Abstract
Staphylococcus aureus is the most frequent cause of bacteremia and hospital-acquired infection, however a vaccine that prevents staphylococcal disease is currently not available. Two sortase-anchored surface proteins, IsdA and IsdB, have been identified as subunit vaccines that, following active immunization, protect experimental animals against intravenous challenge with staphylococci. Here we investigate the molecular basis of this immunity and report that, when passively transferred to naïve mice, purified antibodies directed against IsdA or IsdB protected against staphylococcal abscess formation and lethal intravenous challenge. When added to mouse blood, IsdA- or IsdB-specific antibodies did not promote rapid opsonophagocytic killing of wild-type staphylococci. Antibodies directed against IsdA interfered with heme-binding and IsdB antibodies perturbed the ability of this surface protein to bind hemoglobin. As the structural genes for isdA and isdB are required for heme-iron scavenging during the pathogenesis of infection, we hypothesize that IsdA and IsdB antibodies may at least in part provide protection against staphylococci by interfering with the pathogen's heme-iron scavenging mechanisms.
Keywords: Staphylococcus aureus, IsdA, IsdB, Vaccine, Heme-iron-transport
1. Introduction
Staphylococcus aureus, a commensal of the human skin and nares, is also a common cause of bacteremia, skin and soft tissue infections, pneumonia, osteomyelitis or septic arthritis in addition to toxin mediated disease entities such as toxic shock syndrome, epidermolytic syndromes and gastroenteritis [1]. The remarkable pathogenic potential of this organism has recently been documented by the rapid spread of drug-resistant S. aureus strains, designated methicillin-resistant S. aureus (MRSA) [2]. Although initially described as a hospital-acquired infection, a recent study reported that about 14% of all invasive MRSA disease in the United States originates in the community (CA-MRSA) [3]. Klevens et al. reported that about 94,000 cases of invasive MRSA disease occurred in the United States in 2005, resulting in more than 18,000 deaths [3]. The overall incidence of staphylococcal disease appears to have increased over the past ten years [4]. Thus, there is a pressing need for the development of a vaccine that can prevent S. aureus infections, most importantly the disease entities soft tissue abscesses, bacteremia and lethal infection [5].
Using a mouse model of intravenous challenge, sortase A-anchored surface proteins of S. aureus have been demonstrated to be essential for the microbe's survival in blood [6] and for the pathogenesis of staphylococcal abscess formation [7]. A recent analysis comparing the vaccine potential of sortase-anchored surface proteins identified IsdA and IsdB, surface proteins with NEAT domains that promote heme-iron scavenging from host hemoglobin [8], as protective antigens [9]. Kuklin et al. reported that mice and rhesus macaques actively immunized with purified IsdB generated protective immune responses against staphylococcal challenge, which correlated with the amount of IgG antibody produced in vaccinated animals [10]. Previous work left unresolved by what mechanism antibodies directed against IsdA or IsdB generate protective immunity. These questions are addressed in this report.
2. Materials and methods
2.1. Bacterial strains, media and growth conditions
S. aureus Newman [11] was grown in tryptic soy broth (TSB) at 37 °C. isdA, isdB, isdC, and isdH mutants harboring the bursa aurelis mariner transposon were obtained from the Phoenix (ΦN(x0039E)) library [12]. Transposon insertions were transduced into wild-type S. aureus Newman using bacteriophage φ85 and selected on TSA plates with 10 μg ml−1 erythromycin and 40 mM sodium citrate [12]. Transductants were confirmed by inverse PCR and DNA sequencing, as previously described [13]. Cultures of mutant staphylococci were grown at 37 °C in TSB supplemented with erythromycin (10 μg ml−1). Escherichia coli strains DH5α, BL21 (DE3) (for pET-15b derived plasmids) and CA8000 (for pGEX-2TK derived plasmids) were gown in Luria-Bertani (LB) broth with 100 μg ml−1 ampicillin at 37 °C.
2.2. Animal experiments
Experimental protocols were reviewed, approved and performed under regulatory supervision of The University of Chicago's Institutional Biosafety Committee (IBC) and Institutional Animal Care and Use Committee (IACUC). BALB/c mice and New Zealand white rabbits were purchased from Charles River Laboratories. After confirming that the datasets abide by a normal distribution, mouse renal abscess data were analyzed for statistical significance using the unpaired 2-tailed student's t-test. Statistical significance of the mouse lethal challenge data was calculated with the 2-tailed log-rank test. The results of all animal experiments were examined for reproducibility. One of 2 independent trials is displayed in Figs. 1, 4 and 6 and summarized in Table 2.
Fig. 1.
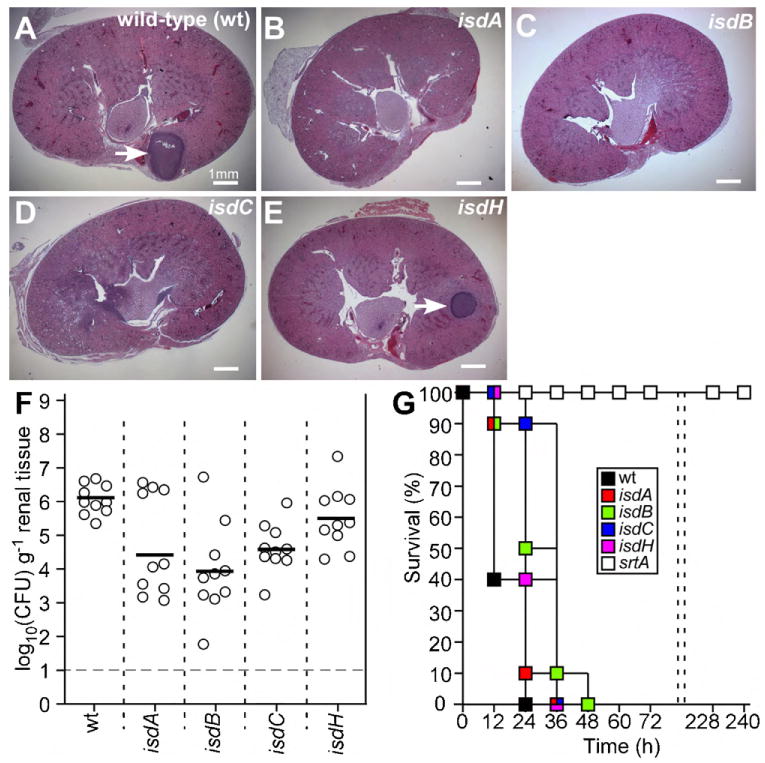
Contribution of iron-regulated surface determinants (Isd) to Staphylococcus aureus abscess formation and lethal infection in mice. (A–F) Six-week-old BALB/c mice were infected by retro-orbital injection with 1 × 107 CFU S. aureus Newman. Four days following challenge, mice were killed and kidneys removed for histopathology (A–E) or bacterial load measurements (F). For histopathology, kidneys were fixed in formaldehyde, thin-sectioned, stained with hematoxylin-eosin and viewed by light microscopy. For tissue homogenization, kidneys were mechanically disrupted in PBS, 1% Triton X-100, homogenate spread on agar plates, and enumerated by colony formation. S. aureus Newman (A) and its isogenic variants carrying transduced bursa aurealis insertions into isdA, isdB, isdC or isdH were analyzed. (G) Six-week-old BALB/c mice were infected by retro-orbital injection with 1.8 × 108 CFU S. aureus Newman or its isogenic variants and survival of BALB/c mice monitored over 10 days (240 h).
Fig. 4.

Purified rabbit antibodies specific for IsdA or IsdB protect mice against staphylococcal abscess formation and lethal challenge. (A) Affinity purified rabbit IgG (85 μg or 5 mg kg−1 body weight) directed against IsdA or IsdB was injected into the peritoneal cavity of BALB/c mice. Twenty-four hours later (Day 0) or 4 days after staphylococcal challenge (Day 4), 5 animals were bled and serum IgG antibody levels against IsdA or IsdB were determined by ELISA. Twenty-four hours following passive immunization, cohorts of ten mice were challenged with 1 × 107 CFU S. aureus Newman via retro-orbital injection. After 4 days, animals were killed, kidneys were removed and analyzed for histopathology (B) or staphylococcal load (C) as described in the legend to Fig. 1. Staphylococcal abscess communities at the center of these lesions are marked with blue arrows. Necrotic immune cells are marked with white arrows. (D) Twenty-four hours following passive immunization, cohorts of ten mice were challenged with 1.5 × 108 CFU S. aureus Newman via retro-orbital injection. The development of lethal infections was monitored over the next 240 h.
