Abstract
Tear proteins are supplied by the regulated fusion of secretory vesicles at the apical surface of lacrimal gland acinar cells, utilizing trafficking mechanisms largely yet uncharacterized. We investigated the role of Rab27b in the terminal release of these secretory vesicles. Confocal fluorescence microscopy analysis of primary cultured rabbit lacrimal gland acinar cells revealed that Rab27b was enriched on the membrane of large subapical vesicles that were significantly colocalized with Rab3D and Myosin 5C. Stimulation of cultured acinar cells with the secretagogue carbachol resulted in apical fusion of these secretory vesicles with the plasma membrane. Evaluation of morphological changes by transmission electron microscopy of lacrimal glands from Rab27b−/− and Rab27ash/ash/Rab27b−/− mice, but not ashen mice deficient in Rab27a, showed changes in abundance and organization of secretory vesicles, further confirming a role for this protein in secretory vesicle exocytosis. Glands lacking Rab27b also showed increased lysosomes, damaged mitochondria, and autophagosome-like organelles. In vitro, expression of constitutively active Rab27b increased the average size but retained the subapical distribution of Rab27b-enriched secretory vesicles, whereas dominant-negative Rab27b redistributed this protein from membrane to the cytoplasm. Functional studies measuring release of a cotransduced secretory protein, syncollin-GFP, showed that constitutively active Rab27b enhanced, whereas dominant-negative Rab27b suppressed, stimulated release. Disruption of actin filaments inhibited vesicle fusion to the apical membrane but did not disrupt homotypic fusion. These data show that Rab27b participates in aspects of lacrimal gland acinar cell secretory vesicle formation and release.
Keywords: actin, Rab3d, exocrine secretion, syncollin, mouse models
a functioning lacrimal gland (LG) is critical for a healthy ocular surface. This exocrine gland is the primary source of tear proteins and fluid, which, released up on the gland’s exposure to sympathetic or parasympathetic agonists provided by innervating nerves, contribute to the middle aqueous layer of the precorneal film. Much of the LG’s secretions originate from acinar cells, which constitute over 80% of the mass of the LG (13). These LG acinar cells produce a diverse array of secretory proteins that are internally sorted into large pools of serous and mucous secretory vesicles (SV) sized ∼1 μm in diameter and stored beneath the apical plasma membrane (APM) in preparation for regulated exocytosis. SV contents include nutrients and growth factors (lacritin and EGF), antibacterial and antiviral factors (secretory IgA and lactoferrin), and an array of proteases and lysosomal hydrolases (10, 39, 47). Despite its physiological importance, however, few studies have focused on the mechanisms of secretory membrane trafficking in LG acinar cells, in part due to the fragility and heterogeneity of these LG acinar SV relative to those in other acinar secretory cells, e.g., pancreatic acini (7).
The current schematic of exocytosis in the LG acinar cell suggests multiple participants. Mature LG acinar cell SV localize beneath an actin-rich network underlying the APM; on stimulation, SV appear to undergo compound fusion followed by exocytosis of their contents at the APM (25). Myosin motors and actin filaments are believed to facilitate terminal aspects of SV fusion with APM and content extrusion; studies in LG acinar cells have collectively suggested that stimulation with the muscarinic agonist, carbachol (CCH), results in transient disassembly of the actin barrier beneath the APM concurrent with the assembly of actin “coats,” which aid in the compound fusion of SV homotypically and with the APM (24, 46, 52). Both the unconventional myosin motor, myosin 5C, and non-muscle myosin II are thought to participate in aspects of terminal SV fusion (24, 29). Microtubules and cytoplasmic dynein have also been shown to play a general role in the maturation and maintenance of SV in the LG (50).
With over 60 members, Rab proteins are strong candidates for the regulation of specific trafficking steps in exocytosis in the LG acinar cells (23). Rabs, which cycle between GTP-bound active states and GDP-bound inactive states as assisted by guanine nucleotide exchange factors or GTPase activating proteins, respectively, are implicated in protein trafficking and targeting and fusion of membranous organelles in all eukaryotic cell types (11, 12, 40). In acinar cells from the LG, pancreas, and parotid gland, the Rab3D isoform is enriched on mature SV and appears to serve as a negative regulator of homotypic fusion (38). In comparison, Rab27 proteins are related but distinct in primary sequence to Rab3 proteins and are one of few Rab proteins directly linked to a human disease, Griscelli Syndrome Type II (1, 31). Mammals express two Rab27 isoforms (Rab27a, Rab27b) that share 71% aa sequence identity (4). Studies have suggested partial compensation of the two isoforms, although their expression varies by tissue (1, 51). Rab27a is primarily expressed in the endocrine pancreas, intestines, and parotid gland and has also been studied in melanocytes, platelets, PC12 cells, and cytotoxic T-lymphocytes. Rab27b is highly expressed in the brain, spleen, parotid gland, platelets, and exocrine pancreas (4, 6, 21, 22, 37, 42, 45, 56).
Previous studies have suggested a role for Rab27 isoforms in intracellular trafficking in many cell types. This study establishes Rab27b as a positive regulator of the LG acinar cell secretory pathway, elucidates some of its mechanisms in exocytosis through detailed imaging studies, and for the first time characterizes morphologically the exocrine acinar cell phenotype associated with a knockout of this Rab protein.
METHODS
Reagents.
Matrigel was obtained from Collaborative Biochemicals (Bedford, MA), carbachol from Sigma-Aldrich (St. Louis, MO), and latrunculin B from EMD Biosciences (San Diego, CA). Commercial antibody for Rab27a was from Santa Cruz Biotechnology (goat polyclonal N20, Santa Cruz, CA) or Novus Biologicals (mouse MAb M02, Littleton, CO), whereas Rab27b antibodies were from Santa Cruz Biotechnology (goat polyclonal C20), IBL America (rabbit polyclonal, Minneapolis, MN), or Novus Biologicals (mouse polyclonal B01P). Cathepsin S Activity Assay Kit was from Abcam (Cambridge, MA). Rabbit polyclonal antibody to recombinant Rab3D was generated by Antibodies (Davis, CA) (14). Other commercial primary antibodies utilized included mouse MAb to GFP (Santa Cruz Biotechnology) and mouse MAb to Xpress tag (Invitrogen, Carlsbad, CA). Rabbit polyclonal antibody to myosin 5C was a gift from Dr. Richard Cheney (University of North Carolina). Secondary antibodies and fluorescent affinity probes for microscopy, including Texas Red 10,000 MW dextran and FM 1–43, were purchased from Molecular Probes/Invitrogen (Carlsbad, CA). Purified recombinant His-tagged Rab27a and Rab27b protein were prepared as described (41). All other chemicals were reagent grade and obtained from standard suppliers.
Primary rabbit LG acinar cell culture and treatments.
All animal procedures were in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication no. 85-23, revised 1996) (9, 17, 18) and were approved by the University of Southern California Institutional Animal Care and Use Committee. Acinar cells isolated from LG from New Zealand White rabbits (1.8–2.2 kg) (Irish Farms, Norco, CA) were sequentially incubated in Mg2+- and Ca2+-free HBSS supplemented with EDTA and Ham’s medium supplemented with collagenase, hyaluronidase, and DNAse, and cultured for 2 days in Peter’s serum-free culture medium (18). These cultured cells reform acinus-like structures displaying distinct apical and basolateral domains (9, 17, 24). For imaging of fixed cells, LG acinar cells were seeded on coverslips at 2 × 106 cells per well in 12-well plates coated with Matrigel. For live-cell imaging, LG acinar cells were seeded on 35-mm dishes with glass coverslipped bottoms at 6 × 106 cells per dish (MatTek, Ashland, MA).
Mouse LG isolation and analysis.
The Rab27b knockout mouse strain, which was then crossed with a naturally occurring Rab27a knockout strain ashen to produce a double knockout strain, was created as described in Ref. 44 by the authors. LG from 3- to 4-mo-old male mice were surgically removed and processed (8). For immunocytochemical and immunofluorescence labeling and analysis, tissue was immediately immersed in 4% paraformaldehyde for 2 h at room temperature, transferred to 30% sucrose overnight, and then frozen in Tissue-Tek OCT (Sakura Finetek, Torrance, CA). The embedded tissue was sectioned to 5- to 8-μm thickness and thaw-mounted onto warm glass slides. For transmission electron microscopy, fresh tissue was carefully minced into 1-mm3 pieces and fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer overnight. Samples were postfixed with 1% osmium/0.8% potassium ferricyanide in 0.1 M cacodylate buffer, dehydrated, and infiltrated in 100% Spurrs resin: by weight, 23.6% ERL4221, 14.2% DER736, 61.5% NSA, 0.7% DMAE (EMS, Hatfield, PA). Thin sections were prepared with an RMC MTX ultra microtome (Boeckeler Instruments, Tucson, AZ) and counterstained with Sato’s lead stain and 2% uranyl acetate.
Production and amplification of recombinant adenovirus.
