Abstract
Epithelial cells have the ability to regulate paracellular permeability dynamically in response to extracellular stimuli. With every respiratory effort, airway epithelial cells are exposed to both physiologic as well as pathologic stimuli, and regulation of the epithelial barrier in response to these stimuli is crucial to respiratory function. We report that increased membrane septin-2 localization mediates decreases in paracellular permeability by altering cortical actin arrangement in human airway epithelial cells. This phenomenon occurs in response to both physiologic levels of shear stress and a pathologic stimulus, particular matter exposure. The resulting changes in barrier function in response to septin-2 redistribution have a significant impact on the ability of the apical ligand, epidermal growth factor, to interact with its receptor, epidermal growth factor receptor, which is segregated to the basolateral side in airway epithelial cells. This suggests that the dynamic regulation of the epithelial barrier function is essential in regulating signaling responses to extracellular stimuli. These findings indicate that septin-2 plays a fundamental role in regulating barrier function by altering cortical actin expression.
Keywords: epithelial barrier function, paracellular permeability, septin-2, actin cytoskeleton
CLINICAL RELEVANCE.
This article shows a novel role for septin-2 in airway epithelial barrier function in response to both a physiologic level of shear stress as well as a pathologic exposure of particulate matter. In addition, it introduces the idea that in response to luminal stimuli, the airway epithelial barrier function dynamically changes and that can translate to changes in receptor–ligand interactions and resultant cell signaling.
Airway epithelial cells form an important barrier that protects the subepithelial tissue from a wide array of noxious substances, including allergens, viruses, inhaled particles and irritants, and luminal microbial pathogens. The regulation of this epithelial barrier depends on a complex of proteins that compose distinct intercellular junctions, tight junctions, adherens junctions, and desmosomes, each of which exhibits cell type–specific regulation by various growth factors, agonists, and second messengers (1). In response to both physiologic and pathologic stimuli in the lumen, a coordinated epithelial response is necessary to regulate barrier function appropriately. Common to the regulation of the intercellular junctions is cortical actin. Modulation of cortical actin alters permeability in multiple cell types, from intestinal epithelial cell lines (2–4) to pulmonary endothelial cells (5, 6), and is intimately associated with regulation of the intercellular junctions (7).
Septin-2 is a member of a highly conserved GTPase family found in fungi and animals. Septins have been implicated in diverse cellular processes, including cytokinesis, formation of diffusion barriers, and vesicle trafficking. In yeast cells, septin-2 is responsible for separating the mother cell contents before division (8). In humans, 12 septin genes have been found so far, many of which also undergo alternative splicing to generate dozens of polypeptides. Septin-2 partially colocalizes with actin bundles in mammalian interphase, and is required for reorganization of the actin cytoskeleton in migrating or ruffling cells (9). Just recently, a new role for septin-2 has been identified in Xenopus oocytes where inhibition of septin-2 with forchlorfenuron (FCF) leads to defective gastrulation, and potential changes in cell shape (10, 11). It has been identified, in association with the protein, Fritz, to be involved in collective cell movement and ciliogenesis (10). However, in differentiated monolayers that are no longer dividing, the role of septin-2 has not been elucidated.
We have previously shown that, in response to physiologic levels of apical shear stress, the paracellular permeability of airway epithelial cells decreases, reflecting barrier enhancement. This barrier enhancement is associated with reorganization of the actin cytoskeleton with increased cortical actin ring (12). However, the molecular mechanisms mediating this cytoskeletal rearrangement are not known. In this study, we show that, in response to both low levels of shear stress and a pathologic stimulus, such as a single dose of particulate matter (PM) (13), the epithelial barrier is enhanced and septin-2 moves to the plasma membrane. In response to shear stress, this septin-2 redistribution is associated with increased septin-2–actin interactions and actin rearrangement, which is required to cause enhancement of the barrier in response to luminal stimuli.
MATERIALS AND METHODS
Materials
Antibody to septin-2 was a gift from Ian Macara (University of Virginia, Charlottesville, VA). Unless specified, all other reagents were purchased from Sigma (St. Louis, MO).
Cell Culture
Primary human bronchial epithelial (NHBE) cells (Lonza, Walkersville, MD) were grown on collagen-coated inserts (Falcon, Franklin Lakes, NJ) at 37°C with 5% CO2 and maintained at an air–liquid interface for 6–9 weeks before study. The transepithelial resistance was greater than 400 ohms when cells were used. Madin Darby Canine Kidney cells with and without yellow fluorescent protein septin-2 plasmid (gift of Elias Spiliotis, Drexel University, Philadelphia, PA) were cultured on inserts (transepithelial resistance > 240 ohms). Human pulmonary artery endothelial cells (HPAECs; Lonza) were grown to confluence in gold microelectrodes containing polycarbonate wells (transendothelial electrical resistance [TER] > 1,000), and TER was measured as previously described (14).
Cells were harvested and lysed in RIPA buffer (12). In certain experiments, membrane preparations were performed as previously described (12). Equal amounts of total protein (10–20 μg) in 1.5% (wt/vol) SDS were loaded per lane, as previously described (15).
