Abstract
Plasmodium falciparum infection during pregnancy results in the sequestration of infected red blood cells (IRBCs) in the placenta, contributing to pregnancy associated malaria (PAM). IRBC adherence is mediated by the binding of a variant Plasmodium falciparum erythrocyte binding protein 1 named VAR2CSA to the low sulfated chondroitin 4-sulfate (C4S) proteoglycan (CSPG) present predominantly in the intervillous space of the placenta. IRBC binding is highly specific to the level and distribution of 4-sulfate groups in C4S. Given the strict specificity of IRBC-C4S interactions, it is better to use either placental CSPG or CSPGs bearing structurally similar C4S chains in defining VAR2CSA structural architecture that interact with C4S, evaluating VAR2CSA constructs for vaccine development or studying structure-based inhibitors as therapeutics for PAM.
Plasmodium falciparum malaria and parasite sequestration
Blood stage infection by the Plasmodium family of protozoan parasites causes malaria, a devastating disease in many countries of the world. Although several species of the parasite infect humans, severe clinical conditions, including cerebral and other organ-related fatal complications, are mainly associated with Plasmodium falciparum infection [1,2]. This is due to the distinctive ability of P. falciparum to sequester in the microvascular endothelia of various organs through the adherence of infected red blood cells (IRBCs) to the endothelial surface molecules such as CD36 (cluster of differentiation 36) and intracellular adhesion molecule 1 [2–4]. The adherence and accumulation of IRBCs lead to the recruitment of immune cells, causing severe inflammation, endothelial damage, and vital organ dysfunction and failure [2–4].
In malaria-endemic areas, through multiple infections, children acquire protective immunity that includes the development of inhibitory antibodies against IRBC adhesion [5,6]. Thus, regardless of gender, adults are generally resistant to malaria. Blocking of IRBC adherence by adhesion-specific antibodies enables the host to efficiently control infection by the clearance of IRBCs through the spleen, thereby avoiding organ-related pathogenesis [3,4]. However, in the case of women, this situation changes when they become pregnant, especially during the first pregnancy [7,8].
In pregnant women, P. falciparum seizes the availability of a new organ, the placenta, as a new opportunity for its survival by overcoming the preexisting protective immunity [7,8]. The placenta contains chondroitin sulfate proteoglycan (CSPG) receptors bearing chondroitin 4-sulfate (C4S) chains to which IRBCs can bind [9]. However, C4S are either not available or scarcely present on the endothelial surface [10]. Hence C4S-adherent IRBCs do not sequester in organs other than the placenta. Since, as in the case of men, women prior to their first pregnancy were not exposed to C4S-adherent parasites, they lack C4S-IRBC adhesion inhibitory antibodies [10–12]. Multiplication of the C4S-selected parasites and accumulation of IRBCs leads to the infiltration of mononuclear cells, causing inflammation and impairment of placental function. Eventually these processes result in pregnancy associated malaria (PAM), which is characterized by stillbirth, spontaneous abortion, premature delivery, low birth weight babies, and severe anemia and mortality in the mother [10–15]. During the first and second pregnancies, women acquire anti-adhesive antibodies against C4S-adherent parasites [10–12,16–18]. Sera from multigravid pregnant women in different malaria endemic regions of the world have been shown to inhibit the binding of various placental IRBC isolates to C4S [11,16–19]. In addition, multigravid women retain immunological memory to the anti-adhesion antibody responses [17]. This universal nature of the anti-adhesion antibodies indicates the involvement of conserved structural elements in the IRBC-C4S interactions. Further, notably, the presence of IRBC-C4S adhesion inhibitory antibodies has been shown to be associated with the reduced risk of PAM [12,18,19]. Thus, there is a considerable interest in understanding the molecular and structural interactions involved in IRBC adherence to placental CSPG from the point of view of developing a vaccine and/or small molecule inhibitor-based therapeutics for PAM.
What parasite protein mediates IRBC sequestration in the placenta?
A family of 200 to 400 kDa antigenically variant proteins called P. falciparum erythrocyte membrane protein 1 (PfEMP1) expressed on the IRBC surface is thought to mediate IRBC adherence to various host receptors (Box 1) [2–4,20,21]. PfEMP1s are encoded by a repertoire of ~60 var (variant) genes, a group of polymorphic genes present in the P. falciparum genome. The expression of var genes is tightly regulated [3]. In a clonal parasite population, only one PfEMP1 variant is expressed, enabling IRBCs to bind to a specific host receptor [22]. In response, the host produces specific anti-adhesive antibodies, thereby blocking IRBC sequestration and allowing for parasite clearance by the spleen. However, as the parasite clonal population expands, it switches at a rate of 0.03 to 2% to different adherent phenotypes by expressing other PfEMP1s [23]. Since the host lacks inhibitory antibodies against these newly expressed PfEMP1s, parasite phenotypes having different adhesive receptor specificity get selected and multiply. Development of antibodies against these adhesive phenotypes eventually results in broad protective immunity thereby preventing the IRBC sequestration and development of organ related severe pathologies.
