Abstract
Studies of epilepsy have mainly focused on the membrane proteins that control neuronal excitability. Recently, attention has been shifting to intracellular proteins and their interactions, signaling cascades and feedback regulation as they relate to epilepsy. The mTOR (mammalian target of rapamycin) signal transduction pathway, especially, has been suggested to play an important role in this regard. These pathways are involved in major physiological processes as well as in numerous pathological conditions. Here, involvement of the mTOR pathway in epilepsy will be reviewed by presenting; an overview of the pathway, a brief description of key signaling molecules, a summary of independent reports and possible implications of abnormalities of those molecules in epilepsy, a discussion of the lack of experimental data, and questions raised for the understanding its epileptogenic mechanism.
Keywords: epilepsy, mTOR, rapamycin
Introduction
In 'On the sacred disease', the first book on epilepsy, Hippocrates correctly described epilepsy as a brain disorder. However, for hundreds of years, epilepsy patients have been considered possessed or contagious, and persons with epilepsy have been stigmatized, prohibited, or even segregated from their communities. Even today, epilepsy still remains a mysterious disease. It is one of the most common neurological problems in the world, and approximately 1% of the general population has epileptic episodes at some point in their lives (WHO, 2005). It has the genetic, environmental, and epigenetic components, and these factors are differentially interwoven in individual patients with various types of epilepsy (Berkovic et al., 2006).
Since the first report of an α4 neuronal nicotinic receptor subunit mutation in humans was linked to epilepsy, the list of epileptic mutations in both voltage- and ligand-gated ion channels has continued to grow (Steinlein et al., 1995; Helbig et al., 2008). Thus, the knowledge of molecular mechanism of seizures caused by those mutations has been deepened over last decade (Reid et al., 2009). However, genetic defects are only a partial, if not minor, cause of epilepsy. The effectiveness of anti-epileptic drugs (AEDs) against ion channels is limited for the treatment and management of 'acquired' epileptic conditions (Beck, 2007). Epileptic seizures result from abnormal synchronous firing of neuronal population (Scharfman, 2007). Since epilepsy show multiple events; cell death, cell survival and ectopic neurogenesis, aberrant axonal sprouting, and synaptic reorganization, the existence of the core signaling pathway involved in these processes should have been expected. However, we have not been able to have the luxury of intracellular signaling mechanism for epilepsy like other neurological diseases until recently (Swiech et al., 2008). Here, I will summarize and frame individual reports of epilepsy-related molecules into the mTOR pathway and try to set the common ground that can be served for the continuing discussion. This may be helpful for researchers especially in the epilepsy field who are not familiar with the intracellular signaling pathway.
Known involvement of the mTOR pathway in epilepsy
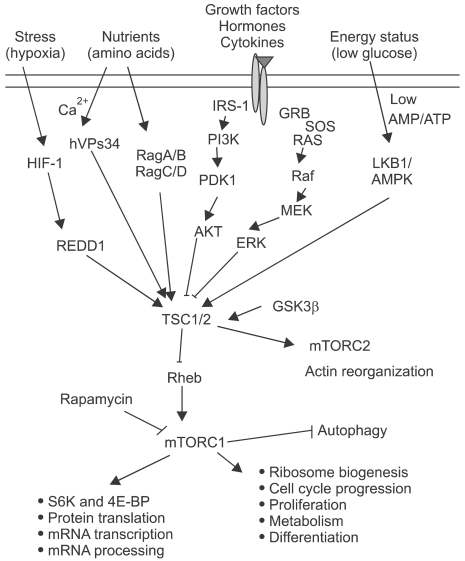
The mTOR pathway has been studied extensively over the last decade and has been involved both in various normal physiological processes (metabolism, cell growth, proliferation, differentiation, longevity, apoptosis, and autophagy) and several disease conditions (tumorigenesis, type 2 diabetes, inflammation, and neurodegenerative diseases) (Figure 1).
Figure 1.
Overview of mTOR signaling pathway. Activation and inhibition of signaling molecules by phosphorylation are shown in red and blue respectively.
Recently, the mTOR pathway has been examined in animal models of medial temporal lobe epilepsy (Buckmaster et al., 2009; Zeng et al., 2009; Huang et al., 2010). In these studies, kainate or pilocarpine was injected into rats to induce status epilepticus (SE) and the animals went on to develop spontaneous seizures. It has been shown that S6, a ribosomal protein involved in translation initiation, and a downstream molecule in the mTOR signaling pathway, became phosphorylated (activated). Treatment of rapamycin, an mTOR kinase inhibitor, given either as a pretreatment or given after SE, reduced both mossy fiber sprouting, an abnormal change in the dentate gyrus and hilus, and seizure frequency (Davenport et al., 1990).
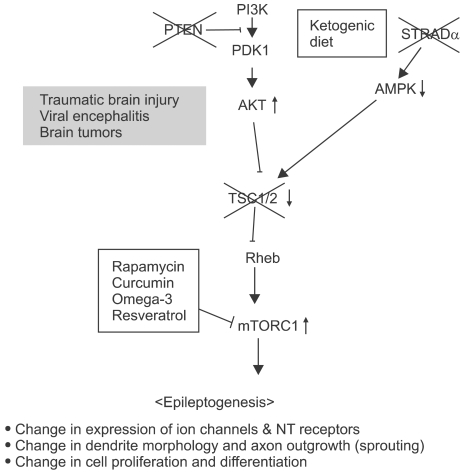
Some diseases caused by genetic mutations in the molecules on the mTOR pathway show epileptic seizures (Figure 2). For example, Tuberous sclerosis complex (TSC), a multi-organ disorder, is mainly caused by mutations in TSC1 and/or TSC2. Its tuber formation is highly associated with mental retardation, autism and epilepsy (Curatolo et al., 2008). TSC2, a tumor suppressor forming a complex with TSC1, has been known as a key regulator of the mTOR kinase, and its functional failure results in uncontrolled mTORC1 activity (Inoki et al, 2005). Treatment with rapamycin reduced the seizure frequency in TSC patients and mouse models of TSC (Meikle et al., 2008; Zeng et al., 2008; Muncy et al., 2009).
Figure 2.
Genetic mutations of signaling molecules implicated in mTOR pathway (red Xs). Red arrows (up- or downward) indicate the changes in activity of particular molecules in epileptic conditions. Causatives for acquired epilepsy are described in gray. Therapeutic intervening possibilities are shown in boxes. NT - neurotransmitter receptor.
Similarly, PTEN (phosphatase and tensin homolog) is a molecule found mutated in autosomal dominant harmatoma and epilepsy-associated glioblastoma, and the conditional knockout mice showed cortical dysplasia, ataxia, and seizures (Backman et al., 2001). PTEN is a negative regulator of phosphoinositide 3-kinase (PI3K) which is located at upstream of mTOR (Cully et al., 2006). Treatment with rapamycin inhibits seizures in this animal model (Ljungberg et al., 2009; Zhou et al., 2009a).
Lafora disease is an autosomal recessive epilepsy which is caused by defective laforin or malin proteins (Ganesh et al., 2006) (Figure 3G). This neurodegenerative disease has lafora bodies, which are polyglucosans masses, are found in neurons, myocytes, and hepatocytes. Polyglucosans, insoluble and abnormally formed glycogen molecules, are produced by failure to regulate glycogen synthase (GS) activity. Laforin (encoded by EPM2A gene) dephosphorylates GSK3β, thus controlling GS. Malin (encoded by EPM2B gene) is an E3 ubiquitin ligase which binds GS and laforin, regulating their degradation (Gentry et al., 2005; Lohi et al., 2005). GSK3β is phosphorylated by AKT and S6K1, and it phosphorylates TSC1, TSC2, and REDD1 which all are on the mTOR pathway (Zhang et al., 2006; Inoki et al., 2006; Allard et al., 2008; Katiyar et al., 2009).
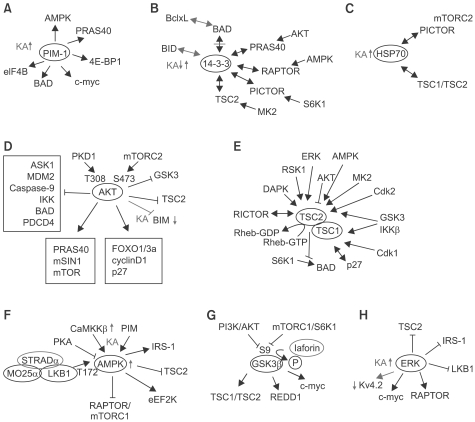
Figure 3.
Signaling molecules implicated in epilepsy (see the text for the detail). Arrows indicate the phosphorylation events. Up- and downward arrows indicate the changes in the expression level or activity of particular molecules. Double arrows indicate the protein-protein interaction. Some interactions were induced by phosphorylation. (A) PIM-1 is increased in kainate model. (B) 14-3-3 interacts with BID and dissociates from BAD in kainate model. (C) HSP70 level is increased in kainate model. (D) AKT decreased BIM expression in epilepsy model. AKT is activated by PDK1 phosphorylation at T308 and mTORC2 phosphorylation at S473. AKT modulates molecules involved in apoptosiss and cell cyle as well as other molecules in the mTOR pathway. (E) Various protein-protein interactions with TSC1 and TSC2. When mutated, TSC1/2 lose control of Rheb activity. (F) AMPK and CaMKKβ are increased in kainate model, causing TSC2 inhibition. STRADα in an epileptic condition was indicated in red. AMPK is phosphorylated at T172 by STRADα-MO25α-LKB1 complex. (G) GSK3β is inhibited by phosphorylation at S9. In lafora disease, GSK3β can not be regulated due to the mutation in laforin, a phosphatase. (H) Activation of ERK decrease the surface expression of Kv4.2 channels in kainate model. KA - kainic acid, T-Threonine, S-Serine.
PMSE (Polyhydramnios, megalencephaly and symptomatic epilepsy) has been recently found having gene deletion in STRADα (Puffenberger et al., 2007) (Figures 2 and 3F). STRADα forms a complex with LKB1 and MO25α, and this complex regulates AMPK which controls mTORC1 and TSC2, an upstream regulator of mTORC1 (Hardie, 2005).
The mTOR pathway overview
The mTOR is a master regulator which integrates multiple upstream signals: both extracellular (e.g., growth factors) and intracellular (e.g., energy status) to regulate gene expression, translational rates and metabolic processes (Hay and Sonenberg, 2004) (Figure 1). When the ligand such as insulin-like growth factor (IGF) binds to its receptor (e.g., IGF receptor) on the plasma membrane, the activated signal (phosphorylation) transduces to PI3K either directly or indirectly via mediator proteins such as Insulin receptor substrate-1 (IRS-1). PI3K makes phosphatidylinositol 3, 4, 5 trisphosphate (PIP3) from phosphatidylinositol 4, 5 bisphosphate (PIP2), PIP3 activates PDK1, and PDK1 phosphorylates and activates AKT/PKB at Thr308. PI3K reaction can be reversed by PTEN and/or SHIP-2, and PI3K can be activated by Ras or PI3K enhancer (PIKE). Activated AKT inhibits TSC2 by phosphorylation, subsequently disinhibits Rheb to activate mTORC1. AKT can be fully activated by phosphorylation at Ser473 by mTORC2, inhibiting mTORC2-regulating molecules such as FOXO and BAD. PHLPP reverses mTORC2-mediated phosphorylation of AKT. TSC2 forms a complex with TSC1 inhibiting Rheb by keeping it GDP bound form (Rheb-GDP), and Rheb-GTP activates mTORC1. Signaling through mTORC1 promotes protein synthesis via phosphorylation which causes the inactivation of translation repressor 4E-BPs and the activation of S6 Kinases and ribosomal protein S6. TSC2/TSC1 complex integrates another inhibitory signal from growth factor-related signaling pathway of Ras/MAPK including ERK/RSK, and activating signals of energy status through LKB1/AMPK, stress or oxygen level via HIF-1/REDD1, and Wnt signaling through GSK3. Signals of amino acid availability are transduced to mTORC1 directly via RagA/B and RagC/D, hVps34 or MAP4K3. AKT, AMPK, and RSK can regulate mTORC1 activity either directly or via TSC2/TSC1 complex.
Feedback regulation is important in the mTOR pathway. TSC1/2-mediated activation of mTORC2 phosphorylates and activates AKT. S6K phosphorylates IRS-1, mTORC1, and GSK3 which is inhibited by AKT and activates TSC2. Cross-talk between signaling pathways is also important; mTORC1 activation induces phosphorylation of ERK1/2 at Thr202, inhibiting its activity via PP2A (Harwood et al., 2008). Ras activates PI3K as well as MAPK pathway, ERK inhibits TSC2 either directly or indirectly via RSK, and it also regulates eIF4B via RSK or MNK1/2.
Components of mTORC1 and mTORC2
mTORC1 and mTORC2 are two different protein complexes that mTOR partners with, executing different but related functions. Substrate specificity of mTOR is therefore determined by the core proteins with which mTOR forms a complex and they are also regulated in distinct ways.
mTORC1 comprises five different components: 3 common proteins that it shares with mTORC2 (mTOR, mLST8, and DEPTOR), and 2 mTORC1-specific proteins (RAPTOR and PRAS40). mTORC1 plays a major role in controlling cell growth in response to amino acids, energy status, stress, oxygen levels, hormones, growth factors and cytokines by regulating several cellular processes, including translation, transcription, ribosome biogenesis, nutrients transport and autophagy (Reiling and Sabatini, 2006; Wullschleger et al., 2006; Dunlop and Tee, 2009; Mizushima, 2010). mTORC1 is known to be rapamycin-sensitive via its FKBP12 interaction (Sabers et al., 1995). Identified downstream targets of mTORC1 for regulating these processes at the translational level are S6K1, 4E-BP1, eEF2K, eIF3F, and eIF4G (Browne and Proud, 2004; Hay and Sonenberg, 2004; Harris et al., 2006; Csibi et al., 2010). At the transcriptional level, SREBP1, Lipin-1, c-Myc and STAT3 interact with mTORC1 to control expression of their specific target genes (Yokogami et al., 2000; Huffman et al., 2002; Porstmann et al., 2008; Zhang et al., 2008). mTORC1 phosphorylates CLIP-170 to reorganize microtubule, and ULK1 (ATG1)/ATG13 to inhibit autophagy (Choi et al., 2002; Jung et al., 2009). PP2A, PIM-1, and 14-3-3 interact closely with mTORC1 (Harwood et al., 2008; Gwinn et al., 2008; Zhang et al., 2009).
mTORC2 has six components: 3 common proteins that it shares with mTORC1 (mTOR, mLST8, and DEPTOR) and 3 mTORC2-specific proteins (RICTOR, mSIN1, and Protor-1). The upstream regulators of mTORC2 are less clearly defined- it is TSC1/2-dependent, possibly via direct TSC2-RICTOR interaction (Huang et al., 2008, 2009a). mTORC2 is generally known as rapamycin-insensitive, and it seems to be regulated only by growth factors (Yang et al., 2006a). However, prolonged treatment of rapamycin (> 12 h) blocks mTORC2 assembly (Sarbassov et al., 2006). AKT/PKB, PKCα, and SGK1 are known downstream targets of mTORC2 (Jacinto et al., 2004; Sarbassov et al., 2005; García-Martínez and Alessi, 2008). By releasing the inhibitory action of TSC via AKT, mTORC2 controls the upstream of mTORC1 activity (Inoki et al., 2002; Sancak et al., 2008). Through AKT activation, mTORC2 controls the expression of transcription factors such as FOXO, and an apoptosis regulator, BAD (Datta et al., 1997; Guertin et al., 2006). mTORC2 also reorganizes actin cytoskeleton through Rho-associated kinase (ROCK1) and PKCα (Jacinto et al., 2004; Sarbassov et al., 2004; Shu and Houghton, 2009). P-REX1, HSP70, and 14-3-3 interact closely with mTORC2 (Hernández-Negrete et al., 2007; Martin et al., 2008; Dibble et al., 2009).
Individual molecules of the mTOR complexes and closely interacting molecules
Here, a brief description will be given of the individual molecules in the mTOR pathway, their phosphorylation patterns and protein-protein interactions, phenotypes of null mice, drugs modulating their activities, and epilepsy-related findings.
mTOR
The mTOR is a Ser/Thr protein kinase of phosphatidylinositide-kinase-related family (Keith and Schreiber, 1995). It was first identified in Saccharomyces cerevisiae, and it is highly conserved among eukaryotes (Jacinto and Hall, 2003; Wullschleger et al., 2006). It is also called FRAP (FKBP-rapamycin associated protein), RAPT (Rapamycin target), RAFT (rapamycin and FKBP12 target) or SEP (sirolimus effector protein). Expression of mTOR is ubiquitous, high expression of mRNA is found in brain, kidney, placenta and skeletal muscle (Kim et al., 2002). Multiple subcellular localization of mTOR has been reported in endoplasmic reticulum, Golgi apparatus, mitochondria, cytoplasm, and nucleus, implicating its multi-functionality (Kim and Chen, 2000; Desai et al., 2002; Liu and Zheng, 2007). Ubiquitination of mTOR by FBXW7 leads to the proteosomal degradation (Mao et al., 2008). Knockout mice (mTOR-/-) die at E5.5 and these embryos show the inability to establish embryonic stem cells (Gangloff et al., 2004; Murakami et al., 2004). Heterozygous mTOR (mTOR+/-) knockout mice did not develop any noticeable abnormality and were fertile (Gangloff et al., 2004; Murakami et al., 2004). mTOR participates in signaling pathways associated with human diseases including tuberous sclerosis complex, lymphangioleiomyomatosis, Cowden disease, Peutz-Jeghers syndrome, neurofibromatosis, familial cardiac hypertrophy, and cancers characterized by hyperactivation of PI3K/AKT (Guertin and Sabatini, 2005; Shaw and Cantley, 2006). There are three phosphorylation sites (Thr2446, Ser2448, and Ser2481) on mTOR: Thr2446 has been shown to be phosphorylated by AMPK and S6K1 (Cheng et al., 2004; Holz and Blenis, 2005), Thr2448 by AKT and S6K1 (Sekulić et al., 2000; Holz and Blenis, 2005), and Ser2481 has been reported to be autophosphorylated by mTOR itself (Peterson et al., 2000). There is a report that Ser2448 is predominantly phosphorylated with mTORC1, whereas Ser2481 with mTORC2 (Copp et al., 2009).
mLST8
mLST8 (mammalian lethal with Sec13 protein 8) is a positive regulator of mTORC1 and mTORC2 (Kim et al., 2003; Guertin et al., 2006). It is also known as GβL, a protein homologous to β subunits of heterotrimeric G proteins (Kim et al., 2003). It has seven WD40 repeats for protein-protein interaction, and it binds near the catalytic domain of mTOR required for the full kinase activity (Kim et al., 2003). mLST8 null mice have defective vascular development and die at E10.5 (Guertin et al., 2006; Shiota et al., 2006).
DEPTOR
DEPTOR (DEP-domain containing mTOR-interacting protein) is a negative regulator of mTOR complexes, and it binds to mTOR via its PDZ domain (Peterson et al., 2009). When DEPTOR is activated by mTORC1 phosphorylation, the mTORC1-DEPTOR interaction became weak. RNAi-mediated knockdown of DEPTOR shows that increased cell size, reduced vulnerability for apoptosis. DEPTOR appears to inhibit mTORC1 more strongly than mTORC2.
RAPTOR
RAPTOR (regulatory-associated protein of mTOR) is a positive regulator of mTORC1, and recruits mTOR substrates (Kim et al., 2002). It has a distinctive amino-terminal region followed by three HEAT motif and seven WD40 repeats (Kim et al., 2002). RAPTOR binds to 4E-BP1 and S6K1 using carboxy-terminal TOR signaling (TOS) motifs, and TOS motifs were also identified in PRAS40, PLD2 and eIF3F (Schalm and Blenis, 2002). RAPTOR is phosphorylated at Ser792 by AMPK, inducing 14-3-3 binding to AMPK-ULK1-mTORC1 complex to inhibit mTORC1 activity (Gwinn et al., 2008; Lee et al., 2010). It is also phosphorylated by ERK1/2 at Ser8, Ser696, and Ser863 and by RSK at Ser719, Ser721 and Ser722, activating mTORC1 activity (Carrière et al., 2008a, 2011). RAPTOR null mice die early in development - between E6.5 and E8.5 (Gangloff et al., 2004; Murakami et al., 2004; Guertin et al., 2006).
PRAS40
PRAS40 (Proline-rich AKT substrate of 40 KDa; also known as AKT1 substrate 1 (AKTS1)) is phosphorylated at Thr246 by AKT and this promotes its binding with 14-3-3, relieving from mTORC1, thus disinhibits mTORC1 (Vander Haar et al., 2007). PIM-1 kinase also phosphorylates PRAS40 at Thr246 (Zhang et al., 2009). PRAS40 has a TOS motif and is phosphorylated at Ser183, Ser212 and Ser221 by mTORC1 (Oshiro et al., 2007; Wang et al., 2008). Ser221 and Thr246 are involved in its binding to 14-3-3 (Wang et al., 2008).
RICTOR
RICTOR (Rapamycin-insensitive companion of mTOR) is a key component of mTORC2 (Sarbassov, et al., 2004). RICTOR null embryos exhibit growth arrest and die at E11.5 and cells deficient of RICTOR showed low proliferation rate and metabolic activity (Shiota et al., 2006). By RNAi-mediated knockdown, RICTOR (thus mTORC2) has been shown to regulate organization of actin cytoskeleton and phosphorylate/activate AKT (Jacinto et al., 2004; Sarbassov et al., 2004, 2005). RICTOR directly interacts with TSC2, stimulating mTORC2 activity (Huang et al., 2009a). Among 21 identified phosphorylation sites of RICTOR, Thr1135 is phosphorylated by SGK1, AKT, or S6K1 via mTORC1, and this phosphorylation is acutely sensitive to rapamycin (Dibble et al., 2009). This phosphorylation dissociates RICTOR/Cullin1 complex, an E3 ubiquitin ligase and stimulates binding of RICTOR to 14-3-3 proteins without affecting mTORC2 kinase activity (Dibble et al., 2009; Gao et al., 2010).
mSIN-1
mSIN-1 (mammalian stress-activated protein kinase interacting protein; also known as MIP1 (MEKK2 interacting protein 1)) is necessary for mTORC2 assembly and for phosphorylation at Ser473 of AKT (Frias et al., 2006). Among five alternative splicing variants (mSin1.1 - mSin1.5), three can assemble into mTORC2 to make distinct mTORC2s (mSin1.1, 1.2, and 1.5). Only two of them (mSin1.1 and mSin1.2) are insulin-responsive (Frias et al., 2006). mSin1-/- mice are embryonic lethal but mSin1+/- appears to develop normally (Jacinto et al., 2006). Knockdown of mSin1 results in decrease of RICTOR phosphorylation and protein levels, and disruption of the RICTOR-mTOR interaction (Yang et al., 2006b). This knockdown also decreases the phosphorylation of AKT substrates and makes cells more sensitive to apoptosis.
PROTOR-1
PROTOR-1 (Protein observed with RICTOR-1, also called PRR5 (Proline-rich protein 5)) was identified to bind to RICTOR, and silencing of its gene inhibits AKT and S6K1 phosphorylation (Pearce et al., 2007; Woo et al., 2007). It has ubiquitous expression including in the brain (Shan et al., 2003). PRR5-like protein (PRR5L, Q6MZQ0) is likely to be PROTOR-2 which also binds to mTORC2 (Pearce et al., 2007; Thedieck et al., 2007).
FKBP12
FKBP12 (FK506 binding protein 12) is an immunophilin which inhibits mTORC1 by forming a complex with rapamycin (Vignot et al., 2005). FKBP12 also has peptidyl-prolyl isomerase activities and regulates intracellular calcium release, cellular trafficking and gene expression by interacting with ryanodine receptors, IP3 receptors, and TGFβ receptors (Harrar et al., 2001). FKBP38 is an endogenous inhibitor of mTORC1 and it is closely related to FKBP12 (Bai et al., 2007). FKBP38 is removed from mTOR when Rheb binds to it, thus activating mTORC1 and this Rheb-FKBP38 interaction is regulated by mitogens and amino acid availability. Its brain expression is very low, therefore, FKBP12 has been considered as the major repressor of mTORC1 activity (Bai et al., 2007). FKBP12 null mice showed severe congenital heart symptoms, and brain-specific deletion of FKBP12 shows the enhancement of long-term potentiation (LTP) in the hippocampus and memory (Shou et al., 1998; Hoeffer et al., 2008).
PIM-1
PIM-1 (provirus integration site for Moloney murine leukemia virus) is a Ser/Thr kinase which localizes to the nucleus and dendrites of activated neurons (Konietzko et al., 1999) (Figure 3A). It phosphorylates PRAS40 on Thr246, and this modification releases PRAS40 from mTORC1, activating mTORC1 kinase (Zhang et al., 2009). It activates AMPK by phosphorylating it at Thr172, thus inhibiting mTORC1 activity (Beharry et al., 2011). It stabilizes c-Myc, an mTORC1 substrate by phosphorylation on Thr62 and Ser329 (Zhang et al., 2008). It also phosphorylates 4E-BP1 on Thr37 and Thr46 and eIF4B on Ser406, enhancing protein synthesis (Chen et al., 2005; Peng et al., 2007). It phosphorylates and inactivates BAD on Ser112 in vitro, improving the cell survival (Aho et al., 2004). Kainate injection induces PIM-1 expression in dentate gyrus in rats (Feldman et al., 1998). PIM-1 expression is induced by and required for LTP (Konietzko et al., 1999). Pim null mice develop normally, and are fertile without any significant abnormality in the brain (Laird et al., 1993). Compound 24 and 4a were developed to inhibit PIM-1 (Grey et al., 2009; Xia et al., 2009).
