Abstract
Pancreatic adenocarcinoma is the fourth leading cause of cancer death with an overall 5-year survival of less than 5%. As there is ample evidence that pancreatic adenocarcinomas elicit antitumor immune responses, identification of pancreatic cancer-associated antigens has spurred the development of vaccination-based strategies for treatment. While promising results have been observed in animal tumor models, most clinical studies have found only limited success. As most trials were performed in patients with advanced pancreatic cancer, the contribution of immune suppressor mechanisms should be taken into account. In this article, we detail recent work in tumor antigen vaccination and the recently identified mechanisms of immune suppression in pancreatic cancer. We offer our perspective on how to increase the clinical efficacy of vaccines for pancreatic cancer.
Keywords: chimeric antigen receptor, immune suppression, immune therapy, MDSC, pancreatic cancer, T cell, Treg, vaccine
Epidemiology of pancreatic cancer & clinical management
Pancreatic adenocarcinoma, henceforth indicated as pancreatic cancer, accounts for the majority of all pancreatic cancers and has the poorest outcome. Pancreatic cancer is highly aggressive and is the fourth leading cause of cancer death in the USA with over 43,000 estimated new cases in 2010 [1]. The prognosis of these patients is dismal with overall survival less than 5% and a median survival of 4–6 months. At the time of diagnosis, 15–20% of patients present with operable disease whereas approximately 40% are found to have locally advanced, unresectable disease and approximately 45% have metastatic disease. Surgical resection is the only known curative treatment for pancreatic cancer [2]. However, even with complete surgical resection, recurrence is common and a majority of patients recur with distant metastasis. Patients who develop recurrence usually present between 9 and 12 months after resection [3]. Median survival after surgery is 15–20 months with a 5-year survival rate of approximately 20% [3,4]. The median survival of patients with locally advanced, unresectable disease is 10–12 months [2,5]. The significance of this malignancy is further illustrated by comparing the estimated 36,800 deaths from pancreatic cancer with the 32,050 and 40,230 deaths from prostate and breast cancer, respectively [1].
The poor prognosis of pancreatic cancer is related to a combination of late detection, as most patients present with locally advanced or metastatic disease, and standards of care that consist of relatively ineffective chemotherapeutic regimens. Gemcitabine is currently approved, and the chemotherapeutic agent of choice, for the treatment of patients with pancreatic cancer, with adjuvant chemoradiation now considered the standard of care in many postoperative pancreatic cancer patients treated in North America. Multiple promising drugs, targeting hallmarks of cancer such as angiogenesis, proliferation and metastasis, have failed to provide any clinically relevant benefit [6].
A role for the immune response in pancreatic cancer
Immune-based treatments for cancer represent a rapidly evolving therapy with great potential. Based on the supposition that the immune system can discriminate tumor from normal self, investigators have pursued immune-based treatments for cancer for over 100 years [7]. Over the past 30 years, a large body of data has been accumulated showing that cancer patients generate B and T cells that recognize antigens expressed on autologous pancreatic tumor cells [8–14]. In addition, evidence has been obtained in animal models showing that mice deficient in genes associated with immunity (e.g., IFN [15] and perforin [16]) are prone to develop cancer. Furthermore, extensive analysis of immune infiltrates in human tumors has demonstrated a positive correlation between prognosis and the presence of a humoral response to pancreatic tumor antigens, such as MUC-1 and mesothelin, and of tumor-infiltrating cytotoxic T cells and Th1 cells [10,11,17,18]. By contrast, in a mouse model in which an activating K-Ras mutation is expressed in the pancreas, preinvasive pancreatic lesions are characterized by the infiltration of immune suppressor cells rather than immune effector cells, suggesting tumor immunity may be blocked from the inception of pancreatic cancer development [19]. All mice with the K-Ras mutation develop pancreatic adenocarcinoma and eventually die of disease. Finally, the finding that antagonism of negative T-cell regulators, such as cytotoxic T-lymphocyte-associated (CTLA) protein-4 and B- and T-lymphocyte attenuator (BTLA), can augment the antitumor immune response confirms that patients mount an immune response to their tumor [20,21]. Despite mounting evidence that an antitumor immune response is elicited in cancer patients, this response is ineffective and does not result in the elimination of tumor. Given that most tumor antigens are self- or mutated self-antigens and that the pancreatic tumor microenvironment is immunosuppressive (discussed at length below), this is not surprising [22]. Of particular note are the findings that both the prevalence of Treg in peripheral blood and tumor, and expression level of programmed death-1 ligand (PD-L1) in tumor are independent predictors of poor survival in pancreatic cancer [23–25]. Tregs that constitute 5–10% of CD4 T cells induce immune tolerance by suppressing host immune responses against self- and nonself-antigens [26–30], thus playing a critical role in tolerance and the immune response to malignancies. These findings strengthen the notion that pancreatic cancers induce antitumor immune responses. Thus, efforts towards improving the clinical efficacy of immune therapy should involve strategies to neutralize or overcome immune suppression.
We discuss in this article preclinical and clinical efforts towards immune therapy of pancreatic cancer.
Vaccination against tumor antigens as a treatment for pancreatic cancer
With the molecular identification of human tumor antigens in the early 1990s [31], the opportunity to specifically sensitize immune cells against tumor antigens became a reality, leading to a multitude of experimental strategies for immune-based therapy of cancer. While multiple clinical studies have documented evidence of treatment-induced, antigen-specific immune responses, few, if any, protective immune responses have been observed in patients with metastatic disease. Despite this setback, there is renewed optimism for immune therapy, as vaccination postsurgery in patients with no or minimal disease was shown to have an impact in breast and pancreatic cancer [32,33]. In addition, vaccination against tumor antigens is an attractive approach to adjuvant treatment postsurgery, when tumor-induced immune suppression is minimal [34–36].
To be considered an ideal tumor vaccine candidate, expression of the antigen – be it mutated or unaltered self – must be restricted to the tumor or only minimally expressed elsewhere in the body. In the following sections, we have restricted ourselves to discussion of a short list of tumor antigens that fit this criterion for pancreatic cancer (Table 1). As such, the use of immunological adjuvants will not be discussed. We have focused on the most recent examples in pancreatic cancer studies, either murine or human, whenever possible. In this article, some tumor antigen targets are discussed to a greater extent than others, reflecting the quantity of reports in the literature. Table 2 lists a number of clinical trials involving pancreatic cancer patients, representing multiple different vaccine platforms (Figure 1) that will be discussed in the following sections.
Table 1.
Overview of candidate pancreas cancer-associated antigens for immune targeting.
| Antigen | Location | Expression in tumor | Prevalence (%) | Description | Ref. |
|---|---|---|---|---|---|
| CEA | Cell surface (GPI-linked) | Overexpressed | 30–100 | Glycoprotein, normally expressed only on oncofetal tissues. Functions as cell-adhesion molecule. First tumor antigen to be described | [185–187] |
| Her2-neu | Transmembrane | Overexpressed | >50 | A receptor tyrosine kinase, member of the EGF−receptor family, involved in cell growth and differentiation | [188–190] |
| K-Ras | Intracellular | Mutated self | 90 | Mutated form of ras, a GTPase important for cell proliferation, differentiation and survival | [191,192] |
| Mesothelin | Cell surface (GPI-linked) | Overexpressed | ~100 | GPI-linked glycoprotein normally expressed on the surface of mesothelial cells lining the pleura, peritoneum and pericardium at low levels. Binding partner of CA125/MUC16 | [40,193–196] |
| MUC-1 | Transmembrane | Overexpressed, hypo-glycosylation | 90 | Type 1 transmembrane glycoprotein, expressed on apical surface of ductal and glandular epithelial cells at low levels. Extracellular domain has a polypeptide core with multiple tandem repeats of 20 amino acids | [197,198] |
| p53 | Intracellular | Mutated self | 50–70 | Tumor suppressor that regulates cell cycle. Normally inhibits survivin at the transcription level and can initiate apoptosis if DNA damage is unrepairable | [199,200] |
| Survivin | Intracellular | Overexpressed | 80 | Member of IAP family. Inhibits caspase activation; is found in most human tumors and fetal tissue, but is completely absent in terminally differentiated cells | [201–204] |
| Telomerase | Intracellular | Overexpressed | 95 | Ribonucleoprotein that is responsible for RNAdependent synthesis of telomeric DNA. TERT is its catalytic subunit | [205,206] |
| VEGFR2 | Transmembrane | Overexpressed | 64 | A tyrosine kinase and member of platelet derived growth factor family. Receptor for VEGF with functions in blood vessel development | [207,208] |
CEA: Carcinoembryonic antigen; GPI: Glycosylphosphatidylinositol; IAP: Inhibitor of apoptosis protein; MUC: Mucin; TERT: Telomerase reverse transcriptase; VEGFR: VEGF receptor.
Table 2.
Selection of current vaccine/immunotherapy clinical trials open to pancreatic cancer patients.
| Treatment | ClinicalTrial.gov ID | Status | Phase |
|---|---|---|---|
| CEA RNA-pulsed DC | NCT00004604 | Active, not recruiting | I |
| Muc-1 α-gal epitope (HyperAcute®) | NCT00569387 | Active, not recruiting | II |
| Muc-1 α-gal epitope (HyperAcute®) | NCT00614601 | Active, not recruiting | II |
| Muc-1 α-gal epitope (HyperAcute®) | NCT01072981 | Recruiting patients | III |
| Pancreatic tumor cell line/GM-CSF | NCT00389610 | Active, not recruiting | II |
| Pancreatic tumor cell line/GM-CSF | NCT01088789 | Recruiting | II |
| Pancreatic tumor cell line/GM-CSF (GVAX) | NCT00727441 | Recruiting | I |
| Pancreatic tumor cell line/GM-CSF ± ipilimumab | NCT00836407 | Recruiting | I |
| Pancreatic tumor cell line/GM-CSF and cetuximab | NCT00305760 | Active, not recruiting | II |
| CEA peptide | NCT00203892 | Active, not recruiting | II |
| Muc-1 peptide | NCT00008099 | Active, not recruiting | I/II |
| Survivin peptide | NCT00108875 | Recruiting patients | I/II |
| Telomerase peptide (GV1001) | NCT00425360 | Recruiting patients | III |
| VEGFR1/VEGFR2 peptide | NCT00639925 | Active, not recruiting | I/II |
| VEGFR1/VEGFR2 peptide | NCT00655785 | Active, not recruiting | I/II |
| ALVAC-CEA, Vaccinia-CEA, GM-CSF, aldesleukin | NCT00003125 | Active, not recruiting | II |
| PANVAC™-VF (CEA, MUC-1, TRICOM) | NCT00088660 | Active, not recruiting | III |
| Fowlpox-CEA (6D), TRICOM, autologous DCs, denileukin diftitox | NCT00128622 | Active, not recruiting | I |
| PANVAC-V, PANVAC-F, GM-CSF (CEA, MUC-1, TRICOM) | NCT00669734 | Recruiting | I |
| Vaccinia Virus Ankara Vaccine (p53) | NCT01191684 | Recruiting | I |
| SC-K-ras (GI-4000) | NCT00300950 | Active, not recruiting | II |
| SC-CEA (GI-6207) | NCT00924092 | Recruiting patients | I |
ALVAC: Canary pox virus; CEA: Carcinoembryonic antigen; DC: Dendritic cell; GM-CSF: Granulocyte–macrophage colony-stimulating factor; MUC: Mucin; PANVAC-F: Vaccine containing fowlpox virus; PANVAC-V: Vaccine containing vaccinia virus; SC: Saccharomyces cerevisiae; TRICOM: Three costimulatory molecules (B7.1, ICAM-1 and LFA-3); VEGFR: VEGF receptor.
