Abstract
Glutathione contributes to thiol-redox control and to extra-mitochondrial iron–sulphur cluster (ISC) maturation. To determine the physiological importance of these functions and sort out those that account for the GSH requirement for viability, we performed a comprehensive analysis of yeast cells depleted of or containing toxic levels of GSH. Both conditions triggered an intense iron starvation-like response and impaired the activity of extra-mitochondrial ISC enzymes but did not impact thiol-redox maintenance, except for high glutathione levels that altered oxidative protein folding in the endoplasmic reticulum. While iron partially rescued the ISC maturation and growth defects of GSH-depleted cells, genetic experiments indicated that unlike thioredoxin, glutathione could not support by itself the thiol-redox duties of the cell. We propose that glutathione is essential by its requirement in ISC assembly, but only serves as a thioredoxin backup in cytosolic thiol-redox maintenance. Glutathione-high physiological levels are thus meant to insulate its cytosolic function in iron metabolism from variations of its concentration during redox stresses, a model challenging the traditional view of it as prime actor in thiol-redox control.
Keywords: glutathione, iron–sulphur cluster, oxidative protein folding, redox homoeostasis, thioredoxin
Introduction
The low-molecular weight thiol glutathione (L-γ-glutamyl-L-cysteinylglycine) (GSH), glutathione reductase and glutaredoxin constitute with the thioredoxin pathway, the two independent arms of the system that assists disulphide bond reduction (Toledano et al, 2007). Because of its high cellular abundance, between 3 and 10 mM, and low redox potential of −240 mV, GSH is also given the special attribute of a redox buffer not only important for reducing oxidized cysteine residues, but also for protecting them from irreversible oxidation and for scavenging various endogenous and exogenous electrophilic compounds (Meister and Anderson, 1983; Grant et al, 1999; Shelton and Mieyal, 2008).
In E. coli, simultaneous inactivation of the GSH and thioredoxin pathways is unviable but inactivation of either pathway is fully viable, which indicate that each can fully operate the essential function of reducing disulphides in the absence of the other (reviewed in Toledano et al (2007)). In S. cerevisiae, inactivating glutathione reductase together with both cytoplasmic thioredoxins (Muller, 1996) or with thioredoxin reductase (Trotter and Grant, 2003) is also lethal, whereas deletion of both cytoplasmic thioredoxins (Muller, 1991) or of both dithiol glutaredoxins (Luikenhuis et al, 1998) is viable. However, unlike in E. coli, deletion of the rate-limiting GSH-biosynthetic enzyme, γ-glutamyl cysteine synthase (gsh1Δ) is unviable on its own (Wu and Moye-Rowley, 1994; Grant et al, 1996). Such a requirement of GSH for yeast viability has prevented evaluating its contribution to thiol-redox control, and constitutes an enigma as to its molecular cause. Attempts at identifying yeast extragenic suppressors of the GSH auxotrophy of gsh1Δ only yielded mutations restoring synthesis of very small GSH amounts, indicating both that no single-gene mutation can replace GSH and that minute amounts of the tripeptide suffice for full viability (Spector et al, 2001). A reactive oxygen species (ROS)-protective function cannot account for the yeast GSH requirement, as anaerobiosis does not rescue GSH auxotrophy (Spector et al, 2001). Ribonucleotide reduction is the function that makes the presence of either the GSH or thioredoxin pathways essential for E. coli aerobic viability (Gon et al, 2006b), but is predominantly operated by thioredoxin in yeast (Camier et al, 2007). In the endoplasmic reticulum (ER), GSH competes with luminal protein-thiols for oxidation by the ER oxidase (Ero1)-protein disulphide isomerase (Pdi1) oxidative folding machinery (Cuozzo and Kaiser, 1999), but ER secretion is not altered by GSH depletion (Frand and Kaiser, 1998; Pollard et al, 1998). If not for thiol-redox maintenance, GSH might otherwise be essential by its requirement, together with the mitochondrial inner-membrane ABC transporter Atm1 and the intermembrane space Erv1 thiol oxidase, for maturation of cytoplasmic iron–sulphur cluster (ISC) by the cytosolic ISC assembly (CIA) system (Kispal et al, 1999; Lange et al, 2001; Sipos et al, 2002), which is a vital process (Lill, 2009).
In addition to its essential nature, GSH impedes growth when at high intracellular levels, as suggests the inability of yeast cells overexpressing Hgt1, a specific GSH transporter (Bourbouloux et al, 2000), to grow on medium containing GSH (Srikanth et al, 2005).
To apprehend the physiological role of GSH, we sought to elucidate the bases of its essential requirement and toxicity by analysing the cellular consequences of its depletion and presence at toxic levels, using the gsh1Δ and HGT1-overexpressing strains, respectively. Both extreme GSH conditions caused major alterations of iron metabolism, but surprisingly did not significantly impact thiol-redox control, except for the effect of high GSH levels blocking oxidative protein folding and secretion. We also evaluated the contribution of GSH to thiol-redox maintenance by the redox load excess of cells with a crippled thioredoxin pathway and, conversely, tested the effect of exogenous iron in rescuing the defects of GSH-depleted cells. Altogether, our data indicate that the essential function of GSH is linked to its involvement in iron metabolism, which requires very limited amounts of the tripeptide, while its cytosolic thiol-redox function only serves as backup of thioredoxin, and requires high levels of the tripeptide. We further show that such split functions of GSH link thiol-redox control and iron metabolism thereby causing redox stresses that consume GSH to impinge on iron metabolism. We propose a model that departs from the notion of GSH as a prime actor in thiol-redox maintenance, while emphasizing its crucial role in iron metabolism.
Results
HGT1 overexpression causes toxic accumulation of GSH
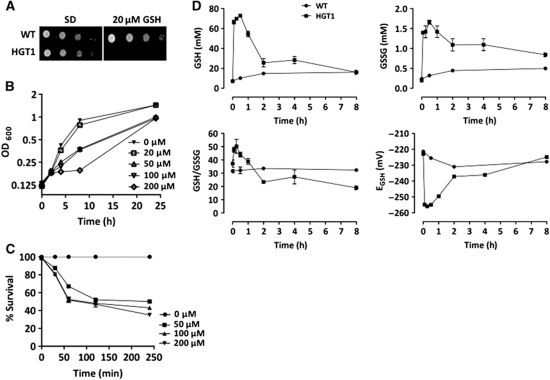
Hgt1 is an exclusive GSH and GSSG transporter (Bourbouloux et al, 2000). Cells that overexpressed HGT1 were unable to grow on minimal medium plates that contained GSH at concentrations as low as 20 μM (Figure 1A). Similarly in liquid medium, HGT1 cells were growth inhibited at GSH concentrations of 50 μM and above (Figure 1B), but then resumed growth after a few hours. These cells died massively within 4 h after GSH addition (Figure 1C). Lack of a dose effect on cell killing at GSH concentration above 100 μM probably reflects saturation of HGT1, the Km of which for GSH is 50 μM (Bourbouloux et al, 2000). Saturation of GSH uptake also explains the lag period before growth resumption to be longer at 200 μM than at 100 μM GSH, as GSH remains longer in the medium under the former condition. In the absence of GSH, HGT1 cells had a wild-type GSH content (7.5 mM); however, on adding GSH (100 μM), this concentration peaked at ∼70 mM after 30 min (Figure 1D; Table I). GSSG levels paralleled the increase in GSH levels peaking at 1.7 mM from a baseline value of 0.2 mM. The increases in GSH and GSSG resulted in a significant decrease of the GSH redox potential (EGSH) from approximately −220 mV to approximately −250 mV at 30 min. GSH levels then decreased after an hour to normalize at 8 h (Figure 1D; Table I). Such decrease, which explains the growth resumption of HGT1 cells in liquid medium and only partial lethality (see Figure 1B and C), is not a consequence of GSH efflux, as the GSH concentration in the culture medium gradually decreased (Supplementary Figure S1A), but of degradation by the γ-glutamyl transpeptidase (γ-GT)-independent Dug1–Dug2–Dug3 pathway (Ganguli et al, 2007; Kaur et al, 2009), as the corresponding mutants maintained elevated GSH levels for a longer period and were sensitive to lower GSH concentrations (Supplementary Figure S1B and C). The decreased GSH tolerance of the Dug pathway mutants indicates that GSH toxicity is caused by GSH itself and not by a GSH catabolite. Fast correction of GSH levels also indicates a fast turnover rate of GSH.
Figure 1.
HGT1 overexpression increases GSH to toxic levels. (A) HGT1 or WT cells serially diluted and spotted onto SD plates containing or not containing GSH (20 μM). (B) HGT1 cells inoculated in fresh SD medium containing GSH, as indicated and growth monitored by turbidity. (C) HGT1 cells incubated in SD medium containing the indicated amount of GSH for the indicated time, and spread onto SD plates lacking GSH. Colony forming units were recorded (% survival relative to untreated cells). (D) Kinetics of cellular content of GSH (upper left), GSSG (upper right), GSH/GSSG (lower left) and GSH redox potential (EGSH) (lower right) in HGT1 and WT cells grown in SD medium supplemented with GSH (100 μM). GSH and GSSG in mM/cell. Values are the mean of three independent experiments. Error bars represent mean values±s.d.
