Abstract
Preeclampsia is associated with increased circulating levels of proinflammatory molecules, such as soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng). On release by an inadequately perfused placenta into the maternal circulation, these molecules cause systemic endothelial dysfunction and the associated hypertension and proteinuria that characterize preeclampsia. We previously showed that glyceryl trinitrate (GTN) inhibits hypoxia/reoxygenation-induced apoptosis in the syncytiotrophoblast of term chorionic villi explants. Herein, we demonstrate that GTN inhibits the release of sFlt-1 and sEng from term chorionic villi explants exposed to hypoxia. Although transcript levels and secretion of sFlt-1 and sEng increased in explants exposed to hypoxia, low concentrations of GTN significantly inhibited the hypoxia-induced expression of these molecules at the mRNA and protein levels. Treatment of explants with GTN also prevented the hypoxia-induced accumulation of hypoxia-inducible factor-1α, a key mediator of cellular adaptations to hypoxia. Furthermore, knockdown of hypoxia-inducible factor-1α inhibited the hypoxia-induced secretion of sFlt-1 and sEng. This study provides evidence that hypoxia induces the release of sFlt-1 and sEng in the placenta via a mechanism that is inhibited by low concentrations of GTN. Our findings indicate that GTN may have potential applications in the treatment and/or prevention of preeclampsia.
Preeclampsia is a leading cause of perinatal and maternal morbidity and mortality, affecting 3% to 7% of pregnant women worldwide.1 It is characterized by the development of maternal hypertension, proteinuria, edema, and systemic coagulopathies. Although the etiology of preeclampsia is not well understood, there is evidence that high levels of antiangiogenic molecules [ie, soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng)] in the maternal circulation are linked to the endothelial dysfunction associated with this pregnancy complication.2–5
Secreted sFlt-1, usually a splice variant of Flt-1, is the soluble form of vascular endothelial growth factor receptor-1 (VEGFR-1). Soluble Flt-1 binds to angiogenic molecules (ie, placental growth factor and VEGF) and prevents them from interacting with VEGFR-1 and VEGFR-2 on the surface of endothelial cells.5,6 Consequently, any increase in blood sFlt-1 levels causes a decrease in bioavailable VEGF and placental growth factor, with corresponding inhibition of angiogenesis and vascular function. There is evidence that the placenta is a primary source of sFlt-1 in human pregnancy,7 and clinical studies have revealed that normal circulating sFlt-1 levels increase during gestation. However, compared with normal pregnancies, blood sFlt-1 levels are substantially higher in preeclamptic pregnancies.2 Clinical signs of preeclampsia may be a direct outcome of high concentrations of serum sFlt-1.5,8 Furthermore, animal experimentation has revealed that abnormally high circulating levels of sFlt-1 lead to hypertension, proteinuria, and endothelial damage.5,9
Eng is a cell membrane glycoprotein that functions as a coreceptor for transforming growth factor (TGF)-β signaling.10 sEng competes with the binding of TGF-β1 to its corresponding cell surface receptors and, consequently, interferes with downstream TGF-β signaling in the vasculature.4 Similar to signaling by VEGF and placental growth factor, TGF-β signaling plays an important role in the maintenance of vascular function and homeostasis. In preeclampsia, high levels of sEng in the maternal circulation correlate with increased expression of Eng in the placenta.4,11 Circulating sEng levels were markedly increased 2 to 3 months before the clinical onset of preeclampsia.12
Placental hypoxia may play a major role in the pathophysiological characteristics of preeclampsia9,13,14; hypoxia increases the secretion of sFlt-1 and sEng from first- and second-trimester chorionic villi explants.9,15–17 Although hypoxia also increases the secretion of sFlt-1 by third-trimester explants,18 its effect on sEng secretion by third-trimester explants has not been determined. Because the clinical signs of preeclampsia only appear in the second half of pregnancy, it is possible that a hypoxia-induced increase in sFlt-1 and sEng secretion in the second and third trimesters of gestation contributes to the pathophysiological characteristics of preeclampsia.
The hypoxia response pathway represents a potential therapeutic target for inhibiting placental secretion of sFlt-1 and sEng. A key player that mediates many adaptive responses to hypoxia is the transcriptional activator hypoxia-inducible factor (HIF)-1. This transcription factor is a basic helix-loop-helix-PAS domain protein composed of α and β subunits. Although the HIF-1β subunit is constitutively expressed, the levels of HIF-1α increase in response to hypoxia.19 The stability of HIF-1α is regulated by hydroxylation of proline residues 402, 564, or both in an oxygen-dependent reaction.20 Proline hydroxylation of HIF-1α promotes the binding of the von Hippel–Lindau protein, leading to ubiquitination and proteasomal degradation of HIF-1α.20,21
There is evidence that relatively low concentrations of nitric oxide (NO) inhibit the accumulation of HIF-1α under hypoxia, thereby interfering with HIF-1–mediated adaptive responses.22,23 Low concentrations (lower than micromolar) of NO mimetics [eg, glyceryl trinitrate (GTN)] inhibit the hypoxia-induced acquisition of malignant phenotypes in cancer cells, such as invasiveness, metastasis, and drug resistance.24,25 Although the precise role of endogenous NO in normal and pathological pregnancy is unclear, there is evidence that decreased endogenous NO availability contributes to the pathophysiological characteristics of preeclampsia.26 A previous study27 demonstrated that low concentrations of GTN inhibit apoptosis of the syncytiotrophoblast in chorionic villi explants exposed to hypoxia/reoxygenation (H/R). In the present study, we determined the effect of administration of low concentrations of GTN on the secretion of sFlt-1 and sEng from term chorionic villi explants exposed to hypoxia.