Phosphatidic acid
Phosphatidic acid (PA) is made by phospholipase D (PLD), diacylglycerol kinase, and lysophosphatidic acid acyltransferase and it transduces mitogenic signals to mTORC1 (Foster, 2009). PA specifically binds to the FKBP12 binding domain of mTOR and it also binds to and activates S6K1 (Fang et al., 2001; Lehman et al., 2007).
14-3-3
14-3-3 proteins are involved in extraordinarily broad cellular process in all eukaryotes, and they function as a dimer by binding to phosphorylated target proteins at the specific site, causing a conformational change (Mackintosh, 2004) (Figure 3B). Among the many proteins that interact with 14-3-3, several are on the mTOR pathway: 1) PRAS40 is binds to 14-3-3 when phosphorylated at Ser221 and Thr246 by AKT (Wang et al., 2008). 2) RAPTOR binds to 14-3-3 when phosphorylated at Ser722 and Ser792 by AMPK (Gwinn et al., 2008). 3) RICTOR binds to 14-3-3 when phosphorylated at Thr1135 by S6K1 (Dibble et al., 2009). 4) TSC2 is phosphorylated at Ser1210 by MK2, enhancing binding to 14-3-3 (Li et al., 2003). 5) REDD1 competes with TSC2 on binding to 14-3-3 under stressed condition (DeYoung et al., 2008). In kainate injected rats, a pro-apoptotic molecule, BAD is dephosphorylated to disrupt binding to 14-3-3, and BAD dimerized with anti-apoptotic molecule BCL-XL (Meller et al., 2003). During seizure-induced neuronal death, another pro-apoptotic molecule, BID (BH3-interacting domain death agonist) is cleaved, increasing its binding to 14-3-3, although level of 14-3-3 was decreased (Shinoda et al., 2003). 14-3-3ε and 14-3-3ζ levels were increased in human temporal lobe epilepsy specimen (chronic period) than control, however, 14-3-3ε and 14-3-3ζ level were decreased in acute kainate injected rats (Schindler et al., 2006). 14-3-3ε and 14-3-3σ null mice appear normal (Steinacker et al., 2005; Su et al., 2011).
P-REX1
P-REX1 (PIP3-dependent Rac exchanger 1) is a guanine nucleotide exchange factor for Rac and it connects G-protein coupled receptors through Gβγ and PI3K to Rac activation (Barber et al., 2007). Through its DEP domains, it interacts with mTORC2, serves as an effector of mTOR to Rac activation and cell migration (Hernández-Negrete et al., 2007). It is also implicated in migration of cortical neurons and neurite differentiation (Yoshizawa et al., 2005; Waters et al., 2008). P-REX1 null mice are healthy except for mild neutrophilia (Welch et al., 2005).
HSP70
HSP70 (heat shock protein 70) has been shown to interact with RICTOR for the formation and activity of mTORC2 in addition to the interaction with TSC1 and TSC2 (Martin et al., 2008; Inoue et al., 2009) (Figure 3C). HSP70-1/HSP70-3 double knockout mice are more susceptible to ischemia-induced damages (Kim et al., 2006). In kainate-induced epileptic rat, gene expression of HSP72, a mammalian homolog, is enhanced (Gass et al., 1995). Overexpression of HSP72 helps the survival of dentate granule cells from cell death induced by kainate (Yenari et al., 1998). It remains to be seen how epileptic insult induces this stress protein to interact with TSC1/2 and/or mTOR complexes for the neuroprotective effect.
Upstream signaling molecules
IRS-1
IRS-1 (Insulin receptor substrate-1) transduces activation signals via tyrosine phosphorylation from Insulin- or IGF receptors to PI3K (Ogawa et al., 1998). Phosphorylation of IRS-1 promotes its proteasomal degradation (Gual et al., 2005). Phosphorylation patterns at multiple sites are complicated in the pathological conditions such as tumorigenesis and diabetes (Gibson et al., 2007). IRS-1 is phosphorylated at Ser 270 and Ser1101 by S6K1, at Ser794 by AMPK, and at Ser636, Ser639, Ser662 and Ser639 by mTORC1 (Tzatsos and Kandror, 2006; Tzatsos and Tsichlis, 2007; Tremblay et al., 2007; Zhang et al., 2008). ERK also phosphorylates IRS-1 at Ser612 (Andreozzi et al., 2004). IRS-1 null mice showed half-size compared to controls and impaired glucose tolerance, and female IRS-1 null mice lived longer than controls (Araki et al., 1994; Selman et al., 2008).
PI3K
PI3K (phosphoinositide 3-kinase, Class I) phosphorylates PIP2 to produce PIP3. It consists of a catalytic (p110α, p110β, p110γ, or p110δ) and a regulatory subunit (p85 or p101 for p110γ) (Zhao and Vogt, 2008). Insulin, IGF, and epidermal growth factor (EGF) use p110α/p85 to make PIP3, further activate mTOR signaling cascade (Knight et al., 2006). PI3K negatively controls FOXO-mediated neuronal excitability, and PI3K activation increases axon size and synapse number in mTOR/S6K-dependent manner (Howlett et al., 2008). Several PI3K-specific inhibitors including LY294002 are available (Kong and Yamori, 2008). Specific inhibitors against both mTORC1 and PI3K are extensively being developed to circumvent the drug resistance (Brachmann et al., 2009). Phenotypes of knockout mice of PI3K isoforms are described in detail elsewhere (Vanhaesebroeck et al., 2005). In epilepsy-associated gangliogliomas, PI3K and other mTOR pathway signaling molecules have been shown to be activated in patients' specimen (Boer et al., 2010).
PTEN
PTEN (phosphatase and tensin homolog) is a lipid phosphatase found mutated in autosomal dominant harmatoma, and it converts PIP3 to PIP2, and further to phosphatidylionitol 5-monophosphate (Cully et al., 2006) (Figure 2). PTEN is transported to the plasma membrane via myosin V by its phosphorylation at Ser380, Thre 382 and Thr 383 by GSK3β (van Diepen et al., 2009). PTEN suppresses transcription of rRNAs and tRNAs by disrupting the binding of transcription factors either to their promoters or other proteins (Zhang et al., 2005a; Woiwode et al., 2008). PTEN has been shown to be involved in NMDA receptor-dependent long-term depression (LTD) (Jurado et al., 2010). Formation of a new growth cone after axotomy and axon regeneration after injury in retinal ganglion cells is modulated by PTEN/mTOR signaling pathway (Verma et al., 2005; Park et al., 2008). PTEN null mice are embryonic lethal and heterozygous null mice have multiple tumors (Di Cristofano et al., 1998; Suzuki et al., 1998). Potassium bisperoxo (1, 10-phenanthroline) oxovanadate inhibits PTEN (Lai et al., 2009).
SHIP-2
SHIP-2 (SH2-domain containing inositol 5-phosphatase 2) is a negative regulator of the insulin signaling pathway and it hydrolyses PIP3 to PIP2, inhibiting PDK1 and AKT activation (Vinciguerra and Foti, 2006). SHIP-2 null mice show the high resistance to the weight gain on high-fat diet (Sleeman et al., 2005).
PIKEs
PIKEs (PI3K enhancers) are a family of GTPase that interacts with and stimulates PI3K and AKT, especially in the brain (Ahn and Ye, 2005). PIKE-S localizes in the nucleus, PIKE-L shows multiple localizations, and PIKE-A directly activates AKT. PIKE-L binds to Homer, connecting mGluR to PI3K (Rong et al., 2003). PIKE-A is important for insulin to modulate AMPK phosphorylation, PIKE null mice are resistant to diabetes (Chan et al., 2010).
PDK1
PDK1 (3-phosphoinositide-dependent protein kinase-1) has been characterized as an essential link between PI3K and AKT by phosphorylating and activating AKT at Thr308 (Wick et al., 2000). Interestingly, PDK1 has been shown to shuttle between cytoplasm and nucleus (Kikani et al., 2005). PDK1 also phosphorylates RSK at Ser227, SGK at Thr256 and S6K at Thr252 (Alessi et al., 1998; Frödin et al., 2000; Biondi et al., 2001). It phosphorylates and stabilizes several PKC isoforms (Balendran et al., 2000). PDK1 null embryos die at E9.5, hypomorphic mice are half-size compared to controls (Lawlor et al., 2002). PDK1 deficient brain showed microcephaly and increased phosphorylation of AKT at Ser473 in glia, not in neurons (Chalhoub et al., 2009). 3-Hydroxyanthranilic acid specifically inhibits PDK1 (Hayashi et al., 2007).
AKT/PKB
AKT/PKB (acutely transforming retrovirus AKT8 in rodent T cell lymphoma/Protein Kinase B) is a Ser/Thr kinase which is a key intracellular mediator of diverse cellular processes, and is activated in a PI3K-dependent manner (Manning and Cantley, 2007) (Figure 3D). AKT phosphorylates TSC2 and suppresses GTPase-activating protein (GAP) activity, thus activating mTORC1 (Inoki et al., 2002). Full activation of AKT requires the phosphorylation at Thr308 by PDK1 and at Ser473 (and Thr450) by mTORC2, a long-sought PDK2 (Sarbassov et al., 2005; Shiota et al., 2006; Facchinetti et al., 2008). When fully activated, AKT acts both as an upstream activator of mTORC1 and as a downstream substrate of mTORC2 (Sarbassov et al., 2006). Phosphorylation at Ser 473 is necessary for FOXO1/3a phosphorylation, but not other AKT targets including TSC2 and GSK3 in vivo (Jacinto et al., 2006). In addition to PRAS40, mSIN1 and mTOR (Sekulić et al., 2000; Frias et al., 2006; Vander Haar et al., 2007), protein substrates of AKT are grouped in two: 1) cell cycle regulation - FOXO1/3a, cyclin D1, and p27 (Liang and Slingerland, 2003), and 2) apoptosis - ASK1, MDM2, caspase-9, IKK, BAD, and PDCD4 (Cardone et al., 1998; Lawlor and Alessi, 2001; Franke et al., 2003; Palamarchuk et al., 2005; Zhang et al., 2005b; Dan et al., 2008). AKT is implicated in suppressing apoptosis, and kainate-injury induces phosphorylation of mTOR and AKT (Zhang et al., 2005b; Shacka et al., 2007). AKT activation may be neuroprotective against kainate-induced epilepsy by inhibiting BIM (Bcl-2-interacting mediator of cell death) expression (Shinoda et al., 2004). AKT1 null mice showed the impairment in adult neurogenesis and LTP in the hippocampus (Balu et al., 2010). AKT2 null mice develop insulin resistance and other abnormality in glucose metabolism (Cho et al., 2001). AKT3 null mice have smaller brains and the phosphorylation level of S6 is reduced via mTOR/S6K (Easton et al., 2005). Interestingly, Akt3Nmf350, dominant mutant mice have enlarged brain, increased phosphorylation of S6, ectopic neurogenesis in the hippocampus and low seizure threshold (Tokuda et al., 2011). AKT1/AKT2 double knockout mice show impaired development of skin, muscle, bone and adipogenesis (Peng et al., 2003). A-443654, perifosine, and triciribine are selective AKT inhibitors with distinctive mechanisms (Han et al., 2007; Dieterle et al., 2009; Gill and Dennis, 2009).
PHLPP1/2
PHLPP1/2 (PH domain leucine-rich repeat protein phosphatase) dephosphorylates AKT at Ser473, the site is important in mTORC2-mediated AKT signaling to promote apoptosis and suppressing cancerous growth (Gao et al., 2005; Brognard et al., 2007). In addition to regulating the common inhibitory effect of AKT on TSC2 and GSK3β, PHLPP1 interacts with AKT2 and AKT3, and PHLPP2 interacts with AKT1 and AKT3, differentially regulating HDM2 and p27 (Brognard et al., 2007). PHLPP1/2 dephosphorylates PKCα at Ser657, promoting their degradation (Gao et al., 2008). One of two PHLPP1 isoforms PHLPP1β, also called SCOP (suprachiasmatic nucleus circadian oscillatory protein), was found that its expression oscillates, increasing during the subjective night (Shimizu et al., 1999). SCOP negatively regulates K-Ras and CREB-mediated transcription, affecting long-term memory in the hippocampus (Shimizu et al., 2007).
TSC1 (hamartin) and TSC2 (tuberin)
TSC1 (hamartin) and TSC2 (tuberin) are well studied tumor suppressors due to the fact that their autosomal dominant mutations cause Tuberous Sclerosis Complex (Jansen et al., 2008). Over 70% of patients who suffer from TSC exhibit epileptic symptoms (Thiele, 2004) (Figure 3E). The disease-causing genes (TSC1 and TSC2) are identified (Consortium E.C.T.S. 1993; van Slegtenhorst and de Hoogt, 1997). The inhibitory function of TSC1/TSC2 obligate heterodimer acts through TSC2's GAP activity which turns Rheb from GTP-bound active state to GDP-bound inactive state (Zhang et al., 2003). TSC2 regulates cell cycle by binding to p27, a cyclin-dependent kinase (cdk) inhibitor and this interaction prevents p27 degradation (Rosner and Hengstschlager, 2004). TSC2 inhibits phosphorylation on Ser126 of BAD by S6K, which induces apoptosis (Freilinger et al., 2006). The growing list of more than fifty TSC1/TSC2-interacting proteins is described in detail elsewhere (Rosner et al., 2008). TSC1 is phosphorylated at Thr310, Ser332, Thr417, Ser584, and Thr1047 by CDK1, at Thr357 and Thr390 by GSK3, and at Ser487 and Ser511 by IKKβ (Astrinidis et al., 2003; Mak et al., 2005; Lee et al., 2007). TSC2 is phosphorylated at Ser939, Ser981 and Thr1462 by AKT, at Thr1227 and Ser1345 by AMPK, at Ser 664 by ERK, at Ser1210 by MK2, at Ser1337 and Ser1341 by GSK3, and at Ser939, Ser1462 and Ser1798 by RSK1 (Inoke et al., 2003a; Li et al., 2003; Tee et al., 2003; Roux et al., 2004; Ma et al., 2005; Cai et al., 2006; Inoki et al., 2006; Carrière et al., 2008b). Serum-activated death-associated protein kinase (DAPK) phosphorylates TSC2 in vitro (Stevens et al., 2009). TSC2 physically interact with RICTOR, activating mTORC2 activity (Huang et al., 2009a). TSC null mice (TSC1-/- and TSC2-/-) die around at E11 (Kobayashi et al., 1999, 2001) and heterozygotes (TSC1+/- and TSC2+/-) have no seizure episode (Onda et al., 1999; Kobayashi et al., 2001). Interestingly, mice with cell-type specific deletion of TSC genes develop epilepsy: astrocyte-specific TSC1 knockout (TSC1GFAP) mice start developing the seizures at 4 week-old, and neuron-specific TSC1 knockout (TSC1synI) mice also show seizure episodes (Uhlman et al., 2002; Meikle et al., 2007). Astrocyte-specific TSC2hGFAP knockout mice showed enlarged cells, megalencephaly and astrocytosis, and start dying after 3 weeks old (Way et al., 2009). Disturbed balance between excitatory and inhibitory synaptic transmission might be linked to seizure incidents in tissues from patients as well as genetically manipulated mouse models (Uhlmann et al., 2002; Meikle et al., 2007; Wang et al., 2007). A rat model carrying a spontaneous TSC2 mutation (Eker rat, TSC2+/-) showed improved performance in episodic-like memory test, and impaired LTP and LTD in the hippocampus (Von der Brelie et al., 2006; Waltereit et al., 2006).
Rheb
Rheb (Ras homolog enriched in brain) is a Ras family small GTPase, an immediate-early gene product, and a direct activator of mTORC1 (Yamagata et al., 1994; Bai et al., 2007). When TSC1/TSC2 inactivated, GTP-bound Rheb activates mTORC1 (Inoki et al., 2003; Zhang et al., 2003). TCTP (Translationally controlled tumor protein) serves as GEF (guanine nucleotide exchange factor) for Rheb that leads to GTP-bound Rheb accumulation (Dong et al., 2009). FKBP38 or BNIP3 binds to Rheb and inhibits mTORC1 (Bai et al., 2007; Li et al., 2007). Rheb also physically associates with NMDA receptor subunit NR3A (Sucher et al., 2010). Recently, a glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been shown to interact with Rheb and inhibits mTORC1 activity when glucose level is low (Lee et al., 2009b). Glyceraldehyde-3-phosphate, an intermediate metabolite of glycolysis, binds to GAPDH, weakening the GAPDH-Rheb interaction, thus activating Rheb/mTORC1 signaling. A cAMP-specific phosphodiesterase, PED4D, interacts with Rheb negatively to control mTORC1 activity (Kim et al., 2010). In this manner, the mTOR pathway may respond to change in the glucose and cAMP levels. Rheb also block aggresome formation by disrupting dynein-mediated transport of misfolded proteins (Zhou et al., 2009b). Although TSC deficient cells do not show aggresome formation but undergo autophagic process, it will be interesting to see how Rheb behave in affected cells on epilepsy-related diseases such as the aggresome forming Lafora disease and microtubule-involved Type 1 lissencephaly (Friocourt et al., 2003; Mittal et al., 2007).
AMPK
AMPK (AMP-activated protein kinase) is a Ser/Thr kinase and composed of a catalytic subunit (α1 or α2), a regulatory subunit (β1 or β2), and an AMP-binding regulatory subunit (γ1, γ2, or γ3). AMPK is activated both by the direct AMP binding and its phosphorylation at Thr172 by LKB1 (Hardie, 2005) (Figure 3F). AMPKα1 is phosphorylated at Ser173 by PKA, impeding LKB1-mediated AMPK activation (Djouder et al., 2010). AMPK phosphorylates IRS-1 at Ser794 to promote apoptosis when cells encounter energy depletion via LKB1 or oxidative stress via CaMKKβ (calcium/calmodulin-dependent protein kinase kinase β) (Tzatsos and Tsichlis, 2007). AMPK can be phosphorylated at Thr172 by CaMKKβ and PIM kinases (Hawley et al., 2005; Woods et al., 2005; Beharry et al., 2011). AMPK activation transmits this energy demand signal to inhibit TSC2 and RAPTOR by phosphorylating at Thr1227 and Ser1345, and at Ser722 and Ser792, respectively (Inoki et al., 2003; Gwinn et al., 2008). AMPK also phosphorylates mTOR at Thr2446 and eEF2K at Ser398 (Browne et al., 2004; Cheng et al., 2004). AMPK activation induces 14-3-3 binding to AMPK-ULK1-mTORC1 complex via Ser792 phosphorylation of RAPTOR (Lee et al., 2010). AMPK activation downregulates the gene expression and the activity of SREBP-1c in mTOR-dependent manner (Zhou et al., 2001). AMPK modulates LTP based on the energy status (Potter et al., 2010). Activation of AMPK and CaMKKβ in the mouse hippocampus has been shown 2 h after kainate injection (Lee et al., 2009a). AMPK binds and phosphorylates GABAB receptors, enhancing synaptic inhibition especially in ischemic injury condition (Kuramoto et al., 2007). AMPKγ1 null mice are viable, and AMPKα2 null mice showed impaired insulin secretion (Viollet et al., 2003; Jørgensen et al., 2004). AMPKγ3 null mice have reduced effect of hypoxia-induced glucose transport in the skeletal muscle (Deshmukh et al., 2009). AICAR (5-amino-4-imidazolecarboxamide ribose) is an AMP mimetic and AMPK agonist (Pruznak et al., 2008; Kwon et al., 2010). Dinitrophenol and 2-deoxy-D-glucose also activates AMPK (Pelletier et al., 2005; Potter et al., 2010). Metformin, a drug commonly used to treat type II diabetes, activates AMPK with less defined mechanism of its action (Leverve et al., 2003; Rotella et al., 2006). Compound-C and ATP mimetic ara-A inhibit AMPK (Potter et al., 2010).
LKB1
LKB1 (also known as STK11 or AMPK Kinase) is a master Ser/Thr kinase and a tumor suppressor that controls at least 13 AMPK subfamily kinases (Lizcano et al., 2004; Hezel and Bardeesy, 2008). Under falling energy status (starvation or low glucose level) or stress conditions (such as hypoxia or ischemia) that facilitates ATP consumption or inhibits ATP production, AMP/ATP ratio rises, then this high ratio activates LKB1 (Hardie et al., 2005). LKB1 forms a heterotetramer with STRADα and a scaffolding protein, MO25α, and it activates AMPK by phosphorylation at Thr172 (Hawley et al., 2003). This LKB1-AMPK activation facilitates glucose uptake and cellular catabolism to have more ATP available and inhibits the cellular biosynthetic processes to save ATP (Hardie, 2004). LKB1 is phosphorylated at Ser431 by ERK1/2, PKA, and RSK (Sapkota et al., 2001). BDNF- or cAMP-induced axonal differentiation is mediated by LKB1-STRADα interaction, and it is induced LKB1's phosphorylation at Ser431, a PKA site (Shelly et al., 2007). LKB1 also associates with BRG1 (brahma-related gene 1), another tumor suppressor on the same chromosome 19 (Marignani et al., 2001). Germline mutation on LKB1 causes the Peutz-Jeghers syndrome, a harmatomatous syndrome similar to PTEN and TSC mutations (Hemminki et al., 1998). LKB1 null embryos are lethal and heterozygous knockout mice developed intestinal polyps identical to the human specimens (Bardeesy et al., 2002).
STRADα
STRADα (STE20-related adaptor protein α) is a pseudo kinase that binds to either MO25α or ATP to stabilize the interaction with LKB1, and it localizes LKB1 to the cytoplasm (Boudeau et al., 2003; Zeqiraj et al., 2009) (Figure 3F). This complex regulates AMPK which then control TSC2 and mTOR (Hardie, 2005). Mutation in STRADα was recently found to cause PMSE, an epileptic disease (Puffenberger et al., 2007).
Rag
Rag (Ras-related GTPase) is required for mTORC1 activation by nutrients level, is independent from PI3K/AKT/TSC/Rheb axis (Shaw, 2008). RagA and RagB are closely related to each other, and it is the same with RagC and RagD (Sekiguchi et al., 2001). RagA or RagB forms a stable heterodimer with RagC or RagD, and these heterodimers interact with RAPTOR directly in an amino-acid dependent fashion (Kim et al., 2008a; Sancak et al., 2008). Only when RagA/B is bound to GTP and RagC/D is bound to GDP, these heterodimers increases its affinity to RAPTOR. Rag-bound mTORC1 then relocalizes to Rab9-containing perinucleolar membrane structure where Rheb resides, thus activates mTORC1 activity (Sancak et al., 2008). However, Rag proteins do not sense the amino acid itself, and Vam6/Vps39 is suggested to be a GEF for RagA or Rag B (Price et al., 2000; Binda et al., 2009). How Vam6/Vps39 activity is controlled in response to the level of amino acids remains to be examined.
hVPS34
hVPS34 (human vacuolar protein sorting 34) is class III PI3K which senses the availability of amino acids (Nobukuni et al., 2005). It forms a complex with Vps15, recruiting either MTM1 or Rab5/7 for the endocytic sorting, or Beclin-1 and UVRAG (UV irradiation resistance-associated gene) for autophagy during nutrient deprivation (Backer, 2008). When a branched amino acid such as leucine increases intracellular concentration of calcium, calcium/calmodulin complex binds to hVps34, activating mTORC1/S6K1 (Gulati et al., 2008). MAP4K3 has been also identified as a kinase that senses and mediates amino acids signals to TOR in Drosophila (Findlay et al., 2007). It remains to be seen how MAP4K3 and hVPS34 are differentially sense and transduce amino acid signals in mammalian brains.
REDD1
REDD1 (regulated in development and DNA damage responses 1, also called DDIT4 (DNA-damage-inducible transcript 4) or RTP801) is a negative regulator of the mTOR signaling by modulating TSC2 activity (Brugarolas et al., 2004). REDD1 is activated in stress condition (e.g., hypoxia) and competes with TSC2 on binding to 14-3-3, thus inhibiting mTORC1 activity (DeYoung et al., 2008). Expression of REDD1 is ubiquitous and regulated by stress proteins, p53 and HIF1 (Ellisen et al., 2002; Jin et al., 2007). The mTORC1 signaling is rapidly modulated due to the fast degradation of REDD1 (Kimball et al., 2008). When REDD1 is phosphorylated by GSK3β, β-TRCP recruits ubiquitin ligase complex DDB1-CUL4A-ROC1 to REDD1 for its degradation and mTORC1 activity restoration (Katiyar et al., 2009). REDD1 null mice are viable (Sofer et al., 2005).
