Abstract
Two related ER oxidation 1 (ERO1) proteins, ERO1α and ERO1β, dynamically regulate the redox environment in the mammalian endoplasmic reticulum (ER). Redox changes in cysteine residues on intralumenal loops of calcium release and reuptake channels have been implicated in altered calcium release and reuptake. These findings led us to hypothesize that altered ERO1 activity may affect cardiac functions that are dependent on intracellular calcium flux. We established mouse lines with loss of function insertion mutations in Ero1l and Ero1lb encoding ERO1α and ERO1β. The peak amplitude of calcium transients in homozygous Ero1α mutant adult cardiomyocytes was reduced to 42.0 ± 2.2% (n=10, P≤0.01) of values recorded in wild-type cardiomyocytes. Decreased ERO1 activity blunted cardiomyocyte inotropic response to adrenergic stimulation and sensitized mice to adrenergic blockade. Whereas all 12 wild-type mice survived challenge with 4 mg/kg esmolol, 6 of 8 compound Ero1l and Ero1lb mutant mice succumbed to this level of β adrenergic blockade (P≤0.01). In addition, mice lacking ERO1α were partially protected against progressive heart failure in a transaortic constriction model [at 10 wk postprocedure, fractional shortening was 0.31±0.02 in the mutant (n=20) vs. 0.23±0.03 in the wild type (n=18); P≤0.01]. These findings establish a role for ERO1 in calcium homeostasis and suggest that modifying the lumenal redox environment may affect the progression of heart failure.—Chin, K. T., Kang, G., Qu, J., Gardner, L. B., Coetzee, W. A., Zito, E., Fishman, G. I., Ron, R. The sarcoplasmic reticulum luminal thiol oxidase ERO1 regulates cardiomyocyte excitation-coupled calcium release and response to hemodynamic load.
Keywords: disulfide bonds, endoplasmic reticulum, heart failure
Protein thiol oxidation in the endoplasmic reticulum stabilizes the tertiary structure of the lumenal domains of secreted and membrane-bound proteins. Thiol oxidases and oxidoreductases generate disulfide bonds in the lumen of the ER and transfer these to unfolded nascent proteins. The ER also maintains a parallel reductive process by which misplaced disulfides are shuffled or reduced (1). Deviation from physiological redox in the ER lumen interferes with proper maturation of secreted proteins, imposes unfolded protein stress in the organelle, and activates stress signal transduction pathways. The latter are known as the unfolded protein response that coordinates a rectifying response enhancing the expression of genes generally involved in ER protein biogenesis and in oxidative protein folding (reviewed in refs. 2, 3).
ER oxidation 1 (ERO1) encodes an enzyme that generates disulfides in the lumen of the ER by transferring electrons from reduced cysteines to molecular oxygen. Simple eukaryotes, such as yeast and worms, require ERO1 for oxidative folding of secreted proteins (4, 5). Mammals have 2 ERO1 genes: a broadly expressed α isoform, encoded by Ero1l (or Ero1α). and a β isoform, encoded by Ero1lb (or Ero1β), whose expression is greatly enriched in the insulin-producing β cells of the endocrine pancreas (6). Homozygous Ero1β mutant mice have a modest defect in oxidative folding of insulin (the major secretory product of β cells) and develop mild glucose intolerance (7). Homozygous Ero1α mutant mice are superficially indistinguishable from wild-type mice (see below) and even compound homozygous Ero1α; Ero1β mutant mice are viable, and their secretory cells are capable of sustained oxidative protein folding (7, 8). Similar observations in flies (9) point to the existence of redundant mechanisms for disulfide bond formation in the endoplasmic reticulum of higher eukaryotes. Together, these suggest the possibility that in more complex eukaryotes, ERO1 enzymes may regulate aspects of ER redox that are relevant to processes beyond folding of secreted proteins.
Calcium metabolism has a pervasive role in cardiac function and dysfunction (reviewed in refs. 10, 11), and redox changes on the cytoplasmic side of the sarcoplasmic reticulum membrane are known to affect calcium channels and pumps (reviewed in ref. 12). However, several studies have also suggested links between ER lumenal thiol redox and calcium metabolism by the organelle: Mixed disulfides between the ER-localized oxidoreductase ERp44 and cysteines on the third luminal loop of the inositol 1,4,5-trisphosphate receptor isoform 1 (IP3R1) have been implicated in inhibiting the activity of this ligand-regulated calcium release channel (13). Cysteine-dependent interactions of the oxidoreductase ERp57 with the fourth lumenal loop of the sarcoplasmic reticulum calcium ATPase isoform 2b (SERCA 2b) inhibits calcium reuptake into the ER by this ATP-driven pump (14). These regulatory events are directly affected by the redox state of thiols in the calcium-handling proteins and their oxidoreductase partners and are thus predicted to be sensitive to the redox state of the ER lumen.
The ER stress-induced transcription factor CHOP, which activates ERO1α (15), is induced in the overloaded and failing heart muscle, and recent evidence suggests that ablation of the CHOP-encoding gene, Ddit3, may be protective in heart failure induced by pressure overload (16). These observations prompted us to examine the role of ERO1 on calcium handling in the heart. Here, we report on the finding that attenuated ERO1α activity diminishes the amplitude of calcium transients in cardiomyocytes, sensitizing hearts to adrenergic blockade in vivo and blunting the progression to heart failure in response to hemodynamic overload.