Fig. 6.

Antibodies against IsdBN or IsdBC protect mice against staphylococcal abscess formation and lethal challenge. (A) Coomassie-stained SDS-PAGE of purified recombinant IsdBN (lane 1) and IsdBC (lane 2). Numbers indicate the migrational position of molecular weight markers in kDa. (B) Affinity purified rabbit IgG (85 μg or 5 mg kg−1 body weight) directed against IsdBN or IsdBC was injected into the peritoneal cavity of BALB/c mice. Twenty-four hours later (Day 0) or 4 days after challenge (Day 4), 5 animals were bled serum IgG antibody levels against IsdBN or IsdBC were determined by ELISA. Twenty-four hours following passive immunization, cohorts of ten mice were challenged with 1 × 107 CFU S. aureus Newman via retro-orbital injection. After 4 days, animals were killed, kidneys were removed and analyzed for histopathology (E–J) or staphylococcal load (C) as described in the legend to Fig. 1. Staphylococcal abscess communities at the center of these lesions are marked with blue arrows. Necrotic immune cells are marked with white arrows. (D) Twenty-four hours following passive immunization, cohorts of ten mice were challenged with 2 × 108 CFU S. aureus Newman via retro-orbital injection. The development of lethal infections was monitored over the next 240 h.
Table 2.
Antibodies against IsdA and IsdB protect against staphylococcal abscess formation.
| Antibodiesa | IgG titerb | Staphylococcal load in kidneysc | Abscess formation | ||||
|---|---|---|---|---|---|---|---|
| Log10 CFUd | Reductione | P valuef | Abscessesg | Reductionh | P valuei | ||
| Mockj | – | 6.59 ± 0.15 | – | – | 4.64 ± 1.17 | – | – |
| V10 | 1300 ± 55 | 6.94 ± 0.34 | −0.35 | 0.2801 | 2.1 ± 1.1 | 2.54 | 0.1432 |
| IsdA | 1400 ± 126 | 5.11 ± 0.79 | 1.48 | 0.0181 | 1.0 ± 0.5 | 3.64 | 0.0268 |
| IsdB | 1640 ± 320 | 2.83 ± 0.68 | 3.76 | 0.0001 | 0.86 ± 0.46 | 3.78 | 0.0395 |
| IsdBN | 875 ± 97 | 5.54 ± 0.69 | 1.05 | 0.0551 | 1.4 ± 0.54 | 3.24 | 0.0382 |
| IsdBC | 910 ± 114 | 3.51 ± 0.99 | 3.08 | 0.0001 | 1.2 ± 0.68 | 3.44 | 0.0327 |
Affinity purified antibodies were injected at IgG concentration of 5 mg kg−1 animal weight in 100 μl PBS into the peritoneal cavity of naïve 6-week-old female BALB/c mice.
Antibody titers were analyzed by ELISA with purified recombinant antigen (1 μg ml−1) by dilution of serum samples derived via retro-orbital bleeding 24 h following passive immunization.
Four days following intravenous challenge with 1 × 107 CFU S. aureus Newman, animals were killed, kidneys excised and tissue homogenate from one randomly chosen kidney spread on agar plates for colony formation and enumeration. Data represent the average and standard error of the means of the log10 CFU from 10 kidneys. Twenty kidneys were analyzed for mock immunized animals injected with 100 μl PBS.
Staphylococcal load was enumerated as the log10 of colony forming units (CFU) per gram of kidney tissue homogenate.
Reduction in log10 CFU compared to the mock control.
Statistical significance was analyzed with the unpaired 2-tailed student's t-test and P values recorded using a cohort of mice (n = 20) for the PBS control group.
Average number (and standard error of the means) of abscesses formed in the kidneys of infected mice was enumerated by histopathology of thin-sectioned hematoxylin-eosin stained tissue samples.
Reduction in the number of abscesses compared to the mock control.
Statistical significance was analyzed with the unpaired 2-tailed student's t-test and P values recorded using a cohort of mice (n = 20) for the PBS control group.
Mock immunized animals received an intraperitoneal injection of 100 μl of PBS.
2.3. Rabbit antibodies
The coding sequences for IsdBN were PCR-amplified with 2 primers, aactcgaggcagctgaagaaacaggt and aaggatcccacttgctcatctaaagc, using S. aureus Newman template DNA [11]. Sequences for IsdBC were amplified with aactcgaggctttagatgagcaagtg and aaggatcctgattttgctttattttc. PCR products were cloned into pET-15b or pGEX-2TK generating N-terminal His6-tagged or N-terminal GST-tagged recombinant proteins, respectively. Plasmids were transformed into BL21(DE3) or CA8000 and overnight cultures of transformants were diluted 1:100 into fresh media and grown at 37 °C to an OD600 0.5, at which point cultures were induced with 1 mM isopropyl β-D-1-thiogalatopyranoside (IPTG) and grown for an additional 3 h. Bacterial cells were sedimented by centrifugation, suspended in column buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl) and disrupted with a French pressure cell at 14,000 psi. Lysates were cleared of membrane and insoluble components by ultracentrifugation at 40,000 × g. Proteins in the soluble lysate were subjected to nickel-nitrilotriacetic acid (Ni-NTA) or GST affinity chromatography. Proteins were eluted in column buffer containing successively higher concentrations of imidazole (100–500 mM) or 30 mM reduced glutathione, respectively. Expression and purification of recombinant V10, IsdA, IsdB, IsdC and IsdH were described previously [8,9,14]. Protein concentrations were determined by bicinchonic acid (BCA) assay (Thermo Scientific). For antibody generation, rabbits (Charles River Laboratories, 6-month-old New Zealand white, female) were immunized with 500 μg protein emulsified in Complete Freund's Adjuvant (Difco) by subscapular injection. For booster immunizations, proteins were emulsified in incomplete Freund's adjuvant and injected 24 or 48 days following the initial immunization. On day 60, rabbits were bled and serum recovered.
Purified antigen (5 mg protein) was covalently linked to HiTrap NHS-activated HP columns (GE Healthcare). Antigen-matrix was used for affinity chromatography of 10–20 ml of rabbit serum at 4 °C. Charged matrix was washed with 50 column volumes of PBS, antibodies eluted with elution buffer (1 M glycine pH 2.5, 0.5 M NaCl) and immediately neutralized with 1 M Tris–HCl, pH 8.5. Purified antibodies were dialyzed overnight against PBS at 4 °C.
2.4. Passive immunization
Affinity purified antibodies prepared in PBS at 5 mg kg−1 of experimental animal weight were delivered via intraperitoneal injection into mice 24 h prior to challenge with S. aureus. Animal blood was collected via periorbital vein puncture. Blood cells were removed with heparinized micro-hematocrit capillary tubes (Fisher) and Z-gel serum separation microtubes (Sarstedt) were used to collect serum antibodies.
Purified antigens (V10, IsdA, IsdB, IsdC, IsdBN and IsdBC) were coated onto MaxiSorp ELISA plates (NUNC) in 0.1 M carbonate buffer (pH 9.5) at 1 μg ml−1 concentration overnight at 4 °C. Plates were blocked with 1% bovine serum albumin (BSA) followed by incubation with serial dilutions of mouse sera (PBS, 1% BSA) for 1 h. Plates were washed and incubated for an additional hour with secondary anti-mouse IgG HRP-conjugated rabbit antibody. Plates were washed and developed using OptEIA ELISA reagents (BD). Reactions were quenched with 1 M phosphoric acid and A450 readings were used to calculate half maximal IgG titers.
