Abstract
Fusion of human immunodeficiency virus (HIV-1) with target cells is mediated by the gp41 transmembrane envelope protein. The loop region within gp41 contains 2 crucial cysteines that play an unknown role in HIV-cell fusion. On the basis of cell-cell fusion assay, using human T-cell lines [Jurkat E6-1 and Jurkat HXBc2(4)], and virus-cell fusion assay, using fully infectious HIV-1 HXBc2 virus and TZM-bl human cell line, we provide evidence that the oxidation state of the disulfide bond within a loop domain peptide determines its activity. The oxidized (closed) form inhibits fusion, while the reduced (opened) form enhances hemifusion. These opposite activities reach 60% difference in viral fusion. Both forms of the loop domain interact with gp41: the opened form enhances gp41 folding into a bundle, whereas the closed form inhibits this folding. Therefore, the transformation of the cysteines from a reduced to an oxidized state enables the loop to convert from opened to closed conformations, which assists gp41 to fold and induces hemifusion. The significant conservation of the loop region within many envelope proteins suggests a general mechanism, which is exploited by viruses to enhance entry into their host cells.—Ashkenazi, A., Viard, M., Wexler-Cohen, Y., Blumenthal, R., Shai, Y. Viral envelope protein folding and membrane hemifusion are enhanced by the conserved loop region of HIV-1 gp41.
Keywords: HIV-1 fusion inhibitor, peptide-membrane interaction
Human immunodeficiency virus (HIV-1) entry into host cells takes place through complex biological processes that involve membrane receptors and conformational changes in proteins, as well as protein-lipid interactions (1–4). HIV infection is mediated by the envelope (ENV) glycoprotein, promoting virus-cell and cell-cell fusion (5–7). HIV-1 ENV is synthesized as a single, glycosylated protein, gp160, which forms a trimeric structure that is cleaved by the cellular protease furin into 2 subunits: a soluble one, gp120, and a transmembrane one, gp41 (8–12). Binding of gp120 to the receptor CD4 and a coreceptor, such as CCR5 or CXCR4, causes major conformational changes that expose gp41 (13–15), which is responsible for the actual fusion between the viral and the host cell membranes (16). During fusion, gp41 is believed to undergo a conformational change from a “prefusion” or extended state, in which its N- and C-heptad repeat (NHR and CHR) regions are not associated, to a “postfusion” state, in which the NHR and CHR regions are integrated into the 6-helix bundle structure (11, 17–21).
The NHR/CHR regions of gp41 are connected by an immunodominant, protease-sensitive loop that reverses the polypeptide chain (22–24). This loop region contains 2 cysteines that are highly conserved among lentiviruses (Fig. 1A) and are believed to form a disulfide bond (25, 26). Whether the conserved cysteines are involved in the mechanical fusion reaction, and if so, in what manner, have yet to be determined. Inhibition of lymphocyte surface-associated protein-disulfide isomerase (PDI) blocks HIV fusion at the post-CD4 binding, suggesting that redox changes within the viral ENV are required for a productive fusion (27–31). The redox changes of the cysteines within the gp41 loop region in HIV-cell fusion have been difficult to assess, because mutating them disrupts gp160 proteolytic processing and trafficking (32, 33), and PDI inhibitors, as well as other agents that interfere with thiol-disulfide interchange, disrupt gp120-CD4 rearrangement (28, 34). The disruption of those early events prevented obtaining any insight into the gp41-mediated fusion that takes place downstream. In addition, the loop region was missing from the crystal structures of the simian immunodeficiency virus (SIV) and HIV gp41 ectodomain (17, 35). Whereas the SIV gp41 NMR studies characterized the dynamic properties of ∼30 residues in this region (36), the conserved and critical cysteines were replaced by alanines, as was the case in membrane binding studies (22). Considering these observations and assuming that the region of the loop, including the 2 cysteines, may change its conformation during the activation of the membrane fusion reaction (37), we set out to study how this region contributed to HIV-mediated fusion.
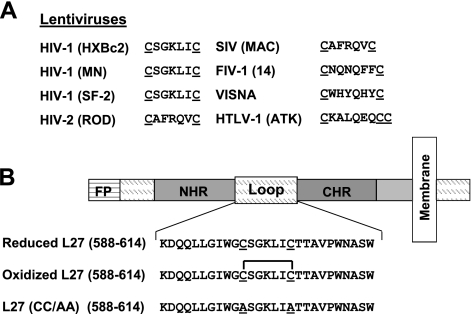
Figure 1.
A) Conservation of the cysteine residues within the loop region of several retroviral transmembrane envelope proteins. In lentiviruses, the cysteines are present in a similar position. SIV, simian immunodeficiency virus; FIV, feline immunodeficiency virus; HTLV, human T-cell lymphotropic virus. B) Scheme of the ectodomain of gp41, the transmembrane subunit of HIV-1 ENV. From the N terminus of gp41 are the fusion peptide (FP) and the NHR and CHR regions, which are connected by the loop region. Sequences and designations of the different loop peptides that were designed for the study are presented.
Synthetic peptides that interact with gp41 domains can interrupt viral fusion. Peptides overlapping with the CHR or NHR regions (C or N peptides, respectively) were shown to serve as excellent tools for exploring the mechanism and mode of action of gp41-mediated fusion, as well as powerful HIV-fusion inhibitors (38–44). Therefore, a peptide mimicking the loop region was designed, namely, L27, with the 2 conserved cysteines: either in a reduced opened form with free thiol groups, or in an oxidized closed form with an intramolecular disulfide bond. A mutant with 2 alanines substituting for the cysteine residues was also prepared (Fig. 1B). The peptides were tested for their ability to modulate the ongoing gp41-mediated fusion event.
Strikingly, the reduced conformation of the loop peptide accelerated HIV ENV-mediated hemifusion, whereas oxidation of the cysteines resulted in fusion inhibition. We addressed the possible mechanism leading to this transformation, and we discuss it in the context of the rearrangements underlying HIV-1-mediated cell fusion.
MATERIALS AND METHODS
Materials
F-moc amino acids, including lysine with an MTT side-chain-protecting group, and F-Moc Rink Amide MBHA resin were purchased from Nova-Biochem AG (Laufelfinger, Switzerland). Fatty acid-hexadecanoic acid (C16), tris-(2-carboxyethyl) phosphine hydrochloride (TCEP), ethanedithiol (EDT), and PBS were purchased from Sigma Chemical (Rehovot, Israel). DiIC18(5) or 1,1′-dioctadecyl-3,3,3′,3′,-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD), 1,1′-dioctadecyl-3,3,3′,3′,0 tetramethylinocarbocyanine perchlorate (DiI), 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) lypophilic fluorescent probes, and 5(6)-carboxyteramethylrhodamine N-succinimidyl ester (Rho) were obtained from Biotium (Hayward, CA, USA). The cytoplasmatic fluorescent probes 5- and 6-{[(4-chloromethyl)benzoyl]amino} tetramethylrhodamine (CMTMR) and calcein AM were purchased from Molecular Probes (Eugene, OR, USA). All other reagents were of analytical grade. Buffers were prepared in double-distilled water.
