Abstract
Background
The prevalence of albuminuria in the general population is high, but awareness of it is low. Therefore, we sought to develop and validate a self-assessment tool that allows individuals to estimate their probability of having albuminuria.
Study Design
Cross-sectional study
Setting & Participants
The population-based REasons for Geographic And Racial Differences in Stroke (REGARDS) study for model development and the National Health and Nutrition Examination Survey 1999-2004 (NHANES 1999-2004) for model validation. US adults ≥ 45 years of age in the REGARDS study (n=19,697) and NHANES 1999-2004 (n=7,168)
[nijsje 1]Factor
Candidate items for the self-assessment tool were collected using a combination of interviewer- and self-administered questionnaires.
Outcome
Albuminuria was defined as a urinary albumin to urinary creatinine ratio ≥ 30 mg/g in spot samples.
Results
Eight items were included in the self-assessment tool (age, race, gender, current smoking, self-rated health, and self-reported history of diabetes, hypertension, and stroke). These items provided a c-statistic of 0.709 (95% CI, 0.699 – 0.720) and a good model fit (Hosmer-Lemeshow chi-square p-value = 0.49). In the external validation data set, the c-statistic for discriminating individuals with and without albuminuria using the self-assessment tool was 0.714. Using a threshold of ≥ 10% probability of albuminuria from the self-assessment tool, 36% of US adults ≥ 45 years of age in NHANES 1999-2004 would test positive and be recommended screening. The sensitivity, specificity, and positive and negative predictive values for albuminuria associated with a probability ≥ 10% were 66%, 68%, 23% and 93%, respectively.
Limitations
Repeat urine samples were not available to assess the persistency of albuminuria.
Conclusions
Eight self-report items provide good discrimination for the probability of having albuminuria. This tool may encourage individuals with a high probability to request albuminuria screening.
Albuminuria, a key subclinical marker for kidney disease, affects over 10 million US adults1. It has been demonstrated to be a strong, independent risk factor for cardiovascular disease, end-stage renal disease (ESRD) and all-cause mortality2-7. The use of renin-angiotensin system blockers in patients with albuminuria has been shown to slow the progression of kidney disease and lower the risk for cardiovascular disease8-10. Despite its high prevalence, prognostic importance, and availability of treatment, many US adults with albuminuria are unaware of their diagnosis11.
Results from a previous analysis suggest that population-wide screening of individuals for albuminuria is not cost-effective12;13. Screening for albuminuria may be beneficial amongst individuals at increased risk for the adverse sequelae of kidney damage 14-16. However, even among such high risk populations, including patients with diabetes, rates of albuminuria screening are low17-19. Therefore, approaches are needed for raising awareness of albuminuria, especially for populations that are likely to demonstrate a high prevalence20.
As several correlates of albuminuria have been identified, we hypothesized that a simple set of self-reported items could be assembled to accurately discriminate between individuals with and without albuminuria21-23. To test this hypothesis, we developed a self-assessment tool for the presence of albuminuria using data from a large nationwide sample of African-American and white US adults. Additionally, we analyzed data from a second nationally representative sample, drawn from the general United States population, to externally validate this tool.
METHODS
Datasets
This analysis was performed using two datasets: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study and the National Health and Nutrition Examination Survey 1999-2004 (NHANES 1999-2004)24-26. Data from the REGARDS study was used to develop the self-assessment tool and data from NHANES 1999-2004 was used to externally validate the tool.
Derivation Dataset: The REGARDS Study
The REGARDS study is a community-based investigation of stroke incidence among US adults ≥ 45 years of age. In brief, 30,239 African-American and white US adults were enrolled between January 2003 and October 2007. Beginning in May 2004, a prior diagnosis of kidney disease and a family history of ESRD were assessed for REGARDS study participants. The current analysis was limited to participants for whom answers to these questions were ascertained (n=21,658). Participants receiving hemodialysis or reporting a prior diagnosis of kidney disease (n=393), without urinary albumin and creatinine measurements (n=1,137), or who were missing information for items in the self-assessment tool (n=431) were excluded, leaving 19,697 participants with complete data for the present analysis. The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided informed consent.
The REGARDS study data were collected through interview- and self-administered questionnaires and during an in-home study visit. A list of self-report items collected as part of the REGARDS study was reviewed by the study authors with the following selected for possible inclusion in the albuminuria self-assessment tool: age, race, gender, education (high school graduate or not), physical activity (any or none), alcohol consumption (none, some but less than two drinks per day and two or more drinks per day), cigarette smoking (current, former, never), family history of ESRD, self-rated health (excellent, very good, good, fair, or poor), and prior diagnosis of diabetes, hypertension, myocardial infarction, stroke and self-reported antihypertensive medication use. Urinary albumin was measured at the Department of Laboratory Medicine and Pathology at the University of Minnesota, using the BN ProSpec Nephelometer from Dade Behring (www.dadebehring.com). Urinary creatinine was measured with a rate-blanked Jaffé procedure, using the Modular-P analyzer (Roche/Hitachi; labsystems.roche.com).
