Abstract
The gastrointestinal (GI) tract is divided into several segments that have distinct functional properties, largely absorptive. The gastric corpus is the only segment thought of as largely secretory. Microarray hybridization of the gastric corpus mucosal epithelial cells was used to compare gene expression with other segments of the columnar GI tract followed by statistical data subtraction to identify genes selectively expressed by the rat gastric corpus mucosa. This provides a means of identifying less obvious specific functions of the corpus in addition to its secretion-related genes. For example, important properties found by this GI tract comparative transcriptome reflect the energy demand of acid secretion, a role in lipid metabolism, the large variety of resident neuroendocrine cells, responses to damaging agents and transcription factors defining differentiation of its epithelium. In terms of overlap of gastric corpus genes with the rest of the GI tract, the distal small bowel appears to express many of the gastric corpus genes in contrast to proximal small and large bowel. This differential map of gene expression by the gastric corpus epithelium will allow a more detailed description of major properties of the gastric corpus and may lead to the discovery of gastric corpus cell differentiation genes and those mis-regulated in gastric carcinomas.
Keywords: transcriptome, stomach
the function of an organ depends on the genes and protein products expressed and their regulation. Much has been learnt by classical physiological or biochemical approaches, but the complexity of biology suggests that many functions may be overlooked by conventional approaches. If a complete and specific gene expression profile were available, a more realistic understanding of a specific organ's capabilities would ensue.
The gastric corpus epithelium is the most complicated region of the gastrointestinal (GI) tract in terms of secretion, endocrine function, and differentiation due to its large variety of cell types. In this article, we present the specific transcriptome of the rat gastric corpus mucosa as obtained by whole rat genome microarray hybridization compared with three other mucosal segments of the columnar GI tract (proximal and distal small bowel, and colon). This is followed by statistical data subtraction to identify genes selectively expressed by the rat gastric corpus mucosa, but not by the other segments. Genes identified with this method most likely reflect functional importance in the stomach when considered in the context of relative rather than absolute expression levels. For example, many receptors are expressed at relatively low levels but their increased expression relative to other regions of the GI tract indicates significant function in the gastric secretory epithelium. The success of this method has been previously shown during transcriptomal analysis of purified suspensions of individual cell types, such as parietal and enterochromaffin-like (ECL) cells compared with the primary mucosal digest of the gastric corpus mucosa, which gave new insights into the interaction between these two cell types, such as apelin and the apelin receptor or the main pathway for regulation of histamine levels in the ECL cell (43–45). Similar approaches have been used to isolate and characterize regulation of gene expression of primary gastrointestinal epithelial cells, especially gastric corpus mucosal cell suspensions (3, 32, 82), purified mouse gastric parietal cells (31, 52), gastric chief cells (51), and gastric epithelial progenitor cells (50).
Furthermore, we confirmed gene expression identified by the above method in specific cases by RT-qPCR. As gene expression may not always result in protein levels, we evaluated protein expression by fluorescent immunohistochemistry in one selected case. This approach shows how statistical subtraction of gene expression profiles from microarrays results in the detection of expression of previously unsuspected proteins in a given organ indicating novel functions. Analysis of the function of specifically expressed genes in the stomach corpus mucosa will result in a more complete picture of gastric function compared with other GI segments being the first digestive organ encountered by food. This analysis will help to explain how the secretory portion of the stomach accomplishes the multitude of its essential processes and may reveal new functions of this interesting epithelium.
MATERIALS AND METHODS
Animals and Experimental Design
Adult male Sprague-Dawley rats (Harlan, San Diego, CA) (body wt 250–300 g) were housed in groups of four animals per cage under conditions of controlled illumination (12-h light, 12-h dark cycle: lights on at 0600 and off at 1800), humidity (60%), and temperature (22 ± 2°C). Animals were fed with a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water ad libitum. Rats were divided into four equal groups of four rats each. Each group was killed using CO2 inhalation followed by cervical dislocation. All experimental protocols are approved by the Research Committee of the VA Greater Los Angeles Healthcare System (#04032-02). Cell isolation from gastric, upper small bowel (duodenum and jejunum), lower small bowel (ileum), and colonic epithelium was performed as described before (43–45). Briefly, a cell suspension was generated by controlled digestion and separated by elutriation, the mRNA isolated and after labeling hybridized to a whole rat genome microarray (Agilent Technologies, Santa Clara, CA).
Gastric Oxyntic Mucosal Cells
Stomachs were removed and everted, and contents washed out. Ligations were placed above the gastric antrum and at the forestomach. Solution A [1–1.5 ml, 50 mM HEPES, 350 μM EDTA, 0.5 mM NaH2PO4, 1 mM Na2HPO4, 20 mM NaHCO3, 70 mM NaCl, 20 mM KCl, and 11 mM D(+)-glucose, pH 7.8] containing 10 mg/ml pronase E from Streptomyces griseus (Roche Diagnostics, Indianapolis, IN) was injected into the inside of the everted stomachs, which were then incubated at 37°C for 30 min in 50 ml solution A to allow inactive pronase to diffuse from the serosal to the mucosal side of the gastric wall. Solution A was discarded, and the stomachs were incubated for 10 min in solution B (same as solution A, but without EDTA and with 10 mM Ca2Cl and 15 mM Mg2Cl, pH 7.4, gentle stirring), which activates pronase to release most of the gastric corpus mucosal cells into the bathing solution. This was confirmed by hematoxylin and eosin (H&E) staining of sections of stomach wall remnants after digestion (data not shown). The cells were filtered through a nylon sieve, washed twice in solution C (140 mM NaCl, 1.2 mM MgSO4, 1 mM CaCl2, 10 mM HEPES, 11 mM glucose, and 0.5 g/l BSA, pH 7.4 containing 300 mg/l dithiothreitol, DTT, to avoid cell clumping), centrifuged at 1,100 rpm for 3 min in a Sorvall centrifuge, and finally concentrated to 10 ml volume. This suspension was injected into a zonal rotor of an elutriator (Beckman Coulter, Fullerton, CA) spinning at 1,400 rpm with a counter flow rate of 8 ml/min. After stabilization of the suspension in the chamber of the rotor the flow rate was increased to 13 ml/min to remove cell debris. Single cells suspensions were removed by reducing rotor speed to 950 rpm and increasing flow rate to 65 ml/min to avoid clumping.
Small and Large Bowel Epithelial Cells
Parts of the proximal duodenum (1.5 cm), jejunum (3 cm), terminal ileum (5 cm), and colon (5 cm) were removed and everted, and contents washed out. Ligations were placed on one end, and the sacs filled with solution A containing 10 mg/ml pronase E. A second ligation was performed to obtain solution A filled fluid-tight sacs. These were incubated for 15 min at 37°C in 50 ml solution A. Solution A was discarded, and the inside out sacs were incubated for 7 min in solution B with gentle stirring. This method of pronase digestion does not damage the basement membrane of the bowel mucosa. H&E-stained sections confirmed that only epithelial cells were released from the villi and crypts of the bowel, but the submucosal villi and folds remained intact (data not shown). The cells were filtered through a nylon sieve, washed twice in solution C (140 mM NaCl, 1.2 mM MgSO4, 1 mM CaCl2, 10 mM HEPES, 11 mM glucose, and 0.5 g/l BSA, pH 7.4 containing 300 mg/l DTT to avoid cell clumping), centrifuged at 1,100 rpm for 3 min in a Sorvall centrifuge and finally concentrated to 10 ml volume. This suspension was injected into a zonal rotor of an elutriator (Beckman) spinning at 1,400 rpm with a counter flow rate of 8 ml/min. After stabilization of the suspension in the chamber of the rotor the flow rate is increased to 13 ml/min to remove cell debris. Single cell suspensions were removed by reducing rotor speed to 950 rpm and increasing flow rate to 25 ml/min.
cRNA Labeling, Quality Assessment, Whole Rat Genome Oligonucleotide Microarray Hybridization
We used 100 μl of the cell suspensions to isolate total RNA using a NucleoSpin RNA II Kit (BD Biosciences, San Jose, CA). The RNA was assessed regarding purity and stability using the Agilent Bioanalyzer 2100 (Agilent Technologies). The typical RNA concentration was 300–500 ng/μl. RNA integrity number (RIN) was 9.9–10. Fluorescently labeled cRNA was generated using 500 ng total RNA in a reverse transcriptase reaction with a poly d(T) - T7 promoter primer followed by T7 polymerase based linear amplification in the presence of fluorophore labeled nucleotides, Cy3- or Cy5-CTP according to the manufacturer's protocol (Low RNA input Fluor Linear Amp Kit, Agilent Technologies). The final cRNA concentration of typically 300–500 ng/μl and the cyanine 3- or cyanine 5-cytidine incorporation of 5–10 pmol/μg cRNA, a measure for efficiency of dye incorporation, were determined using a Nanodrop spectrophotometer ND-1000 (Nanodrop Technologies, Wilmington, DE). Labeled cRNA (3.5 μg) samples were hybridized to a 44K rat oligonucleotide expression array (Agilent Technologies) according to the manufacturer's protocol. Each set of three independent microarray experiments per regional epithelial cell suspension of the GI tract contained one dye swap experiment. All microarrays were scanned and the intensities normalized over background using a microarray scanner from Agilent Technologies including proprietary software.
Subtraction of Whole Rat Gastric Microarray Transcriptome From the Mean of Proximal, Distal Small Bowel and Colonic Transcriptomes Using Statistical Data Analysis
All microarray data sets (single channel) were imported into the microarray data analysis software Genespring 7.3 (Agilent Technologies) and normalized intensities compared. The software is able to calculate the ratios and statistical significance of difference between large sets of data (i.e., four sets with three data points from each regional GI tract epithelial cell suspension). Data presented in the paper are expressed as the mean of three ratios ± SD as analyzed by one-way ANOVA. Differences between the gastric corpus mucosal transcriptome to proximal, distal small intestinal, and colonic mucosal transcriptomes were evaluated by pair-wise multiple comparison procedures (built in statistical analysis by Genespring 7.3).