Cell lines
Cell culture reagents and media were purchased from Biological Industries Israel (Beit Haemek, Israel). The following cell lines were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health: Jurkat E6-1 T cells were from Dr. Arthur Weiss (45), and Jurkat HXBc2(4) T cells expressing HIV-1 HXBc2 Rev and ENV proteins were from Dr. Joseph Sodroski (46). Cells were cultured every 3–4 d and maintained in RPMI 1640 supplemented with the appropriate antibiotics at 37°C with 5% CO2 in a humidified incubator. For ENV expression, Jurkat HXBc2(4) cells were transferred to medium without tetracycline 3 d prior to the experiments. The TZM-bl cells were from the AIDS repository and were provided by Dr. John C. Kappes (University of Alabama, Birmingham, AL, USA). GP4F cells, which constitutively express influenza hemagglutinin (HA), were a gift from Dr. Judith M. White (University of Virginia, Charlottesville, VA, USA). These cells were grown in regular Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS and antibiotics.
Peptide synthesis, fatty acid conjugation, and fluorescent labeling
L27, GCN4 trimer, and C34 peptides were synthesized on Rink Amide MBHA resin by using the F-moc strategy as described previously (47). N-terminally conjugated GCN4 trimer peptides contain a lysine residue at their N terminus with a 4-methyltrityl (MTT) side-chain-protecting group, enabling the conjugation of a rhodamine that requires a special deprotection step under mild acidic conditions (2×1 min of 5% trifluoroacetic acid (TFA) in dichloromethane (DCM) and 30 min of 1%TFA in DCM). Conjugation of a fatty acid to the N terminus was performed using standard F-moc chemistry. Addition of the Rho (emission 580, excitation 530) fluorescent probe to the N terminus of L27, K-GCN4 and C34 peptides was performed in dimethylformamide (DMF) with 2% N,N-diisopropylethylamine (DIEA) solution overnight. Noncontaining cysteine peptides (including the fluorescent ones) were cleaved from the resin by a TFA:DDW:triethylsilane (TES) (93.1:4.9:2 v/v) mixture, and purified by reverse phase high-performance liquid chromatography (RP-HPLC) to >95% homogeneity. The molecular weight of the peptides was confirmed by platform LCA electrospray mass spectrometry.
Formation of the structural forms of the loop peptide
The loop peptides (including the fluorescent ones) that contained the cysteine residues were cleaved by a TFA:DDW:TES:thioanisol:EDT (92.1:3.9:1.25:1.25:2.5 v/v) mixture. Before RP-HPLC purification, samples were dissolved in 5 mM TCEP as a reducing agent, and the peptides were purified under acidic conditions of 0.1% (v/v) TFA to keep the cysteine residues in a reduced form. The purified loop peptides were kept as a powder and were dissolved just before the experiments. To enhance the oxidized closed form, the loop peptides were dissolved in dimethyl sulfoxide (DMSO) or in PBS (pH 7.2) at a concentration of 25 μg/ml. Oxidation was performed under stirring conditions, and the peptides were exposed to air for 24 h. Next, the samples were taken to RP-HPLC to follow the oxidation process. The peptides were eluted with a flow rate of 0.6 ml/min using a linear gradient from CH3CN/H2O 25:75 (v/v) to CH3CN/H2O 50:50 (v/v) in 40 min on analytical C4 column (5-μm particle size, 0.46×25 cm; GraceVydac, Columbia, MD, USA). The retention time of the oxidized peptide and the reduced peptide was 24.9 and 25.3 min, respectively. The formation of intramolecular disulfide bonds was further confirmed by mass spectrometry.
Virus infectivity assay
Fully infectious HIV-1 HXBc2 concentrated virus stock was a kind gift from the AIDS Vaccine Program (SAIC-Frederick). The infectivity of HIV-1 HXBc2 was determined using the TZM-bl cell line as a reporter. Cells were added (2×104 cells/well) to a 96-well clear-bottomed microtiter plate with 10% serum-supplemented DMEM. Plates were incubated at 37°C for 18–24 h to allow the cells to adhere. The medium was then aspirated from each well and replaced with serum-free DMEM containing 40 μg/ml DEAE-dextran. Stock dilutions of each peptide were prepared in DMSO, so that each final concentration was achieved with 1% dilution. On addition of the peptides, the virus was added to the cells diluted in serum-free DMEM containing 40 μg/ml DEAE-dextran. The plate was then incubated at 37°C for 18 h to allow the infection to occur. Luciferase activity was analyzed using the Steady-Glo Luciferase assay kit (Promega, Madison WI, USA).
Cell-cell fusion assay
Cell-cell fusion was assayed by cell-cell lipid mixing or by cell-cell content mixing followed by monitoring the distribution of fluorescent probes (48), both water soluble and lipophilic, between target (Jurkat E6-1) and effector (Jurkat HXBc2) cells on their coincubation with each other.
The cell-cell lipid-mixing assay was previously described (49). In brief, Jurkat E6-1 and Jurkat HXBc2 cells were labeled with DiI and DiD lypophilic fluorescent probes, respectively. The two cell populations were coincubated for 6 h at a ratio of 1:1 in the presence of different concentrations of the peptides. Cells that coincubated without the presence of peptides served as an optimal fusion reference. Unlabeled cells that were handled similarly served as an intrinsic fluorescence control. Cells labeled separately with DiI or DiD were used to compensate for the optimal separation of fluorescent signals. Jurkat HXBc2 cells labeled with DiI were coincubated with Jurkat HXBc2 cells labeled with DiD for a fusion background that was subtracted from the measurements of the experiment. The following alterations were applied to the original protocol: 5 μl of a 1-mg/ml DiI or DiD solution in DMSO was added to 1 ml of 4 × 106 cells/ml Jurkat E6-1 or Jurkat HXBc2 cells, respectively; data from 1.5 ×105 events for each well were collected on FACSort, upgraded to a FACSCalibur cell analyzer (Becton Dickinson, Franklin Lakes, NJ, USA), and further analyzed.
A cell-cell content-mixing assay was previously described (39, 50). In brief, Jurkat E6-1 cells were loaded with the cytoplasmatic dye CMTMR at a concentration of 10 μM, and Jurkat HXBc2 cells were loaded with calcein AM at a concentration of 0.5 μM for 45 min at 37°C. The two cell populations were coincubated for 6 h at a ratio of 1:1 in the presence of different concentrations of the peptides and analyzed by flow cytometry, similarly to the cell-cell lipid-mixing assay.
Cell fusion (lipid mixing) between GP4F cells, which constitutively express influenza HA, and human erythrocytes was monitored and assayed as described previously (50, 51). The erythrocytes were labeled with the red fluorescent lipid dye PKH26, instead of DiI as in the original protocol.
Fusion kinetics
The fusion kinetics of the virus cell infection and the cell-cell lipid mixing was monitored at 37°C by adding the entry inhibitor peptide, C34, to the fusion reaction wells at different time points. C34 was added at a concentration of 0.5 or 1 μM for the virus cell-infection or the cell-cell lipid-mixing systems, respectively, to achieve full inhibition. To determine the effect of the loop peptides on the fusion kinetics, a loop peptide at a concentration of 2 μM was first introduced to the fusion reaction wells at t = 0, and then the C34 peptide was added at different time points. The data were fitted to Eq. 1 to get the fusion curve, as described previously (52):
| (1) |
where Y is the fusion value determined at the time t of the addition of C34, and the kinetic constants of the curve are Ymax, the maximal fusion value, t1/2, the time of half the maximal fusion, and b, the exponential rate constant.