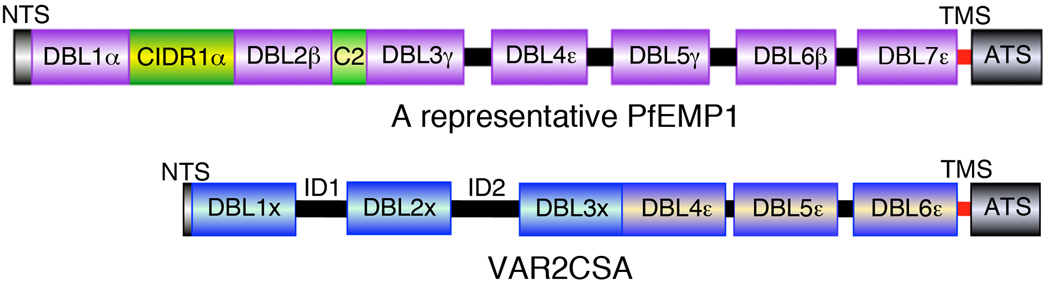
Box 1. Structural features of PfEMP1s.
Each PfEMP1 consists of a large extracellular polypeptide and a highly conserved acidic intracellular segment (ATS) that are joined by a short transmembrane segment (TMS) [2–4,21; Figure I]. The extracellular polypeptide portion consists of multiple Duffy binding-like (DBL) adhesive domains and an N terminal segment (NTS); in addition, several PfEMP1s contain cysteine-rich interdomain region (CIDR) segments and connecting (C2) segments. These DBL domains are homologous to the Duffy-binding domains of the Duffy binding protein of Plasmodium vivax and Plasmodium knowlesi, and of P. falciparum erythrocyte binding antigen-175 (EBA-175) that are involved in erythrocyte invasion [56–58]. The domain arrangements in a representative PfEMP1 are shown in Figure I. It has been reported that DBL1α mediates the agglutination of uninfected and infected erythrocytes, CIDRα binds to CD36, and DBLβ plus C2 is involved in ICAM-1 binding [59–61]. However, in view of the recent findings that a structural architecture of VAR2CSA involving multiple DBL domains is involved in C4S binding [25,33,34], the notion that individual DBL domains of PfEMP1s mediate IRBC binding to endothelial surface receptors requires further investigation.
VAR2CSA is different significantly from other PfEMP1s in that the three N terminal DBL domains are considerably different in the characteristic amino acid sequence features found in DBL domains of other PfEMP1s. Therefore, these DBL domains of VAR2CSA are referred to as DBL1x, DBL2x, and DBL3x. Earlier studies reported that the DBL2x, DBL3x, DBL5ε and DBL6ε domains independently bind C4S [19,62]. However, it is currently thought that a folded architecture in which structural elements from different DBL domains participate to form an extended C4S binding pocket or groove. Crystal structure studies have shown that the DBL3x structure fold is similar to DBL domains of the EBA-175 region II and P. knowlesi α (Pkα) Duffy binding domain [56–58]. DBL3x consists of three subdomains, S1, S2 and S3 [52]. Co-crystallization of DBL3x with C4S oligosaccharides and NMR studies showed that S2 and S3 subdomains together provide a positively charged sulfate-binding pocket. The DBL6ε and other DBL domains have no such sulfate-binding pocket [59–61], Considering that the full-length extracellular portion of VAR2CSA binds to placental CSPG with high affinity and a relatively extended dodecasaccharide motif of C4S is involved in binding, it is logical that various DBL domains interact with different functional groups of C4S, leading to high affinity binding.
Despite being antigenically variant and possessing different adhesive specificity, the PfEMP1s encoded by all 60 or so var genes have similar domain sequence arrangements [21]. PfEMP1s are transmembrane proteins, each consisting of a large extracellular polypeptide and a highly conserved cytoplasmic acidic terminal segment that are linked via a short transmembrane segment. The domain arrangement in a representative PfEMP1 is shown schematically in Figure I in Box 1. A distinct PfEMP1 called VAR2CSA is exclusively expressed on the surface of the C4S-adherent IRBCs and confer specificity to sequester in the placenta [24,25]. The extracellular portion of VAR2CSA consists of six Duffy binding like (DBL) domains and two helical interdomain (ID) regions, ID1 between DBL1x and DBL2x and ID2 between DBL2x and DBL3x (Figure I, Box 1). Recently, ID2 has been reported to resemble CIDR domains of PfEMP1s [26]. Disruption of var2csa results in the loss of IRBC adhesion to C4S [27,28]. The presence of VAR2CSA antibodies correlates with protection against PAM and women having anti-VAR2CSA antibodies deliver healthy babies [12,18,29]. Thus, VAR2CSA is thought to mediate IRBC adherence to placental CSPG and there is a substantial interest for determining the VAR2CSA structural interactions involved in C4S binding, evaluating antibodies raised against full length VAR2CSA and constructs comprising different regions of VAR2CSA for vaccine development or identifying the structure-based small molecule inhibitors as therapeutics for PAM.