Table 1. GSH measurements in HGT1 and gsh1Δ cells.
| Strain | GSH addeda | [GSH] (mM/cell) | [GSSG] (mM/cell) | GSH/GSSG | EGSH (mV) |
|---|---|---|---|---|---|
| WT | 0 | 7.2±0.04 | 0.21±0.02 | 31±1.25 | −221.78±0.65 |
| HGT1 | 0 | 7.59±0.27 | 0.19±0.02 | 38.94±4.94 | −224.02±2.03 |
| HGT1 | 50 μM | 35.13±3.49 | 0.9±0.02 | 39.08±4.49 | −244.03±2.88 |
| HGT1 | 100 μM | 54.53±4.61 | 1.42±0.06 | 38.86±3.75 | −249.56±2.88 |
| gsh1Δ | 100 μM | 2.17±0.07 | 0.08 | 26.72±0.37 | −202.80±0.51 |
| gsh1Δ | 1 μM | 0.43±0.05 | 0.03 | 16.49±1.23 | −175.27±2.61 |
| gsh1Δ | 0 | 0.06±0.002 | 0.02 | 2.92±0.06 | −125.44±0.52 |
| trr1Δ | 0 | 11.46±1.2 | 1.34±0.04 | 8.57±1.11 | −209.69±0.62 |
| aIn HGT1 cells, GSH and GSSG measurements were performed 1 h after GSH addition; in gsh1Δ: in exponentially grown cells in the presence of the indicated GSH amount or in cells grown for six divisions in SD medium lacking GSH; in trr1Δ: in exponentially growing cells. Data are from three replicate samples of independent cultures±s.d. | |||||
GSH toxic levels cause both potent UPR and a full iron starvation-like response
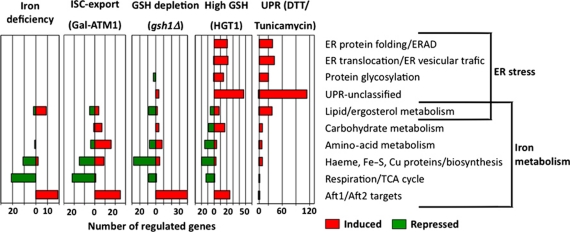
To search for causes of GSH toxicity, we established the mRNA profiles of HGT1 cells cultured with 50 μM GSH during 5, 30 and 240 min. The 5- and 30-min transcript profiles had similarities, but were distinct from the 4-h transcript profile that was not further considered as reflecting a non-specific stress response (Supplementary Figure S2). The 5-min response was modest (70 genes, Supplementary Table S1), and its significance was best appreciated in view of the 30-min response that was concurrent to the GSH peak. The 30-min response was large (375 genes, Supplementary Table S2) and included ∼50% of the genes regulated at 5 min (Supplementary Figure S3). Functional clustering revealed two major components in this response (Figure 2). The first one was highly similar to the response of iron-starved cells (Shakoury-Elizeh et al, 2004; Puig et al, 2005), and even more similar to that of the atm1Δ mutant (Hausmann et al, 2008). ATM1 encodes a mitochondrial inner-membrane ABC transporter required for extra-mitochondrial ISC assembly (Hausmann et al, 2008). The HGT1 cell iron response comprised 25 genes of the iron regulon (Supplementary Table S2; Yun et al, 2000; Foury and Talibi, 2001; Rutherford et al, 2003) controlled by the Aft1 and Aft2 transcription factors, which regulates the uptake, compartmentalization and use of iron. Many repressed genes identified as part of the iron starvation response were also present here. These included genes encoding Fe–S- and haeme-containing proteins of mitochondrial respiration and the tricarboxylic acid cycle and of other pathways, and haeme, ergosterol, biotin and amino-acid/nitrogen biosynthetic enzymes. The second major component of the response to high GSH levels (115 genes, ∼30%) was a typical unfolded protein response (UPR) that is regulated by the Ire1 kinase and Hac1 transcription factor (Figure 2; Supplementary Table S2). It encompassed all major UPR gene categories induced by the model ER stress agents DTT and tunicamycin (Travers et al, 2000; Supplementary Figure S4A and B). Induction of several cytoplasmic chaperone genes could also be considered as satellite of the UPR, as the encoded proteins are known to help cells cope with ER stress (Liu and Chang, 2008). Repression of membrane proteins and proteins involved in ribosome biogenesis and translation might also be considered as part of the ER stress response, as shown in C. albicans (Wimalasena et al, 2008).
Figure 2.
Synthetic view of genome-wide mRNA profile changes induced by toxic GSH levels and GSH depletion. Genes affected by GSH at toxic levels (HGT1 cells cultured with 50 μM GSH during 30 min), and by GSH depletion (gsh1Δ cells grown for three and six divisions in medium devoid of GSH) identified by DNA microarray analysis (see Supplementary Tables S2 and S3), in duplicate independent cultures, and assigned to functional categories (http://www.yeastgenome.org/cgi-bin/GO/goSlimMapper.pl). Numbers indicate the sum of regulated genes in each functional category. Genes represented comprise 61% (228/375) of the total genes regulated by GSH toxic levels and 68% (121/178) of the total genes regulated by GSH depletion. A few genes are present in more than one category. Data of transcriptional responses to ATM1 depletion (Gal-ATM1) were from Hausmann et al (2008), to iron deficiency from Puig et al (2005) and to DTT/tunicamycin (UPR) from Travers et al (2000) and Kimata et al (2006).
The UPR and iron starvation-like responses were independent of each other, as the latter persisted in HGT1 ire1Δ cells that had otherwise lost the UPR (Supplementary Figure S4C and D), but their concurrence probably caused conflicting gene regulation. For instance, haeme and ergosterol biosynthesis genes that are found repressed here are upregulated during the UPR.
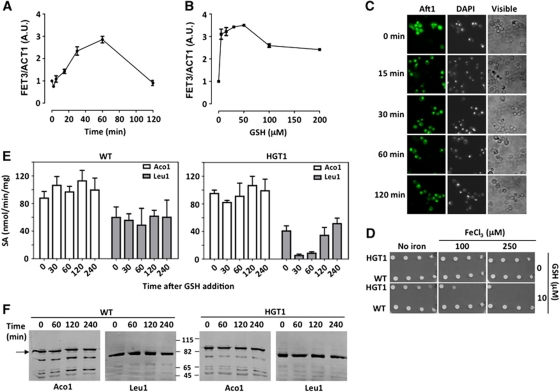
GSH toxic levels inhibit cytoplasmic Fe–S maturation and activate Aft1
We explored the link between toxic GSH levels and iron metabolism. Addition of 100 μM GSH to HGT1 cells induced the Aft1 target-gene FET3, which peaked between 30 and 60 min (Figure 3A), confirming the microarray data. FET3 was maximally induced at concentrations of GSH as low as 10 μM (Figure 3B). Aft1p is cytoplasmic in iron-replete cells, and accumulates in the nucleus in iron-deprived cells (Yamaguchi-Iwai et al, 2002). In accordance with FET3 expression, in HGT1 cells, GFP-Aft1 became nuclear from a diffuse cellular pattern 15 min after addition of GSH (Figure 3C), and at concentrations of 10 μM and above (Supplementary Figure S5A). Although Aft1 was activated, iron was not limiting, as GSH-grown HGT1 cells had a slightly higher iron content than wild-type cells (Supplementary Figure S5B), and excess iron >100 μM worsened GSH toxicity (Figure 3D). Reciprocally, inactivating high-affinity iron uptake by deletion of FET3, which encodes the iron oxidase of the high-affinity iron uptake system, or AFT1, or AFT1 and AFT2 did not decrease GSH toxicity (Supplementary Figure S5C). Aft1 senses iron through the activity of mitochondrial ISC assembly (Chen et al, 2004) and mitochondrial ISC export systems, which are also required for extra-mitochondrial Fe–S protein assembly (Rutherford et al, 2005). In HGT1 cells, the activity of the mitochondrial Fe–S enzyme aconitase (Aco1) was not altered by GSH addition, in contrast to that of the cytoplasmic Fe–S enzyme isopropylmalate isomerase (Leu1), which decreased with a kinetics that inversely paralleled the GSH level changes (Figure 3E; see Figure 1D). Aco1 and Leu1 protein levels remained unchanged under all conditions (Figure 3F).
Figure 3.
