Abstract
Bartonellae are bacterial pathogens for a wide variety of mammals. In humans, bartonellosis can result in angioproliferative lesions that are potentially life threatening to the patient, including bacillary angiomatosis, bacillary peliosis, and verruga peruana. The results of this study show that Bartonella bacilliformis, the agent of Oroya fever and verruga peruana, produces a proteinaceous mitogen for human vascular endothelial cells (HUVECs) that acts in a dose-dependent fashion in vitro with maximal activity at ≥72 h of exposure and results in a 6- to 20-fold increase in cell numbers relative to controls. The mitogen increases bromodeoxyuridine (BrdU) incorporation into HUVECs by almost twofold relative to controls. The mitogen is sensitive to heat and trypsin but is not affected by the lipopolysaccharide inhibitor polymyxin B. The mitogen does not affect caspase 3 activity in HUVECs undergoing serum starvation-induced apoptosis. The Bartonella mitogen was found in bacterial culture supernatants, the soluble cell lysate fraction, and, to a lesser degree, in insoluble cell fractions of the bacterium. In contrast, soluble cell lysate fractions from closely related B. henselae, although possessing significant mitogenicity for HUVECs, resulted in only about a twofold increase in cell numbers. Biochemical and immunological analyses identified GroEL as a participant in the observed HUVEC mitogenicity. A B. bacilliformis strain containing the intact groES-groEL operon on a multicopy plasmid was generated and used to demonstrate a correlation between HUVEC mitogenicity and GroEL levels in the lysate (r2 = 0.85). Antiserum to GroEL significantly inhibited mitogenicity of the lysate. Data also show that GroEL is located in the soluble and insoluble fractions (including inner and outer membranes) of the cell and is actively secreted by B. bacilliformis.
Human infection by Bartonella species can result in angioproliferative lesions that stem from bacterium-induced hyperproliferation of the vasculature (1, 37). This pathology is unique among pathogenic bacteria and resembles Kaposi's sarcoma. The earliest study investigating this phenomenon identified a factor with a size of >12 kDa in the soluble fraction of a Bartonella bacilliformis cell homogenate that was specifically mitogenic for vascular endothelial cells (17). Human umbilical vein endothelial cells (HUVECs) treated with the factor showed proliferation rates approximately threefold greater than those in controls. The absence of cytotoxic effects coupled with heat sensitivity led the authors to conclude that the factor was not lipopolysaccharide (LPS). This study also demonstrated that the factor was angiogenic in a rat model. A subsequent study by this group showed that live B. bacilliformis could stimulate proliferation of HUVECs in cocultures (16).
A study with Bartonella henselae showed enhanced proliferation and migration of HUVECs in coculture and identified a trypsin-sensitive proliferative factor detectable in the insoluble (cell wall) fraction of the bacterium (10). Further work with B. henselae demonstrated that the proliferative factor was secreted into the culture medium and, like the B. bacilliformis factor, was specific to endothelial cells (27). More recently, Kirby and Nekorchuk (23) showed that inhibition of apoptosis, and not mitogenicity, is the predominant means by which several B. henselae strains apparently increase the number of HUVECs in coculture. Their data also show that the antiapoptotic factor is released into the bacterial culture medium and, as previously demonstrated, is specific for endothelial cells.
Two additional studies have shown that B. henselae infection induces expression of potentially angiogenic cytokines and growth factors in vitro that may act in a paracrine and/or autocrine fashion to stimulate growth of endothelial cells. Kempf et al. showed that piliated B. henselae cells induce synthesis of vascular endothelial growth factor (VEGF) by EA.hy 926 or HeLa cells, but not HUVECs, in cocultures (22). HUVEC cultures supplemented with conditioned medium from EA.hy 926-B. henselae cocultures showed proliferation rates 30- to 70-fold greater than those in controls. Kempf et al. also showed that EA.hy 926-B. henselae cocultures induced synthesis of interleukin-8 (IL-8), a second angiogenic factor (39). Finally, this study demonstrated that B. henselae growth correlated with host cell growth rates, suggesting that paracrine stimulation might provide additional host cells for colonization. More recently, Resto-Ruiz et al. (29) demonstrated that VEGF and IL-1β could be detected within 6 to 12 h in medium from THP-1 macrophage cells cocultured with B. henselae. Conditioned medium from these cocultures was mitogenic for human microvascular endothelial cells (HMEC-1). In addition, HMEC-1 cells, but not THP-1 cells, were stimulated to produce IL-8 within 6 h of infection. Taken together, these two studies suggest that proliferation of endothelial cells during Bartonella infection may be enhanced by paracrine stimulation with VEGF, IL-1β, and IL-8 and a possible IL-8 autocrine loop.
In this study, we analyzed a B. bacilliformis proliferative factor to help elucidate its molecular structure and function. Our results suggest that B. bacilliformis produces a proteinaceous, soluble factor that is mitogenic for HUVECs and does not affect apoptosis during a 6-h serum starvation period. Mitogenicity directly correlates with dosage and occurs maximally at ∼72 h, resulting in cell numbers 6- to 20-fold greater than those in controls. Furthermore, our data indicate that B. henselae lysates are much less mitogenic than B. bacilliformis lysates, suggesting these related pathogens use different means of enhancing host cell proliferation. Our biochemical and immunological analyses show that GroEL is involved in the observed HUVEC mitogenicity.
MATERIALS AND METHODS
Bacterial strains, cell lines, and cell culture.
B. bacilliformis KC583 (ATCC 35685) or JB584 (5) cells were grown for 4 days at 30°C in 100% relative humidity on heart infusion agar blood plates (HIAB; heart infusion agar [Difco, Detroit, Mich.] containing 4% sheep erythrocytes and 2% sheep serum). B. henselae strain Houston R1302 (ATCC 49793) was grown for 3 days at 37°C, 5% CO2, and 100% relative humidity on HIAB. Escherichia coli strains were grown overnight in Luria-Bertani (LB) broth or plates with appropriate antibiotics as needed. HUVEC cultures were purchased and grown at 37°C in 5% CO2 and 100% relative humidity in EGM (Clonetics, Walkersville, Md.). HUVECs were only used at passages 2 through 6 to minimize variation between experiments.
Bacterial cell fractionation.
B. bacilliformis and B. henselae cell lysates were prepared by harvesting several HIAB plates of bacteria into ice-cold phosphate-buffered saline (PBS; pH 7.4) and disrupting the cells for 3 min by using 0.1-mm-diameter glass beads and a Mini-Beadbeater 8 (BioSpec, Bartlesville, Okla.). Particulates were removed from the mixture by centrifugation at 16,000 × g for 5 min and/or ultracentrifugation at 100,000 × g for 60 min. The resulting lysate was tested for mitogenicity and served as starting material for chromatographic fractionations. Inner and outer membranes were isolated from total membrane preparations of B. bacilliformis using a sucrose step-gradient ultracentrifugation protocol as previously described (8, 9).
B. bacilliformis was exposed to a temperature upshift to determine if mitogenicity of the lysate could be enhanced. Thirty HIAB plates containing B. bacilliformis were divided into two groups, from which half were maintained at 30°C and the others upshifted to 37°C. Following a 6-h incubation, the plates were removed and harvested into PBS (pH 7.4). The resulting cells were washed twice by centrifugation for 5 min at 4,500 × g and resuspension in PBS. A lysate was prepared from each group as described above.
Culture supernatants were prepared by harvesting Bartonella from HIAB plates (or E. coli DH5α from LB plates), into recovery broth (RB): heart infusion broth containing 5% (wt/vol) bovine serum albumin and 5% (vol/vol) sheep erythrocyte lysate (6). The suspension was centrifuged for 10 min at 4,500 × g, the supernatant was discarded, and the resulting pellet was gently resuspended in RB to an optical density at 600 nm (OD600) of 1.0. The Bartonella and E. coli suspensions were then incubated for 16 h at 30 and 37°C, respectively. Following incubation, the mixtures were centrifuged for 10 min at 4,500 × g, and the resulting supernatants were collected, filter sterilized (0.22-μm-pore-size filter), and immediately tested for HUVEC mitogenicity.
