This study demonstrates that a unique population of lymphocytes called γδ T cells plays a crucial role in both the early and late phases of allergic conjunctivitis.
Abstract
Purpose.
To determine the function of γδ T cells in early- and late-phase responses in allergic conjunctivitis.
Methods.
Wild-type (WT) C57BL/6 and γδ T cell–deficient (TCR-δ−/−) mice were immunized intraperitoneally and challenged topically for 7 consecutive days with short ragweed pollen. Natural killer T (NKT) and γδ T cell–double-deficient mice were generated by treating TCR-δ−/− mice with anti-CD1d antibody. Allergic conjunctivitis was evaluated clinically, and the late-phase response was assessed by histopathology. Cytokine profiles were evaluated by ELISA. The afferent and efferent arms of allergic conjunctivitis were assessed by adoptive transfer of CD4+ T cells from WT or TCR-δ−/− mice into naive TCR-δ−/− or WT mice.
Results.
TCR-δ−/− mice had decreased clinical manifestations of allergic conjunctivitis compared with WT mice. TCR-δ−/− mice had decreased eosinophilic infiltration compared with WT mice. TCR-δ−/− mice produced less Th2-associated cytokines interleukin (IL)-4, -5, and -13 compared with WT mice. Clinical manifestations of allergic conjunctivitis were lowest in NKT cell–depleted TCR-δ−/− mice. However, late-phase allergic conjunctivitis in NKT cell–depleted, TCR-δ−/− mice was the same as TCR-δ−/− mice. Adoptive transfer of CD4+ T cells revealed that γδ T cells are needed for the afferent and efferent arms of allergic conjunctivitis.
Conclusions.
γδ T cells are needed for full expression of both the clinical manifestations and the late phase of allergic conjunctivitis. Thus, γδ T cells have an important impact in the expression of allergic conjunctivitis and are a potential therapeutic target in the management of allergic diseases of the ocular surface.
Allergic conjunctivitis describes a variety of ocular inflammatory diseases that affect the eyelid, conjunctiva, and/or the cornea.1 It has been estimated that allergic conjunctivitis affects 20% of the world's population.2–4 A mild form of allergic conjunctivitis, known as seasonal allergic conjunctivitis, is induced by environmental irritants such as grass, ragweed, and birch pollens.2 Severe forms, such as atopic keratoconjunctivitis or vernal keratoconjunctivitis, display more chronic symptoms that involve the cornea.5,6 The development of allergic conjunctivitis consists of two phases: an early-phase and a late-phase reaction. The early-phase reaction occurs within minutes of allergen exposure and is initiated when the allergen cross-links specific IgE antibodies bound via FcεRI receptors on the surface of ocular mast cells and results in the release of histamine, leukotrienes, proteases, prostaglandins, and cytokines.6,7 The early-phase reaction is characterized by tearing, lid edema, conjunctival edema (chemosis), and vasodilatation of conjunctival blood vessels. The late-phase reaction appears hours after allergen exposure and involves the infiltration of inflammatory cells, especially eosinophils, into the conjunctiva.6
Although Th2 polarized CD4+ T cells have been studied in allergic conjunctivitis, the role of other T cell subsets in this disease has not been determined. It has been found that γδ T cells preferentially express TCR V regions in distinct tissues.8 It is known that in asthma CD4+ T cells participate in the inflammatory process through the release of cytokines. Because γδ T cells can produce Th2 cytokines, they too may participate in the onset of pulmonary allergic reactions. Zuany-Amorin et al.9 showed that γδ T cell–deficient mice exhibited low antigen-specific IgE and interleukin (IL)-5 release and a decrease in T cell infiltration compared with wild-type (WT) mice. This response was restored when IL-4 was administered, suggesting that γδ T cells were necessary for secretion of IL-4 and Th2-mediated inflammation and for allergic airway inflammation. We hypothesized that γδ T cells play a similar role in allergic conjunctivitis. Studies in this report address the role γδ T cells play in allergic conjunctivitis.
Methods
Animals
C57BL/6 (H-2b) mice and TCR-δ−/− mice were purchased from the Jackson Laboratories (Bar Harbor, ME). All mice were used at 6 to 8 weeks of age. Animals were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology statement about the Use of Animals in Ophthalmic and Vision Research.