Validation Dataset: NHANES 1999-2004
NHANES 1999-2000, 2001-2002, and 2003-2004, are serial cross-sectional surveys including nationally representative samples of the non-institutionalized civilian US population identified through a stratified, multi-stage probability sampling design25;26. NHANES 1999-2004 included 8,654 adults, ≥ 45 years of age, who completed the study questionnaires and attended a study examination. Participants with known kidney disease (n=282), missing data for urinary albumin or creatinine excretion (n=1,039), missing information for the items in the self-assessment tool (n=161), or who were pregnant at the time of their NHANES examination (n=4) were excluded from the current analyses. After these exclusions, the external validation in NHANES 1999-2004 was based on data from 7,168 participants. Items used in the self-assessment tool were collected through interview-administered questionnaires. Urinary albumin was measured using a solid-phase fluorescence immunoassay; urinary creatinine was measured using the modified kinetic method of Jaffé (Beckman Coulter Synchron AS/Astra Analyzer).
Definition of albuminuria
For participants in the REGARDS study and NHANES 1999-2004, the urinary albumin-creatinine ratio was calculated. Albuminuria was defined as levels ≥ 30 mg/g27.
Statistical Methods
Model development
Participant characteristics were calculated overall and by albuminuria status. Unadjusted odds ratios for albuminuria associated with each candidate item were calculated using logistic regression. The self-assessment tool was constructed following a multi-step process28. First, all candidate items were included in a multivariable logistic regression model, and backward selection was used to eliminate items with a p-value > 0.20. Linear, quadratic and cubic terms for age were included in this model. All items excluded by the backward selection procedure were re-evaluated by adding them into the model one at a time and assessing improvement in discrimination (i.e., changes in the c-statistic and the relative integrated discrimination improvement index [RIDI])29. Next, interaction terms for age (modelled as a linear term) and diabetes with each other and all other candidate items were considered for inclusion. Finally, to develop a parsimonious self-assessment tool, each candidate item remaining in the model was considered for exclusion based on the change in c-statistic and RIDI when they were removed. The intercept and beta coefficients from a logistic regression model, including the items selected for the self-assessment tool, were used to estimate each individual’s probability of having albuminuria. Using this probability, the sensitivity and specificity of albuminuria was calculated across the full range of possible cut-points for defining high probability. Additionally, using the intercept and beta coefficients, a point scoring system for the probability of albuminuria was developed following a standard approach30.
Model validation
Using the self-assessment tool developed in the REGARDS study (i.e., intercept and beta coefficients from the final logistic regression model), each NHANES 1999-2004 participant was assigned a predicted probability of albuminuria. The c-statistic for discriminating NHANES 1999-2004 participants with and without albuminuria associated with the assigned probability was calculated for those ≥ 45 years of age. The c-statistic is a measure of how well a model discriminates between individuals with and without a condition. For the current study, the c-statistic is the proportion of all pairs of participants, one with albuminuria and one without albuminuria, wherein the participant with albuminuria has a higher predicted probability on the self-assessment tool. The sensitivity, specificity and positive and negative predictive values for albuminuria associated with a probability ≥ 10%, selected a priori, from the self-assessment tool were calculated. This cut-point was chosen based on levels that provided maximum specificity while sensitivity remained above 75% in the derivation data set. Because hypertension and diabetes are major risk factors for albuminuria, for comparative purposes the c-statistic and sensitivity, specificity and positive and negative predictive values for albuminuria were calculated for a model using self-report of a prior diagnosis of hypertension, diabetes, and either of these conditions. Additionally, a recent article proposed albuminuria screening for individuals with diabetes, hypertension or ≥ 60 years of age31. Therefore, the test characteristics of this screening approach were also determined. To assess the calibration of the self-assessment tool, the observed and predicted prevalence of albuminuria were calculated, overall and, by decile of predicted probability in NHANES 1999-2004. Additionally, a slope calibration curve was graphed. To assess the benefit of using the self-assessment tool, we also conducted a decision curve analysis32. Four secondary analyses were conducted in the validation data set (NHANES 1999-2004). First, we calculated the c-statistic, sensitivity, specificity, and positive and negative predictive values for detecting albuminuria in sub-groups defined by the presence or absence of hypertension, diabetes and eGFR < 60 ml/min/1.73 m2, separately. Second, we calculated these statistics for detecting albuminuria defined using gender-specific cut-points (≥17 mg/g in men and ≥25 mg/g in women). Next, the sensitivity, specificity and positive and negative predictive values for albuminuria associated with a probability ≥ 8% on the screening tool were calculated. This cut-point was selected so that all individuals with diabetes are referred for screening. Lastly, the c-statistic, sensitivity, specificity, and positive and negative predictive values for the self-assessment tool point scoring system were calculated.
Analysis of the REGARDS study was performed using SAS 9.2 (SAS Institute, www.sas.com). Calculation of c-statistics in NHANES 1999-2004 were performed using the weighted commands in SAS 9.2 with all other statistics for NHANES 1999-2004 calculated using SUDAAN version 10 (Research Triangle Institute, www.rti.org), accounting for the complex survey design of the study.
