Abstract
The mechanisms by which oscillatory shear stress (OS) induces, while high laminar shear stress (LS) prevents, atherosclerosis are still unclear. Here, we examined the hypothesis that OS induces inflammatory response, a critical atherogenic event, in endothelial cells by a microRNA (miRNA)-dependent mechanism. By miRNA microarray analysis using total RNA from human umbilical vein endothelial cells (HUVECs) that were exposed to OS or LS for 24 h, we identified 21 miRNAs that were differentially expressed. Of the 21 miRNAs, 13 were further examined by quantitative PCR, which validated the result for 10 miRNAs. Treatment of HUVECs with the miR-663 antagonist (miR-663-locked nucleic acids) blocked OS-induced monocyte adhesion, but not apoptosis. In contrast, overexpression of miR-663 increased monocyte adhesion in LS-exposed cells. Subsequent mRNA expression microarray study using HUVECs treated with miR-663-locked nucleic acids and OS revealed 32 up- and 3 downregulated genes, 6 of which are known to be involved in inflammatory response. In summary, we identified 10 OS-sensitive miRNAs, including miR-663, which plays a key role in OS-induced inflammatory responses by mediating the expression of inflammatory gene network in HUVECs. These OS-sensitive miRNAs may mediate atherosclerosis induced by disturbed flow.
Keywords: micro-ribonucleic acid, microarray, blood flow
atherosclerosis is an inflammatory disease that occurs preferentially at particular areas of disturbed flow characterized by low and oscillatory wall shear stress (OS) in branched or curved arteries (10, 31). In contrast, straight arterial regions are exposed to high and stable laminar shear stress (LS) and are well protected from atherosclerosis (31). Recently, our laboratory has shown that disturbed flow caused by partial ligation of mouse carotid artery induces robust atherosclerosis rapidly within 2 wk on a high-fat diet (19), directly demonstrating the causal relationship between disturbed flow and atherosclerosis (19). However, the underlying mechanisms by which disturbed flow induces atherosclerosis still remain unclear.
Gene expression profiles are dramatically altered when endothelial cells (ECs) are exposed to LS or OS. LS is known to increase expression of atheroprotective genes, including Kruppel-like factors 2 and 4 (KLF2 and KLF4), and endothelial nitric oxide synthase (eNOS), while OS stimulates inflammation by overexpression of bone morphogenic protein-4 (BMP4) and adhesion molecules, such as VCAM1, ICAM1, and E-selectin (4, 5, 14, 20, 24, 25). Numerous studies have shown differences between LS- and OS-dependent gene and protein regulation; however, the detailed mechanisms underlying shear dependent gene expression has not been fully elucidated.
MicroRNAs (miRNAs) are a large class of conserved, noncoding, small RNAs that are typically 18–22 nucleotides in length. They repress gene expression posttranscriptionally by interacting mainly with the 3′ untranslated region of specific target mRNAs in a sequence-specific manner (36). Nearly 800 miRNAs are encoded in the human genome, and each targets multiple mRNAs, resulting in mRNA degradation or translational inhibition (30). miRNAs control cell proliferation, differentiation, and apoptosis (2, 15, 35). In ECs, miRNAs regulate cell migration, angiogenesis, and inflammation. In human ECs, knockdown of Dicer or Drosha, two key enzymes for miRNA biogenesis, causes a decrease in angiogenesis (11, 26). More specifically, let-7f and miR-27b have been shown to exert proangiogenic effects (11), while overexpression of miR-221/222 in human umbilical vein EC (HUVEC) inhibits tube formation, migration, and wound healing in response to stem cell factor, suggesting it has an antiangiogenic effect (21). The role of miRNAs in vascular inflammation has been reported. miR-126 was shown to inhibit VCAM-1 expression, a mediator of leukocyte adherence to ECs (8). Also, downregulation of miR-21 decreased neointima formation in the injured rat carotid artery (9).
Although several insights have been made regarding miRNAs governing cellular responses in ECs, the effect of shear stress on miRNA expression is not fully elucidated (22, 33, 34). Given the differential gene regulation between LS and OS, we hypothesized that shear-sensitive miRNAs play critical roles in regulating gene expression and subsequently mediate OS-induced inflammation. To test the hypothesis, we screened the miRNA expression profiles of HUVEC exposed to LS or OS. Through validation studies, we identified 10 OS-sensitive miRNAs. Next, we determined the functional importance of the most OS-induced miRNA, miR-663, and found its specific role in endothelial inflammatory response, but not in apoptosis,. We then carried out an additional genomewide array study to discover the potential target genes of miR-663. This DNA microarray study identified 35 potential miR-663 targets, which include a network of inflammatory genes and transcription factors such as KLF4. Collectively, these results suggest that OS induces inflammatory responses in ECs by altering miRNA expression, such as upregulation of miR-663, which, in turn, mediates expression of networks of genes.