MATERIALS AND METHODS
Animal breeding, genotyping, surgery, and hemodynamic and cardiac measurements
All experiments performed on mice were approved by the New York University Institutional Animal Care and Use Committee, and were carried out in accordance with Public Health Service Guidelines for the Care and Use of Laboratory Animals. Mice breeding and genotyping were performed as described previously (7).
Transaortic constriction (TAC) was performed on wild-type and Ero1α mutant male mice (3 to 4 mo of age), as described previously (17, 18).
Left ventricular dimensions and function were assessed noninvasively by echocardiography using an ATL 5000CV Ultrasound System (Philips Medical, Bothell, WA, USA), and invasively using a Mikro-Tip pressure transducer catheter (Millar Instruments, Houston, TX, USA), as described previously (17, 19), with or without previous intravenous injection of esmolol hydrochloride (Sigma, St. Louis, MO, USA).
Cell culture, hypoxia treatment, and redox analysis
Wild-type, homozygous Ero1α, and compound homozygous Ero1α; Ero1β mutant mouse embryonic fibroblasts (MEFs), isolated at embryonic day 13.5, were immortalized with SV-40 large T antigen and cultured in DMEM supplemented with 25 mM glucose, 10% FCS and nonessential amino acids, as described previously (7).
Prior to experiments, MEFs were plated at 80% confluence and grown at 37°C and 5% CO2. To generate hypoxic conditions, cells were placed in a Plas-Labs environmental chamber (Plas-Labs, Inc., Lansing, MI, USA) for the indicated period of time. The oxygen concentration in the chamber was maintained at <0.1% for all experiments, as assessed by an Alpha Omega oxygen analyzer (Alpha Omega Instruments, Cumberland, RI, USA).
Where indicated, cells were exposed to a brief pulse of dithiothreitol followed by a washout period during which they were lysed in N-ethylmaleimide-containing buffer; the proteins were resolved by gel electrophoresis in reducing or nonreducing conditions and blotted for protein disulfide isomerase, GRP94, BiP (SPA-827; Stressgen, Victoria, BC, Canada), ERO1α (7), and β-actin (clone AC-74; Sigma), as described previously (8).
QM295 (5(4H)-isoxazolone, 4-[(4-hydroxy-3-methoxyphenyl)methylene]-3-phenyl; 5904135; Chembridge, San Diego, CA, USA) was dissolved in DMSO at 10 mM and applied to cells at a final concentration of 50 μM, as described previously (20).
Measurements of the redox state in MEFs transduced with the ER-localized FLAG-roGFP_iE (21) was performed as described previously (8).
Adult cardiomyocyte isolation and calcium transient measurements
Adult cardiomyocytes were isolated using an established enzymatic digestion protocol yielding 60–80% rod-shaped, Ca2+-tolerant myocytes. Intracellular calcium transients were recorded as described previously (22). In brief, cells were exposed to a dye-loading solution consisting of a Tyrode's solution: 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 5.6 mM glucose; pH was adjusted to 7.4 with NaOH at 22°C. Where indicated, the cells had been pretreated for 180 min with QM295 (25 μM), and the inhibitor was included in the Tyrode's solution. The dye-loading solution using Tyrode's supplemented with 0.5 μM Fura-2 acetoxymethyl ester (Fura-2/AM; Invitrogen, Eugene, OR, USA) with or without QM295 (25 μM). Cells were exposed to Fura-2/AM for 6 min at 22°C. The loading solution was removed, and cells were washed and equilibrated in fresh Tyrode's solution for 30 min at 22°C to allow deesterification of the dye before recording. Cells were field-stimulated at 0.5 Hz to achieve steady state, and fluorescent signals were acquired using a ×40 UVF objective (numerical aperture 1.0; Nikon, Tokyo, Japan), and dual excitation wavelength microfluorimetry PMT system (IonOptix Corp., Milton, MA, USA).
Immunoblotting and mRNA analysis of gene expression in tissue
Cells were harvested in PBS with 1 mM EDTA containing 20 mM N-ethylmaleimide (NEM), and were lysed in buffer containing 20 mM HEPES (pH 7.9), 50 mM NaCl, 0.5 M sucrose, 0.1 mM EDTA, 0.5% Triton X-100, and 20 mM NEM plus protease inhibitors in the presence and absence of dithiothreitol, and blotted with rabbit anti-ERO1α (7), mouse monoclonal anti-KDEL (SPA-827; Stressgen, Victoria, BC, Canada), and mouse monoclonal anti-β-actin antibodies (clone AC-74; Sigma).
Total RNA was isolated using RNA STAT-60 reagent (AMS Biotechnology, Abingdon, UK) and then reverse-transcribed using random 12-mer primers. Quantitative PCR was then assessed using SYBR Green 1 by iCycler iQ (Bio-Rad, Hercules, CA, USA) using gene-specific primers purchased from Fisher Scientific (Hampton, NH, USA).
Statistical analysis
All kinetic parameters and results are expressed as means ± se, and compared using unpaired 2-tailed Student's t test. Tests for differences were performed using repeated measures ANOVA with a suitable ANOVA post hoc test or Student's t test where appropriate. A value of P < 0.05 was considered significant.