RESULTS
Development of the self-assessment tool
The mean age of REGARDS study participants was 63.9 ± 9.7 years, 40.6% were African-American and 37.3% were men (Table 1). The prevalence of albuminuria in the REGARDS study was 13.8%. Compared to those without albuminuria, REGARDS study participants with albuminuria were older and more likely to be African-American and men. Additionally, each of the candidate items was significantly associated with albuminuria.
Table 1.
Characteristics of the REGARDS study participants[nijsje 2]
| Albuminuria | ||||
|---|---|---|---|---|
| Candidate item | Overall (n=19,967) |
No (n=16,980) |
Yes (n=2717) | p-value |
| Age, years | 63.9 +/− 9.7 | 63.5 +/− 9.5 | 66.6 +/− 10.1 | <0.001 |
| Race | <0.001 | |||
| Whites, % | 59.4% | 89.0% | 11.0% | |
| African-American, % | 40.6% | 82.0% | 18.0% | |
| Gender | <0.001 | |||
| Women, % | 62.7% | 87.1% | 12.9% | |
| Men, % | 37.3% | 84.6% | 15.4% | |
| Education | <0.001 | |||
| ≥ High school | 88.7% | 87.1% | 12.9% | |
| < High school | 11.3% | 79.2% | 20.8% | |
| Physically inactive | <0.001 | |||
| No | 66.3% | 87.8 | 12.2 | |
| Yes | 33.7% | 83.1 | 16.9 | |
| Alcohol consumption | <0.001 | |||
| No | 63.9% | 85.0% | 15.0% | |
| Yes, < 2 drinks per day | 30.7% | 88.6% | 11.4% | |
| Yes, ≥ 2 drinks per day | 5.4% | 87.3% | 12.7% | |
| Smoking | <0.001 | |||
| Never | 48.1% | 87.7% | 12.3% | |
| Former | 37.3% | 85.8% | 14.2% | |
| Current | 14.6% | 82.4% | 17.6% | |
| Family history of ESRD | <0.001 | |||
| No | 88.2% | 86.8% | 13.2% | |
| Yes | 11.8% | 81.7% | 18.3% | |
| Self-rated health | <0.001 | |||
| Excellent | 15.8% | 92.0% | 8.0% | |
| Very good | 31.1% | 90.0% | 10.0% | |
| Good | 35.6% | 84.7% | 15.3% | |
| Fair | 14.3% | 77.6% | 22.4% | |
| Poor | 3.2% | 76.7% | 23.3% | |
| Self-report of hypertension | <0.001 | |||
| No | 43.1% | 91.7% | 8.3% | |
| Yes | 56.9% | 82.0% | 18.0% | |
| Antihypertensive medication use | <0.001 | |||
| No | 49.4% | 90.8% | 9.2% | |
| Yes | 50.6% | 81.7% | 18.3% | |
| Self-report of MI | <0.001 | |||
| No | 92.9% | 86.8% | 13.2% | |
| Yes | 7.1% | 77.9% | 22.1% | |
| Self-report of stroke | <0.001 | |||
| No | 94.5% | 86.9% | 13.1% | |
| Yes | 5.5% | 74.6% | 25.4% | |
| Self-report of diabetes | <0.001 | |||
| No | 79.0% | 89.6% | 10.4% | |
| Yes | 21.0% | 73.5% | 26.5% | |
Values shown are mean +/− SD or percentage.
ESRD – end-stage renal disease; MI – myocardial infarction; REGARDS, Results from the Reasons for Geographic and Racial Differences in Stroke
The unadjusted odds ratios for albuminuria associated with each of the candidate items are provided in the left column of Table 2. The discrimination for any individual candidate item was low; the highest c-statistic for any single candidate item was for diabetes (c-statistic, 0.613; 95% CI, 0.603 – 0.623). In a backward stepwise model, age (modelled as a linear and a quadratic term but not as a cubic term), race, gender, physical inactivity, current smoking, family history of ESRD, self-rated health, and self-report of diabetes, hypertension, myocardial infarction and stroke remained associated with albuminuria (Table 2 middle column). The c-statistic for the model including all of these items was 0.711 (95% CI, 0.701 – 0.721). No interactions were significant for age or diabetes with any of the other candidate items (all p>0.1). When each excluded item was considered for re-inclusion into the self-assessment tool, no item resulted in a significant improvement in discrimination assessed via c-statistics or the RIDI. Removing physical inactivity, a family history of ESRD, and self-report of a prior myocardial infarction from the self-assessment tool did not significantly reduce the discrimination of the self-assessment tool (c-statistic of 0.709 [95% CI, 0.699 – 0.720] and change in RIDI of −2.6% [95% CI, −5.8% to +11.8%]; Table 2, right column). The final model, consisting of age, race, gender, current smoking, self-rated health, and self-report of a prior diabetes, hypertension and stroke was well calibrated (Hosmer–Lemeshow chi-square statistic, 7.44; P value for lack of fit = 0.49) and thus becomes the REGARDS self-assessment tool for albuminuria. This is shown in algebraic form in Appendix 1 and using the points system for scoring in Appendix 2. Appendix 3 relates the score from Appendix 2 to the probabilities for having albuminuria. For example, a 60 year old (1 point), white, man (1 point) who is a non-smoker, who reports good health (1 point), has a history of hypertension (3 points) but has no history of stroke or diabetes would be assigned 6 points and a probability for albuminuria of 10.1%.
