Abstract
A substantial body of data was reported between 1984 and 2000 demonstrating that the neuropeptide N-acetylaspartylglutamate (NAAG) not only functions as a neurotransmitter but also is the third most prevalent transmitter in the mammalian nervous system behind glutamate and GABA. By 2005, this conclusion was validated further through a series of studies in vivo and in vitro. The primary enzyme responsible for the inactivation of NAAG following its synaptic release had been cloned, characterized and knocked out. Potent inhibitors of this enzyme were developed and their efficacy has been extensively studied in a series of animal models of clinical conditions, including stroke, peripheral neuropathy, traumatic brain injury, inflammatory and neuropathic pain, cocaine addiction, and schizophrenia. Considerable progress also has been made in defining further the mechanism of action of these peptidase inhibitors in elevating synaptic levels of NAAG with the consequent inhibition of transmitter release via the activation of presynaptic mGluR3 by this peptide. Very recent discoveries include identification of two different nervous system enzymes that mediate the synthesis of NAAG from N-acetylaspartate and glutamate and the finding that one of these enzymes also mediates the synthesis of a second member of the NAAG family of neuropeptides, N-acetylaspartylglutamylglutamate (NAAG2).
Keywords: N-acetylaspartylglutamate, N-acetylaspartylglutamylglutamate, NAAG, NAAG2, metabotropic glutamate receptor 3, NAAG synthetase, stroke, traumatic brain injury, inflammatory pain, schizophrenia
Introduction
In the mid 1960s, high concentrations of N-acetylaspartylglutamate (NAAG) were discovered in the mammalian brain by two laboratories on opposite sides of the planet (Curatolo et al., 1965; Miyamoto et al., 1966). As it later was noted with respect to this discovery, “in science as in life timing is everything” (Neale et al., 2000). The 1960s were the wrong time for NAAG to be discovered. GABA had recently been recognized as a transmitter and a role for glutamate in neurotransmission was on slightly stronger footing after more than a decade of dispute as to its physiological relevance. Peptides were not regarded as significant players in chemical neurotransmission and despite the data on glutamate and GABA, transmitters generally were not believed to be present in high concentrations in the nervous system. As a result, the discovery of this peptide remained effectively dormant for nearly 20 years. While the discovery of the opiate peptides set off a stampede to discover and characterize additional neuropeptides, NAAG was left unattended in this “gold rush”. The consequence was that this peptide was overdue for exploration by the mid 1980s and a small number of research groups took up the challenge. As it turns out, proof of NAAG’s function was more complex than first imagined because of the prevalence of extracellular NAAG peptidase activity in the nervous system that converts NAAG to NAA and importantly to glutamate. The complexity was exacerbated by the discovery that NAAG activated a metabotropic glutamate receptor (mGluR3), rather than directly activating an ionotropic receptor, and by some data leading to the incorrect conclusion that this peptide was a low potency agonist, or alternatively antagonist, at NMDA receptors. Sorting this out took more than a decade.
1984 to 1997 – NAAG meets each of the criteria of a neurotransmitter
The first step in defining the function of NAAG came from the discovery of NAAG-like immunoreactivity (IR) in neurons in the rat and cat nervous systems (Anderson et al., 1986; Cangro et al., 1987; Forloni et al, 1987; Tieman et al., 1987). Strikingly, this IR was not restricted to codistribution with a single small amine transmitter but was found in neurons in the brain, spinal cord, sensory ganglia and retina that coexpressed glutamate, GABA, acetylcholine, norepinephrine, dopamine, and serotonin (reviewed in Coyle et al., 1997). Several reports appeared in 1988 that demonstrated the calcium-dependent release of NAAG following depolarization of different neuronal preparations and this was quickly followed by an ultrastructural study in which NAAG-IR was identified within synaptic vesicles (Tsai et al., 1988; Williamson et al., 1988a, b; Zollinger et al., 1988). In parallel, some early studies took the approach of applying NAAG directly to neuronal preparations in order to elucidate a physiological function in spite of the understanding that peptide transmitters traditionally are inactivated by extracellular peptidases that in this case would release glutamate. Once Riveros and Orrego (1984) reported that NAAG was hydrolyzed by a brain peptidase activity, it was realized that some of the early physiologic data obtained by direct application of the peptide to neuronal preparations were severely compromised (Blakely et al., 1988).
Several hundred uM to 1 mM highly purified NAAG activated NMDA receptors in spinal cord neurons, olfactory bulb neurons and oligodendrocytes in culture and (Westbrook et al., 1986; Trombley and Westbrook, 1990; Kolodziejczyk et al., 2009). Due to the high concentration of peptide used in these studies, it is difficult to assess the physiological relevance of these data. Lower concentrations of NAAG have been reported to antagonize the NMDA receptor currents in hippocampal neurons. However, this effect was inexplicable eliminated in the presence of glycine, brining into question its significance (Bergeron et al., 2005; 2007). In contrast, several studies have directly demonstrated that NAAG is not a physiologically relevant NMDA receptor agonist or antagonist when applied to hippocampal and cerebellar granule cell neurons (Lea et al., 2000; Losi et al., 2004; Fricker et al., 2009).
In order to resolve the physiological role of NAAG and indeed to confirm its function as a neurotransmitter, the receptor that it activated needed to be rigorously identified. Here again, timing was important but in a positive way. That is, initial studies on the application of NAAG to cerebellar granule cells and later to astrocytes in cell cultures demonstrated that it reduced forskolin-stimulated levels of cAMP (Wroblewska et al., 1993; 1998). At about the same time, the metabotropic glutamate receptors (mGluRs) were cloned and several were found to be negatively coupled to adenylate cyclase. Following this lead and using cDNA for mGluRs, NAAG was reported to selectively activate mGluR3 in stably transfected cell lines (Wroblewska et al., 1997; 1998; Lea et al., 2001). Later studies revealed that NAAG also negatively regulated cGMP levels via mGluR3 in cerebellar neurons and astrocytes as did group 2 agonists in mGluR3 transfected cells (Wroblewska et al., 2006; 2011)
The conclusion that NAAG selectively activates mGluR3 recently was challenged in two papers reporting data from Xenopus oocytes and HEK cell lines cotransfected with the receptor and a G-protein sensitive potassium channel (Chopra et al., 2009; Fricker, 2009). These studies confirmed an earlier report (Losi et al., 2004) that commercial NAAG often contained from 0.1–0.4% glutamate. Citing studies in which high levels of NAAG were reported to activate mGluR3, these reports concluded that those NAAG samples also might have contained sufficiently high levels of glutamate as to be responsible for the apparent activation of mGluR3 by NAAG. Unfortunately, these two papers failed to fully consider data in a series of reports that directly contradict this conclusion and demonstrate that NAAG, rather than glutamate contamination of NAAG, activates this receptor (Bischofberger and Schild, 1996; Wroblewska et al. 1997; 1998; Lea et al., 2001; Adedoyin et al., 2010; reviewed in Neale, 2011, submitted). Indeed, the laboratory directing all but one of these studies began routinely repurifying commercial NAAG in July of 1996 (Wroblewska and Neale, unpublished observation). However, these two reports that glutamate, but not NAAG, activate a G-protein regulated potassium channel in cells cotransfected with mGluR3 suggest that glutamate and NAAG interact somewhat differently with the ligand binding site of mGluR3 and thus the second messenger coupling. Indeed, different ligands for the same receptor have been well documented to activate different second messenger cascades in the same cells (reviewed in Ambrosio et al., 2011).
