Abstract
Helicobacter pylori infection plays a causal role in the development of gastric mucosa-associated lymphoid tissue (MALT) lymphoma (LG-MALT) and duodenal ulcer (DU). Although many virulence factors have been associated with DU, many questions remain unanswered regarding the evolution of the infection toward this exceptional event, LG-MALT. The present study describes and compares the complexome of two H. pylori strains, strain J99 associated with DU and strain B38 associated with LG-MALT, using the two-dimensional blue native/SDS-PAGE method. It was possible to identify 90 different complexes (49 and 41 in the B38 and J99 strains, respectively); 12 of these complexes were common to both strains (seven and five in the membrane and cytoplasm, respectively), reflecting the variability of H. pylori strains. The 44 membrane complexes included numerous outer membrane proteins, such as the major adhesins BabA and SabA retrieved from a complex in the B38 strain, and also proteins from the hor family rarely studied. BabA and BabB adhesins were found to interact independently with HopM/N in the B38 and J99 strains, respectively. The 46 cytosolic complexes essentially comprised proteins involved in H. pylori physiology. Some orphan proteins were retrieved from heterooligomeric complexes, and a function could be proposed for a number of them via the identification of their partners, such as JHP0119, which may be involved in the flagellar function. Overall, this study gave new insights into the membrane and cytoplasm structure, and those which could help in the design of molecules for vaccine and/or antimicrobial agent development are highlighted.
Helicobacter pylori infection is one of the most common chronic bacterial infections worldwide with up to half of the world's population infected (for a review, see Ref. 1). This infection is involved in the development of various gastroduodenal diseases including two malignant diseases, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT)1 lymphoma (LG-MALT) (2–5). H. pylori infection is associated with ∼80% of extranodal marginal zone B-cell lymphomas of MALT type (6). The causal role of this infection in the lymphomatic process was proven by the beneficial effect of H. pylori eradication on the regression of lymphoma (7–9). Despite this proof, many questions remain unanswered concerning the mechanism involved in the evolution of H. pylori infection toward the development of an LG-MALT. In fact, the development of an LG-MALT is a very exceptional event because fewer than 0.1% of infected patients will develop this cancer. Consequently, few strains are available, and this lymphoma has not been extensively studied as compared with gastric adenocarcinoma or duodenal ulcer (DU) disease. To date, no environmental factor nor genetic host factor has been found, and in contrast to other severe diseases due to H. pylori infection, none of the virulence factors known for this bacterium, including the presence of the cag pathogenicity island (PAI) or the VacA toxin, could be associated with this pathology except for the vacAm2 allele (10–12). However, phylogenic analyses, based on DNA array hybridization, revealed that most of the H. pylori strains associated with LG-MALT, although lacking the main H. pylori virulence factors, cluster separately from strains associated with other pathologies (gastric carcinoma or DU). This, in turn, has led to the assumption that these strains have a specific genetic material content involved in the clinical outcome of LG-MALT (13). Given that the conventional methods used in molecular biology and genetics did not allow the identification of strains with specific virulence genes, it was proposed that other strategies be implemented (11, 12, 14–17). Moreover and despite the availability of 10 different H. pylori genome sequences, there are many “orphan” genes from H. pylori for which no function has been attributed, and few data on protein expression are available.
Certain studies have suggested that nearly all biochemical processes are performed by protein complexes (18). The exploration of protein interactions (protein complexes or complexome) is one of the main challenges of functional genomics to get insight into protein function to understand the physiology and pathogenesis of microorganisms. Among the high throughput technologies used to study complexes, blue native/sodium dodecyl sulfate-polyacrylamide gel electrophoresis (BN/SDS-PAGE) is a highly resolvent separation method (19). It was initially described for the separation under native conditions of the membrane protein complexes of mitochondria (20), chloroplasts (21), and more recently bacteria, such as Paracoccus denitrificans (22), Synechocystis species (23), and Escherichia coli (24). It was later applied to the study of whole complexes of eukaryotic cells (25, 26) and of bacteria, e.g. E. coli (27) and H. pylori reference strain J99 associated with DU (28). This last study led to the description of 13 multiprotein complexes, 11 issued from the cytoplasm and two issued from the membrane that were either partially or totally reported previously in the literature.
In the present study, two-dimensional BN/SDS-PAGE was applied, after technical improvements, to study the whole complexome of two sequenced H. pylori strains to determine whether some complexes were specific to one or the other. Because patients with DU are not predisposed to LG-MALT (29), the complexome of the J99 strain associated with DU (30) was compared with that of the B38 strain chosen to be representative of an LG-MALT-specific cluster (13). Protein identification was performed by using liquid chromatography-mass spectrometry (LC-MS/MS). Purification steps, such as gel filtration, liquid isoelectrofocalization (IEF), and ionic column separation, were used to improve the multiprotein complex separation.
EXPERIMENTAL PROCEDURES
Strains Used
H. pylori strain B38 was isolated from a 62-year-old French male patient with extranodal marginal zone B-cell lymphoma of MALT type whose lymphoma had regressed after eradication of H. pylori. This patient was enrolled in a prospective multicenter study carried out by the Groupe d'Etude Français des Lymphomes Digestifs of the Fédération Française de Cancérologie Digestive (8). The genome of this strain, recently sequenced, is the smallest H. pylori genome described to date (13). The B38 strain is lacking in all known pathogenic determinants because it is negative for the entire cag PAI; it appears that it does not produce a functional cytotoxin (positive for vacAs2m2) and the major adherence factors (absence of babB, babC, sabB, and homB genes). This strain is positive for babA2, iceA1, and hopQII genotypes, and it has a functional hopZ gene and non-functional oipA and sabA genes. The second H. pylori strain, J99 (ATCC 700824), was isolated from a patient with DU in the United States (30). The J99 strain is positive for the entire cag PAI; it is positive for vacAs1m1, babA2, iceA1, and hopQII genotypes and has functional hopZ, oipA, and sabA genes. Another characteristic of both strains is that they do not carry plasmidic DNA. The genomes of the B38 and J99 strains are available at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/sites/entrez?db=genome). More detailed information about the J99 genome (31) is available at the PyloriGene World Wide Web Server (http://genolist.pasteur.fr/PyloriGene/genome.cgi).
Bacterial Growth Conditions
H. pylori B38 and J99 cells were cultured simultaneously and under identical conditions for 48 h on Wilkins-Chalgren agar plates (Oxoid Ltd., Hampshire, UK) supplemented with 10% human blood and the following antibiotics: 1 mg/ml vancomycin (Lilly France S.A., Fergesheim, France), 5 mg/ml cefsulodin (Takeda France S.A., Puteaux, France), 5 mg/ml Fungizone (Bristol-Myers Squibb Co.), and 1 mg/ml trimethoprim (GlaxoSmithKline). The plates were incubated at 37 °C under microaerobic conditions (5% O2, 10% CO2, 85% N2). Bacteria harvested from agar plates were suspended in ice-cold 0.85% NaCl (bioMérieux, Marcy l'Etoile, France). For performance of the two-dimensional BN/SDS-PAGE applied to the H. pylori cytosolic and membrane extracts, a total of 3 g of each H. pylori strain was frozen at −80 °C. All of the bacteria and sample manipulations (cytoplasmic and membrane preparation) were performed at 4 °C (unless otherwise indicated).
Cytoplasmic Extract Preparations
Bacteria were harvested from a 48-h culture by centrifugation at 6,000 × g for 10 min and washed in ultrapure water. Bacteria were suspended in native extraction buffer A (750 mm 6-amino-n-caproic acid, 50 mm Tris) supplemented with a 1 mm final concentration of phenylmethanesulfonyl fluoride and passed through a One Shot disruptor (Constant Systems Ltd., Northants, UK) at 2 kilobars (one shot). The lysate was centrifuged at 6,000 × g for 20 min, and a 0.2 mg/ml final concentration of DNase I was added to the supernatant for 1 h at 25 °C. Then, the supernatant was centrifuged at 100,000 × g for 30 min at 4 °C and filtered with a Miracloth membrane (Calbiochem). The pellet contained membrane (see “Membrane Extract Preparations”). The cytosolic multiprotein complexes contained in the supernatant were desalted. Indeed, for H. pylori cytoplasmic extracts, a preliminary dialysis is necessary to obtain highly resolvent gels. Here dialysis was sometimes replaced by a desalting step, which allows the elimination of small molecules and salts, as was described for the purification of the human embryonic kidney cell line HEK293 (25). The final result was the same for dialysis and the desalting step, but the first technique allows sample concentration using a dialysis membrane (cutoff, 14,000 Da) in buffer A with 30% glycerol.
Membrane Extract Preparations
The pellet was resuspended in buffer A with 1 mm phenylmethanesulfonyl fluoride and passed through a One Shot disruptor at 2 kilobars (one shot). The resulting lysate was centrifuged at 6,000 × g for 20 min, the pellet was discarded, and the supernatant was centrifuged at 100,000 × g for 30 min. The extraction of the protein complexes from the resulting pellet was then carried out by resuspending the membrane in 1 ml of buffer A supplemented with 2% n-dodecyl β-d-maltoside detergent (Sigma-Aldrich). This sample was then centrifuged at 100,000 × g for 30 min, and the membrane multiprotein complexes contained in the supernatant were separated by two-dimensional BN/SDS-PAGE.
Purification Steps
All of the steps were carried out at 4 °C. Therefore, liquid IEF, exclusion filtration methods, and ionic column separation were used as purification steps before applying the two-dimensional BN/SDS-PAGE.
Liquid IEF purification was used to separate the multiprotein complexes according to their pI in a pH range from 3.5 to 10, 4 to 6, 5 to 7, and 6 to 8. An aliquot of a crude cytosolic sample was analyzed in a Rotofor system (Rotofor Prep IEF Cell, Bio-Rad). The protein mixture was prepared according to the manufacturer's recommendations before filling the Rotofor chamber. The IEF method produced many protein precipitates in the most abundant protein fractions with a pI of ∼5–6. Very low protein concentrations were found in the basic fractions, although a concentration step with Vivaspin column (Vivascience, Aubagne, France) was used.
Gel filtration purification was also carried out. An aliquot of crude cytosolic or membrane sample was loaded on a SuperdexTM 200 column (Amersham Biosciences). Buffer A was run at a flow rate of 0.3 ml/min using the FPLC ÄKTA (Amersham Biosciences). Multiprotein complexes were recovered in 250-μl fractions. The cytoplasmic sample was separated into four peaks of major interest: 1,000, 450, 220, and 155 kDa. This method allowed the adaptability of the two-dimensional BN/SDS-PAGE acrylamide gradient according to the mass of interest of the complexes.
Complexes were also separated using an ionic column. Crude extract was loaded on a 1-ml HiTrapTM Q XL column (Amersham Biosciences) at a flow rate of 1 ml/min using the FPLC ÄKTA (Amersham Biosciences) and was washed with 5 ml of buffer A before a two-step elution using 5 ml of buffer A supplemented with 250 mm NaCl and 1 m NaCl. The two last fractions were desalted using a dialysis membrane (cutoff, 14,000 Da) (Medicell International Ltd., London, UK).
First Dimension: BN-PAGE
Sample preparation and BN-PAGE were carried out as described previously (20, 28) with the following minor modifications. The gel dimension was 20 cm × 14.5 cm × 1 mm. Separating gels with a linear 4–12, 4–13, 4–13.5, 3–14.6, 4–14.6, 7–14.6, or 7–18% acrylamide gradient gels were used. Anode and cathode buffers contained 50 mm Tris, 75 mm glycine, and only the cathode buffer was supplemented with 0.004% Serva Blue G (Serva, Heidelberg, Germany). Before loading the sample, 2 μl of buffer B (500 mm 6-amino-n-caproic acid, 5% Serva Blue G) was added. The gel was run overnight at 4 °C at 1 watt. Thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), lactate dehydrogenase (140 kDa) and Albumin (66 kDa) (GE Healthcare, Uppsala, Sweden) were used for each BN-PAGE analysis as molecular mass size standards. Different acrylamide gradients were tested for the BN-PAGE to improve the multiprotein complex separation. A certain balance needs to be found to optimize both focalization and separation of complexes with a mass greater than 60 kDa. A molecular mass could be attributed to the membrane complexes based on a molecular mass marker.
Second Dimension: SDS-PAGE
Individual lanes from BN-PAGE were equilibrated for 5 min in an equilibrating buffer containing 1% SDS (w/v), 125 mm Tris, pH 6.8 and then dipped into equilibrating buffer supplemented with 50 mm dithiothreitol (Sigma-Aldrich) for 15 min. Individual lanes were subsequently soaked in equilibrating buffer supplemented with 125 mm iodoacetamide (Sigma-Aldrich) for 15 min. An ultimate washing step lasting 5 min was performed in the equilibrating buffer without supplement. Individual lanes were placed on a glass plate at the usual position for stacking gels. After covering with the second glass plate, the gel was brought into a vertical position. Then the 10, 13, or 15% acrylamide separating gel mixture was poured. After polymerization, the stacking gel mixture was poured.
Gel Staining
Silver staining was performed using a silver staining kit (Sigma-Aldrich) according to the manufacturer's instructions. Coomassie Brilliant Blue G-250 (Bio-Rad) was also used for gel staining. After two ultrapure water washings of 3 min each, the gels were placed overnight in an incubation solution (10% ammonium sulfate, 0.1% Coomassie Brilliant Blue G-250, 3% orthophosphoric acid, 20% ethanol). Gels were washed twice for 1 min in ultrapure water and twice for 1 h in 5% acetic acid.
In-gel Protein Digestion
Silver-stained proteins separated by SDS-PAGE were excised and destained using the PROTSIL2 silver staining kit (Sigma-Aldrich) according to the manufacturer's instructions. Spots were subsequently washed in ultrapure water until completely destained. The solvent mixture was removed and replaced by acetonitrile. After shrinking of the gel pieces, acetonitrile was removed, and the gel pieces were dried in a vacuum centrifuge. They were then rehydrated in 10 ng/μl trypsin (Sigma-Aldrich) and 50 mm ammonium bicarbonate and incubated overnight at 37 °C. Ammonium bicarbonate (50 mm) was added to the gel pieces, which were incubated for 15 min at room temperature under rotary shaking. The supernatant was collected, and an ultrapure water/acetonitrile/acetic acid (47.5:47.5:5) solution was added to the gel pieces for 15 min. This step was repeated twice. Supernatants were pooled and concentrated in a vacuum centrifuge to a final volume of 25 μl. Digested products were finally acidified by the addition of 1.5 μl of acetic acid and stored at −20 °C.
On-line Capillary HPLC Nanospray Ion Trap MS/MS Analysis
Peptide mixtures were analyzed by on-line capillary HPLC (LC Packings, Amsterdam, The Netherlands) coupled to a nanospray LCQTM ion trap mass spectrometer (ThermoFinnigan, San Jose, CA). Peptides were separated on a 75-μm-inner diameter × 15-cm C18 PepMapTM column (LC Packings). The flow rate was set at 200 nl/min. Peptides were eluted using a 5–50% linear gradient of solvent B for 30 min (solvent A was 0.1% formic acid in 5% acetonitrile, and solvent B was 0.1% formic acid in 80% acetonitrile). The mass spectrometer was operated in positive ion mode at a 2-kV needle voltage and a 38-V capillary voltage. Data acquisition was performed in a data-dependent mode consisting of alternatively in a single run full scan MS over the range m/z 300–2,000 and full scan MS/MS in an exclusion dynamic mode. MS/MS data were acquired using a 3-m/z unit ion isolation window, a 35% relative collision energy, and a 5-min dynamic exclusion duration.
Data Analysis
Data were analyzed by SEQUEST (ThermoFinnigan) against a subset of the NCBI database consisting of H. pylori strain protein sequences. Carbamidomethylation of cysteines (+57 Da) and oxidation of methionines (+16 Da) were considered as differential modifications. Only peptides with an Xcorr greater than 1.5 (single charge), 2 (double charge), and 2.5 (triple charge) were retained. In all cases, ΔCn had to be greater than 0.1.
Bioinformatics Tools
Protein sequences were compared with the GenBankTM database with the Blast program “protein blast” (algorithms: blastp, psi-blast, phi-blast; http://www.ncbi.nih.gov/BLAST/) at the National Center for Biotechnology Information computer server (32). The search tool for interactions of chemicals (STITCH; http://stitch.embl.de/) was used to explore possible interactions between partners of complexes identified with or without chemical intermediaries (33).
RESULTS
Global Presentation of Results
In total, 329 proteins were identified by LC-MS/MS of which 32 were never mentioned in previous proteomics studies (supplemental Table S1). Among these additional proteins, 27 have a “predicted” function deduced from homologs characterized in other organisms (31), and nine proteins correspond to open reading frames (ORFs) annotated as “predicted ORF/hypothetical protein”, demonstrating that these ORFs really do encode proteins, such as JHP0628 (HELPY_0684) or JHP0905 (HELPY_0958) annotated as “predicted coding regions” (31).
The basic condition for the identification of a multiprotein complex is that proteins of the same multiprotein complex co-migrate in the first dimension and are found aligned with a similar shape in the second dimension (20). Multiprotein complexes from the membrane were named “MB” and “MJ” for the B38 and J99 strains, respectively. Those from the cytoplasm were named “CB” and “CJ” for the B38 and J99 strains, respectively. The pattern of most of the complexes presented in this study undoubtedly fulfilled these criteria (20). Examples are provided with complexes MB2, CB29, MJ8, and CJ9 (Figs. 1B, 3M, 2B, and 4B, respectively).
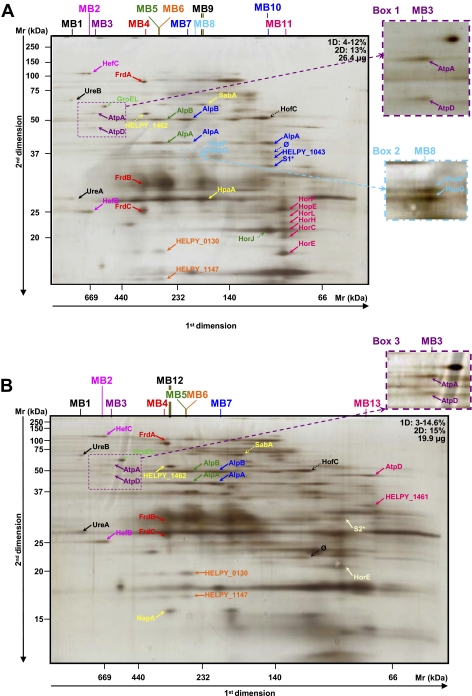
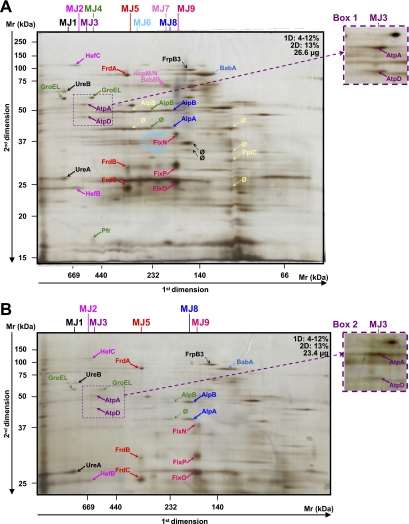
Fig. 1.
Analysis of crude membrane samples of H. pylori strain B38. The first (BN-PAGE) and second dimension gel electrophoreses (SDS-PAGE) were performed with various protein quantities and acrylamide gradients indicated on each gel (A–E). Dotted arrows indicate proteins that were not attributed to heterooligomeric complexes. Enlargement and second migration of the MB3 complex are shown in boxes 1 and 3. Enlargement of the MB8 complex is shown in box 2. Protein identifications are presented in Table I. Multiprotein complexes isolated from the membrane of the B38 strain were named MB. * represents spots where different proteins were identified (see Table I). A mixture of proteins was identified in the following spots: spot number S1: HELPY_0856 (one peptide: K↓DYKDYLTFFEK↓S, coverage = 2.5%, p = 1.01e−7) and SdaC (HELPY_0133, one peptide: K↓EGLEGIIIQSLK↓L, coverage = 2.9%, p = 5.15e−4); and spot number S2: PetC (HELPY_1541, one peptide: K↓GEHGLNVFINDPQK↓L, coverage = 4.9%, p = 2.03e−7) and HELPY_0449 (one peptide: K↓NLFEIQTHTTK↓Q, coverage = 4.3%, p = 8.46e−4). ∅, spots for which identification has failed.