RESULTS
ERO1α contributes to ER redox state in hypoxic cells
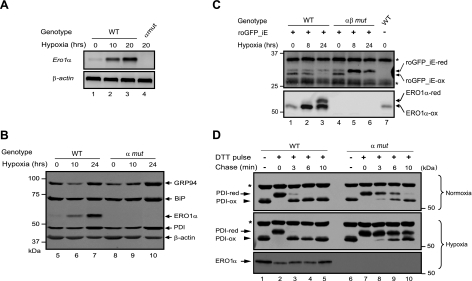
Random mutagenesis of the mouse genome in embryonic stem cells yielded a clone, XST171, with an insertion of a promoter trap into exon 6 of the Ero1α gene (Supplemental Fig. S1). Germline transmission of the mutant allele from chimeric mice yielded lines of mutant mice in whose cells ERO1α expression was reduced >95%, to undetectable levels in most circumstances (refs. 7, 8 and Fig. 1B). Surprisingly, under basal conditions, this mutation had little or no measurable effect on ER redox or on the rate of oxidative folding of insulin or immunoglobulin (7, 8). Therefore, we wondered whether the mutation might gain importance under conditions in which ERO1α expression is induced by acute stress. Ero1α expression increased substantially in MEFs grown under hypoxic conditions (Fig. 1A, B), as reported previously (23). Furthermore, the induced, endogenous protein colocalized with an ER marker (Supplemental Fig. S2), consistent with ERO1α's role as an ER oxidase.
Figure 1.
Hypoxia-induced expression of ERO1α and a reduced ER redox state in hypoxic cells lacking ERO1. A) RT-PCR analysis of mRNA prepared from wild-type (WT) and Ero1α mutant (α mut) MEFs following exposure to hypoxia for the indicated period of time. β-Actin served as a loading control (bottom panel). B) Immunoblots of proteins extracted from WT and α mut cells following exposure to hypoxia for the indicated period of time. GRP94, BiP, and PDI are markers of ER proteins positively regulated by the unfolded protein response; β-actin is a cytosolic loading control. C) Immunoblot of the ER-localized redox marker protein roGFP1_iE immunoprecipitated from extracts of WT and Ero1α; Ero1β compound-mutant (αβ mut) cells and resolved by reducing or nonreducing SDS-PAGE. Positions of the reduced (-red) and oxidized (-ox) forms of roGFP_iE and background bands (asterisks) are indicated. Note the similar distribution of roGFP1_iE between the reduced and oxidized form under basal conditions in both cell types and the accumulation of reduced form in the mutant cells following exposure to hypoxic conditions. Note also the accumulation of reduced ERO1α protein in the hypoxic WT cells, an indication that ERO1α activity is substrate limited under these conditions. D) Immunoblot of endogenous PDI isoforms (reactive with anti-PDI antibody SPA-891; Stressgen), extracted from normoxic or hypoxic WT and α mut MEFs following a reductive pulse with dithiothreitol and washout. Positions of the reduced and oxidized forms of the protein are indicated; asterisk indicates position of a redox-insensitive protein that also reacts with this antibody. Note the marked delay in reoxidation of PDI in the hypoxic mutant cells.
To gauge the effect of ERO1α deficiency on ER redox, we exploited a recently described marker consisting of a modified green fluorescent protein engineered to contain a metastable disulfide bond (21). Presently, optical readout of redox changes in ER-localized roGFP_iE is unreliable, but the presence or absence of the disulfide bond can be assessed by the migration of the alkylated protein on nonreducing SDS-PAGE. In MEFs cultured at atmospheric concentration of oxygen, lack of ERO1α did not measurably affect the steady-state distribution of roGFP_iE between its reduced and oxidized forms (21). However, whereas wild-type MEFs were able to defend the physiological equilibrium between the reduced and oxidized roGFP_iE following exposure to hypoxic conditions, the marker accumulated in its reduced form following similar exposure of the mutant cells (Fig. 1C). Not all disulfide bonds were disrupted in the hypoxic mutant cells; the disulfide detected by an anti-PDI antibody was unaffected by hypoxia in cells of either genotype (compare lanes 1 and 6 in Fig. 1D). Nonetheless in the absence of ERO1α, the reoxidation of the sentinel disulfide in this PDI family member was significantly delayed during the washout period following a pulse of the reducing agent dithiothreitol; a delay that was far more conspicuous in the hypoxic mutant cells (compare upper and middle panels in Fig. 1D). Together, these observations reveal an abnormal ER redox state in stressed cells lacking ERO1α, shifting the redox poise toward the reduced state of lumenal disulfides.