GSH toxic levels activate Aft1 and inhibit cytoplasmic Fe–S maturation. (A) FET3 expression measured by RT–PCR in HGT1 cells at the indicated time after GSH addition (100 μM), or (B) 30 min after adding the indicated amount of GSH. FET3 expression is given as FET3/ACT1 signal ratio. Values are the mean of triplicate samples of the same experiment±s.d. (C) GSH (50 μM) added to wild-type cells co-expressing pRS314-TEF-HGT1 and pTEF-AFT1-GFP, and cellular GFP staining examined at the indicated time (left panel). DAPI nuclear staining (middle panel); visible-light image (right panel). (D) HGT1 and WT cells serially diluted and spotted onto SD plates containing no GSH, or 10 μM GSH, and/or no FeCl3, or 100 or 250 μM FeCl3. (E) GSH (100 μM) added to HGT1 or WT cells and Aco1 and Leu1 enzyme activities measured at the indicated time. Results are in specific activity (SA) (nM/min/mg protein). Data are the average of three independent experiments; error bars represent mean values±s.d. (F) Extracts from the cells used in (E) were processed for western blot with anti-Aco1 or -Leu1 antibodies.
GSH toxic levels thus promptly trigger Aft1 activation and alter extra-mitochondrial but not mitochondrial Fe–S enzyme activity, both events occurring with time courses suggestive of a cause-and-effect relationship. Such alteration in iron metabolism may contribute to the lethality of high GSH levels, as several Fe–S proteins are essential.
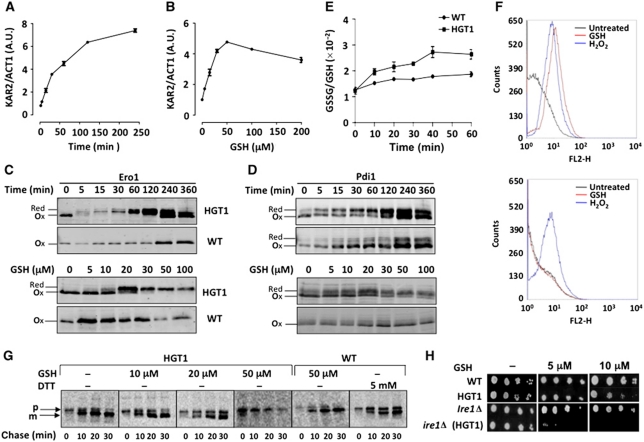
High GSH levels cause an UPR by inhibiting ER oxidative protein folding
We explored the link between GSH toxic levels and the UPR. In HGT1 cells, the Hac1 target-gene Kar2 was induced 5 min after GSH addition (Figure 4A), confirming the microarray data. Induction initially paralleled the increase in GSH levels, but then remained high for up to 4 h while GSH levels had corrected (see Figure 1D). KAR2 induction occurred at GSH concentrations as low as 5 μM and reached a plateau at 50 μM (Figure 4B). As a thiol reductant, GSH might have induced the UPR by interfering with oxidative protein folding. We explored the impact of GSH on the Ero1 and Pdi1 redox states. In untreated HGT1 cells, Ero1 was fully oxidized and Pdi1 was almost fully oxidized. Addition of GSH caused a dose-dependent reduction of these proteins with a kinetics that paralleled the UPR, occurring fast and lasting for up to 1–3 h after GSH levels had corrected, depending on the GSH amount added (Figure 4C and D). Such kinetics suggested that GSH rapidly diffused into the ER and was trapped there, perpetuating Ero1 cycling by concomitantly reducing its negative regulatory disulphide and providing an unlimited reduced substrate supply. The presence of an actively cycling Ero1 was reflected by the GSH-dependent increase in the rate of GSH oxidation (Figure 4E), and by production of ROS (Figure 4F, upper) that are expected to be generated on reduction of oxygen by active Ero1 (Gross et al, 2006). An hour exposure of HGT1 cells to 100 μM GSH caused the ROS-specific probe rhodamine to fluoresce with a signal comparable to that produced by a 15-min exposure to 400 μM H2O2. The nature of this ROS signal was H2O2, as it was detected by the H2O2-specific PF1 probe (Supplementary Figure S6A), and its cellular origin the ER, as suggested its disappearance upon UPR prevention by Ire1 kinase inactivation (Figure 4F, lower). A further indication of Ero1-active cycling was the full reduction of Ero1, but not of Pdi1 (see Figure 4C and D), which indicated a balance between Ero1-mediated Pdi1 oxidation and its reduction by GSH. An active Ero1 was needed to counter the GSH-reductive load as HGT1 cells carrying the Ero1-defective ero1-1 allele was intolerant to very low GSH concentrations (Supplementary Figure S6B).
Figure 4.
GSH toxic levels block secretion by ER reductive load. KAR2 expression monitored by RT–PCR as in Figure 3A in (A) exponentially growing HGT1 cells at the indicated time after adding 100 μM GSH or (B) 30 min after adding the indicated amount of GSH (μM). FET3 expression is given as FET3/ACT1 signal ratio. Values are the mean of triplicate samples of the same experiment±s.d. (C) HGT1 or WT cells expressing Myc-tagged Ero1 exposed to 50 μM GSH for the indicated time (upper panel) or for 30 min with the indicated amount of GSH (lower panel), and processed for redox western with anti-Myc antibodies. (D) As in (C), except that the western blot revealed with Pdi1-specific antibodies. (E) Fifteen minutes after adding GSH (50 μM) to HGT1 or WT cell cultures, 25 mM DTT was added. After 15 min, cells were collected, washed and inoculated in fresh SD medium at the same OD. Samples were collected for GSH and GSSG measurements. Data are the mean of three independent experiments±s.d. (F) HGT1 (upper panel) or ire1Δ cells carrying HGT1 (lower panel) incubated for 1 h into PBS containing dihydrorhodamine 123 and either GSH (100 μM) or H2O2 (400 μM), or left untreated, and analysed by flow cytometry. Data are typical of three independent experiments. (G) HGT1 and WT cells incubated during 30 min with the indicated amount of GSH, pulsed with 35S-methionine for 7 min and chased. Cpy was immunoprecipitated from cells collected at 0, 10, 20 and 30 min after chase initiation, separated by SDS–PAGE and revealed by phosphor technology. Arrows indicate Cpy immature (p) and mature vacuolar (m) forms. In last panel, WT cells treated with 5 mM DTT for 15 min, and processed, as above. (H) Exponentially growing WT or ire1Δ cells carrying pTEF-416-HGT1 or empty vector serially diluted and spotted onto SD plates containing the indicated amount of GSH.
We tested the effect of GSH on ER secretory transport by following the maturation of carboxypeptidase Y (Cpy), a protein requiring five disulphides for proper folding and ER to vacuole transport (Figure 4G). In HGT1 cells, a subtoxic concentration of GSH (20 μM) delayed Cpy maturation and a toxic one (50 μM) totally blocked this process, with accumulation of the ER form (p) and lack of appearance of the matured vacuolar form (m). The impact of GSH at 20 μM was equivalent to that of DTT at 5 mM, a dose–response difference that further supported the notion that GSH but not DTT accumulates into the ER at very high levels. Finally, inactivation of the UPR by IRE1 deletion (ire1Δ) not only eliminated H2O2 production (see Figure 4F, lower), but also made HGT1 cells intolerant to very low and otherwise non-toxic GSH concentrations (Figure 4H; Supplementary Figure S6C). The latter data indicate that the impact of toxic levels of GSH on ER secretion contributes to its lethality, and that this lethality is not caused by the H2O2 produced in this condition.
Alteration of iron metabolism is the pre-eminent defect caused by GSH depletion
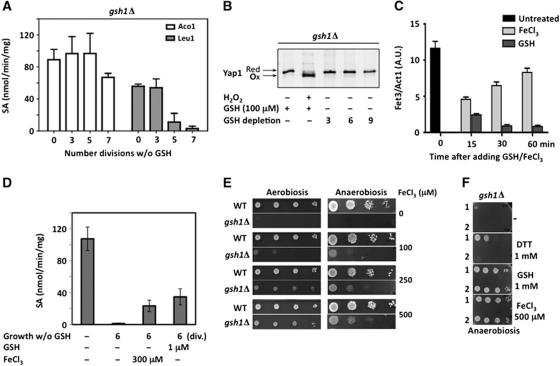
When grown in medium devoid of GSH, yeast cells lacking GSH1 (gsh1Δ) become growth arrested after 8–10 divisions by near-complete GSH depletion (Spector et al, 2001); they activate Aft1 and are defective in extra-mitochondrial but not mitochondrial ISC maturation (Sipos et al, 2002; Rutherford et al, 2005; Figure 5A and C; Supplementary Figure S7A). However, it is not clear whether it is this defect or a defect in thiol-redox control that causes cell inviability by GSH depletion. To answer this question, we established the transcript profiles of gsh1Δ cells withdrawn of GSH for three or six divisions, the latter sample having extremely low GSH cellular levels (60 μM∼0.8% of WT) (Table I). The two GSH-depleted samples had almost identical mRNA profiles (Supplementary Figure S2), and were thus analysed as replicates of the same experiment. The observed response was significant (178 genes), and highly similar to that of the atm1 mutant (Hausmann et al, 2008; Figure 2; Supplementary Table S3). In fact, with few exceptions, most genes responding to GSH depletion were categorized as part of an iron starvation-like response. A few gene targets of the H2O2-reponsive Yap1 transcription factor were also moderately induced, as noticed elsewhere (Wheeler et al, 2003), but their very limited number, several of which are in fact also controlled by Aft1 (see Supplementary Table S3), and lack of perceptible Yap1 oxidative activation (Figure 5B) indicate at best a very minor activation of Yap1. Interestingly, HGT1 was also induced, which fulfilled the demand for increased GSH uptake.