Identification of mGluR3 as the NAAG receptor represented a breakthrough not simply because it advanced understanding of the neurobiology of this peptide, but because of the growing behavioral and neurochemical literature on the efficacy of heterotropic agonists at mGluR2/3 receptors in vitro and in vivo. This literature provided important leads as to potential roles of NAAG in inhibiting transmitter release, including glutamate release, via presynaptic receptors and ultimately in animal models of significant clinical disorders. Among the first reports of physiological actions of group II mGluR agonists was the finding that it functioned presynaptically to reduce transmitter release. Thus, it was not surprising that as little as 1 uM NAAG was shown to reduce voltage dependent calcium currents and transmitter release in olfactory bulb neurons via a group II mGluR (Bischofberger and Schild, 1996). This NAAG-induced inhibition of transmitter release was subsequently confirmed in cerebral cortical nerve cells and in amygdaloid neurons in vitro with both processes being blocked by an mGluR2/3 antagonist (Zhao et al., 2001; Adedoyin et al., 2010).
Critical developments in revealing the functions of endogenous NAAG in the nervous system were the discovery and purification of NAAG peptidase activity and cloning of the genes for NAAG peptidase enzymes, glutamate carboxypeptidase II and III (GCPII, GCPIII) (Riveros and Orrego, 1984; Slusher et al., 1990; Carter et al., 1996; Bzdega et al., 1997; 2004; Luthi-Carter et al., 1998; Bacich et al., 2001). GCPII and GCPIII are zinc metalopeptidases and members of the transferrin superfamily. They share 70% sequence homology with the former being expressed at a much higher level in the brain (Bzdega et al., 1997; 2004). While GCPII appears to be expressed exclusively or nearly so by glia, GCPIII is expressed at a higher level in cerebellar and cerebral cortical neurons than astrocytes (Bzdega et al., 2004), suggesting different sites of action. The crystal structures of both enzymes have been examined and their pharmacophore pockets compared (Barinka et al., 2007; Hlouchova et al., 2009). Several motifs associated with their active sites differ and these differences appear important in their interactions with peptidase inhibitors. For example, the IC50 values for 2-PMPA at GCPII and GCPIII are 7nM and 1 nM respectively (Bzdega et al., 2004). The characterization of these peptidases can be seen as closing the loop on the traditional benchmarks for confirming the status of NAAG as a peptide neurotransmitter.
1998–2008: NAAG Peptidase Inhibitors and Preclinical Models
In order to better understand the role of NAAG as an mGluR3 agonist in vivo, a series of structurally divergent NAAG peptidase inhibitors have been synthesized and characterized with the aim of increasing synaptic levels of this peptide (reviewed in Neale et al., 2005; Zhou et al., 2005; Thomas et al., 2006; Tsukamoto et al., 2007). Important in interpretation of their effects, these peptidase inhibitors do not directly activate any mGluRs (Yamamoto et al., 2004; 2007) but rather increase synaptic levels of NAAG (Slusher et al., 1999; Zhong et al., 2006) that activates presynaptic mGluR3 to inhibit subsequent transmitter release (Figure 1A). Using one of these peptidase inhibitors, 2-PMPA, Slusher et al. (1999) published a breakthrough paper on the efficacy of endogenous NAAG in vivo. Systemic administration of 2-PMPA reduced the elevation of extracellular glutamate levels and consequent nerve cell death following cerebral ischemia in rat brain. Consistent with this result, GCPII knock out mice exhibit no overt differences in standard neurological testing but are less sensitive to ischemic brain injury than their wild type littermates (Bacich et al., 2002; 2005). While these mice clearly lack the full GCPII gene and fail to express GCPII message or protein, another lab inexplicably reported that knocking out GCPII is embryonic lethal (Tsai et al., 2003; Han et al., 2009)
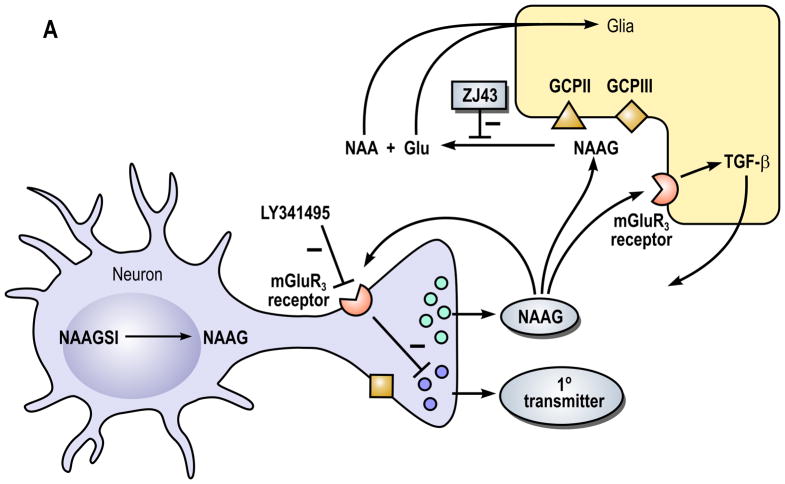
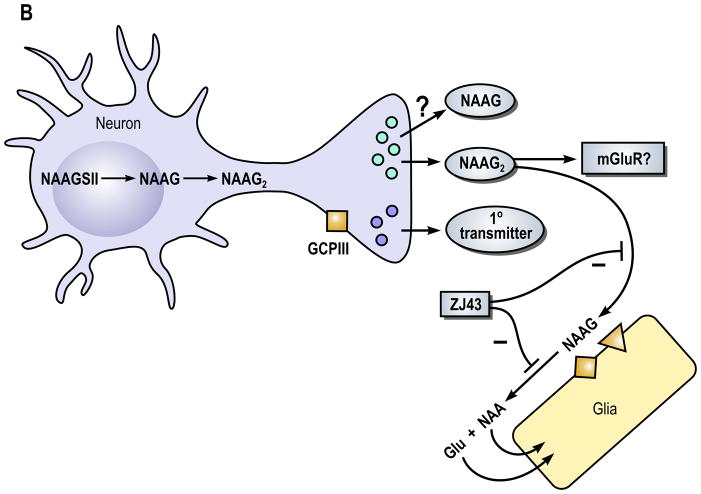
Figure 1. A model of the role of N-acetylaspartylglutamate (NAAG) peptidase inhibition and its influence on (A) NAAG and (B) N-acetylaspartylglutamylglutamate (NAAG2) in the nervous system.