Materials and Methods
Collection and Culture of Chorionic Villi Explants
Human term placentas (n = 22) were obtained from uncomplicated pregnancies immediately after caesarean deliveries at Kingston General Hospital, Kingston, ON. Placentas were collected with the approval of the Queen's University Research Ethics Board. Explants of chorionic villi were prepared as previously described27 and incubated in 1.3 mL of serum-free RPMI 1640 medium (Invitrogen, Burlington, ON). For exposures to hypoxia, explants (approximately 60 per experiment) treated with or without GTN (10 nmol/L or 1 μmol/L; Sabex, Boucherville, QC) were incubated at 37°C for 24 hours in a humidified Plexiglas chamber flushed with a gas mixture of 5% CO2 and 95% N2. Oxygen concentrations within the chamber were maintained at 0.5% (PO2 = 3.8 mm Hg) by oxygen regulators (ProOx 110; Biospherix Inc., Lacona, NY). Controls consisted of explants incubated in either 8% O2 (PO2 = 60 mm Hg, physiological concentrations) or 20% O2 (PO2 = 152 mm Hg) and 5% CO2 for 24 hours. Explants were then collected or exposed to conditions of reoxygenation (20% O2) for 3 additional hours. At the end of all incubations, placental tissues were either flash frozen for molecular analysis or fixed in 4% paraformaldehyde for histological analysis. Media of cultured explants were collected, centrifuged for 10 minutes at 8000 × g, divided into small tubes, and stored at -80°C until further analysis.
Immunohistochemistry
Randomly selected paraffin-embedded sections of chorionic villi were deparaffinized and subjected to antigen retrieval by heating them in a microwave oven for 22 minutes in citrate buffer (0.1 mol/L sodium citrate, pH 6.0). Primary antibodies used were goat polyclonal anti-human Eng or Flt-1 antibody (1 μg/mL; R&D Systems, Minneapolis, MN), mixed with mouse anti–human pan-cytokeratin antibody (1:100; Sigma-Aldrich, St Louis, MO) and incubated at 4°C overnight. Secondary antibodies were a mix of donkey anti-mouse (Alexa Fluor 568) and donkey anti-goat (Alexa Fluor 488) antibodies (1:500 each; Invitrogen, Carlsbad, CA). Slides were mounted with medium (VECTASHIELD Mounting Medium) containing DAPI (Vector Laboratories, Inc., Burlingame, CA). Micrographs were taken using a microscope (Zeiss Imager A1 Microscope; Carl Zeiss Canada Ltd, Toronto, ON) and software (Axiovision v 4.7.1.0; Carl Zeiss Canada Ltd).
Western Blot Analysis of HIF-1α
To assess the ability of chorionic villi to respond to hypoxia, the levels of HIF-1α were determined by Western blot analysis of flash-frozen chorionic villi preexposed to 0.5%, 8%, or 20% O2 for 24 hours (n = 3 to 6 placentas). Primary antibodies used were mouse monoclonal antibody against HIF-1α (1:250; BD Biosciences, San Diego, CA), goat polyclonal anti–TGF-β1 (1:1000, R&D Systems), and monoclonal anti–β-actin antibody (clone AC-15, 1:8000; Bio-Rad Laboratories Ltd, Mississauga, ON, Canada). Goat anti-mouse antibody (1:5000, Vector Laboratories Inc.) labeled with horseradish peroxidase was used as a secondary antibody. The relative intensities of bands were determined by densitometry using software (AlphaErase; α Innotech Corp, San Leandro, CA).
Knockdown of HIF-1α in Chorionic Villi Explants
To down-regulate HIF-1α expression in chorionic villi, HIF-1α small-interfering RNA (siRNA) was introduced into explants using a transfection reagent (siPORT NeoFX; Ambion Inc., Austin, TX). This HIF-1α siRNA was validated and targets exon 5 of the human HIF-1α gene (number 42840; Ambion Inc.). Explants were transfected with 200 nmol/L HIF-1α siRNA or negative control siRNA 2 (Silencer; Ambion Inc.) and then incubated in a standard incubator at 5% CO2 and 20% O2 (151 mm Hg) for 24 hours. After this incubation, the culture medium was replaced with fresh RPMI 1640 medium (Invitrogen, Burlington, ON, Canada) and explants were further incubated for 24 hours in either 20% or 0.5% O2, as previously described.
Determination of sFlt-1 and sEng Levels in the Culture Media
To determine the levels of sFlt-1 or sEng in the culture media, kits (DuoSet ELISA Development System kits; R&D Systems) were used according to the manufacturer's instructions.
Real-Time PCR
Total RNA was isolated using a kit (High Pure RNA Isolation Kit; Qiagen, Valencia, CA), according to the manufacturer's protocol. Total RNA, 1 μg, was reverse transcribed with a kit (Omniscript RT Kit; Qiagen) using a random hexamer (Cortex, Kingston). Real-time PCR was performed with a system (LightCycler 480 Real-Time PCR System; Roche, Mississauga, ON) using a mix (SYBR Green PCR Master Mix; Roche) and primers, as follows: β-actin, 5′-TGGGACGACATGGAGAAAAT-3′ (sense) and 5′-GAGGCGTACAGGGATAGCAC-3′ (antisense); Flt-1, 5′-GCACCTTGGTTGTGGCTGAC-3′ (sense) and 5′-TGGAATTCGTGCTGCTTCCTGGTCC-3′ (antisense); sFlt-1, 5′-GCACTGCAACAAAAAGGC-3′ (sense) and 5′-CCAGGAATGTATACACAGG-3′ (antisense); Eng, 5′-GCTGGATGAGCCGGGAGCTCCCTGCTG-3′ (sense) and 5′-CACAGGCTGAAGGTCACAATGGACTG-3′ (antisense); and HIF-1α, 5′-TGCTTGGTGCTGATTTGTGA-3′ (sense) and 5′-GGTCAGATGATCAGAGTCCA-3′ (antisense). Real-time reaction conditions for all sets of primers were as follows: 94°C for 5 minutes, followed by 60 cycles at 94°C for 20 seconds, 55°C for 20 seconds, and 72°C for 30 seconds. The intensity of dye (FastStart SYBR Green) was analyzed using software (LightCycler 480; Roche). Transcript levels were normalized against β-actin mRNA levels.
Statistical Analysis
For statistical analysis of enzyme-linked immunosorbent assay and quantitative PCR results, one-way analysis of variance was performed and significant differences between groups were determined using Tukey's multiple comparisons post hoc test. All statistical tests were two sided, and differences were considered significant at P < 0.05.