Secreted proteins of B. bacilliformis were identified by harvesting B. bacilliformis cultures grown at 25°C into 10 ml of RB at 25°C to an OD600 of 0.6. The suspension was centrifuged for 10 min at 4,500 × g, and the resulting pellet was gently resuspended in 10 ml of RB and centrifuged again. The final pellet was resuspended in 1 ml of RB and equilibrated for 10 min at 25 or 37°C. Three hundred microcuries of Express [35S]methionine-cysteine mix (Perkin-Elmer, Boston, Mass.) was then added, and the mixture was incubated for an additional 30 min with gentle mixing every 10 min. Cells were subsequently removed by centrifugation at 6,000 × g for 5 min, and the supernatants were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting and autoradiography of the blot.
Endothelial cell growth, proliferation, and apoptosis assays.
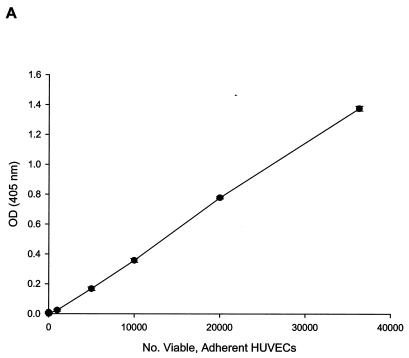
For growth and proliferation experiments, EGM (Clonetics) was replaced with M199 medium (BioWhittaker, Walkersville, Md.) supplemented with penicillin (100 U/ml), streptomycin (0.1 mg/ml), amphotericin B (0.25 μg/ml) (Sigma Chemical, St. Louis, Mo.), and 20% fetal bovine serum (HyClone Laboratories, Logan, Utah). HUVECs were harvested and used to seed 96-well plates with 1,000 cells/well. Each well was brought to a total volume of 100 μl with Bartonella lysate or the fraction to be tested, antiserum, or appropriate controls (e.g., PBS, heated fraction, preimmune serum) plus growth medium. Adherent cell numbers were indirectly determined at 96 h postinoculation by an acid phosphatase assay (11). Strict measurement of cytosolic acid phosphatase was ensured by thoroughly rinsing HUVEC cultures with PBS to remove nonadherent cells and any acid phosphatase released from dead or dying cells. The assay was calibrated with a linear standard curve (Fig. 1A; r2 = 0.99) showing acid phosphatase activity as a function of viable, adherent HUVEC numbers (0 to 36,000 cells by hemocytometer) and encompassed all OD405 values reported in this study (0 to 1.4 OD405 units). A proliferation assay was also performed on 96-h cultures with a bromodeoxyuridine (BrdU) enzyme-linked immunosorbent assay (ELISA) kit per the manufacturer's instructions (Oncogene, La Jolla, Calif.). Mitogenicity of Bartonella lysate on HUVECs was also demonstrated by BrdU incorporation over a 48-h period by ELISA exactly as previously described for B. henselae-HUVEC cocultures (23).
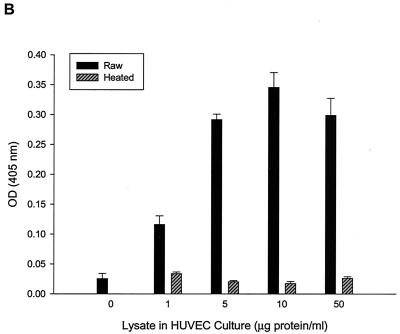
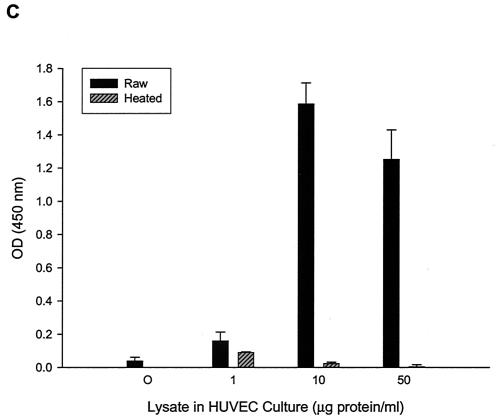
FIG. 1.
HUVEC numbers as a function of increasing doses of B. bacilliformis lysate in the M199-FCS (20% vol/vol) culture medium. (A) Linear (r2 = 0.99) calibration curve showing acid phosphatase activity as a function of viable, adherent HUVEC numbers determined by a hemocytometer. All phosphatase values reported in this study fall within the range of this curve. Dose-response curves were determined by an acid phosphatase assay (B) or a BrdU proliferation assay kit (Oncogene) (C). Data were obtained at 96 h and represent the average of three experiments ± standard error. Raw, raw lysate; heated, lysate pretreated at 100°C for 7 min.
Heat, tryspinization, and polymyxin B were examined for their potential inhibitory effect on lysate mitogenicity. Heated lysates were prepared by incubating for 7 min at 100°C. Trypsinized lysates were prepared by incubating for 40 min at 37°C with trypsin (0 to 1.7 mg/ml). Trypsin was then inactivated with fetal calf serum (FCS; 25% final concentration) prior to addition to HUVEC cultures. Polymyxin B (1,000 U/ml) was mixed with lysate, incubated for 1 h at 37°C, and then added to HUVECs to test for lipopolysaccharide (LPS) inhibition. Control cultures treated with polymyxin B or lysate alone were done in parallel.
A caspase 3 assay kit (Promega, Madison, Wisc.) was used to determine if B. bacilliformis lysate could inhibit apoptosis in HUVECs. HUVECs were grown to 90% confluency in EGM (Clonetics). Apoptosis was induced by a 6-h serum starvation as previously described (23), in the presence of 50 μM Z-VAD-FMK (a cell-permeable pan-caspase inhibitor) or B. bacilliformis lysate (10 μg of protein/ml). Following incubation, treated HUVECs were harvested, and cell extracts were prepared and analyzed by a CaspACE colorimetric assay, as instructed by the manufacturer (Promega).
Protein assay, SDS-PAGE, and immunoblot analysis.
Protein concentrations of samples were determined with a bicinchoninic protein assay kit and a bovine serum albumin standard (Pierce, Rockford, Ill.). SDS-PAGE was done by standard methods (3). Briefly, protein samples were solubilized in an equal volume of Laemmli sample buffer (LSB), boiled for 10 min, and then separated (∼20 μg of protein/lane) on 12.5% (wt/vol) acrylamide SDS-PAGE gels. Resulting gels were fixed and stained with Coomassie brilliant blue or AgCl by standard protocols (3, 38). Immunoblots were prepared by transferring unfixed or unstained gels to supported nitrocellulose (0.45-μm pore size; Osmonics, Minnetonka, Minn.) by the general methods of Towbin et al. (35). Blots were probed with a 1:1,000 dilution of rabbit anti-E. coli GroEL antibodies, rabbit anti-E. coli GroES antibodies (Sigma), rabbit anti-B. bacilliformis whole-cell antiserum (32), or rabbit anti-B. bacilliformis GroEL antiserum prepared as described below. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma) was used at a 1:2,000 dilution to detect the primary antibody-antigen complexes. Bands were visualized by using H2O2 and 4-chloronaphthol as previously described (32). Autoradiographs were prepared by exposing XAR-5 X-ray film (Kodak, Rochester, N.Y.) to the immunoblots overnight.
Column chromatography.
A Biologic high-resolution chromatography system (Bio-Rad, Hercules, Calif.) was used to identify and purify the mitogenic protein from B. bacilliformis lysates. Briefly, lysates were dialyzed overnight against a mixture of 50 mM Tris (pH 8.0), 0.5 mM EDTA, and 10% glycerol and then loaded onto a 1-ml Econo-Pac High Q cartridge (Bio-Rad). The column was then eluted with a 15-ml linear gradient from 0 to 0.5 M NaCl at 1 ml/min, followed by a 5-ml gradient from 0.5 to 1 M NaCl (in 50 mM Tris [pH 8.0], 0.5 mM EDTA, 10% glycerol) at 1 ml/min. Resulting fractions (1 ml) of this and all other chromatographic preparations were individually tested for mitogenic activity in HUVEC cultures, and their protein profiles were analyzed by SDS-PAGE.