Figure I.
Schematic representation of a representative PfEMP1 and VAR2CSA. The arrangements of various Duffy binding like (DBL) domains, and the cysteine-rich interdomain region (CIDR) domains, and the domain-interconnecting segments (black) are indicated. All PfEMP1s are transmembrane proteins with ATS segments are cytoplasmic, and the remainder of the polypeptide portions are extracellular. Abbreviations: NTS, N-terminal segment; C2, a short domain that follows DBL-2β; TMS, transmembrane segment; ATS, acidic terminal segment; ID, interconnecting domain.
What host receptor mediates IRBC adherence in the placenta?
While several PfEMP1s have the potential to bind various receptors such as CD36 and ICAM-1, IRBCs that express VAR2CSA bind C4S with stringent specificity. C4S is a member of the glycosaminoglycan (GAG) family of polysaccharides, which occur as covalent conjugates of proteins called proteoglycans (PGs) and are expressed by almost all animal tissues. PGs are found as secreted molecules in the extracellular matrix and on the cell surface. The structural features of various GAGs are described in Box 2 and are depicted in Figure 1.
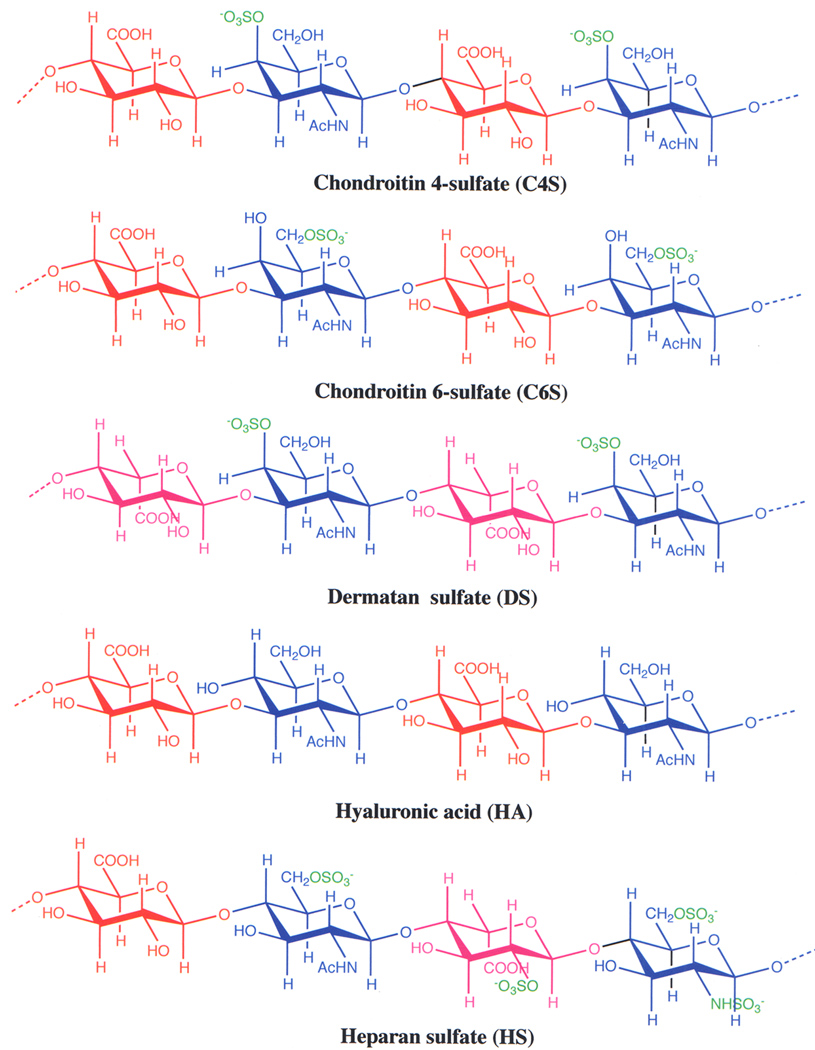
Box 2. The structural features of glycosaminoglycans.
