Abstract
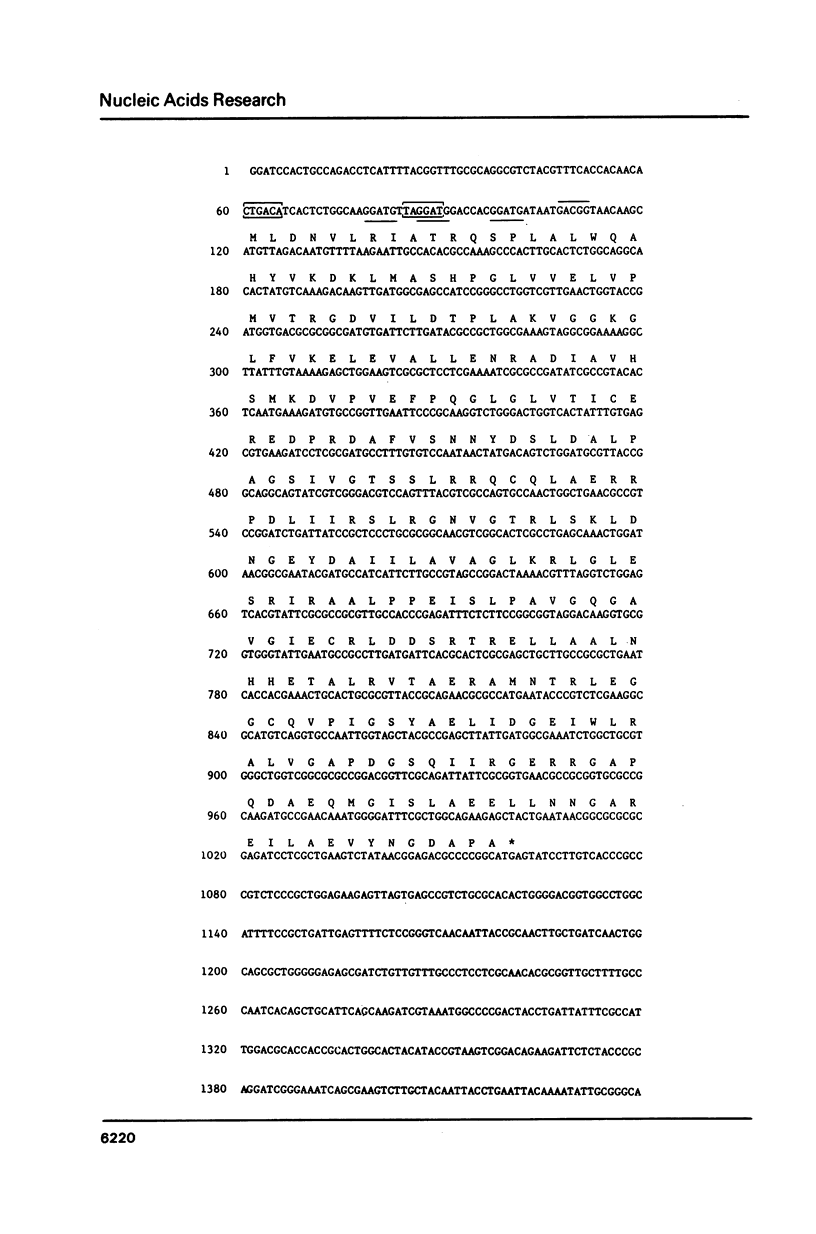
Porphobilinogen deaminase, the product of the hemC locus in Escherichia coli K12, catalyses the tetrapolymerisation of porphobilinogen (PBG) into the hydroxymethylbilane, preuroporphyrinogen. The hemC locus has been subcloned from the Clarke and Carbon plasmid pLC41-4. The sequence of the hemC structural gene and flanking DNA was determined by the dideoxy chain termination method of Sanger. The structural gene for hemC is located within a 942bp sequence encoding the monomeric PBG deaminase, molecular weight 33,857. The extent of the coding region was confirmed by sequencing the N-terminus of the purified enzyme and by determination of the molecular weight. The hemC locus is closely linked to the cyaA locus, the genes being transcribed in a divergent manner. Upstream of the hemC coding region, a possible promoter and three repeated GGATG sequences were identified. This is the first report of a complete DNA sequence for a structural gene specifying an enzyme of the heme biosynthetic pathway in prokaryotes.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Mori K., Tanaka M., Ooi T., Roy A., Danchin A. The complete nucleotide sequence of the adenylate cyclase gene of Escherichia coli. Nucleic Acids Res. 1984 Dec 21;12(24):9427–9440. doi: 10.1093/nar/12.24.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxham D. P., Parmelee D. C., Kumar S., Walsh K. A., Titani K. Complete amino acid sequence of porcine heart citrate synthase. Biochemistry. 1982 Apr 27;21(9):2028–2036. doi: 10.1021/bi00538a009. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Borthwick I. A., Srivastava G., Day A. R., Pirola B. A., Snoswell M. A., May B. K., Elliott W. H. Complete nucleotide sequence of hepatic 5-aminolaevulinate synthase precursor. Eur J Biochem. 1985 Aug 1;150(3):481–484. doi: 10.1111/j.1432-1033.1985.tb09047.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Berry A. Mechanism of action of porphobilinogen deaminase. The participation of stable enzyme substrate covalent intermediates between porphobilinogen and the porphobilinogen deaminase from Rhodopseudomonas spheroides. Biochem J. 1981 Apr 1;195(1):177–181. doi: 10.1042/bj1950177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Shemin D. Purification and properties of uroporphyrinogen I synthetase from Rhodopseudomonas spheroides. J Biol Chem. 1973 Feb 10;248(3):1019–1024. [PubMed] [Google Scholar]