ERO1α promotes calcium homeostasis in adult mouse cardiomyocytes
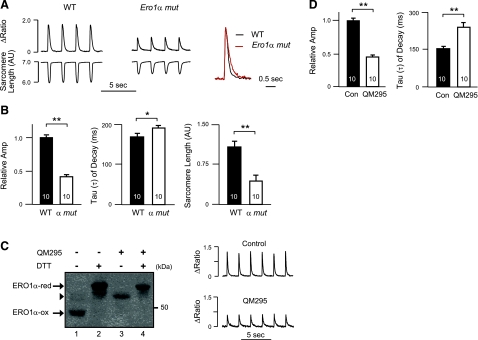
In as much as links have emerged between ER luminal redox and calcium metabolism by the organelle, we examined intracellular calcium homeostasis in ERO1-deficient cardiomyocytes, a cell type with highly regulated intracellular calcium dynamics. Dynamic changes in intracellular calcium concentration are integral to regulation of acto-myosin interactions and cardiac contractility; defects in calcium handling modulate mechanical performance and may also promote electrical instability and cardiac arrhythmias (24). The peak amplitude of depolarization-induced changes in cytosolic calcium concentrations in paced freshly isolated adult cardiomyocytes (i.e., calcium transients) was twofold lower in homozygous Ero1α mutant cells compared with wild-type cells (Fig. 2A, B). In addition, relaxation of the calcium transient (i.e., τ of decay) was prolonged in mutant cardiomyocytes (Fig. 2A, B). Concordant with these abnormalities in the calcium transient, depolarization-induced changes in sarcomere length were attenuated in the mutant cells (Fig. 2B).
Figure 2.
Altered calcium metabolism in freshly isolated cardiomyocytes lacking ERO1α. A) Left panels: representative calcium transients recorded from electrically paced freshly isolated wild-type (WT) or Ero1α mutant (Ero1α mut) adult cardiomyocytes. Right panel: superimposed tracings from WT (black) and Ero1α mut (red) adult cardiomyocytes, normalized to the same peak amplitude, highlighting slower relaxation in the mutant cells. B) Calcium transient amplitude (normalized to an average of 1 in WT cardiomyocytes), rate of decay of cytosolic calcium (τ) and maximum change in sarcomere length in the two genotypes. C) Left panel: immunoblot of endogenous ERO1α in HeLa cells exposed to an ERO1 inhibitor (QM295), the reducing agent dithiothreitol (DTT), or both. Note the accumulation of partially reduced (lower mobility) ERO1α in cells treated with the inhibitor. Right panel: representative tracings showing effect of the ERO1 inhibitor, QM295 (25 μM, 3 h application) on calcium handling in freshly isolated wild-type adult cardiomyocytes. D) Amplitude and rate of decay of calcium transients in untreated and QM295-treated cardiomyocytes, analyzed as in panel A. Data are presented as means ± se; number of cells studied is indicated. *P < 0.05, **P < 0.01.
The aforementioned genotype-dependent difference in calcium handling between wild-type and mutant cardiomyocytes could reflect a developmental role for Ero1α in calcium handling or a direct role for ERO1's enzymatic activity in calcium homeostasis. To discriminate between these possibilities, we measured the affect of acutely applying an ERO1 inhibitor on calcium homeostasis in wild-type cardiomyocytes. The quinone methide ERO1 inhibitor QM295, which traps ERO1 in its reduced form (ref. 20 and Fig. 2C), recapitulated the effects of Ero1α inactivation in terms of lower peak amplitude of the calcium transients (Fig. 2C, D) and prolonged τ (Fig. 2D). With the caveat that QM295 (and the related EN460, which was not studied here) has the potential for reacting with thiols on other proteins, these observations are consistent with an ongoing role for ERO1's enzymatic activity in calcium homeostasis.
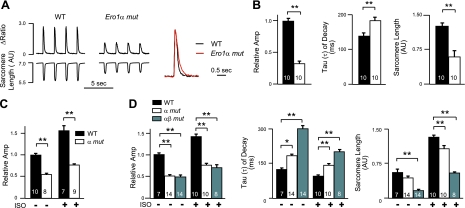
To further explore the scope and physiological significance of the defect in calcium handling, we compared the effect of the β-adrenergic agonist isoproterenol when applied to wild-type and Ero1α mutant cardiomyocytes. The expected stimulation of the peak amplitude of the calcium transients observed in the wild-type cells exposed to isoproterenol was attenuated in mutant cardiomyocytes (Fig. 3A, B), drawing out the differences already observed in the basal state. This defect extended to the effect of isoproterenol on fractional sarcomere shortening (Fig. 3B).
Figure 3.
Blunted inotropic response to adrenergic stimulation in cardiomyocytes lacking ERO1α. A) Left panels: calcium transients in electrically paced (0.5 Hz) freshly isolated wild-type (WT) or Ero1α mutant (Ero1α mut) cardiomyocytes after exposure to the adrenergic agonist isoproterenol (30 nM). Right panel: superimposed tracings from WT (black) and Ero1α mut (red) adult cardiomyocytes, normalized to the same peak amplitude. B) Graphic presentation of the mean peak amplitude of the calcium transient (normalized to an average value of 1 for WT cardiomyocytes), τ, and sarcomere length in the 2 genotypes after adrenergic stimulation. C) Peak amplitude of the caffeine-induced calcium transient (normalized to an average value of 1 for WT cardiomyocytes) in the absence (−) and presence (+) of isoproterenol (ISO). D) Graphic presentation of the mean peak amplitude of the calcium transient, τ, and sarcomere length before (−) and after (+) adrenergic stimulation in cardiomyocytes from WT, Ero1α mutant (α mut), and compound Ero1α; Ero1β mutant mice (αβ mut). Data are presented as means ± se; number of cells studied is indicated. *P < 0.05, **P < 0.01.
