Abstract
Plant evolved a complex profile of responses to cope with changes of nutrient availability in the soil. These are based on a stringent control of expression and/or activity of proteins involved in nutrients transport and assimilation. Furthermore, a sensing and signaling system for scanning the concentration of substrates in the rooted area and for transmitting this information to the plant machinery controlling root development can be extremely useful for an efficient plant response. Ammonium represents for plants either a preferential nitrogen source or the trigger for toxicity symptoms depending by its concentration. We propose a role for the high affinity Lotus japonicus ammonium transporter LjAMT1;3 as an intracellular ammonium sensor to achieve a convenient modulation of the root development in conditions of potentially toxic external ammonium concentration.
Key words: signaling, ammonium, transport, nutrients, root development, Lotus japonicus
Plants are sessile organisms that cope with large variations in soil, apoplastic and intracellular concentrations of mineral nutrients. This justifies the presence of a large number of proteins involved in nutrients uptake, intercellular and/or intracellular transport and sophisticated regulatory arrays to ensure a condition-dependent activity.
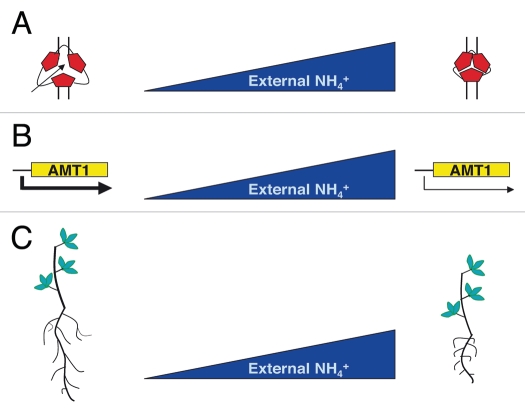
Plants can extract and use various forms of nitrogen (N) from soil, most importantly the inorganic ions ammonium (NH4+) and nitrate (NO3−). NH4+ assimilation requires less energy than that of nitrate,1 but only a few species perform well when NH4+ is the only or predominant source of N, whereas by contrast, most species develop toxicity symptoms in these conditions.2–7 The recent cloning of the genes for a large number of transport proteins and the availability of knockout mutants in Arabidopsis thaliana allowed the dissection of the ammonium transport process in greater detail and the understanding of how this might be modulated to respond to the environment commitments. The high-affinity ammonium transporters (AMT1) isolated in different plant species show a regulation of the expression at the transcriptional level. They are mainly induced in roots of plants deprived of N and downregulated after ammonium re-supply (Fig. 1B).8,9 Recently, a post-translational regulation of the AMT activity has been described for the A. thaliana AMT1;1 protein.10,11 Phosphorylation of the conserved T460, triggered by ammonium in a time- and concentration-dependent manner leads to allosteric inactivation of the AtAMT1;1 trimeric complex. This modification triggers the tuning of the uptake capacity, allowing the quick inactivation of transport in a potentially toxic environment (Fig. 1A).
Figure 1.
Chronology of the repertoire of plant protective responses to high ammonium conditions. (A) Regulation of ammonium uptake through the conformational control of the trimeric AtAMT1;1 protein.10,11 (B) Transcriptional downregulation of AMT1 genes expression.8,9 (C) Block of primary and secondary roots elongation.12,16
The investigation carried out by Rogato et al. allowed the characterization of an additional mechanism of plant response to potential cytotoxic ammonium external concentrations.12 In a high ammonium range of concentration, a specific short root phenotype in wild-type Lotus japonicus plants was reported (Fig. 1C). Primary and secondary roots elongation rates are drastically reduced in plants grown in a ≥10 mM ammonium condition when compared to plants grown in lower ammonium concentration or equivalent high nitrate/glutamine conditions. This is a specific root response that is not associated to any shoot phenotype or pleiotropic stress related symptoms. In addition, ammonium acts as a signal rather than as a nutrient and the effect is exerted locally on Lotus roots. This protective plant response might be needed to avoid energy waste in a potentially unfavorable environmental condition. Such a mechanism might be complementary to those underlying the ammonium transport regulation through the control of the AMT1 activity (Fig. 1A) and expression (Fig. 1B). As an alternative, the root developmental response might occur only in plants with a reduced capability to regulate the ammonium influx rate.6