Each ratio represents the individual normalized intensity of each hybridized oligonucleotide spot of the gastric corpus mucosal transcriptome, divided by the median of all normalized intensities across the data set (12 samples). These ratios reflect the specificity of gene expression in the gastric corpus mucosal segment compared with all other segments (differential gene expression) and not necessarily the quantity of expression (which can be estimated by the total normalized intensities of fluorescence). Absence of genes listed here should not be interpreted as not being expressed at all but should be viewed as lacking sufficient differential expression between the gastric corpus epithelium and the other GI tract epithelia. Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). Equal expression ratios (ratio of 1) are displayed as black. The data are also presented as log ratios in an additional column adjacent to each individual heat map.
Normalized intensities can be obtained from the original data sets which are deposited for public access to the Gene Expression Omnibus (GEO) National Center for Biotechnology Information (NCBI) database with the series number (GSE19313) with the submission of 15 individual data sets (GSM479747, GSM479804, GSM479806, GSM479807, GSM479808, GSM479823, GSM479824, GSM479825, GSM479836, GSM479837, GSM479838, GSM479863, GSM479864, GSM479865, GSM479866).
Gene Validation by RT-qPCR
Based on the data obtained from the microarrays we selected seven genes to validate mRNA expression: colipase, carbonic anhydrase (CAH) 9, CAH11, and Atp10d for gastric epithelium, intestinal alkaline phosphatase for proximal small bowel, matrilysin (also matrix metallopeptidase 7) for distal small bowel, and mucosal pentraxin for colonic epithelial cells. These genes were chosen because they were selectively and highly expressed only in the respective GI segment. Total RNA from each segment was denatured at 65°C for 5 min and used to synthesize first-strand cDNA by reverse transcription with the ThermoScriptTM RT-PCR system (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed in duplicates using DNA Engine Opticon1 2 Detection System interfaced to the Opticon MONITORTM Analysis Software version 2.01 (MJ Research, Waltham, MA) in a 20 μl reaction volume. The optimized reaction contained 10 μl of SYBR1 Premix Ex TaqTM (Perfect Real Time; Takara Mirus Bio, Madison, WI), 1 μl each of oligonucleotide primers (10 mM), 1 μl of the cDNA synthesis reaction, and 7 μl of H2O. Each amplification was followed by a melting curve resulting in only one peak for each amplicon indicative of amplification of only one product. This was confirmed by agarose gel electrophoresis of the RT-PCR products. The cycle of threshold C(T) was determined as the fluorescent signal (binding of SYBR green to double-stranded cDNA) of 1 SD over background. All reactions were carried out in duplicate, and three separate amplifications for each primer pair were performed. Standard curves of efficiency of each primer pair were determined with four serial dilution points of control cDNA (100 ng-100 pg). Relative expression ratios were calculated using the method by Pfaffl (66). HPRT (hypoxanthine guanine ribotransferase) was used as the house keeping (control) gene for all amplifications.
Selected forward (f) and reverse (r) primers were (f)cagtcccagcgtcgtgatta and (r)agcaagtctttcagtcctgtc (HPRT), (f)agatgctgccaacatgacac and (r)cactgcacgatctcactgct (colipase), (f)gtgcaaagaaagcagggaag and (r)tccaccaaggatcacatcaa (intestinal alkaline phosphatase), (f)gagtgccagatgttgcagaa and (r)tctgcagtcccccaactaac (matrilysin), (f)cgcccttacagcatcttctc and (r)agaggcagactcccagttca (mucosal pentraxin), (f)gctcctagtgtccgctcatc and (r)gagggaatcctcctttctgg (CAH9), (f)ttccttagtcgcctcctcaa and (r)accactgaggctctggaaga (CAH11), as well as (f)gcagctgcctgaactttacc and (r)caatagagcggctgtgttca (Atp10d).
Fluorescent Double Immunohistochemistry and Microscopy
At 9 AM ad libitum fed rats (n = 3) were killed by CO2 anesthesia followed by cervical dislocation. The stomach was quickly removed, emptied, washed, and fixed in aqueous Bouin's fixative (5% acetic acid, 9% formaldehyde, 0.9% picric acid) for 2 h, then rinsed 3× in 50% ethanol to remove fixative residues. Thereafter, the tissue was processed following standard procedures and embedded in paraffin. We cut 5 μm paraffin sections of rat stomach corpus tissue using a microtome, deparaffinized and rehydrated with graded xylene-alcohol series and washed with phosphate-buffered saline (PBS) three times for 15 min before immunostaining. Endogenous peroxidase was inactivated by 0.3% hydrogen peroxide in PBS for 30 min and unspecific binding was reduced by pretreatment with 3% normal goat serum. Sections were incubated overnight at 4 °C in polyclonal rabbit anti-Atp10d antibody (1:10,000, SAB2100178, Sigma-Aldrich, Saint Louis, MO) together with monoclonal anti-H,K-ATPase (1:250, 12.18; Ref. 49). Tetramethyl rhodamine iso-thiocyanate (TRITC) conjugated goat anti-rabbit IgG (1:1,000, Jackson ImmunoResearch Laboratories, West Grove, PA) and fluorescein iso-thiocyanate (FITC)-labeled anti-mouse (1:1,000, Jackson ImmunoResearch Laboratories) antibodies were added for 2 h at room temperature. Each step of incubation was followed by a 3 × 5 min washing in PBS. Counterstaining was performed using 4′,6-diamidino-2-phenylindole (DAPI) 1:1,000 for 5 min. The slides were mounted with antifade mounting medium (Vector Laboratory, Burlingame, CA) and visualized by confocal microscopy (Zeiss, LSM 510, Germany). The slides were analyzed with low power view using a ×20 objective and high power view using a ×100 objective. Five high power views were analyzed for coexpression of Atp10d with H,K-ATPase in the gastric corpus mucosa.
Statistical Data Analysis and Data Presentation
All microarray data sets (single channel) were imported into the microarray data analysis software Genespring 7.3 (Agilent Technologies), and normalized intensities compared. The software is able to calculate the ratios and statistical significance of difference between large sets of data (i.e., four sets with three data points from each regional GI tract epithelial cell suspension). Microarray data presented in the paper are expressed as the mean of three ratios ± SD as analyzed by one-way ANOVA. Differences between groups are evaluated by all pair-wise multiple comparison procedures (built in statistical analysis by Genespring 7.3). RT-qPCR data were analyzed similarly using SigmaStat version 3.2. P < 0.05 was considered significant.
Each ratio represents the individual normalized intensity of each hybridized oligonucleotide spot, divided by the median of all normalized intensities across the data set (12 samples). These ratios reflect the specificity of gene expression in a given mucosal segment compared with all other segments (differential gene expression) and not necessarily the quantity of expression (which can be estimated by the total normalized intensities of fluorescence). Absence of genes listed here should not be interpreted as not being expressed but should be viewed as lacking sufficient differential expression between the stomach epithelium and the other GI tract epithelia. Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). Equal expression ratios (ratio of 1) are displayed as black. The data are also presented as log ratios in an additional column adjacent to each individual heat map.
Normalized intensities can be obtained from the original data sets, which are deposited for public access to the GEO NCBI database with the series number (GSE19313) with the submission of 15 individual data sets (GSM479747, GSM479804, GSM479806, GSM479807, GSM479808, GSM479823, GSM479824, GSM479825, GSM479836, GSM479837, GSM479838, GSM479863, GSM479864, GSM479865, GSM479866).
RESULTS AND DISCUSSION
The 44K whole rat genome expression array (G 4131A, Agilent Technologies) contains 41,012 annotated oligonucleotide probes. From these, 23,633 probes hybridized significantly with normalized fluorescent intensities of >1,000 (background fluorescent intensity is <100) with one or more gut segments, indicating substantial mRNA expression of 58% of genes of the whole rat genome in all or some of the assayed samples from these GI tract cell suspensions. The overall mean ratio value (each individual data point divided by the mean of all intensities measured in the entire data set) was 1 with a standard deviation of 0.77 illustrating the relatively low variation of mRNA concentration introduced by cell isolation, hybridization, and normalization of fluorescence intensities between the different GI tract segments. All experiments were performed in triplicate. About 18,000 mRNA probes (normalized fluorescent intensities of >1,000) had statistically similar intensities throughout all mucosal segments indicating equivalent and significant expression throughout the GI tract. The mRNA from gastric corpus showed the highest selectivity of expression compared with the rest of the GI tract and is therefore the focus of this paper.
Of the differentially expressed genes, emphasis is placed on selected members of gene families relatively highly expressed in the gastric oxyntic transcriptome (ratio >2, log ratio >0.3) that are likely to be significant in gastric physiology. Genes without known protein products or proteins with as yet undescribed physiological functions are ignored but are accessible through the publically available data sets at the GEO.
RNA Integrity and Validation of Relative Enrichment of Messages Along the GI Columnar Epithelium by Quantitative RT-qPCR
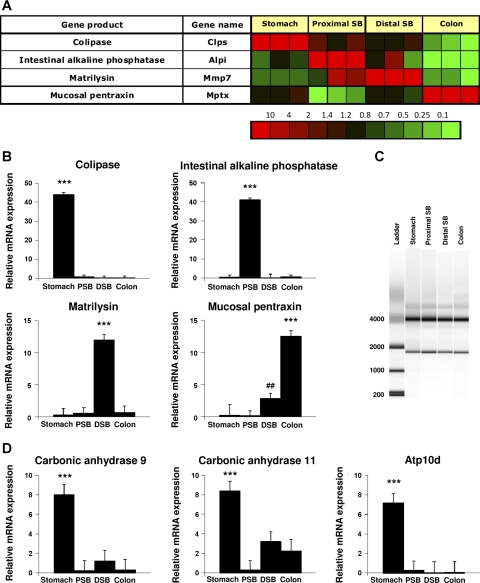
To confirm these microarray data, four genes that were found to be selectively and highly expressed in one particular segment (Fig. 1A) on the array were chosen. These were colipase for the stomach segment, intestinal alkaline phosphatase for the proximal small bowel, matrilysin representing the distal small bowel, and mucosal pentraxin representing the colonic epithelial cells. The microarray data showed a 3,000-, 70-, 20-, and 130-fold increase, respectively. The mRNA expression of these genes was assessed by RT-qPCR. In line with the microarray data, significant enrichment for all four genes was found in the expected segment compared with the other segments (P < 0.001, Fig. 1B). Thus, the selective expression of specific markers of different segments of the digestive system as found on the microarray was confirmed by RT-qPCR.