Figure 5.
The effect of iron on GSH-depleted cells. (A) YPD-grown gsh1Δ cells inoculated in SD medium lacking GSH, grown for the indicated number of divisions, and processed for Aco1 and Leu1 enzyme activities. Data are the mean of three independent cultures±s.d. (B) Same as in (A) with gsh1Δ cells carrying Myc–Yap1, and processed for redox western, using an anti-Myc antibody. (C) YPD-grown gsh1Δ cells inoculated in SD medium lacking GSH and grown for six divisions, and left untreated (black bar), or further incubated with GSH (1 mM) (dark grey bars) or FeCl3 (100 μM) (light grey bars) for the indicated time (min). FET3 expression monitored by RT–PCR as in Figure 3A. Values are the mean of triplicate samples of the same experiment±s.d. (D) YPD-grown gsh1Δ cells re-inoculated in SD medium containing GSH (1 mM) or lacking GSH as indicated, were grown for six divisions, further incubated for 1 h with FeCl3 (300 μM) or GSH (1 μM) and processed for Leu1 enzyme activity. Results are the mean±s.d. of data from three independent cell cultures. (E) Wild-type (WT) and gsh1Δ petite (rho−) cells (Y252 background) depleted of GSH by growth for six divisions in SD medium lacking GSH, serially diluted and spotted onto SD plates containing FeCl3 (μM) at the indicated concentration, and incubated under aerobic (left panel) or anaerobic (right panel) conditions. (F) GSH non-depleted (grown overnight in SD medium with 1-mM GSH) (1) or depleted (grown for six division in SD medium lacking GSH) (2) gsh1Δ petite (rho−) serially diluted and spotted onto SD plates containing DTT (1 mM), GSH (1 mM), FeCl3 (500 μM) or nothing, and incubated under anaerobiosis.
The iron starvation-like response as the only significant genome-wide consequence of GSH depletion did not befit ascribing a thiol-redox control function at the origin of the GSH requirement for viability, which was previously established by the partial growth rescue of GSH-depleted cells by DTT (Sipos et al, 2002). We enquired of the effects of iron on GSH-depleted cells. Ferric iron (100 μM) partially decreased the high FET3 expression of GSH-depleted cells within 15 min, while GSH totally corrected it (Figure 5C). As decrease in FET3 mRNA levels by iron might reflect mRNA degradation rather than ISC assembly remediation (Hassett et al, 1998), we also inspected Leu1 activity (Figure 5D). A 1-h exposure of six-division GSH-depleted cells to FeCl3 (300 μM) increased Leu1 activity from levels that were undetectable to more than half of those that were reached on a 1-h exposure to 1 μM GSH. Ferric iron (100 μM) also enabled some growth of gsh1Δ cells on medium lacking GSH, under anaerobiosis slightly better than aerobiosis (Supplementary Figure S7B). Iron can boost the loss of mitochondrial DNA (mtDNA) occurring during GSH depletion (Kistler et al, 1986; Ayer et al, 2010). We thus also tested its effect on a fully homogeneous petite gsh1Δ cell population, assuming that any adverse influence of iron on growth rate by mtDNA loss would be avoided. Growth of GSH-depleted petite gsh1Δ cells was now better rescued by ferric iron, irrespective of the presence of ambient oxygen, and with a dose-dependent effect up to 500 μM (Figure 5E and F). In comparison, growth rescue by DTT (1 mM) was smaller and was observed with cells less depleted of GSH (Figure 5F). Similar to DTT-rescued cells (Sharma et al, 2000; Spector et al, 2001), iron-rescued ones did not grow upon re-inoculation on medium containing iron but not GSH, indicating only transient, partial rescue.
Thus, the intense iron starvation-like response constituting the only significant genome-wide alteration of GSH-depleted cells suggests that it is a defect in iron metabolism that causes the inviability of these cells. The partial correction by iron of the ISC assembly and growth defects of these cells supports this conclusion.
GSH serves as backup of thioredoxin in thiol-redox maintenance
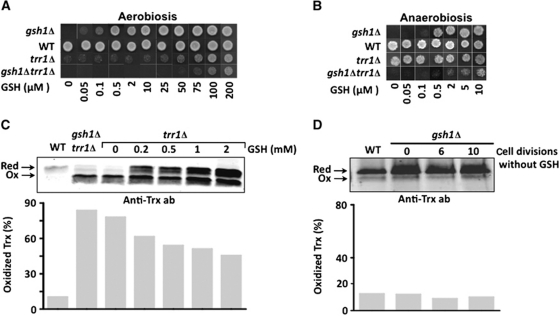
So far, by highlighting its role in iron metabolism while minimizing its impact on thiol-redox control, except for the high GSH levels on the ER, all data that were presented questioned the notion of the primary role of GSH in reducing oxidized Cys residues. Previous studies indicated a quasi-exclusive role of the thioredoxin pathway in peroxide metabolism (Le Moan et al, 2006), and a dominant role in RNR reduction (Camier et al, 2007). As another way to evaluate the actual GSH contribution to thiol-redox control, we evaluated how GSH coped with the redox load increase resulting from inactivation of thioredoxin reductase (trr1Δ). This strain carries a 1.5- to 3-fold increase in total GSH, with proportional increase of GSH and GSSG (Trotter and Grant, 2003; see Table I), presumably indicating a compensatory response to an increased redox load. In medium lacking GSH, trr1Δ cells grew very poorly. Adding high GSH amounts (100–200 μM) improved growth to near wild-type levels (Figure 6A), indicating that the redox peptide was still very limiting despite its 1.5- to 3-fold increase. To rigorously quantify the GSH demand caused by thioredoxin inactivation, we compared the gsh1Δ strain with a strain lacking both GSH1 and TRR1 (gsh1Δtrr1Δ). While the former strain only required 0.5 μM GSH to achieve a wild-type growth, the latter required 400-fold more GSH (200 μM) (Figure 6A). Anaerobiosis did not change the gsh1Δ GSH optimal growth requirement, but totally corrected the trr1Δ one (Figure 6B). Growth of the gsh1Δtrr1Δ strain was also improved, as now requiring 20-fold less GSH (10 versus 200 μM) for optimal growth (compare Figure 6A and B). However, such GSH requirement was still 20-fold higher than that of gsh1Δ (10 versus 0.5 μM), which indicated that an increased cellular redox load was still present in anaerobically grown trr1Δ. In trr1Δ, as in gsh1Δtrr1Δ, thioredoxin was largely oxidized (ox/red ∼70%) compared with its oxidation in wild-type cells (∼10%), and GSH addition decreased this oxidation back to 50% (Figure 6C) indicating that the extra-GSH improved the growth of these cells by balancing their redox load increase. Furthermore, note here (Figure 6C) that trr1Δ had more than a 10-fold increase in thioredoxin abundance, further demonstrating the tremendous compensatory response to an increased redox load. Conversely, however, in the absence of GSH, no increased redox load was applied on the thioredoxin pathway under aerobiosis, as indicated by total preservation of a wild-type redox state (ox/red ∼10%) and levels of thioredoxin in gsh1Δ cells grown for up to 10 divisions in the absence of GSH (Figure 6D).
Figure 6.
GSH serves as a backup of thioredoxin in thiol-redox maintenance. (A, B) gsh1Δ, WT, trr1Δ and gsh1Δtrr1Δ grown in SD medium lacking GSH to an OD600=1; a total of 2 × 106 cells of each culture were spotted onto SD plates containing the indicated amount of GSH (μM), and incubated under aerobic (A) or anaerobic conditions (B). Data are assembled from different plates. (C) WT, gsh1Δtrr1Δ and trr1Δ grown in SD medium lacking GSH, incubated for 1 h in SD medium containing or not containing GSH at the indicated concentration (mM) and processed for redox western with a thioredoxin-specific antibody. For WT cells, 2.5-fold more lysates were loaded. Arrows indicate oxidized (Ox) and reduced (Red) forms of thioredoxin. Lower panel: quantification of oxidized thioredoxin (%). (D) Same as in (C) with WT and gsh1Δ cells grown to saturation in YPD medium, re-inoculated in SD medium lacking GSH and allowed to grow for the indicated number of cell divisions.
In summary, GSH is not capable on its own of efficiently bearing the redox load of the cell, whereas thioredoxin can do so without any increase in levels, which places GSH at best as backup of thioredoxin. As corollary, the contrast between the very high and trace amounts of GSH needed for thiol-redox maintenance and viability, respectively, should disqualify this function as explaining its viability requirement.