Results
Immunolocalization of Flt-1 and Eng in Chorionic Villi Explants
To determine the potential source of secreted Flt-1 and Eng, immunofluorescence using polyclonal antibodies against Flt-1 and Eng was performed on sections of chorionic villi explants incubated in 20% O2 (see Supplemental Figure S1, A and E, at http://ajp.amjpathol.org). Cytokeratin was used as a marker for trophoblast (see Supplemental Figure S1, B and F, at http://ajp.amjpathol.org). Both Flt-1 and Eng colocalized with cytokeratin primarily to the syncytiotrophoblast layer (see Supplemental Figure S1, D and H, at http://ajp.amjpathol.org), indicating that this tissue is a potential source of the secreted molecules. We also observed that Flt-1, but not Eng, is expressed in the fetal vessels within the villous stroma, indicating that fetal vessels are another potential source of sFlt-1 but not of sEng (see Supplemental Figure S1, D versus H, respectively, at http://ajp.amjpathol.org).
Effect of Hypoxia on the Levels of sFlt-1 and Eng mRNA and Secreted Protein
Previous studies14,15,17,28 reported hypoxia-induced sFlt-1 and sEng secretion by trophoblast cell lines and first-trimester chorionic villi explants. Given that preeclampsia is mostly a disease of the second half of pregnancy, we examined the effect of hypoxia on the mRNA levels of Flt-1 variants and Eng by term chorionic villi explants. The secreted form of Flt-1 either can be encoded by a spliced variant of the Flt-1 (spliced Flt-1) gene or, less frequently, is the product of the cleaved ectodomain of the Flt-1 receptor (receptor Flt-1).29,30 To determine whether a specific variant of Flt-1 is responsive to hypoxia in term villous explants, we examined both species of Flt-1 mRNA using real-time PCR. Exposure to 0.5% O2 increased the expression of both the spliced Flt-1 and receptor Flt-1 mRNA species compared with exposure to either 20% or 8% O2 (Figure 1A). However, although the expression of the spliced Flt-1 variant increased several thousandfold, the levels of transcript encoding the receptor Flt-1 variant increased by only approximately twofold. Consequently, the ratio of spliced Flt-1/receptor Flt-1 significantly increased in chorionic villi exposed to hypoxia compared with chorionic villi exposed to either 20% or 8% O2 (Figure 1A: P < 0.001 for both). This ratio is an indication of increased bioavailability of the spliced (secreted) Flt-1 variant compared with nonspliced Flt-1 receptor in chorionic villi exposed to hypoxia. Exposure of chorionic villi to hypoxia (0.5% O2) resulted in a fivefold and a threefold increase in Eng mRNA levels compared with exposure to 20% and 8% O2, respectively (Figure 1B: P < 0.001 for both). Compared with exposure to 20% O2, exposure to 8% O2 did not result in statistically significant increases in the levels of sFlt-1 and sEng mRNA.
Figure 1.
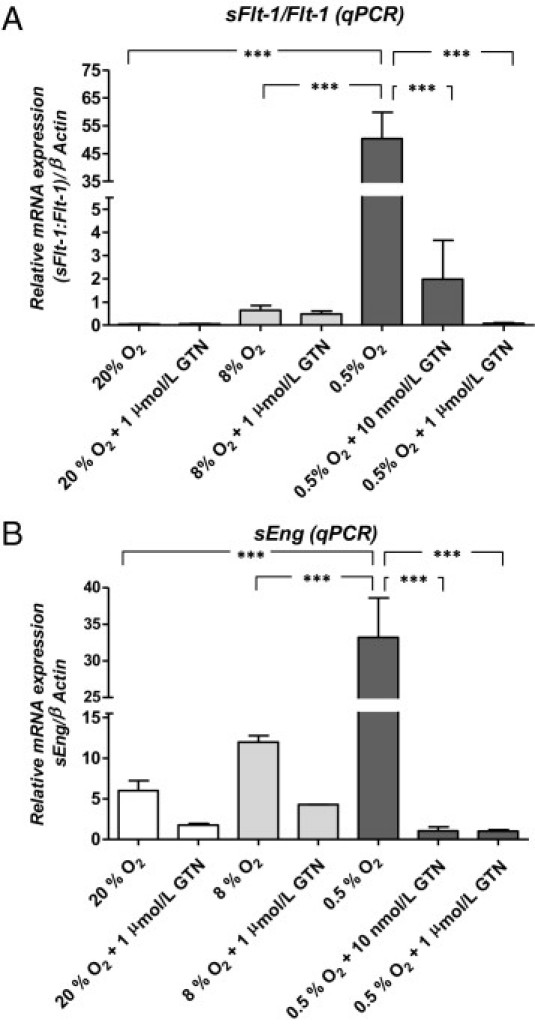
Effect of GTN on hypoxia-induced increases in sFlt-1 and sEng mRNA levels in chorionic villi explants. (minimum of three placentas per treatment) A: Quantitative PCR (qPCR) analysis of Flt-1 mRNA levels (expressed as the ratio of spliced Flt-1/receptor Flt-1 mRNA forms). Transcript levels were normalized against β-actin mRNA levels. B: qPCR analysis of sEng; mRNA levels were quantified relative to β-actin transcript levels. Error bars represent SEM. ***P < 0.001. Transverse lines link two groups to indicate statistical significance.
To further characterize the effect of hypoxia on the secretion of sFlt-1 and sEng, we performed an enzyme-linked immunosorbent assay on the media of explant cultures. Explants exposed to 0.5% O2 for 24 hours secreted significantly higher levels of sFlt-1 and sEng than explants incubated in 20% or 8% O2 (Figure 2, A and B). Although the data shown in Figure 2 are expressed as pictograms of sFlt-1 or sEng per microgram of protein in the media, differences in the reported concentrations of these molecules were not the result of differences in the total amount of protein secreted by chorionic villi; the latter was similar across the different treatment groups (see Supplemental Figure S2 at http://ajp.amjpathol.org). Compared with exposure to 20% O2 alone, exposure of chorionic villi to H/R (24 hours in 0.5% O2, followed by 3 hours in 20% O2) did not significantly increase the detectable levels of sFlt-1 and sEng in the culture medium (see Supplemental Figure S3, A and B, at http://ajp.amjpathol.org). Accordingly, compared with exposure to hypoxia for 24 hours, subsequent reoxygenation of chorionic villi explants (3 hours in 20% O2) resulted in a significant decrease in the levels of detectable sFlt-1 and sEng (see Supplemental Figure S4, A and B, at http://ajp.amjpathol.org; P < 0.05 for both).