Fig. 1.
Gene validation by RT-qPCR of selected genes. Based on microarray gene expression data 4 genes previously known to be selectively expressed in 1 segment (A) were chosen and mRNA expression assessed by RT-qPCR. Significant enrichment (log ratio >1) was found for colipase in gastric epithelial cells, intestinal alkaline phosphatase in proximal small bowel, matrilysin in distal small bowel, and mucosal pentraxin in colonic epithelial cells (B). C: integrity of RNA of gastric, proximal small bowel, distal small bowel, and colon epithelial cell isolations was determined by the electrophoretic trace of the RNA sample. The software algorithm allows for the classification of eukaryotic total RNA, based on a numbering system from 1 to 10, with 1 being the most degraded profile and 10 being the most intact. RNA integrity number (RIN) for the four samples was 9.9–10.0. D: 3 additional, novel genes were validated by RT-qPCR and significant mRNA expression of carbonic anhydrases (CAH) 9, CAH11, and the flippase Atp10d was found in the gastric corpus epithelium. Abbreviations: DSB, distal small bowel; PSB, proximal small bowel. ***P < 0.001 vs. all other groups, ##P < 0.01 vs. stomach and proximal small bowel.
Integrity of RNA of gastric, proximal small bowel, distal small bowel, and colon epithelial cell isolations was determined by capillary electrophoresis. Classification of riboeukaryotic total RNA is based on a numbering system from 1 to 10, with 1 being the most degraded RNA profile and 10 being the most intact RNA. RIN for the four samples was 9.9–10.0 (Fig. 1C) showing that the RNA isolated from the different regions of the gut was of adequate quality for transcriptomal analysis.
Acid Secretion and pH Homeostasis
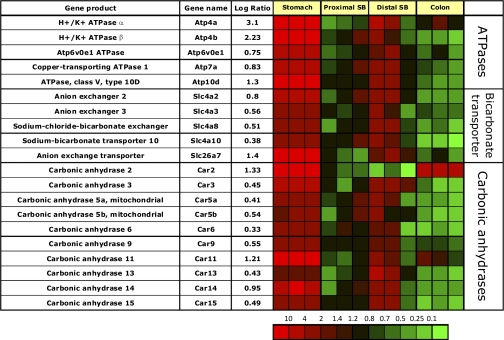
Regional expression of transport ATPases.
There is the expected gastric-specific expression of the parietal cell-specific H,K-ATPase α- and β-subunits (Atp4a and b, Fig. 2). In two of the ileal samples there was also minor expression of the H,K-ATPase α-subunit mRNA, at a 1,000-fold lower level. This might be due to background labeling of another gene since it is not accompanied by a signal for the H,K-ATPase β-subunit but correlates with overlap of gene expression in this segment of the gut with many of the genes in the gastric corpus transcriptome perhaps originating from surface, chief, or stem/progenitor cells. In contrast there is depletion of most of the gastric corpus genes discussed here in the proximal small bowel and in the colon.
Fig. 2.
Segment-specific expression of genes involved in acid secretion and pH homeostasis. This figure illustrates the relative distribution of genes encoding ATPases, bicarbonate transporters, and CAHs that are selectively expressed in epithelial cells of gastric corpus compared with PSB and DSB, or colon in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
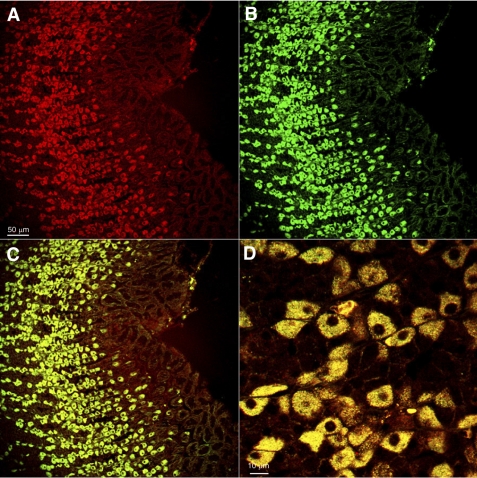
There is high expression of a phospholipid transporting type IV p-type ATPase (lipid flippase, Atp10d), previously implicated in pathophysiology of obesity (14, 15). High expression of this gene was confirmed by RT-qPCR (Fig. 1D), and immunofluorescent staining localized this ATPase to parietal cells as shown by double immunofluorescent labeling with H,K-ATPase (Fig. 3). The presence of this flippase may be required for remodeling of the H,K ATPase membrane after parietal cell transformation between rest and secretion.
Fig. 3.
Protein expression of the flippase Atp10d in the gastric corpus mucosa and colocalization with gastric H,K-ATPase in parietal cells. Confocal microscopy of sections of rat gastric corpus doubly labeled with an antibody against Atp10d (red, A) and an antibody against the H,K-ATPase (green, B), showing that all Atp10d-immunoreactive cells in the gastric corpus colocalize with H,K-ATPase (C, D), a marker for parietal cells. The high-power confocal image (D) shows complete colocalization of this protein with the gastric H,K-ATPase within individual parietal cells (×100 objective, acquisition of fluorescent signals using multitract sequential scan).
Although previously suggested to be expressed in the small bowel (59), there is significant enrichment of message for the copper transporting Menkes gene product (Atp7a), while Wilson's gene product (Atp7b) is not expressed. Atp7a has a housekeeping role for cellular copper homeostasis and may reflect the high mitochondrial content of the parietal cell (25). This ATPase is usually located in the trans-Golgi network and is required for regulation of copper supply to copper requiring enzymes (for review see Ref. 46).
Finally, there is relatively high expression of Atp6v0e1, a member of the Vo component of the lysosomal ATPase, perhaps due to the presence of zymogen granules in the chief cell.
Expression of bicarbonate transporters.
Messages for three of the four anion exchangers are enriched in the gastric corpus mucosa. The significant enrichment of message encoding the Cl−/HCO3− exchanger (anion exchange transporter, Slc26a7) in the gastric mucosa confirms previous gastric parietal cell expression data (65, 86) and likely relates to the need for bicarbonate export that results from the H2CO3 supplying protons for acid secretion.
Segmental enrichment of messages coding for bicarbonate transporters is modest (Fig. 2), indicating these transporters have, in general, a universal function throughout the GI tract. However, some are enriched in the gastric transcriptome.
Expression of carbonic anhydrases.
There are similar levels of expression of sodium proton exchangers across all regions of the GI tract mucosa, indicating that the entire epithelium of the GI tract shares this pH regulatory pathway. By contrast, analysis of messages encoding the 15 known α-CAH shows segment-specific expression patterns (Fig. 2). These carbonic anhydrases can be classified into three functional groups: cytosolic CAH (1, 2, 3, 7 and 13), membrane bound (4, 9, 12, 14, and 15), and cytosolic acatalytic CAH-related proteins (8, 10, and 11). The gastric corpus mucosa is enriched in message for cytosolic CAH2 and CAH11 and membrane-bound CAH9 and CAH14.
The role of CAH in parietal cells is thought to be supply of adequate quantities of H2CO3 for supply of cytoplasmic H+ for the H,K-ATPase. The combination of significant expression of membrane-bound CAH14 (extracellular activity) and cytosolic CAH2 (intracellular activity) supports a model for bicarbonate exchange across the plasma membrane of gastric corpus mucosal cells in combination with anion exchangers AE1–4, as has been shown for CAH14 in hippocampal neurons (79). High gastric corpus expression of CAH9 and CAH11 was confirmed by RT-qPCR (Fig. 3, B and C), but the functional significance of these carbonic anhydrases in the gastric corpus mucosa is unknown. They may be present to supply adequate quantities of H2CO3 for acid secretion or be analogous to the function of CAH8, which has been shown to be involved in inhibition of the IP3 receptor during intracellular calcium activation (87). Both CAH11 and CAH9 have been implicated in the pathophysiology of gastric neoplasia (55, 63). There is also slight gastric epithelial enrichment of the mitochondrial CAH 5a/b probably due to the high concentration of mitochondria in gastric parietal cells.
Therefore, this microarray analysis has confirmed the presence of several genes implicated in gastric acid secretion, the ATPases, two carbonic anhydrases, and one anion exchanger or transporter. In addition, it has revealed expression of other ATPases and anion transporters.
Gastric Expression of Ion and Water Channels
The oxyntic mucosa is a secretory epithelium generating 1.5 l of 0.1 N hydrochloric acid (HCl) per day in humans, as well as pepsinogen, and considerable quantities of mucus and bicarbonate (71). HCl is secreted by the gastric H,K-ATPase dependent on K+ supply by K+/Cl− efflux from the apical surface of the parietal cell, therefore requiring supplies of these ions on the basolateral surface and intracellular pH homeostasis. In addition, this region of the stomach contains multiple neuroendocrine cells that have secretory mechanisms dependent on changes of [Ca2+]in and membrane potential (92). It was anticipated, therefore, that the corpus mucosa would express significantly higher levels of ion and water channels compared with other segments of the intestine.
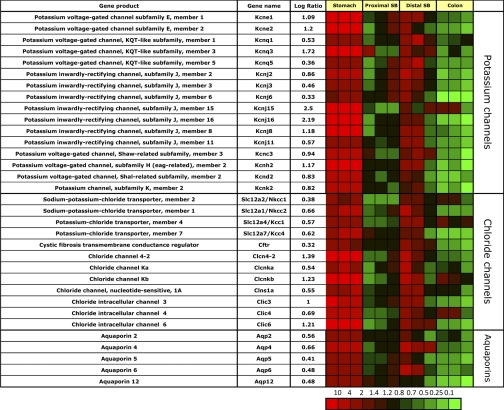
Expression of potassium channels.
Several potassium channel messages are enriched in the gastric corpus mucosa (Fig. 4), and there is also expression of many of these genes in the ileum but not proximal small bowel or colon.