Induction of Allergic Conjunctivitis by Active Immunization
Allergic conjunctivitis was induced as previously described.10 Mice were immunized intraperitoneally (IP) with 50 μg of short ragweed pollen (International Biologicals, Piedmont, OK) in 5 mg of alum (Thermo Fisher Scientific Pierce, Rockford, IL) on day 0. Allergic conjunctivitis was induced by a multi-hit topical challenge in which immunized mice were given 1.5 mg of short ragweed (SRW) pollen in 10 μL PBS in the right eye from days 10 to 16. Mice were examined clinically for signs of immediate hypersensitivity responses 20 minutes after each topical challenge with SRW pollen or PBS. Each parameter (lid edema, tearing, conjunctival vasodilatation, and conjunctival edema) was scored on a scale ranging from 0 to 3.10 A score of 0 indicated that there was no evidence of the respective parameter; 1+ = mild response distinctly greater than the naive control; 2+ = moderate change in respective parameter that could be noted by biomicroscopy, but not with the naked eye; and 3+ = severe response that could be perceived with the naked eye.
In Vivo Antibody Treatment
GL3 antibody was produced from hybridoma cells and was provided by Leo Lefrancois (University of Connecticut, Farmington, CT). The GL3 antibody does not deplete γδ T cells but inhibits their function by blocking the TCR δ-chain.11 Mice were treated with IP injections of hamster anti-mouse GL3 mAb (400 μg/injection) or hamster IgG (Accurate Chemicals & Scientific Corp., Westbury, NY) given two times a week beginning 5 days before immunization with SRW pollen to block γδ T cell activity. This protocol has been used to disable γδ T cell activity.11–13 To block NKT cell function, mice were treated with intravenous (IV) injections of rat anti-mouse CD1d mAb (hybridoma HB323; American Type Culture Collection, Manassas, VA) or rat-IgG (Sigma-Aldrich, St. Louis, MO) isotype control three times a week (50 μg/injection) beginning 7 days before immunization.
Cytokine ELISA
Mice were killed 17 days post-challenge and their spleens removed. Single-cell suspensions of splenocytes were prepared by gently processing between the ends of two sterile frosted slides. Spleen cells (1 × 107 cells/mL) were incubated with 25 μg/mL of soluble SRW pollen extract (Greer Laboratories, Lenoir, NC) for 48 hours in 2 mL of RPMI supplemented with 10% FCS, 2 mM l-glutamine (Cambrex, Charles City, IA), 1 mM sodium pyruvate (Cambrex), 1% penicillin-streptomycin-Fungizone (Cambrex), 1% nonessential amino acids (Cambrex), 1% HEPES buffer (Cambrex), and 5 × 10−5 M 2-mercaptoethanol (2-ME) (Sigma-Aldrich). Six hours before harvest, 1 μg/mL ionomycin (Sigma-Aldrich) and 25 ng/mL phorbol 12-myristate 13-acetate (Sigma-Aldrich) were added to stimulate cytokine release. ELISAs for IL-4, -5, -13, and IFN-γ were performed on culture supernatants according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Induction of Allergic Conjunctivitis by Adoptive Transfer of SRW-Primed CD4+ T Cells
Allergic conjunctivitis was produced by adoptive transfer of sensitized spleen cells as previously described.14 Spleens were removed from Jα18−/− or WT C57BL/6 mice 17 days after sensitization with SRW pollen. CD4+ T cells were isolated using a magnetic microbead system (Miltenyi Biotec, Auburn, CA). Single-cell suspensions of splenocytes were prepared by gently processing between the ends of two sterile frosted slides. Splenocytes were incubated with anti-mouse CD4-coated magnetic beads in bead buffer (0.5% BSA in PBS, pH 7.2) for 15 minutes at 4°C and loaded into separation columns. Unconjugated beads were washed off with bead buffer. CD4+ cells attached to magnetic beads were retained in the column and eluted with bead buffer. Cells not attached to magnetic beads passed through the column and were discarded. One spleen equivalent of enriched CD4+ T cell suspension (6 × 106–12 × 106 cells/recipient) was injected IV into each naive C57BL/6 mouse. Four days later mice were challenged topically with SRW pollen as previously described.
