Abstract
GspB is a serine-rich glycoprotein adhesin of Streptococcus gordonii that is exported to the bacterial surface by the accessory Sec system. This dedicated export pathway is comprised of seven components (SecA2, SecY2, and five accessory Sec proteins [Asp1 to Asp5]). The latter proteins have no known homologs beyond the Asps of other species. Asp1 to Asp3 are absolutely required for export of the substrate GspB, but their roles in this process are unknown. Using copurification analysis and far-Western blotting, we found that Asp2 and Asp3 could individually bind the serine-rich repeat (SRR) domains of GspB. Deletion of both SRR regions of GspB led to a decrease in its export, suggesting that binding of the Asps to the SRR regions is important for GspB transport by the accessory Sec system. The Asps also bound a heterologous substrate for the accessory Sec system containing a slow-folding MalE variant, but they did not bind wild-type MalE. The combined results indicate that the Asps may recognize the export substrate through preferential interactions with its unstructured or unfolded regions. Glycosylation of the SRR domains on GspB prevented Asp binding, suggesting that binding of the Asps to the preprotein occurs prior to its full glycosylation. Together, these findings suggest that Asp2 and Asp3 are likely to function in part as chaperones in the early phase of GspB transport.
INTRODUCTION
The accessory Sec (SecA2/Y2) system is a specialized export pathway of Gram-positive bacteria that mediates the transport of large serine-rich repeat (SRR), cell wall-anchored glycoproteins, such as GspB of Streptococcus gordonii (6), Fap1 of Streptococcus parasanguinis (43), Srr1 of Streptococcus agalactiae (22), PsrP of Streptococcus pneumoniae (23), and SraP of Staphylococcus aureus (31). Several of these glycoproteins are confirmed virulence determinants, as demonstrated in animal models of infection, including streptococcal and staphylococcal endocarditis (32, 44) and pneumococcal pneumonia (23), as well as neonatal sepsis (30) and meningitis (40) due to S. agalactiae. These adhesins are typically encoded within regions of the chromosome that also encode the accessory Sec system, as well as several proteins mediating glycosylation of the substrate (26, 46). Features of these regions also suggest that they are pathogenicity islands, which may explain their widespread prevalence.
The mechanisms for the export of the SRR glycoproteins by the accessory Sec system remain largely uncharacterized. The accessory Sec system resembles the general/canonical Sec system, in that it contains homologs (SecA2 and SecY2) of canonical SecA and SecY (26, 46). SecA is an ATPase motor protein that powers the transport of substrates through the membrane channel (translocon) formed by SecY in complex with SecE and SecG (14). SecA2 is also an ATPase and is likely to play a similar role in the transport of the accessory Sec substrate (7, 13). Although SecY2 has not been studied extensively, it is predicted to share structural similarities to SecY and is likely to function analogous to SecY (6, 8, 42). The accessory Sec system also contains several accessory Sec proteins (Asps) that are important for efficient substrate export (20, 28, 37, 38, 45). Asp1 to Asp3 are conserved across all known species that export SRR glycoproteins via SecA2/Y2, and appear to be essential for transport (20, 28, 37, 45). Some organisms, such as S. gordonii, encode one or more additional proteins (Asp4 and Asp5) that are either essential for substrate export or can enhance translocation efficiency (38).
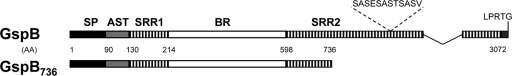
The accessory Sec system of S. gordonii strain M99 is dedicated to the export of GspB, an SRR adhesin that mediates binding to platelets and salivary glycoproteins (4, 34, 35). Like other members of the SRR glycoprotein family (26, 46), GspB consists of several distinct domains (Fig. 1). Located in the N terminus of GspB is an unusually long signal peptide (SP) followed by an accessory Sec transport (AST) domain; both regions are essential for GspB export (8, 9). In the remainder of the polypeptide are the serine-rich repeat domains (SRR1 and SRR2), which flank the basic region (BR). The SRR domains are target sites for glycosylation (36), whereas the basic region confers the adherence property to the glycoprotein (34).
Fig. 1.
Schematic of domain organization within GspB and GspB736. SP, signal peptide; AST, accessory Sec transport domain; SRR1, serine-rich repeat domain 1; BR, basic region; SRR2, serine-rich repeat domain 2. The serine-rich repeat dodecamer sequence is highlighted above the SRR2 region. The C-terminal cell wall anchoring motif (LPRTG) on native GspB is indicated. Numbering indicates the positions of amino acids (AA) marking domain junctions.
GspB export requires Asp1 to Asp3, but the specific functions of these proteins in transport remain unidentified. Asp1 to Asp3 do not have known homologs beyond the accessory Sec system, thus making it difficult to infer their possible roles. Previously, we showed that several of these Asps interact with one another and with SecA2 and that these interactions are required for optimal GspB export (28). These findings suggest that Asp1 to Asp3 may assist GspB export by interacting with the SecA2/Y2 translocon and prompted us to ask whether these Asps interact with the export substrate directly. Here we show that two of the Asps bind directly to distinct regions of GspB and are likely to be the first components of the accessory Sec system to interact with the nascent preprotein.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains, plasmids, and primers used here are listed in Tables 1 and 2. S. gordonii strains were cultured in Todd-Hewitt (TH) broth in 5% CO2 at 37°C. Escherichia coli strains were grown in Luria-Bertani (LB) broth at 37°C with aeration. When indicated, antibiotics were added to LB broth at concentrations of 17 μg/ml of chloramphenicol (Cm), 50 μg/ml of ampicillin (Amp), 50 μg/ml of kanamycin (Kan), or 300 μg/ml of erythromycin (Erm). Selection for transformants harboring pVA891 was made by plating E. coli onto LB agar containing 17 μg/ml of Cm and S. gordonii on TH-sheep blood agar containing 15 μg/ml of Erm. For transformants harboring pMSP3545, selection was made by plating S. gordonii on TH-sheep blood agar containing 100 μg/ml of Erm.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristica | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| BL21(λDE3) | F−ompT hsdSB(rB− mB−)gal dcm (DE3) | Novagen |
