Abstract
The impact of aging on mitochondrial function and the deterministic role of mitochondria on scenescence continue to be topics of vigorous debate. Many studies report that skeletal muscle mitochondrial content and function are reduced with aging and metabolic diseases associated with insulin resistance. However, an accumulating body of literature suggests that physical inactivity typical of aging may be a more important determinant of mitochondrial function than chronological age, per se. Reports of age-related declines in mitochondrial function have spawned a vast body of literature devoted to understanding the underlying mechanisms. These mechanisms include decreased abundance of mtDNA, reduced mRNA levels, as well as decreased synthesis and expression of mitochondrial proteins, ultimately resulting in decreased function of the whole organelle. Effective therapies to prevent reverse, or delay the onset of the aformentioned mitochondrial changes, regardless of their inevitability or precise underlying causes, require an intimate understanding of the processes that regulate mitochondrial biogenesis, which necessitates the coordinated regulation of nuclear and mitochondrial genomes. Herein we review the current thinking on regulation of mitochondrial biogenesis by transcription factors and transcriptional co-activators and the role of hormones and exercise in initiating this process. We review how exercise may help preserve mitochondrial content and functionality across the lifespan, and how physical inactivity is emerging as a major determinant of many age-associated changes at the level of the mitochondrion. We also review evidence that some mitochondrial changes with aging are independent of exercise or physical activity and appear to be inevitable consequences of old age.
INTRODUCTION
There are many features of skeletal muscle that have intrigued inquisitive minds for centuries. Among all cell types, skeletal muscle possesses the unique ability to increase metabolic rate nearly 100-fold during the transition from a basal resting state to near maximal contractile activity. Though perhaps underappreciated by many, this contractile activity of skeletal muscle, combined with the mechanics of tendons and bones, enables us to navigate our environment. The luxury of locomotion comes at a cost. Energy currency in the form of high energy phosphates (ATP, PCr) is necessary to ensure the proper execution of a coordinated series of events, namely 1) the maintenance of the sarcolemmal membrane potential, 2) interaction of the contractile proteins, and 3) calcium handling by the sarcoplasmic reticulum. It is remarkable that myocytes are able to maintain metabolic homeostasis in the face of such a dramatic increase in energetic demand (Dawson et al. 1977). Indeed, depletion of cellular ATP is seldom observed, even under extreme conditions such as sustained high-intensity muscle activity during ischemia (Lanza et al. 2007). Thus, skeletal muscle is quite well-adapted for prolonged periods of energetically costly contractile activity.
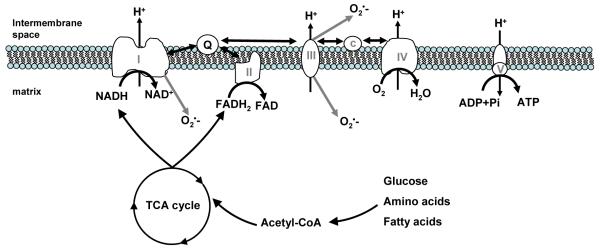
Skeletal muscle owes its metabolic prowess to a defining evolutionary event that occurred approximately 1.5 billion years ago when a eukaryotic cell symbiosed with a Protobacterial ancestor (Balaban et al. 2005,Gray et al. 1999). In what has become known as the serial endosymbiosis theory, the eubacterium evolved into the modern day mitochondrion; an organelle that has been dubbed the “powerhouse of the cell” because of its role in generating a readily usable form of chemical energy; ATP. Mitochondria generate ATP by oxidizing carbon substrates in the tricarboxylic acid cycle, creating a transmembrane proton gradient by the action of the electron transport system, and harnessing this potential energy to phopshorylate ADP (Figure 1).
Figure 1.
Mitochondrial ATP synthesis is coupled to fuel oxidation in the tricarboxylic acid (TCA) cycle. Acetyl CoA, derived from glucose, amino acids and fatty acids, is oxidized in the TCA cycle to produce reducing equivalents in the form of NADH and FADH2. NADH is oxidized by complex I (HADH dehydrogenase), while complex II (succinate dehydrogenase) oxidizes succinate to reduce FADH to FADH2. Electron flow from complexes I and II converge on a mobile electron carrier, Coenzyme Q, which transfers electrons to complex III (coenzyme Q-cytochrome c reductase). A second mobile electron carrier, cytochrome c, transfers electrons to complex IV (cytochrome c oxidase). Passage of electron pairs through respiratory chain complexes I, III, and IV results in proton transport across the inner mitochondrial membrane. The resulting proton gradient is used to drive the phosphorylation of ADP at complex V (ATP synthase). Gray arrows indicate sites of superoxide (O2·−) production at complexes I and III.
Although the precise evolutionary origin of the organelle remains uncertain, the assumption of a protobacterial ancestry stems largely from the fact that mitochondria contain their own genome that is physically and functionally distinct from the nuclear genome. Human mitochondrial DNA is circular and encodes 13 respiratory chain proteins as well as 22 transfer RNAs and 2 ribosomal RNAs essential for the transcription and translation of the mitochondrial genome (Anderson et al. 1981). However, proteins encoded by the mitochondrial genome represent but a small fraction of the thousand or so proteins that make up the mitochondrion, the remainder of which are encoded by nuclear DNA and are imported into the mitochondrion after synthesis in the cytosol. Thus, it seems that over a very long period of time, the nucleus has assumed the majority of the control over mitochondrial biogenesis, though mitochondria retain exclusive governance of a small number of essential proteins, without which the organelle could not function.
Mitochondria have an emergent role in regulating lifespan. From a standpoint of basic survival, highly functional mitochondria in skeletal muscle promote survival by enabling organisms to generate sufficient energy to catch prey or avoid becoming prey. Although physical activity is no longer required to obtain food in most Westernized cultures, mitochondria still appear to play a critical role in the overall health and survival of our species. Mitochondria regulate cellular function in ways that go well beyond their role in energy homeostasis. For example, mitochondria are involved in the process of cell death through the release of apoptogenic factors such as apoptosis inducing factor, endonuclease G, and cytochrome c, which respectively induce DNA condensation, DNA degradation, and apoptosome formation (van Gurp et al. 2003). Mitochondria are also a major source of cellular reactive oxygen species (ROS), originating as superoxide at various sites along the electron transport machinery (Balaban et al. 2005) (figure 1). ROS are generally regarded as being detrimental to cellular function by modifying the structure and function of proteins, lipids, and nucleic acids. However, ROS are also believed to play an important physiological role in cell signaling through pathways that are sensitive to the cellular redox state (Stowe et al. 2006). Thus, although mitochondria were once uninvited guests in the eukaryotic cell, they have since made themselves indispensable by assuming functions that are critical to proper cellular function. Herein we will begin by examining mitochondrial function in the context of aging, followed by discussion of factors that are purported to regulate mitochondrial biogenesis.
AGING MITOCHONDRIA
One of the most profound physical features of human aging is the decline in skeletal muscle mass (sarcopenia), muscle fiber quantity, and preferential loss of type 2 motor units (Lexell 1995). The functional consequences of this loss of muscle mass are profound, including decreased muscle strength, power, and ability to safely perform activities of daily living (Grimby 1995,Wolfson et al. 1995). The decline in muscle strength with aging often exceeds the decline in muscle mass, reflected by reduced force per cross sectional area (i.e., specific strength) (Davies et al. 1986), but not when noncontractile tissue is accounted for (Kent-Braun & Ng 1999). A mechanism underlying the age-related declines in muscle mass is likely to involve decreases in protein synthesis, which have been demonstrated at the level of the whole body (Nair 1995), mixed muscle (Rooyackers et al. 1996,Yarasheski et al. 1993), and myosin heavy chain (Balagopal et al. 2001). The effect of aging on muscle endurance or fatigue, another key determinant of physical function, is more ambiguous (Kent-Braun 2009,Larsson & Karlsson 1978,Lennmarken et al. 1985). Metabolic disturbances such as a progressive decline in whole body peak oxygen uptake have been observed, even after correcting for lean mass (Grimby & Saltin 1966,Rogers et al. 1990,Short et al. 2005). At the cellular level, there has been a strong movement to understand how the mitochondrion changes with age because of its importance in energy metabolism and physical performance. To date, many reports favor the notion that mitochondrial function declines with age, although this remains a topic for vigorous debate. As reviewed below, although there is a great body of evidence to suggest that mitochondrial function is impaired with aging, it is becoming increasingly clear that much of this association is driven by the commensurate changes in physical activity, disease, nutrition, or other environmental factors rather than age per se.
Mitochondrial content
Skeletal muscle mitochondria were once thought to be small, spherical or bean shaped organelles based on their appearance in electron micrographs, but are now known to exist as extensively branched reticula with two distinct sub-populations (Kirkwood et al. 1986). Subsarcolemmal mitochondria are localized within a few microns of the plasma membrane, and intermyofibrillar mitochondria are distributed among the contractile proteins. The ability to sustain a steady-state level of muscular work is a function of the number of mitochondria or, more accurately, the volume density of mitochondria (Chance et al. 1985). Various methods have been used to demonstrate that mitochondrial content decreases with aging, including electron microscopy (Conley et al. 2000), mitochondrial DNA copy number (Barazzoni et al. 2000,Menshikova et al. 2006,Short et al. 2005,Welle et al. 2003), cardiolipin content (Menshikova et al. 2006), and citrate synthase activity (Houmard et al. 1998,Rooyackers et al. 1996,Short et al. 2005,Welle et al. 2000). There is little doubt that reduced mitochondrial content in peripheral tissue such as skeletal muscle, combined with impaired central hemodynamics, is partly to blame for age-related declines in whole-body peak oxygen uptake (Robinson et al. 1973,Rogers et al. 1990).
