Abstract
Receptor-mediated, cell-specific delivery of siRNA enables silencing of target genes in specific tissues, opening the door to powerful therapeutic options for a multitude of diseases. However, development of delivery systems capable of targeted and effective siRNA delivery typically requires multiple steps and use of sophisticated, orthogonal chemistries. Previously, we developed diblock copolymers consisting of dimethaminoethyl methacrylate-b-dimethylaminoethyl methacrylate-co-butyl methacrylate-copropylacrylic acid as potent siRNA delivery systems that protect siRNA from enzymatic degradation and enable its cytosolic delivery through pH-responsive, endosomolytic behavior.1,2 These architectures were polymerized using a living radical polymerization method, specifically reversible addition-fragmentation chain transfer (RAFT) polymerization, which employs a chain transfer agent (CTA) to modulate the rate of reaction, resulting in polymers with low polydispersity and telechelic chain ends reflecting the chemistry of the CTA. Here, we describe the straightforward, facile synthesis of a folate receptor-targeted diblock copolymer siRNA delivery system, as the folate receptor is an attractive target for tumor-selective therapies due to its overexpression in a number of cancers. Specifically, we detail the de novo synthesis of a folate-functionalized CTA, use the folate-CTA for controlled polymerizations of diblock copolymers, and demonstrate efficient, specific cellular folate receptor interaction and in vitro gene knockdown using the folate-functionalized polymer.
Introduction
RNA interference (RNAi) mediated through double stranded small interfering RNA (siRNA) is a promising therapeutic strategy for a variety of diseases.3 Effective siRNA delivery results in highly specific gene knockdown, providing a means to reduce expression of virtually any protein target with lower doses and less toxicity than other RNAi mechanisms.4,5 However, the successful in vivo delivery of siRNA is a formidable challenge. Although a wide variety of carriers for siRNA have been explored and consist of polymers, peptides, and lipids, a bottleneck for efficient siRNA therapy lies in the inability to specifically target active, non-degraded siRNA to tissues of interest.
Typically, cationic carriers have been employed for siRNA delivery.1,2,6–9 Through electrostatically complexation with negatively-charged phosphate groups of nucleic acids, cationic carriers imbibe siRNA with protection against enzymatic degradation and a means to enter cells through endocytosis. However, this approach renders a delivery system that lacks cellular specificity. Folic acid has shown great promise as a targeting ligand for a number of in vivo applications.10–13 While folic acid is internalized by the low affinity reduced folate carrier expressed by nearly all cells (Km ~ 10−6 M)14, it has greater affinity (Kd ~ 10−10 M)15,16 towards the folate receptor, which is overexpressed in a number of tumors and cancer cell lines10–13. Folic acid is internalized via the folate receptor in two steps. First, the folate binds to the receptor and then it is transferred into the cell by receptor-mediated endocytosis, allowing large drug carrier systems, including micelles, proteins, liposomes, and nanoparticles to enter the cell via endo-lysosomal trafficking17. However, irrespective of specificity, once trafficked to the endo-lysosomal pathway, cargo typically becomes degraded in lysosomes. Thus an effective, efficient in vivo siRNA delivery system must be multifunctional and provide protection against enzymatic degradation, be targeted to and internalized by the desired cell type, and result in intracellular trafficking to the intracellular environment where the target is located.
We have previously reported the development of cationic and pH-responsive, endosomolytic diblock copolymers as potent siRNA delivery systems in vitro.1,2,9,18 These carriers were formed using reversible addition-fragmentation chain transfer polymerization (RAFT), a type of living radical polymerization (LRP). A distinct advantage of polymeric carrier systems, and particularly polymers synthesized via LRPs, is the ease with which modularity can be introduced to incorporate many functionalities.1,19–21 LRPs provide polymers with narrow molecular weight distributions and a wide range of functional polymers with defined architectures.19,20,22–25 RAFT, similar to other LRPs, uses a chain transfer agent (CTA), which typically consists of a dithioester or trithiocarbonate, and reactive Rand Z-groups, where the resultant polymer contains the same R and Z functionalities at its chain-ends. Reactive R- and Z-group modified CTAs have been employed to prepare alpha and omega-functional polymers, respectively, that are directly reactive toward biomolecules.26,27 More recently, this approach has been used to exploit pyridyl-disulfide groups to enable reversible conjugation of siRNA23 and proapoptotic peptides18 to polymers for enhanced delivery characteristics. In addition, chain extension strategies have been employed to specifically and reproducibly functionalize the omega-end of RAFT polymers with a multitude of functional groups, including folic acid.28 However, these synthetic schemes requires subsequent conjugation steps to introduce the targeting or drug functionality whereas the current approach achieves functionalization concurrently with RAFT polymer synthesis.
To our knowledge there have been no reports of targeting strategies introduced using the RAFT chain transfer agent directly. Herein, we report the straightforward de novo synthesis of a RAFT CTA with a folate-functionalized R-group. The strategy is different from reported LRP strategies to make targeted polymers29–33 and involves synthesis of a novel chain transfer agent (CTA) for RAFT polymerization. To achieve potent, pH-responsive siRNA carriers with precise presentation of folate and narrow polydispersities, RAFT polymerization was utilized in the synthesis of a diblock copolymer consisting of dimethylaminoethyl methacrylate-b-dimethylaminoethyl methacrylate-co-butyl methacrylate-co-propylacrylic acid (DMAEMA-b-DMAEMA-co-BMA-co-PAA), a terpolymer previously described for its potency for in vitro intracellular siRNA delivery.1,2 The presence of tertiary and partially protonated amines in the first block allows for complexation and protection of siRNA and the second block imbibes pH-responsive endosomolytic behavior. Moreover, each polymer presents targeting moieties meerly through use of the folate-functionalized CTA. As demonstrated through polymer characterization methods, competitive folate binding assays, and gene knockdown studies, this multifunctional polymer provides potent siRNA delivery to cancer cells that overexpress folate receptors.
Experimental Section
Materials
Chemicals and all materials were supplied by Sigma-Aldrich unless otherwise specified.