GSK3β
GSK3β (glycogen synthase kinase 3β) is a ubiquitous multifunctional Ser/Thr kinase which is involved in cell division, proliferation, differentiation and adhesion in addition to regulating glycogen synthase activity (Jope and Johnson, 2004) (Figure 3G). Phosphorylation of Tyr216 is essential for its basal activity, in contrast, phosphorylation at Ser9 inactivates it (Liang and Chuang, 2007). Both PI3K/AKT and mTORC1/S6K1 activation phosphorylates GSK3β at Ser9, thus stimulating glycogen synthesis (Cross et al., 1995; Zhang et al., 2006; van Diepen et al., 2009). Among many substrates of GSK3β PTEN, TSC1, TSC2, and REDD1 are on the mTOR pathway (Mak et al., 2005; Inoki et al., 2006; Katiyar et al., 2009; van Diepen et al., 2009). It also phosphorylates to stabilize c-Myc (Lutterbach and Hann, 1994). GSK3β has been shown to be involved NMDA-dependent LTP and LTD in the hippocampus (Peineau et al., 2008). Epilepsy-related lafora disease is caused by defective mutation in laforin which dephosphorylates GSK3 at Ser9, thus inhibits glycogen synthase (Lohi et al., 2005). GSK3β null embryos die at E14, and heterozygotes were healthy and fertile (Hoeflich et al., 2000). 6-bromoindirubin-3'-oxime has been used as a specific inhibitor of GSK3 (Sato et al., 2005).
DISC1
DISC1 (Disrupted-in-schizophrenia 1) is a susceptibility gene for schizophrenia and other mental disorders, and it is involved in neurogenesis (Brandon et al., 2009). It regulates adult neurogenesis by modulating the mTOR pathway via a protein KIAA1212 (Kim et al., 2009). It also regulates glutamatergic dendritic spines via Karlin-7 and Rac1 interaction (Hayashi-Takagi et al., 2010). DISC1 expression has been shown to be decreased in dentate granule cell layer in kindling model of epilepsy, and downregulation of DISC1 leads to the abnormal neuronal morphology, hyperexcitability, and impaired adult neurogenesis (Duan et al., 2007; Fournier et al., 2010). Conditional knock-in mice with the forebrain-restricted expression of mutant DISC1 associated with schizophrenia show that spontaneous hyperactivity and impaired spatial memory (Pletnikov et al., 2008).
ERK1/2
ERK1/2 (ERK1 = p44 mitogen-activated protein kinase (MAPK); ERK2 = p42 MAPK1) are Ser/Thr kinases of Ras/MAPK signaling pathway particularly involved in neuronal and synaptic plasticity (Roux and Blenis, 2004; Thomas and Huganir, 2004) (Figure 3H). ERK activation increases dendritic protein synthesis at CA1 pyramidal neurons when high frequency stimulation given via PI3K/PDK1/AKT/mTOR pathway (Tsokas et al., 2007). ERK phosphorylates and inhibits TSC2 at Ser 664 and LKB1 at Ser431, possibly by RSK activation, and IRS-1 at Ser612 (Sapkota et al., 2001; Andreozzi et al., 2004; Ma et al., 2005). ERK1/2 also phosphorylate RAPTOR at Ser8, Ser696, and Ser863 and c-myc at Ser63 (Seth et al., 1992; Carrière et al., 2011). Increased activation of ERK has been reported in pilocarpine- or kainate-induced SE and during chronic seizures (Kim et al., 1994; Garrido et al., 1998; Houser et al., 2008). Constitutive ERK activation has been shown to induce spontaneous seizures in mice (Nateri et al., 2007). In traumatic brain injury model ERK has been shown to be activated in hippocampal mossy fiber, possibly mediating mossy fiber reorganization (Hu et al., 2004). Phosphorylation of Kv4.2 by activated ERK decreases the surface expression of the channel and dendritic A current in SE (Lugo et al., 2008). Erk1 null mice showed that ERK1 may be involved in regulating neuronal excitability in hippocampal CA1 area under certain stimulation patterns (Selcher et al., 2003). Erk2 conditional knockout mice have impaired proliferation of neuronal progenitors, fewer neurons and more astrocytes, and deficits in associative learning (Samuels et al., 2008). FR180204 is a specific inhibitor against ERK (Ohori et al., 2005).
RSK1
RSK1 (RPS6K1 ribosomal protein S6 kinase, 90kDa, polypeptide 1) is a Ser/Thr kinase downstream of Ras/Raf/MEK/ERK signaling pathway that regulates diverse cellular processes such as cell growth, motility, survival and proliferation (Anjum and Blenis, 2008). Four isoforms are ubiquitously expressed and they are localized both in the cytoplasm and nucleus. RSK is phosphorylated at Thr573 by ERK1/2 and at Ser227 by PDK1 (Smith et al., 1999; Frödin et al., 2000). It phosphorylates LKB1 at Ser431, RAPTOR at Ser719, Ser721 and Ser722, and TSC2 at Ser939, Ser1462 and Ser1798, regulating mTORC1 activity (Sapkota et al., 2001; Roux et al., 2004; Carrière et al., 2008a). RSK also phosphorylates ribosomal protein S6 at Ser235/236, eEF2K (elongation factor 2 kinase) at Ser366 and eIF4B at Ser422 (Wang et al., 2001; Shahbazian et al., 2006; Roux et al., 2007). Rsk2 null mice show mild impairment of learning and long-term memory deficits, mimicking Coffin-Lowry syndrome associated with RSK2 mutations (Poirier et al., 2007). SL0101, Fmk, and BI-D1870 are specific inhibitors against RSK (Anjum and Blenis, 2008).
PP2A
PP2A (Protein Phosphatase 2A) is a major Ser/Thr phosphatase in mammalian cells that regulates the phosphorylation status of proteins involved in various cellular processes (Westermarck and Hahn, 2008). It is composed of a catalytic subunit (PP2AC), a regulatory subunit (PP2AA), and one of many associate proteins (PP2AB), generating more than 70 combinations. The mTORC1 activation induces phosphorylation of ERK1/2 at Thr202, inhibiting its activity via PP2A (Harwood et al., 2008). This is a cross-talk-between Ras/MAPK and mTOR signaling pathways. PP2A directly dephosphorylates and activates 4E-BP1 at Thr45 and Ser64, thus inhibiting protein synthesis (Guan et al., 2007). It also dephosphorylates c-Myc at Ser62, promoting its ubiquitination-mediated proteosomal degradation (Arnold and Sears, 2006). A pro-apoptotic molecule, BAD is dephosphorylated at Ser112 by PP2A (Hui et al., 2005). Activation of group I mGluR signaling through PP2A to dephosphorylate the fragile X mental retardation protein (FMRP), facilitating protein synthesis, however, the later signals through mTOR inhibit PP2A activity (Narayanan et al., 2007). PP2Aα knockout mice is embryonic lethal (Götz and Schild, 2003). Okadaic acid and calyculin A inhibit PP2A (Garcia et al., 2002).
PLC
PLC (Phospholipase C) hydrolizes phosphatidylinositol-4, 5-bisphosphate (PIP2) to produce inositol triphosphate (IP3) and diacylgylcerol (DAG) which activate downstream signaling cascade including IP3 receptors and PKC, respectively (Rhee, 2001). Among many isoforms, PLCβ3 has been shown to be upregulated by IGF-1 via PI3K and S6K, and PLCγ1-mediated activation of mTOR/S6K pathway (Schnabel et al., 2000; Markova et al., 2010). PLCβ1 null mice showed epilepsy, and age-dependent hippocampal mossy fiber sprouting (Kim et al., 1997; Böhm et al., 2002). PLCβ1 is coupled to muscarinic receptors, and activation of muscarinic receptors induces mTOR-dependent phosphorylation of ribosomal protein S6 (Popova and Rasenick, 2000; Slack and Blusztajn, 2008). Pilocarpine elicits SE through muscarinic receptors and mGluR5/PLCβ1 (el-Etri et al., 1993; Liu et al., 2008b).
PLD
PLD (Phospholipase D) hydrolyzes phosphatidylcholine to generate phosphatidic acid, which can activate the mTOR pathway (Fang et al., 2001; Klein, 2005). PLD1 is activated by Rheb and Cdc42-S6K1 interaction (Fang et al., 2003; Sun et al., 2008). PLD2 has a TOS-like motif and forms a complex with mTOR/RAPTOR (Ha et al., 2006). Elevated PLD activity suppressed binding of PP2A with S6K and 4E-BP1 (Hui et al., 2005). PLD level is increased in reactive astrocytes in kainate model, and interestingly, PLD1 and PLD2 showed differential patterns of gene expression in the hippocampus (Kim et al., 2004). They may be differently involved in epilepsy.
CDC42
CDC42 (cell division cycle 42) is a Rho family GTPase that regulates cytoskeleton organization, and membrane trafficking (Sinha and Yang, 2008). It binds to activate S6K, and it also activates PLD, producing PA which activates mTOR (Chou and Blenis, 1996; Fang et al., 2003). The expression of CDC42 in the hippocampus is increased in kainate model, and its downstream target N-WASP, important in regulating actin cytoskeleton, is also increased in the postmortem brains of human epilepsy patients (Carlier et al., 1999; Xiao et al., 2008; Sharma et al., 2009). Cdc42 null mice are embryonic lethal and CDC42 is essential for PIP2-induced actin reorganization (Chen et al., 2000). Secramine B inhibits CDC42 (Pelish et al., 2006).
Downstream signaling molecules
S6K1/2
S6K1/2 (p70 ribosomal protein S6 kinase 1/2) is a positive regulator of protein translation initiation and one of mTORC1 substrates (Burnett et al., 1998; Park et al., 2002). Ribosomal protein S6, a well-known target of S6K1/2, is a component of the small ribosomal subunit, although the functional significance of phosphorylation of S6 remains to be elucidated in more detail (Ruvinsky et al., 2005). Thr389 of S6K1 is phosphorylated by mTORC1, and Thr229 by PDK1 (Kim et al., 2002; Saitoh et al., 2002). Thr421 and Ser424 are phosphorylated by ERK and Ser411 by CDC2-cyclinB or CDC5-p35 kinase (Papst et al., 1998; Page et al., 2006; Hou et al., 2007). PA binds to activate S6K1 in vitro (Lehman et al., 2007). S6K1 phosphorylates mTOR on Thr2446 and Thr2448, SKAR on Ser383 and Ser385, eIF4B at Ser422, and eEF2K at Ser366 (Wang et al., 2001; Richardson et al., 2004; Holz and Blenis, 2005; Shahbazian et al., 2006). S6K phosphorylates CBP80, a subunit of nuclear RNA cap-binding complex to activate CDC42-mediated pre-mRNA splicing process, and UBF, a transcription factor, to regulate ribosomal gene transcription (Wilson et al., 2000; Hannan et al., 2003). It phosphorylates PDCD4, an eIF4A inhibitor, at Ser67 and subsequently recruits βTRCP, an E3 ubiquitin ligase, to be ubiquitinated and degradated (Dorrello et al., 2006). S6K phosphorylates BAD, a pro-apoptotic molecule, at Ser136, and CREM, a cAMP responsible activator at Ser117, a PKA site (de Groot et al., 1994; Harada et al., 2001). The negative feedback inhibition of PI3K via IRS-1 phosphorylation by S6K1 is very significant in the mTOR pathway (Tremblay et al., 2007; Zhang et al., 2008). Activated S6K1 by mTORC1 activation phosphorylates IRS-1 at multiple sites (Rui et al., 2001). S6K1 null mice showed upregulation of AMPK and similar pattern of gene expression with the effect of caloric restriction on life-span (Aguilar et al., 2007; Selman et al., 2009). S6K2 null mice are slightly larger than wild-type controls in contrast to significantly smaller S6K1 null mice (Pende et al., 2004). mGluR-dependent LTD is normal in S6K1 null mice, but it is enhanced in S6K2 null and S6K1/2 double knockout mice (Antion et al., 2008). Ro31-6045 specifically inhibits S6K (Marmy-Conus et al., 2002).
4E-BPs
4E-BPs (eukaryotic initiation factor 4 (eIF4) binding proteins, also known as PHAS-I) are negative regulators of protein translation initiation and one of mTORC1 substrates (Burnett et al., 1998). 4E-BP1 binds to eIF-4E (a 7-methyl-guanosine mRNA cap-binding protein) to inhibit the formation of eIF-4F via blocking eIF-4E's binding to eIF-4G, a translational scaffolding protein (Ma and Blenis, 2009). Among four phosphorylation sites of 4E-BP1 (Thr36, Thr45, Ser64, and Thr69), Thr36 and Thr45 are preferred by mTORC1 activation (Burnett et al., 1998; Mothe-Staney, 2000). This phosphorylation causes 4E-BP1 to dissociate from eIF4E, which cascades binding eIF4G, eIF3, and eIF4A to initiate translation (Ma and Blenis, 2009). 4E-BP1 can be also phosphorylated by PIM-2, PKCδ, and c-Abl (Kumar et al., 2000; Fox et al., 2003). There are three 4E-BP isoforms, and 4E-BP2 is the major one in the brain whereas expression of 4E-BP1 is low, and that of 4E-BP3 is absent in the brain (Tsukiyama-Kohara et al., 2001). 4E-BP2 null mice showed impaired spatial learning and memory, and altered behavior on several other tests (Banko et al., 2005, 2007).
STAT3
STAT3 (Signal Transducers and Activators of Transcription 3) transduces activation signals from the receptor binding of IL-6, IL-10 and other cytokines families to regulate the expression of genes involved in many different cellular processes (Levy and Lee, 2002). STAT3 is phosphorylated at Ser727 by mTORC1 (Yokogami et al., 2000). Serine phosphorylation of STAT3 regulates mitochondrial energy production by interacting with GRIM-19 and possibly gene transcription (Reich, 2009). STAT3 is also phosphorylated at Tyr705 by Janus kinases (Reich, 2009). In kainate-injected rats, STAT3 is activated in reactive astrocytes in the hippocampus (Choi et al., 2003). Homozygous Stat3 null embryos die at E7 (Takeda et al., 1997).
C-Myc
The c-Myc is a transcription factor that controls the expression of genes involved in cell cycle progression, proliferation, and differentiation where its activity is highly context-dependent (Wierstra and Alves, 2008). The c-Myc inhibits anti-apoptotic molecules, Bcl2 and Bcl-XL and it activates pro-apoptotic molecules, Bak, Bax, Bad and Bim (Hoffman and Liebermann, 2008). The c-Myc represses TSC2 expression and it also controls expression of IRS-1, TSC1, GβL, and S6 (Ravitz et al., 2007). The c-Myc is stabilized by phosphorylation at Thr58 by GSK3 and at Ser63 by ERK, and it is destabilized by dephosphorylation at Ser62 by PP2A (Seth et al., 1992; Lutterbach and Hann, 1994; Arnold and Sears, 2006). The c-Myc is also phosphorylated at Thr62 and Ser329 by PIM-1 (Zhang et al., 2008). c-myc null embryos die at E10.5 and heterozygous c-myc female mice have reduced fertility (Davis et al., 1993).
CLIP-170
CLIP-170 (CAP-GLY domain containing linker protein 1) is phosphorylated by mTOR and its phosphorylation positively regulates its microtubule-binding properties (Choi et al., 2002). Downregulation of CLIP-170 rescued the abnormal microtubule arrangement in Tsc2-/- cells (Jiang and Yeung, 2006).
ULK1/mATG13/FIP200
ULK1/mATG13/FIP200 (unc-51-like kinase 1 = ATG1)/mammalian autophagy related protein 13/focal adhesion kinase interacting protein of 200 KD) complex is essential for autophagy initiation (Mizushima, 2010). ULK1 and mATG13 bind to RAPTOR and they are phosphorylated by mTORC1, inhibiting autophagy process under nutrient-rich condition (Ganley et al., 2009; Hosokawa et al., 2009; Jung et al., 2009). ULK1 has a kinase activity and phosphorylates mATG13 and FIP200 (Ganley et al., 2009; Jung et al., 2009). In C. elegans, Unc51 has been shown to be involved in axonal elongation (Tomoda et al., 2004).
SGK1
SGK1 (Serum- and glucocorticoid-induced kinase 1) regulates diverse effects of extracellular agonist by phosphorylating regulatory proteins that control cellular process such as ion transport and growth (Lang et al., 2006). Changes in cell volume such as dehydration increased SGK1 expression in the hippocampal CA3 neurons, and SGK1 increased Kv1.3 activity (Wärntges et al., 2002). Ser422 of SGK1 is phosphorylated by mTORC2, and Thr256 by PDK1 (Biondi et al., 2001; García-Martínez and Alessi, 2008). SGK1 phosphorylates NDRG1 (N-myc downstream regulated gene 1) at Thr346, Thr356, and Thr366 and FOXO3a at Thr32 and Ser315 (Brunet et al. 2001; Murray et al., 2004). SGK1 null mice showed impaired renal function and increased expression of FOXO3a (Wulff et al., 2002; Nasir et al., 2009).
PKCs
PKCs (Protein kinase Cs) are Ser/Thr protein kinases widely expressed in mammalian cells (Parekh et al., 2000). Among several isoforms, PKCα, PKCδ, PKCε, PKCη and PKCζ are shown to interact with the mTOR pathway (Parekh et al., 1999; Aeder et al., 2004; Guan et al., 2007; Leseux et al., 2008). PKCα is phosphorylated at Ser638 and Ser657 by mTORC2 (Ikenoue et al., 2008). PKCα interacts with mTOR directly that EGFR activation signal can be transduced to activate protein translation (Kumar et al., 2000; Fan et al., 2009). It also hypophosphorylates 4E-BP1 by increasing PP2A activity, which results in the inhibition of protein translation in PI3K/AKT/mTOR-independent manner (Guan et al., 2007). PKCε is required for ET-1-stimulated phosphorylation of S6K1 at Thr389, Thr421 and Ser424, and mTOR at Ser 2448 (Moschella et al., 2007). PKCδ are required for ET-1- and insulin-stimulated phosphorylation of mTOR at Ser2448 and S6K1 at Thr389 (Moschella et al., 2007). PKCζ phosphorylates PKCδ, and this process is rapamycin sensitive, and it also activates mTOR via MAPK activation (Ziegler et al., 1999; Leseux et al., 2008). Downregulation of PKCδ is regulated by PKCε and mTORC2 (Basu et al., 2009). Phosphorylation of PKCδ at Ser662 and PKCε at Ser729 is rapamycin-sensitive (Parekh et al., 1999). PKCη phosphorylates and activates AKT and mTORC1 in glioblastoma (Aeder et al., 2004). Several inhibitors against PKC isoforms are available (Mackay and Twelves, 2007).
FOXO proteins
FOXO proteins (Forkhead box O) are transcription factors that regulate diverse processes (Nakae et al., 2008). FOXO1, FOXO3a, and FOXO4 are phosphorylated and inhibited at Thr32 and Ser253 by AKT and FOXO3a at Thr32 and Ser315 by SGK1 (Brunet et al., 2001; Allard et al., 2008). Phosphorylation at Thr32 and Ser316 of FOXO1 recruits 14-3-3 for the nuclear export (Brunet et al., 2002). Phosphorylation of FOXOs by ERK induces ubiquitination-mediated proteosomal degradation with MDM2 interaction (Fu et al., 2009). FOXO1 interacts with and regulates negatively TSC2 through mTOR/S6K signaling pathway (Cao et al., 2006). Other FOXO-binding proteins are described in detail elsewhere (van der Vos and Coffer, 2008). Under nutrients/insulin limited condition, FOXOs inhibits AKT by inhibiting TRB3 and they also turns on gene expression of insulin receptor, insulin receptor substrate 2 (IRS-2), and 4E-BP1 (Puig et al., 2003; Ide et al., 2004; Naïmi et al., 2007). FOXO1 null mice are embryonic lethal, however, FOXO3a and FOXO4 knockout mice are viable (Castrillon et al., 2003).
mTOR on transcription
Although most of the study of mTOR regulation is focused on translational regulation and post-translational modification (phosphorylation), the mTOR pathway also interacts with nuclear receptors, transcription factors, and splicing factors at the transcriptional level. In addition, mTOR regulates the pre-rRNA processing, expression of ribosomal proteins, the synthesis of 5S rRNA and transcription at large by all three classes of RNA polymerases (Mayer and Grummt, 2006). Some of these molecules interacting with the mTOR pathway are implicated in epilepsy. Transcriptional activation of rRNA genes by RNA polymerase I depends on IGF, PI3K, mTOR and S6K by increased binding of SL1, an essential RNA polymerase I transcription factor, to corresponding promoters (James and Zomerdijk, 2004). PTEN suppresses RNA polymerase I-mediated transcription by disrupting SL1 binding to its promoter (Zhang et al., 2005a). It also suppresses RNA polymerase III-mediated transcription of tRNAs and 5S rRNAs by disrupting TFIIIB binding with TATA-binding protein and BRF1 (Woiwode et al., 2008). PTEN-induced decrease in serine phosphorylation and consequent destabilization of BRF1 may be mediated by AKT (Benjamin et al., 2006).
Estrogen receptor α
Estrogen receptor α (ERα) are nuclear receptors for estrogen (E). E-ER binding changes its conformation, allowing transport to nucleus. This complex binds either to the estrogen response element or to other proteins so that it regulates gene expression as homo- or hetero-dimers (Matthews and Gustafsson, 2003). This is a classical estrogen's genomic effect which has a delayed onset (several minutes to hours) and long-lasting effect. Estrogen's proliferative effect is mediated by ERs, therefore, antagonists against ERs are used for breast cancer treatment. One prognostic biomarker of resistance to anti-estrogen therapy is phosphorylation at Ser167 of ERα, and it is mediated by mTOR/S6K and MAPK/RSK (Yamnik and Holz, 2010). ERαs are expressed in cytoplasm and nucleus of neurons and glia (Mhyre and Dorsa, 2006). Their expression in CA1 and CA3 pyramidal neurons is decreased in kainate-induced acute seizure. In contrast, it appears in the gliotic reactive astrocytes in CA1 (Sakuma et al., 2009). In female animals, estrogen is neuroprotective against SE-induced neuronal damage in the hippocampus (Reibel et al., 2000). However, estrogen's effect on male animals is controversial. Estradiol increases seizure susceptibility but decreases seizure severity by facilitating neuropeptide Y release from inhibitory presynaptic boutons during kainate-induced seizures (Ledoux et al., 2009).
Androgen receptors
Androgen receptors (ARs) are ligand-dependent transcription factors which regulate gene expression by its binding to androgen-responsive promoter elements (Mellinghoff et al., 2004). Protein levels of ARs are rapamycin-sensitive (Cinar et al., 2005). Testosterone is known as neuroprotective, and its metabolites, androgens (As) such as dihydrotestosterone can potentiate GABAA receptors directly, exerting neuroprotective function (Reddy, 2003). Although there is no direct evidence that ARs are involved in epilepsy, there are some indirect reports worth pursuing the possibility. In man with temporal lobe epilepsy major metabolites of testosterone (e.g., 5α-androstan-3α-ol-17-one; 5α3α-A) level is reduced, and A-ARs binding activates ERK1/RSK signaling pathway and neuroprotection (Nguyen et al., 2005). When phenytoin, an AED, is given to animals, testosterone level is decreased, testosterone metabolizing enzymes cytochrome P450 are upregulated. Testosterone metabolites such as 17β-oestradiol are increased, and AR expression is increased in CA1 pyramidal neurons (Meyer et al., 2006). In an epilepsy mouse model, 5α3α-A showed anticonvulsant properties (Kaminski et al., 2005).
SREBP1
SREBP1 (Sterol responsive element binding protein 1; SREBF1) are transcription factors which recognize the sterol responsive element containing promoters that regulate gene expression of lipid and cholesterol biosynthesis (Laplante and Sabatini, 2009; Porstmann et al., 2009). Mammals have three SREBPs: SREBP1a, SREBP1c, and SREBP2. SREBP1c is activated by insulin and involved in fatty acid synthesis (Foufelle and Ferre, 2002). SREBP2 is activated in response to cellular sterol status, and it controls cholesterol and fatty acid biosynthesis (Schmidt et al., 2006). DNA microarray data showed that stearoyl-CoA desaturase 1, a SREBP1a target gene, is upregulated in human cortical specimen of temporal lobe epilepsy (Arion et al., 2006). In kainate model, protein and mRNA levels of SREBP2 are reduced in pyramidal neurons of hippocampal CA1 and CA3 area (Kim and Ong, 2009). mTORC1 activates SREBP1 to upregulate lipid biosynthesis likely by phosphorylation (Porstmann et al., 2008). GSK3β phosphorylates SREBP1 at Ser434, Ser430 and Thr426 sequentially and this phosphorylation cascade recruits a tumor suppressor FBW7 which ubiquitinates mTOR to be degraded proteosomally (Mao et al., 2008; Bengoechea-Alonso and Ericsson, 2009). SREBP-1c is negatively regulated by AMPK directly (Zhou et al., 2001).
