Abstract
Redox reactions are imperative to preserving cellular metabolism yet must be strictly regulated. Imbalances between reactive oxygen species (ROS) and antioxidants can initiate oxidative stress, which without proper resolve, can manifest into disease. In type 1 diabetes (T1D), T-cell-mediated autoimmune destruction of pancreatic β-cells is secondary to the primary invasion of macrophages and dendritic cells (DCs) into the islets. Macrophages/DCs, however, are activated by intercellular ROS from resident pancreatic phagocytes and intracellular ROS formed after receptor-ligand interactions via redox-dependent transcription factors such as NF-κB. Activated macrophages/DCs ferry β-cell antigens specifically to pancreatic lymph nodes, where they trigger reactive T cells through synapse formation and secretion of proinflammatory cytokines and more ROS. ROS generation, therefore, is pivotal in formulating both innate and adaptive immune responses accountable for islet cell autoimmunity. The importance of ROS/oxidative stress as well as potential for redox modulation in the context of T1D will be discussed.
1. Introduction
Oxidation-reduction or redox reactions are pivotal to maintaining life through respiration, metabolism, and energy supply. Mitochondria, which are known to be the powerhouses of the cell, possess the ability to utilize nutrients to generate energy (redox potential) via the electron transport chain, which donates electrons to oxygen to yield ATP and H2O [1, 2]. Consequently, oxygen free radicals, known as superoxide (O2 −), are nonenzymatically leaked from the mitochondria and react with other molecules to create reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), peroxynitrite (ONOO−), and hydroxyl radical (OH−), all of which can alter DNA, proteins, carbohydrates, and nucleic acids [3–5] and may eventually lead to irreversible damage. The inability of a cell's antioxidant defenses to overcome oxidative injury and accretion of severe ROS-mediated damage over time will eventually lead to cell death [5–7]. In order to maintain a reduced environment, several cellular antioxidant defenses are in place, including glutathione, glutathione peroxidase, catalase, and three different superoxide dismutase (SOD) enzymes: SOD1, 2, and 3, located in different subcellular and extracellular locations. A basal level of “accidental” superoxide is accumulated in healthy individuals [1, 8], which has been widely hypothesized to be responsible for aging and the associated pathologies [9–11]. However, oxidative stress occurs from an imbalance between ROS and antioxidant actions. During chronic oxidative stress caused by environmental factors (i.e., UV light, ionizing radiation, toxic substances), infections, or lack of dietary antioxidants, an inequity of cellular reducing equivalents capable of detoxifying the increased burden of ROS has marked effects on normal cellular processes. However, in times of oxidative stress, normal cellular respiration is also still functioning, resulting in dysregulated mitochondrial free radical production and disparity between ROS generation and antioxidant defenses [6, 12]. The combination of stress-induced and conventional mitochondrial dysfunction can manifest into disease states, including cancer [13–15], rheumatoid arthritis [16, 17], neurological disorders [18–21], pulmonary diseases [22], and type 1 diabetes [23–26].
2. Redox and Inflammation
What once was thought to be solely derived from the mitochondria, reactive oxygen species have now been shown to be produced by an important family of primarily immune system-associated enzymes [27–29]. The NADPH oxidase (NOX) family of enzymes is designed to combine NADPH and oxygen to actively generate superoxide. Activated phagocytes, such as macrophages, monocytes, and dendritic cells (DCs), as well as neutrophils, form ROS within the phagosomal membrane for efficient killing of a wide array of invading pathogens [30]. The protection afforded by the phagocytes is crucial, but not without side effects. Production of highly permeable reactive oxygen species (i.e., H2O2) causes leakage of these molecules from phagocytes and therefore, unwanted effects on bystander cells [31, 32]. In an environment high in oxidative stress, these bystander reactions drive increased activation of the immune system, cell damage, and progression to disease. For example, NOX-derived ROS have been shown to stimulate mitogenic signaling and proliferation [33, 34], which can have potential deleterious consequences on the promotion of tumorigenesis [35, 36] and in the context of autoimmunity, can lead to T cell expansion [37]. Additionally, H2O2 can augment monocyte chemokine receptor surface expression critical for migration to sites of infection and inducing inflammation [38] as well as can promote VEGF signaling to trigger angiogenesis, with implications in cancer and tumor progression [39]. Furthermore, ROS generated from both mitochondria and NADPH oxidase complexes can also act intra-cellularly as well as inter-cellularly as signal transduction molecules. Hydrogen peroxide has been suggested to inactivate protein phosphatases [40], as well as to activate protein tyrosine kinases [41, 42] and metalloproteases through the oxidation of critical cysteine residues [43, 44]. Phosphatases such as SHP-1 serve to decrease inflammation by inhibiting tyrosine kinase activity, yet this type of regulation is lost upon cysteine oxidation [45–48]. Similarly, latent metalloproteases require oxidation for activation and, in the presence of hypochlorous acid (HOCL) and H2O2, secretion of chemotactic mediators (L-selectin and proinflammatory TNFα) is highly increased [49], thus enhancing inflammation. In addition, H2O2 has been demonstrated to freely cross the plasma membrane and activate NF-κB, a redox-dependent transcription factor [50, 51]. NF-κB plays a major role in immunity by promoting proinflammatory cytokine production, cell proliferation, and inflammation. In general, receptor-ligand interactions are known to generate ROS [52, 53]. In the immune system specifically, LPS interaction with Toll-like receptor 4 (TLR4) has been shown to facilitate the binding of TLR4 to NADPH oxidase 4 (Nox4) and subsequently release ROS [54], resulting in the activation of NF-κB and generation of proinflammatory cytokines IL-1β and TNFα [53]. In a highly oxidized environment, the binding of pathogens to innate cell receptors can lead to hyperresponsiveness [55], suggesting inflammation is secondary to oxidative stress [25, 56]. Not only are phagocytic cells critical for early pathogen recognition through receptor-ligand interaction, they are also necessary for activation of the adaptive immune response. Following antigen recognition by phagocytic antigen-presenting cells (APC), an adaptive immune response is acquired in secondary lymphoid organs through synapse formation of APCs with lymphocytes, as well as from critical innate-derived ROS and third signal proinflammatory cytokines (TNFα, IL-1β) enhancing T-cell activation, proliferation, and effector function [37, 57]. Within this interaction, the H2O2 made by the phagocytes is able to traverse the synapse and act upon the T cells, at concentrations ranging from 10–100 μM [58, 59], resulting in a feed-forward mechanism stimulating T-cell-specific NF-κB activity and subsequent proinflammatory cytokine production. Similar effects of ROS are also seen on B cells [60]. Moreover, antigen stimulation of the TCR also drives endogenous production of H2O2 through the T cell's own NOX enzyme [28, 61]. Intracellular H2O2 can then signal and lead to T-cell proliferation, apoptosis [61, 62], and in conjunction with proinflammatory cytokines, promote T-cell effector function [37, 52, 63]. Therefore, in the presence of oxidative stress, an inability to balance the oxidation with antioxidant enzymes can drive chronic inflammation from both the innate and adaptive arms of the immune response [64], manifesting into many clinically relevant diseases, particularly type 1 diabetes.
3. Oxidative Stress and Type 1 Diabetes
Type 1 diabetes or insulin-dependent diabetes mellitus (T1D) is an autoimmune disorder involving immune-mediated recognition of islet β-cells by autoreactive T cells, which leads to the liberation of ROS and proinflammatory cytokines, resulting in the destruction of pancreatic β-cells in the islets of Langerhans and loss of insulin secretion. Patients with T1D must constantly prevent hyperglycemia by administering exogenous insulin or in the situation of severe hyperglycemic unawareness, by undergoing islet transplantation. Despite a multitude of efforts in trying to specify the exact etiology, the cause of T1D is still under debate. The combinatorial effects of genetic susceptibility, environmental factors, and dietary deficiencies are known to contribute to disease origin; however, the impact of oxidative stress in a genetically susceptible individual is of particular interest. Oxidative stress, as stated above, occurs when the generation of ROS overcomes the scavenging abilities of antioxidants. Such instances may be mediated by genetic lack of antioxidant enzymes as well as environmental triggers like viral infections. Overall, oxidative stress has been linked to β-cell cytotoxicity [65–67] and has been suggested to play a role in T1D pathology [68–71]. Several studies show that the total serum antioxidant status, as measured by urate, Vitamin C, and total plasma antioxidant levels, of prediabetic and T1D patients is lower in comparison to age-matched controls [72, 73], which inevitably leads to greater oxidative modification of proteins and lipids [74]. Other literature illustrates a connection between viruses, ROS production, and type 1 diabetes onset. Gamble et al. demonstrated a positive correlation between type 1 diabetes onset and Coxsackie B4 virus infection through antibody titer measurements [75, 76]. Furthermore, such infections have been shown to cause indirect [77] and direct β-cell damage [78] and to stimulate the β-cells into secreting inflammatory mediators themselves [79]. ROS are made following viral infection from activated phagocytes [80, 81], as mentioned previously, and work to not only cause cellular injury but also can activate inflammatory, redox-dependent transcription factors, such as NF-κB, perpetuating inflammation. Viral-mediated ROS production or a reduction in antioxidants can have severe consequences as β-cells are more prone to oxidative damage than most other tissues. The β-cell mitochondria have exceptionally low levels of glutathione peroxidase, superoxide dismutase, and catalase activity [24, 82–84]. Because of this low antioxidant defense, β-cells can be clearly disrupted by oxidative stress and, in genetically predisposed individuals, results in easy targets for a subsequent cytokine-mediated autoimmune attack.