Adenovirus (Ad) constructs were amplified in QBI cells at 37°C and 5% CO2 in DMEM (4.5 g/ml glucose, GIBCO/Invitrogen, Carlsbad, CA) containing 10% FBS until cells showed the characteristic cytopathological effect. Cells were then harvested and purified using CsCl gradient ultracentrifugation (50), and viral titers were measured by the formation of viral plaques in sequential dilutions. Replication-deficient Ad constructs were used: Ad-syncollin-GFP (kindly provided by Dr. Christopher Rhodes, University of Chicago) (20, 27) and Ad-GFP (53). Mouse Rab27 sequences, fused to epitope tags on their NH2 termini, were expressed using the following constructs: Ad-Xpress-Rab27bQ78L (constitutively active; Xp-CA), Ad-Xpress-Rab27bN133I (dominant negative; Xp-DN) and Ad-Xpress-Rab27b (wild-type; Xp-WT), which were kind gifts of Dr. John Williams, University of Michigan (6, 56); and Ad-YFP-Rab27b (YFP-WT), Ad-YFP-Rab27bQ78L (YFP-CA), Ad-YFP-Rab27bN133I (YFP-DN) as described (43).
Ad transduction with Rab27b constructs.
Initial studies showed that the Xpress-tagged protein expression yielded better quality images for fixed cell analysis, whereas YFP-tagged protein expression enabled visualization of intact SV in living cells. For imaging of exogenous proteins, cultured LG acinar cells were transduced with Ad constructs at MOI 4–6 and incubated for an additional 18–24 h to optimize expression levels (24). Previous studies have consistently shown a 70–80% transduction efficiency using this low viral titer (53). Transduction efficiency with Rab27b constructs was >80%. For assays analyzing the release of syncollin-GFP, LG acinar cells were doubly transduced with Ad-syncollin-GFP (MOI 2–3) and Xp-WT/CA/DN (MOI 2–5) or with an Ad-GFP control. Ad-syncollin-GFP transduction was 60–70%, but because of the higher transduction efficiency of all other constructs, in dual-transduction experiments most LG acinar cells expressing Ad-syncollin-GFP also expressed the transduced form of the Rab27b construct. Expression levels of epitope-tagged Rab27 constructs were ∼20- to 50-fold that of endogenous protein as determined by Western blot analysis of transduced lysates. Expression of constructs was validated by confocal fluorescence microscopy. Cell viability of the acini expressing the DN Rab27b constructs, which showed loss of epithelial cell polarity, was tested using the LIVE/DEAD Cell Viability Assay Kit for mammalian cells (Invitrogen, Carlsbad, CA).
Confocal fluorescence microscopy.
For immunofluorescence, LG acinar cells were fixed with ethanol, blocked with 1% BSA, and incubated with primary antibody, followed by the appropriate secondary antibody, and mounted on glass slides with Prolong anti-fade mounting medium (Molecular Probes, Eugene, OR). For frozen sections from LG tissue, fresh-cut sections were fixed with 4% paraformaldehyde and permeabilized with 0.1% Tx-100 before blocking with 1% BSA, followed by labeling with the primary and secondary antibodies. Slides were imaged using a Zeiss LSM 510 Meta NLO (Thornwood, NY) confocal imaging system equipped with Argon, red HeNe, and green HeNe lasers and a Coherent Chameleon Ti-Sapphire laser mounted on a vibration-free table. LSM images were converted into tiff images by Adobe Photoshop 8.0 (Adobe Systems, Mountain View, CA). For comparison of relative Rab27b enrichment in the subapical region following stimulation, YFP-Rab27b intensities were measured relative to the region of interest (ROI) around the lumen and compared in the same acini, before and after CCH addition. The subapical ROI was defined as the area within a radius of approximately one-third of the total cell radius from the center of the lumen. Further analysis of colocalization coefficients associated with distinct chromophores linked to proteins of interest was conducted using the Zeiss Enhanced Colocalization Tool software in parallel with non-colocalizing specimens. To distinguish between the close spectral fluorescence emission values of GFP and YFP, the Zeiss META Emission Fingerprinting program was used to acquire a complete lambda stack, followed by linear unmixing.
Time-lapse, live-cell imaging.
Live-cell imaging was conducted in cultured LG acinar cells expressing a tagged protein in a controlled incubation chamber as described (24, 53). For live cell time series studies, reconstituted acini of similar size were analyzed at the resting stage for 10 min and on stimulation after addition of 100 μM CCH (15 min). FM 1–43 and dextran were added at the manufacturer recommended concentrations to cultured acini briefly chilled on ice to minimalize endocytosis of dextran, followed by 30 min of incubation on ice with gentle shaking, before immediate imaging. To analyze the reliance of YFP-Rab27b-enriched vesicle movements on actin-dependent transport, 10 μM latrunculin B was incubated with the cells at 37°C for 60 min before CCH addition. Negative controls for the latrunculin B studies included pretreatment with equivalent amounts of dimethylsulfoxide. DIC images collected in parallel with fluorescence allowed the identification of the apical/luminal and basolateral membranes. Z-stacks were taken to identify relative position of signals within the cell and reconstructed with the Zeiss LSM Projection Tool and with the NIH Image J 1.41a (NIH, Bethesda, MD).
For analysis of vesicle diameter, acini under different experimental conditions were analyzed at specific time points: 5 min before CCH addition and 10 min after CCH addition. Vesicles clearly enriched in Rab27b were measured using the Image J measurement analysis tool. Vesicle morphometry was evaluated in 2–3 random fields from 12 preparations for each condition. Variations in vesicle density prevented usage of high-throughput quantitative analysis programs.
Transmission electron microscopy.
Mouse LG tissue was prepared as described in the section on LG isolation. Samples were analyzed with a digital 11-megapixel JEOL 1011 TEM (JEOL USA, Portland, OR). A large systematic random sampling (55) of mouse sections in randomly oriented samples was used to examine the detailed morphology of the knockout mouse LG tissue compared with that from wild-type (WT) mice. SV, diameters, and distances from the lumen, as well as cell size and organelle positive hits, were measured using an optical dissector of ∼1–3 SV in size, and less for other organelles, per counting frame, following stereological rules (3, 5, 33). Data were then clustered to determine an average of organelle cross section per square micrometer of sampled area, with 70 frames counted per group: WT, DKO: n = 5; 27bKO: n = 4. A per-cell exact count using the Image J (NIH) measurement and counting tools confirmed stereological results. Direct quantitation data of micrographs was collected from 15 to 20 micrographs per mouse: WT: n = 5 mice, 94 cells; DKO: n = 5 mice, 133 cells; 27bKO: n = 4 mice, 96 cells.
Preparation of LG and LG acinar cell lysate for Western blotting.
Cultured rabbit LG acinar cells were lysed in RIPA buffer containing protease inhibitors as previously described (48). LG acinar cell lysate was passed through a 20.5-G syringe needle 20 times, followed by 20 passes through a cell press (H & Y Enterprises, Redwood City, CA). LG tissue from mice was collected and homogenized with three 30-s pulses on ice in 2 ml of RIPA buffer with protease inhibitors using a PT-MR-2100 Polytron tissue homogenizer. Crude tissue homogenate was precleared at 7,700 g for 10 min at 4°C (Hermle Labnet Z216MK, Woodbridge, NJ), and the supernatant was collected. Lysate protein concentrations were determined with the Pierce BCA Assay using albumin as standard protein (Thermo Fisher Scientific, Rockford, IL), and proteins of interest were resolved by SDS-PAGE. Western blot analysis was conducted on nitrocellulose membranes using appropriate primary and IRDye-conjugated secondary antibodies (Rockland, Gilbertsville, PA), the Odyssey infrared imaging system, and the Odyssey imaging software version 2.1 for quantification of immunoreactive band intensities (Li-Cor, Lincoln, NE). Semi-quantitative analysis of the Rab27a and Rab27b antibody reactivities to their respective purified recombinant protein was carried out.
Analysis of LG acinar cell secretion.
As a measure of regulated secretion, syncollin release was measured in LG acinar cell co-transduced with Ad-syncollin-GFP and the Xp-WT, -CA, or -DN constructs as described previously (20, 24, 29). The bathing media, which is continuous with the apical lumina of the cultured cells, was collected at time points after CCH addition. The bathing medium was concentrated on Centricon YM-10 filters (Millipore, Billerica, MA) and resolved by SDS-PAGE. A GFP signal was detected by Western blotting, and its band intensity was measured using the Odyssey imaging software. According to previously established methods (49), secretion into culture medium was normalized to total pellet protein in each well. In previous studies, we showed that only 20–30% of total cellular syncollin-GFP is released during a typical stimulation period with CCH (14, 25). This plotting format thus enables detection of differences between treatment groups and enables clear comparison to previous work in which the role of other effectors in exocytosis of syncollin-GFP (Myo5C, Rab3D, actin filaments) has been probed (14, 25, 29). In data plots, values were expressed as a percentage increase above the total release from stimulated acini of cells expressing WT in the presence of CCH at the maximal time of release (30 min), normalized to 1. Lysate samples were also resolved by SDS-PAGE to ensure equal loading by detection for actin or Xpress by Western blotting.
