contrast to A. nidulans, where loss of the both PKA isoforms has been reported to be lethal (Ni et al., 2005).
PkaC2 has a secondary role to PkaC1 in maintaining colonial growth
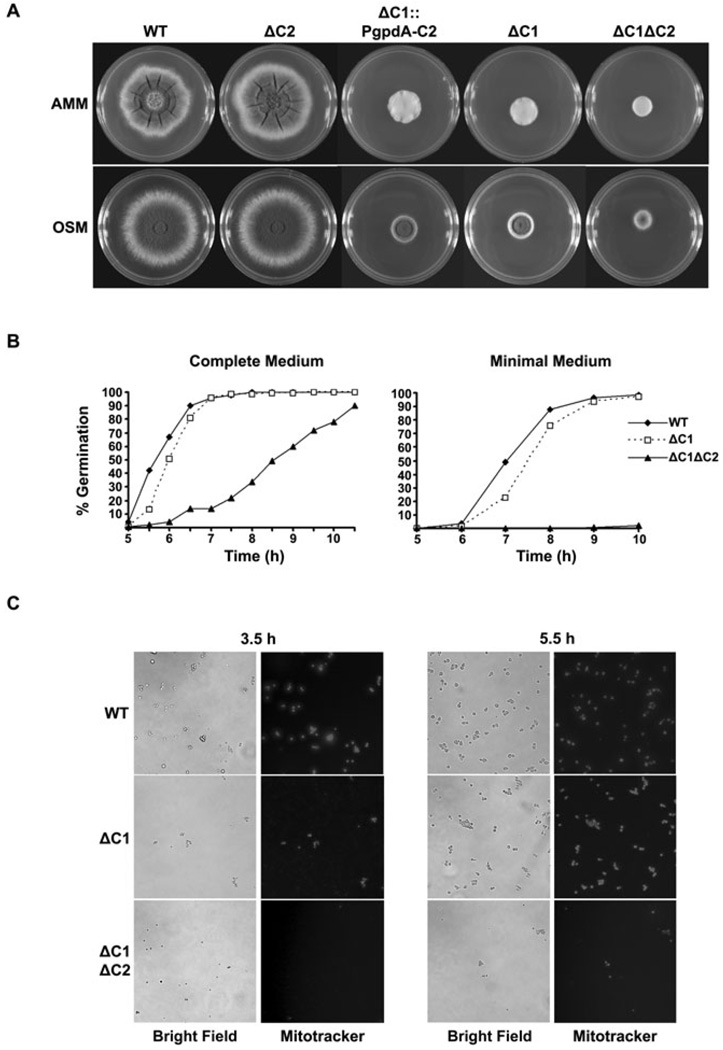
Whereas deletion of pkaC1 resulted in the formation of small compact colonies, the radial growth of the ΔpkaC2 mutant was indistinguishable from wild type (WT). However, the ΔpkaC1ΔpkaC2 mutant was notably growth impaired relative to the ΔpkaC1 strain, indicating that both catalytic subunits contribute to colonial growth of A. fumigatus (Fig. 2A). The normal growth of the ΔpkaC2 strain suggested that PkaC2 functionality could best be elucidated in the context of the loss of PkaC1 activity. Accordingly, in addition to the double-deletion mutant, we overexpressed pkaC2 in the ΔpkaC1 background by placing it under the control of the strong, constitutive promoter of the glyceraldehyde-3-phosphate dehydrogenase (gpdA) gene (Fig. S1). The ΔpkaC1::PgpdA-C2 strain demonstrated a modest increase in radial growth relative to the ΔpkaC1 strain, which further supports a role for PkaC2 in hyphal proliferation. However, pkaC2 overexpression did not fully complement the in vitro growth defects seen in the ΔpkaC1 mutant, which is consistent with previous reports that PkaC1 is the dominant isoform controlling colonial growth in A. fumigatus (Liebmann et al., 2004) (Fig. 2A).
Fig. 2. Growth and germination of the PKA mutants.
A. Colony appearance of the PKA mutants grown on AMM or OSM plates for 3 days at 37°C.
B. PkaC1 and PkaC2 cooperatively regulate germination. Conidia were germinated on coverslips in YG (left) or AMM (right) at 37°C. Germ-tube emergence was scored at the indicated times (n = 200).
C. Conidia of the ΔpkaC1ΔpkaC2 mutant are delayed in metabolic activation. Conidia were incubated in YG at 37°C and stained with Mitotracker Orange. Only conidia harbouring active mitochondria fluoresce.
PkaC2 shares a primary role with PkaC1 in regulating conidial germination
Protein Kinase A is known to regulate fungal morphogenesis in response to extracellular cues in a variety of fungal species (Gold et al., 1994; Adachi and Hamer, 1998; Pan and Heitman, 1999; Bockmühl et al., 2001). As the transition from a conidium to an invasive hypha is critical for the establishment of infection, we analysed the relative contributions of PkaC1 and PkaC2 to germination. Conidia of both the pkaC1 and pkaC2 single gene deletion mutants displayed germination kinetics identical to that of WT, with all three populations reaching 100% germ-tube formation by 8 h incubation in a complete growth medium (Fig. 2B and data not shown). When the ΔpkaC1ΔpkaC2 mutant was tested, only 34% of those conidia had germinated at 8 h, and 100% germ-tube formation was not achieved until about 11 h. In minimal medium, this defect was even more pronounced, with only 2% of the ΔpkaC1ΔpkaC2 conidia producing germ tubes by 11 h (Fig. 2B). By 24 h of incubation, substantial hyphal growth could be appreciated in the ΔpkaC1ΔpkaC2 population, indicating the mutant maintained its capacity to germinate, albeit with a markedly delayed time-course (data not shown).
During germination, polarized growth occurs after the quiescent conidium becomes metabolically activated (d’Enfert, 1997). Therefore, the delay in germ-tube emergence observed for the ΔpkaC1ΔpkaC2 mutant may represent a defect in polarized growth and/or a delay in conidial activation. To resolve this issue, we analysed mitochondrial respiration as a marker for metabolic activation by staining conidia with the dye Mitotracker Orange CMTMRos, as previously described (Fuller et al., 2009) (Fig. 2C). Whereas positively staining conidia of WT and ΔpkaC1 were detected after 3.5 h of incubation in YG, no positive cells within the ΔpkaC1ΔpkaC2 population were evident at that time. Although all ΔpkaC1ΔpkaC2 mutant conidia stained positively at 5.5 h, initiation of germ-tube emergence was further delayed. Because trehalose breakdown provides glucose for energy required for germination, we hypothesized that trehalose levels in resting conidia might vary among the mutants. However, the trehalose content was equivalent among mutant and WT strains (Fig. S2). Nevertheless, loss of phosphorylation of neutral trehalases in the PKA mutants following nutrient stimulation could still have adversely affected the germination rates among the PKAmutants (d’Enfert et al., 1999). These data therefore support a model in which PkaC1 and PkaC2 act in concert to regulate metabolic activation and initiation of polarized growth in A. fumigatus conidia.
PkaC2 has limited influence on asexual development
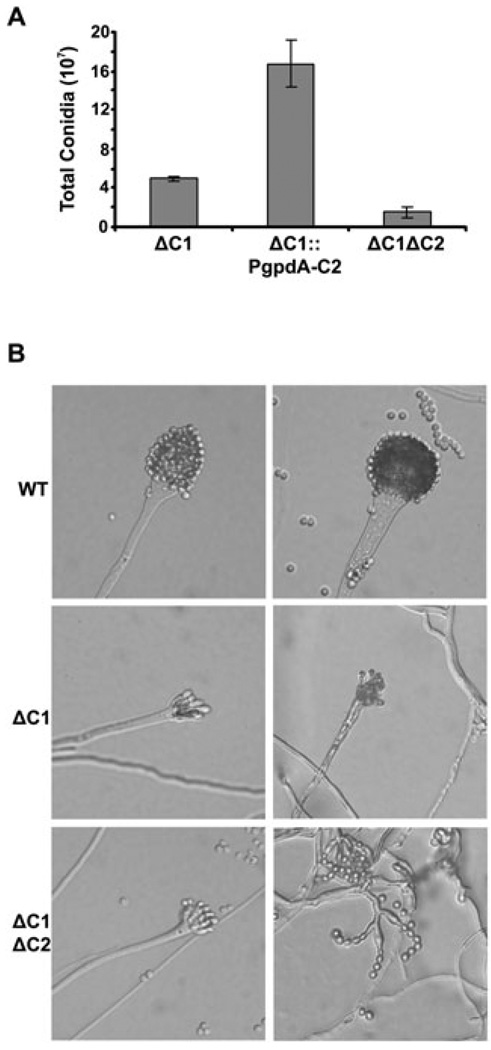
Aspergillus fumigatus produces numerous pigmented asexual conidia, which give the mycelium a blue-green colour when grown on solid medium. The colonial appearance of the ΔpkaC2 mutant was indistinguishable from WT, indicating that PkaC2 is dispensable for asexual development (Fig. 2A). In contrast, all strains harbouring the pkaC1 deletion demonstrated reduced conidiation, as previously demonstrated (Liebmann et al., 2004), which is consistent with a dominant role for PkaC1 in this process. Quantification of conidia revealed that the ΔpkaC1ΔpkaC2 mutant produced even fewer conidia than did ΔpkaC1, whereas the ΔpkaC1::PgpdA-C2 strain produced more, indicating that PkaC2 can positively influence this developmental pathway (Fig. 3A). Furthermore, many conidiophores of the ΔpkaC1 and ΔpkaC1ΔpkaC2 mutants were underdeveloped or displayed aberrant morphologies (Fig. 3B), demonstrating that PKA signalling is required for normal conidiophore formation. Interestingly, osmotic stabilization of the medium largely rescued the conidiation deficiency of each of the pkaC1 mutants, but addition of sorbitol was not able to correct the growth rate (Fig. 2A and data not shown). Sorbitol is an osmostabilizer that has been shown to protect a variety of fungal cell wall mutants from osmotic stress-induced lysis (Fortwendel et al., 2008; Richie et al., 2009). Therefore, these finding suggest that PKA mutants have underlying defects, which influence cell wall integrity, leading to impaired conidiophore development.
