Abstract
The past two decades have witnessed tremendous advances in noninvasive and postmortem neuroscientific techniques, advances that have made it possible, for the first time, to compare in detail the organization of the human brain to that of other primates. Studies comparing humans to chimpanzees and other great apes reveal that human brain evolution was not merely a matter of enlargement, but involved changes at all levels of organization that have been examined. These include the cellular and laminar organization of cortical areas; the higher-order organization of the cortex, as reflected in the expansion of association cortex (in absolute terms, as well as relative to primary areas); the distribution of long-distance cortical connections; and hemispheric asymmetry. Additionally, genetic differences between humans and other primates have proven to be more extensive than previously thought, raising the possibility that human brain evolution involved significant modifications of neurophysiology and cerebral energy metabolism.
Keywords: hominid, evolution, neuroimaging, genomics, cerebral metabolism
Introduction
Until quite recently, it has been impossible for neuroscientists to address in much detail one of the most fundamental questions in the life sciences—how do human brains differ from those of other animals? All we’ve known for sure is that human brains are freakishly large for a mammal of our body size. In most mammals, the portion of the skull devoted to eating is bigger than the part that houses the equipment for thinking, whereas the human brain box dwarfs the machinery of mastication. But what’s in the box? What’s in there that gives us the ability to think and act in specifically human-like ways? The allure of neuroscience surely depends on the conviction that there’s something unusual about the human brain, yet neuroscientists have been unable to specify how the cells and the systems of connections that make up the brain were modified in human evolution.
The main reason for this failure is that neuroscientists have lacked the technical means to explore the human brain in anything like the detail with which they are able to study nonhuman species. For nonhuman species, we have had a range of powerful investigative techniques available to us, such as injecting chemicals into brain tissue to trace neuronal connections, a procedure considered unethical in humans. Such invasive techniques, it is important to note, are also out of bounds for studying rare and endangered species, such as chimpanzees, the animals most closely related to humans. Without the ability to compare humans to chimpanzees and other great apes, we can say little about what’s distinctively human about human brains.
Over the past 20 years, however, and particularly over the last decade, the means available for studying human brains, and for directly comparing humans to chimpanzees and other primates, have improved enormously. Of course, the continual refinement of noninvasive imaging techniques, and their application to nonhuman primates as well as to humans, has been very important. Functional magnetic resonance imaging (MRI) and positron emission tomography (PET) are the imaging techniques that have garnered the most attention, but perhaps the most important development in this area for students of human brain evolution is the introduction of diffusion-tensor imaging (DTI) and related techniques, which can be used to track white-matter pathways between gray-matter regions noninvasively (for a recent review, see Ref. [1]). Compared to conventional tract-tracing techniques, which involve making injections of chemical tracers into the brains of experimental animals, DTI has certain limitations: its resolution is relatively coarse, because under most circumstances it cannot track fibers into the gray matter, and it cannot distinguish between anterograde and retrograde connections. Yet DTI has made it possible to study the major fiber systems in human brains comprehensively and also to compare human fiber systems to those of other primates. Far less eye-catching, but nonetheless enormously important, has been the steadily increasing power of histological techniques for exploring the structure of tissue acquired postmortem, reflecting the proliferation of antibodies and other ligands available for probing the distribution of molecules in the brain. Older histological and histochemical techniques remain very useful, however. The value of all these techniques, and the value of the postmortem tissue to which they can be applied, has been multiplied by the introduction of antifreeze storage solutions that preserve tissue for years in a state suitable for immunohistochemistry and in situ hybridization, avoiding the degrading effects of overfixation [2]. A third source of new information about human brain evolution comes from genomics and comparative molecular biology, including techniques for identifying genes that underwent positive selection or expression changes in human evolution.
The upshot of these technical innovations is that the subject of human brain evolution, long something of a scientific backwater, has become a matter of keen interest, as reflected by a spate of recent books (e.g., Refs. 3–7). Different authors will, of course, have different takes on this subject. Here, I cast my account of current results in the light of hypotheses and expectations about human brain specializations that were posited before the advent of the new techniques just discussed.
Issues and evidence
Was encephalization accompanied by the expansion of association cortex?
There is no question that human brains are much, much larger than would be expected for a primate of our body size, and that most of this difference reflects an enlargement of the neocortex. Classically, it has been supposed that this enlargement resulted from a selective expansion of the higher-order association cortex of the frontal, temporal, and parietal lobes, relative to the primary sensory and motor areas (see, e.g., Refs. 8–11). With the application of structural neuroimaging techniques to the study of human brain evolution, this conclusion has been called into question [12]. The essence of the counterclaim is that association cortex—and prefrontal cortex, in particular—is no larger in humans than would be expected for an ape with a human-sized brain. More specifically, the claim is that if one were to plot the size of prefrontal cortex as a function of the size of the rest of the brain for anthropoid primates, human prefrontal cortex would fall within the expected size range. One can take issue with the methods employed by those who have adopted this view, for they have not measured prefrontal cortex size directly (something that is currently not possible to do using imaging techniques), and have instead measured a morphology proxy for prefrontal cortex—the size of the entire frontal lobe or the size of the frontal cortex anterior to the precentral gyrus. Moreover, other authorities have affirmed the traditional view of association cortex expansion (e.g., Refs. 13–14).
