Abstract
Methionine is the precursor for S-adenosylmethionine (SAM), the major 1-carbon donor involved in >100 transmethylation reactions. Homocysteine produced from SAM must be metabolized either by remethylation for recycling of methionine or transsulfuration to form cystathionine and then cysteine. Pyridoxal 5′-phosphate (PLP) serves as a coenzyme in enzymes involved in transsulfuration as well as for primary acquisition of 1-carbon units used for remethylation and other phases of 1-carbon metabolism. Because the intake of vitamin B-6 is frequently low in humans and metabolic consequences of inadequacy may be amplified in the postprandial state, we aimed to determine the effects of marginal vitamin B-6 deficiency on the postprandial rates of remethylation, transmethylation, overall transsulfuration, and cystathionine synthesis. Healthy, young adults (4 male, 5 female; 20–35 y) received a primed, constant infusion of [1-13C]methionine, [methyl-2H3]methionine, and [5,5,5-2H3]leucine to quantify in vivo kinetics at normal vitamin B-6 status and after a 28-d dietary vitamin B-6 restriction. Vitamin B-6 restriction lowered the plasma PLP concentration from 49 ± 4 nmol/L (mean ± SEM) to 19 ± 2 nmol/L (P < 0.0001). Mean remethylation, transsulfuration, and transmethylation rates did not change in response to vitamin B-6 restriction; however, the responses to vitamin B-6 restriction varied greatly among individuals. The plasma cystathionine concentration increased from 142 ± 8 to 236 ± 9 nmol/L (P < 0.001), whereas the fractional cystathionine synthesis rate increased by a mean of 12% in 8 of 9 participants. Interrelationships among plasma concentrations of glycine and cystathionine and kinetic results suggest that individual variability occurs in normal postprandial 1-carbon metabolism and in the response to vitamin B-6 restriction.
Introduction
Dietary vitamin B-6 is necessary for the formation of pyridoxal 5′-phosphate (PLP),9 which serves as a coenzyme in many reactions of human intermediary metabolism. Marginal vitamin B-6 deficiency, as reflected by plasma PLP concentrations between 20 and 30 nmol/L (1), occurs frequently (2) and has been associated with coronary artery disease (3–5), stroke (6), and elevated risk of Alzheimer’s disease (7). The plasma PLP concentration also is inversely correlated with the risk of colorectal cancer (8) and breast cancer (9). The mechanisms responsible for such linkages between vitamin B-6 status and chronic disease have not been fully characterized.
Relationships among the activities of PLP-dependent metabolic processes that affect the function of the methionine cycle constitute one plausible mechanism connecting vitamin B-6 status and chronic disease. Methionine is a nutritionally indispensable amino acid and has major roles in human metabolism, including as a substrate for protein synthesis, a methyl donor [as S-adenosylmethionine (SAM)], a regulatory outlet of tetrahydrofolate (THF) in 1-carbon transfer reactions and in choline degradation, and a source of sulfur for the formation of cysteine, taurine, and glutathione via the transsulfuration pathway. During transmethylation processes, SAM is transformed to S-adenosylhomocysteine, which is hydrolyzed to form homocysteine. Homocysteine has 2 possible fates: remethylation for recycling of methionine or transsulfuration. Remethylation occurs either by the predominant folate-dependent pathway with a serine-derived 1-carbon unit (10) or by betaine as methyl donor through a folate-independent process (11). The entry of 1-carbon units into the folate pool (as 5,10-methylene-THF) occurs via the glycine cleavage system and serine hydroxymethyltransferase (SHMT), both of which require PLP as coenzyme. Transsulfuration constitutes the catabolic route for homocysteine and is comprised of 2 PLP-dependent reactions. Cystathionine β-synthase (CBS) catalyzes the condensation of homocysteine with serine to form cystathionine. Cystathionine γ-lyase (CGL) then cleaves cystathionine to form cysteine and α-aminobutyrate. Among other metabolic needs for cysteine, it is the limiting amino acid in the synthesis of glutathione, a major component of oxidant defense and xenobiotic metabolism. Previous studies with rats and humans have suggested that the activity of CGL is much more profoundly affected by low vitamin B-6 status than CBS (12, 13). Because elevated plasma total homocysteine concentration has been associated with an increased risk of coronary heart disease (14, 15), cognitive decline (16, 17), and pregnancy complications (18), homocysteine elimination by remethylation and the transsulfuration processes, as balanced against the rate of homocysteine production through transmethylation, is of major importance.
Storch et al. (19) developed a stable isotope tracer protocol for quantifying the overall kinetics of the major components of the methionine cycle in human volunteers, including the fluxes of remethylation, transsulfuration, transmethylation processes, and methionine flux in postprandial and fasting states. This basic protocol has been applied to explore the effect of diets containing different amounts of sulfur-containing amino acids on the methionine cycle in young and elderly participants (20–23). MacCoss et al. (24) modified this tracer model to account more directly for the intracellular metabolism of methionine. Despite the utility of these approaches for investigating global kinetics of the methionine cycle, they have not been previously employed to investigate the potential influence of inadequate supply of relevant vitamins, including vitamin B-6.
We previously developed a tracer protocol utilizing [3-13C]serine, [13C5]methionine, [2,2-2H2]cysteine, and [5,5,5-2H3]leucine to investigate the effect of vitamin B-6 restriction on the in vivo kinetics of overall remethylation, remethylation from serine-derived 1-carbon units (i.e. requiring PLP-dependent SHMT processing), cysteine turnover, and synthesis of cystathionine in transsulfuration, with volunteers maintained in the postprandial state but with minimal amino acid intake during infusions (12, 25). However, we hypothesize that the effects of vitamin B-6 deficiency might have the greatest impact in the postprandial state, because pathways are subjected to higher fluxes due to greater amino acid intake and, thus, substrate availability (19). We report here an application of a stable isotope tracer protocol intended to simultaneously measure the global kinetics of the methionine cycle and the function of the transsulfuration pathway to investigate the effects of vitamin B-6 restriction in the postprandial state with adequate protein intake. Aside from the difference of amino acid intake, this study also differs from our previous work (25) by determining the overall flux of the transsulfuration pathway by measuring methionine oxidation rate.