Transfection
Cells were grown in chamber slides to 50–60% confluence and transiently transfected (1 μg/well) with YFP–septin-2 cDNA using FuGENE 6 (1.5 μl; Roche, Indianapolis, IN) or a control plasmid, according to the manufacturer's recommendations.
Confocal Imaging
Confocal laser microscopy (Leica SP5; Bannockburn, IL) was performed on cells grown on inserts using antibodies against septin-2 or actin and appropriate secondary antibodies (Alexa 488 or Alexa 555; Molecular Probes, Carlsbad, CA). Fluorescence resonance energy transfer (FRET) with acceptor photobleaching (AB) was performed on fixed samples. The donor fluorescence intensity in a region of interest before and after AB was compared. Positive FRET occurred if AB resulted in increased donor fluorescence, as described in the online supplement.
Immunoprecipitation
The crosslinking and immunoprecipitation (IP) was performed as previously described (16), where 2 mM Dithiobis[succinimidyl propionate] in PBS/25 mM Hepes were added to static and shear-exposed monolayers for 30 minutes at room temperature, after which the reaction was quenched with 20 mM Tris (pH 7.5) for 15 minutes. Co-IP of septin-2 and actin was performed, and the samples were analyzed by immunoblotting.
Lentiviral Transduction
Lentivirus expressing either short hairpin RNA directed against septin-2 or a nontargeting control (Sigma) (107 infectious particles/ml) was used at the lowest concentration required for protein knockdown. Cells were incubated with polybrene (8–16 μM) in 1 ml per insert. Apical media were removed, basal media changed, and cells were used 48–72 hours later.
Shear Stress
Fluid flow to generate shear levels of 1.5–3.0 dynes/cm2 was applied as described previously (12). Fluid flow rates of 0.5–1.0 ml/min provide a shear stress consistent with this magnitude for airway epithelial cells in vivo.
Permeability Assay
Paracellular permeability of cells was assessed by the passage of 4-kD FITC-dextran across the monolayer, as previously described (12). The concentration was measured by fluorometry.
PM
Ambient Baltimore PM was collected using a high-volume cyclone collector with a cutoff point of 0.3-μm aerodynamic diameter when operated at a flow rate of 1 m3/min. This has been used to collect Baltimore PM for studies of air pollution impact on airway hyperresponsiveness, and the PM has been well characterized, as previously described (17, 18).
Statistical Analysis
Statistical analysis was performed using STATA 9 (Stata Corporation, College Station, TX).
RESULTS
Airway Epithelial Barrier Enhancement Is Associated with Altered Septin-2 Expression
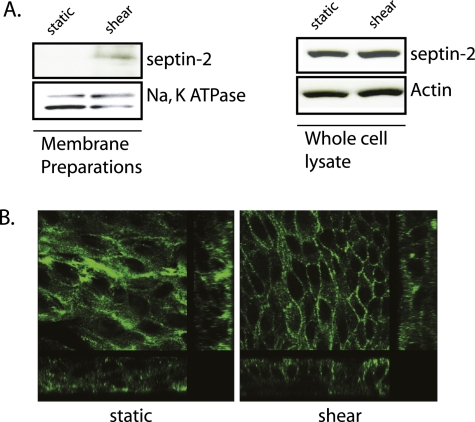
We previously demonstrated that low levels of shear stress lead to increases in airway epithelial barrier function, using both in vivo and in vitro models. Although the in vitro models have used only unidirectional shear stress, we have evidence of similar findings in vivo using both unidirectional (12) and bidirectional flow (unpublished data, V.S.). To dissect potential mechanisms mediating the shear-induced barrier enhancement that we have described (12), membrane preparations of both NHBE cells and 16HBE+adenoAQP5 cells under static and shear conditions were sent to the Johns Hopkins National Heart, Lung, and Blood Institute Proteomics Center for processing. In both of these cell types, we have shown that shear enhances barrier function. Although both shear and static conditions altered over 100 proteins, a comparison of the two conditions revealed only a limited number of proteins that exhibited consistent changes in membrane fraction abundance, one of which was septin-2. With enhanced barrier function, septin-2 increased in the membrane fraction. To confirm that shear increases in membrane expression of septin-2, immunoblotting of membrane fractions (Figure 1A) and immunofluorescence (Figure 1B) of NHBE cells were performed. Both revealed increased membrane expression of septin-2 in response to shear stress. Of note, there was no change in total septin-2 expression in the cells (Figure 1A), and the increase in membrane fraction was due to protein redistribution.
Figure 1.
Shear stress leads to increased membrane-associated Septin-2 in primary human bronchial epithelial (NHBE) cells. (A) NHBE cells were placed under shear stress, as described in Materials and Methods, for 2 hours and membrane preparations were compared with control cells under static condition. Immunoblotting shows that shear stress increases the membrane fraction of septin-2 in NHBE cells (left), whereas there is no change in septin-2 abundance in the whole-cell lysate (47). (B) Confirmation of shear-induced increases in membrane septin-2 expression is visualized using confocal immunofluorescence.
