Abstract
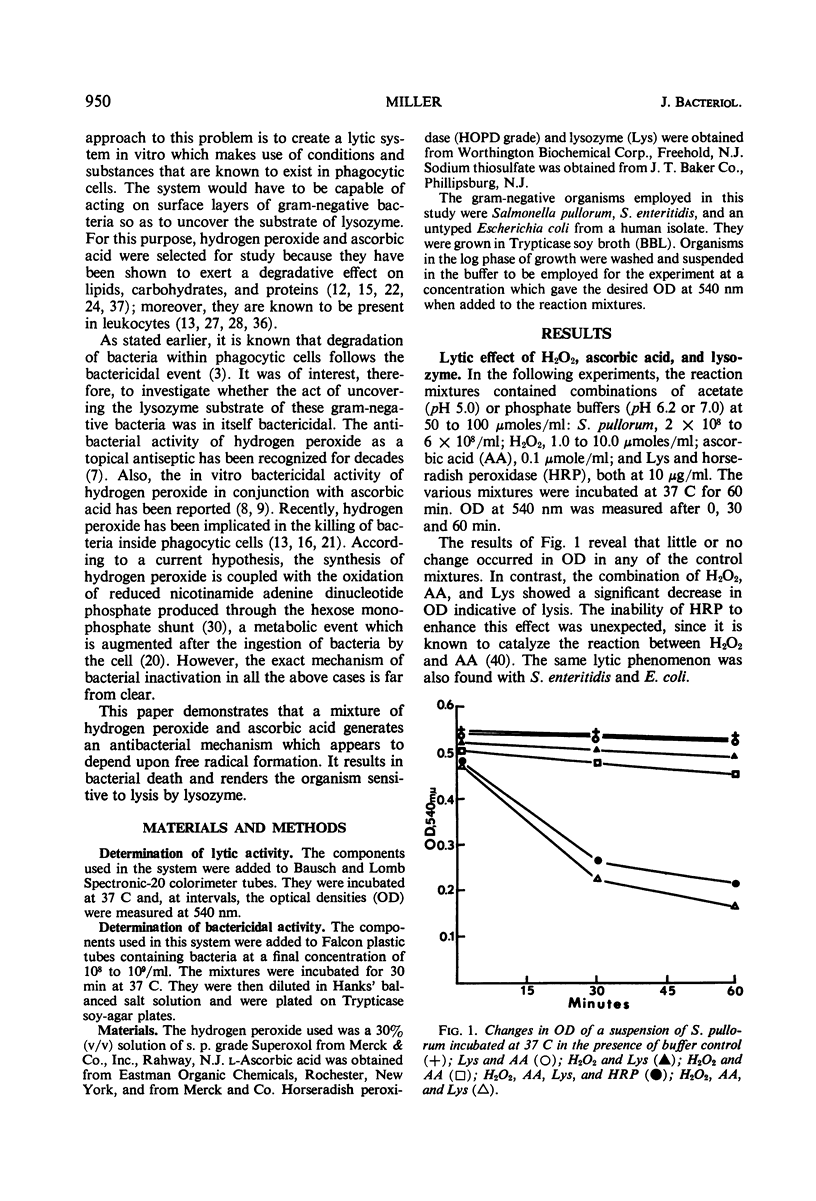
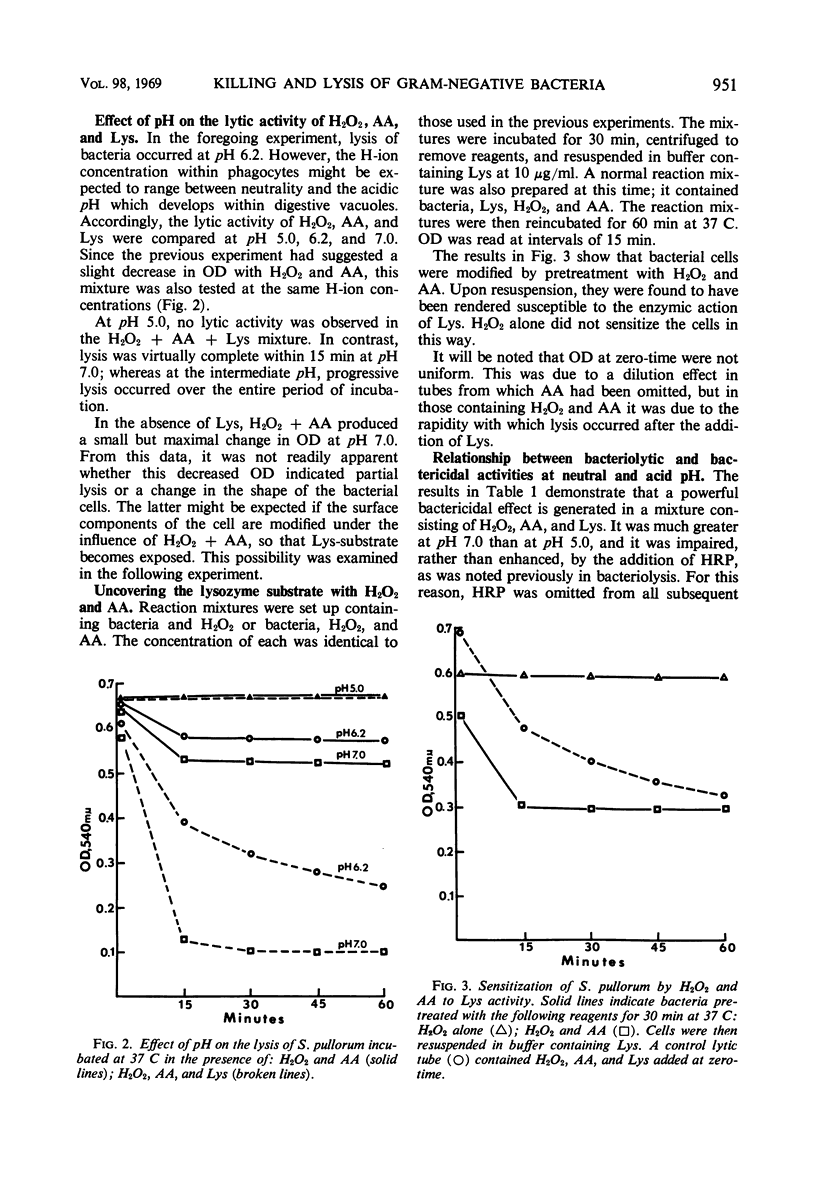
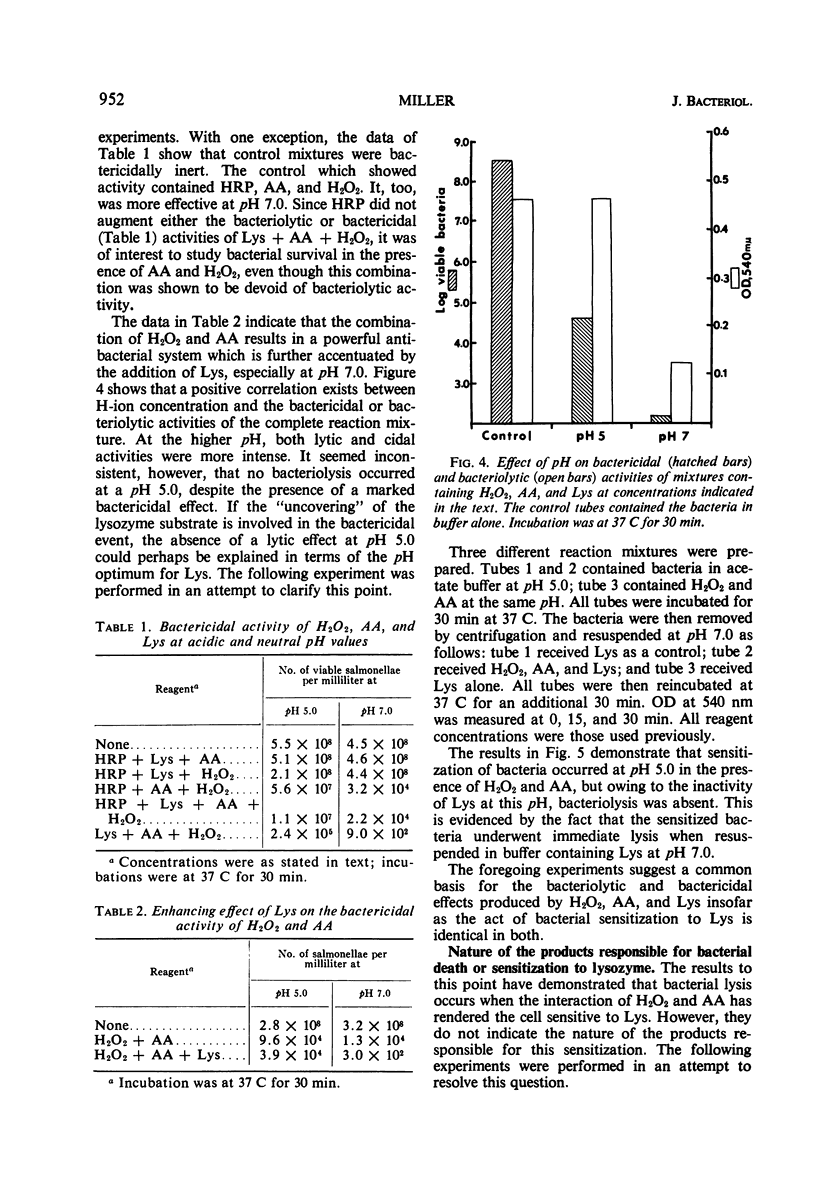
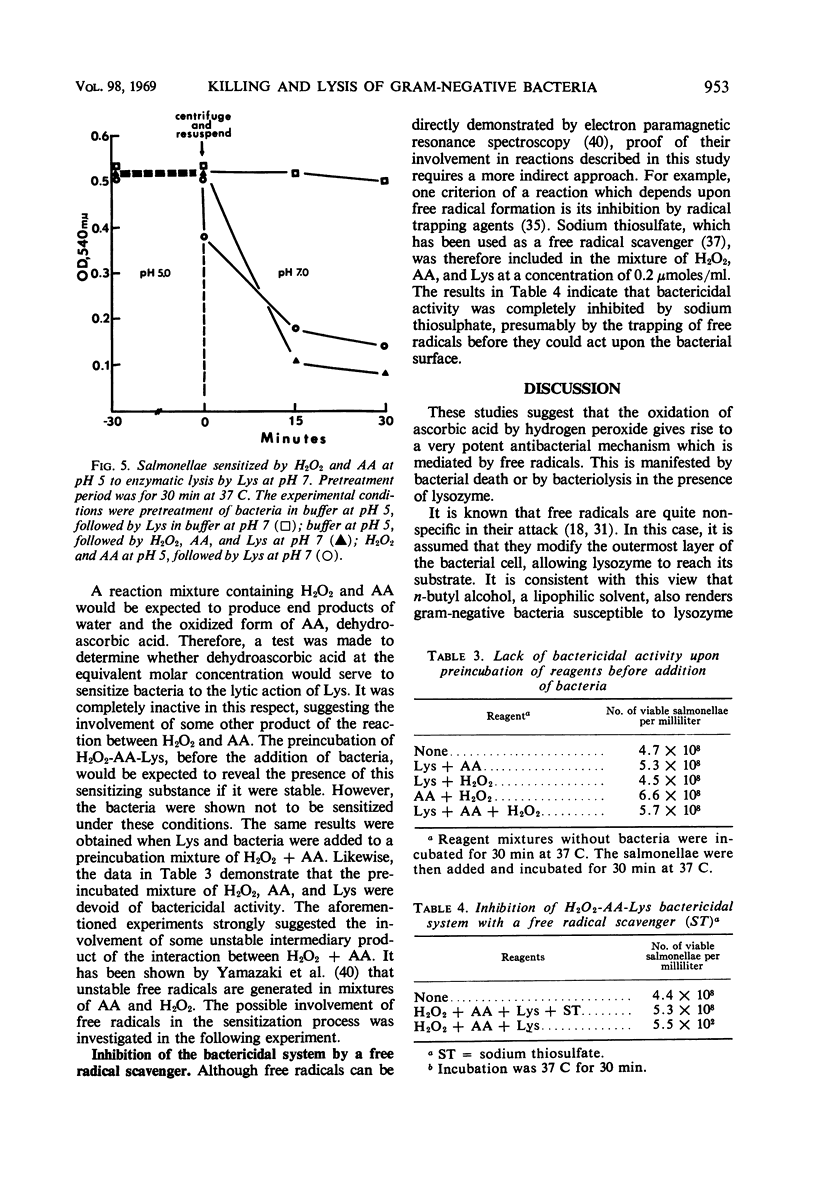
A mixture of hydrogen peroxide and ascorbic acid has been found to generate an antibacterial mechanism which is active against gram-negative bacteria. It results in bacterial death and renders the organism sensitive to lysis by lysozyme. Under the conditions used, horseradish peroxidase did not augment the antibacterial effect. It is suggested that the effector mechanism involves the generation of short-lived free radicals which disturb the integrity of the cell wall. This effect alone might kill bacteria by interfering with selective permeability, but in the presence of lysozyme a further bactericidal activity is accomplished by complete disruption of the cell. It is proposed that a transient antibacterial system such as that described could exist within phagocytic cells. Free radicals would be formed through the interaction of certain oxidizable substances and hydrogen peroxide, which is produced during the enhanced metabolic activity that accompanies ingestion of bacteria. Such a system would help to explain why macrophages, which are apparently devoid of preformed bactericidins, are nonetheless very efficient in killing most phagocytosed bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Benson B. The in vitro differentiation of mononuclear phagocytes. 