Statistical analysis.
Data analysis was conducted to compare between data sets using either Student’s two-sample t-test (for assays of GFP-Ad-syncollin secretion) or a one-way ANOVA followed by post hoc analysis using the Games-Howell test (PHAST TM, by Phillip R. Stanwood, Copyright 2007) (for comparison of organelle abundance in different mouse models) as appropriate. The criterion for significance was at least P < 0.05.
RESULTS
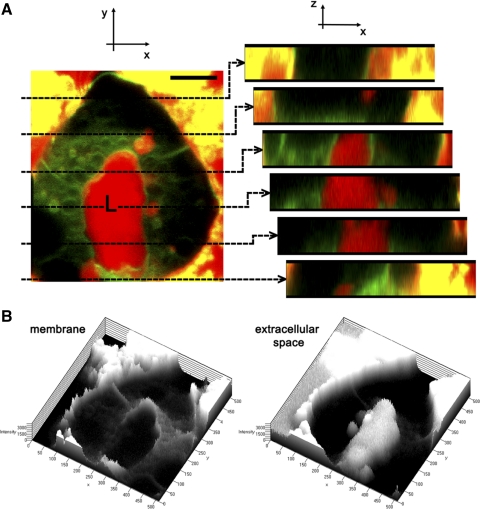
Rab27b is enriched with mature SV located in the subapical region of LG acinar cells. Like exocrine pancreatic acinar cells, the LG acinar cells are secretory epithelial cells, although they contain larger SV with more dilute and heterogeneous content than that of zymogen granules. For orientation, Fig. 1A depicts a three-dimensional reconstruction of the LG acinar cell culture model fluorescently labeled with a membrane dye that outlines individual cell borders and membrane compartments in exchange with plasma membrane, and a fluid phase tracer that, when imaged immediately after addition to the cells, delineates the extracellular space. Single optical sections through the acinus emphasize open exchange between the lumina and the culture media. Topographies of the membrane marker and of the fluid-phase marker are also shown in the same cultured acinus in Fig. 1B.
Fig. 1.
Cultured lacrimal gland (LG) acinar cells reform lumina that are in open exchange with the extracellular culture media. A: a three-dimensional reconstruction of a live, cultured LG acinus stained with FM 1-43 dye (green), which labels cell membranes, and incubated in medium in which a fluid tracer, 10-kDa Texas Red dextran (red), has been added. The membrane marker delineates cell membranes and some apical vesicular structures within the cell that have exchanged with the cell membrane during the incubation period, whereas the fluorescent fluid phase tracer is apparent in the extracellular space, which includes the lumen, but not within the acinar cells. Note that some cell debris adjacent to this acinar cluster in the image nonspecifically absorbs both fluorescent dyes. Optical sections at regular intervals through the lumen are shown beside the three-dimensional reconstruction. Bar = 5 μm. B: the topography of the cell is shown through representations of the signal intensities of both markers, depicting the membrane and extracellular space, respectively.
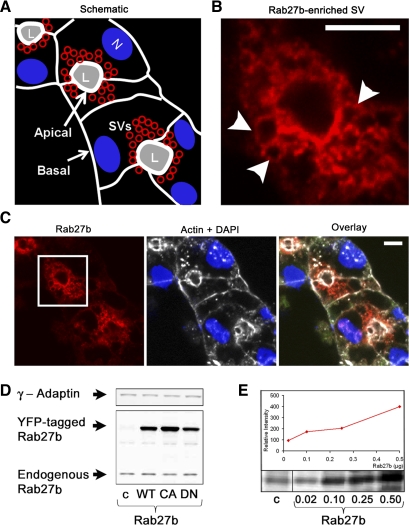
The schematic diagram shown in Fig. 2A shows the approximate orientation of cells within the reconstituted rabbit LG acinus in Fig. 2C and indicates the positioning of basolateral and apical membranes, the latter of which delineate the lumen. Numerous SV with heterogeneous protein content are located in the subapical region (24). We first analyzed the endogenous expression of Rab27 isoforms in LG acinar cells by indirect immunofluorescence in reconstituted acini (Fig. 2, B and C) and similarly in frozen LG tissue samples (data not shown). Rab27b immunofluorescence was abundant and, as seen at higher magnification (Fig. 2B), clearly localized to the membranes of apparent large mature SV located immediately beneath the APM. Although Rab27b immunofluorescence was primarily subapically enriched, traces of Rab27b labeling were also detected at the basolateral regions of the cells. Biochemical data was also consistent with imaging results. A band at ∼29 kDa representing endogenous Rab27b expression was detected in lysates from both untreated as well as virally transduced LG acinar cells expressing YFP-tagged activity variants of Rab27b (∼50 kDa) (Fig. 2, D and E). Endogenous Rab27b represented <0.001% of total protein in lysate, although this is not unreasonable compared with more abundant structural proteins such as tubulin (∼0.6% measured from chicken erythrocytes) (32). Preabsorption of primary Rab27b antibody effectively blocked any positive immunofluorescence signal (see supplement 1 available online at the Am J Physiol-Cell Physiol website). Little or no immunoreactivity was detected for the other isoform, Rab27a (see supplement 2 available online at the Am J Physiol-Cell Physiol website). There was a low level of cross reactivity between the antibodies to the individual isoforms at above-endogenous protein expression levels, with recognition of overexpressed YFP-tagged Rab27b protein by anti-Rab27a antibody at ∼10% of the Rab27b signal intensity and lower nonspecific recognition by the anti-Rab27b antibody. These data suggest that Rab27b is the predominant Rab27 isoform in LG acinar cells and is localized to subapical mature SV.
Fig. 2.
Rab27b expression is enriched on membranous structures in the subapical region of LG acinar cells. A: schematic of the reconstituted LG acinar cluster (in C) shows polarized cells that are delineated by a white outline representing cortical actin-enriched cell boundaries. The APM, which has a more highly enriched subapical actin network than the basolateral membrane, borders the lumen (L). SV (red) are located in the subapical regions, whereas nuclei (N) are more basal. B: high-magnification image of Rab27b immunofluorescence from C shows expression on apparent SV membranes in the subapical region. Arrows point to punctate structures. Bar = 5 μm. C: indirect immunofluorescence of reconstituted LG acinar cells probing for Rab27b (red), with a boxed region representing the image magnified in B. *, lumen. Endogenous Rab27b is highly enriched in the subapical region. Actin (white) and the nuclei (DAPI; blue) are shown for orientation. *, lumen; bar = 5 μm. D: fresh mouse LG lysate was prepared in RIPA buffer with protease inhibitors. Lysate protein (10 μg) from non-transduced (c) or mutant Rab27b-transduced (WT, CA, DN) LG acinar cells were resolved by SDS-PAGE and analyzed by Western blotting. Endogenous Rab27b is detected at its predicted molecular mass (∼29 kDa), and in transduced cells the tagged protein is detected at 52 kDa. γ-Adaptin was used to control for equal loading. E: serial dilution of purified recombinant His-tagged Rab27b protein in micrograms compared with endogenous Rab27b in 20 μg of lysate. Exposure to secondary antibody alone showed no bands in this region (data not shown).
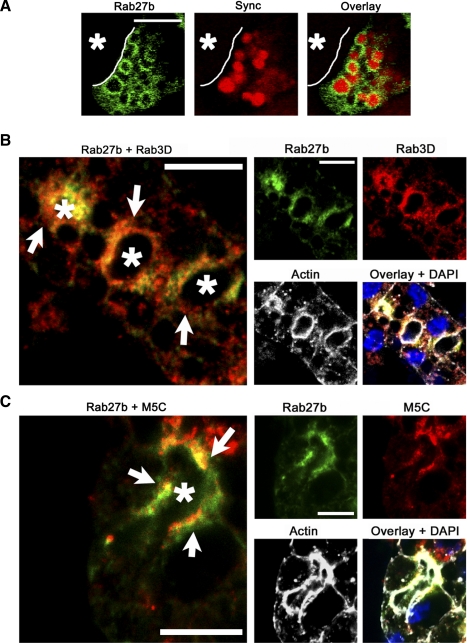
Rab27b is colocalized with SV markers in cultured LG acinar cells. Cultured rabbit LG acinar cells not only retain the polarized organization of epithelial cells but can also be efficiently transduced with replication-deficient adenovirus constructs (Ad). This enables the expression of epitope-tagged wild-type and mutant proteins of interest in these primary cells. Syncollin-GFP is an established SV content marker that has been previously used to probe aspects of SV fusion and content exocytosis in LG acinar cells and other professional secretory cells (20, 24). Although poorly retained in SV after various fixation conditions, it is clearly detectable within SV when analyzed using live-cell imaging. Real-time images of syncollin-GFP co-expressed with YFP-tagged WT Rab27b construct revealed YFP Rab27b-enriched apparent SV that retained syncollin-GFP as vesicle content (Fig. 3A). Dual immunofluorescence labeling of cultured LG acinar cells for endogenous Rab27b and other known LG SV markers revealed further ties between Rab27b and the secretory pathway. As shown in Fig. 3B, Rab27b was colocalized in the subapical region with Rab3D, an established marker of mature SV in LG acinar cells that associates with resting SV and, on stimulation, is released to the cytosol concomitant with SV fusion with the APM (50). Although colocalization between Rab27b and Rab3D was high, analysis of the labeling patterns of each suggested that Rab3D enriched a more diffusely distributed subapical vesicle pool than Rab27b. Consequently, these data reinforced previous findings that the LG acinar cell vesicle pool is heterogeneous in its markers and likely also in SV content, as has been suggested previously by electron microscopy (29).
