Abstract
Aberrant activation of oncogenic signal transducer and activator of transcription 3 (STAT3) protein signaling pathways has been extensively implicated in human cancers. Given STAT3’s prominent dysregulatory role in malignant transformation and tumorigenesis, there has been a significant effort to discover STAT3-specific inhibitors as chemical probes for defining the aberrant STAT3-mediated molecular events that support the malignant phenotype. To identify novel, STAT3-selective inhibitors suitable for interrogating STAT3 signaling in tumor cells, we explored the design of hybrid molecules by conjugating a known STAT3 inhibitory peptidomimetic, ISS610 to the high-affinity STAT3-binding peptide motif derived from the ILR/gp-130. Several hybrid molecules were examined in in vitro biophysical and biochemical studies for inhibitory potency against STAT3. Lead inhibitor 14aa was shown to strongly bind to STAT3 (KD = 900 nM), disrupt STAT3:phosphopeptide complexes (Ki = 5 μM) and suppress STAT3 activity in in vitro DNA-binding activity/ electrophoretic mobility shift assay (EMSA). Moreover, lead STAT3 inhibitor 14aa induced a time-dependent inhibition of constitutive STAT3 activation in v-Src transformed mouse fibroblasts (NIH3T3/v-Src), with 80 % suppression of constitutively-active STAT3 at six hours following treatment of NIH3T3/v-Src. However, STAT3 activity recovered at 24 hours after treatment of cells, suggesting potential degradation of the compound. Results further showed a suppression of aberrant STAT3 activity in NIH3T3/v-Src by the treatment with compound 14aa-OH, which is the non-pTyr version of compound 14aa. The effect of compounds 14aa and 14aa-OH are accompanied by a moderate loss of cell viability.
1. Introduction
STAT3 is a cytosolic transcription factor that becomes activated upon stimulation of cytokine or growth factor receptors.1 Receptor activation leads to intracellular phosphorylation of STAT3 via receptor associated Janus kinases (JAKs) and, as a result, forms a STAT3–STAT3 protein complex.2 STAT3 homodimers associate through reciprocal phosphotyrosine–SH2 domain interactions. In the nucleus, the transcriptionally active protein complex binds to specific DNA response elements and elicits a transcriptional response. Typically, STAT3 signaling is transient and responsive to physiological cues. However, dysregulated STAT3 activity results in the uncontrolled expression of genes involved in cell growth, survival and angiogenesis. Moreover, STAT3-mediated up-regulation of anti-apoptotic and cell survival genes provides an underlying mechanism for apoptotic resistance in many cancer cells.3-7 Since most currently available chemotherapy options aim to initiate apoptosis, cancer cells have an intrinsic resistance to current treatment strategies. Therefore, therapeutics disrupting STAT3-mediated anti-apoptotic gene expression patterns hold significant promise as stand-alone or adjuvant therapeutics.
We herein report a novel family of hybrid peptidomimetic Stat3 inhibitors. The present hybrid inhibitors bind to STAT3’s SH2 domain with a high affinity, disrupt STAT3:phosphopeptide complexation and consequently, inhibit STAT3–STAT3 protein dimerization. Lead inhibitor 14aa exhibited biological activity and inhibited the viability of human breast and prostate cancer.
2. Results and Discussion
2.1 Inhibitor design
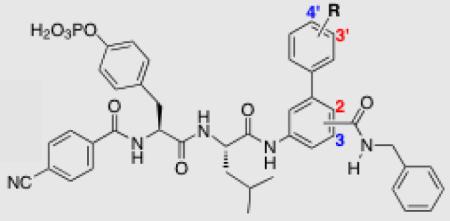
Peptidomimetic inhibitors of STAT3 have played important roles in understanding the key binding interactions required for STAT3 recognition,8-12 and in the development of non-peptidic inhibitors.13-16 Peptidomimetic-inspired antagonists of STAT3 have been derived from both PpYLKTK, the cognate binding sequence of STAT3, and GpYLPQTV-NH2, a truncated peptide from the gp130 receptor that is known to bind the STAT3-SH2 domain.8,17 Given that the GpYLPQTV-NH2 peptide is known to bind Stat3 with high-affinity (KD, 150 nM), we hypothesized that a hybrid peptidomimetic inhibitor, incorporating key structural facets from this peptide and other most potent peptidomimetic designs could furnish an improved STAT3 inhibitor. As part of this combinatorial process, we elected to fuse 1 (Fig. 1A),18 a peptidomimetic inhibitor inspired by the native binding sequence of STAT3, to the C-terminal portion of 2, a gp130-derived peptidomimetic inhibitor, which was derived and previously evaluated by McMurray and co-workers.17 Significantly, in the discovery process of 2, these authors demonstrated that modulating the pY+3 Gln side chain was not tolerated, and that, in particular, the carboxamide hydrogens were critical for inhibitory activity.11, 19 Hence, we were keen to maintain a Gln residue or a Gln mimetic in this position of our peptidomimetics. To this end, and in an effort to achieve more cell-permeable peptidomimetic inhibitors, we elected to substitute the Pro-Gln dipeptide unit with a novel functionalized biphenyl-based amino acid that replicates the positioning of the key glutamine side chain to afford scaffold 3 (Fig. 1). We reasoned that the biphenyl moiety favorably reduced the number of structural conformations, rigidifying the peptidomimetic core, and thus reducing the entropic binding penalty. Moreover, inclusion of the biphenyl amino acid significantly reduces the peptidic character of the inhibitor by replacing two amino acid residues (Pro-Gln) and three peptide bonds with one unnatural amino acid, less susceptible to intracellular peptidases.
Figure 1.
Development of STAT3 hybrid peptidomimetic 3 derived from peptidomimetic inhibitors 1 and 2
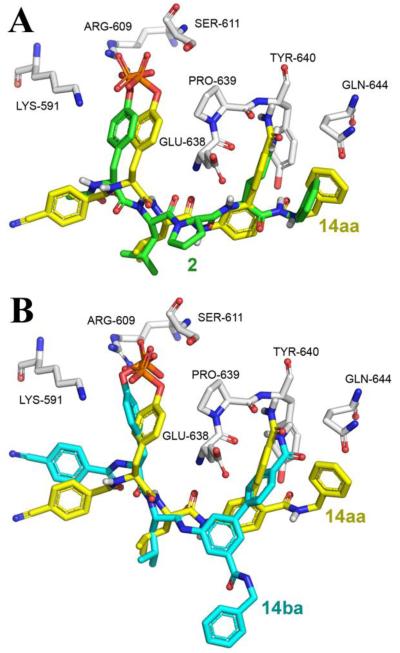
GOLD20 computational docking of inhibitor 14aa (where R = 4′-carboxyamide, Table 1) in the STAT3 SH2 domain,21 showed accurate replication of the segment common to both 3 and the gp130-derived peptidomimetic 2 (Fig. 2A). As part of our structural investigation of the biphenyl motif, we prepared two discrete biphenyl amino acid isomers where the C-terminal benzylcarbamoyl group was attached to the 2 or the 3 position of the “lower” phenyl ring, and two sub-sets of isomers where the functional group on the “upper” phenyl ring was located at the 3′ and the 4′ positions (Fig. 1). As illustrated in Fig. 2B, a high-scoring GOLD docked pose of the 3-substituted benzylcarbamoyl isomer 14ba is overlaid with the docked pose of 14aa taken from Fig. 2A. As anticipated, due to the rigidity of the “lower” phenyl ring, the benzylcarbamoyl moiety cannot access the same hydrophobic region believed to be occupied by the same moiety in 2 and 14aa, although most of the other interactions appear to be maintained. Therefore, in terms of mimicry of the gp130-inspired peptidomimetic 2, we might expect 14ba to bind in a different manner to 14aa.
Table 1.
IC50 inhibitory potencies of novel hybrid peptidomimetic family
| Inhibitor | R Group | Benzyl amide | FP Ki(μM) | EMSA IC50 (μM) |
|---|---|---|---|---|
| 14aa | 4′-amide | 2-benzylcarbamoyl | 5 ± 1 | 73.1 ± 6 |
| 14ab | 4′-cyano | 2-benzylcarbamoyl | 11 ± 4 | 33 ± 2 |
| 14ac | 4′-ester | 2-benzylcarbamoyl | 26 ± 5 | > 200 |
| 14ad | 3′-amide | 2-benzylcarbamoyl | 15 ± 2 | 62 ± 2 |
| 14ae | 3′-cyano | 2-benzylcarbamoyl | 13 ± 1 | 64 ± 6 |
| 14af | 3′-ester | 2-benzylcarbamoyl | 10 ± 2 | 40 ± 2 |
| 14ba | 4′-amide | 3-benzylcarbamoyl | 9 ± 2 | 5 ± 1 |
| 14bb | 4′-cyano | 3-benzylcarbamoyl | 36 ± 8 | 60 ± 2 |
| 14bc | 4′-ester | 3-benzylcarbamoyl | 25 ± 6 | 112 ± 12 |
| 14bd | 3′-amide | 3-benzylcarbamoyl | 38 ± 16 | 188 ± 48 |
| 14be | 3′-cyano | 3-benzylcarbamoyl | 18 ± 3 | 92 ± 10 |
| 14bf | 3′-ester | 3-benzylcarbamoyl | 23 ± 2 | 66 ± 1 |
Figure 2.
Comparative lowest energy GOLD docking results for (A) 14aa and peptidomimetic 2 (B) inhibitors 14aa and 14ba and bound in the SH2 domain of the STAT3βdimer (PDB: 1BG1)
2.2 Synthetic protocols
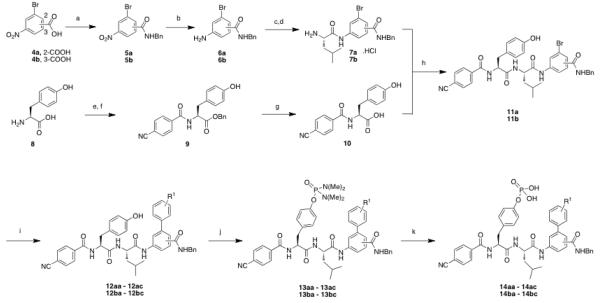
The synthesis of the peptidomimetic inhibitor 14aa is depicted in Scheme 1, and serves as a representative synthesis for the family of peptidomimetics described in this work. Oxidation of the methyl group of 2-bromo-1-methyl-4-nitrobenzene (3) was achieved with KMnO4 in refluxing pyridine/water to afford the corresponding carboxylic acid species (4), which was subsequently condensed with benzylamine to furnish amide 5. Chemoselective reduction of the nitro group was effected with SnCl2 to afford aniline 6 in almost quantitative yield. The poorly nucleophilic amino group in 6 demanded pre-activation of the carboxylic acid of N-Boc-Leu-OH as its mixed anhydride with isobutyl chloroformate, since attempted condensations employing HBTU and EDCI proved unsuccessful. N-Boc-protected analogue of 7 was thus afforded in an excellent yield of 89%, and was quantitatively Boc-deprotected to furnish 7 as its HCl salt. Meanwhile, in this convergent synthetic strategy, the 4-cyanobenzoyl-tyrosyl dipeptide unit of the target molecule was prepared in parallel. Specifically, the carboxylic acid of tyrosine (8) was esterified with benzyl alcohol in excellent yield under acid-catalyzed conditions. Subsequent condensation with p-cyanobenzoic acid, once more enlisting the mixed anhydride method, furnished the dipeptide 9 in 84 % yield. Subsequently, debenzylation of 9 with hydrogen gas over catalytic palladium on carbon (10% Pd/C) delivered carboxylic acid 10. Condensation of amine 7 with acid 10 was accomplished using coupling agent HBTU to afford 11 in 63 % yield. Next, microwave-assisted Suzuki coupling of aryl bromide 11 to (4-carbamoylphenyl)boronic acid using Pd(PPh3)4 and K2CO3 gave the biphenyl species 12 in 57 % yield. The tyrosyl phenol OH group was then phosphorylated using bis(dimethylamino)-phosphoramidic chloride to give 13 in 42 % yield, and then hydrolyzed with aqueous trifluoroacetic acid to furnish phosphoric acid 14aa in quantitative yield.
Scheme 1.
(a) benzylamine, HBTU, DIPEA, DMF, 25 °C, 4 hr, 74 % (b) SnCl2, EtOAc, 70 °C, 2 hr, 95 %; (c) (i) N-Boc-Leu-OH, isobutyl chloroformate, CH2Cl2, N-methyl morpholine, 25 °C, 10 min; (ii) (6a, 6b) N-methyl morpholine, CH2Cl2:THF (1:1), 25 °C, 1.5 hr, 96 %; (d) 4M HCl, dioxane:MeOH (1:1), 25 °C, 1 hr, 99 %; (e) benzyl alcohol, p-TsOH.H2O, 110 °C, 24 hr; 95 %; (f) (i) p-cyanobenzoic acid, isobutyl chloroformate, N-methyl morpholine, 25 °C, 15 min; (ii) 8, N-methyl morpholine, CH2Cl2:THF (1:1), 25 °C, 30 min, 84 %; (g) H2, Pd/C, THF:MeOH (1:1) 25 °C, 1 hr, 95 %; (h) HBTU, DIPEA, DMF, 25 °C, 4 hr, 76 %; (i) ArB(OH)2, Pd(PPh3)4, K2CO3, DMF, 170 °C, 15 mins, 50 %; (j) bis(dimethylamino)phosphoramidic chloride, DMAP, DBU, THF: CH2Cl2 (1:1) 25 °C, 16 hr, 62 %; (k) TFA:H2O (9:1), 25 °C, 16 hr, 99 %.
2.3 Evaluation of STAT3-phosphopeptide inhibition by fluorescence polarization (FP) assay
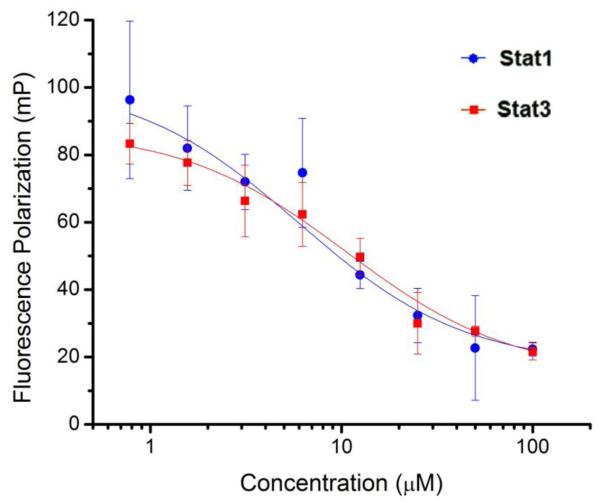
We first investigated inhibitor binding potency for the STAT3-SH2 domain using a fluorescence polarization (FP) assay developed by Berg and co-workers (Table 1, column 4).22 In this assay, inhibitor-mediated displacement of an N-terminus 5-carboxyfluorescein-labeled (F*) gp130 phosphopeptide from the STAT3-SH2 domain results in reduced polarization of the emitted fluorescence and allows binding constants to be calculated. Library screening identified 14aa and 14ba (14aa: R1 = 4′-CONH ; R2 2 = 2-benzylcarbamoyl, and; 14ba: R1 = 4′-CONH ; R2 2 = 3-benzylcarbamoyl) as the most potent inhibitors (14aa: Ki = 5 μM; 14ba: Ki = 9 μM, full FP data shown in supporting information). Given the importance of the Gln side chain in McMurray’s peptidomimetic (2), and considering our docking studies (Fig. 2), it is possible that the biphenyl carrying the para-carboxamide in peptidomimetics 14aa and 14ba is operating as a functional mimetic of the Pro-Gln dipeptide unit in 2, as we had hoped. Interestingly, 14ba, the 3-benzylcarbamoyl isomer of 14aa, was only half as potent as 14aa. Moreover, as can be seen from the data in Table 1, the 3-benzylcarbamoyl isomer of each series shows slightly lower activity than the corresponding 2-isomer in almost every case. McMurray has previously presented encouraging evidence to suggest that the Leu-Pro peptide bond in the gp130 sequence is trans when bound to STAT3. We speculated that the 2-isomer, which would be anticipated to exhibit a larger aryl-aryl twist angle owing to the additional steric hindrance, better mimics the trans peptide configuration than does the 3-isomer and consequently elicits moderately higher potency through improved interactions between the carboxamide group of the peptidomimetic and the STAT3-SH2 domain.11 Moreover, our docking studies demonstrate that the benzylcarbomyl unit in 14aa, in contrast to that unit in 14ba, closely mimics that in 2, suggesting that 14aa makes different binding contacts with the protein than does 14ba (Fig. 2A). Docking studies revealed that 14ba accesses an adjacent hydrophobic sub-domain and makes binding contacts with residues Pro715 and Phe716 (Fig. 2B). To assess for STAT isoform selectivity, 14aa and 14ba were subjected to a series of analogous, previously published, FP-based competitive binding experiments for both the STAT1 and STAT5 isoforms (Fig. 3, 14aa data shown).23,24 We found that both 14aa and 14ba were approximately equipotent against the structurally homologous STAT1 isoform (78 % homology), inhibiting STAT1–phosphopeptide complexes with Ki values of 6.3 μM and 16.5 μM, respectively (14ba data shown in supporting). Against the structurally dissimilar STAT5 isoform (53 % homology), both 14aa and 14ba were found to have no effect (data not shown).
Figure 3.
