Abstract
In this study, we defined the role of peroxisome proliferator-activated receptor β/δ (PPARδ) in metabolic homeostasis by using subtype selective agonists. Analysis of rat L6 myotubes treated with the PPARδ subtype-selective agonist, GW501516, by the Affymetrix oligonucleotide microarrays revealed that PPARδ controls fatty acid oxidation by regulating genes involved in fatty acid transport, β-oxidation, and mitochondrial respiration. Similar PPARδ-mediated gene activation was observed in the skeletal muscle of GW501516-treated mice. Accordingly, GW501516 treatment induced fatty acid β-oxidation in L6 myotubes as well as in mouse skeletal muscles. Administration of GW501516 to mice fed a high-fat diet ameliorated diet-induced obesity and insulin resistance, an effect accompanied by enhanced metabolic rate and fatty acid β-oxidation, proliferation of mitochondria, and a marked reduction of lipid droplets in skeletal muscles. Despite a modest body weight change relative to vehicle-treated mice, GW501516 treatment also markedly improved diabetes as revealed by the decrease in plasma glucose and blood insulin levels in genetically obese ob/ob mice. These data suggest that PPARδ is pivotal to control the program for fatty acid oxidation in the skeletal muscle, thereby ameliorating obesity and insulin resistance through its activation in obese animals.
Keywords: obesity, insulin resistance, thermogenesis, pancreatic β-cell, PGC-1α
Obesity is one of the most prevalent and serious chronic disorders in industrialized societies. Frequently, it clusters with type 2 diabetes, hypertension, and hyperlipidemia in the so-called metabolic syndrome X (1). Obesity causes excess fat accumulation in various tissues; most notoriously in adipose tissues, but also in other insulin-responsive organs, such as skeletal muscle and liver, predisposing to the development of insulin resistance. Especially, insulin resistance in skeletal muscle is a hallmark feature of type 2 diabetes (2, 3). Yet, the molecular mechanisms of the pathogenesis of insulin resistance and obesity have not been fully clarified and effective therapeutic approaches are currently of general interest.
The peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily that function as fatty acid-activated transcription factors (4). There are three related PPAR family members: PPARα, PPARγ, and PPARβ/δ (hereafter referred to as PPARδ), with different ligand specificities and tissue distributions. PPARα is highly expressed in liver, where it controls peroxisomal and mitochondrial fatty acid catabolism, whereas PPARγ is abundant in adipose tissues functioning as the key transcriptional factor for adipogenesis. Synthetic ligands for PPARα, such as the fibrates, are used for the treatment of hyperlipidemia, whereas synthetic PPARγ ligands, such as the thiazolidinediones, are increasingly used to treat type 2 diabetes (5). PPARδ is expressed in many tissues including skeletal muscle and brown adipose tissue (BAT) (5). Although several polyunsaturated fatty acids and eicosanoids have been shown to act as putative ligands for PPARδ, displaying overlapping ligand specificity with PPARα (6), the physiological role of PPARδ is still unclear. Through a gain-of-function approach using adipose tissue-specific PPARδ transgenic mice, Wang et al. (7) demonstrated that PPARδ activation in adipose tissue leads to enhanced fatty acid oxidation, improved lipid profiles, and reduced adiposity. Interestingly, these PPARδ-transgenic mice are completely resistant to both high-fat-diet-induced and genetically predisposed obesity.
Recently potent PPARδ agonists have been developed and shown to increase plasma HDL cholesterol levels in both mice and nonhuman primates (8, 9). The increase in HDL cholesterol levels occurs through an increase of cholesterol efflux from the cells secondary to an increase in the expression of the reverse cholesterol transporter ATP-binding cassette AI (8). Furthermore, when dosed to insulin-resistant middle-aged obese rhesus monkeys, GW501516 lowered the fasting plasma levels of insulin without changing the glucose levels.
Because the skeletal muscle is the major target organ of insulin, we investigated the role of PPARδ in skeletal muscle, especially in relation to the insulin sensitivity. We demonstrated that activation of PPARδ by the selective agonist GW501516 induced the expression of key molecules involved in fatty acid oxidation and energy expenditure, as well as in adaptive thermogenesis, PPARγ coactivator-1α (PGC-1α) (10). Most importantly, GW501516 treatment enhanced fatty acid β-oxidation in the skeletal muscle, protected against diet-induced obesity, and improved glucose tolerance and insulin sensitivity. Here, we present evidence that PPARδ is a key target for the intervention and prevention of obesity and type 2 diabetes.