MATERIALS AND METHODS
Cell culture and shear studies.
HUVECs (more than 5 different lots) were purchased from BD Biosciences, cultured in M199 media (Cellgro) with 20% fetal bovine serum (Hyclone) and used between passages 5 and 6. Confluent cells were exposed to unidirectional LS (15 dyn/cm2) or OS (±5 dyn/cm2 at 1-Hz frequency) for 24 h using a cone-and-plate shear device, as described by our laboratory previously (1, 25).
Microarray analysis of miRNA expression and quantitative PCR validation.
Total RNA was isolated with the miRNeasy Mini Kit (QIAGEN) using HUVEC exposed to LS or OS for 1 day. Microarray assay was performed using a service provider (LC Sciences), as described previously (13). Briefly, total RNA samples were size fractionated, and small RNAs (<300 nt) were 3′-extended with a poly(A) tail. An oligonucleotide tag was then ligated to the poly(A) tail for fluorescent dye staining; two different tags (for Cy3 and Cy5 dyes) were used in dual-sample experiments. Hybridization was performed overnight on a μParaflo microfluidic chip (Chip ID miRHuman 12.0 version, LC Sciences). After the fluorescence images were collected, the ratio (Cy3/Cy5, log2 transformed, balanced) and P values were calculated using Student's t-test. Significant signals were those with <0.05 P values. We then validated the array data by quantitative PCR (qPCR). Briefly, the isolated total RNA was polyadenylated and reverse transcribed for use in a two-step qPCR using the NCode miRNA First-Strand cDNA Synthesis and qPCR kits (Invitrogen). The resulting cDNA was subjected to qPCR using the NCode universal reverse primer, in conjunction with a sequence-specific forward primer for selected miRNAs. A master mix was prepared for each PCR, which included SYBR GreenER qRT-PCR SuperMix, forward primer, Universal qPCR Primer, ROX reference dye, and template cDNA. RNU6B was used as the internal control. The reactions were monitored using a preheated real-time instrument (ABI StepOne Plus). The PCR conditions were 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 95°C for 4 s and 57°C for 30 s.
HUVEC transfection with miR-663-locked nucleic acids antagonist or pre-miR-663 precursor.
HUVECs were transfected with miRCURY locked nucleic acids (LNA) (miR-663 LNA and scrambled miR as a control, Exiqon) or miRNA precursor (pre-miR-663 and pre-miR-control, Ambion) in a dose-dependent manner using Oligofectamine (Invitrogen), as described previously (24).
Monocyte adhesion and caspase-3 activity.
One day posttransfection, HUVECs were exposed to OS or LS for 1 day, and monocyte adhesion was determined using THP-1 cells, as our laboratory has previously described (24, 25). Caspase-3 activity in the cell lysate was determined by using the caspase-3 Fluorescent Assay Kit (BD Biosciences), according to the manufacturer's instructions.
mRNA microarray analysis and qPCR validation.
Total RNA samples were extracted using miReasy mini kit (QIAGEN) from HUVECs transfected with miR-663-LNA or control miR-LNA after 24-h shear exposure (LS or OS). All RNA samples passed quality control using Agilent BioAnalyze NanoChip before the gene chip study was carried out in the Emory Biomarker Service Center at the Emory University. A HumanHT-12 v3 Expression BeadChip array (Illumina) was used in this study, and the data were statistically analyzed by significance analysis of microarrays (29). The differentially expressed genes were identified as significant if expression level in OS-exposed ECs was different by >50% of LS and at the false discovery rate of 10% (Q value). Total RNA of each sample was reverse transcribed into cDNA using SuperScript III and random primers (Invitrogen), as we described. Briefly, qPCR was performed on selected genes using Brilliant II SYBR Green QPCR Master Mix (Stratagene) with custom-designed primers on a Real-Time PCR System (ABI StepOne Plus). All qPCR results were normalized based on 18S RNA expression in each sample. Fold changes between samples were determined using the ΔΔCt method (23).
RESULTS
Identification of miRNAs differentially regulated by OS and LS.
To determine whether miRNA expression is changed in ECs exposed to OS compared with LS, total RNAs obtained from HUVECs exposed to LS or OS were used for miRNA microarray using μParaflo microfluidic chip containing 856 human miRNA probes. This analysis revealed that 244 miRNAs of the 856 examined were detectable in HUVECs. Of the 244 detected miRNAs, 21 miRNAs (9 higher and 12 lower) were differentially expressed by >50% (P < 0.05) in OS-exposed cells compared with LS (Table 1).