STAT3 vs. STAT1 binding potency as assessed by FP assays. For STAT3, FP measured upon titrating the F*pYLPQTV (7.2 nM) with compound 14aa in the presence of STAT3 protein (292 nM). For STAT1, FP measured upon titrating the F*GpYDKPHVL-NH2 (10 nM) with compound 14aa in the presence of STAT1 protein (80 nM). STAT5 data not shown.
2.4 Inhibition of STAT3 DNA-binding activity (EMSA analysis) in nuclear extracts
Next, we examined inhibitor-mediated disruption of STAT3–STAT3:DNA complexation in nuclear extracts obtained from NIH3T3/vSrc transformed cells harboring constitutively activated STAT3 signaling.4,25 The nuclear extracts were treated with increasing concentrations of inhibitors and incubated with the radiolabeled STAT3-specific hSIE oligonucleotide probe, followed by electrophoretic mobility shift assay (EMSA) analysis and densitometry quantification (Table 1, column 5). It was found that STAT3–STAT3:DNA binding was thus suppressed in a dose-dependent manner. Most notably, 14ba showed excellent disruption of STAT3 dimerization (IC50 = 5 ± 1 μM). In contrast, 14aa showed > 50 μM activity in the EMSA cf. 5 μM in the FP assay. In general, we observed lower inhibitory activity in the EMSA assay, most likely due to the presence of significantly more protein targets, including the entire STAT family and in particular, STAT1. Given the relative activities observed for STAT3 cf. STAT1 in the FP assay, this was not an unsurprising result.
It has long being known that the inhibitory activities of SH2 domain binding pTyr-peptide probes have a key requirement for the phosphate ester. To verify that the pTyr moiety was essential for the activity of the hybrid peptides against STAT3 DNA-binding activity, we investigated whether the dephosphorylated phenolic species retained activity in the DNA-binding assay using the dephoshorylated analogs of 14aa and 14ba, 14aa-OH and 14ba-OH, respectively. Interestingly, both 14aa-OH and 14ba-OH inhibited STAT3-STAT3-DNA binding activity, with IC50 values of 103 ± 11 μM and 190 ± 8 μM, respectively (supporting information). This result was not too unsurprising, given that Dourlat et al. reported a similar phenomenon with the STAT3-binding sequence, AYRNRpYRRQYRY, wherein the corresponding non-phosphorylated sequence was found to be equipotent and effectively retained Stat3 inhibitory activity.26 Poor cell permeability and dephosphorylation by intracellular and/or cell surface phosphatases/esterasase of phosphate ester containing inhibitors have led to the development of a number of successful protecting group/prodrug strategies, including difluorophosphonates and POM protecting group strategies.27 However, phosphate group modulation was beyond the scope of this study. Based on the present findings, it is conceivable that the non-phospho-counterparts could similarly inhibit STAT3 and STAT3-mediated events in tumor cells. However, studies are needed to further characterize how the non-phospho-counterparts promote Stat3 inhibition and how well it would induce STAT3-depedent anti-tumor cell effects compared to the phospho-counterparts. To further investigate 14ba and 14aa’s binding to STAT3, and to complement the EMSA and FP analysis, we conducted surface plasmon resonance (SPR) binding experiments with full-length STAT3 protein to determine directly the binding affinity of peptidomimetics for STAT3’s SH2 domain.
2.5 SPR analysis of inhibitor-STAT3 binding
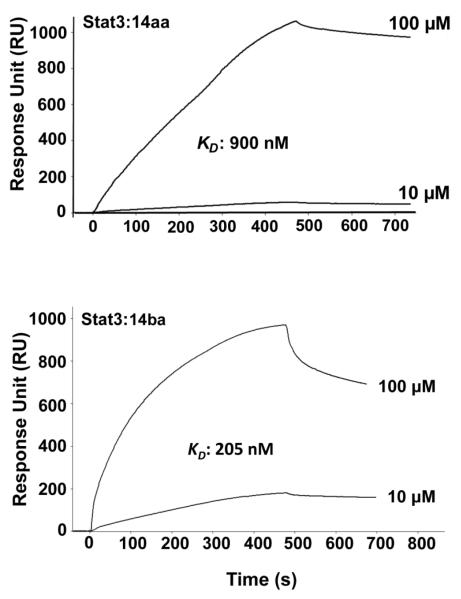
SPR binding experiments with lead inhibitors 14ba and 14aa were conducted against His-tagged STAT3 protein immobilized on a Ni-NTA sensor chip on a Biacore instrument as previously reported.28 SensiQ and its analysis software Qdat (ICX Technologies) were utilized to analyze the interaction between peptidomimetic and STAT3 protein. Various concentrations of 14ba and 14aa, in running buffer (1x PBS, 0.5% DMSO) were passed over the sensor chip to produce response signals. Association and dissociation measurements were recorded and the binding affinities determined. The results showed that 14ba bound to STAT3 with a lower dissociation constant (KD) than the high affinity STAT3 binding sequence GpYLPQTV-NH2, (14ba, KD = 205 nM (Fig. 4) cf. GpYLPQTV-NH2, KD = 24 nM).28 In addition, 14aa was shown to bind to STAT3 protein with good affinity (KD = 900 nM (Fig. 4)). Thus taken in conjunction with the FP and EMSA data, we concluded that inhibitor 14ba and 14aa are potent in vitro STAT3 binders and inhibitors of STAT3 complexation events. Moreover, SPR analysis of the non-phosphorylated analogs, 14aa-OH and 14ba-OH were performed to determine the binding constants and corroborate the EMSA analysis. Encouragingly, both phenolic inhibitors 14aa-OH and 14ba-OH showed binding affinity for STAT3, with KD values of 12 μM and 17 μM, respectively (supporting information). These data showed that the dephosphorylated metabolites bind to the STAT3 protein surface. However, we cannot specify the location of binding by SPR, only that 14aa-OH and 14ba-OH bind STAT3. To determine if 14aa-OH or 14ba-OH bind to STAT3’s SH2 domain, we repeated the STAT3 FP assay, which is an excellent indicator of SH2 domain binding. Most interestingly, we found that neither peptidomimetic inhibited the STAT3–phosphopeptide complex (data not shown). Thus, in addition to the phosphorylated inhibitors acting as STAT3 SH2 domain inhibitors, it is conceivable that the dephosphorylated metabolites may also act as STAT3 inhibitors via a different mode of inhibition.
Figure 4.
SPR analysis of the binding of STAT3 protein to inhibitors 14aa and 14ba.
2.6 Permeability and efflux analysis of STAT3 inhibitors 14aa, 14aa-OH, 14ba and 14ba-OH
Given the pharmacokinetic drawbacks associated for phosphate-containing inhibitors, we examined cell permeability and efflux in Caco-2 human epithelial cells, routinely used to mimic the small intestinal mucosa.29 Experiments were performed at the Ontario Institute for Cancer Research as described in the experimental section. Permeability was classified based on A-B apparent permeability rate coefficient (Papp) values (for absorptive transport) as: low (Papp< 2), medium (Papp 2-10) or high (Papp>10) × 10−6 cm/sec. In summary, as assessed by a narrow window mass extraction LC/MS analysis (Waters Xevo quadrupole time-of-flight MS), both 14aa and 14ba were predominantly dephosphorylated at the end of the 90-minute permeability assay (1-2 % post assay recovery) and showed negligible cell penetration (Papp(A-B) values of < 0.5). Dephosphorylated derivatives, 14aa-OH and 14ba-OH exhibited low cell permeability (Papp(A-B) values of > 0.5), but were significantly more metabolically stable (52 % and 36 % post-assay recovery).
2.7 Inhibition of intracellular Stat3 activation
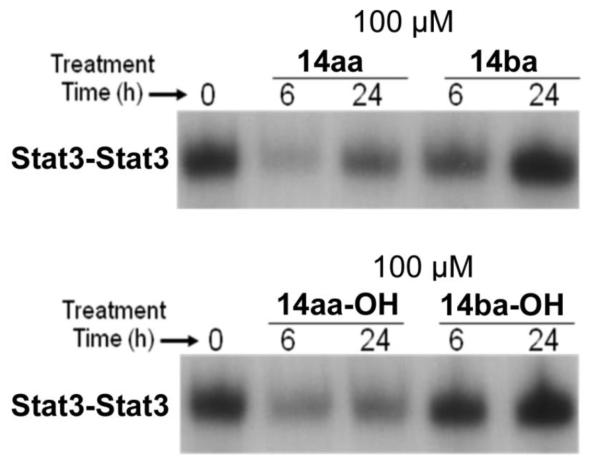
Despite the poor cell permeability results observed in Caco-2 cell permeability studies, we reasoned that the same effects might not be replicated in different cellular assays. Moreover, several Stat3-targeting groups have demonstrated cellular efficacy with phosphorylated compounds in whole cells.12,15,16 Thus, we evaluated 14aa, 14ba, 14aa-OH and 14ba-OH in v-Src transformed mouse fibroblasts (NIH3T3/vSrc) for suppression of STAT3–STAT3:DNA binding activity. After six hours and forty-eight hours of treatment with inhibitors (100 μM), nuclear extracts were prepared from cells and subjected to STAT3 DNA-binding assay in vitro using the radiolabeled hSIE probe and analyzed by EMSA (Fig. 5). We found that both 14aa and 14ba suppressed STAT3-DNA binding activity after 6 h (14aa > 80% cf. 14ba ~ 50 %). However, after 24 h, phosphorylated STAT3 activity was partially recovered, suggesting that both inhibitors exert a temporal suppression of STAT3 dimerization. Interestingly, the dephosphorylated inhibitor, 14aa-OH was found to suppress STAT3-DNA binding activity with almost the same potency as 14aa, suggesting that 14aa may well be dephosphorylated to 14aa-OH. Alternatively, internalized 14aa-OH is phosphorylated by intracellular kinases to 14aa and elicits temporal suppression of STAT3-DNA binding. 14ba-OH showed negligible disruption of STAT3-DNA binding activity at both 6 and 24 hrs of treatment.
Figure 5.
v-Src transformed mouse fibroblasts (NIH3T3/v-Src) treated or untreated with 100 μM 14aa, 14aa-OH, 14ba and 14ba-OH for 6hrs and 24 hrs and assayed for Stat3-DNA binding via EMSA analysis of nuclear extracts.
2.8 Tumor whole cell studies
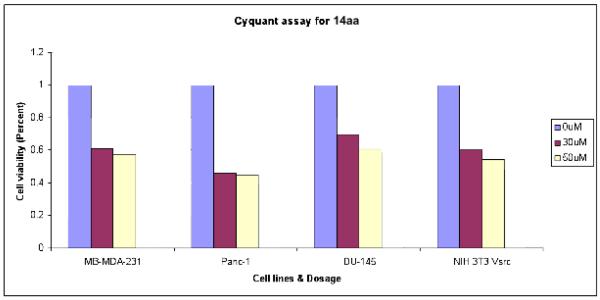
In an effort to evaluate the cellular efficacy of our peptidomimetics in whole cell tumor models, we elected to screen STAT3 inhibitors 14aa and 14ba against a series of cancer cell lines known to harbour constitutively activated STAT3. Specifically, prostate cancer cells (DU145),30 pancreatic cancer cells (Panc-1),31 NIH3T3/vSrc fibroblasts and breast cancer (MDA468) cells32 were treated with a range of inhibitor concentrations and incubated for 24 hrs. Promyelocytic leukemia (HL-60) cells that do not harbor aberrant STAT3 signaling were also treated as a control experiment. Disruption of cell growth and viability were measured by a CyQuant cell viability assay (Invitrogen). From this screen, only 14aa was shown to suppress cell viability as revealed in Fig. 6 (data for 14ba not shown). At 50 μM concentrations of 14aa, there was an approximately 50 % suppression in cell viability in all the cell lines investigated with the exception of HL60 cells, where the IC50 value was > 200 μM. Compared to the cellular activities of our previous STAT3 peptidomimetics (ISS610 (1) and ISS840)9, which required either mM dosages, the present data indicates significantly improved cellular activities of the hybrid peptidomimetics. Corroborating the whole cell STAT3-DNA binding EMSA data, 14ba showed only limited anti-cancer activity at lower concentrations.
Figure 6.
Human breast (MDA-MB-231), pancreatic (Panc-1), and prostate (DU-145) cancer cells, and v-Src transformed mouse fibroblasts (NIH3T3/v-Src) were treated or untreated with 30–50 μM 14aa for 24 hrs and assayed for viability using a CyQuant cell proliferation kit.
3. Conclusions
We have identified a series of novel STAT3-targeting peptidomimetic scaffolds incorporating an unnatural biphenyl-based amino acid residue. These hybrid Stat3 peptidomimetic inhibitors combine strong features of high-affinity binding to and improved potency against Stat3. While lead compound 14aa binds to STAT3 protein, disrupts phosphopeptide–STAT3 protein complexes, inhibits STAT3–STAT3 protein–protein interactions in both nuclear extracts and in whole cells, limited tumor cell cytotoxicity was observed, likely a result of poor cell permeability and metabolic stability. We are currently exploring the application of phosphotyrosine bioisoteres as well as prodrug strategies to improve the whole cell efficacy of lead peptidomimetics. These results shall be reported in due course.
4. Experimental
4.1 Chemical Methods
Anhydrous solvents methanol, DMSO, CH2Cl2, THF and DMF were purchased from Sigma Aldrich and used directly from Sure-Seal bottles. Molecular sieves were activated by heating to 300 °C under vacuum overnight. All reactions were performed under an atmosphere of dry nitrogen in oven-dried glassware and were monitored for completeness by thin-layer chromatography (TLC) using silica gel (visualized by UV light, or developed by treatment with KMnO4 stain or phosphomolybdic acid stain). Low-resolution mass spectrometry (LRMS) was obtained using a Waters Micromass ZQ with an ESI source in methanol. An AB/Sciex QStar mass spectrometer with an ESI source and accurate mass capabilities was used with samples dissolved in methanol to produce high-resolution mass spectrums.1H and 13C NMR spectra were recorded on Bruker 400 MHz and a Varian 500 MHz spectrometers in either CDCl3, CD3OD or d6-DMSO. Chemical shifts (δ) are reported in parts per million after calibration to residual isotopic solvent. Coupling constants (J) are reported in Hz. Before biological testing, inhibitor purity was evaluated by reversed-phase HPLC (rpHPLC). Analysis by rpHPLC was performed using a Microsorb-MV 300 A C18 250 mm × 4.6 mm column run at 1 mL/min, and using gradient mixtures of (A) water with 0.1M CH3COONH4 and (B) methanol. Ligand purity was confirmed using linear gradients from 75 % A and 25 % B to 100 % B after an initial 2 minute period of 100 % A. The linear gradient consisted of a changing solvent composition of either (I) 4.7 % per minute and UV detection at 254nm or (II) 1.4 % per minute and detection at 214nm, each ending with 5 minutes of 100% B. For reporting HPLC data, percentage purity is given in parentheses after the retention time for each condition. All biologically evaluated compounds are > 95 % chemical purity as measured by HPLC. The HPLC traces for all tested compounds are provided in supporting information.
General procedure A (N-benzylation of carboxylic acids)
To a stirring solution of the relevant acid (1.8g, 6.5 mmol) in anhydrous CH2Cl2 (0.1 M) was added (COCl)2 (0.85mL, 9.8mmol) and catalytic DMF under an inert N2 atmosphere at rt. The reaction was completed after 10 mins as assessed by TLC. The product was concentrated under reduced pressure and the resulting residue dissolved in anhydrous CH2Cl2 (0.1 M), followed by the step-wise addition of DIPEA (5 eq) and benzylamine (1.07mL, 9.8 mmol).The reaction was complete after 15 mins as judged by TLC. The solution was diluted with CH2Cl2 and the organics washed consecutively with 0.1 M HCl, saturated NaHCO3, and brine solution. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The resultant product was purified by flash column chromatography.
General procedure B (SnCl mediated nitro group reduction)
To a stirring solution of the appropriate nitro compound (1.7g, 5.0mmol) in EtOAc (0.1 M) was added SnCl dihydrate (5.7g, 25.0mmol) in one portion. The resultant solution was refluxed for 2 hrs at 70 °C before quenching with saturated NaHCO3. The aqueous layer was extracted using EtOAc and the combined organics were dried over anhydrous Na2SO4 and concentrated under reduced pressure to furnish the product.
General procedure C (peptide couplings)
Method A
Under a N2 atmosphere, the relevant carboxylic acid (1.5 eq) (1.6g, 6.5mmol) was added to a stirring solution of NMM (0.74mL, 5.6mmol) in anhydrous THF (0.1 M). Isobutylchloroformate (0.61, 5.6 eq) was added in one portion and the solution was allowed to stir at rt. After 15 mins, the appropriate amine (1.3g, 4.3mmol) was added drop-wise in a solution of THF containing NMM (0.52mL, 4.8mmol). The reaction mixture was left to stir overnight, concentrated and redissolved in distilled water. The water layer was then extracted with CH2Cl2, and the combined organic layers washed with saturated NaHCO3 solution, distilled water, and brine, dried over anhydrous Na2SO4 and concentrated.
Method B
The required carboxylic acid (340mg, 0.74mmol) was added in one portion to a solution of HBTU (334mg, 0.88mmol) and DIPEA (0.18mL, 1.0mmol) in DMF (0.1 M), and the resulting solution stirred at room temperature for 10 minutes. The required amine was then dissolved in a solution of DIPEA (0.18mL, 1.0mmol) in DMF (0.1 M) and added to the activated acid in one portion. The resulting solution was stirred for 4 hours, then diluted with EtOAc (0.1 M) and washed successively with equal volumes of: 2M HCl, saturated bicarbonate and brine. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated.
