Abstract
The neural crest is an induced tissue that is unique to vertebrates. In the clawed frog Xenopus laevis, neural crest induction depends on signals secreted from the prospective dorsolateral mesodermal zone during gastrulation. The transcription factors Snail2 (Slug), Snail1 and Twist1 are expressed in this region. It is known that Snail2 and Twist1 are required for both mesoderm formation and neural crest induction. Using targeted blastomere injection, morpholino-based loss of function and explant studies, we show that: (1) Snail1 is also required for mesoderm and neural crest formation; (2) loss of snail1, snail2 or twist1 function in the C2/C3 lineage of 32-cell embryos blocks mesoderm formation, but neural crest is lost only in the case of snail2 loss of function; (3) snail2 mutant loss of neural crest involves mesoderm-derived secreted factors and can be rescued synergistically by bmp4 and wnt8 RNAs; and (4) loss of snail2 activity leads to changes in the RNA levels of a number of BMP and Wnt agonists and antagonists. Taken together, these results identify Snail2 as a key regulator of the signals involved in mesodermal induction of neural crest.
Keywords: Snail2, Slug, Snail1, Twist1, Mesoderm, Neural crest induction, BMP, Wnt, Xenopus
INTRODUCTION
Neural crest is of interest for both evolutionary and medical reasons. Like the mesoderm, it is an induced tissue, arising at the boundary between the nascent neural plate and the embryonic epidermis (Alfandari et al., 2010; Basch and Bronner-Fraser, 2006; Klymkowsky et al., 2010; Minoux and Rijli, 2010). The neural crest represents an evolutionary innovation (Baker, 2008; Yu, 2010), responsible in part for the diverse cranial morphologies of vertebrates (Hanken and Gross, 2005). It provides a classic example of an epithelial-mesenchymal transition (EMT) and associated apoptotic suppression, and so serves as a model for cancer metastasis (Klymkowsky and Savagner, 2009). Neural crest is also the focus of tetratogenic birth defects in humans, such as those caused by thalidomide (McCredie, 2009) and ethanol (Webster and Ritchie, 1991).
Although there have been reports that mesoderm is not involved in the induction of neural crest in zebrafish (Ragland and Raible, 2004) and chick [(Basch et al., 2006), but see below], there is clear evidence for a role for early mesoderm in neural crest induction in amphibians in general [(Raven and Kloos, 1945), as cited by Baker and Bronner-Fraser (Baker and Bronner-Fraser, 1997)] and, more specifically, in Xenopus laevis (Bonstein et al., 1998; Hong et al., 2008; Marchant et al., 1998; Mayor et al., 1995; Monsoro-Burq et al., 2003; Steventon et al., 2009; Steventon et al., 2005). The situation is somewhat less clear in mouse and human, in part because of the challenges associated with experimental studies in the corresponding early embryonic stages (Aggarwal et al., 2010; Carver et al., 2001; Goh et al., 1997; O'Rourke and Tam, 2002; Xu et al., 2000).
The transcription factors Snail and Twist were first identified in Drosophila melanogaster, where they sit at the end of the Toll/Dorsal (NF-κB) signaling pathway (Stathopoulos and Levine, 2002; Valanne et al., 2011). Mutations in snail and twist lead to defects in mesoderm formation (Leptin, 1991; Thisse et al., 1987). snail-type genes encode C2H2-type zinc-finger transcription factors. Multiple members of this gene family have been characterized from placozoans through humans (Barrallo-Gimeno and Nieto, 2009). twist encodes a basic helix-loop-helix (bHLH)-type transcription factor; these proteins typically act as dimers (Barnes and Firulli, 2009). Both snail- and twist-like genes are found in the primitive chordate Ciona intestinalis (Shi et al., 2005) and in the jellyfish Podocoryne carnea (Spring et al., 2002; Spring et al., 2000). A BLAST analysis indicates that both the Ciona and Podocoryne Snail proteins more closely resemble mammalian Snail2 (Slug) than Snail1 proteins (our unpublished observation). Snail proteins appear to act primarily as transcriptional repressors, binding to DNA E-box (5′-CANNTG-3′) sequences. During Drosophila mesoderm specification and patterning, snail and twist expression are regulated by a molecular cascade involving dorsal, which encodes an ortholog of the NF-κB subunit protein RelA (Huguet et al., 1997; Ip et al., 1992). This network involves negative-feedback regulation of dorsal through the secreted factor WntD, the expression of which is regulated by Snail and Twist (Ganguly et al., 2005; Gordon et al., 2005). Genomic chromatin immunoprecipitation-microarray studies (Sandmann et al., 2007; Zeitlinger et al., 2007) suggest that Snail and Twist regulate a wide array of target genes: Twist targets almost 25% of all annotated Drosophila transcription factors (Sandmann et al., 2007). Interestingly, in the vertebrate X. laevis, snail1, snail2 and twist1 RNAs appear to be `immediate-early' targets of regulation by the NF-κB subunit protein RelA (Zhang et al., 2006).
In vertebrates, there are two distinct twist-like genes: twist1 and twist2 (dermo1) (Li et al., 1995). twist1 has been implicated in mesoderm formation, as well as in a number of developmental events. twist1 haploinsufficiency leads to skeletal dysplasia (Miraoui and Marie, 2010). In the mouse, Twist1 is required for cranial neural crest migration as well as for the suppression of apoptosis (Chen and Behringer, 1995; Soo et al., 2002). In humans, mutations in TWIST have been implicated in mesenchymal stem cell differentiation and skeletal malformations (craniosynostosis) (Miraoui and Marie, 2010). There are two closely related snail-like genes in vertebrates, snail1 and snail2 (previously known as slug), as well as a number of more distantly related genes (Barrallo-Gimeno and Nieto, 2009; Manzanares et al., 2001; Nieto, 2002). In vertebrates, snail gene function was originally studied most intensely in the context of the neural crest (Aybar et al., 2003; Carl et al., 1999; LaBonne and Bronner-Fraser, 2000; Nieto et al., 1994; O'Rourke and Tam, 2002; Tribulo et al., 2004). In the chick, Snail2 is expressed in both mesoderm and premigratory crest, and appears to be involved in the formation and behavior of both tissues (Nieto et al., 1994). In the mouse, the domains of Snail2 and Snail1 expression are switched (Locascio et al., 2002; Sefton et al., 1998) and neither Snail1 nor Snail2 appears to be absolutely necessary for either mesodermal or neural crest formation (Carver et al., 2001; Jiang et al., 1998). That said, Snail1 null mice display a recessive embryonic lethal phenotype with clear gastrulation defects and morphologically abnormal mesoderm (Carver et al., 2001). Whether the roles of Snail1 and Snail2 in the early mouse embryo have been subsumed by other genes, such as Twist1, Zeb1 or Zeb2 (Sip1), which encodes an E-box-binding protein implicated in the regulation of EMT and tumor progression (Bracken et al., 2008; Liu et al., 2009; Schmalhofer et al., 2009; Wellner et al., 2009), remains unclear. That complex interactions may be involved is suggested by a report that Snail2 is required for Twist1-induced EMT in mice (Casas et al., 2011). In the mouse, Snail2 acts downstream of Sox9 in trunk neural crest specification (Cheung et al., 2005). Snail2–/– mice display white forehead blaze, patchy depigmentation of the ventral body, tail and feet, macrocytic anemia, infertility and a deficiency in white adipose tissue mass (Perez-Mancera et al., 2007). The combination of a conditional Snail1 null mutation and Snail2–/– leads to defects in left-right axis formation but not, apparently, to defects in neural crest formation (Murray and Gridley, 2006).
In X. laevis, snail1, snail2 and twist1 are expressed in the blastula stage embryo (Essex et al., 1993; Mayor et al., 1993; Mayor et al., 2000; Sargent and Bennett, 1990; Zhang and Klymkowsky, 2009). Previously, we presented evidence for a role for snail2 and twist1 in mesoderm and neural crest formation (Carl et al., 1999; Zhang et al., 2006; Zhang and Klymkowsky, 2009). The expression of snail2, snail1 and twist1 in both early mesoderm and neural crest raises a number of issues, illustrated in part by the work of Aybar et al. (Aybar et al., 2003). Based on the behavior of dominant-negative mutant forms of Snail2 and Snail1, they claimed that snail (snail1) precedes slug (snail2) in the genetic cascade involved in neural crest specification. Yet morpholino-based studies indicate that inhibition of snail2 expression disrupts mesoderm formation and leads to a decrease in snail1 RNA levels in the early embryo (Zhang et al., 2006; Zhang and Klymkowsky, 2009). To resolve these issues, we extended previous work using morpholinos to examine the role of snail1, snail2 and twist1 in the early Xenopus embryo. Blastomere injection and explant studies enabled us to discover distinct roles for snail2, as compared with snail1 and twist1, in mesoderm-based induction of neural crest.