Figure 2.
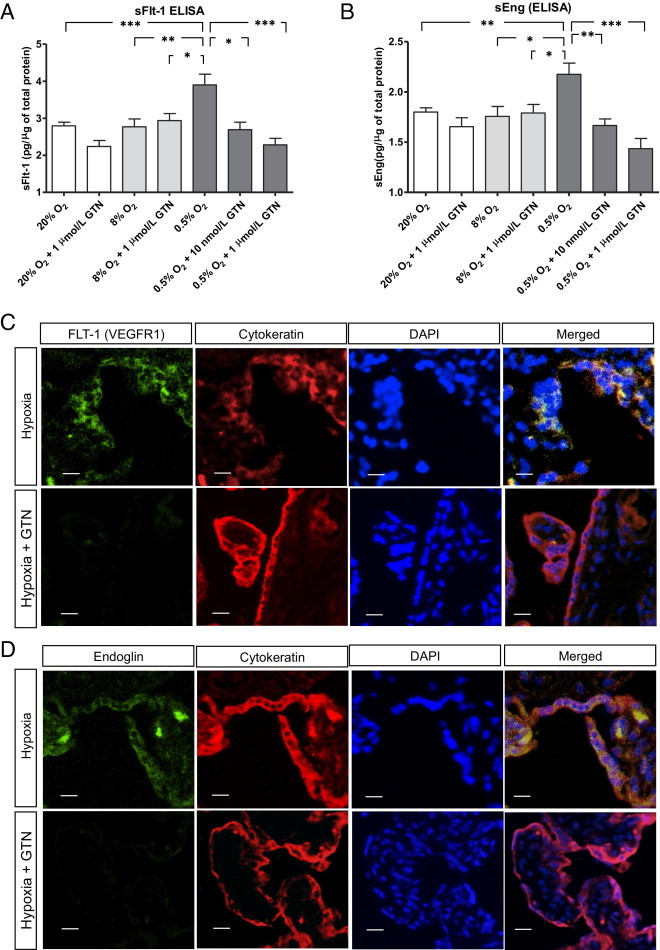
Effect of GTN on the secretion and expression of sFlt-1 and sEng by chorionic villi explants exposed to hypoxia. A and B: Relative sFlt-1 and sEng levels, respectively, in the medium of explant cultures (six explants per placenta, with a minimum of three placentas per treatment). Concentrations (in ng/mL) were divided by the total amount of protein (in μg/mL) in the media. Error bars represent SEM. The lines in A and B identify the comparison groups by the vertical lines pointing to the corresponding bars. C and D: Localization of Flt-1, Eng, cytokeratin (trophoblast marker), and nuclei (DAPI) by fluorescence microscopy. Far right: The merged images with all three markers combined, with yellow representing areas of colocalization of sFlt-1 or Eng with cytokeratin, are shown. Top: Explants exposed to hypoxia (0.5% O2). Bottom: Explants exposed to hypoxia in the presence of 1 μmol/L GTN. *P < 0.05, **P < 0.01, and ***P < 0.001. Transverse lines link two groups to indicate statistical significance. Scale bars = 10 μm. ELISA indicates enzyme-linked immunosorbent assay.
Effect of GTN on the Hypoxia-Induced Increases in sFlt-1 and sEng mRNA Levels and Secretion
The hypoxia-mediated increases in sFlt-1 and sEng mRNA accumulation and protein secretion were significantly inhibited by GTN at concentrations of 10 nmol/L or 1 μmol/L (Figure 1, A and B, and Figure 2, A and B). The inhibitory effect of GTN at 1 μmol/L was confirmed by immunofluorescence microscopy of explants using polyclonal antibodies against Flt-1 or Eng. Compared with exposure to hypoxia alone, Flt-1 and Eng immunofluorescence intensity under hypoxic conditions was substantially decreased after treatment with 1 μmol/L GTN (Figure 2, C and D).
Effect of Hypoxia and GTN on HIF-1α Accumulation
To elucidate the mechanism by which GTN inhibits hypoxia-induced secretion of sFlt-1 and sEng, we examined the effect of GTN on the accumulation of HIF-1α in explants incubated in 0.5% O2. HIF-1α levels were significantly increased in explants exposed to 0.5% O2 compared with HIF-1α levels in explants exposed to 20% O2 (Figure 3, A and B: P < 0.01) or 8% O2 (Figure 3, A and B: P < 0.05). Treatment with GTN at a concentration of 1 μmol/L or 10 nmol/L significantly inhibited the accumulation of HIF-1α in explants exposed to 0.5% O2 (Figure 3, A and B: P < 0.001 and P < 0.01, respectively). Consistent with the inhibitory effects of GTN on sFlt-1 and sEng secretion, the most pronounced suppression of HIF-1α accumulation was achieved with GTN at a concentration of 1 μmol/L.
Figure 3.
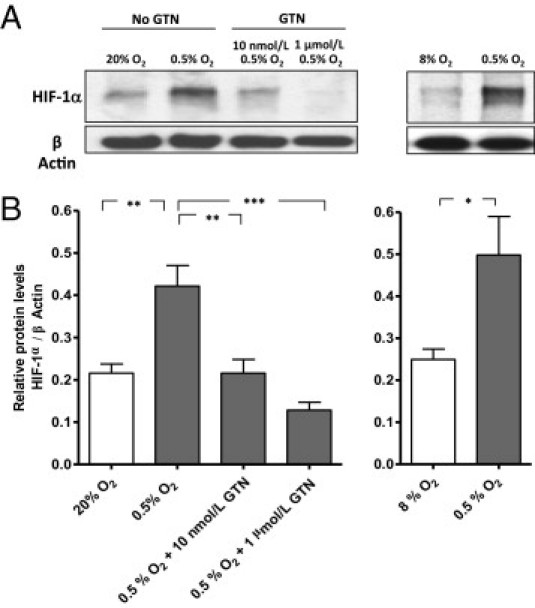
Effect of GTN on the hypoxia-induced accumulation of HIF-1α in chorionic villi explants. A: Western blot analysis of HIF-1α protein in explants cultured in 20%, 8%, or 0.5% O2. The administration of GTN (1 μmol/L or 10 nmol/L) inhibited the accumulation of HIF-1α in explants incubated in 0.5% O2. Incubation of explants in 8% O2 did not lead to accumulation of HIF-1α. B: Densitometric quantification of pooled Western blots from three independent experiments. The lines in A and B identify the comparison groups by the vertical lines pointing to the corresponding bars. *P < 0.05, **P < 0.01, and ***P < 0.001. (Minimum of three placentas per treatment).