Nevertheless, even if one grants for the sake of argument that humans have the expected amount of prefrontal cortex for a primate brain scaled up to human size, there is no getting past the fact that humans have a lot more association cortex in absolute terms than do chimpanzees or other great apes [15]. Human brains are about three times the volume of those of chimpanzees, and whereas the primary sensory and motor regions of humans are, in absolute terms, very similar in size to those of apes, humans have a much, much greater amount of association cortex (Tables 1 and 2) [15]. The same pattern holds for the sensory and association thalamic nuclei (Table 3). I suggest that the most straightforward interpretation of the data is that the primary areas maintained approximately ape-like sizes in human evolution, while association cortex underwent enormous expansion. The mere fact that the magnitude of association cortex expansion is (or might be) in line with what is expected from brain-size scaling might be taken to mean that association cortex expanded in a predictable manner (perhaps reflecting some conserved developmental processes), but it doesn’t negate the fact that association cortex did expand. The result is that humans have a brain dominated by association cortex to an extent unmatched by any other primate, and this difference is likely to have profound implications for the internal organization of the cortex [6, 16], for patterns of long cortical connections [17], and for cortical function.
Table 1.
Absolute and relative sizes of primary motor area (area 4), prefrontal cortex, and other cortex in great apes and humans*
| Area 4 (cm2) | Prefrontal (cm2) | Other cortex (cm2) | ||
|---|---|---|---|---|
| human | 7.34 (65%) | 181.40 (220%) | 499.60 (149%) | |
| great apes | chimp | 8.94 (79%) | 52.84 (87%) | 280.96 (84%) |
| orang | 13.57 (121%) | 68.45 (113%) | 389.22 (116%) |
Data from Blinkov and Glezer [8], Table 196. Values in parentheses represent species’ values expressed as a percentage of the mean value of great apes (i.e., mean of chimpanzee and orangutan).
Table 2.
Absolute and relative size of area striata (primary visual cortex; area 17) in humans and great apes *
| AS volume (ASV) (cm3) | neocortical volume (NV) (cm3) | ASV as % of NV | ASV as % of mean great ape ASV | NV as % of mean great ape NV | ||
|---|---|---|---|---|---|---|
| human | 20228 | 1006525 | 2.0% | 136% | 321% | |
| great apes | chimp | 14597 | 291592 | 5.0% | 98% | 93% |
| orang | 15185 | 341444 | 4.5% | 102% | 107% |
Data from Frahm et al. [96].
Table 3.
Absolute and relative numbers of neurons in thalamic nuclei in humans and great apes*
| Sensory relay | Motor/Premotor | ||||
|---|---|---|---|---|---|
| visual | auditory | somato-sensory | |||
| LGB | MGBp | VB | VL | ||
| human | 2.07 (102%) | 0.87 (107%) | 1.69 (147%) | 3.31 (165%) | |
| great apes | chimp | 2.17 (107%) | 0.91 (113%) | 1.23 (107%) | 2.06 (103%) |
| gorilla | 1.88 (93%) | 0.71 (87%) | 1.07 (93%) | 1.84 (97%) | |
| Association and limbic | |||||
|---|---|---|---|---|---|
| MD | PUL | LD | AP | ||
| human | 7.57 (312%) | 10.11 (202%) | 0.41 (272%) | 1.19 (309%) | |
| great apes | chimp | 2.50 (103%) | 5.45 (109%) | 0.15 (99%) | 0.39 (100%) |
| gorilla | 2.35 (97%) | 4.56 (91%) | 0.15 (101%) | 0.38 (100%) | |
Data from Armstrong [97]. Values represent the numbers of neurons × 106; values in parentheses represent species values expressed as a percentage of the mean value of great apes (i.e., mean of chimpanzee and gorilla).
Abbreviations: LGB - lateral geniculate body; MGBp – principal nucleus of the medial geniculate body; VB – ventrobasal nucleus; VL – ventral lateral nucleus; MD – mediodorsal nucleus; PUL – pulvinar-lateral posterior complex; LD – lateral dorsal nucleus; AP – anterior principal nucleus.
Was the expansion of association cortex accompanied by the addition of new, human-specific areas to support human-specific functions?
This question has long been a matter of contention, with notable authorities lining up on both sides of the issue. To some, it has seemed that the enormous human brain, with its unique psychological functions, must contain human-specific areas [9, 18, 19]. Others, however, have failed to be convinced, arguing that humans have the same set of cortical areas found in our close primate relatives [11, 20, 21].