To our knowledge, this is the first study to specifically determine the effect of dietary vitamin B-6 restriction on postprandial methionine cycle and transsulfuration rate in vivo. The steady-state tracer protocol was a modification of that reported by MacCoss et al. (24) and involved primed, constant infusion of [1-13C]methionine, [methyl-2H3]methionine, and [5,5,5-2H3]leucine, allowing the quantification of remethylation, transmethylation, and transsulfuration rates as well as cystathionine synthesis rates in each healthy male and female volunteer. To determine the effects of marginal vitamin B-6 deficiency on in vivo kinetics, participants were evaluated before and after a dietary vitamin B-6 restriction protocol as previously employed (25–27).
Methods
Materials
l-[1-13C]Methionine, l-[methyl-2H3]methionine, l-[5,5,5-2H3]leucine, and sodium [13C]bicarbonate were purchased from Cambridge Isotopes Laboratories. Parenteral solutions of these compounds were prepared in normal saline, filter sterilized, and analyzed to ensure lack of pyrogenicity and microbial contamination. The concentration of the [1-13C]methionine and [methyl-2H3]methionine tracers in parenteral solution determined as described below were 94.1 and 92.4% of the calculated value.
Human participants
Study participants underwent a physical examination and were screened by standard clinical measures of hematological, hepatic, renal, and thyroid function. Participants also completed a questionnaire on their medical history, dietary habits, and demographic data. Of 16 recruited adult male and nonpregnant female volunteers, 12 met the following inclusion criteria: age between 20 and 40 y; no history of gastrointestinal surgery, abnormal kidney or thyroid function, or any other chronic disease; no smoking or chronic drug use or alcoholism; a BMI < 28 kg/m2; no vitamin, amino acid, or protein supplementation; no chronic consumption of a high-protein diet; and nutritional adequacy indicated by serum folate (>7 nmol/L), serum vitamin B-12 (>200 pmol/L), plasma PLP (>30 nmol/L), and plasma total homocysteine (<12 μmol/L).
During the intervention, 1 participant withdrew for personal reasons. Eleven participants completed the trial; however, the data of 2 participants were excluded from the statistical evaluation due to apparent noncompliance. All participants gave written informed consent. The University of Florida Institutional Review Board and the University of Florida Clinical Research Center (CRC) Scientific Advisory Committee reviewed and approved this protocol.
Dietary treatment
The Bionutrition Unit of the CRC prepared all meals and the nutritive formula administered during infusions. To minimize dietary variation immediately prior to the first infusion, participants consumed nutritionally adequate meals with a standardized composition for 2 d. On the day after the first infusion, participants began consuming a vitamin B-6–restricted diet (<0.5 mg/d vitamin B-6) and continued this regimen for 28 consecutive days (25–28). Mean total protein intake was 1 g · kg−1 · d−1 and mean methionine and cystine intakes were 21 and 17 mg · kg−1 · d−1, respectively, during the vitamin B-6–restricted diet. Participants consumed breakfast in the CRC and were given a take-out lunch and snack to eat at their convenience. In the evening, participants returned to the CRC to consume their evening meal and to receive a take-out snack. A custom-formulated, multivitamin-mineral supplement containing no pyridoxine-HCl administered to participants compensated for any vitamin and mineral inadequacies of the study diets (other than vitamin B-6). As described below, compliance with the dietary regimen was monitored by weekly measurements of plasma PLP. After the second infusion day, i.e. at the end of the intervention, participants received an over-the-counter multivitamin-mineral supplement to facilitate restoration of normal vitamin B-6 status.
Analytical methods
Screening measurements.
Plasma PLP concentration was measured as the semicarbazone-derivative by reverse-phase HPLC with fluorescence detection (29). Serum folate and vitamin B-12 were analyzed with the use of a chemiluminescence-based assay (Elecsys, Roche Diagnostics). The plasma total homocysteine concentration was measured as the ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate derivative by reverse-phase HPLC with fluorescence detection (30).
GC-MS determination of amino acid isotopic enrichment and concentration.
Plasma free amino acids were isolated, derivatized, and analyzed by GC-MS in electron capture negative ionization mode as previously described (10). The relative abundance of specific ions was determined by selected-ion monitoring at the following m/z ratios: leucine (349–352), methionine (367–372), homocysteine (549–553), and cystathionine (677–682). Isotopic enrichments are expressed as molar ratios (mol % excess) of labeled:nonlabeled isotopomers after correction for the natural abundance of stable isotopes, essentially as performed by Storch et al. (19). Methionine, cystathionine, glycine, and serine concentrations in fasting plasma samples of each infusion day were measured by this method except with the use of stable-isotope labeled internal standards: [13C5]methionine, [2H4]cystathionine, [13C2]glycine, and [2H3]serine (Cambridge Isotope Laboratories). The calibration curve was derived from the abundances of unlabeled and labeled isotopomers.
13C isotopic enrichment of breath CO2.
Breath samples were collected in Exetainer tubes (Metabolic Solutions) and analyzed by isotope ratio-MS (Metabolic Solutions) to determine 13C isotopic enrichment of breath CO2. Total CO2 production rate was determined with the use of a metabolic cart (TrueMax 2400; ParvoMedics). Measurements were taken at 30-s intervals for ~5 min until 4 consecutive time points differed by no more than ± 0.01 L/min.
Liquid chromatography-tandem MS analysis of monocyte DNA isotopic enrichment and methyldeoxycytidine concentration.
Monocytes were purified from whole blood using Vacutainer Sodium Heparin CPT tubes (Becton Dickinson) followed by magnetic labeling of the monocytes with human CD14 MicroBeads (Miltenyi Biotec) and isolation using a MACS Separation column (Miltenyi Biotec). DNA was purified from the isolated monocytes using a QIAamp DNA Mini kit (QIAGEN). The concentration of DNA in each sample was quantified by the Qubit Quantitation Platform using Quant-iT fluorescence technology (Invitrogen). DNA was hydrolyzed using a recently developed digest mix containing 250 U Benzonase, 300 mU phosphodiesterase I, and 200 U alkaline phosphatase (31). Isotopic enrichment was determined by liquid chromatography-tandem MS with the use of a Luna 18(2) column (50 × 3.0 mm, 3 μm, Phenomenex) and a Thermo-Finnigan TSQ Quantum mass spectrometer in heated electrospray ionization mode (31) with the mass transitions reported earlier (32). We employed a modification of this procedure with the addition of [15N3]deoxycytidine and [15N3]methyldeoxycytidine as internal standards for the determination of methyldeoxycytidine (MdC) and deoxycytidine to calculate the percentage of methylation of deoxycytidine (MdC%) in monocyte DNA prior to infusion (33).