We next investigated whether the diminution in the magnitude of the calcium transient might reflect altered sarcoplasmic reticulum calcium load, as determined by the caffeine-induced calcium transient amplitude. Indeed, compared to wild-type cardiomyocytes, the peak caffeine-induced calcium transient amplitude was significantly reduced in Ero1α mutant cells, both at baseline and following exposure to isoproterenol (Fig. 3C).
We were unable to detect endogenous ERO1β protein in the heart; however, quantitative RT-PCR showed that Ero1β mRNA is expressed, with a trend toward increased expression in the hearts of the Ero1α mutant mice (Supplemental Fig. S3). Therefore, we determined whether loss of function of both Ero1α and Ero1β led to further compromise in cardiomyocyte intracellular calcium handling. Indeed, freshly isolated compound Ero1α;Ero1β mutant cardiomyocytes showed a significantly slower relaxation of the calcium transient and a depression of fractional sarcomere shortening compared to single ERO1 mutant cardiomyocytes, both at baseline and following β-adrenergic stimulation with isoproterenol (Fig. 3D). These observations are consistent with a concerted role for both genes in affecting sarcoplasmic reticulum calcium homeostasis in cardiomyocytes. Furthermore these studies suggest that this defect might render cardiac function dependent on ongoing adrenergic stimulation in mutant mice.
Mice lacking ERO1 are hypersensitive to inhibition of β-adrenergic stimulation
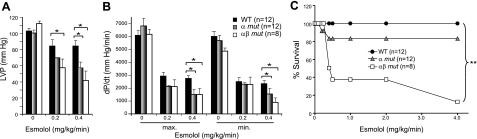
To test the hypothesis that homozygous Ero1α mutant mice and, even more so, compound-mutant mice with impaired expression of both Ero1α and Ero1β, may compensate for the diminished magnitude of calcium transients and associated contractile deficit through augmented adrenergic stimulation in vivo, we examined the response of wild-type and mutant mice to acute exposure to the short-acting β-blocker esmolol. Following exposure to esmolol, Ero1α mutant and, to a greater extent, compound Ero1α; Ero1β mutant mice experienced greater declines in left ventricular peak pressure and the maximum and minimum first derivatives of ventricular pressure (±dP/dt) than wild-type sibling mice (Fig. 4A, B). In contrast, the heart rate response to esmolol was similar in all 3 genotypes. Furthermore, survival of the compound-mutant mice following exposure to esmolol was markedly lower than that of wild-type or Ero1α single-mutant mice (Fig. 4C).
Figure 4.
Hypersensitivity of mice lacking ERO1 to adrenergic blockade. A) Peak left ventricular pressure in anesthetized catheterized mice of wild-type (WT; solid bars), Ero1α (α mut; shaded bars) and compound Ero1α; Ero1β mutant (αβ mut; open bars) genotypes following exposure to the indicated doses of the short-acting β-blocker esmolol. B) Peak positive and negative first derivatives of left ventricular pressure of the mice described in panel A. C) Fraction of surviving animals of each genotype following injection of the indicated dose of esmolol. Data are presented as means ± se; number of animals studied in each group is indicated. *P < 0.05, **P < 0.01.
Ero1α loss of function protects against pressure overload-induced stress
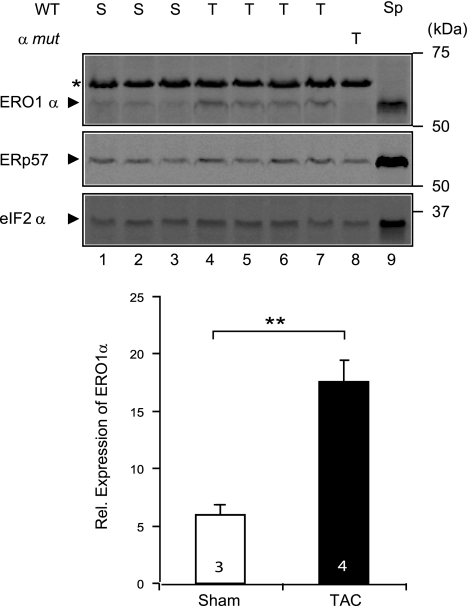
The perturbations in intracellular calcium homeostasis and response to adrenergic blockade of the mutant mice suggested that ERO1α might play a role not only in physiological states, but also in response to imposed stressors, such as surgical TAC. This procedure imposes a sustained hemodynamic pressure overload on the left ventricle, and the augmented workload triggers a cascade of adaptive and maladaptive responses (25). Consistently, we observed elevated levels of ERO1α protein (Fig. 5) in the hearts of mice subjected to TAC (18), an observation that was anticipated by the fact that the level of CHOP, a transcriptional activator of the Ero1α gene, is increased in heart failure (26, 27).
Figure 5.