Fig. 3. PkaC1 and PkaC2 positively regulate asexual development.
A. pkaC2 positively affects conidiation of ΔpkaC1. Total conidia were harvested from AMM plates incubated at 37°C and were counted with a haemocytometer (mean ± SD from an experiment performed in triplicate).
B. PKA affects conidiophore morphogenesis. DIC images (40×) of conidiophores from 48 h cultures on AMM agar at 37°C.
PkaC2 acts in concert with PkaC1 to support cell wall homeostasis
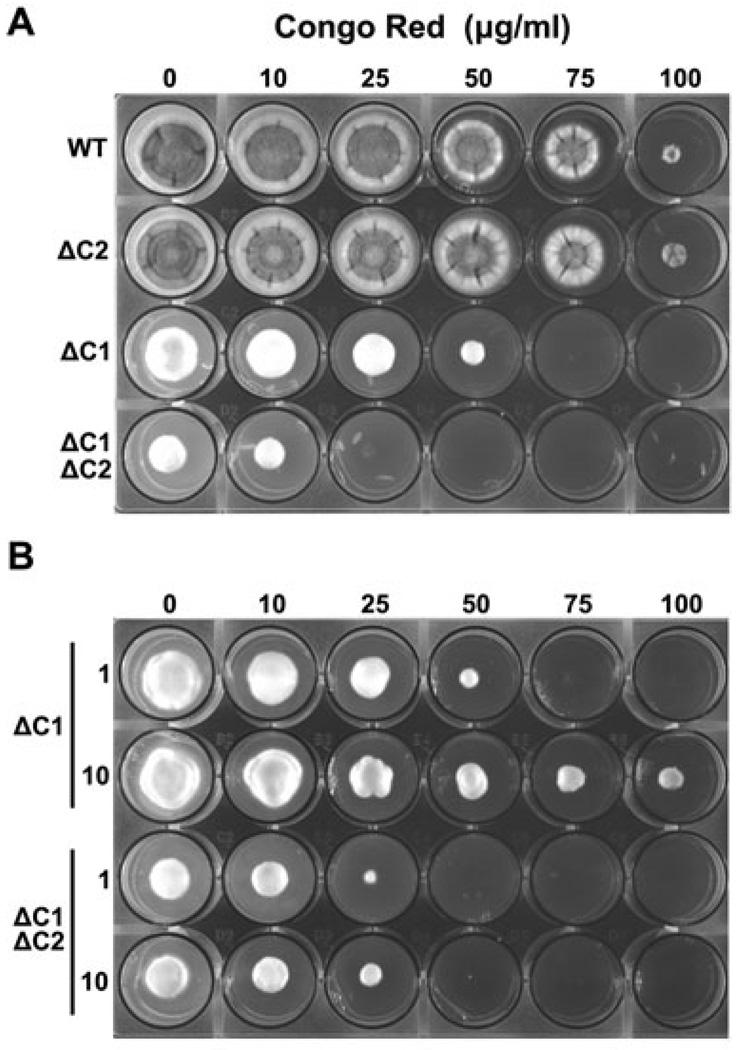
The ability of osmotic stabilization to rescue the conidiation defect of the PKA mutants suggested that PkaC1 and PkaC2 contribute to cell wall homeostasis. To test this, we analysed growth of the various mutants in the presence of the cell wall-damaging agent Congo red. The susceptibility of the ΔpkaC2 mutant was indistinguishable from that of WT, whereas the strains harbouring the pkaC1 deletion were unable to grow at concentrations of Congo red that had limited effect on either the WT or the ΔpkaC2 strains (Fig. 4A). Furthermore, the ΔpkaC1ΔpkaC2 mutant was more sensitive than the pkaC1 single-deletion mutant, suggesting that both isoforms act synergistically to regulate cell wall physiology. Transient exposure to Congo red induced swelling of the hyphal tips in the mutants, which suggests that areas of active cell wall remodelling are more susceptible to the defect imparted by the absence of PKA (data not shown). Similar susceptibility profiles were observed in the presence of SDS, another agent to which fungal cell wall mutants are typically hypersusceptible (Fig. S3). Taken together, these data indicate that both PkaC1 and PkaC2 play active roles in maintaining cell wall homeostasis in A. fumigatus.
Fig. 4. PKA contributes to cell wall homeostasis.
A. Loss of PKA leads to a hypersensitivity to cell wall damage. Conidia were point-inoculated into wells that contained increasing concentrations of Congo red in AMM and were incubated at 37°C for 3 days.
B. Conidia were incubated as above, in the presence of either 1% or 10% glucose.
PkaC2 influences carbohydrate metabolism of the ΔpkaC1 mutant
In S. cerevisiae, PKA mediates carbon catabolite repression by activating glycolytic and fermentative pathways, while concurrently downregulating respiration and alternative carbon utilization pathways in the presence of glucose (Thevelein et al., 2005). Three lines of evidence support a similar role for PKA in A. fumigatus. First, PKA activity is higher in cultures grown in glucose than those grown in glycerol, demonstrating that the pathway is influenced by the available carbon source (Oliver et al., 2002b). Second, the addition of exogenous cAMP, which would be expected to activate PKA, reduces growth of A. fumigatus on glycerol (Oliver et al., 2002b). Finally, overexpression of pkaC1 prevents the organism from growing on acetate as the sole carbon source (Grosse et al., 2008). These findings indicate that PKA signalling in A. fumigatus plays a role in regulating carbon metabolism, although the relative contributions from PkaC1 and PkaC2 in this process are unknown.
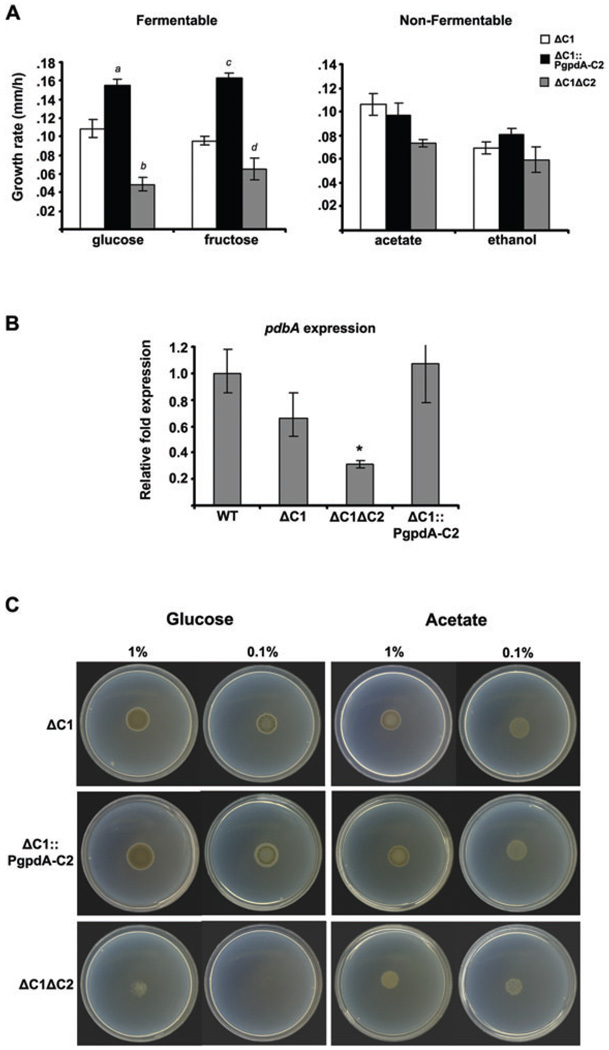
To determine the requirement for PkaC2 in the utilization of carbon sources, the growth rates of the PKA mutants were compared on different nutritional substrates. The ΔpkaC2 mutant was indistinguishable from WT on all carbon sources tested (data not shown), suggesting that PkaC2 is dispensable for regulating carbon metabolism in A. fumigatus. Since functional redundancy between PkaC1 and PkaC2 could account for the apparent lack of activity of PkaC2, we examined whether the deletion or overexpression of pkaC2 in the background of the ΔpkaC1 strain would have discernible influence upon growth on different carbon sources (Fig. 5A). Neither loss (ΔpkaC1ΔpkaC2) nor overexpression (ΔpkaC1::PgpdA-pkaC2) of PkaC2 altered the ability of the ΔpkaC1 mutant to grow on acetate or on glycerol, consistent with the idea that pkaC2 is not involved in regulating growth on non-fermentable carbon sources. However, overexpression of pkaC2 increased the ability of the ΔpkaC1 mutant to grow on both glucose and fructose, whereas the double-deletion mutant was further growth impaired, relative to the pkaC1 mutant, on those fermentable carbon sources.