Table 2.
ORs for albuminuria associated with candidate items for inclusion in a self-assessment tool[nijsje 3]
| Candidate Item | |||
|---|---|---|---|
| OR (95% CI) for albuminuria | |||
| Unadjusted | Backward selection | Final Model | |
| Age (linear term, per 5 y) | 1.18 (1.13 – 1.22) | 1.17 (1.14 – 1.20) | 1.17 (1.15 – 1.19) |
| Age (quadratic term, per 5 y) | 1.014 (1.005 – 1.024) | 1.025 (1.016 – 1.034) | 1.025 (1.016 – 1.034) |
| Age (cubic term, per 5 y) | 0.999 (0.996 – 1.003) | -- | -- |
| African-American | 1.78 (1.64 – 1.93) | 1.40 (1.29 – 1.53) | 1.42 (1.30 – 1.55) |
| Men | 1.23 (1.14 – 1.34) | 1.30 (1.20 – 1.42) | 1.29 (1.18 – 1.40) |
| < High school education | 1.75 (1.56 – 1.95) | -- | -- |
| Physically inactive | 1.47 (1.35 – 1.60) | 1.14 (1.05 – 1.25) | -- |
| Alcohol consumption | |||
| No | 1 (reference) | -- | -- |
| Yes, < 2 drinks per day | 0.73 (0.66 – 0.80) | -- | -- |
| Yes, ≥ 2 drinks per day | 0.82 (0.68 – 0.99) | -- | -- |
| Smoking status | |||
| Never | 1 (reference) | 1 (reference) | 1 (reference) |
| Former | 1.18 (1.08 – 1.29) | ||
| Current | 1.52 (1.35 – 1.70) | 1.53 (1.37 – 1.72) | 1.55 (1.38 – 1.74) |
| Family history of ESRD | 1.48 (1.32 – 1.65) | 1.29 (1.14 – 1.45) | - |
| Self-rated health | |||
| Excellent | 1 (reference) | 1 (reference) | 1 (reference) |
| Very good | 1.28 (1.10 – 1.49) | ||
| Good | 2.06 (1.80 – 2.39) | 1.27 (1.16 – 1.41) | 1.30 (1.19 – 1.44) |
| Fair | 3.30 (2.83 – 3.85) | 1.57 (1.40 – 1.79) | 1.66 (1.48 – 1.87) |
| Poor | 3.47 (2.78 – 4.34) | 1.44 (1.15 – 1.79) | 1.58 (1.30 – 1.95) |
| Self-report of diabetes | 3.10 (2.84 – 3.37) | 2.31 (2.11 – 2.54) | 2.34 (2.14 – 2.57) |
| Self-report of hypertension | 2.43 (2.22 – 2.66) | 1.59 (1.44 – 1.76) | 1.61 (1.46 – 1.78) |
| Antihypertensive medication | 2.20 (2.02 – 2.40) | -- | -- |
| Self-report of MI | 1.88 (1.65 – 2.14) | 1.18 (1.02 – 1.37) | - |
| Self-report of stroke | 2.25 (1.95 – 2.59) | 1.40 (1.20 – 1.63) | 1.42 (1.22 – 1.66) |
| Tool Performance | |||
| C-statistic (95% CI) | |||
|---|---|---|---|
| Unadjusted | Backward selection | Final Model | |
| Correlation | 0.613 (0.603 – 0.623)† | 0.711 (0.701 – 0.721) | 0.709 (0.699 – 0.720) |
-- not included in the regression model[nijsje 4].
ESRD – end-stage renal disease; MI – myocardial infarction; OR, odds ratio; CI, confidence interval
The backward selection model includes all variables associated, after multivariable adjustment, with albuminuria at a p-value <0.20.
Former and never smoking and excellent and very good self-rated health were grouped together as the referent category in the backward selection and multivariable models as the association of these variables with albuminuria was similar.
This c-statistic is for diabetes, the single item with the highest c-statistic in the unadjusted models.
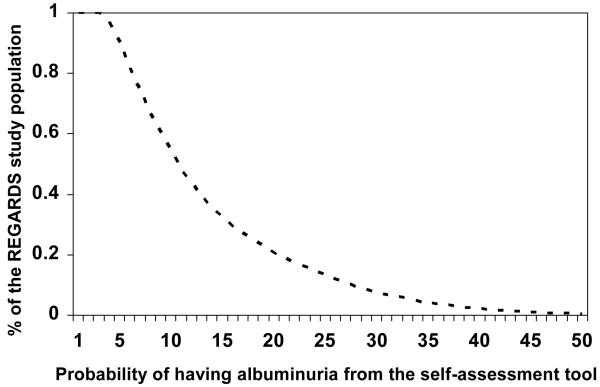
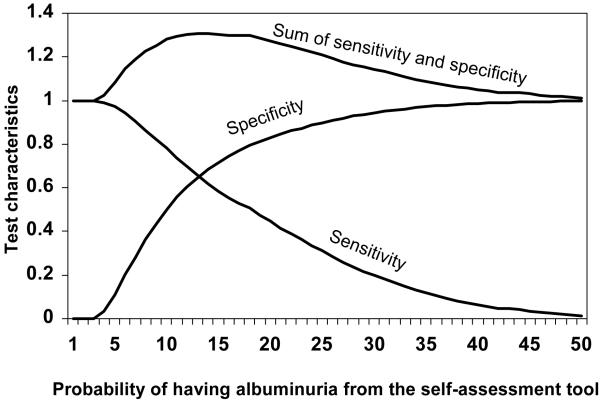
The percent of REGARDS study participants who would be categorized as having a high probability for albuminuria across a range of possible cut-points is provided in Figure 1. In the REGARDS study development data set, 54% of individuals had a probability for albuminuria ≥ 10% via the self-assessment tool. The sensitivity and specificity for albuminuria using the self-assessment tool is provided in Figure 2. A probability ≥ 10% was defined as high in order to capture a high percentage of albuminuria cases (sensitivity = 78%) while maintaining adequate specificity (50%). The positive predictive value and negative predictive value was 20% and 94%, respectively. Using higher cut-points to define high probability for albuminuria would result in higher specificity but lower sensitivity (i.e., missing a higher percentage of albuminuria cases) while lower cut-points would result in substantially more positive test results.