General procedure D (TFA mediated Boc deprotection)
To a stirred solution of Boc protected amine (300mg, 0.44mmol) in CH2Cl2 (2.2mL) under N2 was added trifluoroacetic acid (2.2mL) and allowed to stir at room temperature for 3 hrs. The reaction was monitored via TLC and stopped upon consumption of starting material. The solution was concentrated and purified via silica gel chromatography to yield pure amine.
General procedure E (O-Benzylation of tyrosine)
To a stirring solution of L-tyrosine (2.0g, 13.8mmol) in toluene, was added TsOH-monohydrate (3.2g, 15.2mmol)and benzyl alcohol (28.6mL, 276mmol). The solution was refluxed at 110 °C for 16 hrs before removing the solvent under reduced pressure. The concentrates were dissolved in diethyl ether and refrigerated. Product was precipitated out of solution, vacuumed filtered and subsequently washed with cold ether to furnish pure product. The product was carried over to the next step without further purification
General procedure F (Hydrogenolysis of benzyl ester)
The required benzyl ester (1.6g, 4.0mmol) was dissolved in a stirred solution of MeOH:EtOAc (1:1) and degassed thoroughly before the addition of Pd/C 10% (10 mg/mmol). H2 gas was then bubbled through the solution for 5 mins before the solution was put under an atmosphere of H2 gas and stirred continuously for 3 hrs. The hydrogen gas was excluded from the reaction vessel and the reaction mixture filtered to remove the Pd/C through glass fibre paper. The solution was then concentrated to give pure product.
General procedure G (Suzuki cross-couplings)
A mixture of arylbromide (142mg, 0.2mmol), boronic acid (36mg, 0.22mmol), K2CO3 (69mg, 0.5mmol)and Pd(PPh3)4 (34mg, 0.03mmol) was suspended in DMF (0.1 M) in a sealed tube vessel and irradiated in a Biotage Initiator microwave reactor (17 mins, 170 °C). After cooling to rt, the reaction was diluted with water and repeatedly extracted with CH2Cl2. The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure.
General procedure H (phosphorylation using bis(dimethylamino)-phosphoramidic chloride)
To a stirring solution of phenol (80mg, 0.1mmol) and DMAP (24.4mg, 0.2mmol) in CH2Cl2:THF (0.1 M), was added DBU (22.4μL, 0.15mmol) in one portion, followed by bis(dimethylamino)-phosphoramidic chloride (18.8μL, 0.13mmol).The reaction was left to stir under an inert N2 atmosphere at rt for 6 hrs. The reaction was quenched with distilled water, the product extracted using EtOAc, and the combined organics washed with a 1:1 mixture of distilled water:NaH2PO4, distilled water, and brine. The organics were dried over anhydrous Na2SO4, and concentrated under vacuum.
General procedure I (TFA mediated phosphoramidate hydrolysis)
Phosphoramidate (45mg, 0.051mmol) was added to a 9:1 mixture of TFA:H2O at room temperature. The reaction mixture was left for 16 hrs. Complete transformation into the product was confirmed by TLC. Reaction mixtures were co-evaporated with MeOH to near dryness, then diluted with a mixture of HPLC grade water: acetonitrile (6:1) and lyophilized.
4.2 Detailed synthetic procedures for all compounds
N-benzyl-2-bromo-4-nitrobenzamide (5a)
Reaction of 4a (1.8g, 7.3mmol) according to procedure A, and purified by flash column chromatography (49:1 CH2Cl2:EtOAc) to furnish 5a as a white solid (1.79, 5.3mmol, 73%): δH (400MHz, DMSO-d6), 4.48 (d, J = 5.9 Hz, 2H, CH2Ph), 7.26-7.30 (m, 1H, CH (Ar)), 7.34-7.40 (m, 4H, 4 CH (Ar)), 7.71 (d, J = 8.5 Hz, 1H, 1 CH (Ar)), 8.28 (dd, J = 8.3 Hz and 2.2 Hz, 1H, CH (Ar)), 8.47 (d, J = 2.2 Hz, 1H, CH, (Ar)), 9.22 (t, J = 5.9 Hz, 1H, NH): δC (100 MHz, DMSO-d6) 42.5, 119.5, 122.8, 127.0, 127.4, 127.5, 128.4, 129.8, 138.7, 144.8, 148.1, 166.0; LRMS (MS ES), calcd for C14H11BrN2O3 [M+H] m/z = 335.01, fnd. 335.08.
N-benzyl-3-bromo-5-nitrobenzamide (5b)
Reaction of 4b (1.8g, 7.3mmol) according to procedure A, and purified by flash column chromatography (49:1 CH2Cl2:EtOAc) to furnish 5b as a white solid (1.81g, 5.4mmol, 74%): δH (400 MHz, DMSO-d6) 4.51 (d, J = 5.8 Hz, 2H, CH2), 7.26 (m, 1H, CH (Ar)), 7.34 (m, 4H, 4 CH (Ar)), 8.51 (t, J = 1.5 Hz, 1H, CH (Ar)), 8.55 (t, J = 1.9 Hz, 1H, CH (Ar)), 8.70 (t, J = 1.8 Hz, 1H, CH (Ar)) 9.49 (t, J = 5.8 Hz, 1H, NH): δC (100 MHz, DMSO-d6). 43.1, 121.4, 122.2, 127.0, 127.5, 128.4, 128.6, 136.1, 137.1, 138.9, 148.7, 162.7; LRMS (MS ES), calcd for C14H11BrN2O3Na [M+Na] m/z = 357.00, fnd. 357.13.
4-amino-N-benzyl-2-bromobenzamide (6a)
Reaction of 5a (1.7g, 5.1mmol) according to procedure B, and purified by flash column chromatography (7:2 CH2Cl2:EtOAc) to furnish 6a as a white solid (1.5g, 4.9mmol, 96 %): δH (400MHz, CD3Cl) 4.64 (d, J = 6.0 Hz, 2H, CH2Ph), 5.67 (br s, 2H, NH2), 6.54 (dd, J = 8.3 and 2.0 Hz, 1H, CH (Ar)), 6.80 (d, J = 2.0 Hz, 1H, CH (Ar)), 7.15 (d, J = 8.3 Hz, 1H, 1 CH (Ar)), 7.23 (m, 1H, CH (Ar)), 7.33 (m, 4H, 4 CH (Ar)), 8.58 (t, J = 6.0 Hz, 1H, NHCH2); δC (100 MHz, DMSO-d6) 42.5, 112.0, 116.9, 120.2, 124.9, 126.7, 127.2, 128.2, 130.2, 139.6, 151.1, 167.5; LRMS (MS ES), calcd for C14H13BrN2O [M+H] m/z = 305.03, fnd. 305.18.
3-amino-N-benzyl-5-bromobenzamide (6b)
Reaction of 5b (1.7g, 5.1mmol) according to procedure B, and purified by flash column chromatography (7:2 CH2Cl2:EtOAc) to furnish 6b as a white solid (1.5g, 4.8mmol, 95 %). δH (400 MHz, DMSO-d6) 4.42 (d, J = 6.0 Hz, 2H, CH2Ph), 5.61 (s, 2H, NH2), 6.87 (t, J = 1.9 Hz, 1H, CH (Ar)), 7.05 (t, J = 1.8 Hz, 1H, (Ar)), 7.14 (t, J = 1.5 Hz, 1H, ArBr), 7.23(m, 1H, CH (Ar)), 7.30 (m, 4H, 4 CH (Ar)), 8.94 (t, J = 6.0 Hz, 1H, CONH); δC (100 MHz, DMSO-d6) 42.6, 112.1, 116.3, 118.1, 121.9, 126.7, 127.2, 128.3, 137.2, 139.6, 150.5, 165.5; LRMS (MS ES), calcd for C14H13BrN2ONa [M+Na] m/z = 327.02, fnd. 327.21.
(S)-4-(2-amino-4-methylpentanamido)-N-benzyl-2-bromobenzamide (7a)
Reaction of 6a (1.3g, 4.3mmol) according to procedure C (Method A) followed by Boc deprotection according to procedure D furnished 7a as a white solid (1.7g, 4.1mmol, 97 %): δH (400 MHz, DMSO-d6) 0.91-0.94 (m, 6H, 2 CH3 (Leu)), 1.66-1.77 (m, 3H, CHCH2 (Leu)), 4.12 (s, 1H, CH (Leu)), 4.43 (d, J = 6.0 Hz, 2H, CH2Ph) 7.25-7.26 (m, 1H, CH (Ar)), 7.31-7.38 (m, 4H, 4 CH (Ar)), 7.43 (d, J = 8.4 Hz, 1H, CH (Ar)), 7.71 (dd, J = 8.4 and 1.9 Hz, 1H, CH (Ar)), 8.09 (d, J = 1.9 Hz, 1H, CH (Ar)), 8.50 (br s, 3H, NH3), 8.93 (t, J = 6.0 Hz, 1H, NHBn), 11.5 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 22.2, 22.6, 23.7, 42.5, 51.7, 118.1, 119.1, 122.9, 126.8, 127.3, 128.3, 129.4, 134.1, 139.1, 140.1, 166.9, 168.5 ; LRMS (MS ES), calcd for C20H25BrN3O2 [M+H] m/z = 418.11, fnd. 418.20.
(S)-3-(2-amino-4-methylpentanamido)-N-benzyl-5-bromobenzamide (7b)
Reaction of 6b (1.3g, 4.3mmol) according to procedure C (Method A) followed by Boc deprotection according to procedure D furnished 7b as a white solid (1.7g, 4.2mmol, 98 %): δH (400 MHz, DMSO-d6) 0.91-0.94 (m, 6H, 2 CH3 (Leu)), 1.66-1.73 (m, 3H, CHCH2 (Leu)), 4.09-4.10 (m, 1H, CH (Leu)), 4.43 (d, J = 6.0 Hz, 2H, CH2Ph) 7.21-7.27 (m, 1H, CH (Ar)), 7.30-7.32 (m, 4H, 4 CH (Ar)), 7.86-7.87 (m, 1H, CH (Ar)), 8.13-8.14 (m, 1H, CH (Ar)), 8.16-8.17 (m, 1H, CH (Ar)), 8.52 (br s, 3H, NH3), 9.24-9.26 (t, J = 6.0 Hz, 1H, NHBn), 11.43 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 22.2, 22.6, 23.8, 42.8, 51.7, 118.0, 121.5, 124.2, 124.9, 126.8, 127.3, 128.3, 137.0, 139.3, 139.9, 164.5, 168.5; LRMS (MS ES), calcd for C20H25BrN3O2 [M+H] m/z = 418.11, fnd. 418.28.
(S)-benzyl 2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanoate (9)
Reaction of 8 (1.0g, 5.5mmol) according to procedure E yielded the benzyl ester (not isolated) that was immediately coupled to 4-cyanobenzoic acid according to procedure A (method A) to furnish 9 as a white solid (1.7g, 4.2mmol, 84 %): δH (400 MHz, DMSO-d6) 2.97-3.11 (m, 2H, CH2Ar (Tyr)), 4.61-4.67 (m, 1H, CH (Tyr)), 5.12 (s, 2H, CH2Ph), 6.65 (d, J = 8.44 Hz, 2H (Ar)), 7.08 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.27-7.37 (m, 5H, 5 CH (Ar)), 7.93-7.98 (m, 4H, Ar-CN), 9.14 (d, J = 7.6 Hz, 1H, NH), 9.27 (s, 1H, OH); δC (100 MHz, DMSO-d6) 35.5, 55.0, 66.1, 113.9, 115.1, 118.3, 127.4, 127.8, 128.1, 128.3, 128.4, 130.1, 135.9, 137.7, 156.1, 165.3, 171.4; LRMS (MS ES), calcd for C24H20N2O4Na [M+Na] m/z = 423.14, fnd. 423.23.
(S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanoic acid (10)
Reaction of 9 (1.6g, 4.0mmol) according to procedure F yielded the carboxylate 10 as a white solid in quantitiative yield (1.2g, 4.0 mmol, 99 %): δH (400 MHz, DMSO-d6) 2.90-2.96 (m, 1H, CH, CH2 (Tyr)), 3.06-3.11(m, 1H, CH2 (Tyr)), 4.51-4.57 (m, 1H, CH (Tyr)), 6.34 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.09 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.94 (s, 4H, Ar-CN), 8.92 (d, J = 8.1 Hz, 1H, NH); δC (100 MHz, DMSO-d6) 35.6, 54.8, 113.8, 115.0, 118.4, 128.1, 128.2, 130.0, 132.4, 138.0, 155.9, 165.0, 173.1; LRMS (MS ES), calcd for C17H13N2O4 [M-H] m/z = 309.10, fnd. 309.12.
N-benzyl-2-bromo-4-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)benzamide (11a)
Compound 10 (687mg, 2.2mmol) was coupled to 7a (840mg, 2.0mmol), according to procedure A (method B) to furnish 11a, and then purified by flash chromatography (96.8:2.8:0.4 CH2Cl2:MeOH:NH4OH) to obtain a yellow solid (896mg, 1.3mmol, 63 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 17.0 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.52-1.71 (m, 3H, CH2CH (Leu)), 2.82-2.88 (m, 1H, CH, CH2 (Tyr)), 3.02-3.06 (m, 1H, CH, CH2 (Tyr)), 4.43-4.47 (m, 3H, CH (Leu) and NHCH2Ph), 4.66-4.71 (m, 1H, CH (Tyr)), 6.63 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.15 (d, J = 8.4 Hz, 2H, 2 CH (Ar, Tyr)), 7.23-7.27 (m, 1H, CH (Ar)), 7.32-7.42 (m, 5H, 5 CH (Ar)), 7.59 (d, J = 8.3 Hz, 1H, CH (Ar)), 7.92-7.97 (m, 4H, 4 CH (Ar-CN)), 8.03 (d, J = 2.0 Hz, 1H, CH (Ar)), 8.37 (d, J = 8.0 Hz, 1H, CONH), 8.80 (d, J = 8.4 Hz, 1H, CONH), 8.87 (t, J = 6.0 Hz, 1H, NHBn), 9.15 (s, 1H, OH (Tyr)), 10.24 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.5, 42.5, 52.3, 55.3, 113.7, 114.9, 117.8, 118.3, 119.1, 122.6, 126.8, 127.2, 128.2, 128.2, 128.3, 129.4, 130.1, 132.4, 133.5, 138.0, 139.2, 140.6, 155.7, 165.0, 167.0, 171.4, 171.6; LRMS (MS ES), calcd for C37H36BrN5O5 [M+H] m/z = 710.20, fnd. 710.22.
N-benzyl-3-bromo-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)benzamide (11b)
Compound 10 (512mg, 1.6mmol) was coupled to 7b (630mg, 1.5mmol) according to procedure A (method B) to furnish 11b, and then purified by flash chromatography (3:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to obtain a white solid (672mg, 0.9mmol, 50 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 17.2 and 6.2 Hz, 6H, 2 CH3 (Leu)), 1.53-1.69 (m, 3H, CH2CH (Leu)), 2.80-2.89 (m, 1H, CH, CH2 (Tyr)), 3.02-3.05 (m, 1H, CH, CH2 (Tyr)), 4.43-4.47 (m, 3H, CH (Leu) and NHCH2Ph), 4.65-4.71 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.14 (d, J = 8.4 Hz, 2H, 2 CH (Ar-OH, Tyr)), 7.23-7.26 (m, 1H, CH (Ar)), 7.29-7.35 (m, 4H,4 CH (Ar)), 7.77 (t, J = 1.5 Hz, 1H, CH (Ar)), 7.90-7.96 (m, 4H, 4 CH (Ar-CN)), 8.01 (t, J = 1.5 Hz, 1H, CH (Ar)), 8.14 (t, J = 2.0 Hz, 1H, CH (Ar)), 8.37 (d, J = 7.1 Hz, 1H, CONH), 8.79 (d, J = 8.3 Hz, 1H, CONH) 9.13-9.17 (m, 2H, NHBn and Ar-OH (Tyr)), 10.36 (s, 1H, NHArBr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.7, 42.8, 52.3, 55.3, 113.7, 114.9, 117.7, 118.3, 121.5, 123.9, 124.2, 126.8, 127.3, 128.2, 128.2, 128.3, 130.1, 132.4, 136.9, 138.0, 139.3, 140.5, 155.7, 164.6, 165.0, 171.5, 171.6; LRMS (MS ES), calcd. for C37H36BrN5O5Na [M+Na] m/z = 732.19, fnd. 732.08.
(N2-benzyl-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)biphenyl-2,4′-dicarboxamide (12aa)
Compound 11a (142mg, 0.2mmol) was coupled to 4-aminocarbonylphenyl boronic acid according to general procedure G. Crude material was purified by flash chromatography (92:7:1 CH2Cl2:MeOH:NH4OH) and yield final product 12aa as white solid (86mg, 0.11mmol, 57 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.2 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.52-1.71 (m, 3H, CH2CH (Leu)), 2.82-2.89 (m, 1H, CH, CH2 (Tyr)), 3.01-3.05 (m, 1H, CH, CH2 (Tyr)), 4.27-4.28 (m, 2H, CH2Ph), 4.45-4.50 (m, 1H, CH (Leu)), 4.65- 4.71 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.02 (d, J = 6.4 Hz, 2H, 2 CH (Ar)), 7.14 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.17-7.25 (m, 3H, 3 CH (Ar)), 7.37-7.47 (m, 4H, 4 CH (Ar)), 7.67-7.69 (m, 2H, 2 CONH2), 7.86-7.95 (m, 6H, 6 CH (Ar-CN and CH (Ar)), 8.03 (s, 1H, CH (Ar)), 8.35 (d, J = 7.7 Hz, 1H, CONH), 8.61 (t, J = 6.0 Hz, 1H, NHBn), 8.80 (d, J = 8.4 Hz, 1H, CONH), 9.16 (s, 1H, Ar-OH (Tyr)), 10.23 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.7, 42.4, 52.2, 55.3, 113.7, 114.9, 117.8, 118.3, 120.24, 126.6, 127.1, 127.4, 128.1, 128.2, 128.2, 129.6, 130.0, 132.0, 132.4, 133.0, 138.0, 139.2, 139.4, 139.7, 143.2, 155.7, 165.0, 167.6, 168.6, 171.4, 171.4; LRMS (MS ES), calcd for C44H42N6O6 [M+Na] m/z = 773.31, fnd. 773.33.