Fig. 5. PkaC1 and PkaC2 regulated carbohydrate catabolism.
A. Overexpression of pkaC2 enhances growth of ΔpkaC1 on fermentable substrates (1%), whereas deletion of pkaC2 exacerbates the defect. The data are pooled data from three separate experiments and represent changes in colony diameter between 24 h and 48 h of incubation at 37°C. Error bars represent ± SEM (a, ΔpkaC1 versus ΔpkaC1::PgpdA-pkaC2 P= 0.007; b, ΔpkaC1 versus ΔpkaC1ΔpkaC2 P = 0.005; c, ΔpkaC1 versus ΔpkaC1::PgpdA-pkaC2 P < 0.001; d, ΔpkaC1 versus ΔpkaC1ΔpkaC2 P = 0.016).
B. pdbA expression is reduced in the ΔpkaC1ΔpkaC2 mutant. Quantitative PCR data are displayed as a fold expression (±SE of ΔCt values calculated across triplicate runs) relative to WT in hyphae following overnight incubation in YG at 37°C. Data are normalized to the gpdA reference control. (*P = 0.002).
C. The ΔpkaC1ΔpkaC2 mutant is unable to grow on reduced glucose concentrations. Conidia were point-inoculated onto agarose plates containing glucose or acetate and incubated for 3 days at 37°C.
The above data suggested that PkaC2 plays a specific role in catabolic carbohydrate pathway(s). To test this hypothesis, we analysed the relative transcript levels of the pyruvate dehydrogenase beta subunit gene, pdbA. In filamentous fungi, such as Aspergillus oryzae (Maeda et al., 2004) and Neurospora crassa (Xie et al., 2004), glucose increases the expression of this gene, allowing it to serve as a marker for activation of carbohydrate catabolism. We found that the steady-state levels of pdbA were significantly lower in the ΔpkaC1ΔpkaC2 mutant, relative to the WT, ΔpkaC1 or ΔpkaC1::PgpdA-C2 strains (Fig. 5B). This suggested that there is reduced flux through the carbon catabolic pathway in the double mutant, which may account for the reduced growth on glucose seen in that strain. A defect in glucose utilization would be expected to reduce growth under glucose-limiting conditions. Consistent with that, the double mutant could not grow when the glucose concentration was reduced to 0.1%. In contrast, ΔpkaC1ΔpkaC2 was able to utilize 0.1% and 0.01% acetate as a carbon source for growth (Fig. 5C and data not shown). These data suggest that PkaC1 and PkaC2 act co-ordinately to regulate flux through the glycolytic/pyruvate metabolic pathways and therefore positively regulate carbohydrate utilization in A. fumigatus.
The composition of the fungal cell wall is greater than 90% carbohydrate, consisting of interconnecting chains of modified glucose (glucan) or amino-glucose (chitin) polymers (Latgé, 2007). Therefore, the proper synthesis and maintenance of the cell wall is dependent upon a continual flow of glucose monomers to the site of cell wall assembly. Accordingly, we hypothesized that the cell wall defect observed for the various mutants could be attributed, in part, to the involvement of PKA in controlling glucose assimilation. In support of this possibility, we found that the increased susceptibility of the ΔpkaC1 mutant to Congo red could be recovered by elevating the glucose concentrations of the medium from 1% to 10% (Fig. 4B). Although partial osmotic stabilization by increased solute cannot be ruled out, this result is consistent with a model in which elevated glucose overcomes the reduction of sugar influx into the cell caused by pkaC1 deletion. Interestingly, neither the increase in glucose concentration nor addition of the amino sugar N-acetylglucosamine rescued the hypersensitivity of the ΔpkaC1ΔpkaC2 mutant to Congo red (Fig. 4B and data not shown). It is possible that the defect in the double mutant is simply too severe to be overcome by amending the medium, or that there is insufficient uptake to reach efficacious levels.
PKA signalling through both isoforms is essential for WT virulence
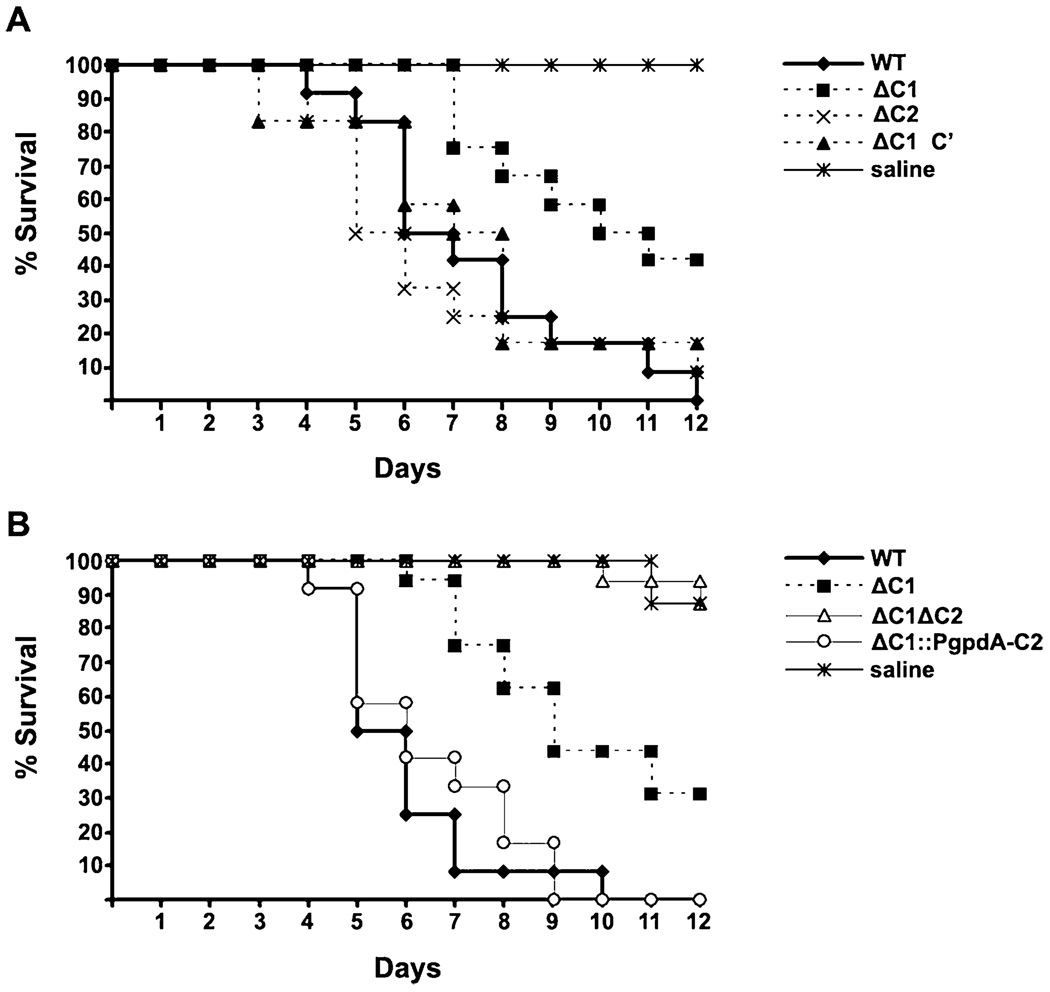
Although loss of PkaC1 has previously been shown to reduce the virulence of A. fumigatus (Liebmann et al., 2004), the contribution to virulence by PkaC2 is unknown. To determine whether the class II isoform also plays a role in regulating virulence, the PkaC2 mutants were analysed in a murine model of IA. The virulence of the ΔpkaC2 strain was indistinguishable from that of WT, indicating that PkaC2 is dispensable for virulence in the presence of PkaC1. This was in contrast to the ΔpkaC1 single mutant, which, consistent with the previous report, demonstrated attenuated virulence (Fig. 6A). However, deletion of both PKA catalytic subunit genes rendered the organism avirulent, and strikingly, overexpression of pkaC2 in the ΔpkaC1 background completely rescued the attenuated virulence of ΔpkaC1. This suggests that there are processes controlled by PkaC2 that are important for regulating virulence-related attributes that are not directly related to radial growth in A. fumigatus (Fig. 6B). We were unable to complement the double mutant due to its poor conidiation and the inability to obtain sufficient numbers of viable protoplasts. Therefore, two additional, independent isolates of the ΔpkaC1ΔpkaC2 mutant were selected and tested for virulence in the murine model; these additional isolates were also avirulent (Fig. S4B).