Figure 1.
Distribution of the REGARDS study population by probability of albuminuria on the self-assessment tool
The curve shows the % of the REGARDS study population with a probability of albuminuria higher than the value on the x-axis. For example, 54% of the REGARDS study population has a probability of albuminuria from the self-assessment tool ≥ 10%.
Figure 2.
Sensitivity and specificity of the self-assessment tool for albuminuria in the derivation study (REGARDS study)
Using a cut-point ≥ 10% from the self-assessment tool, the sensitivity and specificity for albuminuria are 78% and 50%, respectively, in the REGARDS study population.
External Validation of the self-assessment tool
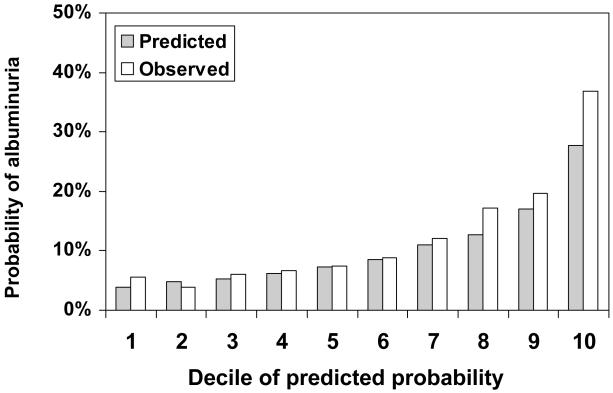
The observed prevalence of albuminuria for US adults ≥ 45 years of age in NHANES 1999-2004 was 12.5% and the mean predicted probability for albuminuria using the self-assessment tool was 10.4%. Participant characteristics for NHANES 1999-2004 are provided in Table S1 (available as online supplementary material). The distribution of scores on the self-assessment tool for individuals with and without albuminuria in NHANES 1999-2004 is provided in Figure S1. The median probability for albuminuria on the self-assessment tool for individuals without and with albuminuria was 0.07 (25th – 75th percentile, 0.05 – 0.12) and 0.13 (25th – 75th percentile, 0.08 – 0.22), respectively. The self-assessment tool demonstrated higher discrimination than a self-report of hypertension, diabetes, either condition, or the combination of hypertension, diabetes or age ≥ 60 years (Table 3). In NHANES 1999-2004, 36% of US adults had a probability ≥ 10% on the self-assessment tool and would, thus, test positive. The probability ≥ 10% cut-point provided a sensitivity of 66%, specificity of 68%, and positive and negative predictive values of 23% and 93%, respectively. The predicted and observed probability for albuminuria was similar for the lower percentiles of risk, but the self-assessment tool under-estimated the probability at higher levels of predicted probabilities (Figure 3 and Figure S2). Using the cut-point of 10% to define high probability for albuminuria, the decision curve in Figure S3 supports the use of the albuminuria screening tool (i.e., the net benefit of the screening tool at 10% is greater than screening everyone and screening no one).
Table 3.
Test characteristics of the self-assessment tool in the external validation set (NHANES 1999-2004)
| Predictive value | ||||||
|---|---|---|---|---|---|---|
| C-statistic | Positive test result |
Sensitivity | Specificity | Positive | Negative | |
| Self-assessment tool ≥ 10% | 0.714 | 36% | 66% | 68% | 23% | 93% |
| Self-report of diabetes | 0.597 | 11% | 28% | 91% | 31% | 90% |
| Self-report of hypertension | 0.574 | 40% | 54% | 62% | 17% | 90% |
| Self report of diabetes or hypertension |
0.638 | 44% | 63% | 59% | 18% | 92% |
| Self report of diabetes or hypertension or age ≥ 60 years |
0.592 | 63% | 84% | 40% | 17% | 94% |
Positive test result is defined as a probability of albuminuria ≥ 10% for the self-assessment tool.
NHANES, National Health and Nutrition Examination Survey
Figure 3.
