Abstract
Under evolutionary pressure to counter the toxicity of iron and to maintain adequate iron supply for hemoglobin synthesis and essential metabolic functions, humans and other vertebrates have effective mechanisms to conserve iron and to regulate its concentration, storage, and distribution in tissues. The iron-regulatory hormone hepcidin, first described 10 years ago, and its receptor and iron channel ferroportin control the dietary absorption, storage, and tissue distribution of iron. Hepcidin causes ferroportin internalization and degradation, thereby decreasing iron transfer into blood plasma from the duodenum, from macrophages involved in recycling senescent erythrocytes, and from iron-storing hepatocytes. Hepcidin is feedback regulated by iron concentrations in plasma and the liver and by erythropoietic demand for iron. Genetic malfunctions affecting the hepcidin-ferroportin axis are a main cause of iron overload disorders but can also cause iron-restricted anemias. Modulation of hepcidin and ferroportin expression during infection and inflammation couples iron metabolism to host defense and decreases iron availability to invading pathogens. This response also restricts the iron supply to erythropoietic precursors and may cause or contribute to the anemia associated with infections and inflammatory disorders.
Introduction
This review, occasioned by the 10th anniversary of the first publications on hepcidin,1–4 focuses on the central role of hepcidin and its receptor/iron exporter ferroportin in the regulation of iron absorption, recycling, and tissue distribution in health and disease. A complementary overview of iron pathobiology was published in this journal 2 years ago.5
Brief history
Already in the 1930s, McCance and Widdowson estimated intestinal iron absorption by subtracting the iron content of feces and urine from the iron content of the diet (for references before year 2000, see the supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). They noted that iron absorption was increased in iron-deficient subjects. There was no change in iron excretion when already replete subjects were given parenteral iron, indicating that excretion was not regulated. Hahn and Whipple analyzed the kinetics of intestinal radioiron absorption and utilization in humans and animal models and confirmed that absorption was regulated and there was no significant excretion of iron. In the 1950s, Finch and Saylor established that iron absorption was also stimulated by increased erythropoietic activity and was suppressed by hypertransfusion. The recycling of hemoglobin from damaged radioiron-labeled erythrocytes into iron was measured by Noyes, Bothwell, and Finch. More iron was released from the reticuloendothelial system when patients or experimental animals were iron-deficient, indicating that the release of iron from macrophages was regu-lated by iron stores. Freireich, Wintrobe, Cartright, Finch, and others showed that inflammation induced the sequestration of iron in macrophages of the liver and the spleen (the “reticuloendothelial” system) and inhibited the supply of iron to erythropoiesis, causing anemia.
Beutler et al anticipated in 1960 that a humoral substance matched the absorption of iron (and by extension, its release from stores) to the iron demands of erythropoiesis, and Krantz et al showed that this substance was not erythropoietin.
In the 1960s, Manis and Schachter and Wheby et al showed in isolated intestinal loops that iron absorption took place in the proximal duodenum and was regulated in 2 steps: uptake of iron into enterocytes (“mucosal uptake”), followed either by cytoplasmic iron storage in ferritin or by the release of iron into the blood circulation (“mucosal transfer”). Because the lifespan of enterocytes is a few days, the fate of dietary iron taken up by enterocytes would be determined by the regulated basolateral iron transporter(s): either the absorbed iron was allowed to enter blood circulation, or it would be returned into the intestinal lumen with the dying enterocytes, as they shed into the fecal stream.
In the 1970s, investigators reexamined the pathogenesis of hereditary hemochromatosis (HH), a syndrome in which excessive iron was deposited in the liver and other tissues, with resulting tissue injury, organ failure, and hepatic carcinogenesis. Powell et al noted that the disorder exhibited features in common with iron deficiency: increased intestinal iron absorption with increased mucosal transfer (ie, increased basolateral iron transport from enterocytes to plasma) and depleted ferritin in enterocytes and macrophages. Weatherall's group reported that similar pathogenic mechanisms were responsible for iron overload in β-thalassemia intermedia whereby hyperabsorption was related to ineffective erythropoiesis and intense erythropoietic stimulation.
The 1990s witnessed a renaissance of iron pathobiology.5 Investigations of patients and animal models with genetic iron disorders led to the identification of genes that encoded iron transporters, transport-related oxidoreductases, and iron regulatory molecules.5
Hepcidin, the iron-regulatory hormone
The iron hormone was not found by the genetic methods, in retrospect because of the small size of its gene, the corresponding rarity of its random mutations, and the lack of sequence motifs shared with known iron-related genes. Multiple serendipitous events helped identify hepcidin and its function in iron homeostasis. While purifying from human urine β-defensin-1, a kidney-produced innate immunity peptide, we found a new defensin-like peptide that contained 25 amino acids and 4 disulfide bonds. In January 1998, we deposited the sequence of this peptide to the Swiss Prot database, and named it hepcidin2 because its mRNA was highly expressed in the liver and the peptide showed weak microbicidal activity in vitro. We also noticed that hepcidin concentration was increased > 100-fold in the urine of a septic donor, linking the peptide to inflammation and innate immunity. Krause et al1 independently isolated the same peptide from hemodialysate. The first iron connection emerged in studies by Pigeon et al,3 who were searching for genes overexpressed in the liver of mice overloaded with iron. They identified hepcidin mRNA as an iron-induced transcript and also showed that the mRNA was increased by inflammation. Soon after, in 2001, the function of hepcidin as an iron regulator was demonstrated by Nicolas et al,4 who noticed that the USF2 knockout mouse, generated for another purpose, had severe iron overload. The targeted USF2 gene was known to be adjacent to the gene encoding hepcidin, and the researchers showed that USF2 disruption also severely suppressed hepcidin transcription. As noted by the researchers and the accompanying editorial,6 hepcidin became the leading candidate for the long-sought iron-regulatory hormone. The function of this peptide in innate immunity, suggested by its connection to antimicrobial peptides and inflammation, still remains to be explored, but it may turn out to be related to its ability to lower the concentration of extracellular iron, a nutrient whose availability can limit the rate of multiplication of invading microbes.