N-benzyl-4′-cyano-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-2-carboxamide (12ab)
Compound 11a (142mg, 0.2mmol) was coupled to 4-cyanophenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) and yield final product 12ab as white solid (81mg, 0.11mmol, 55 %): δH (400 MHz, DMSO-d6)) 0.91 (dd, J = 16.4 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.59-1.71 (m, 3H, CH2CH (Leu)), 2.81-2.89 (m, 1H, CH2 (Tyr)), 3.00-3.05 (m, 1H, CH, CH2 (Tyr)), 4.26 (d, J = 6.0 Hz, 2H, CH2Ph), 4.43-4.50 (m, 1H, CH (Leu)), 4.65-4.71 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.5 Hz, 2H, Ar-OH (Tyr)), 7.06-7.08 (m, 2H, 2 CH (Ar)), 7.13 (d, J = 8.5 Hz, 2H, Ar-OH (Tyr)), 7.23-7.29 (m, 3H, 3 CH (Ar)), 7.45 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.49-7.20 (m, 1H, CH (Ar)), 7.69-7.71 (m, 2H, 2 CH (Ar)), 7.76-7.78 (m, 2H, 2 CH (Ar)), 7.90-7.96 (m, 4H, 4 CH (Ar-CN)), 8.33 (d, J = 7.7 Hz, 1H, CONH), 8.66 (t, J = 6.0 Hz, 1H, NHBn), 8.78 (d, J = 8.4 Hz, 1H, CONH), 9.14 (s, 1H, Ar-OH (Tyr)), 10.25 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.6, 42.4, 52.2, 55.3, 110.0, 113.7, 114.9, 118.3, 118.8, 120.1, 126.7, 127.3, 128.1, 128.2, 128.2, 128.8, 129.2, 130.0, 131.8, 132.0, 132.4, 138.0, 138.6, 139.0, 139.9, 145.1, 145.1 155.7, 165.0, 168.1, 171.4, 171.4; LRMS (MS ES), calcd for C44H40N6O5Na [M+Na] m/z = 755.31, fnd. 755.15.
methyl 2′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxylate (12ac)
Compound 11a (142mg, 0.2mmol) was coupled to 4-methoxycarboxyphenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) and yielded final product 12ac as a white solid (66mg, 0.086mmol, 43 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.4 and 6.3 Hz, 6H, 2 CH3 (Leu)), 1.52-1.75 (m, 3H, CH2CH (Leu)), 2.82-2.88 (m, 1H, CH2 (Tyr)), 3.01-3.08 (m, 1H, CH, CH2 (Tyr)), 3.86 (s, 3H, COOCH3), 4.25 (d, J = 6.0 Hz, 2H, CH2Ph), 4.40-4.50 (m, 1H, CH (Leu)), 4.65-4.71 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.05-7.06 (m, 2H, 2 CH (Ar)), 7.14 (d, J = 8.4 Hz, 2H, Ar-OH), 7.20-7.22 (m, 3H, 3 CH (Ar)), 7.42-7.44 (m, 2H, 2 CH (Ar)), 7.47-7.49 (m, 1H, CH (Ar)), 7.68-7.70 (m, 2H, 2 CH (Ar)), 7.88-7.94 (m, 6H, Ar-CN and Ar), 8.36(d, J = 7.7 Hz, 1H, CONH), 8.64 (t, J = 6.0 Hz, 1H, NHBn), 8.80 (d, J = 8.4 Hz, 1H, CONH), 9.19 (s, 1H, Ar-OH (Tyr)), 10.26 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.4, 36.3, 40.7, 42.5, 52.2, 52.3, 55.4, 113.7, 114.9, 118.1, 118.3, 120.2, 126.7, 127.3, 128.1, 128.2, 128.3, 128.4, 128.7, 128.8, 129.1, 130.0, 132.0, 132.4, 138.0, 139.0, 139.1, 139.9, 145.2, 155.8, 165.1, 166.2, 168.5, 171.5, 171.5; LRMS (MS ES), calcd for C45H43N5O7Na [M+Na] m/z = 788.32, fnd. 788.28.
N2-benzyl-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-2,3′-dicarboxamide (12ad)
Compound 11a (142mg, 0.2mmol) was coupled to 3-amidocarboxyphenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (92:7:1 CH2Cl2:MeOH:NH4OH) and yielded final product 12ad as a white solid (59mg, 0.078mmol, 39 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.2 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.52-1.73 (m, 3H, CH2CH (Leu)), 2.83-2.89 (m, 1H, CH2 (Tyr)), 3.02-3.06 (m, 1H, CH2 (Tyr)), 4.27 (d, J = 6.0 Hz, 2H, CH2Ph), 4.46-4.51 (m, 1H, CH (Leu)), 4.66-4.71 (m, 1H, CH (Tyr)), 6.61 (d, J = 6.8 Hz, 2H, Ar-OH (Tyr)), 7.07 (m, 2H, 2 CH (Ar)), 7.14 (d, J = 8.4 Hz, 1H, CH (Ar)), 7.20-7.27 (m, 3H, 3 CH (Ar)), 7.37-7.48 (m, 4H, 4 CH (Ar)), 7.66-7.67 (m, 2H, 2 CH (Ar)), 7.69-7.72 (m, 1H, CH (Ar)), 7.87 (d, J = 7.4 Hz, 1H, CH (Ar)), 7.91-7.95 (m, 5H, Ar-CN and Ar), 8.03 (s, 1H, CH (Ar)), 8.33 (d, J = 7.5 Hz, 1H, CONH), 8.59 (t, J = 6.0 Hz, 1H, NHBn), 8.80 (d, J = 8.4 Hz, 1H, CONH), 9.14 (s, 1H, Ar-OH (Tyr)), 10.22 (s, 1H, NHAr) ); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.7, 42.4, 52.2, 55.3, 113.7, 114.9, 117.6, 118.3, 120.5, 126.2, 126.5, 127.0, 127.7, 127.9, 128.2, 128.2, 128.7, 128.7, 128.8, 130.0, 131.1, 131.4, 131.5, 131.9, 132.4, 134.3, 138.0, 139.7, 139.8, 140.5, 155.7, 165.0, 167.7, 168.6, 171.4. LRMS (MS ES), calcd. For C44H42N6O6Na [M+Na] m/z = 773.32. fnd. 773.23.
N-benzyl-3′-cyano-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-2-carboxamide (12ae)
Compound 11a (142mg, 0.2mmol) was coupled to 3-cyanophenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) and yielded final product 12ae as a white solid (51mg, 0.070mmol, 35 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.4 and 6.3 Hz, 6H, 2 CH3 (Leu)), 1.51-1.71 (m, 3H, CH2CH (Leu)), 2.82-2.89 (m, 1H, CH2 (Tyr)), 3.00-3.05 (m, 1H, CH2 (Tyr)), 3.86 (s, 3H, COOCH3), 4.27 (d, J = 5.9 Hz, 2H, CH2Ph), 4.44-4.50 (m, 1H, CH (Leu)), 4.65-4.70 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.06-7.07 (m, 2H, 2 CH (Ar)), 7.13 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.20-7.29 (m, 3H, 3 CH (Ar)), 7.50-7.60 (m, 3H, 3 CH (Ar)), 7.66-7.67 (m, 1H, CH (Ar)), 7.70-7.72 (m, 2H, 2 CH (Ar)), 7.82 (d, J = 8.2 Hz, 1H, 1 CH (Ar)), 7.90-7.95 (m, 4H, 4 CH (Ar-CN)), 8.36 (d, J = 7.7 Hz, 1H, CONH), 8.69 (t, J = 6.1 Hz, 1H, NHBn), 8.80 (d, J = 8.2 Hz, 1H, CONH), 9.16 (s, 1H, Ar-OH (Tyr)), 10.26 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.6, 42.4, 52.3, 55.3, 111.3, 113.7, 114.9, 118.2, 118.3, 118.7, 120.4, 126.7, 127.1, 128.2, 128.2, 128.2, 128.8, 129.4, 130.0, 131.0, 131.6, 131.7, 132.4, 133.2, 138.0, 138.2, 139.1, 139.9, 141.6, 155.7, 165.0, 168.2, 171.4, 171.4; LRMS (MS ES), calcd for C44H40N6O5Na [M+Na] m/z = 755.31, fnd. 755.15.
methyl 2′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxylate (12af)
Compound 11a (142mg, 0.2mmol) was coupled to 3-methoxycarboxyphenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) and yielded final product 12af as a white solid (81mg, 0.11mmol, 53 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.4 and 6.3 Hz, 6H, 2 CH3 (Leu)), 1.53-1.72 (m, 3H, CH2CH (Leu)), 2.83-2.89 (m, 1H, CH2 (Tyr)), 3.02-3.06 (m, 1H, CH2 (Tyr)), 3.86 (s, 3H, COOCH3), 4.25 (d, J = 6.0 Hz, 2H, CH2Ph), 4.45-4.50 (m, 1H, CH (Leu)), 4.65-4.71 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.03-7.05 (m, 2H, 2 CH (Ar)), 7.14 (d, J = 8.4 Hz, 2H, Ar-OH), 7.17-7.24 (m, 3H, 3 CH (Ar)), 7.47-7.51 (m, 2H, 2 CH (Ar)), 7.55-7.58 (m, 1H, CH (Ar)), 7.68-7.72 (m, 2H, 2 CH (Ar)), 7.92-7.95 (m, 6H, Ar-CN and Ar), 8.36 (d, J = 7.7 Hz, 1H, CONH), 8.62 (t, J = 6.0 Hz, 1H, NHBn), 8.81 (d, J = 8.4 Hz, 1H, CONH), 9.15 (s, 1H, Ar-OH (Tyr)), 10.25 (s, 1H, NHAr): δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.7, 42.4, 52.2, 52.3, 55.3, 113.7, 114.9, 117.8, 118.3, 120.2, 126.6, 127.1, 128.0, 128.1, 128.2, 128.7, 128.8, 128.9, 129.6, 130.0, 131.8, 132.3, 133.1, 138.0, 139.0, 139.1, 139.9, 140.8, 155.7, 165.0, 166.1, 168.5, 171.4, 171.4; LRMS (MS ES), calcd for C45H43N5O7Na [M+Na] m/z = 788.32, fnd. 788.28.
N3-benzyl-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3,4′-dicarboxamide (12ba)
Compound 11b (142mg, 0.2mmol) was coupled to 4-aminocarbonylphenyl boronic acid according to general procedure G. Crude material was purified by flash chromatography (92:7:1 CH2Cl2:MeOH:NH4OH) and yielded final product 12ba as a white solid (63mg, 0.084mmol, 42 %): δH (400 MHz, DMSO-d6) 0.90 (dd, J = 16.0 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.56-1.75 (br m, 3H, CH2CH (Leu)), 2.83-2.89 (m, 1H, CH2 (Tyr)), 3.02-3.06 (m, 1H, CH2 (Tyr)), 4.48-4.53 (m, 3H, CH and NHCH2), 4.67-4.72 (m, 1H, CH (Tyr)), 6.51 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.14 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.23-7.28 (m, 1H, CH (Ar)), 7.31-7.36 (m, 4H, 4 CH (Ar)), 7.43 (s, 1H, CH (Ar)), 7.77 (d, J = 8.4 Hz, 2H, Ar-COONH2), 7.93-7.95 (m, 5H, Ar-CN and Ar), 8.00 (d, J = 8.0 Hz, 2H, Ar-COONH2), 8.03 (s, 1H, CH, (Ar)), 8.16 (d, J = 5.6 Hz, 2H, COONH2), 8.36 (d, J = 7.4 Hz, 1H, CONH), 8.81 (d, J = 8.2 Hz, 1H, CONH) 9.14 (s, 1H, Ar-OH), 9.19 (t, J = 6.2 Hz, 1H, NHBn), 10.30 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 22.0, 23.6, 26.0, 37.9, 41.7, 44.7, 54.3, 57.2, 116.2, 116.3, 119.1, 119.9, 122.7, 122.8, 128.3, 128.3, 128.7, 129.0, 129.4, 129.5, 129.6, 131.4, 133.5, 134.4, 137.3, 139.4, 140.1, 140.6, 142.4, 144.7, 157.4, 168.6, 169.7, 171.9, 173.4, 174.0; LRMS (MS ES), calcd for C44H42N6O6Na [M+Na] m/z = 773.32, fnd. 773.23.
N-benzyl-4′-cyano-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxamide (12bb)
Compound 11b (142mg, 0.2mmol) was coupled to 4-cyanophenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (3:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) and yielded final product 12bb as a white solid (59mg, 0.080mmol, 40 %): δH (400 MHz, DMSO-d6) 0.92 (dd, J = 15.8 and 6.5 Hz, 6H, 2 CH3 (Leu)), 1.55-1.72 (br m, 3H, CH2CH (Leu)), 2.82-2.89 (m, 1H, CHCH2 (Tyr)), 3.02-3.07 (m, 1H, CHCH2 (Tyr)), 4.46-4.53 (m, 3H, CH and NHCH2), 4.65-4.72 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.14 (d, J = 8.4 Hz, 2H, Ar-OH), 7.22-7.27 (m, 1H, CH (Ar)), 7.31-7.34 (m, 4H, 4 CH (Ar)), 7.89-8.00 (m, 9H, Ar-CN and Ar-CN and (Ar)), 8.17 (s, 1H, CH (Ar)), 8.20 (s, 1H, CH (Ar)), 8.37 (d, J = 7.4 Hz, 1H, CONH), 8.81 (d, J = 8.2 Hz, 1H, CONH), 9.15 (s, 1H, Ar-OH (Tyr)), 9.20 (t, J = 5.7 Hz, 1H, NHBn), 10.34 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.7, 42.7, 52.3, 55.3, 110.5, 113.7, 114.9, 118.3, 118.8, 119.0, 120.2, 120.4, 126.8, 127.3, 127.7, 128.2, 128.2, 128.3, 130.1, 132.4, 133.0, 135.9, 138.0, 138.9, 139.5, 139.9, 144.0, 155.7, 165.1, 165.7, 171.4, 171.5; LRMS (MS ES), calcd for C44H40N6O5Na [M+Na] m/z = 755.31, fnd. 755.28.
methyl 3′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-4-carboxylate (12bc)
Compound 11b (142mg, 0.2mmol) was coupled to 4-methoxycarbonylphenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (98:2 CH2Cl2:MeOH) and yielded final product 12bc as a white solid (86mg, 0.11mmol, 56 %): δH (400 MHz, DMSO-d6) 0.91 (m, 6H, 2 CH3 (Leu)), 1.60-1.72 (br m, 3H, CH2CH (Leu)), 2.85-2.91 (m, 1H, CH2 (Tyr)), 3.04-3.09 (m, 1H, CH2 (Tyr)), 3.88 (s, 3H, COOCH3), 4.49-4.54 (m, 3H, CH (Leu) and CH2 (NHCH2Ph)), 4.70-4.75 (m, 1H, CH (Tyr)), 6.63 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.16 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.23-7.26 (m, 1H, CH (Ar)), 7.31-7.35 (m, 4H, 4 CH (Ar)), 7.86 (d, J = 8.4 Hz, 2H, Ar-COOMe), 7.92-7.98 (m, 5H, 5 CH (Ar-CN) and (Ar)), 8.08 (d, J = 8.4 Hz, 2H, Ar-COOMe), 8.18 (s, 1H, CH, (Ar)), 8.22 (s, 1H, CH, (Ar)), 8.33 (d, J = 8.0 Hz, 1H, CONH), 8.78 (d, J = 8.4 Hz, 1H, CONH) 9.11 (s, 1H, Ar-OH (Tyr)), 9.19 (t, J = 6.5 Hz, 1H, NHBn), 10.30 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.4, 36.3, 40.7, 42.8, 52.2, 52.3, 55.4, 113.7, 114.9, 118.3, 118.7, 120.2, 120.4, 126.8, 127.1, 127.3, 128.3 (2 C), 128.3, 128.9, 130.1, 132.4, 135.9, 138.1, 139.5, 139.5, 139.8, 144.1, 155.8, 165.1, 165.9, 166.0, 171.4, 171.5; LRMS (MS ES), calcd. For C45H43N5O7Na [M+Na] m/z = 788.32, fnd. 788.21.