Experimental Procedures
The methods used in this study are described in detail in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Affymetrix Oligonucleotide Microarray Analysis. Hybridization samples were prepared according to the Affymetrix (Santa Clara, CA) instructions as described (11). Briefly, 10 μg of total RNA was used to generate first-strand cDNA. After second-strand synthesis, biotinylated and amplified RNA were purified by using RNeasy (Qiagen, Valencia, CA) and quantitated by a spectrophotometer. Biotinylated cRNA samples were then hybridized to Affymetrix Rat U34 sub A arrays. These arrays contain probe sets for >8,000 transcripts and EST clones. After hybridization, microarrays were washed, scanned, and analyzed with the GENECHIP software (Affymetrix).
Animals and Diets. C57BL/6J and ob/ob mice (male, 5 weeks of age) were purchased from The Jackson Laboratory. The mice were housed individually in a temperature- and humidity-controlled (26.5°C and 35%) facility with a 12-h light/dark cycle (0900 to 2100 hours). All animals were allowed free access to water and diets. Mice were fed a normal chow diet (CE-2; CLEA Japan, Osaka) or a high-fat diet (HFD) containing 35% fat, 22% casein, 7% dextrin, 30% maltose, and 3.5% minerals. Food consumption was monitored daily and body weight was recorded every other day. PPAR agonists were given at 10 ml/kg of body weight by oral intubation in 3% arabic gum daily between 1030 and 1100 hours. Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed on days 21 and 28, respectively, after administration was started.
Statistical Analyses. The data are presented as means ± SE. The homogeneity in variance was evaluated by a Bartlett test followed by a parametric or nonparametric Dunnett's multiple comparison test (one-sided). The Student or Aspin-Welch t test (one-sided) was used to compare the data between the control and treated groups. A P value <0.05 was considered significant.
Results
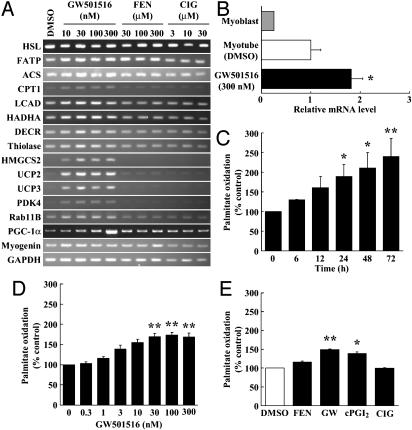
PPARδ Agonists Increase Transcripts for Fatty Acid Oxidation and Energy Dissipation in Cultured Myotubes. To characterize the regulation of gene expression during PPARδ activation in vitro, we performed oligonucleotide microarrays analysis of rat L6 myotube/myoblast cells treated with the PPARδ agonist, GW501516. GW501516 is a subtype-selective ligand for PPARδ with an apparent Kd value of 1 nM, and has >1,000-fold higher affinity toward PPARδ than the other subtypes, PPARα and PPARγ (8). The selection of a muscle cell line was based on the fact that skeletal muscle is the largest tissue determining the insulin sensitivity of the whole body and PPARδ is expressed at a very high level in muscle; the other PPAR family members, PPARα and PPARγ, are expressed to a much lower level in the skeletal muscle (5). We differentiated L6 myoblasts to myotubes under the standard differentiation protocol, added GW501516, and prepared RNA. The abundance of 8,799 transcripts and expressed sequence tags (ESTs) was measured by using oligonucleotide microarrays (Affymetrix). As shown in Table 1, we identified a series of genes that are critical for many aspects of carbohydrate and lipid metabolism, including intracellular fatty acid transport, fatty acid activation, mitochondrial fatty acid β-oxidation, electron transport, and ketogenesis (Fig. 1 A and B). The mRNAs for pyruvate dehydrogenase kinase 4 (PDK4) that spares glucose oxidation and gluconeogenesis, and PGC-1α, a master regulator of the thermogenic program, were also robustly induced. Consistent with the absence of any effect of PPARα and PPARγ on muscle gene expression (8), agonists of PPARα, such as fenofibric acid (FEN), and of PPARγ, such as ciglitazone (CIG), showed very little or no effect on the expression of the genes that were examined above (Fig. 1A).
Table 1. Effects of PPARδ agonist on the expression various genes in L6 myotubes.