Mitogenic fractions from the High Q purification were pooled and dialyzed overnight against 10 mM sodium phosphate (pH 6.8) and 10% glycerol. The mixture was then loaded onto a 5-ml Econo-Pac CHT-II hydroxyapatite column (Bio-Rad). The column was subsequently eluted with a 50-ml linear gradient of 10 to 450 mM sodium phosphate (pH 6.8) in 10% glycerol, followed by a 10-ml linear gradient of 450 to 900 mM sodium phosphate (pH 6.8) in 10% glycerol at 1 ml/min. The resulting fractions (1 ml) were dialyzed overnight against PBS (pH 7.4) and filter sterilized.
Mitogenic fractions obtained from the High Q purification were also dialyzed against a mixture of 10 mM sodium phosphate (pH 6.8), 0.5 mM EDTA, and 10% glycerol and loaded onto a 1.3-ml UNO S1 column (Bio-Rad). This column was eluted with a 15-ml linear gradient of 0 to 0.5 M NaCl and 0.5 to 5 mM EDTA, followed by a 5-ml linear gradient of 0.5 to 1 M NaCl and 5 to 10 mM EDTA (in 10 mM sodium phosphate [pH 6.8], 10% glycerol) at 1 ml/min. Resulting fractions were dialyzed against PBS (pH 7.4) and filter sterilized.
DNA manipulation, cloning, and expression of groEL and the groES-groEL operon.
The B. bacilliformis groEL gene was cloned by a PCR-based strategy. Briefly, PCR primers were designed from a groEL sequence (GenBank accession no. Z15160), including BbGroEL-Fwd (ATGGCTGCTAAAGAAGTAAAATTTG) and BbGroEL-Rev (TTAGAAATCCATTCCGCCCATTC). PCR was done with a GeneAmp PCR reagent kit per the manufacturer's instructions (Applied Biosystems, Branchburg, N.J.) together with 0.1 μg of each primer per 100-μl reaction mixture. Thirty cycles of 1 min at 94°C, 2 min at 55°C, and 2 min at 72°C were done with a GeneAmp 2400 thermal cycler (Perkin-Elmer). The resulting amplicon was purified from an ethidium bromide-stained 1% agarose gel with a GenClean II kit per the manufacturer's instructions (Qbiogene, Carlsbad, Calif.). The purified groEL amplicon was cloned into pCR2.1-TOPO and used to transform E. coli (TOP10) by using a TOPO TA cloning kit per the manufacturer's instructions (Invitrogen, Carlsbad, Calif.). The resulting plasmid, pGROEL-TOPO, was purified to high concentration with a Midi-Prep kit (Qiagen, Valencia, Calif.).
pGROEL-TOPO was digested to completion with PstI and BamHI to yield a ∼1,600-bp fragment of groEL. The fragment was subsequently recloned into compatible sites of pQE31 (Qiagen) by standard protocol (3), to generate an N-terminal six-histidine translational fusion construct, termed pQE31-GROEL. To induce expression of the groEL fusion, E. coli M15-(pREP4) harboring pQE31-GROEL was grown for 1 h at 37°C with shaking (250 rpm). Isopropyl-β-d-thiogalactosidase (IPTG) was added to a final concentration of 1 mM, and the incubation was allowed to continue for an additional 2.75 h. The bacteria were subsequently harvested, and recombinant GroEL was batch purified from the lysates by affinity chromatography with Ni-nitrilotriacetic acid resin per the manufacturer's instructions (Qiagen). The resulting recombinant GroEL was dialyzed overnight against PBS (pH 7.4) prior to use in HUVEC cultures.
A functional groES-groEL operon of B. bacilliformis was also cloned. The amplicon produced by the BbGroEL primers described above was used to probe a λ ZAP Express (Stratagene, La Jolla, Calif.) library of B. bacilliformis. Radiolabeling of the probe and high-stringency DNA hybridizations were done as described before (5). In vivo excision of a λ clone was done per the manufacturer's instructions, resulting in pGROESL-CMV. Automated sequence analysis of this plasmid was done as previously described (8) with divergent primers for the 5′ and 3′ ends of groEL, to verify that the operon was intact. PCR primers for the entire operon were designed from the resulting sequence (BbGroESL Operon-Fwd, TTAAATCGACGTATAAAGAAATTTG; and BbGroESL Operon-Rev, CTTTTACAATGCAGCTCTTTCAATG). PCR with these primers was done as described above, and the amplicon was cloned into pCR2.1-TOPO according to the manufacturer's instructions (Invitrogen), producing pGROESL-TOPO. The operon was subsequently recloned by a standard method (3) into the shuttle vector pBBR1-MCS2 (24) to produce pGRO1. The pGRO1 construct was then electroporated into B. bacilliformis (strain JB584) as previously described (5), to generate strain LSS100. A control strain was made by transforming JB584 with pBBR1-MCS2, to form strain LSS001.
Antibodies.
Recombinant GroEL was prepared as described above, solubilized with LSB, and applied to preparative SDS-PAGE gels (12.5% acrylamide gels without wells). Resulting gels were rinsed twice for 10 min in distilled water and then stained with Coomassie brilliant blue (0.05% [wt/vol] in H2O). Visible GroEL bands were excised from the gels and used to generate rabbit anti-GroEL antiserum as previously described, at a dosage of ∼0.1 mg of GroEL per immunization (32). Antibodies to the B. bacilliformis whole-cell and flagellin protein were previously described and employed in this study (32). Bacteriophage-like particles (BLPs) were prepared as described before (4) and used to generate anti-BLP antiserum as previously detailed (32). To examine antibody-mediated inhibition of mitogenicity, 0.6 μl of lysate and 12 μl of rabbit anti-B. bacilliformis GroEL antiserum or preimmune serum as a control were incubated for 2 h at 25°C. The mixture was then brought to 900 μl with M199-FCS (20%) and aliquoted to HUVEC cultures at 100 μl/well.
Statistical analysis.
Statistical significance was determined with Student's t test. A P value of < 0.05 was considered significant for all studies. Experiments were performed a minimum of three times in triplicate. Mean values ± the standard error of the means are reported.
RESULTS AND DISCUSSION
There are few reports concerning the molecular basis for Bartonella-induced neovascularization in humans. A number of early reports designed to explain these in vivo phenomena focused on identifying bacterial factors that were directly mitogenic for vascular endothelial cells (10, 16, 17, 27). However, none of the studies identified the putative proteins responsible. More recently, work with B. henselae has shown a direct linkage between paracrine-derived VEGF or cytokines (e.g., IL-8 and IL-1β) and enhanced endothelial cell proliferation (22, 29). This proliferative stimulation is also apparently augmented by inhibition of endothelial cell apoptosis by an undescribed, secreted factor from B. henselae (23). Clearly, a complex, multifaceted strategy designed to increase the numbers of endothelial cell hosts is evidently employed by Bartonella.
B. bacilliformis lysate contains a dose-dependent mitogen for HUVECs that does not affect apoptosis.
To identify an optimal concentration range for the B. bacilliformis proliferative factor and to determine if the growth response is dose dependent, various concentrations of soluble cell lysate were added to HUVEC cultures. Adherent, viable HUVEC numbers were indirectly determined after a 96-h culture period by using an acid phosphatase assay calibrated for use with HUVECs according to a linear standard curve (r2 = 0.99) over the entire range of acid phosphatase values reported in this study (Fig. 1A). Data show that the B. bacilliformis lysate is dose dependent and saturating at 5 to 10 μg of lysate protein per ml of culture medium and significantly enhances cell numbers (∼20-fold) relative to those of controls treated with equal volumes of heated lysate (Fig. 1B). Activity was lost, regardless of dose, if the lysate was heated (100°C for 7 min) prior to use. These data are in agreement with those of Garcia et al. (17), who also found that the proliferative factor was heat sensitive. Similar dose-response profiles were obtained if HUVEC numbers were quantified by an ELISA-based BrdU incorporation assay (Fig. 1C).