| DH5α | General cloning strain | Invitrogen |
| Streptococcus gordonii | ||
| M99 | Parental strain | 33 |
| PS846 | M99 ΔgspB::pEVP3; expressing no GspB | 9 |
| PS840 | M99 ΔgspB::cat ΔgtfA::spc; expressing no GspB or GtfA Cmr Spcr | This study |
| PS2865 | PS846 pMSP3545-gspB736FLAG; expressing glycosylated GspB736FLAG | This study |
| PS2866 | PS840 pMSP3545-gspB736FLAG; expressing nonglycosylated GspB736FLAG | This study |
| PS961 | M99 gspB736FLAG::pVA891; expressing glycosylated GspB736FLAG | 5 |
| PS1179 | M99 gspB736FLAG::pVA891, ΔgtfA::spc; expressing nonglycosylated GspB736FLAG | 5 |
| PS2336 | M99 expressing gspB joined domains SP, AST, and BR (codons 1 to 140 and 213 to 598) and a 3×FLAG tag in the C terminus | This study |
| PS1570 | PS846::pBMalE31; expressing GspBflag::MalE31 | 8 |
| PS2413 | PS1570 Δasp2::spc | This study |
| PS2414 | PS1570 Δasp3::spc | This study |
| Plasmids | ||
| pACYCDuet | E. coli expression vector; Cmr | Novagen |
| pETDuet | E. coli expression vector; Ampr | Novagen |
| pGEX-3x | E. coli GST fusion vector; Ampr | GE Healthcare |
| pET28c | E. coli expression vector; Kanr | Novagen |
| pET28b | E. coli expression vector; Kanr | Novagen |
| pMSP3545 | Cloning vector containing replication origin for Gram-positive bacteria and nisin-inducible promoter; Ermr | 11 |
| pMal-c2x | Vector expressing mature MalE (without a signal peptide) | New England Biolabs |
| pVA891-gspB736FLAG | pVA891+gspB736FLAG | 9 |
| pACYCDuet-gspB736FLAG | Vector expressing GspB736FLAG | This study |
| pETDuet-gspB736FLAG | Vector expressing GspB736FLAG | This study |
| pET28c-asp1 | Vector expressing H6Asp1 | This study |
| pET28c-GSTasp1 | pET28C+GSTasp1 | This study |
| pACYCDuet-GSTasp1 | pACYCDuet + GSTasp1 | This study |
| pJSC401asp2 | pJSC401+asp2 | 28 |
| pETDuet-HAasp2 | Vector expressing HAAsp2 carrying silent mutations at codons 346 and 363 | This study |
| pACYCDuet-GSTasp3 | Vector expressing GSTAsp3 | This study |
| pET28c-asp3 | Vector expressing H6Asp3 | This study |
| pET28c-GSTasp3 | pET28c+GSTasp3 | This study |
| pACYCDuet-GSTasp1-gspB736FLAG | Vector coexpressing GSTAsp1 and GspB736FLAG | This study |
| pETDuet-H6asp3-HAasp2 | Vector coexpressing H6Asp3 and HAAsp2 | This study |
| pET28c-asp2 | Vector expressing H6Asp2 | This study |
| pGEX-3x-sp | Vector expressing GST-SP | This study |
| pGEX-3x-ast | Vector expressing GST-AST | This study |
| pGEX-3x-srr1 | Vector expressing GST-SRR1 | This study |
| pGEX-3x-br | Vector expressing GST-BR | This study |
| pGEX-3x-srr2′ | Vector expressing the N terminus of GST-SRR2 | This study |
| pGEX-3x-gspB736 | Vector expressing GST-GspB736 | This study |
| pACYCDuet-sp.ast.s1FLAG | Vector expressing codons 1 to 214 of GspB736FLAG | This study |
| pACYCDuet-sp.astFLAG | Vector expressing codons 1 to 140 of GspB736FLAG | This study |
| pMSP3545-gspB736FLAG | Vector expressing GspB736FLAG | This study |
| pMalE31 | Vector expressing mature MalE with mutated codons 32 to 33 | This study |
| pB117 M | pVA891-gspB736FLAG with codons 118 to 736 replaced by malE31 | 8 |
| pACYCDuet-gspB1-117::malE31 | Vector expressing gspB736FLAG with codons 118 to 736 replaced by malE31 | This study |
| pET28b-sp.ast.br | pET28b+gspB joined domains (codons 1 to 140 and 213 to 598) | This study |
| pVA891-gspB736FLAG.H96L | pVA891+gspB736FLAG with an alteration of the indicated codon | 8 |
| pVA891-[sp.ast.br]FLAG | pVA891+gspB joined domains (codons 1 to 140 and 213 to 598) and a 3×FLAG tag in the C terminus | This study |
| pORF2K | pS326 carrying upstream and downstream fragments of asp2; Spcr | 37 |
| pORF3K | pS326 carrying upstream and downstream fragments of asp3; Spcr | 37 |
| pBMalE31 | pB736flagR with codons 146 to 560 replaced by malE31 | 8 |
Cmr, chloramphenicol resistance; Ermr, erythromycin resistance; Ampr, ampicillin resistance; Spcr, spectinomycin resistance; Kanr, kanamycin resistance.
Table 2.
Primers used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| NdeI-Gf | GGAATTCCATATGTTTTTTAAACGTCAAAAGGGTC |
| Gf-PacI | TAATTTTAATTAATTTTACTTGTCATCGTCATCCTTG |
| asp1N | CCTTGCTAGCATGTATTATTTTATTCCTTCATG |
| NcoI-GST | CCCATGGGATCCCCTATACTAGGTTATTGG |
| asp1-NotI | TTGCGGCCGCTTATTTTTCATCTATAGCCTCC |
| NdeI-HA | CCATATGTCCTACCCTTATGATGTGCC |
| Asp2-XhoI | AAACGTTCTCGAGCTATTTCTTTCTTCCAAATTCTTT |
| asp3N | AAAAGCTAGCATGAAGATTCAAAAACATAAGGAAA |
| asp3C | CTCTGAGCTCTAGTATTTTTAACCATTTGACTCC |
| asp3-NotI | TTGCGGCCGCTTAACCATTTGACTCCTCTAAA |
| NdeI-asp3 | GCATATGAAGATTCAAAAACATAAGGAA |
| asp2N | AAAAGCTAGCATGAAAAATAAGCTGAAGATCTTAC |
| asp2C | CTCTGAGCTCTAGTGAATCTTCATTTCTTTCTTC |
| BamHI-SP | TATAAGGATCCTAATGTTTTTTAAACGTCAAAAGGGTC |
| SP-EcoRI | ACTAGAATTCAATGCTTGTTCCTCTTCAGCATAAAC |
| BamHI-AST | TATAAGGATCCCTGAAGAGGAACAAGCACATG |
| AST-EcoRI | ACTAGAATTCAAACTTGCAGACAAAGTATCTGACAAG |
| BamHI-SRR1 | TATAAGGATCCATGAAAGCACTTCAGCGAGC |
| SRR1-EcoRI | ACTAGAATTCAAACTTGCAGACAAAGTATCTGACAAG |
| BamHI-BR | TATAAGGATCCAGAAATCTACCATCTCTACATCAG |
| BR-EcoRI | ACTAGAATTCAAAATAAATTTACTTCCGTCAATAATGTCTTTTC |
| BamHI-SRR2′ | TATAAGGATCCTTGATACAAGAGCTGGAAGTATATC |
| NcoI-Gf | ACCATCCATGGTTTTTAAACGTCAAAAGGGTC |
| SPT-BamHI | TAATTGGATCCTACTTGCAGACAAAGTATCTGAC |
| BamHI-BR | TATAAGGATCCAGAAATCTACCATCTCTACATCAG |
| SPTBR-NheI | TATGAGCTAGCAATAAATTTACTTCCGTCAATAATGTC |
| Gf-XbaI | TTTATTCTAGAAATTACTTGTCATCGTCATCCTTGTAGTC |
Plasmid construction.
Methods for constructing the plasmids used in these studies are presented in the supplemental material.
Protein expression.
To assess the interaction of GspB with Asp1 to Asp3 by copurification, the proteins were expressed in E. coli BL21(λDE3), transformed with pACYCDuet-GSTasp1-gspB736FLAG and pETDuet-H6asp3-HAasp2. To examine the interaction of GspB with each Asp individually by copurification, pACYCDuet-gspB736FLAG was coexpressed with pETDuet-HAasp2, and pETDuet-gspB736FLAG was coexpressed with pACYCDuet-GSTasp3 in E. coli BL21(λDE3). For far-Western blotting, the H6Asps were expressed from pET28c, the GspB736FLAG or FLAG-tagged GspB domain fusions were expressed from pACYCDuet, whereas glutathione S-transferase (GST)-tagged GspB domains were expressed from pGEX-3x. MalE and MalE31 were expressed from pMal-c2x, whereas GspB1-117::MalE31 was expressed from pACYCDuet in E. coli BL21(λDE3). Protein expression from all constructs was induced by growing cells at 37°C to an optical density of 0.6, adding IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 0.25 mM, and incubation at 25°C for 4 h for constructs expressed from the Duet, pGEX-3x, and pMalc.2x vectors or 2 h for those from pET28c prior to harvesting.