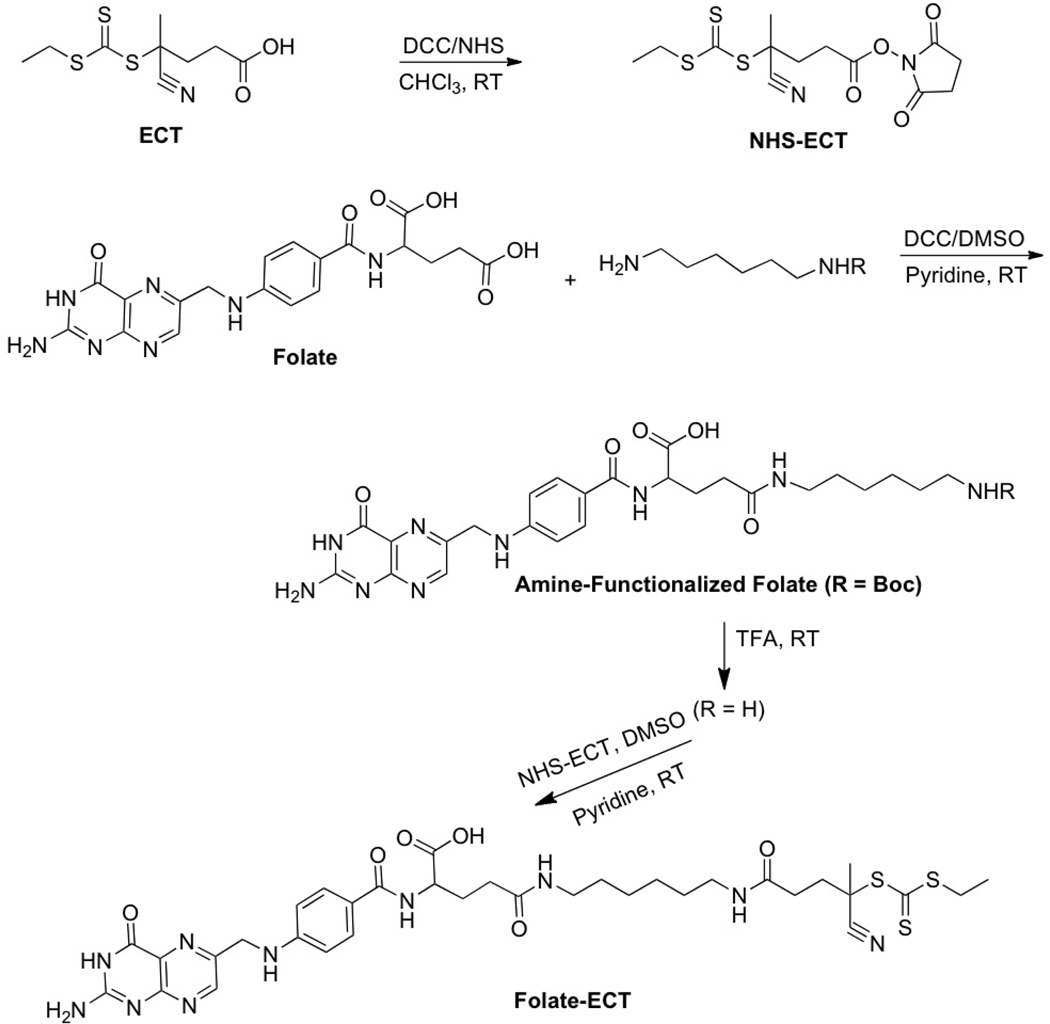
Synthesis of folate-functionalized RAFT chain transfer agent, Folate-ECT
The synthesis of the chain transfer agent (CTA), 4-cyano-4-(ethylsulfanylthiocarbonyl) sulfanylvpentanoic acid (ECT), utilized for the following RAFT polymerizations, was adapted from a procedure by Moad et al.21
Synthesis of NHS-ECT
To a solution of ECT (See Scheme 1, 1.05 g, 4 mmol) in CHCl3 at 0 °C were added N-hydroxysuccinimide (460 mg, 4mmol) and dicyclohexylcarbodimide (865 mg, 4.2 mmol). The reaction mixture was stirred at 0 °C for 1 h and then at room temperature for 22 h. The by-product, dicyclohexylurea (DCU) was filtered off and the filtrate was concentrated using rotary evaporation. The crude product was purified by flash column chromatography using ethyl acetate/hexane (1:1) solvent system. The product after 12 h drying under high vacuum yielded NHS-ECT (1.16 g, 81 %). 1H-NMR (CDCl3) δ 3.35 (q, J = 7.5 Hz, 2H), 2.90–2.96 (m, 2H), 2.85 (s, 4H), 2.47–2.71 (m, 2H), 1.88 s (s, 3H), 1.36 (t, J = 7.5 Hz, 3H) ppm.
Scheme 1.
Synthesis of Folate-CTA for polymerization of folate-functionalized diblock copolymers for siRNA delivery
Coupling of NHS-ECT to amine-functionalized folate
γ-(6-Aminohexyl)folic acid (amine-functionalized folate) was synthesized following the procedure described by Schneider et al.34 Coupling of NHS-ECT with amine-functionalized folate in DMSO/pyridine afforded folate-ECT RAFT chain transfer reagent in 87% yield. To a solution of (270 mg, 0.5 mmol) in dry DMSO (35 mL) and dry pyridine (15 mL) under nitrogen was added NHS-ECT (198 mg, 0.55 mmol) at room temperature. After stirring for 24 h, the reaction mixture in DMSO was slowly dropped into 1 L of ether. The precipitated product was washed several times with ether, dichloromethane and finally with methanol. The product was dried under high vacuum overnight to give folate-ECT as a yellow powder (348 mg, 87%, >95% pure by NMR). 1H NMR (DMSO-d6, 300 MHz): 8.64 (s, 1H), 7.82–8.22 (m, 2H), 7.50–7.80 (m, 1H), 7.65 (d, J = 8.4 Hz, 2H), 6.83–6.95 (m, 3H), 6.63 (d, J = 8.4 Hz, 2H), 4.88 (d, J = 5.7 Hz, 2H), 4.23–4.38 (m, 1H), 3.78 (q, J = 7.2 Hz, 2H), 3.01 (m, 4H), 2.11–2.38 (m, 6H), 1.83 (s, 3H), 1.59–1.93 (m, 2H), 1.14–1.42 (t J = 7.2 Hz 3H, and m, 8H) ppm; ESI-MS m/z 785.4 (M+H)+, 807.4 (M+Na)+, calcd for C34H44N10O6S3 784.3.
Synthesis of poly(dimethylaminoethyl methacrylate) macro chain transfer agent (pDMAEMA macroCTA)
The RAFT polymerization of DMAEMA was conducted in DMF at 30 °C under a nitrogen atmosphere for 10 hours using folate-ECT or ECT and 2,2'-Azobis(4-methoxy-2.4-dimethyl valeronitrile) (V-70) (Wako Chemicals) as the radical initiator. The initial monomer to CTA ratio ([CTA]o/[M]o was such that the theoretical Mn at 100% conversion was 25,000 (g/mol). The initial CTA to initiator ratio ([CTA]o/[I]o) was 10 to 1. The resultant folate-functionalized and control pDMAEMA macro chain transfer agents were isolated by precipitation into 50:50 diethyl ether/pentane. The resultant polymer was redissolved in acetone and subsequently precipitated into pentane (x3) and dried overnight in vacuo.