Fig. 6. PkaC2 modulates the virulence of the ΔpkaC1 mutant.
CF-1 outbred mice were immunosuppressed with triamcinolone acetonide and inoculated intranasally with 105 conidia.
A. ΔpkaC1 is attenuated in virulence relative to WT (P = 0.003), whereas ΔpkaC2 and the ΔpkaC1-complemented strain (ΔpkaC1 C′) are indistinguishable from WT.
B. The five strains can be divided into three statistically distinct groups: (i) WT and ΔpkaC1::PgpdA-pkaC2, (ii) ΔpkaC1 and (iii) ΔpkaC1ΔpkaC2 and saline (n = 12–16 per group). Pairwise log rank values; WT versus ΔpkaC1 (P < 0.001), WT versus ΔpkaC1::PgpdA-pkaC2 (P = 0.6), ΔpkaC1 versus ΔpkaC1ΔpkaC2 (P < 0.001), ΔpkaC1ΔpkaC2 versus saline (P = 0.99).
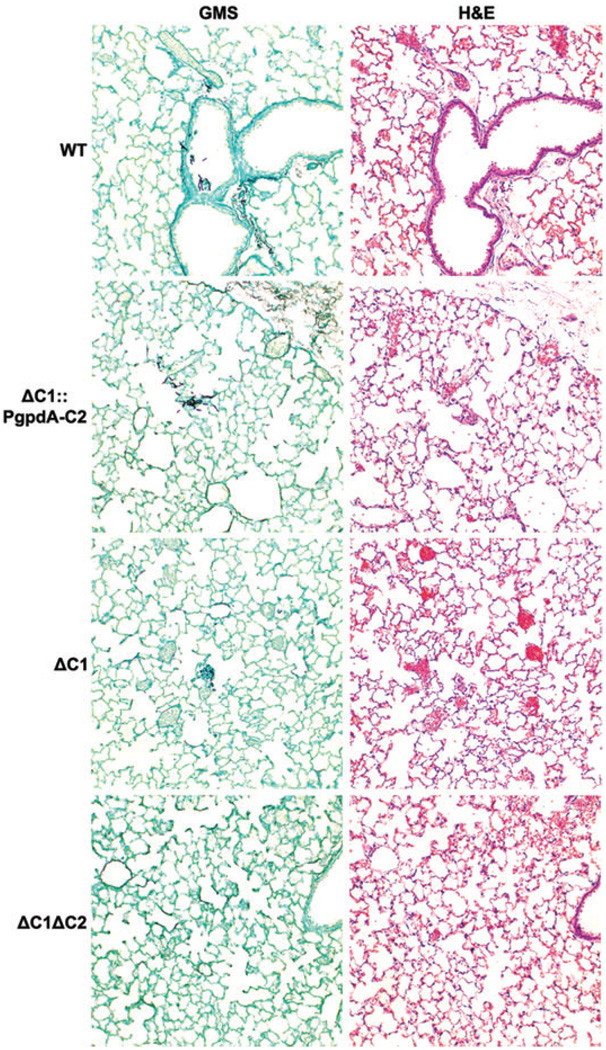
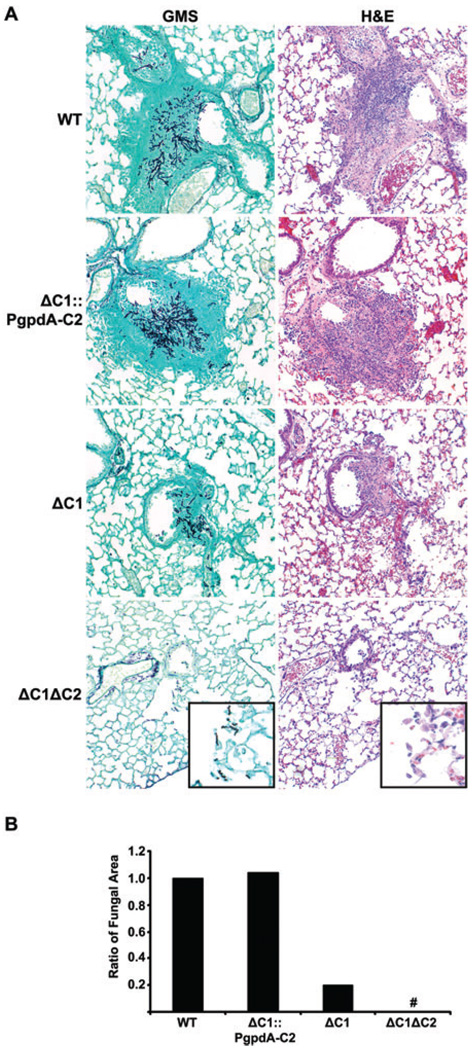
Analysis of the histopathology of the lungs at 24 h post infection revealed no fungal growth in the animals infected with the ΔpkaC1ΔpkaC2 strain, whereas focal fungal colonization was observed in lungs from WT, ΔpkaC1 and ΔpkaC1::PgpdA-pkaC2-infected mice (Fig. 7). At 48 and 72 h post infection, fungal lesions in WT, ΔpkaC1 and ΔpkaC1::PgpdA-pkaC2-infected mice were much more prominent and characterized by abundant fungal hyphae associated with a marked inflammatory infiltrate and focal necrosis. Rarely, fungal hyphae were detected in the ΔpkaC1ΔpkaC2-infected lungs, which verified inoculation; however, the lesions were smaller and not associated with necrosis nor a significant influx of inflammatory cells (Fig 8 and Fig S5). Consistent with the histological findings, morphometric analysis demonstrated that the extent of fungal growth mirrored what would be predicted from cumulative mortality of the strains.
Fig. 7. Histopathology of infected mice at 24 h post infection.
CF-1 mice were immunosuppressed with triamcinolone acetonide and inoculated with 2.0 × 105 conidia. Following staining with Gomori’s methenamine silver (GMS) and haematoxylin and eosin (H&E), small fungal foci were seen in animals from each of the groups, except those infected with the ΔpkaC1ΔpkaC2 mutant. All images are at 10x.
Fig. 8. Histopathology of infected mice at 72 h post infection.
A. Numerous fungal lesions with associated inflammation were seen in mice from each of the infection groups, with the exception of the ΔpkaC1ΔpkaC2-infected mice. The inset shows the only hyphae seen in any of the animals in the ΔpkaC1ΔpkaC2 group.
B. Morphometric analysis of the observable fungus in time-matched histological sections correlated with the survival curves associated with the strains.
Based on the outcome of the virulence studies, the established role of cell wall homeostasis in virulence, and the cell wall defect observed in the PKA mutants, the transcripts of selected cell wall genes were measured to evaluate their possible contributions to the PKA mutant virulence phenotypes. The message levels of ecm33 and ags3 were measured in the WT and in the ΔpkaC1 mutant (Maubon et al., 2006; Romano et al., 2006; Ejzykowicz et al., 2009). Although there was not a significant change in ecm33 transcript, the level of ags3 message was significantly increased in the mutant lacking PkaC1 (Fig. S6). The attenuated virulence of the ΔpkaC1 is compatible with reports of increased virulence-related phenotypes, such as germination rate, in ags3 deletion mutants. Taken as a whole, these data demonstrate that interaction of PkaC1 and PkaC2 plays a critical role in controlling processes that contribute to the success of A. fumigatus as an opportunistic pathogen.
Discussion
In order to successfully establish an invasive infection, A. fumigatus must adjust to and grow within the milieu of the mammalian lung. Although previous studies have implicated the cAMP–PKA pathway in this adaptation (Rhodes et al., 2001; Oliver et al., 2002a; Liebmann et al., 2003; 2004), the relevant processes controlled by PKA within the host remain incompletely understood. A major challenge in understanding PKA signalling in fungi is that the pathway cannot be easily defined through a single catalytic subunit. Rather, most organisms, including all filamentous fungi examined to date, contain multiple isoforms that may display both overlapping and unique functions. A. fumigatus contains a divergent PKA isoform, PkaC2, but its contribution to growth and pathogenesis is unknown. In this report, we demonstrate that PkaC2 acts in concert with PkaC1 to support germination, cell wall homeostasis and carbohydrate catabolism. Furthermore, these functions likely account for the ability of PkaC2 to strongly influence the attenuated virulence of the ΔpkaC1 mutants of A. fumigatus, improving our insight into the PKA-dependent processes required for pathogenesis.
