Abstract
Adult stem cells exist in most mammalian organs and tissues and are indispensable for normal tissue homeostasis and repair. In most tissues, there is an age-related decline in stem cell functionality but not a depletion of stem cells. Such functional changes reflect deleterious effects of age on the genome, epigenome, and proteome, some of which arise cell autonomously and others of which are imposed by an age-related change in the local milieu or systemic environment. Notably, some of the changes, particularly epigenomic and proteomic, are potentially reversible, and both environmental and genetic interventions can result in the rejuvenation of aged stem cells. Such findings have profound implications for the stem cell–based therapy of age-related diseases.
Introduction
Stem cells reside in most adult mammalian tissues where they maintain normal tissue homeostasis and participate in tissue repair and regeneration in response to damage (Weissman, 2000; Li and Clevers, 2010). In general, stem cells represent a diminishingly small proportion of the cells within any tissue, rendering them difficult to identify and even more difficult to study. In the past few years, much effort has been focused on identifying molecular markers that would allow the isolation of different types of tissue-specific stem cells (Relaix et al., 2005; Barker et al., 2007; Yan and Owens, 2008). The development of specific methods to isolate functional stem cells is important not only to study the molecular mechanisms that underlie such important stem cell characteristics as multipotentiality and the ability to self-renew but also for the establishment of stem cell–based therapeutics.
The isolation of stem cells away from other local and systemic influences is essential for characterizing and measuring their intrinsic properties and functionality. However, in vivo labeling and tracing of stem cell lineages are equally important and particularly useful in delineating the influences of environmental factors on stem cell function, such as the switch between quiescence and activation or the determination of the fate of differentiating daughter cells. Environmental cues are transmitted to stem cells by their niches, which are composed of the extracellular matrix, cells in direct contact with stem cells, and soluble factors that are secreted or concentrated locally (Schofield, 1978; Voog and Jones, 2010). The niche is profoundly influenced by the systemic milieu and dynamically changing to regulate stem cell function, a feature that is especially relevant with regard to the process of aging (Gopinath and Rando, 2008).
Aging is accompanied by a decline in the homeostatic and regenerative capacity of all tissues and organs (Kirkwood, 2005; Rando, 2006). With age, wound healing is slower in the skin, hair turns gray or is lost, skeletal muscle mass and strength decrease, the ratio of cellular constituents in the blood is skewed, and there is a decline in neurogenesis (Sharpless and DePinho, 2007). As the homeostatic and regenerative activities of these tissues are attributable to the resident stem cells, these age-related changes are reflections of declines in stem cell function (Bell and Van Zant, 2004; Dorshkind et al., 2009; Jones and Rando, 2011). Clearly, in terms of organismal aging, the focus on stem cells is most relevant for those tissues in which normal cellular turnover is very high, such as epithelia of the skin and gut, as opposed to tissues, such as the cerebral cortex and the heart, in which cellular turnover in adults is exceedingly low (Rando, 2006). There is also an increasing interest in the therapeutic potential of stem cells to treat age-related degenerative diseases or conditions, further highlighting the importance of understanding the relationship between stem cell function and the properties of aged tissues. Within this context, it is essential to understand how the local environment influences stem cells, how aging affects stem cell number and function, and the extent to which aspects of stem cell aging may be reversible. This review focuses on manifestations and underlying mechanisms of age-related changes in stem cells and stem cell functionality.
Aging of somatic stem cells
Adult stem cells are exposed to many of the same factors that lead to age-related changes in their replicative or postmitotic progeny, but stem cells must resist those changes as a self-renewing population to assure proper function and normal tissue homeostasis across the lifespan (Rando, 2006; Sharpless and DePinho, 2007; Jones and Rando, 2011). As a replicative population that may have prolonged periods of quiescence (Fig. 1), stem cells must possess defense and repair mechanisms that are relevant to both highly proliferative cells and to long-lived postmitotic cells (Rando, 2006).
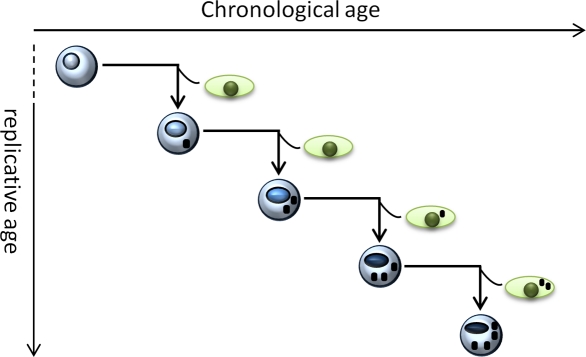
Figure 1.
The chronological and replicative lifespan of stem cells. During prolonged periods of quiescence and by the process of self-renewal to establish a cellular continuum, stem cells experience chronological aging caused by the accumulation of damaged or aberrant intracellular molecules. During the process of asymmetric cell division and self-renewal, stem cells also experience replicative aging, which is particularly important in tissues with high turnover rates.
In long-lived animals, adult stem cells, particularly those in continuously renewing tissues, undergo many rounds of cell division to maintain normal tissue homeostasis (Fuchs et al., 2001; van der Flier and Clevers, 2009). During each round of DNA replication, processes that underlie replicative aging, including telomere shortening, chromosome rearrangements, and single base mutations (Ben-Porath and Weinberg, 2005), can occur and ultimately lead to cellular senescence (Hayflick, 1965; Campisi and d’Adda di Fagagna, 2007). Experimental manipulations, such as serial transplantation, clearly reveal that adult stem cells have a finite replicative lifespan that can be exhausted (Siminovitch et al., 1964; Waterstrat and Van Zant, 2009). However, as serial transplantation experiments subject stem cells to excessive rounds of cell division, it remains to be determined whether replicative aging alone is sufficient to contribute to the decline of stem cell function in long-lived mammals during normal aging.
Adult stem cells are also susceptible to the kinds of age-related changes, namely chronological aging, that occur in nondividing cells, such as neurons and cardiomyocytes (Busuttil et al., 2007). These changes include the accumulation of damaged macromolecules, such as proteins, lipids, and nucleic acids, some of which may, in fact, aggregate and form stable, long-lived complexes that are toxic to the cell (Rajawat et al., 2009; Koga et al., 2011). Adult stem cells exhibit prolonged periods of quiescence in most mammalian tissues (Li and Clevers, 2010). Damaged macromolecules can accumulate in stem cells during this time, just as in long-lived postmitotic cells. Specific macromolecules or macromolecular aggregates may even be selectively retained in stem cells as they undergo the process of self-renewal by asymmetric cell division (Conboy et al., 2007; Knoblich, 2008). In this sense, the self-renewing progeny represent a kind of cellular continuum and only add to the risk that adult stem cells may suffer from the effects of chronological aging.
Functional manifestations of stem cell aging
Aging in stem cells causes changes in the fate or functionality of stem cell progeny. In some cases, such as neural stem cells (NSCs) and melanocyte stem cells (Maslov et al., 2004; Inomata et al., 2009), these changes may lead to a depletion of the stem cell pool (Fig. 2; Kuhn et al., 1996; Maslov et al., 2004). However, in most stem cell compartments, the number of stem cells does not decline significantly with age (Booth and Potten, 2000; Brack and Rando, 2007; Giangreco et al., 2008); rather, these stem cells experience a change in cell fate with age.
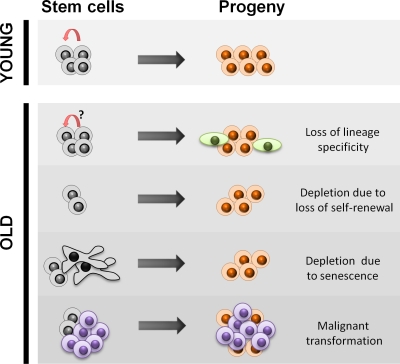
Figure 2.