C4S (also called chondroitin sulfate A, CSA) and other glycosaminoglycan (GAG) polysaccharides such as chondroitin 6-sulfate (C6S; also called chondroitin sulfate C, CSC) and dermatan sulfate (DS, chondroitin sulfate B, CSB), hyaluronic acid (HA), heparan sulfate (HS) widely occur in animal tissues. Structurally, the GAGs share several common features [63]. They are linear polysaccharides having disaccharide repeat units, which consist of an uronic acid (D-glucuronic in HA and CS or L-iduronic acid in DS and HS) and N-acetylhexosamine (either D-acetylgalactosamine in CS and DS or D-acetylglucosamine in HA and HS). All GAGs, except hyaluronic acid, are variously sulfated. Sulfate groups are predominantly found in N-acetylhexosamine residues. In C4S and C6S, N-acetylgalactosamine residues are sulfated at O-4 and O-6, respectively. In C4,6diS, N-acetylhexosamine residues are sulfated both at both O-4 and O-6 positions. In HS, N-acetylglucosamine residues are sulfated at O-6 or both at O-3 and O-6; certain glucosamine residues are N-sulfated instead of N-acetylation. GAGs from different animals or tissues are generally heterogeneous with respect to their chain length, level of sulfation and sulfation pattern. For example, a C4S chain may contain significant levels of sulfation at O-6 or both at O-4 and O-6; in some C4S, substantial levels of disaccharide moieties are not sulfated. Further, some chondroin sulfate chains may contain certain levels of DS structural features (disaccharide moieties contain L-iduronic acid instead of D-glucuronic acid). Conversely, DS from many sources contain variable levels of C4S, C6S or mixed C4S and C6S structural features. Some L-iduronic acid residues in DS are sulfated at O-2. Depending on the level of C4S present within their DS chains, DSPGs from certain sources may exhibit low strength IRBC binding property. Thus, it is important to define the structure of GAG chains of CSPGs that are used for either binding assays or inhibition assessment in studying the specificity of IRBC-C4S and VAR2CSA-C4S interactions.
Figure 1.
The structures of tetrasaccharide segments, representing two repeating units, of various glycosaminoglycans (GAGs). Red, D-glucuronic acid; Pink, L-iduronic acid; blue N-acetyl-D-galactosamine (in C4S, C6S, and DS), N-acetyl-D-glucosamine (in HA and HS) or N-sulfated D-glucosamine (in HS); green, sulfate group. In C4S and C6S, the glucuronic acid residues are linked to N-acetylgalactosamine residues by β(1–3) glycosidic bonds, whereas the N-acetylgalactosamine residues are linked glucuronic acid residues by β(1–4) glycosidic bonds. In DS, the iduronic acid residues are linked to N-acetylgalactosamine residues by α(1–3) glycosidic bonds, while N-acetylgalactosamine residues are linked to iduronic acid residues by β(1–4) glycosidic bonds. In HA, the glucuronic acid residues are linked to N-acetylglucosamine residues by β(1–3) glycosidic bonds, whereas N-acetylglucosamine residues are linked to glucuronic acid residues by β(1–4) glycosidic bonds. In HS, the glucuronic acid residues are linked to N-acetylglucosamine residues by β (1–4) glycosidic bonds, iduronic acid residues are linked to either N-acetylglucosamine or N-sulfated glucosamine residues by α(1–4) glycosidic bonds, and N-acetylglucosamine or N-sulfated glucosamine residues are linked to either glucuronic acid or iduronic acid residues by β(1–4) glycosidic bonds. Note that D-glucuronic acid and L-iduronic acid residues are differed by one another only in the configuration of the carboxyl group present at the C-5 position. In placental C4S chains, not all N-acetylgalactosamine residues are sulfated as shown above for a fully sulfated C4S, but only a few residues are sulfated exclusively at C-4, resulting in low sulfated C4S chains.
Recently, the full ectodomain of VAR2CSA expressed in either the baculovirus system or mammalian cells has been shown to bind placental CSPG with high affinity [7,30–34]. VAR2CSA does not bind other glycosamiglycans such as chondroitin 6-sulfate, dermatan sulfate or hyaluronic acid.
In P. falciparum-infected pregnant women, IRBCs adhere predominantly in the intervillous space of the placenta and moderately to the syncytiotrophoblast lining [8, 35]. Placenta contains a high level of an extracellular CSPG localized in the intervillous space [9]. A unique feature of the chondroitin sulfate chains of this CSPG is that they are extremely lowly sulfated; on average, only 10% of the disaccharide repeat units of the chondroitin sulfate chains are sulfated, exclusively at the C-4 position of N-acetylgalactosamine; the reminder of the disaccharide moieties are nonsulfated [9]. CSPGs containing low sulfated C4S are also present on the syncytiotrophoblast cell lining, but their abundance as compared to CSPG found in the intervillous space is significantly low. About 30% of disaccharide moieties of the chondroitin sulfate chains of placental cell membrane CSPGs are 4-sulfated, ~15% are 6-sulfated, and the remainder are nonsulfated. Both intervillous space and syncytiotrophoblast cell membrane CSPGs efficiently bind IRBCs [9]. The occurrence of CSPGs at high levels in the intervillous space and at low levels on the syncytiotrophoblast cell layer correlates with the high-density accumulation of IRBCs in the intervillous space and moderate level of adherence on the syncytiotrophoblast cell lining seen in the infected placentas [35]. It is important to note that although the sulfate contents of the chondroitin sulfate chains of placental intervillous CSPG are unusually low, the majority of the sulfate groups are clustered at a density of 2 sulfate groups per 6 to 14 disaccharide chain length, corresponding to 20 to 28% sulfate content in the sulfate rich regions of the GAG chains [36]. Further, the oligosaccharides obtained from the sulfate-clustered regions of the placental C4S chains could efficiently inhibit IRBC binding to placental CSPG.
What C4S structural features interact with IRBCs?