2.5. Mouse renal abscess
Overnight cultures of S. aureus Newman were diluted 1:100 into fresh TSB and grown for 2 h at 37 °C. Staphylococci were sedimented, washed and suspended in PBS at OD600 of 0.4 (∼1 × 108 CFU ml−1). Inocula were determined by spreading sample aliquots on TSA and enumerating colony formation. BALB/c mice (6-week-old females, cohorts of 10 animals per experimental group) were anesthetized via intraperitoneal injection with 100 mg ml−1of ketamine and 20 mg ml−1of xylazine per kilogram of body weight. Mice were infected by retro-orbital injection with 1 × 107 CFU of staphylococci. On day 4 following challenge, mice were killed by CO2 inhalation. Both kidneys removed, and the staphylococcal load in the right kidney was analyzed by homogenizing its tissue with PBS, 1% Triton X-100. Serial dilutions of homogenate were spread on TSA and incubated for colony formation. The bacterial load in organ tissue was analyzed in pairwise comparisons between wild-type and mutant strains or between immunized and mock immunized animals. The other kidney of each necropsied animal was examined by histopathology. Briefly, kidneys were fixed in 10% formalin for 24 h at room temperature. Tissues were embedded in paraffin, thin-sectioned, stained with hematoxylin-eosin, and inspected by light microscopy to enumerate abscess lesions per organ tissue. Data were analyzed in pairwise comparisons between wild-type and mutant strains or between immunized and mock immunized animals with the unpaired 2-tailed student's t-test.
2.6. Mouse lethal challenge
Overnight cultures of S. aureus Newman were inoculated 1:100 into fresh TSB and grown for 2 h at 37 °C. Staphylococci were sedimented, washed and suspended in PBS at ∼2 × 109 CFU ml−1. Each inoculum was quantified by spreading sample aliquots on TSA. BALB/c mice (6-week-old females, cohorts of 10 animals per group) were anesthetized via intraperitoneal injection with 100 mg ml−1 of ketamine and 20 mg ml−1 of xylazine per kilogram of body weight. Animals were infected via retro-orbital injection with ∼2 × 108 CFU of staphylococci. Infected animals were monitored for survival over a period of 10 days (240 h). Data were analyzed by pairwise comparison of time-to-death.
2.7. Staphylococcal survival in mouse blood
Overnight cultures of S. aureus Newman were diluted 1:100 into fresh TSB and grown to OD600 of 0.45. Staphylococci of 1 ml culture were sedimented by centrifugation, washed and suspended in PBS. Blood was collected from BALB/c mice via cardiac puncture, treated with lepirudin and then pooled. Blood aliquots (0.45 ml) were infected with 1 × 105 CFU S. aureus Newman suspended in 50 μl PBS, incubated at 37 °C with slow rotation and samples removed as indicated. Each aliquot was treated with 1% saponin for 30 min, diluted in PBS, spread on agar plates and incubated for colony formation. At timed intervals, before and after the addition of 1 μg ml−1 purified antibody specific for IsdA, IsdB, IsdC or V10, 50 μl aliquots were sampled to monitor the impact of antibodies on opsonophagocytic killing of staphylococci. Staphylococcal survival was calculated as percent input and normalized to survival in the absence of specific antibody. Results were analyzed with the unpaired 2-tailed student's t-test using data from 3 independent experimental tests.
2.8. Staphylococcal survival in the presence of human polymorphonuclear leukocytes
PMNs were isolated from fresh blood of human volunteers, counted, examined for viability by trypan blue exclusion, and diluted to 1 × 106 PMN ml−1. Cross-reactive antibodies in infant rabbit serum and normal rabbit sera were removed by incubation with suspensions of S. aureus Newman. Serum was then centrifuged, filter-sterilized, and used as a source of complement and negative control, respectively. S. aureus Newman was adjusted to 1 × 105 CFU ml−1. Equal volumes (100 μl) of PMNs, complement, bacteria and diluted antisera were mixed and incubated at 37 °C for 90 min prior to dilution, agar plating, and bacterial enumeration. Bacterial killing was calculated as the percent difference in CFU between samples without or with complement. Experiments with blood from human volunteers involved protocols that were reviewed, approved and performed under regulatory supervision of The University of Chicago's Institutional Review Board (IRB).
2.9. Heme-binding via spectroscopic absorption
10 μM purified GST-tagged recombinant protein was incubated for 5 min at 25 °C with 1 μM hemin in 100 μl reaction volume (50 mM Tris–HCl pH 7.5, 150 mM NaCl). Spectral changes were monitored between 300 and 800 nm using a Cary 50BIO (Varian, Walnut Creek, CA). Values were calculated as Δ absorbance at 410 nm in the presence and absence of heme [8,15]. The Δ absorbance at 410 nm in the presence and absence of heme for GST alone was determined and used for normalization. All experiments were performed in triplicate.
2.10. SDS-PAGE and immunoblotting
Poly-histidine-tagged recombinant proteins (IsdA, IsdB, IsdH, and sortase A) and GST-tagged recombinant IsdC were boiled in sample buffer, separated by SDS-PAGE, and stained with Coomassie or transferred to PVDF membranes for immunoblot analysis using antigen specific antibodies. Rabbit antibodies directed against IsdA, IsdB, and IsdC were affinity purified. IsdA and IsdB antibodies were pre-absorbed against IsdA and IsdB, respectively. For IsdH, we used mouse immune serum raised against this polypeptide. In the staphylococcal antigen-matrix, affinity purified His6-tagged proteins (including IsdA and IsdB) were immobilized onto nitrocellulose membrane. Antigen concentration was calibrated with His-specific reagents. Immune responses to specific antigens were quantified by measuring the intensities of infra-red signals derived from animal sera with the signals generated by known concentrations of antibodies.
2.11. Hemoglobin-binding via GST pulldown
GST-tagged proteins were purified from E. coli following IPTG induction as described. Cell lysates were applied to a 0.5 ml bed volume of glutathione sepharose beads and washed with 20 bed volumes of column buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl). Two-milligram human hemoglobin (Sigma) was added to the column and washed with 10 bed volumes of column buffer. Bound proteins were eluted with 40 mM reduced glutathione, boiled in sample buffer, separated by SDS-PAGE, and stained with Coomassie brilliant blue.
2.12. Heme-binding via 3,3′,5,5′-tetramethylbenzidine
Hemin-protein complex formation was determined by detection of heme-dependent peroxidase activity on the chromogenic compound 3,3′,5,5′-tetramethylbenzidine [16,17]. Briefly, reactions with 1 μM IsdA or IsdBC were incubated at room temperature for 30 min alone, in the presence of 3 μM αIsdA, αIsdB or αV10. 10 μM hemin-chloride was added and reactions proceeded for an additional hour. Proteins were separated by LDS-PAGE, without prior boiling or exposure to reducing agents. Gels were fixed for 1 h in the dark at 4 °C in a pre-chilled solution of 0.25 M sodium acetate (pH 5.0), methanol, and water (6:3:1). Gels were stained for 35 min in cold TMBZ staining solution, prepared immediately prior to use (12.6 mM TMBZ, 20% methanol, and 70% cold 0.25 M sodium acetate (pH 5.0). 30 mM hydrogen peroxide was added and incubated for an additional 30 mintes. Gels were washed with acetate buffered isopropanol (8:2) and dried. As antibody alone produced signal, values for heme-binding were calculated as the sum of all peroxidation signal in the gel, normalized to antibody alone. Results were analyzed with the unpaired 2-tailed student's t-test using data from 3 independent experimental tests.
2.13. IsdB binding to hemoglobin
Hemoglobin-binding to N-terminal His6-tagged IsdA, IsdB, IsdBN or IsdBc was measured by surface plasmon resonance using a Biacore 3000 instrument (BIAcore AB, Uppsala, Sweden) at 18 °C. A NTA biosensor chip was charged with 500 μM Ni2+ in HBS-P buffer (10 mM HEPES, pH 7.4, 0.15 M NaCl, 50 mM EDTA, 0.05% Tween 20) followed by protein immobilization at 200 nM at a flow rate of 10 μl min−1. 5 μM human hemoglobin was injected at 20 μl min−1 with a dissociation time of 180 s. Hemoglobin-binding was calculated as Rmax hemoglobin/(RmaxIsd protein/MW Isd protein). For antibody inhibition studies, a CM5 α-GST conjugated sensor chip was used to capture GST-tagged IsdBN. Briefly, the chip was prepared by activating with NHS and EDC, followed by 50 μl of 1 μM α-GST in HBS-P buffer, pH 3.6. Unbound esters were neutralized with 1 M ethanolamine hydrochloride pH 8.5. IsdBN-GST (0.5 μM) was first immobilized, followed by treatment with 1 μM αIsdB, αV10 or buffer (mock) and finally 4 μM human hemoglobin, all at a flow rate of 20 μl min−1 and a dissociation time of 180 s. Results were analyzed with the unpaired 2-tailed student's t-test using data from 3 independent experimental tests.
