Abstract
We introduced multiple abasic sites (AP sites) in the chromosome of repair-deficient mutants of Escherichia coli, in vivo, by expressing engineered variants of uracil-DNA glycosylase that remove either thymine or cytosine. After introduction of AP sites, deficiencies in base excision repair (BER) or recombination were associated with strongly enhanced cytotoxicity and elevated mutation frequencies, selected as base substitutions giving rifampicin resistance. In these strains, increased fractions of transversions and untargeted mutations were observed. In a recA mutant, deficient in both recombination and translesion DNA synthesis (TLS), multiple AP sites resulted in rapid cell death. Preferential incorporation of dAMP opposite a chromosomal AP site (‘A rule’) required UmuC. Furthermore, we observed an ‘A rule-like’ pattern of spontaneous mutations that was also UmuC dependent. The mutation patterns indicate that UmuC is involved in untargeted mutations as well. In a UmuC-deficient background, a preference for dGMP was observed. Spontaneous mutation spectra were generally strongly dependent upon the repair background. In conclusion, BER, recombination and TLS all contribute to the handling of chromosomal AP sites in E.coli in vivo.
Keywords: AP-endonuclease/chromosomal abasic site/recombination/translesion DNA synthesis/UmuC
Introduction
Abasic sites (AP sites) in DNA may arise spontaneously by hydrolytic loss of normal or alkylated bases, or by removal of damaged or inappropriate bases by N-gly cosylases (Friedberg et al., 1995). The measured spontaneous depurination rate in double-stranded DNA (Lindahl and Nyberg, 1972) translates to ∼12 events per day in Escherichia coli, while depyrimidination is some 102-fold less frequent. Recent information from knockout mice deficient in 8-oxoguanine-DNA glycosylase (Ogg1) (Klungland et al., 1999) or uracil-DNA glycosylase (Ung) (Nilsen et al., 2000) indicates that the number of AP sites generated by DNA glycosylases may exceed the number resulting from spontaneous depurination, at least in mammals. Thus, AP sites are likely to represent the most common of DNA lesions. In addition to being cytotoxic by stalling DNA replication and transcription, AP sites may promote mutagenesis and result in substitution or frameshift mutations (Loeb and Preston, 1986; Woodgate and Levine, 1996; Rothwell and Hickson, 1997). AP sites generated by DNA glycosylases are shielded by the DNA glycosylase that remains bound to the AP site. These AP sites are, therefore, likely to be less cytotoxic and mutagenic than the AP sites resulting from spontaneous depurination (Parikh et al., 1998; Waters et al., 1999). The general steps of base excision repair (BER) are considered a major mechanism for the repair of AP sites. BER is initiated by an AP-endonuclease, which catalyses the incision of DNA at AP sites, preparing the DNA for subsequent excision, repair synthesis and ligation. BER is believed to be essentially error free (Friedberg et al., 1995). In E.coli, exonuclease III, encoded by the xthA gene, accounts for ∼90% of the AP-endonuclease activity, while endonuclease IV, encoded by the nfo gene, normally contributes <10% (Demple et al., 1986).
Translesion DNA synthesis (TLS) by recently identified DNA polymerases represents a second identified defence mechanism against AP sites. In E.coli, these polymerases are the SOS-induced UmuC protein in complex with activated UmuD (UmuD2′C or pol V) (Reuven et al., 1999; Tang et al., 1999) and the UmuC homologue encoded by the dinB gene (DinB or pol IV) (Wagner et al., 1999). In vitro, pol V rapidly bypasses AP sites, and some other DNA lesions, and replicates undamaged DNA with a high error rate (10–3–10–4), while pol IV bypasses AP sites less efficiently and replicates undamaged DNA with 5- to 10-fold higher fidelity. Pol V has a 2- to 3-fold preference for incorporation of dAMP over dGMP opposite an AP site (Reuven et al., 1999; Tang et al., 1999, 2000), and may therefore be the polymerase responsible for the ‘A rule’ (Lawrence et al., 1990; Strauss, 1991). Due to the low fidelity in in vitro replication, pol V is believed to be involved in untargeted mutagenesis, and the mutation spectra are dominated by transversions (Maor-Shoshani et al., 2000; Tang et al., 2000). However, over-expression of pol IV in E.coli is also reported to cause untargeted mutations, on phage λ (Brotcorne-Lannoye and Maenhaut-Michel, 1986) and F′lac plasmids (mainly as a –1 G change within a G6 cluster sequence, but also base substitutions) (Kim et al., 1997).
Less is known about other mechanisms for the handling of AP sites. However, it has been reported that the UvrABC complex, which is central in nucleotide excision repair (NER) in E.coli, can also remove AP sites from DNA (Lin and Sancar, 1989; Snowden et al., 1990). A role for NER in repair of AP sites was also recently demonstrated in yeast (Torres-Ramos et al., 2000). In addition, it would seem likely that recombination events are involved in repair of AP sites. Thus, reactivation of stalled replication forks may represent a main function for the recombination pathway. Recombination is believed to be non-mutagenic under normal growth conditions (Cox, 1999; Cox et al., 2000). However, stalled replication, with the ultimate formation of single-stranded regions in DNA, activates RecA and thereby also induces the SOS response. The SOS response activates expression of at least 30 genes (Fernandez De Henestrosa et al., 2000), many of which are essential in NER, TLS and recombination. One of the SOS-inducible proteins, RuvA, is involved in the late steps of recombination, and in complex with RuvB it acts as a helicase that translocates Holliday junctions (Friedberg et al., 1995). The SOS response increases the mutation frequency in bacteria, both after exposure to UV light (Friedberg et al., 1995) and in the absence of mutagenic treatment, in strains constitutively expressing activated RecA protein (Miller and Low, 1984; Fijalkowska et al., 1997).
Previous studies on AP site repair have been performed with oligonucleotides in vitro or double-stranded and single-stranded plasmids in vivo. Now, engineered DNA glycosylases that remove either thymine (thymine-DNA glycosylase, TDG) or cytosine (cytosine-DNA glycosylase, CDG) from DNA (Kavli et al., 1996) have enabled studies on the biological consequences of multiple AP sites in the chromosomal DNA in vivo. We have explored the importance of BER, recombination and TLS in the handling of AP sites by analysing the effects of CDG and TDG in the respective repair-deficient E.coli mutants. To limit the complexity, in this study we have concentrated on substitution mutations in the chromosomal RNA polymerase-β-encoding gene.