| Fold change GW/MT
|
||||
|---|---|---|---|---|
| Description | GenBank accession no. | Experiment 1 | Experiment 2 | Experiment 3 |
| Fatty acid transport and activation | ||||
| FATP | U89529 | ≈8.2 | ≈11.3 | ≈1.5 |
| Hormone-sensitive lipase | X51415 | 2.2 | ≈5.2 | ≈2.0 |
| Long-chain acyl-CoA synthetase | AA893242 | 2.4 | 2.5 | 1.5 |
| Carnitine palmitoyltransferase 1 | L07736 | 2.4 | 2.5 | 2.1 |
| Mitochondrial β-oxidation | ||||
| LCAD | J05029 | 2.5 | 2.2 | 1.8 |
| HADHA | D16478 | 1.9 | 1.6 | 1.9 |
| DECR | D00569 | 2.2 | 2.1 | 2.3 |
| Mitochondrial 3-oxoacyl-coenzyme A thiolase | X05341 | 2.1 | 1.8 | 1.8 |
| Peroxisomal β-oxidation-related genes | ||||
| Peroxisomal enoyl hydratase-like protein | U08976 | 14.5 | 3.0 | 3.1 |
| Catalase | AA926149 | 2.5 | 2.3 | 2.0 |
| Electron transport chain | ||||
| UCP2 | AB005143 | 3.8 | 4.3 | 4.1 |
| UCP3 | AF035943 | ≈7.1 | ≈6.3 | ≈12.5 |
| Glucose oxidation | ||||
| PDK4 | AF034577 | ≈9.3 | ≈9.1 | ≈7.2 |
| Others | ||||
| Mitochondrial HMG-CoA synthase | M33648 | 14.0 | ≈17.2 | ≈8.8 |
| RAB11B | D01046 | 2.0 | 2.1 | 1.5 |
| Decorin | X59859 | 1.5 | ≈5.0 | 1.5 |
| Src-related tyrosine kinase | U09583 | 1.8 | 2.0 | 1.6 |
| NT-4 | Y07659 | ≈1.5 | ≈1.5 | ≈1.8 |
| ESTs | ||||
| ESTs | AA891669 | 4.5 | ≈33.1 | ≈5.2 |
| ESTs | AA893870 | ≈2.0 | ≈2.7 | 1.5 |
| ESTs | AA891839 | ≈1.8 | ≈2.2 | 1.5 |
RNA from L6 myotubes (MT) and myotubes treated with 100 nM GW501516 (GW) were hybridized onto Affymetrix oligonucleotide arrays and quantified. For each gene, the fold change was calculated by Affymetrix software; ≈, fold change calculation for which the smaller value is replaced by an estimate of the minimum value for detectable transcripts. A gene was classified as up-regulated if the following three criteria were met: (i) the corresponding ratio was  1.5, (ii) the average difference of gene was >0, and (iii) at least one of the three samples represented as presence.
1.5, (ii) the average difference of gene was >0, and (iii) at least one of the three samples represented as presence.
Fig. 1.
PPARδ agonists increase fatty acid oxidation in L6 myotubes. (A) RT-PCR analyses of PPARδ-regulated genes in L6 myotubes. (B) Real-time PCR analysis of PGC-1α mRNA levels in L6 myoblast/myotube cells exposed to GW501516. (C) Time responses on fatty acid oxidation in L6 myotubes exposed to GW501516. Cells were cultured with 100 nM GW501516 at the indicated times. (D) Dose responses on fatty acid oxidation in L6 myotubes exposed to GW501516. L6 myotubes were cultured with the indicated concentrations of GW501516 for 24 h. (E) The effect of the PPAR agonists on [14C]palmitate oxidation. After incubation in differentiation medium for 7 days, L6 myotubes were treated with DMSO (control), FEN (300 μM), GW501516 (100 nM), cPGI2 (10 μM), or CIG (30 μM) for 24 h. All assays were performed in triplicate, and each bar represents the mean ± SE of three to four independent experiments. *, P < 0.05; **, P < 0.01 compared with vehicle-treated control. HADHA, mitochondrial trifunctional protein; DECR, mitochondrial 2,4-dienoyl CoA reductase 1; HSL, hormone-sensitive lipase.
Next we examined whether GW501516 has the ability to enhance the fatty acid oxidation in L6 myotubes by using [1-14C]palmitate as a substrate. L6 rat myoblasts were therefore differentiated into myotubes, treated with various PPARs agonists, and [1-14C]palmitate oxidation was measured. As shown in Fig. 1 C-E, only PPARδ agonists, such as GW501516 and carbaprostacyclin (cPGI2), increased fatty acid oxidation in L6 myotubes in a dose-dependent manner (1.6-fold at 100 nM; P < 0.002; Fig. 1 C and D). In contrast, PPARα or PPARγ agonists had no effect on fatty acid oxidation in L6 myotubes at the concentration we used (Fig. 1E).