Previous work by Garcia et al. (17) determined that the factor was not cytotoxic for HUVECs, implicating a molecule other than LPS. To build upon these observations, we tested the lysate's sensitivity to trypsin and polymyxin B (LPS inhibitor). Our results indicate that the mitogenic activity is significantly reduced by trypsin in a dose-dependent fashion (e.g., a 25% reduction in activity results when trypsin is used at 1.7 μg/ml) but is not affected by treatment with polymyxin B (data not shown). Like Garcia et al. (17), we did not observe any overt morphological differences between HUVECs treated with lysate and controls in this or any other experiment. Taken together, these four lines of evidence indicated that the mitogen was a lysate protein and not bacterial LPS.
Garcia et al. (17) showed a threefold increase in HUVEC proliferation in response to B. bacilliformis; a value that is considerably lower than those observed in this study. We ascribe this discrepancy to differences in protocol. Specifically, Garcia et al. (17) quantified HUVECs by using a Coulter counter, which enumerates both live and dead cells in control and test wells. As such, their proliferation data are very different from those in the acid phosphatase assay, which indirectly quantifies only viable cells. In addition, their working medium and lysate purification and lysate application protocols differed from those in this study.
We observed considerable variation in HUVEC proliferative responses to the Bartonella lysate or its fractions between experiments. We believe this variation is caused by passage number, because increased HUVEC passage consistently resulted in decreased responsiveness. We therefore employed HUVECs from passages 2 to 6 and conducted internal controls on all assays to establish a baseline for that particular experiment. Regardless of the variation seen between individual experiments, the data trends in this study were consistent and reproducible.
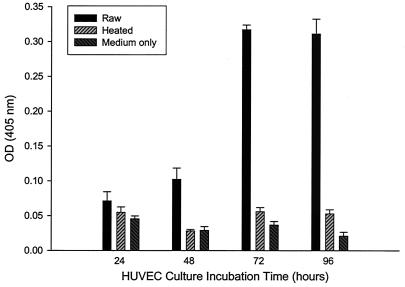
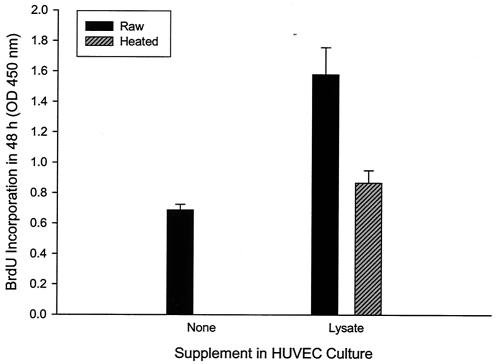
The kinetics of the lysate's proliferative activity was examined over a 96-h period. Data show that HUVEC mitogenicity occurs maximally at approximately 72 h in culture, with cell numbers stable for at least an additional 24 h (Fig. 2). To ensure that the increased number of HUVECs was a result of increased proliferation in response to the lysate and not inhibition of apoptosis as previously reported for HUVECs cocultured with B. henselae (23), we examined BrdU incorporation over a 48-h period. BrdU incorporation was significantly increased (82% greater) in HUVEC cultures treated with lysate compared to that in control cultures grown in the presence of heated lysate (Fig. 3). These data suggest that elevated HUVEC numbers in response to B. bacilliformis lysate are a result of increased proliferation rates, suggesting that the factor is a mitogen.
FIG. 2.
Numbers of HUVECs as a function of incubation time in culture medium containing B. bacilliformis lysate (10 μg of protein/ml). Data were obtained at the indicated times with an acid phosphatase assay and represent the average of three experiments ± standard error. Control experiments with cells grown in M199-FCS (20% vol/vol) medium only or medium supplemented with heated lysate (100°C for 7 min) were also done.
FIG. 3.
Mitogenicity of the B. bacilliformis lysate for HUVECs, as demonstrated by BrdU incorporation over a 48-h incubation. Data were obtained with a BrdU proliferation assay kit (Oncogene) exactly as previously described for B. henselae (23) and represent the average of six experiments ± standard error. Bartonella lysate was used at 10 μg of protein/ml of medium. A control experiment with M199-FCS (20% vol/vol) medium alone was also done.
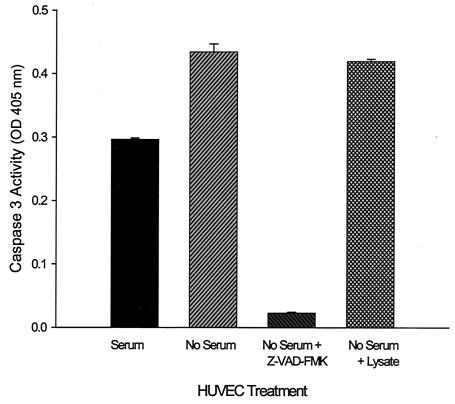
As a corollary to this experiment, we tested the lysate's ability to inhibit HUVEC apoptosis triggered by serum starvation as previously described by Kirby and Nekorchuk (23). For this experiment, we chose to measure caspase 3 because of its central role in programmed cell death (21). As expected, caspase 3 activity was significantly increased (1.5-fold) in response to serum starvation. Z-VAD-FMK, a known inhibitor of apoptosis (2), significantly reduced caspase 3 activity when provided to apoptotic HUVECs (5% of serum-starved cells). However, there was no significant difference in caspase 3 activity between serum-starved cells and serum-starved cells supplemented with Bartonella lysate, suggesting that the proliferative factor does not inhibit apoptosis during a 6-h serum starvation period (Fig. 4).
FIG. 4.
B. bacilliformis lysate does not inhibit caspase 3 activity in serum-starved HUVECs. Caspase 3 activity as a function of HUVEC treatment is shown. Data were obtained by serum starving HUVECs for 6 h in M199 medium containing Z-VAD-FMK (50 μM), B. bacilliformis lysate (10 μg/ml), or no supplement. A negative control experiment with cells grown in M199-FCS (10%) was also done exactly as previously described (23). Caspase 3 activity data were obtained with a CaspACE assay kit (Promega) and represent the average of four independent determinations ± standard error.
B. bacilliformis lysate is significantly more potent than B. henselae as a mitogen for HUVECs.
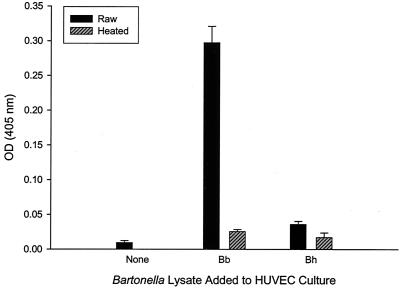
Previous work by Kirby and Nekorchuk (23) showed that elevated HUVEC numbers in cocultures containing B. henselae were primarily caused by inhibition of apoptosis, rather than stimulation of proliferation. To examine the apparent discrepancy between the results of that study and this work, we prepared a B. henselae cell lysate in a manner similar to B. bacilliformis and assayed its mitogenic activity in a HUVEC culture. In confirmation of recent studies (23, 29), B. henselae lysates possessed relatively minor mitogenic activity (∼2-fold greater than heated lysate controls) in contrast to the B. bacilliformis lysate (∼12-fold greater than heated lysate controls) (Fig. 5). Interestingly, although these two bacteria are closely related, the existing data suggest that they may employ different strategies for triggering endothelial cell proliferation.
FIG. 5.
Comparison of B. bacilliformis and B. henselae lysate mitogenicities for HUVECs. Data were obtained at 96 h with an acid phosphatase assay and represent the average of three experiments ± standard error. Bartonella lysates were used at a final concentration of 10 μg of protein/ml of medium. Experiments with HUVECs grown in M199-FCS (20% vol/vol) medium or medium containing heated lysate (100°C for 7 min) were done as controls. Bh, B. henselae; Bb, B. bacilliformis.