This important issue remains unresolved. It needs to be acknowledged, however, that at present, there is no compelling evidence that humans possess more cortical areas than do other primates, nor that human-specific functions require human-specific brain structures. The paradigmatic language areas of Broca and Wernicke provide a case in point. Although there is little doubt that in humans these areas are critically involved in language processing, and that language is a human specialization, there is nonetheless evidence that homologues of Broca’s and Wernicke’s areas exist in apes and monkeys, based on similarities in their location within the cortical mantle, in their histology, and in their other, non-linguistic functions, such as forelimb and orofacial movements and analysis of species-specific calls (see reviews in Refs. 22 and 23). Thus, the claim that Broca’s and Wernicke’s area evolved originally to support functions other than language, and then were “recruited” into a language-processing system (an old claim, in fact; see Ref. [24]), is defensible, and we should not conclude that human-specific functions require human-specific brain areas (see also Refs. 25 and 26).
Mapping studies of other brain regions also tend to identify a common complement of areas in humans and nonhuman primates. For example, if the cytoarchitectonic maps of Petrides and Pandya [27] are correct, each of the prefrontal areas of humans has a homologue in macaque monkeys, despite the much larger prefrontal region of humans. Similarly, mapping studies of extrastriate visual cortex using fMRI in humans and macaques identify an identical complement of visual areas [28], despite the expansion of extrastriate cortex relative to striate cortex in humans.
Recently, areas have been identified in human parietal cortex that have functional properties not found in monkeys. The human intraparietal sulcus (IPS) contains areas that are strongly activated when viewing three dimensional shape-from-motion stimuli; the same stimuli produce minimal activation in macaque IPS [29]. In general, IPS areas are much more sensitive to motion in humans than in macaques [30]. Similarly, humans possess an area in the left anterior inferior parietal lobule (i.e., the supramarginal gyrus; area PF or 7b) that is more strongly activated when human subjects view videos depicting tools being used to grasp or otherwise manipulate objects than when hands are viewed performing the same actions [31]. A comparable, tool-selective enhancement was not seen in the anterior inferior parietal lobule of macaques, even macaques with extensive training in tool use. It is possible, then, that humans possess new motion-selective and tool-use related areas in parietal cortex [29, 31, 32]. It might just as well be the case, however, that the functional differences observed between humans and macaques represent evolutionary changes in the functions of pre-existing areas. That evolution can effect these kinds of changes in visual areas is illustrated by the differences between humans and macaques in the functional properties of dorsal extrastriate areas V3 and V3A, with human V3A exhibiting greater sensitivity to motion, and V3 less so, compared to macaques [33]. We cannot settle the question of new areas without more complete cross-species mappings, so that the homologous areas shared by humans and non-humans are completely accounted for.
Although the data do not so far provide clear indication of human-specific cortical areas, it is the case that large regions of the cortex remain to be explored. Regions that appear to have been modified in human evolution, and may house new areas, include the cortex of the middle temporal gyrus, which is involved in semantic representation and is probably greatly enlarged in humans compared to other primates [34], and also the anterior and dorsal parts of insular cortex, a locus of self representation [35].
Are human brains more “lopsided” than those of other primates?
Human brains are highly lateralized functionally, the left hemisphere being dominant for language and motor control, and the right hemisphere dominant for visuospatial and attentional functions. Apes and monkeys, by contrast, do not exhibit the same degree of right-handedness as do humans at either an individual or population level, and while we know little about visuospatial representation in apes, monkeys do not show the same degree of lateralization as do humans [36]. Differences like these prompted Corballis to characterize the human species as “lopsided” [37] and Annett to postulate that humans underwent an evolutionary “right shift” in hand preference [38]. Hemispheric specializations have thus been regarded as key features of the human phenotype (see especially Refs. 5, 39–41).
Despite the evidence for increased functional laterality, corresponding anatomical asymmetries have proven rather elusive. There are, to be sure, anatomical differences between human left and right hemispheres. The human brain has a kind of torque—the right frontal lobe protruding further interiorly than the left, and the left occipital lobe protruding further posteriorly than the right—and this has been advanced as part of a core specialization of humans [41]. There are, however, studies indicating that apes share this pattern [42, 43], although direct comparison of humans and other hominoids does suggest that is more common and/or more pronounced in humans [44].
Given the uniqueness of human language, and strong left-hemisphere dominance for language, one might reasonably expect the human language areas to be more asymmetrical than their homologues in apes. Surprisingly, the planum temporale (the portion of the superior temporal lobe commonly identified with Wernicke’s area), which is larger on the left than the right in most humans [45], shows a very comparable asymmetry in chimpanzees and other great apes [46, 47], and area Tpt, the main cytoarchitectonic area on the planum temporale, is larger on the left than right in chimpanzees [23]. By contrast, a recent study in which Broca’s area was delineated cytoarchitectonically indicates that this area, which is larger on the left than the right in humans, is not asymmetrical in size in chimpanzees [48].
At an even finer level of resolution, Buxhoeveden and colleagues [49] have reported an asymmetry in histology of cortical area Tpt, an architectonic territory that occupies the planum temporale and is often identified with Wernicke’s area, with humans having more neuropil space between cell columns on the left than on the right; by contrast, no asymmetry was found in chimpanzees or macaques. Similar asymmetries, with higher neuropil fractions on the left than the right, are found in human motor and visual cortex (e.g., Refs. 50 and 51), however. This raises the possibility that the Tpt asymmetry reflects a human-specific, cortex-wide asymmetry [52] that is not specifically related to language.