Mitochondrial function ex vivo
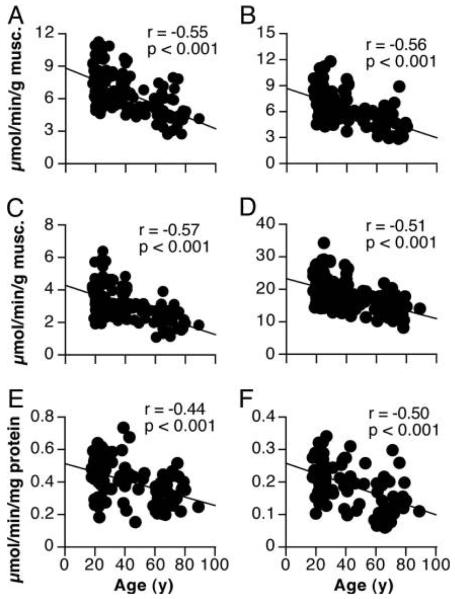
When viewed from a standpoint of bioenergetics, the function of mitochondria is to generate ATP. Thus, the most direct way to assess mitochondrial function in this context is to measure ATP synthesis rates. Recombinant firefly luciferase can be used to measure the rates of ATP production by monitoring light emission as a substrate luciferin is oxidized in an ATP-dependent manner (DeLuca & McElroy 1974). By adding ADP in the presence of specific combinations of substrates, it is possible to measure the rates of ATP synthesis as a result of electron flow through distinct complexes along the cytochrome chain. Using this method, we measured mitochondrial ATP production rates in mitochondria isolated from muscle tissue isolated from humans across a wide age range (Short et al. 2005). Substrates glutamate and malate were used to isolate electron flow exclusively into respiratory chain complex I (NADH dehydrogenase). The combination of succinate and the complex I inhibitor rotenone were used to isolate electron flow exclusively to complex II (succinate dehydrogenase). As shown in figure 2, mitochondrial ATP synthesis rates declined with increasing age at a rate of 8% per decade for respiratory chain complexes I and II. The fact that these ATP production rates are expressed per gram of tissue is of critical importance to the interpretation of the data. When expressed in this way, one must use caution in the use of the term “mitochondrial dysfunction”, which implies that there is an intrinsic defect in the organelle that limits ATP synthesis. As noted above, the decline in mitochondrial content with aging is likely to be a major determinant of the age-related decline in mitochondrial ATP synthesis rates, although impaired function at the level of the organelle may also be a factor. Moreover, the age-related decline in ATP synthesis rates occurs in parallel with decreasing mtDNA copy number (Short et al. 2005). To address whether there is an intrinsic mitochondrial defect with aging, we expressed ATP synthesis rates per milligram of mitochondrial protein (figure 2), which removes the contribution of age-related differences in mitochondrial content. Although normalizing to mitochondrial protein content slightly lessens the age-related decline in ATP synthesis rates, there remains a significant reduction (~5% per decade) that cannot be explained simply by a decline in mitochondrial content. These changes appear to be independent of physical activity as the study included only individuals who exercised less than 30 minutes per day, twice per week for the 9 months preceding the study. Although activity questionnaires revealed no differences in habitual physical activity levels across the age range, it is possible that the results could be impacted by more subtle changes in physical activity patterns that would be undetectable by questionnaire. More sensitive, objective assessments of habitual physical activity such as accelerometry have become essential components of study which aim to assess the independent effects of aging and physical activity patterns on mitochondrial function (Kent-Braun & Ng 2000,Lanza et al. 2005,Lanza et al. 2007). Thus, aging is associated with an intrinsic impairment in mitochondrial function that goes beyond a simple reduction in mitochondrial content. This apparent gradual decline in mitochondrial function across the lifespan is mirrored by a similar rate of decline in whole-body VO2 peak (Short et al. 2005). Although age-related changes to central hemodynamics (e.g., cardiac output) undoubtedly play a major role in the age-related decline in VO2 peak, we find that muscle mitochondrial capacity and VO2 peak are well-correlated (Short et al. 2005), suggesting that peripheral changes at the level of the mitochondria should not be discounted.
Figure 2.
ATP synthesis rates decreased with aging when expressed per muscle tissue weight (Panels A,B,C) or per mg mitochondrial protein (Panel E and F). Citrate synthase activity also decreased with age (Panel D). Figure reproduced with permission from (Short et al., PNAS, 2005).
As an alternative to measuring ATP synthesis rates, it is common to assess mitochondrial function by measuring the rates of oxygen consumption. Oxygen, acting as the final electron acceptor at the level of complex IV (cytochrome c oxidase), is consumed at a rate that is generally proportional to the rate of ATP synthesis, assuming that the two processes are well-coupled. For more than half a century, researchers have been using polarographic oxygen electrodes to study mitochondrial physiology (Chance & WILLIAMS 1955). More recently, several groups have applied this methodology to study the effects of aging on human skeletal muscle mitochondrial function. In general, the results support the notion that mitochondrial respiratory activity declines with age (Boffoli et al. 1994), even after expressing respiration rates relative to mitochondrial protein (Trounce et al. 1989). These observations are in-line with the notion that, irrespective of age-related declines in mitochondrial content, there appears to be impairment in mitochondrial function with aging. It is, however, worth highlighting the recent work of Rasmussen and colleagues, who report more modest changes in mitochondrial function with aging (Rasmussen et al. 2003a). A unique aspect of this work was the meticulous attention to the isolation procedures to maximize the integrity, yield, and purity of the isolated mitochondria. Mitochondrial function was assessed from respiration rates and activities of thirteen different enzymes that were normalized to mitochondrial protein content or citrate synthase activity. Although fatty acid oxidation and respiration rates using complex I substrates declined with age, the activities of several key mitochondrial enzymes (e.g., citrate synthase, cytochrome c oxidase, succinate dehydrogenase) were unaltered. The authors propose that age-related mitochondrial dysfunction is less profound than suggested by many investigators, possibly due to suboptimal isolation procedures and collagen contamination in the mitochondrial fraction. A similar study by the same research group found that mitochondria isolated from skeletal muscle of older humans (70+ years) exhibited some evidence of altered mitochondrial function when compared with young humans (20+ years), but that mitochondrial capacity was related to physical activity and not chronological age (Rasmussen et al. 2003b). More recently, Hutter et al. found no evidence of age-related changes in respiration rates of human permeabilized muscle fibers (Hutter et al. 2007). Furthermore, these investigators also found that the level of reactive oxygen species was higher in muscle tissue from young compared to older humans; findings that are in stark contrast with the free radical theory of aging (Hutter et al. 2007). The extent to which aging per se impacts mitochondrial function continues to be a topic of debate, particularly when consideration is given to physical activity, nutrition, and other lifestyle changes that are likely to influence mitochondrial function independently of chronological age.
Mitochondrial function in vivo
Techniques such as phosphorous magnetic resonance spectroscopy (31P-MRS) allow mitochondrial function to be assessed in vivo. Although in vitro methods are advantageous in probing the intrinsic function of the organelle at distinct stages of oxidative phosphorylation, in vivo methods permit mitochondrial assessment under physiological conditions where all circulatory and regulatory systems are intact. Many, but not all, in vivo 31P-MRS experiments reveal age effects that are consistent with those observed by in vitro measurements from muscle biopsy tissue (table 1). Years after Britton Chance pioneered the use of 31P-MRS to investigate human muscle energetics (Chance et al. 1980), several groups applied this method to show that oxidative capacity decreases with age (Conley et al. 2000,McCully et al. 1991,McCully et al. 1993) or remains unchanged (Chilibeck et al. 1998,Kent-Braun & Ng 2000,Schunk et al. 1999,Taylor et al. 1997). Several years later, Petersen et al. measured steady-state basal oxidative phosphorylation flux using a unique 31P-MRS approach and observed a nearly 50% reduction in the basal rate of mitochondrial ATP synthesis in older compared to young participants (Petersen et al. 2003). This reduction in basal ATP flux was also observed in aging mice (Marcinek et al. 2005). Furthermore, these investigators paired near-infrared spectroscopy and 31P-MRS to demonstrate that mitochondrial coupling was reduced in skeletal muscle of senescent mice. Reduced phosphorylation activity in the basal state is often assumed to reflect mitochondrial dysfunction, but it is unlikely that a decline in resting oxidative phosphorylation flux is consequence of decreased mitochondrial capacity since basal flux represents a small fraction of the overall mitochondrial oxidative capacity. It is important to consider that the findings could also simply reflect reduced mitochondrial content, lower metabolic demand in the resting state (i.e., basal metabolic rate), or reduced oxygen or substrate delivery due to vascular factors. Other groups have measured muscle oxidative capacity by 31P-MRS using a kinetic analysis of phosphocreatine (PCr) recovery following a brief bout of exercise. Oxidative capacity in the vastus lateralis muscle was found to be significantly reduced with age, as evidenced by slower PCr recovery following a bout of femoral nerve stimulation (Conley et al. 2000). Mitochondrial volume density was lower in older participants, which accounted for only a small portion of the age-related decline in oxidative capacity. There remained a significant effect of age after normalizing oxidative capacity for mitochondrial content, indicative of some inherent dysfunction at the level of the organelle. As shown in table 1, not all in vivo studies have reached this conclusion. A series of studies published by the Kent-Braun group have consistently found that oxidative capacity in the human tibialis anterior muscle is well-preserved with age (Kent-Braun & Ng 2000,Lanza et al. 2005,Lanza et al. 2007,Larsen et al. 2009). A unifying strength of these studies is the careful measurement and matching of daily physical activity levels between age groups. As reviewed elsewhere (Russ & Kent-Braun 2004), much of the decline in mitochondrial function observed with old age may be secondary to the sedentary lifestyle that often accompanies aging. The idea that certain muscle groups are more susceptible to the detriments of aging has long been discussed, but only recently has oxidative capacity been measured in vivo in morphologically and functionally distinct muscle groups in well-matched young and older adults (Larsen et al. 2009). The above report indicated that oxidative capacity was reduced with age in the vastus lateralis but not the tibalis anterior muscle (Larsen et al. 2009). In sum, the disparity in the literature concerning the effects of aging on muscle mitochondrial function is likely explained by methodological differences, muscle group specificity of age-related changes, and subject characteristics such as age, health, and physical activity levels.