N3-benzyl-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3,3′-dicarboxamide (12bd)
Compound 11b (142mg, 0.2mmol) was coupled to 3-aminocarbonylphenyl boronic acid according to general procedure G. Crude material was purified by flash chromatography (92:7:1 CH2Cl2:MeOH:NH4OH) and yielded final product 12bc as a white solid (94mg, 0.13mmol, 63 %): δH (400 MHz, DMSO-d6) 0.91 (m, 6H, 2 CH3 (Leu)), 1.55-1.67 (br m, 3H, CH2CH (Leu)), 2.81-2.87 (m, 1H, CHCH2 (Leu)), 3.01-3.05 (m, 1H, CHCH2), 4.48-4.50 (m, 3H, CH (Leu) and CH2 (NHCH2Ph)), 4.66-4.70 (m, 1H, CH (Tyr)), 6.60 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.14 (d, J = 8.4 Hz, 2H, Ar-OH), 7.21-7.25 (m, 1H, CH (Ar)), 7.29-7.35 (m, 4H, 4 CH (Ar)), 7.49 (s, 1H, CH (Ar)), 7.58 (t, J = 7.7 Hz, 1H, CH (Ar)), 7.85 (d, J = 8.4 Hz, 1H, CH (Ar)), 7.93 (m, 7H, 5 CH (Ar-CN) and (Ar) and CONH2), 8.13-8.17 (m, 3H, 3 CH (Ar)), 8.21 (s, 1H, CH (Ar)), 8.36 (d, J = 8.0 Hz, 1H, CONH), 8.81 (d, J = 8.4 Hz, 1H, CONH), 9.15 (s, 1H, Ar-OH), 9.21 (t, J = 6.5 Hz, 1H, NHBn), 10.31 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.4, 36.3, 40.7, 42.8, 52.2, 55.4, 113.7, 114.9, 118.3, 118.7, 120.2, 120.4, 126.8, 127.1, 127.3, 128.3 (2C), 128.3, 128.9, 130.1, 132.4, 135.9, 138.1, 139.5, 139.5, 139.8, 144.1, 155.8, 165.1, 165.9, 166.0, 171.4, 171.5; LRMS (MS ES), calcd. For C45H42N5O7Na [M+Na] m/z = 773.32, fnd. 773.21.
N-benzyl-4′-cyano-5-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxamide (12be)
Compound 11b (142mg, 0.2mmol) was coupled to 3-cyanophenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (3:2 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) and yielded final product 12bc as a white solid (75mg, 0.10mmol, 51 %): δH (400 MHz, DMSO-d6) 0.92 (dd, J = 15.8 and 6.5 Hz, 6H, 2 CH3 (Leu)), 1.55-1.73 (br m, 3H, CH2CH (Leu)), 2.83-2.89 (m, 1H, CHCH2 (Tyr)), 3.02-3.07 (m, 1H, CHCH2 (Tyr)), 4.47-4.58 (m, 3H, CH (Leu) and NHCH2), 4.66-4.72 (m, 1H, CH (Tyr)), 6.61 (d, J = 8.4 Hz, 2H, Ar-OH), 7.15 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.23-7.28 (m, 1H, CH (Ar)), 7.31-7.36 (m, 4H, 4 CH (Ar)), 7.72 (t, J = 7.8Hz, 1H, CH (Ar)), 7.87-7.90 (m, 1H, CH (Ar)), 7.93-7.95 (m, 5H, Ar-CN and Ar), 8.01-8.03 (m, 1H, CH (Ar)), 8.17-8.18 (m, 3H, 3CH (Ar)), 8.38 (d, J = 7.4 Hz, 1H, CONH), 8.82 (d, J = 8.2 Hz, 1H, CONH) 9.15-9.19 (m, 2H, Ar-OH and NHBn), 10.33 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 36.2, 40.7, 42.7, 52.3, 55.3, 110.5, 113.7, 114.9, 118.3, 118.8, 119.0, 120.2, 120.4, 126.8, 127.3, 127.7, 128.2, 128.2, 128.3, 130.1, 132.4, 133.0, 135.9, 138.0, 138.9, 139.5, 139.9, 144.0, 155.7, 165.1, 165.7, 171.4, 171.5; LRMS (MS ES), calcd for C44H40N6O5Ns [M+Na] m/z = 755.31, fnd. 755.28.
methyl 3′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-hydroxyphenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxylate (12bf)
Compound 11b (142mg, 0.2mmol) was coupled to 4-methoxycarbonylphenylboronic-acid according to general procedure G. Crude material was purified by flash chromatography (98:2 CH2Cl2:MeOH) and yielded final product 12bf as a white solid (93mg, 0.12mmol, 61 %): δH (400 MHz, DMSO-d6) δH (400 MHz, DMSO-d6) 0.92 (dd, J = 15.5 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.59.1.69 (br m, 3H, CH2CH (Leu)), 2.84-2.90 (m, 1H, CH2 (Tyr)), 3.05-3.08 (m, 1H, CHCH2), 3.89 (s, 3H, COOCH3), 4.48-4.53 (m, 3H, CH (Leu) and NHCH2), 4.68-4.73 (m, 1H, CH (Tyr)), 6.62 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.16 (d, J = 8.4 Hz, 2H, Ar-OH (Tyr)), 7.22-7.27 (m, 1H, C6H5), 7.31-7.35 (m, 4H, 4 CH (Ar)), 7.67 (t, J = 7.8 Hz, 1H, Ar-COOMe), 7.94-7.95 (m, 5H, Ar-CN and Ar-COOMe), 7.99-8.02 (m, 2H, 2 CH (Ar-COOMe)), 8.17-8.25 (m, 3H, 3 CH (Ar)), 8.37 (d, J = 7.6 Hz, 1H, CONH), 8.81 (d, J = 8.3 Hz, 1H, CONH) 9.15 (s, 1H, Ar-OH (Tyr)), 9.24 (t, J = 6.0 Hz, 1H, NHBn), 10.33 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.4, 36.2, 40.7, 42.7, 52.3, 52.3, 55.3, 113.7, 114.9, 118.3, 119.9, 120.1, 126.8, 127.1, 127.2, 128.3, 128.6, 128.7, 129.7, 130.1, 130.4, 131.6, 131.7, 132.4, 135.9, 138.1, 139.6, 139.6, 139.8, 140.0, 155.73, 165.0, 165.9, 166.1, 171.4, 171.4; LRMS (MS ES), calcd for C45H43N5O7Na [M+Na] m/z = 788.32, fnd. 788.15.
4-((S)-3-(((S)-1-((6-(benzylcarbamoyl)-4′-carbamoyl-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13aa)
Phenol 12aa (50mg, 0.067mmol) was treated according to general procedure H, and purified by flash column chromatography (1:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13aa as a white powder (25mg, 0.028mmol, 42 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.2 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.52-1.70 (m, 3H, CH2CH (Leu)), 2.53-2.55 (dd, J = 10.1 and 1.5 Hz, 12H, N(Me3)2), 2.88-2.97 (m, 1H, CH2 (Tyr)), 3.11-3.15 (m, 1H, CH2 (Tyr)), 4.27 (d, J = 5.9 Hz, 2H, NHCH2Ph), 4.46-4.51 (m, 1H, CH (Leu)), 4.73-4.79 (m, 1H, CH (Tyr)), 7.00-7.03 (m, 4H, 4 CH (Ar)), 7.19-7.25 (m, 3H, CH (Ar)), 7.31-7.33 (m, 2H, CH (Ar)), 7.37-7.41 (m, 3H, CH (Ar)), 7.45-7.47 (m, 1H, CH (Ar)), 7.68-7.70 (m, 2H, 2 CH (Ar)), 7.86-7.95 (m, 6H, 6 CH (Ar)), 8.03 (s, 1H, CH (Ar)), 8.41 (d, J = 7.5 Hz, 1H, CONH), 8.62 (t, J = 6.0 Hz, 1H, NHBn), 8.85 (d, J = 8.4 Hz, 1H, CONH), 10.26 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.4, 36.2, 36.2, 36.2, 40.7, 42.4, 52.3, 54.9, 113.7, 117.8, 118.3, 119.7, 119.7, 120.2, 126.6, 127.1, 127.4, 128.1, 128.1, 128.2, 128.6, 130.2, 132.0, 132.4, 133.0, 133.9, 138.0, 139.2, 139.4, 139.8, 143.2, 149.5, 149.6, 155.7, 165.0, 167.5, 168.6, 171.1, 171.4; LRMS (MS ES), calcd. For C48H53N8O7PNa [M+Na] m/z = 907.37, fnd. 907.48.
4-((S)-3-(((S)-1-((6-(benzylcarbamoyl)-4′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13ab)
Phenol 12ab (50mg, 0.068mmol) was treated according to general procedure H, and purified by flash column chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13ab as a white powder (37mg, 0.042mmol, 62%): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.6 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.53-1.70 (m, 3H, CH2CH (Leu)), 2.52-2.55 (dd, J = 10.1 and 1.5 Hz, 12H, N(CH3)2), 2.90-2.96 (m, 1H, CH2 (Tyr)), 3.11-3.15 (m, 1H, CH2 (Tyr)), 4.28 (d, J = 6.0 Hz, 2H, CH2Ph), 4.45-4.51 (m, 1H, CH (Leu)), 4.73-4.79 (m, 1H, CH (Tyr)), 7.00 (d, J = 8.3 Hz, 2H, 2 CH (Ar)), 7.06-7.08 (m, 2H, 2 CH (Ar)), 7.23-7.32 (m, 5H, 5 CH (Ar)), 7.45 (m, 2H, 2 CH (Ar)), 7.49-7.51 (m, 1H, CH (Ar)), 7.69-7.71 (m, 2H, 3 CH (Ar)), 7.76-7.78 (m, 2H, CH (Ar)), 7.90-7.95 (m, 4H, 4 CH (Ar)), 8.42 (d, J = 7.7 Hz, 1H, CONH), 8.67 (t, J = 6.0 Hz, 1H, NHBn), 8.85 (d, J = 8.4 Hz, 1H, CONH), 10.30 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 22.9, 24.3, 36.2, 36.2, 36.2, 40.6, 42.4, 52.3, 54.9, 110.0, 113.7, 118.3, 118.8, 119.7, 119.7, 120.1, 126.7, 127.3, 128.1, 128.2, 128.8, 129.2, 130.2, 131.8, 132.0, 132.3, 133.9, 138.0, 138.6, 139.0, 139.4, 145.2, 149.5, 149.6, 165.0, 168.1, 171.1, 171.4; LRMS (MS ES), calcd for C48H51N8O6PNa [M+Na] m/z = 889.37, fnd. 889.28.
methyl 2′-(benzylcarbamoyl)-5′-((S)-2-((S)-3-(4-((bis(dimethylamino)phosphoryl)oxy)phenyl)-2-(4-cyanobenzamido)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-4-carboxylate (13ac)
Phenol 12ac (45mg, 0.059mmol) was treated according to general procedure H, and purified by flash column chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13ac as a white powder (41mg, 0.046mmol, 78 %): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.5 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.55-1.70 (m, 3H, CH2CH (Leu)), 2.52-2.55 (dd, J = 10.1 and 1.6 Hz, 12H, N(CH3)2), 2.90-2.96 (m, 1H, CH2 (Tyr)), 3.11-3.15 (m, 1H, CH2 (Tyr)), 3.89 (s, 3H, COOCH3), 4.26 (d, J = 6.0 Hz, 2H, NHCH2Ph), 4.45-4.51(m, 1H, CH (Leu)), 4.73-4.79 (m, 1H, CH (Tyr)), 7.00 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.04-7.06 (m, 2H, 2 CH (Ar)), 7.21-7.24 (m, 3H, 3 CH (Ar)), 7.31 (d, J = 8.4 Hz, 2H, CH (Ar)), 7.41-7.43 (m, 2H, 2 CH (Ar)), 7.47-7.49 (m, 1H, CH (Ar)), 7.68-7.70 (m, 2H, 2 CH (Ar)), 7.88-7.94 (m, 6H, 6 CH (Ar)), 8.39 (d, J = 7.7 Hz, 1H, CONH), 8.64 (t, J = 6.0 Hz, 1H, NHBn), 8.83 (d, J = 8.4 Hz, 1H, CONH), 10.28 (s, 1H, NHAr)); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.4, 36.2, 36.3, 40.7, 42.5, 52.2, 52.3, 54.9, 113.7, 118.1, 118.3, 119.7, 119.7, 120.1, 126.7, 127.3, 128.1, 128.2, 128.4, 128.7, 128.8, 129.1, 130.3, 132.0, 133.9, 138.0, 139.1, 139.1, 139.9, 145.1, 149.5, 149.6, 165.1, 166.1, 168.4, 171.2, 171.4; LRMS (MS ES), calcd for C49H54N7O8PNa [M+Na] m/z = 922.38, fnd. 922.41.
4-((S)-3-(((S)-1-((6-(benzylcarbamoyl)-3′-carbamoyl-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13ad)
Phenol 12ad (45mg, 0.061mmol) was treated according to general procedure H, and purified by flash column chromatography (3:2 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13ad as a white powder (24mg, 0.028mmol, 46 %): δH (400 MHz, DMSO-d6) δH (400 MHz, DMSO-d6) 0.92 (dd, J = 16.5 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.57-1.69 (m, 3H, CH2CH (Leu)), 2.52-2.55 (dd, J = 10.1 and 1.5 Hz, 12H, N(CH3)2), 2.91-2.97 (m, 1H, CH2 (Tyr)), 3.11-3.16 (m, 1H, CH2 (Tyr)), 4.28 (d, J = 6.0 Hz, 2H, CH2Ph) 4.45-4.51 (m, 1H, CH (Leu)), 4.73-4.79 (m, 1H, CH (Tyr)), 7.00 (d, J = 6.8 Hz, 2H, 2 CH (Ar)), 7.05 (d, J = 7.0 Hz, 2H, 2 CH (Ar)), 7.18-7.27 (m, 3H, 3 CH (Ar)), 7.31-7.33 (m, 2H, 2 CH (Ar)), 7.36-7.48 (m, 4H, 4 CH (Ar)), 7.67-7.72 (m, 2H, 2 CH (Ar)), 7.86-7.94 (m, 6H, 6 CH (Ar)), 8.04 (s, 1H, CH (Ar)), 8.42 (d, J = 7.8 Hz, 1H, CONH), 8.61 (t, J = 5.8 Hz, 1H, NHBn), 8.79 (d, J = 8.3 Hz, 1H, CONH), 10.26 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.3, 36.2, 36.2, 36.2, 40.7, 42.4, 52.3, 54.9, 113.7, 117.6, 118.3, 119.7, 119.7, 120.4, 126.2, 126.6, 127.1, 127.7, 127.9, 128.2, 128.2, 128.7, 130.2, 131.1, 131.9, 132.4, 133.9, 134.3, 138.0, 139.2, 139.7, 139.8, 140.5, 149.5, 149.6, 165.0, 167.6, 168.6, 171.1, 171.3; LRMS (MS ES), calcd for C48H53N8O7PNa [M+Na] m/z = 907.38, fnd. 907.23.
4-((S)-3-(((S)-1-((6-(benzylcarbamoyl)-3′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13ae)
Phenol 12ae (35mg, 0.048mmol) was treated according to general procedure H, and purified by flash column chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13ae as a white powder (33mg, 0.038mmol, 80 %): δH (400 MHz, DMSO-d6): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.6 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.55-1.70 (m, 3H, CH2CH (Leu)), 2.52-2.55 (dd, J = 10.1 and 1.5 Hz, 12H, N(CH3)2), 2.90-2.96 (m, 1H, CH2 (Tyr)), 3.11-3.15 (m, 1H, CH2 (Tyr)), 4.28 (d, J = 5.9 Hz, 2H, NHCH2Ph), 4.45-4.51 (m, 1H, CH (Tyr)), 4.73-4.79 (m, 1H, CH (Tyr)), 7.00 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.06-7.07 (m, 2H, 2 CH (Ar)), 7.20-7.33 (m, 5H, 5 CH (Ar)), 7.50-7.57 (m, 2H, 2 CH (Ar)), 7.60-7.62 (m, 1H, CH (Ar)), 7.68-7.72 (m, 3H, 3 CH (Ar)), 7.81-7.83 (m, 1H, 1 CH (Ar)), 7.90-7.95 (m, 4H, 4 CH (Ar)), 8.42 (d, J = 7.7 Hz, 1H, CONH), 8.70 (t, J = 6.0 Hz, 1H, NHBn), 8.85 (d, J = 8.2 Hz, 1H, CONH), 10.29 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 22.9, 24.3, 36.2, 36.2, 36.2, 40.6, 42.4, 52.3, 54.9, 111.2, 113.7, 118.2, 118.3, 118.7, 119.7, 119.7, 120.3, 126.7, 127.0, 128.2, 128.8, 129.4, 130.2, 131.0, 131.6, 131.7, 132.3, 133.2, 133.9, 137.9, 138.2, 139.1, 139.9, 141.6, 149.5, 149.6, 165.0, 168.1, 171.1, 171.3; LRMS (MS ES), calcd for C48H51N8O6PNa [M+Na] m/z = 889.37, fnd. 889.22.
methyl 2′-(benzylcarbamoyl)-5′-((S)-2-((S)-3-(4-((bis(dimethylamino)phosphoryl)oxy)phenyl)-2-(4-cyanobenzamido)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxylate (13af)
Phenol 12af (60mg, 0.078mmol) was treated according to general procedure H, and purified by flash column chromatography (2:1 CH2Cl2:[92:7:1 CH2Cl2:MeOH:NH4OH]) to yield final product 13af as a white powder (45mg, 0.050mmol, 64 %): δH (400 MHz, DMSO-d6): δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.5 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.55-1.70 (m, 3H, CH2CH (Leu)), 2.52-2.55 (dd, J = 10.1 and 1.5 Hz, 12H, N(CH3)2), 2.89-2.97 (m, 1H, CH2 (Tyr)), 3.11-3.16 (m, 1H, CH2 (Tyr)), 3.85 (s, 3H, COOCH3), 4.26 (d, J = 6.0 Hz, 2H, CH2Ph), 4.45-4.51 (m, 1H, CH (Leu)), 4.73-4.79 (m, 1H, CH (Tyr)), 6.99-7.04 (m, 4H, 4 CH (Ar)), 7.20-7.22 (m, 3H, 3 CH (Ar)), 7.32 (d, J = 8.4 Hz, 1H, CH (Ar)), 7.47-7.51 (m, 2H, 2 CH (Ar)), 7.56-7.78 (m, 1H, CH (Ar)), 7.79-7.72 (m, 2H, 2 CH (Ar)), 7.89-7.95 (m, 6H, 6 CH (Ar)), 8.43 (d, J = 7.5 Hz, 1H, CONH), 8.65 (t, J = 6.0 Hz, 1H, NHBn), 8.86 (d, J = 8.3 Hz, 1H, CONH), 10.29 (s, 1H, NHAr)); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.4, 36.2, 36.3, 36.3, 40.7, 42.5, 52.3, 52.4, 54.9, 113.7, 117.9, 118.3, 119.7, 119.8, 120.3, 126.6, 127.1, 128.0, 128.1, 128.2, 128.7, 128.8, 129.0, 129.6, 130.3, 131.9, 132.4, 133.2, 133.9, 138.0, 139.1, 139.1, 139.9, 140.8, 149.5, 149.6, 165.1, 166.1, 168.5, 171.2, 171.4; LRMS (MS ES), calcd for C49H54N7O8PNa [M+Na] m/z = 922.38, fnd. 922.09.