MATERIALS AND METHODS
Embryos and their manipulation
X. laevis embryos were staged, and explants and co-explants were generated following standard procedures (Klymkowsky and Hanken, 1991; Nieuwkoop and Faber, 1967; Sive et al., 2000; Zhang et al., 2004). Similar studies were carried out using X. tropicalis embryos using animals purchased from Xenopus I following methods analogous to those used in X. laevis, and modified as described in Khokha et al. (Khokha et al., 2002). Capped mRNAs were transcribed from linearized plasmid templates using mMessage mMachine kits (Ambion) following the manufacturer's instructions. For 2-cell stage studies, embryos were injected equatorially; for 32-cell stage studies, C2 and C3 blastomeres were co-injected with RNA encoding GFP, and examined at stage 10 to confirm the accuracy of injection. Animal caps were isolated from stage 8-9 blastula embryos in 0.5 MMR (Sive et al., 2000), transferred into wells of a 2% agarose plate (one animal cap per well) and combined with dorsolateral mesodermal zone isolated from stage 10.5 gastrula embryos according to Bonstein et al. (Bonstein et al., 1998). Explant recombinants were harvested when siblings reached stage 18. Images were captured using a Nikon CoolPix 995 camera on a Leica M400 photomicroscope or using a Nikon D5000 camera on a Wild stereomicroscope. Images were manipulated with Fireworks CS4 software (Adobe) using Auto Levels and Curves functions only.
Morpholinos and plasmids
Modified morpholino antisense oligonucleotides (MOs) were purchased from Gene Tools. The MO against snail1 (5′-TTTAGCAGCCGAGCACTGAGTTCCT-3′) was tested for its ability to block the translation of snail1 RNA using an RNA that contains its target site and encodes a Snail1-GFP chimera. Other MOs used were designed to block the translation of antipodean/vegT (5′-ACTTTACATCCGGCAGAGAATGCAT-3′) and xbra (5′-GCGCAGCTCTCGGTCGCACTCATTA-3′) RNAs. snail2 and twist1 MOs were as described previously (Zhang et al., 2006; Zhang and Klymkowsky, 2009). In addition, a new snail2 MO was designed (5-TCTTGACCAGAAAAGATCGTGGCAT-3′) that matches the single identified X. tropicalis snail2 gene perfectly (Vallin et al., 2001).
Plasmids encoding ΔNp63 and ΔNp63R304W (Barton et al., 2009) were generously supplied by Ethan Lee. Plasmids encoding Sizzled (Salic et al., 1997), Cerberus (Bouwmeester et al., 1996), BMP4, Wnt8, Noggin (Smith and Harland, 1992) and Dickkopf (Dkk) (Glinka et al., 1998) were supplied by Eddy DeRobertis and Richard Harland. Snail2, Snail1 and Twist expression plasmids have been described previously (Zhang et al., 2006; Zhang and Klymkowsky, 2009); a GFP-tagged form of Snail1 was constructed for this project. RNA-injected embryos were selected based on GFP fluorescence.
Immunoblot and in situ hybridization studies
For in situ hybridization studies, digoxigenin-UTP-labeled antisense probes were prepared following standard methods; specific probes for sox9, vegT/antipodean, chordin, endodermin, myoD, chd7 and xbra RNAs were used. In many cases, embryos were co-injected with RNA (50 pg/embryo) encoding β-galactosidase, the activity of which was visualized in fixed embryos using a brief Red-Gal (Research Organics) reaction in order to identify successfully injected embryos.
RT-PCR and quantitative RT-PCR (qPCR)
RNA isolation, RT-PCR and qPCR analyses were carried out as described previously (Zhang et al., 2003; Zhang et al., 2006). In brief, total RNA was isolated from embryos or dorsal lateral mesoderm regions; cDNA synthesis was performed from 1 μg purified RNA using a Verso cDNA kit (Thermo Scientific) according to the manufacturer's directions. Real-time PCR was carried out using a Mastercycler Epgradient Realplex device (Eppendorf). PCR reactions were set up using DyNAmo SYBR Green qPCR kits (Finnzymes). Each sample was normalized to the expression level of ornithine decarboxylase (ODC). The cycling conditions used were: 95°C for 5 minutes; then 40 cycles of 95°C for 15 seconds, 56°C for 15 seconds, 60°C for 30 seconds. The ΔΔCT method was used to calculate real-time PCR results. Primers for RT-PCR analyses were (5′ to 3′, forward and reverse):
snail2, GATGCACATCAGGACACACAC and CTGCGAATGCTCTGTTGCAGT;
snail1, AAGCACAATGGACTCCTT and CCAATAGTGATACACACC;
twist1, AGTCCGATCTCAGTGAAGGGC and TGTGTGTGGCCTGAGCTGTAG;
ODC, CAGCTAGCTGTGGTGTGG and CAACATGGAAACTCACAC;
sox9, TGCAATTTTCAAGGCCCTAC and GCTGCCTACCATTCTCTTGC;
sizzled, CATGTCCGGAGTCTTCCTGC and GGATGAACGTGTCCAGGCAG;
cerberus, CCTTGCCCTTCACTCAG and TGGCAGACAGTCCTTT;
wnt8, TGATGCCTTCACTTCTGTGG and TCCTGCAGCTTCTTCTCTCC;
bmp4, TGGTGGATTAGTCTCGTGTCC and TCAACCTCAGCAGCATTCC;
dkk, ACGGAAGATGATGACTGTGC and CTCTTGATCTTGCTCCACAGG;
noggin, AGTTGCAGATGTGGCTCT and AGTCCAAGAGTCT CAGCA;
frzb1, TGGACTCATTCCTGCTACTGG and AATTGCCAGGATAGCATTGG;
sfrp2, TCTGTGTGAGCAGGTGAAGG and GTCATTGTCATCCTCGTTGC;
crescent, GGCTCTCTGCTATGACATTGG and CACACAGACTGCGACATGG;
chordin, CCTCCAATCCAAGACTCCAGCAG and GGAGGAGGAGGAGCTTTGGGACAAG;
fgf8, TGGTGACCGACCAACTAAGC and ACGATTAACTTGGCGTGTGG;
eomes, TCCTCAGGCTACCAGTACAGC and GCCGGTTACAGAGGAAGACC;
gata4a, CTGGACTGCTTCTCCATTCG and ACATTGCTCCACAGTTGACG; and
tbx6, CAGCCAATCAGGAACAAGG and GTTCTGTGCTGCATCTGTGG.
RESULTS
snail1 is required for both mesoderm and neural crest formation
It is clear that snail1 RNA is present in X. laevis embryos following the onset of zygotic transcription (Mayor et al., 2000; Sargent and Bennett, 1990; Zhang and Klymkowsky, 2009). In past studies, it was reported that injection of snail1 RNA could partially rescue the effects of snail2 antisense RNA and snail2 and twist1 morphant phenotypes (Carl et al., 1999; Zhang et al., 2006; Zhang and Klymkowsky, 2009), as well as the effects of dominant-negative versions of Snail2 (LaBonne and Bronner-Fraser, 2000). Levels of snail1 RNA are reduced in snail2 and twist1 morphant embryos (Zhang and Klymkowsky, 2009). To determine whether snail1 expression is involved in early mesoderm formation and/or maintenance, which does not appear to have been examined previously, we designed a morpholino (MO) directed against the 5′ UTR of the single identified snail1 mRNA in X. laevis (see Fig. S1 in the supplementary material). The previously employed snail2 MO (Zhang et al., 2006) has 12 out of 25 mismatches to the analogous region of the snail1 mRNA, whereas the snail1 MO has 17 and 18 out of 25 mismatches to the snail2a and snail2b mRNAs, respectively, as identified by Vallin et al. (Vallin et al., 2001). As we have yet to identify a functional antibody for Xenopus Snail1 (or Snail2 or Twist), we examined the effects of snail1 MO on the translation of an RNA, UTR-Snail1-GFP, that contains the MO 5′ UTR target sequence. When UTR-Snail1-GFP RNA and snail1 MO were co-injected into fertilized eggs, there was a reduction in the level of Snail1-GFP protein accumulation (Fig. 1A) and a reduction in the level of Snail1-GFP fluorescence (Fig. 1B,C). RT-PCR (Fig. 1D) and quantitative RT-PCR (qPCR) (Fig. 1E) analyses indicated that injection of snail1 MO into both blastomeres of a 2-cell embryo led to a reduction in the amounts of both twist1 and snail2 RNA.
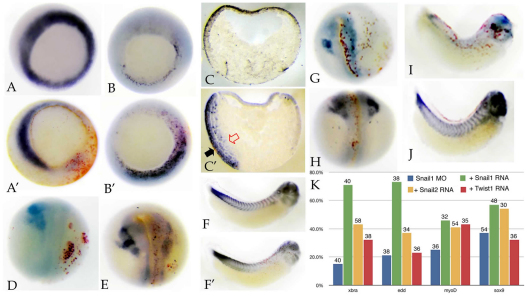
Fig. 1.