Table 1.
Effects of aging on mitochondrial function in humans
| Reference | Muscle group | Measurement | Age effect |
|---|---|---|---|
| In vitro studies | |||
| Barrientos et al., 1996 (Barrientos et al. 1996) |
Vastus lateralis | Isolated mitochondria respiration | ↔ |
| Boffoli et al., 1996 (Boffoli et al. 1996) | Vastus lateralis | Enzyme activity | ↓ |
| Brierley et al., 1996 (Brierley et al. 1996) | Vastus lateralis | Isolated mitochondria respiration | ↔ |
| Chretien et al., 1998 (Chretien et al. 1998) | Deltoid | ↔ | |
| Coggan et al., 1992 (Coggan et al. 1992) | Lateral gastrocnemius |
Enzyme activity | ↓ |
| Houmard et al., 1998 (Houmard et al. 1998) | Vastus lateralis Lateral gastrocnemius |
Enzyme activity |
↓
↔ |
| Hutter et al., 2007 (Hutter et al. 2007) | Vastus Lateralis | Permeabilized fiber respiration | ↔ |
| Lanza et al., 2008 (Lanza et al. 2008) | Vastus lateralis | Enzyme activity Isolated mitochondria ATP synthesis |
↓
↓ |
| McCully et al., 1993 (McCully et al. 1993) | Lateral gastrocnemius |
Enzyme activity | ↓ |
| Meredith et al., 1989 (Meredith et al. 1989) | Vastus lateralis | Tissue homogenate respiration | ↓ |
| Pastoris et al., 2000 (Pastoris et al. 2000) | Vastus lateralis Gluteus maximus Rectus abdominus |
Enzyme activity |
↓
↔ ↔ |
| Proctor et al., 1995 (Proctor et al. 1995) | Vastus lateralis | Enzyme activity | ↓ |
| Rasmussen et al., 2003 (Rasmussen et al. 2003a) |
Vastus lateralis | Isolated mitochondria respiration | ↔ |
| Rasmussen et al., 2003 (Rasmussen et al. 2003b) |
Vastus lateralis | Isolated mitochondria respiration | ↔ |
| Rooyackers et al., 1996 (Rooyackers et al. 1996) |
Vastus lateralis | Enzyme activity | ↓ |
| Short et al., 2005 (Short et al. 2005) | Vastus lateralis | Isolated mitochondria ATP synthesis | ↓ |
| Short et al., 2003 (Short et al. 2003) | Vastus lateralis | Enzyme activity | ↓ |
| Tonkonogi et al., 2003 (Tonkonogi et al. 2003) |
Vastus lateralis | Isolated mitochondria and permeabilized fiber respiration |
↓
↓ |
| Trounce et al., 1989 (Trounce et al. 1989) | Vastus lateralis | Isolated mitochondria respiration | ↔ |
|
| |||
| In vivo studies | |||
| Amara et al., 2007 (Amara et al. 2007) | Tibialis anterior First dorsal interosseus |
31P-MRS PCr kinetics Near-infrared spectroscopy |
↔
↓ |
| Chilibeck et al., 1998 (Chilibeck et al. 1998) |
Gastrocnemius | 31P-MRS PCr kinetics | ↔ |
| Conley et al., 2000 (Conley et al. 2000) | Vastus lateralis | 31P-MRS PCr kinetics | ↓ |
| Kent-braun and Ng, 2000 (Kent-Braun & Ng 2000) |
Tibialis anterior | 31P-MRS PCr kinetics | ↔ |
| Lanza et al., 2005 (Lanza et al. 2005) | Tibialis anterior | 31P-MRS PCr kinetics | ↔ |
| Lanza et al., 2007 (Lanza et al. 2007) | Tibialis anterior | 31P-MRS PCr kinetics | ↔ |
| Larsen et al., 2009 (Larsen et al. 2009) | Tibialis anterior Vastus lateralis |
31P-MRS PCr kinetics |
↔
↓ |
| McCully et al., 1991 (McCully et al. 1991) | Lateral gastrocnemius |
31P-MRS PCr kinetics | ↓ |
| McCully et al., 1993 (McCully et al. 1993) | Lateral Gastrocnemius |
31P-MRS PCr kinetics | ↓ |
| Petersen et al., 2003 (Petersen et al. 2003) | Soleus | Basal oxidation (13C-MRS) and phosphorylation (31P-MRS) |
↓ |
| Schunk et al., 1999 (Schunk et al. 1999) | Vastus lateralis | 31P-MRS PCr kinetics | ↔ |
| Taylor et al., 1997 (Taylor et al. 1997) | Calf | 31P-MRS PCr kinetics | ↔ |
Mechanisms of mitochondrial changes with aging
There is uncertainty concerning the degree to which lifestyle factors versus aging per se contribute to declining mitochondrial content and function with old age. The underlying mechanisms are beginning to emerge as investigators probe various factors between the expression of genes and the assembly of the functional organelle. At the level of gene expression, numerous mRNA transcripts for genes encoding mitochondrial proteins are known to be significantly reduced with aging (Barazzoni et al. 2000,Calleja et al. 1993,Short et al. 2005,Welle et al. 2000). This decline in mRNA template availability may be the result of reduced gene transcription or mRNA instability with aging. Transcripts corresponding to the mitochondrial genome are undoubtedly influenced by declining mtDNA copy number with age (Barazzoni et al. 2000,Lanza et al. 2008,Menshikova et al. 2006,Short et al. 2005). Consequently, the expression of the proteins encoded by these genes would logically be expected to be influenced by the availability of the mRNA template. We recently measured the expression of numerous skeletal muscle proteins using isotopic tags and mass spectrometry and found that mitochondrial proteins encoded by both nuclear and mitochondrial genomes were significantly decreased with aging (Lanza et al. 2008,Short et al. 2005) (figure 5a). Protein expression, reflecting the balance between protein synthesis and degradation, is also influenced by the translational rate of gene transcripts. Using stable isotopes of amino acids, we found that the in vivo synthesis rates of mitochondrial proteins decreased with aging (Rooyackers et al. 1996). It is uncertain whether aging affects the synthesis rates of mitochondrial proteins encoded by both genomes in the same way. Recent advances in measuring the synthesis rates of individual mitochondrial proteins (Jaleel et al. 2008) will allow us to address this question in the future. Although specific rates of mitochondrial protein breakdown have yet to be measured in vivo, it is likely that mitochondrial protein turnover is reduced with aging based on the observations that Lon protease expression, a key enzyme for mitochondrial proteolysis, is reduced in older mice (Bota et al. 2002). In support of this notion we recently reported that whole body proteolysis decreases with aging (Henderson et al. 2009). A potential mismatch between rates of protein synthesis and breakdown may lead to an overall decrease in protein expression, but an important consequence of decreased protein turnover is the accumulation of oxidatively damaged dysfunctional proteins. Indeed, aging is associated with increased protein carbonylation (Hepple et al. 2008)and nitrotyrosine-modified proteins (Fugere et al. 2006). In addition to proteins, nucleic acids also demonstrate increased levels of oxidative damage with aging (Michikawa et al. 1999,Short et al. 2005), particularly mtDNA, whose susceptibility is increased by its proximity to the source of damaging reactive oxygen species and the lack of protection by histones. Mitochondrial fusion and fission appear to be critical for the maintenance of mtDNA (Herlan et al. 2003). At this point, the link between mitochondrial fusion/fission, aging, and mtDNA damage remains in its nascent stages (Lee et al. 2007). Altogether there are multiple factors at the levels of gene expression, protein synthesis, protein quality, and mitochondrial dynamics that can account for reductions in mitochondrial number and function with aging.
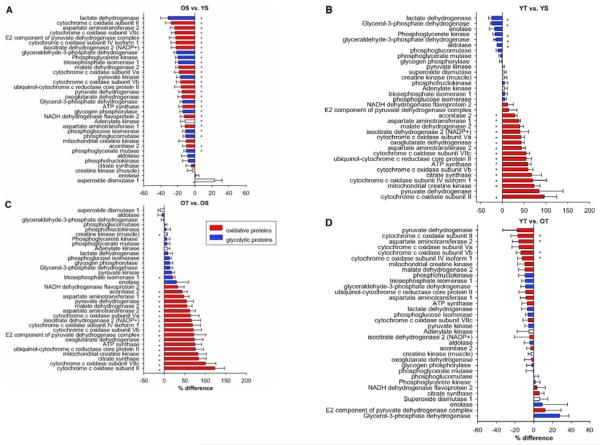
Figure 5.