Mitogenic activity is located in both soluble and insoluble fractions of the B. bacilliformis cell and in culture supernatants.
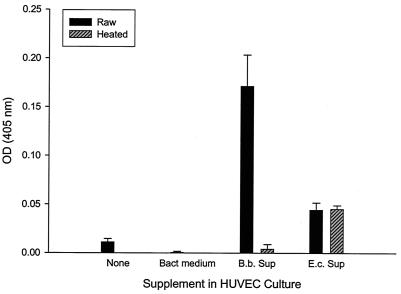
One inconsistent observation from previous studies of the Bartonella proliferative factor concerned its cellular location within the bacterium. Work with B. henselae suggested the factor was located in the insoluble, cell wall fraction of the bacterium (10); however, subsequent work showed it was apparently soluble and secreted (27). In contrast, studies with B. bacilliformis suggested that its factor was located in the soluble fraction of a cell lysate (17). Therefore, we fractionated the B. bacilliformis cell into soluble (lysate) and insoluble (pellet) fractions and also collected supernatants from 16-h bacterial cultures. Each fraction (10 μg/ml) was then tested for its mitogenic activity on HUVECs. Data show that the majority of mitogenic activity was found in the soluble lysate fraction (approximately sixfold increase over heated lysate control), although the insoluble pellet fraction also possessed significant activity (approximately twofold increase over heated pellet control) (data not shown). Culture supernatants were also found to be significantly mitogenic for HUVECs, with a 342-fold increase in cell numbers relative to HUVEC cultures supplemented with an equal volume of bacterial culture medium, suggesting that the factor was secreted during bacterial growth (Fig. 6). It is important to note that the apparent 342-fold increase in adherent cell number was determined relative to controls containing tissue culture medium supplemented with sterile bacterial culture medium and is likely a result of the extensive death observed in the control wells (verified by microscopy), not exaggerated growth stimulated by the supernatant. Parallel experiments using equal concentrations of an E. coli (DH5α) culture supernatant showed a lower but significant (∼89-fold) increase over HUVEC cultures treated with bacterial culture medium only. The E. coli mitogen is heat resistant and thus likely LPS (Fig. 6).
FIG. 6.
Mitogenicity of the B. bacilliformis culture supernatant. HUVECS grown in M199-FCS (20% vol/vol) medium containing equal volumes (45 μl) of a filter-sterile supernatant from a B. bacilliformis or E. coli DH5α culture (OD600 = 1.0 for both) were assayed for HUVEC numbers at 96 h by using an acid phophatase assay. HUVECs grown in M199-FCS (20% vol/vol) medium alone (none), in tissue culture medium containing 45 μl of sterile bacterial culture medium (Bact medium), or with heated B. bacilliformis (B.b.) or E. coli (E.c.) bacterial culture supernatants (Sup; 100°C for 7 min) were also conducted. Data represent the average of three experiments ± standard error.
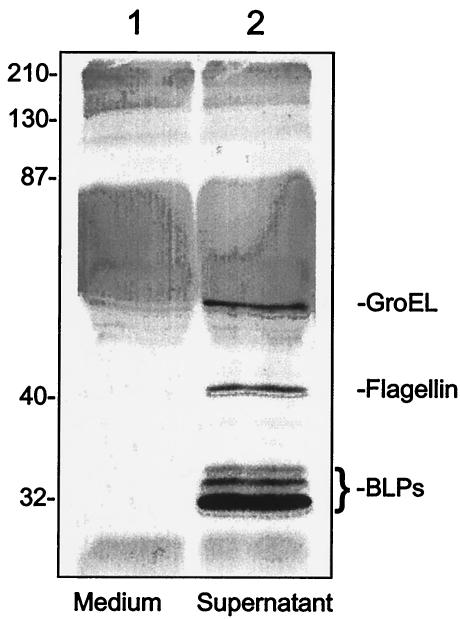
We therefore analyzed Bartonella culture supernatants for any bacterium-derived proteins in hopes of identifying a potential candidate for the mitogen. Silver-stained SDS-PAGE gels (data not shown) and immunoblots developed with anti-B. bacilliformis whole-cell antibody or antibodies specific to particular Bartonella proteins identified five Bartonella polypeptides in the culture supernatant, including the 42-kDa flagellin protein (32); three BLP proteins with sizes of 32, 34, and 36 kDa (4); and GroEL (61 kDa) (Fig. 7).
FIG. 7.
Immunoblot showing B. bacilliformis proteins translocated to the growth medium over a 16-h incubation. Culture supernatants were prepared (see Materials and Methods), separated by SDS-PAGE (12.5% acylamide), and blotted to nitrocellulose, and the immunoblot was developed with anti-B. bacilliformis whole-cell antibody (32). Lanes: 1, medium alone; 2, supernatant from the spent growth medium. The positions of the bacterium-specific proteins, GroEL, flagellin, and the BLPs are indicated. The identity of the proteins was determined by using immunoblots developed with monospecific antibodies (not shown). Molecular mass standards are indicated to the left in kilodaltons.
The presence of GroEL correlates with mitogenic activity, and antibodies to GroEL or GroES inhibit mitogenicity of the lysate.
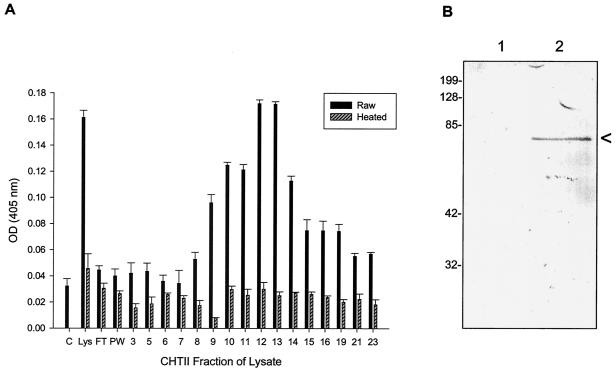
A B. bacilliformis cell lysate preparation was sequentially fractionated by high-resolution column chromatography employing a variety of resins. Resulting fractions from each chromatographic separation were individually tested for HUVEC mitogenicity in vitro. The mitogenic activity in the eluted fractions from a hydroxyapatite (CHT-II) column fractionation shows a broad, heat-sensitive peak (Fig. 8A), a feature also seen in anionic and cationic exchange columns (data not shown). Fractions corresponding to peak mitogenic activities for HUVECs were pooled and used in subsequent chromatographic fractionations. In addition to assaying HUVEC mitogenicity, the protein profile for each fraction was obtained by SDS-PAGE, followed by staining the gel with AgCl to maximize sensitivity of protein detection. A protein of ∼61 kDa was found to be present in the mitogenic fractions and was not detected or was barely detectable in fractions that did not exhibit significant mitogenicity. Given the prominence of this protein band and its molecular mass, we hypothesized that it was GroEL. This was verified by using immunoblots developed with commercial anti-E. coli GroEL IgG (Sigma). An immunoblot comparing GroEL levels in a poorly mitogenic CHT-II fraction (fraction 7 of Fig. 8A) with a significantly mitogenic CHT-II fraction (fraction 12 of Fig. 8A) is shown in Fig. 8B (lanes 1 and 2, respectively).
FIG. 8.
Differential HUVEC numbers in response to CHT-II fractions of the B. bacilliformis lysate. (A) HUVEC cultures were grown in M199-FCS (20% vol/vol) medium containing 40 μl of each chromatographic fraction (no. 3 to 23) and assayed at 96 h with an acid phosphatase assay. The data represent the average of three experiments ± standard error. Control experiments with cells grown in medium alone (C), medium with lysate (Lys) at 10 μg/ml, or heated samples (100°C for 7 min) were also done. FT and PW cultures were treated with flowthrough and prewash from the column, respectively. (B) Immunoblot showing absence of GroEL in a fraction with insignificant mitogenicity (lane 1, fraction 7) and a prominent GroEL band in a highly mitogenic CHT-II fraction (lane 2, fraction 12). A correlation between GroEL presence and mitogenicity was observed in the other fractions as well (not shown).