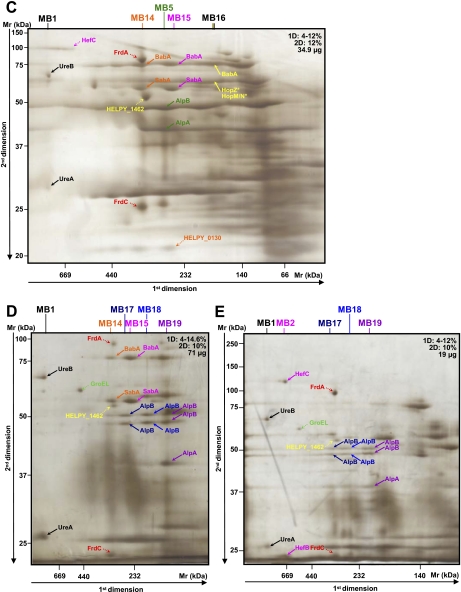
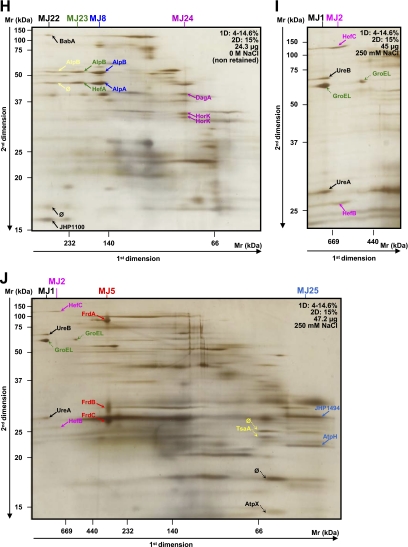
Fig. 3.
Analysis of crude and purified cytoplasmic samples of H. pylori strain B38. The first (BN-PAGE) and second dimension gel electrophoreses (SDS-PAGE) were performed with the various protein quantities and acrylamide gradients indicated on each gel (A–N). Dotted arrows indicate proteins that were not attributed to heterooligomeric complexes. More contrasted pictures of migration of the CB2 and CB16 complexes are shown in boxes 1 and 2, respectively. Protein identifications are presented in Table III. Multiprotein complexes from the cytosol of the B38 strain were named CB. A–C represent the analyses of the crude cytoplasmic samples. D and F represent the analyses of the fractions eluted when the cytoplasmic sample was purified using the gel filtration method (Superdex 200 column) before applying the two-dimensional BN/SDS-PAGE. G–N represent the analyses of the fractions eluted by the isoelectrofocalization method (Rotofor system) before applying the two-dimensional BN/SDS-PAGE. ∅, spots for which identification has failed.
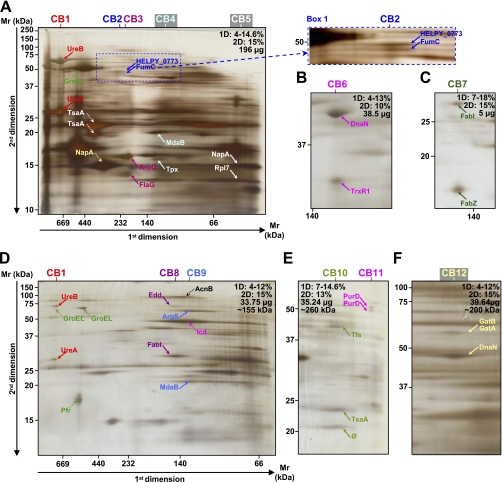
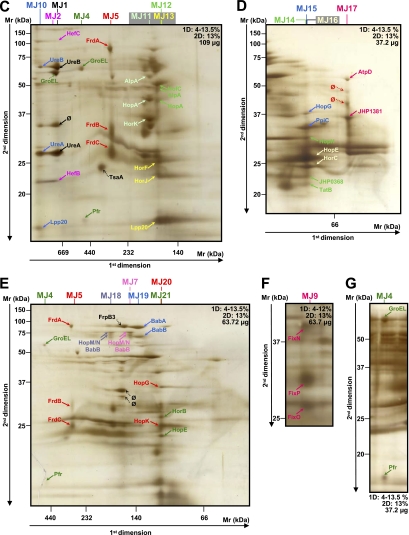
Fig. 2.
Analysis of crude and purified membrane samples of H. pylori strain J99. The first (BN-PAGE) and second dimension gel electrophoreses (SDS-PAGE) were performed with the various protein quantities and acrylamide gradients indicated on each gel (A–J). Dotted arrows indicate proteins that were not attributed to heterooligomeric complexes. More contrasted pictures of migration of the MJ3 complex are shown in boxes 1 and 2. Protein identifications are presented in Table II. Multiprotein complexes from the membrane of strain J99 were named MJ. A–G represent the analyses of the crude membrane samples. H–J represent the analyses of the fractions eluted when the membrane sample was purified using the ionic column (HiTrap Q column) before applying the two-dimensional BN/SDS-PAGE. H corresponds to the directly eluted fraction. I and J correspond to fractions eluted with 250 mm NaCl from different sample preparations. ∅, spots for which identification has failed.
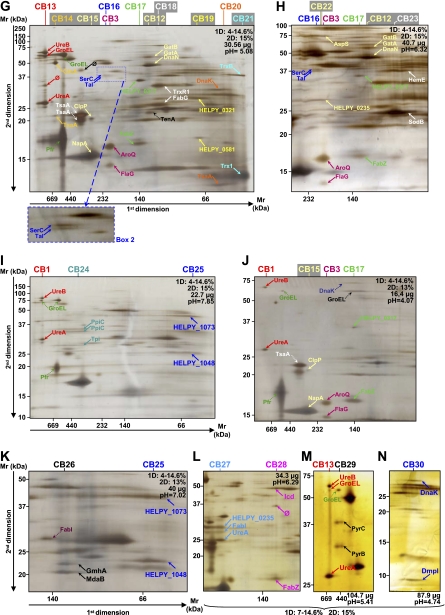
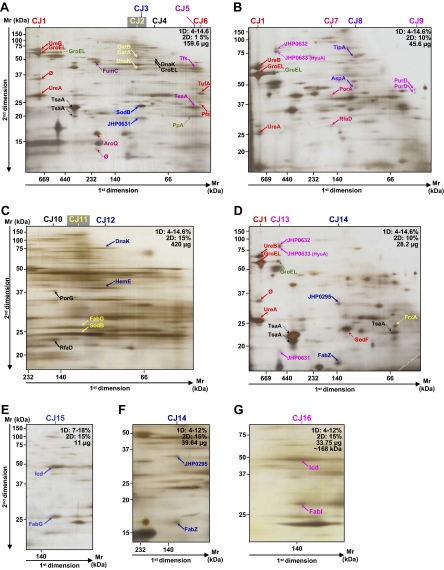
Fig. 4.
Analysis of the crude and purified cytoplasmic samples of H. pylori strain J99. The first (BN-PAGE) and second dimension gel electrophoreses (SDS-PAGE) were performed with the various protein quantities and acrylamide gradients indicated on each gel (A–G). Dotted arrows indicate proteins that were not attributed to heterooligomeric complexes. Protein identifications are presented in Table IV. Multiprotein complexes from the cytosol of the J99 strain were named CJ. A–F represent the analyses of crude cytoplasmic samples. G represents the analysis of the fraction eluted at ∼168 kDa when the cytoplasmic sample was purified using the gel filtration method (Superdex 200 column) before applying the two-dimensional BN/SDS-PAGE. ∅, spots for which identification has failed.
The 90 heterooligomeric complexes comprised 49 protein complexes in the H. pylori B38 strain and 41 in the H. pylori J99 strain. All of these complexes are shown in Figs. 1–4 and are described in Tables I–IV. Fifty proteins whose functions remain to be elucidated appear in heterooligomeric complexes, and 14 of these 50 proteins correspond to genes annotated as predicted coding regions. The presence of these proteins in heterooligomeric complexes, through the identification of their partners, should give more insight into their functions.
As previously pointed out by Schägger et al. (34), the same shape benchmark can sometimes be difficult to apply; consequently, the assignment of spots to a complex becomes difficult. In the present study, spots corresponding to FrdA/B/C (MB4 and MJ5 for the B38 and J99 strains, respectively) were found aligned (Figs. 1, A and B, and 2, A–C, E, and J), but a spot corresponding to FrdA presented a slightly oval shape, whereas spots corresponding to FrdB/C had a rounded and more diffuse shape due to a more diffuse gel migration in the lower approximate Mr range (28). However, these three proteins correspond to the three subunits of the fumarate reductase (FRD) complex of H. pylori (35, 36), and FrdA and FrdB were found previously in a membrane complex of the J99 strain using the two-dimensional BN/SDS-PAGE method (28). Consequently, this complex was included given that its third evident partner, FrdC, was identified on most of the gels (Figs. 1, A and B, and 2, A–C, E, and J). It was also noted that the Mr of FrdB was higher than that of FrdC, whereas their deduced Mr values were in the same range. This apparent migration of FrdB at 31 kDa was reported previously by Birkholz et al. (35). The FRD complex, commonly associated with the membrane of H. pylori (37), is the key enzyme of the Krebs cycle involved in fumarate respiration in the case of anaerobic growth. This enzyme is indeed constitutively expressed under microaerobic conditions and is essential for H. pylori colonization of the mouse stomach (38).
Different migrations of some proteins were observed during the separation in the second dimension electrophoresis as was the case for TsaA (Fig. 3, A and G, Fig. 4, A and D); the outer membrane proteins (OMPs) AlpB (MB17 and MB18, Fig. 1, D and E) and HorK (MJ24, Fig. 2H); the “predicted glycinamide ribonucleotide synthetase” PurD (CB11 and CJ9, Fig. 3E and Fig. 4B), and the peptidyl-prolyl cis-trans isomerase C, PpiC (CB24, Fig. 3I), suggesting that multiple isoforms of these proteins do exist. Their occurrence can be explained by probable post-translational modifications changing their physicochemical criteria (pI, Mr, and binding affinity). In fact, H. pylori proteins are subjected to a high degree of post-translational modification as is the case for TsaA, Pfr, UreA, UreB, and RecA (28, 39, 40) and also for some OMPs such as HopK (41). Different oligomerization states of some complexes were also observed, e.g. the BabA-SabA (MB14 and MB15, Fig. 1C) and HopM/N-BabB (MJ7 and MJ18, Fig. 2E) complexes. This was also previously reported in H. pylori (28).
Compared with the previous study performed on the H. pylori J99 strain (28), four of the 11 cytosolic heterooligomeric complexes could be totally or partially retrieved. Indeed, modifications in sample preparation were made to increase the number of multiprotein complexes and to confirm certain protein-protein interactions. In the present study, purification steps were used, different amounts of proteins were loaded onto the gels, and various acrylamide concentrations in the first and second dimensions were used. Concerning membrane complexes, the urease complex (MJ1) was partially retrieved, and the FRD complex was completed by the third subunit FrdC (see previous paragraph). Moreover, a partner of SodB, AroQ, and FabZ, described previously to belong to homooligomeric complexes (28), was found (Table III). These partners, hardly or not visible during previous stainings (28), were observed and identified in this study. For this reason and to avoid the description of homooligomeric complexes with an inadequate number of subunits, homooligomeric complexes were not reported in the present study. Dotted arrows in Figs. 1–4 indicate proteins that were not attributed to heterooligomeric complexes.
Table III. Description of cytosolic protein complexes identified in H. pylori strain B38 using two-dimensional BN/SDS-PAGE.
The complexes presented in the table were all localized on two-dimensional BN/SDS-PAGE gels performed in this study and are represented in Fig. 3. Multiprotein complexes isolated from the cytosol of the B38 strain were named CB. The experimental approximate molecular mass is given in kDa. GBAN, GenBank accession number (NCBI Reference Sequence); n, the number of peptides; Cov., the protein sequence coverage (percentage) of the peptides.
| Complex no. | Molecular mass | Fig. 3 gel letter | Proteina | Previously identified | Strain B38a |
LC-MS/MS information |
Strain J99b |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HELPY no. | GBAN | Protein annotation | Molecular mass | n | Xcorr | Cov. | JHP no. | GBAN | |||||
| kDa | Da | % | |||||||||||
| CB1 | 800 | A, D, I, J | UreB | + | HELPY_0068 | YP_003056903 | Urease B subunit | 61,553 | 10 | 40.76 | JHP0067 | NP_222789 | |
| UreA | + | HELPY_0069 | YP_003056904 | Urease A subunit | 26,465 | 18 | 38.66 | JHP0068 | NP_222790 | ||||
| CB2 | 230 | A | HELPY_0773 | + | HELPY_0773 | YP_003057512 | Methyl-accepting chemotaxis transmembrane sensory protein (MCP-like protein) | 48,157 | 12 | 58.78 | JHP0546 | NP_223264 | |
| FumC | + | HELPY_1301 | YP_003057973 | Fumarate hydratase class II (fumarase C) | 50,773 | 8 | 27.00 | JHP1245 | NP_223963 | ||||
| CB3 | 200 | A, G, H, J | AroQ (AroD) | + | HELPY_0415 | YP_003057206 | 3-Dehydroquinate dehydratase (3-dehydroquinase) (type II DHQase) | 18,352 | 6 | 49.10 | JHP0386 | NP_223105 | |
| FlaG | − | HELPY_0614 | YP_003057378 | Flagellar protein FlaG (H. pylori B38) | 13,279 | 2 | 3.37 (2+) | 19.33 | JHP0688 | NP_223406 | |||
| 2.29 (1+) | |||||||||||||
| CB4 | 135 | A | MdaB | + | HELPY_0741 | YP_003057488 | NAD(P)H oxidoreductase (NADPH quinone reductase) | 21,524 | 7 | 34.02 | JHP0573 | NP_223291 | |
| Tpx | + | HELPY_1035 | YP_003057724 | Thiol peroxidase | 18,195 | 2 | 3.42 (2+) | 15.06 | JHP0991 | NP_223708 | |||
| 3.37 (2+) | |||||||||||||
| CB5 | 45 | A | NapA | + | HELPY_0248 | YP_003057054 | Neutrophil-activating protein NapA (bacterioferritin) | 16,677 | 9 | 70.83 | JHP0228 | NP_222949 | |
| Rpl7 | + | HELPY_1172 | YP_003057852 | 50 S ribosomal protein L7/L12 | 13,197 | 3 | 41.60 | JHP1122 | NP_223839 | ||||
| CB6 | 110 | B | DnaN | + | HELPY_0852 | YP_003057584 | DNA polymerase III subunit β | 42,002 | 24 | 70.60 | JHP0452 | NP_223170 | |
| TrxR1 (TrxB1) | + | HELPY_0531 | YP_003057307 | Thioredoxin reductase (TRXR) (TR) | 33,378 | 12 | 44.10 | JHP0764 | NP_223482 | ||||
| CB7 | 200 | C | FabI | + | HELPY_0198 | YP_003057011 | Putative enoyl-(acyl-carrier-protein) reductase | 29,868 | 9 | 34.18 | JHP0181 | NP_222902 | |
| FabZ | + | HELPY_1363 | YP_003058021 | (3R)-Hydroxymyristoyl-(acyl-carrier-protein) dehydratase ((3R)-hydroxymyristoyl ACP dehydrase) | 18,079 | 4 | 25.79 | JHP1290 | NP_224008 | ||||
| CB8 | 140 | D | Edd | + | HELPY_1069 | YP_003057758 | Phosphogluconate dehydratase (6-phosphogluconate dehydratase) | 66,713 | 4 | 7.63 | JHP1026 | NP_207891 | |
| FabI | + | HELPY_0198 | YP_003057011 | Putative enoyl-(acyl-carrier-protein) reductase | 29,868 | 7 | 20.73 | JHP0181 | NP_222902 | ||||
| CB9 | 130 | D | ArgS | − | HELPY_0322 | YP_003057123 | Arginyl-tRNA synthetase (arginine-tRNA ligase) (ArgRS)E | 61,946 | 14 | 24.58 | JHP0302 | NP_223022 | |
| MdaB | + | HELPY_0741 | YP_003057488 | NAD(P)H oxidoreductase (NADPH quinone reductase) | 21,524 | 3 | 19.59 | JHP0573 | NP_223291 | ||||
| CB10 | 60 | E | Tfs | + | HELPY_1558 | YP_003058194 | Elongation factor Ts (EF-Ts) | 39,595 | 6 | 18.60 | JHP1444 | NP_224162 | |
| TsaA | + | HELPY_1565 | YP_003058201 | Alkyl hydroperoxide reductase | 22,105 | 4 | 26.26 | JHP1471 | NP_224189 | ||||
| CB11 | 50 | E | PurD | + | HELPY_1194 | YP_003057869 | Phosphoribosylamine-glycine ligase (GAR synthetase) (GARS) (glycinamide ribonucleotide synthetase) (phosphoribosylglycinamide synthetase) | 47,297 | 3 | 8.30 | JHP1140 | NP_223858 | |
| PurD | + | HELPY_1194 | YP_003057869 | Phosphoribosylamine-glycine ligase (GAR synthetase) (GARS) (glycinamide ribonucleotide synthetase) (phosphoribosylglycinamide synthetase) | 47,297 | 2 | 3.98 (2+) | 6.60 | JHP1140 | NP_223858 | |||
| 3.75 (2+) | |||||||||||||
| CB12 | 100 | F–H | GatB | + | HELPY_0713 | YP_003057460 | Aspartyl/glutamyl-tRNA(Asn/Gln) amidotransferase subunit B (Asp/Glu-ADT subunit B) | 52,889 | 5 | 13.30 | JHP0603 | NP_223321 | |
| GatA | + | HELPY_0524 | YP_003057302 | Glutamyl-tRNA(Gln) amidotransferase subunit A (Glu-ADT subunit A) | 49,618 | 6 | 20.30 | JHP0769 | NP_223487 | ||||
| DnaN | + | HELPY_0852 | YP_003057584 | DNA polymerase III subunit β | 42,002 | 12 | 36.60 | JHP0452 | NP_223170 | ||||
| CB13 | 800 | G, M | UreB | + | HELPY_0068 | YP_003056903 | Urease B subunit | 61,553 | 18 | 38.66 | JHP0067 | NP_222789 | |
| GroEL | + | HELPY_0008 | YP_003056851 | 60-kDa chaperonin (protein Cpn60) (groEL protein) | 58,111 | 28 | 48.72 | JHP0008 | NP_222730 | ||||
| UreA | + | HELPY_0069 | YP_003056904 | Urease A subunit | 26,465 | 10 | 40.76 | JHP0068 | NP_222790 | ||||
| CB14 | 600 | G | GlnA | + | HELPY_0840 | YP_003057574 | Glutamine synthetase (glutamate-ammonia ligase) | 54,280 | 21 | 47.40 | JHP0461 | NP_223179 | |
| TsaA | + | HELPY_1565 | YP_003058201 | Alkyl hydroperoxide reductase | 22,105 | 7 | 33.84 | JHP1471 | NP_224189 | ||||
| CB15 | 280 | G, J | ClpP | + | HELPY_0566 | YP_003057337 | ATP-dependent Clp protease proteolytic subunit (endopeptidase Clp) | 21,427 | 2 | 2.29 (2+) | 23.47 | JHP0730 | NP_223448 |
| 5.04 (3+) | |||||||||||||
| NapA | + | HELPY_0248 | YP_003057054 | Neutrophil-activating protein NapA (bacterioferritin) | 16,677 | 8 | 65.28 | JHP0228 | NP_222949 | ||||
| kDa | Da | % | |||||||||||
| CB16 | 210 | G, H | SerC | + | HELPY_0631 | YP_003057392 | Putative aminotransferase, class V | 40,955 | 9 | 51.54 | JHP0673 | NP_223391 | |
| Tal | + | HELPY_1468 | YP_003058112 | Transaldolase | 34,967 | 2 | 2.74 (2+) | 20.3 | JHP1388 | NP_224106 | |||
| 3.28 (2+) | |||||||||||||
| CB17 | 130 | G, H, J | HELPY_0317 | + | HELPY_0317 | YP_003057118 | Putative NodB-like polysaccharide deacetylase | 33,451 | 2 | 2.69 (2+) | 6.48 | JHP0295 | NP_223015 |
| 3.72 (2+) | |||||||||||||
| FabZ | + | HELPY_1363 | YP_003058021 | (3R)-Hydroxymyristoyl-(acyl-carrier-protein) dehydratase ((3R)-hydroxymyristoyl ACP dehydrase) | 18,079 | 2 | 2.57 (2+) | 10.69 | JHP1290 | NP_224008 | |||
| 2.61 (2+) | |||||||||||||
| CB18 | 120 | G | TrxR1 (TrxB1) | + | HELPY_0531 | YP_003057307 | Thioredoxin reductase (TRXR) (TR) | 33,378 | 12 | 42.44 | JHP0764 | NP_223482 | |
| FabG | + | HELPY_0816 | YP_003057552 | 3-Oxoacyl-(acyl-carrier-protein) reductase | 26,629 | 2 | 4.95 (2+) | 12.15 | JHP0508 | NP_223226 | |||
| 2.79 (2+) | |||||||||||||
| CB19 | 75 | G | HELPY_0321 | + | HELPY_0321 | YP_003057122 | Hypothetical protein HELPY_0321 | 28,512 | 2 | 3.46 (2+) | 10.32 | JHP0301 | NP_223021 |
| 3.79 (2+) | |||||||||||||
| HELPY_0581 | − | HELPY_0581 | YP_003057349 | Hypothetical protein HELPY_0581 | 19,797 | 3 | 21.14 | JHP0720 | NP_223438 | ||||
| CB20 | 55 | G | DnaK | + | HELPY_0109 | YP_003056934 | Chaperone protein dnaK (heat shock protein 70) (heat shock 70-kDa protein) (HSP70) | 66,920 | 4 | 11.77 | JHP0101 | NP_222822 | |
| TsaA | + | HELPY_1565 | YP_003058201 | Alkyl hydroperoxide reductase | 22,105 | 8 | 13.10 | JHP1471 | NP_224189 | ||||
| CB21 | 45 | G | TrxB, TrxB_2 | + | HELPY_1138 | YP_003057820 | Thioredoxin reductase | 35,748 | 21 | 59.89 | JHP1091 | NP_223808 | |
| Trx1, TrxA | + | HELPY_0532 | YP_003057308 | Thioredoxin 1 | 11,724 | 2 | 2.56 (2+) | 9.22 | JHP0763 | NP_223481 | |||
| 2.93 (2+) | |||||||||||||
| CB22 | 230 | H | AspS | + | HELPY_0755 | YP_003057498 | Aspartate-tRNA ligase (aspartic acid translase) (aspartyl-tRNA synthetase) | 65,236 | 3 | 5.50 | JHP0560 | NP_223278 | |
| HELPY_0235 | + | HELPY_0235 | YP_003057042 | Hypothetical protein HELPY_0235 | 29,400 | 2 | 3.78 (2+) | 9.10 | JHP0216 | NP_222937 | |||
| 3.73 (2+) | |||||||||||||
| CB23 | 100 | H | HemE | + | HELPY_0768 | YP_003057507 | Uroporphyrinogen decarboxylase | 38,225 | 6 | 21.24 | JHP0551 | NP_223269 | |
| SodB, SodF | + | HELPY_1036 | YP_003057725 | Superoxide dismutase | 24,416 | 9 | 48.36 | JHP0992 | NP_223709 | ||||
| CB24 | 380 | I | PpiC | + | HELPY_0179 | YP_003056994 | Peptidyl-prolyl cis-trans isomerase C (PPIase) (rotamase); putative signal peptide | 33,847 | 4 | 16.05 | JHP0161 | NP_222882 | |
| PpiC | + | HELPY_0179 | YP_003056994 | Peptidyl-prolyl cis-trans isomerase C (PPIase) (rotamase); putative signal peptide | 33,847 | 8 | 26.76 | JHP0161 | NP_222882 | ||||
| Tpi | + | HELPY_0197 | YP_003057010 | Triose-phosphate isomerase (TIM) | 26,495 | 4 | 17.52 | JHP0180 | NP_222901 | ||||
| CB25 | 60 | I, K | HELPY_1073 | + | HELPY_1073 | YP_003057762 | Putative zinc-containing alcohol dehydrogenase | 38,376 | 6 | 25.86 | JHP1030 | NP_223747 | |
| HELPY_1048c | + | HELPY_1048c | YP_003057737 | Hypothetical protein HELPY_1048 | 25,451 | 2 | 3.71 (2+) | 9.42 | JHP1004 | NP_223721 | |||
| 2.70 (2+) | |||||||||||||
| CB26 | 120 | K | GmhA | + | HELPY_0495 | YP_003057281 | Phosphoheptose isomerase (sedoheptulose-7-phosphate isomerase) | 20,883 | 3 | 19.79 | JHP0791 | NP_223509 | |
| MdaB | + | HELPY_0741 | YP_003057488 | NAD(P)H oxidoreductase (NADPH quinone reductase) | 21,524 | 2 | 5.55 (2+) | 16.49 | JHP0573 | NP_223291 | |||
| 4.67 (2+ | |||||||||||||
| CB27 | 380 | L | HELPY_0235 | + | HELPY_0235 | YP_003057042 | Hypothetical protein HELPY_0235 | 29,400 | 2 | 4.39 (2+) | 9.10 | JHP0216 | NP_222937 |
| 3.97 (2+) | |||||||||||||
| FabI | + | HELPY_0198 | YP_003057011 | Putative enoyl-(acyl-carrier-protein) reductase | 29,868 | 2 | 3.71 (2+) | 8.40 | JHP0181 | NP_222902 | |||
| 3.73 (2+) | |||||||||||||
| UreA | + | HELPY_0069 | YP_003056904 | Urease A subunit | 26,465 | 4 | 17.65 | JHP0068 | NP_222790 | ||||
| CB28 | 130 | L | Icd | + | HELPY_0025 | YP_003056867 | Isocitrate dehydrogenase | 47,361 | 5 | 13.60 | JHP0023 | NP_222745 | |
| FabZ | + | HELPY_1363 | YP_003058021 | (3R)-Hydroxymyristoyl-(acyl-carrier-protein) dehydratase ((3R)-hydroxymyristoyl ACP dehydrase) | 18,079 | 3 | 20.75 | JHP1290 | NP_224008 | ||||
| CB29 | 440 | M | PyrC | + | HELPY_0272 | YP_003057074 | Dihydroorotase (DHOase) | 42,096 | 2 | 2.42 (2+) | 5.04 | JHP0251 | NP_222972 |
| 2.81 (2+) | |||||||||||||
| PyrB | + | HELPY_0369 | YP_003057162 | Aspartate carbamoyltransferase (aspartate transcarbamylase) (ATCase) | 34,004 | 4 | 12.38 | JHP0341 | NP_223060 | ||||
| kDa | Da | % | |||||||||||
| CB30 | 40 | N | DnaK | + | HELPY_0109 | YP_003056934 | Chaperone protein dnaK (heat shock protein 70) (heat shock 70-kDa protein) (HSP70) | 66,920 | 5 | 10.97 | JHP0101 | NP_222822 | |
| DmpI | + | HELPY_0908 | YP_003057631 | Putative tautomerase, DmpI-related protein | 7,366 | 2 | 2.52 (2+) | 38.24 | JHP0858 | NP_223576 | |||
| 3.06 (2+) | |||||||||||||
a Gene product/function and protein molecular mass according to the annotation of the strain B38 available in NCBI.