Results
The specificities of TDG and CDG in vitro
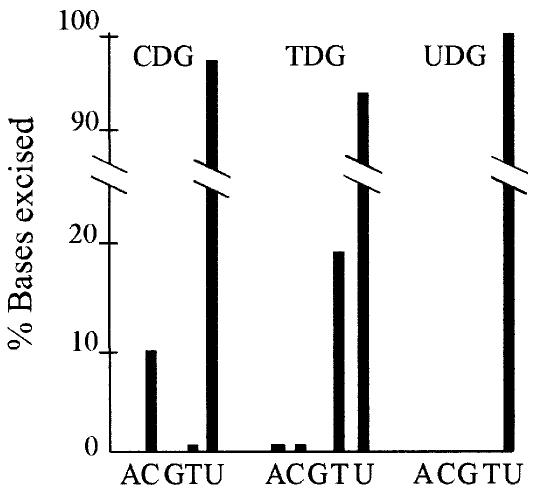
Engineered variants of human uracil-DNA glycosylase (UDG) that remove either thymine (TDG) or cytosine (CDG), expressed from an inducible vector, have been purified and described previously (Kavli et al., 1996). Briefly, replacing active site Asn204 (amide side chain) with Asp (carboxyl side chain) allows binding and excision of cytosine. Replacing Tyr147 with Ala allows binding and excision of thymine that is otherwise prevented from entering the catalytic pocket due to a steric clash between the bulky side chain of tyrosine and the 5-methyl group of thymine. The specificities of purified CDG and TDG were tested on double-stranded DNA substrates containing radiolabelled adenine, thymine, guanine, cytosine or uracil. Figure 1 shows that among the normal DNA bases, CDG has a strong preference for cytosine in DNA, whereas TDG has a very strong preference for thymine, although both release uracil more efficiently. In contrast, the wild-type enzyme (UDG) remains very specific for uracil in DNA. Although not evident from Figure 1, UDG activity of the wild-type enzyme is some 103-fold higher than for CDG and TDG (Kavli et al., 1996). Estimated from 3 h incubations, TDG has a 106-fold preference for thymine over cytosine, and CDG has a 22-fold preference for cytosine over thymine. After prolonged incubation (20 h), we observed that CDG and TDG also excised tiny amounts of the other bases (data not shown). Previously, we have demonstrated that over-expression of human UDG is not cytotoxic and does not change the frequency of substitution mutations in ung+ E.coli cells. This was confirmed in the present study (data not shown). Furthermore, we have previously demonstrated that human UNG (catalytic domain) fully complements an E.coli ung– strain, by lowering spontaneous mutation frequencies to wild-type levels. This was expected considering the very high degree of conservation of the enzymes (Olsen et al., 1991, Kavli et al., 1996). In addition, over-expression of human UNG did not change the mutation spectrum significantly, compared with the spontaneous mutation spectrum in the wild type (Figure 3). Therefore, the mutagenic effects of TDG and CDG are unlikely to be attributed to their residual UDG activities or to effects other than induction of AP sites. This is of importance since another E.coli DNA glycosylase, AlkA, was shown to remove normal bases and induce mutations when over-expressed (Berdal et al., 1998).
Fig. 1. Specificity of engineered DNA glycosylases. In vitro excision of adenine (A), cytosine (C), guanine (G), thymine (T) and uracil (U) by CDG, TDG and UDG, from double-stranded DNA after incubation for 3 h.
Fig. 3. Mutation spectra in the rpoB gene (RNA polymerase β) within codons 509–574 from rifR colonies, spontaneous (VEC) and after expression of UDG (only wild type), CDG or TDG. Codon positions are given on the X axis, and the number of mutations are given on the Y axis. The n value at the top left corner gives the total number of sequences. The mutations are classified as GC transitions (black bars), AT transitions (white bars), GC transversions (checked bars) and AT transversions (striped bars). The type of transitions and transversions, given as numbers as well as percentages, are specified at the top right corner of the spectra. In the recA mutant, only the spontaneous mutation spectrum was analysed.
TDG increases the number of unprocessed chromosomal AP sites in vivo
The number of detectable AP sites will increase if their rate of formation is higher than their rate of processing. To examine whether the engineered enzymes increased the number of unprocessed chromosomal AP sites in vivo, we quantified AP sites before and after TDG or CDG induction in the wild-type strain (AB1157) using an aldehyde reactive probe, ARP (Nakamura et al., 1998; Nakamura and Swenberg, 1999). We found that the number of AP sites increased >20-fold above the detection limit (3 AP sites/106 base pairs) after induced TDG expression for 2.5 h, to 6.1 AP sites per 105 base pairs, or ∼240 AP sites per chromosome. The number of AP sites in chromosomal DNA from bacteria carrying empty vector or induced CDG vector was below the detection limits using this method. However, DNA was fragmented after induction of CDG, as well as after TDG induction, most likely reflecting processing subsequent to formation of AP sites. The ARP method only detects unprocessed AP sites present at any time in DNA, and not the total number of AP sites introduced by CDG or TDG.
Cytotoxic effects of multiple AP sites in E.coli are enhanced in cells deficient in recombination, TLS or BER
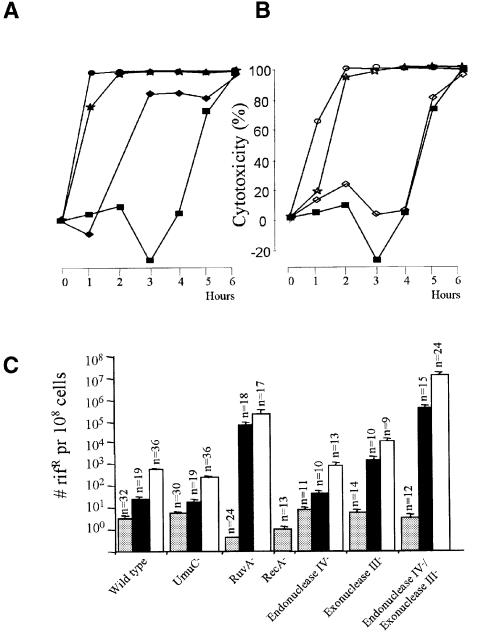
We wanted to explore the relative importance of recombination, TLS and BER in the handling of AP sites. Generally, AP sites generated by CDG or TDG resulted in reduced cell growth. In the wild-type strain, we observed a cytotoxic effect after 3 h of TDG expression (Figure 2A). An enhanced cytotoxic effect of TDG was observed in the UmuC-deficient strain. The RuvA-deficient strain was even more sensitive to AP sites, with RecA-deficient cells being ultra sensitive (Figure 2A). Increasing deficiencies in BER also enhanced cytotoxicity (Figure 2B). Although loss of endonuclease IV activity (minor AP-endonuclease) did not significantly affect the cytotoxicity of AP sites induced by TDG, deficiency in the exonuclease III activity (major AP-endonuclease) had a strong effect. The cytotoxic effect of TDG was further enhanced in the double mutant (endonuclease IV–/exonuclease III–). These experiments were carried out 2–4 times with equivalent results. The order of sensitivity of the strains was always as reported in Figure 2A and B. In addition, similar results were obtained with these strains after expression of TDG and CDG from a toluic acid-inducible vector (Blatny et al., 1997) (data not shown). CDG was generally less cytotoxic than TDG in all strains in this study (data not shown). The levels of expressed protein from the inducible vector were analysed by assaying human UDG activity in all the strains (containing UDG vector). Expressed human UDG accounted for ∼1% of total cell protein, and no significant differences between the strains were found (data not shown). Thus, different expression levels are unlikely to explain the differences in cytotoxicity among the various strains. A low level expression of TDG activity from non-induced vector, insufficient to cause a detectable increase in chromosomal AP sites, lowered the viability of the RuvA-deficient strain slightly and the viability of the RecA-deficient strain moderately, while the wild-type strain was unaffected (data not shown). This may indicate that recombination is important in AP site repair also when BER is not saturated. The higher sensitivity of the RecA-deficient strain suggests the possibility that repair factors dependent on RecA, such as UmuC or DinB, may also be required after relatively low level induction of AP sites.