Fig. 4.
Segment-specific expression of genes for ion and water channels. This figure illustrates the relative distribution of genes encoding potassium and chloride channels as well as aquaporins that are selectively expressed in epithelial cells of gastric corpus, compared with PSB, DSB, and colon in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
The K+ channel, Kcnq1, is highly expressed in gastric parietal cells (45) and has been shown to enable potassium export across the canalicular membrane of the parietal cell (75). This gene is also expressed in the ileum. Two of the regulatory β-subunits of Kcnq1, Kcne1 and especially Kcne2, are all enriched in the gastric corpus mucosa. While Kcne2 is expressed in parietal cells (45), Kcne1 is not, indicating that it is Kcnq1 and Kcne2 that form the parietal cell-specific potassium channel heterodimer related to acid secretion. In addition, another isoform of Kcnq1, Kcnq3, is also enriched in the gastric corpus mucosa and in parietal cells but not ECL cells (45), perhaps indicating additional subtypes of the Kcnq-Kcne potassium channel complexes in parietal cells, perhaps to supply K+ on their basolateral surface to the acid secretion Kcnq1/Kcne2 complex. There is also gastric corpus mucosal and parietal cell-specific expression of Kir4.2 and 5.1 (Kncj15 and Kcnj16), which form a potassium inward-rectifying pH-sensitive heterodimeric channel (64). It has been claimed that Kir4.2 localizes to the secretory membrane upon stimulation (37). The role of these inward rectifying channels in parietal cells is as yet unknown, but some are likely required to replenish cell K+ for the loss due to acid secretion since the H,K-ATPase is not able to recycle all of the K+ exiting the canalicular membrane.
Further gastric-specific expression was found for Kir3.2 and Kir3.1 (Kcnj6 and Kcnj3), which form a channel with IKrAC-like currents and have been shown to be present in the lamina propria muscularis of the GI tract (5). They are regulated by muscarinic receptors and may be involved in contraction of the muscularis mucosae (5).
The strong enrichment of Kir7.1, Kcnh2 (ERG channel), and Kcnk2 (Trek-1) in ECL cells (45) indicates an important role in ECL cell physiology likely related to histamine secretion perhaps counteracting depolarization during histamine exocytosis. Two-pore domain potassium channels such as Kcnk2, have recently been colocalized with ECL cells and with the majority of ghrelin-immunoreactive X/A-like cells in rats (56). These channels therefore probably reflect properties of the neuroendocrine cell population of the gastric corpus.
Kir2.1 (Kcnj2) and Kcnc3 are also enriched in gastric corpus mucosal suspensions but are only slightly enriched in ECL cell suspensions with no parietal cell expression (45), indicating expression in another gastric corpus mucosal cell such as other endocrine, mucus, chief, or stem cells.
Expression of chloride channels.
The gastric corpus mucosa is enriched in messages for many chloride channels. These channels may contribute to the stomach's main function of acid secretion (Fig. 4). There is corpus-specific expression of the intracellular chloride channel (Clic6, parchorin), which has been shown to be specifically expressed in gastric parietal cells and suggested to be responsible for chloride secretion at the stimulated canalicular membrane of parietal cells (58). There is also gastric corpus mucosal specific enrichment of message of the voltage gated chloride channels Clcn4–2 and Clcnkb, and Clcnka. Clcn4–2 and Clcnkb were enriched in a previous expression analysis of gastric parietal cells (44) and may represent candidate proteins involved in basolateral chloride uptake by parietal cells during acid secretion. Other messages enriched in the gastric corpus mucosa encode Clic3 and 4. The latter two genes were not enriched in gastric parietal or ECL cells (data not shown), indicating possible surface or chief cell expression.
There are several genes encoding Na+/Cl− and K+/Cl− transporters, likely reflecting the need for homeostasis of these ions in acid pepsinogen secretion and gastric neuroendocrine cell function. The plethora of cation and chloride transporters in the gastric corpus mucosa suggests very specific functions for most of them that still remain to be discovered, reflecting the heterocellular nature of this region of the stomach. In terms of the remainder of the intestine, only the ileum expresses many of these genes, while the jejunum and colon express very few of these Cl− transporters.
Expression of aquaporins.
Messages for aquaporins are present in all GI epithelia. However, different subtypes are selectively expressed that may determine specific association with other transporters (Fig. 4).
Aquaporins 2, 4, 5, 6, and 12 messages are enriched in the gastric mucosa compared with jejunum and colon, and aquaporins 4 and 6 are also enriched in the ileum. Since aquaporin 2 has been shown to cycle between intracellular vesicles and the apical surface of renal collecting ductal cells (36), its gastric-specific expression suggests that aquaporin 2 and perhaps 4 allows gastric mucosal apical water secretion driven by the outward KCl gradient. The role of the other gastric enriched aquaporins has not been identified.
Endocrine Regulation
Expression of general endocrine mediators and receptors.
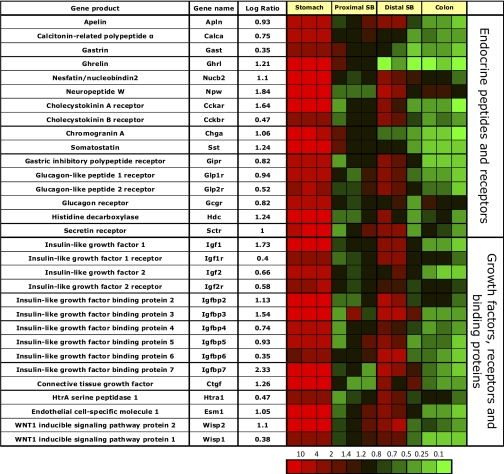
As previously described, the gastric corpus mucosa is enriched in messages coding for calcitonin gene-related polypeptide (CGRP, Ref. 42) and apelin, which is expressed in gastric parietal cells and plays a role in a negative feed-back loop between parietal and gastric ECL cells that express the apelin receptor (45) (Fig. 5). Furthermore, messages coding for gastrin (21), ghrelin and NUCB2/nesfatin-1 (78), and neuropeptide W (6) are all enriched, indicating a significant role of the gastric corpus mucosa in regulating food intake and energy balance (Fig. 5). Ghrelin and chromogranin A messages are selectively enriched in the gastric corpus mucosa due to expression in ghrelin and ECL cells (9). Neither chromogranin B nor C is enriched in any of the intestinal regions (data not shown). Message coding for somatostatin is much higher in the gastric corpus mucosa compared with the other small and large intestinal epithelial segments, indicating that gastric D-cells appear to be the major source of somatostatin in the rat GI corpus mucosa. The enrichment for histidine decarboxylase reflects the need for histamine generation by the ECL cell.
Fig. 5.
Segment-specific expression of genes encoding endocrine mediators, growth factors, and receptors and binding proteins. This figure illustrates the relative distribution of genes encoding endocrine ligands, growth factors, and their receptors and binding proteins that are selectively expressed in epithelial cells of gastric corpus compared with the other GI segments in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
The gastric corpus mucosa is enriched in messages coding for a variety of receptors, whose ligands are mainly expressed in the small bowel mucosa. These include receptors for CCK, gastric inhibitory peptide (Gip), secretin, and glucagon (Fig. 5). The CCK-A receptor is highly enriched due to its role as a secretagogue for chief cells (24). The CCK-B/gastrin receptor is also enriched, but to a much lower degree compared with the CCK-A receptor, due to significant expression in the ileum but not jejunal or colonic mucosa. Messages coding for all three incretin receptors (GIP-R, GLP-1 and 2 receptors) are significantly enriched in the gastric corpus mucosa, indicating a role for this segment in appetite control and glucose homeostasis.
The presence of significant GIP-R as well as lipoprotein lipase messages (see Fig. 4) in the gastric corpus mucosa in conjunction with recently published data (40) suggests endocrine activation of lipoprotein lipase activity by GIP released by the upper small bowel mucosa to increase triglyceride concentration in the stomach. This may represent a novel regulatory feedback loop between small bowel and stomach to meet the unique energy requirements of the gastric corpus mucosa during acid secretion.
Message for the secretin receptor is also seen in the gastric corpus mucosa likely expressed in somatostatin expressing D cells and involved in inhibition of acid secretion (74).
Expression of growth factors (GF), receptors, and binding proteins.
Although thought to be mainly expressed in liver tissue, messages coding for many insulin-like growth factors are highly enriched in the gastric mucosa (Fig. 5).
Messages encoding insulin-like growth factor (Igf)-1, Igf-2 and the two Igf receptors are enriched. This supports recent research showing that glucose levels and ghrelin release appear to increase serum Igf concentration (84). Also, exogenously administered as well as endogenously released Igf-1 has been shown to prevent gastric erosions and ulcers and to promote mucosal proliferation and growth (7, 41). IGF function in tissues is tightly regulated through Igf binding proteins. There is also enrichment of messages for most of the high-affinity Igf-binding proteins (Igfbp) with the exception of Igfbp1 in the gastric mucosa, extending previous data on gastric cell lines (90). Concomitant expression of Igf functional inhibitory (Igfbp4) and stimulatory peptides (Igfbp3 and 5) indicates a finely tuned regulatory system of Igf activity within the gastric mucosa. These receptors likely play an important role in cell differentiation in this region of the gut. As observed before, many of these receptors are also expressed in the ileum but not jejunum or colon.
Messages for a variety of other growth factors are also enriched in the gastric mucosa. These data support the hypothesis that the stomach plays a major role in nutrient homeostasis and glucose metabolism.
Epithelial Protection, Regeneration, and Innate Immune Response
Epithelial protection and regeneration.
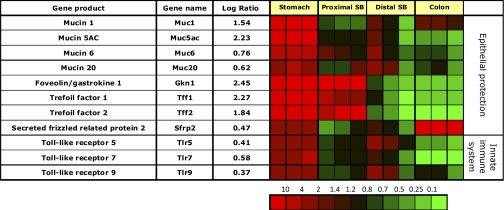
There is gastric enrichment of messages encoding epithelial mucins such as foveolin and trefoil factors. Mucins play an important role in recognition of the innate immune system to sense luminal bacterial and toxic agents. The stomach is the first organ to receive ingesta and thus requires significant protection against possible damage (Fig. 6).