b Information available in NCBI; see also the revised annotation of strain J99 in Boneca et al. (31).
c According to this annotation, HELPY_1048 was annotated “hypothetical protein” whereas its corresponding protein in the J99 strain, JHP1004, was annotated “predicted DsbC-like protein” by Boneca et al. (31) (http://genolist.pasteur.fr/PyloriGene/genome.cgi).
Membrane Protein Complexes
At the membrane level, 19 and 25 heterooligomeric protein complexes composed of 31 and 41 different proteins were identified for H. pylori B38 (Fig. 1 and Table I complexes named MB) and J99 (Fig. 2 and Table II complexes named MJ) strains, respectively. Although gel profiles remained similar, only seven heterooligomeric complexes were common to both strains: UreB-UreA, HefC-HefB, AtpA-AtpD, AtpD-HELPY_1461 (JHP1381), FrdA-FrdB-FrdC, AlpB-AlpA, and HopF-HopG.
Table I. Description of membrane protein complexes identified in H. pylori strain B38 using two-dimensional BN/SDS-PAGE.
The complexes presented in the table were all localized on two-dimensional BN/SDS-PAGE gels performed in this study and are represented in Fig. 1. Multiprotein complexes isolated from the membrane of the B38 strain were named MB. The experimental approximate molecular mass is given in kDa. GBAN, GenBank accession number (NCBI Reference Sequence); n, the number of peptides; Cov., the protein sequence coverage (percentage) of the peptides.
| Complex no. | Molecular mass | Fig. 1 gel letter | Proteina | Previously identified | Strain B38a |
LC-MS/MS information |
Strain J99b |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HELPY no. | GBAN | Protein annotation | Molecular mass | n | Xcorr | Cov. | JHP no. | GBAN | |||||
| kDa | Da | % | |||||||||||
| MB1 | 800 | A–E | UreB | + | HELPY_0068 | YP_003056903 | Urease B subunit | 61,553 | 10 | 25.30 | JHP0067 | NP_222789 | |
| UreA | + | HELPY_0069 | YP_003056904 | Urease A subunit | 26,465 | 5 | 22.30 | JHP0068 | NP_222790 | ||||
| MB2 | 670 | A, B, E | HefC (AcrB) | + | HELPY_0765 | YP_003057504 | Membrane fusion protein of the hefABC efflux system | 113,657 | 13 | 15.50 | JHP0554 | NP_223272 | |
| HefB (MtrC) | + | HELPY_0766 | YP_003057505 | Outer membrane protein of the hefABC efflux system | 25,898 | 8 | 39.74 | JHP0553 | NP_223271 | ||||
| MB3 | 550 | A, B | AtpA | + | HELPY_1106 | YP_003057791 | ATP synthase F1, subunit α | 54,982 | 14 | 32.01 | JHP1062 | NP_223779 | |
| AtpD | + | HELPY_1104 | YP_003057789 | ATP synthase F1, subunit β (ATPase subunit β) | 51,321 | 17 | 47.55 | JHP1060 | NP_223777 | ||||
| MB4 | 320 | A, B | FrdA | + | HELPY_0195 | YP_003057008 | Fumarate reductase, flavoprotein subunit | 79,968 | 2 | 2.43 (2+) | 2.80 | JHP0178 | NP_222899 |
| 2.73 (2+) | |||||||||||||
| FrdB | + | HELPY_0194 | YP_003057007 | Fumarate reductase, iron-sulfur subunit | 27,480 | 4 | 22.40 | JHP0177 | NP_222898 | ||||
| FrdC | + | HELPY_0196 | YP_003057009 | Fumarate reductase, transmembrane subunit; putative membrane protein | 28,671 | 5 | 17.65 | JHP0179 | NP_222900 | ||||
| MB5 | 260 | A–C | AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 7 | 16.17 | JHP0849 | NP_223567 | |
| AlpA (HopC) | + | HELPY_0897 | YP_003057621 | Outer membrane porin and adhesin HopC; putative signal peptide | 55,942 | 6 | 21.08 | JHP0848 | NP_223566 | ||||
| MB6 | 260 | A, B | HELPY_0130 | + | HELPY_0130 | YP_003056952 | Conserved hypothetical protein; putative signal peptide | 32,642 | 4 | 16.80 | JHP0119 | NP_222840 | |
| HELPY_1147 | + | HELPY_1147 | YP_003057829 | Conserved hypothetical protein; putative signal peptide | 20,532 | 5 | 33.90 | JHP1100 | NP_223817 | ||||
| MB7 | 200 | A, B | AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 8 | 20.30 | JHP0849 | NP_223567 | |
| AlpA (HopC) | + | HELPY_0897 | YP_003057621 | Outer membrane porin and adhesin HopC; putative signal peptide | 55,942 | 6 | 18.60 | JHP0848 | NP_223566 | ||||
| MB8 | 180 | A | HopF (HopX) | + | HELPY_0258 | YP_003057062 | Outer membrane protein HopF; putative signal peptide | 53,024 | 3 | 6.17 | JHP0237 | NP_222958 | |
| HopG (HopY) | + | HELPY_0259 | YP_003057063 | Outer membrane protein HopG; putative signal peptide | 51,724 | 2 | 3.56 (2+) | 7.01 | JHP0238 | NP_222959 | |||
| 3.62 (2+) | |||||||||||||
| MB9 | 200 | A | SabA (HopP) | + | HELPY_0642 | YP_003057401 | Outer membrane protein HopP | 72,465 | 5 | 11.30 | JHP0662 | NP_223380 | |
| HpaA | + | HELPY_0563 | YP_003057334 | Neuraminyllactose-binding hemagglutinin | 28,926 | 4 | 19.60 | JHP0733 | NP_223451 | ||||
| MB10c | 130 | A | AlpA (HopC) | + | HELPY_0897 | YP_003057621 | Outer membrane porin and adhesin HopC; putative signal peptide | 55,942 | 5 | 16.8 | JHP0848 | NP_223566 | |
| HELPY_1043 | − | HELPY_1043 | YP_003057732 | Putative metalloprotease; putative membrane protein | 46,146 | 6 | 16.7 | JHP0999 | NP_223716 | ||||
| S1c | + | HELPY_0856 | YP_003057586 | Putative sodium-and chloride-dependent transporter; putative membrane protein | 49,292 | 1 | 3.46 (1+) | 2.5 | JHP0449 | NP_223167 | |||
| + | HELPY_0133 | YP_003056954 | Amino acid transport protein, HAAAP family; putative membrane protein | 46,190 | 1 | 3.46 (1+) | 2.9 | JHP0121 | NP_222842 | ||||
| MB11 | 120 | A | HorF | + | HELPY_0698 | YP_003057447 | Outer membrane protein HorF | 30,257 | 3 | 10.00 | JHP0614 | NP_223332 | |
| HopE | + | HELPY_0660 | YP_003057417 | Outer membrane protein HopE; putative signal peptide | 29,784 | 4 | 20.10 | JHP0645 | NP_223363 | ||||
| HorL | + | HELPY_1515 | YP_003058154 | Outer membrane protein HorL; putative signal peptide | 26,603 | 4 | 22.30 | JHP1432 | NP_224150 | ||||
| HorH | − | HELPY_0393 | YP_003057185 | Conserved hypothetical protein; putative signal peptide | 27,461 | 5 | 30.50 | JHP1034 | NP_223751 | ||||
| HorC | + | HELPY_0327 | YP_003057128 | Outer membrane protein HorC; putative signal peptide | 27,590 | 3 | 19.18 | JHP0307 | NP_223027 | ||||
| HorE | + | HELPY_0456 | YP_003057244 | Outer membrane protein HorE; putative signal peptide | 20,764 | 4 | 26.88 | JHP0424 | NP_223142 | ||||
| MB12 | 300 | B | HELPY_1462 | + | HELPY_1462 | YP_003058106 | Putative outer membrane transporter; putative signal peptide | 56,824 | 9 | 22.75 | JHP1382 | NP_224100 | |
| NapA | + | HELPY_0248 | YP_003057054 | Neutrophil-activating protein NapA (bacterioferritin) | 16,677 | 3 | 26.39 | JHP0228 | NP_222949 | ||||
| kDa | Da | % | |||||||||||
| MB13 | 80 | B | AtpD | + | HELPY_1104 | YP_003057789 | ATP synthase F1, subunit β (ATPase subunit β) | 51,321 | 19 | 57.80 | JHP1060 | NP_223779 | |
| HELPY_1461 | + | HELPY_1461 | YP_003058105 | Conserved hypothetical protein | 36,038 | 11 | 34.65 | JHP1381 | NP_224099 | ||||
| MB14 | 320 | C, D | BabA (HopS) | + | HELPY_0880 | YP_003057607 | Adhesin; putative signal peptide | 80,815 | 7 | 18.36 | JHP0833 | NP_223551 | |
| SabA (HopP) | + | HELPY_0642 | YP_003057401 | Outer membrane protein HopP | 72,465 | 10 | 24.43 | JHP0662 | NP_223380 | ||||
| MB15 | 240 | C, D | BabA (HopS) | + | HELPY_0880 | YP_003057607 | Adhesin; putative signal peptide | 80,815 | 6 | 12.60 | JHP0833 | NP_223551 | |
| SabA (HopP) | + | HELPY_0642 | YP_003057401 | Outer membrane protein HopP | 72,465 | 13 | 28.24 | JHP0662 | NP_223380 | ||||
| MB16d | 180 | C | BabA (HopS) | + | HELPY_0880 | YP_003057607 | Adhesin; putative signal peptide | 80,815 | 5 | 8.85 | JHP0833 | NP_223551 | |
| HopZd | − | HELPY_0007 | YP_003056850 | Outer membrane protein HopZ; putative signal peptide | 73,043 | 6 | 12.69 | JHP0007 | NP_222729 | ||||
| HopM/Nd | + | HELPY_0231/HELPY_1317 | YP_003057038/YP_003057989 | Outer membrane protein HopM/N; putative signal peptide | 75,857 | 8 | 15.09 | JHP0212/JHP1261 | NP_222933/NP_223979 | ||||
| MB17 | 250 | D, E | AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 4 | 11.47 | JHP0849 | NP_223567 | |
| AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 3 | 6.80 | JHP0849 | NP_223567 | ||||
| MB18 | 210 | D, E | AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 2 | 2.37 (2+) | 4.32 | JHP0849 | NP_223567 |
| 3.87 (2+) | |||||||||||||
| AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 3 | 6.80 | JHP0849 | NP_223567 | ||||
| MB19 | 180 | D, E | AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 4 | 11.17 | JHP0849 | NP_223567 | |
| AlpB (HopB) | + | HELPY_0898 | YP_003057622 | Outer membrane porin and adhesin HopB; putative signal peptide | 57,029 | 5 | 6.20 | JHP0849 | NP_223567 | ||||
| AlpA (HopC) | + | HELPY_0897 | YP_003057621 | Outer membrane porin and adhesin HopC; putative signal peptide | 55,942 | 5 | 16.05 | JHP0848 | NP_223566 | ||||
a Gene product/function and protein molecular mass according to the annotation of strain B38 available in NCBI.
b Information available in NCBI; see also the revised annotation of strain J99 in Boneca et al. (31) (http://genolist.pasteur.fr/PyloriGene/genome.cgi).
c MB10 contains putative additional proteins in the spot number S1: HELPY_0856 (one peptide: K↓DYKDYLTFFEK↓S, ΔM = 0.61503, score = 3.46, z = 2, coverage = 2.5%, p = 1.01e−7) and SdaC (HELPY_0133, one peptide: K↓EGLEGIIIQSLK↓L, ΔM = 1.41851, score = 3.46, z = 2, coverage = 2.9%, p = 5.15e−4).
d MB16 contains a mixture of two proteins (HopZ and HopM/N) in the second spot.
Table II. Description of membrane protein complexes identified in H. pylori strain J99 using two-dimensional BN/SDS-PAGE.
The complexes presented in the table were all localized on two-dimensional BN/SDS-PAGE gels performed in this study and are represented in Fig. 2. Multiprotein complexes isolated from the membrane of the J99 strain were named MJ. The experimental approximate molecular mass is given in kDa. GBAN, GenBank accession number (NCBI Reference Sequence); n, the number of peptides; Cov., the protein sequence coverage (percentage) of the peptides; Pred., predicted.