Inhibition studies using C4S containing different sulfate contents (3% to 98%) and oligosaccharides of varying sizes (4 to 18 disaccharide repeats) have shown that a dodecasaccharide (consisting of 6 disaccharide repeats) motif is the minimum chain length required for the optimal binding of IRBCs to placental CSPG [30,37–39). A minimum of two 4-sulfated disaccharide moieties within the dodecasaccharide chain length is sufficient for maximum IRBC binding. The other GAGs, chondroitin 6-sulfate, dermatan sulfate, hyaluronic acid, and heparan sulfate (see Figure 1), do not support IRBC adherence [7,30,37–40].
Detailed C4S structure-inhibitory activity relationship studies have revealed additional structural elements involved in IRBC binding to placental CSPG [38,39,41]. These include: (i) the position and steric orientation of the carboxyl groups - the equatorial orientation and the location at the nonreducing side of the C4S are required, and (ii) the presence of 4-sulfated disaccharide moieties at or proximal to the nonreducing end of the dodecasaccharide motif is also critical [41]. Further, dodecasaccharides consisting of four 4-sulfated disaccharide moieties, but not those containing one, five or six 4-sulfate groups, are efficient inhibitors of IRBC binding to placental CSPG, indicating that, in addition to the C-4 sulfate group, one or more hydroxyl groups at the C-4 of certain N-acetylgalactosamine residues interact with IRBCs [37,39]. The C4S structural elements that do not interact with IRBCs are the N-acetyl groups, and the C-2 and C-4 hydroxyl groups of the glucuronic acid residues [41]. Collectively, these findings agree with the notion that a conformational structure of C4S dodecasaccharide motifs interact with IRBCs, and thus, presumably with VAR2CSA.
In solution, C4S exists as an extended structure having a left-handed single-stranded helical conformation in which one turn (28 Å in length) of the helix corresponds to a hexasaccharide motif [42]. Hence, a dodecasaccharide chain length (56 Å) represents two turns of the helix. Given this and considering that the sulfate and carboxyl groups that are critical for IRBC adhesion are proximal to the nonreducing side of C4S molecule, it appears that these interacting functional groups are on the same one turn of the helix (Figure 2). Therefore, the C-4 hydroxyl of N-acetylgalactosamine that interacts with IRBCs appears to be on the other helical turn of the dodecasaccharide motif. Since the dodecasaccharide minimum motif is 56 Å in length, it is likely that a fairly extended groove/pocket of VAR2CSA structure is involved in IRBC binding to C4S. Given the stringent specificity of IRBC-C4S interactions, it is logical to use placental CSPG (natural receptor) or CSPGs bearing the C4S chains that closely resemble those of the C4S of placental CSPG in defining the specificity of VAR2CSA-C4S interactions.
Figure 2.
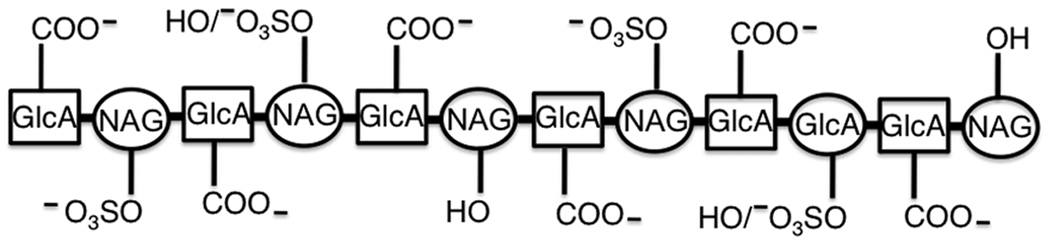
Schematic representation of a C4S dodecasaccharide. Based on the specificity of IRBC binding to C4S, it is inferred that the carboxyl groups of glucuronic acid (GlcA) interact with VAR2CSA. Although the carboxyl group of the GlcA residue at the non-reducing terminal end appears to be required for binding whether all of the reminder carboxyl groups or only some of them interact is not known. Given that dodecasaccharides containing 2, 3, 4 sulfate exhibit similar levels of efficient inhibitory activity whereas those containing 1 or 5 sulfate groups have substantially lower inhibitory activity, it is logical that two hydroxyl groups at the C-4 position of N-acetylgalatosamine (NAG) interact with VAR2CSA. The N-acetyl groups do not interact, but it is possible that the nitrogen lone pair of electrons is also involved. C4S assumes single-stranded helical conformation and therefore, the schematic structure shown here does not accurately represent the spatial orientations of the depicted functional groups.
Are there CSPGs that are alternative to placental CSPG for studying VAR2CSA-C4S interactions?