The modulation of the root developmental program is a typical plant response to the changes of nutrients availability in the rhizosphere.13–15 A specific ammonium-dependent local inhibition of primary and secondary roots elongation has been very recently characterized also in A. thaliana where the effect is exerted mainly on root cell elongation and is associated to an increased efflux rate in the root elongation zone.16 This is consistent with the proposed mechanism explaining the NH4+-induced toxicity as consequence of an NH4+ futile cycle at the plasma membrane of root cells.6
This scenario entails the existence of a plant root derived mechanism for sensing the ammonium external concentration and for transducing this signal to the plant machinery driving primary and secondary roots growth. The results reported in Rogato et al. identify a potential ammonium sensor involved in this root developmental response.12 First, the high affinity ammonium transporter LjAMT1;3 is the only member of the AMT1 family to be induced in the high ammonium conditions, where the root phenotype is observed. Second, its overexpression in independent transgenic lines phenocopy the wild-type phenotype. Interestingly, the phenotype of the overexpressing plants is not related to the increased ammonium uptake, suggesting an additional signaling function of the AMT1;3 protein that is independent by its uptake activity. These features have been already described for different transporter proteins such as the Saccaromyces cerevisiae and Candida albicans methylammonium permease (Mep2), required for pseudohyphal differentiation in N starvation conditions, and the A. thaliana nitrate transporter (NRT1.1), controlling the secondary roots elongation in response to local patches of nitrate.17–19 In both cases a direct mutagenesis approach demonstrated the uncoupling of the transport and signaling functions, confirming their role as transceptors.18,19
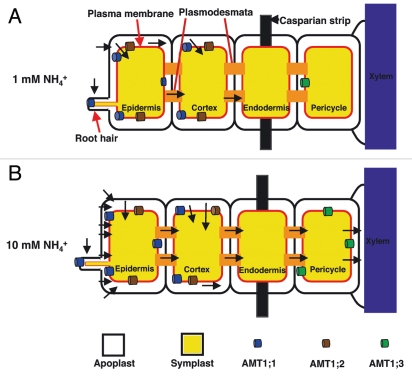
The LjAMT1;3, spatial profile of expression, confined to the root stele, makes unlikely its contribution to high-affinity ammonium uptake in roots. Nutrients such as ammonium are mostly absorbed at the epidermis (rhizodermis) and move symplastically through the cortex to the stele. However, nutrients may also enter the symplast later in cortical and endodermal cells, but the casparian strip provides a major barrier for further apoplastic movement. Yuang et al. proposed a model for a coordinated transport activity of the different AMT1 members in the Arabidopsis roots where AtAMT1 members, with different transport capacities and different root cell layers location, work in a synergic way by taking up ammonium from the soil and apoplastic space to allow NH4+ assimilation (Fig. 2).20 The model shown in Figure 2 to couple external ammonium concentration and short root phenotype proposes that in the presence of increasing external ammonium supply, a linear increase of the amount of un-assimilated ion reaching the xylematic tube occurs21,22 and LjAMT1;3 acts as an internal sensor, leading to the induction of its own expression. Therefore, LjAMT1;3 could perceive the intracellular ammonium transported to the vascular tube, that would reflect the external concentration (Fig. 2). In this model the ammonium acts as an intracellular signal,23,24 whereas in another report the apoplastic ammonium was proposed as the signal triggering the uptake regulation.11
Figure 2.
Model summarizing the putative action of LjAMT1;3 as intracellular ammonium sensor. (A and B) represent moderate and high ammonium external concentrations. The root cell layers, symplastic/apoplastic ammonium routes and AMT members are represented.