| Complex no. | Molecular mass | Fig. 2 gel letter | Proteina | Previously identified | Strain J99a |
LC-MS/MS information |
Strain B38b |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JHP no. | GBAN | Protein annotation | Molecular mass | n | Xcorr | Cov. | HELPY no. | GBAN | |||||
| kDa | Da | % | |||||||||||
| MJ1 | 800 | A–C, I, J | UreB | + | JHP0067 | NP_222789 | Urease B subunit | 61,509.5 | 13 | 35.10 | HELPY_0068 | YP_003056903 | |
| UreA | + | JHP0068 | NP_222790 | Urease A subunit | 26,418.4 | 2 | 3.45 (2+) | 8.80 | HELPY_0069 | YP_003056904 | |||
| 3.30 (2+) | |||||||||||||
| MJ2 | 670 | A–C, I, J | HefC | + | JHP0554 | NP_223272 | Cytoplasmic pump proteins of the hefABC efflux system | 113,554 | 4 | 5.00 | HELPY_0765 | YP_003057504 | |
| HefB | + | JHP0553 | NP_223271 | Membrane fusion protein of the hefABC efflux system | 25,896.7 | 6 | 35.90 | HELPY_0766 | YP_003057505 | ||||
| MJ3 | 550 | A, B | AtpA | + | JHP1062 | NP_223779 | Pred. ATP synthase F1 α chain | 55,061.1 | 1c | 3.03 (2+) | 2.20 | HELPY_1106 | YP_003057791 |
| AtpD | + | JHP1060 | NP_223779 | Pred. ATP synthase F1 β chain | 51,270.5 | 2 | 3.40 (2+) | 6.60 | HELPY_1104 | YP_003057789 | |||
| 3.84 (2+) | |||||||||||||
| MJ4 | 500 | A, C, E, G | GroEL, Hsp60 | + | JHP0008 | NP_222730 | Chaperone and heat shock protein | 58,068.6 | 5 | 10.62 | HELPY_0008 | YP_003056851 | |
| Pfr | + | JHP0598 | NP_223316 | Nonheme iron-containing ferritin | 19,170 | 2 | 4.11 (3+) | 17.40 | HELPY_0718 | YP_003057465 | |||
| 3.20 (2+) | |||||||||||||
| MJ5 | 320 | A–C, E, J | FrdA | + | JHP0178 | NP_222899 | Fumarate reductase, flavoprotein subunit | 79,934.3 | 2 | 3.57 (2+) | 4.22 | HELPY_0195 | YP_003057008 |
| 3.99 (2+) | |||||||||||||
| FrdB | + | JHP0177 | NP_222898 | Fumarate reductase, iron-sulfur subunit | 27,473.6 | 4 | 20.80 | HELPY_0194 | YP_003057007 | ||||
| FrdC | + | JHP0179 | NP_222900 | Fumarate reductase, cytochrome b subunit | 28,722.4 | 3 | 10.20 | HELPY_0196 | YP_003057009 | ||||
| MJ6 | 180 | A | HopF (HopX) | + | JHP0237 | NP_222958 | Outer membrane protein HopF | 52,360 | 5 | 12.30 | HELPY_0258 | YP_003057062 | |
| HopG (HopY) | + | JHP0238 | NP_222959 | Outer membrane protein HopG | 51,837.4 | 2 | 3.18 (2+) | 6.30 | HELPY_0259 | YP_003057063 | |||
| 4.08 (2+) | |||||||||||||
| MJ7 | 140 | A, E | HopM/N | + | JHP0212/JHP1261 | NP_222933/NP_223979 | Pred. outer membrane protein HopM/N | 75,476.5 | 2 | 4.72 (2+) | 5.30 | HELPY_0231/HELPY_1317 | YP_003057038/YP_003057989 |
| 5.46 (2+) | |||||||||||||
| BabB | + | JHP1164 | NP_223882 | Pred. adhesin | 76,516.4 | 3 | 7.30 | / | / | ||||
| MJ8 | 200 | A, B, H | AlpB (HopB) | + | JHP0849 | NP_223567 | Outer membrane porin and adhesin | 56,583 | 5 | 12.90 | HELPY_0898 | YP_003057622 | |
| AlpA (HopC) | + | JHP0848 | NP_223566 | Outer membrane porin and adhesin | 56,235 | 10 | 30.96 | HELPY_0897 | YP_003057621 | ||||
| MJ9 | 160 | A, B, F | FixN | − | JHP0132 | NP_222853 | Pred. cytochrome c oxidase heme b and copper-binding subunit | 55,847.7 | 1c | 3.56 (2+) | 3.07 | HELPY_0147 | YP_003056965 |
| FixP | − | JHP0135 | NP_222856 | Pred. cytochrome c oxidase diheme subunit | 32,451.9 | 3 | 12.07 | HELPY_0151 | YP_003056968 | ||||
| FixO | + | JHP0133 | NP_222854 | Pred. cytochrome c oxidase monoheme subunit | 26,421.3 | 3 | 13.00 | HELPY_0149 | YP_003056966 | ||||
| MJ10 | 800 | C | UreB | + | JHP0067 | NP_222789 | Urease B subunit | 61,509.5 | 10 | 30.10 | HELPY_0068 | YP_003056903 | |
| UreA | + | JHP0068 | NP_222790 | Urease A subunit | 26,418.4 | 6 | 26.50 | HELPY_0069 | YP_003056904 | ||||
| Lpp20 | + | JHP1349 | NP_224067 | Membrane-associated lipoprotein | 18,963.8 | 3 | 19.40 | HELPY_1427 | YP_003058075 | ||||
| MJ11 | 300 | C | AlpA (HopC) | + | JHP0848 | NP_223566 | Outer membrane porin and adhesin | 56,235 | 4 | 15.40 | HELPY_0897 | YP_003057621 | |
| HopA | + | JHP0214 | NP_222935 | Porin protein | 52,915.2 | 4 | 11.80 | HELPY_0233 | YP_003057040 | ||||
| HorK (HopW) | + | JHP1394 | NP_224112 | Outer membrane protein HorK | 42,773.8 | 3 | 12.60 | HELPY_1474 | YP_003058118 | ||||
| MJ12 | 280 | C | HofC | + | JHP0438 | NP_223156 | Pred. outer membrane protein HofC | 59,533 | 12 | 17.30 | HELPY_0469 | YP_003057256 | |
| AlpA (HopC) | + | JHP0848 | NP_223566 | Outer membrane porin and adhesin | 56,235 | 4 | 14.20 | HELPY_0897 | YP_003057621 | ||||
| HopA | + | JHP0214 | NP_222935 | Porin protein | 52,915.2 | 9 | 28.80 | HELPY_0233 | YP_003057040 | ||||
| MJ13 | 280 | C | HorF | + | JHP0614 | NP_223332 | Pred. outer membrane protein HorF | 30,130.7 | 4 | 11.90 | HELPY_0698 | YP_003057447 | |
| HorJ | + | JHP1362 | NP_224080 | Outer membrane protein HorJ | 28,089.4 | 4 | 17.30 | HELPY_1441 | YP_003058088 | ||||
| Lpp20 | + | JHP1349 | NP_224067 | Membrane-associated lipoprotein | 18,963.8 | 4 | 24.00 | HELPY_1427 | YP_003058075 | ||||
| kDa | Da | % | |||||||||||
| MJ14 | 80 | D | HopH (OipA) | + | JHP0581 | NP_223299 | Outer membrane protein HopH | 34,144.3 | 3 | 16.61 | HELPY_0733 | YP_003057480 | |
| JHP0368 | − | JHP0368 | NP_223087 | Pred. coding region JHP0368 with no homolog in the databases | 27,117.6 | 2 | 3.16 (2+) | 9.58 | HELPY_0393 | YP_003057185 | |||
| 4.16 (2+) | |||||||||||||
| TatB | − | JHP0365 | 15611433 | Pred. Sec-independent protein translocase protein | 18,267.4 | 2 | 5.18 (2+) | 12.50 | HELPY_0390 | YP_003057182 | |||
| 3.19 (2+) | |||||||||||||
| MJ15 | 80 | D | HopG (HopY) | + | JHP0238 | NP_222959 | Outer membrane protein HopG | 51,837.4 | 3 | 7.43 | HELPY_0259 | YP_003057063 | |
| PpiC | + | JHP0161 | NP_222882 | Pred. peptidyl-prolyl cis-trans isomerase C | 33,885.9 | 2 | 4.23 (2+) | 9.70 | HELPY_0179 | YP_003056994 | |||
| 4.42 (2+) | |||||||||||||
| MJ16 | 80 | D | HopE | + | JHP0645 | NP_223363 | Porin | 29,400.8 | 2 | 4.47 (2+) | 11.10 | HELPY_0660 | YP_003057417 |
| 2.24 (2+) | |||||||||||||
| HorC | + | JHP0307 | 15611376 | Pred. OMP HorC | 27,595.7 | 1c | 4.879 (+) | 3.70 | HELPY_0327 | YP_003057128 | |||
| MJ17 | 50 | D | AtpD | + | JHP1060 | NP_223777 | Pred. ATP synthase F1 β chain | 51,270.5 | 12 | 36.46 | HELPY_1104 | YP_003057789 | |
| JHP1381 | + | JHP1381 | NP_224099 | Pred. secreted protein | 36,071.9 | 3 | 14.29 | HELPY_1461 | YP_003058105 | ||||
| MJ18 | 180 | E | HopM/N | + | JHP0212/JHP1261 | NP_222933/NP_223979 | Pred. outer membrane protein HopM/N | 75,476.5 | 9 | 20.50 | HELPY_0231/HELPY_1317 | YP_003057038/YP_003057989 | |
| BabB | + | JHP1164 | NP_223882 | Pred. adhesin | 76,516.4 | 4 | 9.10 | ||||||
| MJ19 | 130 | E | BabA (HopS) | + | JHP0833 | NP_223551 | Adhesin | 80,420.8 | 6 | 8.90 | HELPY_0880 | YP_003057607 | |
| BabB | + | JHP1164 | NP_223882 | Pred. adhesin | 76,516.4 | 3 | 6.30 | ||||||
| MJ20 | 120 | E | HopG (HopY) | + | JHP0238 | NP_222959 | Outer membrane protein HopG | 51,837.4 | 2 | 2.56 (2+) | 5.10 | HELPY_0259 | YP_003057063 |
| 4.83 (2+) | |||||||||||||
| HopK | − | JHP0857 | NP_223575 | Pred. outer membrane protein | 41,251.5 | 2 | 3.58 (2+) | 8.60 | HELPY_0461 | YP_003057249 | |||
| 4.36 (2+) | |||||||||||||
| MJ21 | 120 | E | HorB | + | JHP0117 | NP_222838 | Pred. outer membrane protein HorB | 31,702.4 | 1c | 3.15 (2+) | 5.90 | HELPY_0126 | YP_003056949 |
| HopE | + | JHP0645 | NP_223363 | Porin | 29,400.8 | 1c | 2.79 (2+) | 5.90 | HELPY_0660 | YP_003057417 | |||
| MJ22 | 320 | H | BabA (HopS) | + | JHP0833 | NP_223551 | Adhesin | 80,420.8 | 4 | 6.50 | HELPY_0880 | YP_003057607 | |
| JHP1100 | + | JHP1100 | NP_223817 | Pred. coding region JHP1100 with no homolog in the databases | 20,693.6 | 2 | 3.12 (2+) | 13.51 | HELPY_1147 | YP_003057829 | |||
| 3.00 (2+) | |||||||||||||
| MJ23 | 250 | H | AlpB (HopB) | + | JHP0849 | NP_223567 | Outer membrane porin and adhesin | 56,583 | 5 | 12.90 | HELPY_0898 | YP_003057622 | |
| HefA | + | JHP0552 | NP_223270 | Outer membrane protein of the hefABC efflux system | 54,458.3 | 2 | 2.38 (2+) | 5.50 | HELPY_0767 | YP_003057506 | |||
| 2.33 (2+) | |||||||||||||
| MJ24 | 80 | H | DagA | − | JHP0877 | NP_223595 | Pred. sodium/d-alanine-glycine symporter | 48,466.3 | 4 | 13.11 | HELPY_0928 | YP_003057646 | |
| HorK (HopW) | + | JHP1394 | NP_224112 | Outer membrane protein HorK | 42,773.8 | 4 | 13.44 | HELPY_1474 | YP_003058118 | ||||
| HorK (HopW) | + | JHP1394 | NP_224112 | Outer membrane protein HorK | 42,773.8 | 3 | 9.82 | HELPY_1474 | YP_003058118 | ||||
| MJ25 | 40 | J | JHP1494 | + | JHP1494 | NP_224212 | Pred. coding region JHP1494 | 28,325 | 6 | 28.85 | HELPY_1592 | YP_003058226 | |
| AtpH | + | JHP1063 | NP_223780 | Pred. ATP synthase F1 δ chain | 20,154.8 | 3 | 20.56 | HELPY_1107 | YP_003057792 | ||||
a Gene product/function and protein molecular mass according to the revised annotation of strain J99 (31) (http://genolist.pasteur.fr/PyloriGene/genome.cgi).
b Information according the annotation of strain B38 available in NCBI.
c Only one peptide was identified in these spots: AtpA (R↓HALIVYDDLSK↓H, ΔM = 0.86875, score = 3.03; z = 2, coverage = 2.20%, p = 2.02e−7); FixN (-↓MQENVPLSYDYSISK↓L, ΔM = 0.94, score = 3.56, z = 2, coverage = 3.07%, p = 2.38e−7); HorC (K↓ALFVDEHEFEIGFK↓F, ΔM = −0.57033, score = 4.89, z = 2, coverage = 5.70%, p = 6.38e−10); HorB (R↓GSFHPSNFQVLVNGGIR↓L, ΔM = 1.05764, score = 3.15, z = 2, coverage = 5.90%, p = 7.85e−4); and HopE (K↓YANGALNGFGLNVGYK↓K, ΔM = 1.35787, score = 2.79, z = 2, coverage = 5.90%, p = 3.89e−7).
New Insight into H. pylori Membrane Illustrated by Four Examples
Examples of complexes are presented below and classified by function. The complexes MB3 and MJ3 comprised the α and β chains (AtpA and AtpD) of the predicted F1 segment of the ATP synthase. In fact, the approximate Mr of 550 kDA observed for this complex would correspond to the entire complex of the ATP synthase. Indeed, the H. pylori ATP synthase is predicted as a multisubunit enzyme comprising the F0 complex (consisting of three subunits, AtpB, AtpF, and AtpE, forming a proton channel), the F1 complex (consisting of five subunits, AtpA, AtpD, AtpG, AtpH, and AtpC, constituting the catalytic site for ATP synthesis), and an additional subunit named AtpX (predicted ATP synthase F0 B′ chain) (42). The presence of this complex in the membrane is not surprising because the β subunit of bacterial ATP synthases exhibits a tight membrane binding property (43), and AtpA was identified previously in the H. pylori membrane (44). Functionally, the H. pylori ATP synthase would be similar to other bacterial ATPases in that it uses the proton motive force generated by the electron transport chain to synthesize ATP (45, 46). AtpA is very frequently recognized by sera from patients with gastric cancer (47), and AtpD has been shown to be immunogenic (48). This protein, never found in the cytoplasm, was also retrieved with the predicted secreted protein JHP1381 (HELPY_1461) in the membrane fractions from both B38 and J99 strains (MB13 and MJ17). The blastp search for JHP1381 (HELPY_1461) revealed a conserved domain both with the EmrA protein involved in the multidrug resistance efflux pump and the subunit MacA of the macrolide-specific ABC-type efflux transporter from E. coli. In this ABC-type efflux transporter (49), MacA is a membrane fusion protein that stimulates the ATPase activity of MacB, a membrane protein that exports macrolide compounds in cooperation with TolC, a multifunctional outer membrane channel (50). Together, all of these observations suggest that JHP1381 is probably involved in efflux resistance and could stimulate the ATPase activity of its MacB counterpart, which is still unidentified in H. pylori. Another probable subunit of the ATP synthase, the predicted ATP synthase F1 δ chain (AtpH), was found in an interaction with JHP1494 (MJ25) (approximate Mr of 40 kDa), an uncharacterized protein conserved in numerous Gram-negative bacilli.
MB2 and MJ2 complexes comprised the efflux pump HefB and HefC whose genes are homologs to E. coli acrA and acrB, which encode membrane fusion and resistance-nodulation-division cytoplasmic pump proteins, respectively (51). The resistance-nodulation-division family of efflux systems is widespread among Gram-negative bacteria. They are associated with bacterial resistance to antibiotics. In E. coli, AcrA exists as a complex with AcrB on the periplasmic surface of the inner membrane (52). In fact, AcrA, AcrB, and TolC of E. coli form a stable intermembrane multidrug efflux complex (53). In H. pylori, the genes coding for this proposed efflux system are composed of HefB, HefC, and HefA subunits (corresponding to the AcrA, AcrB, and TolC complex in E. coli, respectively) and have been shown to be highly conserved in sequence and organization between multiple H. pylori strains and to be expressed both in vivo and in vitro (54). In H. pylori, HefC is involved in energy-dependent multidrug efflux (55), and HefA, a TolC-like protein (56), plays an important role in multidrug resistance (57, 58). MB2 and MJ2 complexes present an approximate Mr of 670 kDa, which would correspond to three homotrimers of each subunit of this proposed efflux system as reported previously in other bacteria (59). Thus, the HefA subunit was probably lost during the purification of the complex. It therefore remains to be determined whether HefA corresponds to TolC in this complex.
Another TolC-like protein was identified in the H. pylori genome corresponding to JHP1382 (HELPY_1462) (56) and was shown to be active in efflux (57). MB12 is composed of HELPY_1462 and the secreted neutrophil-activating protein NapA (60). Therefore, it is tempting to speculate that the outer membrane efflux protein encoded by JHP1382 (HELPY_1462) participates in the secretion of NapA.
Eight membrane complexes comprising orphan proteins were retrieved (MB6, MB10, MB12, MB13, MJ14, MJ17, MJ22, and MJ25). The MB6 complex comprised two proteins whose genes are present in all of the sequenced H. pylori strains and were annotated as predicted coding regions with no homolog in the databases, i.e. HELPY_0130 (JHP0119) and HELPY_1147 (JHP1100), demonstrating that both of these ORFs encode for proteins present in the membrane. Analysis using the STITCH server (33) also revealed possible interactions between these two proteins and five intermediary proteins: four orphan proteins named JHP1044, HELPY_0788 (JHP0534), HELPY_0795 (JHP0527), JHP0526 and FlbA. FlbA is a membrane protein involved in the coordinated expression of the H. pylori flagellar genes, flaA and flaB, and flbA mutants were aflagellate and completely non-motile (61). Using a blastp search, no putative conserved domains were detected for JHP0119, whereas a 56% identity in a 43-residue overlap was revealed with the dynein heavy chain 6 of Tetrahymena thermophila, a free-living ciliate protozoa. Dyneins are molecular motor complexes involved in cilium and flagellum movement (62, 63). Taken together, these results suggest that these two orphan proteins, in particular JHP0119, could play a role in the flagellar function of H. pylori.
Complexes Involved in H. pylori Adherence
Bacterial adherence is considered to have an important role in the colonization of gastric epithelium by H. pylori. Approximately 4% of the H. pylori genome encodes at least 32 OMPs (64), but the role of these individual OMPs in H. pylori adherence is still poorly understood. The main OMPs associated with H. pylori pathogenicity are BabA, SabA, OipA, AlpA, and AlpB, which were all found in different complexes by two-dimensional BN/SDS-PAGE. However, other OMPs, such as HopF (HopX) and the essential OMP for mouse colonization, HopG (HopY) (65), were found together in both strains (MB8 and MJ6). HopG was found also with the predicted OMPs HopK and PpiC in the MJ20 and MJ15 complexes, respectively. HorJ (HopV) interacts with HorF and Lpp20 in the MJ13 complex, and HorK (HopW) is associated with DagA (MJ24) and with both AlpA and HopA (MJ11). All of these complexes contain at least one of four highly conserved OMPs among H. pylori strains, i.e. HopF, HopG, HorJ, and HorK (HorJ and HopF are porins) (66). The expression of these OMPs/porins does not seem to be regulated by phase variation (67), and they are expressed at the surface of all H. pylori strains and appear to be continuously expressed during all stages of H. pylori infection (66). In fact, these four OMPs/porins are immunogenic in mice, and the resulting sera recognize specifically the corresponding proteins and no other member of the OMP family, suggesting that the conserved regions do not contain immunodominant epitopes (66) and may constitute an excellent vaccine target because they seem to be constitutively expressed in H. pylori.
H. pylori porins are weakly expressed compared with those of other bacteria. In addition to HorJ and HopF, HopE, a non-selective porin allowing the passage of hydrophilic substances by general diffusion (68), was found in complexes MB11, MJ16, and MJ21 comprising proteins rarely studied from the hor family: HorB, HorF, HorL, HorH, HorC, and HorE. This can be explained by the fact that HopE forms large channels (68, 186) compared with other porins described in H. pylori or in other Gram-negative bacteria. HopE was shown to be antigenic in humans and immunologically conserved with both patients' sera and specific monoclonal antibodies (68). Among the proteins of MB11, HorH, HorE, and HorF were reported previously to be present in the membrane; HorE and HorF are also immunoreactive (69). All of these newly characterized OMPs and their interacting partners may constitute attractive targets for a vaccine.
The adherence-associated lipoproteins AlpA (HopC) and AlpB (HopB) are encoded by highly homologous genes (64, 67) and were found in the membrane (69). Both lipoproteins are involved in the adherence of H. pylori to the gastric epithelium (70) in a different pattern than that observed for the BabA-mediated adherence, suggesting that a different receptor may be involved (71). AlpA and AlpB are required for gastric colonization (72, 73) and are especially recognized by sera from H. pylori-infected patients (69, 74). In addition, AlpA/B may induce gastric injury by mediating adherence to gastric epithelial cells and by modulating proinflammatory intracellular signaling cascades (73). Both of these lipoproteins were found in interaction in MB5, MB7, MB19, and MJ8 complexes. Furthermore, AlpA and AlpB are both described as outer membrane porins and adhesins, suggesting that they have multiple activities. In fact, they were retrieved from several complexes (MB10, MB17, MB18, MJ11, MJ12, and MJ23), either alone or together, in association with different OMPs, such as HopA (MJ12); the essential OMP for colonization, HofC (65); HorK; and JHP0999 (HELPY_1043), a putative metalloprotease/putative membrane protein, showing the importance of AlpA and AlpB for the bacteria.
Examples of Membrane Complexes Retrieved from Only One Strain
The proinflammatory OMP OipA (HopH) (75), an adhesin involved in cytoskeleton reorganization (76), was only retrieved from the J99 strain (MJ14), interacting with the predicted Sec-independent protein translocase protein TatB and JHP0368, whose gene was annotated as a predicted coding region with no homolog in the databases. This result is not surprising because the B38 strain has a non-functional oipA status.