A bovine tracheal chondroitin sulfate A (bCSA; copolymer of C4S and C6S), consisting of 53% 4-sulfated, 39% 6-sulfated, and 8% non-sulfated disaccharide moieties, attached to short peptides formed by the degradation of the core protein of bovine tracheal CSPG has been widely used for the selection of C4S-adherent IRBCs and for evaluating IRBC binding specificity. However, the strength of IRBC binding to bCSA is considerably lower than that to the C4S chains of placental CSPGs [43,44]. Some studies have used structurally undefined decorin to study VAR2CSA-C4S interactions [33,34,45,46]. This decorin is a bovine articular cartilage proteoglycan that has been reported to have dermatan sulfate (DS) chains containing some chondroitin sulfate structural features [47]. The chondroitin sulfate portion of this DS/CSPG may contain both 4- and 6-sulfate groups. However, since IRBCs either weakly or do not interact with DS or C6S, the results of IRBC adhesion assays using bCSA and DS/CSPGs are qualitative, i.e., they indicate whether or not IRBCs are C4S-adherent but do not reveal the physiological binding strength. Thus, bCSA can be used for the selection of C4S-adherent IRBCs, qualitative binding/inhibition analysis, assessment of antibody responses in people from endemic areas, analyzing VAR2CSA interactions, and testing of antibody responses to vaccine candidates. However, understanding of the fine details of VAR2CSA interacting elements and validation of vaccine efficacy require placental CSPG or another CSPG that exhibit comparable binding strength.
In this regard, the low sulfated bovine corneal CSPG can be a suitable alternative as the IRBC binding strength is either equal or even higher than that of the placental CSPGs [48,49]. Bovine cornea is readily available and CSPGs can be purified using three standard biochemical procedures that involve salt density gradient centrifugation, ion-exchange chromatography, and size-exclusion chromatography. The corneal CSPG is a mixture of three subpopulations each having decorin core protein substituted with two CS chains consisting of 14%, 31% and 64% overall sulfated disaccharides, respectively; the remainders of the disaccharides are nonsulfated. The CSPG subpopulation with 14% sulfate contains 1% 6-sulfate and has no DS features. The two other CSPG subpopulations, respectively, contain 3% and 13% 6-sulfate, and 5% and 15% DS features. Thus, the total 4-sulfated disaccharide contents in these CSPG subpopulations are 23% and 36%, respectively [48,49]. Comparative evaluations using human placental CSPG revealed that the low levels of 6-sulfate groups and DS elements present in the CS chains of these CSPG subpopulations do not have any adverse effects on their ability to support high strength binding of IRBCs [48,49]. Based on their capacity to bind IRBCs strongly and the presence of low level of 4-sulfate, it appears that the C4S chains of corneal CSPGs have optimal structural features resembling those of the placental CSPG, and hence they can be used for evaluating VAR2CSA binding specificity.
Concluding remarks
The sequestration of P. falciparum IRBCs in the placenta is mediated by the binding of VAR2CSA to uniquely low sulfated CSPGs present in the intervillous space and on the syncytiotrophoblast cell lining. Considering that the IRBC and VAR2CSA binding is highly specific to C4S and that high affinity binding involves specific sulfation pattern in the C4S chains, it is better to use appropriate CSPGs with defined GAG chain structures for evaluating VAR2CSA-C4S interactions. It is also important to use C4S having defined 4-sulfate content and various control GAG inhibitors for meaningfully analyzing the binding specificity and binding strength of VAR2CSA or its constructs. C4S dodecasaccharides containing two, three or 4-sulfate groups represent important reagents for determining C4S-interacting VAR2CSA structural features by crystallographic studies. Crystallography and NMR studies using C4S oligosaccharides have shown that DBL3x domain of VAR2CSA has a positively charged, sulfate-binding pocket (see Box 1 for more details) [50–52]. Such binding pockets are not present in DBL6ε and in other DBL domains [53–55]. The full-length extracellular polypeptide portion is required for specific and avid binding of VAR2CSA to the placental CSPG [33,34]. Given this and considering that a relatively long stretch of C4S interacts with VAR2CSA, it is likely that the 4-sulfate groups, carboxyl groups and C-4 hydroxyl groups of N-acetylgalactosamine in a defined C4S conformational orientation interact with a fairly extended groove or pocket of the VAR2CSA structural architecture. Therefore, it is important that CSPGs having appropriate C4S chains are used for assessing VAR2CSA-C4S interactions.