Block copolymerization of DMAEMA, PAA, and BMA from folate-functionalized and control pDMAEMA macroCTAs
The desired stoichiometric quantities of DMAEMA, PAA, and BMA (25:25:50%, respectively) were added to folate-functionalized or control pDMAEMA macroCTA dissolved in N,N-dimethylformamide (25 wt % monomer and macroCTA to solvent).1,2,18 For all polymerizations [M]o/[CTA]o and [CTA]o/[I]o were 250:1 and 10:1 respectively. Following the addition of V70 the solutions were purged with nitrogen for 30 min and allowed to react at 30 °C for 18 h. The resultant diblock copolymers were isolated by precipitation into 50:50 diethyl ether/pentane. The precipitated polymers were then redissolved in acetone and precipitated into pentane (x3) and dried overnight in vacuo. Gel permeation chromatography (GPC) was used to determine absolute molecular weights and polydispersities (Mw/Mn, PDI) of both the pDMAEMA macroCTAs and diblock copolymer samples (SEC Tosoh TSK-GEL R-3000 (2 columns) and R-4000 (1 column) (Tosoh Bioscience, Montgomeryville, PA) connected in series to a 1200 Series GPC (Agilent Technologies, Santa Clara, CA), miniDAWN TREOS, 3 angle MALS light scattering instrument and Optilab rEX, refractive index detector (Wyatt Technologies, Santa Barbara, CA). HPLC-grade DMF containing 0.1 wt % LiBr at 60 °C was used as the mobile phase at a flow rate of 1 ml/min. Both the pDMAEMA and diblock copolymers were analyzed via 1H NMR spectroscopy (Bruker DRX 499) and UV spectroscopy to determine composition and folate-functionalization, respectively.
Cell culture
Hela cells, human cervical carcinoma cells (ATCC CCL-2), a cell line that overexpresses the folate receptor, were maintained in minimum essential media (MEM) containing L-glutamine (Gibco), 1% penicillin-streptomycin (Gibco), and 10% fetal bovine serum (FBS, Invitrogen) at 37 °C and 5% CO2. Unless otherwise indicated, folate-free RPMI media containing 1% penicillin-streptomycin (Gibco), and 10% fetal bovine serum (FBS, Invitrogen) was used for all siRNA treatments.
Cytocompatibility of folate receptor targeted siRNA/polymer complexes
siRNA/polymer complex cytotoxicity was determined using a lactate dehydrogenase (LDH) cytotoxicity detection kit (Roche). Hela cells were seeded in 96-well plates at a density of 12,000 cells/cm2 and allowed to adhere overnight. Complexes were formed as above at theoretical charge ratios of 4:1 and to attain a concentration of 25 nM siRNA/well. After incubation with the complexes for 24 hours, the media was removed and the cells were washed with PBS twice. Cell lysis buffer was added to the wells (100 µl, 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, and 1 mM sodium orthovanadate) for 1 h at 4 °C to lyse the cells. After mixing with a pipette, 20 µl of the cell lysate was added to 80 µl of PBS to quantify for LDH by reacting with 100 µl of the LDH substrate solution. After a reaction time of 10–20 min, absorbance was measured at 490 nm with the reference at 600 nm.
Assessment of folate receptor binding of folate-functionalized polymer carriers
Competitive binding of free [3H]-folic acid (Amersham, 33 Ci/mmol) and folate-functionalized or control polymers was performed as described by Corona et al.35 Briefly, Hela cells were chilled on ice for 3 min and washed twice with ice-cold HEPES-buffered saline solution (HBSS; 107 mM NaCl, 20 nM HEPES, 26.2 mM NaHCO3, 5.3 mM KCl, 1.9 mM CaCl2, 1 mM MgCl2 and 7 mM glucose adjusted to pH 7.4 with NaOH), followed by an acidic washing with HBSS (pH 3.5 with acetic acid) for 60 sec at 4°C to release folate-receptor-bound folic acid. Cells were re-conditioned with HBSS (pH 7.4) and then incubated on ice with 20 nM [3H]-folic acid in HBSS (pH 7.4) for 30 min at 4°C. Under these conditions, there is no significant folate internalization. After 30 min, either free folic acid or folate-functionalized polymers or control polymers were introduced in equivalent folate concentrations of 0–10 µg/ml and incubated for an additional 30 min. After incubation, cells were washed with ice-cold HBSS, pH 7.4, and then treated with HBSS, pH 3.5, for 60 sec to release [3H]-folate bound to the cells via the folate receptor. The acidic radioactive solution was evaluated by liquid scintillation counting. The specific [3H]-folic acid binding was obtained as the difference between the total cell-bound radioactivity and that measured in the presence of a large excess (10 µM) of unlabeled folic acid.
Measurement of carrier-mediated siRNA uptake
Intracellular uptake of siRNA/polymer complexes was measured using flow cytometry (Becton Dickinson LSR benchtop analyzer). Hela cells were seeded at 12,000 cells/cm2 (24-well plates) in folate-free RPMI media and allowed to adhere overnight. FAM labeled siRNA (Ambion) was complexed with control diblock or folate diblock at a charge ratio of 4:1 for 30 min at room temperature and then added to the plated Hela cells at a final siRNA concentration of 25 nM (500 µl volume). To a subset of the folate diblock/siRNA treatments, 10 µg/ml of free folate was added to the media as a control. After incubation with the complexes for 1 h, the cells were trypsinized and resuspended in PBS with 0.5% BSA and 0.01% trypan blue. Trypan blue was utilized as previously described for quenching of extracellular fluorescence and discrimination of complexes that have been endocytosed by cells.36 Ten thousand cells were analyzed per sample and fluorescence gating was determined using samples receiving no treatment and treated with polymer alone.
Evaluation of GAPDH gene knockdown by folate receptor-targeted siRNA/polymer complexes
The efficacy of the series of polymers for siRNA delivery was screened using a GAPDH siRNA (Ambion). Hela cells (12,000 cells/cm2) were plated in 24-well plates in folate-free RPMI media. After 24 h, complexes (charge ratios = 4:1) were added to the cells at a final siRNA concentration of 25 nM in the presence of 10% serum (100 µl volume). The extent of siRNA-mediated GAPDH mRNA reduction was assessed 24 h after treatment. As a positive control, parallel knockdown experiments were run using HiPerFect (Qiagen) following manufacturer’s conditions. Total RNA was isolated using Qiagen’s Qiashredder and RNeasy mini kit. Any residual genomic DNA in the samples was digested (RNase-Free DNase Set, Qiagen) and RNA was quantified using absorbance at 260 nm.