Mitochondrial and NOX-derived ROS both have implications in β-cell destruction and T1D. Increased glucose causes rapid induction of the tricarboxylic acid (TCA) cycle within the β-cell mitochondria, which can lead to augmented ROS production [85]. The superoxide leaked from mitochondria can then form H2O2 and work to uncouple glucose metabolism from insulin secretion [86]. Ultimately, high levels of mitochondrial ROS can cause β-cell death [87, 88]. Intriguingly, models of T1D induce disease by generating toxic amounts of ROS within the islets (i.e., streptozotocin and alloxan) [89]. Alloxan is easily taken up by β-cells [90], where it is reduced into dialuric acid and subsequently reoxidized to establish a redox cycle [91]. ROS generated by alloxan treatment have been shown to promote islet β-cell DNA fragmentation, culminating in cell death [92]. In contrast, an alloxan-resistant strain of mice, the ALR mouse, shows increased ROS dissipation and resistance to islet destruction [23, 93, 94], further implicating the importance of oxidative stress and T1D. Streptozotocin (STZ), on the other hand, causes β-cell DNA alkylation and eventually drains the cellular NAD+ and ATP source in an effort to repair the DNA [95]. Xanthine oxidase is then able to utilize dephosphorylated ATP as a substrate for superoxide production [96]. Additionally, STZ metabolism increases the levels of islet cell nitric oxide (NO) [97], which together with superoxide can generate peroxynitrite (ONOO−). Detection of peroxynitrite in prediabetic nonobese diabetic mouse (NOD) islets suggests importance of this ROS in β-cell death [71]. Similarly, NOX enzymes have been detected within the pancreatic β-cells [98, 99]. Hyperglycemia can increase the assembly of NOX enzymes through its p47phox subunit, and therefore, enhance superoxide generation [100] and facilitate β-cell death.
4. Immunology of T1D
Autoimmune diabetes onset is preceded by infiltration of immune cells into the pancreatic islets. Ultimately, a breach in tolerance to self-antigens allows for autoreactive T cells to become activated and attack the β-cells, resulting in the loss of insulin secretion. However, innate immune cells, such as macrophages and DCs, are of the first cells to enter the islets during insulitis [101, 102]. Although resident macrophages are present in the pancreas at all times, acquisition of antigen is required for macrophage activation and the production of cytokines. As described above, genetic and environmental factors can lead to cell destruction, releasing β-cell-specific antigens as well as ROS [103]. Macrophages will phagocytose dying β-cells and present antigen in the context of their MHC molecules. In humans, specific HLA molecules HLA-DR3 and DR4 are correlated with a susceptibility to T1D [103, 104]. Moreover, the ROS created by the initial insult to the islets are able to stimulate the activation of redox-dependent NF-κB and other transcription factors within the macrophages [105]. Activated macrophages secrete a mixture of proinflammatory cytokines such as TNFα, IL-6, IL-1β, and ROS, which can start to damage the pancreatic β-cells [106–108]. IL-1β can cause extensive cytolysis in β-cells [109] through the upregulation of iNOS and subsequent generation of nitric oxide (NO) [110, 111], whereas TNFα enhances IL-1β-mediated islet destruction and helps activate APCs and T cells [112–114], but does not cause direct β-cell apoptosis in vivo [114].
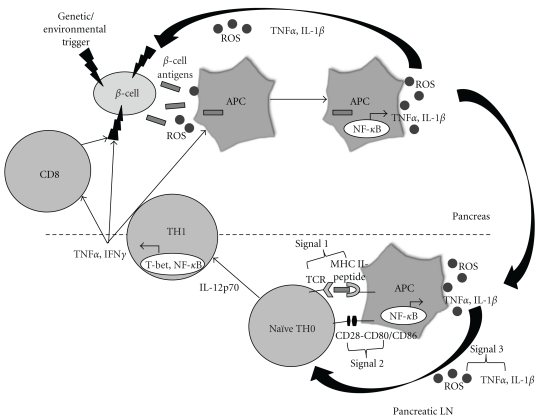
ROS and cytokines released by APCs not only promote β-cell damage, but also help to generate an adaptive immune response, which in T1D, is the crucial step in autoimmune destruction. It is well established that chronic elicitation of antigens to innate immune cells in a highly oxidized environment will lead to MHC-peptide presentation, perpetuating an adaptive immune response [115, 116] (Figure 1).
Figure 1.
Role of redox in the immunopathology of type 1 diabetes. An initial genetic or environmental insult to the beta cell triggers the release of beta cell antigens as well as the production of ROS. Beta cell antigens are phagocytosed, and ROS are able to stimulate redox-dependent transcription factors such as NF-κB, which leads to APC activation and cytokine secretion. ROS and proinflammatory cytokines secreted by APCs act as the third signal within the T-cell-APC immunological synapse, which occurs in the pancreatic lymph node. ROS play a critical role in the progression of naïve TH0 cells to cytokine-secreting TH1 cells. Release of IFNγ by TH1 cells then works directly on the beta cells as well as activates more APCs and CD8 cells, all of which can impart deleterious effects on the islets.
In the context of continuous β-cell ablation, macrophages can phagocytose dying cells and migrate to the pancreatic lymph node where they interact with naïve T-cells. It is this aforementioned synapse that enables T-cell proliferation and effector function to occur. In the presence of all three necessary signals: (1) MHC-peptide, (2) costimulation, and (3) soluble third signal, in this case consisting of ROS, IL-1β, and TNFα, T cells become activated via NFAT and NF-κB [117–121]. Furthermore, IL-12 released from macrophages can differentiate CD4+ T cells into the TH1 lineage via signaling through STAT4 [122–125]. CD4+ TH1 cells then home to the site of antigen production, the β-cells, and call in other T cells and more APCs through the secretion of IFNγ. IFNγ has some indirect effects on β-cells, including potentiating the maturation of pancreatic APCs, which can then elicit an even greater T-cell response [126]. Additionally, neutralization of IFNγ in NOD mice has been shown to reduce both diabetes and insulitis [127], whereas IFNγR-deficient NOD mice demonstrate delayed insulitis, but do not develop T1D [128]. Proinflammatory cytokines TNFα, IL-1β, and IFNγ all play a role in β-cell death primarily through activation of redox-regulated transcription factors NF-κB and STAT1 [129–131]. Combinations of TNFα with IFNγ or IL-1β are necessary for primary murine β-cell death [132], and TNFα/IFNγ act synergistically to activate the stress-activated proapoptotic JNK/SAPK pathway, which promotes β-cell apoptosis via p53 and intracellular ROS [133]. The activation of NF-κB can also increase iNOS and Fas expression, potential inducers of cell death, while downregulating the antiapoptotic Bcl-2 gene [134]. Apoptosis of β-cells is also mediated partially by T-cell expression of Fas ligand, TNFα, and perforin/granzyme [114, 134]. Specifically, CD4+ T cells are thought to be sufficient for T1D onset [135, 136], whereas CD8+ T cells seem to play a lesser role in the final stage of autoimmune destruction [137]. It is known, however, that synergy between both CD4 and CD8 T cells results in absolute transfer of diabetes in rodent models [138, 139]. Although specific to the model of autoimmune diabetes, TNFα secretion from CD4+ T cells can activate TNFR1 on β-cells and cause apoptosis [140], while CD8+ T cells can kill NOD β-cells by a Fas-dependent mechanism [141] or by perforin release [142]. Ultimately, T-cell exacerbation of β-cell death comes from endogenous generation of ROS and cytokines following APC activation [143] that can perpetuate islet destruction through a feed forward mechanism. Overall, ROS are crucial in not only activating the initial infiltrating macrophages and DCs [144] via the common denominator NF-κB, but also for subsequently driving an adaptive TH1 immune response that is necessary for total ablation of β-cells and progression to T1D [134, 136]. Therefore, therapies would be most beneficial if there was not only protection of the β-cells from ROS, but also inhibition of the ROS-mediated autoimmune attack, possibly by preventing NF-κB activation, ensuing inflammation, and the initiation of the adaptive immune response.