Histology
Eyes from mice were removed day 17 post-challenge and fixed in 10% formalin for histology. Paraffin-embedded tissue sections were stained with Geimsa. The number of each category of inflammatory cell was determined by counting the total number of eosinophils, neutrophils, and mononuclear cells in the forniceal conjunctiva. Inflammatory cells were counted in a masked fashion by two investigators and recorded as eosinophils, neutronphils, or mononuclear cells.
Statistics
All results were represented as the mean ± SD. Comparison between the WT immunized and knockout immunized mice were made using Student's t-test. Significance of the histologic data was tested by Student's t-test. P values < 0.05 were considered significant.
Results
γδT Cells Are Needed for the Full Expression of the Early and Late Phases of Allergic Conjunctivitis
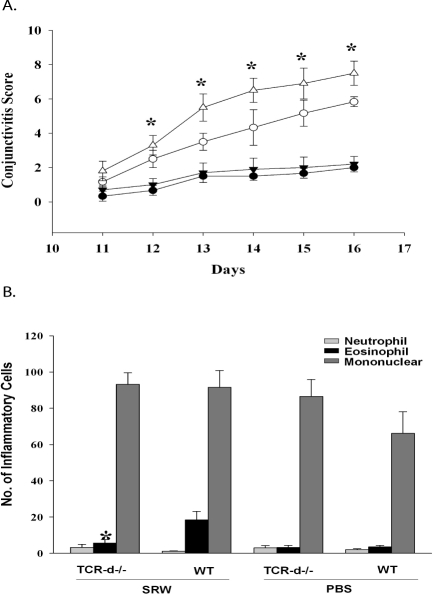
To determine whether γδT cells play a role in the full expression of allergic conjunctivitis, we induced allergic conjunctivitis in TCR-δ−/− and WT C57BL/6 mice by IP sensitization with SRW pollen followed by topical administration of SRW pollen to the right eye for 7 consecutive days. The clinical phenotype of allergic conjunctivitis was evaluated 20 minutes after each daily topical challenge with SRW pollen and by scoring tear production, lid edema, and conjunctival vasodilatation.10 Control mice for each group were challenged with PBS. One of the features of this “multi-hit” model of allergic conjunctivitis is the accumulative effect of repeated topical challenge with SRW pollen. The lid edema that developed in response to the topical challenge with SRW pollen persisted until the next day and gained in intensity with each subsequent topical challenge and accounted for the steady increase in the clinical scores that occurred during the 7-day period of topical challenge (Fig. 1A). The results of the first experiment demonstrate that TCR-δ−/− mice had a reduction in the clinical features of allergic conjunctivitis when compared with WT C57BL/6 mice (Fig. 1A). The late phase of allergic conjunctivitis is characterized by the infiltration of inflammatory cells, primarily eosinophils. We therefore assessed the inflammatory cells present in the conjunctiva of WT and TCR-δ−/− mice by histologic analysis. SRW pollen-sensitized TCR-δ−/− mice had less eosinophillic infiltration in the conjunctiva compared with WT mice, indicating that full expression of the late phase of allergic conjunctivitis requires the participation of γδ T cells (Fig. 1B).
Figure 1.
γδ T cells are needed for the early and late phase of allergic conjunctivitis. (A) Clinical scores for allergic conjunctivitis in TCR-δ−/− mice (○) and WT C57BL/6 mice (▵) that were sensitized and challenged topically with SRW pollen. TCR-δ−/− mice (●) and WT C57BL/6 mice (▾) were challenged topically with PBS and served as negative controls. (B) Eosinophillic infiltration into the conjunctivae of TCR-δ−/− mice and WT C57BL/6 mice (n = 10). Graph is representative of two independent experiments. *P < 0.05.