Reverse transcription was performed using the Omniscript RT kit (Qiagen). A 25 ng total RNA sample was used for cDNA synthesis and PCR was conducted using the ABI Sequence Detection System 7000 using predesigned primer and probe sets (Assays on Demand, Applied Biosystems) for GAPDH and β-actin as the housekeeping gene. Comparative threshold cycle (CT) analysis was used to quantify GAPDH, normalized to β-actin and relative to expression of untreated cells.
Statistical methods
ANOVA was used to test for treatment effects, and Tukey’s test was used for post-hoc pairwise comparisons between individual treatment groups.
Results and Discussion
Synthesis of folate-functionalized RAFT chain transfer agent and folate-functionalized diblock copolymers for siRNA delivery
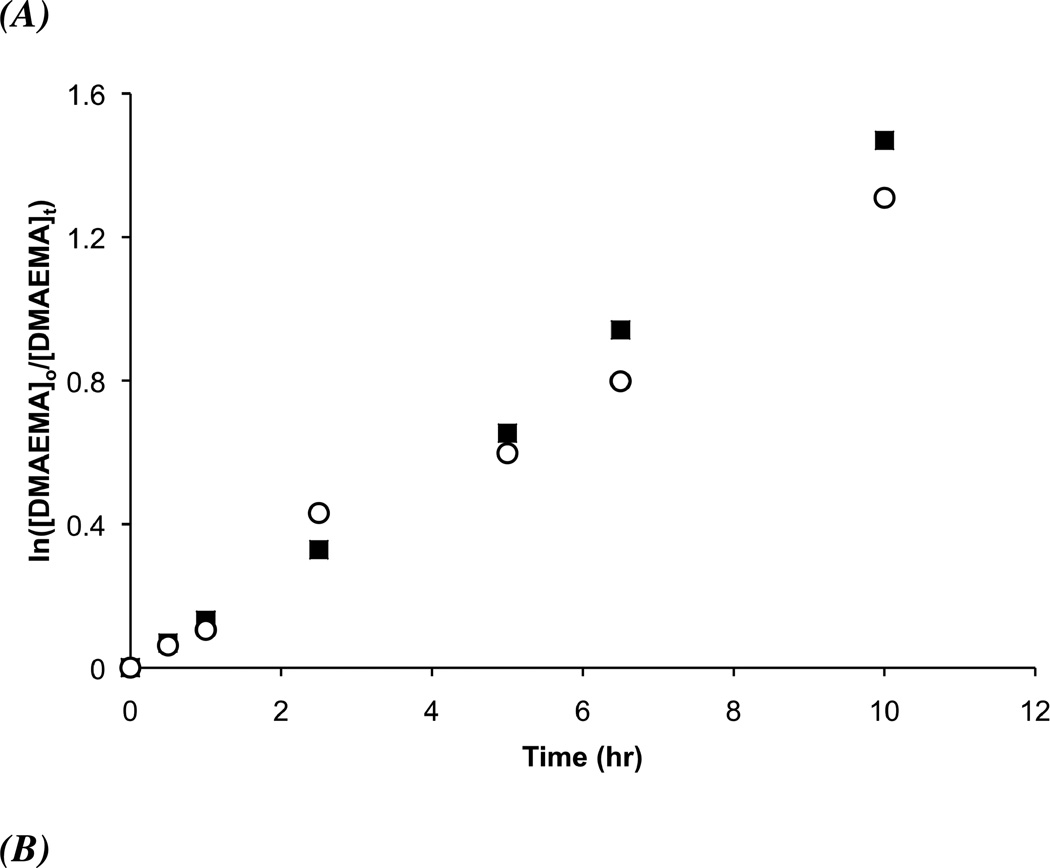
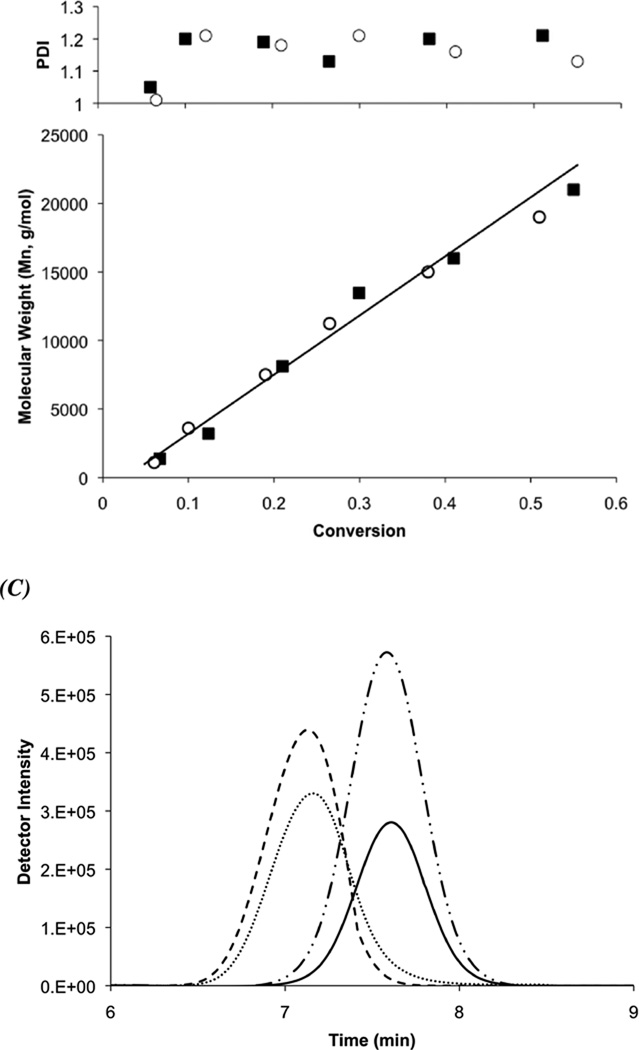
The final coupling step of the synthesis of the folate-CTA is outlined in Scheme 1. The folate-CTA was synthesized with 87% yield through NHS coupling between amine-functionalized folate34 and the NHS-activated CTA, 4-Cyano-4-(ethylsulfanylthiocarbonyl) sulfanylvpentanoic acid (ECT). 1H NMR spectroscopy (see Supporting Information for the spectra) and mass spectrometry indicated formation of the desired CTA (shifts in Experimental Section).21 UV–Vis analysis confirmed that the CTA had been synthesized as the absorbance indicative of the trithiocarbonate and the folate was observed (Amax = 310 and 363 nm, respectively). We employed the folate-CTA to synthesize folate-functionalized diblocks, where the diblocks had previously been discovered as potent siRNA delivery polymers.1,2 These diblocks incorporate a siRNA-condensing, cationic block formed from DMAEMA and a second pH-responsive, ampholytic block, formed from DMAEMA, BMA, and PAA, to enhance endosomal escape.1,2,18 As a control polymer, diblocks were synthesized with the unmodified ECT. To test the general controlling ability of the ECT and folate-functionalized ECT, samples of the DMAEMA polymerization were taken during polymerization and measured by GPC. Figure 1 shows kinetic (a) and average molar weights versus conversion (b) and the corresponding polydispersity indices (PDIs) (c) of two representative reactions utilizing ECT and folate-functionalized ECT. The linear increases in molecular weights both with time and conversion and low PDIs with respect to conversion are concomitant with well-controlled radical polymerizations. Moreover, the macroCTA of DMAEMA is readily reinitiated to enable diblock formation, as highlighted in GPC traces for the DMAEMA macroCTAs and the diblocks utilized herein (Figure 1D). In addition, the diblock too has low polydispersity (1.3 or less, see Table 1), indicating both CTAs are stable and active even after the initial block polymerization. After diblock synthesis, quantification of folate functionalities per polymer chain was achieved using the molar absorbtivity of folate in PBS (ε = 6197 M−1 cm−1, as described37) and GPC-determined molecular weights. The overall diblock characteristics are listed in Table 1.