Lipin-1
Lipin-1 plays a role in lipid biosynthesis by acting as a phosphatidate phosphatase (PAP) in the microsome and cytoplasm as well as an inducible transcriptional coactivator with PPARγ coactivator-1α (PGC1α) and PPARα in the nucleus (Reue and Zhang, 2008). Lipin-1 is shuttled from cytoplasm to nucleus by sumoylation and Lipin-1α is the dominant form in the neurons (Liu and Gerace, 2009). Lipin's PAP activity makes DAG from phosphatidate. Ser106 is the major phosphorylation site by insulin stimulation (Harris et al., 2007). Rapamycin reduces the phosphorylation of lipin-1 by mTORC1 (Huffman et al., 2002). Kainate injection increases the amount of DAG in the brain, which could be an enzymatic product of lipin-1 (Cole-Edwards and Bazan, 2005). The reverse reaction enzyme, DAG kinase ε regulates seizure-susceptibility, and DAG kinase ε null mice show reduced LTP in perforant path-dentate granule cell synapses (Rodriguez de Turco et al., 2001).
SF2/ASF
SF2/ASF (Splicing Factor, Arginine/Serine-rich Factor) is involved in alternative splicing, non-sense-mediated mRNA decay, mRNA export, and translation (Long and Caceres, 2009). It activates mTORC1 and regulates translation initiation by enhancing phosphorylation of 4E-BP1 (Karni et al., 2008; Michlewski et al., 2008). SF2/ASF null embryos die early during development (Xu et al., 2005).
SKAR
SKAR (S6K1 Aly/REF-like target) is a nuclear protein that links pre-mRNA splicing to the enhanced translation efficiency of spliced mRNA mediated by mTOR/S6K1. It is a specific substrate of S6K1 and is phosphorylated at Ser383 and Ser385 (Richardson et al., 2004; Ma et al., 2008a).
Traumatic brain injury, medial temporal lobe epilepsy and mTOR pathway
Traumatic brain injury (TBI) is one of the main causes of medial temporal lobe epilepsy (Lowenstein, 2009). Recently, in fluid-percussion brain injury model, 4E-BP1, S6K, and S6 were phosphorylated by activated mTOR, and eIF4E was phosphorylated by Mnk1 (MAPK-interacting kinase 1) in parietal cortex and hippocampus (Chen et al., 2007). TBI was also shown to induce autophagy by increased expression of LC3-II (microtubule-associated protein light chain 3) and widely redistributed the autophagy-related gene products, ATG12-ATG5 conjugates (Liu et al., 2008a). Rapamycin injection 4 hours after TBI reduced phosphorylation of S6K and reduced activation of microglia/macrophages (Erlich et al., 2007). It remains to be seen that TBI-initiated epilepsy has activated signaling molecules of mTOR pathway.
Viral infection in epilepsy and mTOR pathway
One of the causes of acquired epilepsy and a major cause of febrile seizure could be viral infection of central nervous system and its complications such as high fever and consequential neuronal damages (Eeg-Olofsson, 2003; Getts et al., 2008). Viral encephalitis increases risk of developing seizures and epilepsy even after treatment of infection completed, and its epileptogenic mechanism - both acute and chronic - remains to be solved (Misra et al., 2008). For example, human herpesvirus 6 (HHV6) infection has been associated with febrile seizure and mesial temporal lobe epilepsy (Fotheringham et al., 2007). Its viral antigen is localized to GFAP-positive glia in the hippocampus (Laina et al., 2010). Influenza A virus infection can also cause febrile seizure (Chiu et al., 2001).
Recently, several viral proteins have been shown to interact with the mTOR pathways (Buchkovick et al., 2008). A) Herpes simplex virus type 1 (HSV-1) infection is the most frequently associated with epilepsy (Gannicliffe et al., 1985; Hsieh et al., 2007; Misra et al., 2008). HSV-1 infection reduces depolarizing membrane potential, thus making neurons hyperexcitable (Chen et al., 2004). HSV-1 protein ICP0 directly activates phosphorylation of 4E-BP1, inducing protein synthesis (Walsh and Mohr, 2004). HSV-2 has ICP10 protein which activates PI3K/AKT/mTOR pathway (Smith, 2005). B) Adenovirus infection has been associated with febrile seizure (Chung and Wong, 2007). An adenoviral protein E4-ORF1 directly binds to activate PI3K, and E4-ORF4 does PP2A, inhibiting dephosphorylation of mTORC1 (O'Shea et al., 2005). C) Human immunodeficiency virus (HIV)-positive patients show HIV-associated encephalitis and seizure/epilepsy in some cases (Nardacci et al., 2005; Kellinghaus et al., 2008). In this condition, overexpression and activation of mTOR, and HIV gp120 interaction with mTOR has been reported. This mTOR activation phosphorylates p53 at Ser15, and upregulates and translocates Bax into mitochondria (Castedo et al., 2001; Nardacci et al., 2005). D) Epilepsy has been also reported in congenital cytomegalovirus infection cases (Dunin-Wasowicz et al., 2007; Suzuki et al., 2008). Two immediate-early proteins (72KDa IE1 and 86KDa IE2) of human cytomegalovirus (HCMV) can activate AKT by phosphorylating at Thr308 and Ser473 (Yu and Alwine, 2002). HCMV infection also modulates AMPK activity, thus affecting protein synthesis (Kudchodkar et al., 2007). Under this condition, mTORC2 becomes rapamycin-sensitive and is able to phosphorylate 4E-BP1 and S6K, thus altering mTOR substrate specificities (Kudchodkar et al., 2004, 2006). Another HCMV protein pUL38 inhibits host cells' apoptosis by interacting with TSC1/TSC2 (Moorman et al., 2008). E) Enterovirus infection has been reported in febrile seizure patients and enterovirus-induced autophagy decreases phosphorylated mTOR and phosphorylated S6Ks (Hosoya et al., 1997; Huang et al., 2009b). It is yet to be shown whether, when viral infection is likely the cause of epilepsy, abnormal activation of the signaling molecules of mTOR pathway occurs.
Brain tumors, mTOR pathway and epilepsy
Large number of the patients with dysembryoblastic neuroepithelial tumors, ganglioglioma, low-grade astrocytoma, meningioma, or glioblastoma multiforme has epileptic seizures (van Breemen et al., 2007). Although the molecular mechanism of comorbidity of brain tumors and epilepsy remains to be elucidated, there is enough evidence that abnormal activities of the mTOR pathway prevail in several types of brain tumors. A) Gangliogliomas have been shown that the mTOR pathway (from PDK1 to S6) is activated in patients' specimen (Boer et al., 2010). Reelin has been involved in ganglioglioma as well as granule cell dispersion in temporal lobe epilepsy and cortical dysplasia (Crino, 2009; Haas et al., 2002; Kam, 2004). Reelin binds to VLDL Receptor/ApoER2, and its activation signal is transduced to Dab1 and PI3K/AKT/mTOR (Hiesberger et al., 1999; Kam et al., 2004; Jossin and Goffinet, 2007). Decrease of Reelin expression in subsets of interneurons in the dentate gyrus was reported in human specimen and kainate- and pilocarpine-induced epilepsy models, and Dab1 expression is increased in hilar-ectopic neuroblasts (Heinrich et al., 2006; Gong et al., 2007). Increased methylation in the promoter of reelin was also shown in human temporal lobe epilepsy (Kobow et al., 2009). It will be interesting to see whether interneuron-specific knockout of reelin show the seizure behavior. B) In meningioma, expression of fibroblast growth factor (FGF) and one of its receptors, FGFR-3 are significant (Takahashi, et al., 1990; Johnson et al., 2010). FGFR3 activation transduces signal through PI3K-AKT-mTORC1-STAT3 route as well as AKT-RAF1-MEK1-MAPK (Johnson et al., 2009; 2010). In addition, most sporadic meningiomas have somatic mutations of NF2/Merlin, a negative regulator of mTORC1 (James et al., 2009). NF2/Merlin is a membrane cytoskeleton anchor, and its inactivating mutation causes constitutively activation of mTORC1 signaling, resulting in neurofibromatosis 2 and epilepsy-associated meningioma (Scoles, 2008; López-Lago et al., 2009). Interacting with CD44, a hyaluronan receptor on the plasma membrane, Merlin negatively regulates the mTOR pathway via PIKE/PI3K/AKT (Morrison et al., 2001; James et al., 2009). AKT directly phosphorylates Merlin at Thr230 and Ser315, increasing its binding to CD44 (Tang et al., 2007; Okada et al., 2009). The expression of CD44 is increased in the dentate gyrus 3 days after pilocarpine-induced SE, lasting up to 4 weeks (Borges et al., 2004). It remains to be seen how Merlin's activity is changed in epilepsy. NF2 null mice die around at E7, and heterozygous NF2 knockout mice show highly invasive and metastatic tumors (McClatchey et al., 1997, 1998). C) Glioblastoma multiforme, the most aggressive primary brain cancer, is PI3K-AKT dependent (Knobbe and Reifenberger, 2003). Although rapamycin doesn't work well with patients of this type, it is yet to be studied that it is effective in other brain tumors related to mTOR pathway (Galanis et al., 2005; Albert et al., 2009).
Brain inflammation in epilepsy and mTOR pathway
Reactive gliosis is apparent in the epileptogenic tissues, and The level of inflammatory cytokines such as IL-β, TNF-α, and IL-6 in the area of seizure generation is increased both in clinical specimen and animal models of epilepsy (Vezzani and Granata, 2005; Binder and Steinhäuser, 2006). Although direct evidence of cytokine-mediated mTOR activation in epilepsy is still lacking, the mTOR pathway has been shown to be involved in cytokine-dependent microglial activation (Dello Russo et al., 2009). Activation of the mTOR pathway inhibits the pro-inflammatory cytokines such as TNF-α, and IL-6, and it promotes the release of anti-inflammatory cytokine (IL-10) via NFκB and STAT3 (Weichhart et al., 2008). The mTORC1 activation regulates the activity of NFκB which is associated with IKK (Dan et al., 2008). IKKβ (Inhibitor of NF-κB Kinase β) phosphorylates and inactivates TSC1, thus activating the mTOR pathway (Lee et al., 2007). The expression of NF-κB is increased in human specimen of temporal lobe epilepsy and kainate model (Lerner-Natoli et al., 2000; Crespel et al., 2002). PA, an essential regulator of inflammatory response, activates the mTOR pathway (Lim et al., 2003; Foster, 2009).
Cell death in Epilepsy and mTOR pathway
Epileptogenic insults cause cell deaths which have been classically categorized as apoptosis, necrosis, and autophagy (Edinger and Thompson, 2004; Henshall and Murphy, 2008). However, they are inter-connected and regulated by each other, and their boundaries become overlapped such as in programmed necrosis or necroptosis (Repici et al., 2007; Eisenberg-Lerner et al., 2009; Christofferson and Yuan, 2010). mTOR pathway has been shown to be involved in apoptosis and autophagy. In kainate-induced epilepsy model, both apoptotic and necrotic cell deaths were observed (van Lookeren et al., 1995; Humphrey et al., 2002).
Apoptosis
Apoptosis (also called 'programmed cell death') is the cell's intrinsic suicide process. Apoptosis shows nuclear condensation and fragmentation, and chromosomal DNA cleagage, and it has apoptotic bodies without inflammation. It requires caspase activation either by death-related receptor activation or pro-apoptotic molecules released from mitochondria (Levine et al., 2008). In the sera of children and adolescents with idiopathic epilepsy, a pro-apoptotic molecule, Fas and an anti-apoptotic molecule, Bcl-2 were elevated (El-Hodhod et al., 2006). In temporal lobe epilepsy patients, the levels of tumor necrosis factor receptor 1 (TNFR1), TNFR-associated protein with death domain (TRADD), Fas-associated protein with death domain (FADD), cleaved caspase8, and apoptosis signal-regulating kinase 1 (ASK1) are higher than controls (Yamamoto et al., 2006). ASK1 is activated by rapamycin treatment via reducing PP5 activity and it interacts with FIP200, an autophagic molecules regulated by mTOR (Huang et al., 2004; Gan et al., 2006). TNFα is increased after kainate injection in rodents and its expression has been shown to be regulated by mTORC2 in melanoma B16 cells (de Bock et al., 1996; Wang et al., 2007). TSC2 inhibits the phosphorylation of a pro-apoptotic molecule, BAD on Ser136, and this phosphorylation facilitates BAD/BCL-2 and BAD/BCL-XL interactions which lead to apoptosis (Freilinger et al., 2006). S6K and PIM-1 also phosphorylates BAD (Harada et al., 2001; Aho et al., 2004). PDCD4 (Programmed cell death 4) is phosphorylated on Ser67 and Ser457 by both AKT and S6K1 for nuclear translocation and/or proteosomal degradation (Palamarchuk et al., 2005; Dorrello et al., 2006).
Necrosis
Necrosis is generally conceived as passive form of cell death, resulting from ATP depletion, toxic insults, or physical damage. It is characterized by cytoplasmic vacuolation, breakdown of the plasma membrane, and inflammation surrounding the dying cells. In a kainate model of epilepsy, cytoplasmic shrinkage and nuclear condensation consistent with necrosis are far more significant than apoptosis in the entorhinal cortex (Puig and Ferrer, 2002). In a pilocarpine model, necrotic cell deaths were prominent in hippocampus and other brain regions (Fujikawa et al., 2002). Pilocarpine-induced SE in P14 rat shows activation of caspase-3 and necroptosis (Niquet et al., 2007). RIP3 is a necroptosis-specific marker which forms a complex with RIP1, triggering production of reactive oxygen species (Cho et al., 2009). It remains to be seen how RIP1 and RIP3 interact with each other in epilepsy, and whether a specific inhibitor, necrostatin-1 prevents epilepsy-induced cell death (Degterev et al., 2005).
Autophagy
Autophagy is a catabolic process that cells use autophagosomal/lysosomal machineries to degrade own cytoplasmic components (Ravikumar et al., 2009). It is triggered by starvation (ATP exhaust), oxidative stress, and glutamate. The mTOR is a negative regulator of autophagy, and rapamycin can induce autophagy (Rubinsztein et al., 2007). The mTOR interacts with ULK1-mATG13-FIP200 complex, one of autophagic pathways (Jung et al., 2009). By mTOR-mediated phosphorylation of ULK1 and mATG13, ULK's kinase activity regulates mATG13, FIP200 and ULK itself. FIP200 null mice are embryonic lethal (Gan et al., 2006). Kainate treatment induces phosphorylation and activation of mTOR and AKT (Shacka et al., 2007). The amount of LC3-II, a specific marker of autophagosomes, is increased by kainate or pilocarpine injection (Shacka et al., 2007; Cao et al., 2009). Therefore, it remains to be seen if mTOR-mediated autophagy is induced via ULK1-mATG13-FIP200 in kainate or pilocarpine-induced epileptic models or patients' specimen.
Ectopic neurogenesis and mTOR pathway
Severity of spontaneous seizures is associated with the increased number of ectopic adult neurogenesis of granule cells in the hilus as well as the normal adult neurogenesis in the dentate gyrus (Parent, 2007). DISC1 regulates adult neurogenesis in mTOP-dependent manner and it inhibits AKT activity via DISC1-KIAA1212 binding (Porteous, 2008; Kim et al., 2009). DISC1 has been also shown to have decreased level in kindling model (Fournier et al., 2010). The mTOR is activated both in insulin-activated neuronal differentiation of neural progenitor cells and in EGF/FGF2-mediated maintenance of neural stem cells (Han et al., 2008; Sato et al., 2010). It will be exciting to see how the mTOR pathway contributes to neurogenesis after epileptic insults in detail.
Synaptic plasticity and mTOR pathway
The mTOR pathway has been shown to be involved in both (short-term) activity-dependent local protein synthesis and (long-term) synaptic plasticity (Hoeffer and Klann, 2010). Induction and maintenance of NMDA receptor-mediated LTP in hippocampal CA1 neurons has been shown to be rapamycin-sensitive (Vickers et al., 2005). Brief glutamate stimulation in primary cultured neurons activates PI3K/AKT, ERK1/2, and mTOR/S6K in NMDAR- and CaMKII-dependent manner, although sustained stimulation inhibits ERK, AKT and S6K (Lenz and Avruch, 2005). AMPA receptors-mediated synaptic transmission is not mTOR-dependent, but mGluR- or BDNF-mediated neosynthesis of AMPA receptor subunit (GluR1 and GluR2) is (Mameli et al., 2007; Slipczuk et al., 2009). Metabotrophic glutamate receptor (mGluR)-mediated synaptic plasticity via the mTOR pathway has been relatively well studied (Mameli et al., 2007; Hou and Klann, 2004). Homer 1a couples mGluR1 to PI3K/mTOR pathway via PIKE interaction in mGluR-dependent synaptic plasticity (Rong et al., 2003). Homer 1b/c couples mGluR5 to activate S6K via ERK signaling cascade independent of the mTOR pathway (Mao et al., 2005). Pilocarpine-induced SE decreased protein levels of mGluR5 and Homer, losing mGluR-dependent LTD (Kirschstein et al., 2007). Homer1a mRNA expression is drastically increased in acute phase of kindling- and pilocarpine-models (Potschka et al., 2002; Avedissian et al., 2007). Forskolin, an adenylate cyclase activator, activates PKA and subsequently ERK, and/or activates BDNF/TrkB signaling, thus enhancing mTOR-dependent mRNA translation in hippocampal slice culture (Gobert et al., 2008). ERK activation increases dendritic protein synthesis at CA1 pyramidal neurons via PI3K/PDK1/AKT/mTOR pathway when high frequency stimulation given (Tsokas et al., 2007). D1/D5 dopamine receptor-mediated memory consolidation in auditory cortex has been shown to be rapamycin-sensitive (Tischmeyer et al., 2003; Schicknick et al., 2008). Protein Kinase Mζ, an autonomous brain-specific atypical PKC isoforms which is important in LTP maintenance in hippocampus, must be phosphorylated by PDK1 (Kelly et al., 2007).
Endocannabinoid receptors, epilepsy and mTOR pathway
Endocannabinoid CB1 receptors are localized at glutamatergic terminal in the hippocampus and its activation controls neuronal excitability by the increase of GABA release (Monory et al., 2006). Cannabinoids display anticonvulsive properties and specific CB1 receptor agonists such as marijuana extract Δ9-THCV (tetrahydrocannabinol) or ACEA (arachidonyl-2-chloroethylamide) are being developed as AEDs (Wallace et al., 2003; Ma et al., 2008b; Kozan et al., 2009). Hippocampal long-term memory is transiently modulated by CB1 receptor activation via mTOR/p70S6K signaling pathway (Puighermanal et al. 2009). In this report, this modulation was mediated by CB1Rs expressed on GABAergic interneurons - presumably basket cells - through a NMDA receptor-mediated mechanism. Short-term pilocarpine-injected rats showed increased levels of CB1 receptors and 2-arachidonylglycerol, an endogenous CB1 ligand, in the hippocampus (Wallace et al., 2003). However, long-term effect of SE showed the decreases in CB1 receptors in the pyramidal cell layer neurophil and the inner molecular layer of dentate gyrus, and increases in CA1-3 stratum oriens and stratum radiatum (Falenski et al., 2007). In febrile seizure model, it has been shown that an increase in number of presynaptic CB1 receptors and long-term increase in CB1 receptor-mediated retrograde signaling at GABAergic synapses (Chen et al., 2003). In epileptic patients, hippocampus has reduced expression of CB1 receptors (Ludanyi et al., 2008). Thus, CB1 receptor agonists likely become less effective as AEDs. Therefore, modulating downstream signaling molecule of mTOR pathway seems to be the better approach for the anti-epileptic treatment.
Astrocytes in epilepsy and mTOR pathway
Astrogliosis is a common phenomenon caused by epileptogenic insults such as head trauma, infection, excitotoxic injury (McGraw et al., 2001; Ortinski et al., 2010). Astrogliosis shows a decrease in glutamate and potassium uptake which can aggravate seizure activity (Tian et al., 2005; Binder and Steinhäuser, 2006; Seifert et al., 2010). Astrocyte-specific deletion of TSC2 mice showed astrogliosis and reduced uptake of potassium and glutamate by astrocyte, and the seizures (Uhlmann et al., 2002; Wong et al., 2003). STAT3 has been shown to be activated in reactive astrocytes in the hippocampus in kainate model (Choi et al., 2003). The mTOR pathway is activated in reactive astrocytes in spinal cord injury (Codeluppi et al., 2009). The levels of ERα in CA1 astrocytes and PLD were increased in KA model (Kim et al., 2004; Sakuma et al., 2009). ERK/MAPK is activated in mechanical trauma-induced astrogliosis and human reactive astrocytes (Mandell and VandenBerg, 1999; Mandell et al., 2001). It remains to be seen whether mTOR pathway is activated specifically in astrocytes when epileptogenic insults given.
mTOR inhibitors as new anti-epileptic drugs
More than thirty FDA-approved AEDs are available, and more are in the process of development and approval to treat the epileptic patients, however at least a third of patients respond poorly or become refractory to the current AEDs, particularly people with TSC (Shorvon, 1996; Curatolo et al., 2006; Bialer and White, 2010). Although mTOR inhibitors have not been tested for non-TSC patients with epilepsy, those may have the broader therapeutic effects in treating various types of epilepsy. Therefore, a new class of AEDs should be considered to be developed against the intracellular signaling molecules in the mTOR pathway (Wong, 2010).
Rapamycin (also known as sirolimus) is first developed as antifungal agent but widely used now as immunosuppressant and anticancer agents (Law, 2005). It was originally discovered from Streptomyces hygroscopicus in a soil sample collected from Easter Island (Vézina et al., 1975). It has structural resemblance with a macrolide antibiotic FK506 (Chang et al., 1991). It does not directly inhibit the mTOR kinase activity per se but it binds to FKBP12 and disrupts mTOR-RAPTOR interaction (Vignot et al., 2005). By this disruption, rapamycin fails to phosphorylate S6K1 and 4E-BP1, thus inhibits protein translation. Rapamycin itself does not seem to have any immediate effect on electrophysiological (voltage-gated sodium and potassium currents) properties of neurons in vitro (Rüegg et al., 2007). However, rapamycin has been shown to modulate the protein level of some voltage-gated potassium channels (Raab-Graham et al., 2006; Tyan et al., 2010).
It is noteworthy that rapamycin inhibits axonal sprouting in pilocarpine model only when it was continuously infused, and this abnormal sprouting reoccurs when its administration was terminated (Buckmaster et al., 2009). It might be related to the fact that animals become refractory to the drug or drug's effect subsidizes, and also that prolonged rapamycin treatment inhibits mTORC2 assembly and AKT/PKB (Sarbassov et al., 2006). Therefore, we may need to develop the better strategy of drug administration and/or the new drug candidates against other targets on the mTOR pathway.
There are three types of drug have been developing against mTOR complexes:
Rapalogue
Due to the poor water solubility, high toxicity, and resistance development with rapamycin, rapamycin analogs are being developed to overcome these problems: for example, CCI-779 (Temsirolimus), RAD001 (Everolimus), and AP23573 (deforolimus and MK-8669) (Plas and Thomas, 2009). For the preclinical trial of rapalogues against tumor regression of TSC patients, rapamycin was given to the mice model of TSC to examine the tumor regression (Lee et al., 2009c). Although side effects are tolerable, responses are incomplete, and tumor regrowth is common when rapamycin is stopped. In a small clinical trial, RAD001 reduced seizure frequency in TSC patients with an intractable epilepsy (Krueger et al., 2010).
PI3K/mTOR dual inhibitors
Both PI3K and mTOR belongs to the broad phosphatidylinositide kinase family (Kong and Yamori, 2008). Specific inhibitors against mTORC1 and PI3K are extensively under development to circumvent the drug resistance and to treat inherited hematoma syndromes which show the hyperactivity of both protein kinases (Krymskaya and Goncharova, 2009; Liu et al., 2009). NVP-BEZ235, PI-103, BGT226, XL765, and SF1126 are PI3K/mTOR dual inhibitors (Knight et al., 2006; Brachmann et al., 2009).
Inhibitors against catalytic site of mTOR
These inhibitors are designed to block the catalytic active site of mTOR. Therefore, they could block both mTORC1 and mTORC2 (Feldman et al., 2009; Thoreen et al., 2009). Torin1 is a highly potent and selective ATP-competitive mTOR inhibitor that directly inhibits both complexes (Thoreen et al., 2009). In acute leukemia model, PP242 delays the disease onset but has less effect on the normal lymphocytes' function than rapamycin (Janes et al., 2010). PP30, AZD8055 and OSI-027 are other inhibitors of this kind (Maira et al., 2008; Yap et al., 2008; Brachmann et al., 2009).
Ketogenic diet, anti-epileptic food supplements, and mTOR pathway
Ketogenic diet, a high fat and low carbohydrate diet, has been used especially for children since 1920s, and it has been often effective reducing seizure frequency, but its mechanism of action is poorly understood (Vamecq et al., 2005; Keene, 2006). Although recent small pilot study with TSC patients on tumor growth didn't show any effect of ketogenic diet, ketogenic diet has been shown to attenuate kainate-induced cell death in the hippocampus through AMPK, which activates the mTOR pathway (Jeon et al., 2009; Chu-Shore and Thiele, 2010) (Figure 2). Insulin level has been shown to be reduced on ketogenic diet (Thio et al., 2006). In kainate model, ketogenic diet reduces mossy fiber sprouting and shows fewer and briefer seizures (Muller-Schwarze et al., 1999).