Effect of HIF-1α Knockdown on the Secretion of sFlt-1 and sEng
To determine whether HIF-1 mediates the hypoxia-induced secretion of sFlt-1 and sEng, we knocked down HIF-1α mRNA in chorionic villi explants. Villi exposed to 0.5% O2 significantly expressed higher levels of HIF-1α mRNA compared with villi exposed to 20% O2 or villi exposed to 0.5% O2 and transfected with HIF-1α siRNA (Figure 4A: P < 0.001 for both). Chorionic villi with higher HIF-1α expression levels (ie, exposed to 0.5% O2) secreted more sFlt-1 and sEng than those with lower HIF-1α mRNA levels (ie, exposed to 20% O2; Figure 4, B and C: P < 0.05 and P < 0.01, respectively). Knockdown of HIF-1α with siRNA inhibited the hypoxia-induced secretion of sFlt-1 and sEng (Figure 4, B and C: P < 0.001 for both). Furthermore, explants transfected with HIF-1α siRNA secreted significantly lower levels of sFlt-1 and sEng than explants transfected with scrambled negative control siRNA (Figure 4, B and C: P < 0.05 and P < 0.01, respectively). These results indicate that HIF-1α is a key mediator of the hypoxia-induced secretion of sFlt-1 and sEng. These findings also indicate a hypoxia-induced up-regulation of HIF-1α at the mRNA level, which was previously reported.31 However, our findings do not exclude the possibility that hypoxia may also increase HIF-1α levels by increasing protein stability.23,32
Figure 4.
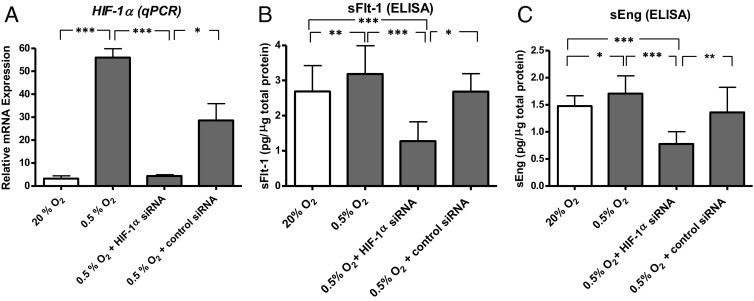
Effect of HIF-1α knockdown on the hypoxia-induced increase in mRNA levels and secretion of sFlt-1 and sEng by third-trimester chorionic villi explants. (minimum of three placentas per treatment). A: Expression of HIF-1α transcript levels relative to β-actin transcript levels as determined by real-time quantitative PCR (qPCR). B and C: Relative levels of sFlt-1 and sEng protein in the tissue culture media. Values (in ng/mL) were divided by the total amount of protein (in μg/mL) in the media. The lines in A and B identify the comparison groups by the vertical lines pointing to the corresponding bars. *P < 0.05, **P < 0.01, and ***P < 0.001. Lines with asterisks denote the comparison groups. ELISA indicates enzyme-linked immunosorbent assay.
Discussion
The main novel finding that we report herein is that hypoxia-induced secretion of sFlt-1 and sEng by chorionic villi explants can be inhibited by low concentrations of GTN. Given that hypoxia and increased circulating levels of sFlt-1 and sEng contribute to the pathophysiological characteristics of preeclampsia, the results of this study indicate that administration of low doses of NO mimetics to women with preeclampsia or at a high risk of developing this complication may be a useful therapeutic or preventative strategy.
Other studies14,17,28 have focused primarily on determining the effect of hypoxia on placental sFlt-1 secretion; increased sFlt-1 secretion during preeclampsia may be part of a placental response to hypoxia, whereby sFlt-1 increases maternal blood pressure to improve placental perfusion. Hypoxia-mediated stimulation of sFlt-1 and sEng secretion by first-trimester chorionic villi explants was previously reported.15,17,28 However, the role of hypoxia in the regulation of sEng secretion is controversial. Although some studies28,33 have described a lack of effect of hypoxia on sEng secretion, others15,34 have found a stimulatory effect in first-trimester explants and preeclamptic term placentas but not in normal-term placentas. In light of our results, it is possible that sEng secretion is dependent on gestational age and length of exposure to hypoxia. In women who later develop preeclampsia, increased circulating sFlt-1 and sEng levels are first detected in the late second and early third trimesters, respectively, before the clinical onset of disease.12,35 Thus, cultures of third-trimester explants may represent a more accurate model to study the effects of hypoxia on placental sFlt-1 and sEng secretion.
In our study, only hypoxia, and not H/R, resulted in increased detection of sFlt-1 and sEng in the culture medium. Although the effect of H/R on the secretion of sFlt-1 and sEng by chorionic villi was not previously examined, Cindrova-Davies36 reported a significant increase in tissue-associated sFlt-1 levels in chorionic villi after exposure to H/R. In our study, compared with exposure to hypoxia alone, exposure to conditions of reoxygenation resulted in decreased detectable levels of sFlt-1 and sEng. We cannot exclude that H/R increased the secretion of sFlt-1 and sEng by chorionic villi explants. It is possible that the decreased detection of these molecules after H/R was the result of sequestration by high levels of secreted VEGF and TGF-β, which can potentially interfere with the detection assay. In support of this concept, Western blot analysis revealed substantial increases in the levels of TGF-β1 in the culture medium of explants exposed to H/R compared with media from explants incubated in 20% or 0.5% O2 alone (see Supplemental Figure S4C at http://ajp.amjpathol.org).