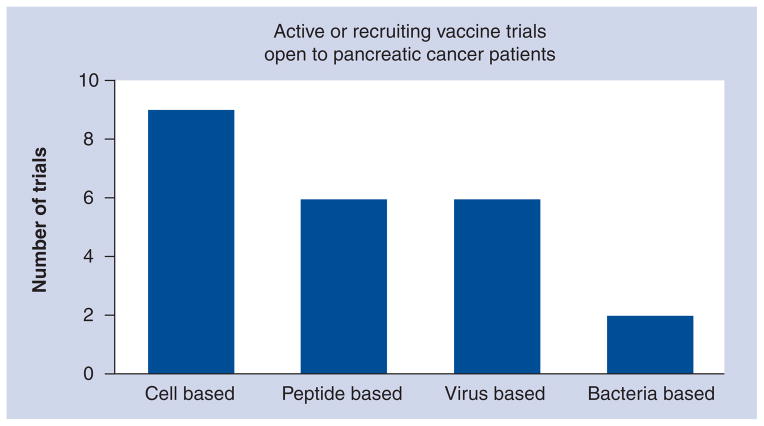
Figure 1. Overview of current immunotherapy trials in pancreatic cancer.
All active trials listed on Clinical Trials.gov were categorized based on the type of vaccine used.
Whole-cell vaccines
The simplest vaccine approach that has been applied to cancer is the inoculation of individuals with irradiated tumor cells. This approach has many advantages:
Specific tumor antigens do not need to be identified or characterized prior to vaccination;
Immune responses to multiple tumor antigens can be generated, which may protect against tumor escape variants [37];
Such vaccines are not limited by patient HLA background owing to cross-presentation of tumor antigens after uptake by dendritic cells (DCs) [37,38] – this is particularly advantageous as tumor cell lines are readily available, while the availability of autologous tumor cells may be restricted;
The tumor cell vaccine platform can be easily modified.
For example, tumor cells can be transduced to express immunomodulatory cytokines such as granulocyte macrophage colony-stimulating factor (GM-CSF), as was performed by Jaffee et al. in a Phase I clinical trial [39]. In their studies, a pancreatic tumor cell vaccine induced a CD8+ T-cell response, specific to mesothelin, regardless of HLA match between the tumor vaccine and recipient – demonstrating that cross-priming had occurred [38,39]. Mesothelin is a particularly promising cancer vaccine target owing to its low level of expression on nontumor tissues and high levels of expression on pancreatic as well as other cancers (i.e., ovarian) [40]. A Phase II trial for this vaccine is ongoing in patients with resectable pancreatic cancer (NCT00389610).
Tumor cell vaccines have also be modified to express epitopes, which increase antibodymediated uptake by DCs. Normally, MUC-1 expressed on tumors is immunogenic owing to overexpression and tumor-restricted hypoglycosylation [41]. The NewLink Genetics Corporation (IA, USA) has developed a whole-cell vaccine expressing MUC-1 modified to express α-gal epitopes, which is the focus of multiple clinical trials (NCT00255827, NCT00614601, NCT00569387 and NCT01072981) [42]. This vaccine takes advantage of anti-α-gal antibodies that are found in most people due to exposure to gastrointestinal flora, resulting in increased uptake of the vaccine in an antibody-dependent manner [43]. In murine studies, the addition of such α-gal epitopes to a Muc-1+ pancreatic cancer whole-cell vaccine resulted in increased production of anti-Muc-1 antibodies; enhanced tumor-specific T-cell responses and increased survival after challenge with Muc-1+ B16 cells in α-gal knockout mice, previously sensitized to α-gal [44]. A study using similarly treated melanoma cells as vaccine resulted in the complete protection against melanoma in mice [45,46].
Autologous DCs have also been used in tumor vaccination when pulsed with tumor lysates or peptides, transfected with whole-tumor mRNA, or transfected with mRNA or cDNA of a specific antigen [47]. Mature DCs have the benefit of expressing high levels of costimulatory molecules in addition to both HLA class I and class II molecules, allowing for direct presentation of tumor antigens to, and enhanced activation of, both CD8+ and CD4+ T cells. For example, Schmidt et al. intratumorally vaccinated with whole tumor mRNA transfected DCs and found an antitumor specific immune response and significantly decreased tumor volume in a murine pancreatic cancer model [48]. In melanoma patients, the whole-tumor mRNA approach has been used to generate antitumor CD4+ and CD8+ T-cell responses [49,50]. Apoptotic pancreatic tumor lysates have also been evaluated as a source of antigen and have been demonstrated to elicit stronger antitumor lytic activity when used to stimulate autologous human CD8+ T cells in vitro compared with those stimulated with tumor lysate-pulsed DCs [51]. Recently, a peptidepulsed autologous DC vaccine has been US FDA approved for the treatment of asymptomatic metastatic castration-resistant prostate cancer. This vaccine, known as Provenge® (Dendreon Corp., WA, USA) or Sipuleucel-T, consists of autologous, patient-derived DCs pulsed with a fusion protein consisting of the prostate tumor antigen prostatic acid phosphatase and GM-CSF [52]. In a Phase III clinical trial, vaccination resulted in a 3-year survival advantage in vaccinated castration- resistant prostate cancer patients (31.7% survival) compared with placebo (23%). Such a result is encouraging and gives hope that pancreatic cancer-targeted DC vaccines could produce similar effects.
In addition, autologous DCs, virally transduced to express IL-12, have also been used in cancer treatment. One pancreatic cancer patient receiving this treatment had a partial response in studies by Mazzolini et al. [53]. As the treatment DCs were not loaded with tumor antigen, cross-presentation of tumor antigens must have occurred.
A variation on the whole-cell approach involves the fusions of cancer cells and DCs, with the resulting cell used as the vaccine. Such vaccines can be made with autologous DCs and autologous tumor, with allogenic DCs and autologous tumor, or with autologous DCs and allogenic tumor [54]. This technique has been used to treat mice in a pancreatic tumor model, resulting in the generation of CD8+ T cells with tumor-specific cytolytic activity and tumor rejection [55].
In cases in which an immunogenic tumor antigen is known, autologous DCs have been transfected with, or virally transduced to express, the mRNA or cDNA of a specific tumor antigen. This technique does not require that the exact immunogenic epitopes of the antigen be identified, as full-length protein is transfected. Such a vaccine consisting of autologous DCs transfected with MUC-1 cDNA was administered to ten patients with advanced breast, pancreatic or papillary cancer in a Phase I/II clinical trial. A MUC-1-specific CD8+ T-cell response was generated in four patients, with a delayed-type hypersensitivity (DTH) response found in three patients [56]. However, all of the pancreatic cancer patients in this study developed progressive disease. Currently, a similar vaccine is in a Phase I/II clinical trial in melanoma patients using DCs transfected with the mRNA of tumor cells along with that of telomerase and survivin (NCT00961844). The use of multiple antigens may protect against tumor escape variants.
For some pancreatic tumor antigens, HLA-restricted immunogenic peptide epitopes have been identified and have been used to pulse DC vaccines. This approach allows the immune system to respond only to the immunologically relevant epitope, although its use is limited to patients expressing the corresponding HLA alleles. In an encouraging study by Lepisto et al., patients with resected pancreatic and biliary tumors were administered MUC-1, peptide-pulsed, autologous DCs in a Phase I/II clinical trial [57]. Although a clear antigen-specific T-cell response was not detectable following vaccination, 25% of the patients were alive at year 4. In similar studies with a telomerase peptide-pulsed DC vaccine, an antigen-specific CD8+ T-cell response was generated in patients who developed various cancers following vaccination. This response was enhanced when DCs were also pulsed with class II telomerase peptides, illustrating the importance of CD4+ T cells in fighting tumor [58]. Pulse can also be performed with peptides from multiple tumor antigens, as was performed in a Phase I clinical study by Carbone et al. Patients with various cancers, including pancreatic cancer, immunized with p53 and K-Ras peptide-pulsed PBMCs saw increased survival [59].
Peptide vaccines
Peptides corresponding to immunogenic tumor antigens or antigen epitopes have been administered as cancer vaccines, many of which have been designed to enhance the CD8+ T-cell response – the most important response for elimination of tumor. Peptide vaccines do not require manipulation of patient tissues, whose availability may be limited. A number of peptide vaccines have been successfully used to produce antigen-specific responses in pancreatic cancer patients. In a Phase I study, vaccination with a 100-mer peptide of the MUC-1 extracellular tandem repeat generated a MUC-1-specific T-cell response in some patients with resected or locally advanced pancreatic cancer, with two of the 15 patients alive at 61 months [60]. In a separate Phase I clinical trial using the same 100-mer peptide vaccine, the production of anti-MUC-1 circulating antibodies was detected in patients with inoperable pancreatic or biliary cancer, although no significant impact on survival was discovered [61]. A telomerase-based vaccine, consisting of the human telomerase reverse transcriptase (also known as GV1001) peptide, was found to induce a telomerase-specific immune response in 63% of evaluable patients, as measured by DTH in nonresectable pancreatic cancer. Those with a positive DTH were found to live longer than those that did not have a positive DTH [62]. More recently, a Phase III clinical trial was performed in which the effect of gemcitabine treatment on survival was compared with gemcitabine treatment in combination with GV1001 therapy in unresectable and metastatic pancreatic cancer patients [63]. However, the trial was terminated when no survival benefit was found. In similar studies, VEGF receptor (VEGFR)2–169, a peptide epitope vaccine, has been administered with gemcitabine to patients with advanced pancreatic cancer. A total of 83% of patients receiving the vaccine had an antigen-specific DTH and VEGFR2-specific CD8+ cells were detected in 61% of those vaccinated, with a median overall survival time of 8.7 months [64]. A randomized, placebo-controlled, multicenter, Phase II/III study of this VEGFR2–169 peptide vaccine therapy, combined with gemcitabine, is currently underway in patients with unresectable advanced or recurrent pancreatic cancer [301].
The most exciting results have come from studies of K-Ras-targeted peptide vaccines. In a pilot vaccine study, pancreatic and colorectal patients were vaccinated with K-Ras peptides containing patient-specific mutations. Three of the five pancreatic cancer patients displayed an antigen-specific immune response to a K-Ras [65]. Disease progression was observed in the two pancreatic cancer patients that did not respond to the vaccine, with the responders having no evidence of disease. Of the pancreatic cancer patients, a mean disease-free survival of 35.2+ months and a mean overall survival of 44.4+ months were observed. In a longer-term study, patients were followed for up 10 years after surgical resection of pancreatic adenocarcinoma and vaccination with a K-Ras peptide vaccine administered concomitantly with GM-CSF [33]. Remarkably, 20% of patients who received the vaccine were still alive at this time point. Immunological tests showed that a memory T-cell response was still present in 75% of the survivors. Such results with peptide vaccines are highly encouraging.
To increase the immunogenicity of peptide vaccines, some groups have mutated key anchor residues in the peptides such that binding to MHC-I molecules is increased. Therefore, presentation to CD8+ T cells is also increased. This is especially important when vaccinating against tumor (self) epitopes, as they are often weak or only intermediate binders to HLA molecules [66–70]. In a murine model of pancreatic cancer, our group has found this strategy to increase survival when applied to a peptide vaccine derived from murine mesothelin [Hamilton N & Hawkins WG: Unpublished Data]. Similarly, a MUC-1 peptide vaccine modified in this way was shown to enhance production of IFN-γ from patient and normal donor T cells. MUC-1-specific T-cell clones, generated via stimulation with this peptide, could lyse targets pulsed with native Muc-1 epitope as well as HLA-A2+ MUC-1+ human tumor cells in vitro [71]. Remarkably, one case has been reported in which vaccination with a modified HLA-A2-restricted survivin peptide resulted in remission of liver metastasis in one individual with pancreatic cancer [72].
An alternative method to enhance the presentation of peptides is to link them to antibodies, or other peptides, which target them to DCs. For example, an antimurine DEC205 antibody fused to either human mesothelin or survivin has been shown to enhance the immune response by directing delivery of tumor antigen to activated DCs in murine studies [73,74]. Similarly, the TAT protein from HIV, which facilitates entry into cells, has been fused to antigens in order to enhance uptake by DCs [75]. However, this technique has been used mainly to introduce antigens, such as carcinoembryonic antigen (CEA), into DCs and not as a peptide vaccine alone [76–79].