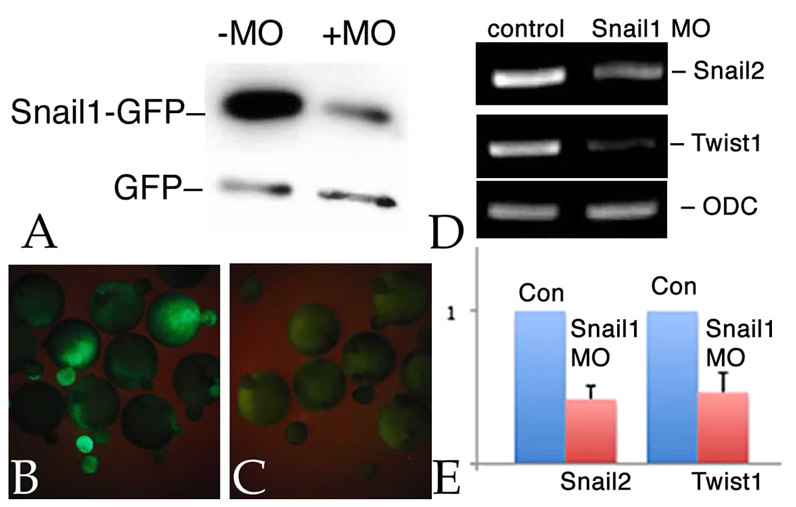
snail1 MO effects. Xenopus embryos were injected with RNAs encoding myc-tagged GFP (mt-GFP; 50 pg/embryo) and UTR-Snail1-GFP (which latter includes the target of the snail1 MO) RNAs (600 pg/embryo), either alone or together with the snail1 MO (7 ng/embryo). (A) Immunoblot analysis of stage 11 embryos using an anti-GFP antibody revealed a clear and specific reduction in the accumulation of Snail1-GFP as compared with GFP in the snail1 MO-injected sample. (B,C) UTR-Snail1-GFP RNA was injected into one cell of 2-cell embryos either alone (B) or together with the snail1 MO (C). At stage 11, the snail1 MO greatly reduced UTR-Snail1-GFP fluorescence. (D,E) RT-PCR (D) and qPCR (E) analyses indicate that the injection of the snail1 MO into both cells of 2-cell embryos led to a specific reduction in the levels of twist1 and snail2 RNAs at stage 11. Ornithine decarboxylase (ODC) RNA was used to control for non-specific effects. Error bars indicate s.d.
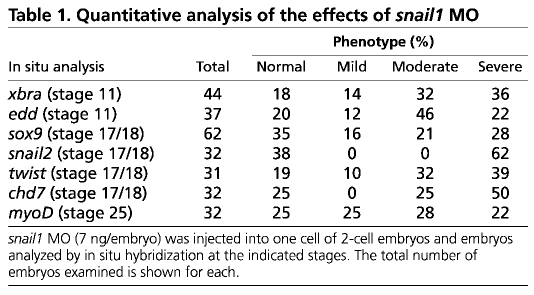
Injection of snail2 antisense RNA, or snail2 or twist1 MOs, into one cell of 2-cell embryos leads to the loss of the mesodermal markers xbra, antipodean/vegT and myoD, an increase in the expression domain of the endodermal marker endodermin (edd), and the loss of expression of the neural crest marker sox9 (Carl et al., 1999; Zhang et al., 2006; Zhang and Klymkowsky, 2009). Injection of the snail1 MO produced similar effects: loss of xbra (Fig. 2A,A′) and antipodean/vegT (data not shown) expression, an increase in edd expression (Fig. 2B,B′) and expansion of the edd expression domain into the underlying mesoderm of stage 11/12 embryos (Fig. 2C,C′), and the loss of expression of sox9 (Fig. 2D,E) and myoD (Fig. 2F,F′) in later stage embryos. Levels of other neural crest markers, specifically snail2, twist and chd7 (Bajpai et al., 2010), were reduced in snail1 morphant embryos (see Fig. S2 in the supplementary material). A quantitative analysis of the effects of snail1 MO are presented in Table 1. As noted in past studies, a number of morphant embryos failed to gastrulate, suggesting that later phenotypes are hypomorphic rather than null (amorphic) (see Zhang et al., 2006; Zhang and Klymkowsky, 2009). The effects of the snail1 MO were rescued by the injection of an RNA encoding Snail1-GFP (Fig. 2G-J), an RNA that does not contain the target sequence of the snail1 MO. As in the case of snail2 and twist1 morphant embryos, the snail1 MO phenotype was rescued, albeit not as efficiently, by snail2 and twist1 RNAs (Fig. 2K). The extent to which snail2, snail1 and twist1 RNAs rescue the expression of `late' genes, such as sox9 and myoD, was lower than their ability to rescue the expression of the `earlier' genes xbra and edd, perhaps because later `differentiation' genes are more redundantly regulated.
Fig. 2.
snail1 MO effects on germ layer markers. (A-B′) snail1 MO injection (into one cell of a 2-cell embryo) led to the loss of expression (as measured by in situ hybridization) of xbra (A′, versus control in A) and to an increase in endodermin (edd) (B′, versus control in B) RNA levels (at stage 11). (C,C′) Section analysis revealed that the edd expression domain, which is restricted to the superficial region in control embryos (C), extends deeper into the mesodermal region in snail1 MO-injected embryos (C′, black arrow indicates lacZ marker staining, and the red arrow indicates the extent of edd staining in deep mesodermal tissue). (D-F′) There was a loss of expression of the neural crest/placodal marker sox9 at stage 17/18 (D, severely affected; E, mildly affected; injected sides to the right), as well as a loss of expression of myoD at stage 25, as expressed in myotomal muscle, a mesodermal derivative (F′, versus control in F). (G-J) The effects on sox9 (G,H) and myoD (I,J) of snail1 MO injection (G,I) were rescued by the injection of the Snail1-GFP RNA (H,J), which lacks the snail1 MO target sequence. Injected sides are shown. (K) The percentage of normal embryos plotted with respect to xbra, edd, myoD and sox9 in situ expression. Blue, snail1 MO injected; green, snail1 MO together with snail1 RNA (600 pg/embryo); yellow, snail1 MO together with Snail2-GFP RNA (600 pg/embryo); red, snail1 MO together with Twist1-GFP RNA (600 pg/embryo). The number of embryos examined is indicated above each bar.
Table 1.
Quantitative analysis of the effects of snail1 MO
Loss of mesoderm leads to loss of neural crest
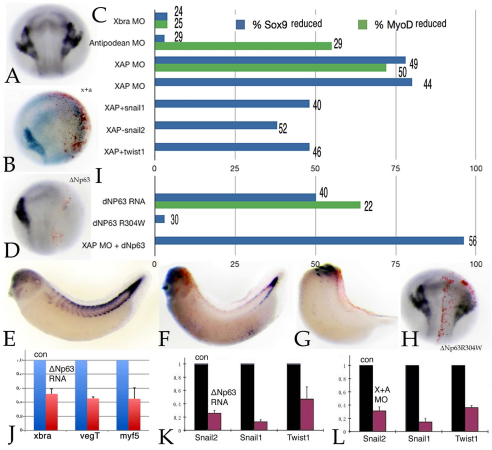
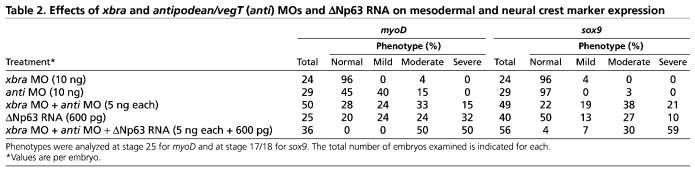
To confirm that loss of mesoderm per se leads to the failure of neural crest induction, as described previously (Bonstein et al., 1998), we disrupted mesoderm formation in two complementary ways. xbra (Smith et al., 1991) and vegT/antipodean (Stennard et al., 1996; Stennard et al., 1999) encode T-box-type transcription factors involved in mesoderm formation in X. laevis. Injection of an xbra MO (10 ng/embryo) into one cell of a 2-cell embryo had little effect on the expression of the myotomal marker myoD, whereas injection of an antipodean/vegT MO led to the loss of myoD expression, as described previously (Fukuda et al., 2010) (see Fig. S3 in the supplementary material); neither had any apparent effect on sox9 expression (data not shown). By contrast, when injected together (5 ng each/embryo), the xbra and antipodean/vegT MOs caused the loss of sox9 expression in ∼80% of embryos (Fig. 3A-C; Table 2).
Fig. 3.
Loss of mesoderm leads to loss of neural crest. (A,B) Injection of the xbra MO has little effect on myoD (stage 25) or sox9 (stage 17/18) expression. Injection of the antipodean/vegT MO causes a decrease in myoD expression (Fukuda et al., 2010), but little effect on sox9 expression (data not shown). Together (XAP), the xbra and antipodean/vegT MOs block sox9 (stage 17/18) (B, versus control in A) and myoD (data not shown) expression. (C) The percentage of embryos with loss of myoD or sox9 staining, with the number of embryos examined shown at the end of each bar. Injection of snail1, snail2 or twist1 RNAs (600 pg/embryo) produced a modest rescue of sox9 expression in XAP morphant embryos. (D-G) Injection of 600 pg ΔNp63 RNA into one cell of a 2-cell embryo led to the loss of sox9 (D), xbra (not shown) and myoD (F, moderate effect; G, severe effect; versus control in E) expression in ∼50% of embryos. (H) Injection of RNA encoding the mutated and inactive ΔNp63R304W protein had no effect on sox9 or myoD (data not shown) expression. (I) Quantitative presentation of the data shown in D-H. (J-L) qPCR analyses of ΔNp63 RNA-injected embryos (both cells of 2-cell embryos injected, analyzed at stage 11) revealed a substantial decrease in the levels of the xbra, vegT/antipodean and myf5 mesodermal marker RNAs (J). In both ΔNp63 RNA (K) and XAP MO (L) injected embryos, there were similar decreases in the levels of snail2, snail1 and twist1 RNAs. Error bars indicate s.d.