Fig. 3.
Rab27b colocalization with SV markers is consistent with a role in LG acinar exocytotic processes. A: reconstituted LG acinar cells co-transduced with Ad constructs encoding YFP-tagged WT (green) (MOI 3–5) and a GFP-tagged syncollin construct (red) (MOI 5) show that, in cells expressing both proteins, Rab27b is enriched on membranes of apparent SV containing syncollin-GFP. Bar = 5 μm. B: immunofluorescence detection of endogenous Rab27b (green) and Rab3D (red) reveal high co-localization (arrows), although Rab3D appeared to label a larger pool of vesicles. C: immunofluorescence detection of endogenous Rab27b (green) and myosin5C (red) in LG acinar cells also showed a high degree of colocalization (arrows). Nuclei are shown in the overlay images and were detected with DAPI (blue). Actin is shown in white. Bar = 10 μm. *, Lumen.
Rab27b was also highly colocalized with the actin-based motor protein myosin 5C, and colocalization was especially prominent in the region of the cytoplasm immediately adjacent to the APM on the side of the vesicle facing the lumen (Fig. 3C). Recent work in our group has shown that myosin 5C is enriched on LG acinar cell SV and that inhibition of myosin 5C impairs CCH-stimulated SV exocytosis (29). Colocalization coefficients were quantified for comparison purposes, and values demonstrated greater colocalization in the resting state than when following CCH stimulation of exocytosis for both Rab3D and myosin 5C (Table 1). This finding, in combination with the diminished intensity of subapical labeling for both markers following CCH treatment, was consistent with the likely dissociation of Rab proteins from SV after exocytosis. For comparison, F-actin, which is a key component of the cytoskeletal network that is enriched at the cell cortex but also detected throughout the cell, was minimally colocalized with Rab27b at the APM.
Table 1.
Rab27b subapical colocalization with proteins involved in SV trafficking
| Rab3D | |
| At rest | 73.6 ± 2.0% (n = 4) |
| +CCH | 39.0 ± 7.4% (n = 4) |
| Myosin 5C | |
| At rest | 58.3 ± 8.1% (n = 6) |
| +CCH | 53.4 ± 6.1% (n = 4) |
| F-actin | |
| At rest | 41.4 ± 6.2% (n = 4) |
| +CCH | 22.0 ± 2.0% (n = 4) |
Fluorescent pixel colocalization of Rab27b with different markers in reconstituted lacrimal gland acinar cells was measured with the Zeiss enhanced colocalization tool in a region of interest defined as the luminal area within a radius equal to half of the maximal distance from the apical to basal membrane. More than four random fields of view per preparation (n) were quantified. Values are given as the percentage of total fluorescent pixels associated with each marker in the region of interest that colocalized with Rab27b. Errors represent SE. CCH, carbachol.
In vivo support of a role for Rab27b in the LG acinar cell secretory pathway.
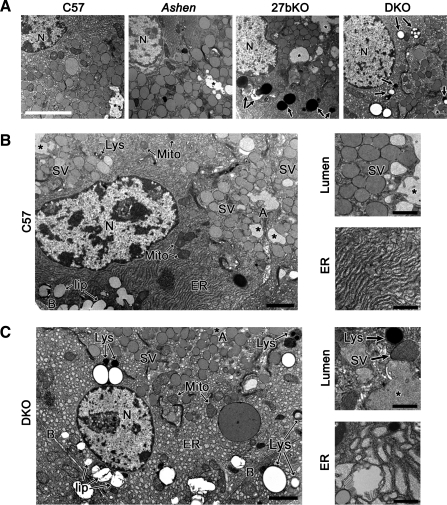
Investigation into the function of Rab27b in the LG in situ confirmed its important role in the secretory pathway. Ultrastructural morphological examinations were conducted in LG from C57BL/6J (C57), Rab27aash/ash (ashen), Rab27b−/− (27bKO), and Rab27 double knockout (Rab27aash/ashRab27b−/−; DKO) mice. In Fig. 4A, LG from C57 mice revealed an abundant array of SV adjacent to lumina, as well as normal endoplasmic reticulum (ER) and mitochondrial structure. In contrast, the LG from the 27bKO mice exhibited an apparent decrease in SV, in parallel with evidence for biosynthetic trafficking abnormalities, which included distended ER. Cell area was measured (in μm2: WT 220.1 ± 8.9; DKO 189.3 ± 5.1; 27bKO 200.1 ± 4.8) and showed no significant differences between the mice. Prominent additional dense degradative organelles, possibly lysosomes or autophagolysosomes, were also evident in the cytoplasm. The same features were observed in the DKO mouse LG but not in LG from ashen mice, further supporting the in vitro data suggesting that Rab27b rather than Rab27a plays a dominant role in the LG acinar cell secretory pathway.
Fig. 4.
27bKO and DKO mouse LG are morphologically distinguishable from those of the C57 background strain. A: electron micrographs of 27bKO and DKO mouse LG showed major morphological differences relative to LG from the C57 background strain. Ashen LG, however, were morphologically akin to those from C57. Notable changes in 27bKO and DKO mouse LG included some loss of polarity and increased expression of degradative organelles (arrows). N, nucleus; *, lumen. Bar = 10 μm. B: LG from C57 mice showed clearly polarized acinar cells with large pools of SV located just beneath the APM (A) delineating the lumen (*). Toward the basolateral membrane (B) lies the nucleus (N), mitochondria (Mito), and lipid droplets (lip). Throughout the cytoplasm, several lysosomes (Lys) and well organized endoplasmic reticulum (ER) can be detected. High magnification images show representations of the dense SV population that lies beneath the APM and ER. C: DKO LG tissue exhibited decreased cellular organization and polarity, which varied in severity from region to region. Individual SV were less apically concentrated, whereas increased lysosomes and other apparent degradative organelles were prominent. Cells also displayed swollen or vesiculated ER as shown at higher magnification along with an example of the less densely populated subapical region beside the lumen. 27bKO morphology was similar to DKO and is not shown here. At lower magnification, bar is 2 μm; at higher magnification, bar is 1 μm.
For the purpose of illustrating the more significant morphological differences, Fig. 4, B and C, compare high-magnification micrographs from C57 and DKO mouse LG. In Fig. 4B, the general organization of the acinar cell depicted from C57 mouse reflects that of a healthy, polarized cell with distinct basolateral and apical membranes, nucleus, SV (apical), ER, lysosomes, healthy mitochondria with intact cristae, and some lipid droplets shown both in the lower and higher magnification images. The high magnifications more clearly delineate the numerous SV located adjacent to the luminal regions as well as the healthy, intact ER. In contrast, DKO mouse displayed a general loss of polarity and organization, the extent of which varied somewhat from section to section (Fig. 4C). Higher magnification images represent subapical areas in the DKO, which show a decrease in the SV pool as well as many regions with either highly vesiculated or swollen ER.
Changes in SV and other organelles were quantified and are shown graphically as well as illustrated in the higher magnification images depicted in Fig. 5. In both 27bKO and DKO mouse LG relative to normal tissue, there was a significant decrease in the cross-sectional area for SV. This finding suggested in LG acinar cells lacking Rab27b that fewer SV were able to appropriately mature and/or to be stabilized and sequestered in the subapical region. Unlike C57 mouse LG samples, SV in 27bKO and DKO mouse LG samples appeared less subapically clustered. Reflecting this loss of clustering, average SV distance from the APM as a percentage of cell diameter was significantly higher in DKO (37 ± 2%) than in C57 (32 ± 2%) (P < 0.001). Cross-sectional areas positive for lysosomes, identified as highly dense structures in our images (arrows), were also significantly increased in 27bKO and DKO compared with WT. Although the total cross-sectional area of mitochondria did not appear to change, we observed across preparations that an increased proportion of the mitochondria in the 27bKO and DKO mouse LG were abnormal and showed varying degrees of either organelle swelling or cristolysis. A large cross-sectional area of dense degenerated structures was also observed in the basal regions in close proximity to the lysosomes in the 27bKO and DKO mouse LG relative to C57 control LG. These may be damaged organelles in the process of being broken down and processed, including those that represent mitochondria and other organelles in later stages of degradation. Aside from SV, there was no difference statistically in the cross-sectional areas between 27bKO and DKO organelles.
Fig. 5.