Curcumin, a polyphenol natural product from the plant Curcuma longa, is in early clinical trial as a potential anti-cancer agent against various types of cancer (Strimpakos and Sharma, 2008). It inhibits cancerous proliferation by disrupting mTOR-RAPTOR complex (Beevers et al., 2009) (Figure 2). It will be very interesting if curcumin inhibits seizure by inhibiting mTOR kinases (Kang et al., 2006; Marcu et al., 2006). It also induces neurogenesis in the hippocampus which is also hampered in medial temporal lobe epilepsy (Hattiangady et al., 2004; Kim et al., 2008b).
Omega-3, a long-chain polyunsaturated fatty acids found in fish oil, has been shown to reduce seizure frequency in patients, and reduce neuronal excitability and promotes neuroprotection in animal models (Schlanger et al., 2002; DeGiorgio et al., 2008; Scorza et al., 2008). Recently, omega-3 has been shown to regulate protein metabolism via the mTOR pathway in bovine muscle (Gingras et al., 2007). It will be interesting to see whether omega-3 supplementation will modulate the mTOR pathway in the brain to have the anti-epileptic activity.
Resveratrol (trans-3',4',5'-trihydroxystibene) is natural polyphenolic substance present in grapes and red wine or isolated from the root of several plants such as Polygonum cuspidatum. It has antioxidant, anti-inflammatory, and anti-cancer properties (Frémont, 2000; Kimura and Okuda, 2001). It suppressed kainate- and NMDA- receptor-mediated synaptic transmission in CA1 pyramidal neurons and inhibited the K channels (Gao and Hu, 2005; Gao et al., 2006). Thus, it can inhibit abnormal kainate-evoked currents at the mossy fiber synapses in epilepsy (Epsztein et al., 2005). As predicted, it has been shown to decrease the seizure frequency, inhibited epileptiform discharges, protected neuronal cell death in the hippocampus, and depressed mossy fiber sprouting in the kainate model (Wu et al., 2009). Resveratrol inhibits mTORC1 activity by promoting mTOR/DEPTOR and by activating LKB1/AMPK or SIRT1-TSC1/2 and inhibiting PI3K (Dasgupta and Milbrandt, 2007; Frojdo et al., 2007; Ghosh et al., 2010; Liu et al., 2010). It will be interesting to see how resveratrol speficically modulates the downstream signaling molecules of mTOR pathway to have the anti-epileptic activity.
Epilepsy-comorbid diseases and mTOR pathway
Among several neurological and neuropsychiatric diseases that are comorbid with epilepsy, autism spectrum disorder and Alzheimer's disease are associated to the abnormal activity of the mTOR pathway.
Autism spectrum disorders
Autism spectrum disorders (ASD) covers heterogeneous population which show the three typical characteristics; poor social interaction, delayed language development, and stereotypical repetitive behavior (Geschwind, 2009). About 0.6% of general population is affected in ASD, up to 40% of ASD individuals show epileptic symptoms (Canitano, 2007). Although genes susceptible to ASD remain largely unknown, some genes (such as TSC1/TSC2 or PTEN when mutated) primarily responsible for ASD are on the mTOR pathway (Bill and Geschwind, 2009). Therefore, it is likely that this common pathway is, at least in part, involved in this developmental disorder. Interestingly, another ASD-related gene product, fragile X mental retardation protein (FMRP) negatively regulates mTOR/S6K1-dependent protein synthesis in neurons as an RNA binding protein, and it inhibits PIKE, then PI3K and mTOR activities (Narayanan et al., 2008; Sharma et al., 2010). It remains to be seen whether modulators of the mTOR pathway improve ASD individuals with epilepsy.
Alzheimer's disease
Alzheimer's disease (AD) has been reported with higher incidence of epileptic seizures than general population (Amatniek et al., 2006). In mouse model overexpressing mutant form of human amyloid precursor protein, high level of amyloid-β peptide, axonal sprouting of dentate granule cells, epileptiform activities in the hippocampus, and behavioral seizures were observed (Palop et al., 2007). In AD patients' specimen and animal models, mTOR, S6K, 4E-BP1 and eEF2K were shown to be activated, and hyperphosphorylation of tau, a pathological biomarker found in neurofibrillary tangles, is mediated by S6K and GSK3 (An et al., 2003; Li et al., 2005; Meske et al., 2008; Pei and Hugon, 2008). It remains to be seen whether mTOR-related therapeutic approach on Alzheimer's disease will reduce the seizure frequency of this subpopulation.
Challenges
One may become convinced of the involvement of the mTOR pathway in epilepsy from this review, however, there are enormous amount of evidences that need to be accumulated. To have a clear insight of intracellular signaling mechanism in epilepsy, the signaling molecules need to be examined to see whether they are activated or suppressed in the relevant types of cells in affected brain region, not just changes in the level of certain proteins described previously. It will be very exciting to figure out distinct patterns of protein-protein interactions in particular cell types at critical points during epileptogenesis. Different types of epilepsy may have different patterns of signaling footprint, and other conditions that people with epilepsy have may show unique signatures. It may also be different at different time points during the brain development or after the incident of epileptogenic insults. In addition, the number of proteins interacts with individual molecules in the mTOR pathway is still growing, and their specific interaction( s) may exclude or recruit other proteins to transduce the unique signals. There are mammalian homologs of components of mTOR complex yet to be identified, such as AVO2 and BIT61, two components of yeast TORC2 complex as well as Tco89, Bit61, Toc1, Tel2, Tti1 and Cka1 in mTOR pathway. Furthermore, we can not simply ignore the possibility of involvement of certain genes in the epilepsy based on the expression pattern in normal condition. It is because epileptogenic mechanism could initiate the expression of certain genes, unique post-translational modifications, or new pattern of protein-protein interaction. It will be fascinating to see if (and how) the mTOR pathways are connected to ion channels that are epileptogenic, and also whether other non-ion channel proteins that are genetically linked to epilepsy (e.g, LGI1, ME2, EFHC1 and BRD2) are implicated in the mTOR pathway (Lucarini et al., 2007).
Summary
It should be noted that the full scope of signaling events in epilepsy is unknown. Neuronal insultdriven epileptogenetic process leading toward chronic spontaneous seizures have multiple aspects including cell death, cell survival and proliferation to recovery, abnormal neuronal differentiation, consequent changes in neuronal network activity. Therefore, there are numerous possibilities of distinct signaling mechanisms could be involved. In this review, I tried to link epileptic causatives to the abnormal activities of signaling molecules in the mTOR pathway. I also briefly summarized the most of individual signaling molecules of the mTOR pathway. It will be exciting to watch (or study) how extensively the mTOR pathways are involved in all different types of epilepsy in years to come.
Acknowledgements
I apologize to those authors whose work is not cited or discussed due to editorial space limitations. I thank Drs. M. McCartney and E. Marsh for the helpful comments on the early version of this manuscript. I declare no conflict of interest in publishing this article. Any communication on the subject of this article will be greatly appreciated.
Abbreviations
- 4E-BP
eukaryotic initiation factor 4 (eIF4) binding proteins
- ACEA
arachidonyl-2-chloroethylamide
- AD
Alzheimer's disease
- AED
anti-epileptic drug
- AICAR
5-amino-4-imidazolecarboxamide ribose
- AKT
acutely transforming retrovirus AKT8 in rodent T cell lymphoma
- AMPK
AMP-activated protein kinase
- AR
androgen receptor
- ASD
autism spectrum disorder
- ASK
apoptosis signal-regulating kinase
- BID
BH3-interacting domain death agonist
- BIM
Bcl-2-interacting mediator of cell death
- BRG1
brahma-related gene 1
- CaMKKβ
calcium/calmodulin-dependent protein kinase kinase β
- Cdc42
cell division cycle 42
- Cdk
cyclin-dependent kinase
- CLIP-170
CAP-GLY domain containing linker protein 1
- CR
cannabinoid receptor
- DAG
diacylgylcerol
- DAPK
death-associated protein kinase
- DDIT4
DNA-damage-inducible transcript 4
- DEPTOR
DEP-domain containing mTOR-interacting protein
- DISC1
disrupted-in-schizophrenia 1
- EGF
epidermal growth factor
- eIF4
eukaryotic initiation factor 4
- ER
estrogen receptor
- FADD
Fas-associated protein with death domain
- FGF
fibroblast growth factor
- FKBP12
FK506 binding protein 12
- FMRP
fragile X mental retardation protein
- FIP200
focal adhesion kinase interacting protein of 200 KD
- FOXO
forkhead box O
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- GSK
glycogen synthase kinase
- HCMV
human cytomegalovirus
- HSP70
heat shock protein 70
- HSV
Herpes simplex virus
- hVPS34
human vacuolar protein sorting 34
- IGF
insulin-like growth factor
- IKKβ
Inhibitor of NF-κB kinase β
- IP3
inositol triphosphate
- IRS-1
insulin receptor substrate-1
- LTD
long-term depression
- LTP
long-term potentiation
- mATG13
mammalian autophagy related protein 13
- mGluR
metabotrophic glutamate receptor
- mLST8
mammalian lethal with Sec13 protein 8
- Mnk1
MAPK-interacting kinase 1
- mSIN-1
mammalian stress-activated protein kinase interacting protein
- mTOR
mammalian target of rapamycin, NDRG1, N-myc downstream regulated gene 1
- PA
phosphatidic acid
- PAP
phosphatidate phosphatase
- PDCD4
programmed cell death 4
- PDK1
3-phosphoinositide-dependent protein kinase-1
- PGC1α
PPARγ coactivator-1α
- PHLPP1/2
PH domain leucine-rich repeat protein phosphatase
- PI3K
phosphoinositide 3-kinase
- PIM-1
provirus integration site for Moloney murine leukemia virus
- PIP2
phosphatidylinositol 4, 5 bisphosphate
- PIP3
phosphatidylinositol 3, 4, 5 trisphosphate
- PMSE
polyhydramnios, megalencephaly and symptomatic epilepsy
- PP2A
protein phosphatase 2A
- PRAS40
proline-rich AKT substrate of 40 KDa
- P-REX1
PIP3-dependent Rac exchanger 1
- Protor-1
protein observed with RICTOR-1
- PTEN
phosphatase and tensin homolog
- Rag
Ras-related GTPase
- RAPTOR
regulatory-associated protein of mTOR
- REDD1
regulated in development and DNA damage responses 1
- Rheb
Ras homolog enriched in brain
- RICTOR
rapamycin-insensitive companion of mTOR
- RSK
RPS6K1 ribosomal protein S6 kinase
- S6K
p70 ribosomal protein S6 kinase
- SCOP
suprachiasmatic nucleus circadian oscillatory protein
- SE
status epilepticus
- SF2/ASF
splicing factor, arginine/serine-rich factor
- SGK1
serum- and glucocorticoid-induced kinase 1
- SHIP-2
SH2-domain containing inositol 5-phosphatase 2
- SKAR
S6K1 Aly/REF-like target
- SREBP
sterol responsive element binding protein
- STAT3
signal transducers and activators of transcription 3
- STRADα
STE20-related adaptor protein α
- TBI
traumatic brain injury
- TCTP
translationally controlled tumor protein
- TNFR
tumor necrosis factor receptor
- TRADD
TNFR-associated protein with death domain
- TSC
tuberous sclerosis complex
- ULK1
unc-51-like kinase 1
References
- 1.Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudière E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ahn JY, Ye K. PIKE GTPase signaling and function. Int J Biol Sci. 2005;1:44–50. doi: 10.7150/ijbs.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Albert L, Katsy M, Murali R, Jhanwar-Uniyal M. Inhibition of mTOR activates the MAPK pathway in glioblastoma multiforme. Cancer Genomics Proteomics. 2009;6:255–262. [PubMed] [Google Scholar]
- 6.Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- 7.Allard D, Figg N, Bennett MR, Littlewood TD. AKT regulates the survival of vascular smooth muscle cells via inhibition of FOXO3a and GSK3. J Biol Chem. 2008;283:19739–19747. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- 8.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 9.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 10.An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimers disease. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreozzi F, D'Alessandris C, Federici M, Laratta E, Del Guerra S, Del Prato S, Marchetti P, Lauro R, Perticone F, Sesti G. Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser307 and Ser612 and impairs the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic beta-cells. Endocrinology. 2004;145:2845–2857. doi: 10.1210/en.2003-0939. [DOI] [PubMed] [Google Scholar]
- 12.Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol Cell Biol. 2008;28:2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki E, Lipes MA, Patti ME, Brüning JC, Haag B, 3rd, Johnson RS, Kahn CR. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 14.Arion D, Sabatini M, Unger T, Pastor J, Alonso-Nanclares L, Ballesteros-Yáñez I, García Sola R, Moñoz A, Mirnics K, DeFelipe J. Correlation of transcriptome profile with electrical activity in temporal lobe epilepsy. Neurobiol Dis. 2006;22:374–387. doi: 10.1016/j.nbd.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol. 2006;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astrinidis A, Senapedis W, Coleman TR, Henske EP. Cell cycle-regulated phosphorylation of hamartin, the product of the tuberous sclerosis complex 1 gene, by cyclin-dependent kinase 1/cyclin B. J Biol Chem. 2003;278:51372–51379. doi: 10.1074/jbc.M303956200. [DOI] [PubMed] [Google Scholar]
- 17.Avedissian M, Longo BM, Jaqueta CB, Schnabel B, Paiva PB, Mello LE, Briones MR. Hippocampal gene expression analysis using the ORESTES methodology shows that homer 1a mRNA is upregulated in the acute period of the pilocarpine epilepsy model. Hippocampus. 2007;17:130–136. doi: 10.1002/hipo.20248. [DOI] [PubMed] [Google Scholar]
- 18.Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 19.Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- 20.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 21.Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000;484:217–223. doi: 10.1016/s0014-5793(00)02162-1. [DOI] [PubMed] [Google Scholar]
- 22.Balu DT, Carlson GC, Talbot K, Kazi H, Hill-Smith TE, Easton RM, Birnbaum MJ, Lucki I. Akt1 deficiency in schizophrenia and impairment of hippocampal plasticity and function. Hippocampus. 2010 doi: 10.1002/hipo.20887. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the Hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banko JL, Merhav M, Stern E, Sonenberg N, Rosenblum K, Klann E. Behavioral alterations in mice lacking the translation repressor 4E-BP2. Neurobiol Learn Mem. 2007;87:248–256. doi: 10.1016/j.nlm.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Barber MA, Donald S, Thelen S, Anderson KE, Thelen M, Welch HC. Membrane translocation of P-REX1 is mediated by G protein betagamma subunits and phosphoinositide 3-kinase. J Biol Chem. 2007;282:29967–29976. doi: 10.1074/jbc.M701877200. [DOI] [PubMed] [Google Scholar]
- 26.Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 27.Basu A, Sridharan S, Persaud S. Regulation of protein kinase C delta downregulation by protein kinase C epsilon and mammalian target of rapamycin complex 2. Cell Signal. 2009;21:1680–1685. doi: 10.1016/j.cellsig.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck H. Plasticity of antiepileptic drug targets. Epilepsia. 2007;48(Suppl 1):14–18. doi: 10.1111/j.1528-1167.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 29.Beevers CS, Chen L, Liu L, Luo Y, Webster NJG, Huang S. Curcumin disrupts the mammalian target of rapamycin RAPTOR complex. Cancer Res. 2009;69:1000–1008. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beharry Z, Mahajan S, Zemskova M, Lin Y-W, Tholanikunnel BG, Xia Z, Smith CD, Kraft AS. The Pim protein kinases regulate energy metabolism and cell growth. Proc Natl Acad Sci USA. 2011;108:528–533. doi: 10.1073/pnas.1013214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bengoechea-Alonso MT, Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1. J Biol Chem. 2009;284:5885–5895. doi: 10.1074/jbc.M807906200. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin D, Schmidlin M, Min L, Gross B, Moroni C. BRF1 protein turnover and mRNA decay activity are regulated by protein kinase B at the same phosphorylation sites. Mol Cell Biol. 2006;26:9497–9507. doi: 10.1128/MCB.01099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trend Neurosci. 2006;29:391–397. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 35.Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19:271–278. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binda M, Péli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Binder DK, Steinhäuser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- 38.Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 2001;20:4380–4390. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boer K, Troost D, Timmermans W, van Rijen PC, Spliet WG, Aronica E. Pi3K-mTOR signaling and AMOG expression in epilepsy-associated glioneuronal tumors. Brain Pathol. 2010;20:234–244. doi: 10.1111/j.1750-3639.2009.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Böhm D, Schwegler H, Kotthaus L, Nayernia K, Rickmann M, Köhler M, Rosenbusch J, Engel W, Flügge G, Burfeind P. Disruption of PLC-beta 1-mediated signal transduction in mutant mice causes age-dependent hippocampal mossy fiber sprouting and neurodegeneration. Mol Cell Neurosci. 2002;21:584–601. doi: 10.1006/mcne.2002.1199. [DOI] [PubMed] [Google Scholar]
- 41.Borges K, McDermott DL, Dingledine R. Reciprocal changes of CD44 and GAP-43 expression in the dentate gyrus inner molecular layer after SE in mice. Exp Neurol. 2004;188:1–10. doi: 10.1016/j.expneurol.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interacts with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brachmann S, Fritsch C, Maira SM, García-Echeverría C. PI3K and mTOR inhibitors: a new generation of targeted anticancer agents. Curr Opin Cell Biol. 2009;21:194–198. doi: 10.1016/j.ceb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci. 2009;29:12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of AKT signaling by regulating distinct AKT isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 47.Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24:2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, Dalal SN, DeCaprio JA, Greenberg ME, Yaffe MB. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol. 2002;156:817–828. doi: 10.1083/jcb.200112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-AKT-mTOR signaling pathway. Nat Rev MicroBiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canitano R. Epilepsy in autism spectrum disorders. Eur Child Adolesc Psychiatry. 2007;16:61–66. doi: 10.1007/s00787-006-0563-2. [DOI] [PubMed] [Google Scholar]
- 56.Cao Y, Kamioka Y, Yokoi N, Kobayashi T, Hino O, Onodera M, Mochizuki N, Nakae J. Interaction of FOXO1 and TSC2 induces insulin resistance through activation of the mammalian target of rapamycin/p70 S6K pathway. J Biol Chem. 2006;281:40242–40251. doi: 10.1074/jbc.M608116200. [DOI] [PubMed] [Google Scholar]
- 57.Cao L, Xu J, Lin Y, Zhao X, Liu X, Chi Z. Autophagy is upregulated in rats with SE and partly inhibited by Vitamin E. Biochem Biophys Res Commun. 2009;379:949–953. doi: 10.1016/j.bbrc.2008.12.178. [DOI] [PubMed] [Google Scholar]
- 58.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 59.Carlier MF, Ducruix A, Pantaloni D. Signalling to actin: the Cdc42-N-WASP-Arp2/3 connection. Chem Biol. 1999;6:R235–R240. doi: 10.1016/s1074-5521(99)80107-0. [DOI] [PubMed] [Google Scholar]
- 60.Carrière A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated RAPTOR phosphorylation. Curr Biol. 2008a;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 61.Carrière A, Ray H, Blenis J, Roux PP. The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci. 2008b;13:4258–4275. doi: 10.2741/3003. [DOI] [PubMed] [Google Scholar]
- 62.Carrière A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP. ERK1/2 phosphorylate raptor to promote ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castedo M, Ferri KF, Blanco J, Roumier T, Larochette N, Barretina J, Amendola A, Nardacci R, Métivier D, Este JA, Piacentini M, Kroemer G. Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J Exp Med. 2001;194:1097–1110. doi: 10.1084/jem.194.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor FOXO3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 65.Chalhoub N, Zhu G, Zhu X, Baker SJ. Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev. 2009;23:1619–1624. doi: 10.1101/gad.1799609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan CB, Liu X, Jung DY, Jun JY, Luo HR, Kim JK, Ye K. Deficiency of PIKE protects mice from diet-induced obesity and insulin resistance. Diabetes. 2010;59:889–893. doi: 10.2337/db09-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang JY, Sehgal SN, Bansbach CC. FK506 and rapamycin: novel pharmacological probes of the immune response. Trends Pharmacol Sci. 1991;12:218–223. doi: 10.1016/0165-6147(91)90555-7. [DOI] [PubMed] [Google Scholar]
- 68.Chen F, Ma L, Parrini MC, Mao X, Lopez M, Wu C, Marks PW, Davidson L, Kwiatkowski DJ, Kirchhausen T, Orkin SH, Rosen FS, Mayer BJ, Kirschner MW, Alt FW. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10:758–765. doi: 10.1016/s0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 69.Chen K, Ratzliff A, Hilgenberg L, Gulyás A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 70.Chen SF, Huang CC, Wu HM, Chen SH, Liang YC, Hsu KS. Seizure, neuron loss, and mossy fiber sprouting in herpes simplex virus type 1-infected organotypic hippocampal cultures. Epilepsia. 2004;45:322–332. doi: 10.1111/j.0013-9580.2004.37403.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen WW, Chan DC, Donald C, Lilly MB, Kraft AS. Pim family kinases enhance tumor growth of prostate cancer cells. Mol Cancer Res. 2005;3:443–451. doi: 10.1158/1541-7786.MCR-05-0007. [DOI] [PubMed] [Google Scholar]
- 72.Chen S, Atkins CM, Liu C, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- 73.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 74.Chiu SS, Tse CY, Lau YL, Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001;108:E63. doi: 10.1542/peds.108.4.e63. [DOI] [PubMed] [Google Scholar]
- 75.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase AKT2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 76.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi JH, Bertram PG, Drenan R, Carvalho J, Zhou HH, Zheng XF. The FKBP12-rapamycin-associated protein (FRAP) is a CLIP-170 kinase. EMBO Rep. 2002;3:988–994. doi: 10.1093/embo-reports/kvf197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi JS, Kim SY, Park HJ, Cha JH, Choi YS, Kang JE, Chung JW, Chun MH, Lee MY. Upregulation of gp130 and differential activation of STAT and p42/44 MAPK in the rat hippocampus following kainic acid-induced seizures. Mol Brain Res. 2003;119:10–18. doi: 10.1016/j.molbrainres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Chou MM, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 80.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung B, Wong V. Relationship between five common viruses and febrile seizure in children. Arch Dis Child. 2007;92:589–593. doi: 10.1136/adc.2006.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu-Shore CJ, Thiele EA. Tumor growth in patients with tuberous sclerosis complex on the ketogenic diet. Brain Dev. 2010;32:318–322. doi: 10.1016/j.braindev.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by mammalian target of rapamycin. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 84.Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M, Marsala M, Pasquale EB. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29:1093–1104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cole-Edwards KK, Bazan NG. Lipid signaling in experimental epilepsy. Neurochem Res. 2005;30:847–853. doi: 10.1007/s11064-005-6878-4. [DOI] [PubMed] [Google Scholar]
- 86.Consortium E.C.T.S. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 87.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crespel A, Coubes P, Rousset MC, Brana C, Rougier A, Rondouin G, Bockaert J, Baldy-Moulinier M, Lerner-Natoli M. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952:159–169. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- 89.Crino PB. Focal brain malformations: seizures, signaling, sequencing. Epilepsia. 2009;50(Suppl 9):3–8. doi: 10.1111/j.1528-1167.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- 90.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 91.Csibi A, Cornille K, Leibovitch MP, Poupon A, Tintignac LA, Sanchez AM, Leibovitch SA. The Translation Regulatory Subunit eIF3f Controls the Kinase-Dependent mTOR Signaling Required for Muscle Differentiation and Hypertrophy in Mouse. PLoS One. 2010;5:e8994. doi: 10.1371/journal.pone.0008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 93.Curatolo P, Bombardieri R, Cerminara C. Current management for epilepsy in tuberous sclerosis complex. Curr Opin Neurol. 2006;19:119–123. doi: 10.1097/01.wco.0000218225.50807.12. [DOI] [PubMed] [Google Scholar]
- 94.Curatolo P, Bombardieri R, Jozwiak S. Tuberous Sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 95.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. AKT-dependent regulation of NF-κB is controlled by mTOR and RAPTOR in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. AKT phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 98.Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990;109:180–190. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- 99.Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- 100.de Bock F, Dornand J, Rondouin G. Release of TNF alpha in the rat hippocampus following epileptic seizures and excitotoxic neuronal damage. Neuroreport. 1996;7:1125–1129. doi: 10.1097/00001756-199604260-00004. [DOI] [PubMed] [Google Scholar]
- 101.de Groot RP, Ballou LM, Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell. 1994;79:81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 102.DeGiorgio CM, Miller P, Meymandi S, Gornbein JA. n-3 fatty acids (fish oil) for epilepsy, cardiac risk factors, and risk of SUDEP: clues from a pilot, double-blind, exploratory study. Epilepsy Behav. 2008;13:681–684. doi: 10.1016/j.yebeh.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 104.Dello Russo C, Lisi L, Tringali G, Navarra P. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem Pharmacol. 2009;78:1242–1251. doi: 10.1016/j.bcp.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 105.Desai BN, Meyers BR, Schreiber SL. FKBP12-rapamycin associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deshmukh AS, Glund S, Tom RZ, Zierath JR. Role of the AMPKgamma3 isoform in hypoxia-stimulated glucose transport in glycolytic skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E1388–E1394. doi: 10.1152/ajpendo.00125.2009. [DOI] [PubMed] [Google Scholar]