Fig. 2. The cytotoxic effect of TDG expression in wild-type E.coli (filled squares) compared with expression in strains deficient in (A) UmuC (filled diamonds), RuvA (filled stars) and RecA (filled circles), or (B) endonuclease IV (open diamonds), exonuclease III (open stars) or endonuclease IV/exonuclease III (open circles). (C) Mutation frequency (rifR bacteria per 108 ampR bacteria) spontaneously (grey bars) and after induction of AP sites with CDG (black bars) or TDG (white bars). The n values indicate the number of experiments for each column and bars indicate standard deviations.
The present results demonstrate that BER is important, but not sufficient for the handling of multiple chromosomal AP sites in vivo, and that UmuC-dependent TLS and recombinational repair are also required. Furthermore, there is an essentially absolute requirement for RecA, for cellular survival after induction of multiple AP sites.
Chromosomal AP sites are highly mutagenic in E.coli strains deficient in recombination or BER
Frequencies of substitution mutations, spontaneous and after the formation of AP sites by CDG or TDG, were scored as the number of bacteria that gained rifampicin resistance (rifR) while retaining their ampicillin resistance (ampR) per 108 cells. The rifR method selects for certain missense mutations, whereas nonsense mutations and deletions/insertions causing frameshifts are not detected (Severinov et al., 1993). TDG was generally more mutagenic than CDG (Figure 2C), probably because it is more active than CDG on dsDNA (Figure 1). The spontaneous mutation frequencies were essentially similar for wild-type cells, the UmuC-deficient strain and the single or double AP-endonuclease mutants. However, the RuvA- and RecA-deficient strains had 10- and 3-fold lower spontaneous mutation frequencies, respectively, when compared with wild type (Figure 2C, note the logarithmic scale of the Y axis). Since RecA-deficient cells are defective in the induction of error-prone pol V (UmuC) and pol IV (DinB), we did not expect a higher degree of mutation suppression in the RuvA-deficient strain than in the RecA-deficient strain. Nevertheless, these data indicate that recombination processes may contribute to generation of spontaneous mutations.
Expression of CDG and TDG enhanced the mutation frequencies 7- and 150-fold, respectively, in the wild-type strain, compared with 4- and 51-fold in the UmuC-deficient strain. This 2- to 3-fold lower, but substantial, yield of mutants in UmuC-deficient cells indicates that UmuC (pol V) is important but not essential for generation of mutations at AP sites. Thus, UmuC increases survival at the cost of moderately enhanced chromosomal mutation frequencies. In the RuvA-deficient strain, mutation frequencies caused by multiple AP sites were 102–103-fold higher than in wild-type cells. These results, and those described in the previous section, demonstrate an important role of RuvA both for survival and for mutation avoidance after induction of multiple AP sites; however, in the absence of exogenous challenge, RuvA apparently increases mutation frequency. The mutation frequencies in the endonuclease IV-deficient strain after AP site induction are only slightly increased (some 1.5-fold), while they increase 20- to 55-fold in the exonuclease III-deficient strain, when compared with the wild type. In the AP-endonuclease double mutant (endonuclease IV–/exonuclease III–), AP site induction increased mutation frequencies 104-fold above wild-type levels.
These results demonstrate that BER and recombination are both important in order to limit the number of substitution mutations from multiple chromosomal AP sites, and that the mutagenicity of multiple AP sites is not only due to UmuC-dependent TLS.
The vast majority of rifR colonies harbour mutations within codons 509–535 and 563–574 in the rpoB gene, and all six types of substitution are detected
To characterize spontaneous and AP site-induced substitution mutations in the E.coli chromosome of different DNA repair-deficient strains, we sequenced the rpoB region encoding amino acids 500–580 in the β subunit of RNA polymerase from rifR colonies. The majority of previously known mutations causing rifR map to this region, located only a few angstroms away from the residues in the active centre (Severinov et al., 1993, 1994, 1995). From a total of 813 rifR colonies analysed in this study, 764 (94%) had mutations within the sequenced region. We identified 46 different amino acid substitutions due to mutations at 27 different sites in 18 codons (Table I). Among these, 25 substitutions are to our knowledge not previously reported to give rifR. As shown in Table I, all six substitution mutations possible were selected for within this region.
Table I. Amino acid substitutions encoded by the rpoB gene in rifR clones.
| Mutation | Amino acid substitution (No. of mutants) |
|---|---|
| GC→AT | S512F (32), D516N (47), S522F (7), H526Y (82), R529H (4), S531F (34), P564L (30), S574F (7) |
| GC→TA | S509R*(1), S512Y*(25), Q513K*(10), D516Y (34), S522Y*(2), H526N*(24), H526Q (5), R529S (1), R529L (2), S531Y (19), G534C*(4), G534V*(4), G534S*(2), G570C*(4), S574Y*(7) |
| GC→CG | S522C*(1), H526D*(7), A532P*(1), G534R*(1), I572M*(1) |
| AT→GC | L511P*(8), S512P (33), Q513R (4), D516G*(177), N518D*(4), L533P (8), I572T*(1) |
| AT→CG | S509R*(2), L511R*(2), Q513P (14), T563P (6), I572L*(27), I572S*(9) |
| AT→TA | Q513L (20), D516V (10), H526L*(13), L533H*(1), I572F (27) |
*Substitutions that are not previously reported.