Unlike humans, mice have 2 hepcidin genes, located, respectively, next to USF2 and its partially duplicated pseudogene. Hepcidin-1 is a gene closely related to human hepcidin and hepcidin-2 is a divergent gene. To clarify the involvement of USF2 and the 2 hepcidin genes in iron regulation, Nicolas et al7 constructed transgenic mice that overexpressed either hepcidin-1 or hepcidin-2 in their hepatocytes. Most mice overexpressing hepcidin-1 died of severe iron-deficiency anemia shortly after birth, indicating that hepcidin-1 overexpression inhibited placental iron transport from the mother to the affected fetus. The few mosaic survivors remained severely iron-deficient after birth, indicating that their duodenal iron absorption was also inhibited. In contrast, mice transgenic for hepcidin-2 and mice with an alternative method for ablating USF2 showed no iron abnormalities, excluding a major role for these genes in iron regulation.8 The researchers next demonstrated that hepcidin-1 was suppressed by phlebotomy and hemolysis, an important indication that it was the hormone that released iron to meet the needs of erythropoeisis.9
Roetto et al10 identified 2 rare families with the severe juvenile form of HH, and the affected members were homozygous for destructive hepcidin mutations, confirming that the hepcidin gene encoded the iron-regulatory hormone in humans. To show that the predicted 25–amino acid mature peptide product of the hepcidin gene possessed iron-regulatory activity, we synthesized and refolded the 4 disulfide form of human hepcidin-25 and showed that its parenteral administration induced prolonged and severe hypoferremia in mice.11
Ferroportin is the hepcidin receptor
Ferroportin, identified in 2000 by 3 groups using distinct approaches, is the iron exporter on macrophages and on the basolateral membrane of duodenal enterocytes.12–14 On the basis of the demonstrated importance of mucosal transfer for the regulation of absorption, ferroportin was a natural candidate for the target of hepcidin, either directly or through a separate receptor and signaling pathway. The Kaplan group constructed a cell line that inducibly expressed a ferroportin-GFP fusion construct that localized to the cell membrane,15 and we collaborated to examine the effect of hepcidin on these cells. After exposure of the cells to hepcidin, the ferroportin-GFP fusion protein was internalized within 1 hour and soon after degraded. Radioiodinated hepcidin specifically bound to ferroportin-expressing but not to control cells, and it could be competed with unlabeled hepcidin but not with truncated hepcidin-20 or an unrelated cationic peptide, protegrin. Ferroportin therefore was not only the hepcidin-regulated iron exporter but was itself the receptor for hepcidin, and the binding of hepcidin to ferroportin directly induced the endocytosis of ferroportin and its proteolysis in lysosomes.15 This mechanism explained how hepcidin decreased the export of iron from enterocytes, macrophages, and placental cells into plasma. The ability of hepcidin to induce the endocytosis of ferroportin and to reduce iron export was confirmed in a macrophage cell line in which iron was delivered by erythrophagocytosis of 59Fe-labeled red cells.16 The structural determinants and details of hepcidin-induced ferroportin endocytosis and signaling are subjects of intense investigation, but a definitive model has not yet emerged, in part because of the difficulties of obtaining structural information on ferroportin. The expression and activity of other iron-transport molecules, such as the apical and vacuolar iron transporter divalent metal transporter 1, is also affected by hepcidin, but these effects may be indirect, and their mechanism remains to be elucidated.
In vivo, iron transfer from cells to plasma completely depended on ferroportin, as demonstrated by Donovan et al in studies of mice with ferroportin inactivation.17 Mice with global ferroportin inactivation died during embryonic development. If ferroportin expression at the maternal-fetal interface was selectively preserved, the mice were born but rapidly developed severe iron-deficiency anemia in proportion to the completeness of ferroportin inactivation. Despite severely iron-restricted erythropoiesis, iron accumulated in enterocytes, macrophages, and hepatocytes, indicating that ferroportin is essential for iron transfer to plasma and that ferroportin decrease allows iron to be safely stored in the cytoplasmic ferritin of macrophages and hepatocytes.
Although ferroportin is found already in plants and worms, hepcidin and the conserved cysteine-containing hepcidin-binding loop in ferroportin are a vertebrate development. Other forms of ferroportin regulation should exist in invertebrates and plants, but these have not yet been analyzed. However, hepcidin-independent regulation is also functionally important in vertebrates. Ferroportin expression was shown to be regulated translationally by intracellular iron through the iron-responsive element/iron-regulatory protein system and transcriptionally by heme, and these responses allow macrophages to match their iron export capacity to the fluctuating iron and heme load caused by episodic erythrophagocytosis.18 There are 2 main isoforms of ferroportin19: ferroportin 1A (FPN1A), with a 5′-iron–responsive element for translational repression in iron-deficient cells, and FPN1B, lacking this motif. FPN1B is expressed in duodenal enterocytes, where it may allow them to export iron to the rest of the organism even if the enterocytes become iron-deficient.
Studies of patients with human ferroportin mutations20 provided important information about this molecule. Ferroportin mutations most often cause autosomal dominant “ferroportin disease,” manifested as loss of ferroportin function, iron accumulation in macrophages with high levels of serum ferritin, but mild if any liver injury. Rare ferroportin mutations cause gain of function because of resistance to hepcidin, with parenchymal iron overload similar to that seen in classic HH. Importantly, so far all mutations in ferroportin disease in humans and in mouse models were missense, suggesting that haploinsufficiency because of heterozygous nonsense mutations does not decrease ferroportin activity enough to result in ferroportin disease. If most loss-of-function mutations give rise to trafficking defects, ferroportin multimerization with mistrafficking of complexes that contain a mutated copy would lead to a dominant negative effect.21 This simple and attractive model has been contested by multiple investigators who did not detect multimerization or a trafficking defect in a variety of loss-of-function mutants, as recently summarized.22,23 Alternatively, some mutations may cause an iron transport defect whose effect could be increased if the mutant ferroportin molecules trigger endocytosis that also entrains wild-type ferroportin.
