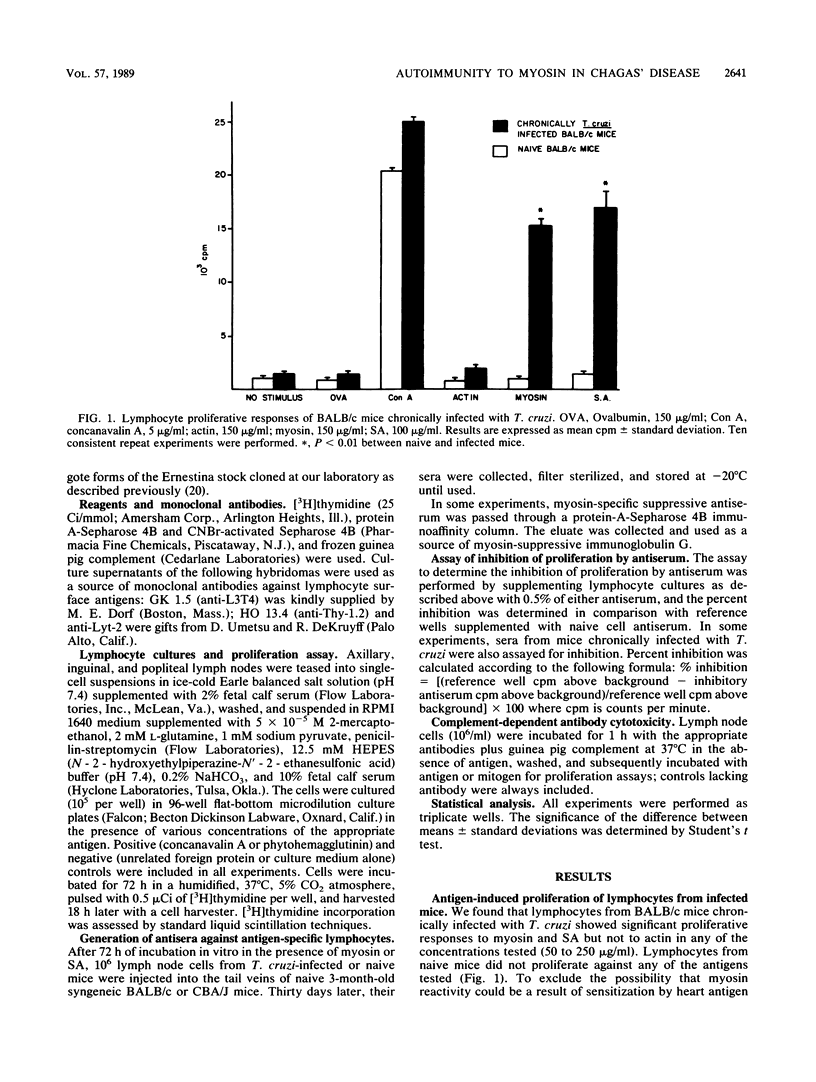
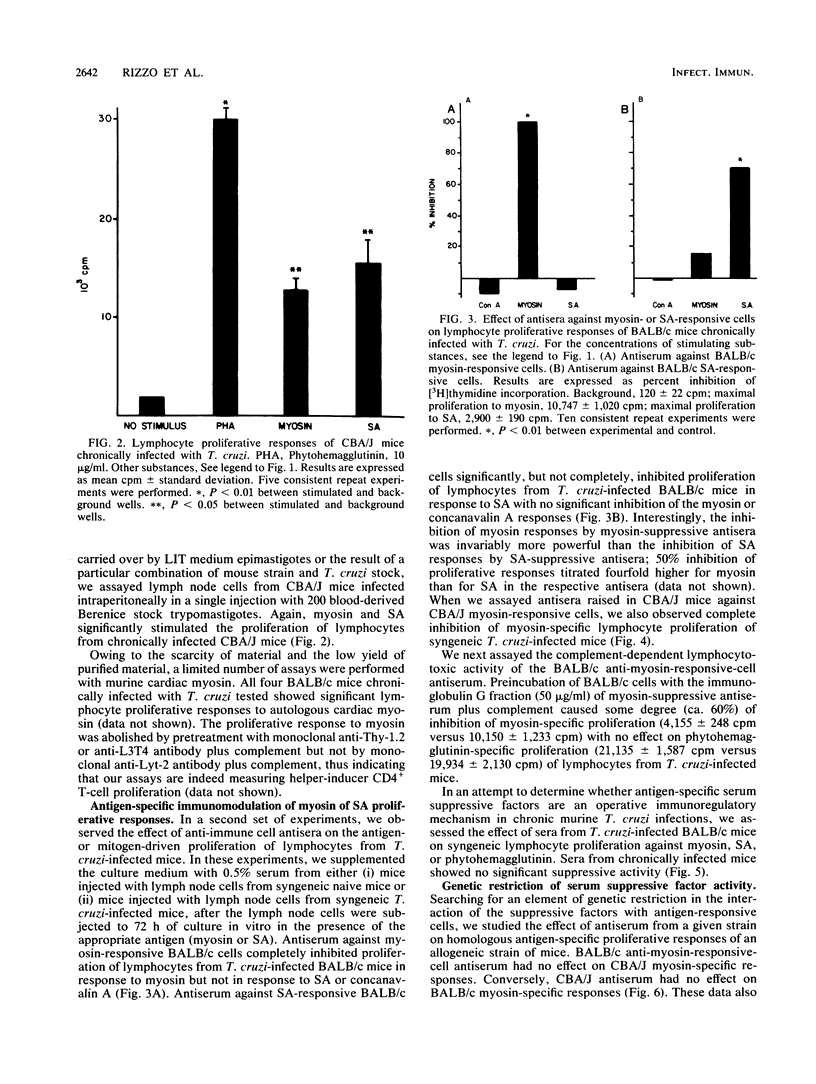
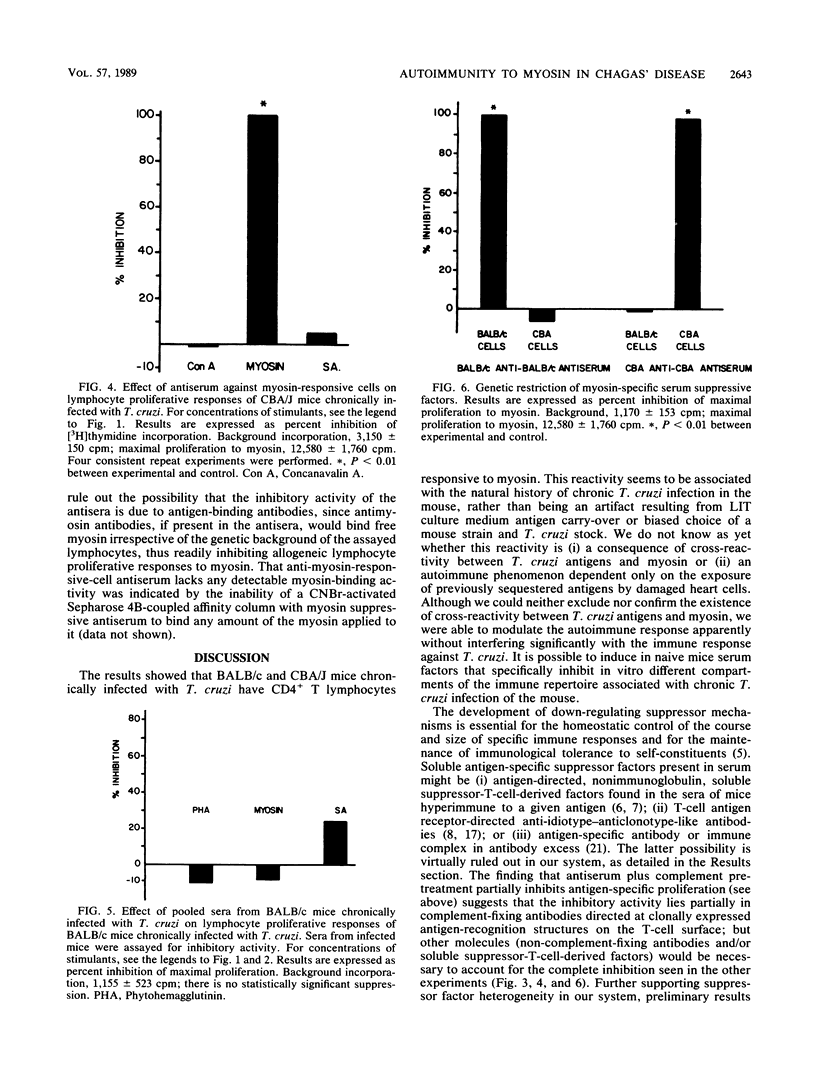
Abstract
In this study, we assessed the proliferative response of T cells from mice chronically infected with Trypanosoma cruzi to actin, myosin, or T. cruzi soluble antigen (SA). We report here that CD4+ T cells from mice chronically infected with T. cruzi proliferated in response to myosin but not to actin, whereas cells from naive mice did not proliferate against any of the antigens tested. Antisera raised against myosin- or SA-activated T cells specifically inhibited respectively, the myosin or SA in vitro proliferative response, whereas the response to unrelated antigen remained unimpaired. Sera from chronically infected mice failed to show any significant inhibitory activity. The above findings suggest that autoreactive and T. cruzi-reactive T cells belong to different, perhaps nonoverlapping, compartments of the immune cell repertoire of mice chronically infected with T. cruzi. The failure of infected mice to trigger the suppressive mechanisms described here might be the primary immune defect leading to breakdown of self-tolerance and unopposed, perhaps tissue-damaging, autoimmunity in experimental Chagas' disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta A. M., Santos-Buch C. A. Autoimmune myocarditis induced by Trypanosoma cruzi. Circulation. 1985 Jun;71(6):1255–1261. doi: 10.1161/01.cir.71.6.1255. [DOI] [PubMed] [Google Scholar]