Detailed comparison and quantification of organelle morphology in mouse LG lacking Rab27b. Electron micrographs of LG acinar cells from C57, DKO, and 27bKO mouse LG sections were individually quantified using stereological guidelines for the cross-sectional areas of specific organelles of interest. High-resolution images are matched with quantifications as examples of the measured organelles. The last two sets of images are examples of abnormal mitochondria and degenerated structures, respectively. The total mitochondrial cross-sectional area is inclusive of abnormal mitochondria. Error bars represent SE. Bar = 1 μm. *Significantly different from C57BL/6 mouse LG (P ≤ 0.003). #Significantly different from DKO (P < 0.005).
The activity of Rab27b plays a role in determining Rab27b-enriched vesicle distribution within LG acinar cells. In addition to observing profound changes in general LG tissue organization and organelles in Rab27b knockout mice, Rab27b localization was also directly affected by its functional state. In LG acinar cells at resting state, endogenous Rab27b-enriched SV were primarily subapical (located immediately beneath the apical plasma membrane and luminal actin) (Fig. 6A). On stimulation with the secretagogue, CCH, these apparent SV were observed to undergo exocytosis. An acinus expressing YFP-tagged WT Rab27b incubated in culture medium containing a fluorescent fluid-phase marker accessible to the luminal space from the extracellular space is shown in supplemental movie 3 (available online at the Am J Physiol-Cell Physiol website). On stimulation with CCH, YFP-Rab27b-enriched vesicles in close proximity could be observed to merge and to become accessible to the luminal fluorescent dye, indicative of the formation of a fusion pore between apical plasma membrane and large SV formed within the cytoplasm by compound fusion. By 10 min, fixed cell images showed that CCH-stimulated cells underwent considerable loss of Rab27b signal concurrent with an increased compression of the remaining Rab27b-enriched vesicles in a region immediately adjacent to the APM (Fig. 6A). This can be more readily detected in the reconstructed three-dimensional image of the same acinus, pre- and post-CCH stimulation, shown in supplemental movie 4 (available online at the Am J Physiol-Cell Physiol website). Finally, quantification of the signal intensities of Rab27b in multiple acini showed that the mean fluorescence intensity in CCH-stimulated acini was significantly diminished to 55.3 ± 0.02% of the intensity of acini at rest (n = 8; P < 0.05), suggestive of release of Rab27b from sites on membrane where the protein was concentrated to the cytosol where the fluorescence signal fell below the threshold of detection.
Fig. 6.
Redistribution of Rab27b-enriched SV occurs when LG acinar cells are stimulated to secrete and when Rab27b function is affected. A: reconstituted LG acinar cells were treated without or with 100 μM CCH for 15 min before fixation and labeling to detect endogenous Rab27b (green), nuclei (blue), and actin (red). CCH stimulation concentrates Rab27b signal in the subapical-most region. Bar = 10 μm. B: expression of mutant Rab27b affects the distribution of SV content. Reconstituted LG acinar cells were transduced with Rab27b-YFP-tagged adenoviral constructs (MOI = 3–5) encoding full-length wild-type Rab27b (WT), a constitutively active form of Rab27b that is GTP-locked (CA), or a dominant negative GDP-locked form (DN). Co-expression of Rab27b constructs (green) with syncollin-GFP (red) showed that Rab27b mutant expression affected the distribution of syncollin-GFP and, by extension, SV content. In DN-expressing acini, little syncollin-GFP appeared as enclosed content within membrane-bounded organelles, and, when they were so encased, these organelles were not clearly subapical (arrowheads). Dashed lines, lumen; arrowheads, syncollin-GFP labeling. Bar = 5 μm.
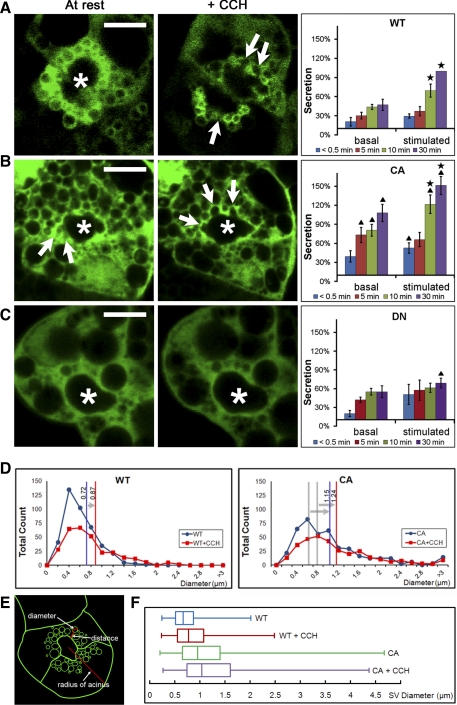
To test Rab27b’s functional role in exocytosis, we established protocols showing equivalent Ad expression of full-length WT, CA, and DN Rab27b fused to Xpress (Xp) or YFP tags as described in methods (43, 56). Although the WT construct encodes the fully functional Rab27b protein, CA proteins are locked in a GTP-bound state, and DN proteins have low affinity for both GTP and GDP.
We found that LG acinar cells transduced with mutant forms of Rab27b altered the localization and fusion of Rab27b-enriched SV. Supplemental movie 5 (available online at the Am J Physiol-Cell Physiol website) shows LG acinar cells expressing each construct pre- and post-CCH stimulation. CCH-stimulated compound fusion and exocytosis seemed comparable in acini expressing WT and CA Rab27b, although CA Rab27b showed significantly higher fluorescence intensity signal in the subapical ROI, comprising a 2.2-fold increase in fluorescence intensity relative to WT Rab27b in both resting and stimulated acini (n = 8; P < 0.05). In contrast, fewer clearly labeled subapical SV were apparent in DN-transduced acini, and little evidence for compound fusion and access of fused vesicles to the apical plasma membrane was seen. CA and DN Rab27b proteins also altered the distribution of SV content (Fig. 6B). In LG acinar cells expressing the WT protein, syncollin-GFP appeared largely enriched in the subapical region within apparent SV. In CA-transduced acini, co-transduced syncollin-GFP also appeared largely localized within subapical Rab27b-enriched SV. In contrast, DN protein expression was largely cytoplasmic and, unlike WT and CA, did not appear to be abundantly enriched with large, subapical vesicles or to demonstrate redistribution on CCH stimulation. In DN-transduced acini, syncollin-GFP labeling appeared more scattered and not clearly associated with vesicular Rab27b (arrowheads).
Expression of CA and DN Rab27b affect secretion of syncollin-GFP in the LG acinar cells.
The previous data confirmed that changes in SV exocytosis were clearly detectable in cultured LG acinar cells expressing mutant Rab27b. We then conducted a functional test for the ability of CA and DN to modulate responses to secretagogue using secretion of syncollin-GFP to assay exocytosis. As previously described, primary LG acinar cells are able to reform acinus-like structures that can be stimulated to promote apical exocytosis of mature SV and release of their contents into the extracellular bathing medium (9). Since the acinar luminal regions are contiguous with the culture medium, syncollin-GFP collected into culture medium can be used as a biochemical marker for exocytosis (25).
Relative content release was measured in LG acinar cells co-transduced with syncollin-GFP and Rab27b constructs. Release of syncollin-GFP into cell media with and without CCH stimulation over a 30-min timeframe was measured as a percentage of maximal release in stimulated cells as detailed in methods. The results are summarized in Fig. 7, A–C, in parallel with still frames from live cell images showing YFP-tagged Rab27b that illustrate the corresponding intracellular events. In Fig. 7A, a low basal level of secretion occured even during resting stages, but stimulated WT-expressing cells demonstrated increased release of syncollin-GFP detectable by 10 min and increasing to 30 min, similar to release patterns seen in previous studies (25). Corresponding still frames from live-cell movies (Fig. 7A) show that, upon CCH stimulation, vesicles immediately acquired a “squeezed” and irregular appearance (arrows) that suggested the application of external compressive force, consistent with previous findings of actin coats assembling around fusing SV (24), followed by sequential compound fusion between vesicles and also apparent fusion of vesicles with the APM. By 15 min, Rab27b-labeled vesicles were noticeably (50%) depleted in the subapical region, likely due to SV fusion and concomitant loss of membrane-associated following fusion Rab27b. Compared with the LG acinar cells expressing WT, CA-expressing LG acinar cells showed a significant increase in basal release of syncollin-GFP and an even greater response to CCH stimulation, relative to WT (Fig. 7B). The corresponding live-cell images revealed that CA in resting LG acinar cells marked subapical vesicle pools of larger average size with compound fusion events noted even in the resting state. Arrows point to the periodic “bursts” by a vesicle, signifying fusion events between vesicles or between vesicles and the APM. With CCH stimulation, the frequency of fusion events increased. In contrast, although there was no significant change in basal syncollin-GFP release, LG acinar cells expressing the DN form showed significantly decreased CCH-stimulated release of syncollin-GFP (Fig. 7C). In corresponding live-cell images, the fluorescence signal of DN in resting LG acinar cells and in CCH-stimulated LG acinar cells appeared dispersed and, unlike WT and CA, did not appear to be strongly enriched around subapically localized SV. Likewise, no movement of Rab27b-enriched membranes or fusion events was detected. A statistically insignificant initial increase in basal release seen in the biochemical data may be due to the efflux of inappropriately sequestered SV proteins, as suggested by the apparent Rab27b-independent sequestration of syncollin-GFP seen in Fig. 6B, through constitutive release pathways. Sample time-lapse videos from LG acinar cells expressing Rab27b WT and mutant forms reveal more information about the dynamics and movement of vesicles before and after CCH addition (see supplemental movie 5 available online at the Am J Physiol-Cell Physiol website).