The structure of ferroportin has not yet been elucidated, in part because it is a multipass transmembrane molecule without any close structurally characterized homologs. The best-supported ferroportin model has 12 transmembrane segments with both termini located intracellularly.22,24 Despite the importance of ferroportin, it is still not known how it transports iron. The dependence of iron export on extracellular ferroxidase activity suggests that ferroportin allows ferrous iron to transit along its gradient of concentration.14
The hepcidin-ferroportin interaction controls systemic iron homeostasis
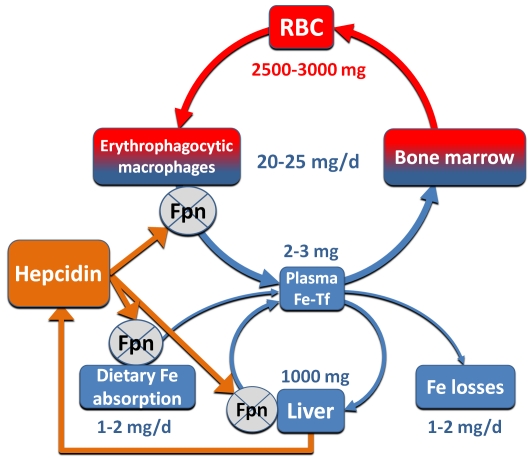
Hepcidin, acting on ferroportin, controls the main inflows of iron into plasma: from duodenal enterocytes absorbing dietary iron, from macrophages involved in the recycling of iron from senescent erythrocytes and other cells, and from hepatocytes involved in iron storage (Figure 1). During pregnancy, fetal hepcidin controls the placental transfer of iron from maternal plasma to the fetal circulation. When hepcidin concentrations are low, iron enters blood plasma at a high rate. When hepcidin concentrations are high, ferroportin is internalized, and iron is trapped in enterocytes, macrophages, and hepatocytes. Plasma iron concentrations and transferrin saturation reflect the difference between the hepcidin/ferroportin-regulated transfer of iron to plasma and iron consumption by the erythropoietic BM and, to a lesser extent, other tissues. The plasma transferrin compartment is relatively small, and its iron content therefore turns over every 3 hours or so, allowing iron concentrations to respond rapidly to changes in hepcidin concentrations.
Figure 1.
Hepcidin interaction with ferroportin controls the main iron flows into plasma. Iron flows and reservoirs are depicted in blue, iron in hemoglobin in red, and hepcidin and its effect in orange. RBC indicates red blood cell; and Fpn, ferroportin.
The role of hepcidin in regulating the absorption of dietary heme, the main form of absorbable iron in human and other carnivore diets, has not been experimentally examined. To the extent that heme is metabolized to ferrous iron within enterocytes, its transfer to plasma would still depend on ferroportin and would therefore be subject to hepcidin regulation.
Hepcidin regulation by iron
Hepcidin is feedback-regulated both by iron concentrations and by the erythropoietic requirements for iron. In mouse models, hepcidin-1 mRNA was shown to be induced by iron loading3,9 and suppressed by anemia and hypoxia.9 Because additional levels of regulation were possible before secretion of the bioactive peptide, it was important to establish how the levels of circulating hepcidin are regulated. However, the production of high-quality antihepcidin antibodies for immunoassays of human or mouse hepcidin proved difficult, so initial immunoassays relied on the estimation of hepcidin in urine. Hepcidin was decreased or absent in iron deficiency, increased by transfusion-induced iron overload and inflammatory diseases, and in general showed high correlation with serum ferritin levels.25 An oral load of 65 mg of iron in healthy volunteers caused > 5-fold increase in hepcidin within 1 day.26 Improvement in immunochemical and mass spectrometric hepcidin assays allowed the detection of hepcidin in serum. The assays established that hepcidin transiently rises ∼ 4-8 hours after oral iron administration and that it is subject to diurnal variation with a midday maximum, perhaps because of dietary iron ingestion.27,28 The responsiveness of blood hepcidin concentrations to dietary iron may be increased by the dual blood supply of the liver from the portal and systemic circulation that may allow diferric transferrin from the portal circulation to be sensed as it delivers boluses of dietary iron.