In part A, the neuron expresses NAAG synthetase I (NAAGS I), an enzyme that mediates the synthesis of NAAG but not NAAG2. In this cell, NAAG is co-released with a primary amine transmitter, such as glutamate, under conditions of elevated neuronal activity. While the primary transmitter is released into the immediate synaptic space, the peptide is released perisynaptically where it activates presynaptic and glial type 3 metabotropic glutamate receptors (mGluR3). NAAG is inactivated by glutamate carboxypeptidases II (GCPII) and III (GCPIII), forming N-acetylaspartate (NAA) and glutamate (Glu), which are transported into glial cells. While GCPIII is expressed by neurons and glia in cell culture (Bzdega et al., 2004), its localization on presynaptic ending is purely speculative. High levels of glutamate-mediated neurotransmission are associated with several clinical disorders including traumatic brain injury, stroke, peripheral neuropathy, inflammatory pain and schizophrenia. NAAG inhibits glutamate release by activation of presynaptic mGluR3 receptors. Inhibition of the peptidases GCPII and GCPIII by a NAAG peptidase inhibitor, such as ZJ43, reduces inactivation of NAAG. In animal models of these disorders, the NAAG peptidase inhibitor-mediated elevation of peptide levels increases the activation of mGlu3 receptors on axon endings, inhibiting further glutamate release and reducing the pathology. In a second neuroprotective pathway, NAAG activation of mGlu3 receptors on glial cells stimulates the release of a trophic factor, transforming growth factor β (TGF-β).
In Part B, the neuron expresses NAAG synthetase II (NAAGSII), an enzyme that mediates the synthesis of NAAG and NAAG2. In this model, we propose that NAAG2 and perhaps NAAG are co-released with a primary amine transmitter, again as it the case for other neuropeptides, under conditions of elevated neuronal activity. The receptor that NAAG2 might activate has not been identified but is likely to be defined in the near future. Since GCPII hydrolyzes both NAAG and NAAG2, peptidase inhibitors such as ZJ43 can be predicted also to elevate levels of NAAG2 and increase its activity.
GCPII was first cloned as prostate specific membrane antigen and used as a marker of prostate hypertrophy before its NAAG peptidase activity was independently discovered. This coincidence has led to a potentially important advance in diagnosis and treatment of prostate cancer as high affinity antagonists of this enzyme, such as ZJ43, are being used to both image the human prostate and to deliver drugs to prostate cells expressing high levels of this surface protein (reviewed in Zhou et al., 2005; Zaheer et al., 2009; Sanna et al., 2011).
NAAG peptidase Inhibition and Peripheral Neuropathy
The initial findings of the neuroprotective effects of a NAAG peptidase inhibitor in the stroke model prompted assessment of these inhibitors in peripheral neuropathies resulting from trauma, diabetes or chemotherapy. Chronic treatment of type 1 diabetic BB/Wor rats with the NAAG peptidase inhibitors GPPI-5232 and 2-MPPA reduced the development of hyperalgesia while improving sciatic nerve function and morphology (Zhang et al., 2002; 2006). Using an in vitro model of hyperglycemia, Berent-Spillson and colleagues found that NAAG acting via mGluR3 blocked glucose induction of caspase activity in sensory neurons, that the NAAG peptidase inhibitor 2-PMPA reversed glucose-induced programmed cell death in these neurons and that these effects were mediated by mGluR3 receptors on Schwann cells (Spillson and Russell, 2003; Berent-Spillson et al., 2004; Berent-Spillson and Russell, 2007). Similarly, NAAG peptidase inhibition attenuates the neurotoxicity induced by several different chemotherapeutic regimens (Carozzi et al., 2010).
In the sciatic nerve crush model of peripheral neuropathology, GCPII knockout mice suffered less injury and faster recovery than their wild type littermates (Bacich et al., 2005), consistent with the concept that NAAG peptidase inhibition is protective in peripheral neuropathy. Similarly, NAAG peptidase inhibition attenuates mechanical allodynia induced by partial sciatic nerve cell ligation (Yamamoto et al., 2004). The expression of NAAG in dorsal sensory ganglion neurons (Cangro et al., 1987), of mGluR3 receptors on these neurons and Schwann cells (Bruno et al., 1998) and of GCPII by Schwann cells (Chiechio et al., 2006; Berger and Schwab, 1996) further support the view that this peptide system plays a role in dorsal sensory neuron function.
Traumatic Brain Injury
Fluid percussion injury to the rat cerebral cortex causes neuron and glial cell death in the hippocampus ipsilateral to the injury. As is known for stroke, percussive brain injury leads to cell death via elevated release of glutamate and a combination of apoptosis and necrosis over the 24-hour interval following injury. Systemic injection of the NAAG peptidase inhibitor ZJ43 just before and 8 and 16 hours after injury reduced neuronal and glial cell death by increasing extracellular NAAG levels and reducing the trauma-induced elevation in release of other transmitter levels, including glutamate, aspartate and GABA (Zhong et al., 2005; 2006). Each of these effects of ZJ43 was blocked by co-administration of the mGluR2/3 antagonist LY341495, a result supporting NAAG-mediated inhibition of transmitter release via a group II receptor. Consistent with NAAG activation of mGluR3 in these studies, neuroprotection induced by group II mGluR agonists appears to be mediated by this receptor rather than mGluR2 (Corti et al., 2007).
Inflammatory and Neuropathic Pain and Hyperalgesia
The analgesic efficacy of group II mGluR agonists (reviewed in Neugebauer, 2001) stimulated testing several NAAG peptidase inhibitors in animal models of inflammatory, neuropathic pain and metastatic cancer pain (Yamamoto et al., 2001; 2004; 2007; Carpenter et al., 2003; Saito et al., 2006). Analgesia induced by systemically administered NAAG peptidase inhibitors appears to be mediated both spinally and via brain pathways. NAAG is expressed at millimolar levels in the spinal cord (Fuhrman et al., 1994) and intrathecal administration of NAAG peptidase inhibitors induces an analgesic response to inflammatory pain in the hindlimb. Similarly, introduction of NAAG peptidase inhibitors directly into the ipsilateral lateral ventricle reduced responses to footpad inflammation (Yamamoto et al., 2008). NAAG peptidase inhibition also has been shown to reduce induction of contralateral hindlimb allodynia 24 hours after an inflammatory insult (Adedoyin et al., 2010). These data suggest that NAAG has a central role in moderating pain perception.
Consistent with the expression of NAAG-immunoreactivity in large and some mid size spinal sensory neurons (Cangro et al., 1987), the expression of mGluR3 by these neurons (Carlton and Hargett, 2007), and the analgesic efficacy of group II mGluR agonists on peripheral neurites (Yang and Gereau, 2003), NAAG and NAAG peptidase inhibitors were shown to be analgesic when injected into the hindpaw prior to induction of an inflammatory insult, raising the possibility of topical analgesia via application of an inhibitor in a medium that facilitates penetration of the skin.