Infusion protocol
On the evening before the infusion protocol, participants were admitted to the CRC and consumed no food or drinks, except water, between 2100 h and the first blood draw. On the morning of the infusion, a catheter was inserted into the antecubital vein of each arm: 1 for the tracer infusion and 1 for blood collection. Fasting blood samples were taken 2 h before infusion (at ~0700 h) for measurement of plasma PLP, serum folate, and vitamin B-12 and plasma amino acid concentrations. Infusions were initiated at ~0900 h with a 5-min, ~20-mL priming dose that delivered 2.4 μmol · kg−1 of [1-13C]methionine, 1.4 μmol · kg−1 of [methyl-2H3]methionine, 1.87 μmol · kg−1 of [5,5,5-2H3]leucine, and 2.15 μmol · kg−1 of NaH13CO3. The 9-h constant infusion followed immediately after the priming dose and delivered ~20 mL infusion solution/h that contained 2.4 μmol · kg−1 of [1-13C]methionine, 1.4 μmol · kg−1 of [methyl-2H3]methionine, and 1.87 μmol · kg−1 of [5,5,5-2H3]leucine.
Blood samples were taken at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7.5, and 9 h of the infusion. They were placed immediately on ice and were centrifuged within 15 min after the blood draw (1500 × g, 4°C, 10 min). Plasma was stored in cryotubes at −80°C.
Breath samples were collected into Exetainer tubes at 0, 1, 2, 3, 4, 6, 7.5, and 9 h of infusion to measure 13CO2 production. Measurements of the total CO2 production rate were conducted at 0, 2, 4, 6, 7.5, and 9 h of infusion. The participants received a nutritive formula hourly starting 2 h before infusion to maintain a fed state (30). This formula provided a balanced composition of amino acids at a rate based on requirements of 0.8 g protein · kg−1 · d−1, which equals an hourly protein dose of ~0.03 g · kg−1 with 5.23 and 5.44 kJ · kg−1 · d−1 for women and men, respectively. The concentration of sulfur-containing amino acids was 4.7 and 0.76 μmol · kg−1 · h−1 for methionine and cystine, respectively. The formula further provided an adequate energy intake according to the requirements of 126 and 130 kJ · kg−1 · d−1 for women and men, respectively.
Blood samples were collected prior to the infusion (0-time) to determine the natural isotopic abundance of monocyte DNA. Because monocytes appear in circulation ~1 d after synthesis and have a half-life of ~2 d, blood samples were collected after 2, 2.5, 3, 3.5, and 4 d of each infusion procedure for monocyte isolation and determination of isotopic enrichment of DNA MdC. MdC% was determined in monocytes collected prior to each infusion (0-time) before and after vitamin B-6 restriction. These measurements of DNA enrichment and MdC% were conducted on a random subsample of 5 participants.
Kinetic principles and analysis
This tracer model is based on simultaneous infusion of [1-13C]methionine and [methyl-2H3]methionine, as described by others (19, 20, 24), with [2H3]leucine included to evaluate any nutritional effects on protein turnover that would be reflected by a change in leucine flux (25, 27, 34). The use of 2 labeled forms of methionine allows determining the recycling of methionine through the remethylation of homocysteine. Because methionine is metabolized (via SAM) in the methylation cycle, the [methyl-2H3]methionine loses its labeled methyl-group during transmethylation. However, the [1-13C]methionine tracer conserves its labeling during transmethylation and remethylation but ultimately loses the 13C-atom during oxidation following transsulfuration. The difference between the fluxes of the carbon-labeled methionine to the methyl-labeled tracer equals the remethylation flux. The only outflow of the carboxyl-label occurs through the transsulfuration pathway (also termed methionine oxidation), which can be quantified by measuring breath 13CO2. The transmethylation rate, i.e. the rate of transfer of the methionine methyl group on a 1-carbon acceptor, is the sum of the remethylation and the transsulfuration rates accounting for both possible fates of homocysteine after transmethylation (19). We employed the approach of MacCoss et al. (24), who amended the dual-methionine tracer protocol of Storch et al. (19) by using plasma homocysteine enrichment as marker of intracellular methionine enrichment.
Plateau enrichment (Ep) for all infused amino acid tracers was calculated as the mean of the isotopic enrichments for the ~1.5- to 9-h time points for the infused [methyl-2H3]methionine, [1-13C]methionine, and [2H3]leucine tracers. The Ep of all labeled metabolic products was determined by fitting enrichment data to single exponential curves defined by the equation
where E is the enrichment at time t (h), Ef the enrichment at infinity (i.e. Ep), and k the rate constant (h−1) from the fitted curve (24) using SigmaPlot 2002 (version 8.02; SPSS).
Steady-state kinetics of amino acid tracers were calculated using standard equations (34), including correction for overestimation of intracellular enrichment from plasma enrichment data (19, 24, 34), as discussed below. The flux of an amino acid is the rate of appearance of that amino acid from endogenous production (de novo synthesis and protein breakdown), absorption, and the tracer infusion and is calculated from the Ep of the corresponding amino acid tracer. Specifically, the flux of leucine (QLeu) in the plasma pool was calculated as:
where ILeu is the [2H3]leucine infusion rate, ELeu is the enrichment of the [2H3]leucine tracer, and EpLeu is the Ep of [2H3]leucine in plasma. The Ep of plasma leucine was not corrected for overestimation of intracellular enrichment, consistent with previous studies using leucine flux as a relative indicator of protein turnover (25, 27, 35, 36).
The steady-state kinetics of the methionine tracers as well as the rates of remethylation, transsulfuration, transmethylation, and methionine uptake for protein synthesis were quantified according to MacCoss et al. (24) and are described in detail in the Supplemental Methods.
The fractional synthesis rate (FSR) represents the fraction of a product (e.g. cystathionine pool) that is produced from its precursor (e.g. methionine or homocysteine) per unit time. The FSR for cystathionine (FSRCsn) was calculated from the initial rate of synthesis using early time points (0.5–2 h) from the linear portion of the enrichment curve of the product, in this case, [13C1]cystathionine. The FSR of cystathionine from infused methionine is:
where the initial rate of CsnM+1 enrichment is the slope of the initial time points (0.5–2 h) of the cystathionine enrichment vs. time curve and Ep13C-Hcy is the Ep of [13C1]homocysteine in plasma.