Untargeted mutations dominate in E.coli strains deficient in BER or recombination after induction of chromosomal AP sites
The expected in vivo effect of TDG is the introduction of AP sites in the chromosome by excision of thymine, resulting in substitution mutations if C, G or T, but not A, is incorporated opposite the AP site. Similarly, the expected effect of CDG is excision of cytosine and mutation if C, A or T, but not G, is incorporated opposite the AP site. Table II demonstrates that after CDG expression most of the mutations occur at GC base pairs, while after TDG expression most mutations are at AT base pairs. Assuming that the specificities of TDG and CDG and the preference of pol V (insertion of dAMP or dGMP opposite AP sites) are similar in vivo and in vitro, targeted mutations after AP site induction by TDG or CDG are represented by AT→GC and GC→AT transitions, respectively. Incorporation of A by pol V opposite an AP site, resulting from removal of T by TDG, prevents mutations in much the same way as pol V does when it inserts A–A opposite a T–T cyclobutane pyrimidine dimer (Tang et al., 2000). From Table II, targeted mutations are most pronounced after TDG expression, and particularly in UmuC-deficient cells. This is consistent with loss of UmuC/pol V, which has been shown to introduce untargeted mutations in vitro (Maor-Shoshani et al., 2000; Tang et al., 2000), or alternatively, it may reflect a different preference for nucleotide insertion opposite an AP site by other polymerases. Deficiency in BER (exonuclease III– and endonuclease IV–/exonuclease III–) or recombination (RuvA–) strongly increased the fraction of untargeted mutations (Table II). This is likely to be explained by enhanced UmuC/pol V activity as a consequence of SOS induction. The low fraction of mutations at GC pairs after CDG expression in the UmuC-deficient strain is probably due to a preference for dGMP insertion in the absence of UmuC (see below and Discussion).
Table II. Mutations at GC pairs or AT pairs after induction of AP sites by CDG or TDG, respectively.
| CDG | TDG | |||
|---|---|---|---|---|
| Strain |
GC (%) |
GC→AT (%) |
AT (%) |
AT→GC (%) |
| Wild type | 74 | 56 | 86 | 84 |
| UmuC– | 24 | 21 | 97 | 94 |
| RuvA– | 54 | 34 | 70 | 40 |
| Endonuclease IV– | 72 | 53 | 88 | 88 |
| Exonuclease III– | 60 | 33 | 74 | 42 |
| Endonuclease IV–/ Exonuclease III– | 65 | 23 | 63 | 18 |
UmuC is essential for the ‘A rule’ after induction of AP sites, and also causes an ‘A rule-like’ pattern of spontaneous base substitutions
A possible ‘A rule’ (incorporation of dAMP opposite an AP site) in vivo can be studied by expression of CDG, because removal of C and insertion of A opposite the resulting AP site result in GC→AT transition mutations. As mentioned above, insertion of dAMP opposite an AP site generated by TDG will restore the normal sequence. A strong reduction in the fraction of GC→AT transitions after CDG expression is observed in the umuC mutant when compared with wild type (21 and 56%, respectively, see Table II and Figure 3), indicating that UmuC is required for the ‘A rule’. In most of the rpoB mutation spectra shown in Figure 3, codons 516 (GAC) and 526 (CAC) are hotspots for mutations. The predominance of the mutation CAC→TAC at codon 526 (GC transition, black bar) in the CDG-induced spectrum of the wild type may be explained by incorporation of dAMP by pol V opposite an AP site resulting from excision of cytosine by CDG. This hotspot (CAC→TAC) is also present in the spontaneous spectrum of the wild type, where it may be a result of incorporation of dAMP opposite AP sites resulting from spontaneous depyrimidination (loss of cytosine) or removal of deaminated cytosine (uracil) by UDG. However, the CAC→TAC mutation at codon 526 is not a hotspot in the spectrum from UmuC-deficient cells, which is in agreement with loss of pol V activity (‘A rule’). Furthermore, it may indicate that dGMP is preferentially incorporated in this strain, thus restoring the normal sequence (Figure 3). Codon 516 (GAC) appears as a hotspot in both the wild-type and the UmuC– spectra. The most frequent spontaneous mutation at codon 516 in the wild type is GAC→AAC (GC transition, black bar), which is in agreement with an ‘A rule’. This mutation is not present in the spontaneous spectrum of the UmuC-deficient strain, where the most frequent mutation at codon 516 is GAC→GGC (AT transition, white bar). This type of transition also occurs in the wild type, but less frequently than in the umuC mutant. Furthermore, this transition is even more pronounced after expression of TDG, and more so in the UmuC-deficient strain than in the wild type, even though the mutation frequency is 3-fold lower. Thus, this AT transition at codon 516 most likely results from incorporation of dGMP opposite an AP site originally carrying a thymine. Incorporation of dAMP opposite such an AP site will not be detected. Together these data demonstrate that UmuC deficiency results in a shift in the preference of the nucleotide incorporated opposite an AP site (or other lesions in spontaneously damaged DNA) from dAMP to dGMP. Loss of ‘A rule’ in the absence of UmuC is in agreement with the reported in vitro preference for pol V (UmuD2′C), which favours incorporation of dAMP some 2- to 3-fold over dGMP opposite an AP site (Reuven et al., 1999; Tang et al., 1999, 2000).
The low fraction of mutations at GC pairs after CDG expression in UmuC-deficient cells may at first seem paradoxical, since CDG in vitro preferentially removes C from DNA (Figure 1). However, incorporation of dGMP opposite AP sites after removal of cytosine in the UmuC-deficient strain will restore the normal sequence and thus will not be detected. This will tend to skew the mutation pattern toward events at AT pairs. In addition, residual untargeted mutations (pol IV?), background mutations (25%) and possibly the lack of absolute specificity of CDG for C may explain the high fraction of transitions at AT base pairs, as seen in Figure 3.
The spontaneous mutation spectra in the wild-type and the UmuC-deficient strains are clearly different, both with regard to the type of base pair mutated (GC versus AT pairs) and the location of hotspots. Calculated from data in Figure 3, 78% of the spontaneous mutations in the wild type are in GC pairs, compared with only 40% in the UmuC-deficient strain. Furthermore, in the wild type, 85% of the spontaneous base substitutions may be explained by incorporation of dAMP opposite an AP site or damaged base (GC→AT, GC→TA, TA→AT), while only 45% of the mutations in the umuC mutant followed an ‘A rule-like’ pattern.
These data demonstrate that UmuC is responsible for the ‘A rule’ in vivo after induction of AP sites. Furthermore, the apparent ‘A rule-like’ pattern of spontaneous mutations is also dependent upon UmuC, even though UmuC does not significantly increase the total frequency of spontaneous substitution mutations. The latter suggests that spontaneous mutation spectra to a larger extent may reflect the capacity of different DNA repair pathways than the pattern of damage induction.
In the absence of RuvA, spontaneous mutations obey the ‘A rule’ more strictly and the frequency of transversions increases
Using data from Figure 3, we have calculated that 94% of the spontaneous mutations in the RuvA-deficient strain may be explained by incorporation of dAMP opposite an AP site or a different DNA lesion, as compared with 85% in wild-type and 71% in RecA-deficient cells. Further more, the fraction of transversions (mainly GC→TA and AT→TA) is increased in the RuvA-deficient strain compared with the wild type and the recA-mutant (47% compared with 17 and 26%, respectively). An increase in transversions in the lacI gene (GC→TA and AT → TA) was reported after continuous induction of SOS in E.coli (recA441) (Miller and Low, 1984), and also recently in the cro gene after in vitro gap filling by pol V (Maor-Shoshani et al., 2000). Thus, in the absence of RuvA-dependent recombination the spontaneous mutation spectrum may, to a larger degree, reflect the activity of pol V.