The major H. pylori adhesin is BabA (HopS), which binds to the fucosylated Lewis b blood group antigen (77) and has a closely related paralog, BabB (HopT), whose function has not yet been determined. BabA and BabB are associated in the complex MJ19, which is undoubtedly specific for J99 strain because the B38 strain does not express BabB. BabB was also found to interact with HopM/N (MJ18) in the J99 strain. In the B38 strain (MB16), BabA interacts with HopM/N as well as with the predicted coding region JHP1100 (MJ22), a protein with no homolog in the databases recently reported to be present and immunoreactive in the H. pylori membrane (69). Recent studies showed that neither BabA nor BabB could induce an immune response in monkeys (78) and that BabA and BabB were not immunodominant antigens in humans (48, 79). One hypothesis is that the proteins interacting with them in the membrane could mask the BabA epitope and could consequently be exposed and therefore be antigenic; this is probably the case of HopM/N previously shown to be immunoreactive to sera from H. pylori-positive patients (44, 80). These BabA/BabB-interacting proteins (JHP1100, HopM/N, and HopZ) represent potential antigen targets for the development of an H. pylori vaccine. In the current study, BabA was also associated with SabA in different oligomerization states in the B38 strain (MB14 and MB15); this complex was never retrieved from the J99 strain. SabA (HopP) is the second most well characterized adhesin of H. pylori; it binds to sialylated Lewis X antigens and is up-regulated during persistent H. pylori infection (81), strengthening the epithelial attachment necessary to achieve successful colonization (82). These BabA-SabA complexes could be potentially implicated in development of malignant diseases because each protein has been associated with gastric cancer (83–86). Indeed, SabA anchors to cellular receptors (81) considered as tumor antigens (87) and gastric dysplasia markers (88). Furthermore, a recent study has shown that BabA-positive strains were associated with an intercellular localization of the bacterium, intestinal metaplasia, and degenerative alterations observed on gastric biopsies (89). Thus, BabA-SabA association could permit strains expressing this complex to reach the intercellular compartment and to persist between host cells even during the development of a malignant disease. The sabA gene is among the most divergent genes in the H. pylori genome (90), and its “on”/”off” expression is regulated by phase variation (67). Although a non-functional status was found for the sabA gene in the B38 strain (11), the corresponding protein is undoubtedly synthesized because it was identified in two complexes. This is not surprising because the SabA expression is frequently switched on or off both in vitro (91) and in vivo (16).
Although never retrieved from the J99 strain, SabA appears in three complexes in the B38 strain (MB14 and MB15) and also in association with the neuraminyllactose-binding hemagglutinin HpaA (MB9), an antigenic lipoprotein present in the flagella sheath of H. pylori and expressed in all strains (69, 92).
Cytosolic Protein Complexes
At the cytosol level, 30 and 16 heterooligomeric protein complexes composed of 47 and 27 different proteins were identified in the H. pylori B38 (Fig. 3 and Table III, complexes named CB) and J99 (Fig. 4 and Table IV, complexes named CJ) strains, respectively. Only five complexes were common to both strains: UreB-GroEL-UreA, GatA-GatB-DnaN, DnaK-GroEL, PurD-PurD, and HELPY_0317 (JHP0295)-FabZ.
Table IV. Description of cytosolic protein complexes identified in H. pylori strain J99 using two-dimensional BN/SDS-PAGE.
The complexes presented in the table were all localized on two-dimensional BN/SDS-PAGE gels performed in this study and are represented in Fig. 4. Multiprotein complexes isolated from the cytosol of the J99 strain were named CJ. The experimental approximate molecular mass is given in kDa. GBAN, GenBank accession number (NCBI Reference Sequence); n, the number of peptides; Cov., the protein sequence coverage (percentage) of the peptides; Pred., predicted.
| Complex no. | Molecular mass | Fig. 4 gel letter | Proteina | Previously identified | Strain J99a |
LC-MS/MS information |
Strain B38b |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JHP no. | GBAN | Protein annotation | Molecular mass | n | Xcorr | Cov. | HELPY no. | GBAN | |||||
| kDa | Da | % | |||||||||||
| CJ1 | 800 | A, B, D | UreB | + | JHP0067 | NP_222789 | Urease B subunit | 61,509.5 | 13 | 35.10 | HELPY_0068 | YP_003056903 | |
| GroEL | + | JHP0008 | NP_222730 | Chaperone and heat shock protein | 58,068.6 | 23 | 45.10 | HELPY_0008 | YP_003056851 | ||||
| UreA | + | JHP0068 | NP_222790 | Urease A subunit | 26,418.4 | 12 | 41.60 | HELPY_0069 | YP_003056904 | ||||
| CJ2 | 110 | A | GatB | + | JHP0603 | NP_223321 | Pred. Glu-tRNA(Gln) amidotransferase subunit B | 52,934.9 | 5 | 12.90 | HELPY_0713 | YP_003057460 | |
| GatA | + | JHP0769 | NP_223487 | Pred. Glu-tRNA(Gln) amidotransferase subunit A | 49,584.7 | 2 | 2.96 (2+) | 8.20 | HELPY_0524 | YP_003057302 | |||
| 3.04 (2+) | |||||||||||||
| DnaN | + | JHP0452 | NP_223170 | Pred. DNA polymerase III β subunit | 41,888.1 | 2 | 2.73 (2+) | 5.60 | HELPY_0852 | YP_003057584 | |||
| 2.78 (2+) | |||||||||||||
| CJ3 | 100 | A | SodB, SodF | + | JHP0992 | NP_223709 | Superoxide dismutase | 24,384.7 | 8 | 41.30 | HELPY_1036 | YP_003057725 | |
| JHP0631 | + | JHP0631 | NP_223349 | Pred. coding region JHP0631 | 19,527.2 | 2 | 3.56 (2+) | 15.70 | HELPY_0673 | YP_003057426 | |||
| 3.82 (2+) | |||||||||||||
| CJ4 | 80 | A | DnaK | + | JHP0101 | NP_222822 | Chaperone and heat shock protein 70/DnaK | 66,943.4 | 2 | 4.30 (2+) | 4.70 | HELPY_0109 | YP_003056934 |
| 3.98 (2+) | |||||||||||||
| GroEL | + | JHP0008 | NP_222730 | Chaperone and heat shock protein | 58,068.6 | 8 | 15 | HELPY_0008 | YP_003056851 | ||||
| CJ5 | 60 | A | Tfs | + | JHP1444 | NP_224162 | Pred. translation elongation factor Ts | 39,700.6 | 2 | 2.50 (2+) | 6.50 | HELPY_1558 | YP_003058194 |
| 2.39 (2+) | |||||||||||||
| TsaA | + | JHP1471 | NP_224189 | Pred. alkyl hydroperoxide reductase | 22,112.8 | 7 | 42.90 | HELPY_1565 | YP_003058201 | ||||
| CJ6 | 50 | A | TufA | + | JHP1128 | NP_223846 | Pred. translation elongation factor Tu | 43,568.9 | 6 | 18.30 | HELPY_1179 | YP_003057859 | |
| Pfs | + | JHP0082 | NP_222804 | Pred. 5′-methylthioadenosine nucleosidase/S-adenosylhomocysteine nucleosidase | 24,872 | 5 | 23.90 | HELPY_0086 | YP_003056917 | ||||
| CJ7 | 150 | B | PorA | + | JHP1037 | NP_223754 | Pyruvate ferredoxin oxidoreductase, α subunit | 44,599.6 | 3 | 12.00 | HELPY_1079 | YP_003057768 | |
| RfaD | + | JHP0793 | NP_223511 | Pred. ADP-l-glycero-d-mannoheptose-6-epimerase | 37,196.4 | 6 | 21.90 | HELPY_0493 | YP_003057279 | ||||
| CJ8 | 110 | B | TipA, YihK | + | JHP0432 | NP_223150 | Pred. GTP-binding protein of the TypA subfamily | 66,471.4 | 8 | 13.00 | HELPY_0464 | YP_003057252 | |
| AspA | + | JHP0594 | NP_223312 | Pred. aspartate ammonia-lyase | 51,771 | 5 | 12.20 | HELPY_0722 | YP_003057469 | ||||
| CJ9 | 40 | B | PurD | + | JHP1140 | NP_223858 | Pred. glycinamide ribonucleotide synthetase | 47,348 | 3 | 7.10 | HELPY_1194 | YP_003057869 | |
| PurD | + | JHP1140 | NP_223858 | Pred. glycinamide ribonucleotide synthetase | 47,348 | 3 | 10.10 | HELPY_1194 | YP_003057869 | ||||
| CJ10 | 150 | C | PorG | + | JHP1035 | NP_223752 | Pyruvate ferredoxin oxidoreductase, γ subunit | 20,892 | 2 | 3.59 (2+) | 10.80 | HELPY_1077 | YP_003057766 |
| 2.50 (2+) | |||||||||||||
| RfaD | + | JHP0793 | NP_223511 | Pred. ADP-l-glycero-d-mannoheptose-6-epimerase | 37,196.4 | 5 | 16.10 | HELPY_0493 | YP_003057279 | ||||
| CJ11 | 130 | C | FabG | + | JHP0508 | NP_223226 | Pred. 3-ketoacyl-acyl carrier protein reductase | 26,446.1 | 3 | 12.60 | HELPY_0816 | YP_003057552 | |
| SodB, SodF | + | JHP0992 | NP_223709 | Superoxide dismutase | 24,384.7 | 4 | 18.30 | HELPY_1036 | YP_003057725 | ||||
| CJ12 | 100 | C | DnaK | + | JHP0101 | NP_222822 | Chaperone and heat shock protein 70 | 66,943.4 | 10 | 21.00 | HELPY_0109 | YP_003056934 | |
| HemE | + | JHP0551 | NP_223269 | Pred. uroporphyrinogen decarboxylase | 38,211.5 | 3 | 9.70 | HELPY_0768 | YP_003057507 | ||||
| CJ13 | 440 | D | JHP0632 | + | JHP0632 | NP_223350 | Pred. N-methylhydantoinase | 86,415.6 | 19 | 26.50 | HELPY_0674 | YP_003057427 | |
| HyuAc | + | JHP0633 | NP_223351 | Pred. N-methylhydantoinase | 78,148 | 3 | 5.80 | ||||||
| JHP0631 | + | JHP0631 | NP_223349 | Pred. coding region JHP0631 | 19,527.2 | 2 | 4.37 (2+) | 15.70 | HELPY_0673 | YP_003057426 | |||
| 4.54 (2+) | |||||||||||||
| CJ14 | 130 | D, F | JHP0295 | + | JHP0295 | NP_223015 | Pred. coding region JHP0295 | 33,489.5 | 8 | 43.30 | HELPY_0317 | YP_003057118 | |
| FabZ | + | JHP1290 | NP_224008 | Pred. (3R)-hydroxymyristoyl-(acyl-carrier-protein) dehydratase | 18,067.8 | 4 | 25.80 | HELPY_1363 | YP_003058021 | ||||
| CJ15 | 120 | E | Icd | + | JHP0023 | NP_222745 | Pred. isocitrate dehydrogenase | 47,298.8 | 6 | 13.18 | HELPY_0025 | YP_003056867 | |
| FabG | + | JHP0508 | NP_223226 | Pred. 3-ketoacyl-acyl carrier protein reductase | 26,446.1 | 6 | 24.30 | HELPY_0816 | YP_003057552 | ||||
| kDa | Da | % | |||||||||||
| CJ16 | 120 | G | Icd | + | JHP0023 | NP_222745 | Pred. isocitrate dehydrogenase | 47,298.8 | 15 | 41.90 | HELPY_0025 | YP_003056867 | |
| FabI | + | JHP0181 | NP_222902 | Pred. enoyl-(acyl-carrier-protein) reductase (NADH) | 29,874.6 | 10 | 33.09 | HELPY_0198 | YP_003057011 | ||||
a Gene product/function and protein molecular mass according to Boneca et al. (31) (http://genolist.pasteur.fr/PyloriGene/genome.cgi).
b Information according the annotation of strain B38 available in NCBI.
c The gene corresponding to HyuA (JHP0633) is not found in the B38 strain; only three “pseudogenes” correspond to HyuA: HELPY_0677 (Part 1), HELPY_0676 (Part 2), and HELPY_0675 (Part 3).
New Insight into H. pylori Cytoplasm Illustrated by Six Examples
In this study, most of the complexes identified in the cytoplasm contained proteins suspected to be involved in metabolism, which is a prerequisite for virulence. For example, the CB29 complex comprised two proteins predicted to catalyze the second and third steps in the de novo pyrimidine biosynthesis pathway, aspartate carbamoyl transferase (PyrB) and dihydroorotase (PyrC), respectively (93). This complex would allow the transformation of carbamoylphosphate into dihydroorotic acid during the de novo synthesis of UTP and CTP (94).
The major route for the generation of acetyl coenzyme A in H. pylori is via the pyruvate:flavodoxin oxidoreductase (POR), an essential heterotetrameric complex composed of PorA, PorB, PorC (ex-PorG), and PorD (95, 96), which has also been implicated in metronidazole resistance (95, 97). Although PorA, PorB, and PorC subunits were found previously using two-dimensional BN/SDS-PAGE (28), modifications were made during sample preparation that did not allow us to retrieve the POR complex. However, PorA and PorC were found separately in 150-kDa complexes with RfaD (CJ7 and CJ10), a “predicted ADP-l-glycero-d-mannoheptose 6-epimerase”. ADP-l-Glycero-d-mannoheptose 6-epimerase is the last enzyme in the pathway for synthesis of ADP-heptose, a precursor of core lipopolysaccharide in Gram-negative bacteria (98). Thus, the interaction of RfaD with PorA and PorC suggests a new activity related to the virulence of H. pylori POR.
Several complexes contain proteins predicted to be involved in fatty acid biosynthesis (FAS). The FAS system is divided into two different pathways named FAS I and FAS II based on the architecture of the enzymes involved. In contrast to the large multifunctional enzymes with multiple domains that catalyze various reactions of the FAS I pathway in fungi and mammals, FAS enzymes for bacteria belong to the FAS II pathway where the acyl chain covalently attached to the acyl carrier protein (ACP) is elongated with five enzymes catalyzing consecutively. Thus, the enzymes involved in the FAS II pathway represent a validated yet unexploited and very promising target for antibacterial agent development (99, 100). In H. pylori, the elongation phase of fatty acid biosynthesis could imply FabF, FabH, FabG, FabI, and FabZ (94). FabG, FabI, and FabZ were found in 10 different complexes (CB17, CB18, CB27, CB28, CB7, CB8, CJ11, CJ14, CJ15, and CJ16). Each of these three enzymes was found with the predicted isocitrate dehydrogenase Icd (CB28, CJ15, and CJ16), suggesting that the enzymes of the FAS II pathway are closely linked with Icd. Such a link was demonstrated in Saccharomyces cerevisiae (101, 102) in which Icd provides NADPH for β-oxidation of polyunsaturated fatty acids. Similarly, Icd could play a role in fatty acid biosynthesis of H. pylori. Complexes including orphan proteins associated with enzymes of the FAS II pathway were found: FabG interacts with HELPY_0235 (JHP0892) in CB27, and FabZ is associated with HELPY_0317 (JHP0295) both in B38 and J99 strains (CB17 and CJ14), suggesting a possible role of these two orphan proteins in FAS. FabZ is an important enzyme for the elongation cycles of both saturated and unsaturated fatty acids in the FAS II pathway. With regard to H. pylori, FabZ was shown to be immunoreactive (69), and its recent x-ray crystal structure revealed that it maintains its unique features and suggests that it could be inhibited either by occupying the entrance of the tunnel or plugging the tunnel to prevent the substrate from accessing the active site (103). FabZ was also found in complex CB7 with FabI, a predicted enoyl-ACP reductase (NADH) catalyzing the reduction of the enoyl-ACP resulting from the FabZ reaction. FabI is highly conserved and widely expressed among bacteria with only a single known isoform. This reductase is essential for the bacterial viability of E. coli (104) and now appears to be an excellent target for the development of narrow spectrum antimicrobial agents that selectively target pathogens, such as Mycobacterium tuberculosis (105, 106) or multidrug-resistant Staphylococcus aureus (107). The presence of multiple targets in the FAS II pathway presents the possibility of developing synergistic chemotherapeutic regimes that could intervene simultaneously at multiple points in the biosynthesis of fatty acids (100). Moreover, the identification of certain multiprotein complexes with enzymes involved in FAS II pathway should help in developing new therapeutic strategies by inhibiting the formation of these complexes. Thus, the partners of FabZ and/or FabI could also be targets for antibacterial drugs.
GmhA, a predicted phosphoheptose isomerase involved in the biosynthesis of inner core lipopolysaccharides, was found to be associated with MdaB (CB26), a predicted modulator of drug activity and an important enzyme to fight against oxidative stress (108), suggesting that this complex may be involved in drug resistance.
The essential thioredoxin system of H. pylori comprises thioredoxin Trx1 (TrxA; JHP0763) and thioredoxin reductase TrxR1 (TrxB; JHP0764) (109). Trx1 and TrxR1 demonstrate specialized catalytic properties because both form a reductase system for H. pylori TsaA/AhpC (110). Trx1 is considered as a stress response element in H. pylori as its expression increases dramatically under conditions of oxidative stress (109). Trx1 also acts as an arginase chaperone capable of renaturing the enzyme to a catalytically active state (111). Another predicted thioredoxin reductase, TrxR2 (also named FqrB), was demonstrated to exhibit an NADPH oxidoreductase activity that is part of the pyruvate:ferredoxin oxidoreductase complex (112). Trx1 and TrxR2 were found in complex CB21, suggesting that these enzymes really have multiple functions in the bacteria: TrxR2 could also be implicated in the H. pylori thioredoxin system, or conversely, Trx1 could be implicated in the pyruvate:ferredoxin oxidoreductase activity.
Chaperones constitute a functionally related group of proteins increasingly synthesized under heat shock conditions to prevent protein aggregation (113), thus protecting the cell from damage caused by the formation of improperly folded polypeptides (114). Many heat shock proteins play a key role in cellular metabolism under all growth conditions, assisting the folding, assembly, and translocation of cellular proteins (115–117). The presence of DnaK (Hsp70) with TsaA (CB20) is not really surprising because TsaA was shown to switch from a peroxide reductase to a stress-dependent molecular chaperone function (118). The best studied examples of such “molecular chaperones” include the ubiquitous GroEL (Hsp60) and DnaK proteins. In H. pylori, the most prominent chaperone is GroEL and its co-chaperone GroES (119), both immunogenic and present in the structure-bound and soluble fractions (48, 120). In the present study, GroEL and DnaK were found in complex CJ4. It was reported recently that the two eukaryotic homologs of these chaperones are able to interact to form a stable complex (121), whereas no similar interaction seems to occur between their prokaryotic counterparts, GroEL and DnaK. However, both of these chaperones are up-regulated by cadmium in Rhodobacter capsulatus (122), and they cooperate in their chaperone functions in E. coli (123). Moreover, their transcription is negatively regulated by the same repressor in H. pylori (124). DnaK was also found with DmpI, a putative tautomerase (CB30), and with HemE, a predicted uroporphyrinogen decarboxylase (CJ12).
Examples of Cytosolic Complexes Retrieved from Only One Strain
The complex including JHP0631, JHP0632, and JHP0633 (HyuA) (28) was again isolated in the J99 strain but not in the B38 strain (CJ13). This was predictable because the protein corresponding to JHP0633 is absent in strain B38. JHP0632 and JHP0633 are annotated as predicted N-methylhydantoinase, and JHP0631 is annotated as a predicted coding region. However, JHP0631, JHP0632, and JHP0633 show homologies with the γ (AcxC), α (AcxB) and β (AcxA) subunits, respectively, of the acetone carboxylase (ACX) of numerous bacteria (Burkholderia species, Thauera species, Ralstonia species, Xanthobacter autotrophicus, etc.) (125). The approximate Mr of 440 kDa observed for CJ13 is relatively close to that observed for the heterohexameric ACX complex of X. autotrophicus, comprising three different polypeptides with Mr values of 86 kDa, 78 kDa, and 19 kDa arranged in an α2 β2 γ2 quaternary structure (126). In H. pylori, the proposed pathway for the conversion of acetone to acetyl-CoA involves three steps. First, ACX (composed of JHP0632, JHP0633, and JHP0631) is functional and may catalyze the conversion of acetone to acetoacetate. The acetoacetate is subsequently converted into acetoacetyl-CoA by the succinyl-CoA-transferase complex, ScoA/B (JHP0636/JHP0637), and finally transformed by FadA (JHP0638) into two molecules of acetyl coenzyme A that would feed into the TCA cycle to provide energy for the bacteria (125). The fact that two complexes involved in the first two steps of acetone utilization (ACX and ScoA/B complexes) were purified from the J99 strain (28) but never retrieved from the B38 strain is an argument in favor of the specificity of ACX toward the J99 strain. In addition, the transcriptional regulator JHP0403 was shown to strongly activate the transcription of the acxABC and scoAB gene cluster (127). ACX is expressed during infection (127) and enhances the ability of H. pylori to colonize the mouse stomach (125). Indeed, strain B38, which should be defective for ACX activity, hardly colonizes the mouse.2 In fact, the acxA gene seems to be absent in strain B38 because it would be truncated into three parts, corresponding to the pseudogenes annotated helpy_0675, helpy_0676, and helpy_0677, localized at the acxBC locus (helpy_0674/helpy_0673). The fact that JHP0631 (AcxC) was retrieved from another complex (CJ3) with SodB, a superoxide dismutase involved in detoxification and oxidative stress resistance, suggests multiple functions for this protein and could explain why its corresponding gene has been conserved in the genome of B38 unlike acxA.