Statistical analysis
Isotopic enrichments are expressed as the ratio of labeled:nonlabeled isotopomers after correction for the natural abundance of stable isotopes. The study was designed based on power calculations assuming variance equivalent to that observed in previous studies of homocysteine remethylation (13). The power of the study was >80% for detecting a difference of at least 30% in measured fluxes with assumed SD of 30%. All data were presented as mean ± SEM. An unpaired t test was performed for determination of differences in baseline characteristics between men and women. A paired t test was used to assess differences between normal vitamin B-6 status and marginal vitamin B-6 deficiency. Two-way repeated-measures ANOVA was performed to calculate differences and interactions between plasma total homocysteine concentrations before and after vitamin B-6 restriction and at the fed and fasting states. Correlations between static and dynamic parameters were determined with the use of the Spearman correlation coefficient. Linear regression was used to determine the influence of static and dynamic variables on in vivo kinetics before and after vitamin B-6 restriction and on the change in in vivo kinetics in response to vitamin B-6 restriction. Data were analyzed using Microsoft Office Excel 2007 (Microsoft) and SigmaStat 3.0 (SPSS).
Results
Indicators of nutritional status and parameters of 1-carbon metabolism
All participants showed adequate nutritional status at baseline. After 28 d of dietary vitamin B-6 restriction, the plasma PLP concentration (Table 1) was significantly reduced by 59 ± 3%. All participants reached the range of plasma PLP associated with marginal vitamin B-6 deficiency (20–30 nmol/L) after 20 ± 2 d of consuming the vitamin B-6–restricted diet. Marginal vitamin B-6 deficiency did not alter serum folate and vitamin B-12 concentrations, although these would be maintained by the RDA amounts of folic acid and cyanocobalamin in the daily supplements. The plasma cystathionine concentration, which is indicative of intracellular vitamin B-6 deficiency, increased (P < 0.001), whereas the plasma glycine concentration tended to increase after vitamin B-6 restriction (P = 0.06). The change in plasma cystathionine concentrations in response to vitamin B-6 restriction correlated inversely with the plasma cystathionine concentration before vitamin B-6 restriction (ρ = −0.758; P < 0.05).
TABLE 1.
Fasting concentrations of plasma or serum PLP, folate, vitamin B-12, glutathione, and amino acids of 1-carbon metabolism before and after dietary vitamin B-6 restriction in healthy men and women1
| Baseline | Restricted | |
| B vitamins | ||
| Plasma PLP, nmol/L | 49 ± 4 | 19 ± 2 #x2021 |
| Serum folate, nmol/L | 33 ± 2 | 36 ± 1 |
| Serum vitamin B-12, pmol/L | 353 ± 37 | 383 ± 40 |
| Amino acids of 1-carbon metabolism and glutathione in plasma | ||
| Methionine, μmol/L | 30 ± 2 | 28 ± 2 |
| Total homocysteine, μmol/L | 7.3 ± 0.4 | 7.4 ± 0.5 |
| Cystathionine, nmol/L | 142 ± 8 | 236 ± 9 #x2021 |
| Total cysteine, μmol/L | 280 ± 10 | 273 ± 11 |
| Glycine, μmol/L | 296 ± 27 | 334 ± 27 |
| Serine, μmol/L | 117 ± 8 | 128 ± 8 |
| Total glutathione, μmol/L | 7.5 ± 0.5 | 7.7 ± 0.4 |
Values are means ± SEM, = 10. Symbols indicate different from baseline: ‡P < 0.0001 (paired t test).
As reported in a previous vitamin B-6 restriction protocol (26, 27), mean fasting concentrations of plasma methionine, homocysteine, cysteine, serine, and glutathione exhibited no change associated with vitamin B-6 restriction in the present study. Similar to the pattern observed for cystathionine, the change in plasma glutathione concentrations in response to vitamin B-6 restriction correlated with the plasma glutathione concentration before vitamin B-6 restriction (ρ = −0.830; P < 0.001). The effect of vitamin B-6 restriction on plasma amino acid and glutathione concentrations varied among individuals, but several consistent relationships emerged from these data. The change in plasma total homocysteine concentration due to vitamin B-6 restriction was related to the change in the plasma glutathione concentration (ρ = 0.636; P < 0.05), the change in plasma methionine to the change in plasma serine (ρ = 0.661; P < 0.05), and the change in plasma cystathionine to the change in plasma glycine concentrations (ρ = 0.697; P < 0.05).
Plasma total homocysteine concentrations had a significant interaction between fasting (preprandial) and postprandial state and before and after vitamin B-6 restriction (Table 2).
TABLE 2.
Plasma total homocysteine concentrations at fasting and fed state before and after dietary vitamin B-6 restriction in healthy men and women1
| Baseline | Restricted | |
| Plasma total homocysteine at fasting state, μmol/L | 7.7 ± 0.5 | 7.5 ± 0.5 |
| Plasma total homocysteine at fed state,2μmol/L | 8.7 ± 0.5#x2021 | 9.2 ± 0.7#x2021# |
| Change from fasting to fed state, % | 13.7 ± 2.5 | 22.3 ± 2.5 |
Values are means ± SEM, = 10. Symbols indicate: ‡different from fasting state, P < 0.001 (2-way repeated-measures ANOVA); # interaction between fasting/fed and before/after vitamin B-6 restriction, P = 0.05 (2-way repeated-measures ANOVA).
Concentration at fed state is the mean of the times 6, 7.5, and/or 9 h of infusion.