GSH links thiol-redox maintenance and iron metabolism
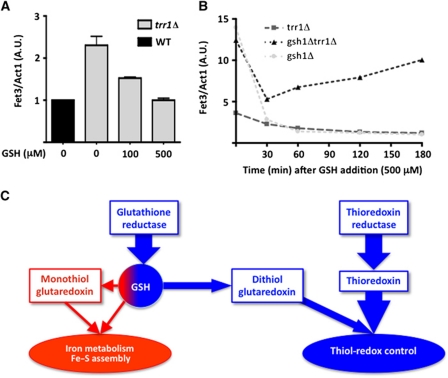
We enquired whether GSH linked thiol-redox and iron metabolism by monitoring FET3 expression in thioredoxin reductase mutants. In trr1Δ, FET3 had a 2.5-fold increased expression, but adding GSH returned this expression to wild-type levels (Figure 7A), which indicated that in these cells GSH is also limiting for iron metabolism despite its 1.5- to 3-fold compensatory increase in levels. In gsh1Δtrr1Δ, FET3 expression was further induced, in keeping with the very low uncompensated GSH levels of these cells. Adding GSH decreased FET3 expression, but only slightly and very transiently, as FET3 mRNA levels increased again after 30 min (Figure 7B). The latter observation indicates that thioredoxin deficiency not only led to an increased GSH demand (see Figure 6A and B), but also caused very fast consumption of the redox peptide.
Figure 7.
GSH establishes a functional crosstalk between iron metabolism and thiol-redox maintenance. (A) WT (black) and trr1Δ (light grey) grown in SD medium lacking GSH, re-inoculated in SD medium containing the indicated amount of GSH and allowed to grow for 1 h. FET3 expression measured by RT–PCR as in Figure 3A. Values are the mean of triplicate samples of the same experiment±s.d. (B) trr1Δ (dark grey square), gsh1Δtrr1Δ (black triangle) and gsh1Δ (light grey circle) grown in SD medium lacking GSH until GSH depletion, at which time GSH (500 μM) was added; cells were further incubated for the indicated time (min) and processed for FET3 expression as in (A). Values are the mean of triplicate samples of the same experiment±s.d. (C) Schematics of the GSH physiological role split between an iron function, which is vital but only requires trace amounts of the tripeptide, and a thiol-redox maintenance function, which appears as a backup of the thioredoxin pathway but requires much higher levels of the tripeptide. In the absence of thioredoxin reductase, GSH takes up on thiol-redox maintenance duties and thus becomes limiting for its vital iron function.
In conclusion, under conditions increasing its thiol-redox maintenance duties, GSH becomes limiting for its function in iron metabolism.
Discussion
We provide here data that question the view of glutathione as the major cellular redox buffer (Grant et al, 1999; Pocsi et al, 2004; Shelton and Mieyal, 2008), and emphasize the importance of its function in iron metabolism. We have analysed the genome-wide consequences of GSH depletion and its presence at toxic levels, using strains lacking GSH1 or overexpressing the GSH-specific HGT1 transporter. Both lethal conditions trigger an intense iron starvation-like response associated with extra-mitochondrial Fe–S enzyme inactivation. Surprisingly, however, none of them impact thiol-redox metabolism, except for the effect of high GSH levels that impair ER oxidative protein folding and trigger the UPR as a vital adaptive measure. We also show, by evaluating the cellular redox load when thioredoxin is absent and conversely, that GSH is not capable of efficiently supporting the thiol-redox duties of the cell, despite a significant increase in its cellular levels, while thioredoxin can. We also contrast the high GSH demand for thiol-redox maintenance with the limited amount of it needed for viability, and show that iron can partially rescue the ISC maturation and growth defects of GSH-depleted cells. We propose that GSH is split between a function in iron metabolism that is vital, but only requires trace amounts of the redox peptide, and a thiol-redox maintenance function that serves as backup of the thioredoxin pathway, but requires much higher levels of the tripeptide (Figure 7C). A conflict between these two functions occurs under redox stresses that consume GSH, which activate Aft1 and may endanger cytoplasmic Fe–S protein maturation. Accordingly, the high GSH physiological levels in the mM range may not be meant for it to function as a cytosolic thiol-redox buffer, but to insulate its essential function in iron metabolism from variations of its concentration during redox stresses.
The redox-independent function of GSH in iron metabolism
Links between GSH and iron metabolism were initially suggested by the presence of increased and decreased levels of the tripeptide in yeast ATM1 (Kispal et al, 1997) and frataxin mutants (Auchere et al, 2008), respectively. The observations that GSH depletion causes impairment of extra-mitochondrial ISC maturation (Sipos et al, 2002) and activates Aft1 (Rutherford et al, 2005), allowed identifying GSH as part of mitochondrial ISC export, a system providing to the CIA machinery a compound of undefined nature emanating from the mitochondrial ISC machinery (Lill, 2009), also serving as a signal that negatively regulates Aft1/2 (Chen et al, 2004). We now show that the near-unique genome-wide consequence of GSH depletion is a major iron starvation-like response remodelling most of iron-dependent mitochondrial and extra-mitochondrial pathways (see Figure 2). We also show that, unexpectedly, toxic GSH levels recapitulate the iron phenotype of GSH depletion. The molecular function of GSH in iron metabolism may not involve a conventional thiol-disulphide reductase activity, as suggests the ability of iron (Figure 5D), but not DTT (Sipos et al, 2002), to partially correct the ISC defect of GSH-depleted cells. GSH probably engages a partnership with Grx3 and Grx4, with which it forms in vitro unusual Fe–S-bridged dimeric complexes that are coordinated by its Cys residue and the glutaredoxin active site ones (Li et al, 2009; Rouhier et al, 2009). A recent report has provided proofs of the in vivo presence of these unusual Fe–S clusters, and has proposed that they deliver iron to all iron-containing proteins and to mitochondria (Muhlenhoff et al, 2010). The slightly higher GSH requirement of cells lacking GSH1, GRX3 and GRX4 in comparison with those only lacking GSH1 (Supplementary Figure S7C) is tantamount of synthetic lethality, and indicate that the corresponding gene products operate in the same essential pathway. Nevertheless, the ability of iron to improve the ISC assembly defect of GSH-depleted cells could also indicate a specific function of GSH in cytosolic iron delivery. However, while Grx3/4 depletion inactivates both mitochondrial and extra-mitochondrial ISC assembly (Ojeda et al, 2006; Muhlenhoff et al, 2010), GSH depletion only inactivates the latter (Sipos et al, 2002) (this study), which does not fit the notion of shared functions of GSH and Grx3/4, by Fe–S cluster coordination. These seeming discrepancies might be, at least in part, linked to the incomplete nature of GSH cellular depletion that causes cell death by affecting first extra-mitochondrial ISC assembly before the full phenotypic scope of GSH deficiency reveals, which could also suggest wider functions of GSH in iron metabolism than Grx3/4.
Toxic GSH levels also impaired Leu1 activity, while sparing Aco1 activity. Impairment of the cytosolic Fe–S enzymes could be caused by disruption of the Grx3/4-GSH Fe–S clusters by titration of Grxs by GSH; however, overexpressing Grx4 in HGT1 cells only corrected GSH-induced high FET3 expression but not growth inhibition (Supplementary Figure S5D, E and F). High GSH levels could also prevent formation of the GSH-Grx3/4 Fe–S clusters by iron sequestration, or could directly affect cytosolic Fe–S enzymes, as suggests the very fast inhibition of Leu1 (see Figure 3). The fact that toxic GSH levels spared mitochondrial iron metabolism was surprising, and might relate to the exclusion from this organelle of the high GSH levels reached in HGT1 cells (not shown). Further work will be needed to establish the molecular function and toxicity of GSH in iron metabolism and its partnership with Grx3/4.
The redox impact of glutathione
The data presented here, all point to a limited cytosolic redox function of GSH. GSH depletion should have caused Cys residue oxidation, but the genome-wide mRNA profiles of GSH-depleted cells did not reveal any other hint of redox imbalance than induction of a very few Yap1 targets (see Figure 2; Supplementary Table S3). This result, and the observation that GSH depletion does not cause any proteome-wide thiol oxidation (Le Moan et al, 2006), suggest that GSH is totally dispensable for cytosolic thiol-redox maintenance. Reciprocally high GSH levels should have caused Cys residue reduction, but this effect was only observed in the ER, in which GSH led to total reduction of Ero1 and Pdi1, which blocked secretion and induced the UPR (see Figure 4). We also show, using cells with inactivation of the thioredoxin pathway, that GSH is not capable of supporting the redox load of the cell on its own. Despite the presence of a 1.5- to 3-fold compensatory increase of total GSH in trr1Δ (Trotter and Grant, 2003; see Table I), the redox peptide was still very limiting for growth (Figure 6A) and was unable to sustain the redox load increase resulting from the lack of thioredoxin reductase. A large part of the redox load increase in trr1Δ was linked to aerobic metabolism, but was also present under anaerobiosis (Figure 6A and B). Reciprocally, cells that were severely depleted of GSH preserved thioredoxin in their wild-type redox state, without any protein compensatory increase, indicating that the thioredoxin pathway could entirely assume the thiol-redox maintenance duties of the cell. These observations and the quasi-exclusive role of the thioredoxin pathway in peroxide metabolism (Le Moan et al, 2006) and a dominant role in RNR reduction (Camier et al, 2007), place GSH as an ancillary system of the thioredoxin pathway in cytosolic thiol-redox control.