Decline in stem cell function with age. In young animals, stem cells divide asymmetrically to self-renew and give rise to lineage-specific differentiated progeny during tissue homeostasis or regeneration. With age, some stem cells lose their lineage specificity and give rise to nonfunctional progeny, resulting in loss of tissue integrity and decline of physiological function, even though the number of stem cells remains unaffected. Some stem cells lose the capacity for self-renewal, resulting in symmetric cell divisions giving rise to two differentiated daughters and a gradual depletion of the stem cell pool. The senescence of stem cells can also contribute to a loss of functional stem cells. The increase in malignancies with age, particularly in epithelia with high turnover rates, has been proposed to arise from within the stem cell compartment or from early progenitors.
Age-related changes in the fate of stem cell progeny.
Adult stem cells may be unipotent, bipotent, or multipotent, giving rise to a restricted diversity of progeny based on the tissue in which they reside (Weissman, 2000; Rando, 2006). The ability of stem cells to produce an appropriate repertoire of tissue-specific progeny is crucial for functional tissue homeostasis and regeneration. The extent to which adult stem cells and their progeny are committed to a particular lineage is determined largely by the epigenome, influencing which genes will be expressed and which will be repressed and ultimately shaping the phenotypic characteristics of the cells (Bernstein et al., 2006; Mikkelsen et al., 2007; Hemberger et al., 2009). The execution of the epigenomic program that influences the fate of stem cell progeny is modulated by environmental factors and mediated by signaling pathways that have important roles in organogenesis during development, including the Wnt, Notch, and Hedgehog pathways (Berger, 2007; Brack et al., 2008; Rittié et al., 2009; van der Flier and Clevers, 2009). With age, untimely activation of these pathways as a result of signals from the “old environment” may lead to aberrant lineage specification of stem cell progeny as has been demonstrated in tissues, such as skeletal muscle, tendon, and the hematopoietic system (Sudo et al., 2000; Taylor-Jones et al., 2002; Brack et al., 2007; Zhou et al., 2010). Accumulation of these abnormal progeny contributes to the gradual deterioration of tissue structure and function associated with aging.
Despite the extremely low turnover rate of cells within the tissue under normal homeostatic conditions, skeletal muscle possesses remarkable regenerative ability upon injury, a process that is mediated by the resident muscle stem cells (satellite cells; Mauro, 1961; Zammit et al., 2006; Le Grand and Rudnicki, 2007). However, satellite cells isolated from aged rodents have a higher propensity to undergo adipogenic or fibrogenic differentiation that may be partially caused by elevated levels of Wnt in the muscle compartment (Taylor-Jones et al., 2002; Brack et al., 2007). As a result, aged animals require a longer time to recover from muscle injury, the regenerated myofibers are smaller in diameter, and there is an increase in tissue fibrosis (Conboy et al., 2005; Brack et al., 2007). Strikingly, this age-related disruption of lineage fidelity can be restored by a youthful systemic milieu generated by parabiotic pairing between old and young mice (heterochronic parabiosis; Conboy et al., 2005; Brack et al., 2007). As such, these age-related changes in satellite cells appear to be driven by an “aged environment” rather than intrinsic changes within the cells. An elevated level of Wnt pathway activators in the circulation may be part of such an aged environment, as inhibition of the Wnt pathway restores the lineage specificity of satellite cells (Brack et al., 2007). Furthermore, the cytokine TGF-β has been found to be expressed at an elevated level in the serum of aged human and mice (Carlson et al., 2009) and may cross talk with the Wnt pathway to influence the satellite cell progeny.
Within the hematopoietic system, the ratios of differentiated progeny change with age. Hematopoietic stem cells (HSCs) from both old humans and old mice show an increased propensity to differentiate along the myeloid rather than the lymphoid lineage (Sudo et al., 2000; Rossi et al., 2005). Such lineage bias is not caused by a change in the differentiation potential of individual HSCs but rather by a preferential selection of distinct subsets of HSCs over time (Cho et al., 2008; Beerman et al., 2010; Challen et al., 2010). The differential responsiveness of these two HSC populations to TGF-β may further enhance the skewed ratio between myeloid versus lymphoid progeny in old individuals (Challen et al., 2010). Although the progeny of the aged HSCs do not include any cells that are not otherwise part of the normal repertoire of cells produced by HSCs, this lineage skewing results in a decreased number of memory B cells and naive T cells (Linton and Dorshkind, 2004; Min et al., 2004) and adversely affects immunological responses (Rink et al., 1998; Grubeck-Loebenstein et al., 2009).
Depletion of stem cell pools.
Certain types of age-related dysfunction of stem cells, such as loss of the ability to self-renew and the activation of senescence pathways, can lead to the depletion of the stem cell pool. Within specific stem cell compartments, such as melanocyte stem cells in the skin and NSCs in the brain, an age-related decline in stem cell number appears to be responsible for specific aging phenotypes in those tissues (Kuhn et al., 1996; Maslov et al., 2004; Nishimura et al., 2005; Inomata et al., 2009; Renault et al., 2009). The mammalian skin contains at least three types of stem cells at the bulge area in hair follicles that orchestrate skin homeostasis: epidermal stem cells that give rise to keratinocytes, hair follicle stem cells that are responsible for hair growth, and melanocyte stem cells that pigment new hair (Blanpain and Fuchs, 2009). Depletion of melanocyte stem cells in the hair follicles and appearance of mature pigmented melanocytes in the stem cell niche have been reported in both aged mice and humans (Nishimura et al., 2005; Inomata et al., 2009), leading to one of the most visible phenotypic changes during aging, hair graying. Aging or genotoxic stress induces the accumulation of DNA damage in melanocyte stem cells that results in the loss of stem cell self-renewal (Inomata et al., 2009). Both daughter cells of a melanocyte stem cell with high levels of DNA damage tend to differentiate into pigment-producing melanocytes, resulting in the gradual depletion of the stem cell pool. Although the loss of melanocyte stem cell self-renewal occurs independently of checkpoint proteins p53, p16INK4a, and p19ARF, defective DNA repair sensitizes melanocyte stem cells to premature differentiation under a much lower level of genotoxic damage (Inomata et al., 2009), highlighting the importance of genome maintenance for stem cell longevity.
Depletion of NSCs, possibly also related to a specific loss of capacity for self-renewal, appears to be responsible for declining neurogenesis with age (Molofsky et al., 2006; Nishino et al., 2008; Renault et al., 2009). In rodents, the number of NSCs significantly decreases with age in both the subventricular zone of the lateral ventricle and the subgranular zone of the dentate gyrus in the hippocampus, correlating with a gradual loss of olfactory function and cognitive function, respectively (Kuhn et al., 1996; Bondolfi et al., 2004; Maslov et al., 2004). Compared with NSCs from young rodents, NSCs from aged rodents exhibit a marked decline in their self-renewal activity as demonstrated in both in vitro neurosphere assays and in vivo tracing studies (Renault et al., 2009; Lugert et al., 2010). Mechanisms for the age-related loss of self-renewal of NSCs have been investigated in mouse models that lack the expression of important regulators of NSC homeostasis, including the Polycomb ring finger protein Bmi-1, the chromatin-associating protein HMGA2, and the longevity-associated transcription factor FoxO3 (Molofsky et al., 2003; Nishino et al., 2008; Renault et al., 2009). As revealed by these studies, the Bmi-1 and HMGA2 proteins regulate NSC self-renewal by suppressing the expression of cell cycle inhibitors p16INK4a and p19ARF (Molofsky et al., 2005; Nishino et al., 2008), and the FoxO proteins affect NSC homeostasis by regulating the expression of cell cycle regulatory proteins, such as cyclin D1, inhibitor of DNA binding 1, and polo-like kinase 2 (Paik et al., 2009). In addition, the FoxO proteins regulate the expression of several soluble Wnt antagonists and fine tune the activity of the Wnt pathway (Paik et al., 2009; Renault et al., 2009), thereby potentially maintaining NSC homeostasis during aging by attenuating Wnt signaling that is likely to be elevated in the aging environment (Brack et al., 2007; Liu et al., 2007). Although these mouse models have proven to be valuable in delineating pathways that are involved in NSC homeostasis and the precocious NSC depletion in these germline knockout mice recapitulates aspects of NSC aging, it remains to be determined whether these proteins play causal roles in NSC depletion during normal aging.
Senescence of stem cells.