Mutation spectra of the AP-endonuclease-deficient strains are dominated by transversions
The spontaneous mutation spectra of strains deficient in endonuclease IV, exonuclease III or both have a higher frequency of transversions when compared with wild type. This applies to GC→TA transversions particularly (Figure 3). The fraction of transversions in the spontaneous mutation spectra increases ∼4-fold from the wild type to the exonuclease III- and the endonuclease IV/exonuclease III-deficient strains. BER is important in the repair of AP sites generated by N-glycosylases and in the repair of AP sites caused by depurination (loss of A or G), which is possibly one of the most frequent spontaneous DNA damaging events in the cells (Lindahl and Nyberg, 1972). When spontaneously depurinated sites are bypassed by the translesion polymerase (pol V), with a preference for dAMP over dGMP, this will result in transversions as observed in these spectra. In the AP-endonuclease-deficient strains, BER is severely compromised, thus the AP sites will probably persist longer. However, after AP site induction at pyrimidines by CDG or TDG, the high fraction of transversions (71% transversions after TDG expression in the AP-endonuclease double mutant, calculated from data in Figure 3) and untargeted mutations (only 18% targeted mutations after TDG expression in the AP-endonuclease double mutant, Table II) probably reflect a highly induced SOS response.
The change in the pattern of spontaneous mutations in the endonuclease IV-deficient strain is interesting, considering that this phenotype does not significantly change the frequency of spontaneous base substitutions. However, after CDG or TDG expression, the mutation spectra as well as the cytotoxicity and the mutation frequency in the endonuclease IV-deficient strain were quite similar when compared with wild type. This indicates that endo nuclease IV is not essential for the repair of CDG- and TDG-induced AP sites. Still, the spontaneous mutation pattern is strongly influenced by the absence of endonuclease IV, consistent with the idea that spontaneous mutations reflect the profile of DNA repair proteins in the cell.
Discussion
Apparently, it is generally more common that alternative mechanisms that complement each other in a cellular function are genetically unrelated, rather than being contributed by related products of gene families (Wagner, 2000). This also appears to be a general strategy in DNA repair, at least for some of the most common types of damage, and this probably enhances the robustness against deleterious phenotypic effects of mutations. Our work demonstrates that at least three distinct mechanisms are important for DNA repair and survival of E.coli after introduction of multiple chromosomal AP sites. These are BER, RuvA-dependent recombination repair and UmuC-dependent TLS. In addition, it was recently demonstrated that NER is involved in the repair of AP sites, at least in yeast (Torres-Ramos et al., 2000). Thus, the cellular mechanisms and networks dealing with AP sites are apparently quite complex.
A prerequisite for studying the cytotoxicity and repair of DNA lesions in chromosomal DNA is the ability to induce the desired type of lesion without introducing additional lesions. Not only do the novel DNA glycosylases specifically induce AP sites, but they also induce AP sites with a known base opposite the AP site (A with TDG and G with CDG). Here, we show that TDG and CDG have very strong, but not absolute, preferences for removal of thymine and cytosine in vitro, respectively. We observed fragmentation of DNA after expression of TDG or CDG, and a >20-fold increase in the number of intact AP sites after TDG expression. This demonstrates that the enzymes have access to chromosomal DNA in the cell. This makes these engineered DNA glycosylases useful tools for studying AP site biology in vivo. In addition, we demonstrate that these DNA glycosylases, particularly TDG, are also powerful mutagens and may find many uses as such.
Recent in vitro studies have demonstrated that pol V is error prone, induces untargeted mutations and preferentially incorporates dAMP opposite AP sites (Reuven et al., 1999; Tang et al., 1999, 2000). Our work strongly indicates that pol V/UmuC also have these functions in vivo, after induction of chromosomal AP sites. Somewhat surprisingly, the spectrum of spontaneous mutations is consistent with an ‘A rule’ as well. Thus, we observe a reduction in mutations consistent with the ‘A rule’ in the spontaneous rpoB mutation spectrum of the umuC-mutant (45%), compared with the wild type (85%). This may indicate that a large fraction of the spontaneous base lesions are AP sites, or alternatively that TLS over other DNA lesions also follows an ‘A rule’. Our results with the UmuC-deficient strain are consistent with the spontaneous lacI mutation spectra in a umuC122::Tn5 mutant compared with wild-type cells (Sargentini and Smith, 1994). However, the authors did not point this out, and the changes were also less pronounced (54% for UmuC-deficient cells and 70% for wild type).
Although the spontaneous mutation frequencies were not significantly different for wild type and strains deficient in either UmuC, endonuclease IV and/or exonuclease III, the spectra of spontaneous mutations were very different. This clearly demonstrates that the pattern of spontaneous mutations in a cell not only reflects the number of different lesions, but also, possibly more importantly, the presence of different repair proteins. We find this highly interesting and it may indicate that a search for ‘fingerprints’ in mutational patterns, indicative of mutagenesis by a certain agent in the environment (usually present at low concentrations) may be futile. We also found that UmuC-deficient cells were significantly more sensitive to AP sites than wild-type cells after induction by TDG, and that the mutation frequency was 2- to 3-fold higher in the wild-type than in the UmuC-deficient cells. Thus, increased survival is at the cost of increased mutations. However, we still find a 50-fold increase in substitution mutations after TDG expression in the UmuC-deficient strain when compared with the spontaneous mutation frequency in the same strain. Apparently, mutagenic pathways independent of UmuC contribute substantially to substitution mutations at chromosomal AP sites. Furthermore, our data suggest that in the UmuC-deficient cells dGMP is preferentially inserted opposite a damaged base or an AP site. Complementary roles of pol IV and pol V were recently proposed, with pol IV extending aberrant primer 3′ ends and pol V catalysing TLS (Tang et al., 2000). However, the exact biological function of pol IV remains unsettled. Thus, mutagenic backup systems may involve pol IV, the Npf pathway (Napolitano et al., 1997) or other, as yet unidentified, mechanisms leading to base substitutions.