Do hepatocytes sense iron and regulate hepcidin production on their own or in response to signals from other cell types that sensed iron concentrations? Although other cell types may influence hepcidin production, in vitro studies indicate that isolated hepatocytes contain iron sensors as well as the transduction apparatus required for regulating hepcidin synthesis. First attempts to replicate hepcidin regulation by iron in isolated hepatocytes showed no stimulation or even suppression of hepcidin mRNA by iron loading,25,29 probably because these cells underwent rapid phenotypic change in culture and lost their ability to express characteristic hepatic proteins. Hepatocyte-derived cell lines also showed a paradoxical decrease in hepcidin mRNA after treatment with iron or transferrin.25 However, freshly harvested mouse hepatocytes did increase their hepcidin-1 mRNA after treatment with physiologic concentrations of holotransferrin.30,31 It appears, however, that apart from diferric transferrin that may induce rapid hepcidin responses on the time scale of hours,27 other forms of iron are involved in hepcidin regulation in response to chronic iron loading, perhaps sensed as hepatic cellular iron stores.32
Molecular mechanisms of hepcidin regulation by iron: a current model
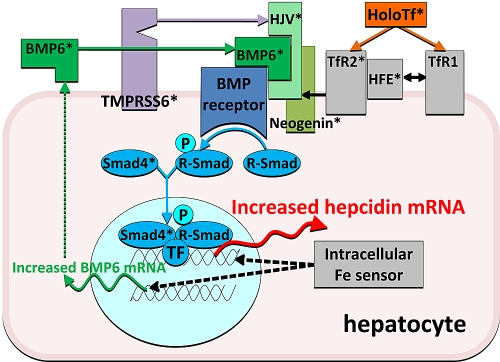
Hepcidin is transcriptionally regulated33; there is no evidence yet of other types of control. Naturally occurring human mutations and transgenic mouse models have provided valuable information about the molecules involved in hepcidin regulation and, together with biochemical studies, have yielded insights into their interactions. In general, mutations in regulatory molecules cause either hepcidin deficiency, resulting in iron overload, or hepcidin excess with consequent iron deficiency and sequestration. Hemochromatosis gene (HFE), transferrin receptor 2 (TfR2), and hemojuvelin (HJV; Table 1 with references) are genes mutated in human hemochromatosis, and their ablation results in decreased hepcidin responsiveness to iron and relative or absolute hepcidin deficiency. Conversely, mutations in the protease TMPRSS6, which are associated with severe iron deficiency, prevent the appropriate decrease of hepcidin in the face of iron deficiency. Mouse models of hemochromatosis showed that components of the bone morphogenetic protein (BMP) pathway are also necessary for hepcidin regulation by iron: BMP6 as well as liver-specific Sma- and Mad-related protein 4 (Smad4) knockout mice also developed severe iron overload and had inappropriately low hepcidin mRNA levels. Furthermore, the production of BMP6 is increased when liver iron concentrations are high, suggesting that BMP6 may be a signal reflecting iron stores. The task of understanding hepcidin regulation by iron is made more difficult by the apparent redundancy in the system. The combined effect of HFE and TfR2 ablation is much more severe than the effect of the loss of either molecule.33,34 Moreover, although each mutation has a profound effect on hepcidin response to acute iron loading, individual ablation of HFE, TfR2, hemojuvelin, or BMP6 only partially impairs the hepcidin increase in response to chronic iron loading. In addition, the ablation of HFE, TfR2, or HJV has no detectable effect on BMP6 regulation by iron, indicating that a separate mechanism is responsible for iron-dependent regulation of BMP6.32 On the basis of the work by many groups, a model of hepcidin regulation by iron has emerged (Figure 2) in which (1) the BMP receptor and its signaling components are at the core of the regulatory mechanism and control the transcription of hepcidin thorough the Smad pathway; (2) HJV is an iron-specific adaptor-ligand of the BMP receptor that increases its sensitivity to BMPs; (3) information about hepatic iron stores is conveyed through increased production of BMP6, an activating ligand of the BMP receptor; (4) extracellular iron concentration is sensed through the interaction of holotransferrin with TfR1 and TfR2, with HFE as an intermediary between the 2 molecules; (5) TfR2 and HFE increase the sensitivity of the BMP receptor to its ligands in an holotransferrin-dependent manner, perhaps by interactions with hemojuvelin; (6) TMPRSS6, perhaps stabilized by iron deficiency, cleaves membrane HJV and inactivates it; and (7) other pathways participate in hepcidin regulation by iron. Although many components of the hepcidin-regulatory apparatus have been identified (Table 1), the list is probably incomplete, and much more remains to be learned about how the various components physically interact to modulate hepcidin transcription.
Table 1.
Human and mouse mutations that cause systemic iron dysregulation
| Component | Putative role |
|---|---|
| Transferrin34,35 | Iron carrier, presents extracellular iron to hepatocytes for sensing |
| TfR1 | Cellular iron uptake, signals holotransferrin concentration through HFE? |
| TfR236,37 | Holotransferrin sensing, partially redundant to TfR1/HFE38,39 |
| HFE40,41 | Binds to TfR142 and TfR2,43 signals TfR1 occupancy42 |
| Hemojuvelin44–46 | BMP coreceptor,47 enhances BMP signaling, target of TMPRSS648,49 |
| Neogenin | Binds to hemojuvelin,50,51 enhances BMP signaling51,52 |
| TMPRSS6 | Negative regulator,35,75 decreases BMP signaling by cleaving hemojuvelin53–55 |
| Smad4 | Conveys BMP signal to the hepcidin transcription complex56 |
| BMP6 | Iron-specific ligand57,58 of the BMP receptor,59 increased by hepatic iron60 |
Figure 2.
A current model of regulation of hepcidin transcription by iron. Iron as holotransferrin is shown in orange, iron sensors and associated molecule in gray, BMP receptor and its transduction pathway in shades of blue, the ligands and coreceptors of the BMP receptor in shades of green, and the negative regulator protease in purple. *Molecules whose ablation was shown to cause iron dysregulation.
Hepcidin regulation by erythropoiesis
In hemorrhagic or hemolytic anemia, both iron absorption in the duodenum and the release of iron from stores are greatly increased, consistent with the effect that would be expected from a hepcidin decrease. Nicolas et al9 observed that hepatic hepcidin mRNA in the mouse model was indeed suppressed by anemia and proposed that the increased absorption and mobilization of iron in anemia was mediated by the hypoxic suppression of hepcidin. In principle, multiple signals could mediate the erythropoietic suppression of hepcidin production. Anemia decreases oxygen tension in organs with high oxygen requirements, potentially regulating hepcidin through hypoxia-inducible factors, similarly to erythropoietin regulation. In addition, increased demand for iron by erythropoietin-stimulated marrow could cause transient hypoferremia and suppress hepcidin through an iron-regulatory mechanism. Third, there is evidence for BM-derived factors (“erythroid regulators”) that communicate the activity of the erythropoietic system to other organs. Although some contribution of hypoxia is probable,61 it now appears that the predominant signal depends on BM activity, because ablation of the BM in anemic mice reverses the hepcidin-suppressive effect of anemia.62,63
Hepcidin regulation by inflammation
Inflammation has a potent effect on iron homeostasis, reducing intestinal iron absorption, sequestering iron in macrophages, and thereby decreasing serum iron levels. There is now substantial evidence that these effects of inflammation are also mediated by hepcidin. Wild-type mice increased hepcidin-1 mRNA and developed hypoferremia in response to turpentine-induced inflammation, but in USF2/Hamp knockout (KO) mice, the hypoferremic response was lost.9 These experiments were confounded by the severe iron overload in Hamp KO mice, which could by itself provide enough iron to relieve hypoferremia, so they need to be replicated in hepcidin-deficient humans or Hamp KO mice that are depleted of iron. In favor of the role of hepcidin in inflammatory hypoferremia, IL-6 and supernatants of lipopolysaccharide-stimulated macrophages readily induced hepcidin in human hepatocytes and hepatic cell lines.25 Moreover, urinary hepcidin level rose within hours of IL-6 or lipopolysaccharide infusion into human volunteers, on the average > 7-fold, and hypoferremia coincided with the rise of hepcidin.26,64 The stimulatory effect of IL-6 on hepcidin is transcriptional and depends on STAT3 interactions with a STAT3-binding element in the hepcidin promoter.65–67 Other cytokines and direct effects of microbial molecules on hepatocytes may also contribute to the inflammatory increase in hepcidin.