In each of these studies, the analgesia induced by peptidase inhibition was blocked by co-administration of the group II mGluR antagonist, LY341495, supporting the conclusion that the process is mediated by NAAG activation of mGluR3. The extent to which the analgesic effects of NAAG are due, if any, to interactions with other transmitters in the ascending and descending pain pathways is not known. Nonetheless, proof of the concept that NAAG peptidase inhibition is an efficacious analgesic strategy is particularly important because it represents a completely novel approach to pain perception.
NAAG Peptidase Inhibition as Drug Abuse Therapy
One element in the behavioral and addictive properties of cocaine is the stimulation of dopamine release in the nucleus accumbens. Based on the codistribution of NAAG with dopamine in some neurons (Forloni et al., 1987) and the efficacy of NAAG peptidase inhibitors in reducing transmitter release (Slusher, et al., 1999; Sanabria et al., 2004; Zhong et al., 2006; Adedoyin et al., 2010), these inhibitors were tested in animal models of cocaine abuse where they inhibited cocaine-induced conditioned place preference, reinstatement of drug seeking behavior and cocaine self-administration under progressive ratio reinforcement conditions (Slusher et al., 2000; 2001;Peng et al., 2010; Xi et al., 2010a; 2010b). Additionally, microinjection of a peptidase inhibitor or NAAG into the nucleus accumbens inhibited cocaine self-administration and drug-induced reinstatement of drug seeking behavior while systemic injection of the inhibitor dose dependently reduced cocaine-induced release of dopamine and glutamate in this nucleus. Reinforcing the conclusion that NAAG mediated these effects via mGluR3, coinjection of a group II mGluR antagonist, systemically or directly into the nucleus accumbens reversed the effects of the peptide and peptidase inhibitor in these studies. The influence of NAAG on the opiate circuits is somewhat different with peptidase inhibition attenuating tolerance but not dependence in mice (Kozela et al., 2005)
Schizophrenia
A decade ago, a substantial body of data emerged on the efficacy of group II mGluR agonists in PCP, dizocilpine and d-amphetamine based animal models of schizophrenia (review in Niswender and Conn, 2010). These open channel NMDA receptor antagonists induce schizophrenia-like behaviors in humans and animals while stimulating the flux of dopamine and glutamate in the prefrontal cortex (Moghaddam and Adams, 1998). Given NAAG’s role as a group II agonist and its efficacy in inhibition of transmitter release, the NAAG peptidase inhibitor ZJ43 was tested in a series of animal models of this disorder (Olszewski et al., 2004, 2008; Takatsu et al., 2011; Profaci et al., 2011). NAAG peptidase inhibition significantly reduced the motor activation and stereotypic movement effects of PCP and MK801 in both rat and mouse models, reduced PCP-induced social withdrawal in the resident-intruder assay and attenuated MK801 but not PCP induced reduction in prepulse inhibition of acoustic startle. In each study, the effects of the peptidase inhibitors were blocked by the co-administration of a group II mGluR antagonist.
Consistent with data on the efficacy of mGluR2 positive allosteric modulators, a heterotropic group II mGluR agonist reduced the effects of PCP in mice that were null mutant for mGluR3, but not in mGluR2 knockout mice, a result that called into question the selectivity of NAAG for mGluR3 in these schizophrenia assays. Using the same strains of mice, however, a NAAG peptidase inhibitor was recently found to be effective in reducing PCP-induced motor activation in the mGluR2 but not mGluR3 knockout mice (Olszewski et al., submitted). These data further strengthen the conclusion that NAAG is mGluR3 selective in vivo and suggest that both mGluR2 and mGluR3 activation have therapeutic relevance in schizophrenia.
NAAG and Astrocytes
The influence of NAAG on mammalian glia remains relatively unexplored. Astrocytes express high levels of mGluR3 message, respond to NAAG via a pertussis toxin sensitive G protein to negatively regulate cAMP and cGMP levels and are the primary, if not exclusive, source of GCPII in the nervous system (Wroblewska et al., 1997; Berger et al., 1999). Yet the primary observations on the role of NAAG and mGluR3 in astrocytes relates to their release of transforming growth factor beta (TGF-β) following activation of mGluR3 (Figure 1A) and the consequent neuroprotection that this provides in culture (Bruno et al., 1998; D’Onofrio et al, 2001; Thomas et al., 2001a; 2001b). Beyond this, Gehl et al. (2004) demonstrated that cortical astrocytes in cell culture had the capacity to synthesize low levels of NAAG from N-acetylaspartate and [3H]-glutamate.
2010–2011 – Two Synthetases and Two Neuropeptides: NAAG and NAAG2
Two breakthrough papers were published in 2010 in which independent research groups identified two nervous system enzymes, NAAG synthetase I and NAAG synthetase II, which mediate the synthesis of NAAG in vitro and in transfected cells (Becker et al., 2010; Collard et al., 2010). Previous reports demonstrated that NAAG is not synthesized via post translational processing as is the case for other mammalian peptides, except carnosine, but rather it is synthesized from N-acetylaspartate and glutamate (Cangro et al., 1987, Gehl et al., 2004; Arun et al., 2006). However, the enzymes mediating NAAG synthesis remained elusive for nearly 50 years. Both NAAG synthetase I and II are expressed in the rat brain and spinal cord with expression patterns that are similar to the distribution of NAAG. Both are members of the ATP grasp family of synthetases. NAAG synthetase I also mediates the synthesis of β-citrylglutamate and has two splice variants with somewhat different relative distributions among brain, spinal cord and testis (Becker et al., 2010).
More stunning was the very recent report that NAAG synthetase II also mediates the synthesis of the tripeptide N-acetylaspartylglutamylglutamate (NAAG2) and that this peptide found in brain at 30–50-fold lower concentrations than NAAG (Lodder-Gadaczek et al, 2011). Due to the low concentration of NAAG2 in brain tissue samples and its poor chromatographic separation from NAAG, its resolution required tandem MS fragmentation. This important discovery significantly advances this field and suggests that, like other neuropeptides, NAAG and NAAG2 are members of a peptide family.
It is possible that previous studies in which antibodies were used to localize NAAG via immunohistochemistry or to assess its synaptic release may also have recognized NAAG2. It seems unlikely, however, that such cross reactivity, if it did occur, would have significantly affected the results, given the 1–2 orders of magnitude difference in concentration of the two peptides in the nervous system and the fact cells transfected with NAAG synthetase II synthesized several fold more NAAG than NAAG2. Rather, this discovery supports the conclusion that some of the cells in which NAAG has been localized also contain NAAG2, albeit at a much lower concentration. These issues will need to be clarified via the development of NAAG2 specific antibodies and assay of microdialysis samples using LS-MS.