RecA-deficient cells were hypersensitive to AP sites and more sensitive than RuvA-deficient cells. The hypersensitivity of RecA-deficient cells is not surprising given the roles of this multifunctional protein in recombination, SOS induction and TLS (Friedberg et al., 1995). RuvA is required for the later steps in recombination, and our results strongly suggest a direct role of recombination in cell survival and DNA repair after induction of multiple AP sites. The mutational spectrum revealed a relative increase in transversions in RuvA-deficient cells, both spontaneously and after induction of AP sites. This is consistent with the evidence indicating chronic SOS induction in ruvA mutants (Asai and Kogoma, 1994), and with the reported SOS-associated increase in transversions (Miller and Low, 1984; Maor-Shoshani et al., 2000). The 10-fold decreased spontaneous mutation frequency in RuvA-deficient cells is not easily reconciled with chronic SOS induction. In addition, the strong increase in spontaneous transversions in the BER-deficient strains may also indicate induction of SOS without an increase in mutation frequencies. However, it should be kept in mind that the rifR assay does not detect frameshifts and nonsense mutations. It is possible that frameshifts may be increased relative to missense mutations when an SOS response is induced. Our data also demonstrate that in RuvA-deficient cells, a larger fraction of the mutations may be explained by the insertions of dAMP opposite AP sites (‘A rule’). This may reflect that the balance between repair by TLS and recombination repair is shifted towards TLS in RuvA-deficient cells. Alternatively, stalled replication forks that initiate recombination that is not completed in the absence of RuvA may result in cell death, selecting for mutants resulting from TLS.
From our mutation spectra, it is very clear that there are hotspots for mutation along the part of the rpoB gene sequenced, and that the appearance of hotspots differs between the AP-endonuclease-deficient strains. One might speculate that at least some of these represent AP sites slowly repaired by BER. In fact, slight differences in the rates of endonucleolytic cleavage by exonuclease III and endonuclease IV have been attributed to the sequence context flanking the AP site (Shida et al., 1996). However, when a high fraction of cells are killed by mutagens, e.g. TDG or CDG, one cannot exclude the possibility that the survivors represent a subfraction of mutagenic events. In the present paper, the largest changes in mutational spectra, compared with wild type, are seen in the mutant strain where the cytotoxicity is lowest (UmuC-deficient strain). Furthermore, the number of mutated sites tends to increase, and hotspots tend to be levelled out with increasing cytotoxicity, especially in the BER-deficient cells. Thus, our data do not give indications of selection for a small subfraction of mutagenic events, although we cannot exclude this possibility.
To our knowledge, the cellular requirements for repair of chromosomal AP sites have not been investigated in vivo previously for any system. Our results demonstrate that the cellular handling of multiple AP sites is complex, but that by using specific tools for damage induction in combination with a collection of DNA repair mutants derived from the same strain, the complexity can be unravelled.
Materials and methods
Strains and constructs
All strains in this study are isogenic, except for the particular mutation of interest. Strains used were: the wild-type strain AB1157; the endonuclease IV-deficient strain BW527 (RPC500 = AB1157 nfo-1::kan) (Cunningham et al., 1986); the exonuclease III-deficient strain BW9101 [AB1157 Δ(xthA-pncA)DE90] (White et al., 1976); the endonuclease IV- and exonuclease III-deficient strain BW528 [RPC501 = AB1157 nfo-1::kanΔ(xthA-pncA)DE90] (Cunningham et al., 1986); the RecA-deficient strain AB2463 (AB1157 recA13) (Kato and Shinoura, 1977); the RuvA-deficient strain N2057 (AB1157 ruvA60::Tn10) (Shurvinton et al., 1984) and the UmuC-deficient strain GW2100 (AB1157 umuC122::Tn5) (Christensen et al., 1988). The strains were generously supplied by Professor Erling Seeberg, Department of Molecular Biology, Institute of Medical Microbiology, University of Oslo, Norway and Professor Robert G.Lloyd, Division of Genetics, Queens Medical Centre, Nottingham, UK (RuvA-deficient strain). The expression constructs for UDG (pTUNGΔ84), CDG (pTUNGΔ84 Asn204Asp) and TDG (pTUNGΔ84Tyr147Ala) are previously described (Slupphaug et al., 1995; Kavli et al., 1996).
3H-labelled substrate-DNA
The 3H-labelled dNTPs were from Amersham UK: [3H]dATP (TRK347), [3H]dTTP (TRK625), [3H]dGTP (TRK350), [3H]dTTP (TRK354), [3H]dUTP (TRK351). The five different DNA substrates containing 3H-radiolabelled bases (A, C, G, T or U) were prepared by PCR amplification of the rpoB fragment. DyNAzyme (Finnzymes) and the PCR primers (Severinov et al., 1993) were used to amplify a 927 bp fragment from chromosomal DNA. 3H-labelled dNTP (2.7–5.0 nmol) was mixed with 5.0 nmol of the three unlabelled dNTPs in a 100 µl reaction mixture. The 3H-labelled PCR products were isolated on QIAquick™ PCR purification columns in order to remove unincorporated radioactivity.
Assay for testing substrate specificities of engineered enzymes
Two hundred and fifty nanograms of enzyme (10 pmol, UDG, CDG or TDG) were incubated with 17–39 pmol of 3H-labelled (A, C, G, T or U) substrate DNA, at 37°C for 3 h under standard conditions (10 mM Tris–HCl pH 7.5, 1 mM EDTA, 10 mM NaCl, 50 mg/ml bovine serum albumin) (Kavli et al., 1996), and excision activity was measured as previously described (Wittwer et al., 1989).
Quantification of AP sites
Quantification of AP sites was carried out using an aldehyde reactive probe (ARP) essentially as described by Swenberg and co-workers using a log-log plot (Nakamura et al., 1998; Nakamura and Swenberg, 1999). By incubating [3H]uracil-containing DNA, with known specific radioactivity, with UDG and measuring the amount of radioactivity released, a standard with known number of AP sites was prepared. In order to prepare a standard curve, serial dilutions were made by diluting the AP-DNA with chromosomal DNA isolated from the wild-type strain (containing empty vector). ARP was then added to the different dilutions. To quantify AP sites in the chromosome after CDG and TDG expression, single colonies were cultured overnight at 30°C in Luria-Bertani (LB) medium containing ampicillin (200 µg/ml) (LBamp), and then diluted to 5% in fresh LBamp medium containing 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to induce expression of CDG and TDG. The expression cultures were incubated at 37°C with shaking for 2.5 h before harvesting. Chromosomal DNA was isolated according to Moore (1998).
Measurements of the cytotoxic effect
Measurements of the cytotoxic effects of TDG and CDG expression were complex, due to self-inactivation of TDG- and CDG-expressing sequences in the plasmids by their gene products, and loss of plasmids (data not shown). Therefore, we defined cytotoxicity as the decrease in viable ampR cells relative to the number of cells at the time of induction. Due to large variations in the cytotoxic effect of CDG and TDG between single colonies, we incubated 10 single colonies from fresh plates separately at 30°C overnight in LBamp. To obtain data on average cytotoxicity, the overnight cultures were mixed before inoculation (5%), and then cultured for 6 h at 37°C in LBamp containing 1 mM IPTG (induction). Every hour, aliquots from the cultures were mixed in 3 ml of soft agar, plated on LBamp-agar plates and incubated at 37°C, overnight before the colonies were counted.