Primary disorders of hepcidin and ferroportin regulation
Overview
The discovery of hepcidin and its role in iron homeostasis remarkably simplified and rationalized our understanding of the pathogenesis of the most common iron disorders (Table 2). In the following discussion, I designate as “primary” those disorders that result from lesions in the genes that encode hepcidin, ferroportin, or their physiologic regulators and as “secondary” those disorders that are caused by diseases that originate outside the iron-homeostatic system.
Table 2.
Hepcidin and iron disorders
| Disorder | Genes mutated | Hepcidin |
|---|---|---|
| Hereditary hemochromatosis (hepcidin deficiency) | HFE, TfR2, HJV, hepcidin | Low or inappropriately normal, despite iron load |
| Hereditary hemochromatosis (hepcidin resistance) | Ferroportin | High, reflects iron load, rare |
| Ferroportin disease | Ferroportin | Insufficiently studied |
| Iron-refractory iron deficiency anemia | TMPRSS6 | High or inappropriately high normal, despite iron deficiency |
| Hypotransferrinemia | Transferrin | Low unless transfused with plasma |
| β-Thalassemia intermedia | β-Globin | Low unless transfused |
| Chronic hepatitis C, alcoholic liver disease | Decreased | |
| Anemia of inflammation | High or inappropriately normal, despite anemia and hypoferremia | |
| Anemia of chronic kidney diseases | High or inappropriately normal, despite anemia and hypoferremia |
Hepcidin deficiency
HHs are a group of genetic disorders characterized by excessive absorption of dietary iron and its deposition in the liver and other organs. Liver injury leading to cirrhosis and hepatocellular carcinoma is a common clinical manifestation of HH, but iron toxicity can also damage other organs. The most common form of HH in populations of Northern or Central European ancestry is caused by homozygous or compound heterozygous mutations in HFE, a gene encoding a membrane protein related to histocompatibility antigens. This form is often mild and incompletely penetrant, with phenotypic expression depending on sex, alcohol consumption, and other genetic and environmental factors. A rare but possibly more severe form is caused by autosomal recessive mutations in TfR2. Also rare but severe, “juvenile” HH is an early-onset disease with prominent cardiac and endocrine involvement, and it is caused by autosomal recessive mutations in HJV or hepcidin (HAMP). The common feature of all these genetic disorders is hepcidin deficiency, mildest in HFE disease and more severe or complete in the others. Hepcidin deficiency and iron accumulation in the liver and other organs are recapitulated in the corresponding mouse models, but mice appear resistant to the toxic effects of iron. Transgenic expression of hepcidin prevented iron overload in the mouse HFE model,68 confirming the pathogenic role of hepcidin deficiency. Apart from the genes involved in human HH, heritable iron overload in mice (as yet without known clinical equivalents in humans) is also caused by ablation of the gene encoding Bmp6 and the gene encoding Smad4, a common signaling component of the BMP and TGF-β pathways.
At first glance it is puzzling why HH hepatocytes are iron-overloaded, despite expressing ferroportin, whereas enterocytes and macrophages are iron-depleted, consistent with high expression of ferroportin and increased iron export from these cells. The iron loading of hepatocytes may be a consequence of their ability to take up increased plasma iron, especially in its non–transferrin-bound forms, in excess of their ability to export it, so hepatocytes end up as the predominant iron storage organ in HH. The distribution of excess iron to other tissues probably reflects their respective avidity for nontransferrin iron.
Resistance to hepcidin
A rare disease similar to HH is caused by autosomal dominant mutations in ferroportin that cause resistance to hepcidin. Of several ferroportin mutations that give rise to this syndrome, the one best characterized is C326S, which causes early-onset parenchymal iron overload with documented high or high-normal hepcidin levels.69 In these patients, the ferroportin mutation interferes with hepcidin binding,70 but other mutations with a similar, but apparently less severe, phenotype may impair hepcidin-induced internalization of ferroportin by an as yet unknown mechanism.