3. Results
3.1. Mutations in isd genes affect the pathogenesis of staphylococcal infections in mice
IsdB binds to hemoglobin and scavenges heme, which is transferred first to IsdA and then to IsdC [8,18,19]. IsdC delivers the tetrapyrrol to IsdEF for transport across the cytoplasmic membrane [20–22]. Once within the bacterial cell, IsdG and IsdI cleave heme and liberate iron as nutrient for staphylococcal growth [15,23]. Previous work asked whether mutations in isdB and isdH, the latter of which encodes a haptoglobin receptor [24–26], affect the pathogenesis of staphylococcal infections in spleen or kidney tissues [27]. Mutations in isdB, but not in isdH, reduced the staphylococcal load 4 days following intravenous challenge of mice [27]. These studies left unresolved whether isdB or isdH mutations also impact abscess formation and the ability of mice to survive intravenous high-dose staphylococcal challenge. We examined abscess formation of S. aureus Newman, a human clinical isolate [28], following intravenous challenge of BALB/c mice with 1 × 107 CFU. Four days after challenge, cohorts of ten mice per group were killed, kidneys removed, fixed in formalin and thin-sectioned tissue was stained with hematoxylin-eosin and viewed by microscopy (Fig. 1). Mice infected with the wild-type parent harbored an average of 3.0 (±1.0) abscesses per kidney. Bursa aurealis insertions in isdA [0.25 (±0.25) abscesses; P = 0.01891], isdB [0.5 (±0.29) abscesses; P = 0.01465] and isdC [0.75 (±0.25) abscesses; P = 0.04189] reduced the ability of staphylococci to form infectious lesions in renal tissue (Fig. 1A–D). We recently observed that these defects in abscess formation of srtA and isd mutant are also a prerequisite for staphylococcal persistence in host tissues [7]. Mutations in isdH [1.25 (±0.75) abscesses; P = 0.1549] did not cause a significant defect in staphylococcal abscess formation (Fig. 1E). A mutation in sortase A, which abolishes cell wall anchoring and surface display of all proteins with LPXTG sorting signals (including IsdA, IsdB and IsdH) [20,29,30], abolishes the ability of staphylococci to form abscesses [7]. Tissue homogenates of kidneys removed 4 days after challenge with wild-type S. aureus Newman revealed a bacterial load of 6.040 (±0.095) log10 CFU g−1 of kidney tissue. Significant reductions in staphylococcal load were observed for mutations in isdA [4.403 (±0.475) log10 CFU g−1; P = 3.83 × 10−4], isdB [3.903 (±0.475) log10 CFU g−1; P = 4.82 × 10−3], and isdC [4.602 (±0.722) log10 CFU g−1; P = 1.34 × 10−2]. The mutation in isdH did not cause a reduction in staphylococcal load [5.515 (±0.962) log10 CFU g−1; P = 4.94 × 10−1] (Fig. 1F).
We sought to study the contributions of Isd factors towards staphylococcal bacteremia and lethal disease, a frequent concern of hospital-acquired infections [31]. Previous work developed an intravenous challenge model for BALB/c mice [9]. Briefly, retro-orbital injection of 1.8 × 108 CFU S. aureus Newman caused animals to be moribund within 4 h and precipitated a lethal infection of mice (n = 10) within 24 h [32]. An isogenic S. aureus Newman mutant that cannot express sortase A (srtA) [30] was completely avirulent and failed to cause death (P < 0.0001) (Fig. 1G). Bursa aurealis insertion mutations in any one of the 4 isd genes (isdA, isdB, isdC and isdH) did not alter the lethal outcome of staphylococcal challenge experiments. Data in Fig. 1G were analyzed with the log-rank test for increased survival, which revealed that variants with insertions in isdA (P = 0.0181), isdB (P = 0.0035), isdC (P < 0.0001) and isdH (P = 0.0017) displayed a delay in time-to-death as compared to the wild-type parent control (Fig. 1G).
In summary, isdA, isdB and isdC, but not isdH, is required for the pathogenesis of staphylococcal abscess formation in mice. Unlike srtA, isd genes are not essential for S. aureus virulence in the murine lethal challenge model. Nevertheless, isdA, isdB, isdC and isdH contribute to the pathogenesis of this disease.
3.2. Antibodies directed against Isd factors
Recombinant IsdA, IsdB, IsdC, and IsdH were expressed in E. coli, purified by affinity chromatography, emulsified in adjuvant and injected into rabbits to generate humoral immune responses (Fig. 2). Antisera were used to blot PVDF membranes with purified proteins, which revealed that IsdA-immune serum recognized IsdA and, to a lesser degree, IsdB (Fig. 2). IsdB immune serum recognized IsdB as well as IsdA, whereas IsdH antiserum reacted with IsdH (Fig. 2). Rabbit antiserum directed against IsdC did not display cross-reactivity to IsdA, IsdB or IsdH (Fig. 2).
Fig. 2.
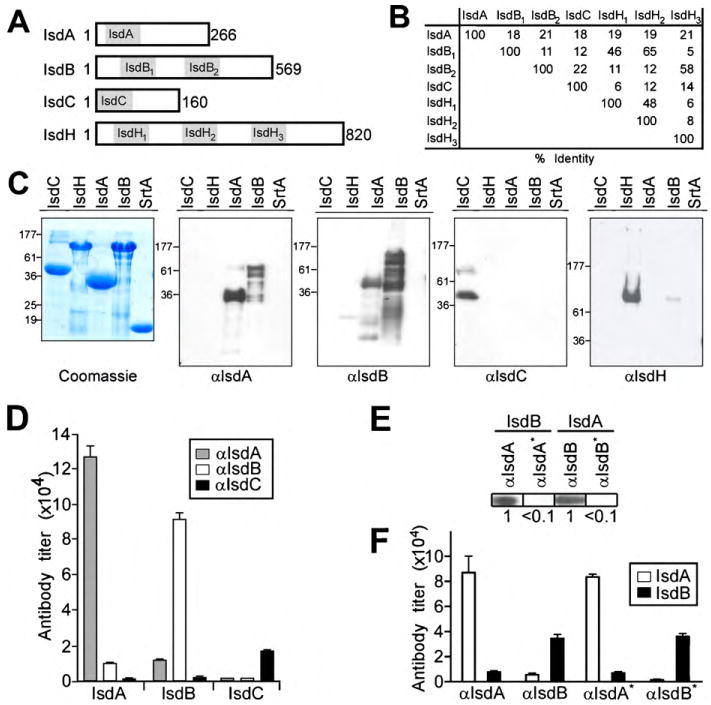
Generation of rabbit antibodies specific for iron-regulated surface determinants. (A) Schematic to illustrate the primary structure of the mature domain (lacking the N-terminal signal peptide and the C-terminal sorting signal) of IsdA, IsdB, IsdC and IsdH. NEAT domains are highlighted in grey and designated for each polypeptide. (B) Amino acid identity between the various NEAT domains of IsdA, IsdB, IsdC and IsdH is designated in percent of its sequence. (C) Recombinant, poly-histidine affinity tagged IsdA, IsdB, IsdC and IsdH or sortase A (SrtA) [43] was purified by affinity chromatography and analyzed by Coomassie-stained SDS-PAGE (C) or by immunoblot with rabbit antisera raised against purified IsdA (αIsdA), IsdB (αIsdB), or IsdC (αIsdC), and mouse serum against IsdH (αIsdH). (D) Cross-reactivity of rabbit antibodies directed against IsdA, IsdB and IsdC was quantified by ELISA using purified antigen. (E and F) Antibodies directed against IsdA and IsdB were purified by affinity chromatography on a matrix with covalently linked IsdA or IsdB. Cross-reactive antibodies in the eluate were removed by chromatography over the reciprocal column (αIsdA* and αIsdB*) and analyzed by immunoblotting (E) or ELISA (F).