The advent of diffusion-tensor imaging (DTI) has opened up new dimensions of organization for the analysis of asymmetries. One measure derived from DTI is the fractional anisotropy (FA) of brain voxels. FA is a measure of the propensity of water to diffuse directionally rather than randomly; differences in FA are thought to reflect differences in the microstructure of white matter—for example, the amount of myelin and the coherence of fibers. One can compare the FA of specific fibers tracts between hemispheres and across species. In a recent report, Li et al. [53] found that the corticospinal tracts of chimpanzees showed higher FA on the left than on the right, consistent with reports from humans. Work in progress, however, suggests that other tracts show marked differences between humans, chimpanzees, and macaques. For example, humans exhibit higher FA on the left than the right in the arcuate fasciculus (the tract that carries fibers between Wernicke’s and Broca’s areas), and the magnitude of asymmetry is greater in humans than in chimpanzees [54].
Was long-distance cortical connectivity modified in human brain evolution?
Cortical areas have extensive systems of long-distance connections; these connections link functionally related areas, and link the cortex to sensory and motor structures in the thalamus and brainstem. Prior to the development of robust methods to study fiber tracts noninvasively, few neuroscientists entertained the possibility that long-distance connectivity might differ in important respects between humans and other primates; Crick and Jones [18] and Deacon [17] are conspicuous exceptions. The only widely cited human specialization of connectivity came from evidence that humans, and not other primates, have direct cortical projections to brainstem nuclei involved in orofacial motor control, a projection thought to be related to language evolution [55, 56]. This evidence, however, was obtained using silver staining for degenerating axons, a technique not considered to be very sensitive or reliable by modern standards.
The recent development of DTI and related in vivo fiber-tracking techniques makes possible systematic comparisons of human and nonhuman primate connectivity (Fig. 1). The first such study demonstrated differences in the composition of the arcuate fasciculus in humans, chimps, and macaques [34]. In humans, but not the other primates, the arcuate fasciculus carries not only fibers that interconnect Broca’s area (in the posterior inferior frontal gyrus) and Wernicke’s area (in the posterior superior temporal gyrus), but also fibers traveling between the inferior frontal lobe and areas in the middle and inferior temporal lobe that are known to represent word meanings.
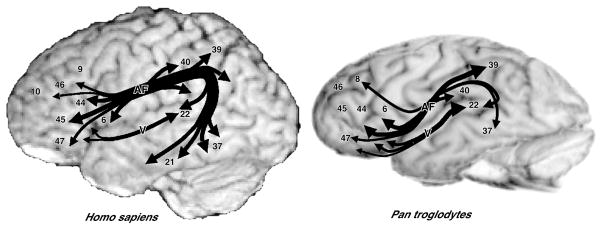
Figure 1.
Summary of results from a DTI study by Rilling et al. [34] comparing the organization of the arcuate fasciculus, a white-matter bundle conveying fibers between the frontal lobe and posterior cortex, in humans (Homo sapiens) and chimpanzees (Pan troglodytes). In both species, the arcuate fasciculus (AF) carries fibers between frontal language cortex, including areas 44, 45, and 47 (Broca’s area), and posterior language cortex, including area 22 (Wernicke’s area) and the inferior parietal lobule (areas 40 and 39). In humans, however, the AF carries fibers from middle temporal cortex (area 21) that represent word meanings. Fibers also pass between the temporal and frontal lobes via a ventral pathway (V), which is relatively prominent in chimpanzees and macaques (not shown).
Was the microstructure of cortical gray matter modified in human evolution?
Until recently, few would have taken seriously the possibility that cortical microstructure underwent important changes in human evolution. According to the doctrine of “basic uniformity“ developed in the 1970s, the cortex of all mammals was thought to be composed of cell columns that were essentially unvarying across species in their cell number, cell types, laminar distribution of afferents and efferents, and intrinsic connectivity (see the reviews in Refs. 57 and 58). In this view, there is little room for human specializations at these levels of organization. While basic uniformity has enjoyed tremendous popularity, however, it simply cannot be squared with evidence from modern comparative studies that highlights the diversity of microstructural organization across mammalian species (reviewed in Refs. 57 and 58). At present, few studies have rigorously compared human microstructure to that of other primates. One area that has been studied in some detail, however, is the primary visual area (area V1; Brodmann’s area 17; striate cortex), and this area exhibits a number of human specializations, particularly in the organization of layer 4A 59–61. The pattern of differences suggests modifications related to motion processing, changes that may underlie the human–macaque differences in the extrastriate and parietal motion-sensitive areas discussed above. Additional specializations of human cortex that have been documented include modifications of neuronal and glial phenotypes, 62–65 the horizontal spacing of neurons [66], and modifications of the laminar organization of afferents.67–69
Do human phenotypic specializations result from only a few genetic changes?