Table 1.
Shear-sensitive miRNAs in human umbilical vein endothelial cells
| miRNA | OS Mean Intensity | LS Mean Intensity | Fold Change (OS/LS), log2 scale | P Value |
|---|---|---|---|---|
| hsa-miR-663 | 7,693 | 1,892 | 2.02 | 0.005 |
| hsa-miR-1275 | 1,579 | 819 | 0.95 | 0.015 |
| hsa-miR-424* | 107 | 80 | 0.42 | 0.026 |
| hsa-miR-1469 | 11,416 | 3,604 | 1.66 | 0.029 |
| hsa-miR-638 | 11,710 | 3,384 | 1.79 | 0.031 |
| hsa-miR-421 | 127 | 66 | 0.95 | 0.032 |
| hsa-miR-939 | 67 | 21 | 1.64 | 0.043 |
| hsa-miR-149* | 855 | 150 | 2.51 | 0.046 |
| hsa-miR-1231 | 125 | 34 | 1.89 | 0.049 |
| hsa-miR-151-3p | 1,476 | 2,144 | −0.54 | 0.005 |
| hsa-miR-320a | 3,726 | 7,600 | −1.03 | 0.006 |
| hsa-miR-320c | 3,445 | 7,303 | −1.08 | 0.006 |
| hsa-miR-320d | 2,325 | 5,269 | −1.18 | 0.006 |
| hsa-miR-139-5p | 113 | 412 | −1.87 | 0.008 |
| hsa-miR-320b | 2,778 | 6,364 | −1.20 | 0.019 |
| hsa-miR-192 | 26 | 70 | −1.41 | 0.023 |
| hsa-miR-125a-3p | 34 | 60 | −0.84 | 0.023 |
| hsa-miR-191 | 2,691 | 3,065 | −0.19 | 0.027 |
| hsa-miR-194 | 19 | 42 | −1.12 | 0.033 |
| hsa-miR-195 | 2,840 | 4,131 | −0.54 | 0.032 |
| hsa-miR-27b | 2,518 | 4,654 | −0.89 | 0.048 |
miRNA, micro-RNA; OS, oscillatory shear stress; LS, laminar shear stress.
Verification of shear-sensitive miRNAs by qPCR.
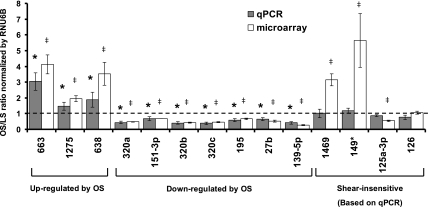
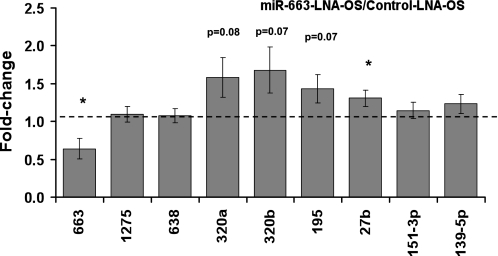
To validate the microarray data, qPCR was used as an independent measure of miRNA expression. Of the 21 miRNAs identified by the array result, we selected the top 13 based on their potential abundance and fold stimulation, as determined by the microarray data (Table 1). In addition, we also examined miR-126 expression, since it is a well-known endothelial-specific miRNA (8), although it was not shear sensitive in our array result. Ten miRNAs of the 13 examined were confirmed by qPCR results as OS sensitive (Fig. 1). These include miR-663, miR-1275, and miR-638, which were upregulated, while miR-320a,b,c, miR-151–3p, miR-195, miR-27b, and miR-139–5p were downregulated by OS compared with LS in HUVECs. As expected, miR-126 was highly expressed in ECs, but its level was not altered by OS compared with LS.
Fig. 1.
Identification and validation of shear-sensitive micro-RNAs (miRNAs). Human umbilical vein endothelial cells (HUVECs) were exposed to laminar shear stress (LS) or oscillatory shear stress (OS) for 24 h, and total RNA was collected for miRNA expression analysis, either by microarray or quantitative RT-PCR (qRT-PCR). miRNA expression was normalized by RNU6B and was shown as means ± SE (n = 4 for qPCR and n = 3 for microarray). P < 0.05: OS vs. *LS by qPCR and ‡LS by microarray.
miR-663 plays a role in OS-induced monocyte adhesion, but not in apoptosis of ECs.