The bases of the essential nature and toxicity of GSH
The GSH requirement for viability could theoretically be linked to either its thiol-redox or iron-metabolism functions, as both these processes are essential (Toledano et al, 2007; Lill, 2009). Notwithstanding, the notion that GSH thiol-redox maintenance only serves as backup of thioredoxin, disqualifies this function as causing the GSH essential requirement. The contrast between the very high GSH levels needed for thiol-redox maintenance in the absence of thioredoxin and the very low intracellular GSH levels required for viability (Figure 6A; Table I), at which GSH should behave as a thiol oxidant, further support this idea. Still, GSH could have one exclusive essential redox target, but this is unlikely, as DTT should have then totally rescued the growth of GSH-depleted cells, as it does in E. coli that is made unviable by inactivation of thioredoxin reductase and glutathione reductase (Ortenberg et al, 2004; Gon et al, 2006a). Contrasting with the negligible importance of its thiol-redox function, the function of GSH in iron metabolism appeared pre-eminent. The intense iron starvation-like response that constituted the near-unique genome-wide consequence of GSH depletion befit the idea that it is this function that makes GSH essential, which is supported by the partial rescue by iron of the ISC and growth defects of GSH-depleted cells. DTT can also partially rescue GSH auxotrophy (Grant et al, 1996; see Figure 5F), presumably by substituting for some of its redox functions, thereby decreasing its consumption (Sharma et al, 2000; Spector et al, 2001; Ayer et al, 2010), or by recruiting GSH by reduction of GSSG, thus temporarily sparing its vital iron function.
The lethal effect of toxic levels of GSH is clearly linked to its impact on secretion, as indicated by the partial growth rescue of HGT1 cells by the thiol oxidant diamide that decreased ER reductive stress (not shown), and by the much lower GSH tolerance of HGT1 ire1Δ. The latter observation also indicates that cell death caused by prolonged ER stress is not linked to H2O2 production (Haynes et al, 2004), as HGT1 ire1Δ cells did not generate H2O2 when exposed to GSH, but were much more sensitive to its toxicity. The GSH impact on iron metabolism may also affect viability, but how it does so will only be deduced from knowledge of its molecular function in this pathway.
Conclusions
The data presented here provide a framework of the cellular organization, compartmentation and functions of thiol-redox pathways in eukaryotic cells that has been lacking. They also establish an unsuspected crosstalk between thiol-redox and iron homeostasis that probably applies to higher eukaryotes, in which GSH is also essential for viability (Shi et al, 2000) and partners with glutaredoxins to assemble Fe–S clusters (Rouhier et al, 2009). Although higher eukaryotes have necessarily more complex thiol-redox systems, as here but not in yeast, GSH is also involved in peroxide scavenging by serving as exclusive proton donor for the peroxide scavengers glutathione peroxidases (Toledano et al, 2007), and by constituting a cellular nitric oxide storage in the form of nitrosoglutathione (GS-NO) (Hess et al, 2005). The redox-iron homeostasis crosstalk described here predicts a novel toxic mechanism for redox stresses caused by peroxides, electrophilic xenobiotics and metals that may endanger fitness not only by direct effect but by consumption of GSH and inhibition of cytoplasmic Fe–S protein maturation. Knowledge of such crosstalk should contribute to elucidating the intricate pathophysiological links between redox imbalance, GSH depletion, alterations of iron metabolism and oxidative stress underlying age-related diseases, such as neurodegeneration and inflammation (Altamura and Muckenthaler, 2009; Bharath et al, 2002; Lee et al, 2009).
Materials and methods
Yeast strains, growth conditions and plasmids
The S. cerevisiae strains ABC 1144 (WT) and ABC 1230 (HGT1 cells) are derived from YPH499 (MATα ura3-52 leu2Δ-1 lys2-801 his3Δ-200 trp1-63 ade2-101) and carry an integrated URA3 cassette or TEF-HGT1, respectively (Srikanth et al, 2005). Y252, isogenic gsh1Δ (Spector et al, 2001) and trr1Δ (Le Moan et al, 2006) were described. The gsh1Δtrr1Δ strain was produced by integrating KanMX4 at the TRR1 locus in gsh1Δ. BY4742 and isogenic derivative ire1Δ are from EUROSCARF. The pTEF-416-HGT1 plasmid (CEN URA3 TEF-HGT1) has been described (Bourbouloux et al, 2000); pRS314-TEF-HGT1 (CEN TRP1 TEF-HGT1) was constructed by subcloning a partial SacI–KpnI digest fragment from pTEF416-HGT1 into pRS314; pAFT1-GFP is a gift from MA de la Torres-Ruiz (Lleida, Spain) (Pujol-Carrion et al, 2006); pTEF-AFT1-GFP was constructed by inserting a pTEF-416 400-bp SacI–XbaI fragment containing the TEF1 promoter in place of the MET25 promoter in pAFT1-GFP; pAF85 (CEN LEU2 ERO1–Myc) is a gift from Chris Kaiser (Sevier et al, 2007). pRS316–Myc–Yap1 was described (Delaunay et al, 2002). Cells were grown in YPD (1% yeast extract, 2% peptone and 2% glucose), YPG (same as YPD, with 2% glycerol instead of glucose), or minimal media (SD) (0.67% yeast nitrogen base w/o amino acids, 2% glucose), or minimal media glycerol (SG) (same as SD with 2% glycerol instead of glucose) with amino-acid supplements as appropriate, at 30°C. Anaerobic growth was performed as described (Spector et al, 2001). For generating respiratory incompetent (petite) gsh1Δ, cells were grown overnight in YPD, and then diluted in SD without GSH until they were growth arrested, re-inoculated in SD+100 μM GSH, and tested for their ability to grow on YPG. GSH, GSSG and ferric chloride (FeCl3) were obtained from Sigma.
Measures of glutathione by LC-tandem MS
The pellet of cold H2O-washed yeast cells was suspended in perchloric acid (0.1%, pH 2), boiled (95°C, 5 min) and centrifuged. An aliquot of the supernatant, which correspond to the metabolic fraction, was mixed with an aliquot of an 15N-labelled reference metabolic fraction, and diluted with formic acid (0.1%) to reach the equivalent of 4 × 107 cells/ml, and injected into the chromatographic system for analysis, as described (Lafaye et al, 2005). GSH and GSSG concentrations in mM/cell were calculated on the basis of an average yeast cell volume of 4.5 × 10−14l, which assumes that yeast cells are spherical, with an average diameter of 4.4 μM, as determined by microscopic measurement of 100 cells. We ensured that these size parameters did not change with strain backgrounds and growth conditions.
Microarray analysis
For each growth condition, microarray experiments were performed in two replicates with RNA isolated from independent cultures grown on different days. Each replicate comprised a dye swap between the experimental and reference samples. Totals RNAs were extracted using the Agilent Total RNA isolation mini kit. Labelled complementary RNAs (cRNAs) were generated using the Agilent Low RNA Input Linear Amplification Kit. The cRNA synthesis and Cy-Dye incorporation efficacies were checked by spectrophotometry. Equal amounts of Cy3- (reference sample) and Cy5 (experimental sample)-labelled probes, and the reverse for the dye swap reaction, were mixed together and hybridized onto the Agilent Yeast Oligo Microarray (V2) slides. Slides were scanned using the GenePix 4000B scanner (Molecular Devices). Raw data of spot intensities corrected for background signals were calculated using GenePix Pro 6.0 (Molecular Devices) and exported to GeneSpring Gx 7.3 (Silicon genetics Agilent) for normalization and statistical analyses (see Supplementary data). Genes with an expression change of ⩾2-fold were considered induced or repressed. A lower cutoff of ⩾1.7-fold was used for the 5-min HGT1 condition.
Real-time quantitative PCR
Unique oligonucleotide primer pairs were designed with the QuantPrime software (http://www.quantprime.de) and assayed on efficiency (>80%) and specificity. Six micrograms of RNeasy Mini kit (Quiagen) purified total RNA, digested with RQ1 RNase-Free DNase (Promega) was reverse-transcribed with SuperScript II reverse transcriptase (Invitrogen) with random hexanucleotides (Roche Diagnostics). Real-time quantitative PCR was performed in triplicate as described (Desaint et al, 2004).