Another protein-based vaccine approach utilizes an anti-idiotypic vaccine that mimics the tumor antigen, CEA. This murine monoclonal, 3H1, has been successfully used to generate anti-CEA humoral and T-cell responses resulting in the rejection of CEA-expressing tumor in a murine model of colon cancer [80–82]. Two Phase II trials involving 3H1 have been completed in lung and colorectal cancer, although the results have yet to be published (NCT00006470 and NCT00033748).
More recently, an effort has been made to generate personalized peptide vaccines based on the tumor-antigen epitopes that are most immunogenic for a particular patient. Yanagimotto et al. applied this approach, in combination with gemcitabine therapy, to pancreatic cancer in a Phase I clinical trial. Prior to vaccination, T cells from patient PBMCs were screened against a panel of tumor antigen-derived peptides. Patients were vaccinated only with the peptides to which they had a response [14]. An increase in tumor antigen-specific T-cell responses was observed from the 13 evaluable patients with no correlation to clinical responses or humoral responses post vaccination, although 11 patients experienced either reduction in tumor size. Median survival time was 7.6 months. In 2010, this group published a similar Phase II study and found a median survival time of 9 months and a 1-year survival of 38% [83].
DNA vaccines
When specific tumor antigens are known, DNA vaccination can be utilized. DNA vaccines can be administered to humans via intramuscular injection with or without electroporation. This technique allows for an immune response to multiple potential epitopes within an antigen to be generated regardless of the recipients’ MHC profile [84]. In pancreatic cancer studies, DNA vaccination has mainly been applied to murine models of cancer, though there are ongoing clinical trials in other cancers (NCT00807781, NCT00859729, NCT00471133 and NCT01064375).
In one such murine pancreatic cancer study, vaccination with either murine or human fulllength survivin DNA generated an antitumor specific response, increased infiltration of tumor with lymphocytes and increased survival [85]. Similar results were found in studies with Muc-1 DNA vaccine when used as a preventative or therapeutic vaccine in a pancreatic cancer model [86]. In a murine ovarian cancer model, a full-length human mesothelin DNA vaccine has also been shown to inhibit growth of human mesothelin-expressing tumors, enhance survival and induce a CD8+ T-cell, CD4+ T-cell and humoral response [87]. Likewise, an oral DNA vaccination with either full-length extracellular domain or regions 1–4 of VEGFR2 resulted in decreased tumor size, intratumoral microvessel and density of liver metastases in murine colon cancer studies. An increase in survival time and VEGFR2 antibody was also observed in serum with both vaccines [88].
DNA vaccination has also been used to express a CEA66, which encodes for CEA linked to a T-helper epitope from tetanus toxin [89]. This vaccine skews the response to a Th1 phenotype and increases IFN-γ production from CEA-specific T cells when boosted with either CEA peptide or with repeat DNA vaccination in mice [90,91]. However, no studies with tumor challenges have been published with the CEA66 vaccine.
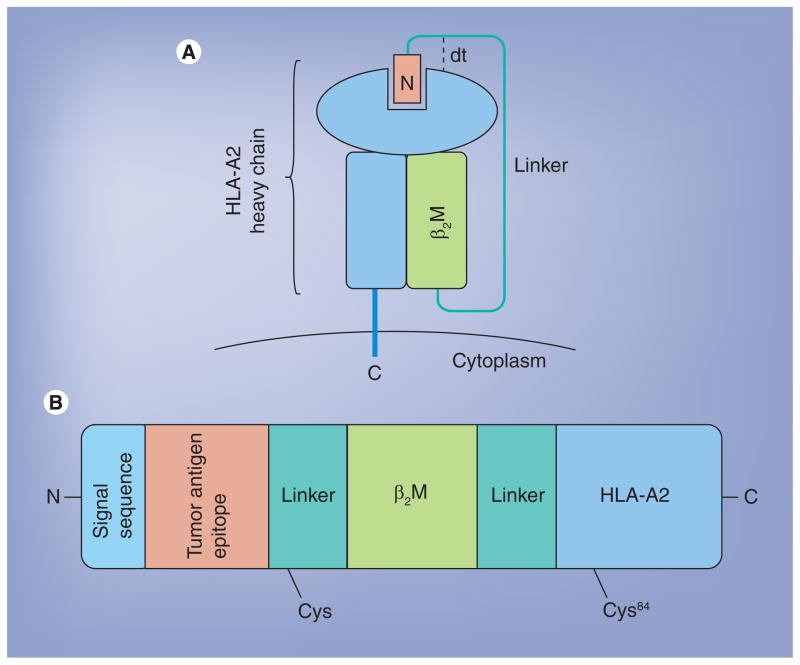
Highly engineered molecules, such as HLA-A2 single-chain trimers (SCTs) can also be expressed by DNA vaccination. HLA SCTs consist of the HLA heavy chain, β2M, and a peptide of interest connected by flexible linker regions (Figure 2). When introduced via DNA vaccination, SCTs bypass antigen processing, leading to direct expression of the encoded HLA/peptide complex on the cell surface [92]. This expression is more stable than that of the endogenous HLA/peptide compound, allowing for increased stimulation of antigen specific CD8+ T cells [93–95]. As such, SCTs are the ideal platform for the presentation of tumor antigens to CD8+ T cells.
Figure 2. Structure of the disulfide trap single-chain trimer.
Representation of a fully folded dtSCT as expressed on the cell surface (A). Note the presence of the Y84C mutation, creating the disulfide trap. SCTs that contain this mutation are more stably expressed on the cell surface. dtSCTs are encoded by a single DNA sequence that, when expressed, produces a peptide–MHC class I complex (B).
β2M: HLA heavy chain; dt: Disulfide trap; SCT: Single-chain trimer.
In fact, SCT DNA vaccines have been utilized for multiple viral and tumor targets and have been shown in in vivo murine studies to be more effective as DNA vaccines than comparable peptide and cDNA vaccines [93,94,96–100]. For example, Hung et al. administered such a DNA vaccine encoding a HLA-A2 SCT presenting a human mesothelin peptide to mice that transgenically expressed HLA-A2 in an ovarian cancer model. A mesothelin specific CD8+ T-cell response was generated and tumor-free survival was enhanced compared with vaccination with an Ova-SCT [99]. However, the HLA-A2 mice used were not tolerized to the foreign human mesothelin peptide presented in the SCT, making these data less translationally relevant. More recently, the SCT platform has been improved upon such that peptide binding to the HLA molecule and interaction with CD8 is enhanced. In addition, the pan-T-cell helper epitope (PADRE) has been included in the SCT DNA vaccines [94]. Such a SCT vaccine presenting a West Nile virus peptide showed increased survival in West Nile virus-infected HLA-A2 transgenic mice [100]. This improved SCT platform shows great promise and is currently in development for testing in patients with breast cancer at our institution.
Vaccines with microorganisms
In addition to more traditional (i.e., peptide-based) vaccine modalities, other groups have utilized various properties of microorganisms or viruses to enhance vaccination. Microorganisms can be used essentially as an antigen delivery system, but also have the added benefit of acting as an adjuvant by stimulating an innate immune response though engagement of pattern recognition receptors, such as Toll-like receptors (TLRs). A number of groups have used virus to deliver pancreatic cancer associated antigens and have tested them in murine models and/or clinical trials. For example, one group has used virus as a scaffold to display MUC-1, administration of which resulted in a robust immune response and a delayed growth of MUC-1-expressing tumors in mice transgenic for human MUC-1 [101].
However, most virally based vaccines are designed to deliver transgenes that enable infected cells to produce the tumor antigen of interest. Adenovirus has been used in this way to deliver CEA and was found to decrease tumor volume in mice bearing CEA-expressing tumors [102]. In human clinical trials, a pox virus-based approach to deliver tumor antigens, as well as costimulatory molecules, has been used. Kaufman et al. have completed a Phase I study of pancreatic cancer patients vaccinated via a vaccinia virus (PANVAC-V) expressing a HLA-A2-restricted CEA peptide, a MUC-1 peptide and three costimulatory molecules (B7.1, ICAM-1 and LFA-3 – also known as TRICOM) with GM-CSF administered as an adjuvant. An additional vaccination with a fowlpox virus (PANVAC-F) expressing the same molecules was also administered [103]. An antigen-specific antibody response was discovered in all ten patients and antigen-specific T-cell responses were found in five out of eight patients tested, with increased survival seen in those who generated an immune response.
Similarly, Morse et al. have generated an alphavirus vector expressing CEA that is packaged in a virus-like particle termed CEA(6D) VRP (AVX701) [104]. The alphavirus was chosen as it preferentially infects DCs [105]. Although only one pancreatic cancer patient was included in this study, the liver metastasis of that individual remarkably resolved after vaccination. A lentiviral system has also been generated that specifically targets DCs and has been shown to inhibit tumor growth in a murine tumor model. However, this virus has not been used to vaccinate against pancreatic tumor antigens, nor has it been utilized in a pancreatic cancer model or clinical studies [106].
Heat-killed yeast, transfected with tumor antigens, have been shown to activate DCs when used as vaccines in murine models [107]. Remondo et al. showed that Saccharomyces cerevisiae (SC), expressing a CEA transgene, activated human DCs, which then successfully activated CEA-specific T-cell clones. The T-cell clones were able to lyse human CEA+ pancreatic tumor lines [108]. In murine studies, vaccination with SC-CEA resulted in the generation of CEA-specific T cells, reduced tumor volume and increased survival when vaccinated hCEA-transgenic mice were challenged with a CEA-expressing tumor [109,110]. When compared with a CEA-pox vaccine platform, similar levels of CEA-specific T cells were generated in response to SC-CEA [110]. However, T-cell receptor (TCR) repertoires differed in their specificity and avidity. The authors suggest taking advantage of this diversity by vaccinating with both modalities, although this has not been performed in humans as of yet. In a murine carcinogen-induced lung tumor model, SC has been used to vaccinate against K-Ras, resulting in tumor regression [111].
Bacteria can also be used in vaccination, with the most popular being the intracellular facultative bacteria Listeria monocytogenes (LM) and Salmonella enterica serovar Typhimurium (ST) [112]. Bacteria have the advantages of being inexpensive to generate, are easily attenuated and can be killed with antibiotics if needed. LM infects phagocytic cells and has the unique ability to escape the phagosome and live intracellularly. Therefore, live-attenuated LM can deliver a protein, mRNA or cDNA of interest directly to the cytosol of antigen-presenting cells such as macrophages. This platform has been applied to human mesothelin, resulting in a mesothelin-specific CD8+ and CD4+ T-cell response in mice and in cynomolgus monkeys, which had a therapeutic effect in tumor-bearing mice [40]. More recently, a Phase I clinical trial found this LM-based mesothelin-expressing vaccine, CRS-207, to be well tolerated [113]. LM vaccines expressing Her-2/neu, p53 and VEGFR2 have been applied to murine breast cancer models, showing antigen-specific T-cell responses and decreased tumor growth [114–117].
Similar to LM, ST infects phagocytic cells but evades lysis by blocking the fusion of the phagosome and lysosome. Most work has focused on developing oral ST-based DNA vaccines. One such vaccine delivered CEA DNA and was shown to prevent growth of lung carcinoma in CEA transgenic mice [118]. Similarly, a ST-CEA/CD40 DNA vaccine resulted in a complete rejection of a CEA-expressing MC38 colon carcinoma in murine studies [119]. In addition, the type III secretion system of ST can be utilized to deliver protein antigen directly into the cytosol of antigen-presenting cells. When this strategy was used to vaccinate against survivin in a murine model, an antitumor immune response against CT26-derived tumors was observed [120].
Monoclonal antibody treatment
The humoral immune response to tumor antigens, such as mesothelin, has been found to be a favorable prognostic factor for pancreatic cancer [10,17,121,122]. The anticancer effect of such antibodies is thought to be primarily mediated via antibody-dependent cell cytotoxicity (ADCC), although complement-mediated lysis may contribute [108,123–126]. A number of groups have generated monoclonal antibodies to cell surface tumor antigens, such as mesothelin, MUC-1, CEA and Her-2/neu. In addition to ADCC, these antibodies can be linked to immunotoxins resulting in direct killing of the tumor cell after internalization or linked to a radioisotope and used as radiopharmaceuticals. We restrict our discussion to antibodies directed at antigens that have been extensively studied as vaccine targets (Table 3). As such, antibodies targeted to blocking angiogensis, such as bevacizumab, will not be discussed.