3. The reversibility of granule and hydrolytic enzyme formation and the turnover of granule constituents. J Exp Med. 1965 Sep 1;122(3):455–466. doi: 10.1084/jem.122.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERICSSON Y., LUNDBECK H. Antimicrobial effect in vitro of the ascorbic acid oxidation. I. Effect on bacteria, fungi and viruses in pure cultures. Acta Pathol Microbiol Scand. 1955;37(6):493–506. doi: 10.1111/j.1699-0463.1955.tb00975.x. [DOI] [PubMed] [Google Scholar]
- ERICSSON Y., LUNDBECK H. Antimicrobial effect in vitro of the ascorbic acid oxidation. II. Influence of various chemical and physical factors. Acta Pathol Microbiol Scand. 1955;37(6):507–527. doi: 10.1111/j.1699-0463.1955.tb00976.x. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, SCOTT A., HOFFSTEN P. E., GUERRA F., WEINSTEIN J., SCHNEIDER A., SCHUTZ B., FINK J., FORD L., SMITH E. STUDIES ON THE MECHANISM OF ASCORBATE-INDUCED SWELLING AND LYSIS OF ISOLATED LIVER MITOCHANDRIA. J Biol Chem. 1964 Feb;239:604–613. [PubMed] [Google Scholar]
- Hirsch J. G. Phagocytosis. Annu Rev Microbiol. 1965;19:339–350. doi: 10.1146/annurev.mi.19.100165.002011. [DOI] [PubMed] [Google Scholar]