To obtain GspB736FLAG from the culture medium of S. gordonii for far-Western blotting, the gspB deletion strain PS846 (9) was transformed with pMSP3545-gspB736FLAG to produce strain PS2865, which was grown overnight to produce the glycosylated protein. The gspB gtfA double-deletion strain PS840 was similarly transformed with pMSP3545-gspB736FLAG to generate strain PS2866, which was grown overnight to obtain the nonglycosylated protein. Note that GspB736FLAG does not contain a cell wall anchoring motif like native GspB (Fig. 1) and is thus freely secreted into the culture medium when expressed by S. gordonii.
Protein purification.
Cells expressing H6Asp1 or H6Asp3 were suspended in lysis buffer (300 mM NaCl, 50 mM sodium phosphate buffer [pH 8.0], 1% Triton X-100, 2 mg of lysozyme/ml, 1 mM phenylmethylsulfonyl fluoride [PMSF], protease inhibitors [Roche], 25 mM imidazole). After 4 min of sonication, lysates were clarified by centrifugation at 11,000 × g for 20 min, passed through a 0.45-μm-pore-size filter, and then incubated with an Ni-NTA resin (Qiagen) for 2 h at 4°C. The resin was loaded onto a gravity flow column (Bio-Rad) and washed at least four times with 10 bed volumes of wash buffer (300 mM NaCl, 50 mM sodium phosphate buffer [pH 8.0], 0.5% Triton X-100, 60 mM imidazole). Proteins bound to the resin was eluted in fractions with 25 mM HEPES containing 100 mM EDTA and 100 mM NaCl. H6Asp2 was purified as described for the other Asps, except that the lysis buffer contained 8 M urea. The bound denatured H6Asp2 was allowed to refold gradually on the resin by washing with 10 bed volumes of buffer containing decreasing concentrations of urea. H6Asp2 was then eluted with 300 mM NaCl, 50 mM sodium phosphate buffer (pH 8.0), 0.5% Triton X-100, and 500 mM imidazole. Protein purity in each eluted fraction was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Selected fractions were pooled, and proteins were quantified on stained gels using molecular weight standards (Bio-Rad).
Purification of GspB736FLAG exported from S. gordonii was carried out by first precipitating proteins from the culture medium with 50% (wt/vol) of ammonium sulfate. The precipitated proteins were then solubilized in phosphate-buffered saline (PBS; Sigma) and desalted by passing through a 10DG chromatography column (Bio-Rad) before affinity purification. FLAG-tagged GspB736 or domain fusion proteins were affinity purified with anti-FLAG antibody immobilized on agarose beads (Sigma). GST-fused GspB736 or GST-GspB domain fusions were affinity purified with glutathione-Sepharose 4B agarose (GE Healthcare). Purification was carried out under conditions recommended by the manufacturers.
Copurification of GspB and the Asps.
E. coli cells expressing GspB736FLAG and Asp1 to Asp3 were harvested by centrifugation at 4,500 × g for 20 min and suspended in BugBuster HT (EMD) supplemented with protease inhibitors (Roche), 2 mg of lysozyme/ml, and 1 mM PMSF. The lysates were clarified as described above and incubated for 3 h at 4°C with anti-FLAG antibody immobilized on agarose beads to precipitate GspB736FLAG. The beads were recovered by centrifugation at 500 × g for 5 min and washed in PBS supplemented with protease inhibitors at room temperature. Precipitated proteins were then eluted with 3×FLAG peptides (Sigma).
To assess the binding of GspB with each Asp individually, GspB736FLAG was coexpressed with each Asp and then purified from lysates, as described above. To purify GST-fused Asp1 or Asp3 and hemagglutinin (HA)-tagged Asp2, whole-cell lysates (WCL) were processed as described above, except that glutathione-Sepharose 4B agarose was used to recover the GST fusion proteins. HAAsp2 was purified with anti-HA antibody immobilized on agarose (Sigma). Purified GST fusion proteins were eluted with 10 mM reduced glutathione (GE Healthcare) in 50 mM Tris-HCl (pH 8.0), and bound HAAsp2 was eluted with HA peptides (Sigma).
Far-Western blotting.
Purified GspB variants were resolved on 4 to 12% Bis-Tris gradient gels (Invitrogen), and lysates of E. coli expressing MalE variants were resolved on 3 to 8% Tris-acetate gradient gels (Invitrogen) by SDS-PAGE under reducing conditions. Proteins were transferred onto nitrocellulose membranes and allowed to refold overnight at 4°C in a blocking solution of 5% milk in PBS containing 5% Tween 20 (PBS-T). Membranes were incubated with 3.5 μg of H6Asp1, H6Asp2, or H6Asp3 per milliliter of PBS-T for 2 h at room temperature. Subsequently, the membranes were incubated in a 1:4,000 dilution of mouse anti-H6 antibody (GE Healthcare) in PBS-T, followed by incubation with a 1:10,000 dilution of rabbit anti-mouse horseradish peroxidase-conjugated antibody (Sigma) in PBS-T for 2 h per incubation at room temperature. Binding of the Asps was detected with SuperSignal West Pico chemiluminescence substrate (Pierce).
Assessing proteins exported by the accessory Sec system.
To monitor the transport of a variant of GspB lacking both SRR domains in S. gordonii, the gspB deletion strain PS846 (9) was transformed with pVA891-[sp.ast.br]FLAG. Recombination of a sequence upstream of the sp.ast.br insert in pVA891-[sp.ast.br]FLAG with the same sequence upstream of gspB in the streptococcal chromosome resulted in a variant, PS2336, expressing the protein under native conditions. To study the Asp-dependent export of GspBflag::MalE31 (8) from S. gordonii, strain PS1570 expressing the heterologous protein was transformed with pORF2K or pORF3K (37) to generate the respective Δasp2 or Δasp3 isogenic strain. After growth for 18 h under the conditions specified above, the cultures were fractionated into the culture medium and cell pellet by centrifugation at 16,000 × g for 4 min. A total of 20 μl of the culture medium was mixed with loading buffer (Invitrogen) containing 5% β-mercaptoethanol and then resolved on a 3 to 8% Tris-acetate gradient gel by SDS-PAGE. The cell pellet was further treated with mutanolysin to produce protoplasts. A 100-μl culture volume equivalent of the protoplasts was resolved per lane. Western blotting with anti-FLAG antibody was carried out for the detection of GspB variants, and polyclonal antibodies specific for the β-subunit of RNA polymerase in group B streptococci (29) were used for the detection of RNA polymerase.
Bioinformatics analysis.
Structure predictions for the GspB domains were performed using the protein homology/analogy recognition engine (17) or PHYRE (http://www.sbg.bio.ic.ac.uk/∼phyre/).
RESULTS
Binding of Asp2 and Asp3 to the GspB preprotein.