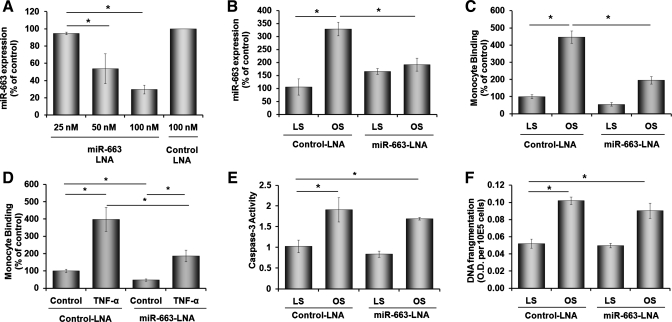
Since miR-663 expression was the most OS-sensitive miRNA, we decided to study its functional significance in ECs by using a specific inhibitor, miR-663-LNA. Transfection of HUVEC with miR-663-LNA decreased endogenous miR-663 level in a concentration-dependent manner compared with the control LNA (Fig. 2A). Furthermore, miR-663-LNA (100 nM) significantly inhibited OS-induced miR-663 expression (Fig. 2B), demonstrating that this is an efficient approach to inhibit miR-663 in HUVECs exposed to shear. Interestingly, however, miR-663-LNA did not lower miR-663 level in cells under LS condition (Fig. 2B). This may be because its expression level in LS condition is already low, and miR-663-LNA could not lower it further. To test whether miR-663 plays a role in endothelial function, we examined whether miR-663-LNA prevents two well-characterized OS-induced events in ECs: inflammation (24, 25) and apoptosis (18), as measured by monocyte adhesion and caspase-3 activity assays, respectively. As shown previously, exposure of HUVECs to OS increased monocyte adhesion to ECs by more than fourfold compared with that of LS in cells treated with control LNA (Fig. 2C). miR-663-LNA treatment inhibited OS-induced monocyte adhesion by ∼70% of the control LNA group (Fig. 2C). Moreover, miR-663-LNA also significantly inhibited monocyte adhesion induced by another well-known proinflammatory cytokine, TNF-α (Fig. 2D).
Fig. 2.
miR-663 mediates OS-induced monocyte adhesion, but not apoptosis. HUVECs were transfected with miR-663-locked nucleic acids (LNA) (antagonist) or control LNA and were exposed to static (A) or LS or OS conditions (B) for 24 h. A and B: miR-663 expression was assayed by qRT-PCR and normalized by RNU6B. HUVECs transfected with miR-663-LNA or control LNA (100 nM) were exposed to LS or OS for 24 h (C, E, and F) or TNF-α (1 ng/ml) for 16 h, followed by THP-1 monocyte binding (C and D), caspase-3 activity (E), or DNA fragmentation by ELISA (F) assays. Values are means ± SE; n = 3 each. OD, outer diameter. *P < 0.05.
Next, we examined the effect of miR-663-LNA on OS-induced caspase-3 activity. OS exposure significantly increased caspase-3 activity compared with LS in HUVEC treated with control LNA, but it was not affected by miR-663-LNA (Fig. 2E). In addition, another independent apoptosis assay, DNA fragmentation assay, showed essentially the same result as the caspase-3 assay (Fig. 2F). These results suggest that miR-663 has a specific role in OS- or TNF-α-induced inflammatory pathways, but not in apoptosis.
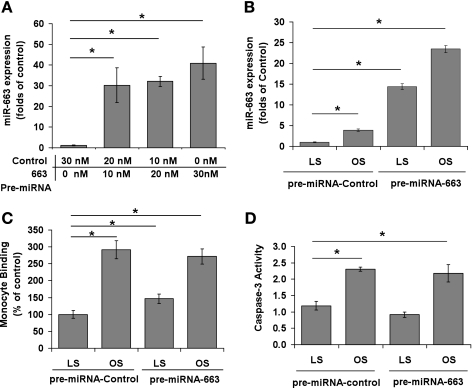
Next, we tested whether overexpression of miR-663 has any effect on monocyte adhesion and apoptosis. Transfection with pre-miR-663 dramatically increased miR-663 expression in HUVECs cultured under static (Fig. 3A) LS or OS condition (Fig. 3B). While pre-miR-663 overexpression in HUVEC increased monocyte adhesion by ∼50% in LS-exposed cells (Fig. 3C), it did not affect OS-induced monocyte adhesion (Fig. 3C). In contrast, pre-miR-663 had no effect on apoptosis of HUVEC exposed to OS or LS (Fig. 3D). Together, these findings suggest a specific role of miR-663 in OS-induced monocyte adhesion, but not in apoptosis.
Fig. 3.