Fig. 6.
Segment-specific expression of genes that code for proteins involved in epithelial protection, regeneration, and innate immune system. This figure illustrates the relative distribution of genes encoding proteins implicated in epithelial protection, regeneration, and innate immune system response that are selectively expressed in epithelial cells of the gastric corpus compared with PSB, DSB, and colon in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
Many of these protective genes encode membrane bound or secreted proteins, and their expression is downregulated in GI carcinomas (20, 54, 60, 61). Muc1, 5ac, and 6 are highly enriched and are main constituents of the mucus barrier in the stomach, which protects the underlying epithelium from proteases, mechanical trauma, and pathogenic microorganisms (35). Accumulating evidence implicates potential roles of Muc1, Muc5ac, and Muc6 genetic variation in the development of stomach cancer (35). In a knockout mouse model, Muc1 has been shown to influence both Helicobacter pylori colonization of the murine gastric mucosa and the associated gastritis (47). Muc5ac is only expressed in gastric foveolar epithelium (73), and is associated with trefoil factor Tff1 (57).
The gastric mucosa is highly enriched in message encoding the gastro-protective peptides foveolin (gastrokine 1 Ref. 60) and two trefoil factors such as Tff2 (spasmolytic polypeptide, SP1). These peptides promote mucosal regeneration (26, 68) and may serve as chaperones in proper protein folding (81). Foveolin and trefoil factor genes remain enriched in the jejunum, but not in ileum or colon. Hence, the corpus and jejunum share these regenerative gene messages.
The Wnt signaling pathway inhibitor, secreted Frizzled-related protein 2 (sFRP2) is enriched in corpus but particularly in colonic epithelium and must be involved in the morphogenesis of these two segments of the GI tract. This protein maintains normal mucosal regeneration by regulating the rate of terminal differentiation of stem cells by inhibition of the β-catenin/Wnt signaling pathway. Inhibition of this signaling pathway in the stomach leads to terminal gastric differentiation of stem cells (38).
Innate immune system.
There is gastric corpus mucosal enrichment of messages for Toll-like receptors (Tlr) 5, 7, and 9 (Fig. 6), which also play a role in activation of the immune system during H. pylori or other bacterial infections (70). A recent report suggests that activation of these receptors by luminal agents induces histamine decarboxylase expression in the gastric corpus mucosa, which may result in increased histamine production and hence acid secretion, perhaps intended to increase the antibacterial effect of acid secretion (19, 67).
Regulation of Transcription
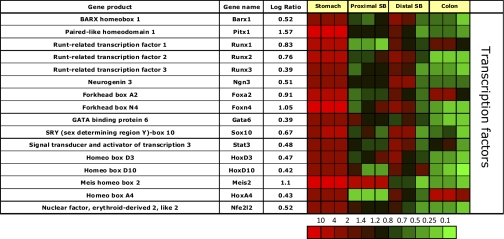
The gastric corpus mucosa is enriched in message for the homeobox transcription factor Barx1, which has been recently described to inhibit expression of secreted Frizzled-related protein 2 (sFRP2, Fig. 7) (38). Inhibition of Wnt signaling by sFRPs in the stomach leads to gastric differentiation of stem cells (38). Presumably this expression is related to regulation of stem cell differentiation.
Fig. 7.
Segment-specific expression of genes coding for transcription factors. This figure illustrates the relative distribution of genes encoding transcription factors that are selectively expressed in epithelial cells of the gastric corpus in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
In addition to sFRP2, Barx1 controls expression of another transcription factor Pitx1, and of one of the Igf binding proteins, Igfbp4 (38). While it has been postulated that Barx1 is only transiently expressed during fetal organogenesis (38), these data show sustained expression, since messages for this transcription factor and its downstream regulated transcripts sFRP2, Pitx1, and Igfbp4 are all enriched in the adult rat gastric corpus mucosa (see Figs. 5 and 6).
The gastric corpus mucosa is also enriched in messages coding for three Runt-related transcription factors, Runx 1–3 (Fig. 7). These factors are crucial for stem cell differentiation in addition to Barx1. Runx3 regulates gastric epithelial cell differentiation (17, 18). Runx1 and 3 are downregulated in intestinal-type gastric cancer cells, which are positive for Cdx2 (69), a transcription factor that is not expressed in the normal gastric corpus mucosa (11, 53), but ubiquitously expressed in the small bowel and colonic mucosa. This indicates that Runx factors and Barx1 differentiate stem cells toward a gastric lineage.
In addition to Barx1 and Runx 1–3, there is also expression of Gata6, a retinoic acid-inducible zinc finger transcription factor, in the gastric corpus mucosa adding to the multifactorial regulation of differentiation of this heterocellular epithelium.
Messages for transcription factors involved in endocrine cell differentiation are enriched in the gastric corpus mucosa, reflecting the presence of numerous endocrine cells in this segment of the GI tract. These include neurogenin 3 and forkhead transcription factor Foxa2, both of which have been shown to control D-cell differentiation (33, 89). The gastric corpus mucosa is also highly enriched in message for another forkhead transcription factor, Foxn4, which has not been described in cell differentiation of the gastric epithelium. It is also enriched in message for the nuclear factor, erythroid-derived 2, like 2 (Nfe2l2). Nfe2l2 is involved in regulation of antioxidant responses by upregulation of transcription of antioxidative stress genes (28). Nfe2l2 has been shown to increase transcription of aldehyde dehydrogenases 1a1 and 1a7 (1), messages of which are enriched in gastric and duodenal mucosa (see Fig. 10).
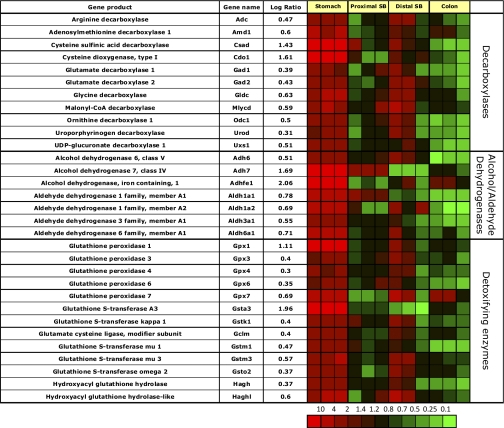
Fig. 10.
Segment-specific expression of genes coding for various enzymes involved in decarboxylation, alcohol dehydrogenation, and detoxification. This figure illustrates the relative distribution of genes encoding various enzymes involved in decarboxylation, alcohol dehydrogenation, and detoxification that are selectively expressed in epithelial cells of gastric corpus compared with PSB and DSB, or colon in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
The gastric corpus mucosa is also significantly enriched in message coding for one of the SRY-related HMG-box family of transcription factors, Sox10. This factor is crucial for the development of the neural crest, and maintains melanocytic differentiation. In the GI tract, it has been shown that Sox10 knockout mice are aganglionic, displaying a phenotype seen in Hirschsprung disease (77). It may be that the gastric corpus mucosal enrichment of the message observed in our data set originates from intramucosal cells of neural origin.
The gastric corpus mucosa is also enriched in Stat3 mRNA (Fig. 7), which has been implicated in the control of the apelin promoter during states of colonic inflammation (23). Apelin is expressed in gastric parietal cells and inhibits gastric acid secretion by inhibiting ECL cell histamine release (45) (see Fig. 4). It is likely that, in the inflamed gastric corpus mucosa, Stat3 induces increased apelin expression, which leads to decreased acid secretion, a likely protective mechanism often seen in chronic gastritis.
The gastric corpus mucosa is highly enriched in message for HoxD3, a homeobox transcription factor implicated in angiogenesis and wound healing. In addition, HoxD3 is also implicated in the control of aquaporin 2 expression in the renal collecting duct (91), which is also significantly expressed in the gastric corpus mucosa (Fig. 4). There is also enrichment of message for HoxD10, which has been shown to control renal renin expression (62). Both of these homeobox proteins require transcriptional cofactors (either Pbx1 or Pbx1-like proteins Meis1 or Meis2) for high-affinity DNA binding (8, 30, 88). Therefore, the gastric corpus mucosa is also highly enriched in message for Meis2. Clearly, there is a complicated pattern of transcription factor expression in the stomach, reflecting its marked cellular heterogeneity and complex morphology. HoxA4 gene expression is enriched in the colon and been shown to be down regulated in ulcerative colitis and colorectal carcinoma (80).
Digestion and Absorption
Expression of digestive enzymes.
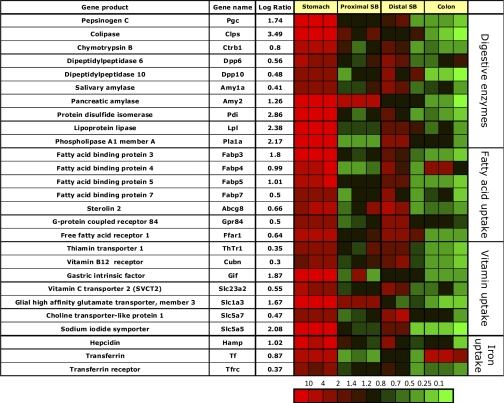
The gastric corpus mucosa shows very high enrichment of message (log ratio between 2 and 3.5) for colipase, lipoprotein lipase, and phosphatidylserine-specific extracellular phospholipase A1 (Pla1a, Fig. 8). The high expression of secreted lipases may indicate a high demand in this mucosa for energy precursors other than glucose most likely to provide the massive amounts of ATP needed for acid secretion. Other gastric corpus mucosal enriched messages coded for chymotrypsin B and the dipeptidylpeptidases 6 and 10 and even salivary and pancreatic amylase, although the latter is also enriched in jejunal epithelial cells.
Fig. 8.
Segment-specific expression of genes coding for various secreted digestive enzymes and involved in fatty acid, vitamin, and iron uptake. This figure illustrates the relative distribution of genes encoding digestive enzymes released into the lumen of different segments as well as proteins involved in uptake of fatty acids, vitamins, and iron in the GI tract that are selectively expressed in epithelial cells of gastric corpus compared with PSB and DSB, and colon in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
Expression of proteins facilitating lipid, cholesterol, and bile acid uptake, and chylomicron synthesis.