Septin-2 Alters Barrier Function in a Confluent Monolayer
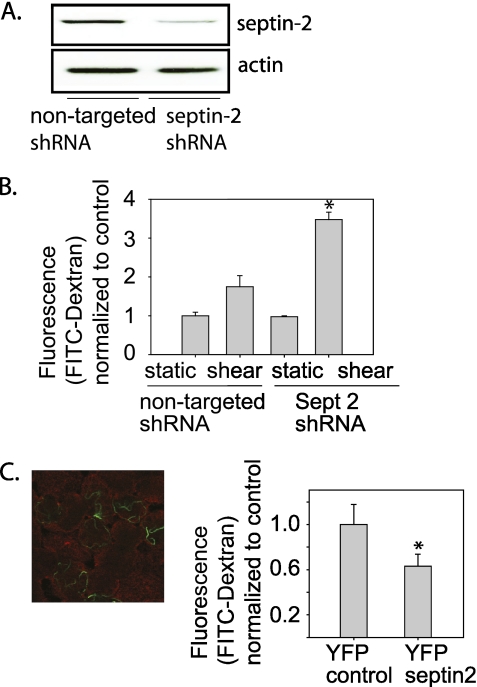
To identify a potential role for septin-2 in regulation of barrier function, we used specific lentiviral shRNA to knock down endogenous septin-2 expression in MDCK cells (Figure 2A). Under static conditions, barrier function was not altered by septin-2 knockdown. In contrast to NHBE cells, shear stress increased paracellular permeability in MDCK cells (Figure 2B). To determine if septin-2 affects barrier function in MDCK cells, we infected MDCK cells with shRNA against septin-2 or control lentivirus. When septin-2 was knocked down, shear induced an even greater increase in paracellular permeability (Figure 2B). To test if overexpression of septin-2 enhanced barrier function, MDCK cells were transiently transfected with YFP–septin-2 (∼40% transfection efficiency). After transient transfection, the majority of YFP–septin-2 was present on the membrane (Figure 2C, right). Cells expressing YFP–septin-2 had decreased paracellular permeability compared with cells transfected with a control plasmid (Figure 2C, left).
Figure 2.
Septin-2 modulates barrier function properties in an Madin Darby Canine Kidney monolayer. (A) Using lentiviral delivery of short hairpin RNA directed against Septin-2, we have caused greater than 80% reduction in protein expression, as confirmed by immunoblotting. (B) After septin-2 knockdown, there is no significant change in barrier properties under static conditions, but when cells are placed under shear stress, there is a significant worsening in barrier function compared with cells transduced with a nontargeting construct. (C) (Left) Of note, when overexpressed, the majority of septin-2 is seen at the membrane (the membrane is shown in red, and yellow fluorescent protein–septin-2 is shown in green); (right) transient transfection of overexpression of yellow fluorescent protein–septin-2 in MDCK cells leads to barrier enhancement (n = 3; *P < 0.05 with one-way ANOVA).
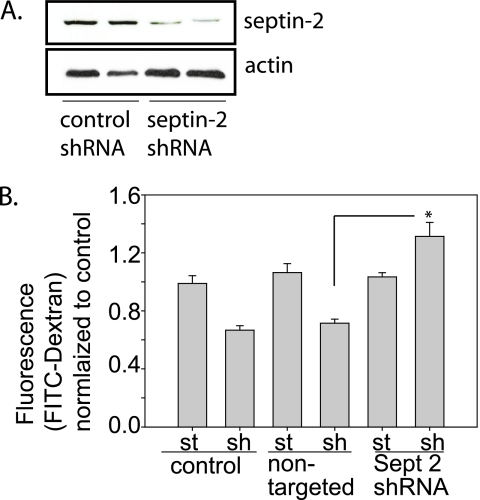
To assess if changes in septin-2 expression altered airway epithelial barrier function, NHBE cells were transduced with septin-2 shRNA or a nontargeting shRNA (Figure 3A). Under static conditions, expression of septin-2 shRNA or the nontargeting shRNA had no effect on barrier function. However, after exposure to shear stress, NHBE cells with septin-2 knockdown no longer exhibited shear-induced barrier enhancement; rather, paracellular permeability was significantly increased (Figure 3B).
Figure 3.
Septin-2 modulates barrier function properties in NHBE cells. (A) NHBE cells treated with lentiviral shRNA directed against septin-2 have more than 85% reduction in protein expression, as confirmed by immunoblotting. (B) After septin-2 knockdown, again there is no significant change in barrier properties under static conditions. In NHBE cells, shear leads to barrier enhancement, which is preserved in cells transduced with the nontargeting lentiviral construct, but exposure to septin-2 shRNA causes significant barrier disruption. (Sept, septin; sh, shear; st, static; n = 4; *P < 0.05 with one-way ANOVA).