Fig. 7.
Rab27b positively regulates SV exocytosis. A: still-frame images of live YFP-WT Rab27b-expressing acinar cells depict Rab27b expression in the subapical region at resting state, when SV are enriched within the subapical region, and 15 min after the addition of CCH, at which time SV fuse with the APM (sites of fusion are shown by arrows). In biochemical secretion studies conducted in parallel in LG acinar cells doubly expressing both Xp-WT Rab27b and syncollin-GFP, CCH stimulation significantly increased syncollin-GFP release into the cell medium (stars). For reference, only 20–30% of total cellular syncollin-GFP is secreted within this stimulation time as we have shown in previous studies (25). *, Lumen; bar = 10 μm; n = 7. B: in still-frame images, SV labeled with YFP-tagged CA Rab27b showed increased evidence for fusion with the APM even at resting state and also after CCH stimulation. Corresponding secretion data showed that LG acinar cells expressing the CA form responded with a significant increase in secretion in response to CCH addition (stars) but also exhibited significantly more secretory activity relative to LG acinar cells expressing the WT Rab27b both in the resting and in the CCH-stimulated state (triangles). *, Lumen; bar = 10 μm; n = 7. C: in still-frame images, YFP-tagged DN Rab27b did not appear to label subapical SV. In the biochemical secretion study, DN-expressing cells also displayed a loss of response to CCH stimulation, and secretion levels were significantly less than WT by 30 min (triangles). *, Lumen; bar = 10 μm; n = 6. D and E: the diameters of Rab27b enriched SV membranes (green) which, in the schematic shown, included all SV apparent in the plane within half the maximal radius of the cell from the center of the lumen, were measured in resting acini and at 10 min after CCH stimulation. DN was not quantified because Rab27b signal was diffuse. WT: 412 SV; WT+CCH: 297 SV; CA: 431 SV; CA+CCH: 324 SV; n = 12. F: SV diameter measurements were summarized in a box-and-whisker plot. CA SV diameter was significantly greater at both resting and stimulated phases compared with WT. For all data, values are means ± SE; P < 0.05.
To further characterize the nature of WT and CA Rab27b-enriched SV, we measured Rab27b-enriched SV diameters from time-lapse videos of LG acinar cells at two time points: 10 min before CCH addition and 15 min after CCH stimulation (Fig. 7, D–F). In LG acinar cells transduced with WT, YFP Rab27b-enriched SV were ∼0.72 μm in diameter and increased in size to an average of 0.87 μm with CCH stimulation, consistent with previous data suggesting compound fusion before extrusion of SV contents in these cells (24). In contrast, YFP-Rab27b-labeled SV in resting LG acinar cells transduced with CA showed a greater average diameter (30%) than WT, although they still responded to CCH with a slight increase in size as did SV in LG acinar cells expressing WT (arrows). Diameters of SV enriched in the DN form of Rab27b were not measurable because the DN protein was not recruited to SV membrane; labeling was significantly dispersed into the cytoplasm.
Evaluation of these imaging and biochemical studies of syncollin-GFP content release led us to believe that the increased release of syncollin-GFP and the movement and fusion events of SV at the APM were collectively tied to the increased apical exocytosis that occurred upon stimulation of these acinar cells.
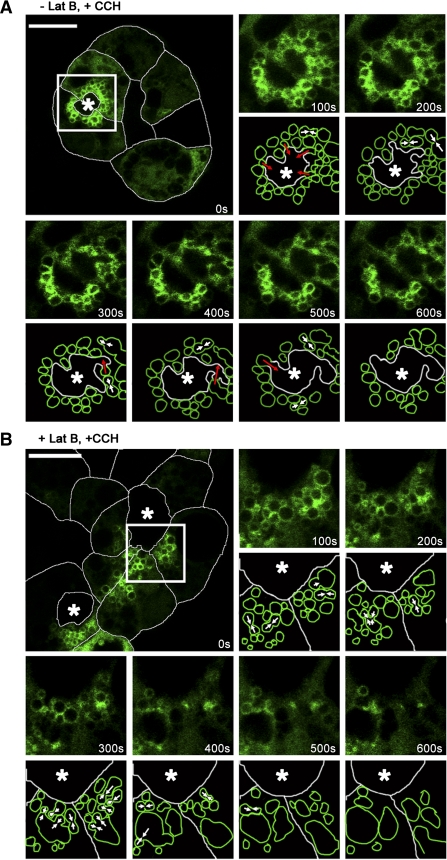
Terminal fusion of Rab27b-enriched SV requires actin filaments. We investigated the role of actin in the trafficking of Rab27b-enriched SV by treating YFP-tagged WT expressing cells with latrunculin B, which disrupts F-actin polymerization (24). Results indicated that latrunculin B had little effect on the ability of the cells to accumulate large subapical SV after depletion of existing SV with CCH stimulation (data not shown). However, the addition of latrunculin B affected the final stages of Rab27b-enriched SV fusion. Live cells were imaged in time-series during resting stages and with CCH stimulation for 15 min. Results shown in Fig. 8 and supplemental movie 6 (available online at the Am J Physiol-Cell Physiol website) show LG acinar cells with and without latrunculin B treatment after CCH stimulation. In untreated cells (Fig. 8A), SV appeared localized within the subapical region at the moment of stimulation. As the insets show, SV immediately began to undergo multiple instances of compound fusion as well as fusion with the APM over a 10-min period. Vesicle fusion with the APM was reflected in the morphological distortion of the lumen over time. In Fig. 8B, latrunculin B-treated cells also showed a large subapical pool of SV at the moment of stimulation. However, differences between treatments were most apparent in the 10 min after stimulation. While homotypic fusion occurred, fusion events occurred without the apical orientation seen in nontreated cells, and few, if any, apparent fusion events were detected with the APM. Over time, compound fusion led to the production of large SV aggregates or possible vacuoles, some of which had only a weak Rab27b labeling on the surface. This suggested that, although Rab27b-enriched SV maturation and trafficking to the APM was intact, the process of docking to the APM was either directly or indirectly impaired by actin depolymerization.
Fig. 8.
Disruption of the actin network alters terminal apical membrane fusion of Rab27b-enriched SV. A: an untreated acinus expressing YFP-tagged Rab27b was imaged in time-series immediately upon and after stimulation. Transduced cells expressing Rab27b (green) are outlined with white to delineate the cell borders. The remaining images in the series are higher magnifications of the boxed region, along with matching schematics that represent the events described. Within 100 s after CCH addition, the region around the lumen began to constrict and SV moved toward the lumen, fusing with each other (white arrows) and with the APM (red arrows). Within 600 s, ∼50% of the SV were in apparent contiguity with the lumen, suggesting discharge of contents. B: an acinus treated with 10 μM latrunculin B for 60 min at 37°C was similarly imaged. With CCH addition, SV failed to move toward the lumen. Although many SV underwent homotypic fusion (white arrows), membrane of internal fused SV failed to fuse with the APM. *, Lumen. Bar = 10 μm.
DISCUSSION
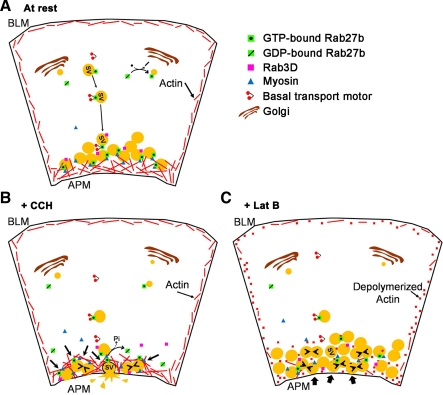
A working model of the role of Rab27b in SV exocytosis of acinar cells is shown in Fig. 9 based on key findings highlighted throughout results including co-localization of Rab27b on vesicles with other SV markers, demonstration that these apparent SV engage in compound fusion and fusion with the APM, and confirmation that changes in Rab27b activity affect secretory protein release. Key features of the model include, in the resting phase (Fig. 9A), some enrichment of active Rab27b on early stage SV located in the basal regions of the cell and co-localization of Rab27b and myosin 5C on apical SV some of which are also labeled with Rab3D. Rab27b appears to be recruited only to a subpool of the total SV population. This is supported by the fact that we see marked, but not complete, co-localization between endogenous Rab27b and other mature SV markers such as Rab3D.