- Laursen R. A., Horn M. J., Bonner A. G. Solid-phase Edman degradation. The use of p-phenyl diisothiocyanate to attach lysine- and arginine-containing peptides to insoluble resins. FEBS Lett. 1972 Mar;21(1):67–70. doi: 10.1016/0014-5793(72)80165-0. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Williams P. H., Ditta G. S. Analysis of the 5' regulatory region of the gene for delta-aminolevulinic acid synthetase of Rhizobium meliloti. Nucleic Acids Res. 1985 Aug 26;13(16):5965–5976. doi: 10.1093/nar/13.16.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. L., Charles H. P. Mutants of Escherichia coli K12 accumulating porphobilinogen: a new locus, hemC. J Gen Microbiol. 1979 Mar;111(1):193–200. doi: 10.1099/00221287-111-1-193. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Roy A., Danchin A. Restriction map of the cya region of the Escherichia coli K12 chromosome. Biochimie. 1981 Aug-Sep;63(8-9):719–722. doi: 10.1016/s0300-9084(81)80220-9. [DOI] [PubMed] [Google Scholar]
- Roy A., Danchin A. The cya locus of Escherichia coli K12: organization and gene products. Mol Gen Genet. 1982;188(3):465–471. doi: 10.1007/BF00330050. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R., McLachlan A. D. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. doi: 10.1093/nar/10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Urban-Grimal D., Volland C., Garnier T., Dehoux P., Labbe-Bois R. The nucleotide sequence of the HEM1 gene and evidence for a precursor form of the mitochondrial 5-aminolevulinate synthase in Saccharomyces cerevisiae. Eur J Biochem. 1986 May 2;156(3):511–519. doi: 10.1111/j.1432-1033.1986.tb09610.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams D. C., Morgan G. S., McDonald E., Battersby A. R. Purification of porphobilinogen deaminase from Euglena gracilis and studies of its kinetics. Biochem J. 1981 Jan 1;193(1):301–310. doi: 10.1042/bj1930301. [DOI] [PMC free article] [PubMed] [Google Scholar]