Since at least the mid-1970s, it has been understood that the amino-acid sequences of proteins, and the nucleotide sequences of the genes that code for them, are very similar in humans and chimpanzees—on the order of 98–99% similar [70]. The magnitude of similarity prompted King and Wilson [70] to conclude that evolutionary changes in gene expression, rather than changes in gene sequences, are the principal source of human phenotypic specializations. Gould [71] popularized this idea, and emphasized the possibility that a small number of expression changes acting early in development could have profound phenotypic consequences. Given this background, and the fact that there are few widely acknowledged specializations of the human brain and cognition other than encephalization and language, it is not surprising that when improved means to identify human genetic specializations became available, attention was focused on genes believed to be related to encephalization and language.72–74 The FOXP2 gene has been especially appealing in this regard, as mutations of the gene result in language and cognitive deficits [75], it underwent positive selection in the human lineage [72], and it codes for a transcription factor that regulates the expression of numerous brain-expressed genes [76, 77].
I certainly don’t disparage the search for genes related to language or brain size, yet I fear that in focusing on these we’ve missed an important lesson of recent genomics research, specifically, that the genetic differences between humans and other primates have proven to be much more extensive than generally supposed [58]. While the protein-coding sequences of humans and chimpanzees are the same at about 98% of nucleotides, the overall DNA sequence similarity is more like 95–96% [78, 79]. There are hundreds of genes, at least, that are differentially expressed in the brains of human and chimpanzees [80, 81]. There are also hundreds of genes, at least, that were targets of positive selection in the human lineage subsequent to the human-chimpanzee divergence [82]. Gene duplications, deletions, and insertions, were common in the human lineage,83–85 in some instances resulting in the evolution of new genes and gene families (e.g., Refs. 86 and 87). There is also evidence of evolutionary changes in human protein chemistry not predicted by differences in genes (e.g., Ref. 88).
Comparisons of humans and nonhuman primates have, in short, yielded an embarrassment of molecular riches—embarrassing, because we currently know a lot more about human genetic specializations than we do about phenotypic specializations of the human mind and brain. Perhaps we should start thinking about genetics differently, and instead of focusing quite so much on identifying genes that influence known phenotypic specializations, use our knowledge of the genes to help us discover previously unknown human phenotypes [81]. Consider, for example, that we have genomic evidence suggesting that humans modified the expression and structure of genes related to synapse formation [89, 90] and aerobic energy metabolism [91–93]. This suggests that human brains evolved so as to support higher levels of neural activity and plasticity than the brains of our closest relatives. Intuitively, this may seem unsurprising, but one would be hard pressed to identify a clear scientific claim for a human specialization of this kind, backed up by comparative phenotypic data. Such data may not be entirely lacking, however. While there are few modern data comparing brain metabolic rates between humans and nonhuman primates, those data that have been published from PET studies suggest that human brains in the awake state have rates of glucose consumption per unit of cortical tissue that are approximately equal in absolute terms to those of Old World monkeys in the awake state (e.g., Refs. 94 and 95). That shouldn’t be—it is a well-established principle of physiology (Kleiber’s law) that larger organs and organisms tend to use less energy per unit of tissue than do smaller ones. On this basis, we would expect the enormous brain of Homo sapiens, while using far more energy overall than that of apes and monkeys, to use less energy per gram of tissue. But the current evidence suggests this is not the case, and that human brains are running hot [89]. If true, this would likely have profound consequences for human psychology, neurophysiology, disease, and life history.
Conclusions
Neuroscientists are now in possession of powerful, noninvasive techniques for understanding the physical basis of the human mind. More than that, we can now determine what the human brain shares with that of other species, as well as what is distinctively human—that is, what we have evolved since our lineage parted ways with the lineage leading to our great-ape relatives. What is novel about this research, however, is not only the noninvasive methods employed, but also the strategy of comparative investigation. As valuable as studies of model species unquestionably are, if we want to understand how humans resemble and differ from other species, there is no substitute for studies that directly compare humans to other primates, and especially to our closest relatives, the chimpanzees.
Acknowledgments
The author would like to thank the organizers for their invitation to participate in the symposium, Chet Sherwood and Emmanuel Gilessen for sharing their views on some of the issues discussed here, and Katherine Bryant, Nicole Taylor, Mary Ann Cree, and Carolyn Suwyn for reviewing earlier drafts of the manuscript. The author’s research is supported by the Yerkes base grant (NIH RR-00165).