- JOLLES P. RECENT DEVELOPMENTS IN THE STUDY OF LYSOZYMES. Angew Chem Int Ed Engl. 1964 Jan;3:28–36. doi: 10.1002/anie.196400281. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEACH S. J. The mechanism of enzymic oxido-reduction. Adv Enzymol Relat Subj Biochem. 1954;15:1–47. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCHEL L. H., CAREY W. F., BARON L. S. Formation of bacterial protoplasts by serum components. J Immunol. 1959 Jan;82(1):38–42. [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XI. Relationship between stimulated oxidative metabolism and hydrogen peroxide formation, and intracellular killing. J Bacteriol. 1967 Nov;94(5):1417–1424. doi: 10.1128/jb.94.5.1417-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel C. E., Kann H. E., Jr, Meriwether W. D. Studies of paroxysmal nocturnal hemoglobinuria erythrocytes: increased lysis and lipid peroxide formation by hydrogen peroxide. J Clin Invest. 1967 Nov;46(11):1715–1723. doi: 10.1172/JCI105662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLLER E. C., HARTSELL S. E. Bacteriolysis of Enterobacteriaceae. I. Lysis by four lytic systems utilizing lysozyme. J Bacteriol. 1961 Mar;81:482–491. doi: 10.1128/jb.81.3.482-491.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLLER E. C., HARTSELL S. E. Bacteriolysis of Enterobacteriaceae. II. Pre- and co-lytic treatments potentiating the action of lysozyme. J Bacteriol. 1961 Mar;81:492–499. doi: 10.1128/jb.81.3.492-499.