Table 3.
Selection of current monoclonal therapy clinical trials open to pancreatic cancer patients.
| Treatment | ClinicalTrials.gov ID |
|---|---|
| MORAb-009 (anti-mesothelin) | NCT01018784, NCT00711191 |
| Trastuzumab (anti-Her2/neu) | NCT00003797 |
| Cetuximab (anti-EGFR) | NCT00923299, NCT00042939, NCT00448838, NCT00305760, NCT00075686, NCT00408564, NCT00467116 |
| Panitumumab (anti-EGFR) | NCT00004879, NCT00601627, NCT00550836 |
| Nimotuzumab (anti-EGFR) | NCT00561990 |
| Trastuzumab and cetuximab | NCT00599833 |
EGFR: EGF-receptor.
A number of antibodies to mesothelin have been studied in both mouse and humans. For example, SS1P is a murine single-chain Fv, specific for human mesothelin, which has been linked to Pseudomonas exotoxin A (PE), which results in cell death via inhibition of EF2 when internalized [127]. In Phase I clinical studies SS1P was found to be well tolerated and administration of a version of SS1P with releasable PEGylation resulted in complete regression of a mesothelin-expressing human carcinoma in mice with only a single dose [127–129]. A mouse–human chimeric IgG1k monoclonal antibody, MORAb-009, utilizes the same Fv as SS1P and was shown to elicit ADCC on mesothelin-positive cells. When combined with gemcitabine, MORAb-009 reduced tumor growth in nude mice compared with either treatment alone and had no adverse effects in nonhuman primates [130]. Two fully human, antihuman mesothelin antibodies, M912 and HN1, have been developed, which bind mesothelin- positive cells and result in their lysis via ADCC [131,132]. Similar to SS1P, HN1 has been fused to truncated PE-A immunotoxin, although its binding site on mesothelin probably binds a distinct but overlapping epitope to that of SS1P [132].
Many anti-CEA antibodies have been generated, with the bulk of studies in radioimmunotherapy, which will not be covered in the scope of this article. However, there are a number of anti-CEA antibodies that have been used for immunotherapy. One such antibody is hPRIA3, a humanized anti-CEA antibody, originally developed in mice, which binds membrane-bound CEA [133,134]. hPRIA3 has been shown in in vitro studies to bind to, and enhance, CEA-specific cytotoxicity of CEA+ human colorectal cancer cell lines when incubated with human PBMCs and NK cells, which can be increased when the Fc portion of the antibody is glycosylated [135,136]. A second anti-CEA monoclonal, hMN-14 (labetuzumab), has also been shown to induce ADCC in vitro with CEA+ colon tumor cells and inhibited growth of lung metastases in nude mice [137]. A Phase I/II trial with hMN-14 in pancreatic cancer patients has been completed but the results have not been published (NCT00041639).
PankoMab™ (Glycotope, Germany) is a murine antihuman MUC-1 antibody that binds to a carbohydrate induced conformational tumor epitope of MUC-1, greatly increasing its tumor specificity [138]. PankoMab can induce ADCC of MUC-1 positive cells and can also induce death following internalization by inhibition of RNA polymerase when linked to β-amanitin. The humanized version of PankoMab has been shown to react to the tumor expressed MUC-1 in multiple human carcinomas, although no clinical trials have been published [139].
An anti-Her-2/neu antibody, know as Herceptin® (Genetech Inc., CA, USA) or trastuzumab, has been used with some success to treat pancreatic cancer in murine models. Treatment with trastuzumab prolonged survival and reduced liver metastasis in nude mice orthotopically challenged with human pancreatic tumor cell lines that expressed Her-2/neu at low levels. The pancreatic lines were sensitive to ADCC lysis by trastuzumab in vitro [140]. Similar results were found when nude mice, challenged with Her-2/neuhigh-expressing human pancreatic cancer cell lines, were treated with both trastuzumab and 5-fluorouracil [141]. The combination of treatment significantly inhibited tumor growth compared with either treatment alone. When combined with matuzumab, an anti-EGFR antibody, trastuzumab treatment resulted in inhibited tumor growth in a nude mouse model of pancreatic cancer [142]. This combined treatment was more than four-times more effective than treatment with either antibody alone. This group also found that the combined antibody treatment was more effective than gemcitabine treatment in orthotopic nude mice studies [143]. A number of clinical trials involving trastuzumab are listed in Table 3.
Passive T-cell therapy
In recent years, much work has focused on adoptive tumor immunotherapy in which the patient’s own T cells are expanded and reinfused into the patient. One method results in the selective expansion of T cells endogenously expressing TCRs specific for the tumor antigen of interest. For example, in a murine ovarian cancer model, Hung et al. vaccinated mice with a murine mesothelin DNA prior to antigen-specific T-cell expansion ex vivo. The mesothelin-specific T-cell clones were transferred to tumor-challenged mice, resulting in decreased tumor volume and increased survival [144]. Similarly, in a clinical study, MUC-1-specific autologous T cells, isolated from patient PBMCs, were expanded by incubation with a MUC-1-presenting cell line prior to administration to pancreatic cancer patients. The mean survival time for unresectable patients in this study was 5 months [145]. However, patients with resectable pancreatic cancer had 1-, 2- and 3-year survival rates of 83.3, 32.4 and 19.4%, respectively, and a mean survival time of 17.8 months. In a similar study by the same group, Kondo et al. isolated adherent cells from patient PBMCs to generate mature DCs that were then pulsed with MUC-1 peptide. The pulsed DCs were administered, along with autologous expanded MUC-1-specific T cells, to patients with unresectable or recurrent pancreatic cancer. Remarkably, a complete response was observed in one patient with lung metastases and the mean survival time of the whole group was 9.8 months, suggesting that the addition of pulsed DCs may have improved the outcome [146].
Chimeric antigen receptors
An alternative to antigen-specific expansion is the transduction of patient T cells, usually via lentivirus, with a chimeric antigen receptor (CAR) specific for a tumor antigen. Such receptors are transmembrane proteins typically comprise an antibody-derived single-chain variable fragment (scFv) specific for a tumor antigen fused to a hinge region, a spacer, a membrane spanning element and signaling domain [147,148]. Often the intracellular signaling domain contains the signaling motifs from multiple molecules, such as 41BB, OX40, CD28 and TCRζ chain. This allows for both TCR and costimulatory signaling cascades to be initiated, leading to T-cell activation. The resulting T cells recognize the tumor antigen in its native form and do not rely on presentation of antigen by MHC I. This is highly advantageous, as tumors often downregulate MHC-I expression.
Chimeric antigen receptors have been created with specificity for mesothelin, CEA, MUC-1 and Her-2/neu. Carpenito et al. have generated a CAR that recognizes mesothelin via the SS1 scFv and utilizes the intracellular signaling domains of CD3ζ, CD28 and CD137 [147]. When human T cells expressing this receptor were transferred to tumor bearing NOD/SCID/IL2rγ−/− mice, a reduced tumor volume was observed. Similar results were generated when T cells expressing CARs, possessing CD28 and TCRζ intracellular motifs and a specificity for human CEAs were administered to CEA+ tumor-challenged mice [149]. In addition, Shirasu et al. generated a CEA-specific CAR utilizing the human monoclonal antibody C2–45 and cytoplasmic motifs of CD28 and CD3ζ [150]. When expressed in Jurkat cells, the CEA-CAR initiated signaling cascades similar to those expected from TCR-mediated activation.
A CAR with specificity to MUC-1 has also been generated using the SM3 antibody with intracellular signaling motifs of CD28, OX40 and CD3ζ [151]. T cells expressing this CAR proliferated and produced cytokine in response to antigen challenge, killed MUC-1+ tumor cells in vitro and delayed growth of Muc-1 tumors in mice. Bakhtiari et al. developed a similar anti-MUC-1 CAR with the intracellular signaling motifs CD28 and CD3ζ [152]. When expressed in Jurkat cells, MUC-1-specific stimulation included cytokine production and killing of a MUC-1+ cell line.
T cells expressing a Her-2/neu-specific CAR with CD28 and CD3ζ chain signaling motifs has been shown to produce cytokines and to lyse a Her-2/neu+ breast cancer tumor line following antigen stimulation [153]. Mice challenged with breast cancer tumors had long-term tumor-free survival after administration of these T cells and were found to have developed a memory response that protected from rechallenge with tumor. Likewise, T cells expressing a Herceptinbased CAR with signaling motifs from CD28, CD3ζ and 41BB was shown to lyse Her-2/neu+ tumor in vitro and suppressed tumor growth in an in vivo murine model [154]. A number of Phase I clinical trial involving Her-2/neu CAR-expressing T cells are currently recruiting breast cancer patients (NCT00889954, NCT00902044 and NCT01109095).
Although this process results in a large population of antigen-specific T cells, adoptive immunotherapy is a very expensive and time consuming process in comparison with the vaccine and antibody therapies discussed in this article. There are no published studies utilizing CAR expressing T cells in pancreatic cancer or in murine models of pancreatic cancer.
Pancreatic cancers induce an immunosuppressive tumor microenvironment
Despite all the progress in vaccine development and increased understanding of basic immune mechanisms, the clinical application of therapeutic cancer vaccines has been characterized by many setbacks. Recent vaccines for melanoma (e.g., Canvaxin™ [CancerVax, CA, USA] and Melacine® [Corixa, WA, USA]), renal cell carcinoma (Oncophage® [Antigenics Inc., MA, USA]) and prostate cancer (G-VAX), tested in Phase III clinical trials, all failed to show clinical efficacy [7,155]. This has increased awareness of gaps in our knowledge regarding tumor-induced immune suppression.
We, and others, have shown that the tumor microenvironment in pancreatic cancer preferentially favors the recruitment of immune suppressor, rather than immune effector, cell types (Figure 3) [30,156]. Pancreatic cancer cells themselves actively contribute to immune suppression through production of immune suppressive cytokines (e.g., TGF-β [157]) and by expressing surface molecules that mediate immune suppression (e.g., FasL [158], PD-L1 [25] and possibly BTLA [159]). In addition, tumor-associated macrophages (TAMs), tumor-associated fibroblasts, plasmacytoid dendritic cells (pDCs) expressing the tryptophan-converting enzyme 2,3-indoleamine dioxygenase (IDO) [160], Treg and soluble factors produced by suppressor cells all contribute to pancreatic cancer-induced immune suppression. Finally, recent studies have described a heterogeneous population of immature cells arising from the myeloid lineage, termed myeloid-derived suppressor cells (MDSCs) [161]. MDSCs express CD11b and myelomonocytic/macrophage markers such as CD14 and CD33, or granulocytic/neutrophilic markers such as CD15. In some reports, the immature status of MDSCs was demonstrated by the absence of markers of terminal differentiation (Lin−DRlo/−) [162]. As such, MDSCs differ from TAMs and pDCs, which are fully differentiated lineages of cells. The accumulation of MDSCs in patients with advanced cancers, including pancreatic cancer, were demonstrated to be closely related to the extent of disease and correlated well with disease stage [163].
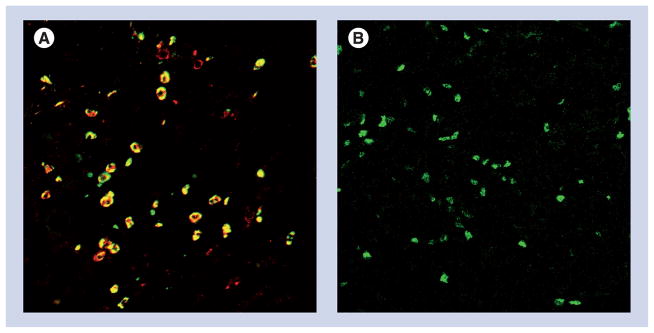
Figure 3. Immunohistology of human pancreatic adenocarcinoma.