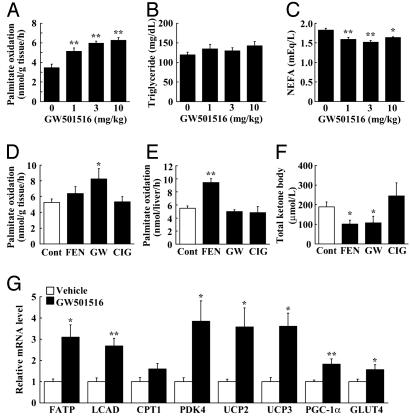
PPARδ Agonists Stimulate Fatty Acid Oxidation in Skeletal Muscles. Next, we examined whether treatment with PPARδ agonists could stimulate fatty acid oxidation in vivo, especially in oxidative tissues including skeletal muscle, liver, and BAT (Fig. 2). Mice fed a normal chow diet were treated with GW501516 for 1 week (see Experimental Procedures), were killed, and were subjected for [1-14C]palmitate oxidation measurement. In the quadriceps muscle, GW501516 enhanced fatty acid β-oxidation in a dose-dependent manner (1.8-fold at 10 mg/kg treatment), whereas little or no induction was observed by PPARα or PPARγ agonists (Fig. 2 A and D). In contrast, hepatic β-oxidation was not induced by GW501516 (Fig. 2E), presumably due to the low expression of PPARδ in the liver. As a control agonist for PPARα, fenofibrate (FEN) strongly increased hepatic β-oxidation (Fig. 2E). We also observed a modest increase of fatty acid oxidation by GW501516 in intrascapular BAT (data not shown). These data demonstrate that GW501516 promotes fatty acid oxidation mainly in the skeletal muscle, but not in the liver.
Fig. 2.
PPARδ agonist increases skeletal muscle fatty acid oxidation in C57BL/6J mice. Data in A-C show the effects of GW501516 on fatty acid oxidation in skeletal muscle (A), plasma TG (B), and NEFA (C). Data in D-F show the effect of PPAR agonists on fatty acid oxidation in skeletal muscle (D) and in liver (E), and serum total ketone body level (F). (G) Quantitative real-time PCR analyses in GW501516-treated skeletal muscle. GW501516 (3 mg/kg), FEN (300 mg/kg), or CIG (30 mg/kg) were orally administrated to mice for 7 days. Each bar represents the mean ± SE of four to five mice. *, P < 0.05; **, P < 0.01; difference from control, respectively.
We then examined whether increased fatty acid oxidation in the skeletal muscle affected the levels of lipid-derived substrates, such as nonesterified fatty acids (NEFAs), triglyceride (TG), and ketone bodies in the circulation. Although plasma TG levels were not altered significantly, the plasma levels of free fatty acids and ketone bodies were significantly decreased (Fig. 2 B, C, and F). This result decrease in the levels of ketone bodies was also confirmed in other experiments (Table 2, which is published as supporting information on the PNAS web site).
To confirm that GW501516-induced β-oxidation in skeletal muscle in vivo was also associated with increased expression of genes involved in β-oxidation (listed in Table 1), we analyzed the expression of some representative genes by real-time quantitative PCR assay (Supporting Materials and Methods and Tables 3 and 4, which are published as supporting information on the PNAS web site). The levels of mRNA expression were determined in individual animals, and the average expression for each group is presented. As expected, GW501516 also significantly increased mRNA expression in vivo in skeletal muscle for proteins involved in fatty acid catabolism, including fatty acid transport protein (FATP) and long-chain acyl-CoA dehydrogenase (LCAD), energy expenditure, such as uncoupling protein (UCP) 2 and UCP3, and the thermogenic cofactor PGC-1α (Fig. 2G). Interestingly, these mRNAs induced by PPARδ agonist both in vitro and in vivo (Table 1 and Fig. 2G) were also up-regulated in skeletal muscle during starvation (J.Y. and J.S., unpublished data).
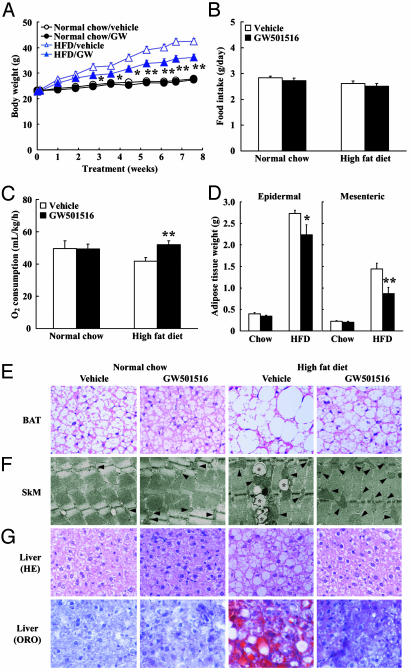
GW501516 Treatment Protects Against Diet-Induced Obesity. We predicted that enhanced fatty acid utilization and energy expenditure would protect against diet-induced obesity; accordingly, we examined the long-term effects of treatment with a PPARδ agonist on the development of obesity. Because prolonged administration of 10 mg/kg GW501516 to animals caused hepatomegaly and stimulated fatty acid oxidation in the liver (data not shown), we selected the dose of 3 mg/kg GW501516 for long-term administration studies. To induce obesity, mice were fed with an HFD containing 35% fat and 30% maltose. On feeding this high-calorie diet, male C57BL/6J mice showed a significant increase in the rate of body weight gain compared with animals fed a normal chow diet (terminal body weight: chow