Effect of miR-663 overexpression on monocyte adhesion and endothelial apoptosis. A–D: HUVECs were transfected with pre-miR-663 or control to overexpress miR-663 as assayed by qRT-PCR and normalized by RNU6B. HUVECs transfected with pre-miR-663 or control (10 nM) were exposed to LS or OS for 24 h (B–D), and THP-1 monocytes binding (C) or caspase-3 activity (D) was determined. Values are means ± SE; n = 3. *P < 0.05.
miRNA-663 altered mRNA expression in ECs exposed to OS.
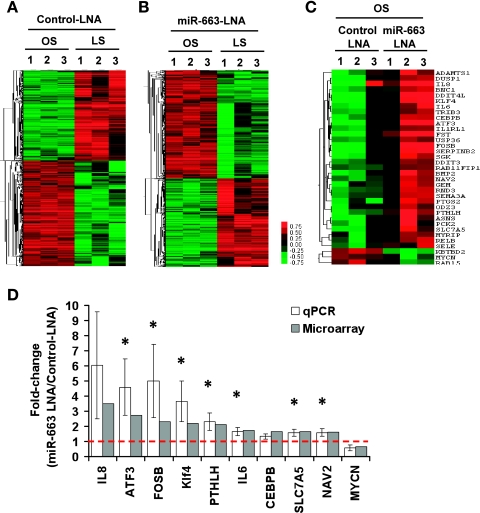
Extensive in silico analysis using web-based search programs, such as TargetScan and miRanda, to search for potential targets of miR-663 produced no obviously identifiable proinflammatory genes. These results suggested that miR-663 may regulate a network of genes, rather than a single or a small number of target genes to regulate inflammatory response. To test this hypothesis, we performed an additional genomewide microarray to identify mRNAs regulated by miR-663 in HUVECs exposed to OS. ECs were transfected with control miR-LNA or miR-663-LNA and then subjected to either LS or OS for 24 h. Microarrays were then performed using Illumina BeadChip, and the data were first analyzed to determine gene expression profiles between cells exposed to OS and LS in control or miR-663-LNA-treated cells. As shown in the heat map (Fig. 4A), OS upregulated 1,056 gene probes, while downregulating 903 compared with LS in control LNA-treated ECs. Among those, many well-studied, shear-sensitive genes identified in our array data, including KLF2, KLF4, eNOS, BMP4, ANGPT2, and VCAM-1, were shown to be regulated in a manner that is consistent with the previous findings (4, 14), providing confidence on our current array data. We also found that 854 and 698 gene probes were up- and downregulated, respectively, by OS compared with LS in miR-663-LNA-treated ECs, as shown in Fig. 4B (gene list deposited to GEO:GSE20739).
Fig. 4.
Effect of miR-663 inhibition on shear-dependent gene expression profiles and qPCR validation. A–D: HUVECs transfected with miR-663-LNA or control-miR-LNA (100 nM) were exposed to LS or OS for 24 h. Total RNAs were collected and analyzed by genomewide DNA array, as shown by the heat maps (A–C), showing differentially expressed genes by >50% at a false discovery rate of 1% (A and B) and 10% (C). Ten of the miR-663-sensitive genes were validated by qPCR, normalized against 18S, and shown as fold change (means ± SE, n = 3), along with the corresponding microarray results. *P < 0.05.
More importantly, we determined which gene expression was altered in a miR-663-dependent manner by comparing miR-663-LNA-treated ECs to control LNA group under OS condition. The results showed that 32 genes were upregulated, while 3 were downregulated in cells treated with OS and the miR-663 inhibitor (Fig. 4C and Table 2). In contrast, only one gene, KBTDB2, exhibited a significant difference between control LNA and miR-663-LNA in LS-treated ECs (Table 2). Next, we validated the microarray results by qPCR for 10 genes. The qPCR results validated 7 of 10 genes, including KLF4, FOSB, SLC7A5, and NAV2 (neuron navigator 2) (Fig. 4D).
Table 2.