(A) Tissue slides were stained with antibodies for CD11b (red), and CD15 (green), and shows dual positive cells (yellow), indicative of MDSC. (B) Intracellular Foxp3 staining as a marker of Treg (green). No immune infiltrate was detectable in normal human pancreas tissue (data not shown).
MDSC: Myeloid-derived suppressor cell.
Images courtesy of David C Linehan and Jonathan B Mitchem, Washington University, Department of Surgery.
Instrumental in shaping the immunosuppressive environment are the tumor cells and tumor-associated fibroblasts, in particular through secretion of chemokines and other cytokines [164]. Chemokines induce motility of endothelial cells and tumor cells. In addition, leukocyte subpopulations recruited to the tumor by chemokines are skewed in the tumor environment towards cells that support tumor growth, promoting angiogenesis, tumor invasion and metastasis formation while suppressing immune effector cells. For example, we recently observed that Treg cells in the tumor microenvironment of human pancreatic adenocarcinoma express CCR5 and the pancreatic tumor cells produce greater than tenfold higher levels of the CCR5 ligands, CCL3, CCL4 and CCL5 [165].
There is clear evidence of cross-talk between the various suppressor cell subsets. For example, MDSCs can promote Treg recruitment and maintenance through TGF-β-dependent and -independent mechanisms. IDO production by tumor-associated DCs and macrophages has also been implicated in Treg activation and expansion. IDO expression can be induced in DCs by reverse signaling through B7–1 and B7–2 (CD80 and CD86, respectively) and CTLA-4 expressed on Tregs. In turn, IDO activates Tregs and induce differentiation of naive CD4 T cells towards Tregs. By converting tryptophan into N-formyl kynurenine, effector T cells that are dependent on tryptophan for proliferation, are inhibited [166,167]. TAMs and MDSCs also skew the CD4 T-cell subset composition towards a Th2 phenotype through cytokines such as IL-10. The interplay between the various subsets of suppressor cells and cytokines creates a powerful barrier against immune attack.
Enhancing vaccine efficacy by targeting immune suppression
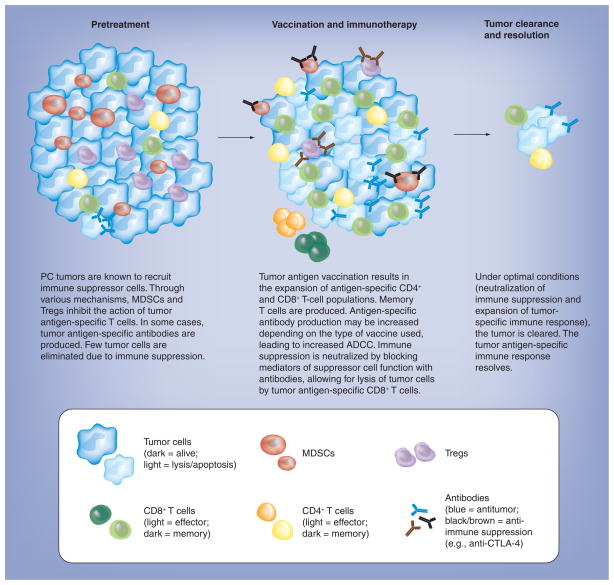
As we make progress in defining the molecular pathways of immune suppression, it becomes more and more relevant to integrate strategies that activate antitumor immunity with those that neutralize or overcome tumor-induced immune suppression (Figure 4) [155]. Just as immune activation strategies should be multifaceted to take advantage of both innate and adaptive immune effector mechanisms, targeting immune suppression may need to be equally comprehensive in order to have an effect. Preclinical studies in animal tumor models have provided proof-of-concept that targeting immune suppression may have an impact on cancer vaccines. For example, a promising antibody treatment involves the use of anti-CTLA-4 [168]. Aimed at preventing the downregulation of activated T cells, anti-CTLA-4 monotherapy has shown encouraging results in a small percentage of patients with advanced cancers [169]. In a recently completed Phase III trial, anti-CTLA-4 antibody with or without gp-100 peptide vaccine significantly prolonged survival of patients with metastatic melanoma. Interestingly, anti-CTLA-4 did not improve the gp-100 peptide vaccine efficacy. The reason for the lack of additive effect is not clear to the investigators of the study but may be due to minimal clinical activity of the gp-100 vaccine [170]. Although disappointing, the clinical benefit of anti-CTLA-4 was statistically significant with or without gp-100 vaccine, supporting the idea that in patients with an existing endogenous antitumor immune response, the use of anti-CTLA-4 may enhance this response and induce a clinically meaningful response. At the same time, it is unlikely that anti-CTLA-4 monotherapy will lead to long-term cures in the majority of patients. In this regard, a recently completed Phase II trial in patients with locally advanced or metastatic pancreatic cancer (NCT00836407) showed that anti-CTLA-4 monotherapy is ineffective in this patient population [171]. Likewise, the most commonly used strategy for neutralization of the PDL1-PD1 pathway is via antibodies that either block signalling through PD1 or that neutralize PDL1. The first clinical studies have been performed with PD-1-blocking antibodies, both in solid tumors (MDX-1106)[172] and in hematologic malignancies (CT-011)[173]. An experimental drug, AMP-224, consisting of a PD-L2/Ig fusion protein that binds to PD-1 on T cells and prevents the inhibitory signal [302] has not yet been tested clinically. Finally, the depletion of MDSCs in murine models has been associated with improved host immune responses, resulting in delayed tumor growth, improved survival and increased efficacy of vaccine therapy. Elimination of MDSCs has been shown to improve antitumor responses, restore CTL and NK function, decrease tumor angiogenesis and enhance immunotherapy [174–176].
Figure 4. Depiction of ideal therapy strategy.
Prior to therapy, the immunological milieu is characterized by high levels of immune suppression and low levels of immune responders, as well as immunological memory. The ideal immune therapy not only augments the antigen-specific immune response, but also reduces immune suppression. Key players such as NK cells, macrophages, B cells, complement and cytokines have been omitted to increase clarity.
ADCC: Antibody-dependent cell cytotoxicity;
MDSC: Myeloid-derived suppressor cell; PC: Pancreatic cancer.
Rather than describe specific strategies in detail, we will offer some comments on this topic:
It can be concluded from our current knowledge of immune suppressor mechanisms in cancers that multiple mechanisms are at play, which act in concert. Some of these, for example the production of certain cytokines such as IL-10, are not restricted to an individual cell type. Thus, successful intervention may require a multipronged attack;
There is heterogeneity among different histological types of tumor with regard to which suppressor mechanisms are present or dominant, and perhaps even within tumors of the same clinical stage and histologic type. This has obvious implications for the type of strategy that will be used;
Vaccination against self-antigens has been associated with induction Tregs and MDSCs. For example, Nishikawa et al. have reported that immunization of mice with plasmids encoding tumor antigens resulted in marked enhancement of in vivo tumor growth in a murine pulmonary metastasis model [177]. Subsequent analysis of their findings revealed that depletion of Tregs reversed this effect and adoptive transfer restored it, suggesting that vaccination promoted the proliferation of autoantigen-reactive Tregs. Similarly, T-cell clones derived from tumor-infiltrating lymphocytes were recently described, which recognized the melanoma antigen, LAGE-1 with characteristics of Tregs [178];
Finally, we reiterate that tumor vaccines may be most effective in a setting of minimal disease (i.e., postsurgery, at a time when tumor-induced immune suppression is likely to also be minimal). In this setting, vaccines that induce immunological memory through tumor-specific CD4 T cells and IgG antibodies may be most effective.
However, as discussed previously, most patients with pancreatic cancer have advanced disease at the time of diagnosis and are not candidates for surgery.
Conclusion
There is ample evidence to suggest that pancreatic cancer induces an antitumor immune response. There is also ample evidence that this response can be enhanced by various immune-based strategies. However, successful clinical application may require a better understanding of how pancreatic cancers evade immune recognition and integration of specific strategies that target immune suppressor mechanisms.
Future perspective
When reviewing the current status of FDA-approved immune-based interventions for cancer, it is relevant to ask whether there is a future for immune therapy for the treatment and prevention of cancer. With the recent FDA approval of Provenge being a prime example [52,179,180] we consider the future to be optimistic for several reasons. First, our understanding of immune mechanisms has accelerated over the past decade and will probably continue. This includes specific knowledge on how to overcome tolerance to self (tumor) antigens, implementation of immunological adjuvants and strategies to induce immunological memory. Combined with the growing emphasis on defining molecular pathways of immune suppression and negative regulatory pathways (CTLA-4, PD-1 and others), the challenge going forward in the near future is to integrate everything we have learned into new generation vaccines. Furthermore, we expect the rapidly evolving techniques, developed to sequence cancer genomes, to have unprecedented consequences for the treatment and prevention of cancer. What was considered a monumental task just several years ago will become a routine and straightforward assay in the next few years. The first such efforts have already been successfully completed in pancreatic cancer [181,182]. These studies not only demonstrated the presence of multiple mutations that can be targeted; they also showed that many of these mutations are already present in primary pancreatic cancers, and suggest vaccination after primary resection may protect against recurrence. The main implication for immune therapy will be that personalized vaccines will no longer be science fiction, but state of the art. The main advantage will be that vaccines can be tailored towards unique mutations found only in the patient’s cancer [183], or, alternatively, applied to certain subtypes of tumors. Such mutations have been identified in the past [184], but in the next 4–6 years we expect the sequencing of a patient’s cancer (and normal cells) to be part of the diagnosis. From an immunological standpoint, the immune response to mutated (nonself) antigens will be much more robust, as tolerance to such antigens should be minimal.
Executive summary.
Epidemiology of pancreatic cancer & clinical management
Pancreatic adenocarcinoma is the fourth leading cause of cancer death in the USA.
No effective therapy exists for pancreatic adenocarcinoma owing to its late discovery and strong resistance to conventional therapy.
A role for the immune response in pancreatic cancer
Pancreatic cancer elicits an adaptive antitumor immune response.
Both the prevalence of Tregs and expression levels of PD-L1 in the tumor are prognostic factors.
Vaccination against tumor antigens as a treatment for pancreatic cancer
Vaccination against pancreatic cancer antigens is an attractive approach to adjuvant treatment postsurgery.
The ideal cancer antigen not only elicits a strong immune response but is exclusively expressed on tumor cells.
Whole-cell vaccines, peptide vaccines, DNA vaccines & vaccines with microorganisms
Pancreatic cancer-associated antigens that are candidates for immune targeting include Her2/neu, Muc-1, CEA, mesothelin, telomerase and survivin.
Multiple vaccine platforms have been used successfully for induction of antigen-specific immune responses in animal tumor models and in in vitro studies.
Monoclonal antibody treatment
Adoptive transfer of antibodies to pancreatic cancer surface antigens such as mesothelin has been used for therapy with promising results in animal tumor models.
The antibodies may induce antibody-dependent cell cytotoxicity or complement-dependent lysis, or block tumor growth, for example when directed to growth factor receptors such as EGF and Her2/neu.
Passive T-cell therapy
Promising clinical results were obtained through adoptive transfer of patient-derived T cells expanded ex vivo.
Chimeric antigen receptors
A variation of passive T-cell therapy involves the genetic engineering and expansion of T cells expressing a chimeric receptor consisting of a single-chain variable fragment antibody chain specific for a pancreas cancer-associated antigen fused to a transmembrane signaling domain essential for T-cell activation such as CD28 and TCRζ.
This strategy has not yet been tested in patients with pancreatic cancer.
Pancreatic cancers induce an immunosuppressive tumor microenvironment
The tumor environment in pancreatic cancer is highly immunosuppressive through increased prevalence of multiple types of suppressor cells.
Enhancing vaccine efficacy by targeting immune suppression
It is key for the treatment of patients with established disease to integrate immunization strategies with those that neutralize immune suppression.