References
- 1.Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman GE, Le WW. Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides. 2004;25:425–431. doi: 10.1016/j.peptides.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Allen JS. The Lives of the Brain: Human Evolution and the Organ of Mind. Belknap Press of Harvard University Press; Cambridge, Mass: 2009. [Google Scholar]
- 4.Cohen J. Almost Chimpanzee: Searching for What Makes Us Human, in Rainforests, Labs, Sanctuaries, and Zoos. Times Books; New York: 2010. [Google Scholar]
- 5.Gazzaniga MS. Human: The Science Behind What Makes Us Unique. Ecco; New York: 2008. [Google Scholar]
- 6.Passingham RE. What is Special About the Human Brain? Oxford University Press; Oxford; New York: 2008. [Google Scholar]
- 7.Taylor J. Not a Chimp: The Hunt to Find the Genes that Make Us Human. Oxford University Press; Oxford; New York: 2009. [Google Scholar]
- 8.Blinkov S, Glezer I. The Human Brain in Figures and Tables. Basic Books; New York: 1968. [Google Scholar]
- 9.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrhinde. In: Garey LJ, editor. Barth (reprinted as Brodmann’s ‘Localisation in the Cerebral Cortex,’. London; Smith-Gordon: Leipzig: 1909. 1994. [Google Scholar]
- 10.Jerison HJ. Evolution of the Brain and Intelligence. Academic Press; New York: 1973. [Google Scholar]
- 11.Lashley KS. Persistent problems in the evolution of mind. Quart Rev Biol. 1949;24:28–42. doi: 10.1086/396806. [DOI] [PubMed] [Google Scholar]
- 12.Semendeferi K, et al. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- 13.Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Med Image Anal. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Deacon TW. Human brain evolution: II. Embryology and brain allometry. In: Jerison HJ, Jerison I, editors. Intelligence and Evolutionary Biology. Springer-Verlag; Berlin: 1988. pp. 383–415. [Google Scholar]
- 15.Preuss TM. What is it like to be a human? In: Gazzaniga MS, editor. The Cognitive Neurosciences. 3. MIT Press; Cambridge, MA: 2004. pp. 5–22. [Google Scholar]
- 16.Passingham RE. The frontal cortex: does size matter? Nat Neurosci. 2002;5:190–192. doi: 10.1038/nn0302-190. [DOI] [PubMed] [Google Scholar]
- 17.Deacon T. Rethinking mammalian brain evolution. Am Zool. 1990;30:629–705. [Google Scholar]
- 18.Crick F, Jones E. Backwardness of human neuroanatomy. Nature. 1993;361:109–110. doi: 10.1038/361109a0. [DOI] [PubMed] [Google Scholar]
- 19.Kaas JH. The organization and evolution of neocortex. In: Wise SP, editor. Higher Brain Function: Recent Explorations of the Brain’s Emergent Properties. John Wiley; New York: 1987. pp. 347–378. [Google Scholar]
- 20.von Bonin G, Bailey P. Patterns of the cerebral isocortex. In: Hofer H, Schultz AH, Starck D, editors. Primatologia. II/2. Vol. 10. Leiferung: 1961. pp. 1–42. [Google Scholar]
- 21.Holloway RL., Jr The evolution of the primate brain: some aspects of quantitative relations. Brain Res. 1968;7:121–172. doi: 10.1016/0006-8993(68)90094-2. [DOI] [PubMed] [Google Scholar]
- 22.Preuss TM. The New Cognitive Neurosciences. 2. MIT Press; Cambridge, MA: 2000. What’s human about the human brain? pp. 1219–1234. [Google Scholar]
- 23.Spocter MA, et al. Wernicke’s area homologue in chimpanzees (Pan troglodytes) and its relation to the appearance of modern human language. Proc Biol Sci. 2010;277:2165–2174. doi: 10.1098/rspb.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Bonin G. The archilecture. In: Bucy PC, editor. The Prutrl/ral Motor Cortex. Urbana: University of Illinois Press; 1944. pp. 7–82. [Google Scholar]; Bucy PC, editor. The precentral motor cortex. University of Illinois Press; Urbana, IL: pp. 7–32. [Google Scholar]
- 25.Arbib MA. Premotor cortex and the mirror neuron hypothesis for the evolution of language. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4: Primates. Elsevier; Oxford: 2007. pp. 417–422. [Google Scholar]
- 26.Deacon TW. The evolution of language systems in the human brain. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4: Primates. Elsevier; Oxford: 2007. pp. 529–547. [Google Scholar]
- 27.Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Booler F, Grafman J, editors. Handbook of Neuropsychology. Vol. 9. Elsevier; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- 28.Kolster H, Peeters R, Orban GA. The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. J Neurosci. 2010;30:9801–9820. doi: 10.1523/JNEUROSCI.2069-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanduffel W, et al. Extracting 3D from motion: Differences in human and monkey intraparietal cortex. Science. 2002;298:413–415. doi: 10.1126/science.1073574. [DOI] [PubMed] [Google Scholar]
- 30.Orban GA, et al. Similarities and differences in motion processing between the human and macaque brain: evidence from fMRI. Neuropsychologia. 2003;41:1757–1768. doi: 10.1016/s0028-3932(03)00177-5. [DOI] [PubMed] [Google Scholar]
- 31.Peeters R, et al. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Tootell RB, et al. Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rilling JK, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11:426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- 35.Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- 36.Gaffan D, Hornak J. Visual neglect in the monkey. Representation and disconnection. Brain. 1997;120:1647–1657. doi: 10.1093/brain/120.9.1647. [DOI] [PubMed] [Google Scholar]