One potential fate of stem cells and their progeny that has a profound negative impact on tissue homeostasis and regeneration is the state of senescence. The expression of senescence markers, such as senescence-associated β-galactosidase, HP-1 (heterochromatin protein-1) foci, and p16INK4a markedly increases with age in many tissues in several mammalian species (Sharpless and DePinho, 2007). Senescent epidermal stem cells, HSCs, NSCs, and pancreatic β cells have been observed in mouse models for segmental progeroid syndromes or those in which critical genes for stem cell homeostasis are deleted (Krishnamurthy et al., 2004; Liu et al., 2007; Burtner and Kennedy, 2010). Considered a biomarker of aging, p16INK4a appears to contribute to this replicative failure of stem cells (Krishnamurthy et al., 2006; Molofsky et al., 2006; Melzer et al., 2007). Overexpression of p16INK4a suppresses HSC function and pancreatic β cell proliferation in young animals, resembling the functional decline observed in aging (Janzen et al., 2006; Krishnamurthy et al., 2006). Likewise, genetic deletion of p16INK4a attenuates the age-related decline in proliferation and function of NSCs, HSCs, and pancreatic β cells (Janzen et al., 2006; Molofsky et al., 2006). Therefore, it has been proposed that specific populations of stem cells may exhibit an age-related decline in number as a result of stem cell senescence (Sahin and Depinho, 2010), although there are few examples in which stem cell senescence has been directly demonstrated during normal aging. Even in the epidermis, where senescent keratinocytes accumulate with age, direct evaluation of the stem cell compartment has not revealed senescent epidermal stem cells even though their transiently amplifying progeny may undergo senescence (Liang et al., 2004; Stern and Bickenbach, 2007; Giangreco et al., 2008; Charruyer et al., 2009).
Whether other types of stem cells in the skin undergo senescence with age has also been investigated (Liu et al., 2007). Normal cycling of hair follicle stem cells is regulated by local induction of Wnt proteins in the niche, and Wnt activity diminishes as the cells return to quiescence (Fuchs et al., 2001; Andl et al., 2002). This temporal regulation of the Wnt pathway appears to be critical in the homeostatic maintenance of hair follicle stem cells. Constitutive activation of the Wnt pathway in transgenic mouse models causes persistent proliferation of hair follicle stem cells followed by detectable signs of premature senescence and disappearance of stem cells that correlate with precocious hair loss (Liu et al., 2007; Castilho et al., 2009). In spite of the lack of direct demonstration, it is possible that hair follicle stem cells may likewise undergo senescence during normal aging, which, as noted in this paragraph, may be associated with increases in Wnt activity (Brack et al., 2007; Liu et al., 2007).
Malignant transformation of stem cells.
Another fate that has been proposed for tissue-specific stem cells, which would not only affect the functionality of the population but could dramatically impact organismal longevity, is malignant transformation. Whether cancers arise from adult stem cell compartments is a great challenge to demonstrate experimentally. This question has particular relevance in the context of stem cell aging, as age is the number one risk factor for cancer (Danaei et al., 2010). At the molecular level, cancer develops from a few cells harboring genomic mutations that enable their escape from the stringent cell cycle control (Hahn and Weinberg, 2002). Whether the cellular precursors for cancer are bona fide tissue-specific stem cells that have acquired such mutations remains to be shown (Gupta et al., 2009).
One indirect line of evidence in support of the hypothesis that stem cells themselves may undergo malignant transformation is that, in many cancers, the molecular pathways that sustain tumor growth are the same as those that support tissue-specific stem cells. For instance, the proliferative capacity of human acute myeloid leukemia stem cells requires Bmi-1, the Polycomb group gene that also regulates the self-renewal of normal HSCs (Bonnet and Dick, 1997; Lessard and Sauvageau, 2003). MicroRNA-200c suppresses the expression of Bmi-1 and inhibits the self-renewal of both normal mammary stem cells and breast cancer cells (Shimono et al., 2009). A Wnt pathway regulator, PLAGL-2 (pleomorphic adenoma gene-like 2), regulates the self-renewal of NSCs by suppressing their differentiation and also promotes the undifferentiated state of glioblastoma cells (Liu et al., 2010b; Zheng et al., 2010). Enforced expression of the nuclear receptor Tailless antagonizes the age-dependent NSC depletion in mice and results in increased incidents of glioma formation in aged mice (Liu et al., 2010b). Although these findings are consistent with the idea that normal stem cells can give rise to certain types of malignant tumors whose incidence increases with age, conclusive experimental evidence for such conversion requires lineage-tracing studies to label stem cells and monitor their activity during aging.
Intrinsic changes in aging stem cells
Among the cell-intrinsic changes that may mediate age-related changes in stem cell function are alterations at the level at the genome, the epigenome, and the proteome, each of which is considered in the following sections.
Genomic changes.
With age, somatic cells in many, if not all, tissues accumulate measurable genomic lesions, including single- and double-strand DNA breaks, chromosomal translocations, telomere shortening, and single base mutations (Akbari and Krokan, 2008; Wang et al., 2009). DNA repair systems have evolved to maintain genomic integrity, and it has been proposed that the intrinsic DNA repair activity and fidelity in different species may influence the rate of aging (Hart and Setlow, 1974). Mutations in proteins involved in DNA repair, such as the WRN (Werner Syndrome ATP-Dependent) helicase and the ATM (Ataxia Telangiectasia Mutated) kinase, have been associated with segmental progeroid syndromes in humans and mice that have features of accelerated aging in multiple tissues and organs (Savitsky et al., 1995; Gray et al., 1997; Kudlow et al., 2007), providing evidence for the crucial role of DNA repair machinery for normal tissue homeostasis.
Because of their replicative nature, various types of stem cells have been evaluated for telomere shortening. In the skin, shorter telomeres in hair follicle stem cells from old mice parallel a decline in their clonogenic potential (Flores et al., 2008). Additionally, telomere shortening has been observed in stem cells in the small intestine, cornea, testis, and brain in 2-yr-old compared with 2-mo-old mice (Flores et al., 2008). Interestingly, telomeres in old stem cells are still longer than those in the other somatic cells in these tissues (Flores et al., 2008; Wang et al., 2009), suggesting that stem cells divide at a much slower rate than their proliferative progeny or that they have evolved mechanisms to protect against telomere shortening.
The importance of intact telomeres in stem cells has been demonstrated in both telomerase knockout mice and mice that carry short telomeres in the presence of telomerase (Lee et al., 1998; Flores et al., 2005; Hao et al., 2005). In the late generation of mice that lack the gene encoding the essential RNA component (Terc) of the telomerase holoenzyme, stem cells in many tissues are depleted or functionally compromised (Lee et al., 1998). Epidermal stem cells in the hair follicles in these mice appear defective in proliferation and migration (Rudolph et al., 1999; Flores et al., 2005). Loss of renewal and differentiation of NSCs and increased apoptosis in the intestinal stem cell compartment are also apparent in these mice (Rudolph et al., 1999; Ferrón et al., 2004). Interestingly, although the enzymatic activity of telomerase is required to maintain telomere integrity, the protein component (Tert) of the telomerase holoenzyme may be able to regulate stem cell function independently of telomere maintenance. Expression of an enzymatically inactive Tert in Terc−/− mice is sufficient to activate hair follicle stem cells and stimulate hair growth (Sarin et al., 2005). Although the studies involving mice with genetically manipulated short telomeres provide strong evidence for the essential role of telomerase in maintaining stem cell function and tissue homeostasis, it remains to be determined whether telomere shortening, with or without a change in telomerase activity, contributes to stem cell dysfunction during normal aging.