On the basis of protein sequence, the N-terminal NEAT domain of IsdB (IsdB1) is most closely related to the second NEAT domain of IsdH (IsdH2 - which also binds hemoglobin) [33] (Fig. 2). The second NEAT domain of IsdB (IsdB2) is most closely related to the NEAT domain of IsdA [34]. The NEAT domain of IsdC, a sortase B anchored product that functions as the central conduit for heme-iron transport in staphylococci [20], appears unique in sequence and structure [8,35,36]. It is conceivable that the NEAT domains of Isd proteins provide the basis for crossreacting antibodies between IsdA and IsdB. To evaluate the functional properties of rabbit humoral immune responses, we purified IsdA-, IsdB- and IsdC-specific antibodies by binding to affinity matrices comprised of purified IsdA, IsdB and IsdC. Cross-reactive IsdA and IsdB antibodies were removed by chromatography on the reciprocal affinity matrix. The specificity of eluted IsdA and IsdB antibodies was verified by immunoblotting and ELISA against purified recombinant protein (Fig. 2E and F).
3.3. Isd antibodies do not promote opsonophagocytic killing of staphylococci in mouse blood
Gram-positive bacteria, such as S. aureus, cannot be killed by complement lysis alone; host clearance of the pathogen requires opsonophagocytic killing by immune cells [37]. Previous work reported that IsdA- or IsdB-specific antibodies promote opsonophagocytic killing of staphylococci by isolated human PMNs [9]. Briefly, staphylococci are incubated with a 10-fold excess of isolated human PMNs as well as rabbit complement and antiserum pre-adsorbed to staphylococci [9]. Because wild-type staphylococci display protein A to capture immunoglobulins via their Fc portion on the bacterial surface [38,39], these experiments examined opsonophagocytosis with a protein A mutant strain [9]. Here we asked whether antibodies against Isd proteins have a similar effect on wild-type staphylococci [40]. In the presence of complement as well as rabbit IgG specific for IsdA and IsdB, PMNs caused a moderate 9% [P = 0.0041; IsdA vs. non-reactive rabbit serum (NRS)] and 6% (P = 0.0368; IsdB vs. NRS) killing of S. aureus Newman (Fig. 3A). We wondered whether a greater amount of opsonophagocytic killing could be observed in blood. Fresh blood was isolated from naïve BALB/c mice via cardiac puncture and blood coagulation was blocked with lepirudin. The absence of staphylococcal antibodies in blood of naïve mice was determined by incubating serum with the staphylococcal antigen-matrix, a collection of 27 recombinant purified proteins that are known to reside in the staphylococcal envelope or to be secreted into the culture medium (data not shown). The survival of wild-type S. aureus Newman rotating in blood was monitored 15, 30 and 60 min after the addition of 1 × 105 CFU S. aureus Newman (Fig. 3). Mock treatment with PBS or incubation with an irrelevant antibody, 1 μg ml−1 purified rabbit anti-V10 [IgG directed against LcrV, a Yersinia pestis protective antigen that is not expressed by staphylococci [14]], did not affect staphylococcal survival in blood. Addition of 1 μg purified rabbit antibodies directed against IsdA, IsdB, or IsdC per ml blood also did not enable immune cells to phagocytose and kill S. aureus Newman (Fig. 3B, Table 1). Of note, antibody reagents and preparation of staphylococci for in vitro opsonophagocytosis in mouse blood were similar to experimental conditions demonstrating protection against staphylococcal abscess formation and/or lethal challenge in vivo (vide infra). We examined whether passive transfer of purified antibodies into the peritoneal cavity of mice (5 mg kg−1 weight administered 24 h prior to challenge) caused a significant reduction of S. aureus Newman in the bloodstream 60 m following intravenous challenge with 1 × 107 CFU. Isd-specific antibodies did not promote opsonophagocytic clearance of staphylococci in this assay (data not shown). We conclude that rabbit antibodies against IsdA, IsdB and IsdC do not promote significant opsonophagocytic clearance of wild-type staphylococci in mouse blood and that the protective value of these antibodies must be derived from another attribute.
Fig. 3.

Role of rabbit antibodies against Isd proteins towards opsonophagocytic killing of staphylococci. (A) S. aureus Newman (1 × 104 CFU) was incubated in the presence of isolated human polymorphonuclear leukocytes (1 × 105 PMNs) and diluted rabbit antisera against Isd proteins or normal rabbit sera in the presence or absence of baby rabbit complement. Differences in the percent survival of staphylococci after 90 min incubation were recorded by plating reactions of 4 independent experimental trials and enumerating colonies. Data were averaged and standard error of the means derived. Statistical significance was calculated with the unpaired 2-tailed student's t-test comparing non-reactive rabbit serum (NRS) with rabbit serum raised against specific antigens: IsdA (P = 0.004), IsdB (P = 0.036), IsdBN (P = 0.810), and IsdBC (P = 0.720). (B) Purified rabbit antibodies specific for iron-regulated surface determinants do not promote opsonophagocytic killing of S. aureus Newman in anti-coagulated blood from naïve mice. Blood of BALB/c mice was drawn by cardiac puncture, treated with lepirudin, pooled and 0.5 ml aliquots infected with 1 × 105 CFU of S. aureus Newman, which was derived from midlog cultures grown in TSB and washed with PBS. Staphylococci and blood were incubated with rotation at 37 °C in the presence of Isd (αIsdA, αIsdB, αIsdC) or control (αV10) antibodies. Aliquots were removed at timed intervals (0, 15, 30 and 60 min), blood cells lysed with saponin and staphylococcal load enumerated by plating on agar and colony formation. Bacterial survival at indicated time intervals was calculated from the average of 3 experimental determinations, divided by the average staphylococcal load at the beginning of the experiment (time 0 min) and standard error of the means calculated.
Table 1.
Survival of S. aureus Newman in mouse blood is not affected by antibodies directed against iron-regulated surface determinants.
| Antibodya | Survivalb | P-valuec | Survivalb | P-valuec | Survivalb | P-valuec |
|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | ||||
| dV10 | 1.76 ± 0.58 | – | 1.28 ± 0.15 | – | 1.39 ± 0.35 | – |
| IsdA | 1.22 ± 0.28 | 0.3474 | 1.43 ± 0.50 | 0.7500 | 1.94 ± 0.51 | 0.2911 |
| IsdB | 1.32 ± 0.25 | 0.4226 | 1.21 ± 0.30 | 0.6977 | 2.00 ± 0.15 | 0.3113 |
| IsdC | 1.59 ± 0.17 | 0.7981 | 1.46 ± 0.27 | 0.3206 | 1.97 ± 0.46 | 0.2914 |
Affinity purified rabbit antibodies (1 μg/ml) was added to 1 ml of mouse blood and infected with 1 × 105 CFU S. aureus strain Newman.
Bacterial survival reported as [(staphylococcal CFU at observation period/staphylococcal CFU input)] in presence of antibody and normalized to PBS alone.
P-values were determined using the unpaired 2-tailed student's t-test.
V10 antibodies are directed against LcrV, a protective antigen of Yersinia pestis that is not expressed in S. aureus [14].