Alternatively, vaccines may be most efficacious when administered as an adjuvant therapy, when immune suppression is minimal.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Lindzy F Dodson is funded through a postdoctoral fellowship from the American Cancer Society, and this work was partially funded by NIH 1R21CA150945-01 to William G Hawkins. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States) Cancer Causes Control. 2006;17(4):403–409. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 3.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. J Oncol Pract. 2008;9(2):99–132. [PubMed] [Google Scholar]
- 5.Roy R, Maraveyas A. Chemoradiation in pancreatic adenocarcinoma: a literature review. Oncologist. 2010;15(3):259–269. doi: 10.1634/theoncologist.2009-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev Anticancer Ther. 2009;9(10):1473–1485. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- 7.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol. 2009;27(2):129–139. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 8.Yokokawa J, Palena C, Arlen P, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11(17):6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;1 61(3):869–872. [PubMed] [Google Scholar]
- 10.Johnston FM, Tan MC, Tan BR, Jr, et al. Circulating mesothelin protein and cellular antimesothelin immunity in patients with pancreatic cancer. Clin Cancer Res. 2009;15(21):6511–6518. doi: 10.1158/1078-0432.CCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54(11):2856–2860. [PubMed] [Google Scholar]
- 12.Kubuschok B, Neumann F, Breit R, et al. Naturally occurring T-cell response against mutated p21 ras oncoprotein in pancreatic cancer. Clin Cancer Res. 2006;12(4):1365–1372. doi: 10.1158/1078-0432.CCR-05-1672. [DOI] [PubMed] [Google Scholar]
- 13.Wenandy L, Sorensen RB, Sengelov L, Svane IM, Thor SP, Andersen MH. The immunogenicity of the hTERT540–548 peptide in cancer. Clin Cancer Res. 2008;14(1):4–7. doi: 10.1158/1078-0432.CCR-07-4590. [DOI] [PubMed] [Google Scholar]
- 14.Yanagimoto H, Mine T, Yamamoto K, et al. Immunological evaluation of personalized peptide vaccination with gemcitabine for pancreatic cancer. Cancer Sci. 2007;98(4):605–611. doi: 10.1111/j.1349-7006.2007.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6(11):836–848. doi: 10.1038/nri1961. Summarizes the evidence for immune surveillance of cancer, in particular through interferons, and offers a model for how tumors escape from immune recognition. [DOI] [PubMed] [Google Scholar]
- 16.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103(1):97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 18.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 19.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279(1):1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26(32):5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 21.Paulos CM, June CH. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. J Clin Invest. 2010;120(1):76–80. doi: 10.1172/JCI41811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 23.Ikemoto T, Yamaguchi T, Morine Y, et al. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas. 2006;33(4):386–390. doi: 10.1097/01.mpa.0000240275.68279.13. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 25.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 26.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. The first description of Treg cells as regulators of autoimmunity. [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 30.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32(1–3):155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 31▪▪.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–1647. doi: 10.1126/science.1840703. The first paper describing the molecular identification of a human tumor antigen recognized by cytolytic T cells. [DOI] [PubMed] [Google Scholar]
- 32.Peoples GE. HER2 vaccination in high-risk breast cancer. Clin Adv Hematol Oncol. 2009;7(11):715–717. [PubMed] [Google Scholar]
- 33▪.Weden S, Klemp M, Gladhaug IP, et al. Long term follow-up of resected pancreatic cancer patients following vaccination against mutant K-RAS. Int J Cancer. 2010;12 doi: 10.1002/ijc.25449. Provides evidence that vaccination against mutated K-Ras peptides offers long-term protection against recurrence in some patients with pancreatic cancer. [DOI] [PubMed] [Google Scholar]
- 34.Hensler T, Hecker H, Heeg K, et al. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65(6):2283–2291. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10(8):972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Weighardt H, Heidecke CD, Emmanuilidis K, et al. Sepsis after major visceral surgery is associated with sustained and interferon-γ resistant defects of monocyte cytokine production. Surgery. 2000;127(3):309–315. doi: 10.1067/msy.2000.104118. [DOI] [PubMed] [Google Scholar]
- 37.Saito H, Dubsky P, Dantin C, Finn OJ, Banchereau J, Palucka AK. Cross-priming of cyclin B1, MUC-1 and survivin-specific CD8+ T cells by dendritic cells loaded with killed allogeneic breast cancer cells. Breast Cancer Res. 2006;8(6):R65. doi: 10.1186/bcr1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200(3):297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a Phase I trial of safety and immune activation. J Clin Oncol. 2001;19(1):145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 40.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44(1):46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beatson RE, Taylor-Papadimitriou J, Burchell JM. MUC1 immunotherapy. Immunotherapy. 2010;2(3):305–327. doi: 10.2217/imt.10.17. [DOI] [PubMed] [Google Scholar]
- 42.Mandell RB, Flick R, Staplin WR, et al. The αGal HyperAcute® technology: enhancing immunogenicity of antiviral vaccines by exploiting the natural αGal-mediated zoonotic blockade. Zoonoses Public Health. 2009;56(6–7):391–406. doi: 10.1111/j.1863-2378.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 43.Macher BA, Galili U. The Galα1,3Galβ1,4GlcNAc-R (α-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780(2):75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deguchi T, Tanemura M, Miyoshi E, et al. Increased immunogenicity of tumor-associated antigen, mucin 1, engineered to express α-Gal epitopes: a novel approach to immunotherapy in pancreatic cancer. Cancer Res. 2010;70:5259. doi: 10.1158/0008-5472.CAN-09-4313. [DOI] [PubMed] [Google Scholar]
- 45.Rossi GR, Unfer RC, Seregina T, Link CJ. Complete protection against melanoma in absence of autoimmune depigmentation after rejection of melanoma cells expressing α(1,3) galactosyl epitopes. Cancer Immunol Immunother. 2005;54(10):999–1009. doi: 10.1007/s00262-005-0667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi GR, Mautino MR, Awwad DZ, et al. Allogeneic melanoma vaccine expressing αGal epitopes induces antitumor immunity to autologous antigens in mice without signs of toxicity. J Immunother. 2008;31(6):545–554. doi: 10.1097/CJI.0b013e31817d2f45. [DOI] [PubMed] [Google Scholar]
- 47.Koido S, Hara E, Homma S, et al. Cancer vaccine by fusions of dendritic and cancer cells. Clin Dev Immunol. 2009;2009:657369. doi: 10.1155/2009/657369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt T, Ziske C, Marten A, et al. Intratumoral immunization with tumor RNA-pulsed dendritic cells confers antitumor immunity in a C57BL/6 pancreatic murine tumor model. Cancer Res. 2003;63(24):8962–8967. [PubMed] [Google Scholar]
- 49.Kyte JA, Mu L, Aamdal S, Kvalheim G, Dueland S, Hauser M, et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther. 2006;13(10):905–918. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- 50.Kyte JA, Kvalheim G, Lislerud K, et al. T cell responses in melanoma patients after vaccination with tumor-mRNA transfected dendritic cells. Cancer Immunol Immunother. 2007;56(5):659–675. doi: 10.1007/s00262-006-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnurr M, Scholz C, Rothenfusser S, et al. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and γδ T cells. Cancer Res. 2002;62(8):2347–2352. [PubMed] [Google Scholar]
- 52.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castrationresistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 53.Mazzolini G, Alfaro C, Sangro B, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23(5):999–1010. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 54▪.Koido S, Homma S, Hara E, et al. Antigen-specific polyclonal cytotoxic T lymphocytes induced by fusions of dendritic cells and tumor cells. J Biomed Biotechnol. 2010:752381. doi: 10.1155/2010/752381. Reviews on the use of dendritic cell–tumor cell fusions and their ability to induce antigen-specific T-cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto M, Kamigaki T, Yamashita K, et al. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep. 2009;22(2):337–343. [PubMed] [Google Scholar]
- 56.Pecher G, Haring A, Kaiser L, Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a Phase I/II clinical trial. Cancer Immunol Immunother. 2002;51(11–12):669–673. doi: 10.1007/s00262-002-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lepisto AJ, Moser AJ, Zeh H, et al. A Phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–964. [PMC free article] [PubMed] [Google Scholar]
- 58.Aloysius MM, Mc Kechnie AJ, Robins RA, et al. Generation in vivo of peptide-specific cytotoxic T cells and presence of regulatory T cells during vaccination with hTERT (class I and II) peptide-pulsed DCs. J Transl Med. 2009;7:18. doi: 10.1186/1479-5876-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carbone DP, Ciernik IF, Kelley MJ, et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol. 2005;23(22):5099–5107. doi: 10.1200/JCO.2005.03.158. [DOI] [PubMed] [Google Scholar]
- 60.Ramanathan RK, Lee KM, McKolanis J, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54(3):254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto K, Ueno T, Kawaoka T, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25(5):3575–3579. [PubMed] [Google Scholar]
- 62.Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating Phase I/II study. Br J Cancer. 2006;95(11):1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buanes T, Maurel J, Liauw W, Hebbar M, Nemunaitis J. A randomized Phase III study of gemcitabine (G) versus GV1001 in sequential combination with G in patients with unresectable and metastatic pancreatic cancer (PC) J Clin Oncol. 2009;27(Suppl 15):Abstract 4601. [Google Scholar]
- 64.Miyazawa M, Ohsawa R, Tsunoda T, et al. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010;101(2):433–439. doi: 10.1111/j.1349-7006.2009.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toubaji A, Achtar M, Provenzano M, et al. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother. 2008;57(9):1413–1420. doi: 10.1007/s00262-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Begley J, Ribas A. Targeted therapies to improve tumor immunotherapy. Clin Cancer Res. 2008;14(14):4385–4391. doi: 10.1158/1078-0432.CCR-07-4804. [DOI] [PubMed] [Google Scholar]
- 67.Chapatte L, Ayyoub M, Morel S, et al. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 2006;15 66(10):5461–5468. doi: 10.1158/0008-5472.CAN-05-4310. [DOI] [PubMed] [Google Scholar]
- 68.Houghton AN, Guevara-Patino JA. Immune recognition of self in immunity against cancer. J Clin Invest. 2004;114(4):468–471. doi: 10.1172/JCI22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiner LM, Surana R, Murray J. Vaccine prevention of cancer: can endogenous antigens be targeted? Cancer Prev Res (Phila) 2010;3(4):410–415. doi: 10.1158/1940-6207.CAPR-10-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu Z, Theoret MR, Touloukian CE, et al. Poor immunogenicity of a self/tumor antigen derives from peptide–MHC-I instability and is independent of tolerance. J Clin Invest. 2004;114(4):551–559. doi: 10.1172/JCI21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsang KY, Palena C, Gulley J, Arlen P, Schlom J. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clin Cancer Res. 2004;10(6):2139–2149. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 72▪.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55(10):1294–1298. doi: 10.1007/s00262-005-0102-x. Clinical evidence that vaccination against a pancreas cancer-associated antigen can induce a protective immune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charalambous A, Oks M, Nchinda G, Yamazaki S, Steinman RM. Dendritic cell targeting of survivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin. J Immunol. 2006;177(12):8410–8421. doi: 10.4049/jimmunol.177.12.8410. [DOI] [PubMed] [Google Scholar]
- 74.Wang B, Kuroiwa JM, He LZ, Charalambous A, Keler T, Steinman RM. The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann NY Acad Sci. 2009;1174:6–17. doi: 10.1111/j.1749-6632.2009.04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shibagaki N, Udey MC. Dendritic cells transduced with protein antigens induce cytotoxic lymphocytes and elicit antitumor immunity. J Immunol. 2002;168(5):2393–2401. doi: 10.4049/jimmunol.168.5.2393. [DOI] [PubMed] [Google Scholar]