NAAG and NAAG2 Model
A central question arising from the discovery of NAAG2 is its role relative to that of NAAG in nervous system function. Extracellular GCPII hydrolyzes NAAG2 to NAAG and glutamate and hydrolyzes NAAG to N-acetylaspartate, releasing a second glutamate (Loder et al., 2011). If NAAG2 is released synaptically, its levels also are likely to be elevated by NAAG peptidase inhibitors with the resulting elevated levels of activation of a still hypothetical NAAG2 receptor (Figure 1B). Despite the observation that NAAG synthetase II produces much more NAAG than NAAG2, it is possible that neurons expressing this enzyme produce NAAG solely to serve as the precursor for the synthesis of NAAG2. In contrast, neurons expressing NAAG synthetase I do not produce NAAG2 (Figure 1A), suggesting the possibility that there are distinct NAAG- and NAAG2-ergic neurons. Emerging from this model (Figure 1B) and from the traditions of neuropeptide peptide families is the hypothesis that NAAG2 also functions in neurotransmission with its likely receptor candidates being the mGluRs. This theory waits testing in neurons, astrocytes and transfected cells.
Important to understanding the role of NAAG and perhaps NAAG2 is the traditional model of peptide release under conditions of high frequency stimulation into the perisynaptic space with subsequent activation of receptors in this space. The presence of mGluR3 receptors as well as GCPII outside the immediate synaptic space supports this model of NAAG’s action as does the peptide’s seemingly redundant coexpression in some glutamatergic neurons. This model is based on the very active transport of glutamate from the synapse leaving NAAG to regulate perisynaptic neuronal and glial mGluR3. The codistribution of NAAG with other small amine transmitters supports a global role in biasing transmitter release to expand the dynamic range of the release process, particularly at high levels of synaptic activity. This is supported by the low basal levels of extracellular NAAG in the brain, the elevated levels induced by peptidase inhibitors in activated brain regions and the efficacy of these inhibitors in reducing extracellular levels of GABA as well as glutamate (Slusher et al., 1999: Zhao, 2001; Zhong et al., 2006) (Figure 1A).
Conclusion
Now nearly 50 years after its initial discovery, the peptide neurotransmitter NAAG remains much less widely recognized than other neuropeptides within the neuroscience community or the texts that are used to educate the newest generation of students. Nonetheless, understanding the functions of NAAG and NAAG2 via the peptidase inhibitors offers substantial promise as this knowledge is translated in preclinical models.
Acknowledgments
This work was supported by grants from NINDS (NS38080) and NIDA (MH79983) (JHN). JCM, KML and CPP were supported by a grant to Georgetown University from the Howard Hughes Medical Institute through the Precollege and Undergraduate Science Education Program. Nancy and Daniel Paduano also provided generous support for this research (JHN).
Abbreviations
- Dizocilpine
an open channel NMDA receptor antagonist
- LY341495
a group II mGluR antagonist
- mGluR
metabotropic glutamate receptor
- mGluR2
type 2 metabotropic glutamate receptor
- mGluR3
type 3 metabotropic glutamate receptor
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartylglutamate
- NAAG2
N-acetylaspartylglutamylglutamate
- NAAGS I
NAAG synthetase I
- NAAGS II
NAAG synthetase II
- PCP
phencyclidine – an open channel NMDA receptor antagonist
- TGF-β
Transforming Growth Factor Beta
- ZJ43, 2-PMPA, GPPI-5232 and 2-MPPA
NAAG peptidase inhibitors
Footnotes
Note: This review focused exclusively on NAAG research in the mammalian nervous system. For NAAG-related research in crayfish, see the very interesting work of A. K. Urazaev, R. M. Grossfeld, and E. M. Lieberman.
References
- Adedoyin MO, Vicini S, Neale JH. Endogenous N-acetylaspartylglutamate (NAAG) inhibits synaptic plasticity/transmission in the amygdala in a mouse inflammatory pain model. Molecular Pain. 2010;6:60–77. doi: 10.1186/1744-8069-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio M, Zurn A, Lohse M. Sensing G protein-coupled protein activation. Neuropharmacology. 2011;60:45–51. doi: 10.1016/j.neuropharm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Anderson KJ, Monaghan DT, Cangro CB, Namboodiri MA, Neale JH, Cotman CW. Localization of N-acetylaspartylglutamate-like immunoreactivity in selected areas of the rat brain. Neurosci Lett. 1986;72:14–20. doi: 10.1016/0304-3940(86)90610-5. [DOI] [PubMed] [Google Scholar]
- Arun P, Madhavarao CN, Moffett JR, Namboodiri MA. Regulation of N-acetylaspartate and N-acetylaspartylglutamate biosynthesis by protein kinase activators. J Neurochem. 2006;98:2034–2942. doi: 10.1111/j.1471-4159.2006.04068.x. [DOI] [PubMed] [Google Scholar]
- Bacich DJ, Pinto JT, Tong WP, Heston WD. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm Genome. 2001;12:117–123. doi: 10.1007/s003350010240. [DOI] [PubMed] [Google Scholar]
- Bacich DJ, Ramadan E, O’Keefe DS, Bukhari N, Wegorzewska I, Ojeifo O, Olszewski R, Wrenn CC, Bzdega T, Wroblewska B, Heston WD, Neale JH. Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate. J Neurochem. 2002;83:20–29. doi: 10.1046/j.1471-4159.2002.01117.x. [DOI] [PubMed] [Google Scholar]
- Bacich DJ, Wozniak KM, Lu XC, O’Keefe DS, Callizot N, Heston WD, Slusher BS. Mice lacking glutamate carboxypeptidase II are protected from peripheral neuropathy and ischemic brain injury. J Neurochem. 2005;95:314–323. doi: 10.1111/j.1471-4159.2005.03361.x. [DOI] [PubMed] [Google Scholar]
- Barinka C, Rovenská M, Mlcochová P, Hlouchová K, Plechanovová A, Majer P, Tsukamoto T, Slusher BS, Konvalinka J, Lubkowski J. Structural insight into the pharmacophore pocket of human glutamate carboxypeptidase II. J Med Chem. 2007;250:3267–3273. doi: 10.1021/jm070133w. [DOI] [PubMed] [Google Scholar]
- Becker I, Lodder J, Gieselmann V, Eckhardt M. Molecular characterization of N-acetylaspartylglutamate synthetase. J Biol Chem. 2010;285:29156–29164. doi: 10.1074/jbc.M110.111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent-Spillson A, Russell JW. Metabotropic glutamate receptor 3 protects neurons from glucose-induced oxidative injury by increasing intracellular glutathione concentration. J Neurochem. 2007;101:342–354. doi: 10.1111/j.1471-4159.2006.04373.x. [DOI] [PubMed] [Google Scholar]