- 107.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 109.Dibble CC, Asara JM, Manning BD. Characterization of RICTOR phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dieterle A, Orth R, Daubrawa M, Grotemeier A, Alers S, Ullrich S, Lammers R, Wesselborg S, Stork B. The Akt inhibitor triciribine sensitizes prostate carcinoma cells to TRAIL-induced apoptosis. Int J Cancer. 2009;125:932–941. doi: 10.1002/ijc.24374. [DOI] [PubMed] [Google Scholar]
- 111.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Mäkelä TP, Wallimann T, Neumann D, Krek W. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong X, Yang B, Li Y, Zhong C, Ding J. Molecular basis of the acceleration of the GDP-GTP exchange of human ras homolog enriched in brain by human translationally controlled tumor protein. J Biol Chem. 2009;284:23754–23764. doi: 10.1074/jbc.M109.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 114.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dunin-Wasowicz D, Kasprzyk-Obara J, Jurkiewicz E, Kapusta M, Milewska-Bobula B. Infantile spasms and cytomegalovirus infection: antiviral and antiepileptic treatment. Dev Med Child Neurol. 2007;49:684–692. doi: 10.1111/j.1469-8749.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 116.Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signaling input, substrates and feedback mechanisms. Cell Signal. 2009;21:827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 117.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for AKT3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 119.Eeg-Olofsson O. Virological and immunological aspects of seizure disorders. Brain Dev. 2003;25:9–13. doi: 10.1016/s0387-7604(02)00162-6. [DOI] [PubMed] [Google Scholar]
- 120.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 121.el-Etri MM, Ennis M, Jiang M, Shipley MT. Pilocarpine-induced convulsions in rats: evidence for muscarinic receptor-mediated activation of locus coeruleus and norepinephrine release in cholinolytic seizure development. Exp Neurol. 1993;121:24–39. doi: 10.1006/exnr.1993.1068. [DOI] [PubMed] [Google Scholar]
- 122.El-Hodhod MA, Tomoum HY, Abd Al-Aziz MM, Samaan SM. Serum Fas and Bcl-2 in patients with epilepsy. Acta Neurol Scand. 2006;113:315–321. doi: 10.1111/j.1600-0404.2006.00592.x. [DOI] [PubMed] [Google Scholar]
- 123.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, Haber DA. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 124.Epsztein J, Represa A, Jorquera I, Ben-Ari Y, Crèpel V. Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats. J Neurosci. 2005;25:8229–8239. doi: 10.1523/JNEUROSCI.1469-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Erlich S, Alexxandrovich A, Shohami E, Pinkas-Kramarshi R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 126.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E. The mammalian target of rapamycin complex 2 controls folding and stability of AKT and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Falenski KW, Blair RE, Sim-Selley LJ, Martin BR, DeLorenzo RJ. SE causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience. 2007;146:1232–1244. doi: 10.1016/j.neuroscience.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, McCormick F, Shokat KM, Weiss WA. EGFR signals to mTOR through PKC and independently of AKT in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 130.Fang Y, Park IH, Wu AL, Du G, Huang P, Frohman MA, Walker SJ, Brown HA, Chen J. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol. 2003;13:2037–2044. doi: 10.1016/j.cub.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 131.Feldman JD, Vician L, Crispino M, Tocco G, Baudry M, Herschman HR. Seizure activity induces PIM-1 expression in brain. J Neurosci Res. 1998;53:502–509. doi: 10.1002/(SICI)1097-4547(19980815)53:4<502::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 132.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signaling. Biochem J. 2007;403:13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Foster DA. Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells. Biochim Biophys Acta. 2009;1791:949–955. doi: 10.1016/j.bbalip.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fotheringham J, Donati D, Akhyani N, Fogdell-Hahn A, Vortmeyer A, Heiss JD, Williams E, Weinstein S, Bruce DA, Gaillard WD, Sato S, Theodore WH, Jacobson S. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 2007;4:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Foufelle F, Ferre P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fournier NM, Andersen DR, Botterill JJ, Sterner EY, Lussier AL, Caruncho HJ, Kalynchuk LE. The effect of amygdala kindling on hippocampal neurogenesis coincides with decreased reelin and DISC1 expression in the adult dentate gyrus. Hippocampus. 2010;20:659–671. doi: 10.1002/hipo.20653. [DOI] [PubMed] [Google Scholar]
- 138.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 140.Freilinger A, Rosner M, Krupitza G, Nishino M, Lubec G, Korsmeyer SJ, Hengstschläger M. Tuberin activates the proapoptotic molecule BAD. Oncogene. 2006;25:6467–6479. doi: 10.1038/sj.onc.1209660. [DOI] [PubMed] [Google Scholar]
- 141.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 142.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for AKT/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Friocourt G, Koulakoff A, Chafey P, Boucher D, Fauchereau F, Chelly J, Francis F. Doublecortin functions at the extremities of growing neuronal processes. Cereb Cortex. 2003;13:620–626. doi: 10.1093/cercor/13.6.620. [DOI] [PubMed] [Google Scholar]
- 144.Frödin M, Jensen CJ, Merienne K, Gammeltoft S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–518. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, Nawaz Z, Shimojima T, Wang H, Yang Y, Shen Z, Zhang Y, Zhang X, Nicosia SV, Zhang Y, Pledger JW, Chen J, Bai W. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem. 2009;284:13987–14000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fujikawa DG, Ke X, Trinidad RB, Shinmei SS, Wu A. Caspase-3 is not activated in seizure-induced neuronal necrosis with internucleosomal DNA cleavage. J Neurochem. 2002;83:229–240. doi: 10.1046/j.1471-4159.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- 148.Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, Jaeckle KA, Scheithauer BW, Dancey J, Hidalgo M, Walsh DJ North Central Cancer Treatment Group. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 149.Gan B, Peng X, Nagy T, Alcaraz A, Gu H, Guan JL. Role of FIP200 in cardiac and liver development and its regulation of TNFalpha and TSC-mTOR signaling pathways. J Cell Biol. 2006;175:121–133. doi: 10.1083/jcb.200604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ganesh S, Puri R, Singh S, Mittal S, Dubey D. Recent advances in the molecular basis of Laforas progressive myoclonus epilepsy. J Hum Genet. 2006;51:1–8. doi: 10.1007/s10038-005-0321-1. [DOI] [PubMed] [Google Scholar]
- 151.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gannicliffe A, Saldanha JA, Itzhaki RF, Sutton RN. Herpes simplex viral DNA in temporal lobe epilepsy. Lancet. 1985;1:214–215. doi: 10.1016/s0140-6736(85)92043-4. [DOI] [PubMed] [Google Scholar]
- 154.Gao D, Wan L, Inuzuka H, Berg AH, Tseng A, Zhai B, Shaik S, Bennett E, Tron AE, Gasser JA, Lau A, Gygi SP, Harper JW, DeCaprio JA, Toker A, Wei W. Rictor forms a complex with Cullin-1 to promote SGK1 ubiquitination and destruction. Mol Cell. 2010;39:797–808. doi: 10.1016/j.molcel.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates AKT, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 156.Gao ZB, Hu GY. Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. Brain Res. 2005;1056:68–75. doi: 10.1016/j.brainres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 157.Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006;1111:41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 158.Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 159.Garcia L, Garcia F, Llorens F, Unzeta M, Itarte E, Gómez N. PP1/PP2A phosphatases inhibitors okadaic acid and calyculin A block ERK5 activation by growth factors and oxidative stress. FEBS Lett. 2002;523:90–94. doi: 10.1016/s0014-5793(02)02950-2. [DOI] [PubMed] [Google Scholar]
- 160.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 161.Garrido YCS, Sanabria ERG, Funke MG, Cavalheiro EA, Naffah-Mazzacoratti MG. Mitogen-activated protein kinase is increased in the limbic structures of the rat brain during the early stages of SE. Brain Res Bull. 1998;47:223–229. doi: 10.1016/s0361-9230(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 162.Gass P, Prior P, Kiessling M. Correlation between seizure intensity and stress protein expression after limbic epilepsy in the rat brain. Neuroscience. 1995;65:27–36. doi: 10.1016/0306-4522(95)92049-p. [DOI] [PubMed] [Google Scholar]
- 163.Gentry MS, Worby CA, Dixon JE. Insights into Lafora disease: malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin. Proc Natl Acad Sci USA. 2005;102:8501–8506. doi: 10.1073/pnas.0503285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Geschwind DH. Advances in Autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Getts DR, Balcar VJ, Matsumoto I, Muller M, King NJC. Viruses and the immune system: their roles in seizure cascade development. J Neurochem. 2008;104:1167–1176. doi: 10.1111/j.1471-4159.2007.05171.x. [DOI] [PubMed] [Google Scholar]
- 166.Ghosh HS, McBurney M, Robbins PD. SITR1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gibson SL, Ma Z, Shaw LM. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle. 2007;6:631–637. doi: 10.4161/cc.6.6.3987. [DOI] [PubMed] [Google Scholar]
- 168.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gingras AA, White PJ, Chouinard PY, Julien P, Davis TA, Dombrowski L, Couture Y, Dubreuil P, Myre A, Bergeron K, Marette A, Thivierge MC. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signaling to the AKT-mTOR-S6K1 pathway and insulin sensitivity. J Physiol. 2007;579:269–284. doi: 10.1113/jphysiol.2006.121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gobert D, Topolnik L, Azzi M, Huang L, Badeaux F, Desgroseillers L, Sossin WS, Lacaille JC. Forskolin induction of late-LTP and up-regulation of 5 TOP mRNAs translation via mTOR, ERK, and PI3K in hippocampal pyramidal cells. J Neurochem. 2008;106:1160–1174. doi: 10.1111/j.1471-4159.2008.05470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic Hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Götz J, Schild A. Transgenic and knockout models of PP2A. Methods Enzymol. 2003;366:390–403. doi: 10.1016/s0076-6879(03)66029-5. [DOI] [PubMed] [Google Scholar]
- 173.Grey R, Pierce AC, Bemis GW, Jacobs MD, Moody CS, JajooR, Mohal N, Green J. Structure-based design of 3-aryl-6-amino-triazolo[4,3-b]pyridazine inhibitors of Pim-1 kinase. Bioorg Med Chem Lett. 2009;19:3019–3022. doi: 10.1016/j.bmcl.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 174.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 175.Guan L, Song K, Pysz MA, Curry KJ, Hizli AA, Danielpour D, Black AR, Black JD. Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/AKT-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial cells. J Biol Chem. 2007;282:14213–14225. doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- 176.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 177.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components RAPTOR, RICTOR, or mLST8 reveals that mTORC2 is required for signaling to AKT-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 178.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metabolism. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of RAPTOR mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ha SH, Kim DH, Kim IS, Kim JH, Lee MN, Lee HJ, Kim JH, Jang SK, Suh PG, Ryu SH. PLD2 forms a functional complex with mTOR/RAPTOR to transduce mitogenic signals. Cell Signal. 2006;18:2283–2291. doi: 10.1016/j.cellsig.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 181.Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, Pollak S, Zentner J, Frotscher M. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci. 2002;22:5797–5802. doi: 10.1523/JNEUROSCI.22-14-05797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Han EK, Leverson JD, McGonigal T, Shah OJ, Woods KW, Hunter T, Giranda VL, Luo Y. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–5661. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 183.Han J, Wang B, Xiao Z, Gao Y, Zhao Y, Zhang J, Chen B, Wang X, Dai J. Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol Cell Neurosci. 2008;39:118–124. doi: 10.1016/j.mcn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 184.Hannan KM, Brandenburger Y, Henkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, Hannan RD. mTOR-dependent regulation of ribosomal S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23:8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA. 2001;98:9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hardie DG. The AMP-activated protein kinase pathway - new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 187.Hardie DG. New roles for the LKB1 --> AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 188.Harrar Y, Bellini C, Faure JD. FKBPs: at the crossroads of folding and transduction. Trends Plant Sci. 2001;6:426–431. doi: 10.1016/s1360-1385(01)02044-1. [DOI] [PubMed] [Google Scholar]
- 189.Harris TE, Chi A, Shabanowitz J, Hunt DF, Rhoads RE, Lawrence JC., Jr mTOR-dependent stimulation of the association of eIF4G and eIF3 by insulin. EMBO J. 2006;25:1659–1668. doi: 10.1038/sj.emboj.7601047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Harris TE, Huffman TA, Chi A, Shabanowitz J, Hunt DF, Kumar A, Lawrence JC., Jr Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J Biol Chem. 2007;282:277–286. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- 191.Harwood FC, Shu L, Houghton PJ. mTORC1 signaling can regulate growth factor activation of p44/42 mitogen-activated protein kinases through protein phosphatase 2A. J Biol Chem. 2008;283:2575–2585. doi: 10.1074/jbc.M706173200. [DOI] [PubMed] [Google Scholar]
- 192.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult Hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 193.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 194.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 196.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, Lee J, Raz E. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci USA. 2007;104:18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Heinrich C, Nitta N, Flubacher A, Müller M, Fahrner A, Kirsch M, Freiman T, Suzuki F, Depaulis A, Frotscher M, Haas CA. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic Hippocampus. J Neurosci. 2006;26:4701–4713. doi: 10.1523/JNEUROSCI.5516-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Helbig I, Scheffer IE, Mulley JC, Berkovic SF. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 2008;7:231–245. doi: 10.1016/S1474-4422(08)70039-5. [DOI] [PubMed] [Google Scholar]
- 200.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Järvinen H, Kristo P, Pelin K, Ridanpää M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–189. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 201.Henshall DC, Murphy BM. Modulators of neuronal cell death in epilepsy. Curr Opin Pharmacol. 2008;8:75–81. doi: 10.1016/j.coph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 202.Hernández-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernández-García R, Calderón-Salinas JV, Reyes-Cruz G, Gutkind JS, Vázquez-Prado J. P-REX1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007;282:23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 203.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 204.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 205.Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTOR-RAPTOR interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory, and diseases. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 208.Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene. 2008;27:6462–6472. doi: 10.1038/onc.2008.312. [DOI] [PubMed] [Google Scholar]
- 209.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 210.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hosoya M, Honzumi K, Suzuki H. Detection of enterovirus by polymerase chain reaction and culture in cerebrospinal fluid of children with transient neurologic complications associated with acute febrile illness. J Infect Dis. 1997;175:700–703. doi: 10.1093/infdis/175.3.700. [DOI] [PubMed] [Google Scholar]
- 212.Hou L, Klann E. Activation of the phosphoinositide 3-kinase-AKT-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hou Z, He L, Qi RZ. Regulation of s6 kinase 1 activation by phosphorylation at Ser-411. J Biol Chem. 2007;282:6922–6928. doi: 10.1074/jbc.M607836200. [DOI] [PubMed] [Google Scholar]
- 214.Houser CR, Huang CS, Peng Z. Dynamic seizure-related changes in extracellular signal regulated kinase activation in a mouse model of temporal lobe epilepsy. Neuroscience. 2008;156:222–237. doi: 10.1016/j.neuroscience.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Howlett E, Lin CC, Lavery W, Stern M. A PI3-kinase-mediated negative feedback regulates neuronal excitability. PLoS Genet. 2008;4:e1000277. doi: 10.1371/journal.pgen.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hsieh WB, Chiu NC, Hu KC, Ho CS, Huang FY. Outcome of herpes simplex encephalitis in children. J Microbiol Immunol Infect. 2007;40:34–38. [PubMed] [Google Scholar]
- 217.Hu B, Liu C, Bramlett H, Sick TJ, Alonso OF, Chen S, Dietrich WD. Changes in trkB-ERK1/2-CREB/Elk-1 pathways in hippocampal mossy fiber organization after traumatic brain injury. J Cereb Blood Flow Metab. 2004;24:934–943. doi: 10.1097/01.WCB.0000125888.56462.A1. [DOI] [PubMed] [Google Scholar]
- 218.Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, Houghton PJ. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 219.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang J, Wu S, Wu C-L, Manning BD. Signaling events downstream of mammalian target of rapamycin compelx 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Res. 2009a;69:6107–6114. doi: 10.1158/0008-5472.CAN-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huang SC, Chang CL, Wang PS, Tsai Y, Liu HS. Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J Med Virol. 2009;81:1241–1252. doi: 10.1002/jmv.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huffman TA, Mothe-Satney I, Lawrence JC., Jr Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Natl Acad Sci USA. 2002;99:1047–1052. doi: 10.1073/pnas.022634399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hui L, Rodrik V, Pielak RM, Knirr S, Zheng Y, Foster DA. mTOR-dependent suppression of protein phosphatase 2A is critical for phospholipase D survival signals in human breast cancer cells. J Biol Chem. 2005;280:35829–35835. doi: 10.1074/jbc.M504192200. [DOI] [PubMed] [Google Scholar]
- 225.Humphrey WM, Dong H, Csernansky CA, Csernansky JG. Immediate and delayed hippocampal neuronal loss induced by kainic acid during early postnatal development in the rat. Brain Res Dev Brain Res. 2002;137:1–12. doi: 10.1016/s0165-3806(02)00344-9. [DOI] [PubMed] [Google Scholar]
- 226.Ide T, Shimano H, Yahagi N, Matsuzaka T, Nakakuki M, Yamamoto T, Nakagawa Y, Takahashi A, Suzuki H, Sone H, Toyoshima H, Fukamizu A, Yamada N. SREBPs suppress IRS-2-mediated insulin signaling in the liver. Nat Cell Biol. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- 227.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and AKT turn motif phosphorylation, maturation and signaling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by AKT and suppresses mTOR signaling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 229.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 231.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennet C, Harada Y, Stankunas K, Wang C-Y, He X, MacDougald OA, You M, Williams BO, Guan K-L. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 232.Inoue H, Ndong M, Suzuki T, Kazami M, Uyama T, Kobayashi K, Tadokoro T, Yamamoto Y. Hamartin-Hsp70 interaction is necessary for AKT-dependent tuberin phosphorylation during heat shock. Biosci Biotechnol Biochem. 2009;73:2488–2493. doi: 10.1271/bbb.90489. [DOI] [PubMed] [Google Scholar]
- 233.Jacinto E, Hall MN. TOR signaling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- 234.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 235.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains RICTOR-mTOR complex integrity and regulates AKT phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 236.James MJ, Zomerdijk JC. Phosphatidylinositol 3-kinase and mTOR signaling pathways regulate RNA polymerase I transcription in response to IGF-1 and nutrients. J Biol Chem. 2004;279:8911–8918. doi: 10.1074/jbc.M307735200. [DOI] [PubMed] [Google Scholar]
- 237.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, Gusella JF, Ramesh V. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, Vu C, Lilly MB, Mallya S, Ong ST, Konopleva M, Martin MB, Ren P, Liu Y, Rommel C, Fruman DA. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jansen FE, Braams O, Vincken KL, Algra A, Anbeek P, Jennekens-Schinkel A, Halley D, Zonnenberg BA, van den Ouweland A, van Huffelen AC, van Nieuwenhuizen O, Nellist M. Overlapping neurologic and cognitive phenotypes in patients with TSC1 or TSC2 mutations. Neurology. 2008;70:908–915. doi: 10.1212/01.wnl.0000280578.99900.96. [DOI] [PubMed] [Google Scholar]
- 240.Jeon BT, Lee DH, Kim KH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Ketogenic diet attenuates kainic acid induced hippocampal cell death by decreasing AMPK/ACC pathway activity and HSP70. Neurosci Lett. 2009;453:49–53. doi: 10.1016/j.neulet.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 241.Jiang X, Yeung RS. Regulation of microtubule-dependent protein transport by the TSC2/mammalian target of rapamycin pathway. Cancer Res. 2006;66:5258–5269. doi: 10.1158/0008-5472.CAN-05-4510. [DOI] [PubMed] [Google Scholar]
- 242.Jin HO, An S, Lee HC, Woo SH, Seo SK, Choe TB, Yoo DH, Lee SB, Um HD, Lee SJ, Park MJ, Kim JI, Hong SI, Rhee CH, Park IC. Hypoxic condition- and high cell density-induced expression of Redd1 is regulated by activation of hypoxia-inducible factor-1alpha and Sp1 through the phosphatidylinositol 3-kinase/AKT signaling pathway. Cell Signal. 2007;19:1393–1403. doi: 10.1016/j.cellsig.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 243.Johnson MD, O'Connell M, Vito F, Bakos RS. Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J Neurooncol. 2009;92:129–136. doi: 10.1007/s11060-008-9746-7. [DOI] [PubMed] [Google Scholar]
- 244.Johnson MD, O'Connell MJ, Pilcher W, Reeder JE. Fibroblast growth factor receptor-3 expression in meningiomas with stimulation of proliferation by the phosphoinositide 3 kinase-Akt pathway. J Neurosurg. 2010;112:934–939. doi: 10.3171/2009.7.JNS09726. [DOI] [PubMed] [Google Scholar]
- 245.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 246.Jørgensen SB, Viollet B, Andreelli F, Frøsig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5'-AMP-activatedprotein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 247.Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and AKT to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27:7113–7124. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jurado S, Benoist M, Lario A, Knafo S, Petrok CN, Esteban JA. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 2010;29:2827–2840. doi: 10.1038/emboj.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kam R, Chen J, Blümcke I, Normann S, Fassunke J, Elger CE, Schramm J, Wiestler OD, Becker AJ. The reelin pathway components disabled-1 and p35 in gangliogliomas--a mutation and expression analysis. Neuropathol Appl Neurobiol. 2004;30:225–232. doi: 10.1046/j.0305-1846.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 251.Kaminski R, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kang SK, Cha SH, Jeon HG. Curcumin-induced hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–174. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- 253.Karni R, Hippo Y, Lowe SW, Krainer AR. The splicing-factor oncoprotein SF2/ASF activates mTORC1. Proc Natl Acad Sci USA. 2008;105:15323–15327. doi: 10.1073/pnas.0801376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Katiyar S, Liu E, Knutzen CA, Lang ES, Lombardo CR, Sankar S, Toth JI, Petroski MD, Ronai Z, Chiang GG. REDD1, an inhibitor of mTOR signaling, is regulated by the CUL4A-DDB1 ubiquitin ligase. EMBO Rep. 2009;10:866–872. doi: 10.1038/embor.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediat Neurol. 2006;35:1–5. doi: 10.1016/j.pediatrneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 256.Keith CT, Schreiber SL. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science. 1995;270:50–51. doi: 10.1126/science.270.5233.50. [DOI] [PubMed] [Google Scholar]
- 257.Kellinghaus C, Engbring C, Kovac S, Möddel G, Boesebeck F, Fischera M, Anneken K, Klönne K, Reichelt D, Evers S, Husstedt IW. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure. 2008;17:27–33. doi: 10.1016/j.seizure.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 258.Kelly MT, Crary JF, Sacktor TC. Regulation of protein kinase Mzeta synthesis by multiple kinases in long-term potentiation. J Neurosci. 2007;27:3439–3444. doi: 10.1523/JNEUROSCI.5612-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kikani CK, Dong LQ, Liu F. New-clear functions of PDK1: Beyond a master kinase in the cytosol? J Cell Biochem. 2005;96:1157–1162. doi: 10.1002/jcb.20651. [DOI] [PubMed] [Google Scholar]
- 260.Kim YS, Hong KS, Seong YS, Park JB, Kuroda S, Kishi K, Kaibuchi K, Takai Y. Phosphorylation and activation of mitogen activated protein kinase by kainic acid-induced seizure in rat Hippocampus. Biochem Biophys Res Comm. 1994;202:1163–1168. doi: 10.1006/bbrc.1994.2050. [DOI] [PubMed] [Google Scholar]
- 261.Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- 262.Kim JE, Chen J. Cytoplasmic-nuclear shuttling of FKBP12-rapamycin associated protein is involved in rapamycin sensitive signaling and translation initiation. Proc Natl Acad Sci USA. 2000;97:14340–14345. doi: 10.1073/pnas.011511898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kim DH, Sabassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage HH, Tempst P, Sabatini DM. mTOR interacts with RAPTOR to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 264.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient sensitive interaction between RAPTOR and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 265.Kim SY, Min DS, Choi JS, Choi YS, Park HJ, Sung KW, Kim J, Lee MY. Differential expression of phospholipase D isozymes in the hippocampus following kainic acid-induced seizures. J Neuropathol Exp Neurol. 2004;63:812–820. doi: 10.1093/jnen/63.8.812. [DOI] [PubMed] [Google Scholar]
- 266.Kim YK, Suarez J, Hu Y, McDonough PM, Boer C, Dix DJ, Dillmann WH. Deletion of the inducible 70-kDa heat shock protein genes in mice impairs cardiac contractile function and calcium handling associated with hypertrophy. Circulation. 2006;113:2589–2597. doi: 10.1161/CIRCULATIONAHA.105.598409. [DOI] [PubMed] [Google Scholar]