Triple-staining flow cytometry fusion assay
The protocol was previously described (53). In brief, for triple staining, the same cell-cell lipid-mixing assay as above was performed using Jurkat T cells in the presence of Rho-labeled peptides. Cells labeled separately with DiO or DiD probes and unlabeled cells in the presence of a Rho-labeled peptide were used to compensate for the optimal separation of the 3 fluorescent signals. For each data point, 5 × 105 events were collected. The 8 possible combinations (triple, Rho, DiO, DiD, Rho+DiO, Rho+DiD, DiO+DiD, no label) were defined in the analysis software, and the percentage of each one was calculated. The analysis of the data enabled us to examine the relative binding of labeled peptides to different cell populations, namely, target or effector cells (43). The percentage of Rho peptide in cells labeled with DiD (effector; Eq. 2) or DiO (target; Eq. 3) was calculated:
| (2) |
| (3) |
Binding of fluorescently labeled peptides to proteins of coincubated target and effector cells detected by SDS-PAGE
Unlabeled Jurkat E6-1 and Jurkat HXBc2 cells (2×106) were coincubated in the presence of 2 μM Rho-labeled loop peptides, as well as with 2 μM Rho-labeled control peptides (C34 and GCN4). The cells were coincubated for 6 h at 37°C, similarly to the cell-cell fusion assay. Next, the cells were washed with PBS and cross-linked with 1% formaldehyde, and after 10 min, a 125 mM glycine solution was added to stop the reaction. The cells were lysed for 30 min on ice in 50 μl lysis buffer followed by a sonication step. Insoluble material was removed by centrifugation at 12,000 g for 10 min at 4°C. The proteins were resolved by 12% SDS-PAGE, and proteins bound to fluorescently labeled peptide were detected by the Typhoon 9400 variable mode imager (Amersham Biosciences, Piscataway, NJ, USA). Excitation was set at 532 nm, and fluorescence emission was collected by a TAMRA filter (580±30 nm) at 100 μm resolution and 600 V.
Western blot
After running the protein supernatant in a 12% SDS-PAGE, the gel was transferred by Western blot to a membrane using iBlot Gel Transfer Stacks Nitrocellulose, Regular (Invitrogen). The membrane was first blocked with 5% of skim milk and probed with anti-actin monoclonal mouse antibody (Molecular Probes) and then with goat anti-mouse secondary antibody conjugated to horseradish peroxidase (HRP). Bound actin antibody was monitored using the ECL-based Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockville, MD, USA).
Cytotoxicity assay (XTT proliferation)
Aliquots of 2.5 × 104 Jurkat E6-1 cells were distributed onto a 96-well plate (Falcon; BD Biosciences, San Jose, CA, USA) in the presence of various peptide concentrations and incubated for 12 h. Wells in the last 2 columns served as blank (medium only), and 100% survival controls (cells and medium only), respectively. After incubation, the XTT reaction solution [sodium 3′-[1-(phenyl-aminocarbonyl)-3,4-tetrazolium]-bis-(4-methoxy-6-nitro) benzene sulfonic acid hydrate and N-methyl dibenzopyrazine methyl sulfate, mixed in a proportion of 50:1], was added for an additional 2 h. Optical density was read at a wavelength of 450 nm in an enzyme-linked immunoabsorbent assay plate reader. LC50 (concentration at which 50% of the cells die) was determined relative to the control, 2.5 × 104 cells in medium. All assays were performed in duplicate.
Ability of the peptides to self-associate using fluorescence spectroscopy
Rho-labeled peptides were dissolved at a concentration of 1 μM in HEPES buffer (5 mM, pH 7.4). The emission of Rho was monitored at 580 nm with the excitation set at 530 nm. All fluorescence measurements were recorded before and after adding proteinase K (used to follow the kinetics of peptide degradation) and then were followed for 20 min at room temperature. Measurements were performed on an SLM-Aminco Bowman series 2-luminescence spectrometer (SLM-Aminco, Rochester, NY, USA) at room temperature. Typical spectral bandwidths were 8 nm for excitation and 8 nm for emission. The sample was placed in a 5- × 5-mm quartz cuvette with constant magnetic stirring. The stability of the peptides to proteinase K degradation was further assessed by the RP-HPLC. Samples were injected to the HPLC before the addition of the enzyme and 20 min after the addition to calculate the percentage of peptide degradation.
RESULTS
Reduced open-loop peptide enhances hemifusion; oxidized closed form inhibits fusion
The reduced form (reduced L27) and the oxidized form with an intramolecular disulfide bond (oxidized L27) of the loop peptide were prepared along with a control peptide, in which we replaced the cysteines with alanines [L27 (CC/AA)]. The effect of the peptides on membrane fusion mediated by the HIV-1 envelope protein was investigated using 3 fusion systems: a virus cell-infection assay utilizing cells infected with fully contagious HIV-1 viruses; a cell-cell lipid-mixing assay, in which we monitored the distribution of lipid dyes between target and effector T cells; and a cell-cell content-mixing assay, in which we monitored the distribution of cytoplasmatic dyes between target and effector T cells. Before the experiments, the peptides were tested for cell toxicity and displayed no toxic effect on T cells up to 15 μM, the maximum concentration tested.
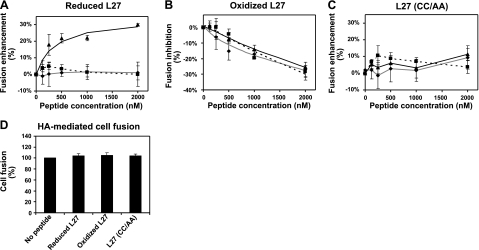
Surprisingly, the reduced L27 peptide enhanced cell-cell lipid mixing in a dose-dependent manner (Fig. 2A). This effect could indicate hemifusion, involving only outer membranes with no cytoplasmic connection between the cells, or complete fusion accompanied by content mixing. To differentiate between these two possibilities, we followed the exchange of cytoplasmic content by virus cell-infection and by cell-cell content-mixing assays. No effect of the peptide was observed for either assay, suggesting that the reduced L27 only enhances hemifusion. On the contrary, the oxidized closed form of the peptide (oxidized L27) inhibited cell-cell lipid mixing, cell-cell content mixing, and virus cell infection in a dose-dependent manner (Fig. 2B). These results therefore indicate that the oxidized L27 inhibits complete fusion. It is important to note that the inhibition rate was similar between the lipid-mixing and content-mixing assays; the inhibition rate was not as high as that of the C peptides (see Discussion for elaboration); and in the lipid-mixing assay, the enhancement rate of the reduced L27 was similar to the inhibition rate of the oxidized L27. This led to a 60% difference in the relative fusion observed in the presence of the reduced vs. the oxidized form of L27. The L27 (CC/AA) control peptide did not affect any of the envelope-mediated fusion reactions (Fig. 2C), indicating that the presence of the cysteines within the loop peptide is crucial for the observed effects.
Figure 2.