- Berent-Spillson A, Robinson AM, Golovoy D, Slusher B, Rojas C, Russell JW. Protection against glucose-induced neuronal death by NAAG and GCP II inhibition is regulated by mGluR3. J Neurochem. 2004;89:90–99. doi: 10.1111/j.1471-4159.2003.02321.x. [DOI] [PubMed] [Google Scholar]
- Berger UV, Schwab ME. N-acetylated alpha-linked acidic dipeptidase may be involved in axon-Schwann cell signalling. J Neurocytol. 1996;25:499–512. doi: 10.1007/BF02284818. [DOI] [PubMed] [Google Scholar]
- Berger UV, Luthi-Carter R, Passani LA, Elkabes S, Black I, Konradi C, Coyle JT. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415:52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Coyle JT, Tsai G, Greene RW. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharm. 2005;30:7–16. doi: 10.1038/sj.npp.1300559. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduced NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100:346–57. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Schield D. Glutamate and N-acetylaspartylglutamate block HVA calcium currents in frog olfactory bulb interneurons via a mGluR2/3-like receptor. J Neurophysiol. 1996;76:2089–2092. doi: 10.1152/jn.1996.76.3.2089. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Robinson MB, Guarda AS, Coyle JT. A re-examination of the interaction of N-acetyl-L-aspartyl-L-glutamate with a subpopulation of rat brain membrane L-[3H]glutamate binding sites. Eur J Pharmacol. 1988;151:419–26. doi: 10.1016/0014-2999(88)90538-9. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Casabona G, Copani A, Caciagli F, Nicoletti F. Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta. J Neurosci. 1998;18:9594–9600. doi: 10.1523/JNEUROSCI.18-23-09594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdega T, Turi T, Wroblewska B, She D, Chung HS, Kim H, Neale JH. Molecular cloning of a peptidase against N-acetylaspartylglutamate from a rat hippocampal cDNA library. J Neurochem. 1997;69:2270–2277. doi: 10.1046/j.1471-4159.1997.69062270.x. [DOI] [PubMed] [Google Scholar]
- Bzdega T, Crowe SL, Ramadan ER, Sciarretta KH, Olszewski RT, Ojeifo OA, Rafalski VA, Wroblewska B, Neale JH. The cloning and characterization of a second brain enzyme with NAAG peptidase activity. J Neurochem. 2004;89:627–635. doi: 10.1111/j.1471-4159.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- Cangro CB, Namboodiri MA, Sklar LA, Corigliano-Murphy A, Neale JH. Immunohistochemistry and biosynthesis of N-acetylaspartylglutamate in spinal sensory ganglia. J Neurochem. 1987;49:1579–1588. doi: 10.1111/j.1471-4159.1987.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501:780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- Carozzi VA, Chiorazzi A, Canta A, Lapidus RG, Slusher BS, Wozniak KM, Cavaletti G. Glutamate carboxypeptidase inhibition reduces the severity of chemotherapy-induced peripheral neurotoxicity in rat. Neurotox Res. 2010;17:380–391. doi: 10.1007/s12640-009-9114-1. [DOI] [PubMed] [Google Scholar]
- Carpenter KJ, Sen S, Matthews EA, Flatters SL, Wozniak KM, Slusher BS, Dickenson AH. Effects of GCP-II inhibition on responses of dorsal horn neurones after inflammation and neuropathy: an electrophysiological study in the rat. Neuropeptides. 2003;37:298–306. doi: 10.1016/j.npep.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci U S A. 1996;93:749–753. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiechio S, Copani A, De Petris L, Morales ME, Nicoletti F, Gereau RW., 4th Transcriptional regulation of metabotropic glutamate receptor 2/3 expression by the NF-kappaB pathway in primary dorsal root ganglia neurons: a possible mechanism for the analgesic effect of L-acetylcarnitine. Mol Pain. 2006;2:20–29. doi: 10.1186/1744-8069-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Yao Y, Blake TJ, Hampson DR, Johnson EC. The neuroactive peptide N-acetylaspartylglutamate is not an agonist at the metabotropic glutamate receptor subtype 3 of metabotropic glutamate receptor. J Pharmacol Exp Ther. 2009;330:212–219. doi: 10.1124/jpet.109.152553. [DOI] [PubMed] [Google Scholar]
- Collard F, Stroobant V, Lamosa P, Kapanda CN, Lambert DM, Muccioli GG, Poupaert JH, Opperdoes F, Van Schaftingen E. Molecular identification of N-acetylaspartylglutamate synthase and beta-citrylglutamate synthase. J Biol Chem. 2010;285:29826–29833. doi: 10.1074/jbc.M110.152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F, Bruno V. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 2007;27:8297–8308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis. 1997;4:231–238. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- Curatolo A, D’Arcangelo P, Lino A, Brancati A. Distribution of N-Acetyl-Aspartic and N-Acetyl-Aspartyl-Glutamic Acids in nervous tissue. J Neurochem. 1965;12:339–342. doi: 10.1111/j.1471-4159.1965.tb06771.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio M, Cuomo L, Battaglia G, Ngomba RT, Storto M, Kingston AE, Orzi F, De Blasi A, Di Iorio P, Nicoletti F, Bruno V. Neuroprotection mediated by glial group-II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J Neurochem. 2001;78:435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- Forloni G, Grzanna R, Blakely RD, Coyle JT. Co-localization of N-acetyl-aspartyl-glutamate in central cholinergic, noradrenergic, and serotonergic neurons. Synapse. 1987;1:455–460. doi: 10.1002/syn.890010509. [DOI] [PubMed] [Google Scholar]
- Fricker AC, Mok MH, de la Flor R, Shah AJ, Woolley M, Dawson LA, Kew JN. Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDAR. Neuropharmacology. 2009;56:1060–1067. doi: 10.1016/j.neuropharm.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fuhrman S, Palkovits M, Cassidy M, Neale JH. The regional distribution of N-acetylaspartylglutamate (NAAG) and peptidase activity against NAAG in the rat nervous system. J Neurochem. 1994;62:275–281. doi: 10.1046/j.1471-4159.1994.62010275.x. [DOI] [PubMed] [Google Scholar]
- Gehl LM, Saab OH, Bzdega T, Wroblewska B, Neale JH. Biosynthesis of NAAG by an enzyme-mediated process in rat central nervous system neurons and glia. J Neurochem. 2004;90:989–997. doi: 10.1111/j.1471-4159.2004.02578.x. [DOI] [PubMed] [Google Scholar]