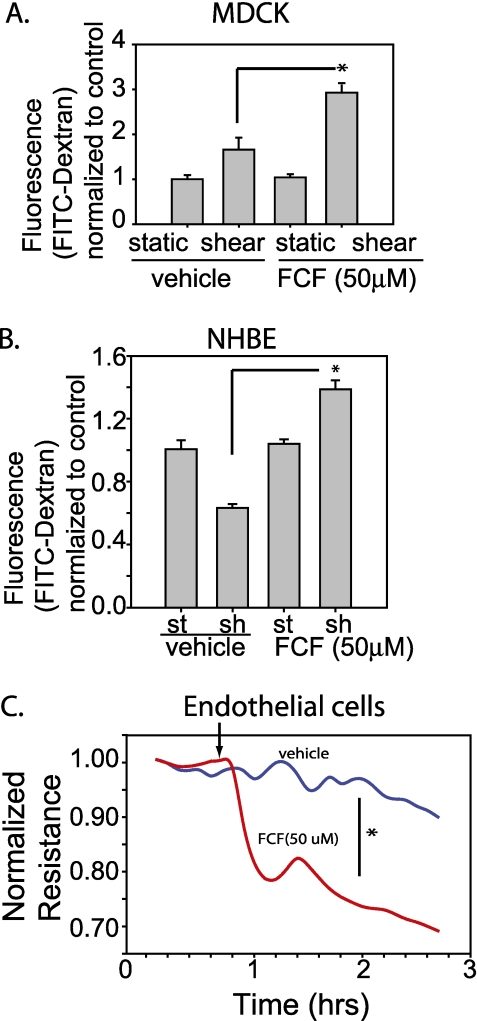
To determine if septin-2 regulation of epithelial barrier function was epithelial cell specific, we assessed the effect of septin-2 on pulmonary endothelial barrier function. The role of cytoskeletal organization in pulmonary endothelial barrier function has been investigated extensively (14, 19–33). We measured TER as a reflection of endothelial monolayer permeability in control HPAECs compared with HPAECs treated with FCF (50 μm), which alters septin-2 assembly and organization in vitro by stabilizing septin filaments (34). Treatment with FCF increased paracellular permeability of both MDCK and NHBE cells under shear stress (Figures 4A and 4B), similar to the effects of septin-2 knockdown on barrier function. Treatment with the septin-2 inhibitor, FCF, caused endothelial barrier dysfunction (Figure 4C).
Figure 4.
Treatment with the septin-2 inhibitor, forchlorfenuron (FCF), decreases barrier function. (A) MDCKs treated with FCF have increased permeability under shear conditions compared with vehicle-treated cells (n = 3). (B) NHBE cells also show evidence of increased permeability under shear conditions in FCF-treated cells (n = 4). (C) Human pulmonary artery endothelial cells (HPAECs) treated with FCF have a significant drop in resistance compared with vehicle control. Arrow indicates the addition of either the drug or vehicle to the cells (n = 5). *P < 0.05.
Septin-2 Directly Binds to Actin and Mediates Cortical Actin Reorganization
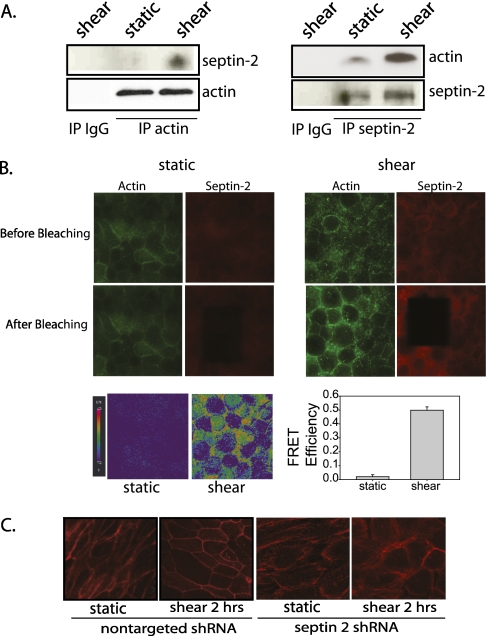
Reduced paracellular permeability can result from cytoskeletal reorganization, and septin-2 expression is involved in actin rearrangements (9). We have shown that shear stress leads to actin rearrangement, contributing to the shear-induced barrier enhancement seen in NHBE cells (12). To determine if direct protein binding was responsible for the increased cortical actin observed in response to shear stress, we assessed for interaction between actin and septin-2. IP of actin under shear conditions also pulled down more septin-2 than observed in static conditions (Figure 5A). Similarly, IP of septin-2 pulled down more actin in shear than in static conditions (Figure 5A). To confirm protein–protein interaction further, we performed FRET AB in fixed static and shear samples probed for actin (Alexa488) and septin-2 (Alexa 555) to determine if the two proteins were spatially located close to one another under either condition. Excitation of one fluorophore results in excitation in the other, as long as the two fluorophores are in nanometer distance of each other, suggesting protein–protein interaction. In both static and shear conditions, the immunofluorescence of actin depicted corresponds specifically to the area that received photobleaching. Under static conditions after photobleaching, there was no increase in donor intensity (Figure 5B, left). However, after shear stress, AB resulted in increased donor intensity (Figure 5B, right). FRET maps were generated to visualize the interaction, with orange-red suggesting increased FRET, and blue with less FRET. After shear stress, there is a significant increase in the FRET sample. A total of 15 regions of interest was randomly preselected in both fields to quantify FRET efficiency. After shear stress, there was a significant increase in FRET efficiency, suggesting actin–septin-2 interaction under shear stress. Both biochemical methods of IP, as well as the fluorescent method of FRET, indicate that, in response to shear stress, there is a significant increase in the interaction of actin and septin-2, and the FRET AB data suggest that this is a direct interaction between the two proteins, and is unlikely to be via interaction with a third protein.