Effect of the different conformations of the loop peptide on HIV-1 ENV-mediated fusion by utilizing 3 fusion systems: virus cell infection, cell-cell lipid mixing, and cell-cell content mixing. A–C) Increasing concentrations of a loop peptide: reduced L27 (A), oxidized L27 (B), and mutant L27 (CC/AA) (C) were tested in the 3 fusion systems. Virus infectivity assay (♦): fully contagious HXBc2 HIV-1 viruses were allowed to infect TZM-bl cells, and the infection rate was determined by luciferase activity. Fitting curve is denoted by a continuous gray line. Cell-cell lipid-mixing assay (▴): Jurkat HXBc2 effector cells and Jurkat E6-1 target cells were labeled with the lipid dyes DID and DiI, respectively. Cells were coincubated, and dye transfer was monitored by flow cytometry. Fitting curve is denoted by a continuous black line. Cell-cell content-mixing assay (■): Jurkat HXBc2 effector cells and Jurkat E6-1 target cells were labeled with the cytoplasmatic dyes calcein AM and CMTMR, respectively. Cells were coincubated, and dye transfer was monitored by flow cytometry. Fitting curve is denoted by a dotted black line. Percentage of fusion enhancement or inhibition was determined by calculating the difference between the fusion rate in the presence or absence of a peptide. Error bars = se; n = 3. D) Effect of each loop peptide (at a concentration of 4 μM) was tested in cell-cell fusion assay mediated by influenza HA. Human erythrocytes were labeled with the lipid dye PKH26 and coincubated with HA-expressing cells. Dye transfer was monitored during the fusion event. Lipid-mixing values in the presence of peptides were normalized to that in the absence of peptides to calculate percentage of change from control (100% lipid mixing). Results are presented as averages ± se; n = 5.
The ability of the gp41-derived loop peptides to modulate viral fusion was also examined in the influenza HA cell-mediated fusion assay. In this assay, we followed lipid mixing during the fusion event between HA-expressing cells and erythrocytes (50, 51) in the absence or presence of the loop peptides (Fig. 2D). No effect of the peptides was observed on HA-mediated cell fusion. This result supports a specific effect of these peptides on HIV-1 ENV-mediated fusion.
Loop peptides affect the prefusion conformations of gp41 during virus-cell and cell-cell fusion
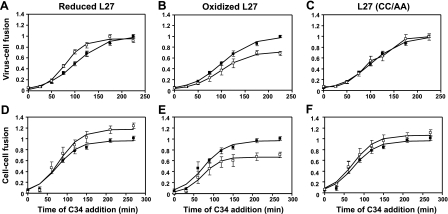
The ability of the loop peptides to modulate HIV progression along the fusion pathway mediated by gp41 was further investigated by following the fusion kinetics. The HIV entry inhibitor C34 peptide was chosen as a reporter to follow the progression of gp41's conformation from a prefusion state to a postfusion state. C34 binds gp41 prefusion intermediates and inhibits the folding into the 6-helix bundle, leading to fusion arrest. The peptide inhibition potency is dependent on the gp41 prefusion intermediates' accessibility to it (54, 55). We added C34, at a concentration that completely blocks fusion, to the gp41-mediated fusion systems (either virus-cell infection or cell-cell lipid mixing) at different time points in the absence or presence of the L27 peptides. The kinetic curves are presented in Fig. 3 for both systems. Each panel presents the fusion kinetic curves of C34 alone and C34 in the presence of a loop peptide. The kinetic parameters for each curve, namely, Ymax and t1/2, were calculated using Eq. 1 (see Materials and Methods) and are presented in Table 1. The kinetic curves of C34 alone in both virus-cell and cell-cell systems were characterized by a lag phase of 15–20 min, as described previously (16). In this lag phase, no fusion occurred, thus allowing C34 to inhibit at full capacity. Later on, membrane fusion increased, creating more postfusion bundles, resulting in diminished accessibility of the prefusion intermediates to C34, thereby reducing its inhibitory potency. A plateau was reached in both systems when fusion was completed and C34 could no longer inhibit, resulting in the same fusion values as without the inhibitor (Ymax=1).
Figure 3.
Loop peptides affect prefusion conformations of gp41 during virus-cell and cell-cell fusion. Fusion kinetics of virus cell infection and cell-cell lipid mixing was monitored at 37°C by adding C34, at a concentration that completely blocked fusion, to the fusion reaction wells at different time points in the absence (■) or presence (□) of a loop peptide: reduced L27 (A, D), oxidized L27 (B, E), and mutant L27 (CC/AA) (C, F). A–C) Kinetics of virus-cell fusion. D–F) Kinetics of cell-cell fusion. For each data series, fusion value at a different time point was divided by the maximal fusion value with no peptide added (relative fusion), and a fusion curve was fitted according to Eq. 1 (see Materials and Methods). Error bars = se; n = 3.
Table 1.
Kinetic constants derived from the fitted fusion curve
| Treatment | Virus-cell fusion |
Cell-cell fusion |
||
|---|---|---|---|---|
| Ymax | t1/2 (min) | Ymax | t1/2 (min) | |
| C34 alone | 0.99 ± 0.02 | 100.9 ± 3.1 | 0.97 ± 0.05 | 74.5 ± 6.9 |
| C34 + reduced L27 | 0.95 ± 0.02 | 78.0 ± 2.5 | 1.20 ± 0.06 | 80.8 ± 6.5 |
| C34 + oxidized L27 | 0.72 ± 0.02 | 97.8 ± 2.7 | 0.67 ± 0.03 | 71.8 ± 4.8 |
| C34 + L27(CC/AA) | 1.02 ± 0.04 | 101.9 ± 4.8 | 1.06 ± 0.03 | 71.5 ± 3.9 |
Values are means ± se of the fitted curve.
In the virus-cell system, the C34 kinetic curve reached a Ymax value of 0.99 and a t1/2 value of 100 min, whereas in the cell-cell system, it reached a Ymax value of 0.97 and a t1/2 value of 74.5 min. The presence of the reduced L27 peptide in the virus-cell system did not affect the maximal fusion value of the C34 curve (Ymax=0.95); however, it decreased the potency of C34 by shortening the inhibition time window by 22 min (Table 1 and Fig. 3A). In the cell-cell lipid-mixing system, the reduced L27 decreased the potency of C34 by increasing the maximal fusion of the curve (Fig. 3D). Given that the Ymax value was not the same as for C34 alone, we could not compare the t1/2 value of both curves (Table 1). As detailed below in Discussion, enhancement of hemifusion by the reduced L27 could explain these results. Inversely, the oxidized L27 peptide amplified the inhibitory potency of C34 in both virus-cell and cell-cell fusion systems by decreasing the maximal fusion of the curve to Ymax values of 0.72 and 0.67, respectively (Table 1 and Fig. 3B, E). The control peptide, L27 (CC/AA), did not affect the fusion kinetic curve (Table 1 and Fig. 3C, F).
Loop peptides interact with gp41 and have an enhanced tendency to bind ENV-expressing effector cells
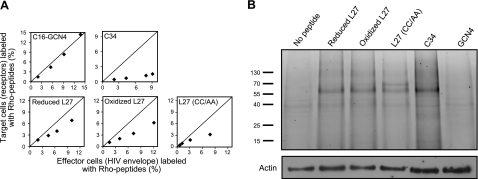
To determine whether the loop peptides have an enhanced tendency to bind the cells with the receptors (target cells), or those with the HIV ENV glycoprotein (effector cells), in a dynamic fusion process, we employed the triple-staining assay (43). Briefly, fluorescently Rho-labeled peptides were incubated with differently labeled effector and target cells, according to the protocol of the cell-cell fusion assay. Further analysis using Eqs. 2 and 3, as specified in Materials and Methods, enabled us to compare the relative binding of the peptides to each cell population (Fig. 4A). A line was drawn in each panel to emphasize where we would expect the data if there were no preference among the different populations. We used two Rho-labeled control peptides: GCN4, a non-HIV coiled-coil peptide attached to palmitic fatty acid (C16) that binds cells without preference to a cell population (43), and C34, a HIV-derived peptide. As expected, C16-GCN4 did not prefer a specific cell population, whereas C34 resided more on effector than target cells (Fig. 4A). The reduced and oxidized form of L27, as well as the control peptide, L27 (CC/AA), exhibited the same tendency as C34.