In vivo amino acid kinetics
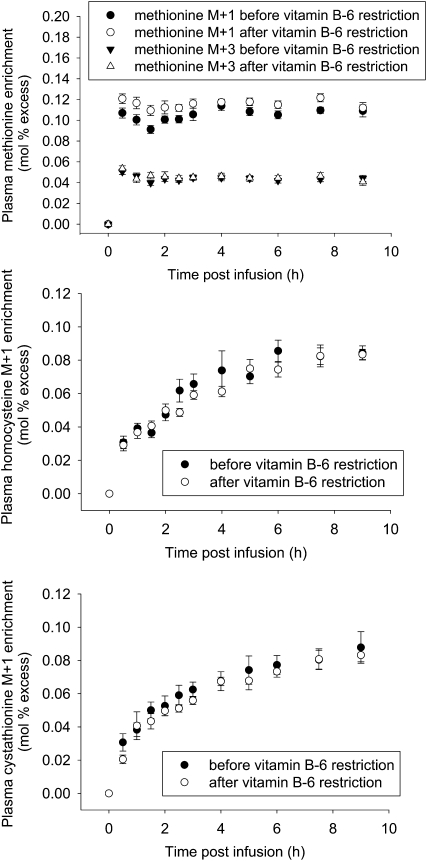
Primed, constant infusion with 2.4 μmol · kg−1 · h−1 of [1-13C]methionine, 1.4 μmol · kg−1 · h−1 of [methyl-2H3]methionine, and 1.87 μmol · kg−1 · h−1 of [5,5,5-2H3]leucine yielded quantifiable time course plots of plasma enrichment of the infused tracers and metabolic products for all participants (Fig. 1). The mean whole body leucine flux did not change as a function of vitamin B-6 restriction (132 ± 6 μmol · kg−1 · h−1 before and 126 ± 8 μmol · kg−1 · h−1 after vitamin B-6 restriction). The absence of a change in leucine flux indicates that this level of vitamin B-6 restriction did not alter the rate of protein turnover.
FIGURE 1.
Plasma methionine M+1 and M+3 (top), homocysteine M+1(middle), and cystathionine M+1 (bottom) enrichment before and after vitamin B-6 restriction in healthy men and women. Values are means ± SEM, n = 9.
The rate of 13CO2 production provided a direct measurement of the methionine oxidation, which was used as indicator of the rate of the transsulfuration pathway. The rate of 13CO2 production differed between males and females before and after vitamin B-6 deficiency (data not shown), apparently due to the higher mean body weight of males (80 ± 7 kg) and females (63 ± 2 kg) (P < 0.05). However, no gender difference in transsulfuration flux existed when expressed per kilogram body weight (Table 3).
TABLE 3.
Rates of TS, QC, QM, RM, methionine synthesis (S), and transmethylation (TM) before and after dietary vitamin B-6 restriction in 9 healthy young men and women1
| Baseline |
Restricted |
||||||||||||||
| n | Gender | TS | QC | QM | RM | S | TM | RM/TS | TS | QC | QM | RM | S | TM | RM/TS |
| μmol⋅kg−1⋅h−1 | |||||||||||||||
| 4 | Male | 5.1 ± 0.3 | 26.4 ± 1.2 | 37.7 ± 3.6 | 11.4 ± 2.7 | 21.3 ± 1.1 | 16.4 ± 2.7 | 2.3 ± 0.5 | 4.9 ± 0.6 | 26.6 ± 2.7 | 41.2 ± 3.8 | 14.6 ± 1.6 | 21.7 ± 2.1 | 19.5 ± 1.9 | 3.1 ± 0.4 |
| 5 | Female | 5.3 ± 0.5 | 23.1 ± 1.8 | 34.3 ± 2.6 | 11.2 ± 1.1 | 17.8 ± 1.4 | 16.5 ± 1.5 | 2.1 ± 0.1 | 5.0 ± 0.2 | 24.6 ± 0.8 | 36.8 ± 2.0 | 12.2 ± 1.5 | 19.6 ± 0.7 | 17.2 ± 1.5 | 2.4 ± 0.3 |
| 9 | All | 5.2 ± 0.3 | 24.5 ± 1.2 | 35.8 ± 2.1 | 11.3 ± 1.2 | 19.4 ± 1.1 | 16.5 ± 1.3 | 2.2 ± 0.2 | 5.0 ± 0.3 | 25.5 ± 1.2 | 38.7 ± 2.0 | 13.3 ± 1.1 | 20.5 ± 1.0 | 18.2 ± 1.2 | 2.7 ± 0.3 |
Values are means ± SEM.
The mean whole body fluxes of carboxyl- (QC) and methyl-labeled (QM) methionine did not change as a function of vitamin B-6 restriction (Table 3). The rate of methionine recycling reflected by remethylation rate comprised 67 ± 2% and 72 ± 2% of the total transmethylation rate, respectively, before and after vitamin B-6 restriction. Substantial variation of kinetics among individuals was observed as well as a variation in the effect of vitamin B-6 restriction (Supplemental Table 1). Transsulfuration flux, QC, and the ratio of remethylation:transsulfuration (RM:TS) at normal vitamin B-6 status correlated with the extent of change in transsulfuration (ρ = −0.70; P < 0.05), QC (ρ = −0.67; P < 0.05), and RM:TS (ρ = −0.67; P < 0.05) in response to vitamin B-6 restriction. Regression analysis showed that the change in transsulfuration (ρ = −0.88; P < 0.0001) and QC (ρ = −0.70; P < 0.05) was negatively associated with the plasma glycine concentration at normal vitamin B-6 status. The change in transsulfuration and the change in the RM:TS correlated with the change in the plasma serine concentration (ρ = 0.68, P < 0.05; and ρ = −0.75, P < 0.05, respectively).
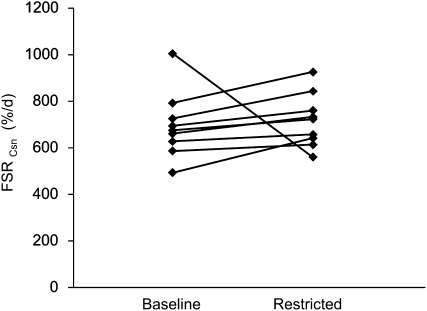
The FSR of cystathionine increased in 8 of 9 participants due to vitamin B-6 restriction (Fig. 2). Mean FSRCsn of all participants was 697 ± 45% d−1 before and 719 ± 36% d−1 after vitamin B-6 restriction. After excluding 1 outlier (the sole participant not showing an increase in FSRCsn but a 44% decrease), vitamin B-6 restriction increased the mean FSRCsn from 658 ± 29% d−1 to 738 ± 33% d−1 (P < 0.01). The change in FSRCsn correlated with the change in the plasma cysteine concentration observed due to vitamin B-6 restriction (ρ = 0.75; P < 0.05).
FIGURE 2.
Individual FSRCsn before and after vitamin B-6 restriction in 9 healthy men and women.