Complex CB5, comprising NapA and the predicted ribosomal protein L7/L12 (RpL7/L12), was only retrieved from the B38 strain. Both of these proteins were reported to be among the 20 most abundant proteins in H. pylori and to be antigenic (48, 79, 119, 128, 129). NapA was found in the cytoplasm (120) and in the membrane (69) and is also secreted (60). RpL7/L12 was found both in the cytoplasm and membrane fractions (120, 130). A recent study showed that RpL7/L12 is overexpressed in LG-MALT-associated strains when compared with DU-associated strains, suggesting that RpL7/L12 could be used as a biomarker for the differential diagnosis of H. pylori-associated clinical outcomes (17). RpL7/L12 was also reported previously to be overexpressed in gastric adenocarcinoma-associated strains (131). Furthermore, NapA was recently proposed as a novel up-regulated biomarker in strains associated with gastric cancer (132) that may play a role in the development of gastric carcinoma (133). Thus, the RpL7/L12-NapA complex would be potentially implicated in the occurrence of gastric cancer. However, it was not possible to determine whether this complex is really specific to the B38 strain associated with LG-MALT because this complex could not be detected in the J99 strain where the RpL7/L12 protein is known to be expressed in smaller quantities (17). Moreover, it was recently demonstrated that NapA is able to prolong the lifespan of monocytes and neutrophils (134), which would contribute to the development of a malignant disease like LG-MALT. This NapA-RplL7/L12 complex is of particular interest because both partners could play a role in the development of LG-MALT. Further studies are necessary to determine the exact role of this complex and of the Rpl7/L12 overexpression in malignant strains.
DISCUSSION
Although H. pylori infection is one of the most common bacterial infections worldwide with up to half of the world's population infected (1), questions still remain concerning the evolution of this infection toward gastroduodenal pathologies and especially toward the development of LG-MALT. The fact that it is possible to cure this lymphoma by an antibiotic-based eradication treatment of H. pylori suggests an important role of the bacterium in the development of this particular cancer. However, no current known H. pylori virulence factors could be associated with the development of this lymphoma (10, 11).
Studies on the H. pylori proteome have intensified in recent years, and various strategies have already been applied to examine the H. pylori proteome, such as the yeast two-hybrid method (135–138), two-dimensional electrophoresis (119, 120, 129, 139–145), tandem affinity purification (146), and in silico analyses (147–150). The extracellular proteome from H. pylori was also investigated (60, 151). Several studies examined the proteome of strains associated with different pathologies (47, 48, 79, 80, 128, 131, 143, 152–155), most of them based on immunoproteomics methods. However, only one of these studies included strains associated with LG-MALT (17).
Because the identification of protein complexes is an important step in interpreting protein-protein interaction data, the two-dimensional BN/SDS-PAGE method combined with mass spectrometry has renewed interest because it can be applied to the study of the whole complexome of an organism (24–28). In the current study, this method was used to study the complexome of two H. pylori sequenced strains, B38 associated with LG-MALT and J99 associated with DU.
Among the 329 proteins identified, only 145 could be grouped in 90 complexes. Many proteins could not be attributed to complexes for different reasons. In some cases, some visible intense spots could not be identified by LC-MS/MS. Indeed, silver staining is not a quantitative method, and the intensity of a spot does not reflect the quantity of proteins present unlike colloidal blue staining. In addition, some proteins are sometimes poorly ionized during the ionization process before mass spectrometry and therefore cannot be identified. Furthermore, different proteins have sometimes been identified in a single spot, and therefore, the Schägger et al. (20, 34) criterion of the “same shape” was not applicable. This is a limit to the method. Moreover, a poor denaturation before the second dimension sometimes occurs and can lead to the identification of a mixture of proteins in the same spot. This descriptive study has nevertheless allowed the identification of genes expressed in vitro in H. pylori and novel complexes that had not yet been described. The main difficulty encountered during this study was to assign the specificity of the complex to a particular strain because subunits or whole complexes can be lost during the sample preparation or can be hardly visible on the gels, which is the case for complexes whose subunits have a low intensity. It was not possible with such a method to achieve a quantitative complexomics study. However, some complexes specific to each strain could be easily described because some of these proteins do not exist in one strain as is the case with HopM/N-BabB and AcxA/B/C, which are specific to the J99 strain. Complexes retrieved from only one strain, such as BabA-SabA and NapA-RplL7/L12 isolated in the LG-MALT strain, open new fields of research to explore the implication of H. pylori in the development of LG-MALT.
Among the 90 heterooligomeric complexes identified in this study (49 in the B38 strain and 41 in the J99 strain), only seven membrane and five cytosolic protein complexes were common to both strains. This result is not surprising because of the huge genetic variability among H. pylori strains. In fact, based on the study of a low number of strains, it appears that 200–400 genes would be variably present in each strain, giving a core of ∼1,100–1,300 genes (156–158). However, as mentioned previously, the eventual loss of certain complexes during the preparation steps of the samples must be kept in mind.
The relevance to study complexes from strain B38 is that it is a type II strain (lacking the cag PAI), whereas many proteomics studies were performed on type I strains (28, 48, 69, 79, 119, 120). However, the choice to study complexes of a type II strain in LG-MALT is relevant because the presence of the cag PAI is not associated with strains isolated from patients with LG-MALT (10, 11). Interestingly, no proteins of the cag PAI were identified in the J99 strain, a type I strain, and the absence of cag PAI proteins does not seem to modify the complexome, especially in the membrane fraction where some of these proteins are expected to be localized (159). In fact, few studies have reported the presence of cag PAI proteins at the membrane level or in culture supernatants (28, 60). Indeed, these proteins are obviously produced in amounts that are below the detection limit of the applied method and are rather detected by immunoblotting (48, 129, 140, 141). Moreover, it was suggested that some proteins of the type IV secretion system contributing to virulence may not be expressed under in vitro culture conditions; rather their expression may be dependent on in vivo stimuli such as bacterium-host cell contact (160, 161).
In most genomes, ∼20–30% of all genes encode membrane proteins (162), and because of their diverse functionality (163–165), they provide one of the most important target groups for drug design (163, 166). However, because of their innate hydrophobic and amphiphilic nature, their low abundance, and their general instability under diverse conditions of purification, membrane proteins are often difficult to purify, produce, and analyze, and as a result, their characterization by proteomics analyses and structural studies has been inadequate (167). Numerous membrane proteins belong to complexes involved in important cellular functions, such as the regulation of energy metabolism, protein trafficking, transport of molecules, and adherence (162, 168). H. pylori virulence is due to unique soluble proteins and membrane proteins that allow its survival at acidic pH (169) and successful colonization of the gastric mucosa (65). Most of the reported virulence factors of H. pylori are in relation to the membrane because they are 1) secreted, such as urease (170) or the VacA cytotoxin; 2) directly associated with the membrane, such as BabA, HopQ, HopZ, OipA, and SabA OMP; or 3) translocated into infected epithelial cells by the type IV secretion system as is the case for CagA. In addition, many orphan genes of H. pylori are believed to be associated with the membrane, i.e. putative adhesins, lipoproteins, and other OMPs (30, 31, 64, 67, 171). The identification of proteins that are part of complexes in the H. pylori membrane contributes to the elucidation of the membrane function; the challenge is to propose a function for ORFs for which no data are available and, in particular, to identify new virulence factors perhaps hitherto unsuspected. This study suggests a role for some proteins, such as JHP0119 (HELPY_0130), which may play a role in the flagellar function, or isocitrate dehydrogenase, which may have a role in fatty acid biosynthesis.
Some proteomics studies have been carried out to study the membrane of H. pylori (69, 120, 139, 142, 172, 173), but few membrane complexes have yet been described (28, 174–177), whereas the yeast two-hybrid method allowed the description of a large set of interactions (135) with a reliability of ∼50% (178). Because of their diverse functionality (163–165), membrane proteins provide one of the most important target protein groups for drug design (163, 166). This study allowed the description of 25 and 19 membrane complexes in the B38 and J99 strains, respectively; some of them were found several times. Most of the proteins identified in this study were reported previously to be associated with the membrane (28, 44, 69, 120, 128, 135, 139), validating the membrane sample preparation. Some proteins, such as HopZ and NapA, were reported to be present both in the extracellular compartment (60) and in the membrane (69, 179), indicating the possibility of variable localizations of these proteins. The membrane complexes reported here, comprising numerous proteins involved in H. pylori adherence, such as the major adhesins BabA and SabA, the lipoproteins AlpA and AlpB, and numerous porins, are reported to be weakly expressed. In various studies aiming to design a vaccine against H. pylori, researchers looked for surface-exposed and/or antigenic proteins (47, 48, 79, 128, 139, 142, 152, 154, 155, 180). Among the antigenic proteins reported in H. pylori, most correspond to housekeeping enzymes rather than to antigens associated with the cell envelope (48). Attempts were made to develop a vaccine against H. pylori with candidate antigens such as urease (181), catalase (182), and CagA and VacA cytotoxins (183). However, none of these vaccines showed satisfactory protection against the infection. Because the OMP family of H. pylori is a very particular family, these OMPs constitute attractive targets for the design of a vaccine. All of the newly characterized OMPs and their interacting partners give new insight into membrane structure. However, a number of the genes encoding these OMPs undergo phase variations in their 5′ region, and therefore, not all strains produce functional proteins (64). To solve this problem, different strategies could be developed. First, conserved regions exist in different H. pylori OMPs (64) and could serve as vaccine targets. Indeed, a recombinant protein constructed from a conserved domain of BabA, AlpA, AlpB, and HopZ was shown to be specifically recognized by the patients' sera (184, 185). A second possibility would be to consider a vaccine targeting several OMPs. Indeed, some H. pylori OMPs are highly specific to H. pylori and would represent potential antigen targets, such as the surface-exposed HorE, HorF, and HopE proteins and their partners, i.e. HorC, HorH, and HorL, as well as the BabA-interacting proteins, i.e. SabA, HopM/N, and HopZ. Otherwise, six (HopA, HopE, HopM/N, FrdA, PyrC, and PpiC) of the 14 best candidate antigens to develop a vaccine against H. pylori (80) were identified, and their partners could also represent new targets. In addition, the characterization of the functions of individual H. pylori OMPs may provide further insight into essential mechanisms for H. pylori colonization and persistence in the human gastric mucosa.
With regard to the cytosol, only 46 complexes were identified, whereas 41 were retrieved from the membranes where ∼300 proteins are expected to be, showing that two-dimensional BN/SDS-PAGE is better suited to the study of membrane complexes. Most of the proteins identified in the cytosol corresponded to proteins involved in H. pylori physiology, i.e. glycolysis, tricarboxylic acid cycle, fatty acid biosynthesis, de novo purine and pyrimidine biosynthesis, amino acid biosynthesis, catabolic pathway of aromatic compounds, LPS biosynthesis, and translation. Enzymes involved in H. pylori metabolism whose structure is very different from their eukaryotic counterparts are very promising targets for the development of new antibacterial molecules. Actually, numerous studies are focusing on such novel targets (99, 100), and it is conceivable to simultaneously target different pathways of bacterial metabolism, a strategy that has remained underexploited in antibacterial molecule development. With regard to H. pylori, numerous enzymes are predicted to be involved in metabolism, but few complexes with metabolic enzymes have been reported. This study described such complexes with enzymes involved 1) in the FAS II pathway (FabG, FabI, and FabZ), 2) in the pathway for synthesis of the core lipopolysaccharide (GmhA and ADP-l-glycero-d-mannoheptose 6-epimerase), and 3) in the major pathway for generation of acetyl coenzyme A (the essential pyruvate:flavodoxin oxidoreductase). All of these proteins involved in H. pylori physiology and their interacting partners may constitute attractive targets for the design of novel antibacterial agents. These metabolic complexes also involved some proteins whose function is unknown because no counterpart exists in other organisms, such as JH0295 (HELPY_0317) retrieved in association with enzymes involved in the FAS II pathway from both of the strains studied. These proteins deserve full attention and should first be studied in more detail, for example using reverse genetic experiments to determine their implication in the physiology of H. pylori.
Conclusions from this study cannot be drawn regarding the pathogenic properties of the strains studied; albeit a hypothesis that two different mechanisms are used by DU- and LG-MALT-associated strains is proposed. In fact, DU strains would be more aggressive via surface expression of certain OMPs via their association in different complexes by mediating adherence to gastric epithelial cells and modulating proinflammatory intracellular signaling cascades. These proteins would therefore be responsible for a strong localized inflammatory response. On the other hand, LG-MALT-associated strains, which seem to be more “insidious” would induce a limited inflammatory response.
CONCLUSION
This study allowed the identification of 329 different proteins of H. pylori as well as 49 protein complexes in H. pylori strain B38 associated with LG-MALT and 41 protein complexes in strain J99 associated with DU. Twelve of these complexes were common to both strains.
With regard to previously published proteomics comparative studies, this study is the first comparative study of the complexome in H. pylori strains. It provides new insight into the membrane and cytoplasm structure that can be used in the design of future molecules for vaccine and/or drug development. Moreover, this is the second study including an H. pylori strain associated with LG-MALT (17) and the first comprehensive study of the complexome of an H. pylori strain associated with LG-MALT. The resulting availability of the genome of the first H. pylori strain associated with LG-MALT should now help pave the way for other studies concerning this very particular cancer.
Supplementary Material
Acknowledgments
We are grateful to Slovénie Pyndiah for technical support. We thank Stéphane Claverol and Anne-Marie Lomenech from the Genomic Platform of the Bordeaux 2 University for technical support in mass spectrometry analysis.
Footnotes
* This study was supported in part by the European consortium Infection and Cancer (INCA) from the 6th Programme Cadre de Recherche et de Développement of the European Union and by the Conseil Régional d'Aquitaine.
 This article contains supplemental Table S1.
This article contains supplemental Table S1.
2 C. Varon, personal communication.
1 The abbreviations used are:
- MALT
- mucosa-associated lymphoid tissue
- LG-MALT
- gastric MALT lymphoma
- BN
- blue native
- DU
- duodenal ulcer
- OMP
- outer membrane protein
- BabA/B
- blood group antigen-binding adhesin A/B
- Hor
- Hop-related
- UreA/B
- urease α/β subunit
- AlpA/B
- adherence-associated lipoprotein AlpA/B
- SabA
- sialic acid-binding adhesin A
- PAI
- pathogenicity island
- FRD
- fumarate reductase
- IEF
- isoelectrofocalization
- POR
- pyruvate:flavodoxin oxidoreductase
- FAS
- fatty acid biosynthesis
- ACP
- acyl carrier protein
- ACX
- acetone carboxylase
- LC-MS/MS
- liquid chromatography mass spectrometry.
REFERENCES
- 1. Suerbaum S., Michetti P. (2002) Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186 [DOI] [PubMed] [Google Scholar]
- 2. Correa P., Fox J., Fontham E., Ruiz B., Lin Y., Zavala D., Taylor N., Mackinley D., de Lima E., Portilla H., Zarama G. (1990) Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer 66, 2569–2574 [DOI] [PubMed] [Google Scholar]
- 3. International Agency for Research on Cancer (1994) in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Schistosomes, Liver Flukes and Helicobacter pylori, Vol. 61, pp. 177–179, World Health Organization, Geneva: [PMC free article] [PubMed] [Google Scholar]
- 4. Forman D., Webb P., Parsonnet J. (1994) H. pylori and gastric cancer. Lancet 343, 243–244 [PubMed] [Google Scholar]
- 5. Parsonnet J., Hansen S., Rodriguez L., Gelb A. B., Warnke R. A., Jellum E., Orentreich N., Vogelman J. H., Friedman G. D. (1994) Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 330, 1267–1271 [DOI] [PubMed] [Google Scholar]
- 6. Isaacson P. G. (1994) Gastric lymphoma and Helicobacter pylori. N. Engl. J. Med. 330, 1310–1311 [DOI] [PubMed] [Google Scholar]
- 7. Wotherspoon A. C., Doglioni C., Diss T. C., Pan L., Moschini A., de Boni M., Isaacson P. G. (1993) Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342, 575–577 [DOI] [PubMed] [Google Scholar]
- 8. Ruskoné-Fourmestraux A., Lavergne A., Aegerter P. H., Megraud F., Palazzo L., de Mascarel A., Molina T., Rambaud J. L. (2001) Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut 48, 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy M., Copie-Bergman C., Traulle C., Lavergne-Slove A., Brousse N., Flejou J. F., de Mascarel A., Hemery F., Gaulard P., Delchier J. C. (2002) Conservative treatment of primary gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue: predictive factors of response and outcome. Am. J. Gastroenterol. 97, 292–297 [DOI] [PubMed] [Google Scholar]
- 10. Koehler C. I., Mues M. B., Dienes H. P., Kriegsmann J., Schirmacher P., Odenthal M. (2003) Helicobacter pylori genotyping in gastric adenocarcinoma and MALT lymphoma by multiplex PCR analyses of paraffin wax embedded tissues. Mol. Pathol. 56, 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lehours P., Ménard A., Dupouy S., Bergey B., Richy F., Zerbib F., Ruskoné-Fourmestraux A., Delchier J. C., Mégraud F. (2004) Evaluation of the association of nine Helicobacter pylori virulence factors with strains involved in low-grade gastric mucosa-associated lymphoid tissue lymphoma. Infect. Immun. 72, 880–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferreira-Chagas B., Lasne G., Dupouy S., Gallois A., Morgner A., Ménard A., Mégraud F., Lehours P. (2007) In vitro proinflammatory properties of Helicobacter pylori strains causing low-grade gastric MALT lymphoma. Helicobacter 12, 616–617 [DOI] [PubMed] [Google Scholar]
- 13. Thiberge J. M., Boursaux-Eude C., Lehours P., Dillies M., Creno S., Coppée J., Rouy Z., Lajus A., Ma L., Burucoa C., Ruskoné-Foumestraux A., Courillon-Mallet A., De Reuse H., Gomperts Boneca I., Lamarque D., Mégraud F., Delchier J., Médigue C., Bouchier C., Labigne A., Raymond J. (2010) From array-based hybridization of Helicobacter pylori isolates to the complete genome sequence of an isolate associated with MALT lymphoma. BMC Genomics 11, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eck M., Schmausser B., Haas R., Greiner A., Czub S., Müller-Hermelink H. K. (1997) MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 112, 1482–1486 [DOI] [PubMed] [Google Scholar]