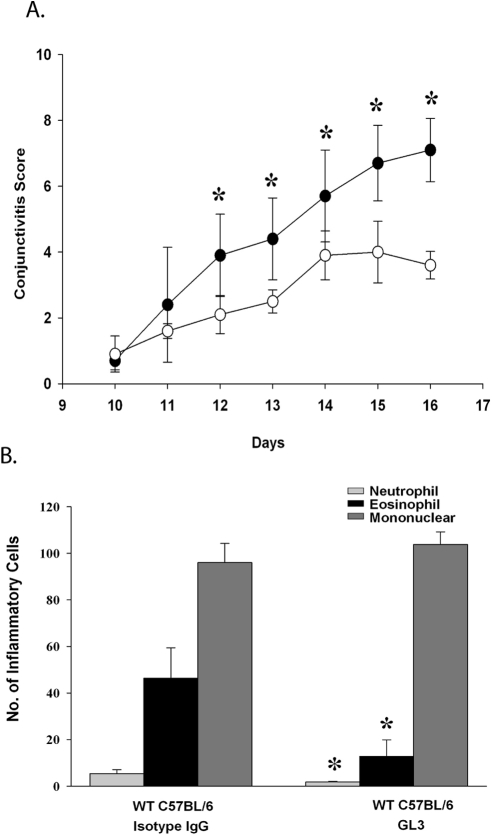
To confirm the role of γδ T cells in allergic conjunctivitis, WT mice were treated with GL3 antibody, which inhibits the function of γδ T cells by blocking the TCRδ chain.12 GL3 or hamster IgG isotype control antibody was administered twice a week beginning 5 days before immunization. WT mice treated with GL3 antibody had a reduction in the clinical manifestations of allergic conjunctivitis as well as in the eosinophillic infiltration into the conjunctiva (Fig. 2). Together, these results support the results from TCR-δ−/− mice and indicate that γδ T cells are needed for the full expression of both the clinical manifestations and the late-phase inflammation of allergic conjunctivitis.
Figure 2.
Inhibiting the function of γδ T cells causes a reduction in allergic conjunctivitis. (A) Clinical scores for allergic conjunctivitis in WT mice treated with GL3 antibody (○) or hamster IgG antibody (●) that were sensitized and challenged topically with SRW pollen. (B) Eosinophillic infiltration into the conjunctivae of WT mice treated with GL3 antibody or hamster IgG antibody.
Effect of Disabling NKT Cells in TCR-δ−/− Mice on Allergic Conjunctivitis
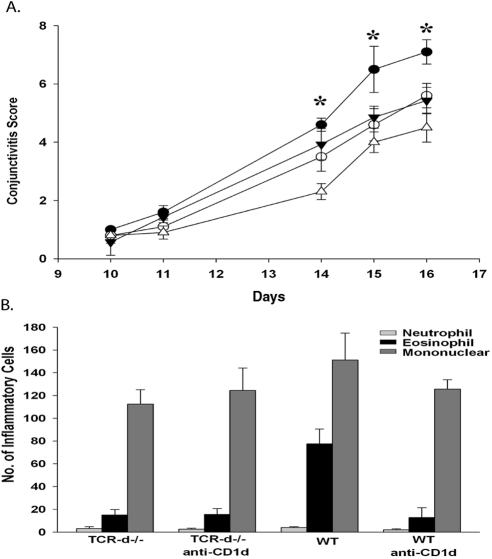
We recently showed that another innate T cell, the natural killer T (NKT) cell, is needed for maximal expression of allergic conjunctivitis.14 Therefore, we wanted to determine what effect disabling NKT cells in γδ T cell–deficient mice would have on the clinical features and the late-phase inflammation in allergic conjunctivitis. Accordingly, we treated TCR-δ−/− mice with blocking anti-CD1d antibody three times a week beginning 7 days before immunization with SRW pollen.
As previously shown, WT mice treated with anti-CD1d had a reduction in the clinical manifestations of the disease when compared with WT mice treated with isotype control antibody. We found that TCR-δ−/− mice treated with anti-CD1d had a further reduction in the clinical features of allergic conjunctivitis compared with TCR-δ−/− mice treated with isotype control antibody (Fig. 3A). This suggests that NKT cells and γδ T cells cooperate to promote full induction of allergic conjunctivitis. When assessing the late phase of allergic conjunctivitis, we found that eosinophillic infiltration into the conjunctiva of anti-CD1d–treated TCR-δ−/− mice was comparable with TCR-δ−/− mice (Fig. 3B). These results indicate that disabling NKT cells in γδ T cell–deficient mice blunts the clinical manifestations of allergic conjunctivitis but does not have an additive effect on the late-phase inflammatory responses.