- 267.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008a;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult Hippocampus. J Biol Chem. 2008b;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kim JH, Ong WY. Localization of the transcription factor, sterol regulatory element binding protein-2 (SREBP-2) in the normal rat brain and changes after kainate-induced excitotoxic injury. J Chem Neuroanat. 2009;37:71–77. doi: 10.1016/j.jchemneu.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 270.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kim HW, Ha SH, Lee MN, Huston E, Kim DH, Jang SK, Suh PG, Houslay MD, Ryu SH. Cyclic AMP controls mTOR through regulation of the dynamic interaction between Rheb and Phosphodiesterase 4D. Mol Cell Biol. 2010;30:5406–5420. doi: 10.1128/MCB.00217-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kimball SR, Do AN, Kutzler L, Cavener DR, Jefferson LS. Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J Biol Chem. 2008;283:3465–3475. doi: 10.1074/jbc.M706643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J Nutr. 2001;131:1844–1849. doi: 10.1093/jn/131.6.1844. [DOI] [PubMed] [Google Scholar]
- 274.Kirschstein T, Bauer M, Müller L, Rüschenschmidt C, Reitze M, Becker AJ, Schoch S, Beck H. Loss of metabotropic glutamate receptor-dependent long-term depression via downregulation of mGluR5 after SE. J Neurosci. 2007;27:7696–7704. doi: 10.1523/JNEUROSCI.4572-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Klein J. Functions and pathophysiological roles of phospholipase D in the brain. J Neurochem. 2005;94:1473–1487. doi: 10.1111/j.1471-4159.2005.03315.x. [DOI] [PubMed] [Google Scholar]
- 276.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Knobbe CB, Reifenberger G. Genetic alterations andaberrant expression of genes related to the phosphatidylinositol-30-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13:507–518. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- 279.Kobayashi T, Minowa O, Sugitani Y, Takai S, Mitani H, Kobayashi E, Noda T, Hino O. A germ-line Tsc1 mutation causes tumor development and embryonic lethality that are similar, but not identical to, those caused by Tsc2 mutation in mice. Proc Natl Acad Sci USA. 2001;98:8762–8770. doi: 10.1073/pnas.151033798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kobow K, Jeske I, Hildebrandt M, Hauke J, Hahnen E, Buslei R, Buchfelder M, Weigel D, Stefan H, Kasper B, Pauli E, Blümcke I. Increased reelin promoter methylation is associated with granule cell dispersion in human temporal lobe epilepsy. J Neuropathol Exp Neurol. 2009;68:356–364. doi: 10.1097/NEN.0b013e31819ba737. [DOI] [PubMed] [Google Scholar]
- 281.Kong D, Yamori T. Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy. Cancer Sci. 2008;99:1734–1740. doi: 10.1111/j.1349-7006.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Konietzko U, Kauselmann G, Scafidi J, Staubli U, Mikkers H, Berns A, Schweizer M, Waltereit R, Kuhl D. Pim kinase expression is induced by LTP stimulation and required for the consolidation of enduring LTP. EMBO J. 1999;18:3359–3369. doi: 10.1093/emboj/18.12.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kozan R, Ayyildiz M, Agar E. The effects of intracerebroventricular AM-251, a CB1-receptor antagonist, and ACEA, a CB1-receptor agonist, on penicillin-induced epileptiform activity in rats. Epilepsia. 2009;50:1760–1767. doi: 10.1111/j.1528-1167.2009.02098.x. [DOI] [PubMed] [Google Scholar]
- 284.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 285.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle. 2009;8:403–413. doi: 10.4161/cc.8.3.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol. 2004;78:11030–11039. doi: 10.1128/JVI.78.20.11030-11039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of RAPTOR- and RICTOR-containing complexes. Proc Natl Acad Sci USA. 2006;103:14182–14187. doi: 10.1073/pnas.0605825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kudchodkar SB, Del Prete GQ, Maguire TG, Alwine JC. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J Virol. 2007;81:3649–3651. doi: 10.1128/JVI.02079-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kumar V, Pandey P, Sabatini D, Kumar M, Majumder PK, Bharti A, Carmichael G, Kufe D, Kharbanda S. Functional interaction between RAFT1/FRAP/mTOR and protein kinase Cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19:1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kwon HJ, Rhim JH, Jang IS, Kim GE, Park SC, Yeo EJ. Activation of AMP-activated protein kinase stimulates the nuclear localization of glyceraldehyde 3-phosphate dehydrogenase in human diploid fibroblasts. Exp Mol Med. 2010;42:254–269. doi: 10.3858/emm.2010.42.4.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lai JP, Bao S, Davis IC, Knoell DL. Inhibition of the phosphatase PTEN protects mice against oleic acid-induced acute lung injury. Br J Pharmacol. 2009;156:189–200. doi: 10.1111/j.1476-5381.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Laina I, Syriopoulou VP, Daikos GL, Roma ES, Papageorgiou F, Kakourou T, Theodoridou M. Febrile seizures and primary human herpesvirus 6 infection. Pediatr Neurol. 2010;42:28–31. doi: 10.1016/j.pediatrneurol.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 294.Laird PW, van der Lugt NM, Clarke A, Domen J, Linders K, McWhir J, Berns A, Hooper M. In vivo analysis of Pim-1 deficiency. Nucleic Acids Res. 1993;21:4750–4755. doi: 10.1093/nar/21.20.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lang F, Bähmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 296.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Law BK. Rapamycin: An anti-cancer immunosuppressant? Crit Rev Oncol Hematol. 2005;56:47–60. doi: 10.1016/j.critrevonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 298.Lawlor MA, Alessi DR. PKB/AKT: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 299.Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L, Prescott AR, Lucocq JM, Alessi DR. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci. 2009;29:1457–1468. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wie Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKKβ suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 302.Lee JY, Jeon BT, Shin HJ, Lee DH, Han JY, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Temporal expression of AMP-activated protein kinase activation during the kainic acid-induced hippocampal cell death. J Neural Transm. 2009a;116:33–40. doi: 10.1007/s00702-008-0158-9. [DOI] [PubMed] [Google Scholar]
- 303.Lee MN, Ha SH, Kim J, Koh A, Lee CS, Kim JH, Jeon H, Kim DH, Suh PG, Ryu SH. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol Cell Biol. 2009b;29:3991–4001. doi: 10.1128/MCB.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lee N, Woodrum CL, Nobil AM, Rauktys AE, Messina MP, Dabora SL. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 2009c;9:8. doi: 10.1186/1471-2210-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lehman N, Ledford B, Di Fulvio M, Frondorf K, McPhail LC, Gomez-Cambronero J. Phospholipase D2-derived phosphatidic acid binds to and activates ribosomal p70 S6 kinase independently of mTOR. FASEB J. 2007;21:1075–1087. doi: 10.1096/fj.06-6652com. [DOI] [PubMed] [Google Scholar]
- 307.Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- 308.Lerner-Natoli M, Montpied P, Rousset MC, Bockaert J, Rondouin G. Sequential expression of surface antigens and transcription factor NFkappaB by hippocampal cells in excitotoxicity and experimental epilepsy. Epilepsy Res. 2000;41:141–154. doi: 10.1016/s0920-1211(00)00132-7. [DOI] [PubMed] [Google Scholar]
- 309.Leseux L, Laurent G, Laurent C, Rigo M, Blanc A, Olive D, Bezombes C. PKC zeta mTOR pathway: a new target for rituximab therapy in follicular lymphoma. Blood. 2008;111:285–291. doi: 10.1182/blood-2007-04-085092. [DOI] [PubMed] [Google Scholar]
- 310.Leverve XM, Guigas B, Detaille D, Batandier C, Koceir EA, Chauvin C, Fontaine E, Wiernsperger NF. Mitochondrial metabolism and type-2 diabetes: a specific target of metformin. Diabetes Metab. 2003;29:6S88–6S94. doi: 10.1016/s1262-3636(03)72792-x. [DOI] [PubMed] [Google Scholar]
- 311.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Li Y, Inoki K, Vacratsis P, Guan KL. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J Biol Chem. 2003;278:13663–13671. doi: 10.1074/jbc.M300862200. [DOI] [PubMed] [Google Scholar]
- 314.Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- 315.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, You M, Guan KL. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem. 2007;282:35803–35813. doi: 10.1074/jbc.M705231200. [DOI] [PubMed] [Google Scholar]
- 316.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 317.Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J Biol Chem. 2007;282:3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- 318.Lim HK, Choi YA, Park W, Lee T, Ryu SH, Kim SY, Kim JR, Kim JH, Baek SH. Phosphatidic acid regulates systemic inflammatory responses by modulating the AKT-mammalian target of rapamycin-p70 S6 kinase 1 pathway. J Biol Chem. 2003;278:45117–45127. doi: 10.1074/jbc.M303789200. [DOI] [PubMed] [Google Scholar]
- 319.Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Liu X, Zheng XF. Endoplasmic recticulum and golgi localization sequences for mammalian target of rapamycin. Mol Biol Cell. 2007;18:1073–1082. doi: 10.1091/mbc.E06-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Liu CL, Chen S, Dietrich D, Hu BR. Changes in autophagy after traumatic brain injury. J Cereb Blood Flow Metab. 2008a;28:674–683. doi: 10.1038/sj.jcbfm.9600587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Liu JX, Tang YC, Liu Y, Tang FR. mGluR5-PLCbeta4-PKCbeta2/PKCgamma pathways in hippocampal CA1 pyramidal neurons in pilocarpine model of SE in mGluR5+/+ mice. Epilepsy Res. 2008b;82:111–123. doi: 10.1016/j.eplepsyres.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 323.Liu GH, Gerace L. Sumoylation regulates nuclear localization of lipin-1alpha in neuronal cells. PLoS One. 2009;4:e7031. doi: 10.1371/journal.pone.0007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D'Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lohi H, Ianzano L, Zhao XC, Chan EM, Turnbull J, Scherer SW, Ackerley CA, Minassian BA. Novel glycogen synthase kinase 3 and ubiquitination pathways in progressive myoclonus epilepsy. Hum Mol Genet. 2005;14:2727–2736. doi: 10.1093/hmg/ddi306. [DOI] [PubMed] [Google Scholar]
- 328.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 329.López-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29:4235–4249. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50(Suppl 2):4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 331.Lucarini N, Verrotti A, Napolioni V, Bosco G, Curatolo P. Genetic polymorphisms and idiopathic generalized epilepsies. Pediatr Neurol. 2007;37:157–164. doi: 10.1016/j.pediatrneurol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 332.Ludanyi A, Eross L, Czirjak S, Vajda J, Jalasz P, Watanabe M, Palkovits M, Magloczky Z, Freund TF, Katona I. Down-regulation of the cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human Hippocampus. J Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lugo JN, Barnwell LF, Ren Y, Lee WL, Johnston LD, Kim R, Hrachovy RA, Sweatt JD, Anderson AE. Altered phosphorylation and localization of the A-type channel, Kv4.2 in SE. J Neurochem. 2008;106:1929–1940. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lutterbach B, Hann SR. Hierarchical phosphorylation at N-terminal transformation-sensitive sites in c-Myc protein is regulated by mitogens and in mitosis. Mol Cell Biol. 1994;14:5510–5522. doi: 10.1128/mcb.14.8.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by ERK: implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 336.Ma XM, Yoon SO, Richardson CJ, Jülich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008a;133:303–313. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 337.Ma YL, Weston SE, Whalley BJ, Stephens GJ. The phytocannabinoid Delta(9)-tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br J Pharmacol. 2008b;154:204–215. doi: 10.1038/bjp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 339.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 340.Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J. 2004;381:329–342. doi: 10.1042/BJ20031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chéne P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, García-Echeverría C. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 342.Mak BC, Kenerson HL, Aicher LD, Barnes EA, Yeung RS. Aberrant beta-catenin signaling in tuberous sclerosis. Am J Pathol. 2005;167:107–116. doi: 10.1016/s0002-9440(10)62958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 344.Mandell JW, VandenBerg SR. ERK/MAP kinase is chronically activated in human reactive astrocytes. Neuroreport. 1999;10:3567–3572. doi: 10.1097/00001756-199911260-00019. [DOI] [PubMed] [Google Scholar]
- 345.Mandell JW, Gocan NC, Vandenberg SR. Mechanical trauma induces rapid astroglial activation of ERK/MAP kinase: Evidence for a paracrine signal. Glia. 2001;34:283–295. doi: 10.1002/glia.1062. [DOI] [PubMed] [Google Scholar]
- 346.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, Neckers L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2006;2:169–174. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- 350.Marignani PA, Kanai F, Carpenter CL. LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest. J Biol Chem. 2001;276:32415–32418. doi: 10.1074/jbc.C100207200. [DOI] [PubMed] [Google Scholar]
- 351.Markova B, Albers C, Breitenbuecher F, Melo JV, Brümmendorf TH, Heidel F, Lipka D, Duyster J, Huber C, Fischer T. Novel pathway in Bcr-Abl signal transduction involves AKT-independent, PLC-gamma1-driven activation of mTOR/p70S6-kinase pathway. Oncogene. 2010;29:739–751. doi: 10.1038/onc.2009.374. [DOI] [PubMed] [Google Scholar]
- 352.Marmy-Conus N, Hannan KM, Pearson RB. Ro 31-6045, the inactive analogue of the protein kinase C inhibitor Ro 31-8220, blocks in vivo activation of p70(s6k)/p85(s6k): implications for the analysis of S6K signalling. FEBS Lett. 2002;519:135–140. doi: 10.1016/s0014-5793(02)02738-2. [DOI] [PubMed] [Google Scholar]
- 353.Martin J, Masri J, Bernath A, Nishimura RN, Gera J. Hsp70 associates with RICTOR and is required for mTORC2 formation and activity. Biochem Biophys Res Commun. 2008;372:578–583. doi: 10.1016/j.bbrc.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 355.Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25:6384–6391. doi: 10.1038/sj.onc.1209883. [DOI] [PubMed] [Google Scholar]
- 356.McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T. The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev. 1997;11:1253–1265. doi: 10.1101/gad.11.10.1253. [DOI] [PubMed] [Google Scholar]
- 357.McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, Jacks T. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12:1121–1133. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res. 2001;63:109–115. doi: 10.1002/1097-4547(20010115)63:2<109::AID-JNR1002>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 359.Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and AKT signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Meller R, Schindler CK, Chu XP, Xiong ZG, Cameron JA, Simon RP, Henshall DC. Seizure-like activity leads to the release of BAD from 14-3-3 protein and cell death in hippocampal neurons in vitro. Cell Death Differ. 2003;10:539–547. doi: 10.1038/sj.cdd.4401206. [DOI] [PubMed] [Google Scholar]
- 362.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 363.Meske V, Albert F, Ohm TG. Coupling of mammalian target of rapamycin with phosphoinositide 3-kinase signaling pathway regulates protein phosphatase 2A- and glycogen synthase kinase-3 -dependent phosphorylation of Tau. J Biol Chem. 2008;283:100–109. doi: 10.1074/jbc.M704292200. [DOI] [PubMed] [Google Scholar]
- 364.Meyer RP, Hagemeyer CE, Knoth R, Kaufmann MR, Volk B. Anti-epileptic drug phenytoin enhances androgen metabolism and androgen receptor expression in murine Hippocampus. J Neurochem. 2006;96:460–472. doi: 10.1111/j.1471-4159.2005.03555.x. [DOI] [PubMed] [Google Scholar]
- 365.Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 366.Michlewski G, Sanford JR, Cáceres JF. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell. 2008;30:179–189. doi: 10.1016/j.molcel.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 367.Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008;49(Suppl 6):13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 368.Mittal S, Dubey D, Yamakawa K, Ganesh S. Lafora disease proteins malin and laforin are recruited to aggresomes in response to proteasomal impairment. Hum Mol Genet. 2007;16:753–762. doi: 10.1093/hmg/ddm006. [DOI] [PubMed] [Google Scholar]
- 369.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 370.Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsh W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wolfel B, Dodt HU, Zieglgansberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the Hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Moorman NJ, Cristea IM, Terhune SS, Rout MP, Chait BT, Shenk T. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe. 2008;3:253–262. doi: 10.1016/j.chom.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H, Herrlich P. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Moschella PC, Rao VU, McDermott PJ, Kuppuswamy D. Regulation of mTOR and S6K1 activation by the nPKC isoforms, PKCepsilon and PKCdelta, in adult cardiac muscle cells. J Mol Cell Cardiol. 2007;43:754–766. doi: 10.1016/j.yjmcc.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Mothe-Satney I, Brunn GJ, McMahon LP, Capaldo CT, Abraham RT, Lawrence JC., Jr Mammalian target of rapamycin-dependent phosphorylation of PHAS-I in four (S/T)P sites detected by phospho-specific antibodies. J Biol Chem. 2000;275:33836–33843. doi: 10.1074/jbc.M006005200. [DOI] [PubMed] [Google Scholar]
- 375.Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport. 1999;10:1517–1522. doi: 10.1097/00001756-199905140-00023. [DOI] [PubMed] [Google Scholar]
- 376.Muncy J, Butler IJ, Koenig MK. Rapamycin reduces seizure frequency in tuberous sclerosis complex. J Child Neurol. 2009;24:477. doi: 10.1177/0883073808324535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Peggie M, Bain J, Bloomberg GB, Grahammer F, Lang F, Wulff P, Kuhl D, Cohen P. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Naïmi M, Gautier N, Chaussade C, Valverde AM, Accili D, Van Obberghen E. Nuclear forkhead box O1 controls and integrates key signaling pathways in hepatocytes. Endocrinology. 2007;148:2424–2434. doi: 10.1210/en.2006-1411. [DOI] [PubMed] [Google Scholar]
- 380.Nakae J, Oki M, Cao Y. The FOXO transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 381.Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and ediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Nardacci R, Antinori A, Larocca LM, Arena V, Amendola A, Perfettini JL, Kroemer G, Piacentini M. Characterization of cell death pathways in human immunodeficiency virus-associated encephalitis. Am J Pathol. 2005;167:695–704. doi: 10.1016/S0002-9440(10)62044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Nasir O, Wang K, Föller M, Gu S, Bhandaru M, Ackermann TF, Boini KM, Mack A, Klingel K, Amato R, Perrotti N, Kuhl D, Behrens J, Stournaras C, Lang F. Relative resistance of SGK1 knockout mice against chemical carcinogenesis. IUBMB Life. 2009;61:768–776. doi: 10.1002/iub.209. [DOI] [PubMed] [Google Scholar]
- 385.Nateri AS, Raivich G, Gebhardt C, Da Costa C, Naumann H, Vreugdenhil M, Makwana M, Brandner S, Adams RH, Jefferys JG, Kann O, Behrens A. ERK activation causes epilepsy by stimulating NMDA receptor activity. EMBO J. 2007;26:4891–4901. doi: 10.1038/sj.emboj.7601911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Nguyen TV, Yao M, Pike CJ. Androgens activates mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 387.Niquet J, Auvin S, Archie M, Seo DW, Allen S, Sankar R, Wasterlain CG. SE triggers caspase-3 activation and necrosis in the immature rat brain. Epilepsia. 2007;48:1203–1206. doi: 10.1111/j.1528-1167.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 388.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/RAPTOR signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Ogawa W, Matozaki T, Kasuga M. Role of binding proteins to IRS-1 in insulin signalling. Mol Cell Biochem. 1998;182:13–22. [PubMed] [Google Scholar]
- 390.Ohori M, Kinoshita T, Okubo M, Sato K, Yamazaki A, Arakawa H, Nishimura S, Inamura N, Nakajima H, Neya M, Miyake H, Fujii T. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem Biophys Res Commun. 2005;336:357–363. doi: 10.1016/j.bbrc.2005.08.082. [DOI] [PubMed] [Google Scholar]
- 391.Okada M, Wang Y, Jang SW, Tang X, Neri LM, Ye K. AKT phosphorylation of merlin enhances its binding to phosphatidylinositols and inhibits the tumor-suppressive activities of merlin. Cancer Res. 2009;69:4043–4051. doi: 10.1158/0008-5472.CAN-08-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(-/-) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.O'Shea CC, Choi S, McCormick F, Stokoe D. Adenovirus overrides cellular checkpoints for protein translation. Cell Cycle. 2005;4:883–888. doi: 10.4161/cc.4.7.1791. [DOI] [PubMed] [Google Scholar]
- 395.Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K. The proline-rich AKT substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Page G, Khidir FA, Pain S, Barrier L, Fauconneau B, Guillard O, Piriou A, Hugon J. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem Int. 2006;49:413–421. doi: 10.1016/j.neuint.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 397.Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y. AKT phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res. 2005;65:11282–11286. doi: 10.1158/0008-5472.CAN-05-3469. [DOI] [PubMed] [Google Scholar]
- 398.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Papst PJ, Sugiyama H, Nagasawa M, Lucas JJ, Maller JL, Terada N. Cdc2-cyclin B phosphorylates p70 S6 kinase on Ser411 at mitosis. J Biol Chem. 1998;273:15077–15084. doi: 10.1074/jbc.273.24.15077. [DOI] [PubMed] [Google Scholar]
- 400.Parekh D, Ziegler W, Yonezawa K, Hara K, Parker PJ. Mammalian TOR controls one of two kinase pathways acting upon nPKCdelta and nPKCepsilon. J Biol Chem. 1999;274:34758–34764. doi: 10.1074/jbc.274.49.34758. [DOI] [PubMed] [Google Scholar]
- 401.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- 403.Park IH, Bachmann R, Shirazi H, Chen J. Regulation of ribosomal S6 kinase 2 by mammalian target of rapamycin. J Biol Chem. 2002;277:31423–31429. doi: 10.1074/jbc.M204080200. [DOI] [PubMed] [Google Scholar]
- 404.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Pearce LR, Huang X, Boudeau J, Pawłowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel RICTOR-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Pei JJ, Hugon J. mTOR-dependent signaling in Alzheimer's disease. J Cell Mol Med. 2008;12(6B):2525–2532. doi: 10.1111/j.1582-4934.2008.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153:5428–5437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Pelish HE, Ciesla W, Tanaka N, Reddy K, Shair MD, Kirchhausen T, Lencer WI. The Cdc42 inhibitor secramine B prevents cAMP-induced K+ conductance in intestinal epithelial cells. Biochem Pharmacol. 2006;71:1720–1726. doi: 10.1016/j.bcp.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Pelletier A, Joly E, Prentki M, Coderre L. Adenosine 5'-monophosphate-activated protein kinase and p38 mitogen-activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology. 2005;146:2285–2294. doi: 10.1210/en.2004-1565. [DOI] [PubMed] [Google Scholar]
- 410.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking AKT1 and AKT2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Peng C, Knebel A, Morrice NA, Li X, Barringer K, Li J, Jakes S, Werneburg B, Wang L. Pim kinase substrate identification and specificity. J Biochem. 2007;141:353–362. doi: 10.1093/jb/mvm040. [DOI] [PubMed] [Google Scholar]
- 413.Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–7423. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]
- 414.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Plas DR, Thomas G. Tubers and tumors: rapamycin therapy for benign and malignant tumors. Curr Opin Cell Biol. 2009;21:230–236. doi: 10.1016/j.ceb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 416.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 417.Poirier R, Jacquot S, Vaillend C, Soutthiphong AA, Libbey M, Davis S, Laroche S, Hanauer A, Welzl H, Lipp HP, Wolfer DP. Deletion of the Coffin-Lowry syndrome gene Rsk2 in mice is associated with impaired spatial learning and reduced control of exploratory behavior. Behav Genet. 2007;37:31–50. doi: 10.1007/s10519-006-9116-1. [DOI] [PubMed] [Google Scholar]
- 418.Popova JS, Rasenick MM. Muscarinic receptor activation promotes the membrane association of tubulin for the regulation of Gq-mediated phospholipase Cbeta(1)signaling. J Neurosci. 2000;20:2774–2782. doi: 10.1523/JNEUROSCI.20-08-02774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to AKT-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Porstmann T, Santos CR, Lewis C, Griffiths B, Schulze A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochem Soc Trans. 2009;37:278–283. doi: 10.1042/BST0370278. [DOI] [PubMed] [Google Scholar]
- 421.Porteous D. Genetic causality in schizophrenia and bipolar disorder: out with the old and in with the new. Curr Opin Genet Dev. 2008;18:229–234. doi: 10.1016/j.gde.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 422.Potschka H, Krupp E, Ebert U, Gümbel C, Leichtlein C, Lorch B, Pickert A, Kramps S, Young K, Grüne U, Keller A, Welschof M, Vogt G, Xiao B, Worley PF, Löscher W, Hiemisch H. Kindling-induced overexpression of Homer 1A and its functional implications for epileptogenesis. Eur J Neurosci. 2002;16:2157–2165. doi: 10.1046/j.1460-9568.2002.02265.x. [DOI] [PubMed] [Google Scholar]
- 423.Potter WB, O'Riordan KJ, Barnett D, Osting SMK, Wagoner M, Burger C, Roopra A. Metabolic Regulation of Neuronal Plasticity by the Energy Sensor AMPK. PLoS One. 2010;5:e8996. doi: 10.1371/journal.pone.0008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Price A, Wickner W, Ungermann C. Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion. J Cell Biol. 2000;148:1223–1229. doi: 10.1083/jcb.148.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr. 2008;138:1887–1894. doi: 10.1093/jn/138.10.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Puffenberger EG, Strauss KA, Ramsey KE, Craig DW, Stephan DA, Robinson DL, Hendrickson CL, Gottlieb S, Ramsay DA, Siu VM, Heuer GG, Crino PB, Morton DH. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 427.Puig B, Ferrer I. Caspase-3-associated apoptotic cell death in excitotoxic necrosis of the entorhinal cortex following intraperitoneal injection of kainic acid in the rat. Neurosci Lett. 2002;321:182–186. doi: 10.1016/s0304-3940(01)02518-6. [DOI] [PubMed] [Google Scholar]