Figure 1.
(A) Pseudo first-order kinetic plots of pDMAEMA and (B) evolution of molecular weight and PDI with respect to conversion utilizing a conventional CTA (closed squares) and folate-functionalized ECT (open circles). The polymerizations were carried out at 40 wt% monomer and 30 °C using [M]o/[CTA]o = 150 and [CTA]o/[I]o = 10 with V-70 as the radical initiator. (C) GPC traces of pDMAEMA first block (ECT-DMAEMA: _____, Folate-DMAEMA: --___--) and diblock copolymer (ECT-Diblock: …….., Folate-Diblock: ------) showing reinitiated, living pDMAEMA first block synthesis.
Table 1.
Molecular weights, polydispersities, and final monomer compositions for control and folate diblock copolymers
| Diblock | Folate-Diblock | |
|---|---|---|
| Mn (1st block) (g/mol) | 19600 | 21600 |
| Mn (2nd block) (g/mol) | 60000 | 66000 |
| PDI | 1.25 | 1.3 |
| % BMA 2nd block | 48 | 52 |
| % PAA 2nd block | 24 | 23 |
| % DMAEMA 2nd block | 28 | 25 |
| Folate/ polymer | - | 1.01 ± 0.04 |
Both the folate-CTA and ECT enabled synthesis of similar molecular weight diblock copolymers with narrow polydispersities (PDI), as determined by gel permeation chromatography (GPC). 1H-NMR (CDCl3) and GPC data was utilized to determine copolymer composition. The second block compositions were very similar to the theoretical compositions, namely 50% BMA and 25% DMAEMA and PAA, irrespective of CTA employed. Thus, diblock copolymer polymerizations using both the folate-functionalized and unfunctionalized CTAs resulted in narrowly dispersed copolymers with similar, precise architectures for subsequent in vitro characterizations.
Cytocompatibility of siRNA/polymer complexes
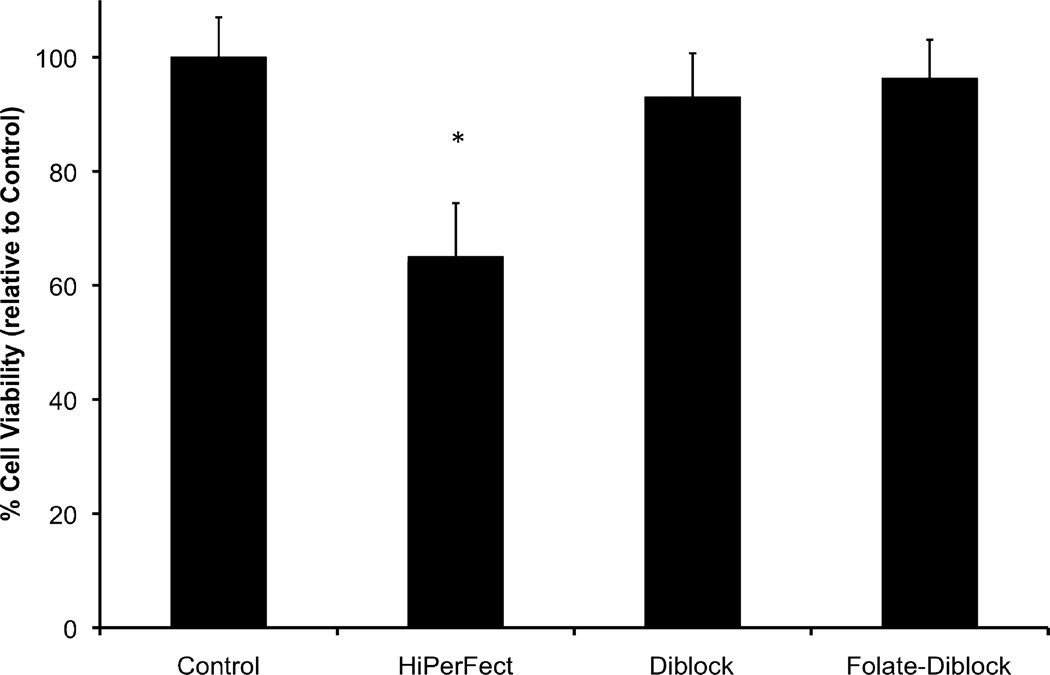
To analyze the cytocompatibility of the folate-targeted or untargeted siRNA delivery polymers, cells were incubated in the presence of the polymer:siRNA (control #1 siRNA, Ambion) complexes at 4:1 charge ratios and 25 nM siRNA concentrations. As seen in Figure 2 and as previously reported1,2, high cell survivability (~100%) was found for cells treated with the diblock or the folate-functionalized diblock. However, the commercially-available carrier, HiPerFect, induces robust cell death, where only ~60% of the cells survived after the 24 hour treatment.
Figure 2.