5. Controlling Redox in T1D
Glutathione peroxidase (GPX), superoxide dismutase (SOD), and catalase are categorized as the most crucial antioxidant enzymes; however, islets inherently contain only a fraction of the enzymatic activities in comparison to liver, which possesses the highest abundance [145]. Because of the low antioxidant defenses present in pancreatic islets, therapeutic strategies to enhance antioxidants and reducing capabilities are of utmost importance. Studies utilizing overexpression of GPX1, SOD1 (Cu/Zn SOD), SOD2 (MnSOD), or SOD mimetic administration in insulinoma cell lines such as NIT-1 and INS-1 afforded protection from ROS and reactive nitrogen species (RNS) in vitro [25, 146, 147]. Usage of SOD mimetics in other inflammatory models has also demonstrated diminutions in proinflammatory cytokines [148, 149]. Furthermore, stable transfection of insulin-producing RINm5F cells with GPX, catalase, and Cu/Zn SOD resulted in defenses against cytokine toxicity imparted by the combination of IL-1β, TNFα, and IFNγ [150]. Antioxidant overexpression has been linked to not only protection against ROS and cytokines, but also to enhanced cell proliferation and decreased death. PDX1, a transcription factor necessary for β-cell differentiation, survival, and insulin synthesis [151], is also very responsive to ROS [152], where high oxidation causes a cytoplasmic relocation of PDX1 out of the nucleus, increased degradation of the protein, and subsequent dysfunction of β-cells [153, 154]. By alleviating ROS within the islets, PDX1 protein has exhibited stability and enhanced function in type 2 diabetes models [155], which can also have implications in T1D for stabilizing β-cell function and survival. Other experiments utilize transgene or adenoviral technology to overexpress antioxidant genes within the β-cells to specifically show islet-mediated versus autoimmune protection from T1D. These studies have elicited conflicting results. For example, overexpression of metallothionein and catalase in β-cells was unable to delay or inhibit spontaneous diabetes onset within NOD mice and promoted reduced activation of the PDX1 survival pathway [156]. Metallothionein proteins are intracellular, cysteine-rich molecules with high redox potential [157]. Similarly, transgenic expression of extracellular SOD in β-cells does not confer any difference in T1D incidence in comparison to control NOD mice [158]. These results suggest that basal levels of ROS production are necessary for β-cell function, possibly by triggering appropriate insulin signaling and regulating cell survival [159]. In contrast, overexpression of thioredoxin, a redox-regulated protein which helps repair ROS-damaged proteins and DNA, constitutes protection of β-cells from autoimmune and STZ-induced diabetes [160]. β-cell-specific transgenic expression of catalase and metallothionein is also able to shield isolated islets from hydrogen peroxide and reduce the effects of STZ treatment [161–163]. Transgenic expression of heme oxygenase-1, which has crucial cytoprotective functions against oxidative stress and inflammation, can improve insulitis and spontaneous diabetes in NOD mice [164], and alloxan-induced diabetes is also reduced following overexpression of Cu/Zn SOD in β-cells [165]. Moreso, precedence for the importance of enhancing islet-associated antioxidant levels has been demonstrated at the genetic level, in which mice resistant to alloxan treatment (ALR mice) exhibit protection from diabetes [94, 166]. This finding particularly helps further justify the need for therapeutic discovery and necessary experiments to determine druggable targets based upon modulation of antioxidant function.
Systemic administration of antioxidants, in comparison to overexpression studies, shows more consistency in ameliorating T1D. Administration of 16 mg/kg/day of a potent antioxidant to young NOD mice resulted in a reduction of diabetes incidence from 89% in controls to 44% in the treated animals [167]. Furthermore, after a multiple low dose administration of STZ, addition of zinc sulphate to the drinking water of animals was able to increase metallothionein levels, inhibiting the onset of T1D [168], whereas intraperitoneal injections of butylated hydroxyanisole (BHA) antioxidant were able to attenuate the production of proinflammatory cytokines by islets and macrophages, thereby lowering insulitis and hyperglycemia [169]. Such uniformity in these results versus the transgenic expression of multiple antioxidants, as discussed above, may relate to the ability of systemic therapies to not only protect the β-cells but to also inhibit immune system activation and inflammation. Adenoviral delivery of systemic heme oxygenase to NOD mice decreased insulitis and T1D incidence; however, this alleviation was associated with a decrease in mature DCs and TH1 effector function [170]. Additionally, ALR mice resistant to alloxan-induced diabetes contain specific genetic modifications conferring systemic elevation of antioxidants, resulting in neutrophils with reduced superoxide bursts [171]. In an in vitro system using the antioxidant probucol, which can delay alloxan-induced [172] and spontaneous diabetes in rats [173], macrophages exhibit decreased H2O2 production, thus maintaining islet viability [174]. Further reports on the effects of systemic antioxidants on innate immunity include studies from our lab utilizing metalloporphyrin-based catalytic antioxidants (CA) with bone marrow-derived macrophages. The CA houses a metal center that catalyzes superoxide dismutation, mimicking SOD activity [175, 176], and is able to scavenge a broad range of ROS including O2 −, H2O2, ONOO−, and lipid peroxyl radicals [53, 177, 178]. Following treatment with CA and LPS stimulation of macrophages, the production of nitrite (NO2 −), O2 − TNFα, and IL-1β was significantly reduced in comparison to control [25, 53]. This effect was mediated by the ability of CA to oxidize the p50 subunit of NF-κB within the nucleus, inhibiting its binding to DNA and subsequent transcription of proinflammatory cytokines [53]. Redox modulation of transcription factor DNA binding has previously been demonstrated for NF-κB as well as other eukaryotic molecules [179, 180]. Inhibition of NF-κB has been well established as an effective method of thwarting the immune response and resolving inflammation to maintain β-cell integrity [181, 182]; however, we are the first to illustrate a link between metalloporphyrin catalytic antioxidants, blockade of NF-κB activation, and delayed autoimmune diabetes, as described below.
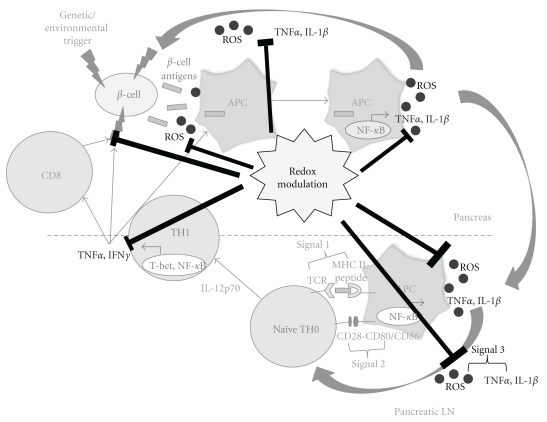
The activation of macrophages and T cells relies on oxidative stress, which ultimately leads to the progression of T1D. Based upon this fact, CA was also investigated in the context of CD4 and CD8 T cells. The BDC-2.5 TCR-Tg TH1 cell clone, which has recently been described as specific for the protein ChgA, a member of the granin family of neuroendocrine secretory proteins [183], causes rapid transfer of diabetes into NOD.scid recipients [184]. By utilizing this method, pretreatment of NOD.scid mice with CA prior to adoptive transfer of the BDC-2.5 clone inhibits the infiltration of T cells into the pancreas, significantly delaying T1D onset. Moreover, APC-dependent BDC-2.5 T cell proliferation and IFNγ production are also reduced after in vitro CA treatment [25]. To further delineate the mechanism of diminished T-cell effector function, in vivo treatment of NOD and BDC-2.5 TCR-Tg mice with CA was able to decrease innate-derived third signal synthesis, primarily consisting of TNFα, resulting in antigen-specific T cell hyporesponsiveness [37]. Similar results were found upon CA treatment in the context of CD8 T cells, reducing proliferation, cytokine production, and cytolytic effector molecules of CTLs [185]. Interestingly, by inhibiting NADPH oxidase in NOD animals (NOD.Ncf1m1J) in an effort to genetically mimic systemic CA administration, not only is NOX-derived superoxide production eliminated, but T cells show reduced TH1 responses, granting protection from T1D onset [120]. Earlier studies by Chaudhri et al. supported our experimentation by demonstrating attenuation of T-cell proliferation and IL-2R expression following antioxidant treatment [186, 187]. Such findings point to the possibility and importance of redox modulation in not only regulating the innate immune cells, but also impacting the T cells which formulate an adaptive immune response crucial for the autoimmune attack in T1D (Figure 2).
Figure 2.
Role of redox modulation in controlling ROS-mediated beta cell destruction. Redox modulation has shown promise in blocking the production of ROS and its ability to activate APCs, resulting in diminished TH1 cell activation and effector function, which ultimately may help regulate beta-cell destruction.