4-((S)-3-(((S)-1-((5-(benzylcarbamoyl)-4′-carbamoyl-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13ba)
Phenol 12ba (45mg, 0.061mmol) was treated according to general procedure H, and purified by flash column chromatography (1:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13ba as a white solid (34mg, 0.039mmol, 63%): δH (400 MHz, DMSO-d6) 0.90-0.96 (m, 6H, 2 CH3 (Leu)), 1.58-1.73 (br m, 3H, CH2CH (Leu)), 2.52-2.55 (m, 12H, N(CH3)2), 2.92-2.98 (m, 1H, CH2 (Tyr)), 3.13-3.17 (m, 1H, CH2 (Tyr)), 4.48-4.53 (m, 3H, Leu-CH and NHCH2), 4.75-4.81 (m, 1H, CH (Tyr)), 7.01 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.23-7.27 (m, 1H, CH (Ar)), 7.32-7.35 (m, 6H, 6 CH (Ar)), 7.43 (s, 1H, CH (Ar)), 7.77 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.93-7.95 (m, 5H, 5 CH (Ar)), 8.00 (d, J = 8.0 Hz, 2H, Ar-COONH2), 8.05 (s, 1H, CH (Ar)), 8.17 (m, 2H, CH (Ar)), 8.41 (d, J = 7.4 Hz, 1H, CONH), 8.85 (d, J = 8.2 Hz, 1H, CONH), 9.20 (t, J = 6.2 Hz, 1H, NHBn), 10.32 (s, 1H, NHAr); δC (100 MHz, MeOD-d4) 22.1, 23.6, 26.0, 36.8, 36.9, 37.9, 41.8, 44.7, 54.3, 56.7, 116.2, 119.0, 119.89, 121.3, 121.4, 122.7, 128.2, 128.3, 128.7, 129.4, 129.5, 129.9, 131.7, 132.4, 133.5, 133.7, 134.4, 135.0, 137.3, 139.3, 140.2, 140.7, 142.3, 144.6, 151.2, 151.3, 168.5, 169.4, 169.6, 171.8, 173.4, 173.7; LRMS (MS ES), calcd for C48H53N8O7PNa [M+Na] m/z = 907.38, fnd. 907.10.
4-((S)-3-(((S)-1-((5-(benzylcarbamoyl)-4′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13bb)
Phenol 12bb (45mg, 0.061mmol) was treated according to general procedure H, and purified by flash column chromatography (1:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13bb as a white solid (42mg, 0.047mmol, 77 %): δH (400 MHz, DMSO-d6) 0.92 (dd, J = 16 and 6.4 Hz , 6H, 2 CH3 (Leu)), 1.55-1.74 (br m, 3H, CH2CH (Leu)), 2.52-2.55 (m, 12H, (N(CH3)2), 2.91-2.97 (m, 1H, CH2 (Tyr)), 3.12-3.17 (m, 1H, CH2 (Tyr)), 4.47-4.53 (m, 3H, Leu-CH and NHCH2), 4.74-4.80 (m, 1H, CH (Tyr)), 7.00 (d, J = 8.0 Hz, 2H, 2 CH (Ar)), 7.23-7.27 (m, 1H, CH (Ar)), 7.31-7.34 (m, 6H, 6 CH (Ar)), 7.89-8.00 (m, 9H, 9 CH (Ar)), 8.18 (s, 1H, CH (Ar)), 8.21 (s, 1H, CH (Ar)), 8.41 (d, J = 7.4 Hz, 1H, CONH), 8.86 (d, J = 8.2 Hz, 1H, CONH), 9.21 (t, J = 5.7 Hz, 1H, NHBn), 10.35 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 22.9, 24.3, 36.2, 36.2, 36.2, 40.6, 42.4, 52.3, 54.9, 110.0, 113.7, 118.3, 118.8, 119.7, 119.7, 120.1, 126.7, 127.3, 128.1, 128.2, 128.8, 129.2, 130.2, 131.8, 132.0, 132.3, 133.9, 138.0, 138.6, 139.0, 139.9, 145.2, 149.5, 149.6, 165.0, 168.1, 171.1, 171.4; LRMS (MS ES), calcd for C48H51N8O6PNa [M+Na] m/z = 889.37, fnd. 889.22.
methyl 3′-(benzylcarbamoyl)-5′-((S)-2-((S)-3-(4-((bis(dimethylamino)phosphoryl)oxy)phenyl)-2-(4-cyanobenzamido)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-4-carboxylate (13bc)
Phenol 12bc (60mg, 0.078mmol) was treated according to general procedure H, and purified by flash column chromatography (2:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13bc as a white solid (48mg, 0.053mmol, 68 %): δH (400 MHz, DMSO-d6) 0.92 (dd, J = 16.0 and 6.0 Hz, 6H, 2 CH3 (Leu)), 1.58-1.73 (br m, 3H, CH2CH (Leu)), 2.52-2.55 (m, 12H, N(CH3)2), 2.89-2.97 (m, 1H, CH2 (Tyr)), 3.11-3.17 (m, 1H, CH2 (Tyr)), 3.88 (s, 3H, COOCH3), 4.48-4.53 (m, 3H, Leu-CH and NHCH2C6H5), 4.73-4.81 (m, 1H, CH (Tyr)), 7.01 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.22-7.28 (m, 1H, CH (Ar)), 7.32-7.34 (m, 6H, 6 CH (Ar)), 7.86 (d, J = 8.4 Hz, 2H, 2 CH (Ar-COOMe)), 7.93 (s, 4H, 4 CH (Ar-CN)), 7.97 (s, 1H, CH, (Ar)), 8.09 (d, J = 8.4 Hz, 2H, 2 CH (Ar-COOMe)), 8.17 (s, 1H, CH (Ar)), 8.22 (s, 1H, CH (Ar)), 8.42 (d, J = 8.0 Hz, 1H, CONH), 8.86 (d, J = 8.4 Hz, 1H, CONH), 9.22 (t, J = 6.5 Hz, 1H, NHBn), 10.35 (s, 1H, NHAr); δC (100 MHz, CCl3D) 22.3, 22.7, 24.8, 36.5, 36.5, 36.8, 40.9, 44.0, 52.1, 53.1, 55.6, 114.9, 117.9, 118.4, 120.1, 120.1, 121.3, 121.5, 127.0, 127.4, 127.6, 128.0, 128.6, 129.3, 130.0, 130.3, 132.0, 132.5, 136.0, 137.3, 138.3, 139.1, 140.9, 144.1, 150.0, 150.1, 166.1, 166.7, 167.6, 170.8, 171.8; LRMS (MS ES), calcd for C49H54N7O8PNa [M+Na] m/z = 922.38, fnd. 922.15.
(4-((S)-3-((S)-1-((5-(benzylcarbamoyl)-3′-carbamoyl-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13bd)
Phenol 12bd (70mg, 0.096mmol) was treated according to general procedure H, and purified by flash column chromatography (1:1 CH2Cl2:(92:7:1 CH2Cl2:MeOH:NH4OH)) to yield final product 13bd as a white solid (41mg, 0.047mmol, 49%): δH (400 MHz, DMSO-d6) 0.90-0.96(m , 6H, 2 CH3 (Leu)), 1.56-1.71 (br m, 3H, CH2CH (Leu)), 2.52-2.55 (m, 12H, N(CH3)2), 2.92-2.98 (m, 1H, tyr-CHCH2), 3.14-3.16 (m, 1H, tyr-CHCH2), 4.46-4.53 (m, 3H, Leu-CH and NHCH2), 4.76-4.80 (m, 1H, tyr-CH), 7.01 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.24-7.26 (m, 1H, CH (Ar)), 7.32-7.34 (m, 6H, 6 CH (Ar)), 7.47 (s, 1H, CH (Ar)), 7.58 (t, J = 7.5 Hz, 1H, CH (Ar)), 7.84 (d, J = 8.4 Hz, 2H, Ar-COONH2), 7.90-7.94 (m, 5H, 5 CH (Ar)), 8.11-8.20 (m, 4H, CH (Ar)), 8.39 (d, J = 7.6 Hz, 1H, CONH), 8.84 (d, J = 8.2 Hz, 1H, CONH), 9.20 (t, J = 6.2 Hz, 1H, NHBn), 10.31 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.7, 23.0, 24.3, 30.7, 36.2, 36.2, 36.2, 40.7, 42.7, 55.3, 113.7, 113.9, 118.1, 118.3, 119.7, 120.2, 126.0, 126.8, 127.2, 128.2, 128.2, 128.3, 128.7, 129.0, 129.5, 130.2, 132.3, 133.9, 135.1, 135.8, 138.1 139.6, 139.7, 140.2, 151.2, 151.3, 165.0, 166.0, 167.7, 171.1; LRMS (MS ES), calcd for C48H53N8O7PNa [M+Na] m/z = 907.38, fnd. 907.36.
4-((S)-3-(((S)-1-((5-(benzylcarbamoyl)-3′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl bis(dimethylamino) phosphordiamidate (13be)
Phenol 12be (50mg, 0.068mmol) was treated according to general procedure H, and purified by flash column chromatography (97.5:2.5 CH2Cl2:MeOH) to yield final product 13be as a white solid (45mg, 0.052mmol, 76 %): δH (400 MHz, MeOD-d4) 0.95 (dd, J = 15.7 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.67-1.77 (br m, 3H, CH2CH (Leu)), 2.60-2.62 (dd, J = 15.1 and 1.0 Hz, 12H, N(CH3)2), 3.02-3.08 (m, 1H, CH2 (Tyr)), 3.27-3.28 (m, 1H, CH2 (Tyr)), 4.59-4.63 (m, 3H, CH and CH2 (Leu)), 4.89-4.92 (m, 1H, CH (Tyr)), 6.99 (d, J = 8.4 Hz, 2H, CH (Ar)), 7.22-7.38 (m, 7H, 7 CH (Ar)), 7.61-7.65 (m, 1H, CH (Ar)), 7.72-7.77 (m, 3H, 3 CH (Ar)), 7.82.7.87 (m, 3H, 3 CH (Ar)), 7.94-7.96 (m, 1 H, CH (Ar-COOMe)), 8.01-8.02 (m, 1H, CH (Ar)), 8.05-8.06 (m, 1H, CH (Ar)), 8.11-8.12 (s, 1H, CH (Ar)); δC (100 MHz, MeOD-d4) 22.0, 23.5, 26.0, 36.8, 36.9, 37.9, 41.8, 44.8, 54.3, 56.7, 114.3, 126.2, 119.0, 119.6, 120.2, 121.4, 121.4, 122.5, 128.3, 128.7, 129.4, 129.6, 131.3, 131.7, 131.8, 133.5, 135.0, 137.5, 139.4, 140.1, 140.9, 141.2, 142.7, 151.2, 151.3, 168.5, 169.5, 173.4, 173.7; LRMS (MS ES), calcd for C48H51N8O6PNa [M+Na] m/z = 889.37, fnd. 889.16.
methyl 3′-(benzylcarbamoyl)-5′-((S)-2-((S)-3-(4-((bis(dimethylamino)phosphoryl)oxy)phenyl)-2-(4-cyanobenzamido)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxylate (13bf)
Phenol 12bf (70mg, 0.091mmol) was treated according to general procedure H, and purified by flash column chromatography (92:7:1 CH2Cl2:MeOH:NH4OH) to yield final product 13bf as a white solid (47mg, 0.052mmol, 57 %): δH (400 MHz, DMSO-d6) 0.92 (dd, J = 16.0 and 6.5 Hz, 6H, 2 CH3 (Leu)), 1.59-1.71 (br m, 3H, CH2CH (Leu)), 2.52-2.55 (m, 12H, N(CH3)2), 2.91-2.98 (m, 1H, CH2 (Tyr)), 3.13-3.17 (m, 1H, CH2 (Tyr)), 3.90 (s, 3H, COOCH3), 4.47-4.53 (m, 3H, Leu-CH and NHCH2 (Leu)), 4.74-4.80 (m, 1H, CH (Tyr)), 7.00 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.23-7.27 (m, 1H, CH (Ar)), 7.31-7.34 (m, 6H, 6 CH (Ar)), 7.66-7.70 (m, 1H, CH (Ar-CN)), 7.93-7.94 (m, 5H, 5 CH (Ar)), 7.99-8.02 (m, 2H, CH (Ar)), 8.16-8.17 (m, 1H, CH (Ar)), 8.19-8.20 (m, 1H, CH (Ar)), 8.24-8.25 (m, 1H, CH (Ar)), 8.43 (d, J = 7.5 Hz, 1H, CONH), 8.89 (d, J = 8.4 Hz, 1H, CONH), 9.17 (t, J = 6.0 Hz, 1H, NHBn), 10.35 (s, 1H, NHAr); δC (100 MHz, CCl3D) 22.2, 22.7, 24.7, 36.5, 36.5, 36.8, 40.8, 44.0, 52.2, 53.1, 55.6, 115.0, 117.9, 117.9, 120.2, 120.2, 121.1, 121.4, 127.4, 127.7, 128.1, 128.6, 128.9, 128.9, 130.3, 130.7, 131.6, 132.1, 132.4, 135.9, 137.4, 138.2, 139.1, 140.1, 141.1, 150.1, 150.1, 166.1, 166.8, 167.5, 170.7, 171.7; LRMS (MS ES), calcd for C49H54N7O8PNa [M+Na] m/z = 922.38, fnd. 922.15.
4-((S)-3-((S)-1-(6-(benzylcarbamoyl)-4′-carbamoylbiphenyl-3-ylamino)-4-methyl-1-oxopentan-2-ylamino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14aa)
Phosphoramidate 13aa (20mg, 0.022mmol) was treated according to general procedure H, to yield final product 14aa as a white lyophilized powder (18mg, 0.021mmol, 95 %): m.p. 254-259 °C; δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.1 and 6.2 Hz, 6H, 2 CH3 (Leu)), 1.53-1.71 (m, 3H, CH2CH (Leu)), 2.88-2.95 (m, 1H, CH2 (Tyr)), 3.07-3.11 (m, 1H, CH2 (Tyr)), 4.27 (d, J = 5.9 Hz, 2H, NHCH2Ph), 4.44-4.50 (m, 1H, CH (Leu)), 4.68-4.73 (m, 1H, CH (Tyr)), 7.00-7.03 (m, 4H, Ar and Ar-OH (Tyr)), 7.19-7.24 (m, 5H, 5 CH (Ar)), 7.37-7.39 (m, 3H, CH (Ar)), 7.44-7.46 (m, 1H, CH (Ar)), 7.68-7.69 (m, 2H, 2 CH (Ar)), 7.86-7.92 (m, 6H, 6 CH (Ar)), 8.05 (s, 1H, CH (Ar)), 8.43 (d, J = 7.6 Hz, 1H, CONH), 8.66 (t, J = 5.9 Hz, 1H, NHBn), 8.87 (d, J = 8.1 Hz, 1H, CONH), 10.27 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.4, 34.3, 40.6, 42.4, 52.4, 55.2, 113.7, 117.8, 118.4, 118.9, 119.5, 119.5, 120.2, 126.6, 127.1, 127.4, 128.1, 128.2, 128.2, 128.7, 129.6, 132.0, 132.4, 133.0, 138.0, 139.2, 139.4, 139.8, 143.2, 165.1, 167.6, 168.6, 171.3, 171.4. HRMS (MS- ES), calcd for C44H44N6O9P [M+H] m/z = 831.2901, fnd. 831.2866; rpHPLC tR: condition (I) 17.361 (II) 30.160 minutes, purity 97.2 %and 97.6%.