Accumulation of DNA mutations may also be a common feature of stem cell aging. With age, elevated levels of DNA damage have been reported in epidermal stem cells and HSCs (Rossi et al., 2007; Sotiropoulou et al., 2010). DNA repair processes are important for maintaining stem cell homeostasis during aging, as demonstrated in several genetically modified mouse models that are defective for DNA repair (Ito et al., 2004; Ben-Porath and Weinberg, 2005; Nijnik et al., 2007; Rossi et al., 2007; Ruzankina et al., 2007). Stem cells from these mice exhibit a diminished capacity for self-renewal and proliferation with age, resulting in increased apoptosis or senescence in the stem cell compartment and depletion of functional stem cells (Ruzankina et al., 2007). On the other hand, quiescent stem cells appear to preferentially use nonhomologous end joining to repair DNA breaks (Mohrin et al., 2010), which is an error-prone mechanism as its action does not rely on a DNA template (Weinstock et al., 2006). The chromosomal deletions and insertions that result from misrepaired double-strand DNA breaks can lead to increased genomic instability and aberrant activation or inactivation of functional genes (Akbari and Krokan, 2008). As such, DNA repair processes may paradoxically lead to an increase in the accumulation of genomic mutations in quiescent stem cells with age. In addition, DNA repair causes recruitment of certain chromatin-remodeling enzymes to foci of damage and, thus, results in redistribution of these enzymes across the genome (Oberdoerffer et al., 2008; Conde et al., 2009). Therefore, the impact of genomic changes can be amplified by the subsequent changes in the epigenome.
The immortal strand hypothesis was proposed as a mechanism for stem cells to minimize the accumulation of replication-induced mutations (Cairns, 1975). According to this hypothesis, concurrent with the asymmetrical division of a stem cell, segregation of sister chromatids occurs nonrandomly. For each pair, the chromatid with the older (“immortal”) template strand is selectively inherited by the self-renewing daughter stem cell, whereas the chromatid with the newer template strand is inherited by the differentiating daughter cell. Therefore, the original genetic information that arose with the formation of a stem cell pool will be selectively retained and preserved in the stem cells, minimizing the accumulation of replication-induced mutations in the stem cell population (Rando, 2006). Such asymmetrical segregation of sister chromatids according to template strand age has been observed in certain stem cell populations, including muscle satellite cells and NSCs (Karpowicz et al., 2005; Shinin et al., 2006; Conboy et al., 2007; Ferron et al., 2010). The mechanisms that regulate nonrandom DNA segregation, the specific physiological conditions in which it occurs, and the effect it has on cell fate decisions remain to be determined (Falconer et al., 2010; Charville and Rando, 2011).
Epigenomic changes.
Unlike acquired DNA mutations, epigenomic changes, including DNA methylation and posttranslational modifications of histones, are dynamically maintained by a balance among chromatin-remodeling complexes and are, thus, reversible (Goldberg et al., 2007). Given the influence of cell extrinsic factors on the epigenome and the reversibility of chromatin modifications, epigenomic changes may underlie the stochastic aspects of aging (Herndon et al., 2002; Fraga et al., 2005; Kirkwood, 2005) and certain environmental influences that delay or even apparently reverse aging, such as the lifespan-extending effect of dietary restriction and the rejuvenation of aged stem cells by exposure to a young environment (Conboy et al., 2005; Dorshkind et al., 2009; Fontana et al., 2010). In yeast, lifespan extension by dietary restriction appears to require Sir2 (Lin et al., 2000), a histone deacetylase that has been shown to extend the lifespan in several model organisms (Longo and Kennedy, 2006). In Caenorhabditis elegans, members of the H3K4 methyltransferase complex affect lifespan in a germline-dependent manner (Greer et al., 2010). Although such data suggest that aging can be modulated at the epigenetic level, it should be noted that some epigenomic modifications are secondary to DNA damage itself. For example, phosphorylation of H2AX is induced by double-strand DNA breaks (Rogakou et al., 1998). There is also evidence that levels of acetylation of H3K56 (histone 3 lysine 56) and methylation of H3K79 (histone 3 lysine 79) increase after DNA damage (Conde et al., 2009; Tjeertes et al., 2009). Thus, the presence of particular histone marks can be indicative of permanent genomic changes.
Recent technological advances, particularly in ultra high-throughput sequencing, have allowed detailed studies of epigenomic changes across the genome (Bernstein et al., 2006; Hemberger et al., 2009). Changes in both DNA methylation and histone modifications have been investigated as epigenomic markers of aging (Sedivy et al., 2008; Calvanese et al., 2009). Age-associated global changes in DNA methylation are found in human, rat, and mouse tissues, including liver, spleen, lung, brain, fat, and different sections of the gastrointestinal tract (Christensen et al., 2009; Maegawa et al., 2010; Thompson et al., 2010). Despite the global decrease found in various cell types during aging, hypermethylation has also been found to occur in specific genes whose promoters are rich in CpG islands (Zhang et al., 2002; Maegawa et al., 2010). In the colon, age-related increases in the methylation of CpG islands have been linked to the silencing of tumor suppressor genes and neoplasia (Jones and Baylin, 2007). Recently, dysregulation of acetylation of H4K12 (histone 4 lysine 12) has been found to be associated with cognitive decline (Peleg et al., 2010). Although these studies have identified detectable epigenomic changes in mammalian cells with age, no direct evidence indicates whether they may play a causal role in cellular aging of whether they are merely consequences of the aging process.
Direct measurement of epigenomic changes in stem cells with age is challenging, as most current assays that detect epigenetic modifications require a large number of cells. The link between aging and epigenomic changes has been investigated in some tissue compartments in which stem cells reside. Therefore, such measurements may reflect changes in the stem cells themselves but may also reflect changes in cells within the stem cell niche. Increased DNA methylation was reported in both intestinal and colon crypts in the gut epithelium from old humans and mice (Yatabe et al., 2001; Maegawa et al., 2010). As noted previously, a significant portion of this hypermethylation occurs at CpG islands in the promoter regions of genes (Yatabe et al., 2001). Interestingly, quantitative simulation of the experimental DNA methylation data revealed that age-related DNA methylation appeared to increase more rapidly in the colon than the small intestine, correlating with the faster cell division rate in the former (Kim et al., 2005). This observation suggests that DNA methylation, as one important determinant of the epigenome, may be an indicator of the replicative age of cells.
The age-related epigenomic changes in stem cells can be a result of altered expression of chromatin-modifying enzymes or their cofactors (Chambers et al., 2007). Microarray analysis of HSCs from young and old mice has revealed an age-dependent reduction in the expression of subunits in the SWI/SNF (switch/sucrose nonfermentable) chromatin-remodeling complex, histone deacetylases HDAC1, 5, and 6, and a DNA methyltransferase, DNMT3b (Chambers et al., 2007). Increased genomic instability and inappropriate transcription have been historically associated with aging (Maslov and Vijg, 2009). Several genes clustered on chromosomal regions coordinately change their expression with age, suggesting an overall loss of transcriptional regulation at these regions (Chambers et al., 2007). The age-related change in the expression of chromatin-remodeling proteins, in combination with loss of nucleosome occupancy and redistribution of histone-modifying enzymes on the chromatin caused by DNA damage in old cells (Oberdoerffer et al., 2008; Dang et al., 2009; Feser et al., 2010), can collectively lead to alterations in chromatin configuration that affect the accessibility of large chromosomal regions and, thus, introduce transcriptional noise (Busuttil et al., 2007; Feser et al., 2010). It will be interesting to determine whether and to what degree the rejuvenation of old stem cells, as by exposure to a young environment (Conboy et al., 2005), is mediated by restoring the balance among different chromatin-remodeling complexes.
Proteomic changes.
Maintenance of the intracellular proteome requires timely removal of improperly folded or damaged proteins that can otherwise impede normal cellular function (Koga et al., 2011). Autophagosomes, chaperones, lysosomes, and the ubiquitin–proteasome system are all important cellular processes and machineries that maintain protein homeostasis (Rajawat et al., 2009; Koga et al., 2011). Together, they sense and remove misfolded or aberrant proteins in cells and ensure a functional proteome. With age, the protein homeostatic machinery becomes less efficient and less effective (Rodriguez et al., 2010; Koga et al., 2011), and these functional declines would only accentuate the negative effect of proteomic changes during aging.