mRNAs regulated by miR-663
| Gene ID | Gene Name | Fold Change (miR-663-LNA/Control LNA) | Q Value, % | Direct Target Predicted by TargetScan |
|---|---|---|---|---|
| Gene differentially expressed (control-LNA-OS vs. miR-663-LNA-OS) | ||||
| ILMN_1668125 | MYRIP | 1.55 | 0.00 | No |
| ILMN_1676984 | DDIT3 | 1.53 | 0.00 | No |
| ILMN_1692219 | RAB11FIP1 | 1.52 | 0.00 | No |
| ILMN_1700081 | FST | 5.48 | 6.14 | No |
| ILMN_1666733 | IL8 | 3.51 | 6.14 | No |
| ILMN_2374865 | ATF3 | 2.72 | 6.14 | No |
| ILMN_1696537 | DDIT4L | 2.61 | 6.14 | No |
| ILMN_1751607 | FOSB | 2.31 | 6.14 | Yes |
| ILMN_2137789 | KLF4 | 2.20 | 6.14 | No |
| ILMN_2314169 | PTHLH | 2.13 | 6.14 | No |
| ILMN_2150851 | SERPINB2 | 1.97 | 6.14 | No |
| ILMN_1677511 | PTGS2 | 1.79 | 6.14 | No |
| ILMN_1702487 | SGK | 1.78 | 6.14 | No |
| ILMN_1699651 | IL6 | 1.73 | 6.14 | No |
| ILMN_1722718 | BMP2 | 1.70 | 6.14 | No |
| ILMN_1693014 | CEBPB | 1.65 | 6.14 | Yes |
| ILMN_1811258 | RELB | 1.65 | 6.14 | No |
| ILMN_1720373 | SLC7A5 | 1.64 | 6.14 | Yes |
| ILMN_1759513 | RND3 | 1.63 | 6.14 | No |
| ILMN_1739393 | SELE | 1.61 | 6.14 | No |
| ILMN_2399300 | NAV2 | 1.61 | 6.14 | Yes |
| ILMN_1677092 | GEM | 1.58 | 6.14 | No |
| ILMN_1765641 | SEMA3A | 1.57 | 6.14 | No |
| ILMN_2336094 | ODZ3 | 1.55 | 6.14 | No |
| ILMN_1781285 | DUSP1 | 1.53 | 6.14 | No |
| ILMN_1697227 | USP36 | 1.52 | 6.14 | No |
| ILMN_1787815 | TRIB3 | 1.52 | 6.14 | No |
| ILMN_2242900 | IL1RL1 | 1.50 | 6.14 | No |
| ILMN_1796417 | ASNS | 1.73 | 7.52 | No |
| ILMN_1751465 | BNC1 | 1.51 | 7.52 | No |
| ILMN_1673566 | ADAMTS1 | 1.54 | 9.52 | No |
| ILMN_1671791 | PCK2 | 1.52 | 9.52 | No |
| ILMN_1784540 | KBTBD2 | 0.52 | 0.00 | No |
| ILMN_1731699 | RAB15 | 0.67 | 10.58 | No |
| ILMN_2219767 | MYCN | 0.64 | 10.58 | No |
| Gene differentially expressed (control-LNA-LS vs. miR-663-LNA-LS) | ||||
| ILMN_1784540 | KBTBD2 | 0.52 | 0 | No |
LNA, locked nucleic acids.
Furthermore, we determined which of the 35 genes (32 upregulated and 3 downregulated) in OS-treated cells were potential targets of miR-663 by in silico analysis using TargetScan (Table 2) and MiRanda (data not shown). This analysis revealed 4 of 35 genes are potential direct targets of miR-663: SLC7A5, NAV2, and two transcription factors, FOSB and CEBPB. To test our hypothesis whether these 35 genes regulated by miR-663 play a role in OS-mediated inflammatory response, we carried out Database for Annotation, Visualization, and Integrated Discovery analysis. The functional annotation result showed that inflammatory responses were indeed affected by miR-663 (Table 3). Moreover, additional cellular processes, such as regulation of transcription and cell proliferation, were also regulated by miR-663 (Table 3). A total of seven transcription factors (FOSB, CEBPB, DDIT3, ATF3, KLF4, BNC1, and MYCN) were identified as direct or indirect targets of miR-663 in OS-treated cells. These results suggest that miR-663 is a shear-sensitive miRNA, regulating expression of many genes, including the transcription factors, which, in turn, induce inflammatory response in ECs.
Table 3.
Functional annotation for genes regulated by miR-663 under OS condition
| Term | Count | % | P Value |
|---|---|---|---|
| Negative regulation of cellular process | 13 | 37.14 | 2.31E-06 |
| Organ development | 13 | 37.14 | 8.50E-06 |
| Regulation of cell proliferation | 9 | 25.71 | 9.24E-06 |
| negative regulation of cell proliferation | 7 | 20.00 | 1.27E-05 |
| Inflammatory response | 6 | 17.14 | 4.75E-04 |
| Tissue development | 6 | 17.14 | 8.15E-04 |
| Epidermis development | 4 | 11.43 | 0.004192 |
| Ectoderm development | 4 | 11.43 | 0.005121 |
| Negative regulation of cellular metabolic process | 5 | 14.29 | 0.010025 |
| Regulation of transcription, DNA dependent | 12 | 34.29 | 0.011825 |
We then examined whether miR-663-LNA altered expression of other OS-sensitive miRNAs. This study was designed to test our additional hypothesis that miR-663-LNA altered expression of OS-sensitive miRNAs, which, in turn, regulated expression of the 31 indirect targets of miR-663 identified above (Table 2). While miR-663-LNA inhibited its own expression level in OS-exposed ECs, as expected, it showed no significant effect on any of the examined shear-sensitive miRNAs, except for a modest (∼30%) increase for miR-27b.