Predicted and observed probability of albuminuria according to decile of predicted probability assigned by the self-assessment tool in the validation cohort (NHANES 1999-2004)
Sensitivity Analyses
The test characteristics for the self-assessment tool among individuals with and without hypertension, diabetes mellitus, or an eGFR < 60 ml/min/1.73 m2, separately, are provided in Table S2. Only 1.7% of NHANES 1999-2004 participants who reported a prior diagnosis of diabetes had a probability of albuminuria < 10% on the self-assessment tool and would test negative. Test characteristics of the screening tool were virtually identical when all individuals with diabetes were automatically categorized as high risk (i.e., grouping them with their peers with a probability ≥ 10%). Defining a positive test result as ≥ 8% on the screening tool resulted in 49% of US adults testing positive, including all individuals with diabetes. Using this cut-point, the sensitivity, specificity, and positive and negative predictive values of the screening tool were 75%, 55%, 19% and 94%, respectively.
Among adults ≥ 45 years of age in NHANES 1999-2004, 17.2% had albumuinuria defined using gender-specific cut-points (≥17 mg/g for men and ≥25 mg/g for women). Using this definition for albuminuria, the c-statistic for the self-assessment tool was 0.713. Additionally, the sensitivity, specificity, positive and negative predictive values were 63%, 70%, 30%, and 90%, respectively.
Using the point scoring system for the self-assessment tool, the c-statistic was 0.706. The mean predicted probability for albuminuria was 11.4%. The sensitivity, specificity, and positive and negative predictive values using the point scoring system and a probability ≥ 10% cut-point were 73%, 59%, 20%, and 94%, respectively.
DISCUSSION
Albuminuria is a common marker of kidney disease and affects more than 10 million US adults1. However, the majority of individuals with kidney disease are unaware of their diagnosis8;9;33. Using data from two large studies, we developed and validated a simple self-assessment tool designed to identify individuals with a high probability of albuminuria. This tool maintained good discrimination as indicated by a c-statistic of 0.709 and 0.714 in the REGARDS study and in NHANES 1999-2004, respectively. Additionally, using a cut-point of ≥ 10% to define a high probability of albuminuria, 66% of those with albuminuria were properly identified as having a high probability while 68% of those without albumuniria were properly identified as having a low probability for albuminuria.
The self-assessment tool we present used a structured approach to produce a parsimonious model comprised of age, six yes/no questions and assessment of self-rated health. Each of these items has been previously reported, individually, to be associated with albuminuria21;22;34;35. The current study extends prior reports by grouping items into a screening tool that could be used to identify individuals in whom urinary albumin should be measured. This tool provided significantly better discrimination than any of these items individually. Although population-wide albuminuria screening is not cost-effective, screening in selected high risk populations may be12; this tool could be used to inform individuals with a high risk so they can talk to their doctors about further testing. Given the sensitivity and specificity of the self-assessment tool were 66% and 68%, respectively future studies may endeavour to identify screening tools with improved test characteristics.
Diabetes is considered a major risk factor for albuminuria36-38. While diabetes was associated with a significantly increased odds ratio for albuminuria in the REGARDS study, its ability to distinguish between individuals with and without albuminuria was low. Additionally, in the NHANES 1999-2004 data set, the c-statistic was only 0.597 for self-reported diabetes and the sensitivity was 28%. This was markedly lower than for the self-assessment tool.
Over 98% of individuals with diabetes were defined as “high risk” for albuminuria in NHANES 1999-2004 (the external data set) based on the a priori chosen cut-point of ≥ 10%. Even though patients with diabetes are recommended annual screening for albuminuria by the National Kidney Foundation and American Diabetes Association, prior studies indicate that routine screening does not occur17;19;39;40. Given the low rate of screening for individuals with diabetes as well as the goal of producing a screening tool aimed at increasing albuminuria to the highest degree possible, we think the self-assessment tool developed in the current study is applicable to individuals with and without diabetes. As demonstrated in sensitivity analyses, the self-assessment tool can be easily adapted, by choosing a lower cut-point to define “high risk” or automatically triaging persons reporting a prior diagnosis of diabetes, such that all individuals with diabetes are recommended albuminuria screening.
Many clinical characteristics beyond the self-reported items evaluated in the current analysis are associated with albuminuria23;41-43. We purposefully chose to limit the present analysis to items that individuals can report. This will allow for broader dissemination and application of the tool developed in the present study. The self-assessment tool does not require a healthcare visit and can be completed by individuals at home (e.g., via the internet) or in community settings (e.g., at health fairs). It is likely that the addition of clinical information (e.g., eGFR) would increase the discrimination of the self-assessment tool. However, such data would limit the broad number of settings in which this tool can be utilized.
While self-assessment tools, such as the one developed in the current analysis, allow for widespread use, they do not provide clinical diagnoses. Individuals with a high probability for albuminuria based on the self-assessment tool should be recommended for follow-up with a healthcare provider for further screening via dipstick or spot urine testing for albuminuria. The challenges of translating risk into patient action are well documented. The approach for effectively communicating the need for screening for individuals who test positive on the albuminuria self-assessment tool requires further study and testing.