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- ROBERTSON W. V. The biochemical role of ascorbic acid in connective tissue. Ann N Y Acad Sci. 1961 Apr 21;92:159–167. doi: 10.1111/j.1749-6632.1961.tb46115.x. [DOI] [PubMed] [Google Scholar]
- Roberts J., Camacho Z. Oxidation of NADPH by polymorphonuclear leucocytes during phagocytosis. Nature. 1967 Nov 11;216(5115):606–607. doi: 10.1038/216606a0. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. The properties of lysozyme and its action on microorganisms. Bacteriol Rev. 1957 Jun;21(2):82–100. doi: 10.1128/br.21.2.82-100.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Wilson L. A. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. I. Loss of label, death, and ultrastructural damage. J Bacteriol. 1966 Jan;91(1):393–400. doi: 10.1128/jb.91.1.393-400.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. A., Spitznagel J. K. Molecular and structural damage to Escherichia coli produced by antibody, complement, and lysozyme systems. J Bacteriol. 1968 Oct;96(4):1339–1348. doi: 10.1128/jb.96.4.1339-1348.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAZAKI I., MASON H. S., PIETTE L. Identification, by electron paramagnetic resonance spectroscopy, of free radicals generated from substrates by peroxidase. J Biol Chem. 1960 Aug;235:2444–2449. [PubMed] [Google Scholar]