In addition to decreasing oxidative stress imposed on the islets, which can directly damage β-cells or indirectly stimulate the autoreactive immune response to become activated, redox modulation may also be useful for decreasing the unyielding ER stress within the β-cells. Because the β-cells are a constant source of insulin and insulin must be folded properly for secretion, the importance of balancing a high protein-folding load with survival of the cells increases substantially in comparison to other nonsecretory cells [188]. An overload of misfolded proteins may eventually result in cell death, if not properly resolved. An early study by Lo et al. highlighted the susceptibility of β-cells to ER stress by overexpressing MHC class II proteins in islets, essentially overwhelming the protein folding machinery and leading to apoptosis [189]. Other more recent studies show biochemical connections between ER stress-induced apoptosis and β-cell death, through both calcium-dependent and independent molecules [190–192]. To reconcile protein misfolding within the ER, the unfolded protein response, or UPR, is consequently triggered [193, 194]. The UPR acts as a backup mechanism to protect cells from accumulating unfolded proteins and to restore the balance between the protein folding machinery and the secretory pathway [195]. However, an accumulation of unfolded proteins during severe ER stress is sometimes unable to be resolved by the UPR, as characterized in the Akita mouse which contains a mutation in the proinsulin 2 gene that disrupts insulin folding, retains it within the ER, activates UPR, yet still eventually leads to β-cell death [196, 197]. Moreover, ROS have been suggested in supporting the UPR towards a more proapoptotic than proadaptive level [198], further illustrating the importance of regulating oxidative stress to maintain β-cell survival. Although the UPR paradoxically utilizes an oxidative environment within the ER to correctly fold proteins (i.e., disulfide bond formation), sustained oxidative stress can perpetuate the UPR to a level that promotes apoptosis [198, 199]. Additionally, the abundance of ROS present during continued unadapted ER stress can trigger apoptosis in neighboring cells as well. This is especially critical in islet β-cells, where their ability to handle oxidative stress is already reduced because of low levels of antioxidants [24, 82–84]. More pertinent is when unresolved ER stress leads to dying β-cells containing the misfolded proteins. These cells can be taken up by resident pancreatic APCs and presented to autoreactive T cells within the pancreatic lymph nodes. This type of event may stimulate the reactivity of T cells to formerly tolerated “neo-autoantigens,” which can ultimately promote more β-cell destruction and eventual development of autoimmune diabetes [200, 201]. A study conducted by Malhotra, et al. shows that antioxidant treatment of CHO cells results in not only decreased oxidative stress, but also decreased misfolded proteins, reduced activation of the UPR, and enhanced secretion of proteins [188]. Thus, it appears that a temporal or redox balance is essential for optimal β-cell function. In situations where the β-cell may experience environmental stressors that lead to disruption of the ER-machinery, the results may set in motion both ER-stress-induced UPR and the expression of misfolded proteins in an oxidative environment, further providing an optimal milieu for driving autoreactive T cells to become activated. Therefore, redox modulation may serve yet another purpose: to help reduce ER stress and subsequently maintain β-cell viability.
Although the ability to predict susceptibility to type 1 diabetes is becoming increasingly accurate [202], and therefore, prophylactic treatment of patients with antioxidant therapeutics is not out of the realm of possibilities, currently a more feasible option for individuals with chronic hyperglycemia is to undergo islet transplantation. Islets, like any other transplantable organ, are in short supply; however, maintaining function and viability of transplanted islets is the major drawback of the procedure [161]. Not only are islets susceptible to immune rejection, but hypoxia during isolation and transplantation is the primary cause of β-cell death [203]. Because of their low resistance to ROS [24, 82–84], β-cells are especially vulnerable to oxidative damage and ischemia-reperfusion injury [204, 205]. In order to combat this weakness, the application of antioxidants seems a suitable alternative, as they have shown promise in liver and kidney transplantations [206, 207]. Longer allograft survival times have been demonstrated with mouse islets soaked with hydroxyl-radical inhibitors prior to transplantation [208] and with multiple in vivo administrations of SOD and catalase prior to and after islet transplantation [209]. Likewise, transduction of islets with heme oxygenase-1 or SOD2 genes was able to improve viability and insulin secretion in vitro [210] and elicit greater functionality upon transplantation in comparison to controls [211], respectively. Furthermore, we have also demonstrated benefit using the catalytic antioxidant approach, whereby adding CA during and after human islet isolation enhanced cell survival and function, allowing for normalization of STZ-induced diabetic NOD.scid mice [212]. Additionally, CA is not only able to protect human islets from STZ cell damage, but can also protect murine islets from both antigen-independent innate-mediated inflammation and antigen-dependent T-cell-mediated allograft rejection [204]. Overall, unlike common antirejection drugs, which are outstanding at protecting against the adaptive immune response but fail to shield islets from ROS/inflammation [213, 214], our CA treatment is nontoxic to islets and can alleviate both the alloimmune [204] and autoimmune responses [25, 37, 53, 185].
6. Conclusion
Although redox has been extensively studied in the context of both T1D and type 2 diabetes [85, 215], the plethora of literature discussed above shows the implications of ROS in all stages of autoimmune T1D, including the primary “trigger”, the initiation of insulitis by the innate immune system, and the acquisition of T-cell-mediated autoreactivity. These studies open the door to novel ideas of redox modulation, such as targeting ROS-dependent immunological metalloproteases [43, 44, 49] or disrupting the autoreactive T-cell pool, as described [37, 120, 185]. Moreover, a study evaluating self-antigen-primed T cells demonstrates how NO is able to reduce FOXP3 expression and subsequently decrease Tregs in autoimmune disorders [216], illustrating how intricate and vast the role of redox is in the immune response and where future studies may focus. In addition to effects on the target organ(s) and the immune system, autoimmunity also gives rise to systemic problems, and in the context of diabetes, ROS have been characterized as crucial elements promoting hyperglycemia-induced diabetic complications, especially those involving the vasculature [6, 217]. One important study conducted by Ling et al. provided evidence of oxidative stress-mediated vascular complications in prediabetic NOD mice [218], which exemplifies the importance of ROS in not only exacerbation of disease, but also on initiation of T1D and nonhyperglycemic associated pathologies. Furthermore, utilizing antioxidants, such as Vitamin E, cannot only assuage vascular activation [219], but can also grant protection from the loss of secondary target organ function, such as the kidneys [220]. Therefore, oxidative stress affects every aspect of T1D and the benefit of redox modulation may be more important than once thought. Optimal treatments may have to incorporate antioxidants with anti-inflammatory agents, such as inhibitors of NF-κB activation, and must also take into consideration the limitations associated with utilization of intact enzyme/protein therapies, including bioavailability, immunogenicity-limited cellular accessibility, and cost of production. However, with the advent of newer nonpeptidyl small compounds, alleviating oxidative stress through antioxidant therapy appears to be a plausible druggable target. This therapy should restore balance between oxidation and reduction, leading to resolution of inflammation, thus reducing the autoimmune destruction of the islet β-cells.
References
- 1.McCord JM. The evolution of free radicals and oxidative stress. American Journal of Medicine. 2000;108(8):652–659. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 2.Rich P. Chemiosmotic coupling: the cost of living. Nature. 2003;421(6923):p. 583. doi: 10.1038/421583a. [DOI] [PubMed] [Google Scholar]
- 3.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochemical Journal. 1972;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Bioscience Reports. 1997;17(1):3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 5.Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96(2):291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- 6.Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutation Research. 2009;674(1-2):137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radical Biology and Medicine. 2000;29(3-4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 8.Harman D. The aging process. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 10.Mitsui A, Hamuro J, Nakamura H, et al. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxidants and Redox Signaling. 2002;4(4):693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 11.Schriner SE, Linford NJ, Martin GM, et al. Medecine: extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B. Antioxidants in human health and disease. Annual Review of Nutrition. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 13.Klaunig JE, Xu Y, Isenberg JS, et al. The role of oxidative stress in chemical carcinogenesis. Environmental Health Perspectives. 1998;106(1):289–295. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Research. 1979;39(4):1141–1149. [PubMed] [Google Scholar]