Although exposure of chorionic villi to hypoxia alone can lead to apoptosis,27,37 thereby resulting in the release of vasoactive molecules, we showed herein that the hypoxia-induced release of sFlt-1 and sEng can also be transcriptionally regulated. This conclusion is based on the observation that the hypoxia-induced expression and release of these molecules was mediated by HIF-1α. In our study, both HIF-1α protein and mRNA levels increased in chorionic villi exposed to hypoxia. Although HIF-1α levels are mostly regulated at the level of protein stabilization, there is evidence that hypoxia can increase the levels of HIF-1α mRNA in chorionic villi exposed to hypoxia.31
Our results also revealed that the increased ratio of spliced Flt-1/receptor Flt-1 mRNA and the increase in Eng mRNA levels were much higher than the secreted protein levels. To our knowledge, this is the first report on the ratio of spliced Flt-1/receptor Flt-1 mRNA in the placenta and may, in part, explain the observed large increase in sFlt-1 mRNA levels. Differences between secreted protein and mRNA levels may be explained at the level of RNA stability and efficiency of the translational machinery.
Our findings support the results of previous studies34,38 showing that the syncytiotrophoblast is a major source of Flt-1 and Eng. We have demonstrated that the expression and secretion of Flt-1 and Eng from syncytiotrophoblast exposed to hypoxia can be inhibited by GTN. The rationale for testing the effect of GTN on the expression and release of these molecules was based on a previous study27 in which H/R-mediated apoptosis of term chorionic villi was significantly attenuated by low concentrations of GTN. It is possible that the effects of GTN, as an NO mimetic, reflect the well-known actions of NO. At lower concentrations, such as those used in our study, NO activates soluble guanylyl cyclase to generate cGMP. Under certain conditions involving low oxygenation, NO can also inhibit mitochondrial respiration so that oxygen becomes available for the activation of prolyl hydroxylases and the degradation of HIF-1α.23,32 Herein, we showed that GTN was able to inhibit HIF-1α protein accumulation. The precise mechanism by which GTN inhibited HIF-1α accumulation requires further investigation. However, based on this observation, together with the finding that siRNA-mediated knockdown of HIF-1α prevented the hypoxia-induced release of sFlt-1 and sEng, we conclude that the inhibitory effect of GTN on the hypoxia-induced secretion of sFlt-1 and sEng was likely the result of inhibition of HIF-1 activity.
The pathophysiological characteristics of preeclampsia and other complications of pregnancy have been linked to a maternal immune imbalance with greater secretion of proinflammatory cytokines and a relative decrease in the levels of anti-inflammatory molecules, such as IL-10.39,40 Although the administration of IL-10 to chorionic villi explants did not affect the secretion of sFlt-1,41 treatment of explants with exogenous sFlt-1 increased IL-10 and tumor necrosis factor-α secretion.42 It is possible that, by increasing the secretion of tumor necrosis factor-α in the placenta, sFlt-1 contributes to the maternal inflammatory state characteristic of severe preeclampsia.
Women who have had preeclampsia are at increased risk of recurrence in a subsequent pregnancy.43,44 Despite the fact that the cause of preeclampsia is largely unknown, the increase in sFlt-1 and sEng levels in the maternal circulation is tightly linked to the pathophysiological characteristics of preeclampsia. Current therapeutic strategies have relied primarily on managing symptoms, with little evidence that any intervention alters the underlying pathophysiological characteristics.43 For example, antihypertensive and antiplatelet drugs have been used to treat severe hypertension and coagulopathies associated with preeclampsia.43 However, it is unclear that such approaches have an impact on other maternal and fetal comorbidities. Furthermore, a recent study45 revealed that antihypertensive drugs had no effect on placental secretion of sFlt-1 and sEng. There are few published studies on the use of GTN for the treatment of preeclampsia. Some studies46–48 have reported beneficial effects of GTN in preeclamptic patients with deficient uteroplacental blood flow. Other studies49 did not reveal the beneficial effects of this drug. In those studies, it is possible that patients developed tolerance to GTN because of continuous use of relatively high doses of this drug. It is also possible that only a subset of patients with preeclampsia benefit from GTN therapy (specifically, patients with hypoxic placentas and elevated serum levels of sFlt-1 and/or sEng).
In summary, our current study reveals a novel strategy to inhibit the secretion of sFlt-1 and sEng from placental tissues exposed to hypoxia and, therefore, has the potential of leading to novel modalities for the management and prevention of preeclampsia.
Acknowledgment
We thank Shannyn Macdonald-Goodfellow (BAH, Queen's University, Kingston, ON, Canada) for her help with the processing and culturing of placental explants.
Footnotes
Supported by a grant from the Heart and Stroke Foundation of Ontario (T-6046).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi:10.1016/j.ajpath.2011.02.013.
Current address of S.J.R., Institute of Maternal-Fetal Biology, University of Kansas Medical Center, Kansas City, Kansas.
Supplementary data
Immunolocalization of Flt-1 (VEGFR-1) and Eng in term chorionic villi explants. A and E: Localization of Flt-1 (A, green) and Eng (E, green) by immunofluorescence. B and F: Immunolocalization of cytokeratin as a marker for trophoblast (red). C and G: Nuclei are highlighted by DAPI fluorescence (blue). D and H: The merged image of all three fluorescent labels; yellow fluorescence reveals areas where Flt-1 or Eng colocalize with cytokeratin. The figures are representative of three independent experiments.
Differences in total protein secreted in the medium of cultured chorionic villi explants. For different O2 concentrations (six explants per placenta, with a minimum of three placentas per treatment), the total amount of proteins secreted in the medium was determined. No significant differences in the levels of released protein were observed between any of the groups. Error bars represent SEM.
Effect of GTN on the secretion of sFlt-1 and sEng by chorionic villi explants exposed to H/R. A and B: Relative sFlt-1 and sEng levels, respectively, in the media of explant cultures (six explants per placenta, with a minimum of three placentas per treatment). Values (in pg/mL) were divided by the total amount of protein (in μg/mL) in the media. No significant differences in the levels of sFlt-1 and sEng in the media were observed.