diet group, 27.8 ± 0.7 g vs. HFD group, 42.4 ± 1.2 g, P < 0.0001, n = 5; Fig. 3A). In contrast, GW501516 treatment of mice resulted in significantly reduced body weight gain compared with the vehicle-treated mice on an HFD: the rate of body weight gain on feeding an HFD in GW501516-treated mouse group was approximately half that of the vehicle group (Fig. 3A). Statistical differences were observed as early as 19 days after initiation of the treatment. Importantly, daily food consumption of GW501516-treated mice was similar to that of vehicle-treated mice (Fig. 3B). Normal-chow-fed mice did not exhibit a reduced body weight gain by GW501516 administration (Fig. 3A and Table 2). To verify whether GW501516 affects the total body metabolic rate, we measured oxygen consumption in whole animals (Fig. 3C). GW-treated mice on an HFD displayed higher rates of oxygen consumption than that of vehicle-treated mice on an HFD in the resting state (P < 0.05; Fig. 3C). These results indicate that GW501516 increases metabolic rate and contributes to the protective effect on diet-induced obesity. Anatomical analysis revealed that this reduced body weight in GW501516-treated mice on feeding an HFD was largely due to the reduced mass of visceral and epidermal fat depots (xFig. 3D, see below). Histological analysis revealed significant amelioration of diet-induced fat cell hypertrophy by GW501516 in both epididymal white adipose tissue (WAT) and BAT. The morphological change of fat cells by GW501516 treatment was more profound in BAT than that in WAT (Fig. 3E). These changes in adipose tissue morphology attenuated by GW501516 treatment indicate that HFD-induced adiposity is curtailed by PPARδ agonism. Consistent with the reduced fat mass and morphological changes in adipose tissues, GW501516-treated animals did show an attenuation of HFD-induced hyperleptinemia (Table 2).
Fig. 3.
PPARδ agonist increases metabolic rate and attenuates body weight gain and adiposity in HFD-fed C57BL/6J mice. (A) Body weight change. (B) Food intake. (C) Oxygen consumption measurement. (D) Adipose tissue weight. (E) Morphological characterization of BAT (×40 magnification). (F) Electron microscopic analysis of mitochondrial biogenesis and lipid accumulation in skeletal muscle (×2,500 magnification). Asterisks, lipid droplets; arrows, mitochondria. (G) Morphological characterization of liver (×20 magnification). Each symbol or bar represents the mean ± SE of five mice. *, P < 0.05; **, P < 0.01; compared with vehicle-treated control. ORO, Oil red O.
GW501516 Treatment Prevents HFD-Induced Hepatic and Intramuscular Lipid Accumulation. Liver and skeletal muscle are the two most important insulin-responsive organs in the body, and it is generally believed that lipid accumulation in these tissues profoundly contributes to the establishment of insulin resistance (12). Electron microscopic comparison of skeletal muscles from GW501516- and vehicle-treated mice fed an HFD showed that GW501516 treatment almost completely eliminated intramuscular lipid droplets around the mitochondrial region that were induced by HFD (Fig. 3F). Importantly, we also observed an appreciable proliferation of mitochondria in the skeletal muscle of GW501516-treated mice (Fig. 3F). HFD is known to induce hepatic steatosis. Histological analysis indicated that mice maintained on an HFD accumulated higher levels of hepatic lipid compared with those from normal-chow-diet-fed mice (Fig. 3G). In contrast, GW501516 treatment reduced hepatic lipid accumulation induced by HFD to the normal levels. Consistent with the reduced hepatic lipid accumulation, plasma alanine aminotransferase and aspartate aminotransferase activities were lowered to the normal levels by GW501516 treatment, indicating that the fatty liver induced by HFD was almost completely cured (Table 2). Real-time PCR analysis revealed that gene transcripts for proteins involved in β-oxidation including acyl-CoA oxidase were elevated, whereas that for fatty acid synthetase were reduced between GW501516- and vehicle-treated mice on an HFD (data not shown), suggesting that the reduction in liver lipid accumulation was subsequent to an increase in fatty acid oxidation and lowered TG synthesis.