4-((S)-3-(((S)-1-((6-(benzylcarbamoyl)-4′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14ab)
Phosphoramidate 13ab (25mg, 0.029mmol) was treated according to general procedure H, to yield final product 14ab as a white lyophilized powder (22mg, 0.027mmol, 94 %): m.p. 232-234 °C; δH (400 MHz, DMSO-d6) 0.90 (dd, J = 15.7 and 6.3 Hz, 6H, 2 CH3 (Leu)), 1.54-1.70 (m, 3H, CH2CH (Leu)), 2.89-2.95 (m, 1H, CH2 (Tyr)), 3.07-3.11 (m, 1H, CH2 (Tyr)), 4.26 (d, J = 5.8 Hz, 2H, NHCH2Ph), 4.44-4.49 (m, 1H, CH (Leu)), 4.68-4.76 (m, 1H, CH (Tyr)), 7.00-7.07 (m, 4H, 4 CH (Ar)), 7.23-7.28 (m, 5H, 5 CH (Ar)), 7.44-7.51 (m, 3H, 3 CH (Ar)), 7.69-7.77 (m, 4H, 4 CH (Ar)), 7.91 (m, 4H, 4 CH (Ar-CN)), 8.42 (d, J = 7.5 Hz, 1H, CONH), 8.72 (t, J = 5.8 Hz, 1H, NHBn), 8.87 (d, J = 8.3 Hz, 1H, CONH), 10.31 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 22.9, 24.3, 34.3, 40.6, 42.4, 52.4, 55.2, 110.0, 113.7, 118.3, 118.8, 119.6, 119.6, 120.1, 126.7, 127.3, 128.1, 128.2, 128.8, 129.3, 129.7, 131.8, 132.0, 132.4, 138.0, 138.6, 139.1, 140.0, 145.2, 165.2, 168.2, 171.3, 171.5; HRMS (MS ES), calcd for C44H42N6O8P [M+H] m/z = 813.2796. fnd. 813.2790; rpHPLC tR: condition (I) 19.446 (II) 35.840 minutes, purity 94.5 % and 96.3 %.
methyl 2′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-(phosphonooxy) phenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-4-carboxylate (14ac)
Phosphoramidate 13ac (30mg, 0.033mmol) was treated according to general procedure H, to yield final product 14ac as a white lyophilized powder (26mg, 0.031mmol, 94 %): m.p. 253-259 °C; δH (400 MHz, DMSO-d6) 0.90 (dd, J = 16.5 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.55-1.69 (m, 3H, CH2CH (Leu)), 2.88-2.94 (m, 1H, CH2 (Tyr)), 3.07-3.10(m, 1H, CH2 (Tyr)), 3.88 (s, 3H, COOCH3), 4.25 (d, J = 5.8 Hz, 2H, NHCH2Ph), 4.43-4.49 (m, 1H, CH (Leu)), 4.68-4.73 (m, 1H, CH (Tyr)), 7.00-7.06 (m, 4H, 4 CH (Ar)), 7.20-7.24 (m, 5H, 5 CH (Ar)), 7.41-7.43 (m, 2H, 2 CH (Ar)), 7.46-7.49 (m, 1H, CH (Ar)), 7.68-7.72 (m, 2H, 2 CH (Ar)), 7.87-7.91 (m, 6H, 6 CH (Ar)), 8.43 (d, J = 7.5 Hz, 1H, CONH), 8.69 (t, J = 5.8 Hz, 1H, NHBn), 8.89 (d, J = 8.4 Hz, 1H, CONH), 10.30 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.4, 34.3, 40.6, 42.4, 52.2, 52.4, 55.3, 113.7, 118.1, 118.3, 119.6, 120.1, 126.6, 127.3, 128.1, 128.2, 128.4, 128.7, 129.0, 129.7, 131.9, 132.4, 138.0, 139.0, 139.1, 139.9, 145.2, 165.2, 166.1, 168.4, 171.3, 171.5; HRMS (MS ES), calcd for C45H45N5O10P [M+H] m/z = 846.2898, fnd. 846.2906; rpHPLC tR: condition (I) 19.673 (II) 36.585 minutes, purity 95.6% and 97.5%.
4-((S)-3-(((S)-1-((6-(benzylcarbamoyl)-3′-carbamoyl-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14ad)
Phosphoramidate 13ad (18mg, 0.020mmol) was treated according to general procedure H, to yield final product 14ad as a white lyophilized powder (16mg, 0.019mmol, 95 %): m.p. 196-201 °C; δH (400 MHz, DMSO-d6) 0.91 (dd, J = 16.4 and 6.2 Hz, 6H, 2 CH3 (Leu)), 1.57-1.70 (m, 3H, CH2CH (Leu)), 2.90-2.96 (m, 1H, CH2 (Tyr)), 3.09-3.12 (m, 1H, CH2 (Tyr)), 4.27 (d, J = 5.8 Hz, 2H, NHCH2Ph), 4.47-4.48 (m, 1H, CH (Leu)), 4.69-4.75 (m, 1H, CH (Tyr)), 7.01-7.06 (m, 4H, 4 CH (Ar)), 7.19-7.29 (m, 5H, 5 CH (Ar)), 7.36-7.48 (m, 4H, 4 CH (Ar)), 7.68-7.72 (m, 2H, 2 CH (Ar)), 7.86-7.94 (m, 6H, 6CH (Ar)), 8.07 (s, 1H, CH (Ar)), 8.44 (d, J = 7.4 Hz, 1H, CONH), 8.65 (t, J = 5.8 Hz, 1H, NHBn), 8.90 (d, J = 7.9 Hz, 1H, CONH), 10.28 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.4, 34.3, 40.7, 42.4, 52.4, 55.3, 113.7, 117.7, 118.4, 119.6, 119.6, 120.5, 126.2, 126.6, 127.1, 127.7, 128.0, 128.3, 128.7, 129.8, 131.2, 131.9, 132.4, 132.6, 134.3, 138.0, 139.3, 139.7, 140.5, 151.1, 151.1, 158.2, 158.5, 165.2, 167.8, 168.7, 171.3, 171.4; HRMS (MS ES), calcd for C44H44N6O9P [M+H] m/z = 831.2901, fnd. 831.2870; rpHPLC tR: condition (I) 17.854 (II) 31.489 minutes, purity 95.9 % and 97.4%.
4-((S)-3-(((S)-1-((6-(benzylcarbamoyl)-3′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14ae)
Phosphoramidate 13ae (25mg, 0.029mmol) was treated according to general procedure H, to yield final product 14ae as a white lyophilized powder (22mg, 0.027mmol, 94 %): m.p. 179-183 °C; δH (400 MHz, DMSO-d6) 0.90 (dd, J = 15.7 and 6.3 Hz, 6H, 2 CH3 (Leu)), 1.56-1.67 (m, 3H, CH2CH (Leu)), 2.89-2.95 (m, 1H, CH2 (Tyr)), 3.07-3.10 (m, 1H, CH2 (Tyr)), 4.27 (d, J = 5.9 Hz, 2H, NHCH2Ph), 4.44-4.50 (m, 1H, CH (Leu)), 4.68-4.74 (m, 1H, CH (Tyr)), 7.00-7.07 (m, 4H, 4 CH (Ar)), 7.19-7.28 (m, 5H, 5 CH (Ar)), 7.49-7.56 (m, 2H, 2 CH (Ar)), 7.60-7.62 (m, 1H, CH (Ar)), 7.67-7.72 (m, 3H, 3 CH (Ar)), 7.80-7.82 (m, 1H, CH (Ar)), 7.91 (s, 4H, 4 CH (Ar)), 8.42 (d, J = 7.5 Hz, 1H, CONH), 8.73 (t, J = 5.9 Hz, 1H, NHBn), 8.88 (d, J = 8.0 Hz, 1H, CONH), 10.30 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 22.9, 24.3, 34.3, 40.6, 42.4, 52.4, 55.2, 111.2, 113.7, 118.2, 118.3, 118.7, 119.6, 119.6, 120.3, 126.7, 127.0, 128.2, 128.8, 129.4, 129.6, 131.0, 131.6, 131.7, 132.3, 133.2, 138.0, 138.2, 139.2, 140.0, 141.6, 165.2, 168.2, 171.3, 171.4; HRMS (MS ES), calcd for C44H42N5O8P [M+H] m/z = 813.2796, fnd. 813.2814; rpHPLC tR: condition (I) 19.314 (II) 35.499 minutes, purity 96.4 % and 97.5%.
methyl 2′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-(phosphonooxy) phenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxylate (14af)
Phosphoramidate 13af (35mg, 0.039mmol) was treated according to general procedure H, to yield final product 14af as a white lyophilized powder (31mg, 0.037mmol, 94 %): m.p. 188-195 °C; δH (400 MHz, DMSO-d6) 0.90 (dd, J = 14.2 and 6.0 Hz, 6H, 2 CH3 (Leu)), 1.56-1.68 (m, 3H, CH2CH (Leu)), 2.89-2.95 (m, 1H, CH2 (Tyr)), 3.07-3.11 (m, 1H, CH2 (Tyr)), 3.85 (s, 3H, COOCH3), 4.25 (d, J = 5.5 Hz, 2H, NHCH2Ph), 4.46-4.48 (m, 1H, CH (Leu)), 4.69-4.71 (m, 1H, CH (Tyr)), 7.02-7.04 (m, 4H, 4 CH (Ar)), 7.19-7.25 (m, 5H, 5 CH (Ar)), 7.46-4.49 (m, 2H, 2 CH (Ar)), 7.55-7.57 (m, 1H, CH (Ar)), 7.68-7.72 (m, 2H, 2 CH (Ar)), 7.90-7.94 (m, 6H, 6 CH (Ar)), 8.42 (d, J = 6.6 Hz, 1H, CONH), 8.66 (t, J = 5.5 Hz, 1H, NHBn), 8.89 (d, J = 8.3 Hz, 1H, CONH), 10.29 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.4, 34.3, 40.6, 42.4, 52.2, 52.4, 55.3, 113.7, 117.9, 118.3, 119.6, 119.6, 120.3, 126.6, 127.1, 128.0, 128.1, 128.2, 128.7, 128.8, 129.0, 129.6, 129.7, 131.9, 132.4, 133.2, 138.0, 139.1, 139.1, 139.1, 139.9, 140.8, 158.0, 158.3, 165.2, 166.1, 168.5, 171.4, 171.5; HRMS (MS ES), calcd for C45H45N5O8P [M+H] m/z = 846.2898, fnd. 846.2904; rpHPLC tR: condition (I) 19.806 (II) 36.905 minutes, purity 95.9 % and 97.0%.
4-((S)-3-(((S)-1-((5-(benzylcarbamoyl)-4′-carbamoyl-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14ba)
Phosphoramidate 13ba (25mg, 0.028mmol) was treated according to general procedure H, to yield final product 14ba as a white lyophilized powder (23mg, 0.027mmol, 97 %): m.p. 167-171 °C; δH (400 MHz, DMSO-d6) 0.92 (dd, J = 15.4 and 6.2 Hz, 6H, 2 CH3 (Leu)), 1.65-1.73 (br m, 3H, CH2CH (Leu)), 2.92-2.95 (m, 1H, CH2 (Tyr)), 3.07-3.11 (m, 1H, CH2 (Tyr)), 4.46-4.51 (m, 3H, 3 CH (Leu)), 4.69-4.73 (m, 1H, CH (Tyr)), 7.01 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.21-7.26 (m, 3H, CH (Ar)), 7.31-7.35 (m, 5H, 5 CH (Ar)), 7.40 (s, 1H, CH, (Ar)), 7.77 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.90-7.94 (m, 5H, 5 CH (Ar)), 8.01 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 8.10 (s, 1H, CH (Ar)), 8.16-8.18 (m, 2H, 2 CH (Ar)), 8.41 (d, J = 7.4 Hz, 1H, CONH), 8.88 (d, J = 8.0 Hz, 1H, CONH), 9.20 (m, 1H, NHBn), 10.33 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 34.3, 40.7, 42.7, 52.4, 55.2, 113.6, 118.3, 119.5, 119.9, 120.2, 126.5, 126.8, 127.2, 128.2, 128.3, 128.6, 129.5, 130.4, 131.7, 132.3, 133.5, 138.0, 139.6, 139.8, 142.0, 157.6, 157.9, 165.1, 167.0, 167.4, 171.3, 171.4; HRMS (MS ES), calcd for C44H44N6O9P [M+H] m/z = 831.2901, fnd. 831.2897; rpHPLC tR: condition (I) 19.490 (II) 36.040 minutes, purity 93.1% and 97.8%.
4-((S)-3-(((S)-1-((5-(benzylcarbamoyl)-4′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14bb)
Phosphoramidate 13bb (30mg, 0.035mmol) was treated according to general procedure H, to yield final product 14bb as a white lyophilized powder (27mg, 0.034mmol, 98 %): m.p. 150-157 °C; δH (400 MHz, DMSO-d6) 0.91 (dd, J = 14.7 and 6.2 Hz), 6H, 2 CH3 (Leu)), 1.55-1.72 (br m, 3H, CH2CH (Leu)), 2.89-2.95 (m, 1H, CH2 (Tyr)), 3.07-3.11 (m, 1H, CH2 (Tyr)), 4.46-4.52 (m, 3H, CH (Leu) and CH2 (NHCH2Ph)), 4.68-4.74 (m, 1H, CH (Tyr)), 7.01 (d, J = 8.1 Hz, 2H, 2 CH (Ar)), 7.22-7.24 (m, 3H, 3 CH (Ar)), 7.31-7.34 (m, 4H, 4 CH (Ar)), 7.88-7.98 (m, 9H, 9 CH (Ar)), 8.20-8.21 (m, 2H, CH (Ar)), 8.45 (d, J = 7.8 Hz, 1H, CONH), 8.91 (d, J = 8.2 Hz, 1H, CONH), 9.25 (t, J = 5.8 Hz, 1H, NHBn), 10.43 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 34.2, 40.7, 42.7, 52.4, 55.4, 110.5, 113.6, 118.3, 118.8, 119.0, 119.5, 120.2, 120.4, 126.8, 127.3, 127.7, 128.2, 128.3, 129.5, 131.4, 132.3, 133.0, 135.9, 138.0, 138.8, 139.5, 139.9, 144.0, 157.9, 158.2, 165.1, 165.7, 171.4, 171.5; HRMS (MS ES), calcd for C44H42N6O8P [M+H] m/z = 813.2796, fnd. 813.2774; rpHPLC tR: condition (I) 20.975 (II) 40.195 minutes, purity 73.8% and 90.3%.
methyl 3′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-(phosphonooxy) phenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-4-carboxylate (14bc)
Phosphoramidate 13bc (35mg, 0.039mmol) was treated according to general procedure H, to yield final product 14bc as a white lyophilized powder (31mg, 0,037mmol, 95 %): m.p. 138-145 °C; δH (400 MHz, DMSO-d6) 0.92 (dd, J = 16.2 and 6.2 Hz, 6H, 2 CH3 (Leu)), 1.57-1.73 (m, 3H, CH2CH (Leu)), 2.89-2.95 (m, 1H, CH2 (Tyr)), 3.07-3.12 (m, 1H, CH2 (Tyr)), 3.89 (s, 3H, COOCH3), 4.49-4.52 (m, 3H, CH (Leu) and CH2 (NHCH2Ph)), 4.69-4.75 (m, 1H, CH (Tyr)), 7.01-7.03 (m, 2H, 2 CH (Ar)), 7.22-7.24 (m, 3H, 3 CH (Ar)), 7.33-7.34 (m, 4H, 4 CH (Ar)), 7.65-7.69 (m, 1H, CH (Ar)), 7.89-7.94 (m, 5H, 5 CH (Ar)), 7.99-8.00 (m, 2H, 2 CH (Ar)), 8.16 (s, 1H, CH (Ar)), 8.20 (s, 1H, CH (Ar)), 8.24 (s, 1H, CH (Ar)), 8.42 (d, J = 7.3 Hz, 1H, CONH), 8.88 (d, J = 8.7 Hz, 1H, CONH), 9.25 (t, J = 6.0 Hz, 1H, NHBn), 10.38 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.4, 34.4, 40.7, 42.7, 52.3, 52.3, 55.3, 113.6, 118.3, 119.5, 119.5, 119.9, 120.1, 126.8, 127.1, 127.2, 128.2, 128.3, 129.5, 129.6, 130.4, 131.6, 132.3, 135.9, 138.0, 139.6, 139.8, 140.0 157.7, 158.0, 165.1, 165.9, 166.1, 171.4, 171.4; HRMS (MS ES), calcd. For C45H45N5O10P [M+H] m/z = 846.2898, fnd. 846.290; rpHPLC tR: condition (I) 21.573 (II) 41.866 minutes, purity 96.5% and 96.6%.
4-((S)-3-(((S)-1-((5-(benzylcarbamoyl)-3′-carbamoyl-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14bd)
Phosphoramidate 13bd (30mg, 0.034mmol) was treated according to general procedure H, to yield final product 14bd as a white lyophilized powder (27mg, 0.034mmol, 96 %): m.p. 152-157 °C; δH (400 MHz, DMSO-d6) 0.90-0.95 (m, 6H, 2 CH3 (Leu)), 1.57-1.73 (br m, 3H, CH2CH (Leu)), 2.88-2.94 (m, 1H, CHCH2 (Tyr)), 3.07-3.11 (m, 1H, CHCH2 (Tyr)), 4.47-4.52 (m, 3H, CH (Leu) and CH2 (NHCH2Ph)), 4.69-4.74 (m, 1H, CH (Tyr)), 7.01 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.20-7.26 (m, 3H, 3 CH (Ar)), 7.31-7.35 (m, 4H, 4 CH (Ar)), 7.47 (s, 1H, CH, (Ar)), 7.56-7.60 (m, 1H, CH (Ar)), 7.83-7.85 (m, 1H, CH (Ar)), 7.89-7.95 (m, 6H, 6 CH (Ar)), 8.14-8.20 (m, 4H, 4 CH (Ar)), 8.40 (d, J = 7.4 Hz, 1H, CONH), 8.86 (d, J = 8.2 Hz, 1H, CONH), 9.22-9.25 (m, 1H, NHBn), 10.35 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.4, 34.2, 40.7, 42.7, 52.4, 55.3, 113.7, 118.1, 118.8, 119.5, 119.5, 120.1, 126.0, 126.8, 126.9, 127.3, 128.2, 128.3, 129.0, 129.5, 131.3, 132.3, 132.6, 135.1, 135.8, 138.0, 139.6, 139.7, 140.2, 158.0, 158.3, 165.1, 166.0, 167.8, 171.3, 171.4; HRMS (MS ES), calcd. For C44H44N6O9P [M+H] m/z = 831.2901, fnd. 831.2921; rpHPLC tR: condition (I) 19.665 (II) 36.574 minutes, purity 87.6 % and 88.1%.