Acknowledgements
We thank Dr. Momcilo Miljkovic for his help in drawing the structures of glycosaminoglycans shown in Figure 1. Due to size restriction by the journal we could not cite many original articles. We apologize for authors whose contributions could not be cited. We also thank NIAID, NIH, for funding (Grant AI45086).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snow RW, et al. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LH, et al. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 3.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Clin. Opin. Microbiol. 2006;9:373–380. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Rowe JA, et al. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. doi: 10.1017/S1462399409001082. ( http://journals.cambridge.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doolan DL, et al. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hviid L. The role of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Hum. Vac. 2010;6:84–86. doi: 10.4161/hv.6.1.9602. [DOI] [PubMed] [Google Scholar]
- 7.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;271:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 8.Brabin BJ, et al. The sick placenta-the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Achur RN, et al. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 2000;275:40344–40356. doi: 10.1074/jbc.M006398200. [DOI] [PubMed] [Google Scholar]
- 10.Duffy PE, Desowitz RS. Pregnancy malaria throughout history: Dangerous labors. In: Duffy PE, Fried M, editors. Malaria in Pregnancy: Deadly parasite, Susceptible Host. Taylor and Francis; 2001. pp. 1–25. [Google Scholar]
- 11.Fried M, et al. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 12.Hviid L. The immuno-epidemiology of pregnancy-associated malaria: a variant surface antigen-specific perspective. Parasite Immunol. 2004;26:477–486. doi: 10.1111/j.0141-9838.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 13.Mcgregor IA, et al. Malaria infection of the placenta in The Gambia West Africa; its incidence and relationship to still birth, birth weight and placental weight. Trans. R. Soc. Trop. Med. Hyg. 1983;77:232–244. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 14.Ismail MR, et al. Placental pathology in malaria: a histological, immunohistochemical and quantitative study. Hum. Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 15.Rogerson SJ, et al. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 16.Ricke CH, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 2000;165:3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 17.O’Neil-Dunne I, et al. Gravidity dependent production of antibodies that inhibit the binding of Plasmodium falciparum- infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect. Immun. 2001;69:7487–7492. doi: 10.1128/IAI.69.12.7487-7492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamain B, et al. Pregnancy-associated malaria: parasite binding, natural immunity and vaccine development. Intl. J. Parasitol. 2007;37:273–283. doi: 10.1016/j.ijpara.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Baruch DI, et al. Plasmodium falciparum erythrocyte membrane protein is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule-1. Proc. Natl. Acad. Sci. USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JD, et al. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 2000;110:293–310. doi: 10.1016/s0166-6851(00)00279-6. [DOI] [PubMed] [Google Scholar]
- 22.Dzikowski R, et al. Mechanisms underlying mutually exclusive expression of virulence genes by malaria parasites. EMBO Rep. 2007;8:959–965. doi: 10.1038/sj.embor.7401063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatton ML, et al. Switching rates of Plasmodium falciparum var genes: faster than we thought? Trends Parasitol. 2003;19:202–208. doi: 10.1016/s1471-4922(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 24.Salanti A, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salanti A, et al. Selective upregulation of a single distinctively structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 26.Rask TS, et al. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes- divide and conquer. PLoS Comput. Biol. 2010;6:e1000983. doi: 10.1371/journal.pcbi.1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viebig NK, et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Reports. 2005;6:775–781. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffy MF, et al. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogenic isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 2006;148:117–124. doi: 10.1016/j.molbiopara.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Hviid L, Salanti A. VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria. Parasitol. 2007;134:1871–1876. doi: 10.1017/S0031182007000121. [DOI] [PubMed] [Google Scholar]
- 30.Alkhalil A, et al. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J. Biol. Chem. 2000;275:40357–40364. doi: 10.1074/jbc.M006399200. [DOI] [PubMed] [Google Scholar]
- 31.Beeson JG, et al. Structural basis for binding of Plasmodium falciparum erythrocyte membrane protein 1 to chondroitin sulfate and placental tissue and the influence of protein polymorphisms on binding specificity. J. Biol. Chem. 2007;282:22426–22436. doi: 10.1074/jbc.M700231200. [DOI] [PubMed] [Google Scholar]
- 32.Goel S, et al. Dual stage synthesis and crucial role of cytoadherence-linked asexual gene 9 in the surface expression of malaria parasite var proteins. Proc. Natl. Acad. Sci. USA. 2010;107:17333–17338. doi: 10.1073/pnas.1002568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava A, et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl. Acad. Sci. USA. 2010;107:4884–4889. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khunrae P, et al. Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J. Mol. Biol. 2010;397:826–834. doi: 10.1016/j.jmb.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthusamy A, et al. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. Am. J. Pathol. 2004;164:2013–2025. doi: 10.1016/S0002-9440(10)63761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achur RN, et al. The low sulfated chondroitin sulfate proteoglycans of human placenta have sulfate group-clustered domains that can efficiently bind Plasmodium falciparum-infected erythrocytes. J. Biol. Chem. 2003;278:11705–11713. doi: 10.1074/jbc.M211015200. [DOI] [PubMed] [Google Scholar]