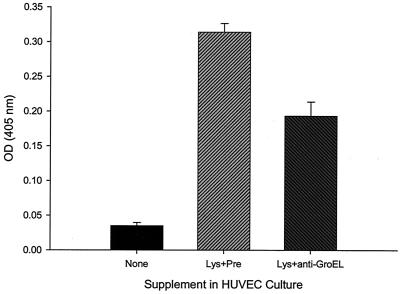
After determining that the protein in the mitogenic fractions was GroEL, we generated monospecific rabbit anti-B. bacilliformis GroEL antiserum and assayed for its inhibitory effect on the lysate's mitogenicity. Data from this assay showed that the anti-B. bacilliformis GroEL antiserum significantly reduces the lysate's mitogenicity by ∼40% when compared to that in controls treated with an equal volume of preimmune rabbit serum (Fig. 9). In fact, preimmune serum, containing growth factors and multiple irrelevant antibody idiotypes, actually enhanced HUVEC growth. Our inability to completely abrogate mitogenic activity with anti-GroEL antiserum suggests that other proliferative factors may be present in the lysate. Substitution with PBS-dialyzed commercial anti-E. coli GroEL or GroES IgG (Sigma) also yielded significant reductions (∼30 and ∼39%, respectively) in mitogenicity of the B. bacilliformis lysate relative to that of PBS controls (data not shown). That anti-GroES antibodies also inhibited activity of the lysate is not surprising, because GroEL would likely be complexed with GroES in the Bartonella cell lysate. Taken together, these data implicate GroEL and potentially the GroEL-GroES complex of this bacterium as a participant in the observed HUVEC mitogenicity.
FIG. 9.
Significant inhibition of HUVEC mitogenicity by pretreatment of the lysate with anti-GroEL antiserum. Pretreatment of B. bacilliformis lysate with rabbit anti-GroEL antiserum for 2 h prior to adding it to the M199-FCS (20% vol/vol) culture medium (10 μg of lysate protein/ml) resulted in a significant reduction in mitogenic activity compared to that of parallel controls treated with equal volumes of rabbit preimmune serum (Pre). Samples were assayed at 96 h with an acid phosphatase assay. The data represent the average of six experiments ± standard error.
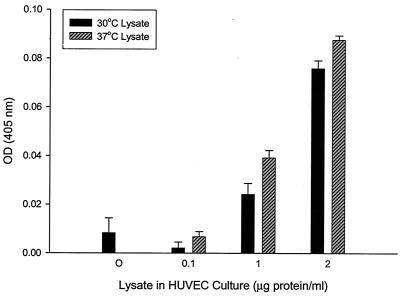
Given the inducibility of groEL during cellular stress, we hypothesized that temperature upshift to a B. bacilliformis culture would augment the mitogenicity of its respective lysate. To examine this possibility, Bartonella cultures were grown for 4 days at 30°C and then upshifted to 37°C for a period of 6 h. Cell lysates were subsequently prepared from these and parallel cultures maintained at 30°C. A dose-response comparison of the resulting lysates showed that at 0.1 to 2.0 μg of protein/ml, lysates from the upshifted cultures produced a significant increase (15 to 224%) in cell numbers relative to 30°C culture-lysate counterparts (Fig. 10). There was also a 9% increase at a 10-μg/ml dosage, but the difference was not statistically significant from the level in the corresponding 30°C control (data not shown).
FIG. 10.
Significant increase in mitogenic activity of B. bacilliformis lysate in response to temperature upshift of the source culture. Shown are HUVEC numbers in response to increasing doses of B. bacilliformis lysates obtained from a 30°C-grown culture and from a culture shifted to 37°C for 6 h prior to lysate preparation. Acid phosphatase assays were done after 96 h of HUVEC growth in M199-FCS (20% vol/vol) supplemented with lysates from cultures grown under the two temperature regimens. Data represent the average of three experiments ± standard error.
GroEL is found in the inner and outer membranes of B. bacilliformis and is actively secreted.
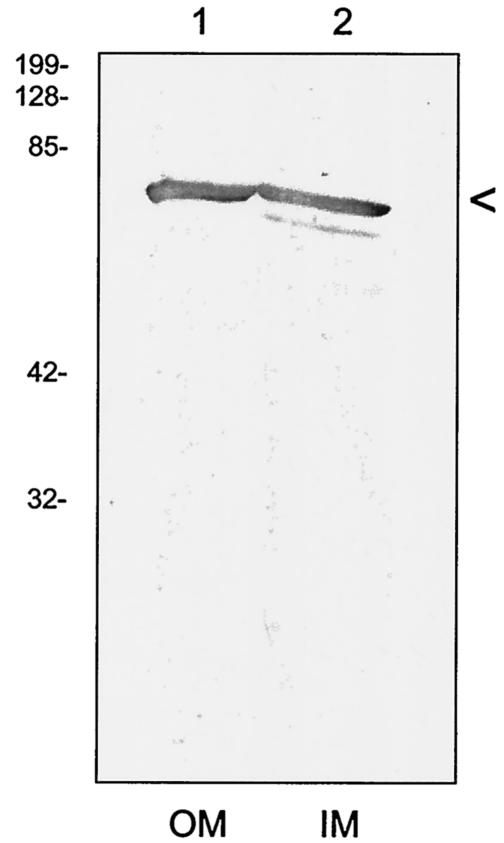
Although our data revealed that the majority of mitogenicity was located in the soluble fraction of the B. bacilliformis cell, we also found significant activity in the insoluble cell pellet (not shown). This contradiction is reminiscent of earlier reports suggesting that the B. henselae proliferative factor was found in both soluble and insoluble fractions of the bacterium (10, 27). We used immunoblots to determine if GroEL was present in membranes of B. bacilliformis prepared by sucrose gradient density ultracentrifugations. The results show that GroEL is present in both inner and outer membranes of the bacterium and is found in similar quantities by visual inspection (Fig. 11). The compiled data show that GroEL is present in a variety of cellular locations within B. bacilliformis, including its soluble fraction, insoluble fraction, and culture supernatant.
FIG. 11.
GroEL is present in both inner and outer membranes of B. bacilliformis. Inner and outer membranes (IM and OM, respectively) of B. bacilliformis were prepared as previously described (8, 9), separated by SDS-PAGE (12.5% acrylamide), and then blotted to nitrocellulose. The resulting immunoblot was developed with an anti-GroEL antiserum. The positions of GroEL are indicated for outer membranes in lane 1 and inner membranes in lane 2. Molecular mass standards are given to the left in kilodaltons.