Polymer/siRNA complex cytotoxicity as a function of carrier composition. Hela cells were treated using complexes at 4:1 charge ratios and 25 nM Control #1 siRNA (Ambion) concentrations. After 24 hours, cell lysate was collected and assayed for lactate dehydrogenase, a measure of cell viability and data is shown relative to control cells (untreated). Data are from three independent experiments conducted in triplicate with error bars representing standard deviation. Statistical significance was evaluated at a level of P < 0.05 with * indicating statistical differences versus control cells.
Assessment of folate receptor binding of folate-functionalized polymer carriers
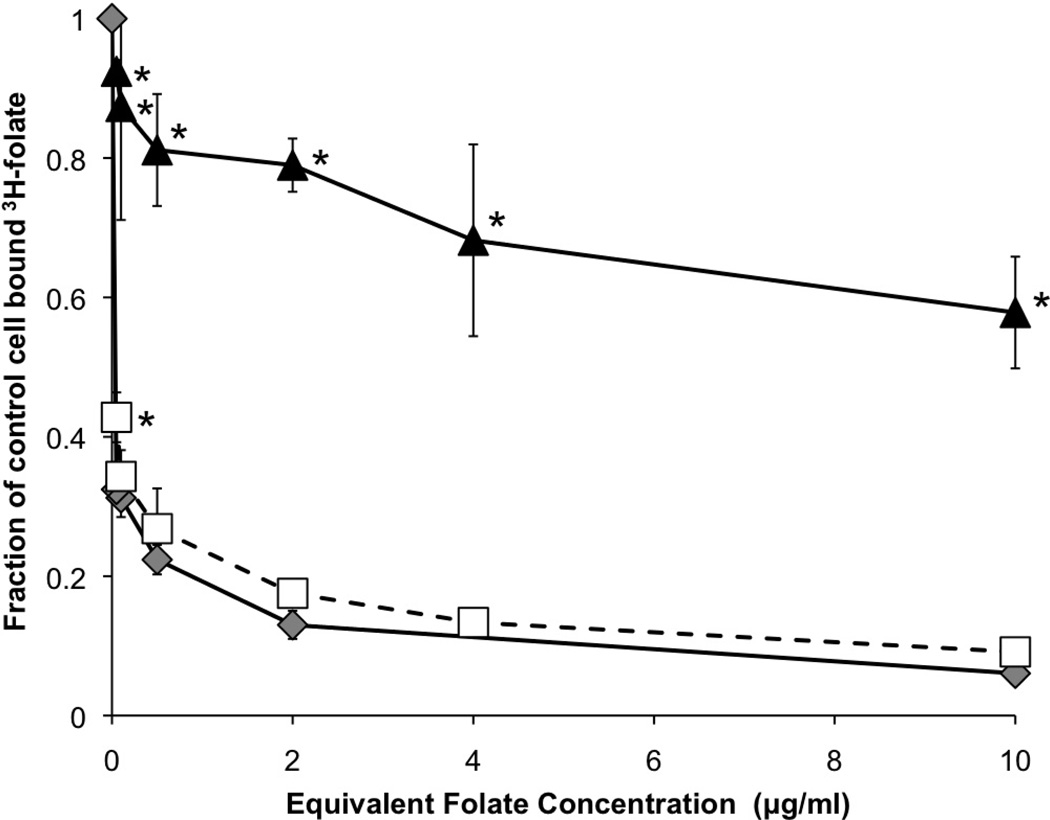
To verify the folate-functionalized diblock copolymers were capable of interacting with folate receptor, a competitive binding assay was performed.35 Briefly, Hela cells, cervical carcinoma cells that overexpress the folate receptor, were washed and stripped of any receptor-bound folate. The cells were exposed to 3H-folate for 30 min then either free folate, folate-targeted diblock copolymers, or non-targeted diblock copolymers were introduced at increasing equivalent folate concentrations. After 30 min, the amount of bound 3H-folate was quantified using scintillation counting to reveal the remaining amount bound to folate receptor expressing cells and the data is shown in Figure 3. The introduction of folate-targeted diblocks or free folate showed a similar competition for binding of folate receptor, where even at very low equivalent concentrations of folate (0.1–0.2 µg/ml), nearly half of the 3H-folate was replaced. At 2 µg/ml, 80% and at 10 µg/ml over 95% of the 3H-folate has been replaced by the free or folate-functionalized diblocks. Importantly, there is no statistically significant difference between free folate and folate-functionalized diblocks at the different equivalent folate concentrations, indicating that folate bound to the diblock interacts with the folate receptor similarly to free folate. Interestingly, the diblock alone, without any folate functionalities, shows some non-specific competition with 3H-folate, reducing bound folate by 20% at 2 µg/ml and 40% at 10 µg/ml. Note that these equivalent concentrations represent equivalent polymer concentrations to the folate-functionalized polymer as there are no folates to equate in the control polymer. We attribute this non-specific competition for folate to electrostatic interactions owing to the cationic nature of DMAEMA in the polymer delivery system and the anionic nature of cellular membranes.
Figure 3.
Folate-diblock (open squares) competitively inhibits 3H-folate binding to folate receptor positive cells. * denotes statistical significance (p < 0.05) versus free folate (grey diamonds). Diblock alone (black triangles) results in non-specific reduction in bound 3H-folate. Data are from three independent experiments conducted in triplicate with error bars representing standard deviation.