- 37.Corballis MC. The Lopsided Ape: Evolution of the Generative Mind. Oxford University Press; New York: 1991. [Google Scholar]
- 38.Annett M. Handedness and Brain Asymmetry: The Right Shift Theory. Psychology Press; Taylor & Francis; Hove, East Sussex, New York: 2002. [Google Scholar]
- 39.Corballis MC. The evolution of hemispheric specializations of the human brain. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4: Primates. Elsevier; Oxford: 2007. pp. 379–394. [Google Scholar]
- 40.Corballis PM, Funnell MG, Gazzaniga MS. An evolutionary perspective on hemispheric asymmetries. Brain Cogn. 2000;43:112–117. [PubMed] [Google Scholar]
- 41.Crow TJ. Nuclear schizophrenic symptoms as the key to the evolution of the human brain. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4: Primates. Elsevier; Oxford: 2007. pp. 549–567. [Google Scholar]
- 42.Hopkins WD, Marino L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI) Neuropsychologia. 2000;38:493–499. doi: 10.1016/s0028-3932(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 43.LeMay M, Billig M, Geschwind N. Asymmetries of the brains and skulls of honhuman primates. In: Armstrong E, Falk D, editors. Primate Brain Evolution. Plenum Press; New York and London: 1982. pp. 263–277. [Google Scholar]
- 44.Holloway RL, de la Coste-Lareymondie MC. Brain endocast asymmetry in pongids and hominids: some preliminary findings on the paleontology of cerebral dominance. Am J Phys Anthropol. 1982;58:101–110. doi: 10.1002/ajpa.1330580111. [DOI] [PubMed] [Google Scholar]
- 45.Geschwind N, Levitsky W. Left-right asymmetry in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 46.Gannon PJ, et al. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke’s brain language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- 47.Hopkins WD, et al. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) Neuroreport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- 48.Schenker NM, et al. Broca’s area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry, and comparison to humans. Cereb Cortex. 2010;20:730–742. doi: 10.1093/cercor/bhp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buxhoeveden DP, et al. Lateralization of minicolumns in human planum temporale is absent in nonhuman primate cortex. Brain Behav Evol. 2001;57:349–358. doi: 10.1159/000047253. [DOI] [PubMed] [Google Scholar]
- 50.Amunts K, et al. Gender-specific left-right asymmetries in human visual cortex. J Neurosci. 2007;27:1356–1364. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amunts K, et al. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- 52.Sherwood CC, Subiaul F, Zawidzki TW. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J Anat. 2008;212:426–454. doi: 10.1111/j.1469-7580.2008.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, et al. Chimpanzee (Pan troglodytes) precentral corticospinal system asymmetry and handedness: a diffusion magnetic resonance imaging study. PLoS ONE. 2010;5:e12886. doi: 10.1371/journal.pone.0012886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Errangi BK, et al. Brain white matter asymmetries in humans and non-human primates – a comparative diffusion magnetic resonance imaging (MRI) study. Soc Neurosci Abstr 2009 [Google Scholar]
- 55.Iwatsubo T, et al. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology. 1990;40:309–312. doi: 10.1212/wnl.40.2.309. [DOI] [PubMed] [Google Scholar]
- 56.Kuypers HG. Corticobular connexions to the pons and lower brain-stem in man: an anatomical study. Brain. 1958;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- 57.Preuss TM. The discovery of cerebral diversity: An unwelcome scientific revolution. In: Falk D, Gibson K, editors. Evolutionary Anatomy of the Primate Cerebral Cortex. Cambridge University Press; Cambridge: 2001. pp. 138–164. [Google Scholar]
- 58.Preuss TM. Reinventing primate neuroscience for the twenty-first century. In: Platt ML, Ghazanfar AA, editors. Primate Neuroethology. Oxford University Press; Oxford: 2010. pp. 422–453. [Google Scholar]
- 59.Preuss TM. Specializations of the human visual system: The monkey model meets human reality. In: Kaas JH, Collins CE, editors. The Primate Visual System. CRC Press; Boca Raton, FL: 2004. pp. 231–259. [Google Scholar]
- 60.Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cereb Cortex. 2002;12:671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- 61.Preuss TM, Qi H, Kaas JH. Distinctive compartmental organization of human primary visual cortex. Proc Natl Acad Sci USA. 1999;96:11601–11606. doi: 10.1073/pnas.96.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allman JM, et al. Intuition and autism: A possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Hof PR, et al. An unusual population of pyramidal neurons in the anterior cingulate cortex of hominids contains the calcium-binding protein calretinin. Neurosci Lett. 2001;307:139–142. doi: 10.1016/s0304-3940(01)01964-4. [DOI] [PubMed] [Google Scholar]
- 64.Nimchinsky EA, et al. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oberheim NA, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semendeferi K, et al. Spatial Organization of Neurons in the Frontal Pole Sets Humans Apart from Great Apes. Cereb Cortex. 2010 Nov 23; doi: 10.1093/cercor/bhq191. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Raghanti MA, et al. Cholinergic innervation of the frontal cortex: differences among humans, chimpanzees, and macaque monkeys. J Comp Neurol. 2008;506:409–424. doi: 10.1002/cne.21546. [DOI] [PubMed] [Google Scholar]