The results from the current analysis need to be interpreted in the context of certain limitations. All data were collected during a single study visit. As such, neither the REGARDS study nor NHANES 1999-2004 had data on the persistence of albuminuria. Analysis of NHANES III, from 1988-1994, indicate a substantial proportion of adults with albuminuria on a single measure may not have it on subsequent measurements44. Also, the REGARDS study was limited to adults ≥ 45 years of age. Although albuminuria is not common among younger adults, future work may be useful to develop a self-assessment tool for this population21. Finally, the self-assessment tool was developed through self- and interview administered questionnaires. Future studies evaluating its test characteristics through other modes of administration are warranted. Despite these limitations, the current analysis has many strengths. Both the REGARDS study and NHANES 1999-2004 included nationwide samples of US adults, extensive data collection, and were conducted following a standardized protocol which included rigorous procedures for data collection. Also, the availability of an external data set (NHANES 1999-2004) allowed for assessing the independent validity of the self-assessment tool. External validation is necessary before a prediction models can be recommended for widespread use. It is noteworthy that discrimination between those with and without albuminuria remained high in the external validation data set.
In conclusion, albuminuria is emerging as an important risk factor for ESRD, cardiovascular disease, and all-cause mortality and early treatment may reduce the risk of these events. Despite its prognostic importance and availability of effective treatments, the awareness of albuminuria in the general population is low. The self-assessment tool developed and validated in the current analysis maintains strong screening test characteristics and may be helpful for educating adults about their risk for albuminuria. Using this tool in the general population may increase the awareness, detection and treatment for albuminuria.
Supplementary Material
ACKNOWLEDGEMENTS
Support: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation or approval of the manuscript.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Appendix 1
The estimated probability of having albuminuria obtained from a logistic regression model with the components of the albuminuria self-assessment screening tool as predictor variables in the REGARDS study.
Let X = −5.1062 + 0.0318 * (age in years) + 0.000994 * (age in years - 65)2 + 0.3476 * (1 for African-Americans, 0 for other race-ethnicities) + 0.2515 * (1 for men, 0 for women) + 0.4383 * (1 for current smokers, 0 for non-smokers) + 0.2615 * (1 if self-rated health is good) + 0.5043 * (1 if self-rated health is fair) + 0.4545 * (1 if self-rated health is poor) + 0.8515 * (1 for self-report of a diagnosis of diabetes, 0 for those not reporting diabetes) + 0.4755 * (1 if self-report of a diagnosis of hypertension, 0 for those not reporting hypertension) + 0.3536 * (1 for self-report of a prior stroke, 0 for those without a prior stroke)
Then the estimated probability of having albuminuria = exp(X)/(1+exp(X))
Appendix 2
Appendix 2.
Point scoring system for the albuminuria self-assessment screening tool
| Points assigned | ENTER APPROPRIATE POINTS |
|
|---|---|---|
| Age group | Age group | |
| <45 years | 0 | |
| 45 to 54 years | 0 | |
| 55 to 64 years | 1 | |
| 65 to 74 years | 3 | |
| ≥ 75 years | 6 | |
| White | 0 | Race |
| African-American | 2 | |
| Women | 0 | Gender |
| Men | 1 | |
| Non-smoker | 0 | Smoking |
| Current smoking | 3 | |
| Self-rated health | Self-rated health | |
| Excellent | 0 | |
| Very good | 0 | |
| Good | 1 | |
| Fair | 3 | |
| Poor | 3 | |
| Self-report of diabetes | Diabetes | |
| No | 0 | |
| Yes | 5 | |
| Self-report of hypertension | Hypertension | |
| No | 0 | |
| Yes | 3 | |
| Self-report of stroke | Stroke | |
| No | 0 | |
| Yes | 2 | |
| TOTAL | MAXIMUM = 25 |
Appendix 3
Appendix 3.
Probability of having albuminuria based on the point scores on the self-assessment screening tool from Appendix 2
| Total Points | Probability for ACR ≥ 30 mg/g |
|---|---|
| 0 | 0.036 |
| 1 | 0.043 |
| 2 | 0.051 |
| 3 | 0.061 |
| 4 | 0.072 |
| 5 | 0.085 |
| 6 | 0.101 |
| 7 | 0.119 |
| 8 | 0.139 |
| 9 | 0.163 |
| 10 | 0.189 |
| 11 | 0.219 |
| 12 | 0.252 |
| 13 | 0.288 |
| 14 | 0.327 |
| 15 | 0.369 |
| 16 | 0.413 |
| 17 | 0.458 |
| 18 | 0.504 |
| 19 | 0.550 |
| 20 | 0.595 |
| 21 | 0.638 |
| 22 | 0.679 |
| 23 | 0.718 |
| 24 | 0.754 |
| 25 | 0.786 |
ACR, albumin-creatinine ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: The supplementary material accompanying this article (doi:-------) is available at www.ajkd.org.