Figure 3.
Blocking activation of NKT cells in γδ T cell-deficient mice further reduces the allergic conjunctivitis. (A) Clinical scores of allergic conjunctivitis in TCR-δ−/− mice treated with anti-CD1d antibody (▵) or hamster IgG antibody (▾) as well at WT C57BL/6 mice treated with anti-CD1d (○) or hamster IgG antibody (●). (B) Eosinophillic infiltration into the conjunctivae (n = 10). Graph is representative of two independent experiments. *P < 0.05.
Production of Th2 Cytokines in γδ T Cell–Deficient Mice
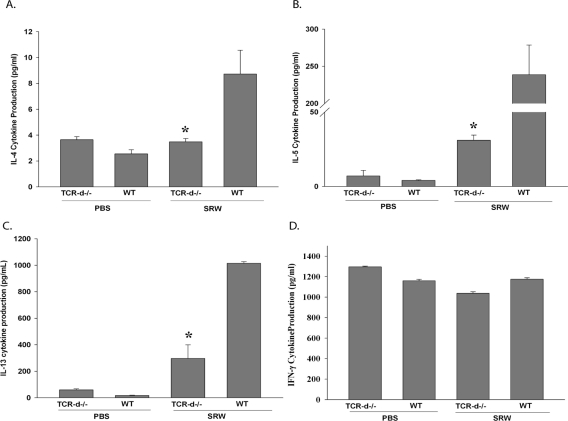
The production of Th2 cytokines (IL-4, -5, and -13) plays a critical role in the induction of allergic conjunctivitis.15,16 We hypothesized that the reduction in the clinical manifestations and the late-phase inflammation of allergic conjunctivitis was due to a decrease in cytokine production by SRW-specific Th2 cells. To assess this, splenocytes from SRW pollen-sensitized TCR-δ−/− and WT C57BL/6 mice were stimulated in vitro with SRW extract for 48 hours, and the production of Th2 cytokines was quantified by ELISA.
SRW-stimulated spleen cells from TCR-δ−/− mice displayed decreased production of the Th2 cytokines IL-4, -5, and -13 compared with WT mice, while the production of the Th1 cytokine, IFN-γ, was comparable with WT levels (Fig. 4). This suggests that the presence of γδ T cells is necessary for the generation of allergen-specific Th2 cells and the optimal expression of allergic conjunctivitis.
Figure 4.
Decreased severity of allergic conjunctivitis in γδ T cell-deficient mice is associated with diminished production of IL-4, -5, and -13. Bulk splenocytes were incubated with APCs pulsed with SRW extract. Supernatants were collected 48 hr after in vitro culture and analyzed by ELISA (n = 10). Graph is representative of two independent experiments. *P < 0.05.
Role of γδ T Cells in the Afferent and Efferent Arms of Allergic Conjunctivitis
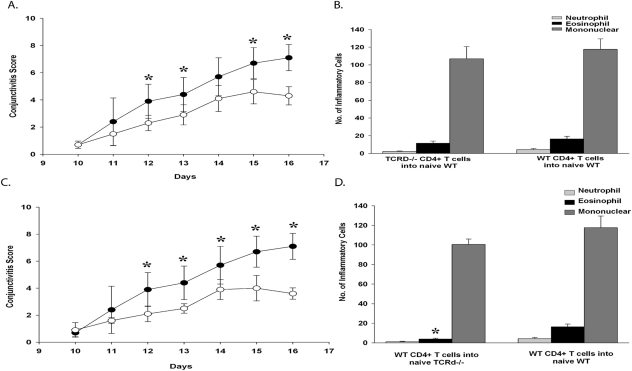
The adaptive immune response consists of two fundamental phases: the initiating or afferent phase and the effector or efferent phase. The afferent phase involves antigen presenting cells (APCs) capturing antigen and traveling to regional lymph nodes, where they present antigen to T cells, which become activated, undergo clonal expansion, and differentiate into the various categories of T helper cells.17 The antigen-specific T cells function as effector cells in the efferent phase of the immune response and, in the case of allergic conjunctivitis, enhance IgE antibody production, secrete cytokines, and participate in inflammation.18 The present results indicate that γδ T cells are needed for activating the afferent phase of the Th2 immune response as shown by the diminished production of Th2 cytokines that occurred in the TCR-δ−/− mice. We hypothesized that γδ T cells were needed for the generation of SRW pollen-specific Th2 cells in the afferent phase of allergic conjunctivitis. Accordingly, we adoptively transferred CD4+ T cells from SRW pollen-sensitized TCR-δ−/− or WT donors into naive WT C57BL/6 recipients. Four days after the adoptive transfer, both groups of mice were challenged topically with daily applications of SRW pollen for 7 consecutive days. The clinical manifestation and the number of inflammatory cells present in the conjunctiva (late-phase reaction of allergic conjunctivitis) were assessed clinically and by histopathology, respectively.