Hepcidin excess
A primary human iron disorder with the opposite phenotype to HH is caused by mutations in the gene encoding the membrane serine protease matriptase 2 (also called TMPRSS6). Genetic impairment of this enzyme in mice or humans causes iron-refractory iron-deficiency anemia (IRIDA). The defining feature of this disorder are high-normal or high serum hepcidin concentrations54,55,71 despite severe iron deficiency. Increased hepcidin levels are presumably because of increased BMP signaling, resulting from the inability of mutated TMPRSS6 to cleave membrane hemojuvelin.53 Milder heterozygous defects in TMPRSS6 may be common and could predispose to iron deficiency.72,73
Hypotransferrinemia
Although transferrin deficiency affects predominantly the delivery of iron to erythropoiesis, a process entirely dependent of transferrin-mediated iron uptake, certain features of this disorder point to the direct involvement of transferrin in hepcidin regulation. The affected patients and mouse models (hpx) show severe microcytic hypochromic anemia with parenchymal iron overload and severe hepcidin deficiency, with transferrin infusions correcting the anemia and raising hepcidin levels.34 In principle, hepcidin deficiency in this disease could be caused either by decreased stimulation of the iron sensors that regulate hepcidin production or by the suppressive effects of erythropoietin-stimulated BM on hepcidin production. Experiments in which the contribution of the erythropoietic factor is negated by transfusions or BM ablation support the existence of both mechanisms.35
Secondary disorders of hepcidin and ferroportin regulation
Iron-loading anemias
Iron overload is the main cause of morbidity and mortality in anemias with ineffective erythropoiesis, including β-thalassemias and congenital dyserythropoietic anemias. In these disorders, ineffective but greatly expanded erythropoiesis is stimulated by high levels of erythropoietin. Severe iron overload resembling juvenile HH can develop even in patients who rarely or never receive a transfusion, indicating that dietary iron is hyperabsorbed in these conditions. Hepcidin concentrations in the patients not receiving a transfusion with these disorders were very low,74 despite high serum ferritins and liver biopsies indicative of severe iron overload.75 Transfusions raise hepcidin probably because of the lowering of erythropoietin and erythroid activity in the BM and further iron loading.75 Hepcidin synthesis in the liver is thought to be suppressed by one or more mediators produced during ineffective erythropoiesis. Recent studies provide evidence for the hepcidin-suppressive role of the BMP family member GDF-15 in iron-loading anemias,76,77 which may be generated during apoptosis of erythroid progenitors. GDF-15, however, is not the physiologic mediator of iron hyperabsorption after blood loss.78
Chronic liver diseases
Hepcidin deficiency with hepatic iron overload can complicate several chronic liver diseases, most prominently chronic hepatitis C and alcoholic liver disease.79 In chronic hepatitis C, hepatic iron accumulation may worsen prognosis, and iron depletion has been reported to be beneficial. The mechanisms that impair hepcidin production in these disorders are being investigated.
Chronic kidney diseases
Hepcidin is in part eliminated by glomerular filtration and degradation in the proximal tubules, causing blood hepcidin concentrations to rise with diminishing renal function.80 The mechanism may contribute to the characteristic anemia as well as to erythropoietin resistance in chronic kidney disease.27,81,82
Anemia of inflammation
The combination of mild-to-moderate anemia and hypoferremia is often seen in chronic infections, inflammatory disorders, hematologic malignancies, and some solid tumors. Anemia of inflammation (AI) has also been called “anemia of chronic disease,” although it often develops rapidly83 and is not seen in most chronic diseases. At its core, AI is a systemic iron disorder in which iron is sequestered in macrophages, intestinal iron absorption is decreased, and hemoglobin synthesis is impaired because of limitations of iron delivery to the maturing erythrocytes. The condition in which iron stores are adequate but not available for hemoglobin synthesis is referred to as “iron restriction,” in contrast to iron deficiency, in which stores are depleted. The inflammatory redistribution of iron can be explained by cytokine-stimulated hepcidin increase that leads to the loss of ferroportin from macrophage and enterocyte cell membranes, and the consequent trapping of iron in these cells. Among the cytokines that stimulate hepcidin synthesis, IL-626,84,85 and BMP-286 may be of particular clinical importance. Increased serum hepcidin concentrations have been found in acute infections, malaria,87 inflammatory diseases,27,88 multiple myeloma,86 Hodgkin disease,89 Castleman disease,85 and in a subset of patients with solid tumors.88,90 It is notable that in the IRIDA syndrome, relatively mild increases in hepcidin concentration are sufficient to sustain an iron-restricted anemia, even with parenteral iron supplementation (T.G., unpublished data, May 2010). Similar mild increases in hepcidin could perpetuate AI once it is established. The differences in erythrocyte morphology in these disorders (AI is usually normocytic, IRIDA is severely microcytic) may be because of the direct effects of inflammatory cytokines on erythropoiesis. Depending on the underlying disease, other mechanisms, including shortened erythrocyte lifespan or mildly impaired erythropoietin production may also contribute to this disorder.
Diagnostic and therapeutic implications
Immunochemical and mass spectrometric assays of serum hepcidin are available for research purposes, and an international effort is under way to achieve their standardization.91 Hepcidin assays would be particularly attractive for the diagnosis of iron-refractory iron-deficiency anemia and the diagnosis and risk stratification of HH because hepcidin dysregulation is the cause of these disorders, and genetic tests are often inconclusive. Hepcidin assays may aid in the diagnosis of iron-deficiency anemia and anemia of inflammation,92 probably in combination with existing diagnostic methods. The assays may also help guide the treatment of anemias with iron, erythropoietin, anticytokine therapies, intensification of hemodialysis, as well as future drugs targeted at hepcidin or ferroportin. Future uses of the assay in clinical medicine may be facilitated by the clinical validation of indices (eg, hepcidin/ferritin ratio for the diagnosis of HH) that assess whether alterations in hepcidin levels are “appropriate” physiologic responses to iron load, erythropoietic activity, or inflammation or result from the pathologic impairment of hepcidin responses to one of its regulators (Table 2). Such indices may also avoid the diagnostic uncertainties resulting from the wide normal range of hepcidin, which is caused by variations in iron stores and perhaps inflammation even in apparently healthy subjects.27
On the therapeutic front, hepcidin agonists93 or stimulators of hepcidin production94 are being developed for the treatment or prevention of iron overload in hepcidin-deficiency states, including HH and β-thalassemias. In the mouse model of β-thalassemia, transgenic hepcidin therapy improved iron overload as well as erythropoiesis,95 suggesting that hepcidin deficiency or iron overload may adversely affect erythropoiesis in this disease. Hepcidin antagonists96 and inhibitors of hepcidin production84,97–100 may find utility in the treatment of iron-restricted anemias, alone or in combination with erythropoiesis-stimulating agents.