Elevated ERO1α expression in the overloaded heart. Top panels: immunoblot of ERO1α in extracts of heart from wild-type (WT; lanes 1–7) or Ero1α mutant mice (lane 8) obtained 8 wk after a sham operation (S) or a TAC procedure (T). ERp57, an ER marker protein, and eIF2α, a cytosolic marker protein, serve as loading controls. Lysate of spleen from a wild-type animal (Sp), a rich source of ERO1α, was applied to lane 9 to clearly demarcate its position on the blot. Note the absence of ERO1α signal from the mutant heart in lane 8. Asterisk marks an irrelevant protein detected by the ERO1α antiserum in heart lysates. Bottom panel: relative levels of ERO1α protein, normalized to the ER loading marker. Values are means ± se. **P < 0.01.
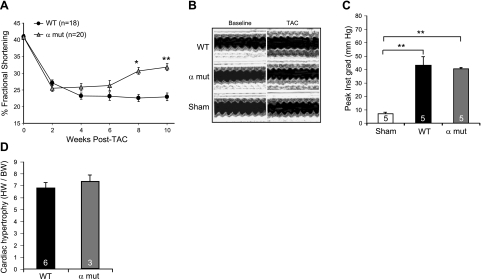
To examine the effects of altered ER lumenal redox on the outcome of a heart failure model, we subjected an additional cohort of wild-type male mice and their siblings homozygous for the Ero1α mutation to the TAC procedure. In surviving mice of both genotypes, there was a progressive decline in fractional shortening. However, in the Ero1α mutant mice, a clear trend for recovery of contractility was observed beginning at 6 wk after TAC, which reached statistical significance by 8 wk after imposition of the hemodynamic load (Fig. 6A). A representative series of M-mode echocardiograms illustrates the persistent defect in the wild-type animals and the recovery of fractional shortening in the mutant (Fig. 6B).
Figure 6.
Partial protection against progressive heart failure in Ero1α mutant mice. A) Fractional shortening of the left ventricle, measured by M-mode echocardiography, in wild-type (WT) and Ero1α mutant (α mut) mice at the indicated time after TAC. B) Representative trace of M-mode echocardiogram at baseline and 8–10 wk following TAC or sham operation in WT and α mut mice. C) Plot of the peak pressure gradient across the TAC in a sample of WT and α mut mice. D) Plot of heart weight to body weight ratios determined 6-wk after TAC in WT and α mut mice. Data are presented as means ± se; number of animals in each group is indicated. *P < 0.05; **P < 0.01.
The extent of TAC, as assessed by peak pressure gradient across the surgical lesion, appeared similar in the two genotypes (Fig. 6C). Also similar was the extent of cardiac hypertrophy, measured by the ratio of heart weight to body weight (Fig. 6D). The small size of the compound Ero1α; Ero1β mutant mice renders them unsuited for TAC; therefore, we were unable to examine the effect of more profound reduction in ERO1 activity on the outcome of this progressive heart failure model.
DISCUSSION
Either genetic or chemical interference with ERO1 function markedly alters intracellular calcium transients in electrically active cardiomyocytes. The observed defects in the magnitude of calcium transients are associated with in vivo hypersensitivity to withdrawal of adrenergic stimulation and correlate with an abnormally reducing ER in freshly isolated cardiomyocytes. Together, these observations point to a role for lumenal redox-regulated calcium transport in the heart. The finding that mice with an abnormally reducing ER are relatively resistant to the long-term consequences of excessive cardiac pressure load suggests that the molecular mechanisms may also be relevant to cardiac pathophysiology.
Recent studies have uncovered a surprising level of redundancy in the pathways to disulfide bond formation in the ER of complex eukaryotes. Under basal conditions, loss of function mutations in both ERO1 genes have an unexpectedly modest effect on secretory protein production (7, 8). This suggests that under basal conditions, and in the mainly secretory tissues examined, the modest decrease of the rate at which disulfides form in the mutant is not crucial to survival and well-being. By contrast, the study of mouse embryonic fibroblasts here has uncovered a role for ERO1 in promoting an oxidizing environment in the ER under conditions of oxygen deprivation. Though the set-point of the various redox buffers that operate in the ER is not believed to be affected by ERO1 activity (28), our observations suggest that the enhanced rate of disulfide bond formation catalyzed by ERO1 affects the partitioning of some lumenal proteins between their disulfide bonded and reduced configurations (redox poise) kinetically. Currently, we lack tools to measure sarcoplasmic reticulum redox in the intact heart (or in freshly isolated cardiomyocytes). Our attempts at end-point assays that monitor disulfide bonding in the active site of oxidoreductases like Erp57 have been frustrated by technical difficulties; therefore, we cannot exclude the possibility that ERO1's role in cardiac calcium homeostasis also reflects other activities of the protein that are unrelated to its function as an ER oxidase in stressed cells. Nonetheless, given the genetic evidence for the functionally hypoxic state of stressed cardiomyocytes (29) and evidence that ERO1α is induced by hypoxia, a role for ERO1-mediated lumenal redox in calcium handling seems a parsimonious explanation for our findings.