- 68.Raghanti MA, et al. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb Cortex. 2008;18:584–597. doi: 10.1093/cercor/bhm089. [DOI] [PubMed] [Google Scholar]
- 69.Raghanti MA, et al. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Neuroscience. 2008;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.King MC, Wilson AC. Evolution at two levels in humans and chimpanzees. Science. 1975;188:107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- 71.Gould SJ. Ontogeny and Phylogeny. Belknap Press of Harvard University Press; Cambridge, Mass: 1977. [Google Scholar]
- 72.Enard W, et al. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- 73.Evans PD, et al. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science. 2005;309:1717–1720. doi: 10.1126/science.1113722. [DOI] [PubMed] [Google Scholar]
- 74.Mekel-Bobrov N, et al. Ongoing adaptive evolution of ASPM, a brain size determinant in Homo sapiens. Science. 2005;309:1720–1722. doi: 10.1126/science.1116815. [DOI] [PubMed] [Google Scholar]
- 75.Vargha-Khadem F, et al. Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci USA. 1995;92:930–933. doi: 10.1073/pnas.92.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Enard W, et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 77.Konopka G, et al. Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature. 2009;462:213–217. doi: 10.1038/nature08549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Britten RJ. Divergence between samples of chimpanzee and human DNA sequences is 5%, counting indels. Proc Natl Acad Sci U S A. 2002;99:13633–13635. doi: 10.1073/pnas.172510699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varki A, Altheide TK. Comparing the human and chimpanzee genomes: searching for needles in a haystack. Genome Res. 2005;15:1746–1758. doi: 10.1101/gr.3737405. [DOI] [PubMed] [Google Scholar]
- 80.Konopka G, et al. Comparative gene expression in primate brain. Soc Neurosci Abstr 2009 [Google Scholar]
- 81.Preuss TM, et al. Human brain evolution: Insights from microarrays. Nat Rev Genet. 2004;5:850–860. doi: 10.1038/nrg1469. [DOI] [PubMed] [Google Scholar]
- 82.Bustamante CD, et al. Natural selection on protein-coding genes in the human genome. Nature. 2005;437:1153–1157. doi: 10.1038/nature04240. [DOI] [PubMed] [Google Scholar]
- 83.Bailey JA, Eichler EE. Primate segmental duplications: crucibles of evolution, diversity and disease. Nat Rev Genet. 2006;7:552–564. doi: 10.1038/nrg1895. [DOI] [PubMed] [Google Scholar]
- 84.Cheng Z, et al. A genome-wide comparison of recent chimpanzee and human segmental duplications. Nature. 2005;437:88–93. doi: 10.1038/nature04000. [DOI] [PubMed] [Google Scholar]
- 85.Fortna A, et al. Lineage-specific gene duplication and loss in human and great ape evolution. PLoS Biol. 2004;2:E207. doi: 10.1371/journal.pbio.0020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayakawa T, et al. A human-specific gene in microglia. Science. 2005;309:1693. doi: 10.1126/science.1114321. [DOI] [PubMed] [Google Scholar]
- 87.Popesco MC, et al. Human lineage-specific amplification, selection, and neuronal expression of DUF1220 domains. Science. 2006;313:1304–1307. doi: 10.1126/science.1127980. [DOI] [PubMed] [Google Scholar]
- 88.Rosen RF, Walker LC, Levine H., 3rd PIB binding in aged primate brain: Enrichment of high-affinity sites in humans with Alzheimer’s disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cáceres M, et al. Elevated gene expression levels distinguish human from non-human primate brains. Proc Natl Acad Sci USA. 2003;100:1330–1335. doi: 10.1073/pnas.2135499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cáceres M, et al. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17:2312–2321. doi: 10.1093/cercor/bhl140. [Epub 2006 Dec 2320] [DOI] [PubMed] [Google Scholar]
- 91.Goodman M, Sterner KN. Colloquium paper: phylogenomic evidence of adaptive evolution in the ancestry of humans. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8918–8923. doi: 10.1073/pnas.0914626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oldham MC, Horvath S, Geschwind DH. Conservation and evolution of gene coexpression networks in human and chimpanzee brains. Proc Natl Acad Sci USA. 2006;103:17973–17978. doi: 10.1073/pnas.0605938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uddin M, et al. Molecular evolution of the cytochrome c oxidase subunit 5A gene in primates. BMC evolutionary biology. 2008;8:8. doi: 10.1186/1471-2148-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eberling JL, et al. Cerebral glucose metabolism and memory in aged rhesus macaques. Neurobiol Aging. 1997;18:437–443. doi: 10.1016/s0197-4580(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 95.Noda A, et al. Age-related changes in cerebral blood flow and glucose metabolism in conscious rhesus monkeys. Brain Res. 2002;936:76–81. doi: 10.1016/s0006-8993(02)02558-1. [DOI] [PubMed] [Google Scholar]
- 96.Frahm HD, Stephan H, Baron G. Comparison of brain structure volumes in Insectivora and Primates. V. Area striata (AS) J Hirnforsch. 1984;25:537–557. [PubMed] [Google Scholar]
- 97.Armstrong E. Mosaic evolution in the primate brain: Differences and similarities in the hominoid thalamus. In: Armstrong E, Falk D, editors. Primate Brain Evolution. Plenum Press; New York and London: 1982. pp. 131–161. [Google Scholar]