Mutation frequency measured as rifR
After pilot experiments, in which we followed the frequency of rifR cells in the different strains over 6 h (data not shown), we found that the expression of CDG or TDG for 5 h was optimal for calculating mutation frequencies. Single colonies from the E.coli strains, containing empty vector (pTrc99A) and plasmids expressing CDG or TDG, were cultured in LBamp overnight at 30°C. The overnight cultures were inoculated (5%) in LBamp containing 1 mM IPTG and cultured for 5 h at 37°C. Aliquots from the cultures were mixed in 3 ml of soft agar and plated on LBamp-agar plates and LBamp-agar plates containing rifampicin (100 µg/ml). The numbers of ampR colonies and rifR colonies were counted after incubation at 37°C overnight or for 2 days, respectively. Mutation frequency was calculated as the number of rifR colonies per 108 ampR colonies.
Determination of mutation spectra by DNA sequencing
Chromosomal DNA was isolated according to the protocol (Moore, 1998), from rifR colonies that had been cultured overnight at 37°C in LB medium containing 50 µg/ml rifampicin (only one mutant was characterized from each start culture). A DyNAzyme kit (Finnzymes) and the PCR primers (Severinov et al., 1993) were used to amplify a 927 bp rpoB fragment from chromosomal DNA, according to the manufacturer’s protocol. The PCR products were purified by QIAquick™ PCR purification kit before sequencing. The sequence encoding the β subunit of RNA polymerase (amino acids 500–580) in rifR colonies was obtained by use of TaqPRISM™ Ready reaction DyeDeoxy™ terminator Cycle Sequence Kit on an Applied Biosystems Model 373A DNA sequencer. For sequencing we used the following forward sequence primer, 5′-AGCGTCTGTCTCTGGGCG-3′ (rpoB, bp 4398–4415), and reverse sequence primer, 5′-CGATACGGAGTCTCAAGG-3′ (rpoβ, bp 4727–4774).
Acknowledgments
Acknowledgements
We thank Ruth Krokan for excellent technical assistance. This work was supported by grants from the Norwegian Cancer Society, the Norwegian Research Council, The Svanhild and Arne Must Fund for Medical Research and the Cancer Fund at the Regional Hospital, Trondheim, Norway.
References
- Asai T. and Kogoma,T. (1994) Roles of ruvA, ruvC and recG gene functions in normal and DNA damage-inducible replication of the Escherichia coli chromosome. Genetics, 137, 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdal K.G., Johansen,R.F. and Seeberg,E. (1998) Release of normal bases from intact DNA by a native DNA repair enzyme. EMBO J., 17, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatny J.M., Brautaset,T., Winther-Larsen,H.C., Karunakaran,P. and Valla,S. (1997) Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid, 38, 35–51. [DOI] [PubMed] [Google Scholar]
- Brotcorne-Lannoye A. and Maenhaut-Michel,G. (1986) Role of RecA protein in untargeted UV mutagenesis of bacteriophage lambda: evidence for the requirement for the dinB gene. Proc. Natl Acad. Sci. USA, 83, 3904–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J.R., LeClerc,J.E., Tata,P.V., Christensen,R.B. and Lawrence,C.W. (1988) UmuC function is not essential for the production of all targeted lacI mutations induced by ultraviolet light. J. Mol. Biol., 203, 635–641. [DOI] [PubMed] [Google Scholar]
- Cox M.M. (1999) Recombinational DNA repair in bacteria and the RecA protein. Prog. Nucleic Acid Res. Mol. Biol., 63, 311–366. [DOI] [PubMed] [Google Scholar]
- Cox M.M. Goodman,M.F., Kreuzer,N., Sherratt,D.J., Sandler,S.J. and Marians,K.J. (2000) The importance of repairing stalled replication forks. Nature, 404, 37–41. [DOI] [PubMed] [Google Scholar]
- Cunningham R.P., Saporito,S.M., Spitzer,S.G. and Weiss,B. (1986) Endonuclease IV (nfo) mutant of Escherichia coli. J. Bacteriol., 168, 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Johnson,A. and Fung,D. (1986) Exonuclease III and endonuclease IV remove 3′ blocks from DNA synthesis primers in H2O2-damaged Escherichia coli. Proc. Natl Acad. Sci. USA, 83, 7731–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez De Henestrosa A.R., Ogi,T., Aoyagi,S., Chafin,D., Hayes,J.J., Ohmori,H. and Woodgate,R. (2000) Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol., 35, 1560–1572. [DOI] [PubMed] [Google Scholar]
- Fijalkowska I.J., Dunn,R.L. and Schaaper,R.M. (1997) Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J. Bacteriol., 179, 7435–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis, ASM Press, Washington, DC. [Google Scholar]
- Kato T. and Shinoura,Y. (1977) Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol. Gen. Genet., 156, 121–131. [DOI] [PubMed] [Google Scholar]
- Kavli B., Slupphaug,G., Mol,C.D., Arvai,A.S., Peterson,S.B., Tainer,J.A. and Krokan,H.E. (1996) Excision of cytosine and thymine from DNA by mutants of human uracil- DNA glycosylase. EMBO J., 15, 3442–3447. [PMC free article] [PubMed] [Google Scholar]
- Kim S.R., Maenhaut-Michel,G., Yamada,M., Yamamoto,Y., Matsui,K., Sofuni,T., Nohmi,T. and Ohmori,H. (1997) Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc. Natl Acad. Sci. USA, 94, 13792–13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klungland A., Rosewell,I., Hollenbach,S., Larsen,E., Daly,G., Epe,B., Seeberg,E., Lindahl,T. and Barnes,D.E. (1999) Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl Acad. Sci. USA, 96, 13300–13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C.W., Borden,A., Banerjee,S.K. and Le Clerc,J.E. (1990) Mutation frequency and spectrum resulting from a single abasic site in a single-stranded vector. Nucleic Acids Res., 18, 2153–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.J. and Sancar,A. (1989) A new mechanism for repairing oxidative damage to DNA: (A)BC excinuclease removes AP sites and thymine glycols from DNA. Biochemistry, 28, 7979–7984. [DOI] [PubMed] [Google Scholar]
- Lindahl T. and Nyberg,B. (1972) Rate of depurination of native deoxyribonucleic acid. Biochemistry, 11, 3610–3618. [DOI] [PubMed] [Google Scholar]
- Loeb L.A. and Preston,B.D. (1986) Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet., 20, 201–230. [DOI] [PubMed] [Google Scholar]
- Maor-Shoshani A., Reuven,N.B., Tomer,G. and Livneh,Z. (2000) Highly mutagenic replication by DNA polymerase V (UmuC) provides a mechanistic basis for SOS untargeted mutagenesis. Proc. Natl Acad. Sci. USA, 97, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.H. and Low,K.B. (1984) Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell, 37, 675–682. [DOI] [PubMed] [Google Scholar]