Effect of reoxygenation on chorionic villi explants previously exposed to hypoxia for 24 hours. A and B: Levels of sFlt-1 and sEng in the culture media from hypoxia-exposed explants before and after reexposure to 20% O2 (H/R). Media from six to seven explants per placenta from two placentas were used. Statistical significance was measured using a paired Student's t-test. C: Representative Western blot (of three different analyses) of TGF-β1 in the media of explants exposed to 20%, 0.5%, or 0.5% O2, followed by reoxygenation at 20% O2 (H/R). D: Densitometric analysis of the Western blot shown in C. Error bars represent SEM. *P < 0.05.
References
- 1.Redman C.W., Sargent I.L. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Koga K., Osuga Y., Yoshino O., Hirota Y., Ruimeng X., Hirata T., Takeda S., Yano T., Tsutsumi O., Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 3.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F., Schisterman E.F., Thadhani R., Sachs B.P., Epstein F.H., Sibai B.M., Sukhatme V.P., Karumanchi S.A. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesha S., Toporsian M., Lam C., Hanai J., Mammoto T., Kim Y.M., Bdolah Y., Lim K.H., Yuan H.T., Libermann T.A., Stillman I.E., Roberts D., D'Amore P.A., Epstein F.H., Sellke F.W., Romero R., Sukhatme V.P., Letarte M., Karumanchi S.A. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 5.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., Epstein F.H., Sukhatme V.P., Karumanchi S.A. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad S., Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 7.Banks R.E., Forbes M.A., Searles J., Pappin D., Canas B., Rahman D., Kaufmann S., Walters C.E., Jackson A., Eves P., Linton G., Keen J., Walker J.J., Selby P.J. Evidence for the existence of a novel pregnancy-associated soluble variant of the vascular endothelial growth factor receptor: Flt-1. Mol Human Reprod. 1998;4:377–386. doi: 10.1093/molehr/4.4.377. [DOI] [PubMed] [Google Scholar]
- 8.Tsatsaris V., Goffin F., Munaut C., Brichant J.-F., Pignon M.-R., Noel A., Schaaps J.-P., Cabrol D., Frankenne F., Foidart J.-M. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 9.Makris A., Thornton C., Thompson J., Thomson S., Martin R., Ogle R., Waugh R., McKenzie P., Kirwan P., Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFlt-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 10.Cheifetz S., Bellon T., Cales C., Vera S., Bernabeu C., Massague J., Letarte M. Endoglin is a component of the transforming growth factor-β receptor system in human endothelial cells. J Biol Chem. 1992;267:19027. [PubMed] [Google Scholar]
- 11.Masuyama H., Nakatsukasa H., Takamoto N., Hiramatsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in preeclampsia. J Clin Endocrinol Metab. 2007;92:2672–2679. doi: 10.1210/jc.2006-2349. [DOI] [PubMed] [Google Scholar]
- 12.Levine R.J., Lam C., Qian C., Yu K.F., Maynard S.E., Sachs B.P., Sibai B.M., Epstein F.H., Romero R., Thadhani R., Karumanchi S.A. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 13.Sandau K.B., Zhou J., Kietzmann T., Brune B. Regulation of the hypoxia-inducible factor 1alpha by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol Chem. 2001;276:39805–39811. doi: 10.1074/jbc.M107689200. [DOI] [PubMed] [Google Scholar]
- 14.Soleymanlou N., Jurisica I., Nevo O., Ietta F., Zhang X., Zamudio S., Post M., Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yinon Y., Nevo O., Xu J., Many A., Rolfo A., Todros T., Post M., Caniggia I. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am J Pathol. 2008;172:77–85. doi: 10.2353/ajpath.2008.070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamudio S., Wu Y., Ietta F., Rolfo A., Cross A., Wheeler T., Post M., Illsley N.P., Caniggia I. Human placental hypoxia-inducible factor-1α expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007;170:2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nevo O., Soleymanlou N., Wu Y. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajakumar A., Powers R., Hubel C., Shibata E., von Versen-Hoynck F., Plymire D., Jeyabalan A. Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Placenta. 2009;30:25–34. doi: 10.1016/j.placenta.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 21.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., Kriegsheim A., Hebestreit H.F., Mukherji M., Schofield C.J., Maxwell P.H., Pugh C.W., Ratcliffe P.J. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 22.Brune B., Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275–282. doi: 10.1016/j.cardiores.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Hagen T., Taylor C.T., Lam F., Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 24.Frederiksen L.J., Sullivan R., Maxwell L.R., Macdonald-Goodfellow S.K., Adams M.A., Bennett B.M., Siemens D.R., Graham C.H. Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clin Cancer Res. 2007;13:2199–2206. doi: 10.1158/1078-0432.CCR-06-1807. [DOI] [PubMed] [Google Scholar]
- 25.Frederiksen L.J., Siemens D.R., Heaton J.P., Maxwell L.R., Adams M.A., Graham C.H. Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate. J Urol. 2003;170:1003–1007. doi: 10.1097/01.ju.0000081126.71235.e0. [DOI] [PubMed] [Google Scholar]
- 26.Lowe D.T. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide. 2000;4:441–458. doi: 10.1006/niox.2000.0296. [DOI] [PubMed] [Google Scholar]
- 27.Belkacemi L., Bainbridge S.A., Dickinson M.A., Smith G.N., Graham C.H. Glyceryl trinitrate inhibits hypoxia/reoxygenation-induced apoptosis in the syncytiotrophoblast of the human placenta: therapeutic implications for preeclampsia. Am J Pathol. 2007;170:909–920. doi: 10.2353/ajpath.2007.060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munaut C., Lorquet S., Pequeux C., Blacher S., Berndt S., Frankenne F., Foidart J.-M. Hypoxia is responsible for soluble vascular endothelial growth factor receptor-1 (VEGFR-1) but not for soluble endoglin induction in villous trophoblast. Hum Reprod. 2008;23:1407–1415. doi: 10.1093/humrep/den114. [DOI] [PubMed] [Google Scholar]
- 29.Kishuku M., Nishioka Y., Abe S., Kishi J., Ogino H., Aono Y., Azuma M., Kinoshita K., Rentsenhand B., Makino H., Ranjan P., Minakuchi K., Sone S. Expression of soluble vascular endothelial growth factor receptor-1 in human monocyte-derived mature dendritic cells contributes to their antiangiogenic property. J Immunol. 2009;183:8176–8185. doi: 10.4049/jimmunol.0803849. [DOI] [PubMed] [Google Scholar]