- 37.Chai W, et al. The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid. J. Biol. Chem. 2002;277:22438–22446. doi: 10.1074/jbc.M111401200. [DOI] [PubMed] [Google Scholar]
- 38.Gowda DC. Role of chondroitin 4-sulfate in pregnancy-associated malaria. Adv. Pharmacol. 2006;53:375–400. doi: 10.1016/S1054-3589(05)53018-7. [DOI] [PubMed] [Google Scholar]
- 39.Achur RN, et al. Structural interactions in chondroitin 4-sulfate mediated adherence of Plasmodium falciparum infected erythrocytes in human placenta during pregnancy-associated malaria. Biochemistry. 2008;47:12635–12643. doi: 10.1021/bi801643m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthusamy A, et al. Chondroitin sulfate proteoglycan but not hyaluronic acid is the receptor for the adherence of Plasmodium falciparum-infected erythrocytes in human placenta, and infected red blood cell adherence up-regulates the receptor expression. Am. J. Pathol. 2007;170:1989–2000. doi: 10.2353/ajpath.2007.061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gowda AS, et al. Structural basis for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin 4-sulfate and design of novel photoactivable reagents for the identification of parasite adhesive proteins. J. Biol. Chem. 2007;282:916–928. doi: 10.1074/jbc.M604741200. [DOI] [PubMed] [Google Scholar]
- 42.Cael JJ, et al. Calcium chondroitin 4-sulfate: modular conformation and organization of polysaccharide chains in proteoglycan. J. Mol. Biol. 1978;125:21–42. doi: 10.1016/0022-2836(78)90252-8. [DOI] [PubMed] [Google Scholar]
- 43.Goyal A, et al. Plasmodium falciparum: Assessment of parasite-infected red blood cell binding to placental chondroitin proteoglycan and bovine tracheal chondroitin sulfate A. Exp. Parasitol. 2009;123:105–110. doi: 10.1016/j.exppara.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthusamy A, et al. Structural characterization of the bovine tracheal chondroitin sulfate chains and binding of Plasmodium falciparum-infected erythrocytes. Glycobiol. 2004;14:635–645. doi: 10.1093/glycob/cwh077. [DOI] [PubMed] [Google Scholar]
- 45.Salanti A, et al. Several domains from VAR2CSA can induce Plasmodium falciparum adhesion-blocking antibodies. Malaria J. 2010;9:11. doi: 10.1186/1475-2875-9-11. ( http://www.malariajournal.com/content/9/1/11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen MA, et al. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect. Immun. 2009;77:2482–2487. doi: 10.1128/IAI.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi HU, et al. Characterization of the Dermatan Sulfate Proteoglycans, DS-PGI and DS-PGII, from Bovine Articular Cartilage and Skin Isolated by Octyl-Sepharose Chromatography. J. Biol. Chem. 1989;264:2876–2884. [PubMed] [Google Scholar]
- 48.Achur RN, et al. Chondroitin sulfate proteoglycans of bovine cornea: structural characterization and assessment for the adherence of Plasmodium falciparum-infected erythrocytes. Biochim. Biophys. Acta. 2004;1701:109–119. doi: 10.1016/j.bbapap.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Muthusamy A, et al. Plasmodium falciparum: adherence of the parasite-infected erythrocytes to chondroitin sulfate proteoglycans bearing structurally distinct chondroitin sulfate chains. Exp. Parasitol. 2004;107:183–188. doi: 10.1016/j.exppara.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Singh K, et al. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat. Struct. Biol. 2008;15:932–938. doi: 10.1038/nsmb.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins MK. The structure of a chondroitin sulfate-binding domain important in placental malaria. J. Biol. Chem. 2008;283:21842–21846. doi: 10.1074/jbc.C800086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh K, et al. Subdomain 3 of Plasmodium falciparum VAR2CSA DBL3x is identified as a minimal chondroitin sulfate A-binding region. J. Biol. Chem. 2010;285:24855–24862. doi: 10.1074/jbc.M110.118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khunrae P, et al. Structural comparison of two CSPG-binding DBL domains from the VAR2CSA protein important in malaria during pregnancy. J. Mol. Biol. 2009;393:202–213. doi: 10.1016/j.jmb.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahlbäck M, et al. Epitope mapping and topographic analysis of VAR2CSA DBL3x involved in P. falciparum placental sequestration. PLoS Pathog. 2006;2:e124. doi: 10.1371/journal.ppat.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gill J, et al. Structural insights into chondroitin sulphate A binding Duffy-binding-like domains from Plasmodium falciparum: implications for intervention strategies against placental malaria. Malaria J. 2009;8:67. doi: 10.1186/1475-2875-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte. J. Exp. Med. 1984;180:486–507. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tolia NH, et al. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell. 2005;122:183–193. doi: 10.1016/j.cell.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 58.Singh SK, et al. Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. Nature. 2006;439:741–744. doi: 10.1038/nature04443. [DOI] [PubMed] [Google Scholar]
- 59.Mayor A, et al. Functional and immunological characterization of a Duffy binding-like alpha domain from Plasmodium falciparum erythrocyte membrane protein 1 that mediates rosetting. Infect. Immun. 2009;77:3857–3863. doi: 10.1128/IAI.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mo M, et al. The C-terminal segment of the cysteine-rich interdomain of Plasmodium falciparum erythrocyte membrane protein 1 determines CD36 binding and elicits antibodies that inhibit adhesion of parasite-infected erythrocytes. Infect. Immun. 2008;76:1837–1847. doi: 10.1128/IAI.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith JD, et al. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA. 2000;97:1766–1771. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gamain B, et al. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J. Infect. Dis. 2005;191:1010–1013. doi: 10.1086/428137. [DOI] [PubMed] [Google Scholar]
- 63.Bhavanandan VP, Davidson EA. Proteoglycans: Structure, synthesis, function. In: Allen HJ, Kissilus EC, editors. Glycoconjugates: Structure, Synthesis, and Function. Marcel Dekker; 1992. pp. 167–202. [Google Scholar]