Reference List
- (1).Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- (2).Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Perkovic V, Verdon C, Ninomiya T, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008;5:e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hermans MM, Henry R, Dekker JM, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol. 2007;18:1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- (5).Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- (6).Ninomiya T, Perkovic V, Verdon C, et al. Proteinuria and stroke: a metaanalysis of cohort studies. Am J Kidney Dis. 2009;53:417–425. doi: 10.1053/j.ajkd.2008.08.032. [DOI] [PubMed] [Google Scholar]
- (7).Warnock DG, Muntner P, McCullough PA, et al. Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis. 2010;56:861–871. doi: 10.1053/j.ajkd.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110:921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- (9).Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensinconverting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- (10).Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–2033. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- (11).Whaley-Connell A, Sowers JR, McCullough PA, et al. Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) Am J Kidney Dis. 2009;53:S11–S21. doi: 10.1053/j.ajkd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- (12).Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- (13).Hoerger TJ, Wittenborn JS, Segel JE, et al. A health policy model of CKD: 2. The cost-effectiveness of microalbuminuria screening. Am J Kidney Dis. 2010;55:463–473. doi: 10.1053/j.ajkd.2009.11.017. [DOI] [PubMed] [Google Scholar]
- (14).Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283:889–896. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- (15).Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ. 1995;311:1595–1599. doi: 10.1136/bmj.311.7020.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Carel RS, Silverberg DS, Kaminsky R, Aviram A. Routine urinalysis (dipstick) findings in mass screening of healthy adults. Clin Chem. 1987;33:2106–2108. [PubMed] [Google Scholar]
- (17).Mainous AG, III, Gill JM. The lack of screening for diabetic nephropathy: evidence from a privately insured population. Fam Med. 2001;33:115–119. [PubMed] [Google Scholar]
- (18).Frazee LA, Samandari S, Tanphaichitr N, Bourguet CC, Pfister EW. Screening for nephropathy and antiangiotensin use among diabetic patients in an academic community medical center. Am J Ther. 2006;13:18–23. doi: 10.1097/01.mjt.0000174345.59177.9b. [DOI] [PubMed] [Google Scholar]
- (19).Hueston WJ, Scibelli S, Mainous AG., III Use of microalbuminuria testing in persons with type 2 diabetes: are the right patients being tested? J Fam Pract. 2001;50:669–673. [PubMed] [Google Scholar]
- (20).de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: Public health perspectives. J Am Soc Nephrol. 2006;17:2120–2126. doi: 10.1681/ASN.2006010097. [DOI] [PubMed] [Google Scholar]
- (21).Islam TM, Fox CS, Mann D, Muntner P. Age-related associations of hypertension and diabetes mellitus with chronic kidney disease. BMC Nephrol. 2009;10:17. doi: 10.1186/1471-2369-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- (23).Sechi LA, Zingaro L, Catena C, Perin A, De Marchi S, Bartoli E. Lipoprotein(a) and apolipoprotein(a) isoforms and proteinuria in patients with moderate renal failure. Kidney Int. 1999;56:1049–1057. doi: 10.1046/j.1523-1755.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- (24).Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- (25). [Accessed September 7, 2004];NHANES 1999-2002 addendum to the NHANES III analytic guidelines. 2004 Available at http://www.cdc.gov/nchs/data/nhanes/guidelines1.pdf. serial online.
- (26).National Center for Health Statistics [Accessed September 1, 2006];NHANES 2003-2004. 2009 http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/nhanes03_04 [serial online]
- (27).KDOQI Working Group Definition and classification of stages of chronic kidney disease. Am J Kidney Dis. 2002;39:S46–S75. [Google Scholar]
- (28).Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- (29).Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- (30).Sullivan LM, Massaro JM, D’Agostino RB., Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- (31).Collins AJ, Vassalotti JA, Wang C, et al. Who should be targeted for CKD screening? Impact of diabetes, hypertension, and cardiovascular disease. Am J Kidney Dis. 2009;53:S71–S77. doi: 10.1053/j.ajkd.2008.07.057. [DOI] [PubMed] [Google Scholar]
- (32).Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168:2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hogan SL, Vupputuri S, Guo X, et al. Association of cigarette smoking with albuminuria in the United States: the third National Health and Nutrition Examination Survey. Ren Fail. 2007;29:133–142. doi: 10.1080/08860220601098888. [DOI] [PubMed] [Google Scholar]
- (35).Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- (36).Mogensen C. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. New England Journal of Medicine. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- (37).Ritz E. Nephropathy in type 2 diabetes. J Intern Med. 1999;245:111–126. doi: 10.1046/j.1365-2796.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- (38).Viberti G. Etiology and prognostic significance of albuminuria in diabetes. Diabetes Care. 1988;11:840–845. doi: 10.2337/diacare.11.10.840. [DOI] [PubMed] [Google Scholar]
- (39).Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- (40).American Diabetes Association Diabetic nephropathy. Diabetes Care. 2002;25:S85–S89. [Google Scholar]
- (41).Kshirsagar AV, Bomback AS, Bang H, et al. Association of C-reactive protein and microalbuminuria (from the National Health and Nutrition Examination Surveys, 1999 to 2004) Am J Cardiol. 2008;101:401–406. doi: 10.1016/j.amjcard.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Jylha M, Volpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population sample. J Clin Epidemiol. 2006;59:465–471. doi: 10.1016/j.jclinepi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- (43).Jenkins AJ, Steele JS, Janus ED, Santamaria JD, Best JD. Plasma apolipoprotein (a) is increased in type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1992;35:1055–1059. doi: 10.1007/BF02221681. [DOI] [PubMed] [Google Scholar]
- (44).Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.