The gastric corpus mucosa shows enrichment in messages of a specific set of fatty acid binding proteins, Fabp 3–5 and 7, suggesting a high demand of this mucosa for energy precursors other than glucose to produce the ATP needed for acid secretion. This hypothesis is also supported by the high expression of secreted lipolytic enzymes in gastric corpus mucosa particularly Pla1a, colipase and lipoprotein lipase (Fig. 8).
In addition, the gastric corpus mucosa expresses two of the known five free fatty acid receptors (Ffar) 1 and Gpr84. These receptors are important luminal sensors of food intake (29) and are most likely expressed on entero-endocrine cells, where they stimulate the release of peptide hormones including incretins glucagon-like peptide (GLP) 1 and 2 (12). Ffar 1 (Gpr40), which is expressed in pancreatic beta cells, is shown to be involved in diabetes mellitus type 2. It is activated by medium to long chain fatty acids (29) and is present in ghrelin-containing X/A cells of the gastric corpus mucosa (12). A similar expression pattern is seen for Gpr84, which is activated largely by medium chain free fatty acids (29). The stomach is thus responsive to various food stimuli, especially for lipids and glucose.
High expression was found for the pancreas-specific isoform of protein disulfide isomerase Pdi (Pdip). Pdi catalyzes the formation of disulfide bonds in secretory proteins (22) and can serve as a molecular chaperone (83). A recent study described high expression of the pancreas-specific isoform of Pdi, Pdip, in mouse gastric mucosal cells, but not submucosa or muscle layers. Pdip expression level was highest in the corpus and appeared to be localized to gastric chief cells (16).
Expression of proteins involved in essential nutrient and vitamin uptake.
Although many of the vitamin and essential nutrient transport proteins are predominantly expressed in the distal small bowel mucosa, there are a few noticeable exceptions observed to be expressed in the gastric mucosa (Fig. 8).
Three genes involved in essential nutrient uptake are highly expressed in the gastric mucosa. There is significant enrichment of mRNA coding for gastric intrinsic factor, which binds cobalamin (vitamin B12) and of the sodium iodide symporter (Slc5a5), confirming immunohistochemical detection of the latter in the gastric mucosa (2, 13, 76). Whether this represents the principal transporter of dietary iodine uptake, given the relative absence of expression in the rest of the gut, is not known but probable. There is also high expression of message for the glial high-affinity glutamate transporter (Slc1a3). This novel finding implies high expression by a gastric neuroendocrine cell that is as yet undefined.
Expression of proteins involved in iron uptake.
The gastric corpus mucosa does not show enrichment of messages coding for iron transport proteins. However, enriched messages for transferrin, the serum transport protein for iron, and the transferrin receptor are found at higher levels in the gastric mucosa (Fig. 8). Transferrin message is also highly expressed in the colon. The gastric corpus mucosa is also highly enriched in message coding for the regulatory protein hepcidin (Fig. 8), shown to inhibit apical iron uptake by DMT-1 in intestinal epithelial Caco-2 cells (48). This may indicate a feed-back loop between the gastric and duodenal mucosa in iron uptake regulation. As before, expression of many of these genes is seen in ileum but not jejunum or colon.
Glucose Homeostasis
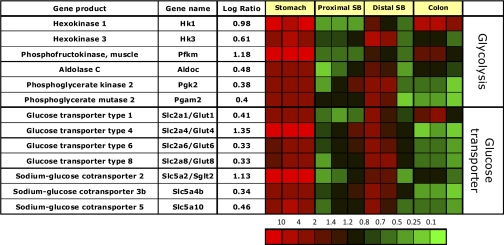
Expression of enzymes of the glycolytic pathway.
The gastric corpus mucosa is enriched in message for hexokinase 1 and 3, muscle phosphofructokinase, aldolase C, phosphoglycerate kinase 2, and phosphoglycerate mutase 2, implying a major role for the glycolytic pathway in the stomach (Fig. 9).
Fig. 9.
Segment-specific expression of genes coding for various enzymes of glycolysis and glucose transporters. This figure illustrates the relative distribution of genes encoding various enzymes involved in glycolysis and glucose transport that are selectively expressed in epithelial cells of the gastric corpus in 3 independent experiments (log of the mean of 3 ratios ± SD). Ratios are presented as heat maps showing gradients of highest (red) to lowest (green). The color scale at the bottom indicates the expression ratios (fold-change).
While hexokinase 1 and 3 are highly enriched in gastric epithelial cells, hexokinase 2 is specifically expressed in ileal epithelial cells (data not shown). The results may explain earlier studies on segment-specific hexokinase enzyme activities along the GI tract (85).
Expression of facilitated sugar transporters and glucosensors.
The gastric corpus mucosa is highly enriched in messages coding for the insulin-regulated basolateral glucose transporter Glut4 (Slc2a4) and the apical glucose transporter Sglt2 (Slc5a2), indicating the presence of a specific glucose uptake pathway in the stomach (Fig. 9). Messages coding for other glucose transporters including Glut1, Glut6, Glut8, and Slc5a10 are found to be slightly enriched in the gastric corpus and also the distal small bowel epithelium, indicating some degree of similarity in sugar transport in the gastric and ileal mucosa compared with the proximal small bowel and colonic epithelium.
Expression of Proteins Involved in Detoxification of Food Contents
Expression of decarboxylases.
The family of amino acid and other decarboxylases are involved in synthesis of neuroendocrine factors and important metabolic intermediates. Many of these enzymes are enriched in the gastric and to a lesser extent in ileal mucosa (Fig. 10).
There is high enrichment of gastric mucosal mRNA for cysteine dioxygenase (34), the enzyme committing cysteine conversion to cysteine sulfinic acid (CSA), and of cysteine sulfinic acid decarboxylase (Csad), which decarboxylates CSA to ultimately yield taurine and CO2. Taurine has been implicated in osmoregulation (4), which is a vital component of gastric parietal cell physiology and is also an anti-oxidant.
The gastric mucosa expresses high amounts of malonyl-CoA decarboxylase. Malonyl CoA is a potent endogenous inhibitor of fatty acid oxidation, secondary to inhibition of carnitine palmitoyl transferase-I, the gatekeeper of mitochondrial fatty acid uptake. Degradation by malonyl CoA decarboxylase leads to increased fatty acid oxidation, important to provide energy precursors for acid secretion in the gastric mucosa.
Expression of alcohol and aldehyde dehydrogenases.
The gastric mucosa expresses high levels of the class IV alcohol dehydrogenase Adh7 (Fig. 10). This enzyme has the highest affinity for ethanol and represents a metabolic barrier [first pass metabolism for dietary ethanols (27, 72)]. There is also significant enrichment of message coding for the novel iron containing alcohol dehydrogenase (Adhfe1), which was recently cloned from human fetal brain (10) and has been found to play a role in adipocyte function (39). There is also slight gastric mucosal enrichment of messages coding for a variety of aldehyde dehydrogenases (Aldh1a1, 1a2, 3a1, and 6a1), confirming previous data (1). This probably reflects the function of the stomach in preventing intoxication due to damaging factors in food.
Expression of glutathione transferases and peroxidases involved in detoxification.
Most enzymes involved in detoxification of food contents are preferentially expressed in gastric mucosa. Knowledge of segment-specific expression of different isoforms of these classes of enzymes is important in the understanding of the bio-availability and metabolism of orally administered drugs.
Enrichment of message for several glutathione peroxidases (Gpx), namely Gpx 1, 3, 4, 6, and 7 is significant in the stomach (Fig. 10). Messages for glutathione transferases are also highly enriched. These also reflect an important role for the stomach in dealing with potentially damaging agents in food. Again, there is overlap of expression in ileum but generally not in jejunum or colon.
Summary
This survey of the rat gastric corpus identifies genes selectively expressed by the gastric oxyntic mucosa compared with the proximal and distal (ileal) small bowel and colonic mucosae. It is striking that the gastric mucosa appears to express the most extensive set of genes that reflect unique functions. The highlights of the results are summarized here and shown in Table 1.
Table 1.
Summary of novel and interesting genes specifically expressed by the gastric corpus mucosa
| ATPases | Carbonic Anhydrases | Potassium and Chloride Channels | Growth Factors and Binding Proteins |
|---|---|---|---|
| Atp6v0e1 | Cah4 | Clcn4-2 | Igf1 |
| Atp7a | Cah9 | Clcnkb | Igfbp7 |
| Atp10d | Cah11 | Clic3 | Ctgf |
| Kcnj8 | Esm1 | ||
| Kcnh2 | Wisp2 | ||
| Wisp1 |
| Epithelial Protection | Transcription Factors | Lipid Metabolism | Vitamin and Iron Uptake |
|---|---|---|---|
| Muc1 | Foxn4 | Lpl | Slc1a3 |
| Muc5ac | Meis2 | Pla1a | Slc5a5 |
| Muc6 | Pitx2 | Fabp3-5 | Hepcidin |
| Gkn1 | Sox10 | Fabp7 | |
| Tff1 | |||
| Tff2 |
| Glucose Transporter | Dehydrogenases | Decarboxylases | Detoxifying Enzymes |
|---|---|---|---|
| Slc2a4 | Adh7 | Csad | Gpx1 |
| Slc5a2 | Adhfe1 | Cdo1 | Gsta3 |
The gastric corpus uniquely expresses the machinery of genes for acid secretion, digestive enzyme release, and for their regulation by gastric enteroendocrine cells. Associated with acid secretion is the presence of both K+ and Cl− channels. Kcnq1/Kcne2 and Clic6 are known to be involved in supplying K+ and Cl− to the luminal face of the H,K-ATPase, but the role of the high expression of the voltage-sensitive chloride channel Clcn4–2 in this part of the stomach is not clear. Kir4.2 and 5.1 (Kcnj15 and Kcnj16) form an inward-rectifying pH-sensitive heterodimeric K+ channel in parietal cells (44). This channel most likely maintains intracellular K+ homeostasis replacing that lost during acid secretion given its location on the apical surface of the parietal cell.