Figure 4.
Loop peptides interact with gp41 and have an enhanced tendency to bind ENV-expressing effector cells. A) Relative binding of Rho-labeled peptides to specific cell populations. In each panel, x axis represents the percentage of effector cells (with HIV ENV) labeled with Rho peptide that was calculated using Eq. 2; y axis represents the percentage of target cells (with receptors) labeled with Rho peptide that was calculated using Eq. 3 (see Materials and Methods). Line in each panel indicates where we would expect the data if there is no preference between the different populations. Different data points represent rising peptide concentrations (up to 1 μM for the L27 peptides and C34; up to 250 nM for the C16-GCN4 peptide). B) Binding of fluorescently labeled peptides to the proteins of coincubated target and effector cells. Unlabeled Jurkat E6-1 and Jurkat HXBc2 cells (2×106) were coincubated in the presence of 2 μM Rho-labeled loop peptides, as well as with 2 μM Rho-labeled control peptides (C34 and GCN4), cross-linked, and lysed. Cell proteins were resolved by SDS-PAGE, and proteins bound to fluorescently labeled peptide were detected by measuring the fluorescent signal of Rho. Protein mass (kDa) is indicated at left. Next, the amount of actin in each run was checked by Western blot analysis with anti-actin antibody. Experiments with fluorescent peptides were performed similarly to the cell-cell fusion assay; results shown were obtained from a representative experiment.
GP41-derived peptides that altered HIV-cell fusion were shown to interact with gp41 during the fusion event (19, 38, 40, 41, 56, 57). We tested whether this property is also shared by the loop peptides. To this end, we incubated loop peptides, fluorescently labeled by Rho, with unlabeled target and effector cells, similarly to the protocol of the cell-cell fusion assay. We also incubated the cells with 2 Rho-labeled control peptides, GCN4 and C34. After coincubation, the cells were cross-linked using formaldehyde. This step formed a cross-linked complex of a fluorescently labeled peptide with its target protein. The cell lysates were loaded on SDS-PAGE to identify this peptide-protein complex by measuring the Rho fluorescence (Fig. 4B). Target and effector cells that coincubated without a Rho-labeled peptide presented no fluorescent signal. Notably, the oxidized and reduced L27, as well as the mutated peptide, L27 (CC/AA), mainly interacted with one protein with the same size. The gp41-derived C peptide, C34, also interacted with a protein of the same size, suggesting that the identity of the protein is gp41. Given that gp41 was in a cross-linked complex with the peptides, the size of the complex was not the same as the native protein alone. In contrast, the irrelevant peptide, GCN4, did not interact with this protein. These findings indicate that the loop peptides, when compared to the well-defined C34 peptide, could bind gp41 in a specific way.
Reduced loop peptide only transforms to its oxidized closed form
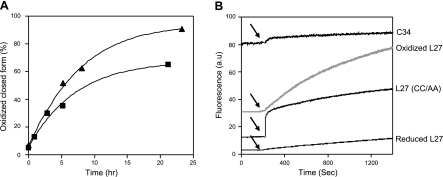
Theoretically, the two free thiol groups within the loop domain peptide could form several oxidative products: a closed form with an intramolecular bond, a dimer, and a polypeptide chain. The ability of the reduced L27 peptide to form disulfide bonds was monitored in buffer solution (PBS, pH 7.2) and in DMSO. To follow the oxidation kinetics, samples were taken to RP-HPLC at different time points and analyzed for the different oxidative products (elaborated in Materials and Methods). Surprisingly, L27 transformed only to its oxidized closed form, which was eluted 0.4 min before the reduced form. Monitoring the oxidation kinetics revealed that after 6 h, 55% of the peptide underwent oxidation in DMSO and 40% in buffer solution (Fig. 5A).
Figure 5.
Loop peptide transforms over time into its oxidized closed form and self-assembles. A) Reduced loop peptide (10 μM) was allowed to form a disulfide bond when it was dissolved in DMSO under stirring (▴) or in PBS (pH 7.2; ■). Oxidation kinetics was monitored by injecting samples to the RP-HPLC at different time points. Oxidized form of the peptide eluted 0.4 min before the reduced form. For each time point, percentage of peptide oxidation was determined by calculating amount of the oxidized closed form divided by total amount of the peptide injected. B) Analysis of loop peptide interaction and its stability. Fluorescence of Rho-labeled peptides [C34; reduced L27; oxidized L27; and L27 (CC/AA)] was monitored before and after the addition of proteinase K (denoted by arrows).
Oxidation-reduction of the cysteines affects the ability of the loop peptides to self-assemble
To analyze the self-assembly properties of the different peptides, L27 peptides and C34 were labeled with Rho, a fluorescent probe known to undergo self-quenching when the probe is in proximity. C34 is a peptide that does not tend to self-assemble in solution (17) and, therefore, used as a control in the experiment. We measured the fluorescence, before and after adding the proteolytic enzyme proteinase K to the labeled peptide (Fig. 5B). The loop peptides displayed much lower initial fluorescence as compared to C34, suggesting that they tend to self-assemble. The addition of proteinase K to the loop peptides recovered the fluorescent signal as the degradation of the peptide-complex by the enzyme induced a dequenching of the fluorescent probe. Twenty minutes on addition of the proteinase, different values of signal recovery were observed for the loop peptides, suggesting a different stability of each complex to enzymatic degradation. The sensitivity to enzymatic degradation (from high to low percentage of degradation) was further determined by RP-HPLC (elaborated in Materials and Methods): C34 (100%) = oxidized L27 (100%) > L27 (CC/AA) (90%) > reduced L27 (60%).
DISCUSSION
Since the discovery of HIV-1-gp41 glycoprotein, a great effort has been made to explore the intermediate steps that take place between the prefusion and postfusion states. Most of these studies used N- and C-inhibitory peptides with sequences that overlapped the NHR and the CHR, respectively (19, 21, 38, 41, 56, 57). It is reasonable to assume that the loop region, located between the NHR and CHR, participates in this process. This hypothesis led us to explore how peptides derived from the loop region affect HIV-cell fusion. For this purpose, we synthesized and investigated by a variety of assays 3 peptides: a reduced open L27, an oxidized closed L27, and an L27 mutant with 2 alanine residues replacing the cysteines.
Action of the loop peptides on HIV-cell fusion
The reduced open form of L27 enhanced hemifusion (Fig. 2A) and decreased the inhibitory potency of C34 (Fig. 3A, D), suggesting that the induction of hemifusion was accompanied by the induction of gp41 folding into bundles. This would diminish the accessibility of C34 to the prefusion intermediates and would decrease its inhibitory potency. The mechanism by which the reduced L27 induces gp41 folding into bundles might be explained by the following events: the open peptide binds cells, giving preference to the HIV ENV effector cells, and interacts with gp41 (Fig. 4). It strongly self-assembles (Fig. 5B), suggesting that the peptide can bind to the endogenous loop region. The self-assembled peptide can then form intramolecular disulfide bonds (Fig. 5A). Since it is bound to the endogenous loop, it can assist in the formation of intramolecular disulfide bonds within gp41 that should contribute to its folding into a bundle. In this model, the loop region serves as a hinge between its open and closed conformations.