Age-related increases in the levels of damaged proteins have been well documented in long-lived postmitotic cells, such as neurons, cardiomyocytes, and skeletal myofibers, and in some cases, these damaged proteins form aggregates or inclusion bodies that can cause proteotoxicity to cells (Rodriguez et al., 2010). It has been postulated that proliferative cells are less prone than are postmitotic cells to impairment caused by the accumulation of aberrant proteins and metabolic wastes, as their accumulation is predicted to be diluted by cell division and the synthesis of new cellular constituents during mitosis (Kirkwood, 2005). One example is the age-related deterioration of nuclear pore complexes (NPCs) that results in an increase in nuclear permeability and the leakage of cytoplasmic proteins into the nucleus in neurons (D’Angelo et al., 2009). This NPC deterioration is caused by oxidation of the scaffold nucleoporin complex Nup107–160, which is not replaced in postmitotic cells because of its inactive transcription. However, in proliferating cells, the active expression of Nup107–160 allows replacement of this damaged nucleoporin during cell division when the NPCs reassemble (D’Angelo et al., 2009).
Stem cells with low turnover rates, such as satellite cells in skeletal muscle (Morgan and Partridge, 2003), are likely to be more susceptible to proteotoxicity than stem cells with a higher turnover rate. During replicative aging of yeast, damaged protein aggregates are selectively retained in mother cells during budding to ensure the youthfulness of daughter cells (Erjavec et al., 2008; Kaeberlein, 2010). The polarizome, which is composed of the formin Bni1p and myosin motor protein Myo2pa, is a key component of the cellular machinery that ensures asymmetric segregation of protein aggregates during cytokinesis associated with yeast budding (Liu et al., 2010a). Adult mammalian stem cells may use similar mechanisms to protect themselves from the accumulation of damaged protein aggregates, perhaps selectively segregating damaged proteins to their differentiating rather than their self-renewing progeny.
Conclusion
Although stem cells exhibit age-related changes at the genomic, epigenomic, and proteomic levels, the functional consequences of these changes remains to be determined. In contrast, age-related changes in the local or systemic environment have been directly implicated in the decline in stem cell function with age. Ultimately, understanding the age-related changes in stem cell functionality will require a distinction among intrinsic irreversible changes (such as genomic mutations), intrinsic reversible changes (such as epigenomic alterations), and extrinsic influences from the local and systemic environment (Fig. 3). These distinctions are further complicated by the fact that stem cells, in turn, influence the niche in which they reside. All of these issues are brought to bear in the consideration of stem cell therapeutics for diseases and disorders of aging. Whether focusing on the potential of endogenous stem cells or of exogenous transplanted stem cells as therapeutic vehicles, the complex interactions between the cells and their environment are likely to be critical determinants of the success of such therapeutic approaches. Understanding the mechanisms by which stem cell functionality declines with age will be essential to enhance tissue repair in the elderly and to solve the challenges of stem cell therapeutics for age-related diseases.
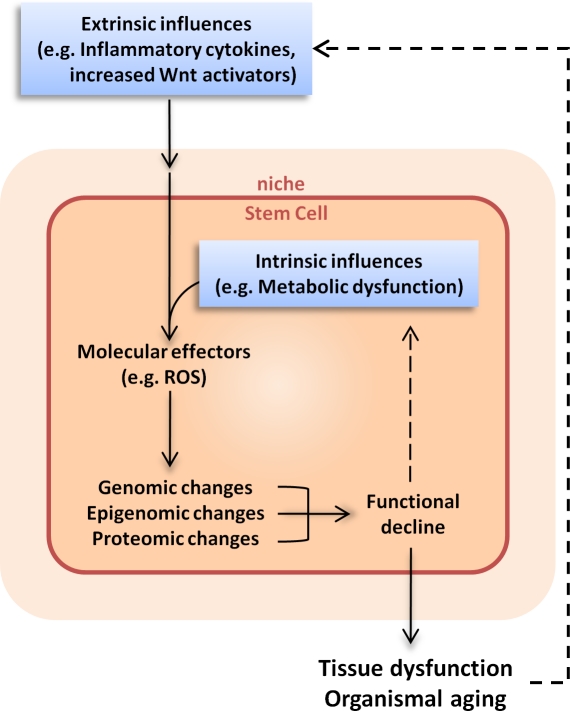
Figure 3.
Extrinsic and intrinsic influences on stem cell aging. Age-related changes in the systemic milieu, for example, an increased level of inflammatory cytokines or Wnt pathway activators in the circulation, can lead to changes of signaling cascades and molecular changes within cells. For stem cells, the effects of extrinsic influences may also be transmitted indirectly via detrimental changes in the niche. Meanwhile, intrinsic changes, such as changes in mitochondrial activity or metabolic rate, can also occur during cellular aging. The extrinsic and intrinsic influences converge at intracellular molecular effectors, such as reactive oxygen species (ROS), which can cause either reversible changes (such as protein oxidation) or irreversible changes (such as DNA mutations) to macromolecules in the cell. The combinatorial effects of genomic, epigenomic, and proteomic changes lead to a decline in cellular function, which in turn contributes to tissue dysfunction and organismal aging. Because dysfunctional stem cells give rise to abnormal differentiated cells in the tissue, stem cell aging exacerbates the extrinsic influences of aging, thereby also contributing to the aging process of the tissue and organism.
Acknowledgments
T.A. Rando is funded by the National Institutes of Health (grants R37 AG23806 and R01 AR056849 and a National Institutes of Health Director’s Pioneer Award), the Glenn Foundation for Medical Research, the Department of Veterans Affairs (Merit Review), and the American Federation for Aging Research (Breakthroughs in Gerontology Award).
Footnotes
Abbreviations used in this paper:
- HSC
- hematopoietic stem cell
- NPC
- nuclear pore complex
- NSC
- neural stem cell
References
- Akbari M., Krokan H.E. 2008. Cytotoxicity and mutagenicity of endogenous DNA base lesions as potential cause of human aging. Mech. Ageing Dev. 129:353–365 10.1016/j.mad.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Andl T., Reddy S.T., Gaddapara T., Millar S.E. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2:643–653 10.1016/S1534-5807(02)00167-3 [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449:1003–1007 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., Rossi D.J. 2010. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA. 107:5465–5470 10.1073/pnas.1000834107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D.R., Van Zant G. 2004. Stem cells, aging, and cancer: inevitabilities and outcomes. Oncogene. 23:7290–7296 10.1038/sj.onc.1207949 [DOI] [PubMed] [Google Scholar]
- Ben-Porath I., Weinberg R.A. 2005. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37:961–976 10.1016/j.biocel.2004.10.013 [DOI] [PubMed] [Google Scholar]
- Berger S.L. 2007. The complex language of chromatin regulation during transcription. Nature. 447:407–412 10.1038/nature05915 [DOI] [PubMed] [Google Scholar]
- Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 125:315–326 10.1016/j.cell.2006.02.041 [DOI] [PubMed] [Google Scholar]
- Blanpain C., Fuchs E. 2009. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10:207–217 10.1038/nrm2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L., Ermini F., Long J.M., Ingram D.K., Jucker M. 2004. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging. 25:333–340 10.1016/S0197-4580(03)00083-6 [DOI] [PubMed] [Google Scholar]
- Bonnet D., Dick J.E. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3:730–737 10.1038/nm0797-730 [DOI] [PubMed] [Google Scholar]
- Booth C., Potten C.S. 2000. Gut instincts: thoughts on intestinal epithelial stem cells. J. Clin. Invest. 105:1493–1499 10.1172/JCI10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A.S., Rando T.A. 2007. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 3:226–237 10.1007/s12015-007-9000-2 [DOI] [PubMed] [Google Scholar]
- Brack A.S., Conboy M.J., Roy S., Lee M., Kuo C.J., Keller C., Rando T.A. 2007. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 317:807–810 10.1126/science.1144090 [DOI] [PubMed] [Google Scholar]
- Brack A.S., Conboy I.M., Conboy M.J., Shen J., Rando T.A. 2008. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2:50–59 10.1016/j.stem.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Burtner C.R., Kennedy B.K. 2010. Progeria syndromes and ageing: what is the connection? Nat. Rev. Mol. Cell Biol. 11:567–578 10.1038/nrm2944 [DOI] [PubMed] [Google Scholar]
- Busuttil R., Bahar R., Vijg J. 2007. Genome dynamics and transcriptional deregulation in aging. Neuroscience. 145:1341–1347 10.1016/j.neuroscience.2006.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. 1975. Mutation selection and the natural history of cancer. Nature. 255:197–200 10.1038/255197a0 [DOI] [PubMed] [Google Scholar]