Fig. 9.
Schematic of Rab27b-enriched SV trafficking in the LG acinar cell. A: active Rab27b enriched on SV are transported to the subapical cytoplasm where they accumulate in a subapical pool as mature SV, some of which are also enriched in Rab3D. B: upon stimulation to secrete, Rab27b-enriched SV interact with actin filaments through an associated myosin motor, possibly myosin 5C. Since actin remodeling occurs as a result of stimulation to secrete, SVs are compressed with each other and undergo compound fusion, concurrent with a loss of Rab3D and release of Rab27b upon conversion of bound GTP to GDP. Simultaneously, actin compression toward the APM enables SV docking and fusion with the APM, followed by release of vesicle content into the lumen. C: when actin polymerization is disrupted, trafficking from the basal region to the subapical membrane appears intact. Upon stimulation to secrete, SV appear to accumulate beneath the APM and come into close contact with each other, which allows for some compound fusion (small arrowheads). More significantly, SV do not appear to be able to undergo fusion with the APM and the lumen shape does not change. Bold arrows represent blocked steps of the trafficking pathway.
Rab27b-enriched SV fuse with each other and with the APM in CCH-stimulated acini (Fig. 9B), whereas depolymerization of actin affects the final APM fusion event but not the homotypic fusion (Fig. 9C). Previous work in LG acinar cells has suggested that CCH increases apical actin filament turnover and promotes the transient assembly of actin coats around SV fusion intermediates during exocytosis (24, 35). We propose that, in CCH-treated acini, actin is responsible for the subapical compression of Rab27b-enriched SV, which results in the “squeezed” appearance of SV seen in live-cell videos, concurrent with the actin restructuring associated with fusion and release of vesicle contents. There are several explanations for how latrunculin B affects SV fusion, including the possibility that the resulting accumulation of primed and activated SV unable to access the APM undergo homotypic fusion simply by proximity. Non-exclusively, an intact actin structural network may also be necessary for SV docking. A more convoluted explanation is based on a previous study in which latrunculin B increased basal secretion of syncollin (24). Although there were no definite indications for this in this study, it is possible that the sequestration of actin filaments somehow promotes a higher level of basal release of Rab27b-enriched SV. Perhaps increased accessibility to fusion intermediates in the accumulating SV pools results in prestimulated release of the SV pool.
There has been speculation regarding overlapping functional characteristics between specific Rabs. Several studies indicate related roles for Rab27 and Rab3 family proteins in diverse systems (19, 28, 36) while conversely other work indicates that Rab27a and Rab3A have different functions in insulin secretion (54). Our previous exploration of Rab3D, which is highly expressed in LG acinar cells, found that expression of DN Rab3D only modestly affected secretion and that CA Rab3D did not significantly alter secretion (14). Analogous to work published by Riedel et al. in exocrine pancreas (38), our findings in LG from Rab3DKO mouse demonstrated a completely different series of morphological changes relative to those seen for 27bKO, most notably a significant increase in SV size and no other evidence for altered cellular homeostasis (unpublished data; Chiang L, Ngo J, and Hamm-Alvarez SF). Although Rab3D and Rab27b appear to co-localize on LG acinar cells SV (Fig. 3), there must clearly be functional distinctions between these different Rabs.
The loss of functional Rab27b appeared to affect cell morphology beyond the secretory pathway. In addition to a general loss of cell polarity in both DKO and 27bKO tissue (Fig. 4) and in DN-Rab27b transduced cells (Figs. 6 and 7), we also found evidence of induced degradative compartments in 27bKO and DKO tissue including lysosomes and autophagosomal-like membrane-enfolded structures consistent with the induction of autophagy. We offer two hypotheses to explain this possible induction of autophagy that are not mutually exclusive. First, dysfunction of Rab27b impairs the formation and budding of nascent SV from biosynthetic compartments, resulting in an accumulation of improperly sorted secretory protein within these compartments and causing biosynthetic stress. Compensatory mechanisms, namely autophagy, are activated to degrade the excess protein, perhaps in a similar mechanism to that seen in the knockout of Rab3A in pancreatic islets (30). Alternatively, the loss of Rab27b may result in a more general failure of cellular energy homeostasis. The major function of LG acinar cells is to exocytose critical tear proteins and fluid, and so a significant proportion of the cell’s volume and energy is dedicated to the production, sorting, and maturation of SV. Rab27b disfunction impairs not only the SV trafficking route but may result in an imbalance in the entire cell system. In attempts to reestablish homeostasis by increasing the rate of metabolism to compensate for the apparent inability to exocytose and/or to facilitate post-Golgi sorting, mitochondria may become stressed, thus inducing higher organelle turnover rates. This second hypothesis is evidenced by the significant number of swollen or cristolysed mitochondria, as well as swollen ER in many areas of these LG acinar cells. A final possibility is that Rab27b actually plays a role in maintenance of mitochondria in some capacity, although this has never been suggested in previous studies.
Although we did not extensively explore the specific role of the Rab27a isoform for which detection was low in LGAC (see supplement 2 available online at the Am J Physiol-Cell Physiol website), it was notable that deficiency of Rab27a alone did not result in the same profound changes in the secretory pathway and LG acinar cell homeostasis. However, in our quantitative analyses of organelle abundances, the significant decreases observed in 27bKO mouse LG were accentuated in DKO mouse LG. This change was statistically significant for SV and a notable trend for other parameters, possibly indicating some unidirectional isoform compensation. These changes in LG acinar cells associated with the knockout of Rab27b contribute to the continuing discussion in the literature regarding compensatory vs. distinct functions that have been brought up in studies of other cell types (1, 34, 51).
The direct link between the clinical manifestations of dry eye and the decreased secretory function of the LG suggest that fundamental studies on secretory mechanisms within the LG acinar cells are necessary for the development of better therapeutic strategies for treatment of dry eye (15, 52). Although we focused mainly on the morphological changes in LG of 27bKO and DKO mice, preliminary studies characterizing DKO mice suggest that these mice exhibit alterations in tears that may be indicative of dry eye disease, although the mechanism is likely complex. In our initial study, we collected both basal and stimulated tears from several C57 and DKO mice. Although basal tear volumes were similar, the average stimulated tear volume, collected by a topical application of CCH to the exposed glands, from DKO mice was almost twice that of C57 (n = 5; P < 0.05). Dry eye disorders in humans can include symptoms of increased tear flow through compensatory changes in the ocular surface, which compensate for decreased LG acinar cell function, although the tears are of reduced quality (16, 26). The protein composition and quality of tears (appropriate balance between aqueous and lipid layers), as much as the actual aqueous tear flow, are true hallmarks of dry eye disease. We also analyzed IgG, which is present only at low levels in normal tears (2). As shown in supplement 7 (available online at the Am J Physiol-Cell Physiol website), DKO mice had significantly high levels of IgG (n = 4; P < 0.05) in stimulated tears compared with matched tear samples from C57 mice. This suggests the possibility of abnormal trafficking of non-tear proteins into tears of DKO mice and/or the development of compensatory mechanisms to sustain fluid flow including altered transcellular or paracellular transport. To definitively classify DKO mice as a model for dry eye disease, however, more extensive studies involving significant numbers of mice will be needed to examine both the detailed composition of the tears as well as the functional changes in the ocular surface.
This study establishes Rab27b as a positive regulator of LG acinar cell exocytosis through detailed imaging systems supported by biochemical and functional studies. This is also the first demonstration of a phenotype associated with loss of Rab27b in any exocrine tissue, substantiating its role in exocrine secretory trafficking.
GRANTS
This work was supported by the National Eye Institute Grants RO1-EY-011386 (to S. F. Hamm-Alvarez) and RO1-EY-10550 (to J. E. Schechter). L. Chiang was also supported in 2007 by a Student Fellowship from the Sjögren’s Syndrome Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Francie Yarber for cell preparations and Ad purification, Srikanth Janga for contributing to mouse tear collection and analysis of data, John Williams for recombinant Ad-Xpress-tagged Rab27b constructs, Chris Rhodes for the recombinant Ad-syncollin-GFP constructs, Mike Pidgeon for expert help with transmission electron microscopy, Kaijin Wu for valuable input in molecular biology and unpublished microarray data, and Roberto Weigert for appreciated guidance on stereological methods.