DNA methylation labeling
We measured in monocyte DNA the MdC M+3 enrichment over the several-day time period after each infusion to assess the in vivo rate of DNA methylation from [methyl-2H3]methionine. Neither the MdC M+3 enrichment nor the global DNA methylation (MdC%) changed in response to vitamin B-6 restriction (Table 4).
TABLE 4.
MdC in monocyte DNA in the fasted state and after infusion with stable isotope labeled methionine before and after dietary vitamin B-6 restriction in healthy men and women1
| Baseline | Restricted | |
| Methylation of deoxycytidine in monocyte DNA, MdC as % of total dC | 4.56 ± 0.06 | 4.42 ± 0.07 |
| Emax MdC M+3, mol % excess | 0.57 ± 0.03 | 0.56 ± 0.03 |
Values are means ± SEM, = 5
Discussion
Marginal vitamin B-6 deficiency has little or no effect on fasting plasma concentrations of methionine, total homocysteine, serine, and cysteine, as observed in this study and previously (12, 25, 27, 37). In contrast, plasma cystathionine is a sensitive biomarker for vitamin B-6 deficiency even at the marginal level we induced (plasma PLP ~20 nmol/L). Whereas fasting plasma total homocysteine concentrations are unaltered at marginal vitamin B-6 deficiency, the effect of vitamin B-6 deficiency on homocysteine metabolism is most pronounced in the fed state (38) or after methionine load test (39). Vitamin B-6–deficient rats had normal concentrations of plasma fasting total homocysteine but had a substantial increase in homocysteine concentrations after administration of dietary methionine compared with their vitamin B-6–replete controls (38). We observed greater elevation of plasma homocysteine in the fed state after vitamin B-6 restriction, which is consistent with the finding by Miller et al. (37) that vitamin B-6 deficiency accentuates the postprandial rise of homocysteine.
There was no consistent effect of vitamin B-6 restriction on the mean postprandial rates of transsulfuration, transmethylation, and remethylation or DNA methylation in the healthy men and women of the present study. Although 6 of 9 participants showed an increase in remethylation and transmethylation rates in response to vitamin B-6 restriction, overall transsulfuration fluxes remained rather constant. Previous studies from our group that examined in vivo kinetics in participants fed an energy-adequate nutritive formula with minimal protein content showed that marginal vitamin B-6 deficiency did not significantly alter in vivo overall methionine turnover or remethylation flux (25, 35). The provision of dietary amino acids during the infusions in the present study accelerated amino acid turnover as reflected by the whole-body leucine flux (132 ± 6 μmol · kg−1 · h−1) compared with 80 ± 3 μmol · kg−1 · h−1 reported by Davis et al. (25). We previously observed that vitamin B-6 restriction had little or no effect on either the in vivo flux of total remethylation or the flux of remethylation involving 1-carbon units derived solely from serine (25) despite an ~40% reduction in lymphocyte SHMT activity assayed in vitro (40). Because remethylation from serine-derived 1-carbon units accounts for the majority of remethylation flux and requires an adequate supply of PLP for SHMT activity, those results suggested that cells had sufficient excess SHMT activity to maintain adequate 5-methyl-THF formation and support remethylation needs (25). Mathematical modeling of vitamin B-6 restriction and its effects on 1-carbon metabolism showed little impact on SHMT flux (41). In the present study, which measured only total remethylation, some degree of compensation by the alternative remethylation process catalyzed by betaine-homocysteine methyltransferase cannot be ruled out.
Determination of the fractional cystathionine synthesis rate allowed quantification of the CBS reaction, the first step of the transsulfuration pathway. Under the steady-state assumption, the total transsulfuration flux is expected to be approximately equal to both CBS flux and CGL flux; however, the expansion of the cystathionine pool in plasma and liver (13) during vitamin B-6 restriction is evidence of a partial bottleneck at CGL (12). The majority of participants in this study exhibited increased FSR of cystathionine after vitamin B-6 restriction, which may be due to enhanced postprandial CBS flux caused by the marginally greater substrate (i.e. homocysteine) concentration. Although an allosteric effect of SAM also could be postulated as stimulating CBS, the lack of difference in the plasma methionine concentration observed here plus the lack of an effect of deficiency on rat liver SAM (42) suggest that allosteric stimulation is unlikely. In the earlier study by our group in which participants received minimal amino acid intake during infusions (12), dietary vitamin B-6 restriction did not significantly affect the FSR of cystathionine (12). The greater amino acid intake provided in this study yielded a fractional cystathionine synthesis rate of ~30%/h compared with ~15%/h in the earlier study (12). As observed previously (12, 27), the plasma cystathionine concentration increased in all participants in response to vitamin B-6 restriction. Liver CBS activity has been shown to be largely unaffected by vitamin B-6 (13). Severe vitamin B-6 deficiency would deplete hepatic CGL, causing reduced formation of cysteine and thereby an accumulation of cystathionine. However, plasma cysteine is maintained in the marginal deficiency of this and other studies (12, 27), as is cysteine flux (12). Similarly, rat liver cysteine concentration is maintained over a broad range of insufficient vitamin B-6 intake (13), which suggests a kinetic compensatory mechanism with the elevated cellular cystathionine as the driving force (13). Mathematical modeling results (41) support this conclusion regarding moderate vitamin B-6 deficiency as occurs in our human participants. The Km of CBS for homocysteine is higher than for remethylation enzymes (43), i.e. removal of homocysteine via transsulfuration is enhanced at high homocysteine concentrations. In contrast to this hypothesis (43), examination of the ratio of RM:TS fluxes (Table 3; Supplemental Table 1) indicates that vitamin B-6 restriction actually favored remethylation in 6 of the 9 participants.
We observed substantial individual variation in the postprandial rates of remethylation, transmethylation, and transsulfuration and in the effect of vitamin B-6 restriction thereon, a finding similar to that reported in a study of fasting men (24). Thus, we do not think that potential differences in methionine uptake or splanchnic utilization substantially contributed to the variation in kinetic rates. Examination of additional men and women in this type of study would be needed to more fully determine the degree of heterogeneity of responses to vitamin B-6 restriction. Although further determination of the behavior of typical individuals is of great importance, of particular interest is the metabolic characterization of individuals having the greatest sensitivity to the consequences of inadequate vitamin B-6 intake. The metabolic factors responsible for the heterogeneity of response are unclear at present and are being examined further.