3.4. IsdA and IsdB antibodies protect against staphylococcal abscess formation and lethal challenge
Affinity purified antibodies or mock control (PBS) were injected into the peritoneal cavity of naïve BALB/c mice (5 mg IgG kg−1 body weight). Twenty-four hours following passive immunization, serum antibody concentrations were examined by ELISA, revealing significant amounts of serum IgG for IsdA [1400 (±126)] or IsdB [1640 (±320)] (Table 2). Serum IgG specific for IsdA or IsdB were not detected in mock immunized animals. Passively immunized animals were challenged by intravenous injection with 1 × 107 CFU S. aureus Newman. Animals were killed 4 days following challenge and kidneys removed. At the time of necropsy, we also examined serum antibody titers, which had dropped by 10–20% (Fig. 4A). To enumerate staphylococcal load in renal tissue, homogenate from the right kidney of each animal was spread on agar media and incubated for colony formation. Compared to a PBS and anti-V10 controls, passive immunization with IsdA or IsdB antibodies caused a significant reduction in staphylococcal load (Fig. 4B and Table 2). To quantify abscess formation, the left kidney of each necropsied animal was fixed in formalin, embedded, thin-sectioned, and stained with hematoxylin-eosin. For each kidney, 4 sagittal sections at 200 μM intervals were viewed by microscopy to determine the number of abscess lesions for each organ. In mock immunized mice, S. aureus Newman caused an average of 4.64 (±1.17) abscesses per kidney (Fig. 4D and E and Table 2). There were no significant changes in staphylococcal load or abscess formation for animals immunized with V10 specific antibody (Table 2). Animals immunized with IsdA or IsdB-specific IgG harbored an average of 1.0 (±0.5) and 0.86 (±0.46) abscesses per organ, respectively (Fig. 4F–I and Table 2). Although IsdA- and IsdB-specific antibodies reduce staphylococcal load and abscess formation in mouse kidneys, the size of residual abscesses in this organ was not significantly altered compared to the abscesses of mock immunized animals: average diameter (±SEM) of abscesses in mock immunized animals was 911 (±84) μm as compared to 659 (±138) μm for IsdA (P = 0.1567) and 728 (±68) μm for IsdB (P = 0.4891) immunized mice.
To test whether passive transfer of purified IsdA and IsdB-specific antibodies confers protection against lethal disease, cohorts of mice were injected with 1.5 × 108 CFU S. aureus Newman and monitored for survival over the next 240 h (Fig. 4C). Most of the mock immunized animals (80%) survived staphylococcal challenge for up to 48 h and the remaining animals lived for up to 100 h (Fig. 4C). No significant protection against lethal challenge was achieved with anti-V10 control antibodies (data not shown). Animals immunized with IsdA-specific IgG survived for a longer time interval (P = 0.028): 80% of animals died 96 h following challenge, whereas the death of the remaining animals occurred by 124 h. Animals immunized with IsdB-specific IgG displayed the most significant protection (P = 0.0001): 10% of mice in this cohort did not suffer from lethal disease at the end of the 240-h observation period. Most of the IsdB immunized animals (80%) survived for at least 144 h (Fig. 4C).
3.5. Antibodies that interfere with heme-iron scavenging
To address the molecular mechanism of antibody protection, recombinant IsdA and IsdB were purified with an N-terminal His- or GST tag and their ability to bind hemoglobin was measured by surface plasmon resonance (SPR). As reported previously, IsdB, but not IsdA, bound to purified human hemoglobin [8]. The ability to bind hemoglobin can be attributed to the first of the 2 NEAT domains, IsdB1 and IsdB2. To distinguish the effect of antibodies on the 2 biochemical activities of IsdB, we engineered recombinant DNA fragments and cut the IsdB molecule into halves, IsdBN (including IsdB1) and IsdBC (with IsdB2) (Fig. 5A). Purified IsdBN bound hemoglobin, but not heme, whereas IsdBC captured heme as a ligand, but not hemoglobin (Fig. 5B–D). These findings are in agreement with the general property of IsdB1 for removing heme from hemoglobin and transferring the iron-tetrapyrrol first to IsdB2 and then to IsdA [18]. Affinity purified rabbit antibodies directed against IsdB blocked the ability of IsdBN to bind hemoglobin (Fig. 5C). As a control, purified rabbit antibodies directed against the plague vaccine antigen rV10 [14] did not affect the binding of IsdBN to hemoglobin (Fig. 5C).
Fig. 5.
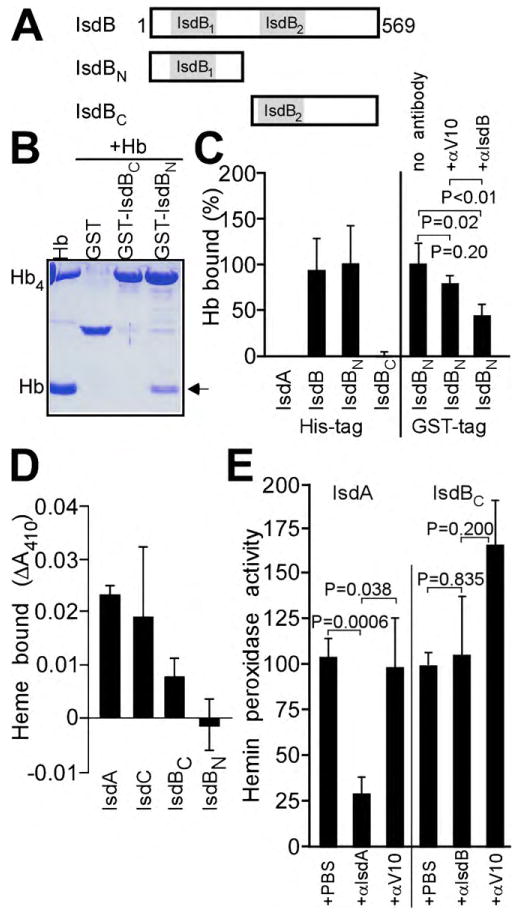
Antibodies against IsdA and IsdB interfere with heme-iron scavenging of staphylococci. (A) Schematic illustrating the primary structure of IsdBN and IsdBC, the approximate 2 halves of IsdB encompassing its hemoglobin-binding (IsdBN) and heme-transfer (IsdBC) domains, respectively. (B) Purified hemoglobin (Hb) was incubated with glutathione S-transferase (GST) or its fusions to IsdBN (GST-IsdBN) and IsdBC (GST-IsdBC) and possible association between polypeptides (arrow) detected by separating affinity chromatography eluates on Coomassie-stained SDS-PAGE. (C) Association of purified poly-histidine tagged IsdA, IsdB, IsdBN or IsdBC with hemoglobin (Hb) was measured by surface plasmon resonance in 3 experimental determinations; data for IsdB were used as calibration (100%). Average measurements and standard error of the means are recorded. On the right panel, the association of GST-tagged IsdBN with hemoglobin (Hb) was measured by surface plasmon resonance. Interaction of GST-IsdBN with Hb was perturbed with irrelevant IgG antibodies (αV10) or with αIsdB, which led to a significant reduction in hemoglobin-binding. (D) The ability of poly-histidine tagged IsdA, IsdC, IsdBC or IsdBN to bind hemin was measured as sample absorbance at 410 nm (Soret). (E) The binding of IsdA and IsdBC to hemin was perturbed by the addition of αV10 (control) or αIsdA and αIsdB. Hemin peroxidase activity was determined as a measure for the association between the NEAT domains of IsdA and IsdBC with hemin. Statistical significance was recorded with the unpaired 2-tailed student's t-test. Data generated for IsdBC were used for calibration (100%).
The ability of IsdA, IsdBN, IsdBC and IsdC to bind heme was measured as an increase in absorbance at 410 nm (Soret) following incubation of purified proteins with hemin [8]. As expected, IsdA, IsdBC and IsdC bound to hemin, whereas IsdBN did not (Fig. 5D). We sought to address the possibility that antibodies against IsdA or IsdB interfere with the heme-binding properties of purified IsdA and IsdBC, respectively. This could not be accomplished measuring 410 nm absorbance, owing to the limited availability of purified antibody. We therefore measured the heme-dependent peroxidation of TMBZ by IsdA and IsdBC in the presence or absence of specific antibody [16]. IsdA-specific antibodies reduced the peroxidation of TMBZ, whereas V10 control antibodies did not (P = 0.0006 αIsdA vs. αV10) (Fig. 5E). IsdB-specific antibodies did not cause a reduction in heme-binding of IsdBC (P = 0.835; αIsdB vs. αV10) (Fig. 5E). The inhibition of IsdA specific antibodies was not due to the formation of ternary complexes (heme-IsdA and antibodies) on LDS-PAGE, as peroxidase measurements for immune complexes did not reveal concomitant increases in signal intensity for samples treated with αIsdA or αIsdB (data not shown). From these data we conclude that IsdB antibodies interfere with the heme-scavenging attributes of this surface protein, i.e. the capture of hemoglobin. Antibodies against IsdA perturb heme-iron traffic, where IsdB and IsdH donate heme to IsdA and IsdA transfers heme to IsdC.