Similar to the ΔpkaB mutant of A. nidulans (Ni et al., 2005), the ΔpkaC2 strain of A. fumigatus displayed no discernable phenotype, indicating that the class II isoform is dispensable for WT growth in vitro. Surprisingly, we were able to delete both pkaC1 and pkaC2, demonstrating that a complete loss of PKA activity is also dispensable in A. fumigatus. Since PKA represents an essential pathway among ascomycetous fungi that have been tested, including A. nidulans, it is interesting to speculate that A. fumigatus has developed novel signalling redundancies that allow other pathways to suffice in the absence of PKA (Toda et al., 1987; Bockmühl et al., 2001; Ni et al., 2005).
Although pkaC2 alone was dispensable for growth, its deletion or overexpression modulated various phenotypes of the ΔpkaC1 mutant, thereby demonstrating the functional capacity of the PkaC2 isoform. Interestingly, however, we did not detect phosphorylation of the synthetic PKA substrate kemptide in any of the strains in which pkaC1 was deleted (Fig. S7). Loss of kemptide phosphorylation in a ΔpkaC1 mutant of A. fumigatus led previous authors to conclude that PkaC2 is nonfunctional, perhaps due to neutral mutations in the nucleotide-binding loop (Liebmann et al., 2004; Grosse et al., 2008). However, an S. cerevisiae TPK1/TPK2 deletion strain (tpk1tpk2TPK3) similarly failed to display kemptide phosphorylation, despite the capability of Tpk3p activity alone to support normal vegetative growth (Mazon et al., 1993). This result, along with our data, suggests that the level of kinase activity required to detect phosphorylation of the kemptide substrate in vitro does not predict physiologic relevance in vivo.
If the results of the kemptide assay are indicative of reduced intrinsic kinase activity of the PkaC2 isoform, what then can account for this finding? One possibility is that PkaC2, and the fungal class II kinases in general, are slowly losing function over evolutionary time. In Cryptococcus neoformans (serotype A), the loss of function of the Pka2 isoform illustrates this point (Hicks et al., 2004). After duplication of the ancestral gene, it has been proposed that one allele is under selective pressure to maintain its function, whereas the other is free to accumulate mutations and lose activity, ultimately becoming a pseudogene (Lynch and Conery, 2000; Hicks and Heitman, 2007; Hahn, 2009). Comparative sequence analysis among various class II isoforms reveals conserved point mutations in the nucleotide-binding loop (S201 to T86 and G203 to A88), mentioned above, and in the catalytic site (I311 to V201 and L320 to I211), both of which result in neutral amino acid changes (Fig. 1). Although the impact of these conservative mutations has not been substantiated experimentally, they may explain, at least in part, the reduced activity of these class II kinases. Another intriguing possibility is that PkaC2 acts upon a unique phosphorylation site and therefore maintains a separate phosphoproteome from that of PkaC1. Although perhaps less likely, based on overlapping functions described herein, experiments are currently underway to explore this exciting possibility.
The proper maintenance of the cell wall is a crucial aspect of fungal morphogenesis and stress resistance (Latgé, 2007). Our data demonstrate that both PKA isoforms contribute to the regulation of cell wall homeostasis in A. fumigatus. To our knowledge, this is the first report describing the involvement of PKA in cell wall homeostasis of a filamentous fungus. In both S. cerevisiae and Candida albicans, constitutive cAMP–PKA signalling, resulting from deletion of the high-affinity phosphodiesterase PDE2, leads to altered expression of genes involved in cell wall biogenesis and architecture. Consequently, these pde2Δ mutants demonstrate hypersensitivity to several cell wall modulating agents (Jones et al., 2003; Jung et al., 2005). Although we did not detect a cell wall phenotype in the A. fumigatus ΔpkaR mutant, which also displays constitutive PKA activity (data not shown), we do show that reduced PKA signalling leads to hypersensitivity to cell wall agents (Fig. 4). This suggests that PKA is a positive regulator of cell wall homeostasis in A. fumigatus, and that the relationship between PKA and the cell wall is fundamentally different between this organism and S. cerevisiae.
The basis for the cell wall defect among the PKA-deficient strains is incompletely understood. However, we demonstrate that the central role for PKA in carbohydrate metabolism may be an important contributing factor. Recently, an A. fumigatus mutant deficient in the glucose phosphorylating enzymes, glucokinase and hexokinase, was shown to be hypersensitive to cell wall perturbation, thereby underscoring the relationship between glucose utilization and the cell wall (Fleck and Brock, 2010). In the case of the PKA mutants, however, there was no detectable difference in hexokinase activity between the WT and the mutants lacking PkaC1 or PkaC1 and PkaC2 (data not shown). Furthermore, efforts to relieve the cell wall stress by providing exogenous N-acetyl glucosamine were not successful, which suggests that limiting quantities of amino sugars were not responsible for the cell wall defect. Indeed, other than osmotic stabilization, only high levels of glucose were able to ameliorate cell wall hypersensitivity in the PKA mutants (Fig. 4). In addition, it is interesting to speculate that the cAMP–PKA pathway co-ordinately regulates the response to cell wall stress along with the classical cell wall integrity (PKC–MAP kinase) pathway (Valiante et al., 2008), since both pathways may be activated by similar upstream signals, e.g. nutrients, and can regulate similar downstream processes, such as morphogenesis (Pandey et al., 2004; Xue et al., 2004; Reyes et al., 2006).
The cell wall phenotypes associated with the PKA mutants of A. fumigatus may contribute to their attenuated virulence. A variety of A. fumigatus mutants harbouring mutations of specific cell wall biosynthetic genes, including a glucanosyltransferase and an α-mannosyltransferase, display cell wall abnormalities that ultimately manifest as changes in virulence (Mouyna et al., 2005; Wagener et al., 2008). Although these examples illustrate that cell wall aberrations may be detrimental for the fungus during infection, the precise relationship between the cell wall and A. fumigatus virulence is unclear. For instance, several glucan and chitin synthase mutants with morphological defects, as well as a MAPK mutant that is hypersensitive to cell wall stress, were all reported to have WT-like virulence (Aufauvre-Brown et al., 1997; Beauvais et al., 2005; Valiante et al., 2008). Indeed, cell wall mutant strains of A. fumigatus in which ecm33 or ags3 have been deleted or downregulated actually have increased virulence-related properties, when compared with WT (Maubon et al., 2006; Romano et al., 2006; Ejzykowicz et al., 2009). The increased level of ags3 message seen in the ΔpkaC1 mutant would be compatible with the decreased virulence seen in strains lacking PkaC1, suggesting that loss of cell wall homeostasis may contribute to the pathogenic defect in these strains.
The germination of inhaled conidia represents the first essential step in the development of IA. Our data revealed that PkaC2 signalling alone is sufficient to support germination in A. fumigatus, as a germination defect was only observed in the ΔpkaC1ΔpkaC2 mutant. Therefore, a major impact of PkaC2 on virulence could be through its involvement in germination. The trehalose pathway has recently been linked to virulence in A. fumigatus, and since trehalose is the primary storage carbohydrate in the conidia, it is possible that PKA may influence germination (d’Enfert et al., 1999; Al-Bader et al., 2010; Puttikamonkul et al., 2010). Although trehalose levels in conidia were similar among the PKA mutants (Fig. S2), we are unable to rule out the role that regulation of the neutral trehalase by PKA phosphorylation may play in trehalose catabolism, which provides glucose to drive early steps in germination (d’Enfert et al., 1999). Indeed, PKA phosphorylation has been shown to regulate the rate of germination in A. nidulans. So, whereas fungal foci were visible in WT, ΔpkaC1 and ΔpkaC1::PgpdA-pkaC2-infected groups within 24 h post infection, no growth was observed in the ΔpkaC1ΔpkaC2-infected mice until 48 h, suggesting that germination was delayed, but not blocked. However, since 48 h also correlated with the influx of neutrophils at sites of fungal growth, it is likely that residual or recovering immune effector cells are better able to clear the germlings of ΔpkaC1ΔpkaC2, relative to the other strains that have established a more advanced infection.
Following germination, A. fumigatus must utilize nutrients available within the host environment. Indeed, transcriptional profiling of germlings recovered from murine bronchoalveolar lavage fluid demonstrated an upregulation of genes involved in carbohydrate transport and a downregulation of respiratory genes (McDonagh et al., 2008), which supports the critical role for metabolic reprogramming of carbohydrate utilization in vivo. We show here that the ΔpkaC1ΔpkaC2 mutant has reduced message level of pdbA, a gene encoding the beta subunit of pyruvate dehydrogenase, which catalyses oxidative decarboxylation of pyruvate to acetyl-coA, thus linking glycolysis with the citric acid cycle. The effect on pbdA, then, suggests that both PKA isoforms function to regulate flow through the carbohydrate catabolic pathway. This finding is consistent with the inability of the ΔpkaC1ΔpkaC2 mutant to grow in the presence of low glucose concentrations, such as what would be expected within the lung (de Prost and Saumon, 2007). Moreover, although the ΔpkaC1::PgpdA-pkaC2 strain grew better than the ΔpkaC1 mutant on glucose, all three mutants grew equally well on acetate. The large disparity in in vivo fungal burden among the mutants may reflect that the fungus must use glucose within the host. Since recent evidence suggests that A. fumigatus is under hypoxic stress in vivo (Willger et al., 2008), limiting oxygen levels may lead to downregulation of respiratory pathways that increases the reliance upon glycolysis for ATP generation. Thus, the infected lung likely provides an environment of reduced glucose and reduced oxygen, in which the disparity in growth rates between ΔpkaC1, ΔpkaC1ΔpkaC2 and ΔpkaC1::PgpdA-pkaC2 would be accentuated.