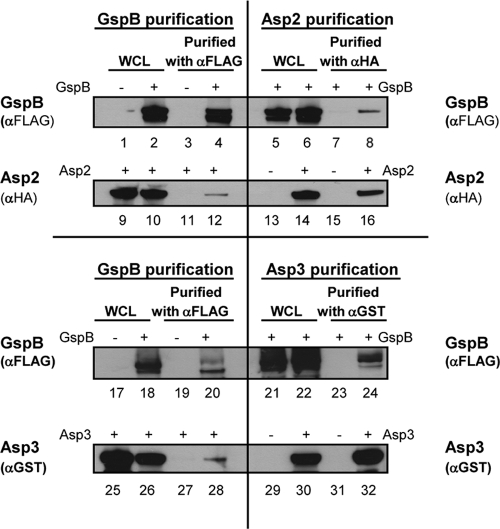
Disruption of the gene encoding Asp1, Asp2, or Asp3 abolishes the export of GspB (28, 37), indicating that these intracellular proteins are required for this process and suggesting that one or more of these intracellular proteins might interact directly with the GspB preprotein in the cytoplasm to facilitate its transport. One possibility is that one or more of the Asps might function as molecular chaperones by binding to GspB and assisting in its targeting to the translocon. To investigate whether the Asps and GspB can interact directly, GSTAsp1, HAAsp2, H6Asp3, and GspB736FLAG were coexpressed collectively in E. coli and assessed for binding by copurification. GspB736FLAG is a truncated, C-terminally FLAG-tagged variant of GspB (Fig. 1) that has export requirements similar to those of the full-length substrate (9). After affinity purification of GspB736FLAG from the WCL, the recovered material was probed for Asps that copurified with GspB. We found that Asp2 and Asp3 copurified with GspB, but Asp1 did not (Fig. 2). In control studies, none of the Asps bound to the immobilized anti-FLAG antibody in the absence of GspB (Fig. 2, lane 3), indicating that the interaction of Asp2 and Asp3 with GspB was specific.
Fig. 2.
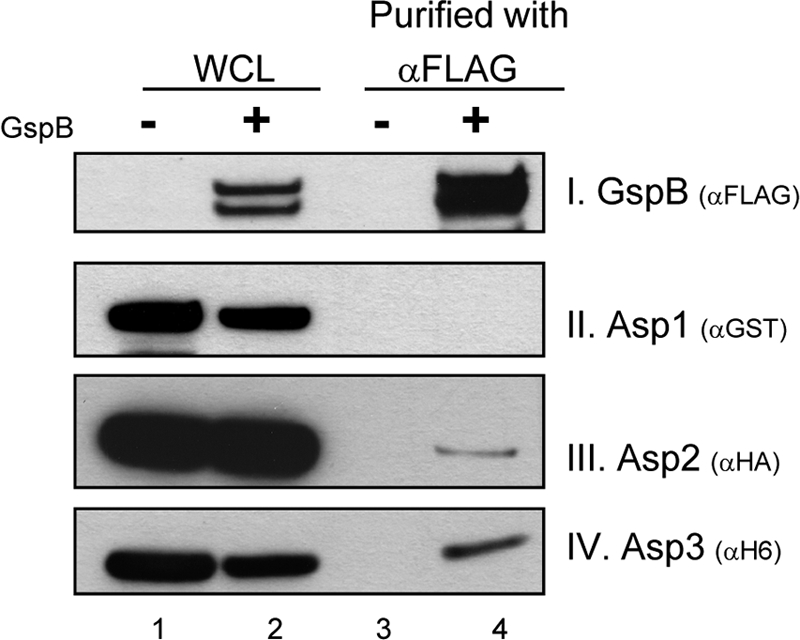
Asp2 and Asp3 copurify with GspB736FLAG. Asp1 to Asp3 and GspB736FLAG were coexpressed in E. coli, and GspB736FLAG was purified from bacterial whole-cell lysates (WCL) using anti-FLAG agarose. The recovered material was then probed for Asp1, Asp2, or Asp3 by blotting with antibody specific for each epitope tag, as indicated. Lane 1, WCL from E. coli expressing Asp1 to Asp3 but not GspB; lane 2, WCL of E. coli expressing the Asps and GspB; lane 3, proteins recovered by anti-FLAG affinity purification from material in lane 1 (lysates of E. coli expressing Asp1 to Asp3), a negative control; lane 4, proteins recovered by purification of GspB736FLAG from material in lane 2 (lysates of E. coli expressing Asp1 to Asp3 and GspB).
To address whether Asp2 or Asp3 could bind GspB independently and directly, each Asp was individually coexpressed with GspB736FLAG, and the subsequent WCL were then subjected to affinity purification as described above. Purification of GspB736FLAG from the WCL containing Asp2 plus GspB736FLAG or Asp3 plus GspB736FLAG identified Asp2 and Asp3 individually copurifying with GspB736FLAG (Fig. 3, lanes 12 and 28, respectively). Similarly, purification of Asp2 or Asp3 from the coexpressing strains also identified GspB736FLAG as a copurifying partner (Fig. 3, lanes 8 and 24, respectively).
Fig. 3.
Asp2 and Asp3 individually bind to GspB. GspB736FLAG and HAAsp2 or GSTAsp3 were coexpressed in E. coli, and each protein was purified from the WCL by affinity purification. The material recovered by purification of GspB736FLAG was probed for Asp2 or Asp3 by Western blotting, with antibody specific for each epitope tag (lanes 12 and 28, respectively). Similarly, the recovered material from purification of Asp2 or Asp3 was probed for GspB736FLAG with anti-FLAG antibody (lanes 8 and 24, respectively). Serving as negative controls in GspB purification were WCL containing Asp2 without GspB (lanes 9 and 1) and Asp3 without GspB (lanes 25 and 17) that were subjected to αFLAG affinity purification (lanes 3, 11, 19, and 27). Negative controls in the Asp2 or Asp3 purification were WCL containing GspB alone (lanes 5, 13, 21, and 29) that were subjected to αHA or αGST affinity purification (lanes 7, 15, 23, and 31), respectively.
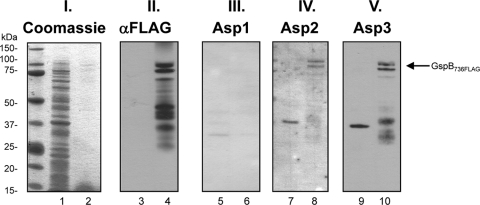
To validate these findings, we also assessed the interactions of GspB with the Asps by far-Western blotting. After SDS-PAGE, purified GspB736FLAG was transferred onto nitrocellulose membranes and probed with purified hexahistidine (H6)-tagged Asp1, Asp2, or Asp3. As shown in Fig. 4, Asp2 and Asp3 bound GspB (lanes 8 and 10, respectively), but Asp1 did not (lane 6). Note that purified GspB736FLAG was typically fragmented (lane 4), and Asp2 and Asp3 bound the full-length protein and several degradation fragments (lanes 8 and 10). In control studies, Asp2 and Asp3 did not bind most other proteins present in WCL (lanes 7 and 9), thus further validating a specific interaction of Asp2 and Asp3 with GspB. Together, these studies demonstrate that individually Asp2 or Asp3 can bind the GspB preprotein.
Fig. 4.
Analysis of Asp binding to GspB736FLAG by far-Western blotting. Purified GspB736FLAG (even number lanes) was blotted on membranes and probed with purified H6Asps. WCL of E. coli carrying the empty expression vector pACYCDuet served as a negative control (odd number lanes). Panel I, Coomassie stain of WLC and purified GspB736FLAG; panel II, immunoblotting of WCL and purified GspB736FLAG with anti-FLAG antibody; panels III, IV, and V, probing of GspB736FLAG with Asp1, Asp2, and Asp3, respectively. Binding of the H6Asps to GspB736FLAG was detected with anti-H6 antibody. The arrow indicates the position of full-length GspB736FLAG.
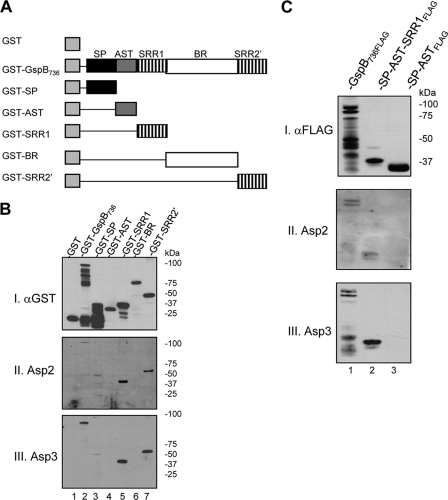
Asp2 and Asp3 bind SRR1 and SRR2 of GspB.