3.6. Passive immunization with IsdBN and IsdBC-specific antibodies
Rabbits were immunized with purified IsdBN or IsdBC and antigen specific antibodies were purified by affinity chromatography. Antibodies against IsdBN, IsdBC or V10 as well as a PBS control were injected into the peritoneal cavity of naïve BALB/c mice (5 mg IgG kg−1 body weight). Twenty-four hours following passive immunization, serum antibody concentrations were examined by ELISA for IsdBN [900 (±126)], IsdBC [950 (±320)] and V10 [1300 (±55)] (Fig. 6). IgG specific for IsdA or IsdB was not detected in the serum of animals that had been mock immunized or passively transferred with V10 antibodies. Additional animals in the same cohorts were challenged by retro-orbital injection with 1 × 107 CFU S. aureus Newman. Animals were killed 4 days following challenge and kidneys removed. Compared to the V10 control, IsdBN and IsdBC-specific antibodies led to a significant reduction in bacterial load 4 days after challenge (Fig. 6C and Table 2). Immunization with IsdBN and IsdBC antibodies also reduced the number of abscess lesions quantified during histopathology of kidney tissue (Fig. 6E–J and Table 2). Of note, the size of residual abscesses in kidneys was not significantly altered for IsdBN [diameter 792 (±188) μm; P = 0.5716 IsdBN vs. mock] or IsdBC antibodies [diameter 754 (±176) μm; P = 0.5716 IsdBN vs. mock]. When tested for the ability to protect against intravenous inoculation of 2.0 × 108 CFU S. aureus Newman, antibodies directed against IsdBN (P = 0.0245) and IsdBC (P = 0.003) alone both extended the survival of passively immunized mice (Fig. 6D). Of note, the combined administration of antibodies against IsdBN and IsdBC generated increased survival as compared to mice that received each of the 2 antibodies alone (P < 0.001) (Fig. 6). In summary, IsdB-specific antibodies directed against the hemoglobin receptor domain or the heme-transfer domain generate moderate levels of protection against staphylococcal abscess formation or lethal challenge. Disease protection is increased by combining antibodies specific for each of the 2 domains. This result is in agreement with the possibility that IsdB immunization may interfere with heme-iron scavenging of S. aureus in host tissues.
4. Discussion
We show here that 2 iron-regulated surface determinants, IsdA and IsdB, are required for the pathogenesis of mouse kidney abscess formation and lethal disease caused by S. aureus Newman infection. If one based the selection of vaccine antigens on their genetic requirement for disease establishment and the conservation of that gene in many, if not all, clinical isolates of a pathogen, our experiments provide validation for IsdA and IsdB as targets for staphylococcal vaccine development. Affinity purified rabbit antibodies directed against either IsdA or IsdB generated significant protection against S. aureus abscess formation or lethal challenge, 2 murine disease models that involve intravenous inoculation of virulent staphylococci. Pathogenic processes that underwrite both diseases occur over a period of 2–4 days. Our experiments made no attempts to optimize the expression of IsdA and IsdB at the time of staphylococcal challenge [20]; we deliberately sought to avoid a bias enabling host clearance of challenge strains by antibodies immediately following inoculation.
Monitoring the in vitro opsonophagocytosis of staphylococci in mouse blood or the in vivo survival of S. aureus Newman in the bloodstream of mice, we could detect only modest clearance effects of IsdA and IsdB antibodies on the invading pathogen over 60 min following inoculation. Earlier studies, utilizing humans PMNs and baby rabbit complement, reported that IsdA- and IsdB-specific antibodies can promote opsonophagocytic killing of protein A mutant staphylococci in vitro [9]. We recently examined the role of isogenic spa variants derived from S. aureus Newman in mice and observed that protein A is required for abscess formation as well as lethal challenge [7] (Kim et al., unpublished observation). The protein A requirement for disease pathogenesis is likely borne out of the overall strategy of staphylococci to modulate phagocyte responses and B cell proliferation [41]. We presume further that the sophisticated immune evasion strategies of staphylococci, i.e. the expression of adenosine synthase A (AdsA) [6] and protein A on the bacterial surface [41], may also be responsible for blocking opsonophagocytic killing of wild-type staphylococci by IsdA and IsdB-specific antibodies. In agreement with this conjecture, antibodies against IsdA and IsdB (which do provide protection against staphylococcal challenge of mice over the course of several days) did not promote the rapid killing of the invading pathogen when tested with in vitro assays using human PMNs and rabbit complement or mouse blood. Although still speculative, it seems plausible to us that the ability of anti-IsdA and anti-IsdB to inhibit the physiological function of Isd proteins, i.e. the scavenging of heme-iron from hemoglobin, may contribute to their disease protective attributes. Experimental proof for this model may be obtained through the isolation of IsdA- and IsdB-specific monoclonal antibodies with disease protective attributes and the characterization of their neutralizing activities.
Purified rabbit antibodies hindered the ability of IsdB to bind hemoglobin for subsequent heme-iron scavenging via a transport cascade in the staphylococcal envelope involving IsdA, IsdC as well as IsdE/IsdF [8,18], and culminating in the cleavage of the tetrapyrrol ring by IsdG/IsdI and the release of iron [15]. IsdB is currently being explored as a vaccine antigen to prevent staphylococcal infection of humans [10,42]. If so, the development of assays that monitor the attributes of IsdB-specific antibodies in blocking heme-iron transport may be considered as a correlate for immunity in humans. Our experiments have not mapped the epitopes of IsdB antibodies that are protective against staphylococcal challenge, however data in Fig. 6 indicate that antibodies directed against the IsdBN (IsdB1) and IsdBC (IsdB2) halves of the protein each generate protection against staphylococcal abscess formation and lethal challenge. Combining antibodies specific for IsdBN and IsdBC clearly increased protection against lethal challenge. Future work will need to map the binding sites and protective properties of a large spectrum of IsdB-specific antibodies in an effort to analyze the molecular basis of protective immunity.
IsdA, a sortase A-anchored NEAT domain protein, transfers heme from the 2 hemophores IsdB and IsdH to IsdC, the central conduit of staphylococcal heme-iron scavenging [8,21]. IsdC is anchored to the cell wall by sortase B and its unique position in the envelope enables the transfer of heme to IsdEF for import into the bacterial cytoplasm. Active immunization with IsdA antigen [9] and, as demonstrated here, passive transfer of antibodies against IsdA provide experimental mice with a significant level of protection against staphylococcal abscess formation and lethal challenge. When compared with IsdB, antibodies against IsdA performed equally well in the renal abscess model. In contrast, IsdA antibodies did not achieve the same level of protection as IsdB antibodies in the lethal challenge model. We have not yet compared IsdC antibodies with IsdA- and IsdB-specific IgG, as it is not clear that IsdC is accessible to antibodies on the staphylococcal surface [8,21]. Nevertheless, earlier work revealed a significant level of protection in mice following active immunization with IsdC, albeit that protection from abscess formation was not as high as measured for IsdA and IsdB [9]. Our future work will need to analyze the level of protection derived from antibodies against IsdC and IsdH, and establish whether combinations of these antibodies provide increased protection over individual vaccine components.
Experiments in Fig. 1 indicate that isdH (harA) is not required for the pathogenesis of S. aureus Newman abscess formation in mice. Nevertheless, the phenotypic defect of the isdH mutant in the lethal challenge model was similar to that of the isdB mutant. In agreement with the conjecture that isdH may be dispensable for abscess formation in the kidney of mice, active immunization with IsdH generated only a moderate level of protection as compared with IsdA and IsdB vaccines [9]. Thus, at least in S. aureus Newman, IsdA and IsdH do not appear to fulfill functionally redundant roles in their hemophore activities. Guided by the hypothesis stated above – vaccine antigens must be essential for disease establishment – we chose not to pursue IsdH as a vaccine candidate in mice. The genetic requirements for the pathogenesis of staphylococcal infections may of course differ between host species. Therefore, future work should investigate whether the IsdH (HarA) haptoglobin hemophore can be a useful vaccine antigen in non-human primates or humans.