Several aquaporin isoforms are also highly expressed in the gastric corpus and are likely to control water flow during acid and/or pepsinogen secretion. Bicarbonate is the source of the protons needed for acid secretion and is also the major intracellular buffer compensating for proton extrusion, and levels are maintained by the bicarbonate transporters Slc26a7 and the three anion exchangers (AE2–4), which also supply Cl−. The reason for expression of multiple subtypes of anion exchangers in these cells is unknown but may reflect temporal differences in pH or Cl− homeostasis as a function of the rate of acid secretion. In addition, there are several isoforms of carbonic anhydrase expressed in this region of the gut to maintain cellular pH by bicarbonate generation.
The gastric corpus is also the site of extensive enteroendocrine regulation expressing receptors whose ligands are mainly expressed in the small bowel mucosa, resulting in feedback circuits between small bowel and gastric corpus. In addition, the presence of ghrelin, neuropeptide W, and NUCB2/nesfatin-1, involved in appetite regulation, emphasizes the importance of the gastric corpus mucosa in weight control. Trefoil peptides and foveolin are important for gastric corpus mucosal repair, and their high expression in gastric mucosa attests to the likelihood of injury in this part of the GI tract. Of the multitude of transcription factors expressed in the gut, Barx1 and Runx appear to be involved in gastric epithelial cell differentiation, while Foxa2 and Foxn4 transcription factors are involved in the differentiation of enteroendocrine cells.
There is selective expression of various lipases and pancreatic amylase in addition to pepsinogen. Also of note is the expression of a specific subset of glucose transporters, Sglt2 and Glut4, resulting in a gastric-specific pathway for glucose uptake and utilization. The presence of the glucose sensor Sglt3b and the free fatty acid receptors Ffar1 and Gpr84 indicates glucose and fatty acid sensing, thereby enabling regulation of appetite and food ingestion.
A specific set of fatty acid binding proteins implies a role of lipids as energy precursors for the metabolic demands in this region. The gastric corpus mucosa also uniquely expresses the sodium iodide cotransporter, suggesting that gastric absorption is the major route for iodide supply to the body.
An unexpected finding is the prominent presence of a pathway for taurine generation (cysteine dioxygenase and cysteine sulfinic acid decarboxylase), perhaps involved in osmoregulation as a vital component of gastric parietal cell isosmotic secretion of HCl. Taurine is also an antioxidant and this may be the major role for taurine generation in the stomach complementing other antioxidant pathways such as those involving glutathione.
Throughout this analysis the overlap between gene expression in gastric corpus and ileum but not jejunum and colon was noted. It is clearly of interest to discern the meaning of this overlap, since the ileum is not a secretory epithelium.
In summary, this extensive analysis of gene expression by stomach epithelial cells compared with the intestine provides a starting point for investigation of new and unexplored functions of this organ and emphasizes the integration of several different functions along the length of the GI tract.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-058333 (G. Sachs) and DK-053642 (G. Sachs), the US Department of Veterans Affairs (G. Sachs), and German Research Foundation Grants STE 1765/1-1 (A. Stengel) and Grant GO 1718/1-1 (M. Goebel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alnouti Y, Klaassen CD. Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (Aldh) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol Sci 101: 51–64, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Altorjay A, Dohan O, Szilagyi A, Paroder M, Wapnir IL, Carrasco N. Expression of the Na+/I− symporter (NIS) is markedly decreased or absent in gastric cancer and intestinal metaplastic mucosa of Barrett esophagus. BMC Cancer 7: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson N, Skrtic SM, Hakanson R, Ohlsson C. A gene expression fingerprint of mouse stomach ECL cells. Biochem Biophys Res Commun 332: 404–410, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrient? Curr Opin Clin Nutr Metab Care 9: 728–733, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bradley KK, Hatton WJ, Mason HS, Walker RL, Flynn ER, Kenyon JL, Horowitz B. Kir3.1/3.2 encodes an I(KACh)-like current in gastrointestinal myocytes. Am J Physiol Gastrointest Liver Physiol 278: G289–G296, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Caminos JE, Bravo SB, Garcia-Rendueles ME, Ruth Gonzalez C, Garces MF, Cepeda LA, Lage R, Suarez MA, Lopez M, Dieguez C. Expression of neuropeptide W in rat stomach mucosa: regulation by nutritional status, glucocorticoids and thyroid hormones. Regul Pept 146: 106–111, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Ceranowicz P, Warzecha Z, Dembinski A, Sendur R, Cieszkowski J, Ceranowicz D, Pawlik WW, Kuwahara A, Kato I, Konturek PC. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J Physiol Pharmacol 60: 87–98, 2009 [PubMed] [Google Scholar]
- 8. Charboneau A, East L, Mulholland N, Rohde M, Boudreau N. Pbx1 is required for Hox D3-mediated angiogenesis. Angiogenesis 8: 289–296, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev 12: 181–187, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Deng Y, Wang Z, Gu S, Ji C, Ying K, Xie Y, Mao Y. Cloning and characterization of a novel human alcohol dehydrogenase gene (ADHFe1). DNA Seq 13: 301–306, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, Sugano K. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol 37: 94–100, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farnedi A, Eusebi LH, Poli F, Foschini MP. Immunohistochemical expression of the human sodium/iodide symporter distinguishes malignant from benign gastric lesions. Int J Surg Pathol 17: 327–334, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Flamant S, Pescher P, Lemercier B, Clement-Ziza M, Kepes F, Fellous M, Milon G, Marchal G, Besmond C. Characterization of a putative type IV aminophospholipid transporter P-type ATPase. Mamm Genome 14: 21–30, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Folmer DE, Elferink RP, Paulusma CC. P4 ATPases - lipid flippases and their role in disease. Biochim Biophys Acta 1791: 628–635, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Fu XM, Dai X, Ding J, Zhu BT. Pancreas-specific protein disulfide isomerase has a cell type-specific expression in various mouse tissues and is absent in human pancreatic adenocarcinoma cells: implications for its functions. J Mol Histol 40: 189–199, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Fukamachi H. Runx3 controls growth and differentiation of gastric epithelial cells in mammals. Dev Growth Differ 48: 1–13, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Fukamachi H, Ito K. Growth regulation of gastric epithelial cells by Runx3. Oncogene 23: 4330–4335, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Funayama H, Huang L, Asada Y, Endo Y, Takada H. Enhanced induction of a histamine-forming enzyme, histidine decarboxylase, in mice primed with NOD1 or NOD2 ligand in response to various Toll-like receptor agonists. Innate Immun 16: 265–272, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Ghosh S, Ghosh A, Maiti GP, Alam N, Roy A, Roychoudhury S, Panda CK. Alterations of ROBO1/DUTT1 and ROBO2 loci in early dysplastic lesions of head and neck: clinical and prognostic implications. Hum Genet 125: 189–198, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Gregory RA, Tracy HJ. The constitution and properties of two gastrins extracted from hog antral mucosa. Gut 5: 103–114, 1964 [PMC free article] [PubMed] [Google Scholar]
- 22. Gruber CW, Cemazar M, Heras B, Martin JL, Craik DJ. Protein disulfide isomerase: the structure of oxidative folding. Trends Biochem Sci 31: 455–464, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Han S, Wang G, Qi X, Englander EW, Greeley GH., Jr Involvement of a Stat3 binding site in inflammation-induced enteric apelin expression. Am J Physiol Gastrointest Liver Physiol 295: G1068–G1078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heim HK, Elhoft A, Wittstock H, Sewing KF. Characterization of CCK receptor-mediated effects on intracellular calcium of porcine chief cells. J Physiol Pharmacol 46: 489–501, 1995 [PubMed] [Google Scholar]
- 25. Helander HF, Leth R, Olbe L. Stereological investigations on human gastric mucosa: I. Normal oxyntic mucosa. Anat Rec 216: 373–380, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 62: 2932–2938, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmes RS. Alcohol dehydrogenases: a family of isozymes with differential functions. Alcohol Alcohol Suppl 2: 127–130, 1994 [PubMed] [Google Scholar]
- 28. Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci USA 97: 12475–12480, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat 89: 82–88, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Jacobs Y, Schnabel CA, Cleary ML. Trimeric association of Hox and TALE homeodomain proteins mediates Hoxb2 hindbrain enhancer activity. Mol Cell Biol 19: 5134–5142, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jain RN, Brunkan CS, Chew CS, Samuelson LC. Gene expression profiling of gastrin target genes in parietal cells. Physiol Genomics 24: 124–132, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Jain RN, Samuelson LC. Transcriptional profiling of gastrin-regulated genes in mouse stomach. Physiol Genomics 29: 1–12, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 21: 6338–6347, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jerkins AA, Jones DD, Kohlhepp EA. Cysteine sulfinic acid decarboxylase mRNA abundance decreases in rats fed a high-protein diet. J Nutr 128: 1890–1895, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Jia Y, Persson C, Hou L, Zheng Z, Yeager M, Lissowska J, Chanock SJ, Chow WH, Ye W. A comprehensive analysis of common genetic variation in MUC1, MUC5AC, MUC6 genes and risk of stomach cancer. Cancer Causes Control 21: 313–321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamsteeg EJ, Duffield AS, Konings IB, Spencer J, Pagel P, Deen PM, Caplan MJ. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA 104: 16696–16701, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaufhold MA, Krabbenhoft A, Song P, Engelhardt R, Riederer B, Fahrmann M, Klocker N, Beil W, Manns M, Hagen SJ, Seidler U. Localization, trafficking, and significance for acid secretion of parietal cell Kir4.1 and KCNQ1 K+ channels. Gastroenterology 134: 1058–1069, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Kim BM, Buchner G, Miletich I, Sharpe PT, Shivdasani RA. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell 8: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Kim JY, Tillison KS, Zhou S, Lee JH, Smas CM. Differentiation-dependent expression of Adhfe1 in adipogenesis. Arch Biochem Biophys 464: 100–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim SJ, Nian C, McIntosh CH. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. J Biol Chem 282: 8557–8567, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Korolkiewicz RP, Tashima K, Fujita A, Kato S, Takeuchi K. Exogenous insulin-like growth factor (IGF)-1 improves the impaired healing of gastric mucosal lesions in diabetic rats. Pharmacol Res 41: 221–229, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Lambrecht N, Burchert M, Respondek M, Muller KM, Peskar BM. Role of calcitonin gene-related peptide and nitric oxide in the gastroprotective effect of capsaicin in the rat. Gastroenterology 104: 1371–1380, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Lambrecht NW, Yakubov I, Sachs G. Fasting-induced changes in ECL cell gene expression. Physiol Genomics 31: 183–192, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Lambrecht NW, Yakubov I, Scott D, Sachs G. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol Genomics 21: 81–91, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Lambrecht NW, Yakubov I, Zer C, Sachs G. Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiol Genomics 25: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Linz R, Lutsenko S. Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins. J Bioenerg Biomembr 39: 403–407, 2007 [DOI] [PubMed] [Google Scholar]