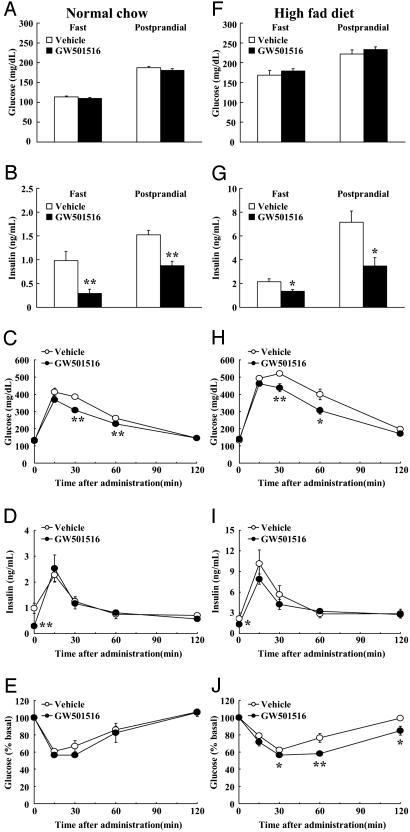
Effects of GW501516 Treatment on Insulin Sensitivity in Diet-Induced Obese Mice. GW501516 treatment had no effects on fasting or postprandial levels of blood glucose in mice fed a normal diet or an HFD. In contrast, both fasting and postprandial levels of plasma insulin were decreased by GW501516 treatment in mice on a normal diet as well as on an HFD (Fig. 4 A, B, F, and G). Furthermore, GTT and ITT revealed that GW501516 treatment improved glucose tolerance and insulin sensitivity, respectively, induced by HFD feeding (Fig. 4 C-E and H-J). These data suggest that the increased fatty acid β-oxidation in the skeletal muscle and subsequent reduction in hepatic and intramuscular lipid content by GW501516 could, at least in part, explain the improved insulin sensitivity.
Fig. 4.
PPARδ agonist ameliorates insulin resistance in HFD-fed mice. (A and F) Plasma glucose. (B and G) Plasma insulin. (C and H) Plasma glucose levels during GTT. (D and I) Plasma insulin levels during GTT. (E and J) ITT. Each bar and symbol represents the mean ± SE of five mice. *, P < 0.05; **, P < 0.01; compared with vehicle-treated control.
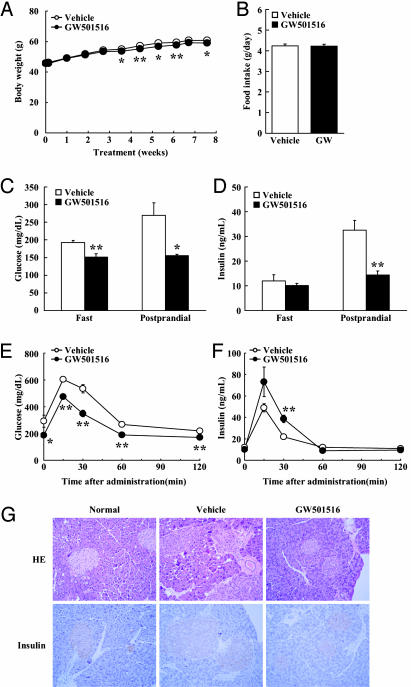
GW501516 Treatment of ob/ob Mice Improves Glucose Tolerance and Reduces Plasma Insulin Levels in Genetically Obese Mice. Next we studied the antiobese and antidiabetic actions of PPARδ in genetically predisposed obese ob/ob mice. ob/ob mice display hyperphagia, severe obesity, insulin resistance, and diabetes, due to the lack of leptin, a key WAT-derived signaling factor regulating body weight and energy balance (13). Treatment of ob/ob mice with GW501516 did not affect food intake (4.23 ± 0.1 g/day; Fig. 5B and Table 2). Interestingly, despite a very modest, yet significant, change in body weight, GW501516-treated ob/ob mice showed a significantly improved glucose tolerance (Fig. 5 C and D). Despite similar insulin levels, GW501516-treated animals had lower glucose levels than the vehicle group in the fasting state (vehicle, 192.7 ± 5.9 mg/dl vs. GW501516, 151.8 ± 9.4 mg/dl;Fig.5 C and D). One-hour postprandial levels of plasma glucose and insulin in GW501516-treated mice were much lower than those in vehicle-treated animals (blood glucose: vehicle, 269.9 ± 35.4 mg/dl vs. GW501516, 155.4 ± 4.2 mg/dl; and plasma insulin: vehicle, 32.5 ± 4.1 ng/ml vs. GW501516, 14.4 ± 1.6 ng/ml). GTT also showed that glucose tolerance was improved by GW501516 administration; however, unexpectedly, GW501516-treated mice showed increased plasma insulin levels during the GTT as compared with vehicle-treated ob/ob mice, pointing to an eventual contribution of the pancreas (Fig. 5 E and F). Histological analysis revealed a reduction both in number and size of lipid droplets in skeletal muscle and the replacement of large lipid droplets with smaller droplets in BAT (data not shown). Pancreatic islets from ob/ob mice were severely hypertrophic. The islet hypertrophy was normalized by GW501516 administration (Fig. 5G), presumably as a consequence of the decreased insulin requirements caused by the increased insulin sensitivity. Plasma TG, NEFA, and ketone bodies were decreased in GW501516-treated ob/ob mice (Table 2), reflecting a higher use of fatty acids in PPARδ-expressing oxidative tissues, including skeletal muscle and BAT.
Fig. 5.