Table 2.
Effects of xbra and antipodean/vegT (anti) MOs and ΔNp63 RNA on mesodermal and neural crest marker expression
That the antipodean/vegT MO alone had no effect on sox9 expression suggested possible compensatory processes. Mesoderm formation involves multiple pathways that act in independent, interdependent and cooperative ways (Koide et al., 2005; Loose and Patient, 2004). A second mesoderm pathway active in X. laevis involves the transcription factor p53 and its modulation of TGFβ signaling through interactions with SMAD proteins (Cordenonsi et al., 2003; Cordenonsi et al., 2007; Sasai et al., 2008; Takebayashi-Suzuki et al., 2003). Barton et al. (Barton et al., 2009) described a splice variant of the p53-related protein p63, ΔNp63, that inhibits the p53-SMAD interaction and blocks mesoderm formation in X. laevis. Injection of ΔNp63 RNA (∼600 pg/embryo) into one cell of a 2-cell embryo led to loss of the neural crest marker sox9 and of the mesoderm/muscle marker myoD in ∼50% of embryos (Fig. 3D-G; Table 2). Injection of ΔNp63 RNA into both cells of a 2-cell embryo led to a reduction in xbra and vegT RNAs, as determined by qPCR, as well as to the reduction of another mesoderm marker, myf5 (Fig. 3J) (Hopwood et al., 1991). Injection of RNA encoding ΔNp63R304W, a mutated form of ΔNp63 that no longer binds to DNA (Barton et al., 2009), had no effect on sox9 expression (Fig. 3H,I). When injected together, xbra and antipodean/vegT MOs and ΔNp63 RNA led to the near complete loss of sox9 expression (Fig. 3C; Table 2), suggesting that the two pathways (T-box and p53/SMAD) cooperate in terms of mesodermal induction of neural crest (Table 2).
qPCR studies of xbra and antipodean/vegT morphant (Fig. 3K) and ΔNp63 RNA-injected (Fig. 3L) embryos (both cells of 2-cell embryos injected) revealed decreased levels of snail1, snail2 and twist1 RNAs. Given that loss of snail1, snail2 or twist1 expression inhibits mesoderm formation and neural crest induction (see above), we examined whether xbra and antipodean/vegT MO-induced loss of sox9 expression could be rescued by the injection of snail2, snail1 or twist1 RNAs. Such studies produced a modest rescue of sox9 expression (Fig. 3C). The loss of sox9 expression in the xbra and antipodean/vegT morphant and ΔNp63 RNA-injected embryos supports an active role of the mesoderm in neural crest induction in Xenopus (Bonstein et al., 1998).
Snail2 is a key regulator of dorsolateral mesoderm-dependent neural crest induction
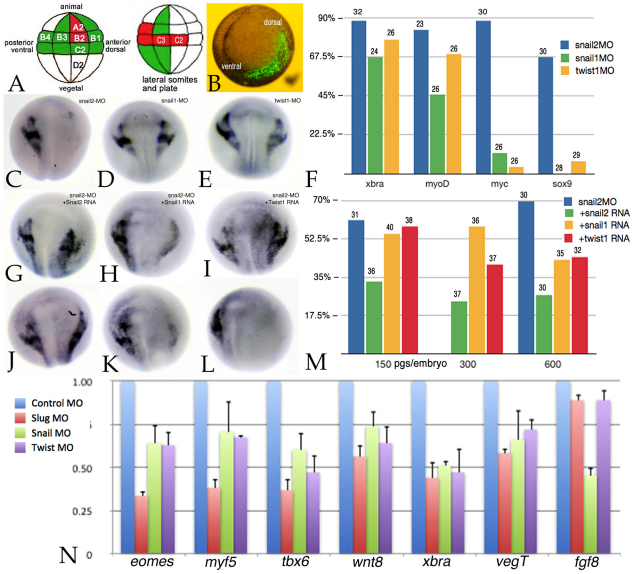
A limitation of whole- and half-embryo studies is that the injected reagents often influence a range of developing tissues. These studies are also complicated by the fact that the rates of diffusion of different reagents (e.g. antibodies, MOs and RNAs) within the embryo differ (Zhang et al., 2004) (our unpublished observations). To circumvent this effect, we used two complementary methods: targeted blastomere injection and combinatorial (sandwich) explant studies. Although it is certainly the case that there is no blastomere within the 32-cell embryo that contributes to a single tissue type or lineage, owing in part to the slow intermixing of cells (Dale and Slack, 1987; Moody, 1987; Nakamura et al., 1978; Wetts and Fraser, 1989), it is possible to favor certain tissues. For example, in the 32-cell embryo, the C2 and C3 blastomeres give rise primarily to paraxial mesoderm and make only moderate contributions to the neural crest. In our studies, we injected the C2/C3 blastomeres (Fig. 4A) with MOs against snail1, snail2 or twist1 RNAs together with RNA encoding GFP as a lineage tracer. Embryos were sorted at stage 10/10.5 (Fig. 4B) to select those accurately injected. When fixed at stage 18 and stained in situ, we found that sox9 expression was dramatically reduced in snail2 morphant embryos, but not in snail1 or twist1 morphant embryos (Fig. 4C-F). Subsequent studies indicated that whereas all three MOs led to a reduction in the levels of the mesodermal markers xbra (stage 11) and myoD (stage 25), only the snail2 MO produced a decrease in the neural border/early neural crest marker c-myc (stage 16) (Bellmeyer et al., 2003) and in the later neural crest marker sox9 (stage 18) (Lee et al., 2004; Spokony et al., 2002) (Fig. 4F). Preliminary studies in X. tropicalis indicate that the same pattern holds true there: C2/C3 snail2, but not snail1 or twist1, morphant embryos show a loss of sox9 expression (see Fig. S4 in the supplementary material).
Fig. 4.
Target blastomere injection effects. (A) Fate map of the 32-cell X. laevis embryo, lateral to front, with blastomeres marked with respect to tiers (A-D) and position (1, dorsal; 4, ventral). Blastomeres are marked that make a major (red) or moderate (green) contribution to neural crest (left) or to lateral somites and plate mesoderm (right). (B) At the 32-cell stage, this embryo was injected with RNA encoding GFP; the embryo was photographed under bright-field and epifluorescence illumination at stage 11. Ventral pole to front (dorsal and ventral are marked). (C-E) C2/C3 blastomeres were injected with snail2, snail1 or twist1 MOs; at stage 17/18 the embryos were fixed and stained in situ for sox9 RNA. sox9 expression was lost in snail2 morphant embryos (C), but was present in snail1 (D) and twist1 (E) morphants. (F) The phenotypes of C2/C3 morphant embryos with respect to xbra, myoD, c-myc and sox9 expression. Bars indicate percentage loss of expression; the number of embryos is indicated above each bar. (G-I) The effects of the snail2 MO could be partially rescued by injection of high levels (600 pg/embryo) of snail2 (G), snail1 (H) or twist1(I) RNAs. (J-L) snail2 RNA was more effective at rescuing the snail2 C2/C3 morphant sox9 phenotype than either snail1 or twist1 RNAs. snail2 C2/C3 morphant embryos were co-injected with 150 pg/embryo of snail2 (J), snail1 (K) or twist1 (L) RNA. (M) The rescue of the C2/C3 snail2 morphant sox9 phenotype by 150, 300 and 600 pg/embryo snail2, snail1 and twist1 RNAs. Bars indicate percentage loss of expression; the number of embryos is indicated above each bar. (N) The DLMZ of C2/C3 MO-injected embryos was dissected at stage 10.5 and subject to qPCR analysis. The effects on the mesodermal markers xbra, eomes, tbx6, myf5, as well as on wnt8 and fgf8 RNA levels were analyzed. The results shown are representative of studies carried out in triplicate. Error bars indicate s.d.
The effects of C2/C3 snail2 MO injection on sox9 expression could be partially rescued by injection of high levels (600 pg/embryo) of snail2, snail1 or twist1 RNAs (Fig. 4G-I). RNA titration studies indicated that snail2 RNA was more effective at every dose (150 to 600 pg/embryo) than snail1 or twist1 RNAs at rescuing the snail2 morphant phenotype (Fig. 4J-M).
At stage 11, the dorsolateral mesodermal zone (DLMZ) of C2/C3 injected morphant embryos was dissected and analyzed by quantitative RT-PCR (Fig. 4N). The levels of RNA encoding the mesodermal markers eomes, myf5 and tbx6 (Hug et al., 1997; Li et al., 2006; Lou et al., 2006; Ryan et al., 1996) were preferentially reduced in snail2 morphant DLMZ. Interestingly, fgf8 RNA levels (Fletcher et al., 2006; Fletcher and Harland, 2008; Monsoro-Burq et al., 2003) appeared to be selectively regulated by Snail1. To resolve this and other issues, we have begun RNA SEQ analysis of morphant X. tropicalis embryos.