Figure 5.
Septin-2 directly binds to actin under shear conditions, and is required for actin rearrangements in NHBE cells. (A) With immunoprecipitation (IP) of actin, there is increased pulldown of septin-2 under shear conditions (46). Similarly, immunoprecipitating septin-2 results in increased pulldown of actin under shear conditions compared with static (left). (B) Fluorescence resonance energy transfer (FRET) acceptor photobleaching (AB) was performed on fixed NHBE monolayers after exposure to static or shear condition. The areas of actin immunofluorescence depicted specifically correlates to the area that received photobleaching. Under static condition after AB (red), there is minimal change in the donor intensity (47). A FRET map (bottom panel) suggests minimal FRET efficiency. However, under shear conditions, there is an increase in donor intensity (47) after AB (red), and the FRET map suggests significant FRET efficiency on the cell boundaries. FRET efficiency was quantified in 15 preselected regions of interest in both static and shear cells with a significant increase in FRET AB between actin and septin-2 after exposure to shear stress (P < 0.05, one-way ANOVA; n = 3). (C) NHBE cells under specified conditions were fixed, permeabilized, and imaged using confocal immunofluorescence. Shear stress leads to actin rearrangement in NHBE cells. In cells treated with lentiviral septin-2 shRNA, there is a change in actin arrangement even under static conditions (third panel from left). In response to shear stress, cortical actin (first and second panels from left) is less well formed (fourth panel from left).
To determine if this interaction was necessary for the shear-induced actin reorganization, we examined the effect of septin-2 knockdown on cortical actin expression. After knockdown of septin-2, there was a decrease in shear-induced cortical actin (Figure 5C). Of note, changes in actin arrangement due to septin-2 knockdown were only associated with altered barrier function when the cells were exposed to a second stressor, such as shear, and not under static control conditions. When exposed to shear, however, the actin rearrangements that were evident in control cells and are associated with barrier enhancement were not seen in the cells treated with septin-2 shRNA (Figure 5C). In these cells, shear stress significantly increased paracellular permeability, as described previously here.
Altered Epithelial Barrier Function Changes Cell Signaling
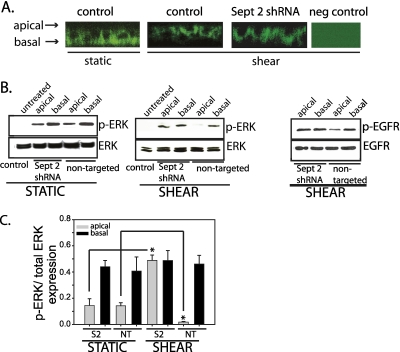
Humlicek and colleagues (35) have described polarized epidermal growth factor (EGF) signaling in airway epithelial cells that is affected by altered paracellular permeability in the context of a wound model or neutralizing antibody to E-cadherin. However, the effect of altering paracellular permeability on compartmentalization of signals in the context of a physiologic stress, or a less overt pathologic insult, is not known. To examine the functional consequences of altered epithelial paracellular permeability on cell signaling, we stimulated NHBE cells transduced with either septin-2 shRNA or a nontargeting shRNA with exogenous EGF on either the apical or basolateral surface. EGF receptor (EGFR) is localized to the basolateral surface of NHBE cells, and the application of shear stress in cells treated with a nontargeting shRNA or septin-2 shRNA does not change this distribution (Figure 6A). Without the addition of EGF ligand to the apical surface, no extracellular signal–regulated kinase (ERK) phosphorylation was observed in NHBE cells. Under static conditions, the addition of EGF to the basolateral chamber increased ERK phosphorylation both in cells transduced with septin-2 shRNA and a nontargeting control (Figure 6B). When control NHBE cells were exposed to shear, barrier function was enhanced, and the addition of apical EGF caused very little ERK phosphorylation; again, basolateral addition of EGF significantly increased ERK phosphorylation. In contrast, after septin-2 knockdown, epithelial barrier function decreased, and addition of EGF to either apical or basolateral surfaces produced high levels of ERK phosphorylation, indicating that the increased permeability resulting from septin-2 knockdown in sheared cells allows for an apically placed EGF to have increased access to the basolateral EGFR. Changes in ERK phosphorylation are quantified by densitometry in Figure 6C. To confirm that increased ERK phosphorylation was due to increased EGFR activation, we looked at the effects of the addition of EGF in cells under shear stress. After septin-2 knockdown, there was increased EGFR phosphorylation after apical exposure of EGF compared with cells treated with a nontargeting control (Figure 6B, right), confirming that the barrier disruption in sheared cells after septin-2 knockdown allowed for enhanced interaction between the apically placed EGF ligand and the basally positioned receptor.