The capacity of A. fumigatus and other pathogenic fungi to grow rapidly at 37°C is considered to be an essential virulence trait. Indeed, in vitro growth rate has been shown to correlate with virulence in a number of studies (Latgé, 2001; Rhodes et al., 2001; Paisley et al., 2005). It is therefore striking that the mortality curve of the ΔpkaC1::PgpdA-pkaC2 strain was indistinguishable from WT, whereas the ΔpkaC1ΔpkaC2 was avirulent, even though both strains have significantly impaired growth phenotypes. This observation adds the ΔpkaC1::PgpdA-pkaC2 mutant to an emerging list of A. fumigatus mutants in which in vitro growth rate does not predict the virulence phenotype. For example, the A. fumigatus ΔgpaB mutant displays WT-like growth in vitro, yet is markedly attenuated for virulence (Liebmann et al., 2003; 2004). In contrast, mutants of two genes involved in MAPK signalling of A. fumigatus, ΔmpkA and Δsho1, display WT-like virulence, despite the strong inhibition of radial outgrowth on plates (Ma et al., 2008; Valiante et al., 2008). Although several scenarios could be constructed to explain these discrepant results, we favour one in which growth in the host environment is the critical determinant of virulence. This is consistent with our observation that the fungal burden of the ΔpkaC1::PgpdA-pkaC2 mutant is indistinguishable from that of the WT. Because our in vitro systems are, at very least, inaccurate predictors of the in vivo environment, the in vitro growth phenotype alone does not necessarily predict virulence. Taken together, our results suggest that PkaC2, in cooperation with PkaC1, regulates additional processes that are critical to growth within the host.
In conclusion, we have characterized the relative contributions of the two PKA isoforms, PkaC1 and PkaC2, in the mould pathogen A. fumigatus. We show that whereas PkaC1 is the predominant isoform with respect to radial growth and asexual development, PkaC2 maintains important roles in regulating germination, cell wall homeostasis and carbohydrate metabolism. Furthermore, the processes controlled by PkaC1 and PkaC2 play a critical role in adaptation to the host niche, and have expanded upon our understanding of how PKA contributes to pathogenesis. Future studies analysing the downstream targets of the two isoforms and how they may be differentially regulated will provide important insight into the complex and important pathway.
Experimental procedures
Strains and culture conditions
Aspergillus fumigatus strain H237, a clinical isolate, was used to generate the pkaC1 and pkaC2 deletion strains. Conidia were harvested from osmotically stabilized medium (OSM), which consisted of Aspergillus minimal medium (AMM) (Cove, 1966) supplemented with 1.2 M sorbitol. Colony images were taken after 72 h incubation at 37°C on either AMM or OSM media, as indicated.
Construction of the various mutant strains
All primers used for vector construction are listed in Table S1 and all products were amplified using PFU Turbo polymerase (Stratagene).
The A. fumigatus pkaC2 gene was deleted using the splitmarker method (Catlett et al., 2002). Briefly, the first two-thirds of the phleomycin resistance cassette were amplified from pBCphleo using primers 398 and 408. This product was combined with the pkaC2 left arm product by overlap PCR using primers 100 and 408 to yield the left deletion construct. The second two-thirds of the phleomycin cassette were amplified using primers 410 and 409. This product was combined with the pkaC2 right arm product with primers 410 and 104 to generate the right deletion construct. Both the left and right deletion constructs were cloned separately into pCR-Blunt II-TOPO (Invitrogen). The inserts were liberated from their plasmids using an EcoRI and NotI/SpeI digestions respectively. Ten micrograms of each was used for transformation of H237 protoplasts, as previously described (Bhabhra et al., 2004). The probe used for the Southern blot was amplified from H237 genomic DNA with primers 109 and 110.
The pkaC1 gene was similarly deleted in both the H237 and ΔpkaC2 strains by a split-marker approach. The first two-thirds of the hygromycin resistance cassette were amplified form pan7-1 using primers 398 and 395. This product was combined with the pkaC1 left arm product by overlap PCR using primers 105 and 395 to generate the left deletion construct. The second two-thirds of the hygromycin cassette were amplified using 396 and 399. This product was combined with the pkaC1 right arm using primers 396 and 108 to generate the right deletion construct. Both the left and right deletion constructs were cloned into pCR-Blunt II-TOPO (Invitrogen). Following amplification from their respective plasmids, 10 µg of each insert was co-transformed into the H237 WT strain to generate the ΔpkaC1 mutant or the ΔpkaC2 strain to generate the ΔpkaC1ΔpkaC2 mutant. The probe used for the Southern blots was isolated by digesting the pkaC1 right deletion plasmid with BamHI and HindIII and purifying the 568 bp fragment.
The construct for the overexpression of pkaC2 was generated by overlap PCR of three products. The first product is the pkaC2 left arm, which was amplified with primers 201 and 202 from genomic DNA. The second product is the promoter of the glyceraldehyde-3-phosphate dehydrogenase gene (gpdA), which was amplified from pBC-phleo with primers 203 and 204. Primers 202 and 203 contain 20 base pairs of reverse complementarity to mediate fusion of the two pieces by PCR. The third product is the pkaC2 ORF amplified with primers 205 and 206 from genomic DNA. Primers 205 and 204 contain a 20-base-pair region of reverse complementarity to mediate fusion of the two pieces by PCR. All three products were fused by a single overlap PCR reaction with primers 201 and 206. The overlap construct was cloned into pCR-Blunt II-TOPO. Ten micrograms of linearized plasmid (SmaI digest) along with 1 µg of linearized p402R (containing the phleomycin resistance cassette) was co-transformed into protoplasts of the ΔpkaC1 mutant. Phleomycin resistance colonies were screened for the presence of the construct with primers 203 and 206.
Analyses of conidial germination and mitochondrial activation
For the germination assay, 5.0 × 105 conidia ml−1 were inoculated onto coverslips in 3 ml of YG (2% glucose, 0.5% yeast extract) or AMM and then incubated at 37°C. At the indicated time points, coverslips were mounted onto a microscope slide and a total of 200 conidia were scored for the presence or absence of a germ tube. Experiments were repeated with similar results.
To analyse mitochondrial activation, conidia were stained with Mitotracker Orange CM-H2 TMRos (Invitrogen), as previously described (Fuller et al., 2009). Briefly, conidia were inoculated onto coverslips in YG medium and incubated for the indicated times at 37°C. Following incubation, the medium was aspirated and the samples were then stained (Fuller et al., 2009). Samples were observed by fluorescence microscopy (TRITC filter) and images were taken with Adobe ImageReady 7.0.1 and analysed with Adobe Photoshop 6.0 software.
Asexual developmental studies
Conidia were streaked onto AMM plates and incubated at 37°C for several days. Total conidia were harvested from the plates, diluted and counted with a haemocytometer. The experiment was performed in triplicate and mean values were analysed statistically with a Student’s t-test.
To analyse conidiophore morphology, conidia were inoculated along the sides of AMM plugs and a coverslip was placed on top of the plug. The samples were then incubated at 37°C for 48 h. Following incubation, coverslips were photographed (40×) using differential interference contrast (DIC) microscopy. Images were analysed using Adobe Photoshop 6.0.
Susceptibility assays to cell wall modulating agents
Two microlitres of a suspension of 4.0 × 103 conidia was point-inoculated across wells that contained the indicated concentrations of Congo red or SDS inAMM solid medium. In some experiments, the concentration of glucose in the medium was increased to 10%. Plates were incubated for 3 days at 37°C.
Analysis of growth on different carbon sources
To determine radial growth rates on the various carbon sources, 1.0 × 104 conidia in a 5 ml suspension were point-inoculated onto AMM plates that contained 1% (w/v) dextrose, fructose or sodium acetate or 1% (v/v) ethanol. The plates were made with 1.5% noble agar (Difco Laboratories) to reduce potential utilization of the agar components as a nutrient source. Plates were incubated at 37°C and colony diameter was measured every 24 h. Experiments were performed in triplicate and changes in colony diameter between 24 h and 48 h were subject to statistical analysis (Student’s t-test).
To analyse the ability of the strains to grow on reducing carbon concentrations, 1.0 × 104 conidia were spotted onto AMM plates that contained the indicated concentrations (w/v) of dextrose or sodium acetate. The media contained 1.5% agarose. Plates were incubated for 3 days at 37°C. The WT organism was included in the study and could grow on all carbon sources and concentrations tested. However, growth was diffuse and was difficult to visualize by photography.