- 30.Kendall R.L., Thomas K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caniggia I., Mostachfi H., Winter J., Gassmann M., Lye S.J., Kuliszewski M., Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Inves. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palacios-Callender M., Quintero M., Hollis V.S., Springett R.J., Moncada S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A. 2004;101:7630–7635. doi: 10.1073/pnas.0401723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeyabalan A., McGonigal S., Gilmour C., Hubel C., Rajakumar A. Circulating and placental endoglin concentrations in pregnancies complicated by intrauterine growth restriction and preeclampsia. Placenta. 2008;29:555–563. doi: 10.1016/j.placenta.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y., Lewis D.F., Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93:260–266. doi: 10.1210/jc.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vivo A., Baviera G., Giordano D., Todarello G., Corrado F., D'Anna R. PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:837–842. doi: 10.1080/00016340802253759. [DOI] [PubMed] [Google Scholar]
- 36.Cindrova-Davies T. Gabor Than Award Lecture 2008: Pre-eclampsia – from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30:55–65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Burton G.J., Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Helske S., Vuorela P., Carpen O., Hornig C., Weich H., Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Human Reprod. 2001;7:205–210. doi: 10.1093/molehr/7.2.205. [DOI] [PubMed] [Google Scholar]
- 39.Saito S., Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59:161–173. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 40.Raghupathy R., Makhseed M., Azizieh F., Hassan N., Al-Azemi M., Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999;196:122–130. doi: 10.1006/cimm.1999.1532. [DOI] [PubMed] [Google Scholar]
- 41.Royle C., Lim S., Xu B., Tooher J., Ogle R., Hennessy A. Effect of hypoxia and exogenous IL-10 on the pro-inflammatory cytokine TNF-alpha and the anti-angiogenic molecule soluble Flt-1 in placental villous explants. Cytokine. 2009;47:56–60. doi: 10.1016/j.cyto.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Xu B., Thornton C., Tooher J., Hennessy A. Exogenous soluble VEGF receptor-1 (sFlt-1) regulates Th1/Th2 cytokine production from normal placental explants via intracellular calcium. Hypertens Pregnancy. 2009;28:448–456. doi: 10.3109/10641950902777721. [DOI] [PubMed] [Google Scholar]
- 43.Duley L., Meher S., Abalos E. Management of pre-eclampsia. BMJ. 2006;332:463–468. doi: 10.1136/bmj.332.7539.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts D.J., Post M.D. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61:1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 45.Xu B., Thornton C., Tooher J., Ogle R., Lim S., Makris A., Hennessy A. Effects of anti-hypertensive drugs on production of soluble fms-like tyrosine kinase 1 and soluble endoglin from human normal and pre-eclamptic placentas in vitro. Clin Exp Pharmacol Physiol. 2009;36:839–842. doi: 10.1111/j.1440-1681.2009.05155.x. [DOI] [PubMed] [Google Scholar]
- 46.Cacciatore B., Halmesmaki E., Kaaja R., Teramo K., Ylikorkala O. Effects of transdermal nitroglycerin on impedance to flow in the uterine, umbilical, and fetal middle cerebral arteries in pregnancies complicated by preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1998;179:140–145. doi: 10.1016/s0002-9378(98)70264-9. [DOI] [PubMed] [Google Scholar]
- 47.Grunewald C., Kublickas M., Carlstrom K., Lunell N., Nisell H. Effects of nitroglycerin on the uterine and umbilical circulation in severe preeclampsia. Obstet Gynecol. 1995;86:600–604. doi: 10.1016/0029-7844(95)00197-y. [DOI] [PubMed] [Google Scholar]
- 48.Cetin A., Yurtcu N., Guvenal T., Imir A., Duran B., Cetin M. The effect of glyceryl trinitrate on hypertension in women with severe preeclampsia: HELLP syndrome, and eclampsia. Hypertens Pregnancy. 2004;23:37–46. doi: 10.1081/PRG-120028280. [DOI] [PubMed] [Google Scholar]
- 49.Lees C., Valensise H., Black R., Harrington K., Byiers S., Romanini C., Campbell S. The efficacy and fetal-maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre-eclampsia and its complications: a randomized double-blind placebo-controlled trial. Ultrasound Obstet Gynecol. 1998;12:334–338. doi: 10.1046/j.1469-0705.1998.12050334.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunolocalization of Flt-1 (VEGFR-1) and Eng in term chorionic villi explants. A and E: Localization of Flt-1 (A, green) and Eng (E, green) by immunofluorescence. B and F: Immunolocalization of cytokeratin as a marker for trophoblast (red). C and G: Nuclei are highlighted by DAPI fluorescence (blue). D and H: The merged image of all three fluorescent labels; yellow fluorescence reveals areas where Flt-1 or Eng colocalize with cytokeratin. The figures are representative of three independent experiments.
Differences in total protein secreted in the medium of cultured chorionic villi explants. For different O2 concentrations (six explants per placenta, with a minimum of three placentas per treatment), the total amount of proteins secreted in the medium was determined. No significant differences in the levels of released protein were observed between any of the groups. Error bars represent SEM.
Effect of GTN on the secretion of sFlt-1 and sEng by chorionic villi explants exposed to H/R. A and B: Relative sFlt-1 and sEng levels, respectively, in the media of explant cultures (six explants per placenta, with a minimum of three placentas per treatment). Values (in pg/mL) were divided by the total amount of protein (in μg/mL) in the media. No significant differences in the levels of sFlt-1 and sEng in the media were observed.
Effect of reoxygenation on chorionic villi explants previously exposed to hypoxia for 24 hours. A and B: Levels of sFlt-1 and sEng in the culture media from hypoxia-exposed explants before and after reexposure to 20% O2 (H/R). Media from six to seven explants per placenta from two placentas were used. Statistical significance was measured using a paired Student's t-test. C: Representative Western blot (of three different analyses) of TGF-β1 in the media of explants exposed to 20%, 0.5%, or 0.5% O2, followed by reoxygenation at 20% O2 (H/R). D: Densitometric analysis of the Western blot shown in C. Error bars represent SEM. *P < 0.05.