PPARδ agonist improves insulin resistance and pancreatic islet hyperplasia in leptin-deficient ob/ob mice. (A) Body weight gain. (B) Food intake. (C) Plasma glucose. (D) Plasma insulin. (E and F) Plasma glucose and insulin levels during GTT. (G) Morphological changes in pancreatic islet in GW501516-treated ob/ob mice (×20 magnification). Each symbol or bar represents the mean ± SE of five mice. *, P < 0.05; **, P < 0.01; compared with vehicle-treated control.
Discussion: PPARδ as a Key Target for Metabolic Syndrome
In this study, we highlighted, by using PPARδ agonists, an important role of PPARδ in skeletal muscle, which is in addition to the previously reported role of PPARδ in adipose tissues (7). Through a comprehensive transcriptional analysis in cultured myotube, we discovered that the PPARδ agonist stimulates almost the entire program for fatty acid use and energy dissipation in skeletal muscle (Table 1 and Figs. 2G and 3C). This increase in fatty acid β-oxidation and energy expenditure in skeletal muscle underpinned the protective effect of PPARδ agonists on the development of obesity in C57BL/6J mice on an HFD (Figs. 2 A, D, and G, and 3A).
Administration of GW501516 to both HFD and ob/ob mice also significantly improved glucose tolerance and insulin sensitivity. The mechanism by which PPARδ agonist improved insulin sensitivity in obese mice is not fully clear at present, but the marked reduction in lipid content in both skeletal muscle and liver (Fig. 3 F and G), resulting from enhanced energy expenditure, can, at least in part, account for the improved insulin sensitivity. Accumulation of excess lipid in skeletal muscle or liver disturbs insulin signaling. In skeletal muscle, this disturbance has been linked to impaired activity of the insulin receptor substrate (IRS)-1-associated phosphatidyl inositol 3-kinase (PI3-kinase), whereas in the liver this was attributed to the impaired activity of IRS-2-associated PI3-kinase subsequent to altered levels of adipose tissue-derived hormones such as leptin or adiponectin/ACRP30 (12). The reduction of visceral obesity (Fig. 3D) may also explain in part the amelioration of insulin resistance through a reduction in adipose tissue-derived hormone such as TNFα (14). Interestingly, and in contrast to the effect in HFD mice, glucose tolerance improved, despite rather modest weight changes in the ob/ob mice (Fig. 5 A-E). Amelioration of diabetes in the ob/ob mice was associated with reduced pancreatic islet hypertrophy (Fig. 5G). This finding indicates that PPARδ activation has a potential positive effect on glucose-induced insulin secretion and raises the possibility that PPARδ may prevent or delay β cell loss (15, 16). In fact, GW501516 treatment of the pancreatic islets isolated from wild-type mice on normal chow diet significantly stimulated glucose-induced insulin release in vitro (Y.I. and J.S., unpublished observation).
During starvation, glucose uptake and oxidation are reduced rapidly in muscle, which shifts to use free fatty acids and ketone bodies. It was interesting to note that the changes in gene expression induced by GW501516 in vitro in cultured myotubes (Table 1 and Fig. 1A) and in skeletal muscle in vivo (Fig. 2G) were very similar to the gene expression profile induced by fasting in skeletal muscle (J.Y. and J.S., unpublished data). On challenges such as cold exposure, fasting, or prolonged exercise, lipolysis in WAT occurs. The free fatty acids released into the circulation are taken up by thermogenic tissues, such as BAT and skeletal muscle, where they serve as fuel for adaptive thermogenesis. Hence, we are speculating that one of the physiological roles of PPARδ is to direct metabolism from utilization of glucose to utilization of lipids in the fatty acid β-oxidation pathway. It is, therefore, conceivable that the free fatty acids released from adipose tissue on fasting or exercise provide PPARδ with natural ligands to stimulate fatty acid oxidation and thermogenesis in skeletal muscle. This idea is consistent with the hypothesis that PPARδ acts as a pivotal thermogenic transcription factor (7).
Recent studies showed that PPARδ directly interacts with PGC-1α (7), and activates the CPT1 gene together with PGC-1α (17). We take this observation one step further by demonstrating that PPARδ not only activates PGC-1α but also stimulates PGC-1α mRNA levels both in vitro and in vivo (Figs. 1A and 2G), an effect accompanied by mitochondrial biogenesis (Fig. 3F). In fact, the PGC-1α promoter contains a highly conserved PPAR-responsive element that is conserved among human, mouse, and rat, where PPARδ can directly bind (T.T. and J.S., unpublished observation), suggesting that PPARδ directly stimulates the PGC-1α gene expression. Mitochondrial oxidative and phosphorylation activity is strongly correlated with insulin sensitivity (18). Exercise, starvation, and cold increase the expression of PGC-1α, subsequently increasing the capacity for mitochondrial energy production through fatty acid β-oxidation. The induction of PGC-1α expression by PPARδ may also improve physical performance by an enhancement of skeletal muscle respiratory capacity (1), a factor that could contribute to the improved insulin sensitivity and obesity.
