Abstract
Opioid analgesics elicit their effects via activation of the mu-opioid receptor (MOR), a G protein-coupled receptor known to interact with Gαi/o-type G proteins. Work in vitro has suggested that MOR couples preferentially to the abundant brain Gαi/o isoform, Gαo. However, studies in vivo evaluating morphine-mediated antinociception have not supported these findings. The aim of the present work was to evaluate the contribution of Gαo to MOR-dependent signaling by measuring both antinociceptive and biochemical endpoints in a Gαo null transgenic mouse strain. Male wild-type and Gαo heterozygous null (Gαo +/−) mice were tested for opioid antinociception in the hot plate test or the warm-water tail withdrawal test as measures of supraspinal or spinal antinociception, respectively. Reduction in Gαo levels attenuated the supraspinal antinociception produced by morphine, methadone, and nalbuphine, with the magnitude of suppression dependent on agonist efficacy. This was explained by a reduction in both high-affinity MOR expression and MOR agonist-stimulated G protein activation in whole brain homogenates from Gαo +/− and Gαo homozygous null (Gαo −/−) mice, compared with wild-type littermates. On the other hand, morphine spinal antinociception was not different between Gαo +/− and wild-type mice and high-affinity MOR expression was unchanged in spinal cord tissue. However, the action of the partial agonist nalbuphine was compromised, showing that reduction in Gαo protein does decrease spinal antinociception, but suggesting a higher Gαo protein reserve. These results provide the first in vivo evidence that Gαo contributes to maximally efficient MOR signaling and antinociception.
Keywords: morphine, antinociception, mu-opioid receptor, G protein, signaling, transgenic mice
INTRODUCTION
Opioid analgesics are prescribed for the management of moderate to severe pain. Clinically used opioids elicit their effects by stimulation of the mu-opioid receptor (MOR), a member of the G protein-coupled receptor superfamily that interacts with heterotrimeric G proteins (Gαβγ), which are defined in terms of the Gα subunit. Specifically, MOR couples to Gα proteins of the pertussis toxin-sensitive Gαi/o family, which comprises Gαo (including splice variants Gαo1 and Gαo2), Gαi1, Gαi2, and Gαi3 (Laugwitz et al, 1993; Chakrabarti et al, 1995), as well as pertussis toxin-insensitive Gαz (Garzon et al, 1997). In the inactive state, Gαβγ exists in complex with the receptor. On agonist stimulation, GDP bound to the Gα subunit is exchanged for GTP, resulting in dissociation of active Gα-GTP from the Gβγ heterodimer (reviewed in Brown and Sihra, 2008); both Gα-GTP and Gβγ modulate effectors downstream of MOR, including adenylyl cyclase (AC) (Yu and Sadee, 1988) and calcium channels (Hescheler et al, 1987; Moises et al, 1994). It has been shown that specific Gαi/o subunits differentially contribute to MOR-dependent behavioral responses, including morphine-mediated antinociception (Raffa et al, 1994; Sanchez-Blazquez et al, 2001). However, findings are inconsistent because of the variety of methods and models utilized in previous work, such that the contribution of each Gα subunit to these responses is controversial.
Gαo is highly expressed in brain (Gierschik et al, 1986). Multiple lines of evidence suggest that opioid agonists can activate MOR-G protein complexes in a non-selective manner, especially in heterologous expression systems (Laugwitz et al, 1993; Clark et al, 2006a; Clark and Traynor, 2006b). On the other hand, the MOR-selective agonist [-Ala2,N-MePhe4,Gly-ol5]enkephalin (DAMGO) was found to activate Gαo to a greater extent than either Gαi2 or Gαi3 (Clark et al, 2008). Furthermore, in cultured neurons or neuronal-like cells, MOR has been shown to couple to AC (Carter and Medzihradsky, 1993) and N-type Ca2+ channels (Hescheler et al, 1987; Moises et al, 1994) primarily via activation of Gαo.
Despite the abundance of Gαo in the brain and evidence from in vitro studies that Gαo modulates signaling downstream of MOR, together with a recent report that Gαo may be involved in opioid dependence (Kest et al, 2009), findings in vivo have primarily implicated Gαi2 and/or Gαz proteins as mediators of opioid agonist antinociception (Raffa et al, 1994; Sanchez-Blazquez et al, 1995, 2001; Standifer et al, 1996). These studies utilized mice administered i.c.v. antisense oligodeoxynucleotides (ODNs) against a specific Gα subunit before antinociceptive testing of opioid agonists (i.c.v.) in the tail flick test (reviewed in Garzon et al, 2000). However, there are a number of inherent difficulties with this technique, including proper verification of the extent of protein knockdown. For most of these studies, knockdown of Gα protein did not exceed ∼50% in peri-ventricular regions (eg, periaqueductal gray) (Sanchez-Blazquez et al, 1995), while ODNs were less effective in brain regions more distal to the site of infusion (eg, thalamus), presumably due to poor diffusion (Sanchez-Blazquez et al, 1995; Standifer et al, 1996). However, in one study, in which greater (∼60–80%) knockdown of Gα subunits was achieved, ODNs directed against Gαo, in addition to other Gα isoforms, suppressed morphine antinociception (Standifer et al, 1996). Clearly, inconsistencies in the efficacy and selectivity of Gα protein knockdown complicate the interpretation of these studies. This previous work is further limited in that only a single measure of opioid antinociception was evaluated.