At present, the molecular events linking the altered redox of mutant cardiomyocytes to altered calcium flux remain unknown. The isoforms of SERCA and IP3R previously shown to be subject to regulation by ER-localized oxidoreductases are not expressed in the heart. Nonetheless, the critical cysteines on their lumenal loops are conserved in cardiac isoforms of these enzymes, suggesting that redox-mediated regulation of their function could contribute to the observed differences in calcium handling by wild-type and mutant cardiomyocytes. Indeed, the diminished sarcoplasmic reticulum calcium load observed in Ero1α mutant cardiomyocytes is consistent with impaired SERCA2a function. However, in freshly isolated cardiomyocytes, pharmacological blockade of SERCA markedly prolongs calcium transients but has a more modest effect on releasable calcium stores (30), whereas in ERO1-deficient cardiomyocytes, attenuated calcium transients dominate the picture, with τ prolongation being less conspicuous. Given the diversity of protein species with structurally important disulfide bonds, the effects of deviations in redox are likely to be multifactorial and may affect proteins other than SERCA. Thus, characterizing the molecular targets of altered redox in the cardiomyocyte sarcoplasmic reticulum is an important task for the future.
Furthermore, ERO1 activity generates hydrogen peroxide (31) that could diffuse across the ER membrane and affect cytoplasmic processes. Recently, an ER-localized peroxiredoxin (PRDX4) has been identified that can exploit lumenal hydroperoxides as electron acceptors to generate disulfide bonds on the lumenal side of the ER membrane (8, 32). Thus, genetic manipulation of PRDX4 may discriminate between the lumenal and cytoplasmic effects of altered ERO1 activity on calcium metabolism.
It is intriguing to consider how the existing relationship between redox and calcium metabolism evolved. The dependence of calcium homeostasis on ER redox may simply reflect structural constraints for certain constitutive disulfide bonds in the lumenal portions of ion channels and pumps. According to this model, the impaired contractility of the mutant cardiomyocytes and their hypersensitivity to adrenergic receptor blockade is a consequence of an altered pattern of constitutive disulfide bonding in the ER of the mutant. In this model, structural alterations adversely affect fitness by constitutively enfeebling the machinery for excitation-contraction coupling. However, it is notable that under the severe and sustained load on the heart imposed by TAC, the mutant fares better than the wild type. Cardiac load and failure are associated with activation of ER stress-induced gene expression programs (the unfolded protein response) that include the induction of CHOP, an upstream activator of Ero1α. Compromise of the CHOP arm of unfolded protein response ameliorates the outcome of pressure overload on the heart (16). Together, these observations support a more nuanced model whereby stress-induced, ERO1-dependent changes to ER redox may affect calcium dynamics as part of a homeostatic mechanism that involves alterations in regulatory disulfide bonds.
It is unclear whether the benefit of attenuated Ero1α expression in chronic pressure overload is played out at the level of altered calcium handling, i.e., by ameliorating the defects in calcium transients normally seen in failing cardiomyocytes, or instead, reflects some other benefit accrued long term by an altered ER redox. Altered calcium dynamics have been implicated in ER stress-mediated death of macrophages (33) and have been proposed to play a role in ER stress-mediated outcomes in the overloaded heart (16), but the importance of cell death to the TAC model and the effect of the Ero1α mutation on it remain to be studied. Nor is it clear whether this effect is mediated by the mutation acting exclusively in cardiomyocytes or in some other cell type. Nonetheless, the functional effect of lowered ERO1 activity in this heart failure model is sufficiently compelling to recommend further mechanistic studies of the links between redox and calcium metabolism.
Supplementary Material
Acknowledgments
The authors thank S. James Remington (University of Oregon, Eugene, OR, USA) for the roGFP_iE.
This study was supported by U.S. National Institutes of Health grants ES08681, DK47119, and DK075311 to D.R. and R01HL82727, R01HL081336, and S10RR026881 to G.I.F., and EMBO long-term fellowship ALTF649-2008 to E.Z. D.R. is a Wellcome Trust Principal Research Fellow.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Hatahet F., Ruddock L. W. (2009) Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 11, 2807–2850 [DOI] [PubMed] [Google Scholar]
- 2. Sevier C. S., Kaiser C. A. (2008) Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim. Biophys. Acta 1783, 549–556 [DOI] [PubMed] [Google Scholar]
- 3. Tu B. P., Weissman J. S. (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frand A. R., Kaiser C. A. (1998) The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1, 161–170 [DOI] [PubMed] [Google Scholar]
- 5. Pollard M. G., Travers K. J., Weissman J. S. (1998) Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell 1, 171–182 [DOI] [PubMed] [Google Scholar]
- 6. Pagani M., Fabbri M., Benedetti C., Fassio A., Pilati S., Bulleid N. J., Cabibbo A., Sitia R. (2000) Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J. Biol. Chem. 275, 23685–23692 [DOI] [PubMed] [Google Scholar]