Figure 6.
Epithelial barrier modulates cell signaling. (A) Epidermal growth factor (EGF) receptor (EGFR) is localized to the basolateral membrane of NHBE cells, and the application of shear stress or the knockdown of septin-2 does not alter its localization. (B) Under static conditions, placement of EGF in the basolateral chamber leads to more phosphorylated–extracellular signal–regulated kinase (ERK) than when placed on the apical membrane. Knockdown of septin-2 does not alter this. (Left) Under shear conditions, placement of EGF in either the apical or basolateral chamber leads to similar p-ERK after septin-2 knockdown (middle), as well as similar p-EGFR (46). However, in cells treated with a nontargeting construct, there is significantly less p-ERK (middle), as well as p-EGFR (46), in cells treated with apical EGF compared with cells treated with basolateral EGF. (C) Bar graph of densitometric analysis from three separate experiments of ERK phosphorylation (S2, septin 2 shRNA; NT, nontargeting shRNA; shaded bars, apically placed ligand; closed bars, basolaterally placed ligand; n = 3; *P < 0.05 with one-way ANOVA).
PM Effects on Barrier Are Mediated by Septin-2
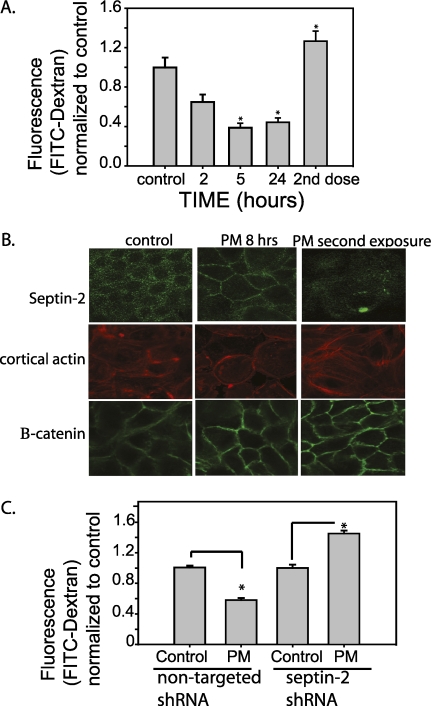
PM is known to produce airway inflammation and respiratory disease, as well as cardiovascular disease (13). PM has been well characterized, and has been reported to induce airway hyperresponsiveness and inflammation in mice (36), and induces a mucosal immune response in airway epithelial cells (37). In endothelial cells, a single exposure of PM has been reported to lead to barrier disruption (17, 38). In contrast, a single dose of PM (150 μg/ml) caused barrier enhancement (decreased paracellular permeability) in NHBE cells (Figure 7A). The initial barrier enhancement of NHBE cells in response to a single dose of PM is associated with increased membrane localization of septin-2, and a corresponding increase in cortical actin. A second exposure of NHBE cells to PM reduced membrane septin-2, decreased cortical actin, and increased paracellular permeability, suggesting that regulation of septin-2 could be responsible for pathologic changes in barrier function in airway epithelial cells after exposure to PM (Figure 7B). The effect of septin-2 on cortical actin was at least relatively specific, as B-catenin labeling in the membrane was not altered, also indicating that the cells were not overtly disrupted after multiple PM exposures (Figure 7B). To assess the role of septin-2 in PM-mediated barrier enhancement, NHBE cells treated with lentiviral septin-2 shRNA or a control nontargeted virus were exposed to a single dose of PM. A single dose of PM caused barrier enhancement in control cells expressing nontargeted virus. In contrast, after knockdown of septin-2, a single treatment of PM caused barrier disruption (Figure 7C), again indicating that septin-2 plays a necessary role in PM-induced barrier enhancement.
Figure 7.
Particulate matter (PM) exposure alters NHBE barrier function and septin-2 translocation. (A) Single exposure of 8 hours to PM placed on the apical surface of NHBE cells causes barrier enhancement in NHBE cells, whereas a second treatment (8-h exposure, followed by washes and no treatment overnight, with a second 8-h exposure the following day) leads to barrier disruption (n = 3; *P < 0.05 with one-way ANOVA). (B) Initial exposure to PM causes increases in septin-2 translocation to the membrane, as well as increased cortical actin. After a second exposure, very little membrane septin-2 is visualized, associated with a change in cortical actin organization. As a comparison, B-catenin is not altered by PM exposure with intact adherens junctions. (C) In cells transduced with septin-2 shRNA, there is no barrier enhancement seen after a single exposure to PM (n = 3; *P < 0.05 with one-way ANOVA).