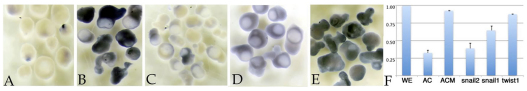
Because C2/C3 blastomeres give rise not only to mesoderm but also to other tissues, including neural crest, we examined inductive interactions between ectoderm (animal cap) and DLMZ explants. On its own, the wild-type animal cap remains as atypical ectoderm, expressing only low levels of the neural crest marker sox9 compared with whole embryos analyzed at the same stage (Fig. 5A,F). As demonstrated previously, DLMZ induces a number of markers of neural crest, including snail2, foxD3, zic5 and sox9 (Bonstein et al., 1998; Monsoro-Burq et al., 2003). Fig. 5B,F illustrates the effect of wild-type DLMZ on sox9 expression in explants. snail2 morphant DLMZ induced a much reduced level of sox9 expression (Fig. 5C,F), whereas twist1 morphant DLMZ behaved very much like wild-type DLMZ (Fig. 5E,F). snail1 morphant DLMZ explants produced a reduced, but readily detectable, level of sox9 expression (Fig. 5D,F).
Fig. 5.
Dorsal mesoderm-animal cap explant studies. (A) When cultured alone, wild-type ectoderm (animal cap) contained little sox9 RNA, as visualized by in situ hybridization (analysis carried out when control embryos had reached stage 17/18). (B) When cultured together with DLMZ from wild-type Xenopus embryos, sox9 RNA accumulated (in the ectodermal region). (C) By contrast, DLMZ from snail2 C2/C3 morphant embryos failed to induce sox9 RNA. (D,E) snail1 morphant DLMZ appears to be intermediate in its ability to induce sox9 expression (D), whereas twist1 morphant DLMZ retained the ability of induce sox9 expression (E). (F) qPCR analysis of sox9 RNA levels supports this general trend. For each condition, 5-15 explants were analyzed when unmanipulated embryos had reached stage 17/18. ODC RNA was used as a standard. WE, whole embryo at stage 17/18 (level set to 1); AC, animal cap/ectodermal explant; ACM, animal cap plus DLMZ from uninjected control embryo; snail2, wild-type animal cap plus snail2 morphant DLMZ; snail1, wild-type animal cap plus snail1 morphant DLMZ; twist1, wild-type animal cap plus twist1 morphant DLMZ. The results shown are representative of more than three replications. Error bars indicate s.d.
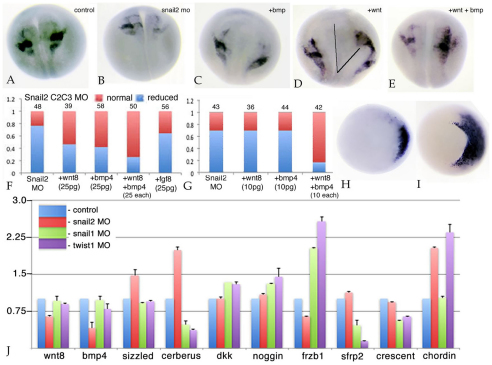
Previous studies have implicated FGF, Wnt and BMP signaling in mesodermal induction of the neural crest in X. laevis (Monsoro-Burq et al., 2003; Monsoro-Burq et al., 2005; Steventon et al., 2009). We examined this situation in the context of C2/C3 snail2 morphant embryos. Injection of RNAs (25 pg/embryo) encoding either BMP4 or Wnt8 were able to substantially rescue sox9 expression (Fig. 6A-D,F). When wnt8 and bmp4 RNAs were injected together, there was an improved (although not dramatic) rescue response (Fig. 6E,F). Injection of fgf8 RNA (25 pg/embryo) alone had little effect on the sox9 expression phenotype (Fig. 6F), and was not studied further. At lower RNA levels (10 pg/embryo), neither bmp4 nor wnt8 RNAs rescued the snail2 C2/C3 morphant sox9 phenotype, but together they produced a strong rescue (Fig. 6G). Levels of chordin RNA, which encodes a secreted BMP signaling inhibitor (Sasai et al., 1995; Sasai et al., 1994), were upregulated in snail2 C2/C3 morphant embryos (Fig. 6H,I). qPCR analyses of C2/C3 injected, and subsequently dissected, DLMZ indicate that levels of wnt8, bmp4 and frzb1 RNAs were decreased, whereas levels of cerberus, sizzled and chordin RNAs were increased in snail2 morphant tissue; a distinctly different pattern of changes in RNA levels was observed in snail1 and twist1 morphant tissues (Fig. 6J).
Fig. 6.
Further characterization of the snail2 morphant phenotype. (A-E) Xenopus C2/C3 blastomeres were injected with snail2 MO. Compared with uninjected embryos (A), snail2 morphants displayed a reduction in sox9 expression at stage 17/18 (B). This reduction was rescued by the injection of 25 pg/embryo bmp4 RNA (C), wnt8 RNA (D), or the two RNAs together (E). In the case of wnt8 RNA injection, there was often evidence of the formation of a secondary axis (D, line to the right). (F) The percentage of snail2 MO C2/C3 injected embryos rescued (red bar) using 25 pg/embryo fgf8, wnt8, bmp4 or wnt8 and bmp4 (25 pg each) RNAs is shown. (G) In a similar study, lower amounts (10 pg/embryo) of bmp4 and wnt8 RNAs were used. Rescue was observed only when bmp4 and wnt8 RNAs were injected together. (H,I) In situ analysis indicated that the levels and extent of chordin expression increased in stage 11 C2/C3 snail2 morphant embryos (I, versus control in H). (J) At stage 10.5-11, DLMZ from snail2, snail1 or twist1 morphant C2/C3 dorsolateral zones were dissected, RNA was isolated and subjected to qPCR analysis. This revealed reproducible increases in the levels of sizzled, cerberus and chordin RNAs and decreases in the levels of wnt8, bmp4 and frzb1 RNAs; distinct patterns of change were observed in snail1 and twist1 morphant DLMZ. Error bars indicate s.d.
DISCUSSION
The role of mesoderm in neural crest induction in X. laevis is well established (Bonstein et al., 1998; Green et al., 1997; Mayor et al., 2000; Monsoro-Burq et al., 2003; Steventon et al., 2009; Zhang et al., 2006; Zhang and Klymkowsky, 2009). Besides demonstrating that snail1 is involved in mesoderm formation/maintenance and neural crest induction, here we report that Snail2 plays a distinct role in the DLMZ of the late blastula/early gastrula stage embryo. Loss of Snail2 in this embryonic region led to the loss of expression of the early neural border/neural crest marker c-myc and of the late neural crest marker sox9. That the effect on neural crest is mediated by inductive signals is supported by `sandwich'-type explant studies between wild-type ectoderm and morphant DLMZ. Rescue studies suggest that both BMP4 and Wnt8 are essential components of this inductive signal; although this appears to partially contradict the conclusions of Steventon et al. (Steventon et al., 2009), it is likely that the details of the different experimental scenarios influence the behavior of the embryo (for example, differences in the concentrations and potency of the RNAs used, which can be influenced by the efficiency of the in vitro capping reaction.) Our conclusion is bolstered by the observation that the loss of snail2 activity led to a decrease in the levels of bmp4 and wnt8 RNAs, as well as to a reduction in the RNA encoding the selective Wnt inhibitor Frzb1 (Fig. 6J) (Leyns et al., 1997; Wang et al., 1997a; Wang et al., 1997b) and to increases in the levels of RNAs encoding the BMP4 antagonist Chordin, the Wnt and BMP antagonist Sizzled, and the Wnt, BMP and Nodal antagonist Cerberus. cerberus is an apparent `immediate-early' target of Snail2 regulation (Zhang and Klymkowsky, 2009). Together, these observations suggest that it is a balance between Wnt and BMP signaling that is critical for mesodermal induction of neural crest.
Although often considered in isolation, there is growing evidence that the BMP and Wnt signaling pathways interact (Itasaki and Hoppler, 2010; Katoh, 2010; Row and Kimelman, 2009). For example, it appears that the secreted Wnt inhibitors Dkk1 and Sost are regulated by BMP signaling (Kamiya et al., 2010) and that BMP signaling requires functional Wnt signaling (Tang et al., 2009). The Wnt signaling modulator Lpr4 interacts with Wise, a secreted and endoplasmic reticulum-localized protein that can either enhance or inhibit Wnt signaling (Guidato and Itasaki, 2007; Guidato et al., 1996; Itasaki et al., 2003). The Lrp4-Wise complex can, in turn, modulate BMP activity (Lintern et al., 2009; Ohazama et al., 2010). It is worth noting that the DLMZ of the late blastula/early gastrula stage X. laevis embryo is characterized by the expression of a number of other secreted signaling agonists and antagonists, in addition to chordin, sizzled, cerberus, frzb1, bmp4 and wnt8; these include other canonical and non-canonical acting Wnts (Kuhl, 2002), the TGFβ inhibitor Follistatin (Fainsod et al., 1997; Iemura et al., 1998; Tashiro et al., 1991), the BMP inhibitor Noggin (Smith and Harland, 1992) and the Wnt inhibitors Dkk, SFRP2, Sizzled2 and Crescent (Bradley et al., 2000; Glinka et al., 1998; Semenov et al., 2001; Shibata et al., 2000), as well as membrane-bound signaling regulators such as Kremen (Nakamura and Matsumoto, 2008). qPCR analyses support the view that the differences in the effects of snail1, snail2 and twist1 MOs in the C2/C3 lineage are due to differences in their regulatory targets (Fig. 6J).