- 15.Toyokuni S. Persistent oxidative stress in cancer. FEBS Letters. 1995;358(1):1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 16.Bashir S, Harris G, Denman MA, Blake DR, Winyard PG. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Annals of the Rheumatic Diseases. 1993;52(9):659–666. doi: 10.1136/ard.52.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clinica Chimica Acta. 2003;338(1-2):123–129. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radical Biology and Medicine. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 19.Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47(6):S161–S170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 20.Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Experimental Neurology. 1999;160(1):28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. Journal of Neuroscience. 1997;17(8):2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. European Journal of Pharmacology. 2006;533(1–3):222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Gusdon AM, Thayer TC, Mathews CE. Role of increased ROS dissipation in prevention of T1D: lessons from the ALR mouse. Annals of the New York Academy of Sciences. 2008;1150:157–166. doi: 10.1196/annals.1447.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenzen S. Oxidative stress: the vulnerable β-cell. Biochemical Society Transactions. 2008;36(3):343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- 25.Piganelli JD, Flores SC, Cruz C, et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes. 2002;51(2):347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 26.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxidants and Redox Signaling. 2010;12(4):537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. Journal of Clinical Investigation. 1973;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nature Immunology. 2004;5(8):818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 29.Suh YA, Arnold RS, Lassegue B, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401(6748):79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 30.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology. 2004;4(3):181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 31.McCord JM. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Federation Proceedings. 1987;46(7):2402–2406. [PubMed] [Google Scholar]
- 32.Petrone WF, English DK, Wong K, McCord JM. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnold RS, Shi J, Murad E, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(10):5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murrell GAC, Francis MJO, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochemical Journal. 1990;265(3):659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arbiser JL, Petros J, Klafter R, et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(2):715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brar SS, Kennedy TP, Sturrock AB, et al. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. American Journal of Physiology. 2002;282(6):C1212–C1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 37.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. Journal of Immunology. 2007;178(2):908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 38.Saccani A, Saccani S, Orlando S, et al. Redox regulation of chemokine receptor expression. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2761–2766. doi: 10.1073/pnas.97.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxidants and Redox Signaling. 2007;9(6):731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 40.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Current Opinion in Cell Biology. 2005;17(2):183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 42.Whisler RL, Goyette MA, Grants IS, Newhouse YG. Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Archives of Biochemistry and Biophysics. 1995;319(1):23–35. doi: 10.1006/abbi.1995.1263. [DOI] [PubMed] [Google Scholar]
- 43.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radical Biology and Medicine. 2004;37(6):768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. Journal of Immunology. 2009;182(4):2449–2457. doi: 10.4049/jimmunol.0802770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You SC, Sun YO, Zhu Z. Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation. American Journal of Respiratory Cell and Molecular Biology. 2008;39(4):412–419. doi: 10.1165/rcmb.2007-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunnick JM, Dorsey JF, Mei L, Wu J. Reversible regulation of SHP-1 tyrosine phosphatase activity by oxidation. Biochemistry and Molecular Biology International. 1998;45(5):887–894. doi: 10.1002/iub.7510450506. [DOI] [PubMed] [Google Scholar]
- 47.Lee K, Esselman WJ. Inhibition of PTPs by H2O2 regulates the activation of distinct MAPK pathways. Free Radical Biology and Medicine. 2002;33(8):1121–1132. doi: 10.1016/s0891-5849(02)01000-6. [DOI] [PubMed] [Google Scholar]
- 48.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nature Immunology. 2002;3(12):1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 49.Pietri M, Schneider B, Mouillet-Richard S, et al. Reactive oxygen species-dependent TNF-α converting enzyme activation through stimulation of 5-HT and α autoreceptors in neuronal cells. FASEB Journal. 2005;19(9):1078–1087. doi: 10.1096/fj.04-3631com. [DOI] [PubMed] [Google Scholar]
- 50.Meyer M, Pahl HL, Baeuerle PA. Regulation of the transcription factors NF-κB and AP-1 by redox changes. Chemico-Biological Interactions. 1994;91(2-3):91–100. doi: 10.1016/0009-2797(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 51.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO Journal. 1991;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radical Biology and Medicine. 1996;22(1-2):269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 53.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radical Biology and Medicine. 2004;36(2):233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 54.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-κB. Journal of Immunology. 2004;173(6):3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 55.Crapo JD. Oxidative stress as an initiator of cytokine release and cell damage. European Respiratory Journal, Supplement. 2003;44:4s–6s. doi: 10.1183/09031936.03.00000203a. [DOI] [PubMed] [Google Scholar]
- 56.Ho E, Bray TM. Antioxidants, NFκB activation, and diabetogenesis. Proceedings of the Society for Experimental Biology and Medicine. 1999;222(3):205–213. doi: 10.1046/j.1525-1373.1999.d01-137.x. [DOI] [PubMed] [Google Scholar]
- 57.Curtsinger JM, Schmidt CS, Mondino A, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. Journal of Immunology. 1999;162(6):3256–3262. [PubMed] [Google Scholar]
- 58.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB Journal. 1997;11(2):118–124. [PubMed] [Google Scholar]
- 59.Nathan CF, Root RK. Hydrogen peroxide release from mouse peritoneal macrophages. Dependence on sequential activation and triggering. Journal of Experimental Medicine. 1977;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Wienands J, Zürn C, Reth M. Induction of the antigen receptor expression on B lymphocytes results in rapid competence for signaling of SLP-65 and Syk. EMBO Journal. 1998;17(24):7304–7310. doi: 10.1093/emboj/17.24.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and Fas ligand expression. Journal of Experimental Medicine. 2002;195(1):59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Los M, Dröge W, Stricker K, Baeuerle PA, Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. European Journal of Immunology. 1995;25(1):159–165. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 63.Karin M. The NF-κB activation pathway: its regulation and role in inflammation and cell survival. Cancer Journal from Scientific American. 1998;4(1):S92–S99. [PubMed] [Google Scholar]
- 64.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochemical Pharmacology. 2006;72(11):1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53(10):2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 66.Rabinovitch A. Free radicals as mediators of pancreatic islet β-cell injury in autoimmune diabetes. Journal of Laboratory and Clinical Medicine. 1992;119(5):455–456. [PubMed] [Google Scholar]
- 67.Tran POT, Parker SM, LeRoy E, et al. Adenoviral overexpression of the glutamylcysteine ligase catalytic subunit protects pancreatic islets against oxidative stress. Journal of Biological Chemistry. 2004;279(52):53988–53993. doi: 10.1074/jbc.M404809200. [DOI] [PubMed] [Google Scholar]
- 68.West IC. Radicals and oxidative stress in diabetes. Diabetic Medicine. 2000;17(3):171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 69.Horio E, Fukuda M, Katoh H, et al. Reactive oxygen intermediates in autoimmune islet cell destruction of the NOD mouse induced by peritoneal exudate cells (rich in macrophages) but not T cells. Diabetologia. 1994;37(1):22–31. doi: 10.1007/BF00428773. [DOI] [PubMed] [Google Scholar]
- 70.Nerup J, Mandrup-Poulsen T, Molvig J, Helqvist S, Wogensen L, Egeberg J. Mechanisms of pancreatic β-cell destruction in type I diabetes. Diabetes Care. 1988;11(supplement 1):16–23. [PubMed] [Google Scholar]
- 71.Suarez-Pinzon WL, Szabó C, Rabinovitch A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet β-cells. Diabetes. 1997;46(5):907–911. doi: 10.2337/diab.46.5.907. [DOI] [PubMed] [Google Scholar]
- 72.Maxwell SRJ, Thomason H, Sandler D, et al. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. European Journal of Clinical Investigation. 1997;27(6):484–490. doi: 10.1046/j.1365-2362.1997.1390687.x. [DOI] [PubMed] [Google Scholar]
- 73.Ročić B, Vučić M, Knežević-Ćuća J, et al. Total plasma antioxidants in first-degree relatives of patients with insulin-dependent diabetes. Experimental and Clinical Endocrinology and Diabetes. 1997;105(4):213–217. doi: 10.1055/s-0029-1211754. [DOI] [PubMed] [Google Scholar]
- 74.Santini SA, Marra G, Giardina B, et al. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997;46(11):1853–1858. doi: 10.2337/diab.46.11.1853. [DOI] [PubMed] [Google Scholar]