- Calvanese V., Lara E., Kahn A., Fraga M.F. 2009. The role of epigenetics in aging and age-related diseases. Ageing Res. Rev. 8:268–276 10.1016/j.arr.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Campisi J., d’Adda di Fagagna F. 2007. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8:729–740 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- Carlson M.E., Conboy M.J., Hsu M., Barchas L., Jeong J., Agrawal A., Mikels A.J., Agrawal S., Schaffer D.V., Conboy I.M. 2009. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 8:676–689 10.1111/j.1474-9726.2009.00517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho R.M., Squarize C.H., Chodosh L.A., Williams B.O., Gutkind J.S. 2009. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 5:279–289 10.1016/j.stem.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen G.A., Boles N.C., Chambers S.M., Goodell M.A. 2010. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 6:265–278 10.1016/j.stem.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Shaw C.A., Gatza C., Fisk C.J., Donehower L.A., Goodell M.A. 2007. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5:e201 10.1371/journal.pbio.0050201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charruyer A., Barland C.O., Yue L., Wessendorf H.B., Lu Y., Lawrence H.J., Mancianti M.L., Ghadially R. 2009. Transit-amplifying cell frequency and cell cycle kinetics are altered in aged epidermis. J. Invest. Dermatol. 129:2574–2583 10.1038/jid.2009.127 [DOI] [PubMed] [Google Scholar]
- Charville G.W., Rando T.A. 2011. Stem cell ageing and non-random chromosome segregation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:85–93 10.1098/rstb.2010.0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho R.H., Sieburg H.B., Muller-Sieburg C.E. 2008. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 111:5553–5561 10.1182/blood-2007-11-123547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B.C., Houseman E.A., Marsit C.J., Zheng S., Wrensch M.R., Wiemels J.L., Nelson H.H., Karagas M.R., Padbury J.F., Bueno R., et al. 2009. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 5:e1000602 10.1371/journal.pgen.1000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 433:760–764 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- Conboy M.J., Karasov A.O., Rando T.A. 2007. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 5:e102 10.1371/journal.pbio.0050102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F., Refolio E., Cordón-Preciado V., Cortés-Ledesma F., Aragón L., Aguilera A., San-Segundo P.A. 2009. The Dot1 histone methyltransferase and the Rad9 checkpoint adaptor contribute to cohesin-dependent double-strand break repair by sister chromatid recombination in Saccharomyces cerevisiae. Genetics. 182:437–446 10.1534/genetics.109.101899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G., Rimm E.B., Oza S., Kulkarni S.C., Murray C.J., Ezzati M. 2010. The promise of prevention: the effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States. PLoS Med. 7:e1000248 10.1371/journal.pmed.1000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W., Steffen K.K., Perry R., Dorsey J.A., Johnson F.B., Shilatifard A., Kaeberlein M., Kennedy B.K., Berger S.L. 2009. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 459:802–807 10.1038/nature08085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo M.A., Raices M., Panowski S.H., Hetzer M.W. 2009. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 136:284–295 10.1016/j.cell.2008.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Montecino-Rodriguez E., Signer R.A. 2009. The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 9:57–62 10.1038/nri2471 [DOI] [PubMed] [Google Scholar]
- Erjavec N., Cvijovic M., Klipp E., Nyström T. 2008. Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc. Natl. Acad. Sci. USA. 105:18764–18769 10.1073/pnas.0804550105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E., Chavez E.A., Henderson A., Poon S.S., McKinney S., Brown L., Huntsman D.G., Lansdorp P.M. 2010. Identification of sister chromatids by DNA template strand sequences. Nature. 463:93–97 10.1038/nature08644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrón S., Mira H., Franco S., Cano-Jaimez M., Bellmunt E., Ramírez C., Fariñas I., Blasco M.A. 2004. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development. 131:4059–4070 10.1242/dev.01215 [DOI] [PubMed] [Google Scholar]
- Ferron S.R., Pozo N., Laguna A., Aranda S., Porlan E., Moreno M., Fillat C., de la Luna S., Sánchez P., Arbonés M.L., Fariñas I. 2010. Regulated segregation of kinase Dyrk1A during asymmetric neural stem cell division is critical for EGFR-mediated biased signaling. Cell Stem Cell. 7:367–379 10.1016/j.stem.2010.06.021 [DOI] [PubMed] [Google Scholar]
- Feser J., Truong D., Das C., Carson J.J., Kieft J., Harkness T., Tyler J.K. 2010. Elevated histone expression promotes life span extension. Mol. Cell. 39:724–735 10.1016/j.molcel.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores I., Cayuela M.L., Blasco M.A. 2005. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 309:1253–1256 10.1126/science.1115025 [DOI] [PubMed] [Google Scholar]
- Flores I., Canela A., Vera E., Tejera A., Cotsarelis G., Blasco M.A. 2008. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 22:654–667 10.1101/gad.451008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Partridge L., Longo V.D. 2010. Extending healthy life span—from yeast to humans. Science. 328:321–326 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga M.F., Ballestar E., Paz M.F., Ropero S., Setien F., Ballestar M.L., Heine-Suñer D., Cigudosa J.C., Urioste M., Benitez J., et al. 2005. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA. 102:10604–10609 10.1073/pnas.0500398102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Merrill B.J., Jamora C., DasGupta R. 2001. At the roots of a never-ending cycle. Dev. Cell. 1:13–25 10.1016/S1534-5807(01)00022-3 [DOI] [PubMed] [Google Scholar]
- Giangreco A., Qin M., Pintar J.E., Watt F.M. 2008. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 7:250–259 10.1111/j.1474-9726.2008.00372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A.D., Allis C.D., Bernstein E. 2007. Epigenetics: a landscape takes shape. Cell. 128:635–638 10.1016/j.cell.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Gopinath S.D., Rando T.A. 2008. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 7:590–598 10.1111/j.1474-9726.2008.00399.x [DOI] [PubMed] [Google Scholar]
- Gray M.D., Shen J.C., Kamath-Loeb A.S., Blank A., Sopher B.L., Martin G.M., Oshima J., Loeb L.A. 1997. The Werner syndrome protein is a DNA helicase. Nat. Genet. 17:100–103 10.1038/ng0997-100 [DOI] [PubMed] [Google Scholar]
- Greer E.L., Maures T.J., Hauswirth A.G., Green E.M., Leeman D.S., Maro G.S., Han S., Banko M.R., Gozani O., Brunet A. 2010. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 466:383–387 10.1038/nature09195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Della Bella S., Iorio A.M., Michel J.P., Pawelec G., Solana R. 2009. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 21:201–209 [DOI] [PubMed] [Google Scholar]
- Gupta P.B., Chaffer C.L., Weinberg R.A. 2009. Cancer stem cells: mirage or reality? Nat. Med. 15:1010–1012 10.1038/nm0909-1010 [DOI] [PubMed] [Google Scholar]
- Hahn W.C., Weinberg R.A. 2002. Rules for making human tumor cells. N. Engl. J. Med. 347:1593–1603 10.1056/NEJMra021902 [DOI] [PubMed] [Google Scholar]
- Hao L.Y., Armanios M., Strong M.A., Karim B., Feldser D.M., Huso D., Greider C.W. 2005. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 123:1121–1131 10.1016/j.cell.2005.11.020 [DOI] [PubMed] [Google Scholar]
- Hart R.W., Setlow R.B. 1974. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc. Natl. Acad. Sci. USA. 71:2169–2173 10.1073/pnas.71.6.2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. 1965. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37:614–636 10.1016/0014-4827(65)90211-9 [DOI] [PubMed] [Google Scholar]
- Hemberger M., Dean W., Reik W. 2009. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat. Rev. Mol. Cell Biol. 10:526–537 10.1038/nrm2727 [DOI] [PubMed] [Google Scholar]
- Herndon L.A., Schmeissner P.J., Dudaronek J.M., Brown P.A., Listner K.M., Sakano Y., Paupard M.C., Hall D.H., Driscoll M. 2002. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 419:808–814 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]