WT naive recipients of CD4+ T cells from TCR-δ−/− SRW pollen-sensitized donors displayed decreased clinical signs of allergic conjunctivitis compared with WT naive recipients of CD4+ T cells from SRW pollen-sensitized WT donors. The reduced severity in the clinical responses in allergic conjunctivitis was consistent with the previously observed diminished production of Th2 cytokines by CD4+ T cells from the TCR-δ−/− mice (Figs. 5A, 5B). By contrast, WT recipients of CD4+ T cells from TCR-δ−/− donors had eosinophillic cell infiltration into the conjunctiva that was similar to that found in WT recipients of CD4+ T cells from WT donors, suggesting that the host's γδ T cell repertoire (presumably in the conjunctiva) can support the expression of the late inflammatory phase of allergic conjunctivitis that is mediated by adoptively transferred Th2 cells. Together, these results suggest that γδ T cells are needed for the maximal generation of Th2 cells and for the expression of the allergic conjunctivitis early-phase reaction.
Figure 5.
γδ T cells participate in the afferent and efferent arms of allergic conjunctivitis. CD4+ T cells isolated from SRW-sensitized donors were adoptively transferred into naive recipients. (A) Clinical scores of allergic conjunctivitis in naive WT recipients that received CD4+ T cells from either WT donors (●) or TCR-δ−/− donors (○) or that were sensitized with SRW pollen. (B) Eosinophillic infiltration into the conjunctivae of WT recipients of CD4+ T cells from SRW-sensitized TCR-δ−/− or WT donors. (C) Clinical scores of allergic conjunctivitis in naive WT (●) or TCR-δ−/− (○) recipients of CD4+ T cells from WT donors that were sensitized with SRW pollen. (D) Eosinophillic infiltration into the conjunctivae of TCR-δ−/− and WT C57BL/6 mice that received CD4+ T cells from WT C57BL/6 mice that were sensitized with SRW pollen (n = 10). Graph is representative of two independent replicate experiments. *P < 0.05.
The capacity of Th2 cells from TCR-δ−/− donor mice to produce late-phase inflammation in hosts with an intact γδ T cell repertoire suggested that the previously observed diminution of allergic conjunctivitis in TCR-δ−/− mice sensitized with SRW pollen was due to impaired expression of Th2-based inflammation in the afferent arm of the immune response. However, it was also important to determine whether γδ T cells affected the late phase of allergic conjunctivitis. This hypothesis was tested by adoptively transferring CD4+ T cells from SRW pollen-sensitized WT donors into TCR-δ−/− recipients and assessing both the clinical manifestations and late-phase inflammation in allergic conjunctivitis. The results of this adoptive cell transfer experiment demonstrated that the absence of an intact γδ T cell repertoire prevented full expression of both the clinical expression and late-phase inflammatory reactions of allergic conjunctivitis by the SRW pollen-sensitized CD4+ T cells from WT mice (Figs. 5C, 5D). These results suggest that γδ T cells also function at the end-stage organ (i.e., conjunctiva).