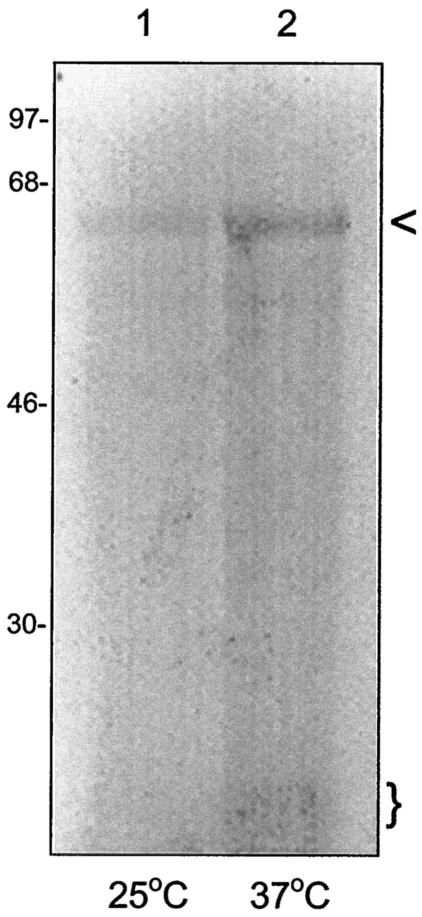
Our experiments during this study showed that GroEL was one of five detectable proteins in culture supernatants analyzed by SDS-PAGE and immunoblotting (Fig. 7); however, the results did not directly demonstrate whether GroEL is actively secreted by B. bacilliformis. To examine this possibility, freshly harvested bacteria from a 25°C culture were pulse-labeled for 30 min at 25 or 37°C in HIAB broth containing 300 μCi of [35S]Met/Cys per ml. An aliquot of the culture supernatant was then separated by SDS-PAGE and blotted to nitrocellulose. The resulting blot was developed by using anti-E. coli GroEL IgG (Sigma) and subsequently autoradiographed. The resulting autoradiograph shows that within the 30-min incubation time, a 61-kDA polypeptide (identified as GroEL by the immunoblotting prior to autoradiography) was the only radiolabeled protein in the supernatant, although a minor band of ∼10 kDa was also detectable after temperature upshift to 37°C (Fig. 12, lane 2). This small protein may be GroES based upon its conserved molecular mass among bacteria and the fact that it complexes with GroEL. As expected, the quantity of secreted GroEL was greater following a temperature upshift relative to the 25°C sample (Fig. 12). The mechanism for secretion of GroEL by Bartonella is unknown, and we are currently investigating the possible involvement of the Bartonella VirB/VirD type IV secretion system (33). Secretion of GroEL has been previously reported in Helicobacter pylori, where the protein can be found extracellularly in culture supernatants (36) and may serve to protect secreted urease enzyme (13). In addition, GroEL secretion has been demonstrated in Legionella pneumophila, where the protein is mainly associated with the cell envelope of the bacterium (18).
FIG. 12.
GroEL is secreted by B. bacilliformis. B. bacilliformis was harvested and radiolabeled for 30 min at 25 or 37°C in HIAB containing 300 μCi of 35S-Express per ml (NEN-Dupont). Culture supernatants were subsequently isolated, separated by SDS-PAGE, and blotted to nitrocellulose, and the immunoblot was developed with anti-GroEL antibody. The blot was then autoradiographed. The resulting autoradiograph is shown and reveals a single radiolabeled 61-kDa protein identified as GroEL (arrow) in the 25°C sample (lane 1) and GroEL plus an ∼10-kDa radiolabeled protein in the 37°C sample (lane 2, parentheses). Molecular mass values are given to the left in kilodaltons.
Recombinant GroEL and HUVEC proliferation.
Although in vitro GroEL expression, synthesis, and purification of a recombinant GroEL fusion protein were successful in our hands as determined by SDS-PAGE (data not shown), significant mitogenic activity in HUVEC cultures treated with the fusion protein was not observed, regardless of dosage (4 ng/ml to 5 μg/ml). Possible reasons for these negative results include inhibition of activity from the six-His tag on the N terminus of the fusion protein, lack of the GroES subunit, or an improperly formed GroEL complex due to overexpression in E. coli.
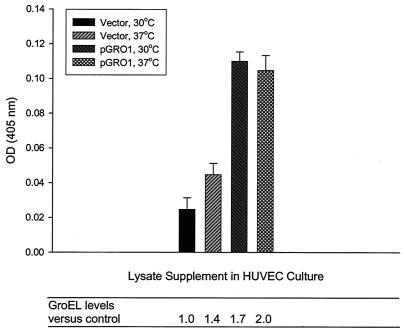
To overcome these problems, a functional B. bacilliformis groES-groEL operon was cloned into a multicopy, broad-host-range shuttle vector to form the plasmid pGRO1. Functional expression of the operon was verified by complementing a temperature-sensitive groEL mutant strain of E. coli (NRK117) (25) with pGROESL-TOPO according to the general methods of Radulovic et al. (28; data not shown). A Bartonella strain transformed with pGRO1 was generated and designated LSS100. A control strain containing the cloning vector was also produced and designated LSS001. The relative level of GroEL in lysates prepared from these strains was quantified by immunoblot densitometry (not shown) and indicated that levels of GroEL were 70 and 43% greater in LSS100 than in the control strain at treatment temperatures of 30 and 37°C, respectively (Fig. 13). HUVEC mitogenicity assays with these lysates showed a direct correlation between GroEL levels and HUVEC mitogenicity (r2 = 0.85) (Fig. 13). HUVEC numbers following treatment with the LSS100 lysate (1.0 μg/ml) were significantly greater than those treated with control LSS001 lysates: at 30°C, an approximately 4.5-fold increase in HUVEC number was obtained, and at 37°C, an ∼2.3-fold increase was observed (Fig. 13). Similar profiles were also obtained with lysate supplements of 0.5, 1.5, and 2.0 μg/ml, whereas saturation occurred at concentrations greater than 5 μg/ml (data not shown). Lack of a significant difference between LSS100 lysates from the two temperature treatments suggests that GroEL saturation occurred.
FIG. 13.
HUVEC numbers correlate with GroEL levels in the Bartonella lysate treatment. HUVEC numbers in response to treatment with B. bacilliformis lysates from strains LSS100 (containing pGRO1, a multicopy plasmid with the groES-groEL operon) and LSS001 (containing the pBBR1-MCS2 vector) cultivated at 30°C or shifted to 37°C for 6 h prior to lysate preparation. Acid phosphatase assays were done after 96 h of HUVEC growth in M199-FCS (20% vol/vol) supplemented with lysates (1.0 μg/ml) from cultures subjected to the two temperature regimens. GroEL levels in the lysates, relative to those in the 30°C vector control, were determined by immunoblot densitometry and are indicated below the corresponding bars of the graph. Data represent the average of three experiments ± standard error.
Although GroEL has been shown to activate endothelial cells by inducing a variety of cytokines (e.g., granulocyte-macrophage colony-stimulating factor, IL-6, and IL-1) and adhesion molecules (ICAM-1, VCAM-1, and E-selectin) (15, 34), this report is the first, to our knowledge, to implicate bacterial GroEL in the proliferation of human vascular endothelial cells. Interestingly, GroEL mitogenicity for other cell types has been reported from various pathogenic bacteria. For example, GroEL from Chlamydia pneumoniae is mitogenic for human vascular smooth muscle cells in culture (31). GroEL from Actinobacillus actinomycetemcomitans is mitogenic for epithelial cells at low dose and is cytotoxic at higher concentrations (20, 40). GroEL from A. actinomycetemcomitans apparently triggers epithelial cell growth via the ERK1/2 and mitogen-activated protein kinase signaling pathways (40).
The mechanism whereby B. bacilliformis GroEL serves as a proliferative factor is unknown, and its role may be indirect. For example, GroEL from Mycobacterium tuberculosis is a potent stimulator of cytokine synthesis (26). In L. pneumophila, GroEL can induce IL-1β in macrophages during infection (30) and correlates with virulence (14). B. bacilliformis GroEL could possibly stimulate synthesis of angiogenic cytokines by HUVECs (e.g., IL-6, IL-8, or IL-1β), which could act in an autocrine fashion to stimulate endothelial cell proliferation in vitro. Given its chaperonin function, GroEL could conceivably protect or even help fold a mitogenic protein, thereby contributing to its activity.
Although we did not observe inhibition of HUVEC apoptosis in cultures treated with B. bacilliformis lysate, inhibition of cell death via an alternative pathway that does not involve caspase 3 is possible. Previous work has shown that bacterial GroEL or GroEL fragments can inhibit apoptosis in a variety of mammalian cells (7, 19). In addition, GroEL is suspected of playing a role in conferring apoptosis resistance to HeLa 229 cells that are persistently infected with Chlamydia trachomatis (12).