4-((S)-3-(((S)-1-((5-(benzylcarbamoyl)-3′-cyano-[1,1′-biphenyl]-3-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-2-(4-cyanobenzamido)-3-oxopropyl)phenyl dihydrogen phosphate (14be)
Phosphoramidate 13be (30mg, 0.035mmol) was treated according to general procedure H, to yield final product 14be as a white lyophilized powder (27mg, 0.034mmol, 97 %): m.p. 125-133 °C; δH (400 MHz, DMSO-d6) 0.92 (dd, J = 14.5 and 6.5 Hz, 6H, 2 CH3 (Leu)), 1.57-1.72 (br m, 3H, CH2CH (Leu)), 2.89-2.95 (m, 1H, CHCH2 (Tyr)), 3.08-3.11 (m, 1H, CHCH2 (Tyr)), 4.46-4.53 (m, 3H, CH (Leu) and CH2 (NHCH2Ph)), 4.68-4.74 (m, 1H, CH (Ar)), 7.01 (d, J = 7.7 Hz, 2H, 2 CH (Ar)), 7.22-7.24 (m, 3H, 3 CH (Ar)), 7.31-7.36 (m, 4H, 4 CH (Ar)), 7.68-7.73 (m, 1H, CH (Ar)), 7.86-7.94 (m, 6H, 6 CH (Ar)), 8.00-8.03 (m, 1H, CH (Ar)), 8.16-8.17 (m, 2H, 2 CH (Ar)), 8.21 (s, 1H, CH (Ar)), 8.46 (d, J = 7.5 Hz, 1H, CONH), 8.82 (d, J = 8.4 Hz, 1H, CONH), 9.22 (t, J = 6.0 Hz, 1H, NHBn), 10.42 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.3, 34.2, 40.7, 42.7, 52.4, 55.4, 112.2, 113.6, 118.3, 118.7, 119.5, 119.5, 120.1, 120.3, 126.8, 127.2, 128.2, 128.3, 129.5, 130.3, 131.3, 131.5, 131.6, 132.3, 135.8, 138.0, 138.6, 139.5, 139.9, 140.7, 157.9, 158.2, 165.1, 165.7, 171.4, 171.5; HRMS (MS ES), calcd for C44H42N6O8P [M+H] m/z = 813.2796, fnd. 813.2777; rpHPLC tR: condition (I) 20.965 (II) 40.147 minutes, purity 93.3% and 95.9%.
methyl 3′-(benzylcarbamoyl)-5′-((S)-2-((S)-2-(4-cyanobenzamido)-3-(4-(phosphonooxy) phenyl)propanamido)-4-methylpentanamido)-[1,1′-biphenyl]-3-carboxylate (14bf)
Phosphoramidate 13bf (30mg, 0.033mmol) was treated according to general procedure H, to yield final product 14bf as a white lyophilized powder (28mg, 0.033mmol, 98 %): m.p. 136-144 °C; δH (400 MHz, DMSO-d6) 0.92 (dd, J = 15.7 and 6.4 Hz, 6H, 2 CH3 (Leu)), 1.59-1.71 (br m, 3H, CH2CH (Leu)), 2.92-2.98 (m, 1H, CHCH2 (Tyr)), 3.11-3.16 (m, 1H, CH2 (Tyr)), 3.88 (s, 3H, COOCH3), 4.47-4.522 (m, 3H, CH (Leu) and CH2 (NHCH2Ph)), 4.72-4.78 (m, 1H, CH (Tyr)), 7.03-7.05 (m, 4H, 4 CH (Ar)), 7.17 (m, 1H, CH (Ar)), 7.30-7.34 (m, 5H, 5 CH (Ar)), 7.85 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 7.92-7.93 (m, 3H, 3 CH (Ar)), 7.97 (s, 1H, CH (Ar)), 8.09 (d, J = 8.4 Hz, 2H, 2 CH (Ar)), 8.17 (s, 1H, CH (Ar)), 8.21 (s, 1H, CH (Ar)), 8.41 (d, J = 8.0 Hz, 1H, CONH), 8.89 (d, J = 8.4 Hz, 1H, CONH), 9.23 (t, J = 6.5 Hz, 1H, NHBn), 10.36 (s, 1H, NHAr); δC (100 MHz, DMSO-d6) 21.6, 23.0, 24.4, 34.3, 40.7, 42.7, 52.2, 52.3, 55.0, 113.7, 118.3, 118.7, 119.6, 119.7, 120.1 120.3, 126.8, 127.1, 127.3, 128.2, 128.3, 128.9, 129.9, 130.0, 132.4, 133.6, 135.9, 138.0, 139.4, 139.5, 139.8, 144.0, 157.9, 158.2, 166.1, 166.8, 167.5, 170.7, 171.7; HRMS (MS ES), calcd for C45H45N5O10P [M+H] m/z = 846.2898, fnd. 846.2911; rpHPLC tR: condition (I) 21.525 (II) 41.764 minutes, purity 98.3% and 98.3 %.
4.3. Biophysical and Biological Assays
4.3.1 Cells and reagents
Normal mouse fibroblasts (NIH3T3) and counterparts transformed by v-Src (NIH3T3/v-Src) or overexpressing the human epidermal growth factor (EGF) receptor (NIH3T3/hEGFR), the murine thymus epithelial stromal cells, and the human breast cancer (MDA-MB-231) and pancreatic cancer (Panc-1) cells have all been previously reported.28,33 Antibodies against STAT3, pY705STAT3, Erk1/2, and pErk1/2 are from Cell Signaling Technology (Danvers, MA).
4.3.2 Cloning and Protein Expression
Coding regions for the murine STAT3 protein and STAT3 SH2 domain were amplified by PCR and cloned into vectors pET-44 Ek/LIC (Novagen) and pET SUMO (Invitrogen), respectively. The primers used for amplification were: STAT3 Forward: GACGACGACAAGATGGCTCAGTGGAACCAGCTGC; STAT3 Reverse: GAGGAGAAGCCCGGTTATCACATGGGGGAGGTAGCACACT; STAT3-SH2 Forward: ATGGGTTTCATCAGCAAGGA; STAT3-SH2 Reverse: TCACCTACAGTACTTTCCAAATGC. Clones were sequenced to verify the correct sequences and orientation. His-tagged recombinant proteins were expressed in BL21(DE3) cells, and purified on Ni-ion sepharose column.
4.3.3 STAT1, STAT3, and STAT5 fluorescence polarization binding assays
A fixed concentration of the fluorescently-labeled peptide probe (10 nM) was incubated with increasing concentration of the STAT1, STAT3, and STAT5 protein for 30 min at room temperature in the buffer, 50 mM NaCl, 10 mM HEPES, 1 mM EDTA, 0.1% Nonidet P-40, and the fluorescent polarization measurements were determined using an M1000 TECAN Infinite (TECAN, NC), with the set gain adjustment at 35 mP. The Z’ value was derived per the equation Z’=1–(3SDbound+3SDfree)/(mPbound–mPfree), where SD is the standard deviation and mP is the average of fluorescence polarization. The bound state for STAT1 was achieved using 10nM 5-carboxyfluorescein-GpYDKPHVL-NH2 incubated with 80nM STAT1 protein; the unbound state used the same mixture except 10 μM of unlabeled probe (Ac-GpYDKPHVL-NH2) was added. In the bound state, 10 nM 5-carboxyfluorescein-GpYLPQTV-NH2 was incubated with 150 nM purified STAT3 protein, while the free (unbound) state was the same mixture, but incubated with an additional 10 μM unlabeled Ac-GpYLPQTV-NH2. The use of 10nM 5-carboxyfluorescein-GpYLVLDKW-NH2 incubated with 105 nM STAT5 protein achieved the STAT5 bound state; the same mixture plus 10 μM of STAT5 unlabeled probes (Ac-GpYLVLDKW-NH2) produced the free state following incubation. For evaluating agents, STAT1 (80nM), STAT3 (150 nM), and STAT5 (105nM) proteins were individually incubated with serial concentrations of inhibitors at 30 °C for 60 min in the indicated assay buffer conditions. Prior to the addition of their respective fluorescent probes, the protein:inhibitor mixtures were allowed to equilibrate at room temperature for 15 min. Probes were added at a final concentration of 10 nM and incubated for 30 min at room temperature following which the FP measurements were taken using the TECAN M1000 polarimeter, with the set gain adjustment at 35 mP.
4.3.4 Nuclear extract preparation and gel shift assays
Nuclear extract preparation from v-Src-transformed fibroblasts (NIH3T3/v-Src) or mouse fibroblasts overexpressing the human epidermal growth factor receptor (NIH3T3/hEGFR) and electrophoretic mobility shift assay (EMSA) were carried out as previously described.8 Nuclear extracts of equal total protein content were pre-incubated with different concentrations of compounds for 30 min at room temperature prior to incubation with the radiolabeled probe. The 32P-labeled oligonucleotide probe used is hSIE (high affinity sis-inducible element from the c-fos gene, m67 variant, 5′-AGCTTCATTTCCCGTAAATCCCTA) that binds STAT1 and STAT3.37
4.3.5 Surface Plasmon Resonance (SPR) binding experiments
SensiQ and its analysis software Qdat (ICX Technologies, Oklahoma City, OK) were used to analyze the interaction between agents and the STAT3 protein and to determine the binding affinity. Purified STAT3 was immobilized on a HisCap Sensor Chip by injecting 50 μg/ml of STAT3 onto the chip. Various concentrations of inhibitors in running buffer (1X PBS, 0.5% DMSO) were passed over the sensor chip to produce response signals. The association and dissociation rate constants were calculated using the Qdat software. The ratio of the association and dissociation rate constants was determined as the affinity (KD).
4.3.6 Permeability and efflux analysis in Caco-2 Models
Human, epithelial Caco-2 cells were seeded at a density of 75,000 cells/cm2, on high-density PET membrane inserts, (1.0 μm pore size, 0.31 cm2 surface area) and utilized on day 25 (post-seeding). At this stage of growth, cell monolayers were fully polarized and differentiated. All compounds were tested at a final concentration of 10 μM, under non-gradient pH conditions (pH 7.4/7.4) for 90 minutes as previously described1. Narrow-window mass extraction LC/MS analysis was performed for all samples from this study using a Waters Xevo quadrupole time-of-flight (QTof) mass spectrometer and an ACQUITY UPLC system, to determine relative peak areas of parent compound. The percent of transported drug was calculated based on these peak areas, relative to the initial, dosing concentration.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the OICR medicinal chemistry group for generously performing pharmacological experiments. For financial support of this work, the authors would like to thank the University of Toronto (PG), NSERC (PG) NIH Grants CA106439 and CA128865 (JT & PG) and the LLSC (PG & JT). Dr. Aaron Schimmer is a Leukemia and Lymphoma Society Scholar in Clinical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Zhong Z, Wen Z, Darnell JE., Jr. Science. 1994;264:95. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 2.Darnell JE, Jr., Kerr IM, Stark GR. Science. 1994;264:1415. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 3.Garcia R, Yu C, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R. Cell Growth and Differentiation. 1997;8:1267. [PubMed] [Google Scholar]
- 4.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Mol. Cell. Biol. 1998;18:2545. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman T, Garcia R, Turkson J, Jove R. Oncogene. 2000;19:2474. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 6.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr. Cell. 1999;98:295. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 7.Siddiquee KAZ, Turkson J. Cell Res. 2008;18:254. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. J. Biol. Chem. 2001;276:45443. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 9.Gunning PT, Katt WP, Glenn M, Siddique K, Kim JS, Jove R, Sebti SM, Turkson J, Hamilton AD. Bioorg. Med. Chem. Lett. 2007;17:1875. doi: 10.1016/j.bmcl.2007.01.077. [DOI] [PubMed] [Google Scholar]
- 10 (a).Fletcher S, Turkson J, Gunning PT. ChemMedChem. 2008;3:1159. doi: 10.1002/cmdc.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fletcher S, Drewry JA, Shahani VM, Gunning PT. Biochem. Cell Biol. 2009;87:825–833. doi: 10.1139/o09-044. [DOI] [PubMed] [Google Scholar]; (c) Avadisian M, Haftchenary S, Gunning PT. Anti-Cancer Drugs. 2010 doi: 10.1097/CAD.0b013e328341185b. DOI:10.1097/CAD.0b013e328341185b. [DOI] [PubMed] [Google Scholar]; (d) Page BDG, Ball D, Gunning PT. Expert Opin. Ther. Patents. 2011;21:1–19. doi: 10.1517/13543776.2011.539205. [DOI] [PubMed] [Google Scholar]
- 11.Coleman DR, IV, Kaluarachchi K, Ren Z, Chen X, McMurray JS. Int. J. Pept. Res. Ther. 2008;14:1. doi: 10.1007/s10989-012-9313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Bai L, Bernard D, Nikolovska-Coleska Z, Gomez C, Zgang J, Yi H, Wang S. ACS Med. Chem. Lett. 2010;1:85. doi: 10.1021/ml100010j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Chem. & Biol. 2006;13:1235. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher S, Singh J, Zhang X, Yue PB, Page BDG, Sharmeen S, Shahani VM, Zhao W, Schimmer AD, Turkson J, Gunning PT. ChemBioChem. 2009;10:1959. doi: 10.1002/cbic.200900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiquee KAZ, Gunning PT, Glenn M, Katt WP, Zhang S, Schroeck C, Sebti SM, Jove R, Hamilton AD, Turkson J. ACS. Chem. Biol. 2007;2:787. doi: 10.1021/cb7001973. [DOI] [PubMed] [Google Scholar]
- 16.Gunning PT, Glenn MP, Siddiquee KAZ, Katt WP, Masson E, Sebti SM, Turkson J, Hamilton AD. ChemBioChem. 2008;9:2800. doi: 10.1002/cbic.200800291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandal PK, Ren Z, Chen X, Xiong C, McMurray JS. J. Med. Chem. 2009;52:6126. doi: 10.1021/jm901105k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turkson J, Kim JS, Zhang SM, Yuan J, Huang M, Glenn M, Haura E, Sebti S, Hamilton AD, Jove R. Mol. Cancer Ther. 2004;3:261. [PubMed] [Google Scholar]
- 19.Coleman DR, IV, Ren Z, Mandal PK, Cameron AG, Dyer GA, Muranjan S, Campbell M, Chen X, McMurray JS. J. Med. Chem. 2005;48:6661. doi: 10.1021/jm050513m. [DOI] [PubMed] [Google Scholar]
- 20.Jones G, Willett P, Glen RC, Leach AC, Taylor R. J. Mol. Biol. 1997;267:727. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 21.Becker S, Groner B, Muller CW. Nature. 1998;394:145. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 22.Schust J, Berg T. Anal. Biochem. 2004;330:114. doi: 10.1016/j.ab.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Pengguang W, Brasseur M, Schindler U. Anal. Biochem. 1997;249:29. doi: 10.1006/abio.1997.2158. [DOI] [PubMed] [Google Scholar]
- 24.Muller J, Schust J, Berg T. Anal. Biochem. 2008;375:249. doi: 10.1016/j.ab.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Turkson J. Expert Opin. on Ther. Targets. 2004;8:409. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- 26.Dourlat J, Liu WQ, Sancier F, Edmonds T, Pamonsinlapatham P, Cruzalegui F, Garbay C. Biochimie. 2009;91:996. doi: 10.1016/j.biochi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Mandal PK, Liao WSL, McMurray JS. Org. Lett. 2009;11:3394. doi: 10.1021/ol9012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Yue P, Fletcher S, Zhao W, Gunning PT, Turkson J. Biochem. Pharm. 2010;79:1398. doi: 10.1016/j.bcp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29 (a).Hidalgo IJ, Raub TJ, Borchardt RT. Gastroenterology. 1989;96:736. [PubMed] [Google Scholar]; (b) Artursson P. J. Pharm. Sci. 1990;79:476. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- 30.Alimirah F, Chen J, Basrawala Z, Xin H, Choubey D. FEBS Lett. 2006;580:2294. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Lieber M, Mazzetta J, Nelson-Rees W, Kaplan M, Todaro G. Int. J. Cancer. 1975;15:741. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- 32 (a).Filmus J, Trent JM, Pollak MN, Buick RN. Mol. Cell. Biol. 1987;7:251. doi: 10.1128/mcb.7.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Garcia R, Bowman TL, Nui G, Yu H, Minton S, Muro-Cacho AA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Oncogene. 2001;20:2499. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]; (c) Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Cancer Res. 2002;22:6659. [PubMed] [Google Scholar]
- 33.Wagner M, Kleeff J, Friess H, Buchler MW, Korc M. Pancreas. 1999;19:370. doi: 10.1097/00006676-199911000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.