Abstract
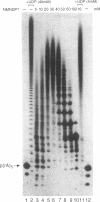
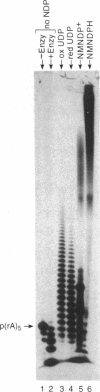
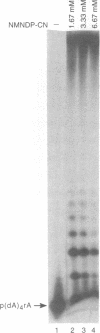
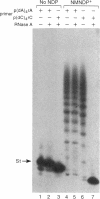
Nicotinamide mononucleoside 5'-diphosphate in its reduced form is an excellent substrate for polynucleotide phosphorylase from Micrococcus luteus both in de novo polymerization reactions and in primer extension reactions. The oxidized form of the diphosphate is a much less efficient substrate; it can be used to extend primers but does not oligomerize in the absence of a primer. The cyanide adduct of the oxidized substrate, like the reduced substrate, polymerizes efficiently. Loss of cyanide yields high molecular weight polymers of the oxidized form. Terminal transferase from calf thymus accepts nicotinamide mononucleoside 5'-triphosphate as a substrate and efficiently adds one residue to the 3'-end of an oligodeoxynucleotide. T4 polynucleotide kinase accepts oligomers of nicotinamide mononucleotide as substrates. However, RNA polymerases do not incorporate nicotinamide mononucleoside 5'-triphosphate into products on any of the templates that we used.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN M., HUGHES T. R., Jr Phosphorus magnetic resonance spectra of adenosine di- and triphosphate. I. Effect of pH. J Biol Chem. 1960 Nov;235:3250–3253. [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., CIOTTI M. M. The reaction of pyridine nucleotide with cyanide and its analytical use. J Biol Chem. 1951 Aug;191(2):447–459. [PubMed] [Google Scholar]
- Dai X., De Mesmaeker A., Joyce G. F. Cleavage of an amide bond by a ribozyme. Science. 1995 Jan 13;267(5195):237–240. doi: 10.1126/science.7809628. [DOI] [PubMed] [Google Scholar]
- Deng G., Wu R. Terminal transferase: use of the tailing of DNA and for in vitro mutagenesis. Methods Enzymol. 1983;100:96–116. doi: 10.1016/0076-6879(83)00047-6. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Harvey R. A., Grunberg-Manago M. Identification of the nucleoside monophosphate end-group on the product of the polynucleotide phosphorylase reaction. Biochem Biophys Res Commun. 1966 May 25;23(4):448–452. doi: 10.1016/0006-291x(66)90748-0. [DOI] [PubMed] [Google Scholar]
- Joyce G. F., Inoue T., Orgel L. E. Non-enzymatic template-directed synthesis on RNA random copolymers. Poly(C, U) templates. J Mol Biol. 1984 Jun 25;176(2):279–306. doi: 10.1016/0022-2836(84)90425-x. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., BARNES C. C. Effect of alkali on diphosphopyridine nucleotide. J Biol Chem. 1951 Aug;191(2):461–472. [PubMed] [Google Scholar]
- Kruger K., Grabowski P. J., Zaug A. J., Sands J., Gottschling D. E., Cech T. R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982 Nov;31(1):147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., ROCK M. K. The stability of pyridine nucleotides. J Biol Chem. 1961 Oct;236:2756–2759. [PubMed] [Google Scholar]
- Liu R., Visscher J. A novel preparation of nicotinamide mononucleotide. Nucleosides Nucleotides. 1994;13(5):1215–1216. doi: 10.1080/15257779408011891. [DOI] [PubMed] [Google Scholar]
- Marlier J. F., Benkovic S. J. On the mechanism of de novo polymerization by form I polynucleotide phosphorylase of Micrococcus luteus. Biochemistry. 1982 May 11;21(10):2349–2356. doi: 10.1021/bi00539a012. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Hoffarth V., Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science. 1992 Jun 5;256(5062):1416–1419. doi: 10.1126/science.1604315. [DOI] [PubMed] [Google Scholar]
- Orgel L. E. Evolution of the genetic apparatus. J Mol Biol. 1968 Dec;38(3):381–393. doi: 10.1016/0022-2836(68)90393-8. [DOI] [PubMed] [Google Scholar]
- Piccirilli J. A., McConnell T. S., Zaug A. J., Noller H. F., Cech T. R. Aminoacyl esterase activity of the Tetrahymena ribozyme. Science. 1992 Jun 5;256(5062):1420–1424. doi: 10.1126/science.1604316. [DOI] [PubMed] [Google Scholar]
- Sulewski M., Marchese-Ragona S. P., Johnson K. A., Benkovic S. J. Mechanism of polynucleotide phosphorylase. Biochemistry. 1989 Jul 11;28(14):5855–5864. doi: 10.1021/bi00440a023. [DOI] [PubMed] [Google Scholar]
- White H. B., 3rd Coenzymes as fossils of an earlier metabolic state. J Mol Evol. 1976 Mar 29;7(2):101–104. doi: 10.1007/BF01732468. [DOI] [PubMed] [Google Scholar]
- Wu T., Orgel L. E. Nonenzymatic template-directed synthesis on oligodeoxycytidylate sequences in hairpin oligonucleotides. J Am Chem Soc. 1992;114(1):317–322. doi: 10.1021/ja00027a040. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. The Tetrahymena intervening sequence ribonucleic acid enzyme is a phosphotransferase and an acid phosphatase. Biochemistry. 1986 Aug 12;25(16):4478–4482. doi: 10.1021/bi00364a002. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Bilsker M. Phosphorylation of N-protected deoxyoligonucleotides by T4 polynucleotide kinase. Biochemistry. 1973 Dec 4;12(25):5056–5062. doi: 10.1021/bi00749a005. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Kleppe K., Khorana H. G. Reversal of bacteriophage T4 induced polynucleotide kinase action. Biochemistry. 1973 Dec 4;12(25):5050–5055. doi: 10.1021/bi00749a004. [DOI] [PubMed] [Google Scholar]