- 15. Lehours P., Dupouy S., Bergey B., Ruskoné-Foumestraux A., Delchier J. C., Rad R., Richy F., Tankovic J., Zerbib F., Mégraud F., Ménard A. (2004) Identification of a genetic marker of Helicobacter pylori strains involved in gastric extranodal marginal zone B cell lymphoma of the MALT-type. Gut 53, 931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamaoka Y., Ojo O., Fujimoto S., Odenbreit S., Haas R., Gutierrez O., El-Zimaity H. M., Reddy R., Arnqvist A., Graham D. Y. (2006) Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut 55, 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernarde C., Khoder G., Lehours P., Burucoa C., Fauchère J. L., Delchier J. C., Mégraud F., Atanassov C. (2009) Proteomic Helicobacter pylori biomarkers discriminative of low-grade gastric MALT lymphoma and duodenal ulcer. Proteomics Clin. Appl. 3, 672–681 [DOI] [PubMed] [Google Scholar]
- 18. Alberts B. (1998) The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell 92, 291–294 [DOI] [PubMed] [Google Scholar]
- 19. Reisinger V., Eichacker L. A. (2007) How to analyze protein complexes by 2D blue native SDS-PAGE. Proteomics 7, Suppl. 1, 6–16 [DOI] [PubMed] [Google Scholar]
- 20. Schägger H., von Jagow G. (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 199, 223–231 [DOI] [PubMed] [Google Scholar]
- 21. Neff D., Dencher N. A. (1999) Purification of multisubunit membrane protein complexes: isolation of chloroplast FoF1-ATP synthase, CFo and CF1 by blue native electrophoresis. Biochem. Biophys. Res. Commun. 259, 569–575 [DOI] [PubMed] [Google Scholar]
- 22. Stroh A., Anderka O., Pfeiffer K., Yagi T., Finel M., Ludwig B., Schägger H. (2004) Assembly of respiratory complexes I, III, and IV into NADH oxidase supercomplex stabilizes complex I in Paracoccus denitrificans. J. Biol. Chem. 279, 5000–5007 [DOI] [PubMed] [Google Scholar]
- 23. Battchikova N., Zhang P., Rudd S., Ogawa T., Aro E. M. (2005) Identification of NdhL and Ssl1690 (NdhO) in NDH-1L and NDH-1M complexes of Synechocystis sp. PCC 6803. J. Biol. Chem. 280, 2587–2595 [DOI] [PubMed] [Google Scholar]
- 24. Stenberg F., Chovanec P., Maslen S. L., Robinson C. V., Ilag L. L., von Heijne G., Daley D. O. (2005) Protein complexes of the Escherichia coli cell envelope. J. Biol. Chem. 280, 34409–34419 [DOI] [PubMed] [Google Scholar]
- 25. Camacho-Carvajal M. M., Wollscheid B., Aebersold R., Steimle V., Schamel W. W. (2004) Two-dimensional Blue native/SDS gel electrophoresis of multi-protein complexes from whole cellular lysates: a proteomics approach. Mol. Cell. Proteomics 3, 176–182 [DOI] [PubMed] [Google Scholar]
- 26. Claeys D., Geering K., Meyer B. J. (2005) Two-dimensional Blue Native/sodium dodecyl sulfate gel electrophoresis for analysis of multimeric proteins in platelets. Electrophoresis 26, 1189–1199 [DOI] [PubMed] [Google Scholar]
- 27. Lasserre J. P., Beyne E., Pyndiah S., Lapaillerie D., Claverol S., Bonneu M. (2006) A complexomic study of Escherichia coli using two-dimensional blue native/SDS polyacrylamide gel electrophoresis. Electrophoresis 27, 3306–3321 [DOI] [PubMed] [Google Scholar]
- 28. Pyndiah S., Lasserre J. P., Ménard A., Claverol S., Prouzet-Mauléon V., Mégraud F., Zerbib F., Bonneu M. (2007) Two-dimensional blue native/SDS gel electrophoresis of multiprotein complexes from Helicobacter pylori. Mol. Cell. Proteomics 6, 193–206 [DOI] [PubMed] [Google Scholar]
- 29. Suzuki T., Matsuo K., Ito H., Hirose K., Wakai K., Saito T., Sato S., Morishima Y., Nakamura S., Ueda R., Tajima K. (2006) A past history of gastric ulcers and Helicobacter pylori infection increase the risk of gastric malignant lymphoma. Carcinogenesis 27, 1391–1397 [DOI] [PubMed] [Google Scholar]
- 30. Alm R. A., Ling L. S., Moir D. T., King B. L., Brown E. D., Doig P. C., Smith D. R., Noonan B., Guild B. C., deJonge B. L., Carmel G., Tummino P. J., Caruso A., Uria-Nickelsen M., Mills D. M., Ives C., Gibson R., Merberg D., Mills S. D., Jiang Q., Taylor D. E., Vovis G. F., Trust T. J. (1999) Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397, 176–180 [DOI] [PubMed] [Google Scholar]
- 31. Boneca I. G., de Reuse H., Epinat J. C., Pupin M., Labigne A., Moszer I. (2003) A revised annotation and comparative analysis of Helicobacter pylori genomes. Nucleic Acids Res. 31, 1704–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuhn M., von Mering C., Campillos M., Jensen L. J., Bork P. (2008) STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 36, D684–D688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schägger H., Cramer W. A., von Jagow G. (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217, 220–230 [DOI] [PubMed] [Google Scholar]
- 35. Birkholz S., Knipp U., Lemma E., Kröger A., Opferkuch W. (1994) Fumarate reductase of Helicobacter pylori—an immunogenic protein. J. Med. Microbiol. 41, 56–62 [DOI] [PubMed] [Google Scholar]
- 36. Ge Z., Jiang Q., Kalisiak M. S., Taylor D. E. (1997) Cloning and functional characterization of Helicobacter pylori fumarate reductase operon comprising three structural genes coding for subunits C, A and B. Gene 204, 227–234 [DOI] [PubMed] [Google Scholar]
- 37. Chang H. T., Marcelli S. W., Davison A. A., Chalk P. A., Poole R. K., Miles R. J. (1995) Kinetics of substrate oxidation by whole cells and cell membranes of Helicobacter pylori. FEMS Microbiol. Lett. 129, 33–38 [DOI] [PubMed] [Google Scholar]
- 38. Ge Z. (2002) Potential of fumarate reductase as a novel therapeutic target in Helicobacter pylori infection. Expert Opin. Ther. Targets 6, 135–146 [DOI] [PubMed] [Google Scholar]
- 39. Lock R. A., Cordwell S. J., Coombs G. W., Walsh B. J., Forbes G. M. (2001) Proteome analysis of Helicobacter pylori: major proteins of type strain NCTC 11637. Pathology 33, 365–374 [PubMed] [Google Scholar]
- 40. Fischer W., Haas R. (2004) The RecA protein of Helicobacter pylori requires a posttranslational modification for full activity. J. Bacteriol. 186, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim J. S., Chang J. H., Seo W. Y., Yu G. J., Chung S. I., Yum J. S. (2000) Cloning and characterization of a 22 kDa outer-membrane protein (Omp22) from Helicobacter pylori. Mol. Cells 10, 633–641 [DOI] [PubMed] [Google Scholar]
- 42. Doig P., de Jonge B. L., Alm R. A., Brown E. D., Uria-Nickelsen M., Noonan B., Mills S. D., Tummino P., Carmel G., Guild B. C., Moir D. T., Vovis G. F., Trust T. J. (1999) Helicobacter pylori physiology predicted from genomic comparison of two strains. Microbiol. Mol. Biol. Rev. 63, 675–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aris J. P., Simoni R. D. (1985) The beta subunit of the Escherichia coli ATP synthase exhibits a tight membrane binding property. Biochem. Biophys. Res. Commun. 128, 155–162 [DOI] [PubMed] [Google Scholar]
- 44. Oleastro M., Cordeiro R., Ferrand J., Nunes B., Lehours P., Carvalho-Oliveira I., Mendes A. I., Penque D., Monteiro L., Mégraud F., Ménard A. (2008) Evaluation of the clinical significance of homB, a novel candidate marker of ulcer strains. J. Infect. Dis. 198, 1379–1387 [DOI] [PubMed] [Google Scholar]
- 45. Matin A., Zychlinsky E., Keyhan M., Sachs G. (1996) Capacity of Helicobacter pylori to generate ionic gradients at low pH is similar to that of bacteria which grow under strongly acidic conditions. Infect. Immun. 64, 1434–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McGowan C. C., Cover T. L., Blaser M. J. (1997) Analysis of F1F0-ATPase from Helicobacter pylori. Infect. Immun. 65, 2640–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin Y. F., Wu M. S., Chang C. C., Lin S. W., Lin J. T., Sun Y. J., Chen D. S., Chow L. P. (2006) Comparative immunoproteomics of identification and characterization of virulence factors from Helicobacter pylori related to gastric cancer. Mol. Cell. Proteomics 5, 1484–1496 [DOI] [PubMed] [Google Scholar]
- 48. Kimmel B., Bosserhoff A., Frank R., Gross R., Goebel W., Beier D. (2000) Identification of immunodominant antigens from Helicobacter pylori and evaluation of their reactivities with sera from patients with different gastroduodenal pathologies. Infect. Immun. 68, 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobayashi N., Nishino K., Yamaguchi A. (2001) Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183, 5639–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tikhonova E. B., Devroy V. K., Lau S. Y., Zgurskaya H. I. (2007) Reconstitution of the Escherichia coli macrolide transporter: the periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol. Microbiol. 63, 895–910 [DOI] [PubMed] [Google Scholar]
- 51. Ma D., Cook D. N., Alberti M., Pon N. G., Nikaido H., Hearst J. E. (1995) Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16, 45–55 [DOI] [PubMed] [Google Scholar]
- 52. Kawabe T., Fujihira E., Yamaguchi A. (2000) Molecular construction of a multidrug exporter system, AcrAB: molecular interaction between AcrA and AcrB, and cleavage of the N-terminal signal sequence of AcrA. J. Biochem. 128, 195–200 [DOI] [PubMed] [Google Scholar]
- 53. Tikhonova E. B., Zgurskaya H. I. (2004) AcrA, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J. Biol. Chem. 279, 32116–32124 [DOI] [PubMed] [Google Scholar]
- 54. Bina J. E., Alm R. A., Uria-Nickelsen M., Thomas S. R., Trust T. J., Hancock R. E. (2000) Helicobacter pylori. uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob. Agents Chemother. 44, 248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kutschke A., de Jonge B. L. (2005) Compound efflux in Helicobacter pylori. Antimicrob. Agents Chemother. 49, 3009–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnson J. M., Church G. M. (1999) Alignment and structure prediction of divergent protein families: periplasmic and outer membrane proteins of bacterial efflux pumps. J. Mol. Biol. 287, 695–715 [DOI] [PubMed] [Google Scholar]
- 57. van Amsterdam K., Bart A., van der Ende A. (2005) A Helicobacter pylori TolC efflux pump confers resistance to metronidazole. Antimicrob. Agents Chemother. 49, 1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Z. Q., Zheng P. Y., Yang P. C. (2008) Efflux pump gene hefA of Helicobacter pylori plays an important role in multidrug resistance. World J. Gastroenterol. 14, 5217–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sennhauser G., Bukowska M. A., Briand C., Grütter M. G. (2009) Crystal structure of the multidrug exporter MexB from Pseudomonas aeruginosa. J. Mol. Biol. 389, 134–145 [DOI] [PubMed] [Google Scholar]
- 60. Smith T. G., Lim J. M., Weinberg M. V., Wells L., Hoover T. R. (2007) Direct analysis of the extracellular proteome from two strains of Helicobacter pylori. Proteomics 7, 2240–2245 [DOI] [PubMed] [Google Scholar]
- 61. Schmitz A., Josenhans C., Suerbaum S. (1997) Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J. Bacteriol. 179, 987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Porter M. E. (1996) Axonemal dyneins: assembly, organization, and regulation. Curr. Opin. Cell Biol. 8, 10–17 [DOI] [PubMed] [Google Scholar]
- 63. Wilkes D. E., Rajagopalan V., Chan C. W., Kniazeva E., Wiedeman A. E., Asai D. J. (2007) Dynein light chain family in Tetrahymena thermophila. Cell Motil. Cytoskeleton 64, 82–96 [DOI] [PubMed] [Google Scholar]
- 64. Alm R. A., Bina J., Andrews B. M., Doig P., Hancock R. E., Trust T. J. (2000) Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect. Immun. 68, 4155–4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kavermann H., Burns B. P., Angermuller K., Odenbreit S., Fischer W., Melchers K., Haas R. (2003) Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J. Exp. Med. 197, 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peck B., Ortkamp M., Nau U., Niederweis M., Hundt E., Knapp B. (2001) Characterization of four members of a multigene family encoding outer membrane proteins of Helicobacter pylori and their potential for vaccination. Microbes Infect. 3, 171–179 [DOI] [PubMed] [Google Scholar]
- 67. Tomb J. F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleischmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A., Nelson K., Quackenbush J., Zhou L., Kirkness E. F., Peterson S., Loftus B., Richardson D., Dodson R., Khalak H. G., Glodek A., McKenney K., Fitzegerald L. M., Lee N., Adams M. D., Hickey E. K., Berg D. E., Gocayne J. D., Utterback T. R., Peterson J. D., Kelley J. M., Cotton M. D., Weidman J. M., Fujii C., Bowman C., Watthey L., Wallin E., Hayes W. S., Borodovsky M., Karp P. D., Smith H. O., Fraser C. M., Venter J. C. (1997) The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388, 539–547 [DOI] [PubMed] [Google Scholar]
- 68. Doig P., Exner M. M., Hancock R. E., Trust T. J. (1995) Isolation and characterization of a conserved porin protein from Helicobacter pylori. J. Bacteriol. 177, 5447–5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Baik S. C., Kim K. M., Song S. M., Kim D. S., Jun J. S., Lee S. G., Song J. Y., Park J. U., Kang H. L., Lee W. K., Cho M. J., Youn H. S., Ko G. H., Rhee K. H. (2004) Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Helicobacter pylori strain 26695. J. Bacteriol. 186, 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Odenbreit S., Till M., Hofreuter D., Faller G., Haas R. (1999) Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol. Microbiol. 31, 1537–1548 [DOI] [PubMed] [Google Scholar]
- 71. Odenbreit S., Faller G., Haas R. (2002) Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int. J. Med. Microbiol. 292, 247–256 [DOI] [PubMed] [Google Scholar]
- 72. de Jonge R., Durrani Z., Rijpkema S. G., Kuipers E. J., van Vliet A. H., Kusters J. G. (2004) Role of the Helicobacter pylori outer-membrane proteins AlpA and AlpB in colonization of the guinea pig stomach. J. Med. Microbiol. 53, 375–379 [DOI] [PubMed] [Google Scholar]
- 73. Lu H., Wu J. Y., Beswick E. J., Ohno T., Odenbreit S., Haas R., Reyes V. E., Kita M., Graham D. Y., Yamaoka Y. (2007) Functional and intracellular signaling differences associated with the Helicobacter pylori AlpAB adhesin from Western and East Asian strains. J. Biol. Chem. 282, 6242–6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xue J., Bai Y., Chen Y., Wang J. D., Zhang Z. S., Zhang Y. L., Zhou D. Y. (2005) Expression of Helicobacter pylori AlpA protein and its immunogenicity. World J. Gastroenterol. 11, 2260–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yamaoka Y., Kwon D. H., Graham D. Y. (2000) A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. U.S.A. 97, 7533–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tabassam F. H., Graham D. Y., Yamaoka Y. (2008) OipA plays a role in Helicobacter pylori-induced focal adhesion kinase activation and cytoskeletal re-organization. Cell. Microbiol. 10, 1008–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ilver D., Arnqvist A., Ogren J., Frick I. M., Kersulyte D., Incecik E. T., Berg D. E., Covacci A., Engstrand L., Borén T. (1998) Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279, 373–377 [DOI] [PubMed] [Google Scholar]
- 78. Solnick J. V., Hansen L. M., Salama N. R., Boonjakuakul J. K., Syvanen M. (2004) Modification of Helicobacter pylori outer membrane protein expression during experimental infection of rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 101, 2106–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haas G., Karaali G., Ebermayer K., Metzger W. G., Lamer S., Zimny-Arndt U., Diescher S., Goebel U. B., Vogt K., Roznowski A. B., Wiedenmann B. J., Meyer T. F., Aebischer T., Jungblut P. R. (2002) Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2, 313–324 [DOI] [PubMed] [Google Scholar]
- 80. Meinke A., Storm M., Henics T., Gelbmann D., Prustomersky S., Kovács Z., Minh D. B., Noiges B., Stierschneider U., Berger M., von Gabain A., Engstrand L., Nagy E. (2009) Composition of the ANTIGENome of Helicobacter pylori defined by human serum antibodies. Vaccine 27, 3251–3259 [DOI] [PubMed] [Google Scholar]
- 81. Mahdavi J., Sondén B., Hurtig M., Olfat F. O., Forsberg L., Roche N., Angstrom J., Larsson T., Teneberg S., Karlsson K. A., Altraja S., Wadström T., Kersulyte D., Berg D. E., Dubois A., Petersson C., Magnusson K. E., Norberg T., Lindh F., Lundskog B. B., Arnqvist A., Hammarström L., Borén T. (2002) Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297, 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marcos N. T., Magalhães A., Ferreira B., Oliveira M. J., Carvalho A. S., Mendes N., Gilmartin T., Head S. R., Figueiredo C., David L., Santos-Silva F., Reis C. A. (2008) Helicobacter pylori induces beta3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J. Clin. Investig. 118, 2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gerhard M., Lehn N., Neumayer N., Borén T., Rad R., Schepp W., Miehlke S., Classen M., Prinz C. (1999) Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. U.S.A. 96, 12778–12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Oleastro M., Gerhard M., Lopes A. I., Ramalho P., Cabral J., Sousa Guerreiro A., Monteiro L. (2003) Helicobacter pylori virulence genotypes in Portuguese children and adults with gastroduodenal pathology. Eur. J. Clin. Microbiol. Infect. Dis. 22, 85–91 [DOI] [PubMed] [Google Scholar]
- 85. Peek R. M., Jr. (2005) Events at the host-microbial interface of the gastrointestinal tract IV. The pathogenesis of Helicobacter pylori persistence. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G8–G12 [DOI] [PubMed] [Google Scholar]
- 86. Prinz C., Schöniger M., Rad R., Becker I., Keiditsch E., Wagenpfeil S., Classen M., Rösch T., Schepp W., Gerhard M. (2001) Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res. 61, 1903–1909 [PubMed] [Google Scholar]
- 87. Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V., Blaszczyk M., Mitchell K. F., Steplewski Z., Koprowski H. (1981) A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science 212, 55–56 [DOI] [PubMed] [Google Scholar]
- 88. Sipponen P., Lindgren J. (1986) Sialylated Lewis determinant CA 19–9 in benign and malignant gastric tissue. Acta Pathol. Microbiol. Immunol. Scand. A 94, 305–311 [DOI] [PubMed] [Google Scholar]
- 89. Azevedo M., Eriksson S., Mendes N., Serpa J., Figueiredo C., Resende L. P., Ruvoën-Clouet N., Haas R., Borén T., Le Pendu J., David L. (2008) Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J. Pathol. 215, 308–316 [DOI] [PubMed] [Google Scholar]
- 90. Saunders N. J., Boonmee P., Peden J. F., Jarvis S. A. (2005) Inter-species horizontal transfer resulting in core-genome and niche-adaptive variation within Helicobacter pylori. BMC Genomics 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sheu B. S., Odenbreit S., Hung K. H., Liu C. P., Sheu S. M., Yang H. B., Wu J. J. (2006) Interaction between host gastric Sialyl-Lewis X and H. pylori SabA enhances H. pylori density in patients lacking gastric Lewis B antigen. Am. J. Gastroenterol. 101, 36–44 [DOI] [PubMed] [Google Scholar]
- 92. Yan J., Mao Y. F., Shao Z. X. (2005) Frequencies of the expression of main protein antigens from Helicobacter pylori isolates and production of specific serum antibodies in infected patients. World J. Gastroenterol. 11, 421–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mendz G. L. (2001) Nucleotide metabolism, in Helicobacter pylori: Physiology and Genetics (Mobley H. L. T., Mendz G. L., Hazell S. L. eds) ASM Press, Washington D. C. Chapter 13, pp. 147–158 [PubMed] [Google Scholar]
- 94. Marais A., Mendz G. L., Hazell S. L., Mégraud F. (1999) Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol. Mol. Biol. Rev. 63, 642–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hughes N. J., Chalk P. A., Clayton C. L., Kelly D. J. (1995) Identification of carboxylation enzymes and characterization of a novel four-subunit pyruvate:flavodoxin oxidoreductase from Helicobacter pylori. J. Bacteriol. 177, 3953–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hughes N. J., Clayton C. L., Chalk P. A., Kelly D. J. (1998) Helicobacter pylori porCDAB and oorDABC genes encode distinct pyruvate:flavodoxin and 2-oxoglutarate:acceptor oxidoreductases which mediate electron transport to NADP. J. Bacteriol. 180, 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hoffman P. S., Goodwin A., Johnsen J., Magee K., Veldhuyzen van Zanten S. J. (1996) Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J. Bacteriol. 178, 4822–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kneidinger B., Marolda C., Graninger M., Zamyatina A., McArthur F., Kosma P., Valvano M. A., Messner P. (2002) Biosynthesis pathway of ADP-L-glycero-beta-D-manno-heptose in Escherichia coli. J. Bacteriol. 184, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wright H. T., Reynolds K. A. (2007) Antibacterial targets in fatty acid biosynthesis. Curr. Opin. Microbiol. 10, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lu H., Tonge P. J. (2008) Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc. Chem. Res. 41, 11–20 [DOI] [PubMed] [Google Scholar]