- 428.Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonade R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 430.Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 431.Ravikumar B, Futter M, Jahreiss L, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Narayanan U, Renna M, Jimenez-Sanchez M, Sarkar S, Underwood B, Winslow A, Rubinsztein DC. Mammalian macroautophagy at a glance. J Cell Sci. 2009;122:1707–1711. doi: 10.1242/jcs.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Ravitz MJ, Chen L, Lynch M, Schmidt EV. c-myc Repression of TSC2 contributes to control of translation initiation and Myc-induced transformation. Cancer Res. 2007;67:11209–11217. doi: 10.1158/0008-5472.CAN-06-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev NeuroBiol. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- 434.Reibel S, André V, Chassagnon S, André G, Marescaux C, Nehlig A, Depaulis A. Neuroprotective effects of chronic estradiol benzoate treatment on hippocampal cell loss induced by SE in the female rats. Neurosci Lett. 2000;281:79–82. doi: 10.1016/s0304-3940(00)00784-9. [DOI] [PubMed] [Google Scholar]
- 435.Reich NC. STAT3 Revs Up the Powerhouse. Sci Signal. 2009;2:pe61. doi: 10.1126/scisignal.290pe61. [DOI] [PubMed] [Google Scholar]
- 436.Reid CA, Berkovic SF, Petrou S. Mechanism of human inherited epilepsies. Prog NeuroBiol. 2009;87:41–57. doi: 10.1016/j.pneurobio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 437.Repici M, Mariani J, Borsello T. Neuronal death and neuroprotection: a review. Methods Mol Biol. 2007;399:1–14. doi: 10.1007/978-1-59745-504-6_1. [DOI] [PubMed] [Google Scholar]
- 438.Reue K, Zhang P. The lipin protein family: dual roles in lipid biosynthesis and gene expression. FEBS Lett. 2008;582:90–96. doi: 10.1016/j.febslet.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 440.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Richardson CJ, Bröenstrup M, Fingar DC, Jülich K, Ballif BA, Gygi S, Blenis J. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol. 2004;14:1540–1549. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 442.Rodriguez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, Taketomi A, Prescott SM, Bazan NG. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl-inositol lipid signaling. Proc Natl Acad Sci USA. 2001;98:4740–4745. doi: 10.1073/pnas.081536298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- 444.Rosner M, Hengstschläger M. Tuberin binds p27 and negatively regulates its interaction with the SCF component Skp2. J Biol Chem. 2004;279:48707–48715. doi: 10.1074/jbc.M405528200. [DOI] [PubMed] [Google Scholar]
- 445.Rosner M, Hanneder M, Siegel N, Valli A, Hengstschlager M. The tuberous sclerosis gene product hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutation Res. 2008;658:234–246. doi: 10.1016/j.mrrev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 446.Rotella CM, Monami M, Mannucci E. Metformin beyond diabetes: new life for an old drug. Curr Diabetes Rev. 2006;2:307–315. doi: 10.2174/157339906777950651. [DOI] [PubMed] [Google Scholar]
- 447.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Rüegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res. 2007;77:85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 452.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Investig. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 455.Saitoh M, Pullen N, Brennan P, Cantrell D, Dennis PB, Thomas G. Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J Biol Chem. 2002;277:20104–20112. doi: 10.1074/jbc.M201745200. [DOI] [PubMed] [Google Scholar]
- 456.Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor α and β in reactive astrocytes at the male rat hippocampus after SE. Neuropathology. 2009;29:55–62. doi: 10.1111/j.1440-1789.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- 457.Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci. 2008;28:6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini SM. The Rag GTPases bind RAPTOR and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- 460.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. RICTOR, a novel binding partner of mTOR, defines a rapamycin-insensitive and RAPTOR-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 461.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of AKT/PKB by the RICTOR-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 462.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and AKT/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 463.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 464.Sato A, Sunayama J, Matsuda KI, Tachibana K, Sakurada K, Tomiyama A, Kayama T, Kitanaka C. Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci Lett. 2010;470:115–120. doi: 10.1016/j.neulet.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 465.Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- 466.Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Schicknick H, Schott BH, Budinger E, Smalla KH, Riedel A, Seidenbecher CI, Scheich H, Gundelfinger ED, Tischmeyer W. Dopaminergic modulation of auditory cortex-dependent memory consolidation through mTOR. Cereb Cortex. 2008;18:2646–2658. doi: 10.1093/cercor/bhn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Schindler CK, Heverin M, Henshall DC. Isoform- and subcellular fraction-specific differences in hippocampal 14-3-3 levels following experimentally evoked seizures and in human temporal lobe epilepsy. J Neurochem. 2006;99:561–569. doi: 10.1111/j.1471-4159.2006.04153.x. [DOI] [PubMed] [Google Scholar]
- 469.Schlanger S, Shinitzky M, Yam D. Diet enriched with omega-3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia. 2002;43:103–104. doi: 10.1046/j.1528-1157.2002.13601.x. [DOI] [PubMed] [Google Scholar]
- 470.Schnabel P, Mies F, Nohr T, Geisler M, Böhm M. Differential regulation of phospholipase C-beta isozymes in cardiomyocyte hypertrophy. Biochem Biophys Res Commun. 2000;275:1–6. doi: 10.1006/bbrc.2000.3255. [DOI] [PubMed] [Google Scholar]
- 471.Schmidt RJ, Ficorilli JV, Zhang Y, Bramlett KS, Beyer TP, Borchert K, Dowless MS, Houck KA, Burris TP, Eacho PI, Liang G, Guo LW, Wilson WK, Michael LF, Cao G. A 15-ketosterol is a liver X receptor ligand that suppresses sterol-responsive element binding protein-2 activity. J Lipid Res. 2006;47:1037–1044. doi: 10.1194/jlr.M500526-JLR200. [DOI] [PubMed] [Google Scholar]
- 472.Scoles DR. The merlin interacting proteins reveal multiple targets for NF2 therapy. Biochim Biophys Acta. 2008;1785:32–54. doi: 10.1016/j.bbcan.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 473.Scorza FA, Cysneiros RM, Arida RM, Terra-Bustamante VC, de Albuquerque M, Cavalheiro EA. The other side of the coin: Beneficiary effect of omega-3 fatty acids in sudden unexpected death in epilepsy. Epilepsy Behav. 2008;13:279–283. doi: 10.1016/j.yebeh.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 474.Seifert G, Carmignoto G, Steinhäuser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 475.Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem. 2001;276:7246–7257. doi: 10.1074/jbc.M004389200. [DOI] [PubMed] [Google Scholar]
- 476.Sekulić A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 477.Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn Mem. 2003;10:26–39. doi: 10.1101/lm.51103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 479.Selman C, Tullet JMA, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson ICA, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenaug K, Thornton JM, Partridge L, Gems D, Whithers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Seth A, Gonzalez FA, Gupta S, Raden DL, Davis RJ. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992;267:24796–24804. [PubMed] [Google Scholar]
- 481.Shacka JJ, Lu J, Xie ZL, Uchiyama Y, Roth KA, Zhang J. Kainic acid induces early and transient autophagic stress in mouse Hippocampus. Neurosci Lett. 2007;414:57–60. doi: 10.1016/j.neulet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–2791. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Shan Z, Haaf T, Popescu NC. Identification and characterization of a gene encoding a putative mouse Rho GTPase activating protein gene 8, Arhgap8. Gene. 2003;303:55–61. doi: 10.1016/s0378-1119(02)01143-5. [DOI] [PubMed] [Google Scholar]
- 484.Sharma AK, Searfoss GH, Reams RY, Jordan WH, Snyder PW, Chiang AY, Jolly RA, Ryan TP. Kainic acid-induced F-344 rat model of mesial temporal lobe epilepsy: gene expression and canonical pathways. Toxicol Pathol. 2009;37:776–789. doi: 10.1177/0192623309344202. [DOI] [PubMed] [Google Scholar]
- 485.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Shorvon SD. The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia. 1996;37(Suppl 2):S1–S3. doi: 10.1111/j.1528-1157.1996.tb06027.x. [DOI] [PubMed] [Google Scholar]
- 487.Shaw RJ. mTOR signaling : RAG GTPases transmit the amino acid signal. Trends Biochem Sci. 2008;33:565–568. doi: 10.1016/j.tibs.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signaling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 489.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 490.Shimizu K, Okada M, Takano A, Nagai K. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999;458:363–369. doi: 10.1016/s0014-5793(99)01190-4. [DOI] [PubMed] [Google Scholar]
- 491.Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Shinoda S, Schindler CK, Meller R, So NK, Araki T, Yamamoto A, Lan JQ, Taki W, Simon RP, Henshall DC. Bim regulation may determine hippocampal vulnerability after injurious seizures and in temporal lobe epilepsy. J Clin Invest. 2004;113:1059–1068. doi: 10.1172/JCI19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Shinoda S, Schindler CK, Quan-Lan J, Saugstad JA, Taki W, Simon RP, Henshall DC. Interaction of 14-3-3 with Bid during seizure-induced neuronal death. J Neurochem. 2003;86:460–469. doi: 10.1046/j.1471-4159.2003.01860.x. [DOI] [PubMed] [Google Scholar]
- 494.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the RICTOR gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 495.Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng MJ, Mathews LM, Schneider MD, Hamilton SL, Matzuk MM. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- 496.Shu L, Houghton PJ. The mTORC2 complex regulates terminal differentiation of C2C12 myoblasts. Mol Cell Biol. 2009;29:4691–4700. doi: 10.1128/MCB.00764-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Sinha S, Yang W. Cellular signaling for activation of Rho GTPase Cdc42. Cell Signal. 2008;20:1927–1934. doi: 10.1016/j.cellsig.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 498.Slack BE, Blusztajn JK. Differential regulation of mTOR-dependent S6 phosphorylation by muscarinic acetylcholine receptor subtypes. J Cell Biochem. 2008;104:1818–1831. doi: 10.1002/jcb.21745. [DOI] [PubMed] [Google Scholar]
- 499.Sleeman MW, Wortley KE, Lai KM, Gowen LC, Kintner J, Kline WO, Garcia K, Stitt TN, Yancopoulos GD, Wiegand SJ, Glass DJ. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat Med. 2005;11:199–205. doi: 10.1038/nm1178. [DOI] [PubMed] [Google Scholar]
- 500.Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Smith CC. The herpes simplex virus type 2 protein ICP10PK: a master of versatility. Front Biosci. 2005;10:2820–2831. doi: 10.2741/1738. [DOI] [PubMed] [Google Scholar]
- 502.Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 503.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25:5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Steinacker P, Schwarz P, Reim K, Brechlin P, Jahn O, Kratzin H, Aitken A, Wiltfang J, Aguzzi A, Bahn E, Baxter HC, Brose N, Otto M. Unchanged survival rates of 14-3-3gamma knockout mice after inoculation with pathological prion protein. Mol Cell Biol. 2005;25:1339–1346. doi: 10.1128/MCB.25.4.1339-1346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, Scheffer IE, Berkovic SF. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–203. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 506.Stevens C, Lin Y, Harrison B, Burch L, Ridgway RA, Sansom O, Hupp T. Peptide combinatorial libraries identify TSC2 as a death-associated protein kinase (DAPK)death domain-binding protein and reveal a stimulatory role for DAPK in mTORC1 signaling. J Biol Chem. 2009;284:334–344. doi: 10.1074/jbc.M805165200. [DOI] [PubMed] [Google Scholar]
- 507.Strimpakos AS, Sharma RA. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 508.Su YW, Hao Z, Hirao A, Yamamoto K, Lin WJ, Young A, Duncan GS, Yoshida H, Wakeham A, Lang PA, Murakami K, Ohashi P, Mak TW. 14-3-3σ regulates B-cell homeostasis through stabilization of FOXO1. Proc Nat Acad Sci USA. 2011;108:1555–1560. doi: 10.1073/pnas.1017729108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sucher NJ, Yu E, Chan SF, Miri M, Lee BJ, Xiao B, Worley P, Jensen FE. Association of the small GTPase Rheb with the NMDA receptor subunit NR3A. NeuroSignal. 2010 doi: 10.1159/000322206. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Sun Y, Fang Y, Yoon MS, Zhang C, Roccio M, Zwartkruis FJ, Armstrong M, Brown HA, Chen J. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci U S A. 2008;105:8286–8291. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 512.Suzuki Y, Toribe Y, Mogami Y, Yanagihara K, Nishikawa M. Epilepsy in patients with congenital cytomegalovirus infection. Brain Dev. 2008;30:420–424. doi: 10.1016/j.braindev.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 513.Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 514.Takahashi JA, Mori H, Fukumoto M, Igarashi K, Jaye M, Oda Y, Kikuchi H, Hatanaka M. Gene expression of fibroblast growth factors in human gliomas and meningiomas: demonstration of cellular source of basic fibroblast growth factor mRNA and peptide in tumor tissues. Proc Natl Acad Sci USA. 1990;87:5710–5714. doi: 10.1073/pnas.87.15.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, Brat D, Gutmann DH, Ye K. AKT phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9:1199–1207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]
- 517.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/AKT-dependent and -independent phosphorylation of tuberin. J Biol Chem. 2003;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 518.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jenö P, Arrieumerlou C, Hall MN. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol. 2004;19:680–686. doi: 10.1177/08830738040190090801. [DOI] [PubMed] [Google Scholar]
- 520.Thio LL, Erbayat-Altay E, Rensing N, Yamada KA. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatr Res. 2006;60:413–417. doi: 10.1203/01.pdr.0000238244.54610.27. [DOI] [PubMed] [Google Scholar]
- 521.Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 522.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Tischmeyer W, Schicknick H, Kraus M, Seidenbecher CI, Staak S, Scheich H, Gundelfinger ED. Rapamycin-sensitive signaling in long-term consolidation of auditory cortex-dependent memory. Eur J Neurosci. 2003;18:942–950. doi: 10.1046/j.1460-9568.2003.02820.x. [DOI] [PubMed] [Google Scholar]
- 525.Tokuda S, Mahaffey CL, Monks B, Faulkner CR, Birnbaum MJ, Danzer SD, Frankel WN. A novel Akt3 mutation associated with enhanced kinase activity and seizure susceptibility in mice. Hum Mol Genet. 2011;20:988–999. doi: 10.1093/hmg/ddq544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Tomoda T, Kim JH, Zhan C, Hatten ME. Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev. 2004;18:541–558. doi: 10.1101/gad.1151204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Tremblay F, Brûlé S, Hee-Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 530.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Tzatsos A, Tsichlis PN. Energy depletion inhibits phosphatidylinositol 3-kinase/AKT signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J Biol Chem. 2007;282:18069–18082. doi: 10.1074/jbc.M610101200. [DOI] [PubMed] [Google Scholar]
- 532.Tyan L, Sopjani M, Dermaku-Sopjani M, Schmid E, Yang W, Xuan NT, Shumilina E, Lang F. Inhibition of voltage-gated K channels in dendritic cells by rapamycin. Am J Physiol Cell Physiol. 2010;299:C1379–C1385. doi: 10.1152/ajpcell.00367.2010. [DOI] [PubMed] [Google Scholar]
- 533.Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 534.Vamecq J, Vallée L, Lesage F, Gressens P, Stables JP. Antiepileptic popular ketogenic diet: emerging twists in an ancient story. Prog NeuroBiol. 2005;75:1–28. doi: 10.1016/j.pneurobio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 535.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 536.van der Vos KE, Coffer PJ. FOXO-binding partners: It takes two to tango. Oncogene. 2008;27:2289–2299. doi: 10.1038/onc.2008.22. [DOI] [PubMed] [Google Scholar]
- 537.van Diepen MT, Parsons M, Downes CP, Leslie NR, Hindges R, Eickholt BJ. MyosinV controls PTEN function and neuronal cell size. Nat Cell Biol. 2009;11:1191–1196. doi: 10.1038/ncb1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.van Lookeren Campagne M, Lucassen PJ, Vermeulen JP, Balázs R. NMDA and kainate induce internucleosomal DNA cleavage associated with both apoptotic and necrotic cell death in the neonatal rat brain. Eur J Neurosci. 1995;7:1627–1640. doi: 10.1111/j.1460-9568.1995.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 539.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 540.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signaling to mTOR mediated by the AKT/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 541.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signaling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 542.Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 544.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 545.Vickers CA, Dickson KS, Wyllie DJ. Induction and maintenance of late-phase long-term potentiation in isolated dendrites of rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;568:803–813. doi: 10.1113/jphysiol.2005.092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 547.Vinciguerra M, Foti M. PTEN and SHIP2 phosphoinositide phosphatases as negative regulators of insulin signalling. Arch Physiol Biochem. 2006;112:89–104. doi: 10.1080/13813450600711359. [DOI] [PubMed] [Google Scholar]
- 548.Viollet B, Andreelli F, Jørgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.von der Brelie C, Waltereit R, Zhang L, Beck H, Kirschstein T. Impaired synaptic plasticity in a rat model of tuberous sclerosis. Eur J Neurosci. 2006;23:686–692. doi: 10.1111/j.1460-9568.2006.04594.x. [DOI] [PubMed] [Google Scholar]
- 550.Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- 551.Walsh D, Mohr I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004;18:660–672. doi: 10.1101/gad.1185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Waltereit R, Welzl H, Dichgans J, Lipp HP, Schmidt WJ, Weller M. Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem. 2006;96:407–413. doi: 10.1111/j.1471-4159.2005.03538.x. [DOI] [PubMed] [Google Scholar]
- 553.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90RSK1 and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Wang P, Yang X, Wu P, Zhang J, Sato T, Yamagata S, Yamagata T. GM3 signals regulating TNF-alpha expression are mediated by RICTOR and Arhgdib in mouse melanoma B16 cells. Oncology. 2007;73:430–438. doi: 10.1159/000136801. [DOI] [PubMed] [Google Scholar]
- 555.Wang L, Harris TE, Lawrence JC., Jr Regulation of prolinerich AKT substrate of 40 kDa (PRAS40) function bymammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Wärntges S, Friedrich B, Henke G, Duranton C, Lang PA, Waldegger S, Meyermann R, Kuhl D, Speckmann EJ, Obermüller N, Witzgall R, Mack AF, Wagner HJ, Wagner A, Bröer S, Lang F. Cerebral localization and regulation of the cell volume-sensitive serum- and glucocorticoid-dependent kinase SGK1. Pflugers Arch. 2002;443:617–624. doi: 10.1007/s00424-001-0737-1. [DOI] [PubMed] [Google Scholar]
- 557.Waters JE, Astle MV, Ooms LM, Balamatsias D, Gurung R, Mitchell CA. P-REX1 - a multidomain protein that regulates neurite differentiation. J Cell Sci. 2008;121:2892–2903. doi: 10.1242/jcs.030353. [DOI] [PubMed] [Google Scholar]
- 558.Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Hörl WH, Hengstschläger M, Müller M, Säemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 560.Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, Jones GE, Hawkins PT, Stephens LR. P-REX1 regulates neutrophil function. Curr Biol. 2005;15:1867–1873. doi: 10.1016/j.cub.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 561.Westermarck J, Hahn WC. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med. 2008;14:152–160. doi: 10.1016/j.molmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 562.WHO. The Atlas: Epilepsy Care in the World. 2005. [Google Scholar]
- 563.Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/AKT by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 2000;275:40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- 564.Wierstra I, Alves J. The c-myc promoter: still mystery and challenge. Adv Cancer Res. 2008;99:113–333. doi: 10.1016/S0065-230X(07)99004-1. [DOI] [PubMed] [Google Scholar]
- 565.Wilson KF, Wu WJ, Cerione RA. Cdc42 stimulates RNA splicing via the S6 kinase and a novel S6 kinase target the nuclear Cap binding complex. J Biol Chem. 2000;275:37307–37310. doi: 10.1074/jbc.C000482200. [DOI] [PubMed] [Google Scholar]
- 566.Woiwode A, Johnson SA, Zhong S, Zhang C, Roeder RG, Teichmann M, Johnson DL. PTEN represses RNA polymerase III-dependent transcription by targeting the TFIIIB complex. Mol Cell Biol. 2008;28:4204–4214. doi: 10.1128/MCB.01912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Wong M, Ess KC, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 568.Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Woo SY, Kim DH, Jun CB, Kim YM, Haar EV, Lee SI, Hegg JW, Bandhakavi S, Griffin TJ, Kim DH. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem. 2007;282:25604–25612. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- 570.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 571.Wu Z, Xu Q, Zhang L, Kong D, Ma R, Wang L. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res. 2009;34:1393–1400. doi: 10.1007/s11064-009-9920-0. [DOI] [PubMed] [Google Scholar]
- 572.Wulff P, Vallon V, Huang DY, Völkl H, Yu F, Richter K, Jansen M, Schlünz M, Klingel K, Loffing J, Kauselmann G, Bösl MR, Lang F, Kuhl D. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest. 2002;110:1263–1268. doi: 10.1172/JCI15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 574.Xia Z, Knaak C, Ma J, Beharry ZM, McInnes C, Wang W, Kraft AS, Smith CD. Synthesis and evaluation of novel inhibitors of Pim-1 and Pim-2 protein kinases. J Med Chem. 2009;52:74–86. doi: 10.1021/jm800937p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Xiao F, Wang XF, Li JM, Xi ZQ, Lu Y, Wang L, Zeng Y, Guan LF, Yuan J. Overexpression of N-WASP in the brain of human epilepsy. Brain Res. 2008;1233:168–175. doi: 10.1016/j.brainres.2008.07.101. [DOI] [PubMed] [Google Scholar]
- 576.Xu X, Yang D, Ding JH, Wang W, Chu PH, Dalton ND, Wang HY, Bermingham JR, Jr, Ye Z, Liu F, Rosenfeld MG, Manley JL, Ross J, Jr, Chen J, Xiao RP, Cheng H, Fu XD. ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell. 2005;120:59–72. doi: 10.1016/j.cell.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 577.Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D, Worley PF. Rheb, a growth factor- and synaptic activity regulated gene, encodes a novel Ras-related protein. J Biol Chem. 1994;269:16333–16339. [PubMed] [Google Scholar]
- 578.Yamamoto A, Schindler CK, Murphy BM, Bellver-Estelles C, So NK, Taki W, Meller R, Simon RP, Henshall DC. Evidence of tumor necrosis factor receptor 1 signaling in human temporal lobe epilepsy. Exp Neurol. 2006;202:410–420. doi: 10.1016/j.expneurol.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 579.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor α serine 167 phosphorylation. FEBS Lett. 2010;584:124–128. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Yang Q, Inoki K, Kim E, Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A. 2006a;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Yang Q, Inoki K, Ikenoue T, Guan K-L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006b;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 583.Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44:584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- 584.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 585.Yoshizawa M, Kawauchi T, Sone M, Nishimura YV, Terao M, Chihama K, Nabeshima Y, Hoshino M. Involvement of a Rac activator, P-REX1, in neurotrophin-derived signaling and neuronal migration. J Neurosci. 2005;25:4406–4419. doi: 10.1523/JNEUROSCI.4955-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Yu Y, Alwine JC. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3-OH kinase pathway and the cellular kinase AKT. J Virol. 2002;76:3731–3738. doi: 10.1128/JVI.76.8.3731-3738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Zeqiraj E, Filippi BM, Goldie S, Navratilova I, Boudeau J, Deak M, Alessi DR, van Aalten DM. ATP and MO25alpha regulate the conformational state of the STRADalpha pseudokinase and activation of the LKB1 tumour suppressor. PLoS Biol. 2009;7:e1000126. doi: 10.1371/journal.pbio.1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 591.Zhang C, Comai L, Johnson DL. PTEN represses RNA Polymerase I transcription by disrupting the SL1 complex. Mol Cell Biol. 2005a;25:6899–6911. doi: 10.1128/MCB.25.16.6899-6911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. Hsp90-AKT phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005b;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- 593.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR dependent feedback inhibition of AKT. Mol Cell. 2006;24:185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27:4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 595.Zhang P, Wang H, Min X, Wang Y, Tang J, Cheng J, Li D, Chen X, Cheng F, Wang N, Yang H. PIM-1 protein kinase regulates PRAS40 phosphorylation and mTOR activity in FDCP1 cells. J Cell Physiol. 2009;220:82–90. [Google Scholar]
- 596.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009a;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Zhou X, Ikenoue T, Chen X, Li L, Inoki K, Guan KL. Rheb controls misfolded protein metabolism by inhibiting aggresome formation and autophagy. Proc Natl Acad Sci U S A. 2009b;106:8923–8928. doi: 10.1073/pnas.0903621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Ziegler WH, Parekh DB, Le Good JA, Whelan RD, Kelly JJ, Frech M, Hemmings BA, Parker PJ. Rapamycin-sensitive phosphorylation of PKC on a carboxy-terminal site by an atypical PKC complex. Curr Biol. 1999;9:522–529. doi: 10.1016/s0960-9822(99)80236-x. [DOI] [PubMed] [Google Scholar]