Taken together, we demonstrate PPARδ agonists have a profound antiobese and antidiabetic action in animal models. PPARδ is now a key target for metabolic syndrome, and our current data may provide molecular bases for the intervention and prevention of metabolic syndrome by highly effective PPARδ agonists.
Supplementary Material
Acknowledgments
We thank Dr. Tokuo Yamamoto and Dr. Iichiro Shimomura for helpful discussion and Aoi Uchida and Yasuyo Urashima for excellent technical assistance. This work was supported through Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology of the Japanese Government, and a Joint Research and Development Project with the Academic Institute and Private Companies. This work was also supported in part by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, and Hopiteaux Universiatire de Strasbourg, Exploratory Research for Advanced Technology/Japan Science and Technology Organization (Yanagisawa orphan receptor project). M.Y. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: PPAR, peroxisome proliferator-activated receptor; PGC-1α, PPARγ coactivator-1α; BAT, brown adipose tissue; GTT, glucose tolerance test; HFD, high-fat diet; ITT, insulin tolerance test; TG, triglyceride; CIG, ciglitazone.
References
- 1.Spiegelman, B. M. & Flier, J. S. (2001) Cell 104, 531-543. [DOI] [PubMed] [Google Scholar]
- 2.Taylor, S. I. (1999) Cell 97, 9-12. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet, P., Alberti, K. G. & Shaw, J. (2001) Nature 414, 782-787. [DOI] [PubMed] [Google Scholar]
- 4.Willson, T. M., Brown, P. J., Sternbach, D. D. & Henke, B. R. (2000) J. Med. Chem. 43, 527-550. [DOI] [PubMed] [Google Scholar]
- 5.Braissant, O., Foufelle, F., Scotto, C., Dauca, M. & Wahli, W. (1996) Endocrinology 137, 354-366. [DOI] [PubMed] [Google Scholar]
- 6.Forman, B. M., Chen, J. & Evans, R. M. (1997) Proc. Natl. Acad. Sci. USA 94, 4312-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, Y. X., Lee, C. H., Tiep, S., Yu, R. T., Ham, J., Kang, H. & Evans, R. M. (2003) Cell 113, 159-170. [DOI] [PubMed] [Google Scholar]
- 8.Oliver, W. R., Jr., Shenk, J. L., Snaith, M. R., Russell, C. S., Plunket, K. D., Bodkin, N. L., Lewis, M. C., Winegar, D. A., Sznaidman, M. L., Lambert, M. H., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 5306-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibowitz, M. D., Fievet, C., Hennuyer, N., Peinado-Onsurbe, J., Duez, H., Bergera, J., Cullinan, C. A., Sparrow, C. P., Baffic, J., Berger, G. D., et al. (2000) FEBS Lett. 473, 333-336. [DOI] [PubMed] [Google Scholar]
- 10.Puigserver, P., Wu, Z., Park, C. W., Graves, R., Wright, M. & Spiegelman, B. M. (1998) Cell 92, 829-839. [DOI] [PubMed] [Google Scholar]
- 11.Saiura, A., Sugawara, Y., Harihara, Y., Sata, M., Hamakubo, T., Kodama, T. & Makuuchi, M. (2002) Transpl. Int. 15, 535-540. [DOI] [PubMed] [Google Scholar]
- 12.Kim, J. K., Fillmore, J. J., Chen, Y., Yu, C., Moore, I. K., Pypaert, M., Lutz, E. P., Kako, Y., Velez-Carrasco, W., Goldberg, I. J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 7522-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature 372, 425-432. [DOI] [PubMed] [Google Scholar]
- 14.Masuzaki, H., Paterson, J., Shinyama, H., Morton, N. M., Mullins, J. J., Seckl, J. R. & Flier, J. S. (2001) Science 294, 2166-2170. [DOI] [PubMed] [Google Scholar]
- 15.Conarello, S. L., Li, Z., Ronan, J., Roy, R. S., Zhu, L., Jiang, G., Liu, F., Woods, J., Zycband, E., Moller, D. E., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 6825-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujino, T., Asaba, H., Kang, M. J., Ikeda, Y., Sone, H., Takada, S., Kim, D. H., Ioka, R. X., Ono, M., Tomoyori, H., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dressel, U., Allen, T. L., Pippal, J. B., Rohde, P. R., Lau, P. & Muscat, G. E. (2003) Mol Endocrinol., in press. [DOI] [PubMed]
- 18.Petersen, K. F., Befroy, D., Dufour, S., Dziura, J., Ariyan, C., Rothman, D. L., DiPietro, L., Cline, G. W. & Shulman, G. I. (2003) Science 300, 1140-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.