It is very likely that these signaling factors interact. For example, Steventon et al. (Steventon et al., 2009) reported that snail2 RNA levels are reduced in chordin morphant embryos. Assuming a simple (and certainly incorrect) linear model for the BMP-Chordin interaction, loss of chordin expression would increase BMP signaling activity, thereby mimicking the ventral region of the embryo, where snail2 (as well as snail1 and twist1) RNA levels are low compared with the dorsal region (Zhang and Klymkowsky, 2009). Dissecting the interactions involved in the mesodermal induction of the neural crest network demands a much more global analysis – an analysis that we have begun by exploiting the recently released genomic data for X. tropicalis (Hellsten et al., 2010). Together with RNA and ChIP SEQ data, it should be possible to identify and study, in detail, the differences in the regulatory targets of Snail2, Snail1 and Twist1 in the DLMZ. In this light, it is worth noting that preliminary studies indicate that loss of snail2 function in the C2/C3 lineage in X. tropicalis (see Fig. S3 in the supplementary material) causes a loss of sox9 expression similar to that observed in X. laevis, suggesting that a similar regulatory network is involved in the two species.
Supplementary Material
Acknowledgments
We thank the Xenopus community, particularly Ethan Lee, Kristin Kroll, Hazel Sive, Eddy DeRobertis, J. Wysocka and Richard Harland for reagents; Mustafa Khokha and Richard Harland for advice on X. tropicalis; and the NIH (GM84133) for financial support. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.064394/-/DC1
Present address: Tongji Hospital, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China
References
- Aggarwal V. S., Carpenter C., Freyer L., Liao J., Petti M., Morrow B. E. (2010). Mesodermal Tbx1 is required for patterning the proximal mandible in mice. Dev. Biol. 344, 669-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfandari D., Cousin H., Marsden M. (2010). Mechanism of Xenopus cranial neural crest cell migration. Cell Adh. Migr. 4, 553-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybar M. J., Nieto M. A., Mayor R. (2003). Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 130, 483-494 [DOI] [PubMed] [Google Scholar]
- Bajpai R., Chen D. A., Rada-Iglesias A., Zhang J., Xiong Y., Helms J., Chang C. P., Zhao Y., Swigut T., Wysocka J. (2010). CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463, 958-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. V. (2008). The evolution and elaboration of vertebrate neural crest cells. Curr. Opin. Genet. Dev. 18, 536-543 [DOI] [PubMed] [Google Scholar]
- Baker C. V., Bronner-Fraser M. (1997). The origins of the neural crest. Part I: embryonic induction. Mech. Dev. 69, 3-11 [DOI] [PubMed] [Google Scholar]
- Barnes R. M., Firulli A. B. (2009). A twist of insight-the role of Twist-family bHLH factors in development. Int. J. Dev. Biol. 53, 909-924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrallo-Gimeno A., Nieto M. A. (2009). Evolutionary history of the Snail/Scratch superfamily. Trends Genet. 25, 248-252 [DOI] [PubMed] [Google Scholar]
- Barton C. E., Tahinci E., Barbieri C. E., Johnson K. N., Hanson A. J., Jernigan K. K., Chen T. W., Lee E., Pietenpol J. A. (2009). DeltaNp63 antagonizes p53 to regulate mesoderm induction in Xenopus laevis. Dev. Biol. 329, 130-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch M. L., Bronner-Fraser M. (2006). Neural crest inducing signals. Adv. Exp. Med. Biol. 589, 24-31 [DOI] [PubMed] [Google Scholar]
- Basch M. L., Bronner-Fraser M., Garcia-Castro M. I. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441, 218-222 [DOI] [PubMed] [Google Scholar]
- Bellmeyer A., Krase J., Lindgren J., LaBonne C. (2003). The protooncogene c-myc is an essential regulator of neural crest formation in Xenopus. Dev. Cell 4, 827-839 [DOI] [PubMed] [Google Scholar]
- Bonstein L., Elias S., Frank D. (1998). Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev. Biol. 193, 156-168 [DOI] [PubMed] [Google Scholar]
- Bouwmeester T., Kim S., Sasai Y., Lu B., De Robertis E. M. (1996). Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature 382, 595-601 [DOI] [PubMed] [Google Scholar]
- Bracken C. P., Gregory P. A., Kolesnikoff N., Bert A. G., Wang J., Shannon M. F., Goodall G. J. (2008). A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 68, 7846-7854 [DOI] [PubMed] [Google Scholar]
- Bradley L., Sun B., Collins-Racie L., LaVallie E., McCoy J., Sive H. (2000). Different activities of the frizzled-related proteins frzb2 and sizzled2 during Xenopus anteroposterior patterning. Dev. Biol. 227, 118-132 [DOI] [PubMed] [Google Scholar]
- Carl T. F., Dufton C., Hanken J., Klymkowsky M. W. (1999). Inhibition of neural crest migration in Xenopus using antisense slug RNA. Dev. Biol. 213, 101-115 [DOI] [PubMed] [Google Scholar]
- Carver E. A., Jiang R., Lan Y., Oram K. F., Gridley T. (2001). The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell. Biol. 21, 8184-8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas E., Kim J., Bendesky A., Ohno-Machado L., Wolfe C. J., Yang J. (2011). Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 71, 245-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. F., Behringer R. R. (1995). twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 9, 686-699 [DOI] [PubMed] [Google Scholar]
- Cheung M., Chaboissier M. C., Mynett A., Hirst E., Schedl A., Briscoe J. (2005). The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 8, 179-192 [DOI] [PubMed] [Google Scholar]
- Cordenonsi M., Dupont S., Maretto S., Insinga A., Imbriano C., Piccolo S. (2003). Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell 113, 301-314 [DOI] [PubMed] [Google Scholar]
- Cordenonsi M., Montagner M., Adorno M., Zacchigna L., Martello G., Mamidi A., Soligo S., Dupont S., Piccolo S. (2007). Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science 315, 840-843 [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. (1987). Fate map for the 32-cell stage of Xenopus laevis. Development 99, 527-551 [DOI] [PubMed] [Google Scholar]
- Essex L. J., Mayor R., Sargent M. G. (1993). Expression of Xenopus snail in mesoderm and prospective neural fold ectoderm. Dev. Dyn. 198, 108-122 [DOI] [PubMed] [Google Scholar]
- Fainsod A., Deissler K., Yelin R., Marom K., Epstein M., Pillemer G., Steinbeisser H., Blum M. (1997). The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech. Dev. 63, 39-50 [DOI] [PubMed] [Google Scholar]
- Fletcher R. B., Harland R. M. (2008). The role of FGF signaling in the establishment and maintenance of mesodermal gene expression in Xenopus. Dev. Dyn. 237, 1243-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R. B., Baker J. C., Harland R. M. (2006). FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development 133, 1703-1714 [DOI] [PubMed] [Google Scholar]
- Fukuda M., Takahashi S., Haramoto Y., Onuma Y., Kim Y. J., Yeo C. Y., Ishiura S., Asashima M. (2010). Zygotic VegT is required for Xenopus paraxial mesoderm formation and is regulated by Nodal signaling and Eomesodermin. Int. J. Dev. Biol. 54, 81-92 [DOI] [PubMed] [Google Scholar]
- Ganguly A., Jiang J., Ip Y. T. (2005). Drosophila WntD is a target and an inhibitor of the Dorsal/Twist/Snail network in the gastrulating embryo. Development 132, 3419-3429 [DOI] [PubMed] [Google Scholar]
- Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357-362 [DOI] [PubMed] [Google Scholar]
- Goh K. L., Yang J. T., Hynes R. O. (1997). Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development 124, 4309-4319 [DOI] [PubMed] [Google Scholar]
- Gordon M. D., Dionne M. S., Schneider D. S., Nusse R. (2005). WntD is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature 437, 746-749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. B., Cook T. L., Smith J. C., Grainger R. M. (1997). Anteroposterior neural tissue specification by activin-induced mesoderm. Proc. Natl. Acad. Sci. USA 94, 8596-8601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidato S., Itasaki N. (2007). Wise retained in the endoplasmic reticulum inhibits Wnt signaling by reducing cell surface LRP6. Dev. Biol. 310, 250-263 [DOI] [PubMed] [Google Scholar]
- Guidato S., Tsai L. H., Woodgett J., Miller C. C. (1996). Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J. Neurochem. 66, 1698-1706 [DOI] [PubMed] [Google Scholar]
- Hanken J., Gross J. B. (2005). Evolution of cranial development and the role of neural crest: insights from amphibians. J. Anat. 207, 437-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten U., Harland R. M., Gilchrist M. J., Hendrix D., Jurka J., Kapitonov V., Ovcharenko I., Putnam N. H., Shu S., Taher L., et al. (2010). The genome of the Western clawed frog Xenopus tropicalis. Science 328, 633-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C. S., Park B. Y., Saint-Jeannet J. P. (2008). Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development 135, 3903-3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B. (1991). Xenopus Myf-5 marks early muscle cells and can activate muscle genes ectopically in early embryos. Development 111, 551-560 [DOI] [PubMed] [Google Scholar]