To identify the specific region of GspB that interacts with the two Asps, individual domains of GspB were expressed as GST fusion proteins (Fig. 5A). After affinity purification, the proteins were assessed for Asp binding by far-Western blotting with H6Asp2 or H6Asp3. As shown in Fig. 5B, the Asps were found to bind the SRR1 (lane 5) and SRR2 (lane 7) domains of GspB. No binding to the AST domain (lane 4) or BR (lane 6) was detected. Likewise, no binding to the SP was detected (GST-SP migrates at the predicted mass of 37 kDa), although some binding to an unidentified 50-kDa protein contaminant in the purified SP fraction was evident (blots II and III, lane 3). To reexamine the possibilities that Asp2 or Asp3 could bind the SP of GspB and that the presence of a SP did not indirectly influence binding, two protein constructs were made: one containing the fused SP and AST domain of GspB and the other containing the SP, AST, and SRR1 joined together. These constructs were blotted on membranes and probed with H6Asp2 or H6Asp3. We found that the two Asps bound to the construct containing the SRR1 domain (Fig. 5C, lane 2) but not to the one containing the SP and AST alone (lane 3). Together, our results indicate that Asp2 and Asp3 bind the SRR regions of the GspB polypeptide, but not the SP, AST domain, or BR. Furthermore, the presence of the SP had no apparent effect on binding of Asp2 or Asp3 to the SRR domains.
Fig. 5.
Asp2 and Asp3 bind the serine-rich repeat regions of GspB. (A) Schematic of GST-GspB736 and GST-GspB truncates containing specific GspB domains. (B) The GST-GspB fusion proteins were affinity purified and probed by far-Western blotting with purified H6Asp2 or H6Asp3. Lane 1, GST; lane 2, GST-GspB736; lane 3, GST-SP; lane 4, GST-AST; lane 5, GST-SRR1; lane 6, GST-BR; lane 7, GST-SRR2′. Each fusion protein was detected with anti-GST antibody (blot I). Binding of the Asps to the GST fusion proteins was detected with anti-H6 antibody (blots II and III). (C) Far-Western blotting assessing binding of the Asps to SRR1. Lane 1, purified Gsp736FLAG served as a positive control; lane 2, purified SP-AST-SRR1FLAG fusion protein; lane 3, purified SP-ASTFLAG fusion protein. The membranes were probed with purified H6Asp2 or H6Asp3, followed by detection of the bound Asp with anti-H6 antibody (blots II and III). The amount of GspB or fusion protein present in each lane was detected with anti-FLAG antibody (blot I).
Glycosylation of GspB prevents Asp binding.
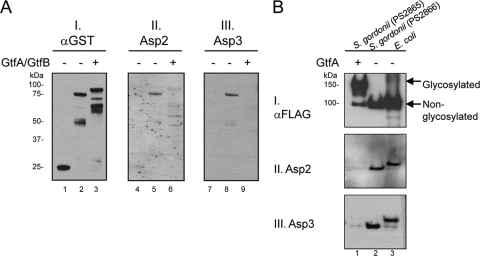
The studies described above examined the binding of Asp1 to Asp3 to a nonglycosylated variant of GspB. In the native S. gordonii strain M99, GspB undergoes glycosylation at both SRR domains prior to export (3, 36). For this reason, we investigated whether this posttranslational modification of GspB altered its interaction with the Asps. Glycosylated and nonglycosylated forms of an SRR1-BR fusion protein (36) were compared for their ability to bind H6Asp2 or H6Asp3. When assessed by far-Western blotting, both Asp2 and Asp3 had higher levels of binding to nonglycosylated SRR1-BR (Fig. 6A, lanes 5 and 8), compared to the glycosylated form (Fig. 6A, lanes 6 and 9). These results thus indicate that glycosylation blocks the interactions between the Asps and the SRRs and suggest that Asp binding probably occurs before GspB is fully glycosylated.
Fig. 6.
Glycosylation of GspB prevents Asp binding. (A) A GST-SRR1-BR fusion protein was expressed in E. coli, either with or without the coexpression of the streptococcal glycosyltransferases GtfA and GtfB (35). After purification, the constructs were probed by far-Western blotting with purified H6Asp2 or H6Asp3. Lanes 1, 4, and 7, GST; lanes 2, 5, and 8, nonglycosylated GST-SRR1-BR; lanes 3, 6, and 9, glycosylated GST-SRR1-BR. The amount of fusion protein present on the blots was assessed with anti-GST antibody (blot I). GspB binding by Asp2 or Asp3 was detected with anti-H6 antibody (blots II and III). (B) GspB736FLAG was expressed from S. gordonii in the presence or absence of GtfA (lanes 1 and 2, respectively) and purified from the culture medium. The proteins were probed by far-Western blotting with purified H6Asp2 or H6Asp3, as indicated. Nonglycosylated GspB736FLAG purified from the WCL of E. coli served as a positive control (lane 3). The amount of protein present on the blot was assessed with anti-FLAG antibody (blot I). Note that nonglycosylated GspB736FLAG secreted into the culture medium of M99 lacks the signal peptide and thus has a lower molecular mass than the recombinant form obtained from E. coli.
We also assessed the binding of Asp2 and Asp3 to GspB variants expressed and secreted by the native S. gordonii host. Glycosylated and nonglycosylated forms of GspB736FLAG were obtained from the culture medium of S. gordonii, and binding by H6Asp2 or H6Asp3 was assessed by far-Western blotting. Binding of the Asps to nonglycosylated GspB736FLAG purified from the E. coli WCL served as a positive control. We found that the Asps did not bind glycosylated GspB736FLAG expressed by the native organism (Fig. 6B, lane 1) but bound the nonglycosylated form expressed by both the native and the recombinant organisms (Fig. 6B, lanes 2 and 3, respectively). The data thus corroborate our findings using recombinant proteins and suggest that the Asps are likely to interact with the SRR domains before they are glycosylated.
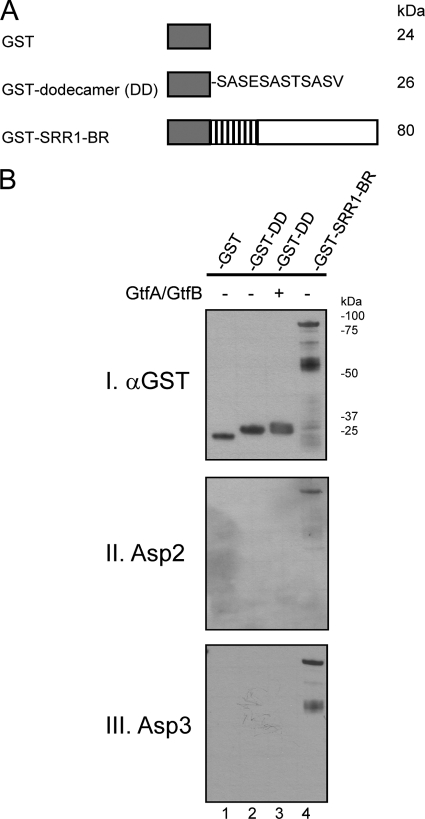
The Asps do not bind a single serine-rich dodecamer.