Discussion
Allergic conjunctivitis is an immediate hypersensitivity reaction mediated by a Th2 immune response. In the early-phase response, the allergen cross-links IgE antibodies bound via FcεRI on the surface of mast cells.6 This event occurs within minutes of allergen exposure and triggers the degranulation of mast cells and the subsequent release of mediators such as histamine. The late-phase reaction in allergic conjunctivitis is evident 6 to 12 hours after allergen challenge and is characterized by an inflammatory infiltrate consisting of neutrophils and a preponderance of eosinophils.6 Although antigen-specific Th2 cells are considered key in the pathogenesis of allergic diseases,17 recent findings indicate that innate T cells such as γδ T cells also have an important role in allergic diseases.9,19
In allergic asthma, γδ T cells are necessary for the development of AHR. Mice lacking γδ T cells have a decrease in antigen-specific IgE and IgG1, pulmonary IL-5, as well as in eosinophil and T cell infiltration compared with WT mice.9 All these responses can be restored by the administration of IL-4 to γδ T cell–deficient mice.9 It has also been shown that γδ T cells affect the IL-10 levels in the airways, and blocking their function results in an increase in CD4+CD25+ cells in the lung, which suggests that γδ T cells might inhibit regulatory T cell function.20 Since the conjunctiva, like the lung, is a mucosal tissue, we hypothesized that γδ T cells play a similar role in allergic conjunctivitis as they do in allergic asthma.
To assess if γδ T cells were needed for the full expression of allergic conjunctivitis, we used γδ T cell–deficient mice as well as mice treated with GL3 antibody, which inhibits γδ T cell function. TCR-δ−/− mice had decreased clinical signs of allergic conjunctivitis and reduced eosinophillic infiltration into the conjunctiva compared with WT mice. We also found that WT mice treated with GL3 antibody had a reduction in the early and late phases of allergic conjunctivitis. These results confirmed that γδ T cells were needed for the full expression of the early and late phases of allergic conjunctivitis.
Since it has been shown that another innate T cell, the NKT cell, also plays a role in the development of allergic conjunctivitis,15 we wanted to determine whether blocking NKT cell activation in TCR-δ−/− mice would have an additive effect in the suppression of allergic conjunctivitis. These studies demonstrate that the absence of both NKT and γδ innate T cells causes a cumulative reduction in the clinical features of allergic conjunctivitis but does not cause further reduction in the late-phase inflammation in allergic conjunctivitis reaction. The additive effect of NKT cells and γδ T cells has been noted in other allergic diseases. Using an adoptive cell transfer approach, Jin et al.21 found that NKT and γδ T cells were necessary for the acute stages of AHR, but not for the later airway eosinophillic inflammation. Another study on AHR demonstrated that NKT cells secreted IL-4 and -13 to produce their effector function, but γδ T cells did not have this effect.22 These data suggest that collaboration between innate T cells may be important in allergic diseases of mucosal surfaces.
The present findings indicate that the decrease in allergic conjunctivitis in mice deficient in innate T cells correlates with a decrease in the production of Th2 (IL-4, -5, and -13) but not Th1 (IFN-γ) cytokines and suggest that γδ T cells are needed for the optimal generation of Th2 cells. Results from experiments involving the adoptive transfer of SRW-primed CD4+ T cells from TCR-δ−/− mice into WT mice demonstrated reduced allergic conjunctivitis in WT hosts, providing further support that the optimal development of inflammatory Th2 cells also required an intact γδ T cell repertoire. We also hypothesized that γδ T cells may also be needed for the expression of Th2-based inflammation at the end-stage organ (i.e., the conjunctiva). To address this, we adoptively transferred CD4+ T cells from WT mice into TCR-δ−/− mice and found that these mice also had a reduction in the expression of allergic conjunctivitis.
In conclusion, this study demonstrates that γδ T cells are needed for development of allergic conjunctivitis. We suggest that γδ T cells are needed for the optimal generation of Th2 cells and the inflammatory responses of Th2 cells that are needed for the maximal expression of allergic conjunctivitis. Further studies need to be performed to identify the underlying molecular mechanisms that γδ T cells employ for the development of allergic conjunctivitis and determine whether disabling γδ T cells at the ocular surface is a viable therapeutic option for the treatment of allergic conjunctivitis.
Footnotes
Supported by National Institute of Health Grants EY0007641 and EY020799, and an unrestricted grant from Research to Prevent Blindness.