Fluorescence microscopy and flow cytometry
Aft1-GFP subcellular localization used cells carrying pTEF-AFT1-GFP stained with DAPI, washed, suspended into DABCO and visualized as described (Delaunay et al, 2002). For detection of H2O2, cells were collected, washed, incubated for 10 min in PBS containing Peroxyfluor-1 (PF1; 10 μM; a gift from Chris Chang, California, USA) (Miller et al, 2005), collected, washed, suspended into PBS containing the indicated amount of GSH or H2O2, and analysed by fluorescence microscopy. Alternatively, cells were collected and suspended into PBS containing the indicated amount of GSH and dihydrorhodamine 123 (20 μM) (Invitrogen), incubated for 1 h at 30°C, and analysed by flow cytometry as described (Desaint et al, 2004).
Leu1 and aconitase enzyme assays
The pellet of yeast cell cultures was suspended in lysis buffer (Tris–HCl (10 mM, pH 7.4), EDTA (2.5 mM), NaCl (150 mM), glycerol (10%, v/v) and Triton X-100 (0.5%, v/v)), and lysed by agitation with glass beads. Cell extracts were centrifuged and supernatants were used for enzyme assays. Aco1 activity was measured by the disappearance of cis-Aconitate at 240 nm, and Leu1 by the decrease in absorbance at 235 nm using citraconate as described (Kispal et al, 1999). Aco1 and Leu1 protein levels were quantified by western blot using polyclonal anti-Aco1, a gift from Anne Bulteau (Paris, France), and anti-Leu1, a gift from Rolland Lill (Marburg, Germany), antibodies. Total cellular iron was determined in mole/cell from a 250-ml culture of exponentially growing HGT1 cells (OD600=0.3–0.4) as described (Doeg and Ziegler, 1962).
Pulse-chase labelling and immunoprecipitation
Exponential phase cells were suspended in 10-ml SD medium lacking methionine (∼6 × 107 cells/ml), pulse-labelled with 500 μCi of [35S] methionine (NEN, Perkin-Elmer) for 7 min and chased with excess cold methionine. A volume of 1.5 ml samples (1 × 108 cells) were collected in 20 mM NaN3, lysed in 100 μl of lysis buffer (Tris–HCl (50 mM, pH 7.5), EDTA (1 mM), sodium dodecyl sulphate (SDS; 1%), urea (6 M) protease inhibitor complete (Roche) (1 ×), PMSF (1 mM)) by agitation with glass beads, and by boiling. Cell extracts were suspended in 1 ml of IP buffer (Tris–HCl (50 mM, pH 7.5), NaCl (150 mM), EDTA (1 mM), Tween-20 (0.5%), protease inhibitor complete (1 ×) and PMSF (1 mM)), mixed with Sheep anti-Mouse IgG Dynabeads® (Invitrogen) coupled to mouse monoclonal anti-Cpy1 antibodies. The mix was tumbled for 2 h at 4°C, washed three times in IP buffer, recovered in 20 μl 1 × Laemmli buffer, boiled and separated by 8% SDS–PAGE. Gels were analysed with a Storm PhosphorImager and ImageQuant TL Software (GE Healthcare).
Redox western
Trichloroacetic acid-precipitated cell extracts (Delaunay et al, 2002) were dissolved in SB buffer (Tris–Cl (100 mM, pH 8.8), EDTA (10 mM) and SDS (1%)) containing 30 mM 4-acetamido-4′-maleimidylstilbene-2,2′-disulphonic acid (AMS, Invitrogen), or 50 mM N-ethylmaleimide and incubated at 37°C for 2 h. Protein concentration was measured by bicinchoninic acid-based colorimetric detection (micro BCA kit, Pierce). For Ero1 and Pdi1, 20 μg protein extracts were mixed with 3 × Laemmli buffer without β-mercaptoethanol, boiled and deglycosylated by incubation with 250 units of endoglycosidase H (NEB) for 60 min at 37°C after addition of sodium citrate (67 mM, pH 5.5). Proteins were resolved by non-reducing SDS–PAGE, and revealed by western blot with 9E10 anti-Myc monoclonal (a gift from C Créminon, Saclay, France), anti-Pdi1 rabbit polyclonal (a gift from J Winther, Denmark), rabbit polyclonal anti-thioredoxin (a gift from C Grant, UK) or anti-His antibodies, anti-mouse IgG or anti-rabbit IgG secondary antibodies labelled with fluorophores of different wavelengths, followed by Infrared Imaging technology (Odyssey, LI-COR).
Supplementary Material
Acknowledgments
We thank F Tacnet for help with fluorescence microscopy and scientific discussions, and Marie-Elyse Lancelot for the microarray analysis. We acknowledge A Bulteau, C Chang, C Grant, C Kaiser, R Lill, MA de la Torres-Ruiz and J Winther for gifting reagents. This work was funded by grant from ANR SOLEMA to MBT, and from the Department of Biotechnology, government of India to AKB. MBT is the recipient of a programme grant from La Ligue Contre le Cancer (LNCC).
Author contributions: CK, AI and MBT planned the experiments; CK and AI performed most experiments; BD′A and AI the enzyme assays; BD′A the iron measurements; EG and CJ the GSH measurements and AGP the ROS measurements; AKB provided reagents; CK, AI, BD′A, ADM and MBT analysed the results; MBT wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Altamura S, Muckenthaler MU (2009) Iron toxicity in diseases of aging: Alzheimer′s disease, Parkinson′s disease and atherosclerosis. J Alzheimers Dis 16: 879–895 [DOI] [PubMed] [Google Scholar]
- Auchere F, Santos R, Planamente S, Lesuisse E, Camadro JM (2008) Glutathione-dependent redox status of frataxin-deficient cells in a yeast model of Friedreich's ataxia. Hum Mol Genet 17: 2790–2802 [DOI] [PubMed] [Google Scholar]
- Ayer A, Tan SX, Grant CM, Meyer AJ, Dawes IW, Perrone GG (2010) The critical role of glutathione in maintenance of the mitochondrial genome. Free Radic Biol Med 49: 1956–1968 [DOI] [PubMed] [Google Scholar]
- Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK (2002) Glutathione, iron and Parkinson's disease. Biochem Pharmacol 64: 1037–1048 [DOI] [PubMed] [Google Scholar]
- Bourbouloux A, Shahi P, Chakladar A, Delrot S, Bachhawat AK (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J Biol Chem 275: 13259–13265 [DOI] [PubMed] [Google Scholar]
- Camier S, Ma E, Leroy C, Pruvost A, Toledano M, Marsolier-Kergoat MC (2007) Visualization of ribonucleotide reductase catalytic oxidation establishes thioredoxins as its major reductants in yeast. Free Radic Biol Med 42: 1008–1016 [DOI] [PubMed] [Google Scholar]
- Chen OS, Crisp RJ, Valachovic M, Bard M, Winge DR, Kaplan J (2004) Transcription of the yeast iron regulon does not respond directly to iron but rather to iron-sulfur cluster biosynthesis. J Biol Chem 279: 29513–29518 [DOI] [PubMed] [Google Scholar]
- Cuozzo JW, Kaiser CA (1999) Competition between glutathione and protein thiols for disulphide-bond formation. Nat Cell Biol 1: 130–135 [DOI] [PubMed] [Google Scholar]
- Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB (2002) A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111: 471–481 [DOI] [PubMed] [Google Scholar]
- Desaint S, Luriau S, Aude JC, Rousselet G, Toledano MB (2004) Mammalian antioxidant defenses are not inducible by H2O2. J Biol Chem 279: 31157–31163 [DOI] [PubMed] [Google Scholar]
- Doeg KA, Ziegler DM (1962) Simplified methods for the estimation of iron in mitochondria and submitochondrial fractions. Arch Biochem Biophys 97: 37–40 [DOI] [PubMed] [Google Scholar]
- Foury F, Talibi D (2001) Mitochondrial control of iron homeostasis. A genome wide analysis of gene expression in a yeast frataxin-deficient strain. J Biol Chem 276: 7762–7768 [DOI] [PubMed] [Google Scholar]
- Frand AR, Kaiser CA (1998) The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell 1: 161–170 [DOI] [PubMed] [Google Scholar]
- Ganguli D, Kumar C, Bachhawat AK (2007) The alternative pathway of glutathione degradation is mediated by a novel protein complex involving three new genes in Saccharomyces cerevisiae. Genetics 175: 1137–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gon S, Camara JE, Klungsoyr HK, Crooke E, Skarstad K, Beckwith J (2006a) A novel regulatory mechanism couples deoxyribonucleotide synthesis and DNA replication in Escherichia coli. EMBO J 25: 1137–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gon S, Faulkner MJ, Beckwith J (2006b) In vivo requirement for glutaredoxins and thioredoxins in the reduction of the ribonucleotide reductases of Escherichia coli. Antioxid Redox Signal 8: 735–742 [DOI] [PubMed] [Google Scholar]
- Grant CM, MacIver FH, Dawes IW (1996) Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr Genet 29: 511–515 [DOI] [PubMed] [Google Scholar]
- Grant CM, Quinn KA, Dawes IW (1999) Differential protein S-thiolation of glyceraldehyde-3-phosphate dehydrogenase isoenzymes influences sensitivity to oxidative stress. Mol Cell Biol 19: 2650–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C, Fass D (2006) Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci USA 103: 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett RF, Romeo AM, Kosman DJ (1998) Regulation of high affinity iron uptake in the yeast Saccharomyces cerevisiae. Role of dioxygen and Fe. J Biol Chem 273: 7628–7636 [DOI] [PubMed] [Google Scholar]