The SRR domains of GspB are comprised of inexact repeats of 12 amino acids, SASESASTSASV (3). The SRR1 domain contains six semiconserved repeats, and the SRR2 contains 200 repeats. This dodecamer is recognized by glycosyltransferases GtfA and GtfB as a target for glycosylation (36). To determine whether Asp-GspB binding was mediated through recognition of the same dodecameric sequence, the GST-tagged SASESASTSASV peptide (Fig. 7A) was purified and probed by far-Western blotting with H6Asp2 or H6Asp3 to assess binding. As shown in Fig. 7B, neither Asp bound the nonglycosylated or glycosylated SRR dodecamer (lanes 2 and 3, respectively), but both interacted with the nonglycosylated SRR1-BR construct (lane 4) containing six semiconserved dodecameric repeats. The data thus indicate that binding of the two Asps to a SRR region requires it to be longer than 12 amino acids and also suggest that the binding is not mediated through recognition of a single serine-rich dodecamer.
Fig. 7.
Assessment of Asp2 and Asp3 binding to the SRR peptides. (A) Schematic of generated GST-GspB fusion proteins containing either a full-length SRR1 region (GST-SRR1-BR) or a single SRR dodecamer sequence stretch (GST-DD). (B) Purified nonglycosylated and glycosylated SRR dodecamers were probed by far-Western blotting with purified H6Asp2 or H6Asp3. Lane 1, GST; lane 2, nonglycosylated SRR dodecameric peptide; lane 3, glycosylated SRR dodecamer peptide; lane 4, nonglycosylated GST-SRR1-BR fusion protein. The amount of peptide or protein present per lane was assessed with anti-GST antibody (panel I). Binding of Asp2 (panel II) or Asp3 (panel III) to GST-SRR1-BR or the dodecamers was detected with anti-H6 antibody.
Deletion of the SRRs affects GspB export.
In previous studies, we found that deleting either the SRR1 or SRR2 domain had no apparent effect on the export of GspB (9). To better determine whether these Asp-binding domains are essential for GspB export, we deleted both SRR regions of GspB736FLAG and expressed the variant protein from the chromosome of M99. The export of this double-SRR deletion mutant was compared to that of the glycosylated and nonglycosylated forms of GspB736FLAG. Although the expression of the double-SRR deletion protein was comparable to GspB736FLAG, export of the variant was reduced, as indicated by lower levels in the culture medium and its accumulation in protoplasts (Fig. 8). Thus, deleting both of the preferred Asp binding domains of GspB led to a reduction in its export, indicating that Asp binding to the SRR regions is important for GspB export.
Fig. 8.
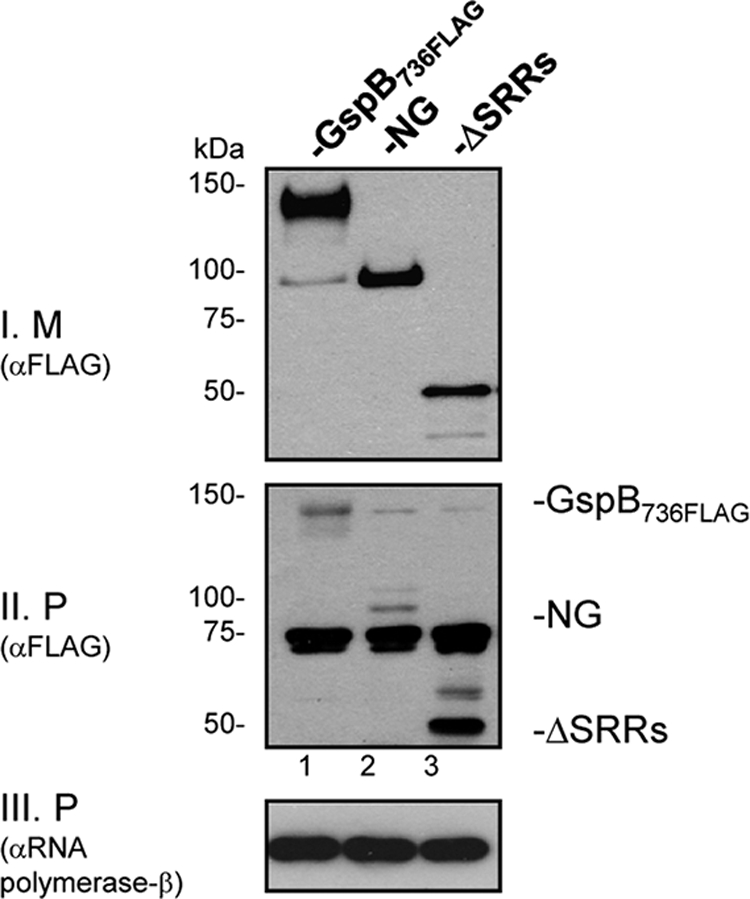
The SRR domains of GspB are important for optimal export. Variants of S. gordonii strain M99 expressing glycosylated GspB736FLAG, nonglycosylated GspB736FLAG (NG), or a GspB736FLAG lacking both SRR regions (ΔSRRs) were cultured overnight. Export of each protein from S. gordonii was assessed by comparing levels of GspB secreted into the culture medium (M) or retained in the protoplast (P) by Western blotting with anti-FLAG antibody (blots I and II). The β-subunit of RNA polymerase in the protoplasts (blot III) served as a loading control. For clarity, the GspB variants on blot II are indicated. Note that three proteins of 150, 75, and 70 kDa that cross-react with the anti-FLAG antibody are present in all lanes on this blot.
The Asps can bind a heterologous, slow-folding accessory Sec export substrate.
Bioinformatics analysis of GspB using PHYRE (17) indicates that the nonglycosylated SRR regions are predicted to be disordered, which suggests that these regions lack a distinct structure or fold (15). In the canonical Sec system, binding of the chaperone SecB to its transport substrates is based on its affinity for unfolded forms of proteins (2, 16), rather than through recognition of a consensus sequence (18, 19, 39). In view of these findings, we investigated whether Asp2 and Asp3 are capable of interacting with unfolded protein substrates. We have previously shown that the streptococcal accessory Sec system is able to transport the heterologous protein MalE31 (a slow-folding variant of MalE [27]), if it is fused to the GspB signal peptide and AST domain (8). To determine whether Asp2 and Asp3 can interact with this heterologous accessory Sec protein substrate, we examined the binding of these Asps to a GspB-MalE31 fusion (GspB1-117::MalE31). As shown in Fig. 9, both Asps bound GspB1-117::MalE31, as measured by far-Western blotting (lane 1, blots II and III). Since the Asps do not bind the SP or AST domain (Fig. 5B and C), these results indicate that the Asps interacted directly with the MalE31 region. To assess this possibility, we compared the binding of Asp2 and Asp3 to MalE and MalE31. Binding of Asp2 and Asp3 to MalE31 could be detected, but no binding was observed to the native MalE protein (compare lane 2 to lane 3). The data thus suggest that the Asps can bind unfolded proteins more readily than folded ones and imply that they might be functionally similar to the SecB chaperone in this respect.
Fig. 9.