DISCUSSION
The airway epithelium senses both physiologic and pathologic stimuli in the luminal airstream, and dynamic responses to these exposures are part of the host defense system. The epithelial barrier is the first line of defense preventing access of inhaled particles to subepithelial tissues (39–42). In addition, the epithelium segregates the apical and basal compartments (35, 43), so that regulation of epithelial barrier properties can influence cell signaling (35). As an example, the EGFR, located on the basolateral membrane and the ligand, on the apical membrane, are spatially separated by the epithelial barrier. Overt disruption of the barrier by creation of a wound or treatment with antibodies that inhibit cell–cell contact eliminates the normal separation between receptor and ligand, allowing increased binding and activation of the EGFR (44). We propose that dynamic regulation of the epithelial barrier in response to luminal stimuli may be an integral part of normal epithelial function.
Although a potential role for septin-2 regulation of barrier function has been hypothesized since its discovery in separating the mother–daughter contents in yeast (8), to our knowledge, this is the first description of septin-2 regulating monolayer barrier properties, both in epithelial cells and in endothelial cells. Septin-2 regulates the actin cytoskeleton and cellular morphology in HeLa cells, where septin-2 depletion significantly alters stress fiber orientation and leads to disordered clusters (45). We find that knockdown of septin-2 in the absence of other exposures does not alter barrier function. However, in response to shear stress, there is increased interaction between actin and septin-2 that regulates the rearrangement of cortical actin, and decreases paracellular permeability of NHBE cells. Pharmacologic intervention to inhibit septin-2 function similarly alters regulation of barrier function in HPAECs, suggesting a more general role for septin-2 in cytoskeletal remodeling and barrier regulation.
We have previously shown that shear generated by airflow over the epithelium is a homeostatic mechanism that enhances the epithelial barrier (12). Constituents of the airstream can also provoke epithelial responses, including transient and dynamic change in barrier function. We found that an initial exposure to PM enhances the barrier function, whereas additional exposure causes barrier disruption. This potentially is a protective mechanism to prevent access of the PM to the vasculature, where it has known inflammatory effects (17, 38). In our model, this barrier enhancement was not maintained after additional doses of PM. This could be a maladaptive response, allowing exposure of subepithelial tissues to PM, but could also mediate altered cell signaling, as increased permeability would increase receptor–ligand access across the epithelium. Although our study focused on EGF ligand–receptor separation, Humlicek and colleagues (35) described that the IFN-α, -β, and -γ, as well as IL-4 ligands and receptors are all also spatially separated. Increases in permeability resulting from multiple exposures to PM (or other luminal stimuli) could facilitate generation of inflammatory signals. Dynamic regulation of the epithelial barrier in response to luminal stimuli may play a key role in regulating host defenses, and our data indicate that septin-2 localization is important in this barrier regulation.
Collectively these studies suggest that septin-2 localization and its interaction with actin allows for dynamic modulation of airway epithelial barrier function in response to both physiologic and pathologic luminal stimuli.
Supplementary Material
Acknowledgments
The authors thank Dr. Rachel Damico and Dr. Mahendra Damarla for the HPAECs, and for their technical assistance with ECIS (electrical cell substrate impedance sensing). In addition, they thank the Johns Hopkins National Heart, Lung, and Blood Institute Proteomics Center for technical assistance.
This work was supported by the Flight Attendant's Medical Research Institution Young Investigator Award and K08HL085763 (V.K.S.) and the Johns Hopkins Bayview Scholars Program (L.S.K.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0235OC on September 24, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol 1995;269:G467–G475. [DOI] [PubMed] [Google Scholar]
- 2.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 1999;276:G1279–G1288. [DOI] [PubMed] [Google Scholar]
- 3.Nusrat A, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA 1995;92:10629–10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nusrat A, Madara JL. Modulation of intercellular junctions of epithelia by scatter factor (hepatocyte growth factor). EXS 1995;74:69–87. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J 2002;16:950–962. [DOI] [PubMed] [Google Scholar]
- 7.Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J 2002;16:1835–1837. [DOI] [PubMed] [Google Scholar]
- 8.Longtine MS, Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol 2003;13:403–409. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt K, Nichols BJ. Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol 2004;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, Wallingford JB. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 2010;329:1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larisch S, Yi Y, Lotan R, Kerner H, Eimerl S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP, Danielpour D, Book-Melamed N, et al. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol 2000;2:915–921. [DOI] [PubMed] [Google Scholar]
- 12.Sidhaye V, Schweitzer K, Caterina MJ, Shimoda LA, King LS. Shear stress regulates AQP5 and airway epithelial barrier function. Proc Natl Acad Sci U S A 2008;105:3345–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimmeler S, Haendeler J, Rippmann V, Nehls M, Zeiher AM. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett 1996;399:71–74. [DOI] [PubMed] [Google Scholar]
- 14.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 2004;279:24692–24700. [DOI] [PubMed] [Google Scholar]