In contrast, the oxidized closed peptide inhibited fusion (Fig. 2B) and amplified the inhibitory potency of C34 (Fig. 3B, E). This could be explained by the stabilization of the prefusion intermediates of gp41 and inhibition of the bundle formation. The mechanism leading to this inhibition might be explained by the following events: the closed peptide binds cells, giving preference to the HIV ENV effector cells, and interacts with gp41 (Fig. 4). It can also self-assemble, but with weaker interactive complexes (Fig. 5B), suggesting that it can also bind to the endogenous loop region. Because the peptide is already oxidized, it cannot enhance the formation of intramolecular disulfide bonds, but it can disrupt the proper conformation of the endogenous loop region, thereby preventing the conversion into the closed conformation. This can stabilize the intermediate prefusion conformation of gp41, which, in turn, inhibits fusion.
We further demonstrated the importance of the cysteines in L27 by mutating them to alanines. Experiments with the mutant peptide, L27 (CC/AA), show that this peptide binds to gp41, giving preference to the HIV ENV effector cells (Fig. 4). It self-assembles (Fig. 5B) and presumably binds the endogenous loop region. Nevertheless, it is inactive in virus-cell and cell-cell fusion assays (Fig. 2C) and does not affect the prefusion conformations of gp41 (Fig. 3C, F).
Comparison to the fusion inhibitory properties of gp41 C peptides
The difference between the inhibition rates of cell-cell lipid mixing vs. cell-cell content mixing can hint at which stage along the fusion pathway a peptide inhibits. For the C peptides, C34 was previously shown to have the same inhibition rates in lipid mixing as content mixing, suggesting that it inhibits prior to the lipid-mixing stage. However, T-20 showed increased inhibitory potency at the post-lipid-mixing stage (39). The inhibition rates of cell-cell lipid mixing vs. cell-cell content mixing induced by the loop peptide were similar (Fig. 2B), indicating that the peptide inhibited prior to the lipid-mixing stage. Another important property of gp41-derived peptides is the rate of fusion inhibition. For the C peptides, this rate is much higher than that of the loop peptide. However C peptides are capable only of inhibiting fusion, whereas the loop peptide can enhance or inhibit fusion, depending on the cysteine conformation. The difference between these opposite effects reached 60% difference in viral fusion (Fig. 2A), which could drastically alter HIV infection.
Importance of the hemifusion state in HIV entry and pathogenesis
Generally, inhibition of hemifusion (lipid mixing) would subsequently block complete fusion (content mixing), since hemifusion is upstream to fusion. However, studies from the influenza HA show that the formation of a hemifusion intermediate does not necessarily accompany complete fusion (58, 59). A productive fusion requires transformation of the hemifusion intermediate into an expanding fusion pore. For both viral fusion and cell content mixing, posthemifusion is the rate-limiting step of the fusion reaction, and theoretical analysis confirms that fusion pore expansion is more energetically demanding than hemifusion (60). Notably, these observations could explain why the enhancement of the fusion reaction by the loop peptide was arrested in the lipid-mixing stage.
Recent studies indicate that hemifusion is a major cause for the cytopathy of HIV through apoptosis of bystander cells by ENV-expressing cells. This cytopathic effect is often called “the kiss of death” (61, 62). One of the most prominent HIV ENV mutants presenting a hemifusion phenotype is D589L within the loop region (23). To date, there has been no evidence from the family of the lentiviruses that peptides derived from their ENV can induce virus-cell or cell-cell hemifusion. Therefore, peptides originating from the loop region can serve as a novel tool for studying the mechanism underlying the hemifusion state in HIV entry and pathogenesis.
Disulfide bond rearrangement in the ENV of HIV and other viruses is essential for a productive membrane fusion
Several viral envelope proteins depend on a specific thiol/disulfide balance to mediate viral membrane fusion. Reduction of disulfide bonds in envelope proteins by cell-associated oxidoreductase activity is an example of a mechanism for triggering the conformational change needed to activate fusogenicity (34). For the ENV of murine leukemia virus (MLV) and human T-cell lymphotropic virus (HTLV-1), isomerization of the disulfide bond by activating a thiol group in a Cys-X-X-Cys motif in peripheral subunit is required for membrane fusion (63–65). HIV-1 ENV-mediated fusion is also modulated by redox changes. The ectodomain of gp41 subunit has only one disulfide bond, which is located within the loop region, whereas gp120 subunit comprises multiple disulfides (34). Most of the studies focused on the redox changes within the gp120 modulated by the oxidoreductase, PDI. The reduction of gp120 by PDI after CD4 binding and before the activation of gp41 is essential for membrane fusion (27–30).
Here, we suggest that the ectodomain of gp41 subunit also changes its redox state by transforming from reduced to oxidized states during the lipid-mixing stage. This contrasts with current thinking that the cysteines within gp41 loop region are already oxidized prior to membrane fusion (26, 66, 67). In HTLV-1 and HIV-1, the loop region within the transmembrane subunit senses conformational changes from the peripheral subunit to induce a fusion active conformation (66, 68). In HIV-1, the gp41 loop region was established as a major contact site to gp120 that is needed for fusion activation (68–70). Therefore, we speculate that changes in the gp120 redox state have an indirect result for the gp41 disulfide bond, leading to its oxidation during membrane fusion.
CONCLUSIONS
To the best of our knowledge, we have provided the first evidence that the loop region and its conserved cysteines play a role in gp41-mediated fusion. We conclude that the loop region of gp41 serves as a hinge between its opened and closed conformations during HIV-cell fusion. The transformation of the cysteines from a reduced to an oxidized state enables the loop to convert from open to closed conformation, thereby assisting gp41 to fold into a bundle. This conversion is formed during the lipid-mixing stage and may also assist in stabilizing the postfusion complex, but further research is needed to determine its specific role following the lipid-mixing stage. The significant similarity of the loop region within the lentiviral transmembrane envelope proteins suggests a general mechanism that is exploited by viruses to enhance entry into their host cells.
Acknowledgments
The authors thank Batya Zarmi for her valuable help with peptide purification, Dr. Ayala Sharp and Eitan Ariel, and the staff of the flow cytometry unit at the Weizmann Institute of Science for their valuable technical assistance and advice.
This study was supported by the Israel Science Foundation and also has been funded, in part, with federal funds from the National Cancer Institute, U.S. National Institutes of Health (NIH), under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does it mention any trade names, commercial products, or organizations or imply endorsement by the U.S. Government. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Y.S. is the incumbent of the Harold S. and Harriet B. Brady Professorial Chair in Cancer Research.