- 76.Bae MY, Cho NH, Seong SY. Protective anti-tumour immune responses by murine dendritic cells pulsed with recombinant Tat-carcinoembryonic antigen derived from Escherichia coli. Clin Exp Immunol. 2009;157(1):128–138. doi: 10.1111/j.1365-2249.2009.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka Y, Dowdy SF, Linehan DC, Eberlein TJ, Goedegebuure PS. Induction of antigen-specific CTL by recombinant HIV trans-activating fusion protein-pulsed human monocyte-derived dendritic cells. J Immunol. 2003;170(3):1291–1298. doi: 10.4049/jimmunol.170.3.1291. [DOI] [PubMed] [Google Scholar]
- 78.Viehl CT, Tanaka Y, Chen T, et al. Tat mammaglobin fusion protein transduced dendritic cells stimulate mammaglobinspecific CD4 and CD8 T cells. Breast Cancer Res Treat. 2005;91(3):271–278. doi: 10.1007/s10549-005-0450-4. [DOI] [PubMed] [Google Scholar]
- 79.Viehl CT, Becker-Hapak M, Lewis JS, et al. A Tat fusion protein-based tumor vaccine for breast cancer. Ann Surg Oncol. 2005;12(7):517–525. doi: 10.1245/ASO.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 80.Saha A, Chatterjee SK, Foon KA, Bhattacharya-Chatterjee M. Anti-idiotype antibody induced cellular immunity in mice transgenic for human carcinoembryonic antigen. Immunology. 2006;118(4):483–496. doi: 10.1111/j.1365-2567.2006.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saha A, Baral RN, Chatterjee SK, et al. CpG oligonucleotides enhance the tumor antigen-specific immune response of an anti-idiotype antibody-based vaccine strategy in CEA transgenic mice. Cancer Immunol Immunother. 2006;55(5):515–527. doi: 10.1007/s00262-005-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saha A, Chatterjee SK, Foon KA, Celis E, Bhattacharya-Chatterjee M. Therapy of established tumors in a novel murine model transgenic for human carcinoembryonic antigen and HLA-A2 with a combination of anti-idiotype vaccine and CTL peptides of carcinoembryonic antigen. Cancer Res. 2007;67(6):2881–2892. doi: 10.1158/0008-5472.CAN-06-3045. [DOI] [PubMed] [Google Scholar]
- 83.Yanagimoto H, Shiomi H, Satoi S, et al. A Phase II study of personalized peptide vaccination combined with gemcitabine for non-resectable pancreatic cancer patients. Oncol Rep. 2010;24(3):795–801. doi: 10.3892/or_00000923. [DOI] [PubMed] [Google Scholar]
- 84.Bodles-Brakhop AM, Draghia-Akli R. DNA vaccination and gene therapy: optimization and delivery for cancer therapy. Expert Rev Vaccines. 2008;7(7):1085–1101. doi: 10.1586/14760584.7.7.1085. [DOI] [PubMed] [Google Scholar]
- 85.Zhu K, Qin H, Cha SC, et al. Survivin DNA vaccine generated specific antitumor effects in pancreatic carcinoma and lymphoma mouse models. Vaccine. 2007;25(46):7955–7961. doi: 10.1016/j.vaccine.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 86.Rong Y, Jin D, Wu W, et al. Induction of protective and therapeutic anti-pancreatic cancer immunity using a reconstructed MUC1 DNA vaccine. BMC Cancer. 2009;9:191. doi: 10.1186/1471-2407-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang CL, Wu TC, Hung CF. Control of human mesothelin-expressing tumors by DNA vaccines. Gene Ther. 2007;14(16):1189–1198. doi: 10.1038/sj.gt.3302974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong Y, Qian J, Ibrahim R, Berzofsky JA, Khleif SN. Identification of H-2Db-specific CD8+ T-cell epitopes from mouse VEGFR2 that can inhibit angiogenesis and tumor growth. J Immunother. 2006;29(1):32–40. doi: 10.1097/01.cji.0000175494.13476.56. [DOI] [PubMed] [Google Scholar]
- 89.Johansson S, Ek M, Wahren B, Stout R, Liu M, Hallermalm K. Intracellular targeting of CEA results in Th1-type antibody responses following intradermal genetic vaccination by a needle-free jet injection device. ScientificWorldJournal. 2007;7:987–999. doi: 10.1100/tsw.2007.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brave A, Hallengard D, Gudmundsdotter L, et al. Late administration of plasmid DNA by intradermal electroporation efficiently boosts DNA-primed T and B cell responses to carcinoembryonic antigen. Vaccine. 2009;27(28):3692–3696. doi: 10.1016/j.vaccine.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 91.Hallermalm K, Johansson S, Brave A, et al. Pre-clinical evaluation of a CEA DNA prime/protein boost vaccination strategy against colorectal cancer. Scand J Immunol. 2007;66(1):43–51. doi: 10.1111/j.1365-3083.2007.01945.x. [DOI] [PubMed] [Google Scholar]
- 92.Yu YY, Netuschil N, Lybarger L, Connolly JM, Hansen TH. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168(7):3145–3149. doi: 10.4049/jimmunol.168.7.3145. [DOI] [PubMed] [Google Scholar]
- 93.Huang CH, Peng S, He L, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12(15):1180–1186. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li L, Herndon JM, Truscott SM, et al. Engineering superior DNA vaccines: MHC class I single chain trimers bypass antigen processing and enhance the immune response to low affinity antigens. Vaccine. 2010;23 28(8):1911–1918. doi: 10.1016/j.vaccine.2009.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Li S, Shan M, et al. Hepatitis B virus core antigen epitopes presented by HLA-A2 single-chain trimers induce functional epitope-specific CD8+ T-cell responses in HLA-A2.1/Kb transgenic mice. Immunology. 2007;121(1):105–112. doi: 10.1111/j.1365-2567.2007.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheung YK, Cheng SC, Sin FW, Chan KT, Xie Y. Induction of T-cell response by a DNA vaccine encoding a novel HLA-A*0201 severe acute respiratory syndrome coronavirus epitope. Vaccine. 2007;25(32):6070–6077. doi: 10.1016/j.vaccine.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheung YK, Cheng SC, Ke Y, Xie Y. Two novel HLA-A*0201 T-cell epitopes in avian H5N1 viral nucleoprotein induced specific immune responses in HHD mice. Vet Res. 2010;41(2):24. doi: 10.1051/vetres/2009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang B, Mao CP, Peng S, He L, Hung CF, Wu TC. Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine. 2007;25(45):7824–7831. doi: 10.1016/j.vaccine.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hung CF, Calizo R, Tsai YC, He L, Wu TC. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007;25(1):127–135. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 100.Kim S, Li L, McMurtrey CP, et al. Single-chain HLA-A2 MHC trimers that incorporate an immundominant peptide elicit protective T cell immunity against lethal West Nile virus infection. J Immunol. 2010;184(8):4423–4430. doi: 10.4049/jimmunol.0903955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pejawar-Gaddy S, Rajawat Y, Hilioti Z, et al. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immunother. 2010;59(11):1685–1696. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gabitzsch ES, Xu Y, Balint JP, Jr, Hartman ZC, Lyerly HK, Jones FR. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol Immunother. 2010;59(7):1131–1135. doi: 10.1007/s00262-010-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaufman HL, Kim-Schulze S, Manson K, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104▪.Morse MA, Hobeika AC, Osada T, et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J Clin Invest. 2010;120(9):3234–3241. doi: 10.1172/JCI42672. Describes the successful clinical intervention in patients with advanced cancer, including pancreatic cancer, by inducing a protective immune response despite an immunosuppressive environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ryman KD, Klimstra WB. Host responses to alphavirus infection. Immunol Rev. 2008;225:27–45. doi: 10.1111/j.1600-065X.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 106.Yang L, Yang H, Rideout K, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26(3):326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bernstein MB, Chakraborty M, Wansley EK, et al. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008;26(4):509–521. doi: 10.1016/j.vaccine.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 108.Remondo C, Cereda V, Mostbock S, et al. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27(7):987–994. doi: 10.1016/j.vaccine.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boehm AL, Higgins J, Franzusoff A, Schlom J, Hodge JW. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother. 2010;59(3):397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wansley EK, Chakraborty M, Hance KW, et al. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14(13):4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu Y, Bellgrau D, Dwyer-Nield LD, et al. Mutation-selective tumor remission with Ras-targeted, whole yeast-based immunotherapy. Cancer Res. 2004;64(15):5084–5088. doi: 10.1158/0008-5472.CAN-04-1487. [DOI] [PubMed] [Google Scholar]
- 112.Paterson Y, Guirnalda PD, Wood LM. Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin Immunol. 2010;22(3):183–189. doi: 10.1016/j.smim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Le DT, Nir-Paz R, Hampl J, et al. Results of Phase I studies testing two live-attenuated listeria vaccines, ANZ-100 and CRS-207, for the treatment of cancer. Tumor Immunology: Basic and Clinical Advances AACR Program and Proceedings. 2010:Abstract B12. [Google Scholar]
- 114.Ishizaki H, Song GY, Srivastava T, et al. Heterologous prime/boost immunization with p53-based vaccines combined with Toll-like receptor stimulation enhances tumor regression. J Immunother. 2010;33(6):609–617. doi: 10.1097/CJI.0b013e3181e032c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seavey MM, Maciag PC, Al-Rawi N, Sewell D, Paterson Y. An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes antiangiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J Immunol. 2009;182(9):5537–5546. doi: 10.4049/jimmunol.0803742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seavey MM, Pan ZK, Maciag PC, et al. A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res. 2009;1 15(3):924–932. doi: 10.1158/1078-0432.CCR-08-2283. [DOI] [PubMed] [Google Scholar]
- 117.Shahabi V, Seavey MM, Maciag PC, Rivera S, Wallecha A. Development of a live and highly attenuated Listeria monocytogenes-based vaccine for the treatment of Her2/neu-overexpressing cancers in human. Cancer Gene Ther. 2010;18:53–62. doi: 10.1038/cgt.2010.48. [DOI] [PubMed] [Google Scholar]
- 118.Niethammer AG, Primus FJ, Xiang R, et al. An oral DNA vaccine against human carcinoembryonic antigen (CEA) prevents growth and dissemination of Lewis lung carcinoma in CEA transgenic mice. Vaccine. 2001;20(3–4):421–429. doi: 10.1016/s0264-410x(01)00362-0. [DOI] [PubMed] [Google Scholar]
- 119.Xiang R, Primus FJ, Ruehlmann JM, et al. A dual-function DNA vaccine encoding carcinoembryonic antigen and CD40 ligand trimer induces T cell-mediated protective immunity against colon cancer in carcinoembryonic antigen-transgenic mice. J Immunol. 2001;167(8):4560–4565. doi: 10.4049/jimmunol.167.8.4560. [DOI] [PubMed] [Google Scholar]
- 120.Xiong G, Husseiny MI, Song L, et al. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int J Cancer. 2010;126(11):2622–2634. doi: 10.1002/ijc.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hellstrom I, Friedman E, Verch T, et al. Anti-mesothelin antibodies and circulating mesothelin relate to the clinical state in ovarian cancer patients. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1520–1526. doi: 10.1158/1055-9965.EPI-08-0039. [DOI] [PubMed] [Google Scholar]
- 122.Ho M, Hassan R, Zhang J, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11(10):3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 123.Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6(5):349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 124.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2- positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94(2):259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci USA. 1998;95(2):652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126▪.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. Comprehensive overview on the role of antibodies in immune therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelinexpressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13(17):5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 128.Filpula D, Zhao H. Releasable PEGylation of proteins with customized linkers. Adv Drug Deliv Rev. 2008;60(1):29–49. doi: 10.1016/j.addr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 129.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15(16):5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hassan R, Ebel W, Routhier EL, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 131.Feng Y, Xiao X, Zhu Z, et al. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol Cancer Ther. 2009;8(5):1113–1118. doi: 10.1158/1535-7163.MCT-08-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ho M, Feng M, Fisher RJ, Rader C, Pastan I. A novel high affinity human monoclonal antibody to mesothelin. Int J Cancer. 2011;128(9):2020–2030. doi: 10.1002/ijc.25557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Richman PI, Bodmer WF. Monoclonal antibodies to human colorectal epithelium: markers for differentiation and tumour characterization. Int J Cancer. 1987;39(3):317–328. doi: 10.1002/ijc.2910390309. [DOI] [PubMed] [Google Scholar]