Trehalose concentration in conidia
Conidia were harvested from AMM plates and counted in a haemocytometer. Equal quantities were heated at 100°C for 20 min to lyse the conidia. The concentration of trehalose, as glucose, was measured in the conidial lysate, as described by Puttikamonkul et al. (2010).
RNA isolation and quantitative RT-PCR analysis
Strains were grown overnight (13–16 h) in YG media at 37°C, shaking at 200 r.p.m. Total RNA was isolated (RNeasy minikit, Qiagen) from vacuum-filtered mycelia that were crushed with liquid nitrogen. Five micrograms of total RNA were then DNased (RQ1 Dnase, Qiagen) for 45 min at 37°C. Following the DNase incubation, 6 µl of sample was used for first-strand cDNA synthesis (Superscript III, Invitrogen) or for a no reverse transcriptase control. For quantitative PCR analysis of pdbA or ags3 mRNA levels, 1 µl of the cDNA was used in a 25 µl Sybr Green reaction (SYBR GreenER, Invitrogen) (SmartCycler II, Cepheid). ΔCt values for each strain were determined based on the mean of three to four separate quantitative PCR runs. The relative expression levels are based on the 2−ΔΔCt values comparing the each mutant strain with WT. Error bars are based on standard error for the ΔCt values of the mutant strain. Statistical analysis was performed comparing the ΔCt values by Student’s t-test. Primers used are listed in Table S1.
Virulence studies
Conidia were harvested from OSM plates and viable conidia were enumerated by dilution plating and colony-forming unit (cfu) counting. Samples were then diluted in sterile saline prior to inoculation. Groups of 12–16 CF-1 outbred, female mice (20–28 g) were immunosuppressed with a dose of 40 mg kg−1 triamcinolone acetonide (Kenalog-10, Bristol-Myers Squibb) subcutaneously on day −1 and with a dose of 20 mg kg−1 on day +4. The mice were anaesthetized with 3.5% isofluorane and inoculated intranasally with 1.0 × 105 viable conidia in a 20 µl suspension. Mortality was monitored for 12 days and statistical analysis was assessed by the log rank test using Sigma Stat 3.5. For testing of additional strains of ΔpkaC1ΔpkaC2, groups of eight were used.
Histopathology and morphometric analysis of fungal burden
For the histopathology studies, additional groups of CF-1, female mice were infected as described above and sacrificed at the 0, 1, 2 or 3 days post infection (n = 2 mice per group per day). Lungs were removed and inflated with 10% phosphate-buffered formalin on the day of sacrifice. Lung tissues were then embedded in paraffin, sectioned and stained with haematoxylin and eosin (H&E) or Grocott’s methenamine silver (GMS). All pictures are at 10×.
Semi-quantitative analysis of fungal burden was performed by morphometric analysis of the histological sections described above. First, the GMS sections of all strains at the 72 h time point were randomly assigned a number by an outside person, such that the investigator was blinded to the strain associated with the section. Second, the sections were observed by microscopy and all regions that contained observable hyphae were photographed at 10× magnification. The photomicrographs were then analysed using the Volocity 4 software (Improvision). Briefly, a selection tool was used to outline the hyphae (stained black) and the area of the selections was calculated to determine the hyphal area associated with each photomicrograph. The hyphal areas associated with a particular section were then added together to give the total hyphal area of the section. The average area associated with WT was set to 1.
Supplementary Material
Acknowledgements
The authors would like to thank Jay Card for his excellent assistance with photography. We also thank the Pathology Research Core at Cincinnati Children’s Hospital Medical Center for the help with histology. We acknowledge support from the National Institutes of Health (J.C.R. – AI061497, D.S.A. – AI072297, K.A.W.-B. – HL079193, CA102357), the American Cancer Society (K.A.W.-B. – RSG-10-194-01-TBG) and St. Baldrick’s Foundation (K.A.W.-B. – 18033).
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adachi K, Hamer JE. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell. 1998;10:1361–1374. doi: 10.1105/tpc.10.8.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bader N, Vanier G, Liu H, Gravelat FN, Urb M, Hoareau CM-Q, et al. Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect Immun. 2010;78:3007–3018. doi: 10.1128/IAI.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew DS. Aspergillus fumigatus: virulence genes in a street-smart mold. Curr Opin Microbiol. 2008;11:331–337. doi: 10.1016/j.mib.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufauvre-Brown A, Mellado E, Gow NAR, Holden DW. Aspergillus fumigatus chse: a gene related to chs3 of Saccharomyces cerevisiae and important for hyphal growth and conidiophore development but not pathogenicity. Fungal Genet Biol. 1997;21:141–152. doi: 10.1006/fgbi.1997.0959. [DOI] [PubMed] [Google Scholar]
- Beauvais A, Maubon D, Park S, Morelle W, Tanguy M, Huerre M, et al. Two alpha(1–3) glucan synthases with different functions in Aspergillus fumigatus. Appl Environ Microbiol. 2005;71:1531–1538. doi: 10.1128/AEM.71.3.1531-1538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffa T, Staib F, Lott Fischer J, Lyon P, Gumowski P, Marfenina O, et al. Myl and surveillance of biological waste and compost. Med Mycol. 1998;36 Suppl. I:137–145. [PubMed] [Google Scholar]
- Bhabhra R, Miley MD, Mylonakis E, Boettner D, Fortwendel J, Panepinto JC, et al. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect Immun. 2004;72:4731–4740. doi: 10.1128/IAI.72.8.4731-4740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmühl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol. 2001;42:1243–1257. doi: 10.1046/j.1365-2958.2001.02688.x. [DOI] [PubMed] [Google Scholar]
- Catlett NL, Lee BN, Yoder OC, Turgeon BG. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet Newsl. 2002;50:9–11. [Google Scholar]
- Chen D, Janganan TK, Chen G, Marques ER, Kress MR, Goldman GH, et al. The cAMP pathway is important for controlling the morphological switch to the pathogenic yeast form of Paracoccidioides brasiliensis. Mol Microbiol. 2007;65:761–779. doi: 10.1111/j.1365-2958.2007.05824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- D’Souza CA, Heitman J. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol Rev. 2001;25:349–364. doi: 10.1111/j.1574-6976.2001.tb00582.x. [DOI] [PubMed] [Google Scholar]
- D’Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, Heitman J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger F, Wong K, Kronstad JW. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc Natl Acad Sci USA. 1998;95:5684–5689. doi: 10.1073/pnas.95.10.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejzykowicz DE, Cunha MM, Rozental S, Solis NV, Gravelat FN, Sheppard DC, Filler SG. The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol Microbiol. 2009;72:155–169. doi: 10.1111/j.1365-2958.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Enfert C. Fungal spore germination: insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet Biol. 1997;21:163–172. [Google Scholar]
- d’Enfert C, Bonini BM, Zapella PDA, Fontaine T, da Silva AM, Terenzi HF. Neutral trehalases catalyse intracellular trehalose breakdown in the filamentous fungi Aspergillus nidulans and Neurospora crassa. Mol Microbiol. 1999;32:471–483. doi: 10.1046/j.1365-2958.1999.01327.x. [DOI] [PubMed] [Google Scholar]
- Fleck CB, Brock M. Aspergillus fumigatus catalytic glucokinase and hexokinase: expression analysis and importance for germination, growth, and conidiation. Eukaryot Cell. 2010;9:1120–1135. doi: 10.1128/EC.00362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortwendel JR, Fuller KK, Stephens TJ, Bacon WC, Askew DS, Rhodes JC. Aspergillus fumigatus RasA regulates asexual development and cell wall integrity. Eukaryot Cell. 2008;7:1530–1539. doi: 10.1128/EC.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller KK, Zhao W, Askew DS, Rhodes JC. Deletion of the protein kinase A regulatory subunit leads to deregulation of mitochondrial activation and nuclear duplication in Aspergillus fumigatus. Eukaryot Cell. 2009;8:271–277. doi: 10.1128/EC.00391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S, Duncan G, Barrett K, Kronstad J. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994;8:2805–2816. doi: 10.1101/gad.8.23.2805. [DOI] [PubMed] [Google Scholar]
- Grosse C, Heinekamp T, Kniemeyer O, Gehrke A, Brakhage AA. Protein kinase A regulates growth, sporulation, and pigment formation in Aspergillus fumigatus. Appl Environ Microbiol. 2008;74:4923–4933. doi: 10.1128/AEM.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. Distinguishing among evolutionary models for the maintenance of gene duplicates. J Hered. 2009;5:605–617. doi: 10.1093/jhered/esp047. [DOI] [PubMed] [Google Scholar]
- van Heerden I, Cronje C, Swart S, Kotze J. Microbial, chemical and physical aspects of citrus waste composting. Bioresour Technol. 2002;81:71–76. doi: 10.1016/s0960-8524(01)00058-x. [DOI] [PubMed] [Google Scholar]