- Hausmann A, Samans B, Lill R, Muhlenhoff U (2008) Cellular and mitochondrial remodeling upon defects in iron-sulfur protein biogenesis. J Biol Chem 283: 8318–8330 [DOI] [PubMed] [Google Scholar]
- Haynes CM, Titus EA, Cooper AA (2004) Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15: 767–776 [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166 [DOI] [PubMed] [Google Scholar]
- Kaur H, Kumar C, Junot C, Toledano MB, Bachhawat AK (2009) Dug1p Is a Cys-Gly peptidase of the gamma-glutamyl cycle of Saccharomyces cerevisiae and represents a novel family of Cys-Gly peptidases. J Biol Chem 284: 14493–14502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y, Ishiwata-Kimata Y, Yamada S, Kohno K (2006) Yeast unfolded protein response pathway regulates expression of genes for anti-oxidative stress and for cell surface proteins. Genes Cells 11: 59–69 [DOI] [PubMed] [Google Scholar]
- Kispal G, Csere P, Guiard B, Lill R (1997) The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett 418: 346–350 [DOI] [PubMed] [Google Scholar]
- Kispal G, Csere P, Prohl C, Lill R (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18: 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler M, Summer KH, Eckardt F (1986) Isolation of glutathione-deficient mutants of the yeast Saccharomyces cerevisiae. Mutat Res 173: 117–120 [DOI] [PubMed] [Google Scholar]
- Lafaye A, Labarre J, Tabet JC, Ezan E, Junot C (2005) Liquid chromatography-mass spectrometry and 15N metabolic labeling for quantitative metabolic profiling. Anal Chem 77: 2026–2033 [DOI] [PubMed] [Google Scholar]
- Lange H, Lisowsky T, Gerber J, Muhlenhoff U, Kispal G, Lill R (2001) An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep 2: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moan N, Clement G, Le Maout S, Tacnet F, Toledano MB (2006) The Saccharomyces cerevisiae proteome of oxidized protein thiols: contrasted functions for the thioredoxin and glutathione pathways. J Biol Chem 281: 10420–10430 [DOI] [PubMed] [Google Scholar]
- Lee DW, Kaur D, Chinta SJ, Rajagopalan S, Andersen JK (2009) A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: implications for Parkinson′s disease. Antioxid Redox Signal 11: 2083–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mapolelo DT, Dingra NN, Naik SG, Lees NS, Hoffman BM, Riggs-Gelasco PJ, Huynh BH, Johnson MK, Outten CE (2009) The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry 48: 9569–9581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R (2009) Function and biogenesis of iron-sulphur proteins. Nature 460: 831–838 [DOI] [PubMed] [Google Scholar]
- Liu Y, Chang A (2008) Heat shock response relieves ER stress. EMBO J 27: 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikenhuis S, Perrone G, Dawes IW, Grant CM (1998) The yeast Saccharomyces cerevisiae contains two glutaredoxin genes that are required for protection against reactive oxygen species. Mol Biol Cell 9: 1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52: 711–760 [DOI] [PubMed] [Google Scholar]
- Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ (2005) Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc 127: 16652–16659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, Lillig CH, Lill R (2010) Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab 12: 373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller EG (1991) Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem 266: 9194–9202 [PubMed] [Google Scholar]
- Muller EG (1996) A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol Biol Cell 7: 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda L, Keller G, Muhlenhoff U, Rutherford JC, Lill R, Winge DR (2006) Role of glutaredoxin-3 and glutaredoxin-4 in the iron regulation of the Aft1 transcriptional activator in Saccharomyces cerevisiae. J Biol Chem 281: 17661–17669 [DOI] [PubMed] [Google Scholar]
- Ortenberg R, Gon S, Porat A, Beckwith J (2004) Interactions of glutaredoxins, ribonucleotide reductase, and components of the DNA replication system of Escherichia coli. Proc Natl Acad Sci USA 101: 7439–7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocsi I, Prade RA, Penninckx MJ (2004) Glutathione, altruistic metabolite in fungi. Adv Microb Physiol 49: 1–76 [DOI] [PubMed] [Google Scholar]
- Pollard MG, Travers KJ, Weissman JS (1998) Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol Cell 1: 171–182 [DOI] [PubMed] [Google Scholar]
- Puig S, Askeland E, Thiele DJ (2005) Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120: 99–110 [DOI] [PubMed] [Google Scholar]
- Pujol-Carrion N, Belli G, Herrero E, Nogues A, de la Torre-Ruiz MA (2006) Glutaredoxins Grx3 and Grx4 regulate nuclear localisation of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J Cell Sci 119(Part 21): 4554–4564 [DOI] [PubMed] [Google Scholar]
- Rouhier N, Couturier J, Johnson MK, Jacquot JP (2009) Glutaredoxins: roles in iron homeostasis. Trends Biochem Sci 35: 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford JC, Jaron S, Winge DR (2003) Aft1p and Aft2p mediate iron-responsive gene expression in yeast through related promoter elements. J Biol Chem 278: 27636–27643 [DOI] [PubMed] [Google Scholar]
- Rutherford JC, Ojeda L, Balk J, Muhlenhoff U, Lill R, Winge DR (2005) Activation of the iron regulon by the yeast Aft1/Aft2 transcription factors depends on mitochondrial but not cytosolic iron-sulfur protein biogenesis. J Biol Chem 280: 10135–10140 [DOI] [PubMed] [Google Scholar]
- Sevier CS, Qu H, Heldman N, Gross E, Fass D, Kaiser CA (2007) Modulation of cellular disulfide-bond formation and the ER redox environment by feedback regulation of Ero1. Cell 129: 333–344 [DOI] [PubMed] [Google Scholar]
- Shakoury-Elizeh M, Tiedeman J, Rashford J, Ferea T, Demeter J, Garcia E, Rolfes R, Brown PO, Botstein D, Philpott CC (2004) Transcriptional remodeling in response to iron deprivation in Saccharomyces cerevisiae. Mol Biol Cell 15: 1233–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KG, Sharma V, Bourbouloux A, Delrot S, Bachhawat AK (2000) Glutathione depletion leads to delayed growth stasis in Saccharomyces cerevisiae: evidence of a partially overlapping role for thioredoxin. Curr Genet 38: 71–77 [DOI] [PubMed] [Google Scholar]
- Shelton MD, Mieyal JJ (2008) Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells 25: 332–346 [PMC free article] [PubMed] [Google Scholar]
- Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, Lieberman MW (2000) Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci USA 97: 5101–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos K, Lange H, Fekete Z, Ullmann P, Lill R, Kispal G (2002) Maturation of cytosolic iron-sulfur proteins requires glutathione. J Biol Chem 277: 26944–26949 [DOI] [PubMed] [Google Scholar]
- Spector D, Labarre J, Toledano MB (2001) A genetic investigation of the essential role of glutathione: mutations in the proline biosynthesis pathway are the only suppressors of glutathione auxotrophy in yeast. J Biol Chem 276: 7011–7016 [DOI] [PubMed] [Google Scholar]
- Srikanth CV, Vats P, Bourbouloux A, Delrot S, Bachhawat AK (2005) Multiple cis-regulatory elements and the yeast sulphur regulatory network are required for the regulation of the yeast glutathione transporter, Hgt1p. Curr Genet 47: 345–358 [DOI] [PubMed] [Google Scholar]
- Toledano MB, Kumar C, Le Moan N, Spector D, Tacnet F (2007) The system biology of thiol redox system in Escherichia coli and yeast: differential functions in oxidative stress, iron metabolism and DNA synthesis. FEBS Lett 581: 3598–3607 [DOI] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101: 249–258 [DOI] [PubMed] [Google Scholar]
- Trotter EW, Grant CM (2003) Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep 4: 184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Trotter EW, Dawes IW, Grant CM (2003) Coupling of the transcriptional regulation of glutathione biosynthesis to the availability of glutathione and methionine via the Met4 and Yap1 transcription factors. J Biol Chem 278: 49920–49928 [DOI] [PubMed] [Google Scholar]
- Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin Z, Brown AJ, Archer DB (2008) Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans. Fungal Genet Biol 45: 1235–1247 [DOI] [PubMed] [Google Scholar]
- Wu AL, Moye-Rowley WS (1994) GSH1, which encodes gamma-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol Cell Biol 14: 5832–5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Ueta R, Fukunaka A, Sasaki R (2002) Subcellular localization of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J Biol Chem 277: 18914–18918 [DOI] [PubMed] [Google Scholar]
- Yun CW, Ferea T, Rashford J, Ardon O, Brown PO, Botstein D, Kaplan J, Philpott CC (2000) Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J Biol Chem 275: 10709–10715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.