REFERENCES
- 1. White J. M. (1992) Membrane fusion. Science 258, 917–924 [DOI] [PubMed] [Google Scholar]
- 2. Chernomordik L. V., Zimmerberg J., Kozlov M. M. (2006) Membranes of the world unite! J. Cell Biol. 175, 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bomsel M., Alfsen A. (2003) Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell. Biol. 4, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viard M., Parolini I., Rawat S. S., Fecchi K., Sargiacomo M., Puri A., Blumenthal R. (2004) The role of glycosphingolipids in HIV signaling, entry and pathogenesis. Glycoconj. J. 20, 213–222 [DOI] [PubMed] [Google Scholar]
- 5. Furuta R. A., Wild C. T., Weng Y., Weiss C. D. (1998) Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5, 276–279 [DOI] [PubMed] [Google Scholar]
- 6. Clapham P. R., McKnight A. (2002) Cell surface receptors, virus entry and tropism of primate lentiviruses. J. Gen. Virol. 83, 1809–1829 [DOI] [PubMed] [Google Scholar]
- 7. Poranen M. M., Daugelavicius R., Bamford D. H. (2002) Common principles in viral entry. Annu. Rev. Microbiol. 56, 521–538 [DOI] [PubMed] [Google Scholar]
- 8. Okumura Y., Yano M., Murakami M., Mori S., Towatari T., Kido H. (1999) The extracellular processing of HIV-1 envelope glycoprotein gp160 by human plasmin. FEBS Lett. 442, 39–42 [DOI] [PubMed] [Google Scholar]
- 9. Center R. J., Leapman R. D., Lebowitz J., Arthur L. O., Earl P. L., Moss B. (2002) Oligomeric structure of the human immunodeficiency virus type 1 envelope protein on the virion surface. J. Virol. 76, 7863–7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu M., Blacklow S. C., Kim P. S. (1995) A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 2, 1075–1082 [DOI] [PubMed] [Google Scholar]
- 11. Weissenhorn W., Dessen A., Harrison S. C., Skehel J. J., Wiley D. C. (1997) Atomic structure of the ectodomain from HIV-1 gp41. Nature 387, 426–430 [DOI] [PubMed] [Google Scholar]
- 12. Chernomordik L. V., Kozlov M. M. (2008) Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 15, 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwong P. D., Wyatt R., Robinson J., Sweet R. W., Sodroski J., Hendrickson W. A. (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393, 648–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rizzuto C. D., Wyatt R., Hernandez-Ramos N., Sun Y., Kwong P. D., Hendrickson W. A., Sodroski J. (1998) A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280, 1949–1953 [DOI] [PubMed] [Google Scholar]
- 15. Finzi A., Xiang S. H., Pacheco B., Wang L., Haight J., Kassa A., Danek B., Pancera M., Kwong P. D., Sodroski J. (2010) Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol. Cell 37, 656–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gallo S. A., Puri A., Blumenthal R. (2001) HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process. Biochemistry 40, 12231–12236 [DOI] [PubMed] [Google Scholar]
- 17. Chan D. C., Fass D., Berger J. M., Kim P. S. (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89, 263–273 [DOI] [PubMed] [Google Scholar]
- 18. Melikyan G. B., Markosyan R. M., Hemmati H., Delmedico M. K., Lambert D. M., Cohen F. S. (2000) Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151, 413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eckert D. M., Kim P. S. (2001) Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70, 777–810 [DOI] [PubMed] [Google Scholar]
- 20. Gallo S. A., Finnegan C. M., Viard M., Raviv Y., Dimitrov A., Rawat S. S., Puri A., Durell S., Blumenthal R. (2003) The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 1614, 36–50 [DOI] [PubMed] [Google Scholar]
- 21. Ashkenazi A., Shai Y. (2011) Insights into the mechanism of HIV-1 envelope induced membrane fusion as revealed by its inhibitory peptides. [E-pub ahead of print] Eur. Biophys. J. doi: 10.1007/s00249-010-0666-z [DOI] [PubMed] [Google Scholar]
- 22. Peisajovich S. G., Blank L., Epand R. F., Epand R. M., Shai Y. (2003) On the interaction between gp41 and membranes: the immunodominant loop stabilizes gp41 helical hairpin conformation. J. Mol. Biol. 326, 1489–1501 [DOI] [PubMed] [Google Scholar]
- 23. Bar S., Alizon M. (2004) Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process. J. Virol. 78, 811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colman P. M., Lawrence M. C. (2003) The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4, 309–319 [DOI] [PubMed] [Google Scholar]
- 25. Schulz T. F., Jameson B. A., Lopalco L., Siccardi A. G., Weiss R. A., Moore J. P. (1992) Conserved structural features in the interaction between retroviral surface and transmembrane glycoproteins? AIDS Res. Hum. Retroviruses 8, 1571–1580 [DOI] [PubMed] [Google Scholar]
- 26. Caffrey M. (2001) Model for the structure of the HIV gp41 ectodomain: insight into the intermolecular interactions of the gp41 loop. Biochim. Biophys. Acta. 1536, 116–122 [DOI] [PubMed] [Google Scholar]
- 27. Ryser H. J., Levy E. M., Mandel R., DiSciullo G. J. (1994) Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc. Natl. Acad. Sci. U. S. A. 91, 4559–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barbouche R., Miquelis R., Jones I. M., Fenouillet E. (2003) Protein-disulfide isomerase-mediated reduction of two disulfide bonds of HIV envelope glycoprotein 120 occurs post-CXCR4 binding and is required for fusion. J. Biol. Chem. 278, 3131–3136 [DOI] [PubMed] [Google Scholar]
- 29. Markovic I., Stantchev T. S., Fields K. H., Tiffany L. J., Tomic M., Weiss C. D., Broder C. C., Strebel K., Clouse K. A. (2004) Thiol/disulfide exchange is a prerequisite for CXCR4-tropic HIV-1 envelope-mediated T-cell fusion during viral entry. Blood 103, 1586–1594 [DOI] [PubMed] [Google Scholar]
- 30. Ou W., Silver J. (2006) Role of protein disulfide isomerase and other thiol-reactive proteins in HIV-1 envelope protein-mediated fusion. Virology 350, 406–417 [DOI] [PubMed] [Google Scholar]
- 31. Billington J., Hickling T. P., Munro G. H., Halai C., Chung R., Dodson G. G., Daniels R. S. (2007) Stability of a receptor-binding active human immunodeficiency virus type 1 recombinant gp140 trimer conferred by intermonomer disulfide bonding of the V3 loop: differential effects of protein disulfide isomerase on CD4 and coreceptor binding. J. Virol. 81, 4604–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Syu W. J., Lee W. R., Du B., Yu Q. C., Essex M., Lee T. H. (1991) Role of conserved gp41 cysteine residues in the processing of human immunodeficiency virus envelope precursor and viral infectivity. J. Virol. 65, 6349–6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sen J., Jacobs A., Jiang H., Rong L., Caffrey M. (2007) The disulfide loop of gp41 is critical to the furin recognition site of HIV gp160. Protein Sci. 16, 1236–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fenouillet E., Barbouche R., Jones I. M. (2007) Cell entry by enveloped viruses: redox considerations for HIV and SARS-coronavirus. Antioxid. Redox Signal. 9, 1009–1034 [DOI] [PubMed] [Google Scholar]
- 35. Tan K., Liu J., Wang J., Shen S., Lu M. (1997) Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. U. S. A. 94, 12303–12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caffrey M., Cai M., Kaufman J., Stahl S. J., Wingfield P. T., Covell D. G., Gronenborn A. M., Clore G. M. (1998) Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 17, 4572–4584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weissenhorn W., Wharton S. A., Calder L. J., Earl P. L., Moss B., Aliprandis E., Skehel J. J., Wiley D. C. (1996) The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 15, 1507–1514 [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang S., Lin K., Strick N., Neurath A. R. (1993) HIV-1 inhibition by a peptide. Nature 365, 113 [DOI] [PubMed] [Google Scholar]