- 75.Gamble DR, Taylor KW. Seasonal incidence of diabetes mellitus. British medical journal. 1969;3(671):631–633. doi: 10.1136/bmj.3.5671.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gamble DR, Taylor KW, Cumming H. Coxsackie viruses and diabetes mellitus. British Medical Journal. 1973;4(5887):260–262. doi: 10.1136/bmj.4.5887.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coleman TJ, Gamble DR, Taylor KW. Diabetes in mice after Coxsackie B 4 virus infection. British medical journal. 1973;3(5870):25–27. doi: 10.1136/bmj.3.5870.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon JW, Onodera T, Notkins AL. Virus-induced diabetes mellitus. XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with Coxsackie virus B4. Journal of Experimental Medicine. 1978;148(4):1068–1080. doi: 10.1084/jem.148.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berg AK, Korsgren O, Frisk G. Induction of the chemokine interferon-gamma-inducible protein-10 in human pancreatic islets during enterovirus infection. Diabetologia. 2006;49(11):2697–2703. doi: 10.1007/s00125-006-0429-7. [DOI] [PubMed] [Google Scholar]
- 80.Xie B, Zhou JF, Lu Q, Li CJ, Chen P. Oxidative stress in patients with acute coxsackie virus myocarditis. Biomedical and Environmental Sciences. 2002;15(1):48–57. [PubMed] [Google Scholar]
- 81.Peterhans E, Grob M, Bürge T, Zanoni R. Virus-induced formation of reactive oxygen intermediates in phagocytic cells. Free Radical Research Communications. 1987;3(1-5):39–46. doi: 10.3109/10715768709069768. [DOI] [PubMed] [Google Scholar]
- 82.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Biology and Medicine. 1996;20(3):463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 83.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46(11):1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 84.Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochemical Journal. 1981;199(2):393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Newsholme P, Haber EP, Hirabara SM, et al. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. Journal of Physiology. 2007;583(1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. Journal of Biological Chemistry. 1999;274(39):27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 87.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 88.Welsh N, Hellerstrom C. In vitro restoration of insulin production in islets from adult rats treated neonatally with streptozotocin. Endocrinology. 1990;126(4):1842–1848. doi: 10.1210/endo-126-4-1842. [DOI] [PubMed] [Google Scholar]
- 89.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 90.Weaver DC, McDaniel ML, Lacy PE. Alloxan uptake by isolated rat islets of langerhans. Endocrinology. 1978;102(6):1847–1855. doi: 10.1210/endo-102-6-1847. [DOI] [PubMed] [Google Scholar]
- 91.Munday R. Dialuric acid autoxidation: effects of transition metals on the reaction rate and on the generation of “active oxygen” species. Biochemical Pharmacology. 1988;37(3):409–413. doi: 10.1016/0006-2952(88)90207-9. [DOI] [PubMed] [Google Scholar]
- 92.Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T. Streptozocin- and alloxan-induced H2O2 generation and DNA fragmentation in pancreatic islets: HO as mediator for DNA fragmentation. Diabetes. 1991;40(9):1141–1145. doi: 10.2337/diab.40.9.1141. [DOI] [PubMed] [Google Scholar]
- 93.Ino T, Kawamoto Y, Sato K, et al. Selection of mouse strains showing high and low incidences of alloxan-induced diabetes. Jikken dobutsu. Experimental animals. 1991;40(1):61–67. doi: 10.1538/expanim1978.40.1_61. [DOI] [PubMed] [Google Scholar]
- 94.Mathews CE, Graser RT, Savinov A, Serreze DV, Leiter EH. Unusual resistance of ALR/Lt mouse β cells to autoimmune destruction: role for β cell-expressed resistance determinants. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(1):235–240. doi: 10.1073/pnas.98.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sandler S, Swenne I. Streptozotocin, but not alloxan, induces DNA repair synthesis in mouse pancreatic islets in vitro. Diabetologia. 1983;25(5):444–447. doi: 10.1007/BF00282526. [DOI] [PubMed] [Google Scholar]
- 96.Nukatsuka M, Sakurai H, Yoshimura Y, Nishida M, Kawada J. Enhancement by streptozotocin of O2 radical generation by the xanthine oxidase system of pancreatic β-cells. FEBS Letters. 1988;239(2):295–298. doi: 10.1016/0014-5793(88)80938-4. [DOI] [PubMed] [Google Scholar]
- 97.Kroncke KD, Fehsel K, Sommer A, Rodriguez ML, Kolb-Bachofen V. Nitric oxide generation during cellular metabolization of the diabetogenic N-methyl-N-nitroso-urea streptozotozin contributes to islet cell DNA damage. Biological Chemistry Hoppe-Seyler. 1995;376(3):179–185. doi: 10.1515/bchm3.1995.376.3.179. [DOI] [PubMed] [Google Scholar]
- 98.Morgan D, Oliveira-Emilio HR, Keane D, et al. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 2007;50(2):359–369. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- 99.Oliveira HR, Verlengia R, Carvalho CRO, Britto LRG, Curi R, Carpinelli AR. Pancreatic β-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52(6):1457–1463. doi: 10.2337/diabetes.52.6.1457. [DOI] [PubMed] [Google Scholar]
- 100.Gyurko R, Siqueira CC, Caldon N, Gao LI, Kantarci A, Van Dyke TE. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. Journal of Immunology. 2006;177(10):7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Reilly LA, Hutchings PR, Crocker PR, et al. Characterization of pancreatic islet cell infiltrates in NOD mice: effect of cell transfer and transgene expression. European Journal of Immunology. 1991;21(5):1171–1180. doi: 10.1002/eji.1830210512. [DOI] [PubMed] [Google Scholar]
- 102.Lee K, Amano K, Yoon J. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes. 1988;37(7):989–991. doi: 10.2337/diab.37.7.989. [DOI] [PubMed] [Google Scholar]
- 103.Nerup J, Mandrup-Poulsen T, Helqvist S, et al. On the pathogenesis of IDDM. Diabetologia. 1994;37(supplement 2):S82–S89. doi: 10.1007/BF00400830. [DOI] [PubMed] [Google Scholar]
- 104.Tiwari JL, Terasaki PI. HLA-DR and disease associations. Progress in Clinical and Biological Research. 1981;58:151–163. [PubMed] [Google Scholar]
- 105.Staal FJT, Roederer M, Herzenberg LA, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor κB and transcription of human immunodeficiency virus. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Held W, MacDonald HR, Weissman IL, Hess MW, Mueller C. Genes encoding tumor necrosis factor alpha and granzyme A are expressed during development of autoimmune diabetes. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2239–2243. doi: 10.1073/pnas.87.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang Z, Woda BA. Cytokine gene expression in the islets of the diabetic Biobreeding/Worcester rat. Journal of Immunology. 1991;146(9):2990–2994. [PubMed] [Google Scholar]
- 108.Mandrup-Poulsen T, Spinas GA, Prowse SJ, et al. Islet cytotoxicity of interleukin 1. Influence of culture conditions and islet donor characteristics. Diabetes. 1987;36(5):641–647. doi: 10.2337/diab.36.5.641. [DOI] [PubMed] [Google Scholar]
- 109.Mandrup-Poulsen T, Egeberg J, Nerup J. Ultrastructural studies of time-course and cellular specificity of interleukin-1 mediated islet cytotoxicity. Acta Pathologica Microbiologica et Immunologica Scandinavica C. 1987;95(2):55–63. doi: 10.1111/j.1699-0463.1987.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 110.Southern C, Schulster D, Green IC. Inhibition of insulin secretion by interleukin-1β and tumour necrosis factor-α via an L-arginine-dependent nitric oxide generating mechanism. FEBS Letters. 1990;276(1-2):42–44. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- 111.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits β cell function. Journal of Clinical Investigation. 1998;102(3):516–526. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic β-cell cytotoxicity. Journal of Immunology. 1987;139(12):4077–4082. [PubMed] [Google Scholar]
- 113.Campbell IL, Oxbrow L, West J, Harrison LC. Regulation of MHC protein expression in pancreatic beta-cells by interferon-gamma and tumor necrosis factor-alpha. Molecular Endocrinology. 1988;2:101–107. doi: 10.1210/mend-2-2-101. [DOI] [PubMed] [Google Scholar]
- 114.Thomas HE, Kay TWH. Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes/Metabolism Research and Reviews. 2000;16(4):251–261. doi: 10.1002/1520-7560(200007/08)16:4<251::aid-dmrr126>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 115.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors. 2003;17(1-4):287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 116.Murata Y, Shimamura T, Hamuro J. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. International Immunology. 2002;14(2):201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- 117.Dinarello CA, Cannon JG, Mier JW. Multiple biological activities of human recombinant interleukin 1. Journal of Clinical Investigation. 1986;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Green EA, Eynon EE, Flaveirti RA. Local expression of TNFα in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity. 1998;9(5):733–743. doi: 10.1016/s1074-7613(00)80670-6. [DOI] [PubMed] [Google Scholar]
- 119.Huang C, Li J, Costa M, et al. Hydrogen peroxide mediates activation of nuclear factor of activated T cells (NFAT) by nickel subsulfide. Cancer Research. 2001;61(22):8051–8057. [PubMed] [Google Scholar]
- 120.Tse HM, Thayer TC, Steele C, et al. NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. Journal of Immunology. 2010;185(9):5247–5258. doi: 10.4049/jimmunol.1001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmitz ML, Bacher S, Dienz O. NF-κB activation pathways induced by T cell costimulation. FASEB Journal. 2003;17(15):2187–2193. doi: 10.1096/fj.02-1100rev. [DOI] [PubMed] [Google Scholar]
- 122.Chung Y-H, Jun H-S, Kang Y, et al. Role of macrophages and macrophage-derived cytokines in the pathogenesis of Kilham rat virus-induced autoimmune diabetes in diabetes-resistant BioBreeding rats. Journal of Immunology. 1997;159(1):466–471. [PubMed] [Google Scholar]
- 123.Holz A, Adrian B, Coon B, Wolfe T, Grusby MJ, Von Herrath MG. Disruption of the STAT4 signaling pathway protects from autoimmune diabetes while retaining antiviral immune competence. Journal of Immunology. 1999;163(10):5374–5382. [PubMed] [Google Scholar]
- 124.Jacobson NG, Szabo SJ, Weber-Nordt RM, et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. Journal of Experimental Medicine. 1995;181(5):1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Macatonia SE, Hsieh C-S, Murphy KM, O'Garra A. Dendritic cells and macrophages are required for Th1 development of CD4+ t cells from αβ TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-γ production is IFN-γ-dependent. International Immunology. 1993;5(9):1119–1128. doi: 10.1093/intimm/5.9.1119. [DOI] [PubMed] [Google Scholar]