Acknowledgments
We thank members of our laboratories as well as Guido Grandi and colleagues at Novartis Vaccines and Diagnostics for discussion. This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (AI52474 to O.S.) and by Novartis Vaccines and Diagnostics (Siena, Italy). A.G.C. was a trainee of the NIH Medical Scientist Training Program at The University of Chicago (GM07281). M.M. was a trainee of the Graduate Training in Growth and Development program at The University of Chicago (HD009007). O.S. and D.M.M. acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (GLRCE, National Institute of Allergy and Infectious Diseases Award 1-U54-AI-057153).
References
- 1.Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Tenover FC, McDougal LK, Goering RV, Killgore G, Projan SJ, Patel JB, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44:108–18. doi: 10.1128/JCM.44.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci USA. 2008;105:1327–32. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Projan SJ, Nesin M, Dunman PM. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr Opin Pharmacol. 2006;6:473–9. doi: 10.1016/j.coph.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206:2417–27. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:1–12. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, et al. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–9. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 9.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Nat Acad Sci USA. 2006;103:16942–7. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74:2215–23. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes. J Bacteriol. 2007;190:300–10. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae T, Banger AK, Wallace A, Glass EM, Aslund F, Schneewind O, et al. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci USA. 2004;101:12312–7. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae T, Baba T, Hiramatsu K, Schneewind O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol Microbiol. 2006;62:1035–47. doi: 10.1111/j.1365-2958.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- 14.Overheim KA, Depaolo RW, Debord KL, Morrin EM, Anderson DM, Green NM, et al. LcrV plague vaccine with altered immunomodulatory properties. Infect Immun. 2005;73:5152–9. doi: 10.1128/IAI.73.8.5152-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skaar EP, Gaspar AH, Schneewind O. IsdG and IsdI, heme degrading enzymes in the cytoplasm of Staphylococcus aureus. J Biol Chem. 2004;279:436–43. doi: 10.1074/jbc.M307952200. [DOI] [PubMed] [Google Scholar]
- 16.Stugard CE, Daskaleros PA, Payne SM. A 101-kilodalton heme-binding protein associated with congo red binding and virulence of Shigella flexneri and enteroinvasive Escherichia coli strains. Infect Immun. 1989;57:3534–9. doi: 10.1128/iai.57.11.3534-3539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen CE, Schmitt MP. HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtheriae. J Bacteriol. 2009;191:2638–48. doi: 10.1128/JB.01784-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muryoi N, Tiedemann MT, Pluym M, Cheung J, Heinrichs DE, Stillman MJ. Demonstration of the iron-regulated surface determinant (Isd) heme transfer pathway in Staphylococcus aureus. J Biol Chem. 2008;283:28125s–36s. doi: 10.1074/jbc.M802171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Tanaka WN, Zhu H, Dooley DM, Lei B. Direct hemin transfer from IsdA to IsdC in the iron-regulated surface determinant (Isd) heme acquisition system of Staphylococcus aureus. J Biol Chem. 2008;283:6668–76. doi: 10.1074/jbc.M708372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazmanian SK, Ton-That H, Su K, Schneewind O. An iron-regulated sortase enzyme anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci USA. 2002;99:2293–8. doi: 10.1073/pnas.032523999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marraffini LA, Schneewind O. Anchor structure of staphylococcal surface proteins. V. Anchor structure of the sortase B substrate IsdC. J Biol Chem. 2005;280:16263–71. doi: 10.1074/jbc.M500071200. [DOI] [PubMed] [Google Scholar]
- 22.Zhu H, Xie G, Liu M, Olson JS, Fabian M, Dooley DM, et al. Pathway for heme uptake from human methemoglobin by the iron-regulated surface determinants system of Staphylococcus aureus. J Biol Chem. 2008;283:18450–60. doi: 10.1074/jbc.M801466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu R, Skaar EP, Zhang R, Joachimiak G, Gornicki P, Schneewind O, et al. Staphylococcus aureus IsdG and IsdI, heme degrading enzymes with structural similarity to monooxygenases. J Biol Chem. 2005 Oct 31;:280, 2840–6. doi: 10.1074/jbc.M409526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dryla A, Gelbmann D, von Gabain A, Nagy E. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol Microbiol. 2003;49:37–53. doi: 10.1046/j.1365-2958.2003.03542.x. [DOI] [PubMed] [Google Scholar]
- 25.Dryla A, Hoffmann B, Gelbmann D, Giefing C, Hanner M, Meinke A, et al. High-affinity binding of the staphylococcal HarA protein to haptoglobin and hemoglobin involves a domain with an antiparallel eight-stranded β-barrel fold. J Bacteriol. 2007;189:254–64. doi: 10.1128/JB.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilpa RM, Robson SA, Villareal VA, Wong ML, Phillips M, Clubb RT. Functionally distinct NEAT (NEAr Transporter) domains within the Staphylococcus aureus IsdH/HarA protein extract heme from methemoglobin. J Biol Chem. 2009;284:1166–76. doi: 10.1074/jbc.M806007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme-iron utilization. J Bacteriol. 2006;188:8421–9. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duthie ES, Lorenz LL. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 29.Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–3. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 30.Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O. Staphylococcus aureus mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci USA. 2000;97:5510–5. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–6. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss WJ, Lenoy E, Murphy T, Tardio L, Burgio P, Projan SJ, et al. Effect of srtA and srtB gene expression on the virulence of Staphylococcus aureus in animal infection. J Antimicrob Chemother. 2004;53:480–6. doi: 10.1093/jac/dkh078. [DOI] [PubMed] [Google Scholar]
- 33.Pilpa RM, Fadeev EA, Villareal VA, Wong ML, Phillips M, Clubb RT. Solution structure of the NEAT (NEAr Transporter) domain from IsdH/HarA: the human hemoglobin receptor in Staphylococcus aureus. J Mol Biol. 2006;360:435–47. doi: 10.1016/j.jmb.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Grigg JC, Vermeiren CL, Heinrichs DE, Murphy ME. Haem recognition by a Staphylococcus aureus NEAT domain. Mol Microbiol. 2007;63:139–49. doi: 10.1111/j.1365-2958.2006.05502.x. [DOI] [PubMed] [Google Scholar]
- 35.Sharp KH, Schneider S, Cockayne A, Paoli M. Crystal structure of the heme-IsdC complex, the central conduit of the Isd iron/heme uptake system in Staphylococcus aureus. J Biol Chem. 2007;282:10625–31. doi: 10.1074/jbc.M700234200. [DOI] [PubMed] [Google Scholar]
- 36.Villareal VA, Pilpa RM, Robson SA, Fadeev EA, Clubb RT. The IsdC protein from Staphylococcus aureus uses a flexible binding pocket to capture heme. J Biol Chem. 2008;283:31591–600. doi: 10.1074/jbc.M801126200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lancefield R. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–13. [PubMed] [Google Scholar]
- 38.Jensen K. A normally occuring staphylococcus antibody in human serum. Acta Path Microbiol Scand. 1958;44:421–8. doi: 10.1111/j.1600-0463.2007.apm_731a.x. [DOI] [PubMed] [Google Scholar]
- 39.Sjöquist J, Meloun B, Hjelm H. Protein A isolated from Staphylococcus aureus after digestion with lysostaphin. Eur J Biochem. 1972;29:572–8. doi: 10.1111/j.1432-1033.1972.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 40.Lancefield RC. The antigenic complex of Streptococcus hemolyticus. I. Demonstration of a type-specific substance in extracts of Streptococcus hemolyticus. J Exp Med. 1928;47:91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, et al. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc Nat Acad Sci USA. 2000;97:5399–404. doi: 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raedler MD, Heyne S, Wagner E, Shalkowski SK, Secore S, Anderson AS, et al. A serologic assay to quantify human IgG antibodies to Staphylococcus aureus iron surface determinant B antigen. Clin Vacc Immunol. 2009;16:739748. doi: 10.1128/CVI.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ton-That H, Liu G, Mazmanian SK, Faull KF, Schneewind O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc Natl Acad Sci USA. 1999;96:12424–9. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]