- Moore D. (1998) Preparation of genomic DNA from bacteria. In Ausubel,F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (eds), Current Protocols in Molecular Biology, Vol. 1, John Wiley & Sons, Inc., 2.41–2.42. [Google Scholar]
- Nakamura J. and Swenberg,J.A. (1999) Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer Res., 59, 2522–2526. [PubMed] [Google Scholar]
- Nakamura J., Walker,V.E., Upton,P.B., Chiang,S.Y., Kow,Y.W. and Swenberg,J.A. (1998) Highly sensitive apurinic/apyrimidinic site assay can detect spontaneous and chemically induced depurination under physiological conditions. Cancer Res., 58, 222–225. [PubMed] [Google Scholar]
- Napolitano R.L., Lambert,I.B. and Fuchs,R.P. (1997) SOS factors involved in translesion synthesis. Proc. Natl Acad. Sci. USA, 94, 5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen H. et al. (2000) Uracil-DNA glycosylase (UNG)-deficient mice reveal a primary role of the enzyme during DNA replication. Mol. Cell, 5, 1059–1065. [DOI] [PubMed] [Google Scholar]
- Olsen L.C., Aasland,R., Krokan,H.E. and Helland,D.E. (1991) Human uracil-DNA glycosylase complements E.coli ung mutants. Nucleic Acids Res., 19, 4473–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S.S., Mol,C.D., Slupphaug,G., Bharati,S., Krokan,H.E. and Tainer,J.A. (1998) Base excision repair initiation revealed by crystal structures and binding kinetics of human uracil-DNA glycosylase with DNA. EMBO J., 17, 5214–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuven N.B., Arad,G., Maor-Shoshani,A. and Livneh,Z. (1999) The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA and SSB and is specialized for translesion replication. J. Biol. Chem., 274, 31763–31766. [DOI] [PubMed] [Google Scholar]
- Rothwell D.G. and Hickson,I.D. (1997) Repair of apurinic/apyrimidinic (AP) sites in DNA by AP endonucleases. In Hickson,I.D. (ed.), Base Excision Repair of DNA Damage, Landes Bioscience, Austin, TX, pp. 67–80. [Google Scholar]
- Sargentini N.J. and Smith,K.C. (1994) DNA sequence analysis of spontaneous and γ-radiation (anoxic)-induced lacId mutations in Escherichia coli umuC122::Tn5: differential requirement for umuC at G.C vs. A.T sites and for the production of transversions vs. transitions. Mutat. Res., 311, 175–189. [DOI] [PubMed] [Google Scholar]
- Severinov K., Soushko,M., Goldfarb,A. and Nikiforov,V. (1993) Rifampicin region revisited. New rifampicin-resistant and streptolydigin-resistant mutants in the β subunit of Escherichia coli RNA polymerase. J. Biol. Chem., 268, 14820–14825. [PubMed] [Google Scholar]
- Severinov K., Soushko,M., Goldfarb,A. and Nikiforov,V. (1994) RifR mutations in the beginning of the Escherichia coli rpoB gene. Mol. Gen. Genet., 244, 120–126. [DOI] [PubMed] [Google Scholar]
- Severinov K., Mustaev,A., Severinova,E., Kozlov,M., Darst,S.A. and Goldfarb,A. (1995) The β subunit Rif-cluster I is only angstroms away from the active center of Escherichia coli RNA polymerase. J. Biol. Chem., 270, 29428–29432. [DOI] [PubMed] [Google Scholar]
- Shida T., Noda,M. and Sekiguchi,J. (1996) Cleavage of single- and double-stranded DNAs containing an abasic residue by Escherichia coli exonuclease III (AP endonuclease VI). Nucleic Acids Res., 24, 4572–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurvinton C.E., Lloyd,R.G., Benson,F.E. and Attfield,P.V. (1984) Genetic analysis and molecular cloning of the Escherichia coli ruv gene. Mol. Gen. Genet., 194, 322–329. [DOI] [PubMed] [Google Scholar]
- Slupphaug G., Eftedal,I., Kavli,B., Bharati,S., Helle,N.M., Haug,T., Levine,D.W. and Krokan,H.E. (1995) Properties of a recombinant human uracil-DNA glycosylase from the UNG gene and evidence that UNG encodes the major uracil-DNA glycosylase. Biochemistry, 34, 128–138. [DOI] [PubMed] [Google Scholar]
- Snowden A., Kow,Y.W. and van Houten,B. (1990) Damage repertoire of Escherichia coli UvrABC nuclease complex includes abasic sites, base damage analogues and lesions containing adjacent 5′ or 3′ nicks. Biochemistry, 29, 7251–7259. [DOI] [PubMed] [Google Scholar]
- Strauss B.S. (1991) The “A rule” of mutagen specificity: a consequence of DNA polymerase bypass of non-instructional lesions? BioEssays, 13, 79–84. [DOI] [PubMed] [Google Scholar]
- Tang M., Shen,X., Frank,E.G., O’Donnell,M., Woodgate,R. and Goodman,M.F. (1999) UmuD′(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl Acad. Sci. USA, 96, 8919–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Pham,P., Shen,X., Taylor,J.S., O’Donnell,M., Woodgate,R. and Goodman,M.F. (2000) Roles of E.coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature, 404, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Torres-Ramos C.A., Johnson,R.E., Prakash,L. and Prakash,S. (2000) Evidence for the involvement of nucleotide excision repair in the removal of abasic sites in yeast. Mol. Cell. Biol., 20, 3522–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. (2000) Robustness against mutations in genetic networks of yeast. Nature Genet., 24, 355–361. [DOI] [PubMed] [Google Scholar]
- Wagner J., Gruz,P., Kim,S.R., Yamada,M., Matsui,K., Fuchs,R.P. and Nohmi,T. (1999) The dinB gene encodes a novel E.coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol. Cell, 4, 281–286. [DOI] [PubMed] [Google Scholar]
- Waters T.R., Gallinari,P., Jiricny,J. and Swann,P.F. (1999) Human thymine DNA glycosylase binds to apurinic sites in DNA but is displaced by human apurinic endonuclease 1. J. Biol. Chem., 274, 67–74. [DOI] [PubMed] [Google Scholar]
- White B.J., Hochhauser,S.J., Cintron,N.M. and Weiss,B. (1976) Genetic mapping of xthA, the structural gene for exonuclease III in Escherichia coli K-12. J. Bacteriol., 126, 1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer C.U., Bauw,G. and Krokan,H.E. (1989) Purification and determination of the NH2-terminal amino acid sequence of uracil-DNA glycosylase from human placenta. Biochemistry, 28, 780–784. [DOI] [PubMed] [Google Scholar]
- Woodgate R. and Levine,A.S. (1996) Damage inducible mutagenesis: recent insights into the activities of the Umu family of mutagenesis proteins. Cancer Surv., 28, 117–140. [PubMed] [Google Scholar]