- 15.Sidhaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem 2005;280:3590–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrlich A, Leitch V, King LS. Role of proneuregulin 1 cleavage and human epidermal growth factor receptor activation in hypertonic aquaporin induction. Proc Natl Acad Sci USA 2004;101:15799–15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Chiang ET, Moreno-Vinasco L, Lang GD, Pendyala S, Samet JM, Geyh AS, Breysse PN, Chillrud SN, Natarajan V, Garcia JG. Particulate matter disrupts human lung endothelial barrier integrity via ROS- and p38 MAPK-dependent pathways. Am J Respir Cell Mol Biol 2010;42:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TM, Yamamoto M, Breysse P, Biswal S, Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol 2008;181:4545–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arce FT,Whitlock JL, Birukova AA, Birukov KG, Arnsdorf MG, Lal R, Garcia JG, Dudek SM. Regulation of the micromechanical properties of pulmonary endothelium by S1P and thrombin: role of cortactin. Biophys J 2008;95:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birukov KG, Birukova AA, Dudek SM, Verin Ad, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 2002;26:453–464. [DOI] [PubMed] [Google Scholar]
- 21.Birukova AA, Arce AA, Moldobaeva N, Dudek SM, Garcia JG, Lal R, Birukov KG. Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: atomic force microscopy force mapping of pulmonary endothelial monolayer. Nanomedicine 2009;5:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown MD, Adyshev D, Bindokas V, Moitra J, Garcia JG, Dudek SM, et al. Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium. Microvasc Res 2010;80:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, Alverdy JC, Garcia JG. Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: role of actin cytoskeletal rearrangement. Microvasc Res 2009;77:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 2007;19:1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 2001;91:1487–1500. [DOI] [PubMed] [Google Scholar]
- 26.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem 2005;280:17286–17293. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol 2004;30:662–670. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol 2006;291:L289–L295. [DOI] [PubMed] [Google Scholar]
- 29.Ma SF, Flores C, Wade MS, Dudek SM, Nicolae DL, Ober C, Garcia JG. A common cortactin gene variation confers differential susceptibility to severe asthma. Genet Epidemiol 2008;32:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J 2005;19:1646–1656. [DOI] [PubMed] [Google Scholar]
- 31.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation: novel role for hyaluronan and CD44 receptor family. J Biol Chem 2006;281:34381–34393. [DOI] [PubMed] [Google Scholar]
- 32.Usatyuk PV, Romer LH, He D, Parinandi NL, Kleinberg ME, Zhan S, Jacobson JR, Dudek SM, Pendyala S, Garcia JG, et al. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem 2007;282:23284–23295. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 2009;77:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Q, Nelson WJ, Spiliotis ET. Forchlorfenuron alters mammalian septin assembly, organization, and dynamics. J Biol Chem 2008;283:29563–29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humlicek AL, Manzel LJ, Chin CL, Shi L, Excoffon KJ, Winter MC, Shasby DM, Look DC. Paracellular permeability restricts airway epithelial responses to selectively allow activation by mediators at the basolateral surface. J Immunol 2007;178:6395–6403. [DOI] [PubMed] [Google Scholar]
- 36.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect 2010;118:640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Usatyuk PV, Gorshkova IA, He D, Wang T, Moreno-Vinasco L, Geyh AS, Breysse PN, Samet JM, Spannhake EW, et al. Regulation of COX-2 expression and IL-6 release by particulate matter in airway epithelial cells. Am J Respir Cell Mol Biol 2009;40:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Moreno-Vinaasco L, Huang Y, Lang GD, Linares JD, Goonwardena SN, Grabavoy A, Samet JM, Geyh AS, Breysse PN, et al. Murine lung responses to ambient particulate matter: genomic analysis and influence on airway hyperresponsiveness. Environ Health Perspect 2008;116:1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrielson EW, Yu XY, Spannhake EW. Comparison of the toxic effects of hydrogen peroxide and ozone on cultured human bronchial epithelial cells. Environ Health Perspect 1994;102:972–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu XY, Schofield BH, Croxton T, Takahashi N, Gabrielson EW, Spannhake EW. Physiologic modulation of bronchial epithelial cell barrier function by polycationic exposure. Am J Respir Cell Mol Biol 1994;11:188–198. [DOI] [PubMed] [Google Scholar]
- 41.Yu XY, Takahashi N, Croxton TL, Spannhake EW. Modulation of bronchial epithelial cell barrier function by in vitro ozone exposure. Environ Health Perspect 1994;102:1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothen-Rutishauser B, Blank F, Muhlfeld C, Gehr P. In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Expert Opin Drug Metab Toxicol 2008;4:1075–1089. [DOI] [PubMed] [Google Scholar]
- 43.Winter MC, Shasby SS, Ries DR, Shasby DM. PAR2 activation interrupts E-cadherin adhesion and compromises the airway epithelial barrier: protective effect of beta-agonists. Am J Physiol Lung Cell Mol Physiol 2006;291:L628–L635. [DOI] [PubMed] [Google Scholar]
- 44.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003;422:322–326. [DOI] [PubMed] [Google Scholar]
- 45.Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell 2007;130:837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krause JE, Chenard BL, Cortright DN. Transient receptor potential ion channels as targets for the discovery of pain therapeutics. Curr Opin Investig Drugs 2005;6:48–57. [PubMed] [Google Scholar]
- 47.Green AS. Modelling of peak-flow wall shear stress in major airways of the lung. J Biomech 2004;37:661–667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.