- Inomata K., Aoto T., Binh N.T., Okamoto N., Tanimura S., Wakayama T., Iseki S., Hara E., Masunaga T., Shimizu H., Nishimura E.K. 2009. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 137:1088–1099 10.1016/j.cell.2009.03.037 [DOI] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Matsuoka S., Takubo K., Hamaguchi I., Nomiyama K., Hosokawa K., Sakurada K., Nakagata N., et al. 2004. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 431:997–1002 10.1038/nature02989 [DOI] [PubMed] [Google Scholar]
- Janzen V., Forkert R., Fleming H.E., Saito Y., Waring M.T., Dombkowski D.M., Cheng T., DePinho R.A., Sharpless N.E., Scadden D.T. 2006. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 443:421–426 [DOI] [PubMed] [Google Scholar]
- Jones D.L., Rando T.A. 2011. Emerging models and paradigms for stem cell aging. Nat. Cell Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.A., Baylin S.B. 2007. The epigenomics of cancer. Cell. 128:683–692 10.1016/j.cell.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. 2010. Lessons on longevity from budding yeast. Nature. 464:513–519 10.1038/nature08981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P., Morshead C., Kam A., Jervis E., Ramunas J., Cheng V., van der Kooy D. 2005. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J. Cell Biol. 170:721–732 (published erratum appears in J. Cell Biol. 2005. 170:1169) 10.1083/jcb.200502073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Siegmund K.D., Tavaré S., Shibata D. 2005. Age-related human small intestine methylation: evidence for stem cell niches. BMC Med. 3:10 10.1186/1741-7015-3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood T.B. 2005. Understanding the odd science of aging. Cell. 120:437–447 10.1016/j.cell.2005.01.027 [DOI] [PubMed] [Google Scholar]
- Knoblich J.A. 2008. Mechanisms of asymmetric stem cell division. Cell. 132:583–597 10.1016/j.cell.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Koga H., Kaushik S., Cuervo A.M. 2011. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 10:205–215 10.1016/j.arr.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J., Torrice C., Ramsey M.R., Kovalev G.I., Al-Regaiey K., Su L., Sharpless N.E. 2004. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114:1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J., Ramsey M.R., Ligon K.L., Torrice C., Koh A., Bonner-Weir S., Sharpless N.E. 2006. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 443:453–457 10.1038/nature05092 [DOI] [PubMed] [Google Scholar]
- Kudlow B.A., Kennedy B.K., Monnat R.J., Jr 2007. Werner and Hutchinson-Gilford progeria syndromes: mechanistic basis of human progeroid diseases. Nat. Rev. Mol. Cell Biol. 8:394–404 10.1038/nrm2161 [DOI] [PubMed] [Google Scholar]
- Kuhn H.G., Dickinson-Anson H., Gage F.H. 1996. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16:2027–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.W., Blasco M.A., Gottlieb G.J., Horner J.W., II, Greider C.W., DePinho R.A. 1998. Essential role of mouse telomerase in highly proliferative organs. Nature. 392:569–574 10.1038/33345 [DOI] [PubMed] [Google Scholar]
- Le Grand F., Rudnicki M.A. 2007. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 19:628–633 10.1016/j.ceb.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J., Sauvageau G. 2003. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 423:255–260 10.1038/nature01572 [DOI] [PubMed] [Google Scholar]
- Li L., Clevers H. 2010. Coexistence of quiescent and active adult stem cells in mammals. Science. 327:542–545 10.1126/science.1180794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Chinnathambi S., Stern M., Tomanek-Chalkley A., Manuel T.D., Bickenbach J.R. 2004. As epidermal stem cells age they do not substantially change their characteristics. J. Investig. Dermatol. Symp. Proc. 9:229–237 10.1111/j.1087-0024.2004.09309.x [DOI] [PubMed] [Google Scholar]
- Lin S.J., Defossez P.A., Guarente L. 2000. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 289:2126–2128 10.1126/science.289.5487.2126 [DOI] [PubMed] [Google Scholar]
- Linton P.J., Dorshkind K. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 5:133–139 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- Liu B., Larsson L., Caballero A., Hao X., Oling D., Grantham J., Nyström T. 2010a. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 140:257–267 10.1016/j.cell.2009.12.031 [DOI] [PubMed] [Google Scholar]
- Liu H., Fergusson M.M., Castilho R.M., Liu J., Cao L., Chen J., Malide D., Rovira I.I., Schimel D., Kuo C.J., et al. 2007. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 317:803–806 10.1126/science.1143578 [DOI] [PubMed] [Google Scholar]
- Liu H.K., Wang Y., Belz T., Bock D., Takacs A., Radlwimmer B., Barbus S., Reifenberger G., Lichter P., Schütz G. 2010b. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 24:683–695 10.1101/gad.560310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo V.D., Kennedy B.K. 2006. Sirtuins in aging and age-related disease. Cell. 126:257–268 10.1016/j.cell.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M., Haas C.A., Kempermann G., Taylor V., Giachino C. 2010. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 6:445–456 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Maegawa S., Hinkal G., Kim H.S., Shen L., Zhang L., Zhang J., Zhang N., Liang S., Donehower L.A., Issa J.P. 2010. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 20:332–340 10.1101/gr.096826.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov A.Y., Vijg J. 2009. Genome instability, cancer and aging. Biochim. Biophys. Acta. 1790:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov A.Y., Barone T.A., Plunkett R.J., Pruitt S.C. 2004. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 24:1726–1733 10.1523/JNEUROSCI.4608-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. 1961. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9:493–495 10.1083/jcb.9.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D., Frayling T.M., Murray A., Hurst A.J., Harries L.W., Song H., Khaw K., Luben R., Surtees P.G., Bandinelli S.S., et al. 2007. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech. Ageing Dev. 128:370–377 10.1016/j.mad.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T.S., Ku M., Jaffe D.B., Issac B., Lieberman E., Giannoukos G., Alvarez P., Brockman W., Kim T.K., Koche R.P., et al. 2007. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 448:553–560 10.1038/nature06008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Montecino-Rodriguez E., Dorshkind K. 2004. Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 173:245–250 [DOI] [PubMed] [Google Scholar]
- Mohrin M., Bourke E., Alexander D., Warr M.R., Barry-Holson K., Le Beau M.M., Morrison C.G., Passegué E. 2010. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 7:174–185 10.1016/j.stem.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., Pardal R., Iwashita T., Park I.K., Clarke M.F., Morrison S.J. 2003. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 425:962–967 10.1038/nature02060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., He S., Bydon M., Morrison S.J., Pardal R. 2005. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 19:1432–1437 10.1101/gad.1299505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., Slutsky S.G., Joseph N.M., He S., Pardal R., Krishnamurthy J., Sharpless N.E., Morrison S.J. 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 443:448–452 10.1038/nature05091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.E., Partridge T.A. 2003. Muscle satellite cells. Int. J. Biochem. Cell Biol. 35:1151–1156 10.1016/S1357-2725(03)00042-6 [DOI] [PubMed] [Google Scholar]
- Nijnik A., Woodbine L., Marchetti C., Dawson S., Lambe T., Liu C., Rodrigues N.P., Crockford T.L., Cabuy E., Vindigni A., et al. 2007. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 447:686–690 10.1038/nature05875 [DOI] [PubMed] [Google Scholar]
- Nishimura E.K., Granter S.R., Fisher D.E. 2005. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 307:720–724 10.1126/science.1099593 [DOI] [PubMed] [Google Scholar]