- 126.Sarvetnick N, Shizuru J, Liggitt D, et al. Loss of pancreatic islet tolerance induced by β-cell expression of interferon-γ . Nature. 1990;346(6287):844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- 127.Debray-Sachs M, Carnaud C, Boitard C, et al. Prevention of diabetes in NOD mice treated with antibody to murine IFNγ . Journal of Autoimmunity. 1991;4(2):237–248. doi: 10.1016/0896-8411(91)90021-4. [DOI] [PubMed] [Google Scholar]
- 128.Wang BO, André I, Gonzalez A, et al. Interferon-γ impacts at multiple points during the progression of autoimmune diabetes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cnop M, Welsh N, Jonas J-C, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(supplement 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 130.Eizirik DL, Moore F, Flamez D, Ortis F. Use of a systems biology approach to understand pancreatic β-cell death in Type 1 diabetes. Biochemical Society Transactions. 2008;36(3):321–327. doi: 10.1042/BST0360321. [DOI] [PubMed] [Google Scholar]
- 131.Moore F, Naamane N, Colli ML, et al. STAT1 is a master regulator of pancreatic β-cell apoptosis and islet inflammation. Journal of Biological Chemistry. 2011;286(2):929–941. doi: 10.1074/jbc.M110.162131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stephens LA, Thomas HE, Ming L, Darwiche MGR, Volodin L, Kay TWH. Tumor necrosis factor-α-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic β cells. Endocrinology. 1999;140(7):3219–3227. doi: 10.1210/endo.140.7.6873. [DOI] [PubMed] [Google Scholar]
- 133.Kim WH, Lee JW, Gao B, Jung MH. Synergistic activation of JNK/SAPK induced by TNF-α and IFN-γ: apoptosis of pancreatic β-cells via the p53 and ROS pathway. Cellular Signalling. 2005;17(12):1516–1532. doi: 10.1016/j.cellsig.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 134.Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44(12):2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 135.Kurrer MO, Pakala SV, Hanson HL, Katz JD. β cell apoptosis in T cell-mediated autoimmune diabetes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Suri A, Katz JD. Dissecting the role of CD4+ T cells autoimmune diabetes through the use of TCR transgenic mice. Immunological Reviews. 1999;169:55–65. doi: 10.1111/j.1600-065x.1999.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 137.DiLorenzo TP, Graser RT, Ono T, et al. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor α chain gene rearrangement. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(21):12538–12543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ cells. Journal of Experimental Medicine. 1987;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice: relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1 donors. Diabetes. 1993;42(1):44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 140.Pakala SV, Chivetta M, Kelly CB, Katz JD. In autoimmune diabetes the transition from benign to pernicious insulitis requires an islet cell response to tumor necrosis factor α . Journal of Experimental Medicine. 1999;189(7):1053–1062. doi: 10.1084/jem.189.7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amrani A, Verdaguer J, Anderson B, Utsugi T, Bou S, Santamaria P. Perforin-independent β-cell destruction by diabetogenic CD8+ T lymphocytes in transgenic nonobese diabetic mice. Journal of Clinical Investigation. 1999;103(8):1201–1209. doi: 10.1172/JCI6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kägi D, Odermatt B, Seiler P, Zinkernagel RM, Mak TW, Hengartner H. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. Journal of Experimental Medicine. 1997;186(7):989–997. doi: 10.1084/jem.186.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suarez-Pinzon WL, Rabinovitch A. Approaches to type 1 diabetes prevention by intervention in cytokine immunoregulatory circuits. International Journal of Experimental Diabetes Research. 2001;2(1):3–17. doi: 10.1155/EDR.2001.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39(9):1005–1029. doi: 10.1007/BF00400649. [DOI] [PubMed] [Google Scholar]
- 145.Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on beta cells and diabetes. doi: 10.1089/ars.2010.3416. Antioxid Redox Signal. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lepore DA, Shinkel TA, Fisicaro N, et al. Enhanced expression of glutathione peroxidase protects islet β cells from hypoxia-reoxygenation. Xenotransplantation. 2004;11(1):53–59. doi: 10.1111/j.1399-3089.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- 147.Moriscot C, Richard MJ, Favrot MC, Benhamou PY. Protection of insulin-secreting INS-1 cells against oxidative stress through adenoviral-mediated glutathione peroxidase overexpression. Diabetes and Metabolism. 2003;29:145–151. doi: 10.1016/s1262-3636(07)70021-6. [DOI] [PubMed] [Google Scholar]
- 148.Cuzzocrea S, Mazzon E, Dugo L, et al. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. British Journal of Pharmacology. 2001;132(1):19–29. doi: 10.1038/sj.bjp.0703775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Salvemini D, Mazzon E, Dugo L, et al. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. British Journal of Pharmacology. 2001;132(4):815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lortz S, Tiedge M, Nachtwey T, Karlsen AE, Nerup J, Lenzen S. Protection of insulin-producing RINm5F cells against cytokine-mediated toxicity through overexpression of antioxidant enzymes. Diabetes. 2000;49(7):1123–1130. doi: 10.2337/diabetes.49.7.1123. [DOI] [PubMed] [Google Scholar]
- 151.Sander M, German MS. The β cell transcription factors and development of the pancreas. Journal of Molecular Medicine. 1997;75(5):327–340. doi: 10.1007/s001090050118. [DOI] [PubMed] [Google Scholar]
- 152.Kaneto H, Kajimoto Y, Miyagawa J, et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes. 1999;48(12):2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 153.Kawamori D, Kajimoto Y, Kaneto H, et al. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor Pdx-1 through activation of c-jun NH2-terminal kinase. Diabetes. 2003;52(12):2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- 154.Boucher MJ, Selander L, Carlsson L, Edlund H. Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. Journal of Biological Chemistry. 2006;281(10):6395–6403. doi: 10.1074/jbc.M511597200. [DOI] [PubMed] [Google Scholar]
- 155.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51(8):1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 156.Li X, Chen H, Epstein PN. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice: reactive oxygen species may have a protective role in pancreatic β-cells. Diabetes. 2006;55(6):1592–1604. doi: 10.2337/db05-1357. [DOI] [PubMed] [Google Scholar]
- 157.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cellular and Molecular Life Sciences. 2002;59(4):627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sandström J, Jonsson LM, Edlund H, Holmberg D, Marklund SL. Overexpression of extracellular-SOD in islets of nonobese diabetic mice and development of diabetes. Free Radical Biology and Medicine. 2002;33(1):71–75. doi: 10.1016/s0891-5849(02)00859-6. [DOI] [PubMed] [Google Scholar]
- 159.Goldstein BJ, Kalyankar M, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54(2):311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hotta M, Tashiro F, Ikegami H, et al. Pancreatic β cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. Journal of Experimental Medicine. 1998;188(8):1445–1451. doi: 10.1084/jem.188.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu BO, Moritz JT, Epstein PN. Overexpression of catalase provides partial protection to transgenic mouse beta cells. Free Radical Biology and Medicine. 1999;27(7-8):830–837. doi: 10.1016/s0891-5849(99)00130-6. [DOI] [PubMed] [Google Scholar]
- 162.Benhamou PY, Moriscot C, Richard MJ, et al. Adenovirus-mediated catalase gene transfer reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia. 1998;41(9):1093–1100. doi: 10.1007/s001250051035. [DOI] [PubMed] [Google Scholar]
- 163.Chen H, Carlson EC, Pellet L, Moritz JT, Epstein PN. Overexpression of metallothionein in pancreatic β-cells reduces streptozotocin-induced DNA damage and diabetes. Diabetes. 2001;50(9):2040–2046. doi: 10.2337/diabetes.50.9.2040. [DOI] [PubMed] [Google Scholar]
- 164.Huang SH, Chu CH, Yu JC, et al. Transgenic expression of haem oxygenase-1 in pancreatic beta cells protects non-obese mice used as a model of diabetes from autoimmune destruction and prolongs graft survival following islet transplantation. Diabetologia. 2010;53(11):2389–2400. doi: 10.1007/s00125-010-1858-x. [DOI] [PubMed] [Google Scholar]
- 165.Kubisch HM, Wang J, Bray TM, Phillips JP. Targeted overexpression of Cu/Zn superoxide dismutase protects pancreatic β-cells against oxidative stress. Diabetes. 1997;46(10):1563–1566. doi: 10.2337/diabetes.46.10.1563. [DOI] [PubMed] [Google Scholar]
- 166.Mathews CE, Leiter EH. Constitutive differences in antioxidant defense status distinguish alloxan-resistant and alloxan-susceptible mice. Free Radical Biology and Medicine. 1999;27(3-4):449–455. doi: 10.1016/s0891-5849(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 167.Rabinovitch A, Suarez WL, Power RF. Lazaroid antioxidant reduces incidence of diabetes and insulitis in nonobese diabetic mice. Journal of Laboratory and Clinical Medicine. 1993;121(4):603–607. [PubMed] [Google Scholar]
- 168.Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43(8):1020–1030. doi: 10.1007/s001250050009. [DOI] [PubMed] [Google Scholar]
- 169.Stosic-Grujicic SD, Miljkovic DM, Cvetkovic ID, Maksimovic-Ivanic DD, Trajkovic V. Immunosuppressive and anti-inflammatory action of antioxidants in rat autoimmune diabetes. Journal of Autoimmunity. 2004;22(4):267–276. doi: 10.1016/j.jaut.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 170.Hu CM, Lin HH, Chiang MT, Chang PIF, Chau LY. Systemic expression of heme oxygenase-1 ameliorates type 1 diabetes in NOD mice. Diabetes. 2007;56(5):1240–1247. doi: 10.2337/db06-0495. [DOI] [PubMed] [Google Scholar]
- 171.Mathews CE, Dunn BD, Hannigan MO, Huang CK, Leiter EH. Genetic control of neutrophil superoxide production in diabetes-resistant ALR/Lt mice. Free Radical Biology and Medicine. 2002;32(8):744–751. doi: 10.1016/s0891-5849(02)00747-5. [DOI] [PubMed] [Google Scholar]