The relative abundance of numerous proteins involved in oxidative ATP synthesis in skeletal muscle decreased with age in sedentary adults (panel A) and increased with exercise training in young (panel B) and older (panel C). A comparison of trained young and older adults revealed that the majority, but not all, age-related differences in protein expression were resolved (panel D). Data are presented as means ± SEM. *Significant (P < 0.05) differences in relative expression. Reproduced with permission from Lanza et al., Diabetes, 2008.
Insulin sensitivity, aging, and the role of mitochondria
Insulin stimulates glucose uptake in peripheral tissues such as skeletal muscle and suppresses hepatic glucose production. The term “insulin sensitivity” is used to qualify the vigorousness of the response of target tissues to changing insulin levels. Insulin sensitivity is generally believed to decline with old age (Defronzo 1979,Houmard et al. 1995,Petersen et al. 2003,Short et al. 2003) but in much the same way as the aging mitochondrion, the reported effects of age vary considerably depending on the sample demographics. Studies in relatively lean individuals often show that insulin sensitivity is well maintained across the lifespan (Broughton et al. 1991,Lanza et al. 2008,Seals et al. 1984). Accumulating evidence supports the notion that physical inactivity and adiposity, not chronological age, are strong predictors of the decline in insulin sensitivity with age (Basu et al. 2003,Kohrt et al. 1993,Szoke et al. 2008).
Mitochondria have been implicated in the age-related decline in insulin sensitivity. This notion is largely driven by correlative evidence, specifically in obese (Simoneau & Kelley 1997), type 2 diabetic (Kelley et al. 2002), and elderly individuals (Petersen et al. 2003,Short et al. 2005). A reasonable hypothesis has been proposed whereby impaired mitochondrial function leads to decreased insulin signaling as a result of lipid metabolite accumulation (Shulman 2000). Although championed by many, this relationship has become clouded by numerous instances where mitochondrial function and insulin sensitivity are clearly dissociated (Boushel et al. 2007,Lanza et al. 2008,Pospisilik et al. 2007,Short et al. 2003,Toledo et al. 2008). A recent observation from our lab demonstrates that insulin sensitivity is similar in young and older men in spite of a significant age-related reduction in mitochondrial oxidative capacity (Lanza et al. 2008). Although mitochondria are frequently bestowed a causative role in the pathogenesis of insulin resistance, this remains a controversial topic that merits further investigation. As we will discuss below, there is some intriguing evidence that mitochondrial abnormalities observed in certain insulin resistant populations may be a consequence of impaired insulin signaling given that mitochondrial biogenesis has been shown to be regulated, in part, by insulin.
EFFECTS OF EXERCISE ON MITOCHONDRIAL FUNCTION
Numerous physiological stimuli (e.g., temperature, stress, hormones, hypoxia, caloric intake) are known to affect mitochondrial physiology, but endurance exercise remains one of the most robust, simple, cost-effective, and well-studied stimuli for mitochondrial biogenesis. Holloszy, having noted that the muscles of wild animals exhibited higher mitochondrial content than their domesticated counterparts, followed up on these observations by showing that prolonged treadmill running in rats increased mitochondrial oxidative capacity (Holloszy 1967), thus opening the floodgates for decades of research into mitochondrial adaptations to exercise. Countless studies have documented the beneficial effects of endurance exercise in a wide variety of species. Specifically, endurance training increased mitochondrial content, mitochondrial enzyme activities, and muscle oxidative capacity (Baldwin et al. 1972,Bizeau et al. 1998,Chow et al. 2007,Davies et al. 1981,Demirel et al. 1999,Dudley et al. 1982,Gollnick & King 1969,Holloszy 1967,Howald et al. 1985,Kent-Braun et al. 1990,Short et al. 2003,Zamora et al. 1995). We recently conducted a study in mice in an effort to better define the precise molecular and cellular mechanisms underlying the effects of exercise on mitochondrial content and function (Chow et al. 2007). Mice performed treadmill running for 5 days per week for 8 weeks at 80% of peak O2 consumption. As expected, endurance training improved mitochondrial function in skeletal muscle, as evidenced by increased whole body peak O2 consumption, mitochondrial enzyme activities (citrate synthase, B-hydroxyacyl Coenzyme A dehydrogenase, cytochrome c oxidase), and maximal mitochondrial ATP synthesis rates. Microarray analyses revealed that these training adaptations were accompanied by increased gene transcripts corresponding to mitochondrial proteins encoded by both genomes. These exercise-induced changes in mitochondrial gene transcripts occurred in conjunction with increases in mtDNA copy number and the expression of peroxisome proliferative-activated receptor-γ coactivator-1α (PGC-1α), a transcriptional co-activator that is known to be a key regulator of mitochondrial biogenesis. This and other cellular signals which are believed to be responsible for mitochondrial biogenesis with exercise will be discussed next.
Signaling pathways linking endurance exercise to mitochondrial biogenesis
What is the cellular signal that links endurance exercise with proliferation of mitochondria? Several comprehensive review articles address this question in detail (Holloszy 2008,Lin et al. 2005,Scarpulla 2002), so the forthcoming paragraphs will focus primarily on the acute and chronic signals that mediate mitochondrial biogenesis in response to endurance exercise. As mentioned earlier, the vast majority of mitochondrial proteins are encoded by the nuclear genome, but there are a handful of proteins encoded by the mitochondrial genome that are essential to the proper function of the organelle. Thus, successful proliferation of mitochondria requires coordinated control of the expression of 2 distinct genomes. Significant advances in understanding this complex regulation have been made over the past 20 years with the discovery of several transcription factors which regulate the transcription of mitochondrial proteins. Mitochondrial transcription factor A (TFAM) regulates the transcription and expression of the mitochondrial genome (Fisher et al. 1987), while nuclear respiratory factors (NRF-1, NRF-2) modulate the transcription of nuclear-encoded mitochondrial proteins (Evans & Scarpulla 1989,Scarpulla 2002,Virbasius et al. 1993). TFAM is a product of the nuclear genome, whose promoter includes binding sites for NRF-1 and NRF-2 (Virbasius & Scarpulla 1994). Thus, by demonstrating that NRF-1 and NRF-2 activate the TFAM promoter, Scarpulla’s group established a link between the expression of the two genomes. However, the binding of transcription factors to gene promoters is generally not sufficient to activate gene transcription, which often requires enzymatic activity of coactivator proteins (Spiegelman & Heinrich 2004). This role is fulfilled by the protein coactivator PGC-1α, which activates NRF-1, NRF-2, and several additional transcription factors involved with mitochondrial biogenesis, fatty acid oxidation, and gluconeogenesis (Lin et al. 2005,Puigserver et al. 1998). Thus, as a result of the pioneering work of Scarpulla’s and Spiegelman’s groups we now have a clearer picture of the coordinated transcriptional control of mitochondrial biogenesis and an upstream inducible coactivator which has become widely recognized as an important regulator of mitochondrial biogenesis.
Endurance exercise initiates mitochondrial biogenesis through PGC-1α in two distinct ways: a rapid increase in PGC-1α activity followed by a more chronic increase in PGC-1α expression. The initial rapid phase of adaptation was first revealed from 3 key observations by Wright and colleagues (Wright et al. 2007). First, gene transcripts and expression of certain mitochondrial proteins increased at similar or faster rates compared to PGC-1α expression in response to exercise. Second, NRF-1 and NRF-2 were bound to their respective promoters in advance of any increase in PGC-1α expression. Third, most PGC-1α is found within the cytosol of resting skeletal muscle but moves into the nuclei in response to exercise. The authors propose that in advance of any changes in PGC-1α expression, exercise rapidly initiates the process of mitochondrial biogenesis by activating PGC-1α (Wright et al. 2007).
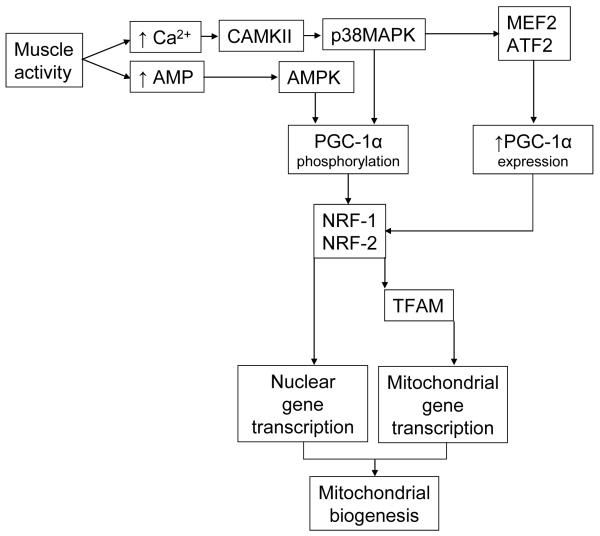
There are currently two protein kinases that are believed to activate (i.e., phosphorylate) PGC-1α in response to exercise, namely p38 mitogen activated protein kinase (p38 MAPK) and AMP-activated protein kinase (AMPK) (Jager et al. 2007,Knutti et al. 2001,Puigserver et al. 2001). During muscle activity, the neural excitation of the sarcolemmal t-tubules and contraction of the sarcomeres are coupled by the release of calcium from the sarcoplasmic reticulum. Increased cytosolic Ca2+ leads to the activation of calcium/calmodulin-dependent protein kinase (CAMKII) (Rose & Hargreaves 2003). CAMKII appears to be an upstream activator of p38MAPK (Wright et al. 2007), providing a link between muscle activity and phosphorylation of PGC-1α. Another important intracellular signal is the increase in AMP concentration that occurs as ATP and ADP are hydrolyzed during contractile activity. AMPK, which is activated in response to elevated cellular AMP, induces mitochondrial biogenesis (Winder et al. 2000) and phosphorylates PGC-1α (Jager et al. 2007). The aforementioned work represents a sampling of the body of literature that has led to an emerging pathway by which exercise acutely induces mitochondrial biogenesis in advance of changes in PGC-1α expression (figure 3).