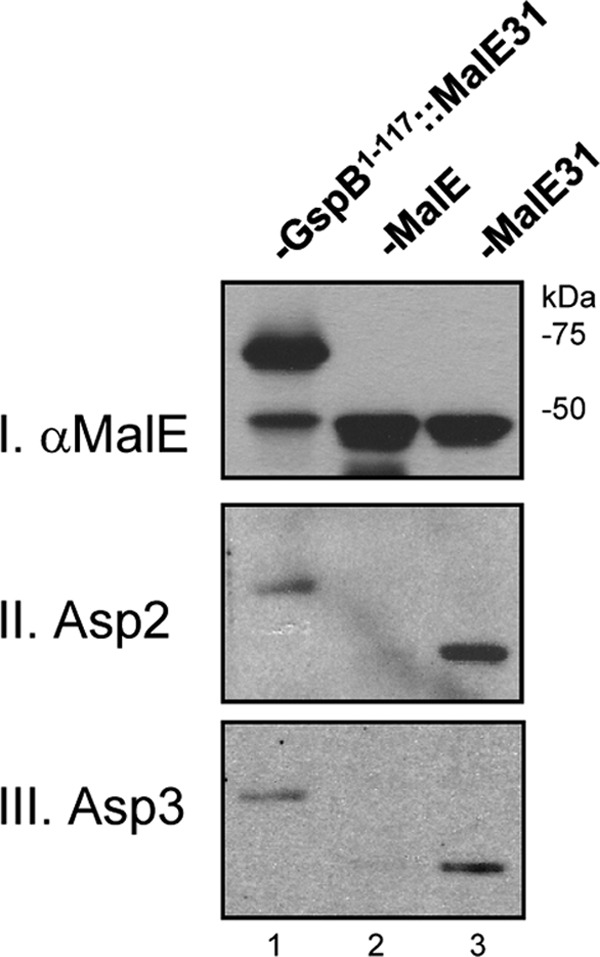
Binding of Asp2 and Asp3 to unfolded variants of MalE. WCL of E. coli expressing wild-type MalE, a slow-folding variant (MalE31), or MalE31 fused to amino acids 1 to 117 of GspB (GspB1-117::MalE31) were probed for Asp binding by far-Western blotting with H6Asp2 or H6Asp3. Lane 1, GspB1-117::MalE31; lane 2, wild-type MalE; lane 3, MalE31. Blot I shows probing of the above WCL with anti-MalE to confirm protein expression. Blots II and III show probing with H6Asp2 or H6Asp3 revealing binding to MalE31 but not the wild-type MalE protein.
We further reasoned that if Asp2 and Asp3 could bind the slow-folding MalE variant, the export of the GspB-MalE31 fusion protein from S. gordonii would likely occur in an Asp-dependent manner. To determine whether this was the case, GspBflag::MalE31 (8) was expressed from the chromosome of S. gordonii in an asp2 or asp3 deletion background, and its export was assessed. The results showed that no GspB-MalE31 fusion protein was exported in either asp deletion background (see Fig. S1 in the supplemental material), indicating that both Asp2 and Asp3 are essential for the export of GspB-MalE31 from the accessory Sec system.
DISCUSSION
The accessory Sec system is dedicated to the export of SRR glycoproteins and contains a number of proteins unique to this transport pathway (26, 46). Previous studies on Asp4 and Asp5 of S. gordonii suggest that these proteins might function as SecE and SecG homologs, which can stabilize the translocon and/or increase the efficiency of substrate export (38). In contrast, the functions for Asp1 to Asp3 are unknown. Asp1 to Asp3 are essential for the accessory Sec substrate export (20, 28, 37, 45) and can interact with one another (20, 28). Furthermore, Asp3 can bind SecA2 (28), suggesting that Asp3 might have a function targeting the substrate to the SecA2/Y2 translocon or modulating translocon activities.
In view of these recent findings, we assessed whether Asp1 to Asp3, either individually or as a multimeric complex, could directly bind the GspB preprotein, the accessory Sec substrate of S. gordonii M99. Using copurification analysis and Far Western blotting, we found that Asp2 and Asp3, but not Asp1, could interact with distinct regions of GspB. The presence of the signal peptide did not affect binding of Asp2 or Asp3 to the remainder of the preprotein. Moreover, Asp2 and Asp3 did not detectably interact with the signal peptide or AST domains of GspB, even though N-terminal regions of preproteins are common mediators of trafficking. Instead, Asp2 and Asp3 could independently bind the SRR domains of GspB. Neither Asp2 nor Asp3 bound a serine-rich dodecamer sequence, but both proteins were able to interact with a longer (∼80-residue) serine-rich polypeptide (SRR1). The Asps also bound a slow-folding variant of MalE, MalE31, which has been shown to be a suitable heterologous substrate for the accessory Sec system (8), but did not readily bind wild-type MalE. Since the nonglycosylated SRR regions are predicted to lack a defined structure, the combined results indicate that binding of Asp2 and Asp3 to GspB is likely due to their ability to interact with unstructured or unfolded preprotein segments.
Although our studies indicate that the Asps readily bind the SRR regions, it is unclear whether they may also bind other regions of GspB, albeit with lower affinity. When both SRRs were removed from GspB, export of the remaining domain (the BR) was less efficient, which provides corroborative evidence that Asp binding to the SRR regions is important for GspB transport. However, deletion of both SRR domains did not fully abolish the export of the variant substrates. It is possible that, although the SRR domains are the preferred binding sites for the Asps, Asp2 or Asp3 might be able to bind regions of GspB outside of the SRR domains, such as the BR, prior to their folding and with weaker affinities. Consistent with this possibility, some binding of Asp3 to the BR was detected by far-Western blotting, if the BR was first denatured with urea (unpublished data). Precedent for such preferential binding can be found in SecB, which binds some regions of its substrates more readily than others (18, 19, 39) and tends to bind segments that are rich in basic (19, 25, 39) or aromatic (19) amino acids.
The combined findings suggest that Asp2 and Asp3 might function as molecular chaperones for the accessory Sec substrate. Precisely how they do so is yet unknown. However, some insights might be found in comparison to other cytoplasmic chaperones of various other export systems. Some examples include the redox enzyme maturation proteins (REMPs) of the twin-arginine translocation system (10, 12), the class IA chaperones of the type III secretion system (41), and SecB of the canonical Sec system (1). The REMPs and the type III secretion class IA chaperones are considered specific chaperones because they typically recognize certain substrates within the export system (10, 12, 41). A number of REMPs bind the preproteins through interactions with the twin-arginine motif located in the signal peptide (12, 24). The class IA chaperones also bind the secretion signal located in the N terminus of the substrate polypeptides; however, no sequence consensus was found among these signals (41). In contrast, SecB belongs to a class of general chaperones that can bind a variety of preproteins (1, 19). SecB does not bind the signal peptide but instead interacts with the mature regions of the substrates (18, 19, 21, 39). Of note, SecB preferentially binds unfolded proteins and can block their folding in order to maintain them in a translocation-competent (partially folded) state (2, 16). Despite these differences in substrate specificity, all of the chaperones bind noncovalently and directly to the preproteins and target the substrate to the transmembrane channel complex through direct interactions with other export system components (1, 10, 41). In comparison, the Asps seem to exhibit features of both the general and specific chaperones in that they are capable of binding to unfolded polypeptides but are dedicated to the transport of only a single glycoprotein substrate.