REFERENCES
- 1. Barral DC, Ramalho JS, Anders R, Hume AN, Knapton HJ, Tolmachova T, Collinson LM, Goulding D, Authi KS, Seabra MC. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest 110: 247–257, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berman ER. Biochemistry of the Eye (1991 ed.) New York: Plenum, 1991, p. 479 [Google Scholar]
- 3. Charleston JS. Estimating cell number in the central nervous system by stereological methods: the optical disector and fractionator. Curr Protocols Toxicol: 12.6.1–12.6.19, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Chen D, Guo J, Miki T, Tachibana M, Gahl WA. Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem Mol Med 60: 27–37, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Edwards JA, Logsdon CD, Ernst SA, Williams JA. Dominant negative Rab3D inhibits amylase release from mouse pancreatic acini. J Biol Chem 277: 18002–18009, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chen X, Li C, Izumi T, Ernst SA, Andrews PC, Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem Biophys Res Commun 323: 1157–1162, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics 5: 306–312, 2006 [DOI] [PubMed] [Google Scholar]
- 8. da Costa SR, Wu K, Veigh MM, Pidgeon M, Ding C, Schechter JE, Hamm-Alvarez SF. Male NOD mouse external lacrimal glands exhibit profound changes in the exocytotic pathway early in postnatal development. Exp Eye Res 82: 33–45, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Costa SR, Yarber FA, Zhang L, Sonee M, Hamm-Alvarez SF. Microtubules facilitate the stimulated secretion of beta-hexosaminidase in lacrimal acinar cells. J Cell Sci 111: 1267–1276, 1998 [DOI] [PubMed] [Google Scholar]
- 10. de Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol 7: R72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deacon SW, Gelfand VI. Of yeast, mice, men. Rab proteins and organelle transport. J Cell Biol 152: F21–F24, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deneka M, Neeft M, van der Sluijs P. Regulation of membrane transport by rab GTPases. Crit Rev Biochem Mol Biol 38: 121–142, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Edman MC, Marchelletta RR, Hamm-Alvarez SF. Ocular surface: lacrimal gland overview. In: Encyclopedia of the Eye ( 1st ed.), edited by Besharse J, Dana R, Dartt DA. New York: Elsevier, 2010 [Google Scholar]
- 14. Evans E, Zhang W, Jerdeva G, Chen CY, Chen X, Hamm-Alvarez SF, Okamoto CT. Direct interaction between Rab3D and the polymeric immunoglobulin receptor and trafficking through regulated secretory vesicles in lacrimal gland acinar cells. Am J Physiol Cell Physiol 294: C662–C674, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox RI, Stern M. Sjogren’s syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand J Rheumatol Suppl: 3–13, 2002 [PubMed] [Google Scholar]
- 16. Gaffney EA, Tiffany JM, Yokoi N, Bron AJ. A mass and solute balance model for tear volume and osmolarity in the normal and the dry eye. Prog Retin Eye Res 29: 59–78, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Gierow JP, Lambert RW, Mircheff AK. Fluid phase endocytosis by isolated rabbit lacrimal gland acinar cells. Exp Eye Res 60: 511–525, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Gierow JP, Yang T, Bekmezian A, Liu N, Norian JM, Kim SA, Rafisolyman S, Zeng H, Okamoto CT, Wood RL, Mircheff AK. Na-K-ATPase in lacrimal gland acinar cell endosomal system: correcting a case of mistaken identity. Am J Physiol Cell Physiol 271: C1685–C1698, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Handley MT, Burgoyne RD. The Rab27 effector Rabphilin, unlike Granuphilin and Noc2, rapidly exchanges between secretory granules and cytosol in PC12 cells. Biochem Biophys Res Commun 373: 275–281, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Hodel A, Edwardson JM. Targeting of the zymogen-granule protein syncollin in AR42J and AtT-20 cells. Biochem J 350: 637–643, 2000 [PMC free article] [PubMed] [Google Scholar]
- 21. Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol 152: 795–808, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J Cell Sci 117: 1945–1953, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Izumi T, Gomi H, Kasai K, Mizutani S, Torii S. The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct Funct 28: 465–474, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Jerdeva GV, Wu K, Yarber FA, Rhodes CJ, Kalman D, Schechter JE, Hamm-Alvarez SF. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci 118: 4797–4812, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jerdeva GV, Yarber FA, Trousdale MD, Rhodes CJ, Okamoto CT, Dartt DA, Hamm-Alvarez SF. Dominant-negative PKC-epsilon impairs apical actin remodeling in parallel with inhibition of carbachol-stimulated secretion in rabbit lacrimal acini. Am J Physiol Cell Physiol 289: C1052–C1068, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res 23: 449–474, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Ma L, Bindokas VP, Kuznetsov A, Rhodes C, Hays L, Edwardson JM, Ueda K, Steiner DF, Philipson LH. Direct imaging shows that insulin granule exocytosis occurs by complete vesicle fusion. Proc Natl Acad Sci USA 101: 9266–9271, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahoney TR, Liu Q, Itoh T, Luo S, Hadwiger G, Vincent R, Wang ZW, Fukuda M, Nonet ML. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell 17: 2617–2625, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marchelletta RR, Jacobs DT, Schechter JE, Cheney RE, Hamm-Alvarez SF. The Class V myosin motor, Myosin 5c, localizes to mature secretory vesicles and facilitates exocytosis in lacrimal acini. Am J Physiol Cell Physiol 295: C13–C28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marsh BJ, Soden C, Alarcon C, Wicksteed BL, Yaekura K, Costin AJ, Morgan GP, Rhodes CJ. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol 21: 2255–2269, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25: 173–176, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Murphy DB, Wallis KT. Isolation of microtubule protein from chicken erythrocytes and determination of the critical concentration for tubulin polymerization in vitro and in vivo. J Biol Chem 258: 8357–8364, 1983 [PubMed] [Google Scholar]
- 33. Nyengaard JR. Stereologic methods and their application in kidney research. JASN 10: 1100–1123, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12: 19–30, 11–13, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Ota I, Zoukhri D, Hodges RR, Rios JD, Tepavcevic V, Raddassi I, Chen LL, Dartt DA. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. Am J Physiol Cell Physiol 284: C168–C178, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 313: 889–901, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Ramalho JS, Tolmachova T, Hume AN, McGuigan A, Gregory-Evans CY, Huxley C, Seabra MC. Chromosomal mapping, gene structure and characterization of the human and murine RAB27B gene. BMC Genet 2: 2, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riedel D, Antonin W, Fernandez-Chacon R, Alvarez de Toledo G, Jo T, Geppert M, Valentijn JA, Valentijn K, Jamieson JD, Sudhof TC, Jahn R. Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol Cell Biol 22: 6487–6497, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanghi S, Kumar R, Lumsden A, Dickinson D, Klepeis V, Trinkaus-Randall V, Frierson HF, Jr, Laurie GW. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol 310: 127–139, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic 5: 393–399, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Seabra MC, Ho YK, Anant JS. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J Biol Chem 270: 24420–24427, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol 152: 825–834, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suda J, Zhu L, Okamoto C, Karvar S. Rab27b localizes to the tubulovesicle membranes of gastric parietal cells and regulates acid secretion. Gastroenterology 140: 868–878, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci USA 104: 5872–5877, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. A general role for Rab27a in secretory cells. Mol Biol Cell 15: 332–344, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Valentijn JA, Valentijn K, Pastore LM, Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci USA 97: 1091–1095, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Setten GB, Viinikka L, Tervo T, Pesonen K, Tarkkanen A, Perheentupa J. Epidermal growth factor is a constant component of normal human tear fluid. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmology 227: 184–187, 1989 [DOI] [PubMed] [Google Scholar]
- 48. Vilalta PM, Zhang L, Hamm-Alvarez SF. A novel taxol-induced vimentin phosphorylation and stabilization revealed by studies on stable microtubules and vimentin intermediate filaments. J Cell Sci 111: 1841–1852, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Chiu CT, Nakamura T, Walker AM, Petridou B, Trousdale MD, Hamm-Alvarez SF, Schechter JE, Mircheff AK. Elevated prolactin redirects secretory vesicle traffic in rabbit lacrimal acinar cells. Am J Physiol Endocrinol Metab 292: E1122–E1134, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Jerdeva G, Yarber FA, da Costa SR, Xie J, Qian L, Rose CM, Mazurek C, Kasahara N, Mircheff AK, Hamm-Alvarez SF. Cytoplasmic dynein participates in apically targeted stimulated secretory traffic in primary rabbit lacrimal acinar epithelial cells. J Cell Sci 116: 2051–2065, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Westbroek W, Lambert J, De Schepper S, Kleta R, Van Den Bossche K, Seabra MC, Huizing M, Mommaas M, Naeyaert JM. Rab27b is up-regulated in human Griscelli syndrome type II melanocytes and linked to the actin cytoskeleton via exon F-Myosin Va transcripts. Pigment Cell Res 17: 498–505, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Wu K, Jerdeva GV, da Costa SR, Sou E, Schechter JE, Hamm-Alvarez SF. Molecular mechanisms of lacrimal acinar secretory vesicle exocytosis. Exp Eye Res 83: 84–96, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Xie J, Chiang L, Contreras J, Wu K, Garner JA, Medina-Kauwe L, Hamm-Alvarez SF. Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini. J Virol 80: 11833–11851, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol 22: 1858–1867, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. Monitoring autophagy by electron microscopy in mammalian cells. Methods Enzymol 452: 143–164, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Zhao S, Torii S, Yokota-Hashimoto H, Takeuchi T, Izumi T. Involvement of Rab27b in the regulated secretion of pituitary hormones. Endocrinology 143: 1817–1824, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.