- 7. Zito E., Chin K. T., Blais J., Harding H. P., Ron D. (2010) ERO1β, a pancreas-specific disulfide oxidase promotes insulin biogenesis and glucose homeostasis. J. Cell Biol. 188, 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zito E., Melo E. P., Yang Y., Wahlander A., Neubert T. A., Ron D. (2010) Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol. Cell 40, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tien A. C., Rajan A., Schulze K. L., Ryoo H. D., Acar M., Steller H., Bellen H. J. (2008) Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster. J. Cell Biol. 182, 1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bers D. M. (2008) Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 70, 23–49 [DOI] [PubMed] [Google Scholar]
- 11. Lompre A. M., Anger M., Levitsky D. (1994) Sarco(endo)plasmic reticulum calcium pumps in the cardiovascular system: function and gene expression. J. Mol. Cell. Cardiol. 26, 1109–1121 [DOI] [PubMed] [Google Scholar]
- 12. Giordano F. J. (2005) Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 115, 500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higo T., Hattori M., Nakamura T., Natsume T., Michikawa T., Mikoshiba K. (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120, 85–98 [DOI] [PubMed] [Google Scholar]
- 14. Li Y., Camacho P. (2004) Ca2+-dependent redox modulation of SERCA 2b by ERp57. J. Cell Biol. 164, 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu H. Y., Okada K., Liao Y., Tsukamoto O., Isomura T., Asai M., Sawada T., Okuda K., Asano Y., Sanada S., Asanuma H., Asakura M., Takashima S., Komuro I., Kitakaze M., Minamino T. (2010) Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 122, 361–369 [DOI] [PubMed] [Google Scholar]
- 17. Qu J., Volpicelli F. M., Garcia L. I., Sandeep N., Zhang J., Marquez-Rosado L., Lampe P. D., Fishman G. I. (2009) Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart. Circ. Res. 104, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rockman H. A., Ross R. S., Harris A. N., Knowlton K. U., Steinhelper M. E., Field L. J., Ross J., Jr., Chien K. R. (1991) Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 88, 8277–8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyers M. B., Fischer A., Sun Y. J., Lopes C. M., Rohacs T., Nakamura T. Y., Zhou Y. Y., Lee P. C., Altschuld R. A., McCune S. A., Coetzee W. A., Fishman G. I. (2003) Sorcin regulates excitation-contraction coupling in the heart. J. Biol. Chem. 278, 28865–28871 [DOI] [PubMed] [Google Scholar]
- 20. Blais J. D., Chin K. T., Zito E., Zhang Y., Heldman N., Harding H. P., Fass D., Thorpe C., Ron D. (2010) A small molecule inhibitor of endoplasmic reticulum oxidation 1 (ERO1) with selectively reversible thiol reactivity. J. Biol. Chem. 285, 20993–21003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lohman J. R., Remington S. J. (2008) Development of a family of redox-sensitive green fluorescent protein indicators for use in relatively oxidizing subcellular environments. Biochemistry 47, 8678–8688 [DOI] [PubMed] [Google Scholar]
- 22. Kang G., Giovannone S. F., Liu N., Liu F. Y., Zhang J., Priori S. G., Fishman G. I. (2010) Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ. Res. 107, 512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. May D., Itin A., Gal O., Kalinski H., Feinstein E., Keshet E. (2005) Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene 24, 1011–1020 [DOI] [PubMed] [Google Scholar]
- 24. Dibb K. M., Graham H. K., Venetucci L. A., Eisner D. A., Trafford A. W. (2007) Analysis of cellular calcium fluxes in cardiac muscle to understand calcium homeostasis in the heart. Cell Calcium 42, 503–512 [DOI] [PubMed] [Google Scholar]
- 25. Nass R. D., Aiba T., Tomaselli G. F., Akar F. G. (2008) Mechanisms of disease: ion channel remodeling in the failing ventricle. Nat. Clin. Pract. Cardiovasc. Med. 5, 196–207 [DOI] [PubMed] [Google Scholar]
- 26. Hamada H., Suzuki M., Yuasa S., Mimura N., Shinozuka N., Takada Y., Nishino T., Nakaya H., Koseki H., Aoe T. (2004) Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol. Cell. Biol. 24, 8007–8017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okada K., Minamino T., Tsukamoto Y., Liao Y., Tsukamoto O., Takashima S., Hirata A., Fujita M., Nagamachi Y., Nakatani T., Yutani C., Ozawa K., Ogawa S., Tomoike H., Hori M., Kitakaze M. (2004) Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110, 705–712 [DOI] [PubMed] [Google Scholar]
- 28. Appenzeller-Herzog, Riemer J., Zito E., Chin K. T., Ron D., Speiss M., Ellgaard L. (2010) Disulfide production by Ero1a-PDI relay is rapid and effectively regulated. EMBO J 29, 3318–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krishnan J., Suter M., Windak R., Krebs T., Felley A., Montessuit C., Tokarska-Schlattner M., Aasum E., Bogdanova A., Perriard E., Perriard J. C., Larsen T., Pedrazzini T., Krek W. (2009) Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 9, 512–524 [DOI] [PubMed] [Google Scholar]
- 30. O'Neill S. C., Miller L., Hinch R., Eisner D. A. (2004) Interplay between SERCA and sarcolemmal Ca2+ efflux pathways controls spontaneous release of Ca2+ from the sarcoplasmic reticulum in rat ventricular myocytes. J. Physiol. 559, 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gross E., Sevier C. S., Heldman N., Vitu E., Bentzur M., Kaiser C. A., Thorpe C., Fass D. (2006) Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc. Natl. Acad. Sci. U. S. A. 103, 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tavender T. J., Bulleid N. J. (2010) Peroxiredoxin IV protects cells from oxidative stress by removing H2O2 produced during disulphide formation. J Cell Sci. 123, 2672–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li G., Mongillo M., Chin K. T., Harding H., Ron D., Marks A. R., Tabas I. (2009) Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell. Biol. 186, 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.