- Hicks JK, Heitman J. Divergence of protein kinase A catalytic subunits in Cryptococcus neoformans and Cryptococcus gattii illustrates evolutionary reconfiguration of a signaling cascade. Eukaryot Cell. 2007;6:413–420. doi: 10.1128/EC.00213-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JK, D’Souza CA, Cox GM, Heitman J. Cyclic AMP-dependent protein kinase catalytic subunits have divergent roles in virulence factor production in two varieties of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2004;3:14–26. doi: 10.1128/EC.3.1.14-26.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Petty J, Hoyle DC, Hayes A, Ragni E, Popolo L, et al. Transcriptome profiling of a Saccharomyces cerevisiae mutant with a constitutively activated ras/cAMP pathway. Physiol Genomics. 2003;16:107–118. doi: 10.1152/physiolgenomics.00139.2003. [DOI] [PubMed] [Google Scholar]
- Jung WH, Warn P, Ragni E, Popolo L, Nunn CD, Turner MP, Stateva L. Deletion of PDE2, the gene encoding the high-affinity cAMP phosphodiesterase, results in changes of the cell wall and membrane in Candida albicans. Yeast. 2005;22:285–294. doi: 10.1002/yea.1199. [DOI] [PubMed] [Google Scholar]
- Latge J-P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé J-P. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–389. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- Latgé J-P. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Liebmann B, Gattung S, Jahn B, Brakhage AA. cAMP signaling in Aspergillus fumigatus is involved in the regulation of the virulence gene pksP and in defense against killing by macrophages. Mol Genet Genomics. 2003;269:420–435. doi: 10.1007/s00438-003-0852-0. [DOI] [PubMed] [Google Scholar]
- Liebmann B, Muller M, Braun A, Brakhage AA. The cyclic AMP-dependent protein kinase a network regulates development and virulence in Aspergillus fumigatus. Infect Immun. 2004;72:5193–5203. doi: 10.1128/IAI.72.9.5193-5203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Conery J. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- Ma Y, Qiao J, Liu W, Wan Z, Wang X, Calderone R, Li R. The Sho1 sensor regulates growth, morphology, and oxidant adaptation in Aspergillus fumigatus but is not essential for development of invasive pulmonary aspergillosis. Infect Immun. 2008;76:1695–1701. doi: 10.1128/IAI.01507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh A, Fedorova N, Crabtree J, Yu Y, Kim S, Chen D, et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008;4:e1000154. doi: 10.1371/journal.ppat.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Sano M, Maruyama Y, Tanno T, Akao T, Totsuka Y, et al. Transcriptional analysis of genes for energy catabolism and hydrolytic enzymes in the filamentous fungus Aspergillus oryzae using cDNA microarrays and expressed sequence tags. Appl Environ Microbiol. 2004;65:74–83. doi: 10.1007/s00253-004-1608-4. [DOI] [PubMed] [Google Scholar]
- Maubon D, Park S, Tanguy M, Huerre M, Schmitt C, Prévost MC, et al. ags3, an a(1–3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet Biol. 2006;43:366–375. doi: 10.1016/j.fgb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Mazon M, Behrens M, Morgado E, Portillo F. Low activity of the yeast cAMP-dependent protein kinase catalytic subunit Tpk3 is due to the poor expression of the TPK3 gene. Eur J Biochem. 1993;213:501–506. doi: 10.1111/j.1432-1033.1993.tb17787.x. [DOI] [PubMed] [Google Scholar]
- Mouyna I, Morelle W, Vai M, Monod M, Léchenne B, Fontaine T, et al. Deletion of gel2 encoding for a beta(1–3)glucanosyltransferase affects morphogenesis and virulence in Aspergillus fumigatus. Mol Microbiol. 2005;56:1675–1688. doi: 10.1111/j.1365-2958.2005.04654.x. [DOI] [PubMed] [Google Scholar]
- Ni M, Rierson S, Seo J-A, Yu J-H. The pkaB gene encoding the secondary protein kinase A catalytic subunit has a synthetic lethal interaction with pkaA and plays overlapping and opposite roles in Aspergillus nidulans. Eukaryot Cell. 2005;4:1465–1476. doi: 10.1128/EC.4.8.1465-1476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver B, Panepinto J, Fortwendel J, Smith D, Askew D, Rhodes J. Cloning and expression of pkaC and pkaR, the genes encoding the cAMP-dependent protein kinase of Aspergillus fumigatus. Mycopathologia. 2002a;154:85–91. doi: 10.1023/a:1015533406565. [DOI] [PubMed] [Google Scholar]
- Oliver BG, Panepinto JC, Askew DS, Rhodes JC. cAMP alteration of growth rate of Aspergillus fumigatus and Aspergillus niger is carbon-source dependent. Microbiology. 2002b;148:2627–2633. doi: 10.1099/00221287-148-8-2627. [DOI] [PubMed] [Google Scholar]
- Paisley D, Robson G, Denning D. Correlation between in vitro growth rate and in vivo virulence in Aspergillus fumigatus. Med Mycol. 2005;43:397–401. doi: 10.1080/13693780400005866. [DOI] [PubMed] [Google Scholar]
- Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, Roca MG, Read ND, Glass NL. Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot Cell. 2004;3:348–358. doi: 10.1128/EC.3.2.348-358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L, Komander D, Alessi D. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- Puttikamonkul S, Willger SD, Grahl N, Perfect JR, Movahed N, Bothner B, et al. Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol Microbiol. 2010;77:891–911. doi: 10.1111/j.1365-2958.2010.07254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Prost N, Saumon G. Glucose transport in the lung and its role in liquid movement. Respir Physiol Neurobiol. 2007;159:331–337. doi: 10.1016/j.resp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Reyes G, Romans A, Nguyen CK, May GS. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot Cell. 2006;5:1934–1940. doi: 10.1128/EC.00178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JC, Oliver BG, Askew DS, Amlung TW. Identification of genes of Aspergillus fumigatus up-regulated during growth on endothelial cells. Med Mycol. 2001;39:253–260. doi: 10.1080/mmy.39.3.253.260. [DOI] [PubMed] [Google Scholar]
- Richie D, Hartl L, Aimanianda V, Winters M, Fuller K, Miley M, et al. A role for the unfolded protein response (upr) in virulence and antifungal susceptibility in Aspergillus fumigatus. PLoS Pathog. 2009;5:e1000258. doi: 10.1371/journal.ppat.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LS, Fink GR. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc Natl Acad Sci USA. 1998;95:13783–13787. doi: 10.1073/pnas.95.23.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LS, Causton HC, Young RA, Fink GR. The yeast A kinases differentially regulate iron uptake and respiratory function. Proc Natl Acad Sci USA. 2000;97:5984–5988. doi: 10.1073/pnas.100113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano J, Nimrod G, Ben-Tal N, Shadkchan Y, Baruch K, Sharon H, Osherov N. Disruption of the Aspergillus fumigatus ECM33 homologue results in rapid conidial germination, antifungal resistance and hypervirulence. Microbiology. 2006;152:1919–1928. doi: 10.1099/mic.0.28936-0. [DOI] [PubMed] [Google Scholar]
- Thevelein JM, Geladé R, Holsbeeks I, Lagatie O, Popova Y, Rolland F, et al. Nutrient sensing systems for rapid activation of the protein kinase A pathway in yeast. Biochem Soc Trans. 2005;33:253–256. doi: 10.1042/BST0330253. [DOI] [PubMed] [Google Scholar]
- Toda T, Cameron S, Sass P, Zoller M, Wigler M. Three different genes in S. cerevisiae encode the catalytic subunit of the cAMP-dependent protein kinase. Cell. 1987;50:277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- Valiante V, Heinekamp T, Jain R, Härtl A, Brakhage AA. The mitogen-activated protein kinase MpkA of Aspergillus fumigatus regulates cell wall signaling and oxidative stress response. Fungal Genet Biol. 2008;45:618–627. doi: 10.1016/j.fgb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wagener J, Echtenacher B, Rohde M, Kotz A, Krappmann S, Heesemann J, Ebel F. The putative {alpha}-1,2-mannosyltransferase AfMnt1 of the opportunistic fungal pathogen Aspergillus fumigatus is required for cell wall stability and full virulence. Eukaryot Cell. 2008;7:1661–1673. doi: 10.1128/EC.00221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willger S, Puttikamonkul S, Kim K, Burritt J, Grahl N, Metzler L, et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200. doi: 10.1371/journal.ppat.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wilkinson HH, Correa A, Lewis ZA, Bell-Pedersen D, Ebbole DJ. Transcriptional response to glucose starvation and functional analysis of a glucose transporter of Neurospora crassa. Fungal Genet Biol. 2004;41:1104–1119. doi: 10.1016/j.fgb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Xue T, Nguyen CK, Romans A, May GS. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot Cell. 2004;3:557–560. doi: 10.1128/EC.3.2.557-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Dickman MB. Colletotrichum trifolii mutants disrupted in the catalytic subunit of cAMP-dependent protein kinase are nonpathogenic. Mol Plant Microbe Interact. 1999;12:430–439. doi: 10.1094/MPMI.1999.12.5.430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.