DISCUSSION
In this study, we identified 10 OS-sensitive miRNAs in cultured ECs by performing a genomewide miRNA microarray and subsequent validation by qRT-PCR study (Fig. 1). We next determined the functional importance of the most OS-sensitive miRNA, miR-663, as a proinflammatory gene. Using the miR-663-specific inhibitor (miR-663-LNA), we found that miR-663 specifically mediated OS-induced monocyte adhesion to ECs, while it did not have any effect on OS-induced apoptosis (Fig. 2). We then performed an additional genomewide DNA microarray study to identify potential gene targets regulated by miR-663 in HUVECs. From the study, we found 35 potential genes regulated (Table 2); however, in silico analysis, such as Targetscan and MiRanda screening of these 35 genes, showed that only four genes are predicted to be the potential target of miR-663. Among those miR-663-regulated genes, several are transcription factors, including KLF4, C/EBPB, and ATF3, and could subsequently regulate a number of genes that are related to inflammatory responses.
Through microarray analysis, we identified four genes (FOSB, CEBPB, SLC7A5, and NAV2) that were upregulated in miR-663-LNA-treated ECs and were also predicted as potential targets of miR-663 by TargetScan (Table 2). Interestingly, all four genes contain poorly conserved miR-663 binding sites. In addition, miR-663 is expressed only in human and nonhuman primates. These findings suggest that the miR-663 effect targeting these four genes is species specific, and the same mechanism may not work in other species, such as mouse and rats. FOSB and CEBPB are transcription factors known to play roles in cell proliferation and inflammatory responses, respectively (12, 17). SLC7A5 acts as a L-type amino acid exchanger (6), and NAV2 is involved in neuronal development (3). Since their function in ECs is unclear, further studies need to be conducted. In addition, the rest of the miR-663-regulated genes that were not predicted as direct targets of miR-663 include several inflammatory genes (IL6, IL8, E-selectin), ER stress-related genes (ATF3 and DDIT3), and additional transcription factor (KLF4). Although they may not be direct targets of miR-663, some of these genes are likely to be important inflammatory and stress mediators. In addition, KLF4 may play an important role in mediating the effects of miR-663. KLF2 and KLF4 are key regulators of endothelial function and are induced by atheroprotective shear stress (27). Overexpression of KLF4 in human ECs significantly reduced TNF-α induction of E-selectin and VCAM-1, suggesting KLF4 has an anti-inflammatory effect through inhibiting adhesion molecules (16). Recently, Villarreal et al. (32) also showed that a significant degree of mechanistic and functional conservation between KLF4 and KLF2. Collectively, these transcription factors have been shown to coordinate transcriptional programs important for vasodilation, anti-inflammation, and antithrombotic effect in vascular ECs. Our data show inhibiting miR-663 with miR-663-LNA restores KLF4 expression in ECs under OS. This warrants further investigation of the role of miR-663 and KLF4 in OS-induced inflammation.
Recently, miR-19a and miR-23b were shown to be upregulated by LS compared with static condition, regulating shear-dependent proliferation pathway in ECs (22, 33). However, these miRNAs were not identified in our study, which compared LS vs. OS (rather than static), and that may be a reason for the discrepancy.