A notable finding of this investigation was that glycosylation of GspB can prevent its interaction with Asp2 and Asp3. This suggests that binding of the Asps to the preprotein may occur early in the course of GspB expression, before or concomitant with its glycosylation. Thus, Asp2 and Asp3 are likely to be the first components of the accessory Sec system that interact with the nascent preprotein. Since Asp3 is predicted to be cytoplasm localized, whereas Asp2 may be membrane associated (28), it is possible that Asp3 binds the preprotein first and then relays it to Asp2 docked at or near the SecA2/Y2 translocon. Moreover, our findings that Asp3 can bind SecA2 (28) and GspB directly are consistent with the possibility that this protein might function in both targeting the substrate and in modulating SecA2 activity. Studies to address these possibilities are now in progress.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Department of Veterans Affairs, the VA Merit Review program, the American Heart Association, and grants R01 AI41513 and R01 AI057433 from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Bechtluft P., Nouwen N., Tans S. J., Driessen A. J. 2010. SecB: a chaperone dedicated to protein translocation. Mol. Biosyst. 6:620–627 [DOI] [PubMed] [Google Scholar]
- 2. Bechtluft P., et al. 2007. Direct observation of chaperone-induced changes in a protein folding pathway. Science 318:1458–1461 [DOI] [PubMed] [Google Scholar]
- 3. Bensing B. A., Gibson B. W., Sullam P. M. 2004. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J. Bacteriol. 186:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bensing B. A., Lopez J. A., Sullam P. M. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 72:6528–6537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bensing B. A., Siboo I. R., Sullam P. M. 2007. Glycine residues in the hydrophobic core of the GspB signal sequence route export toward the accessory Sec pathway. J. Bacteriol. 189:3846–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bensing B. A., Sullam P. M. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081–1094 [DOI] [PubMed] [Google Scholar]
- 7. Bensing B. A., Sullam P. M. 2009. Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA. J. Bacteriol. 191:3482–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bensing B. A., Sullam P. M. 2010. Transport of preproteins by the accessory Sec system requires a specific domain adjacent to the signal peptide. J. Bacteriol. 192:4223–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bensing B. A., Takamatsu D., Sullam P. M. 2005. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 58:1468–1481 [DOI] [PubMed] [Google Scholar]
- 10. Berks B. C., Palmer T., Sargent F. 2005. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 8:174–181 [DOI] [PubMed] [Google Scholar]
- 11. Bryan E. M., Bae T., Kleerebezem M., Dunny G. M. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183–190 [DOI] [PubMed] [Google Scholar]
- 12. Chan C. S., Chang L., Rommens K. L., Turner R. J. 2009. Differential Interactions between Tat-specific redox enzyme peptides and their chaperones. J. Bacteriol. 191:2091–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Q., Wu H., Fives-Taylor P. M. 2004. Investigating the role of secA2 in secretion and glycosylation of a fimbrial adhesin in Streptococcus parasanguis FW213. Mol. Microbiol. 53:843–856 [DOI] [PubMed] [Google Scholar]
- 14. Driessen A. J., Nouwen N. 2008. Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 77:643–667 [DOI] [PubMed] [Google Scholar]
- 15. Dunker A. K., Silman I., Uversky V. N., Sussman J. L. 2008. Function and structure of inherently disordered proteins. Curr. Opin. Struct. Biol. 18:756–764 [DOI] [PubMed] [Google Scholar]
- 16. Hardy S. J., Randall L. L. 1991. A kinetic partitioning model of selective binding of nonnative proteins by the bacterial chaperone SecB. Science 251:439–443 [DOI] [PubMed] [Google Scholar]
- 17. Kelley L. A., Sternberg M. J. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371 [DOI] [PubMed] [Google Scholar]
- 18. Khisty V. J., Munske G. R., Randall L. L. 1995. Mapping of the binding frame for the chaperone SecB within a natural ligand, galactose-binding protein. J. Biol. Chem. 270:25920–25927 [DOI] [PubMed] [Google Scholar]
- 19. Knoblauch N. T., et al. 1999. Substrate specificity of the SecB chaperone. J. Biol. Chem. 274:34219–34225 [DOI] [PubMed] [Google Scholar]
- 20. Li Y., et al. 2008. A conserved domain of previously unknown function in Gap1 mediates protein-protein interaction and is required for biogenesis of a serine-rich streptococcal adhesin. Mol. Microbiol. 70:1094–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu G., Topping T. B., Randall L. L. 1989. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc. Natl. Acad. Sci. U. S. A. 86:9213–9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mistou M. Y., Dramsi S., Brega S., Poyart C., Trieu-Cuot P. 2009. Molecular dissection of the secA2 locus of group B streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J. Bacteriol. 191:4195–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Obert C., et al. 2006. Identification of a candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect. Immun. 74:4766–4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oresnik I. J., Ladner C. L., Turner R. J. 2001. Identification of a twin-arginine leader-binding protein. Mol. Microbiol. 40:323–331 [DOI] [PubMed] [Google Scholar]
- 25. Randall L. L. 1992. Peptide binding by chaperone SecB: implications for recognition of nonnative structure. Science 257:241–245 [DOI] [PubMed] [Google Scholar]
- 26. Rigel N. W., Braunstein M. 2008. A new twist on an old pathway–accessory Sec systems. Mol. Microbiol. 69:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saul F. A., et al. 2003. Crystal structure of a defective folding protein. Protein Sci. 12:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seepersaud R., Bensing B. A., Yen Y. T., Sullam P. M. 2010. Asp3 mediates multiple protein-protein interactions within the accessory Sec system of Streptococcus gordonii. Mol. Microbiol. 78:490–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seepersaud R., Needham R. H., Kim C. S., Jones A. L. 2006. Abundance of the delta subunit of RNA polymerase is linked to the virulence of Streptococcus agalactiae. J. Bacteriol. 188:2096–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seifert K. N., et al. 2006. A unique serine-rich repeat protein (Srr-2) and novel surface antigen (epsilon) associated with a virulent lineage of serotype III Streptococcus agalactiae. Microbiology 152:1029–1040 [DOI] [PubMed] [Google Scholar]
- 31. Siboo I. R., Chaffin D. O., Rubens C. E., Sullam P. M. 2008. Characterization of the accessory Sec system of Staphylococcus aureus. J. Bacteriol. 190:6188–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siboo I. R., Chambers H. F., Sullam P. M. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 73:2273–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sullam P. M., Valone F. H., Mills J. 1987. Mechanisms of platelet aggregation by viridans group streptococci. Infect. Immun. 55:1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takamatsu D., et al. 2005. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibα. Mol. Microbiol. 58:380–392 [DOI] [PubMed] [Google Scholar]
- 35. Takamatsu D., Bensing B. A., Prakobphol A., Fisher S. J., Sullam P. M. 2006. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect. Immun. 74:1933–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takamatsu D., Bensing B. A., Sullam P. M. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 186:7100–7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takamatsu D., Bensing B. A., Sullam P. M. 2004. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52:189–203 [DOI] [PubMed] [Google Scholar]
- 38. Takamatsu D., Bensing B. A., Sullam P. M. 2005. Two additional components of the accessory sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 187:3878–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Topping T. B., Randall L. L. 1994. Determination of the binding frame within a physiological ligand for the chaperone SecB. Protein Sci. 3:730–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Sorge N. M., et al. 2009. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J. Infect. Dis. 199:1479–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilharm G., Dittmann S., Schmid A., Heesemann J. 2007. On the role of specific chaperones, the specific ATPase, and the proton motive force in type III secretion. Int. J. Med. Microbiol. 297:27–36 [DOI] [PubMed] [Google Scholar]
- 42. Wu H., Bu S., Newell P., Chen Q., Fives-Taylor P. 2007. Two gene determinants are differentially involved in the biogenesis of Fap1 precursors in Streptococcus parasanguis. J. Bacteriol. 189:1390–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu H., Fives-Taylor P. M. 1999. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguinis. Mol. Microbiol. 34:1070–1081 [DOI] [PubMed] [Google Scholar]
- 44. Xiong Y. Q., Bensing B. A., Bayer A. S., Chambers H. F., Sullam P. M. 2008. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 45:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou M., Peng Z., Fives-Taylor P., Wu H. 2008. A conserved C-terminal 13-amino-acid motif of Gap1 is required for Gap1 function and necessary for the biogenesis of a serine-rich glycoprotein of Streptococcus parasanguinis. Infect. Immun. 76:5624–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou M., Wu H. 2009. Glycosylation and biogenesis of a family of serine-rich bacterial adhesins. Microbiology 155:317–327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.