- Han L, Picker JD, Schaevitz LR, Tsai G, Feng J, Jiang Z, Chu HC, Basu AC, Berger-Sweeney J, Coyle JT. Phenotypic characterization of mice heterozygous for a null mutation of glutamate carboxypeptidase II. Synapse. 2009;63:625–635. doi: 10.1002/syn.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlouchova K, Barinka C, Konvalinka J, Lubkowski J. Structural insight into the evolutionary and pharmacologic homology of glutamate carboxypeptidases II and III. FEBS J. 2009;276:4448–4462. doi: 10.1111/j.1742-4658.2009.07152.x. [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk K, Hamilton NB, Wade A, Káradóttir R, Attwell D. The effect of N-acetyl-aspartyl-glutamate and N-acetyl-aspartate on white matter oligodendrocytes. Brain. 2009;132:1496–508. doi: 10.1093/brain/awp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, Wrobel M, Kos T, Wojcikowski J, Daniel WA, Wozniak KM, Slusher BS, Popik P. 2-MPPA, a selective glutamate carboxypeptidase II inhibitor, attenuates morphine tolerance but not dependence in C57/Bl mice. Psychopharmacology (Berl) 2005;183:275–284. doi: 10.1007/s00213-005-0182-5. [DOI] [PubMed] [Google Scholar]
- Lea PM, Wroblewska B, Sarvey JM, Neale JH. β-NAAG rescues LTP from blockade by NAAG in the rat dentate gyrus via the type 3 metabotropic glutamate receptor. J Neurophysiol. 2001;85:1097–1106. doi: 10.1152/jn.2001.85.3.1097. [DOI] [PubMed] [Google Scholar]
- Lodder-Gadaczek J, Becker I, Gieselmann V, Wang-Eckhardt L, Eckhardt M. N-Acetylaspartylglutamate synthetase-II synthesizes N-acetylaspartyl-glutamylglutamate. J Biol Chem. 2011;286:16693–16706. doi: 10.1074/jbc.M111.230136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi G, Vicini S, Neale JH. NAAG fails to antagonize synaptic and extrasynaptic NMDA receptors in cerebellar granule neurons. Neuropharmacol. 2004;46:490–496. doi: 10.1016/j.neuropharm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Berger UV, Barczak AK, Enna M, Coyle JT. Isolation and expression of a rat brain cDNA encoding glutamate carboxypeptidase II. Proc Natl Acad Sci U S A. 1998;95:3215–3220. doi: 10.1073/pnas.95.6.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E, Kakimoto Y, Sano I. Identification of N-acetyl-alpha-aspartylglutamic acid in the bovine brain. J Neurochem. 1966;13:999–1003. doi: 10.1111/j.1471-4159.1966.tb10297.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Neale JH. N-Acetylaspartylglutamate (NAAG) IS an agonist at mGluR3 in Vivo and in Vitro. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07380.x. invited commentary, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477–484. doi: 10.1016/j.tips.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Metabotropic glutamate receptors: novel targets for pain relief. Expert Rev Neurother. 2001;1:207–224. doi: 10.1002/1615-9861(200102)1:2<207::AID-PROT207>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski RT, Bukhari N, Zhou J, Kozikowski AP, Wroblewski JT, Shamimi-Noori S, Wroblewska B, Bzdega T, Vicini S, Barton FB, Neale JH. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89:876–85. doi: 10.1111/j.1471-4159.2004.02358.x. [DOI] [PubMed] [Google Scholar]
- Olszewski RT, Bzdega T, Neale JH. The metabotropic glutamate receptor type 3 (mGluR3), not mGluR2, mediates the efficacy of NAAG peptidase inhibition in a PCP model of schizophrenia. 2011 Submitted. [Google Scholar]
- Olszewski RT, Wegorzewska MM, Monteiro AC, Krolikowski KA, Zhou J, Kozikowski AP, Long K, Mastropaolo J, Deutsch SI, Neale JH. Phencyclidine and dizocilpine induced behaviors reduced by N-acetylaspartylglutamate peptidase inhibition via metabotropic glutamate receptors. Biol Psychiatry. 2008;63:86–91. doi: 10.1016/j.biopsych.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XQ, Li J, Gardner EL, Ashby CR, Jr, Thomas A, Wozniak K, Slusher BS, Xi ZX. Oral administration of the NAALADase inhibitor GPI-5693 attenuates cocaine-induced reinstatement of drug-seeking behavior in rats. Eur J Pharmacol. 2010;627:156–161. doi: 10.1016/j.ejphar.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profaci CP, Krolikowski KA, Olszewski RT, Neale JH. Group II mGluR agonist LY354740 and NAAG peptidase inhibitor effects on prepulse inhibition in PCP and D-amphetamine models of schizophrenia. Psychopharmacology (Berl) 2011 Feb 16; doi: 10.1007/s00213-011-2200-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveros N, Orrego F. A study of possible excitatory effects of N-acetylaspartylglutamate in different in vivo and in vitro brain preparations. Brain Res. 1984;299:393–395. doi: 10.1016/0006-8993(84)90727-3. [DOI] [PubMed] [Google Scholar]
- Saito O, Aoe T, Kozikowski A, Sarva J, Neale JH, Yamamoto T. Ketamine and N-acetylaspartylglutamate peptidase inhibitor exert analgesia in bone cancer pain. Can J Anaesth. 2006;53:891–898. doi: 10.1007/BF03022832. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, Wozniak KM, Slusher BS, Keller A. GCP II (NAALADase) inhibition suppresses mossy fiber-CA3 synaptic neurotransmission by a presynaptic mechanism. J Neurophysiol. 2004;91:182–193. doi: 10.1152/jn.00465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna V, Pintus G, Roggio AM, Punzoni S, Posadino AM, Arca A, Marceddu S, Bandiera P, Uzzau S, Sechi M. Targeted biocompatible nanoparticles for the delivery of (-)-epigallocatechin 3-gallate to prostate cancer cells. J Med Chem. 2011;10:1321–1332. doi: 10.1021/jm1013715. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-alpha-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000;38:161–166. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- Spillson AB, Russell JW. Metabotropic glutamate receptor regulation of neuronal cell death. Exp Neurol. 2003;184:S97–105. doi: 10.1016/j.expneurol.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Robinson MB, Tsai G, Simmons ML, Richards SS, Coyle JT. Rat brain N-acetylated alpha-linked acidic dipeptidase activity. Purification and immunologic characterization. J Biol Chem. 1990;265:21297–21301. [PubMed] [Google Scholar]
- Takatsu Y, Fujita Y, Tsukamoto T, Slusher BS, Hashimoto K. Orally active glutamate carboxypeptidase II inhibitor 2-MPPA attenuates dizocilpine-induced prepulse inhibition deficits in mice. Brain Res. 2011;1371:82–86. doi: 10.1016/j.brainres.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Liu W, Olkowski JL, Tang Z, Lin Q, Lu XC, Slusher BS. Neuroprotection mediated by glutamate carboxypeptidase II (NAALADase) inhibition requires TGF-beta. Eur J Pharmacol. 2001a;430:33–40. doi: 10.1016/s0014-2999(01)01239-0. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Olkowski JL, Slusher BS. Neuroprotection afforded by NAAG and NAALADase inhibition requires glial cells and metabotropic glutamate receptor activation. Eur J Pharmacol. 2001b;426:35–38. doi: 10.1016/s0014-2999(01)01198-0. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Wozniak KM, Tsukamoto T, Calvin D, Wu Y, Rojas C, Vornov J, Slusher BS. Glutamate carboxypeptidase II (NAALADase) inhibition as a novel therapeutic strategy. Adv Exp Med Biol. 2006;576:327–337. doi: 10.1007/0-387-30172-0_24. [DOI] [PubMed] [Google Scholar]