A likely connection can be hypothesized between the nutrient signaling governing root development and hormones-induced regulatory arrays. In the case of nitrate signaling the high affinity transporter NRT1.1 is itself a facilitator of the auxin uptake involved in secondary roots elongation,25 while the correlation between auxin and ammonium signaling pathways is not clear yet and needs further investigation.16,26
Acknowledgements
This work was supported by a grant from the Italian Ministry of Education (Progetti di Rilevanza Nazionale, PRIN 2008, Prot. 2008WKPAWW) and National Council of Research, Agrofood Department (award for funding Research of excellence).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/13856
References
- 1.Reisenauer HM. Absorption and utilization of ammonium by plants. In: Nielsen DR, Macdonald JG, editors. Nitrogen in the Environment. New York: Academic; 1978. pp. 157–189. [Google Scholar]
- 2.Barker AV, Volk RJ, Jackson WA. Root environment acidity as a regulatory factor in ammonium assimilation by the bean plant. Plant Physiol. 1966;41:1193–1199. doi: 10.1104/pp.41.7.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marschner H. Mineral Nutrition of Higher Plants. London: Academic Press; 1995. [Google Scholar]
- 4.Herbert J, Kronzucker M, Yaeesh MY, Siddiqi MY, Glass ADM. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature. 1997;385:59–61. [Google Scholar]
- 5.Kronzucker HJ, Schjoerring JK, Erner Y, Guy JD, Kirk M, Siddiqi Y, et al. Dynamic interactions between root NH4+ influx and long-distance N translocation in rice: Insights into feedback processes. Plant Cell Physiol. 1998;39:1287–1293. [Google Scholar]
- 6.Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ. Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA. 2001;98:4255–4258. doi: 10.1073/pnas.061034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronzucker HJ, Britto DT, Davenport RJ, Tester M. Ammonium toxicity and the real cost of transport. Trends Plant Sci. 2001;6:335–337. doi: 10.1016/s1360-1385(01)02022-2. [DOI] [PubMed] [Google Scholar]
- 8.Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MY, Glass AD. AtAMT1 gene expression and NH4+ uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. Plant J. 1999;19:143–152. doi: 10.1046/j.1365-313x.1999.00505.x. [DOI] [PubMed] [Google Scholar]
- 9.Howitt SM, Udvardi MK. Structure, function and regulation of ammonium transporters in plants. Biochim Biophys Acta. 2000;1465:152–170. doi: 10.1016/s0005-2736(00)00136-x. [DOI] [PubMed] [Google Scholar]
- 10.Loquè D, Lalonde S, Looger LL, von Wiren N, Frommer WB. A cytosolic trans-activation domain essential for ammonium uptake. Nature. 2007;446:195–198. doi: 10.1038/nature05579. [DOI] [PubMed] [Google Scholar]
- 11.Lanquar V, Loquè D, Hormann F, Yuan L, Bohner A, Engelsberger WR, et al. Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. Plant Cell. 2009;21:3610–3622. doi: 10.1105/tpc.109.068593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogato A, D'Apuzzo E, Barbulova A, Omrane S, Parlati A, Carfagna S, et al. Characterization of a developmental root response caused by external ammonium supply in Lotus japonicus. Plant Physiol. 2010;154:784–795. doi: 10.1104/pp.110.160309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forde BG, Lorenzo H. The nutritional control of root development. Plant and Soil. 2001;232:51–68. [Google Scholar]
- 14.Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, et al. Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet. 2007;39:792–796. doi: 10.1038/ng2041. [DOI] [PubMed] [Google Scholar]
- 15.Walch-Liu P, Forde BG. Nitrate signalling mediated by the Ntr1.1 nitrate transporter antagonizes L-glutamate-induced changes in root architecture. Plant J. 2008;54:820–828. doi: 10.1111/j.1365-313X.2008.03443.x. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Li BH, Kronzucker HJ, Shi WM. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environm. 2010:1529–1542. doi: 10.1111/j.1365-3040.2010.02162.x. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz MC, Heitman J. Regulators of pseudohyphal differentiation in Saccharomyces cerevisiae identified through multicopy suppressor analysis in ammonium permease mutant strains. Genetics. 1998;150:1443–1457. doi: 10.1093/genetics/150.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas K, Morschhauser J. The mep2p ammonium permease controls nitrogen starvation-induced filamentous growth in Candida albicans. Mol Microbiol. 2005;56:649–669. doi: 10.1111/j.1365-2958.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- 19.Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Yuan L, Loquè D, Kojima S, Rauch S, Ishiyama K, Inoue E, et al. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell. 2007;19:2636–2652. doi: 10.1105/tpc.107.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang MY, Siddiqi MY, Ruth TJ, Glass A. Ammonium uptake by rice roots. II. Kinetics of 13NH4+ influx across the plasmalemma. Plant Physiol. 1993;103:1259–1267. doi: 10.1104/pp.103.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finnemann J, Schjoerring JK. Translocation of NH4+ in oilseed rape plants in relation to glutamine synthetase isogene expression and activity. Physiol Plant. 1999;105:469–477. [Google Scholar]
- 23.Walden R. The alphabet soup of plant intracellular signalling: enter cyclic nucleotides. Curr Opin Plant Biol. 1998;1:419–423. doi: 10.1016/s1369-5266(98)80266-5. [DOI] [PubMed] [Google Scholar]
- 24.Tor M, Lotze MT, Holton N. Receptor-mediated signaling in plants: molecular patterns and programmes. J Exp Bot. 2009;60:3645–3654. doi: 10.1093/jxb/erp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 2010;18:927–937. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Cao Y, Glass ADM, Crawford NM. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1 and axr2. Plant Physiol. 1993;102:983–989. doi: 10.1104/pp.102.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]