- Nishino J., Kim I., Chada K., Morrison S.J. 2008. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 135:227–239 10.1016/j.cell.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdoerffer P., Michan S., McVay M., Mostoslavsky R., Vann J., Park S.K., Hartlerode A., Stegmuller J., Hafner A., Loerch P., et al. 2008. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 135:907–918 10.1016/j.cell.2008.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J.H., Ding Z., Narurkar R., Ramkissoon S., Muller F., Kamoun W.S., Chae S.S., Zheng H., Ying H., Mahoney J., et al. 2009. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 5:540–553 10.1016/j.stem.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S., Sananbenesi F., Zovoilis A., Burkhardt S., Bahari-Javan S., Agis-Balboa R.C., Cota P., Wittnam J.L., Gogol-Doering A., Opitz L., et al. 2010. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 328:753–756 10.1126/science.1186088 [DOI] [PubMed] [Google Scholar]
- Rajawat Y.S., Hilioti Z., Bossis I. 2009. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res. Rev. 8:199–213 10.1016/j.arr.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Rando T.A. 2006. Stem cells, ageing and the quest for immortality. Nature. 441:1080–1086 10.1038/nature04958 [DOI] [PubMed] [Google Scholar]
- Relaix F., Rocancourt D., Mansouri A., Buckingham M. 2005. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 435:948–953 10.1038/nature03594 [DOI] [PubMed] [Google Scholar]
- Renault V.M., Rafalski V.A., Morgan A.A., Salih D.A., Brett J.O., Webb A.E., Villeda S.A., Thekkat P.U., Guillerey C., Denko N.C., et al. 2009. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 5:527–539 10.1016/j.stem.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink L., Cakman I., Kirchner H. 1998. Altered cytokine production in the elderly. Mech. Ageing Dev. 102:199–209 10.1016/S0047-6374(97)00153-X [DOI] [PubMed] [Google Scholar]
- Rittié L., Stoll S.W., Kang S., Voorhees J.J., Fisher G.J. 2009. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 8:738–751 10.1111/j.1474-9726.2009.00526.x [DOI] [PubMed] [Google Scholar]
- Rodriguez K.A., Gaczynska M., Osmulski P.A. 2010. Molecular mechanisms of proteasome plasticity in aging. Mech. Ageing Dev. 131:144–155 10.1016/j.mad.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou E.P., Pilch D.R., Orr A.H., Ivanova V.S., Bonner W.M. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273:5858–5868 10.1074/jbc.273.10.5858 [DOI] [PubMed] [Google Scholar]
- Rossi D.J., Bryder D., Zahn J.M., Ahlenius H., Sonu R., Wagers A.J., Weissman I.L. 2005. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA. 102:9194–9199 10.1073/pnas.0503280102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D.J., Bryder D., Seita J., Nussenzweig A., Hoeijmakers J., Weissman I.L. 2007. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 447:725–729 10.1038/nature05862 [DOI] [PubMed] [Google Scholar]
- Rudolph K.L., Chang S., Lee H.W., Blasco M., Gottlieb G.J., Greider C., DePinho R.A. 1999. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 96:701–712 10.1016/S0092-8674(00)80580-2 [DOI] [PubMed] [Google Scholar]
- Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G., Zediak V.P., Velez M., Bhandoola A., Brown E.J. 2007. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 1:113–126 10.1016/j.stem.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E., Depinho R.A. 2010. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 464:520–528 10.1038/nature08982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin K.Y., Cheung P., Gilison D., Lee E., Tennen R.I., Wang E., Artandi M.K., Oro A.E., Artandi S.E. 2005. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 436:1048–1052 10.1038/nature03836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D.A., Smith S., Uziel T., Sfez S., et al. 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 268:1749–1753 10.1126/science.7792600 [DOI] [PubMed] [Google Scholar]
- Schofield R. 1978. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 4:7–25 [PubMed] [Google Scholar]
- Sedivy J.M., Banumathy G., Adams P.D. 2008. Aging by epigenetics—a consequence of chromatin damage? Exp. Cell Res. 314:1909–1917 10.1016/j.yexcr.2008.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N.E., DePinho R.A. 2007. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 8:703–713 10.1038/nrm2241 [DOI] [PubMed] [Google Scholar]
- Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E., et al. 2009. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 138:592–603 10.1016/j.cell.2009.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinin V., Gayraud-Morel B., Gomès D., Tajbakhsh S. 2006. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat. Cell Biol. 8:677–687 10.1038/ncb1425 [DOI] [PubMed] [Google Scholar]
- Siminovitch L., Till J.E., McCulloch E.A. 1964. Decline in colony-forming ability of marrow cells subjected to serial transplantation into irradiated mice. J. Cell. Physiol. 64:23–31 10.1002/jcp.1030640104 [DOI] [PubMed] [Google Scholar]
- Sotiropoulou P.A., Candi A., Mascré G., De Clercq S., Youssef K.K., Lapouge G., Dahl E., Semeraro C., Denecker G., Marine J.C., Blanpain C. 2010. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 12:572–582 10.1038/ncb2059 [DOI] [PubMed] [Google Scholar]
- Stern M.M., Bickenbach J.R. 2007. Epidermal stem cells are resistant to cellular aging. Aging Cell. 6:439–452 10.1111/j.1474-9726.2007.00318.x [DOI] [PubMed] [Google Scholar]
- Sudo K., Ema H., Morita Y., Nakauchi H. 2000. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192:1273–1280 10.1084/jem.192.9.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Jones J.M., McGehee R.E., Rando T.A., Lecka-Czernik B., Lipschitz D.A., Peterson C.A. 2002. Activation of an adipogenic program in adult myoblasts with age. Mech. Ageing Dev. 123:649–661 10.1016/S0047-6374(01)00411-0 [DOI] [PubMed] [Google Scholar]
- Thompson R.F., Atzmon G., Gheorghe C., Liang H.Q., Lowes C., Greally J.M., Barzilai N. 2010. Tissue-specific dysregulation of DNA methylation in aging. Aging Cell. 9:506–518 10.1111/j.1474-9726.2010.00577.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjeertes J.V., Miller K.M., Jackson S.P. 2009. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 28:1878–1889 10.1038/emboj.2009.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier L.G., Clevers H. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71:241–260 10.1146/annurev.physiol.010908.163145 [DOI] [PubMed] [Google Scholar]
- Voog J., Jones D.L. 2010. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 6:103–115 10.1016/j.stem.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Jurk D., Maddick M., Nelson G., Martin-Ruiz C., von Zglinicki T. 2009. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 8:311–323 10.1111/j.1474-9726.2009.00481.x [DOI] [PubMed] [Google Scholar]
- Waterstrat A., Van Zant G. 2009. Effects of aging on hematopoietic stem and progenitor cells. Curr. Opin. Immunol. 21:408–413 10.1016/j.coi.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Weinstock D.M., Richardson C.A., Elliott B., Jasin M. 2006. Modeling oncogenic translocations: distinct roles for double-strand break repair pathways in translocation formation in mammalian cells. DNA Repair (Amst.). 5:1065–1074 10.1016/j.dnarep.2006.05.028 [DOI] [PubMed] [Google Scholar]
- Weissman I.L. 2000. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 100:157–168 10.1016/S0092-8674(00)81692-X [DOI] [PubMed] [Google Scholar]
- Yan X., Owens D.M. 2008. The skin: a home to multiple classes of epithelial progenitor cells. Stem Cell Rev. 4:113–118 10.1007/s12015-008-9022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatabe Y., Tavaré S., Shibata D. 2001. Investigating stem cells in human colon by using methylation patterns. Proc. Natl. Acad. Sci. USA. 98:10839–10844 10.1073/pnas.191225998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P.S., Partridge T.A., Yablonka-Reuveni Z. 2006. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 54:1177–1191 10.1369/jhc.6R6995.2006 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Deng C., Lu Q., Richardson B. 2002. Age-dependent DNA methylation changes in the ITGAL (CD11a) promoter. Mech. Ageing Dev. 123:1257–1268 [DOI] [PubMed] [Google Scholar]
- Zheng H., Ying H., Wiedemeyer R., Yan H., Quayle S.N., Ivanova E.V., Paik J.H., Zhang H., Xiao Y., Perry S.R., et al. 2010. PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell. 17:497–509 10.1016/j.ccr.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Akinbiyi T., Xu L., Ramcharan M., Leong D.J., Ros S.J., Colvin A.C., Schaffler M.B., Majeska R.J., Flatow E.L., Sun H.B. 2010. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 9:911–915 10.1111/j.1474-9726.2010.00598.x [DOI] [PMC free article] [PubMed] [Google Scholar]