- Tieman SB, Cangro CB, Neale JH. N-acetylaspartylglutamate immunoreactivity in neurons of the cat’s visual system. Brain Res. 1987;420:188–193. doi: 10.1016/0006-8993(87)90259-9. [DOI] [PubMed] [Google Scholar]
- Tsai G, Dunham KS, Drager U, Grier A, Anderson C, Collura J, Coyle JT. Early embryonic death of glutamate carboxypeptidase II (NAALADase) homozygous mutants. Synapse. 2003;50:285–292. doi: 10.1002/syn.10263. [DOI] [PubMed] [Google Scholar]
- Tsai G, Forloni G, Robinson MB, Stauch BL, Coyle JT. Calcium-dependent evoked release of N-[3H]acetylaspartylglutamate from the optic pathway. J Neurochem. 1988;51:1956–1959. doi: 10.1111/j.1471-4159.1988.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today. 2007;12:767–776. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Westbrook GL. Excitatory synaptic transmission in cultures of rat olfactory bulb. J Neurophysiol. 1990;64:598–606. doi: 10.1152/jn.1990.64.2.598. [DOI] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML, Namboodiri MA, Neale JH. High concentrations of N-acetylaspartylglutamate (NAAG) selectively activate NMDA receptors on mouse spinal cord neurons in cell culture. J Neurosci. 1986;6:3385–3392. doi: 10.1523/JNEUROSCI.06-11-03385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Neale JH. Calcium-dependent release of N-acetylaspartylglutamate from retinal neurons upon depolarization. Brain Res. 1988a;475:151–155. doi: 10.1016/0006-8993(88)90209-0. [DOI] [PubMed] [Google Scholar]
- Williamson LC, Neale JH. Ultrastructural localization of N-acetylaspartylglutamate in synaptic vesicles of retinal neurons. Brain Res. 1988b;456:375–381. doi: 10.1016/0006-8993(88)90243-0. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Santi MR, Neale JH. N-acetylaspartylglutamate activates cyclic-AMP coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia. 1998;24:172–180. doi: 10.1002/(sici)1098-1136(199810)24:2<172::aid-glia2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wegorzewska IN, Bzdega T, Olszewski RT, Neale JH. Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J Neurochem. 2006;96:1071–1077. doi: 10.1111/j.1471-4159.2005.03569.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-Acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–182. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Saab O, Neale JH. N-acetylaspartylglutamate inhibits forskolin-stimulated cyclic AMP levels via a metabotropic glutamate receptor in cultured cerebellar granule cells. J Neurochem. 1993;61:943–948. doi: 10.1111/j.1471-4159.1993.tb03606.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wegorzewska IN, Bzdega T, Neale JH. Type 2 metabotropic glutamate receptor (mGluR2) fails to negatively couple to cGMP in stably transfected cells. Neurochem Int. 2011;58:176–179. doi: 10.1016/j.neuint.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Kiyatkin M, Li X, Peng XQ, Wiggins A, Spiller K, Li J, Gardner EL. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacol. 2010a;58:304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010b;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Sakashita Y. Spinal N-acetyl-alpha-linked acidic dipeptidase (NAALADase) inhibition attenuates mechanical allodynia induced by paw carrageenan injection in the rat. Brain Res. 2001;909:138–144. doi: 10.1016/s0006-8993(01)02650-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hirasawa S, Wroblewska B, Grajkowska E, Zhou J, Kozikowski A, Wroblewski J, Neale JH. Antinociceptive effects of N-acetylaspartylglutamate (NAAG) peptidase inhibitors ZJ-11, ZJ-17 and ZJ-43 in the rat formalin test and in the rat neuropathic pain model. Eur J Neurosci. 2004;20:483–494. doi: 10.1111/j.1460-9568.2004.03504.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kozikowski A, Zhou J, Neale JH. Intracerebroventricular administration of N-acetylaspartylglutamate (NAAG) peptidase inhibitors is analgesic in inflammatory pain. Mol Pain. 2008;4:31. doi: 10.1186/1744-8069-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Saito O, Aoe T, Bartolozzi A, Sarva J, Zhou J, Kozikowski A, Wroblewska B, Bzdega T, Neale JH. Local administration of N-acetylaspartylglutamate (NAAG) peptidase inhibitors is analgesic in peripheral pain in rats. Eur J Neurosci. 2007;25:147–158. doi: 10.1111/j.1460-9568.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Gereau RW., 4th Peripheral group II metabotropic glutamate receptors mediate endogenous anti-allodynia in inflammation. Pain. 2003;106:411–417. doi: 10.1016/j.pain.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Zaheer A, Cho SY, Pomper MG. New agents and techniques for imaging prostate cancer. J Nucl Med. 2009;50:1387–1390. doi: 10.2967/jnumed.109.061838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Murakawa Y, Wozniak KM, Slusher B, Sima AA. The preventive and therapeutic effects of GCPII (NAALADase) inhibition on painful and sensory diabetic neuropathy. J Neurol Sci. 2006;247:217–223. doi: 10.1016/j.jns.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Zhang W, Slusher B, Murakawa Y, Wozniak KM, Tsukamoto T, Jackson PF, Sima AA. GCPII (NAALADase) inhibition prevents long-term diabetic neuropathy in type 1 diabetic BB/Wor rats. J Neurol Sci. 2002;194:21–28. doi: 10.1016/s0022-510x(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ramadan E, Cappiello M, Wroblewska B, Bzdega T, Neale JH. NAAG inhibits KCl-induced [(3)H]-GABA release via mGluR3, cAMP, PKA and L-type calcium conductance. Eur J Neurosci. 2001;13:340–346. [PubMed] [Google Scholar]
- Zhong C, Zhao X, Sarva J, Kozikowski A, Neale JH, Lyeth BG. NAAG peptidase inhibitor reduces acute neuronal degeneration and astrocyte damage following lateral fluid percussion TBI in rats. J Neurotrauma. 2005;22:266–276. doi: 10.1089/neu.2005.22.266. [DOI] [PubMed] [Google Scholar]
- Zhong C, Zhao X, Van KC, Bzdega T, Smyth A, Zhou J, Kozikowski AP, Jiang J, O’Connor WT, Berman RF, Neale JH, Lyeth BG. NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J Neurochem. 2006;97:1015–1025. doi: 10.1111/j.1471-4159.2006.03786.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4:1015–1026. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- Zollinger M, Amsler U, Do KQ, Streit P, Cuénod M. Release of N-acetylaspartylglutamate on depolarization of rat brain slices. J Neurochem. 1988;51:1919–1923. doi: 10.1111/j.1471-4159.1988.tb01178.x. [DOI] [PubMed] [Google Scholar]