- 172.Matsushita M, Yoshino G, Iwai M, et al. Protective effect of probucol on alloxan diabetes in rats. Diabetes Research and Clinical Practice. 1989;7(4):313–316. doi: 10.1016/0168-8227(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 173.Drash AL, Rudert WA, Borquaye S, Wang R, Lieberman I. Effect of probucol on development of diabetes mellitus in BB rats. American Journal of Cardiology. 1988;62(3):27B–30B. doi: 10.1016/s0002-9149(88)80047-x. [DOI] [PubMed] [Google Scholar]
- 174.Fukuda M, Ikegami H, Kawaguchi Y, Sano T, Ogihara T. Antioxidant, probucol, can inhibit the generation of hydrogen peroxide in islet cells induced by macrophages and prevent islet cell destruction in NOD mice. Biochemical and Biophysical Research Communications. 1995;209(3):953–958. doi: 10.1006/bbrc.1995.1590. [DOI] [PubMed] [Google Scholar]
- 175.Batinić-Haberle I, Benov L, Spasojević I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium- 2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. Journal of Biological Chemistry. 1998;273(38):24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 176.Pasternack RF, Banth A, Pasternack JM, Johnson CS. Catalysis of the disproportionation of superoxide by metalloporphyrins. III. Journal of Inorganic Biochemistry. 1981;15(3):261–267. doi: 10.1016/s0162-0134(00)80161-0. [DOI] [PubMed] [Google Scholar]
- 177.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radical Biology and Medicine. 1999;26(5-6):730–736. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 178.Ferrer-Sueta G, Vitturi D, Batinić-Haberle I, et al. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. Journal of Biological Chemistry. 2003;278(30):27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 179.Huang Y, Domann FE. Redox modulation of AP-2 DNA binding activity in vitro. Biochemical and Biophysical Research Communications. 1998;249(2):307–312. doi: 10.1006/bbrc.1998.9139. [DOI] [PubMed] [Google Scholar]
- 180.Sun YI, Oberley LW. Redox regulation of transcriptional activators. Free Radical Biology and Medicine. 1996;21(3):335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 181.Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1β by adenoviral gene transfer of an IκB repressor. Journal of Biological Chemistry. 2000;275(47):36509–36513. doi: 10.1074/jbc.M005943200. [DOI] [PubMed] [Google Scholar]
- 182.Heimberg H, Heremans Y, Jobin C, et al. Inhibition of cytokine-induced NF-κB activation by adenovirus-mediated expression of a NF-κB super-repressor prevents β-cell apoptosis. Diabetes. 2001;50(10):2219–2224. doi: 10.2337/diabetes.50.10.2219. [DOI] [PubMed] [Google Scholar]
- 183.Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nature Immunology. 2010;11(3):225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dobbs CM, Haskins K. Comparison of a T cell clone and of T cells from a TCR transgenic mouse: TCR transgenic T cells specific for self-antigen are atypical. Journal of Immunology. 2001;166(4):2495–2504. doi: 10.4049/jimmunol.166.4.2495. [DOI] [PubMed] [Google Scholar]
- 185.Sklavos MM, Tse HM, Piganelli JD. Redox modulation inhibits CD8+ T cell effector function. Free Radical Biology and Medicine. 2008;45(10):1477–1486. doi: 10.1016/j.freeradbiomed.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 186.Chaudhri G, Clark IA, Hunt NH. Effect of antioxidants on primary alloantigen-induced T cell activation and proliferation. Journal of Immunology. 1986;137(8):2646–2652. [PubMed] [Google Scholar]
- 187.Chaudhri G, Hunt NH, Clark IA, Ceredig R. Antioxidants inhibit proliferation and cell surface expression of receptors for interleukin-2 and transferrin in T lymphocytes stimulated with phorbol myristate acetate and ionomycin. Cellular Immunology. 1988;115(1):204–213. doi: 10.1016/0008-8749(88)90174-8. [DOI] [PubMed] [Google Scholar]
- 188.Malhotra JD, Miao H, Zhang K, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(47):18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lo D, Burkly LC, Widera G, et al. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988;53(1):159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- 190.Jiang L, Allagnat F, Nguidjoe E, et al. Plasma membrane Ca2+-ATPase overexpression depletes both mitochondrial and endoplasmic reticulum Ca2+ stores and triggers apoptosis in insulin-secreting BRIN-BD11 cells. Journal of Biological Chemistry. 2010;285(40):30634–30643. doi: 10.1074/jbc.M110.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lei X, Zhang S, Barbour SE, et al. Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A expression: a role for regulation by SREBP-1. Journal of Biological Chemistry. 2010;285(9):6693–6705. doi: 10.1074/jbc.M109.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ramanadham S, Hsu F-F, Zhang S, et al. Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2 beta) and suppressed by inhibition of iPLA2 beta. Biochemistry. 2004;43(4):918–930. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Papa FR, Zhang C, Shokat K, Waiter P. Bypassing a kinase activity with an ATP-competitive drug. Science. 2003;302(5650):1533–1537. doi: 10.1126/science.1090031. [DOI] [PubMed] [Google Scholar]
- 194.Ron D. Stressed cells cope with protein overload. Science. 2006;313(5783):52–53. doi: 10.1126/science.1130469. [DOI] [PubMed] [Google Scholar]
- 195.Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Current Opinion in Cell Biology. 2001;13(3):349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 196.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52(2):409–416. doi: 10.2337/diabetes.52.2.409. [DOI] [PubMed] [Google Scholar]
- 197.Wang J, Takeuchi T, Tanaka S, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. Journal of Clinical Investigation. 1999;103(1):27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 199.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. Journal of Clinical Investigation. 2002;110(10):1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. Journal of Experimental Medicine. 1995;182(6):1625–1634. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in β-cells and development of diabetes. Current Opinion in Pharmacology. 2009;9(6):763–770. doi: 10.1016/j.coph.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bonifacio E, Ziegler AG. Advances in the prediction and natural history of type 1 diabetes. Endocrinology and Metabolism Clinics of North America. 2010;39(3):513–525. doi: 10.1016/j.ecl.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 203.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period: dynamic changes in structure and function. Diabetes. 1996;45(9):1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 204.Sklavos MM, Bertera S, Tse HM, et al. Redox modulation protects islets from transplant-related injury. Diabetes. 2010;59(7):1731–1738. doi: 10.2337/db09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Menger MD, Vajkoczy P, Leiderer R, Jager S, Messmer K. Influence of experimental hyperglycemia on microvascular blood perfusion of pancreatic islet isografts. Journal of Clinical Investigation. 1992;90(4):1361–1369. doi: 10.1172/JCI116002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Land W, Schneeberger H, Schleibner S, et al. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57(2):211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 207.Petrowsky H, Dippe B, Geck P, et al. Do oxygen radicals play a role in primary dysfunction of transplanted livers following preservation in University of Wisconsin solution? Transplantation Proceedings. 1995;27(1):729–731. [PubMed] [Google Scholar]
- 208.Mendola J, Wright JR, Lacy PE. Oxygen free-radical scavengers and immune destruction of murine islets in allograft rejection and multiple low-dose streptozocin-induced insulitis. Diabetes. 1989;38(3):379–385. doi: 10.2337/diab.38.3.379. [DOI] [PubMed] [Google Scholar]
- 209.Nomikos IN, Wang Y, Lafferty KJ. Involvement of O2 radicals in ’autoimmune’ diabetes. Immunology and Cell Biology. 1989;67(1):85–87. doi: 10.1038/icb.1989.12. [DOI] [PubMed] [Google Scholar]
- 210.Li YX, Li GE, Dong WP, Lu DAR, Tan JM. Protection of human islets from induction of apoptosis and improved islet function with HO-1 gene transduction. Chinese Medical Journal. 2006;119(19):1639–1645. [PubMed] [Google Scholar]
- 211.Bertera S, Crawford ML, Alexander AM, et al. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes. 2003;52(2):387–393. doi: 10.2337/diabetes.52.2.387. [DOI] [PubMed] [Google Scholar]
- 212.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 2002;51(8):2561–2567. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- 213.Balamurugan AN, Bottino R, Giannoukakis N, Smetanka C. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas. 2006;32(3):231–243. doi: 10.1097/01.mpa.0000203961.16630.2f. [DOI] [PubMed] [Google Scholar]
- 214.Zhang N, Su D, Qu S, et al. Sirolimus is associated with reduced islet engraftment and impaired β-cell function. Diabetes. 2006;55(9):2429–2436. doi: 10.2337/db06-0173. [DOI] [PubMed] [Google Scholar]
- 215.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators of Inflammation. 2010;2010 doi: 10.1155/2010/453892. Article ID 453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brahmachari S, Pahan K. Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. Journal of Immunology. 2010;184(4):1799–1809. doi: 10.4049/jimmunol.0804394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stirban A, Rösen P, Tschoepe D. Complications of type 1 diabetes: new molecular findings. Mount Sinai Journal of Medicine. 2008;75(4):328–351. doi: 10.1002/msj.20057. [DOI] [PubMed] [Google Scholar]
- 218.Ling X, Cota-Gomez A, Flores NC, et al. Alterations in redox homeostasis and prostaglandins impair endothelial-dependent vasodilation in euglycemic autoimmune nonobese diabetic mice. Free Radical Biology and Medicine. 2005;39(8):1089–1098. doi: 10.1016/j.freeradbiomed.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 219.Yoshikawa T, Yoshida N. Vitamin E and leukocyte-endothelial cell interactions. Antioxidants and Redox Signaling. 2000;2(4):821–825. doi: 10.1089/ars.2000.2.4-821. [DOI] [PubMed] [Google Scholar]
- 220.Haidara MA, Mikhailidis DP, Rateb MA, et al. Evaluation of the effect of oxidative stress and vitamin E supplementation on renal function in rats with streptozotocin-induced Type 1 diabetes. Journal of Diabetes and its Complications. 2009;23(2):130–136. doi: 10.1016/j.jdiacomp.2008.02.011. [DOI] [PubMed] [Google Scholar]