- 39. Kliger Y., Gallo S. A., Peisajovich S. G., Munoz-Barroso I., Avkin S., Blumenthal R., Shai Y. (2001) Mode of action of an antiviral peptide from HIV-1. Inhibition at a post-lipid mixing stage. J. Biol. Chem. 276, 1391–1397 [DOI] [PubMed] [Google Scholar]
- 40. Judice J. K., Tom J. Y., Huang W., Wrin T., Vennari J., Petropoulos C. J., McDowell R. S. (1997) Inhibition of HIV type 1 infectivity by constrained alpha-helical peptides: implications for the viral fusion mechanism. Proc. Natl. Acad. Sci. U. S. A. 94, 13426–13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan D. C., Kim P. S. (1998) HIV entry and its inhibition. Cell 93, 681–684 [DOI] [PubMed] [Google Scholar]
- 42. Eckert D. M., Kim P. S. (2001) Design of potent inhibitors of HIV-1 entry from the gp41 N-peptide region. Proc. Natl. Acad. Sci. U. S. A. 98, 11187–11192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wexler-Cohen Y., Shai Y. (2009) Membrane-anchored HIV-1 N-heptad repeat peptides are highly potent cell fusion inhibitors via an altered mode of action. PLoS Pathog. 5, e1000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu J., Deng Y., Li Q., Dey A. K., Moore J. P., Lu M. (2010) Role of a putative gp41 dimerization domain in human immunodeficiency virus type 1 membrane fusion. J. Virol. 84, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weiss A., Wiskocil R. L., Stobo J. D. (1984) The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J. Immunol. 133, 123–128 [PubMed] [Google Scholar]
- 46. Cao J., Park I. W., Cooper A., Sodroski J. (1996) Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 70, 1340–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Merrifield R. B., Vizioli L. D., Boman H. G. (1982) Synthesis of the antibacterial peptide cecropin A (1–33). Biochemistry 21, 5020–5031 [DOI] [PubMed] [Google Scholar]
- 48. Blumenthal R., Gallo S. A., Viard M., Raviv Y., Puri A. (2002) Fluorescent lipid probes in the study of viral membrane fusion. Chem. Phys. Lipids 116, 39–55 [DOI] [PubMed] [Google Scholar]
- 49. Huerta L., Lamoyi E., Baez-Saldana A., Larralde C. (2002) Human immunodeficiency virus envelope-dependent cell-cell fusion: a quantitative fluorescence cytometric assay. Cytometry 47, 100–106 [DOI] [PubMed] [Google Scholar]
- 50. Munoz-Barroso I., Durell S., Sakaguchi K., Appella E., Blumenthal R. (1998) Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 140, 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morris S. J., Sarkar D. P., White J. M., Blumenthal R. (1989) Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. Measurement by dequenching of fluorescence. J. Biol. Chem. 264, 3972–3978 [PubMed] [Google Scholar]
- 52. Reeves J. D., Gallo S. A., Ahmad N., Miamidian J. L., Harvey P. E., Sharron M., Pohlmann S., Sfakianos J. N., Derdeyn C. A., Blumenthal R., Hunter E., Doms R. W. (2002) Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. U. S. A. 99, 16249–16254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wexler-Cohen Y., Shai Y. (2007) Demonstrating the C-terminal boundary of the HIV 1 fusion conformation in a dynamic ongoing fusion process and implication for fusion inhibition. FASEB J. 21, 3677–3684 [DOI] [PubMed] [Google Scholar]
- 54. Liu S., Lu H., Niu J., Xu Y., Wu S., Jiang S. (2005) Different from the HIV fusion inhibitor C34, the anti-HIV drug fuzeon (T-20) inhibits HIV-1 entry by targeting multiple sites in gp41 and gp120. J. Biol. Chem. 280, 11259–11273 [DOI] [PubMed] [Google Scholar]
- 55. Miyauchi K., Kozlov M. M., Melikyan G. B. (2009) Early steps of HIV-1 fusion define the sensitivity to inhibitory peptides that block 6-helix bundle formation. PLoS Pathog. 5, e1000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. He Y., Cheng J., Li J., Qi Z., Lu H., Dong M., Jiang S., Dai Q. (2008) Identification of a critical motif for the human immunodeficiency virus type 1 (HIV-1) gp41 core structure: implications for designing novel anti-HIV fusion inhibitors. J. Virol. 82, 6349–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu S., Jing W., Cheung B., Lu H., Sun J., Yan X., Niu J., Farmar J., Wu S., Jiang S. (2007) HIV gp41 C-terminal heptad repeat contains multifunctional domains. Relation to mechanisms of action of anti-HIV peptides. J. Biol. Chem. 282, 9612–9620 [DOI] [PubMed] [Google Scholar]
- 58. Kemble G. W., Danieli T., White J. M. (1994) Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell 76, 383–391 [DOI] [PubMed] [Google Scholar]
- 59. Leikina E., LeDuc D. L., Macosko J. C., Epand R., Shin Y. K., Chernomordik L. V. (2001) The 1–127 HA2 construct of influenza virus hemagglutinin induces cell-cell hemifusion. Biochemistry 40, 8378–8386 [DOI] [PubMed] [Google Scholar]
- 60. Chernomordik L. V., Kozlov M. M. (2005) Membrane hemifusion: crossing a chasm in two leaps. Cell 123, 375–382 [DOI] [PubMed] [Google Scholar]
- 61. Garg H., Joshi A., Freed E. O., Blumenthal R. (2007) Site-specific mutations in HIV-1 gp41 reveal a correlation between HIV-1-mediated bystander apoptosis and fusion/hemifusion. J. Biol. Chem. 282, 16899–16906 [DOI] [PubMed] [Google Scholar]
- 62. Perfettini J. L., Castedo M., Roumier T., Andreau K., Nardacci R., Piacentini M., Kroemer G. (2005) Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 12(Suppl. 1), 916–923 [DOI] [PubMed] [Google Scholar]
- 63. Pinter A., Kopelman R., Li Z., Kayman S. C., Sanders D. A. (1997) Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J. Virol. 71, 8073–8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wallin M., Ekstrom M., Garoff H. (2004) Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 23, 54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li K., Zhang S., Kronqvist M., Wallin M., Ekstrom M., Derse D., Garoff H. (2008) Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus Env. J. Virol. 82, 7135–7143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maerz A. L., Center R. J., Kemp B. E., Kobe B., Poumbourios P. (2000) Functional implications of the human T-lymphotropic virus type 1 transmembrane glycoprotein helical hairpin structure. J. Virol. 74, 6614–6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Poumbourios P., Maerz A. L., Drummer H. E. (2003) Functional evolution of the HIV-1 envelope glycoprotein 120 association site of glycoprotein 41. J. Biol. Chem. 278, 42149–42160 [DOI] [PubMed] [Google Scholar]
- 68. Maerz A. L., Drummer H. E., Wilson K. A., Poumbourios P. (2001) Functional analysis of the disulfide-bonded loop/chain reversal region of human immunodeficiency virus type 1 gp41 reveals a critical role in gp120-gp41 association. J. Virol. 75, 6635–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jacobs A., Sen J., Rong L., Caffrey M. (2005) Alanine scanning mutants of the HIV gp41 loop. J. Biol. Chem. 280, 27284–27288 [DOI] [PubMed] [Google Scholar]
- 70. Bellamy-McIntyre A. K., Bar S., Ludlow L., Drummer H. E., Poumbourios P. (2010) Role for the disulfide-bonded region of human immunodeficiency virus type 1 gp41 in receptor-triggered activation of membrane fusion function. Biochem. Biophys. Res. Commun. 394, 904–908 [DOI] [PubMed] [Google Scholar]