Disclosure: N.J. Reyes, None; E. Mayhew, None; P.W. Chen, None; J.Y. Niederkorn, None
References
- 1. Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153(suppl 1):17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stern ME, Siemasko K, Gao J, et al. Role of interferon-gamma in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2005;46:3239–3246 [DOI] [PubMed] [Google Scholar]
- 3. Foster CS. The pathophysiology of ocular allergy: current thinking. Allergy. 1995;50:6–9, discussion 34–38 [DOI] [PubMed] [Google Scholar]
- 4. Ehlers WH, Donshik PC. Allergic ocular disorders: a spectrum of diseases. CLAO J. 1992;18:117–124 [PubMed] [Google Scholar]
- 5. Liu G, Keane-Myers A, Miyazaki D, Tai A, Ono SJ. Molecular and cellular aspects of allergic conjunctivitis. Chem Immunol. 1999;73:39–58 [DOI] [PubMed] [Google Scholar]
- 6. Niederkorn JY. Immune regulatory mechanisms in allergic conjunctivitis: insights from mouse models. Curr Opin Allergy Clin Immunol. 2008;8:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galatowicz G, Ajayi Y, Stern ME, Calder VL. Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. Clin Exp Allergy. 2007;37:1648–1656 [DOI] [PubMed] [Google Scholar]
- 8. Scotet E, Nedellec S, Devilder MC, Allain S, Bonneville M. Bridging innate and adaptive immunity through gammadelta T-dendritic cell crosstalk. Front Biosci. 2008;13:6872–6885 [DOI] [PubMed] [Google Scholar]
- 9. Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280:1265–1267 [DOI] [PubMed] [Google Scholar]
- 10. Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin Immunol Immunopathol. 1998;87:75–84 [DOI] [PubMed] [Google Scholar]
- 11. Ke Y, Pearce K, Lake JP, Ziegler HK, Kapp JA. Gamma delta T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–3618 [PubMed] [Google Scholar]
- 12. Ashour HM, Niederkorn JY. Gammadelta T cells promote anterior chamber-associated immune deviation and immune privilege through their production of IL-10. J Immunol. 2006;177:8331–8337 [DOI] [PubMed] [Google Scholar]
- 13. Skelsey ME, Mellon J, Niederkorn JY. Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–4333 [DOI] [PubMed] [Google Scholar]
- 14. Reyes NJ, Mayhew E, Chen PW, Niederkorn JY. NKT cells are necessary for maximal expression of allergic conjunctivitis. Int Immunol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukushima A, Ozaki A, Jian Z, et al. Dissection of antigen-specific humoral and cellular immune responses for the development of experimental immune-mediated blepharoconjunctivitis in C57BL/6 mice. Curr Eye Res. 2005;30:241–248 [DOI] [PubMed] [Google Scholar]
- 16. Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281 [DOI] [PubMed] [Google Scholar]
- 17. Ozaki A, Ishida W, Fukata K, Fukushima A, Ueno H. Phenotypic changes and inflammatory cell distribution in the cornea during development of experimental immune-mediated blepharoconjunctivitis. Jpn J Ophthalmol. 2004;48:333–339 [DOI] [PubMed] [Google Scholar]
- 18. Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ, Larkin DF. Effect of allergic conjunctival inflammation on the allogeneic response to donor cornea. Invest Ophthalmol Vis Sci. 2007;48:4044–4049 [DOI] [PubMed] [Google Scholar]
- 19. Yokozeki H, Watanabe K, Igawa K, Miyazaki Y, Katayama I, Nishioka K. Gammadelta T cells assist alphabeta T cells in the adoptive transfer of contact hypersensitivity to para-phenylenediamine. Clin Exp Immunol. 2001;125:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hahn YS, Ji XY, Woo SI, et al. Vgamma1+ gammadelta T cells reduce IL-10-producing CD4+CD25+ T cells in the lung of ovalbumin-sensitized and challenged mice. Immunol Lett. 2008;121:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jin N, Miyahara N, Roark CL, et al. Airway hyperresponsiveness through synergy of gammadelta T cells and NKT cells. J Immunol. 2007;179:2961–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin N, Roark CL, Miyahara N, et al. Allergic airway hyperresponsiveness-enhancing gammadelta T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells. J Immunol. 2009;182:2002–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]