Figure 3.
Muscle activity stimulates mitochondrial biogenesis through a rapid increase in PGC-1α activity as well as a more chronic increase in PGC-1α expression. Increased intracellular calcium and AMP levels during exercise respectively activate calcium-calmodulin dependent protein kinase (CAMKII) and AMP-activated protein kinase (AMPK). The activation of both protein kinases leads to phosphorylation and activation of PGC-1α subsequent activation of nuclear transcription factors NRF-1 and NRF-2, which increase the transcription of nuclear-encoded mitochondrial genes. NRFs also stimulate the expression of the mitochondrial transcription factor (TFAM) which increases the expression of mitochondrial genes encoded by the mitochondrial genome. The more chronic increase in PGC-1α expression occurs as a result of activation of MEF2 and ATF2 by P38MAPK.
In addition to the acute changes in PGC-1α activity, exercise also induces a delayed but sustained signal for mitochondrial biogenesis through increased PGC-1α mRNA and protein expression (Irrcher et al. 2003,Pilegaard et al. 2003). p38MAPK not only phosphorylates and activates PGC-1α, but also drives the increased expression of PGC-1α in response to exercise. This conclusion was reached by observing increased PGC-1α promoter activity when the p38 MAPK pathway was stimulated but not in the presence of specific p38 MAPK inhibitors (Akimoto et al. 2005). Activating transcription factor 2 (ATF2) and myocyte enhancer factor 2 (MEF2) are upstream transcription factors of the PGC-1α gene, both of which are stimulated by phosphorylation by p38 MAPK (Cao et al. 2004,Zhao et al. 1999). As illustrated in figure 3, exercise stimulates p38 MAPK, thereby activating PGC-1α transcription factors, leading to increases PGC-1α expression and subsequent mitochondrial biogenesis.
Exercise and Aging
Amidst the plethora of documented detriments of aging, physical inactivity represents a less-well studied but no less important hallmark of the aging process. The nematode worm may seem like an unlikely model for human aging, but we now know that Caenorhabditis elegans, like humans, exhibit a progressive deterioration in muscle as they age (Herndon et al. 2002). Remarkably, the nervous system of the worms remains intact, and it seems that sarcopenia occurs in parallel and possibly as a consequence of decreased locomotor activity as they age. Age-related declines in physical activity have also been reported in rodents (Holloszy 1997) and humans (Black et al. 1996), leading investigators to posit that physical inactivity is a major determinant of many physiological detriments ascribed to aging. The issue of physical activity is particularly important in human studies of aging because of substantial inter-individual variability, but free living habitual physical activity patterns are difficult to accurately quantify and are seldom reported or used as covariates.
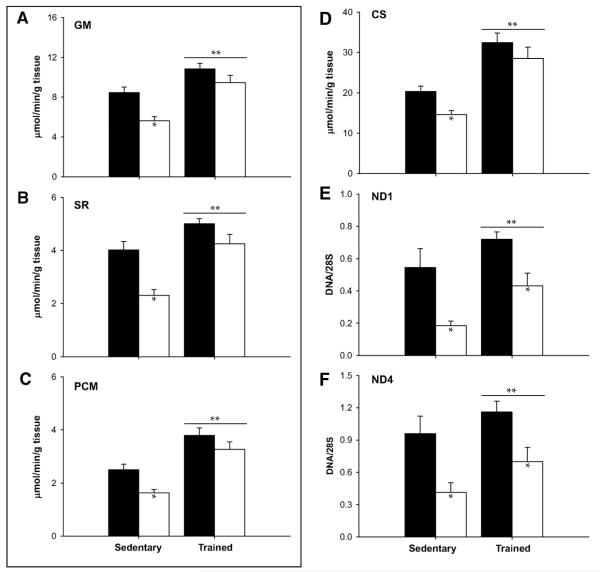
Physical activity, being both a predictor of age-related mitochondrial dysfunction and potent activator of mitochondrial biogenesis, may be an effective tool to prevent, reverse, or delay the onset of sarcopenia. Although exercise has never been shown to increase maximal lifespan, the potential benefit in combating age-related morbidities has prompted many investigators to examine adaptations to endurance training in older adults. The general consensus is that older adults maintain their ability to adapt to endurance exercise, as evidenced by robust increases in VO2 peak (Coggan et al. 1992,Short et al. 2003), mitochondrial enzyme activities (Coggan et al. 1992,Menshikova et al. 2006,Short et al. 2003), mitochondrial content (Jubrias et al. 2001,Menshikova et al. 2006), protein synthesis rates (Balagopal et al. 2001), mtDNA copy number (Menshikova et al. 2006), and gene transcripts for mitochondrial proteins (Short et al. 2003). However, the overall enthusiasm for exercise as a prescription for aging is somewhat dampened by some reports showing more modest effects of exercise in older adults that are blunted when compared to responses in young (Aniansson et al. 1980,Jubrias et al. 2001,Orlander & Aniansson 1980). As discussed elsewhere (Coggan et al. 1992), these data may reflect decreased adaptation of older muscle to a training stimulus or simply insufficient training volume to reveal their full potential. Additional studies are needed to assess whether older adults are able to adapt to low-volume intense interval training, which has been recently shown to induce adaptations similar to endurance training in young humans (Gibala et al. 2009). Cross-sectional studies of chronically endurance trained young and older adults provide some insight into this issue. Proctor et al. found that oxidative capacity, determined by activities of citrate synthase and succinate dehydrogenase, was lower with age in sedentary individuals, but similar in endurance trained young and older men (Proctor et al. 1995). Similarly, mitochondrial respiratory chain activity was similar in young and elderly endurance athletes (Brierley et al. 1996). These seminal observations were recently confirmed by our lab in a study of young (18-30 years) and older (59-76) men who were either sedentary or highly endurance trained (Lanza et al. 2008). Mitochondria were isolated from biopsy tissue from vastus lateralis muscle, and ATP synthesis rates were measured by luciferase bioluminescence. The data in Figure 4 show that mitochondrial oxidative capacity was lower with age in sedentary but not in endurance trained individuals, consistent with the notion that physical (in)activity is a major determinant of mitochondria-based changes with age. However, of interest, exercise could not completely prevent the age-related reduction in mtDNA abundance (Figure 4). Since genes are ultimately expressed as proteins, we measured the relative abundance of numerous proteins related to muscle energy metabolism by first labeling peptides using iTRAQ reagent, followed by separation and analysis by liquid chromatography tandem mass spectrometry. We found that 27 proteins involved in oxidative and non-oxidative ATP production in skeletal muscle were significantly reduced with age in sedentary individuals, but this age effect was nearly absent in endurance trained young and older men (Figure 5). Three subunits of cytochrome c oxidase and an anapleurotic TCA cycle enzyme remained significantly reduced in endurance trained older adults. Interestingly, this study also found that the expression of the class III histone deacetylase SIRT3, was reduced with aging, but similarly elevated in young and older endurance trained individuals (Lanza et al. 2008). Thus, it seems that exercise may activate some of the signaling pathways that are linked with the lifespan-extending effects of caloric restriction. In sum, although exercise may delay or mask some of the functional changes to the aging mitochondrion, there are some changes at the level of mtDNA abundance and protein expression that even regular vigorous endurance exercise cannot entirely prevent. This notion is confirmed by a recent study in vivo in which oxidative capacity (by 31P-MRS) was found to be well-preserved with old age in the tibialis anterior but 35% lower in older compared with young runners (Larsen et al. 2009). Thus, it seems that while physical activity is an important determinant of sarcopenia and related dysfunctions, there is a component of aging that is an inevitable function of chronological age. The less robust increase in PGC-alpha and TFAM expression in highly trained older people than younger people (49) may explain why older people could not regain all mitochondrial capacity as young people by exercise.
Figure 4.
Mitochondrial ATP production rates were decreased in sedentary older compared to young subjects, but were similar in highly endurance trained young and older adults using substrates glutamate + malate (GM, panel A), succinate + rotenone (SR, panel B), and palmitoyl-l-carnitine + malate (PCM, panel C). Similar results were observed for citrate synthase activity (panel D). Mitochondrial DNA copy number corresponding to NADH dehydrogenase subunits 1 (panel E) and 4 (panel F) were lower in sedentary older adults. Endurance exercise increased mtDNA copy number in both age groups, but could not resolve the age-related decrement. Data are presented as means ± SEM. *Pairwise comparisons revealed significant (P < 0.05) effects of age within activity groups; **significant (P < 0.05) main effects of training. ■, young; □, older. Reproduced with permission from Lanza et al., Diabetes, 2008.
HORMONES AND MITOCHONDRIAL BIOGENESIS
Thus far our discussion has focused mainly on the role of exercise in stimulating mitochondrial biogenesis through intracellular signals such as AMP and calcium. It is important to also consider the role of hormones in maintaining energetic homeostasis in their target tissues. Hormones such as insulin, growth hormone, and thyroid hormone accomplish this homeostasis, in part, by stimulating mitochondrial biogenesis.