Conclusion and perspectives
The discovery of hepcidin and its role in iron homeostasis have revolutionized our understanding of the pathogenesis of iron overload and iron-restricted anemias and have stimulated the development of new diagnostic and therapeutic methods for these disorders. Important scientific questions still remain to be answered. Further work is required to elucidate the mechanisms of hepcidin regulation by iron and erythroid activity, to delineate the specific contribution of hepcidin excess to different forms of anemia of inflammation, to understand the structure and transport function of the hepcidin receptor ferroportin, to identify the mechanisms by which hepcidin binding triggers ferroportin internalization and degradation, and to define any activities of hepcidin beyond its role in iron homeostasis.101
Supplementary Material
Acknowledgments
I am grateful for the advice and editorial assistance of Elizabeta Nemeth during the preparation of this manuscript. Our contribution to this field would not have been possible without the collaboration of many colleagues all over the world.
This work was supported by the National Institutes of Health and especially the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The online version of this article contains a data supplement.
Authorship
Contribution: T.G. researched and prepared the manuscript and its figures.
Conflict-of-interest disclosure: T.G. is an officer and part-owner of Intrinsic LifeSciences LLC (La Jolla, CA), a company involved in the development of hepcidin-related diagnostics. He holds a patent dealing with hepcidin regulation by soluble HJV and has filed for a patent on hepcidin agonists for therapeutic use. In the past 2 years, he provided consultations for Xenon Pharmaceuticals and Ortho/Centocor. He has received grant funding from Amgen, Baxter, and Roche Foundation for Anemia Research. He declares no other competing financial interests.
Correspondence: Tomas Ganz, Departments of Medicine and Pathology, David Geffen School of Medicine at UCLA, 10833 Le Conte Ave, CHS 37-055, Los Angeles, CA 90095-1690; e-mail: tganz@mednet.ucla.edu.
References
- 1.Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 2.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 3.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 4.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98(15):8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112(2):219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming RE, Sly WS. Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc Natl Acad Sci U S A. 2001;98(15):8160–8162. doi: 10.1073/pnas.161296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99(7):4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lou DQ, Nicolas G, Lesbordes JC, et al. Functional differences between hepcidin 1 and 2 in transgenic mice. Blood. 2004;103(7):2816–2821. doi: 10.1182/blood-2003-07-2524. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33(1):21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 11.Rivera S, Nemeth E, Gabayan V, et al. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106(6):2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 13.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275(26):19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 14.McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5(2):299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 16.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102(5):1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411(1):123–131. doi: 10.1042/BJ20071474. [DOI] [PubMed] [Google Scholar]
- 19.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9(5):461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32(1):131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 21.De Domenico I, Ward DM, Musci G, Kaplan J. Evidence for the multimeric structure of ferroportin. Blood. 2007;109(5):2205–2209. doi: 10.1182/blood-2006-06-032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace DF, Harris JM, Subramaniam VN. Functional analysis and theoretical modeling of ferroportin reveals clustering of mutations according to phenotype. Am J Physiol Cell Physiol. 2010;298(1):C75–C84. doi: 10.1152/ajpcell.00621.2008. [DOI] [PubMed] [Google Scholar]
- 23.Rice AE, Mendez MJ, Hokanson CA, Rees DC, Bjorkman PJ. Investigation of the biophysical and cell biological properties of ferroportin, a multipass integral membrane protein iron exporter. J Mol Biol. 2009;386(3):717–732. doi: 10.1016/j.jmb.2008.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35(1):33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7):2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 26.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 28.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53(4):620–628. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 29.Gehrke SG, Kulaksiz H, Herrmann T, et al. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102(1):371–376. doi: 10.1182/blood-2002-11-3610. [DOI] [PubMed] [Google Scholar]
- 30.Lin L, Valore EV, Nemeth E, et al. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110(6):2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94(6):765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos E, Kautz L, Rodriguez R, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading. Hepatology. 2011 doi: 10.1002/hep.24178. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flanagan JM, Truksa J, Peng H, Lee P, Beutler E. In vivo imaging of hepcidin promoter stimulation by iron and inflammation. Blood Cells Mol Dis. 2007;38(3):253–257. doi: 10.1016/j.bcmd.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trombini P, Coliva T, Nemeth E, et al. Effects of plasma transfusion on hepcidin production in human congenital hypotransferrinemia. Haematologica. 2007;92(10):1407–1410. doi: 10.3324/haematol.11377. [DOI] [PubMed] [Google Scholar]
- 35.Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2011;117(2):630–637. doi: 10.1182/blood-2010-05-287359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawabata H, Fleming RE, Gui D, et al. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105(1):376–381. doi: 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- 37.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105(4):1803–1806. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 38.Pietrangelo A, Caleffi A, Henrion J, et al. Juvenile hemochromatosis associated with pathogenic mutations of adult hemochromatosis genes. Gastroenterology. 2005;128(2):470–479. doi: 10.1053/j.gastro.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 39.Wallace DF, Summerville L, Crampton EM, et al. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50(6):1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad KA, Ahmann JR, Migas MC, et al. Decreased liver hepcidin expression in the Hfe knockout mouse. Blood Cells Mol Dis. 2002;29(3):361–366. doi: 10.1006/bcmd.2002.0575. [DOI] [PubMed] [Google Scholar]
- 41.Bridle KR, Frazer DM, Wilkins SJ, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- 42.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci U S A. 1998;95(4):1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Chen J, Kramer M, et al. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9(3):217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36(1):77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 45.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115(8):2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115(8):2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 48.Truksa J, Gelbart T, Peng H, et al. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. Br J Haematol. 2009;147(4):571–581. doi: 10.1111/j.1365-2141.2009.07873.x. [DOI] [PubMed] [Google Scholar]