- Contreras V. T., Salles J. M., Thomas N., Morel C. M., Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985 Sep;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Cunningham M. W., Hall N. K., Krisher K. K., Spanier A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986 Jan;136(1):293–298. [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Ferguson T. A., Beaman K. D., Iverson G. M. Isolation and characterization of a T suppressor factor by using a monoclonal antibody. J Immunol. 1985 May;134(5):3163–3171. [PubMed] [Google Scholar]
- Ferguson T. A., Iverson G. M. Isolation and characterization of an antigen-specific suppressor inducer molecule from serum of hyperimmune mice by using a monoclonal antibody. J Immunol. 1986 Apr 15;136(8):2896–2903. [PubMed] [Google Scholar]
- Kaufmann S. H., Eichmann K., Müller I., Wrazel L. J. Vaccination against the intracellular bacterium Listeria monocytogenes with a clonotypic antiserum. J Immunol. 1985 Jun;134(6):4123–4127. [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Coutinho A. Lymphocyte subpopulations and clonal repertoires participate in immune response to acute T. cruzi infection. Mem Inst Oswaldo Cruz. 1988 Nov;83 (Suppl 1):356–359. doi: 10.1590/s0074-02761988000500022. [DOI] [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Rossi M. A., Gonçalves S., Ribeiro-dos-Santos R. Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice. The potential role of intravascular platelet aggregation in its genesis. Am J Pathol. 1984 Feb;114(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Santos-Buch C. A., Teixeira A. R. The immunology of experimental Chagas' disease. 3. Rejection of allogeneic heart cells in vitro. J Exp Med. 1974 Jul 1;140(1):38–53. doi: 10.1084/jem.140.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Qian J. H., Kokudo S., Ikegami R., Suda T., Hamaoka T., Fujiwara H. Studies on the induction of tolerance to alloantigens. III. Induction of antibodies directed against alloantigen-specific delayed-type hypersensitivity T cells by a single injection of allogeneic lymphocytes via portal venous route. J Immunol. 1988 Feb 1;140(3):717–722. [PubMed] [Google Scholar]
- Teixeira A. R., Cunha Neto E., Rizzo L. V. Auto-imunidade não é impeditivo para vacinaço contra doença de Chagas. Rev Soc Bras Med Trop. 1987 Apr-Jun;20(2):123–127. doi: 10.1590/s0037-86821987000200011. [DOI] [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira G., Macêdo V., Prata A. Trypanosoma cruzi-sensitized T-lymphocyte mediated 51CR release from human heart cells in Chagas' disease. Am J Trop Med Hyg. 1978 Nov;27(6):1097–1107. doi: 10.4269/ajtmh.1978.27.1097. [DOI] [PubMed] [Google Scholar]