- Hug B., Walter V., Grunwald D. J. (1997). tbx6, a Brachyury-related gene expressed by ventral mesendodermal precursors in the zebrafish embryo. Dev. Biol. 183, 61-73 [DOI] [PubMed] [Google Scholar]
- Huguet C., Crepieux P., Laudet V. (1997). Rel/NF-kappa B transcription factors and I kappa B inhibitors: evolution from a unique common ancestor. Oncogene 15, 2965-2974 [DOI] [PubMed] [Google Scholar]
- Iemura S., Yamamoto T. S., Takagi C., Uchiyama H., Natsume T., Shimasaki S., Sugino H., Ueno N. (1998). Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc. Natl. Acad. Sci. USA 95, 9337-9342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip Y. T., Park R. E., Kosman D., Yazdanbakhsh K., Levine M. (1992). dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 6, 1518-1530 [DOI] [PubMed] [Google Scholar]
- Itasaki N., Hoppler S. (2010). Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Dev. Dyn. 239, 16-33 [DOI] [PubMed] [Google Scholar]
- Itasaki N., Jones C. M., Mercurio S., Rowe A., Domingos P. M., Smith J. C., Krumlauf R. (2003). Wise, a context-dependent activator and inhibitor of Wnt signalling. Development 130, 4295-4305 [DOI] [PubMed] [Google Scholar]
- Jiang R., Lan Y., Norton C. R., Sundberg J. P., Gridley T. (1998). The Slug gene is not essential for mesoderm or neural crest development in mice. Dev. Biol. 198, 277-285 [PubMed] [Google Scholar]
- Kamiya N., Kobayashi T., Mochida Y., Yu P. B., Yamauchi M., Kronenberg H. M., Mishina Y. (2010). Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J. Bone Miner. Res. 25, 200-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. (2010). Network of WNT and other regulatory signaling cascades in pluripotent stem cells and cancer stem cells. Curr. Pharm. Biotechnol. 12, 160-170 [DOI] [PubMed] [Google Scholar]
- Khokha M. K., Chung C., Bustamante E. L., Gaw L. W., Trott K. A., Yeh J., Lim N., Lin J. C., Taverner N., Amaya E., et al. (2002). Techniques and probes for the study of Xenopus tropicalis development. Dev. Dyn. 225, 499-510 [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Hanken J. (1991). Whole-mount staining of Xenopus and other vertebrates. In Xenopus laevis: Practical Uses in Cell and Molecular Biology, Vol. 36 (ed. Kay B. K., Peng H. B.), pp. 419-441 San Diego, California: Academic Press; [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Savagner P. (2009). Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am. J. Pathol. 174, 1588-1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W., Cortez-Rossi C., Artinger K. B. (2010). Mechanisms driving neural crest induction and migration in the zebrafish and Xenopus laevis. Cell Adh. Migr. 4, 595-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide T., Hayata T., Cho K. W. (2005). Xenopus as a model system to study transcriptional regulatory networks. Proc. Natl. Acad. Sci. USA 102, 4943-4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M. (2002). Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Semin. Cell Dev. Biol. 13, 243-249 [DOI] [PubMed] [Google Scholar]
- LaBonne C., Bronner-Fraser M. (2000). Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev. Biol. 221, 195-205 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Aoki Y., Hong C. S., Saint-Germain N., Credidio C., Saint-Jeannet J. P. (2004). Early requirement of the transcriptional activator Sox9 for neural crest specification in Xenopus. Dev. Biol. 275, 93-103 [DOI] [PubMed] [Google Scholar]
- Leptin M. (1991). twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5, 1568-1576 [DOI] [PubMed] [Google Scholar]
- Leyns L., Bouwmeester T., Kim S. H., Piccolo S., De Robertis E. M. (1997). Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88, 747-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Y., Bourdelas A., Carron C., Gomez C., Boucaut J. C., Shi D. L. (2006). FGF8, Wnt8 and Myf5 are target genes of Tbx6 during anteroposterior specification in Xenopus embryo. Dev. Biol. 290, 470-481 [DOI] [PubMed] [Google Scholar]
- Li L., Cserjesi P., Olson E. N. (1995). Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev. Biol. 172, 280-292 [DOI] [PubMed] [Google Scholar]
- Lintern K. B., Guidato S., Rowe A., Saldanha J. W., Itasaki N. (2009). Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J. Biol. Chem. 284, 23159-23168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ye F., Li Q., Tamiya S., Darling D. S., Kaplan H. J., Dean D. C. (2009). Zeb1 represses Mitf and regulates pigment synthesis, cell proliferation, and epithelial morphology. Invest. Ophthalmol. Vis. Sci. 50, 5080-5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A., Manzanares M., Blanco M. J., Nieto M. A. (2002). Modularity and reshuffling of Snail and Slug expression during vertebrate evolution. Proc. Natl. Acad. Sci. USA 99, 16841-16846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose M., Patient R. K. (2004). A genetic regulatory network for Xenopus mesendodermal formation. Dev. Biol. 271, 467-478 [DOI] [PubMed] [Google Scholar]
- Lou X., Fang P., Li S., Hu R. Y., Kuerner K. M., Steinbeisser H., Ding X. (2006). Xenopus Tbx6 mediates posterior patterning via activation of Wnt and FGF signalling. Cell Res. 16, 771-779 [DOI] [PubMed] [Google Scholar]
- Manzanares M., Locascio A., Nieto M. A. (2001). The increasing complexity of the Snail gene superfamily in metazoan evolution. Trends Genet. 17, 178-181 [DOI] [PubMed] [Google Scholar]
- Marchant L., Linker C., Ruiz P., Guerrero N., Mayor R. (1998). The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev. Biol. 198, 319-329 [PubMed] [Google Scholar]
- Mayor R., Essex L. J., Bennett M. F., Sargent M. G. (1993). Distinct elements of the xsna promoter are required for mesodermal and ectodermal expression. Development 119, 661-671 [DOI] [PubMed] [Google Scholar]
- Mayor R., Morgan R., Sargent M. G. (1995). Induction of the prospective neural crest of Xenopus. Development 121, 767-777 [DOI] [PubMed] [Google Scholar]
- Mayor R., Guerrero N., Young R. M., Gomez-Skarmeta J. L., Cuellar C. (2000). A novel function for the Xslug gene: control of dorsal mesendoderm development by repressing BMP-4. Mech. Dev. 97, 47-56 [DOI] [PubMed] [Google Scholar]
- McCredie J. (2009). History, heresy and radiology in scientific discovery. J. Med. Imaging Radiat. Oncol. 53, 433-441 [DOI] [PubMed] [Google Scholar]
- Minoux M., Rijli F. M. (2010). Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137, 2605-2621 [DOI] [PubMed] [Google Scholar]
- Miraoui H., Marie P. J. (2010). Pivotal role of Twist in skeletal biology and pathology. Gene 468, 1-7 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Fletcher R. B., Harland R. M. (2003). Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development 130, 3111-3124 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Wang E., Harland R. (2005). Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell 8, 167-178 [DOI] [PubMed] [Google Scholar]
- Moody S. A. (1987). Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev. Biol. 122, 300-319 [DOI] [PubMed] [Google Scholar]
- Murray S. A., Gridley T. (2006). Snail family genes are required for left-right asymmetry determination, but not neural crest formation, in mice. Proc. Natl. Acad. Sci. USA 103, 10300-10304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura O., Takasaki H., Nagata A. (1978). Further studies of the prospective fates of blastomeres at the 32-cell stage of Xenopus laevis embryos. Med. Biol. 56, 355-360 [PubMed] [Google Scholar]
- Nakamura T., Matsumoto K. (2008). The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J. Cell. Mol. Med. 12, 391-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A. (2002). The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3, 155-166 [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. (1994). Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835-839 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1967). Normal Table of Xenopus laevis (Daudin). Amsterdam: North-Holland; [Google Scholar]
- O'Rourke M. P., Tam P. P. (2002). Twist functions in mouse development. Int. J. Dev. Biol. 46, 401-413 [PubMed] [Google Scholar]
- Ohazama A., Porntaveetus T., Ota M. S., Herz J., Sharpe P. T. (2010). Lrp4: A novel modulator of extracellular signaling in craniofacial organogenesis. Am. J. Med. Genet. A 152A, 2974-2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mancera P. A., Bermejo-Rodriguez C., Gonzalez-Herrero I., Herranz M., Flores T., Jimenez R., Sanchez-Garcia I. (2007). Adipose tissue mass is modulated by SLUG (SNAI2). Hum. Mol. Genet. 16, 2972-2986 [DOI] [PubMed] [Google Scholar]