In conclusion, the most important findings from this study are (i) the B. bacilliformis proliferative factor is a dose-dependent, proteinaceous mitogen for HUVECS that does not inhibit apoptosis by the capase 3 pathway within a 6-h serum starvation period; (ii) the mitogen is found in bacterial culture supernatants, the soluble cell fraction, and to a lesser degree in insoluble cell fractions of the bacterium; (iii) soluble cell fractions from B. henselae possess significantly less mitogenicity for HUVECs than those from B. bacilliformis; (iv) B. bacilliformis GroEL is a participant in the observed HUVEC mitogenicity; and (v) GroEL is located in the soluble and membrane fractions of the cell and is actively secreted by B. bacilliformis.
Acknowledgments
This work was made possible by American Heart Association Established Investigator grant 9940002N to M.F.M. M.F.M was also supported by NIH grants AI52101 and AI053111. D.S.S. was supported by NSF grant MCB-9722408.
We gratefully acknowledge Jean Pfau's helpful discussions. Special thanks to Scott Knight and Corbin Schwanke for assistance with chromatography and Christopher Coker for E. coli NRK117.
Editor: B. B. Finlay
REFERENCES
- 1.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson, E. A., M. Barry, A. J. Darmon, I. Shostak, P. C. Turner, R. W. Moyer, and R. C. Bleackley. 1998. Cytotoxic T lymphocyte-assisted suicide. Caspase 3 activation is primarily the result of the direct action of granzyme B. J. Biol. Chem. 273:21261-21266. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 4.Barbian, K. D., and M. F. Minnick. 2000. A bacteriophage-like particle from Bartonella bacilliformis. Microbiology 146:599-609. [DOI] [PubMed] [Google Scholar]
- 5.Battisti, J. M., and M. F. Minnick. 1999. Development of a system for genetic manipulation of Bartonella bacilliformis. Appl. Eviron. Microbiol. 65:3441-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson, L. A., S. Kar, G. McLaughlin, and G. M. Ihler. 1986. Entry of Bartonella bacilliformis into erythrocytes. Infect. Immun. 54:347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmichael, J., J. Chatellier, A. Woolfson, C. Milstein, A. R. Fersht, and D. C. Rubinsztein. 2000. Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington's disease. Proc. Natl. Acad. Sci. USA 97:9701-9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll, J. A., S. A. Coleman, L. S. Smitherman, and M. F. Minnick. 2000. Hemin-binding surface protein from Bartonella quintana. Infect. Immun. 68:6750-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman, S. A., and M. F. Minnick. 2001. Establishing a direct role for the Bartonella bacilliformis invasion-associated locus B (IalB) protein in human erythrocyte parasitism. Infect. Immun. 69:4373-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conley, T., L. Slater, and K. Hamilton. 1994. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J. Lab. Clin. Med. 124:521-528. [PubMed] [Google Scholar]
- 11.Connolly, D. T., M. B. Knight, N. K. Harakas, A. J. Wittwer, and J. Feder. 1986. Determination of the number of endothelial cells in culture using an acid phosphatase assay. Anal. Biochem. 152:136-140. [DOI] [PubMed] [Google Scholar]
- 12.Dean, D., and V. C. Powers. 2001. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect. Immun. 69:2442-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans, D. J., Jr., D. G. Evans, L. Engstrand, and D. Y. Graham. 1992. Urease-associated heat shock protein of Helicobacter pylori. Infect. Immun. 60:2125-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez, R. C., S. M. Logan, S. H. S. Lee, and P. S. Hoffman. 1996. Elevated levels of Legionella pneumophila stress protein Hsp60 early in infection of human monocytes and L929 cells correlate with virulence. Infect. Immun. 64:1968-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galdiero, M., G. C. de l'Ero, and A. Marcatili. 1997. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect. Immun. 65:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, F. U., J. Wojta, and R. L. Hoover. 1992. Interactions between live Bartonella bacilliformis and endothelial cells. J. Infect. Dis. 165:1138-1141. [DOI] [PubMed] [Google Scholar]
- 17.Garcia, F. U., J. Wojta, K. N. Broadley, J. M. Davidson, and R. L. Hoover. 1990. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am. J. Pathol. 136:1125-1135. [PMC free article] [PubMed] [Google Scholar]
- 18.Garduño, R. A., G. Faulkner, M. A. Trevors, N. Vats, and P. S. Hoffman. 1998. Immunolocalization of Hsp60 in Legionella pneumophila. J. Bacteriol. 180:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrido, C., S. Gurbuxani, L. Ravagnan, and G. Kroemer. 2001. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 286:433-442. [DOI] [PubMed] [Google Scholar]
- 20.Goulhen, F., A. Hafezi, V.-J. Uitto, D. Hinode, R. Nakamura, D. Grenier, and D. Mayrand. 1998. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 66:5307-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 22.Kempf, V. A. J., B. Volkmann, M. Schaller, C. A. Sander, K. Alitalo, T. Rieβ, and I. B. Autenrieth. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol. 3:623-632. [DOI] [PubMed] [Google Scholar]
- 23.Kirby, J. E., and D. M. Nekorchuk. 2002. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 99:4656-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 25.Kusukawa, N., T. Yura, C. Ueguchi, Y. Akiyama, and K. Ito. 1989. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 8:3517-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewthwaite, J. C., A. R. M. Coates, P. Tormay, M. Singh, P. Mascagni, S. Poole, M. Roberts, L. Sharp, and B. Henderson. 2001. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (Hsp 65) and contains a CD14-binding domain. Infect. Immun. 69:7349-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeno, N., H. Oda, K. Yoshiie, M. R. Wahid, T. Fujimura, and S. Matayoshi. 1999. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb. Pathog. 27:419-427. [DOI] [PubMed] [Google Scholar]
- 28.Radulovic, S., M. S. Rahman, M. S. Beier, and A. F. Azad. 2002. Molecular and functional analysis of the Rickettsia typhi groESL operon. Gene 18:41-48. [DOI] [PubMed] [Google Scholar]
- 29.Resto-Ruiz, S. I., M. Schmiederer, D. Sweger, C. Newton, T. W. Klein, H. Friedman, and B. E. Anderson. 2002. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect. Immun. 70:4564-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retzlaff, C., Y. Yamamoto, S. Okubo, P. S. Hoffman, H. Friedman, and T. W. Klein. 1996. Legionella pneumophila heat-shock protein-induced increase of interleukin-1 beta mRNA involves protein kinase C signaling in macrophages. Immunology 89:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasu, S., D. LaVerda, N. Qureshi, D. T. Golenbock, and D. Beasley. 2001. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ. Res. 89:244-250. [DOI] [PubMed] [Google Scholar]
- 32.Scherer, D. C., I. DeBuron-Connors, and M. F. Minnick. 1993. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect. Immun. 61:4962-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulein, R., and C. Dehio. 2002. The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol. Microbiol. 46:1053-1067. [DOI] [PubMed] [Google Scholar]
- 34.Tabona, P., K. Reddi, S. Khan, S. P. Nair, S. J. V. Crean, S. Meghji, M. Wilson, M. Preuss, A. D. Miller, S. Poole, S. Carne, and B. Henderson. 1998. Homogeneous Escherichia coli chaperonin 60 induces IL-1β and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J. Immunol. 161:1414-1421. [PubMed] [Google Scholar]
- 35.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanet, A., and A. Labigne. 1998. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect. Immun. 66:1023-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster, G. F., C. J. Cockerell, and A. E. Friedman-Kien. 1992. The clinical spectrum of bacillary angiomatosis. Br. J. Dermatol. 126:535-541. [DOI] [PubMed] [Google Scholar]
- 38.Wray, W., T. Boulikas, V. P. Wray, and R. Hancock. 1981. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 118:197-203. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida, S., M. Ono, T. Shono, H. Izumi, T. Ishibashi, H. Suzuki, and M. Kuwano. 1997. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell. Biol. 17:4015-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, L., S. L. Pelech, D. Mayrand, D. Grenier, J. Heino, and V.-J. Uitto. 2001. Bacterial heat shock protein-60 increases epithelial cell proliferation through the ERK1/2 MAP kinases. Exp. Cell Res. 266:11-20. [DOI] [PubMed] [Google Scholar]