- 47. McGuckin MA, Every AL, Skene CD, Linden SK, Chionh YT, Swierczak A, McAuley J, Harbour S, Kaparakis M, Ferrero R, Sutton P. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133: 1210–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Mena NP, Esparza A, Tapia V, Valdes P, Nunez MT. Hepcidin inhibits apical iron uptake in intestinal cells. Am J Physiol Gastrointest Liver Physiol 294: G192–G198, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Mercier F, Bayle D, Besancon M, Joys T, Shin JM, Lewin MJ, Prinz C, Reuben MA, Soumarmon A, Wong H, Walsh JH, Sachs G. Antibody epitope mapping of the gastric H+/K+-ATPase. Biochim Biophys Acta 1149: 151–165, 1993. [DOI] [PubMed] [Google Scholar]
- 50. Mills JC, Andersson N, Hong CV, Stappenbeck TS, Gordon JI. Molecular characterization of mouse gastric epithelial progenitor cells. Proc Natl Acad Sci USA 99: 14819–14824, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mills JC, Andersson N, Stappenbeck TS, Chen CC, Gordon JI. Molecular characterization of mouse gastric zymogenic cells. J Biol Chem 278: 46138–46145, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Mills JC, Syder AJ, Hong CV, Guruge JL, Raaii F, Gordon JI. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc Natl Acad Sci USA 98: 13687–13692, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mizoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T, Kato T, Joh T, Itoh M, Tatematsu M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa–with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer 4: 185–191, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Mollenhauer J, Herbertz S, Helmke B, Kollender G, Krebs I, Madsen J, Holmskov U, Sorger K, Schmitt L, Wiemann S, Otto HF, Grone HJ, Poustka A. Deleted in malignant brain tumors 1 is a versatile mucin-like molecule likely to play a differential role in digestive tract cancer. Cancer Res 61: 8880–8886, 2001 [PubMed] [Google Scholar]
- 55. Morimoto K, Nishimori I, Takeuchi T, Kohsaki T, Okamoto N, Taguchi T, Yunoki S, Watanabe R, Ohtsuki Y, Onishi S. Overexpression of carbonic anhydrase-related protein XI promotes proliferation and invasion of gastrointestinal stromal tumors. Virchows Arch 447: 66–73, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Needham K, Pontell L, Hunne B, Thacker M, McHugh D, Furness JB. Identification of endocrine cells of the stomach that express acid-sensitive background potassium (K(2P)9.1/TASK3) channels. J Mol Histol 41: 403–409, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Newton JL, Allen A, Westley BR, May FE. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut 46: 312–320, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nishizawa T, Nagao T, Iwatsubo T, Forte JG, Urushidani T. Molecular cloning and characterization of a novel chloride intracellular channel-related protein, parchorin, expressed in water-secreting cells. J Biol Chem 275: 11164–11173, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am J Physiol Gastrointest Liver Physiol 292: G1181–G1194, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S, Burns S, Keith WN. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol 203: 789–797, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Oue N, Mitani Y, Aung PP, Sakakura C, Takeshima Y, Kaneko M, Noguchi T, Nakayama H, Yasui W. Expression and localization of Reg IV in human neoplastic and non-neoplastic tissues: Reg IV expression is associated with intestinal and neuroendocrine differentiation in gastric adenocarcinoma. J Pathol 207: 185–198, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Pan L, Glenn ST, Jones CA, Gross KW. Activation of the rat renin promoter by HOXD10. PBX1b PREP1, Ets-1, and the intracellular domain of notch. J Biol Chem 280: 20860–20866, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Pastorekova S, Parkkila S, Parkkila AK, Opavsky R, Zelnik V, Saarnio J, Pastorek J. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 112: 398–408, 1997 [DOI] [PubMed] [Google Scholar]
- 64. Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir42 potassium channels and their modulation by heteropolymerisation with Kir51. J Physiol 532: 359–367, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Petrovic S, Ju X, Barone S, Seidler U, Alper SL, Lohi H, Kere J, Soleimani M. Identification of a basolateral Cl−/HCO3− exchanger specific to gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 284: G1093–G1103, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pohl D, Fox M, Fried M, Goke B, Prinz C, Monnikes H, Rogler G, Dauer M, Keller J, Lippl F, Schiefke I, Seidler U, Allescher HD. Do we need gastric acid? Digestion 77: 184–197, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology 139: 2018–2027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sakakura C, Hagiwara A, Miyagawa K, Nakashima S, Yoshikawa T, Kin S, Nakase Y, Ito K, Yamagishi H, Yazumi S, Chiba T, Ito Y. Frequent downregulation of the runt domain transcription factors RUNX1, RUNX3 and their cofactor CBFB in gastric cancer. Int J Cancer 113: 221–228, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, Eck M. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol 136: 521–526, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schubert ML. Gastric exocrine and endocrine secretion. Curr Opin Gastroenterol 25: 529–536, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Seitz HK, Oneta CM. Gastrointestinal alcohol dehydrogenase. Nutr Rev 56: 52–60, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Shaoul R, Marcon P, Okada Y, Cutz E, Forstner G. The pathogenesis of duodenal gastric metaplasia: the role of local goblet cell transformation. Gut 46: 632–638, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Solomon TE, Varga G, Zeng N, Wu SV, Walsh JH, Reeve JR., Jr Different actions of secretin and Gly-extended secretin predict secretin receptor subtypes. Am J Physiol Gastrointest Liver Physiol 280: G88–G94, 2001 [DOI] [PubMed] [Google Scholar]
- 75. Song P, Groos S, Riederer B, Feng Z, Krabbenhoft A, Smolka A, Seidler U. KCNQ1 is the luminal K+ recycling channel during stimulation of gastric acid secretion. J Physiol 587: 3955–3965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Spitzweg C, Joba W, Schriever K, Goellner JR, Morris JC, Heufelder AE. Analysis of human sodium iodide symporter immunoreactivity in human exocrine glands. J Clin Endocrinol Metab 84: 4178–4184, 1999 [DOI] [PubMed] [Google Scholar]
- 77. Stanchina L, Baral V, Robert F, Pingault V, Lemort N, Pachnis V, Goossens M, Bondurand N. Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev Biol 295: 232–249, 2006 [DOI] [PubMed] [Google Scholar]
- 78. Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Lambrecht NW, Tache Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology 150: 4911–4919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Svichar N, Waheed A, Sly WS, Hennings JC, Hubner CA, Chesler M. Carbonic anhydrases CA4 and CA14 both enhance AE3-mediated Cl–HCO3− exchange in hippocampal neurons. J Neurosci 29: 3252–3258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Toiyama Y, Mizoguchi A, Kimura K, Araki T, Yoshiyama S, Sakaguchi K, Miki C, Kusunoki M. Persistence of gene expression changes in noninflamed and inflamed colonic mucosa in ulcerative colitis and their presence in colonic carcinoma. World J Gastroenterol 11: 5151–5155, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Torres LF, Karam SM, Wendling C, Chenard MP, Kershenobich D, Tomasetto C, Rio MC. Trefoil factor 1 (TFF1/pS2) deficiency activates the unfolded protein response. Mol Med 8: 273–282, 2002 [PMC free article] [PubMed] [Google Scholar]
- 82. Van Landeghem L, Mahe MM, Teusan R, Leger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics 10: 507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang CC, Tsou CL. Protein disulfide isomerase is both an enzyme and a chaperone. FASEB J 7: 1515–1517, 1993 [DOI] [PubMed] [Google Scholar]
- 84. Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Konturek SJ, Polus A, Pawlik WW, Kuwahara A, Kato I, Konturek PC. Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes in young mature rats. J Physiol Pharmacol 57: 425–437, 2006 [PubMed] [Google Scholar]
- 85. Weiser MM, Quill H, Isselbacher KJ. Effects of diet on rat intestinal soluble hexokinase and fructokinase activities. Am J Physiol 221: 844–849, 1971 [DOI] [PubMed] [Google Scholar]
- 86. Xu J, Song P, Nakamura S, Miller M, Barone S, Alper SL, Riederer B, Bonhagen J, Arend LJ, Amlal H, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem 284: 29470–29479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yan J, Jiao Y, Jiao F, Stuart J, Donahue LR, Beamer WG, Li X, Roe BA, LeDoux MS, Gu W. Effects of carbonic anhydrase VIII deficiency on cerebellar gene expression profiles in the wdl mouse. Neurosci Lett 413: 196–201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang Y, Hwang CK, D'Souza UM, Lee SH, Junn E, Mouradian MM. Three-amino acid extension loop homeodomain proteins Meis2 and TGIF differentially regulate transcription. J Biol Chem 275: 20734–20741, 2000 [DOI] [PubMed] [Google Scholar]
- 89. Ye DZ, Kaestner KH. Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology 137: 2052–2062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer 37: 2257–2263, 2001 [DOI] [PubMed] [Google Scholar]
- 91. Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zanner R, Hapfelmeier G, Gratzl M, Prinz C. Intracellular signal transduction during gastrin-induced histamine secretion in rat gastric ECL cells. Am J Physiol Cell Physiol 282: C374–C382, 2002 [DOI] [PubMed] [Google Scholar]