- 101. Henke B., Girzalsky W., Berteaux-Lecellier V., Erdmann R. (1998) IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the beta-oxidation of unsaturated fatty acids. J. Biol. Chem. 273, 3702–3711 [DOI] [PubMed] [Google Scholar]
- 102. van Roermund C. W., Hettema E. H., Kal A. J., van den Berg M., Tabak H. F., Wanders R. J. (1998) Peroxisomal beta-oxidation of polyunsaturated fatty acids in Saccharomyces cerevisiae: isocitrate dehydrogenase provides NADPH for reduction of double bonds at even positions. EMBO J. 17, 677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang L., Liu W., Hu T., Du L., Luo C., Chen K., Shen X., Jiang H. (2008) Structural basis for catalytic and inhibitory mechanisms of beta-hydroxyacyl-acyl carrier protein dehydratase (FabZ). J. Biol. Chem. 283, 5370–5379 [DOI] [PubMed] [Google Scholar]
- 104. Bergler H., Fuchsbichler S., Högenauer G., Turnowsky F. (1996) The enoyl-[acyl-carrier-protein] reductase (FabI) of Escherichia coli, which catalyzes a key regulatory step in fatty acid biosynthesis, accepts NADH and NADPH as cofactors and is inhibited by palmitoyl-CoA. Eur. J. Biochem. 242, 689–694 [DOI] [PubMed] [Google Scholar]
- 105. Davis M. C., Franzblau S. G., Martin A. R. (1998) Syntheses and evaluation of benzodiazaborine compounds against M. tuberculosis H37Rv in vitro. Bioorg. Med. Chem. Lett. 8, 843–846 [DOI] [PubMed] [Google Scholar]
- 106. Slayden R. A., Lee R. E., Barry C. E., 3rd (2000) Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol. Microbiol. 38, 514–525 [DOI] [PubMed] [Google Scholar]
- 107. Miller W. H., Seefeld M. A., Newlander K. A., Uzinskas I. N., Burgess W. J., Heerding D. A., Yuan C. C., Head M. S., Payne D. J., Rittenhouse S. F., Moore T. D., Pearson S. C., Berry V., DeWolf W. E., Jr., Keller P. M., Polizzi B. J., Qiu X., Janson C. A., Huffman W. F. (2002) Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI). J. Med. Chem. 45, 3246–3256 [DOI] [PubMed] [Google Scholar]
- 108. Olczak A. A., Wang G., Maier R. J. (2005) Up-expression of NapA and other oxidative stress proteins is a compensatory response to loss of major Helicobacter pylori stress resistance factors. Free Radic. Res. 39, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 109. Windle H. J., Fox A., Ní Eidhin D., Kelleher D. (2000) The thioredoxin system of Helicobacter pylori. J. Biol. Chem. 275, 5081–5089 [DOI] [PubMed] [Google Scholar]
- 110. Baker L. M., Raudonikiene A., Hoffman P. S., Poole L. B. (2001) Essential thioredoxin-dependent peroxiredoxin system from Helicobacter pylori: genetic and kinetic characterization. J. Bacteriol. 183, 1961–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. McGee D. J., Kumar S., Viator R. J., Bolland J. R., Ruiz J., Spadafora D., Testerman T. L., Kelly D. J., Pannell L. K., Windle H. J. (2006) Helicobacter pylori thioredoxin is an arginase chaperone and guardian against oxidative and nitrosative stresses. J. Biol. Chem. 281, 3290–3296 [DOI] [PubMed] [Google Scholar]
- 112. St Maurice M., Cremades N., Croxen M. A., Sisson G., Sancho J., Hoffman P. S. (2007) Flavodoxin:quinone reductase (FqrB): a redox partner of pyruvate:ferredoxin oxidoreductase that reversibly couples pyruvate oxidation to NADPH production in Helicobacter pylori and Campylobacter jejuni. J. Bacteriol. 189, 4764–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Beissinger M., Buchner J. (1998) How chaperones fold proteins. Biol. Chem. 379, 245–259 [PubMed] [Google Scholar]
- 114. Lindquist S., Craig E. A. (1988) The heat-shock proteins. Annu. Rev. Genet. 22, 631–677 [DOI] [PubMed] [Google Scholar]
- 115. Craig E. A., Gambill B. D., Nelson R. J. (1993) Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol. Rev. 57, 402–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Georgopoulos C., Welch W. J. (1993) Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 9, 601–634 [DOI] [PubMed] [Google Scholar]
- 117. Hartl F. U., Hlodan R., Langer T. (1994) Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biochem. Sci. 19, 20–25 [DOI] [PubMed] [Google Scholar]
- 118. Chuang M. H., Wu M. S., Lo W. L., Lin J. T., Wong C. H., Chiou S. H. (2006) The antioxidant protein alkylhydroperoxide reductase of Helicobacter pylori switches from a peroxide reductase to a molecular chaperone function. Proc. Natl. Acad. Sci. U.S.A. 103, 2552–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jungblut P. R., Bumann D., Haas G., Zimny-Arndt U., Holland P., Lamer S., Siejak F., Aebischer A., Meyer T. F. (2000) Comparative proteome analysis of Helicobacter pylori. Mol. Microbiol. 36, 710–725 [DOI] [PubMed] [Google Scholar]
- 120. Backert S., Kwok T., Schmid M., Selbach M., Moese S., Peek R. M., Jr., König W., Meyer T. F., Jungblut P. R. (2005) Subproteomes of soluble and structure-bound Helicobacter pylori proteins analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 5, 1331–1345 [DOI] [PubMed] [Google Scholar]
- 121. Cuéllar J., Martín-Benito J., Scheres S. H., Sousa R., Moro F., López-Viñas E., Gómez-Puertas P., Muga A., Carrascosa J. L., Valpuesta J. M. (2008) The structure of CCT-Hsc70 NBD suggests a mechanism for Hsp70 delivery of substrates to the chaperonin. Nat. Struct. Mol. Biol. 15, 858–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mohamed Fahmy Gad El-Rab S., Abdel-Fattah Shoreit A., Fukumori Y. (2006) Effects of cadmium stress on growth, morphology, and protein expression in Rhodobacter capsulatus B10. Biosci. Biotechnol. Biochem. 70, 2394–2402 [DOI] [PubMed] [Google Scholar]
- 123. Phillips G. J., Silhavy T. J. (1990) Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins GroEL and DnaK. Nature 344, 882–884 [DOI] [PubMed] [Google Scholar]
- 124. Spohn G., Scarlato V. (1999) The autoregulatory HspR repressor protein governs chaperone gene transcription in Helicobacter pylori. Mol. Microbiol. 34, 663–674 [DOI] [PubMed] [Google Scholar]
- 125. Brahmachary P., Wang G., Benoit S. L., Weinberg M. V., Maier R. J., Hoover T. R. (2008) The human gastric pathogen Helicobacter pylori has a potential acetone carboxylase that enhances its ability to colonize mice. BMC Microbiol. 8, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nocek B., Boyd J., Ensign S. A., Peters J. W. (2004) Crystallization and preliminary X-ray analysis of an acetone carboxylase from Xanthobacter autotrophicus strain Py2. Acta. Crystallogr. D Biol. Crystallogr. 60, 385–387 [DOI] [PubMed] [Google Scholar]
- 127. Pflock M., Bathon M., Schär J., Müller S., Mollenkopf H., Meyer T. F., Beier D. (2007) The orphan response regulator HP1021 of Helicobacter pylori regulates transcription of a gene cluster presumably involved in acetone metabolism. J. Bacteriol. 189, 2339–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bumann D., Jungblut P. R., Meyer T. F. (2004) Helicobacter pylori vaccine development based on combined subproteome analysis. Proteomics 4, 2843–2848 [DOI] [PubMed] [Google Scholar]
- 129. Mini R., Figura N., D'Ambrosio C., Braconi D., Bernardini G., Di Simplicio F., Lenzi C., Nuti R., Trabalzini L., Martelli P., Bovalini L., Scaloni A., Santucci A. (2005) Helicobacter pylori immunoproteomes in case reports of rosacea and chronic urticaria. Proteomics 5, 777–787 [DOI] [PubMed] [Google Scholar]
- 130. Voland P., Weeks D. L., Vaira D., Prinz C., Sachs G. (2002) Specific identification of three low molecular weight membrane-associated antigens of Helicobacter pylori. Aliment. Pharmacol. Ther. 16, 533–544 [DOI] [PubMed] [Google Scholar]
- 131. Zhang J., Ding S. G., Zhong L. J., Lin S. R., Yang B., Peng J. R., Lou Y. X. (2006) Difference analysis on proteome of Helicobacter pylori in patients with peptic ulcer, gastritis, and gastric cancer. Zhonghua Yi Xue Za Zhi 86, 2690–2694 [PubMed] [Google Scholar]
- 132. Khoder G., Yamaoka Y., Fauchère J. L., Burucoa C., Atanassov C. (2009) Proteomic Helicobacter pylori biomarkers discriminating between duodenal ulcer and gastric cancer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Long M., Luo J., Li Y., Zeng F. Y., Li M. (2009) Detection and evaluation of antibodies against neutrophil-activating protein of Helicobacter pylori in patients with gastric cancer. World J. Gastroenterol. 15, 2381–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cappon A., Babolin C., Segat D., Cancian L., Amedei A., Calzetti F., Cassatella M. A., D'Elios M. M., de Bernard M. (2010) Helicobacter pylori-derived neutrophil-activating protein increases the lifespan of monocytes and neutrophils. Cell. Microbiol. 12, 754–764 [DOI] [PubMed] [Google Scholar]
- 135. Rain J. C., Selig L., De Reuse H., Battaglia V., Reverdy C., Simon S., Lenzen G., Petel F., Wojcik J., Schächter V., Chemama Y., Labigne A., Legrain P. (2001) The protein-protein interaction map of Helicobacter pylori. Nature 409, 211–215 [DOI] [PubMed] [Google Scholar]
- 136. Terradot L., Durnell N., Li M., Li M., Ory J., Labigne A., Legrain P., Colland F., Waksman G. (2004) Biochemical characterization of protein complexes from the Helicobacter pylori protein interaction map: strategies for complex formation and evidence for novel interactions within type IV secretion systems. Mol. Cell. Proteomics 3, 809–819 [DOI] [PubMed] [Google Scholar]
- 137. Busler V. J., Torres V. J., McClain M. S., Tirado O., Friedman D. B., Cover T. L. (2006) Protein-protein interactions among Helicobacter pylori cag proteins. J. Bacteriol. 188, 4787–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kutter S., Buhrdorf R., Haas J., Schneider-Brachert W., Haas R., Fischer W. (2008) Protein subassemblies of the Helicobacter pylori Cag type IV secretion system revealed by localization and interaction studies. J. Bacteriol. 190, 2161–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sabarth N., Lamer S., Zimny-Arndt U., Jungblut P. R., Meyer T. F., Bumann D. (2002) Identification of surface proteins of Helicobacter pylori by selective biotinylation, affinity purification, and two-dimensional gel electrophoresis. J. Biol. Chem. 277, 27896–27902 [DOI] [PubMed] [Google Scholar]
- 140. Bumann D., Meyer T. F., Jungblut P. R. (2001) Proteome analysis of the common human pathogen Helicobacter pylori. Proteomics 1, 473–479 [DOI] [PubMed] [Google Scholar]
- 141. Krah A., Schmidt F., Becher D., Schmid M., Albrecht D., Rack A., Büttner K., Jungblut P. R. (2003) Analysis of automatically generated peptide mass fingerprints of cellular proteins and antigens from Helicobacter pylori 26695 separated by two-dimensional electrophoresis. Mol. Cell. Proteomics 2, 1271–1283 [DOI] [PubMed] [Google Scholar]
- 142. Sabarth N., Hurvitz R., Schmidt M., Zimny-Arndt U., Jungblut P. R., Meyer T. F., Bumann D. (2005) Identification of Helicobacter pylori surface proteins by selective proteinase K digestion and antibody phage display. J. Microbiol. Methods 62, 345–349 [DOI] [PubMed] [Google Scholar]
- 143. Park S. A., Lee H. W., Hong M. H., Choi Y. W., Choe Y. H., Ahn B. Y., Cho Y. J., Kim D. S., Lee N. G. (2006) Comparative proteomic analysis of Helicobacter pylori strains associated with iron deficiency anemia. Proteomics 6, 1319–1328 [DOI] [PubMed] [Google Scholar]
- 144. Pereira D. R., Martins D., Winck F. V., Smolka M. B., Nishimura N. F., Rabelo-Gonçalves E. M., Hara N. H., Marangoni S., Zeitune J. M., Novello J. C. (2006) Comparative analysis of two-dimensional electrophoresis maps (2-DE) of Helicobacter pylori from Brazilian patients with chronic gastritis and duodenal ulcer: a preliminary report. Rev. Inst. Med. Trop. Sao Paulo 48, 175–177 [DOI] [PubMed] [Google Scholar]
- 145. Jungblut P. R., Schiele F., Zimny-Arndt U., Ackermann R., Schmid M., Lange S., Stein R., Pleissner K. P. (2010) Helicobacter pylori proteomics by 2-DE/MS, 1-DE-LC/MS and functional data mining. Proteomics 10, 182–193 [DOI] [PubMed] [Google Scholar]
- 146. Stingl K., Schauer K., Ecobichon C., Labigne A., Lenormand P., Rousselle J. C., Namane A., de Reuse H. (2008) In vivo interactome of Helicobacter pylori urease revealed by tandem affinity purification. Mol. Cell. Proteomics 7, 2429–2441 [DOI] [PubMed] [Google Scholar]
- 147. Martin S., Roe D., Faulon J. L. (2005) Predicting protein-protein interactions using signature products. Bioinformatics 21, 218–226 [DOI] [PubMed] [Google Scholar]
- 148. Sharan R., Ideker T., Kelley B., Shamir R., Karp R. M. (2005) Identification of protein complexes by comparative analysis of yeast and bacterial protein interaction data. J. Comput. Biol. 12, 835–846 [DOI] [PubMed] [Google Scholar]
- 149. Liang Z., Xu M., Teng M., Niu L. (2006) NetAlign: a web-based tool for comparison of protein interaction networks. Bioinformatics 22, 2175–2177 [DOI] [PubMed] [Google Scholar]
- 150. Dutta A., Singh S. K., Ghosh P., Mukherjee R., Mitter S., Bandyopadhyay D. (2006) In silico identification of potential therapeutic targets in the human pathogen Helicobacter pylori. In Silico Biol. 6, 43–47 [PubMed] [Google Scholar]
- 151. Bumann D., Aksu S., Wendland M., Janek K., Zimny-Arndt U., Sabarth N., Meyer T. F., Jungblut P. R. (2002) Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70, 3396–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McAtee C. P., Lim M. Y., Fung K., Velligan M., Fry K., Chow T., Berg D. E. (1998) Identification of potential diagnostic and vaccine candidates of Helicobacter pylori by two-dimensional gel electrophoresis, sequence analysis, and serum profiling. Clin. Diagn. Lab. Immunol. 5, 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Govorun V. M., Moshkovskii S. A., Tikhonova O. V., Goufman E. I., Serebryakova M. V., Momynaliev K. T., Lokhov P. G., Khryapova E. V., Kudryavtseva L. V., Smirnova O. V., Toropyguine I. Y., Maksimov B. I., Archakov A. I. (2003) Comparative analysis of proteome maps of Helicobacter pylori clinical isolates. Biochemistry 68, 42–49 [DOI] [PubMed] [Google Scholar]
- 154. Mini R., Bernardini G., Salzano A. M., Renzone G., Scaloni A., Figura N., Santucci A. (2006) Comparative proteomics and immunoproteomics of Helicobacter pylori related to different gastric pathologies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 833, 63–79 [DOI] [PubMed] [Google Scholar]
- 155. Lin Y. F., Chen C. Y., Tsai M. H., Wu M. S., Wang Y. C., Chuang E. Y., Lin J. T., Yang P. C., Chow L. P. (2007) Duodenal ulcer-related antigens from Helicobacter pylori: immunoproteome and protein microarray approaches. Mol. Cell. Proteomics 6, 1018–1026 [DOI] [PubMed] [Google Scholar]
- 156. Salama N., Guillemin K., McDaniel T. K., Sherlock G., Tompkins L., Falkow S. (2000) A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. U.S.A. 97, 14668–14673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gressmann H., Linz B., Ghai R., Pleissner K. P., Schlapbach R., Yamaoka Y., Kraft C., Suerbaum S., Meyer T. F., Achtman M. (2005) Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet. 1, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Josenhans C., Beier D., Linz B., Meyer T. F., Suerbaum S. (2007) Pathogenomics of Helicobacter. Int. J. Med. Microbiol. 297, 589–600 [DOI] [PubMed] [Google Scholar]
- 159. Backert S., Meyer T. F. (2006) Type IV secretion systems and their effectors in bacterial pathogenesis. Curr. Opin. Microbiol. 9, 207–217 [DOI] [PubMed] [Google Scholar]
- 160. Backert S., Ziska E., Brinkmann V., Zimny-Arndt U., Fauconnier A., Jungblut P. R., Naumann M., Meyer T. F. (2000) Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2, 155–164 [DOI] [PubMed] [Google Scholar]
- 161. Odenbreit S., Püls J., Sedlmaier B., Gerland E., Fischer W., Haas R. (2000) Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287, 1497–1500 [DOI] [PubMed] [Google Scholar]
- 162. Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 163. Russell R. B., Eggleston D. S. (2000) New roles for structure in biology and drug discovery. Nat. Struct. Biol. 7, (suppl.) 928–930 [DOI] [PubMed] [Google Scholar]
- 164. Hopkins A. L., Groom C. R. (2002) The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 [DOI] [PubMed] [Google Scholar]
- 165. Cachau R. E., Podjarny A. D. (2005) High-resolution crystallography and drug design. J. Mol. Recognit. 18, 196–202 [DOI] [PubMed] [Google Scholar]
- 166. Orry A. J., Abagyan R. A., Cavasotto C. N. (2006) Structure-based development of target-specific compound libraries. Drug Discov. Today 11, 261–266 [DOI] [PubMed] [Google Scholar]
- 167. White S. H. (2004) The progress of membrane protein structure determination. Protein Sci. 13, 1948–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Daley D. O., Rapp M., Granseth E., Melén K., Drew D., von Heijne G. (2005) Global topology analysis of the Escherichia coli inner membrane proteome. Science 308, 1321–1323 [DOI] [PubMed] [Google Scholar]
- 169. Eaton K. A., Brooks C. L., Morgan D. R., Krakowka S. (1991) Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 59, 2470–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Marshall B. J., Barrett L. J., Prakash C., McCallum R. W., Guerrant R. L. (1990) Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology 99, 697–702 [DOI] [PubMed] [Google Scholar]
- 171. Oh J. D., Kling-Bäckhed H., Giannakis M., Xu J., Fulton R. S., Fulton L. A., Cordum H. S., Wang C., Elliott G., Edwards J., Mardis E. R., Engstrand L. G., Gordon J. I. (2006) The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc. Natl. Acad. Sci. U.S.A. 103, 9999–10004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Psakis G., Nitschkowski S., Holz C., Kress D., Maestre-Reyna M., Polaczek J., Illing G., Essen L. O. (2007) Expression screening of integral membrane proteins from Helicobacter pylori 26695. Protein Sci. 16, 2667–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kim K. M., Lee S. G., Joo J. S., Kwon Y. C., Bea D. W., Song J. Y., Kang H. L., Lee W. K., Cho M. J., Rhee K. H., Youn H. S., Baik S. C. (2008) Proteomic analysis of Helicobacter pylori J99 outer membrane protein by tandem mass spectrometry. J. Bacteriol. Virol. 38, 53–60 [Google Scholar]
- 174. Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. (1990) Purification and characterization of urease from Helicobacter pylori. J. Biol. Chem. 265, 9464–9469 [PubMed] [Google Scholar]
- 175. Evans D. J., Jr., Evans D. G., Engstrand L., Graham D. Y. (1992) Urease-associated heat shock protein of Helicobacter pylori. Infect. Immun. 60, 2125–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hare S., Fischer W., Williams R., Terradot L., Bayliss R., Haas R., Waksman G. (2007) Identification, structure and mode of action of a new regulator of the Helicobacter pylori HP0525 ATPase. EMBO J. 26, 4926–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Karasawa A., Mitsui K., Matsushita M., Kanazawa H. (2007) Functional assembly of the Na+/H+ antiporter of Helicobacter pylori from partial fragments in vivo. Biochemistry 46, 14272–14283 [DOI] [PubMed] [Google Scholar]
- 178. Sprinzak E., Sattath S., Margalit H. (2003) How reliable are experimental protein-protein interaction data? J. Mol. Biol. 327, 919–923 [DOI] [PubMed] [Google Scholar]
- 179. Peck B., Ortkamp M., Diehl K. D., Hundt E., Knapp B. (1999) Conservation, localization and expression of HopZ, a protein involved in adhesion of Helicobacter pylori. Nucleic Acids Res. 27, 3325–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. McAtee C. P., Fry K. E., Berg D. E. (1998) Identification of potential diagnostic and vaccine candidates of Helicobacter pylori by “proteome” technologies. Helicobacter 3, 163–169 [PubMed] [Google Scholar]
- 181. Fujii R., Morihara F., Fukushima K., Oku T., Hifumi E., Uda T. (2004) Recombinant antigen from Helicobacter pylori urease as vaccine against H. pylori-associated disease. Biotechnol. Bioeng. 86, 737–746 [DOI] [PubMed] [Google Scholar]
- 182. Radcliff F. J., Hazell S. L., Kolesnikow T., Doidge C., Lee A. (1997) Catalase, a novel antigen for Helicobacter pylori vaccination. Infect. Immun. 65, 4668–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rossi G., Ruggiero P., Peppoloni S., Pancotto L., Fortuna D., Lauretti L., Volpini G., Mancianti S., Corazza M., Taccini E., Di Pisa F., Rappuoli R., Del Giudice G. (2004) Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: safety, immunogenicity, and efficacy. Infect. Immun. 72, 3252–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bai Y., Zhang Y. L., Wang J. D., Lin H. J., Zhang Z. S., Zhou D. Y. (2002) Conservative region of the genes encoding four adhesins of Helicobacter pylori: cloning, sequence analysis and biological information analysis. Di Yi Jun Yi Da Xue Xue Bao 22, 869–871 [PubMed] [Google Scholar]
- 185. Bai Y., Zhang Y. L., Wang J. D., Zhang Z. S., Zhou D. Y. (2003) Cloning and immunogenicity of conservative region of adhesin gene of Helicobacter pylori. Zhonghua Yi Xue Za Zhi 83, 736–739 [PubMed] [Google Scholar]
- 186. Bina J., Bains M., Hancock R. E. (2000) Functional expression in Escherichia coli and membrane topology of porin HopE, a member of a large family of conserved proteins in Helicobacter pylori. J. Bacteriol. 182, 2370–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.