A single-pool model using plasma amino acid enrichment for calculation of whole body amino acid kinetics often overestimates the whole body amino acid pool. This overestimation can be compensated for by using a correction factor, as applied by Storch et al. (19), or by using the enrichment of a plasma metabolite that derives from intracellular turnover of the infused tracer. MacCoss et al. (24) used plasma total homocysteine enrichment as an indicator of intracellular methionine turnover, as was done in this study. In a tracer protocol using [U-13C5]methionine, Davis et al. (10) applied both the approaches of Storch et al. (19) and MacCoss et al. (24) for determining methionine kinetics and obtained very similar results, probably because the enrichment ratio of homocysteine:methionine used by Davis et al. (10) was 0.9 and, therefore, close to the correction factor of 0.8 suggested by Storch et al. (19). The enrichment ratio of homocysteine:methionine in this study was 0.8 at normal vitamin B-6 status and 0.7 at marginal vitamin B-6 deficiency. Using cystathionine as an indicator for intracellular methionine enrichment yielded enrichment ratios at normal vitamin B-6 status and marginal vitamin B-6 deficiency of 0.8 and 0.7, respectively. Although the correction factor chosen by Storch et al. (19) was close to the “true” value, use of this factor needs to be carefully considered. In the fasting state, MacCoss et al. (24) determined an enrichment ratio of homocysteine:methionine of 0.58, indicating that the intracellular free methionine pool measured is derived ∼58% from plasma methionine and 42% of methionine from protein breakdown. This lower enrichment ratio suggests a lower turnover of methionine in the cell at fasting state. In contrast, we showed that in the fed state only 20% of intracellular methionine is due to protein breakdown. Further, in fasting participants, homocysteine remethylation accounted for only ~10% of the total methionine methyl group flux, whereas in this study and that by Davis et al. (10), homocysteine remethylation accounted for 30% of methyl-labeled methionine flux.
Examination of correlations among kinetic and static metabolite concentration data allowed investigation of potential interrelationships affecting the metabolic response to vitamin B-6 restriction. The change in transsulfuration and in methionine flux in response to vitamin B-6 restriction was inversely related to the plasma glycine concentrations measured in the vitamin B-6–replete state (Supplemental Fig. 1). Despite these significant relationships observed here, in an identical dietary protocol, dietary vitamin B-6 restriction did not affect whole body glycine flux and formation of glycine-derived 1-carbon units (i.e. 5,10-methylene-THF) via mitochondrial glycine cleavage system but did increase the rate of glycine-to-serine formation (27). Despite the modest increase in glycine concentration and altered kinetics of serine formation from glycine in vitamin B-6 restriction (27), the observed effects on whole body methionine kinetics were subtle. The relationship between prerestriction plasma glycine and the response of transsulfuration and methionine (QC) fluxes to vitamin B-6 restriction may imply that prerestriction plasma glycine is a biomarker indicative of the vulnerability of these processes to vitamin B-6 deficiency.
In conclusion, the mean in vivo rates of remethylation, total transsulfuration, transmethylation, and DNA methylation were unaffected by dietary vitamin B-6 restriction in healthy, fed participants. Perhaps of greater interest is the fact that the metabolic response to vitamin B-6 restriction varies among individuals. Further examination of these effects will yield better insight into “metabolic phenotypes” and perhaps individualized nutritional recommendations.
Supplementary Material
Acknowledgments
J.F.G., Y.L., and P.W.S. designed research; Y.L. conducted research; B.C. was responsible for study coordination, participant recruitment, and screening; Y.L. and M.R. conducted analytical methods; E.P.Q. analyzed data of monocyte DNA enrichment; Y.L. performed statistical analysis; P.W.S. had clinical oversight; J.F.G. was principal investigator; and Y.L. and J.F.G. wrote the paper. All authors have read and approved the final manuscript.
Footnotes
Supported by NIH grant DK072398 and by NIH National Center for Research Resources CTSA grant 1UL1RR029890.
Supplemental Figure 1, Supplemental Table 1, and Supplemental Methods are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: CBS, cystathionine β-synthase; CGL, cystathionine γ-lyase; CRC, University of Florida Clinical Research Center; Ep, plateau enrichment; FSR, fractional synthesis rate; FSRCsn, fractional synthesis rate for cystathionine; MdC, methyldeoxycytidine; Q, a measurement of flux (e.g. leucine flux = QLeu); QC, carboxyl-labeled methionine flux; QM, methyl-labeled methionine flux; PLP, pyridoxal 5′-phosphate; RM:TS, ratio of remethylation:transsulfuration; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate.