- 134.Stewart LM, Young S, Watson G, et al. Humanisation and characterisation of PR1A3, a monoclonal antibody specific for cell-bound carcinoembryonic antigen. Cancer Immunol Immunother. 1999;47(6):299–306. doi: 10.1007/s002620050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ashraf SQ, Umana P, Mossner E, et al. Humanised IgG1 antibody variants targeting membrane-bound carcinoembryonic antigen by antibody-dependent cellular cytotoxicity and phagocytosis. Br J Cancer. 2009;101(10):1758–1768. doi: 10.1038/sj.bjc.6605355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Conaghan P, Ashraf S, Tytherleigh M, et al. Targeted killing of colorectal cancer cell lines by a humanised IgG1 monoclonal antibody that binds to membrane-bound carcinoembryonic antigen. Br J Cancer. 2008;98(7):1217–1225. doi: 10.1038/sj.bjc.6604289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blumenthal RD, Osorio L, Hayes MK, Horak ID, Hansen HJ, Goldenberg DM. Carcinoembryonic antigen antibody inhibits lung metastasis and augments chemotherapy in a human colonic carcinoma xenograft. Cancer Immunol Immunother. 2005;54(4):315–327. doi: 10.1007/s00262-004-0597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Danielczyk A, Stahn R, Faulstich D, et al. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol Immunother. 2006;55(11):1337–1347. doi: 10.1007/s00262-006-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fan XN, Karsten U, Goletz S, Cao Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TA-MUC1) with various human carcinomas. Pathol Res Pract. 2010;206(8):585–589. doi: 10.1016/j.prp.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 140.Pratesi G, Petrangolini G, Tortoreto M, et al. Antitumor efficacy of trastuzumab in nude mice orthotopically xenografted with human pancreatic tumor cells expressing low levels of HER-2/neu. J Immunother. 2008;31(6):537–544. doi: 10.1097/CJI.0b013e31817c37ff. [DOI] [PubMed] [Google Scholar]
- 141.Saeki H, Yanoma S, Takemiya S, et al. Antitumor activity of a combination of trastuzumab (Herceptin) and oral fluoropyrimidine S-1 on human epidermal growth factor receptor 2-overexpressing pancreatic cancer. Oncol Rep. 2007;18(2):433–439. [PubMed] [Google Scholar]
- 142.Larbouret C, Robert B, Navarro-Teulon I, et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13(11):3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 143.Larbouret C, Robert B, Bascoul-Mollevi C, et al. Combined cetuximab and trastuzumab are superior to gemcitabine in the treatment of human pancreatic carcinoma xenografts. Ann Oncol. 2010;21(1):98–103. doi: 10.1093/annonc/mdp496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene Ther. 2007;14(12):921–929. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawaoka T, Oka M, Takashima M, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep. 2008;20(1):155–163. [PubMed] [Google Scholar]
- 146.Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. AntiCancer Res. 2008;28(1B):379–387. [PubMed] [Google Scholar]
- 147.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davies DM, Maher J. Adoptive T-cell immunotherapy of cancer using chimeric antigen receptor-grafted T cells. Arch Immunol Ther Exp (Warsz) 2010;58(3):165–178. doi: 10.1007/s00005-010-0074-1. [DOI] [PubMed] [Google Scholar]
- 149.Emtage PC, Lo AS, Gomes EM, Liu DL, Gonzalo-Daganzo RM, Junghans RP. Second-generation anti-carcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor activity in vivo: a preclinical evaluation. Clin Cancer Res. 2008;14(24):8112–8122. doi: 10.1158/1078-0432.CCR-07-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shirasu N, Shibaguci H, Kuroki M, Yamada H, Kuroki M. Construction and molecular characterization of human chimeric T-cell antigen receptors specific for carcinoembryonic antigen. AntiCancer Res. 2010;30(7):2731–2738. [PubMed] [Google Scholar]
- 151.Wilkie S, Burbridge SE, Chiapero-Stanke L, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010;285(33):25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bakhtiari SH, Rahbarizadeh F, Hasannia S, Ahmadvand D, Iri-Sofla FJ, Rasaee MJ. Anti-MUC1 nanobody can redirect T-body cytotoxic effector function. Hybridoma (Larchmt) 2009;28(2):85–92. doi: 10.1089/hyb.2008.0079. [DOI] [PubMed] [Google Scholar]
- 153.Wang H, Wei H, Zhang R, et al. Genetically targeted T cells eradicate established breast cancer in syngeneic mice. Clin Cancer Res. 2009;15(3):943–950. doi: 10.1158/1078-0432.CCR-08-2381. [DOI] [PubMed] [Google Scholar]
- 154.Zhao Y, Wang QJ, Yang S, et al. A herceptinbased chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183(9):5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koos D, Josephs SF, Alexandrescu DT, et al. Tumor vaccines in 2010: need for integration. Cell Immunol. 2010;263(2):138–147. doi: 10.1016/j.cellimm.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 156.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 157.Tian M, Neil JR, Schiemann WP. Transforming growth factor-β and the hallmarks of cancer. Cell Signal. 2011;23(6):951–962. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.von Bernstorff W, Spanjaard RA, Chan AK, et al. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery. 1999;125(1):73–84. doi: 10.1067/msy.2099.93570. [DOI] [PubMed] [Google Scholar]
- 159.Derre L, Rivals JP, Jandus C, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120(1):157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg. 2008;206(5):849–854. doi: 10.1016/j.jamcollsurg.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 161▪.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. Comprehensive overview of the suppressor cells population, myeloid-derived supressor cells, and how they can be targeted clinically. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 163.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloidderived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J Leukoc Biol. 2011;89(1):31–39. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 165.Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;1 182(3):1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 168.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer – preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 169.Agarwala SS, Ribas A. Current experience with CTLA4-blocking monoclonal antibodies for the treatment of solid tumors. J Immunother. 2010;33(6):557–569. doi: 10.1097/CJI.0b013e3181dcd260. [DOI] [PubMed] [Google Scholar]
- 170.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic Ppancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 174.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Aminobiphosphonate- mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67(23):11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 176.Zhang B, Zhang Y, Bowerman NA, et al. Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res. 2008;68(5):1563–1571. doi: 10.1158/0008-5472.CAN-07-5324. [DOI] [PubMed] [Google Scholar]
- 177.Nishikawa H, Kato T, Tanida K, et al. CD4+ CD25+ T cells responding to serologically defined autoantigens suppress antitumor immune responses. Proc Natl Acad Sci USA. 2003;100(19):10902–10906. doi: 10.1073/pnas.1834479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20(1):107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 179▪▪.Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464(7291):999–1005. doi: 10.1038/nature08989. Describes the successful sequencing of a breast cancer genome, opening up the near-future development of personalized treatments for solid tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ley TJ, Mardis ER, Ding L, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456(7218):66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68(3):889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 184.Sensi M, Anichini A. Unique tumor antigens: evidence for immune control of genome integrity and immunogenic targets for T cell-mediated patient-specific immunotherapy. Clin Cancer Res. 2006;12(17):5023–5032. doi: 10.1158/1078-0432.CCR-05-2682. [DOI] [PubMed] [Google Scholar]
- 185.Huang EH, Kaufman HL. CEA-based vaccines. Expert Rev Vaccines. 2002;1(1):49–63. doi: 10.1586/14760584.1.1.49. [DOI] [PubMed] [Google Scholar]
- 186.Beatty JD, Romero C, Brown PW, Lawrence W, Jr, Terz JJ. Clinical value of carcinoembryonic antigen: diagnosis, prognosis, and follow-up of patients with cancer. Arch Surg. 1979;114(5):563–567. doi: 10.1001/archsurg.1979.01370290013002. [DOI] [PubMed] [Google Scholar]
- 187.Ona FV, Zamcheck N, Dhar P, Moore T, Kupchik HZ. Carcinoembryonic antigen (CEA) in the diagnosis of pancreatic cancer. Cancer. 1973;31(2):324–327. doi: 10.1002/1097-0142(197302)31:2<324::aid-cncr2820310208>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 188.Ladjemi MZ, Jacot W, Chardes T, Pelegrin A, Navarro-Teulon I. Anti-HER2 vaccines: new prospects for breast cancer therapy. Cancer Immunol Immunother. 2010;59(9):1295–1312. doi: 10.1007/s00262-010-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lei S, Appert HE, Nakata B, Domenico DR, Kim K, Howard JM. Overexpression of HER2/neu oncogene in pancreatic cancer correlates with shortened survival. Int J Pancreatol. 1995;17(1):15–21. doi: 10.1007/BF02788354. [DOI] [PubMed] [Google Scholar]
- 190.Yamanaka Y, Friess H, Kobrin MS, et al. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol. 1993;24(10):1127–1134. doi: 10.1016/0046-8177(93)90194-l. [DOI] [PubMed] [Google Scholar]
- 191.Downward J. Targeting RAS and PI3K in lung cancer. Nat Med. 2008;14(12):1315–1316. doi: 10.1038/nm1208-1315. [DOI] [PubMed] [Google Scholar]
- 192.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 193.Li M, Bharadwaj U, Zhang R, et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7(2):286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124(6):838–845. [PubMed] [Google Scholar]
- 195.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7(12):3862–3868. [PubMed] [Google Scholar]
- 196.Kaneko O, Gong L, Zhang J, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;6 284(6):3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tang CK, Katsara M, Apostolopoulos V. Strategies used for MUC1 immunotherapy: human clinical studies. Expert Rev Vaccines. 2008;7(7):963–975. doi: 10.1586/14760584.7.7.963. [DOI] [PubMed] [Google Scholar]
- 198.Qu CF, Li Y, Song YJ, et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. Br J Cancer. 2004;91(12):2086–2093. doi: 10.1038/sj.bjc.6602232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen F, Wang W, El-Deiry WS. Current strategies to target p53 in cancer. Biochem Pharmacol. 2010;80(5):724–730. doi: 10.1016/j.bcp.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 200.Scarpa A, Capelli P, Mukai K, et al. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993;142(5):1534–1543. [PMC free article] [PubMed] [Google Scholar]
- 201.Ryan BM, O’Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35(7):553–562. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 202.Kanwar RK, Cheung CH, Chang JY, Kanwar JR. Recent advances in anti-survivin treatments for cancer. Curr Med Chem. 2010;17(15):1509–1515. doi: 10.2174/092986710790979935. [DOI] [PubMed] [Google Scholar]
- 203.Qiao JG, Zhang YQ, Yin YC, Tan Z. Expression of Survivin in pancreatic cancer and its correlation to expression of Bcl-2. World J Gastroenterol. 2004;10(18):2759–2761. doi: 10.3748/wjg.v10.i18.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92(2):271–278. doi: 10.1002/1097-0142(20010715)92:2<271::aid-cncr1319>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 205.Liu JP, Chen W, Schwarer AP, Li H. Telomerase in cancer immunotherapy. Biochim Biophys Acta. 2010;1805(1):35–42. doi: 10.1016/j.bbcan.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 206.Hiyama E, Kodama T, Shinbara K, et al. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res. 1997;57(2):326–331. [PubMed] [Google Scholar]
- 207.Shibuya M. Vascular endothelial growth factor (VEGF)-receptor2: its biological functions, major signaling pathway, and specific ligand VEGF-E. Endothelium. 2006;13(2):63–69. doi: 10.1080/10623320600697955. [DOI] [PubMed] [Google Scholar]
- 208.Itakura J, Ishiwata T, Friess H, et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3(8):1309–1316. [PubMed] [Google Scholar]
Websites
- 301.The International Clinical Trials Registry offers a registration of all interventional clinical trials conforming to WHO standards International Clinical Trials registry ID number. JPRN-UMIN000001664 http://apps.who.int/trialsearch/trial.aspx?trialid=JPRN-UMIN000001664
- 302.Amplimmune Inc. is a company that is involved in developing immune-based biologics to treat patients in the fields of cancer, autoimmunity, transplantation and infectious disease www.amplimmune.com