- Ragland J. W., Raible D. W. (2004). Signals derived from the underlying mesoderm are dispensable for zebrafish neural crest induction. Dev. Biol. 276, 16-30 [DOI] [PubMed] [Google Scholar]
- Row R. H., Kimelman D. (2009). Bmp inhibition is necessary for post-gastrulation patterning and morphogenesis of the zebrafish tailbud. Dev. Biol. 329, 55-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K., Garrett N., Mitchell A., Gurdon J. B. (1996). Eomesodermin, a key early gene in Xenopus mesoderm differentiation. Cell 87, 989-1000 [DOI] [PubMed] [Google Scholar]
- Salic A. N., Kroll K. L., Evans L. M., Kirschner M. W. (1997). Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development 124, 4739-4748 [DOI] [PubMed] [Google Scholar]
- Sandmann T., Girardot C., Brehme M., Tongprasit W., Stolc V., Furlong E. E. (2007). A core transcriptional network for early mesoderm development in Drosophila melanogaster. Genes Dev. 21, 436-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Bennett M. F. (1990). Identification in Xenopus of a structural homologue of the Drosophila gene snail. Development 109, 967-973 [DOI] [PubMed] [Google Scholar]
- Sasai N., Yakura R., Kamiya D., Nakazawa Y., Sasai Y. (2008). Ectodermal factor restricts mesoderm differentiation by inhibiting p53. Cell 133, 878-890 [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. (1994). Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, 779-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336 [DOI] [PubMed] [Google Scholar]
- Schmalhofer O., Brabletz S., Brabletz T. (2009). E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 28, 151-166 [DOI] [PubMed] [Google Scholar]
- Sefton M., Sanchez S., Nieto M. A. (1998). Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 125, 3111-3121 [DOI] [PubMed] [Google Scholar]
- Semenov M. V., Tamai K., Brott B. K., Kuhl M., Sokol S., He X. (2001). Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11, 951-961 [DOI] [PubMed] [Google Scholar]
- Shi W., Levine M., Davidson B. (2005). Unraveling genomic regulatory networks in the simple chordate, Ciona intestinalis. Genome Res. 15, 1668-1674 [DOI] [PubMed] [Google Scholar]
- Shibata M., Ono H., Hikasa H., Shinga J., Taira M. (2000). Xenopus crescent encoding a Frizzled-like domain is expressed in the Spemann organizer and pronephros. Mech. Dev. 96, 243-246 [DOI] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M., Harland R. M. (2000). Early Development of Xenopus laevis: a Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. (1991). Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67, 79-87 [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. (1992). Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70, 829-840 [DOI] [PubMed] [Google Scholar]
- Soo K., O'Rourke M. P., Khoo P. L., Steiner K. A., Wong N., Behringer R. R., Tam P. P. (2002). Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev. Biol. 247, 251-270 [DOI] [PubMed] [Google Scholar]
- Spokony R. F., Aoki Y., Saint-Germain N., Magner-Fink E., Saint-Jeannet J. P. (2002). The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development 129, 421-432 [DOI] [PubMed] [Google Scholar]
- Spring J., Yanze N., Middel A. M., Stierwald M., Groger H., Schmid V. (2000). The mesoderm specification factor twist in the life cycle of jellyfish. Dev. Biol. 228, 363-375 [DOI] [PubMed] [Google Scholar]
- Spring J., Yanze N., Josch C., Middel A. M., Winninger B., Schmid V. (2002). Conservation of Brachyury, Mef2, and Snail in the myogenic lineage of jellyfish: a connection to the mesoderm of bilateria. Dev. Biol. 244, 372-384 [DOI] [PubMed] [Google Scholar]
- Stathopoulos A., Levine M. (2002). Dorsal gradient networks in the Drosophila embryo. Dev. Biol. 246, 57-67 [DOI] [PubMed] [Google Scholar]
- Stennard F., Carnac G., Gurdon J. B. (1996). The Xenopus T-box gene, Antipodean, encodes a vegetally localised maternal mRNA and can trigger mesoderm formation. Development 122, 4179-4188 [DOI] [PubMed] [Google Scholar]
- Stennard F., Zorn A. M., Ryan K., Garrett N., Gurdon J. B. (1999). Differential expression of VegT and Antipodean protein isoforms in Xenopus. Mech. Dev. 86, 87-98 [DOI] [PubMed] [Google Scholar]
- Steventon B., Carmona-Fontaine C., Mayor R. (2005). Genetic network during neural crest induction: from cell specification to cell survival. Semin. Cell Dev. Biol. 16, 647-654 [DOI] [PubMed] [Google Scholar]
- Steventon B., Araya C., Linker C., Kuriyama S., Mayor R. (2009). Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development 136, 771-779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi-Suzuki K., Funami J., Tokumori D., Saito A., Watabe T., Miyazono K., Kanda A., Suzuki A. (2003). Interplay between the tumor suppressor p53 and TGF beta signaling shapes embryonic body axes in Xenopus. Development 130, 3929-3939 [DOI] [PubMed] [Google Scholar]
- Tang N., Song W. X., Luo J., Luo X., Chen J., Sharff K. A., Bi Y., He B. C., Huang J. Y., Zhu G. H., et al. (2009). BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J. Cell. Mol. Med. 13, 2448-2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro K., Yamada R., Asano M., Hashimoto M., Muramatsu M., Shiokawa K. (1991). Expression of mRNA for activin-binding protein (follistatin) during early embryonic development of Xenopus laevis. Biochem. Biophys. Res. Commun. 174, 1022-1027 [DOI] [PubMed] [Google Scholar]
- Thisse B., el Messal M., Perrin-Schmitt F. (1987). The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 15, 3439-3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulo C., Aybar M. J., Sanchez S. S., Mayor R. (2004). A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of the neural crest. Dev. Biol. 275, 325-342 [DOI] [PubMed] [Google Scholar]
- Valanne S., Wang J. H., Ramet M. (2011). The Drosophila Toll signaling pathway. J. Immunol. 186, 649-656 [DOI] [PubMed] [Google Scholar]
- Vallin J., Thuret R., Giacomello E., Faraldo M. M., Thiery J. P., Broders F. (2001). Cloning and characterization of three Xenopus Slug promoters reveal direct regulation by Lef/beta-catenin signaling. J. Biol. Chem. 287, 30350-30358 [DOI] [PubMed] [Google Scholar]
- Wang S., Krinks M., Lin K., Luyten F. P., Moos M., Jr (1997a). Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88, 757-766 [DOI] [PubMed] [Google Scholar]
- Wang S., Krinks M., Moos M. J. (1997b). Frzb-1, an antagonist of Wnt-1 and Wnt-8, does not block signaling by Wnts -3A, -5A, or -11. Biochem. Biophys. Res. Commun. 236, 502-504 [DOI] [PubMed] [Google Scholar]
- Webster W. S., Ritchie H. E. (1991). Teratogenic effects of alcohol and isotretinoin on craniofacial development: an analysis of animal models. J. Craniofac. Genet. Dev. Biol. 11, 296-302 [PubMed] [Google Scholar]
- Wellner U., Schubert J., Burk U. C., Schmalhofer O., Zhu F., Sonntag A., Waldvogel B., Vannier C., Darling D., zur Hausen A., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487-1495 [DOI] [PubMed] [Google Scholar]
- Wetts R., Fraser S. E. (1989). Slow intermixing of cells during Xenopus embryogenesis contributes to the consistency of the blastomere fate map. Development 105, 9-15 [DOI] [PubMed] [Google Scholar]
- Xu W., Edmondson D. G., Evrard Y. A., Wakamiya M., Behringer R. R., Roth S. Y. (2000). Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nat. Genet. 26, 229-232 [DOI] [PubMed] [Google Scholar]
- Yu J. K. (2010). The evolutionary origin of the vertebrate neural crest and its developmental gene regulatory network-insights from amphioxus. Zoology (Jena) 113, 1-9 [DOI] [PubMed] [Google Scholar]
- Zeitlinger J., Zinzen R. P., Stark A., Kellis M., Zhang H., Young R. A., Levine M. (2007). Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev. 21, 385-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Klymkowsky M. W. (2009). Unexpected functional redundancy between Twist and Slug (Snail2) and their feedback regulation of NF-kappaB via Nodal and Cerberus. Dev. Biol. 331, 340-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Basta T., Jensen E. D., Klymkowsky M. W. (2003). The beta-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development 130, 5609-5624 [DOI] [PubMed] [Google Scholar]
- Zhang C., Basta T., Hernandez-Lagunas L., Simpson P., Stemple D. L., Artinger K. B., Klymkowsky M. W. (2004). Repression of nodal expression by maternal B1-type SOXs regulates germ layer formation in Xenopus and zebrafish. Dev. Biol. 273, 23-37 [DOI] [PubMed] [Google Scholar]
- Zhang C., Carl T. F., Trudeau E. D., Simmet T., Klymkowsky M. W. (2006). An NF-kappaB and slug regulatory loop active in early vertebrate mesoderm. PLoS ONE 1, e106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.