Literature Cited
- 1.Leklem JE. Vitamin B-6: a status report. J Nutr. 1990;120 Suppl 11:1503–7 [DOI] [PubMed] [Google Scholar]
- 2.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1446–54 [DOI] [PubMed] [Google Scholar]
- 3.Friso S, Girelli D, Martinelli N, Olivieri O, Lotto V, Bozzini C, Pizzolo F, Faccini G, Beltrame F, et al. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr. 2004;79:992–8 [DOI] [PubMed] [Google Scholar]
- 4.Lin PT, Cheng CH, Liaw YP, Lee BJ, Lee TW, Huang YC. Low pyridoxal 5′-phosphate is associated with increased risk of coronary artery disease. Nutrition. 2006;22:1146–51 [DOI] [PubMed] [Google Scholar]
- 5.Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, Rubba P, Palma-Reis R, Meleady R, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation. 1998;97:437–43 [DOI] [PubMed] [Google Scholar]
- 6.Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, Furie KL. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke. 2003;34:e51–4 [DOI] [PubMed] [Google Scholar]
- 7.Miller JW, Green R, Mungas DM, Reed BR, Jagust WJ. Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology. 2002;58:1471–5 [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077–83 [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Lee IM, Cook NR, Selhub J, Manson JE, Buring JE, Zhang SM. Plasma folate, vitamin B-6, vitamin B-12, and risk of breast cancer in women. Am J Clin Nutr. 2008;87:734–43 [DOI] [PubMed] [Google Scholar]
- 10.Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF., III Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–9 Erratum in: Am J Physiol Endocrinol Metab. 2004;286: E674 [DOI] [PubMed] [Google Scholar]
- 11.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46 [DOI] [PubMed] [Google Scholar]
- 12.Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF III. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr. 2006;136:373–8 [DOI] [PubMed] [Google Scholar]
- 13.Lima CP, Davis SR, Mackey AD, Scheer JB, Williamson J, Gregory JF III. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J Nutr. 2006;136:2141–7 [DOI] [PubMed] [Google Scholar]
- 14.Verhoef P. Hyperhomocysteinemia and risk of vascular disease in women. Semin Thromb Hemost. 2000;26:325–34 [DOI] [PubMed] [Google Scholar]
- 15.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62 [DOI] [PubMed] [Google Scholar]
- 16.Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr. 2004;80:114–22 [DOI] [PubMed] [Google Scholar]
- 17.Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ. Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr. 2002;75:908–13 [DOI] [PubMed] [Google Scholar]
- 18.Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: a systematic review. Placenta. 1999;20:519–29 [DOI] [PubMed] [Google Scholar]
- 19.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol. 1988;255:E322–31 [DOI] [PubMed] [Google Scholar]
- 20.Storch KJ, Wagner DA, Burke JF, Young VR. [1-13C; methyl-2H3]methionine kinetics in humans: methionine conservation and cystine sparing. Am J Physiol. 1990;258:E790–8 [DOI] [PubMed] [Google Scholar]
- 21.Fukagawa NK, Yu YM, Young VR. Methionine and cysteine kinetics at different intakes of methionine and cysteine in elderly men and women. Am J Clin Nutr. 1998;68:380–8 [DOI] [PubMed] [Google Scholar]
- 22.Raguso CA, Pereira P, Young VR. A tracer investigation of obligatory oxidative amino acid losses in healthy, young adults. Am J Clin Nutr. 1999;70:474–83 [DOI] [PubMed] [Google Scholar]
- 23.Raguso CA, Regan MM, Young VR. Cysteine kinetics and oxidation at different intakes of methionine and cystine in young adults. Am J Clin Nutr. 2000;71:491–9 [DOI] [PubMed] [Google Scholar]
- 24.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280:E947–55 [DOI] [PubMed] [Google Scholar]
- 25.Davis SR, Scheer JB, Quinlivan EP, Coats BS, Stacpoole PW, Gregory JF III. Dietary vitamin B-6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am J Clin Nutr. 2005;81:648–55 [DOI] [PubMed] [Google Scholar]
- 26.Lamers Y, O'Rourke B, Gilbert LR, Keeling C, Matthews DE, Stacpoole PW, Gregory JF III. Vitamin B-6 restriction tends to reduce the red blood cell glutathione synthesis rate without affecting red blood cell or plasma glutathione concentrations in healthy men and women. Am J Clin Nutr. 2009;90:336–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamers Y, Williamson J, Ralat M, Quinlivan EP, Gilbert LR, Keeling C, Stevens RD, Newgard CB, Ueland PM, et al. Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr. 2009;139:452–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coburn SP, Ziegler PJ, Costill DL, Mahuren JD, Fink WJ, Schaltenbrand WE, Pauly TA, Pearson DR, Conn PS, et al. Response of vitamin B-6 content of muscle to changes in vitamin B-6 intake in men. Am J Clin Nutr. 1991;53:1436–42 [DOI] [PubMed] [Google Scholar]
- 29.Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr. 1985;342:277–84 [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–2 [PubMed] [Google Scholar]
- 31.Quinlivan EP, Gregory JF III. DNA digestion to deoxyribonucleoside: a simplified one-step procedure. Anal Biochem. 2008;373:383–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinlivan EP, Davis SR, Shelnutt KP, Henderson GN, Ghandour H, Shane B, Selhub J, Bailey LB, Stacpoole PW, et al. Methylenetetrahydrofolate reductase 677C>T polymorphism and folate status affect one-carbon incorporation into human DNA deoxynucleosides. J Nutr. 2005;135:389–96 [DOI] [PubMed] [Google Scholar]
- 33.Quinlivan EP, Gregory JF III. DNA methylation determination by liquid chromatography-tandem mass spectrometry using novel biosynthetic [U-15N]deoxycytidine and [U-15N]methyldeoxycytidine internal standards. Nucleic Acids Res. 2008;36:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe RR. Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. New York: Wiley Liss; 1992 [Google Scholar]
- 35.Cuskelly GJ, Stacpoole PW, Williamson J, Baumgartner TG, Gregory JF III. Deficiencies of folate and vitamin B6 exert distinct effects on homocysteine, serine, and methionine kinetics. Am J Physiol Endocrinol Metab. 2001;281:E1182–90 [DOI] [PubMed] [Google Scholar]
- 36.Gregory JF III, Cuskelly GJ, Shane B, Toth JP, Baumgartner TG, Stacpoole PW. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am J Clin Nutr. 2000;72:1535–41 [DOI] [PubMed] [Google Scholar]
- 37.Miller JW, Ribaya-Mercado JD, Russell RM, Shepard DC, Morrow FD, Cochary EF, Sadowski JA, Gershoff SN, Selhub J. Effect of vitamin B-6 deficiency on fasting plasma homocysteine concentrations. Am J Clin Nutr. 1992;55:1154–60 [DOI] [PubMed] [Google Scholar]
- 38.Miller JW, Nadeau MR, Smith D, Selhub J. Vitamin B-6 deficiency vs folate deficiency: comparison of responses to methionine loading in rats. Am J Clin Nutr. 1994;59:1033–9 [DOI] [PubMed] [Google Scholar]
- 39.Ubbink JB, van der Merwe A, Delport R, Allen RH, Stabler SP, Riezler R, Vermaak WJ. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest. 1996;98:177–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheer JB, Mackey AD, Gregory JF III. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J Nutr. 2005;135:233–8 [DOI] [PubMed] [Google Scholar]
- 41.Nijhout HF, Gregory JF, Fitzpatrick C, Cho E, Lamers Y, Ulrich CM, Reed MC. A mathematical model gives insights into the effects of vitamin B-6 deficiency on 1-carbon and glutathione metabolism. J Nutr. 2009;139:784–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez M, Cuskelly GJ, Williamson J, Toth JP, Gregory JF III. Vitamin B-6 deficiency in rats reduces hepatic serine hydroxymethyltransferase and cystathionine beta-synthase activities and rates of in vivo protein turnover, homocysteine remethylation and transsulfuration. J Nutr. 2000;130:1115–23 [DOI] [PubMed] [Google Scholar]
- 43.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.