Measurement of carrier-mediated siRNA uptake
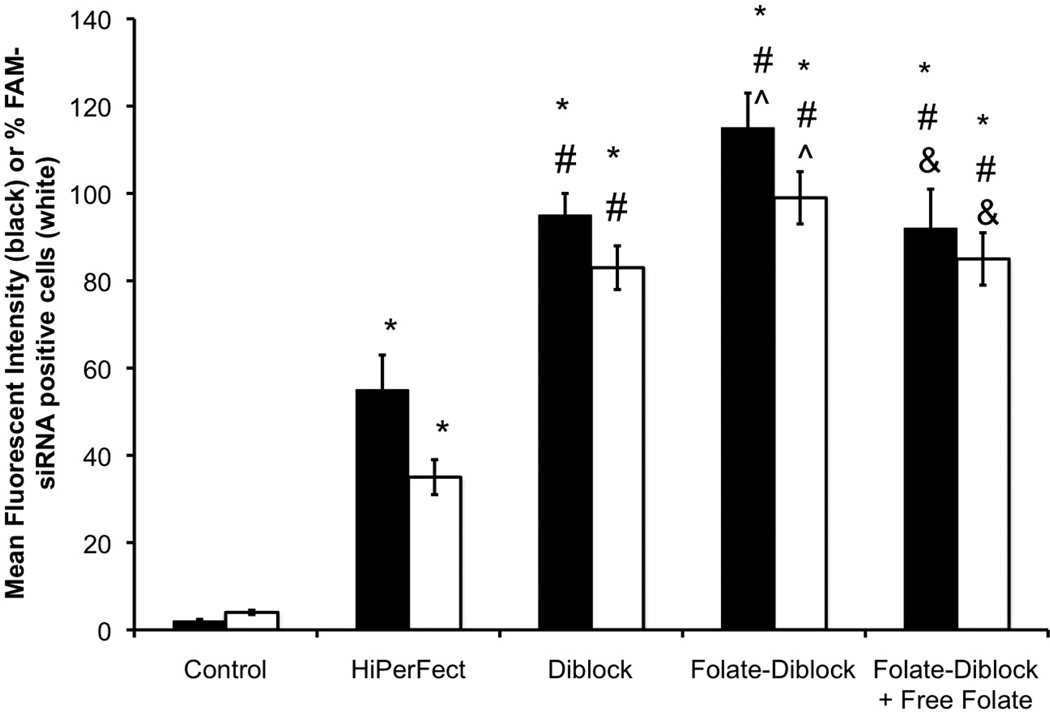
Next, we assessed how folate-targeting affected polymer-mediated siRNA uptake by Hela cells. Cells were treated with FAM (fluorescently-labeled)-siRNA complexed with the diblocks and allowed to incubate for 1 hour. As a control, free folate (at 10 µg/ml) was added to the incubation medium to compete with folate-functionalized diblocks for receptor-mediated endocytosis. In addition, comparison of uptake with a commercially available siRNA delivery reagent, HiPerFect, is also shown in Figure 4.
Figure 4.
Hela cell internalization (mean fluorescence (black) and percent of total cells (white)) of FAM-labeled siRNA is enhanced through diblock functionalization. Error bars represent standard error. *, #, ^, & denote statistical significance (p < 0.05) versus control, HiPerFect, diblock, and folate-diblock, respectively. Data are from three independent experiments conducted in triplicate with error bars representing standard deviation.
Uptake (both mean fluorescent intensity, black and %FAM-siRNA positive cells, white) of siRNA is superior with the polymeric delivery system, as outlined previously1,2 versus no carrier (control) or the commercially available reagent, HiPerFect. However, both mean fluorescent intensity and percent siRNA-positive cells were increased by 20 A.U. and 15%, respectively, by introducing folate functionalities, statistically significant, albeit modest, increases. However, and very important looking towards in vivo applications, the targeted carrier mediated delivery of siRNA to 100% of treated cells. Thus, all cells contain therapeutic siRNA for subsequent gene knockdown. In addition, and equally important, folate carrier-mediated uptake is reduced to diblock control levels when free folate is introduced into the incubation medium, outlining the specificity of folate-enhanced uptake.
Evaluation of GAPDH gene knockdown by folate receptor-targeted siRNA/polymer complexes
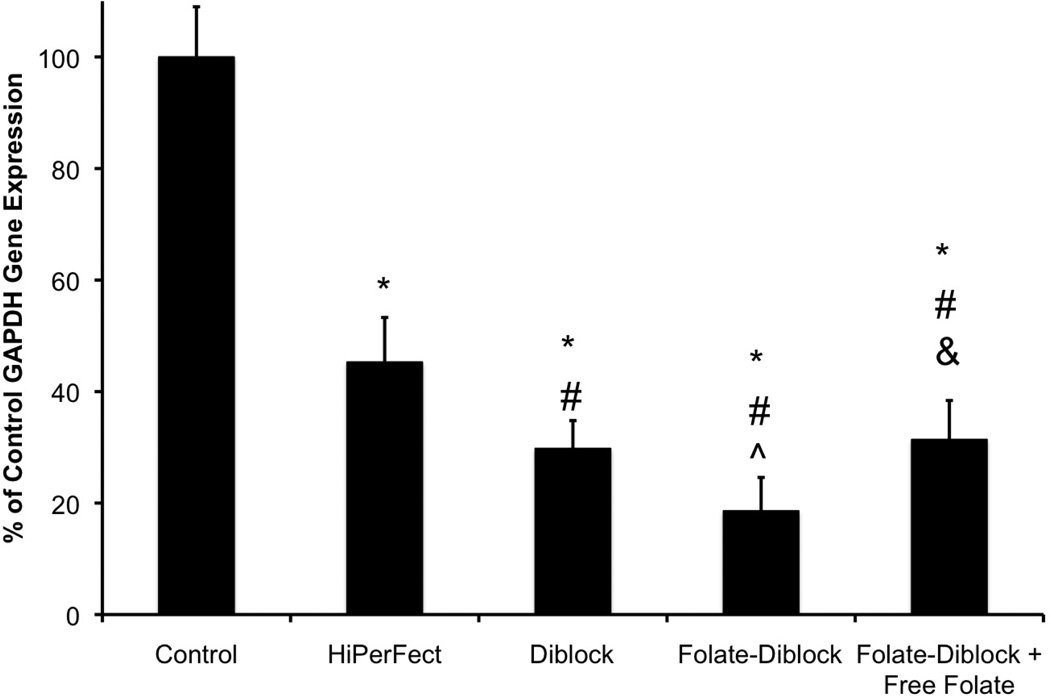
To assess siRNA activity using the folate-targeted delivery system, in vitro gene knockdown experiments were undertaken. Hela cells were treated with GAPDH siRNA complexed with a commercially available carrier, HiPerFect, control, or folate-diblocks were used to treat the cells and resulting gene expression was examined after 24 h. As described in Figure 5, GAPDH gene knockdown was greatest using folate-functionalized diblock copolymer siRNA delivery, where GAPDH gene expression was reduced to 18% of untreated cells.
Figure 5.
siRNA-mediated GAPDH gene knockdown is enhanced by folate targeting. Untreated cells and a commercially available transfection reagent, HiPerFect (Qiagen), were used as negative (‘Control’) and positive controls, respectively. *, #, ^, & denote statistical significance (p < 0.05) versus control, HiPerFect, control diblock, and folate-diblock, respectively. Data are from three independent experiments conducted in triplicate with error bars representing standard deviation.