- 49.Finberg KE, Whittlesey RL, Fleming MD, Andrews NC. Down-regulation of Bmp/Smad signaling by Tmprss6 is required for maintenance of systemic iron homeostasis. Blood. 2010;115(18):3817–3826. doi: 10.1182/blood-2009-05-224808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang AS, Anderson SA, Meyers KR, et al. Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J Biol Chem. 2007;282(17):12547–12556. doi: 10.1074/jbc.M608788200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang AS, Yang F, Wang J, Tsukamoto H, Enns CA. Hemojuvelin-neogenin interaction is required for bone morphogenic protein-4-induced hepcidin expression. J Biol Chem. 2009;284(34):22580–22589. doi: 10.1074/jbc.M109.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee DH, Zhou LJ, Zhou Z, et al. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood. 2010;115(15):3136–3145. doi: 10.1182/blood-2009-11-251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silvestri L, Pagani A, Nai A, et al. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melis MA, Cau M, Congiu R, et al. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93(10):1473–1479. doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- 56.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Meynard D, Kautz L, Darnaud V, et al. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 58.Andriopoulos B, Jr., Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111(10):5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kautz L, Meynard D, Monnier A, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112(4):1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 61.Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7(1):28–32. doi: 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- 62.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vokurka M, Krijt J, Sulc K, Necas E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol Res. 2006;55(6):667–674. doi: 10.33549/physiolres.930841. [DOI] [PubMed] [Google Scholar]
- 64.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106(5):1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 65.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pietrangelo A, Dierssen U, Valli L, et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132(1):294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Verga Falzacappa MV, Vujic SM, Kessler R, et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 68.Nicolas G, Viatte L, Lou DQ, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34(1):97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 69.Sham RL, Phatak PD, Nemeth E, Ganz T. Hereditary hemochromatosis due to resistance to hepcidin: high hepcidin concentrations in a family with C326S ferroportin mutation. Blood. 2009;114(2):493–494. doi: 10.1182/blood-2009-04-216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandes A, Preza GC, Phung Y, et al. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood. 2009;114(2):437–443. doi: 10.1182/blood-2008-03-146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chambers JC, Zhang W, Li Y, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. 2009;41(11):1170–1172. doi: 10.1038/ng.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benyamin B, Ferreira MA, Willemsen G, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. 2009;41(11):1173–1175. doi: 10.1038/ng.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papanikolaou G, Tzilianos M, Christakis JI, et al. Hepcidin in iron overload disorders. Blood. 2005;105(10):4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 76.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13(9):1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 77.Tamary H, Shalev H, Perez-Avraham G, et al. Elevated growth differentiation factor 15 expression in patients with congenital dyserythropoietic anemia type I. Blood. 2008;112(13):5241–5244. doi: 10.1182/blood-2008-06-165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanno T, Rabel A, Lee YT, et al. Expression of growth differentiation factor 15 is not elevated in individuals with iron deficiency secondary to volunteer blood donation. Transfusion. 2010;50(7):1532–1535. doi: 10.1111/j.1537-2995.2010.02601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: New insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis. 2011;43(2):89–95. doi: 10.1016/j.dld.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Zaritsky J, Young B, Wang HJ, et al. Hepcidin–a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1051–1056. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ashby DR, Gale DP, Busbridge M, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75(9):976–981. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 82.Bansal SS, Abbate V, Bomford A, et al. Quantitation of hepcidin in serum using ultra-high-pressure liquid chromatography and a linear ion trap mass spectrometer. Rapid Commun Mass Spectrom. 2010;24(9):1251–1259. doi: 10.1002/rcm.4512. [DOI] [PubMed] [Google Scholar]
- 83.Corwin HL, Krantz SB. Anemia of the critically ill: “acute” anemia of chronic disease. Crit Care Med. 2000;28(8):3098–3099. doi: 10.1097/00003246-200008000-00079. [DOI] [PubMed] [Google Scholar]
- 84.Hashizume M, Uchiyama Y, Horai N, Tomosugi N, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol Int. 2010;30(7):917–923. doi: 10.1007/s00296-009-1075-4. [DOI] [PubMed] [Google Scholar]
- 85.Song SN, Tomosugi N, Kawabata H, et al. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood. 2010;116(18):3627–3634. doi: 10.1182/blood-2010-03-271791. [DOI] [PubMed] [Google Scholar]
- 86.Maes K, Nemeth E, Roodman GD, et al. In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2. Blood. 2010;116(18):3635–3644. doi: 10.1182/blood-2010-03-274571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Mast Q, Syafruddin D, Keijmel S, et al. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica. 2010;95(7):1068–1074. doi: 10.3324/haematol.2009.019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butterfield AM, Luan P, Witcher DR, et al. A dual-monoclonal sandwich ELISA specific for hepcidin-25. Clin Chem. 2010;56(11):1725–1732. doi: 10.1373/clinchem.2010.151522. [DOI] [PubMed] [Google Scholar]
- 89.Hohaus S, Massini G, Giachelia M, et al. Anemia in Hodgkin's lymphoma: the role of interleukin-6 and hepcidin. J Clin Oncol. 2010;28(15):2538–2543. doi: 10.1200/JCO.2009.27.6873. [DOI] [PubMed] [Google Scholar]
- 90.Sasu BJ, Li H, Rose MJ, et al. Serum hepcidin but not prohepcidin may be an effective marker for anemia of inflammation (AI). Blood Cells Mol Dis. 2010;45(3):238–245. doi: 10.1016/j.bcmd.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Kroot JJ, Kemna EH, Bansal SS, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94(12):1748–1752. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 93.Preza GC, Ruchala P, Nemeth E, Ganz T. Minihepcidins: small peptides involved in disulfide exchange with ferroportin act as agonists [abstract]. FASEB J. 2010;24:1011, 1. [Google Scholar]
- 94.Corradini E, Schmidt PJ, Meynard D, et al. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. 2010;139(5):1721–1729. doi: 10.1053/j.gastro.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest. 2010;120(12):4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sasu BJ, Cooke KS, Arvedson TL, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 97.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106(8):2884–2889. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 98.Babitt JL, Huang FW, Xia Y, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117(7):1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poli M, Girelli D, Campostrini N, et al. Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood. 2011;117(3):997–1004. doi: 10.1182/blood-2010-06-289082. [DOI] [PubMed] [Google Scholar]
- 101.De Domenico I, Zhang TY, Koening CL, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120(7):2395–2405. doi: 10.1172/JCI42011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.