A critical unexplored aspect of miRNA function is the subtlety and complexity of gene regulatory networks. In a setting in which one miRNA can regulate hundreds of genes, and one gene can be regulated by a number of miRNAs, lack of knowledge in the mechanisms that govern miRNA-mRNA, as well as miRNA-miRNA interactions, is a major issue. Current prediction methods of miRNA targets are based on the seeding region of miRNAs that bind to the target site(s) on a given mRNA by sequence complementarities. These methods also consider additional pairing by other nucleotides, which has been hypothesized to be important in miRNA binding (7). The tolerance for extensive mismatches outside the seed region is indeed to increase the number of potential targets under miRNA control. Unfortunately, this complexity of interactions hinders the discovery of actual miRNA targets. In addition, it was interesting to note a complex relationship between the endogenous miR-663 levels regulated by OS or LS to the miR-663 levels regulated by forced overexpression using pre-miR633 in our present study. While inhibition of endogenous miR-663 by using miR-663-LNA significantly attenuated OS-induced monocyte adhesion, forced overexpression of miR-663 by using pre-miR663 showed only a marginal effect on it. The reason for this discrepancy is not clear, but one possibility is that miR-663 expression in HUVEC may have reached a sufficient level by OS alone, and that additional forced expression by pre-miR-663 does not contribute to further inflammatory pathway activation for some reason. In addition, factors contributing to the control of inducible or repressible miRNA expression and miRNA-coordinated expression with other regulatory molecules are not well known and need to be investigated. For instance, depleting miRNA 221 and 222 in HUVEC affects the miRNA profile, showing 9 upregulated and 23 downregulated miRNAs (28). This observation demonstrates the complex network involving coexpression of miRNAs and transcription factors that can be altered by a single miRNA variation (28). In our study, we examined whether miR-663 inhibition alters expression of other shear-sensitive miRNAs. As shown in Fig. 5, while miR-663-LNA inhibited its own expression level in OS-exposed ECs, it showed no significant effect on any of the examined shear-sensitive miRNAs, except for a modest (∼30%) increase for miR-27b. This result suggests that the proinflammatory effect of miR-663 is not likely to be mediated by regulating expression of other shear-sensitive miRNAs. Given its typical subtlety, any individual biological process mediated by miRNA may require a number of different factors that contribute to the final outcome. This type of subtlety makes it difficult to identify a single gene as a direct mediator responsible for the effect of miRNA on any given biological function.
Fig. 5.
Shear-sensitive miRNAs are partially regulated by miR-663. HUVECs were transfected with miR-663-LNA or control miR-LNA (100 nM) 1 day before shear experiments (OS for 24 h). Total RNA samples were collected, and miRNA expression was analyzed by qPCR. miRNA expression was normalized by RNU6B. Values are means ± SE (n = 3). *P < 0.05, control LNA-OS vs. miR-663-LNA-OS.
In summary, this is the first report identifying oscillatory shear-sensitive miRNAs in HUVECs and demonstrating miR-663 involvement in OS-induced cellular inflammation. We also demonstrate a gene list regulated by miR-663 under OS condition. We have recently demonstrated that disturbed flow conditions, such as OS, are causally linked to atherosclerosis development. The shear-sensitive miRNAs, discovered in this study, could be potential therapeutic targets for the treatment of atherosclerosis.
GRANTS
This research project was supported in part by the Emory Biomarker Service Center. This work was supported by funding from National Heart, Lung, and Blood Institute Grants HL87012 (H. Jo), a Program of Excellence in Nanotechnology Award HHSN268201000043C (H. Jo), and HL75209 (H. Jo), and a World Class University Project (H. Jo) from the Ministry of Science, Technology and Education of S. Korea.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
We thank Randy Ankeny and Casey Holliday for help in this study.
REFERENCES
- 1. Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol 285: C499– C508, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science 303: 83– 86, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Clagett-Dame M, McNeill EM, Muley PD. Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J Neurobiol 66: 739– 756, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A 101: 14871– 14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689– 1698, 2002 [DOI] [PubMed] [Google Scholar]
- 6. del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci 35: 161– 174, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91– 105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A 105: 1516– 1521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res 100: 1579– 1588, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5: 293– 302, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 101: 59– 68, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Li H, Gade P, Xiao W, Kalvakolanu DV. The interferon signaling network and transcription factor C/EBP-beta. Cell Mol Immunol 4: 407– 418, 2007 [PMC free article] [PubMed] [Google Scholar]
- 13. Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of microRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem 284: 7903– 7913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A 98: 8955– 8960, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mendell JT. miRiad roles for the miR-17–92 cluster in development and disease. Cell 133: 217– 222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Methe H, Balcells M, Alegret Mdel C, Santacana M, Molins B, Hamik A, Jain MK, Edelman ER. Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am J Physiol Heart Circ Physiol 292: H2167– H2175, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer 41: 2449– 2461, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Mueller CF, Widder JD, McNally JS, McCann L, Jones DP, Harrison DG. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ Res 97: 637– 644, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297: H1535– H1543, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood 116: e66– e73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 108: 3068– 3071, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A 107: 3240– 3244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101– 1108, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773– 779, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 278: 31128– 31135, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 100: 1164– 1173, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol 25: 1135– 1141, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Tuccoli A, Poliseno L, Rainaldi G. miRNAs regulate miRNAs: coordinated transcriptional and post-transcriptional regulation. Cell Cycle 5: 2473– 2476, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98: 5116– 5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest 117: 2369– 2376, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 24: 12– 22, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Villarreal G, Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, Garcia-Cardena G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun 391: 984– 989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A 107: 3234– 3239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun 393: 643– 648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu P, Guo M, Hay BA. MicroRNAs and the regulation of cell death. Trends Genet 20: 617– 624, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci 32: 189– 197, 2007 [DOI] [PubMed] [Google Scholar]