Moreover, siRNA delivery using the diblock also showed robust gene knockdown (~30% control), although not as great of levels as the folate-functionalized diblock. In addition, gene knockdown resulting from delivery of siRNA using the folate diblock with 10 µg/ml of free folate in the media was statistically the same as knockdown with the untargeted diblock, again supporting the specificity with which the folate-functionalized carrier is capable of eliciting a reduction in gene expression due to interactions with the folate receptor. All siRNA delivery using diblock copolymer architectures resulted in greater (by 15–25%) gene knockdown than delivery using a commercially available siRNA delivery system, HiPerFect, as decribed previously.1,2
Conclusions
Herein we describe the development of a facile approach to provide folate receptor-specific delivery of polymer therapeutics by employing a de novo synthesized folate-functionalized CTA. Specifically for the polymers employed, folate-functionalization provided specific polymer therapeutic-folate receptor interactions and elicited gene knockdown through delivery of siRNA. Gene knockdown was reduced in the presence of free folate, outlining receptor specificity. Many polymer therapeutics can be functionalized using this strategy, and this approach is highly versatile for functionalizing RAFT polymers.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the National Institute of Health (EB2991-01) and the Damon Runyon Cancer Research Foundation (DSWB, Merck Fellow) for funding.
Footnotes
Supporting Information Available
1H-NMR spectra of the folate functionalized CTA is available as supporting information. This information is available free of charge via the internet at http://pubs.acs.org/.
References
- 1.Convertine AJ, Benoit DS, Duvall CL, Hoffman AS, Stayton PS. J Control Release. 2009;133:221. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. Biomacromolecules. 2010 doi: 10.1021/bm100652w. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aigner A. Curr Pharm Des. 2008;14:3603. doi: 10.2174/138161208786898815. [DOI] [PubMed] [Google Scholar]
- 4.Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. Biochemistry. 2003;42:7967. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- 5.Dorsett Y, Tuschl T. Nat Rev Drug Discov. 2004;3:318. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- 6.Bisht HS, Manickam DS, You Y, Oupicky D. Biomacromolecules. 2006;7:1169. doi: 10.1021/bm0509927. [DOI] [PubMed] [Google Scholar]
- 7.Lam JK, Ma Y, Armes SP, Lewis AL, Baldwin T, Stolnik S. J Control Release. 2004;100:293. doi: 10.1016/j.jconrel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 8.York AW, Kirkland SE, McCormick CL. Adv Drug Deliv Rev. 2008;60:1018. doi: 10.1016/j.addr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Benoit DS, Henry SM, Shubin AD, Hoffman AS, Stayton PS. Mol Pharm. 2010;7:442. doi: 10.1021/mp9002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattes MJ, Major PP, Goldenberg DM, Dion AS, Hutter RV, Klein KM. Cancer Res. 1990;50:880s. [PubMed] [Google Scholar]
- 11.Ross JF, Chaudhuri PK, Ratnam M. Cancer. 1994;73:2432. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Weitman SD, Frazier KM, Kamen BA. J Neurooncol. 1994;21:107. doi: 10.1007/BF01052894. [DOI] [PubMed] [Google Scholar]
- 13.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, Kamen BA. Cancer Res. 1992;52:3396. [PubMed] [Google Scholar]
- 14.Kamen BA, Capdevila A. Proc Natl Acad Sci U S A. 1986;83:5983. doi: 10.1073/pnas.83.16.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antony AC, Kane MA, Portillo RM, Elwood PC, Kolhouse JF. J Biol Chem. 1985;260:14911. [PubMed] [Google Scholar]
- 16.Rothberg KG, Ying YS, Kolhouse JF, Kamen BA, Anderson RG. J Cell Biol. 1990;110:637. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leamon CP, Low PS. Proc Natl Acad Sci U S A. 1991;88:5572. doi: 10.1073/pnas.88.13.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvall CL, Convertine AJ, Benoit DS, Hoffman AS, Stayton PS. Mol Pharm. 2010;7:468. doi: 10.1021/mp9002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barner L, Davis TP, Stenzel MH, Barner-Kowollik C. Macromol. Rapid Commun. 2007;28:539. [Google Scholar]
- 20.Matyjaszewski K, Xia J. Chem Rev. 2001;101:2921. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 21.Moad G, Chong YK, Postma A, Rizzardo E, Thang SH. Polymer. 2005;46:8458. [Google Scholar]
- 22.Hawker CJ, Bosman AW, Harth E. Chem Rev. 2001;101:3661. doi: 10.1021/cr990119u. [DOI] [PubMed] [Google Scholar]
- 23.Heredia KL, Nguyen TH, Chang C-W, Bulmus V, Davis TP, Maynard HD. Chemical Communications. 2008;28:3245. doi: 10.1039/b804812f. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Bulmus V, Barner-Kowollik C, Stenzel MH, Davis TP. Macromol. Rapid Commun. 2007;28:305. [Google Scholar]
- 25.Moad G, izzardo E, Thang SH. Aust. J. Chem. 2005;58:379. [Google Scholar]
- 26.Boyer C, Bulmus V, Liu JQ, Davis TP, Stayton PS, Barner-Kowollik C. J. Am. Chem. Soc. 2007;129:7145. doi: 10.1021/ja070956a. [DOI] [PubMed] [Google Scholar]
- 27.Zhao YL, Perrier S. Chem Commun. 2007:4294. doi: 10.1039/b708293b. [DOI] [PubMed] [Google Scholar]
- 28.Henry SM, Convertine AJ, Benoit DS, Hoffman AS, Stayton PS. Bioconjug Chem. 2009;20:1122. doi: 10.1021/bc800426d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barz M, Canal F, Koynov K, Zentel R, Vicent MJ. Biomacromolecules. 2010;11:2274. doi: 10.1021/bm100338x. [DOI] [PubMed] [Google Scholar]
- 30.De P, Gondi SR, Sumerlin BS. Biomacromolecules. 2008;9:1064. doi: 10.1021/bm701255v. [DOI] [PubMed] [Google Scholar]
- 31.Oh JK, Siegwart DJ, Matyjaszewski K. Biomacromolecules. 2007;8:3326. doi: 10.1021/bm070381+. [DOI] [PubMed] [Google Scholar]
- 32.York AW, Huang F, McCormick CL. Biomacromolecules. 2010;11:505. doi: 10.1021/bm901249n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.York AW, Zhang YL, Holley AC, Guo YL, Huang FQ, McCormick CL. Biomacromolecules. 2009;10:936. doi: 10.1021/bm8014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider R, Schmitt F, Frochot C, Fort Y, Lourette N, Guillemin F, Muller JF, Barberi-Heyob M. Bioorg Med Chem. 2005;13:2799. doi: 10.1016/j.bmc.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Corona G, Giannini F, Fabris M, Toffoli G, Boiocchi M. Int J Cancer. 1998;75:125. doi: 10.1002/(sici)1097-0215(19980105)75:1<125::aid-ijc19>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.Sahlin S, Hed J, Runfquist I. Journal of Immunological Methods. 1983;60:115. doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Murthy N. Biochem Biophys Res Commun. 2007;360:275. doi: 10.1016/j.bbrc.2007.06.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.