Insulin
Insulin is a major postprandial hormone with strong influence on energy metabolism (Nair et al. 1984). One of the first clues pointing to insulin as a mediator of mitochondrial function came in a study of muscle protein synthesis in miniature swine (Boirie et al. 2001). An 8 hour infusion of insulin (0.7 mU/kg/min) significantly increased the fractional synthesis rate of mitochondrial proteins in skeletal muscle, measured using L-[1-13C]leucine as a tracer. In contrast, the synthesis rates of contractile and sarcoplasmic proteins remained unchanged. Insulin’s effect on mitochondrial protein synthesis is mediated, at least in part, through its influence on gene expression as it has been shown to increase mRNA transcripts for cytochrome oxidase 1 and NADH dehydrogenase 1 (Huang et al. 1999). As protein synthesis rates increase in response to insulin, so too should the abundance of mitochondrial proteins and overall muscle oxidative capacity. Shortly after Boirie’s initial report from swine, these findings were confirmed in humans (Stump et al. 2003). As with swine, insulin infusion increased muscle mitochondrial protein synthesis rates, and consistent with Huang et al., gene transcript levels of NADH dehydrogenase and cytochrome c oxidase also increased. Furthermore, mitochondrial oxidative capacity was clearly elevated with insulin infusion, as indicated by increased citrate synthase activity, cytochrome oxidase activity, and mitochondrial ATP production rates (Stump et al. 2003). However, this insulin effect in humans has been shown only when amino acids and glucose levels are maintained. This functional response may be attributed to increased expression of mitochondrial proteins; however, it is also possible that ATP production rates could increase acutely as a result of changes in the efficiency of the respiratory chain or allosteric interactions that increase mitochondrial enzyme activities. Subsequent studies in c-peptide negative type 1 diabetic subjects revealed that insulin deprivation significantly reduced many mitochondrial gene transcripts and ATP synthesis rates (Karakelides et al. 2007). Although the precise molecular mechanism requires further study, these studies uncover a new role for insulin as a regulator of skeletal muscle mitochondrial function. Thus far, our discussion has focused largely on the role of insulin in stimulating gene expression, the fractional synthesis rates of mitochondrial proteins, and acute stimulation of mitochondrial ATP synthesis. In addition to insulin’s anabolic role in regulating mitochondrial protein expression, there is evidence to support a role for insulin in suppressing proteolysis independently of the presence of amino acids (Gelfand & Barrett 1987); an effect which seems to be diminished with age (Rennie 2009). Thus, insulin’s ability to enhance mitochondrial function is likely to be due to the combined effects of acute activation of mitochondrial enzymes, increased mitochondrial protein synthesis, and suppressed mitochondrial protein degradation.
Insulin’s role in regulating mitochondrial function has provided some inroads to understanding the tenuous relationship between insulin sensitivity and mitochondrial function. Altered mitochondrial physiology is often proposed to be a cause of insulin resistance in populations such as obese (Simoneau & Kelley 1997), type 2 diabetic (Kelley et al. 2002), and elderly (Petersen et al. 2003,Short et al. 2005). Now that insulin has emerged as a regulator of mitochondrial biogenesis, it is not unreasonable to expect that this signaling pathway may be blunted in insulin resistant individuals. In other words, decreased mitochondrial content and function may be a consequence of impaired insulin signaling rather than the reverse. This alternative view is supported by a report that insulin increased muscle mitochondrial ATP synthesis in non-diabetic controls but not in individuals with type 2 diabetes (Stump et al. 2003). Similarly, insulin increased mitochondrial protein synthesis in non-diabetic people (Stump et al. 2003) but not in people with type 2 diabetes (Halvatsiotis et al. 2002). A follow-up study revealed that mitochondrial ATP synthesis rates were similar in type 2 diabetic and nondiabetic people at lower (i.e., postabsorptive) insulin concentrations, but increasing insulin to postabsorptive levels stimulated mitochondrial ATP synthesis in non-diabetic people only (Asmann et al. 2006). These results were mirrored by insulin’s effect on PGC-1α expression, which was blunted in type 2 diabetic individuals at high physiological insulin levels (Asmann et al. 2006). In sum, these data are consistent with the notion that mitochondrial function is altered in type 2 diabetes because of altered insulin signaling pathways related to mitochondrial biogenesis rather than an intrinsic mitochondrial abnormality. Several other reports demonstrate complete dissociation between mitochondrial function and insulin sensitivity, casting even more shadows on the precise relationship between the two variables. For example, Asian Indians with severe insulin resistance have higher mitochondrial DNA abundance and ATP production rate (Nair et al. 2008) and experimental fat overfeeding increases mitochondrial biogenesis while causing insulin resistance (Hancock et al. 2008). These studies underscore that there must be many factors other than insulin that are involved in mitochondrial biogenesis and function.
Thyroid hormone and growth hormone
Thyroid hormones have long been recognized as potent mediators of mitochondrial function (Winder & Holloszy 1977). Much of the early work in this area was conducted by the Italian group of Paradies and Ruggiero who found that mitochondria from hyperthyroid rats exhibited increased enzyme activities, substrate oxidation, and respiration rates (Paradies et al. 1994,Paradies & Ruggiero 1988,Paradies & Ruggiero 1990). Exogenous thyroid hormone T4 increases mitochondrial volume density increases in both liver and muscle in rats (Goldenthal et al. 2004,Wooten & Cascarano 1980). Thyroid hormone also increases the expression of uncoupling proteins (UCP2, UCP3) (Jekabsons et al. 1999,Lanni et al. 1997), which are believed to alter mitochondrial efficiency by dissipating the proton gradient across the inner mitochondrial membrane. It is logical that UCPs and other mitochondrial proteins would be co-regulated to maintain energetic homeostasis in the face of increased proton leak. Until recently, it was unclear whether thyroid hormone increased ATP production, or simply increased fuel oxidation to maintain an adequate proton motive force. We found that 14 days of T3 treatment in rats increased UCP2 and UCP3 expression as well as mitochondrial ATP synthesis rates in soleus muscle, heart, and liver (Short et al. 2001). These data demonstrate that thyroid hormone increases mitochondrial capacity for ATP synthesis in oxidative tissues, rather than simply increasing substrate oxidation to account for dissipation of the transmembrane proton gradient. Thyroid hormone appears to regulate mitochondrial function, in part, by increasing the expression of mitochondrial proteins. A follow-up study from our group showed that T3 administration robustly increased mitochondrial protein synthesis rates (Short et al. 2007); an effect that could be explained by increased transcription of genes encoding mitochondrial proteins such as cytochrome c oxidase (Short et al. 2001,Wiesner et al. 1992). Since mitochondrial DNA contains promoter regions with response elements for thyroid hormone receptors (Enriquez et al. 1999), it is probable that thyroid hormone affects mitochondrial protein expression by regulating the transcription of mitochondrial genes. Thyroid hormone also appears to influence mitochondrial function independently of protein expression. Our studies of T3 administration in rats showed that T3 increases mitochondrial protein synthesis in the soleus and plantaris muscles, but ATP synthesis rates increased in the oxidative but not the glycolytic tissues (Short et al. 2001,Short et al. 2007), suggesting that thyroid hormone may regulate mitochondrial function through other mechanisms. Cardiolipin, which is increased by T3 (Brand et al. 1992) is known to exert allosteric control of several enzymes involved in substrate transport such as carnitine acylcarnitine translocase (Paradies et al. 1996). Thyroid hormone 3,5-Diiodothyronine (T2) also has been suggested abolish the allosteric inhibition of cytochrome oxidase by ATP (Arnold & Kadenbach 1999). Thus, although thyroid hormone clearly influenced mitochondrial function, the underlying mechanism is multifaceted and involved regulation of the translational rate of gene transcripts as well as post-translational effects on the activities of numerous enzymes that require further study.
The effects of growth hormone (GH) on mitochondrial function are less well-studied, but there is accumulating evidence that similar to thyroid hormone, GH may exhibit some regulatory control over mitochondrial biogenesis and function. We measured muscle mitochondrial function in humans infused for 14 hours with either saline or GH (Short et al. 2008). Acute GH infusion significantly increased mitochondrial function, measured by citrate synthase activity and ATP synthesis rates in mitochondria isolated from skeletal muscle. These functional changes were accompanied by significantly increased mRNA transcripts for cytochrome c oxidase and TFAM, and non-significant increases in PGC-1α and NRF-1. Unlike thyroid hormone, these effects occurred without changing mitochondrial protein synthesis rates (Short et al. 2008). Additional studies are needed to fully elucidate the effects of GH on mitochondrial function, particularly in consideration of previous findings that acute GH administration to trained cyclists results in decreased exercise performance and increased plasma lactate levels (Lange et al. 2002). Although it is difficult to draw conclusions from studies of sedentary versus highly trained subjects, these paradoxical observations suggest that GH may induce mitochondrial biogenesis without a commensurate increase in exercise capacity, possibly because of effects of GH on insulin action and substrate metabolism that may limit exercise performance..
Conclusion
For more than a billion years, mitochondria have resided within their host cells, providing regulatory control of energy homeostasis, cellular signaling, and apoptosis. Reports of age-related declines in mitochondrial function have spawned a vast body of literature devoted to understanding the underlying mechanisms. These mechanisms involve decreased abundance of mtDNA, reduced expression of mRNA encoded by both nuclear and mitochondrial genes, and reduced synthesis and expression of mitochondrial proteins, ultimately resulting in decreased function of the whole organelle. Research into potential therapies to prevent, reverse, or delay the onset of age-related detriments have been fueled by recent advances in understanding the regulation of mitochondrial biogenesis by transcription factors and transcriptional co-activators that govern the coordinated expression of two distinct genomes. As with many physiological processes that must be tightly regulated, mitochondrial biogenesis appears to be controlled in part by hormones such as insulin, thyroid hormone, and growth hormone. Other intracellular signals linked with exercise (i.e., Ca2+, AMP) initiate a signaling pathway involving PGC-1α to induce mitochondrial proliferation and increased function of existing mitochondria. The beneficial effects of exercise on combating age-related mitochondrial dysfunction are well-documented, and although physical inactivity is emerging as a major determinant of mitochondrial dysfunction, exercise cannot ameliorate all signs of aging in the mitochondrion.
ACKNOWLEDGEMENTS
The authors are greatly indebted to the skillful assistance of Maureen Bigelow, Jill Schimke, Katherine Klaus, Dawn Morse, Bushra Ali, Jane Kahl, Dan Jakaitis, Roberta Soderberg, Beth Will, Deborah Sheldon, and Melissa Aakre. We are also grateful for support from the Mayo Clinic Center for Translational Science Activities and the National Institutes of Health R01-AG09531, R01-DK41973 (KSN) and T32-DK007198 (IRL). Additional support was provided by the Mayo Foundation and the Murdock-Dole Professorship (to KSN).
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest
References
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. %20. [DOI] [PubMed] [Google Scholar]
- Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci U S A. 2007;104:1057–1062. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Aniansson A, Grimby G, Rundgren A, Svanborg A, Orlander J. Physical training in old men. Age Ageing. 1980;9:186–187. doi: 10.1093/ageing/9.3.186. [DOI] [PubMed] [Google Scholar]
- Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280:E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA, Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972;222:373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Casademont J, Rotig A, Miro O, Urbano-Marquez A, Rustin P, Cardellach F. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem Biophys Res Commun. 1996;229:536–539. doi: 10.1006/bbrc.1996.1839. [DOI] [PubMed] [Google Scholar]
- Basu R, Breda E, Oberg AL, Powell CC, Dalla MC, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- Bizeau ME, Willis WT, Hazel JR. Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J Appl Physiol. 1998;85:1279–1284. doi: 10.1152/jappl.1998.85.4.1279. [DOI] [PubMed] [Google Scholar]
- Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- Boffoli D, Scacco SC, Vergari R, Persio MT, Solarino G, Laforgia R, Papa S. Ageing is associated in females with a decline in the content and activity on the b-c1 complex in skeletal muscle mitochondria. Biochim Biophys Acta. 1996;1315:66–72. doi: 10.1016/0925-4439(95)00107-7. [DOI] [PubMed] [Google Scholar]
- Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226:73–82. doi: 10.1016/0925-4439(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50:2652–2658. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Steverding D, Kadenbach B, Stevenson PM, Hafner RP. The mechanism of the increase in mitochondrial proton permeability induced by thyroid hormones. Eur J Biochem. 1992;206:775–781. doi: 10.1111/j.1432-1033.1992.tb16984.x. [DOI] [PubMed] [Google Scholar]
- Brierley EJ, Johnson MA, James OF, Turnbull DM. Effects of physical activity and age on mitochondrial function. QJM. 1996;89:251–258. doi: 10.1093/qjmed/89.4.251. [DOI] [PubMed] [Google Scholar]
- Broughton DL, James OW, Alberti KG, Taylor R. Peripheral and hepatic insulin sensitivity in healthy elderly human subjects. Eur J Clin Invest. 1991;21:13–21. doi: 10.1111/j.1365-2362.1991.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Calleja M, Pena P, Ugalde C, Ferreiro C, Marco R, Garesse R. Mitochondrial DNA remains intact during Drosophila aging, but the levels of mitochondrial transcripts are significantly reduced. J Biol Chem. 1993;268:18891–18897. [PubMed] [Google Scholar]
- Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Eleff S, Leigh JS., Jr. Noninvasive, nondestructive approaches to cell bioenergetics. Proc Natl Acad Sci U S A. 1980;77:7430–7434. doi: 10.1073/pnas.77.12.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Leigh JS, Jr., Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985;82:8384–8388. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, WILLIAMS GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- Chilibeck PD, McCreary CR, Marsh GD, Paterson DH, Noble EG, Taylor AW, Thompson RT. Evaluation of muscle oxidative potential by 31P-MRS during incremental exercise in old and young humans. Eur J Appl Physiol Occup Physiol. 1998;78:460–465. doi: 10.1007/s004210050446. [DOI] [PubMed] [Google Scholar]
- Chow LS, Greenlund LJ, Asmann YW, Short KR, McCrady SK, Levine JA, Nair KS. Impact of endurance training on murine spontaneous activity, muscle mitochondrial DNA abundance, gene transcripts, and function. J Appl Physiol. 2007;102:1078–1089. doi: 10.1152/japplphysiol.00791.2006. [DOI] [PubMed] [Google Scholar]
- Chretien D, Gallego J, Barrientos A, Casademont J, Cardellach F, Munnich A, Rotig A, Rustin P. Biochemical parameters for the diagnosis of mitochondrial respiratory chain deficiency in humans, and their lack of age-related changes. Biochem J. 1998;329:249–254. doi: 10.1042/bj3290249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–10. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. 203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CT, Thomas DO, White MJ. Mechanical properties of young and elderly human muscle. Acta Med Scand Suppl. 1986;711:219–26. doi: 10.1111/j.0954-6820.1986.tb08954.x. 219-226. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981;209:539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977;267:703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- DeLuca M, McElroy WD. Kinetics of the firefly luciferase catalyzed reactions. Biochemistry. 1974;13:921–925. doi: 10.1021/bi00702a015. [DOI] [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Naito H, Hughes M, Coombes JS. Exercise-induced alterations in skeletal muscle myosin heavy chain phenotype: dose-response relationship. J Appl Physiol. 1999;86:1002–1008. doi: 10.1152/jappl.1999.86.3.1002. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53:844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- Enriquez JA, Fernandez-Silva P, Garrido-Perez N, Lopez-Perez MJ, Perez-Martos A, Montoya J. Direct regulation of mitochondrial RNA synthesis by thyroid hormone. Mol Cell Biol. 1999;19:657–670. doi: 10.1128/mcb.19.1.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Scarpulla RC. Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter. Characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. J Biol Chem. 1989;264:14361–14368. [PubMed] [Google Scholar]
- Fisher RP, Topper JN, Clayton DA. Promoter selection in human mitochondria involves binding of a transcription factor to orientation-independent upstream regulatory elements. Cell. 1987;50:247–258. doi: 10.1016/0092-8674(87)90220-0. [DOI] [PubMed] [Google Scholar]
- Fugere NA, Ferrington DA, Thompson LV. Protein nitration with aging in the rat semimembranosus and soleus muscles. J Gerontol A Biol Sci Med Sci. 2006;61:806–812. doi: 10.1093/gerona/61.8.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol. 2009;106:929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- Goldenthal MJ, Weiss HR, Marin-Garcia J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol Cell Biochem. 2004;265:97–106. doi: 10.1023/b:mcbi.0000044321.17680.a2. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, King DW. Effect of exercise and training on mitochondria of rat skeletal muscle. Am J Physiol. 1969;216:1502–1509. doi: 10.1152/ajplegacy.1969.216.6.1502. [DOI] [PubMed] [Google Scholar]
- Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- Grimby G. Physical performance, physical activity and quality of life in elderly people. Scand J Med Sci Sports. 1995;5:127–128. doi: 10.1111/j.1600-0838.1995.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Grimby G, Saltin B. Physiological analysis of physically well-trained middle-aged and old athletes. Acta Med Scand. 1966;179:513–526. doi: 10.1111/j.0954-6820.1966.tb07968.x. [DOI] [PubMed] [Google Scholar]
- Halvatsiotis P, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–2404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008;105:7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O’Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009;23:631–641. doi: 10.1096/fj.08-117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Qin M, Nakamoto H, Goto S. Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1231–R1237. doi: 10.1152/ajpregu.90478.2008. [DOI] [PubMed] [Google Scholar]
- Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS. Processing of Mgm1 by the rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J Biol Chem. 2003;278:27781–27788. doi: 10.1074/jbc.M211311200. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol. 2008;59(Suppl 7):5–18. 5-18. [PubMed] [Google Scholar]
- Houmard JA, Weidner MD, Dolan PL, Leggett-Frazier N, Gavigan KE, Hickey MS, Tyndall GL, Zheng D, Alshami A, Dohm GL. Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes. 1995;44:555–560. doi: 10.2337/diab.44.5.555. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85:1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. 1985;403:369–376. doi: 10.1007/BF00589248. [DOI] [PubMed] [Google Scholar]
- Huang X, Eriksson KF, Vaag A, Lehtovirta M, Hansson M, Laurila E, Kanninen T, Olesen BT, Kurucz I, Koranyi L, Groop L. Insulin-regulated mitochondrial gene expression is associated with glucose flux in human skeletal muscle. Diabetes. 1999;48:1508–1514. doi: 10.2337/diabetes.48.8.1508. [DOI] [PubMed] [Google Scholar]
- Hutter E, Skovbro M, Lener B, Prats C, Rabol R, Dela F, Jansen-Durr P. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007;6:245–256. doi: 10.1111/j.1474-9726.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol. 2003;284:C1669–C1677. doi: 10.1152/ajpcell.00409.2002. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleel A, Short KR, Asmann YW, Klaus KA, Morse DM, Ford GC, Nair KS. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab. 2008;295:E1255–E1268. doi: 10.1152/ajpendo.90586.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekabsons MB, Gregoire FM, Schonfeld-Warden NA, Warden CH, Horwitz BA. T(3) stimulates resting metabolism and UCP-2 and UCP-3 mRNA but not nonphosphorylating mitochondrial respiration in mice. Am J Physiol. 1999;277:E380–E389. doi: 10.1152/ajpendo.1999.277.2.E380. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol. 2001;90:1663–1670. doi: 10.1152/jappl.2001.90.5.1663. [DOI] [PubMed] [Google Scholar]
- Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS. Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes. 2007;56:2683–2689. doi: 10.2337/db07-0378. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev. 2009;37:3–9. doi: 10.1097/JES.0b013e318190ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA, McCully KK, Chance B. Metabolic effects of training in humans: a 31P-MRS study. J Appl Physiol. 1990;69:1165–1170. doi: 10.1152/jappl.1990.69.3.1165. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87:22–29. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kirkwood SP, Munn EA, Brooks GA. Mitochondrial reticulum in limb skeletal muscle. Am J Physiol. 1986;251:C395–C402. doi: 10.1152/ajpcell.1986.251.3.C395. [DOI] [PubMed] [Google Scholar]
- Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci U S A. 2001;98:9713–9718. doi: 10.1073/pnas.171184698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. [PubMed] [Google Scholar]
- Lange KH, Larsson B, Flyvbjerg A, Dall R, Bennekou M, Rasmussen MH, Orskov H, Kjaer M. Acute growth hormone administration causes exaggerated increases in plasma lactate and glycerol during moderate to high intensity bicycling in trained young men. J Clin Endocrinol Metab. 2002;87:4966–4975. doi: 10.1210/jc.2001-011797. [DOI] [PubMed] [Google Scholar]
- Lanni A, De Felice M, Lombardi A, Moreno M, Fleury C, Ricquier D, Goglia F. Induction of UCP2 mRNA by thyroid hormones in rat heart. FEBS Lett. 1997;418:171–174. doi: 10.1016/s0014-5793(97)01375-6. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol. 2007;583:1093–1105. doi: 10.1113/jphysiol.2007.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Effects of Old Age and Physical Activity on Oxidative Capacity in Human Skeletal Muscle. Med Sci Sports Exerc. 2009;41:S97–S98. [Google Scholar]
- Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand. 1978;104:129–136. doi: 10.1111/j.1748-1716.1978.tb06259.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- Lennmarken C, Bergman T, Larsson J, Larsson LE. Skeletal muscle function in man: force, relaxation rate, endurance and contraction time-dependence on sex and age. Clin Physiol. 1985;5:243–255. [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. Spec No:11-6. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569:467–473. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr., Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75:813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- McCully KK, Forciea MA, Hack LM, Donlon E, Wheatley RW, Oatis CA, Goldberg T, Chance B. Muscle metabolism in older subjects using 31P magnetic resonance spectroscopy. Can J Physiol Pharmacol. 1991;69:576–580. doi: 10.1139/y91-084. [DOI] [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith CN, Frontera WR, Fisher EC, Hughes VA, Herland JC, Edwards J, Evans WJ. Peripheral effects of endurance training in young and old subjects. J Appl Physiol. 1989;66:2844–2849. doi: 10.1152/jappl.1989.66.6.2844. [DOI] [PubMed] [Google Scholar]
- Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- Nair KS. Muscle protein turnover: methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci. 1995;50:107–112. doi: 10.1093/gerona/50a.special_issue.107. Spec No:107-12. [DOI] [PubMed] [Google Scholar]
- Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes. 2008;57:1166–1175. doi: 10.2337/db07-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984;27:13–16. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- Orlander J, Aniansson A. Effect of physical training on skeletal muscle metabolism and ultrastructure in 70 to 75-year-old men. Acta Physiol Scand. 1980;109:149–154. doi: 10.1111/j.1748-1716.1980.tb06580.x. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM. Effect of hyperthyroidism on the transport of pyruvate in rat-heart mitochondria. Biochim Biophys Acta. 1988;935:79–86. doi: 10.1016/0005-2728(88)90110-7. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM. Stimulation of phosphate transport in rat-liver mitochondria by thyroid hormones. Biochim Biophys Acta. 1990;1019:133–136. doi: 10.1016/0005-2728(90)90134-p. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Enhanced cytochrome oxidase activity and modification of lipids in heart mitochondria from hyperthyroid rats. Biochim Biophys Acta. 1994;1225:165–170. doi: 10.1016/0925-4439(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Stimulation of carnitine acylcarnitine translocase activity in heart mitochondria from hyperthyroid rats. FEBS Lett. 1996;397:260–262. doi: 10.1016/s0014-5793(96)01190-8. [DOI] [PubMed] [Google Scholar]
- Pastoris O, Boschi F, Verri M, Baiardi P, Felzani G, Vecchiet J, Dossena M, Catapano M. The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects. Exp Gerontol. 2000;35:95–104. doi: 10.1016/s0531-5565(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78:2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. %20. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol. 2003a;38:877–886. doi: 10.1016/s0531-5565(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch. 2003b;446:270–278. doi: 10.1007/s00424-003-1022-2. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab. 2009;34:377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- Robinson S, Dill DB, Ross JC, Robinson RD, Wagner JA, Tzankoff SP. Training and physiological aging in man. Fed Proc. 1973;32:1628–1634. [PubMed] [Google Scholar]
- Rogers MA, Hagberg JM, Martin WH, III, Ehsani AA, Holloszy JO. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol. 1990;68:2195–2199. doi: 10.1152/jappl.1990.68.5.2195. [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303–309. doi: 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age? Sports Med. 2004;34:221–229. doi: 10.2165/00007256-200434040-00002. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Schunk K, Pitton M, Duber C, Kersjes W, Schadmand-Fischer S, Thelen M. Dynamic phosphorus-31 magnetic resonance spectroscopy of the quadriceps muscle: effects of age and sex on spectroscopic results. Invest Radiol. 1999;34:116–125. doi: 10.1097/00004424-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Seals DR, Hagberg JM, Allen WK, Hurley BF, Dalsky GP, Ehsani AA, Holloszy JO. Glucose tolerance in young and older athletes and sedentary men. J Appl Physiol. 1984;56:1521–1525. doi: 10.1152/jappl.1984.56.6.1521. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS. Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab. 2008;93:597–604. doi: 10.1210/jc.2007-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Nygren J, Barazzoni R, Levine J, Nair KS. T(3) increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and -3. Am J Physiol Endocrinol Metab. 2001;280:E761–E769. doi: 10.1152/ajpendo.2001.280.5.E761. [DOI] [PubMed] [Google Scholar]
- Short KR, Nygren J, Nair KS. Effect of T(3)-induced hyperthyroidism on mitochondrial and cytoplasmic protein synthesis rates in oxidative and glycolytic tissues in rats. Am J Physiol Endocrinol Metab. 2007;292:E642–E647. doi: 10.1152/ajpendo.00397.2006. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1997;83:166–171. doi: 10.1152/jappl.1997.83.1.166. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Heinrich R. Biological control through regulated transcriptional coactivators. Cell. 2004;119:157–167. doi: 10.1016/j.cell.2004.09.037. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol. 2006;290:H434–H440. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoke E, Shrayyef MZ, Messing S, Woerle HJ, van Haeften TW, Meyer C, Mitrakou A, Pimenta W, Gerich JE. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31:539–543. doi: 10.2337/dc07-1443. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Kemp GJ, Thompson CH, Radda GK. Ageing: effects on oxidative function of skeletal muscle in vivo. Mol Cell Biochem. 1997;174:321–324. [PubMed] [Google Scholar]
- Toledo FG, Menshikova EV, Azuma K, Radikova Z, Kelley CA, Ritov VB, Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–497. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- Virbasius CA, Virbasius JV, Scarpulla RC. NRF-1, an activator involved in nuclear-mitochondrial interactions, utilizes a new DNA-binding domain conserved in a family of developmental regulators. Genes Dev. 1993;7:2431–2445. doi: 10.1101/gad.7.12a.2431. [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA. Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol. 2003;94:1479–1484. doi: 10.1152/japplphysiol.01061.2002. [DOI] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Thornton CA. High-abundance mRNAs in human muscle: comparison between young and old. J Appl Physiol. 2000;89:297–304. doi: 10.1152/jappl.2000.89.1.297. [DOI] [PubMed] [Google Scholar]
- Wiesner RJ, Kurowski TT, Zak R. Regulation by thyroid hormone of nuclear and mitochondrial genes encoding subunits of cytochrome-c oxidase in rat liver and skeletal muscle. Mol Endocrinol. 1992;6:1458–1467. doi: 10.1210/mend.6.9.1331777. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holloszy JO. Response of mitochondria of different types of skeletal muscle to thyrotoxicosis. Am J Physiol. 1977;232:C180–C184. doi: 10.1152/ajpcell.1977.232.5.C180. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50:64–67. doi: 10.1093/gerona/50a.special_issue.64. Spec No:64-7. [DOI] [PubMed] [Google Scholar]
- Wooten WL, Cascarano J. The effect of thyroid hormone on mitochondrial biogenesis and cellular hyperplasia. J Bioenerg Biomembr. 1980;12:1–12. doi: 10.1007/BF00745009. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- Zamora AJ, Tessier F, Marconnet P, Margaritis I, Marini JF. Mitochondria changes in human muscle after prolonged exercise, endurance training and selenium supplementation. Eur J Appl Physiol Occup Physiol. 1995;71:505–511. doi: 10.1007/BF00238552. [DOI] [PubMed] [Google Scholar]
- Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]