This study was designed to test the hypothesis that MOR coupling to Gαo is necessary for opioid antinociception using a constitutive Gαo knockout mouse strain (Duan et al, 2007). To probe the role of Gαo in MOR-mediated antinociception, opioid spinal and supraspinal antinociception were evaluated in response to noxious thermal stimuli; this is the first time that mice null for Gαo have been evaluated for alterations in MOR-dependent antinociception. Furthermore, to directly relate changes in opioid antinociception to alterations in MOR function, membrane homogenates from either whole brain or spinal cord of Gαo transgenic mice were evaluated for MOR expression and MOR agonist-stimulated G protein activity. These studies demonstrate that the abundant brain G protein, Gαo, is the primary Gα subtype responsible for MOR-mediated signaling and antinociception.
MATERIALS AND METHODS
Transgenic Mice
Transgenic mice null for Gnao1 (Gαo −/−), Gnai2 (Gαi2 −/−), or Gnai3 (Gαi3 −/−) were generated as previously described (Mortensen et al, 1992; Sowell et al, 1997; Duan et al, 2007) and were backcrossed onto the 129S6/SvEvTac strain for four generations. Transgenic mice and wild-type littermates were obtained by heterozygous breeding to control for genetic background. Adult, opioid-naïve male mice, matched for age, were utilized for all experiments. Mice were group-housed with food and water available ad libitum. Lights were maintained on a 12-h light–dark cycle (lights on at 0700 hours), and all testing was performed during the light phase. Studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and all experimental protocols were approved by the University of Michigan Committee on the Use and Care of Animals.
Antinociceptive Tests
The hot plate test was used to evaluate supraspinal antinociception. Mice were given two injections of saline (i.p.) to determine baseline latency, followed by three cumulative doses of agonist (i.p.) in 15-min intervals (nalbuphine) or 30-min intervals (morphine and methadone). Where four doses of drug were used, dose-effect curves were generated by pooling data from two overlapping, cumulative dose-effect measurements. Mice were placed on a 52 or 55 °C hot plate at the appropriate interval following each injection and the latency to lick forepaw(s) or jump was measured with a cutoff time of 60 or 45 s for the 52 or 55 °C hot plate temperatures, respectively, in order to prevent tissue damage.
The warm-water tail withdrawal test was used to evaluate spinal antinociception. Mice were given a single injection of saline (i.p.) to determine baseline latency, followed by four cumulative doses of agonist (i.p.) in 15-min intervals (nalbuphine) or 30-min intervals (morphine). The distal tip of the mouse's tail was placed in a 50 or 55 °C warm-water bath at the appropriate interval following each injection and the latency to tail flick was measured with a cutoff time of 20 or 15 s for 50 or 55 °C water, respectively, in order to prevent tissue damage.
For both antinociceptive tests, agonist-stimulated antinociception is expressed as a percentage of maximum possible effect (% MPE), where % MPE=(post-drug latency−baseline latency) ÷ (cutoff latency−baseline latency) × 100.
Membrane Preparation
Mice were euthanized by cervical dislocation. Whole brain tissue, minus cerebellum, or thoracic and lumbar spinal cord was removed, immediately chilled in ice-cold 50 m Tris base, pH 7.4, and membrane homogenates were prepared as previously described (Lester and Traynor, 2006). Final membrane pellets were resuspended in 50 m Tris base, pH 7.4, aliquoted and stored at −80 °C. Protein content was determined using the method of Bradford (1976).
Western Blot Analysis
Membranes from whole brain (20 μg protein) were mixed with sample buffer (63 m Tris base, pH 6.8, 2% SDS, 10% glycerol, 0.008% bromophenol blue, and 50 m dithiothreitol) and separated by SDS-PAGE on 10% (for detection of Gαo, Gαz, Gαi1, Gαi2, or Gβ1−4) or 15% polyacrylamide gels (for detection of Gγ2). Proteins were then transferred to nitrocellulose membranes (Pierce, Rockford, IL) and probed with either rabbit polyclonal anti-Gαo (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-Gαz (1 : 200; Santa Cruz), rabbit polyclonal anti-Gαi1 (1 : 100; Santa Cruz), mouse monoclonal anti-Gαi2 (1 : 1000; Millipore, Billerica, MA), rabbit polyclonal anti-Gβ1−4 (1 : 500; Santa Cruz), or rabbit polyclonal anti-Gγ2 (1 : 200; Santa Cruz). Membranes from spinal cord (20 μg protein) were also evaluated for Gαo protein content, as above. All membranes were probed with mouse monoclonal anti-α-tubulin (1 : 1000; Sigma-Aldrich, St Louis, MO) as a loading control. Membranes were then incubated with horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibody (1 : 10 000; Santa Cruz). Antibody immunoreactivity was detected by enhanced chemiluminesence using an EpiChem3 Benchtop Darkroom (UVP, Upland, CA) and band densities were quantified using Image J software (http://rsbweb.nih.gov/ij/index.html). Specifically, after background chemiluminesence was subtracted, G protein band densities were normalized to respective α-tubulin band densities and used to calculate expression relative to wild type for each G protein.
Radioligand-Binding Assays
For [3H]diprenorphine ([3H]DPN) binding, membranes from whole brain (100 μg protein) or spinal cord (100–200 μg protein) were incubated for 60 min at 25 °C with 4 n [3H]DPN in 50 m Tris base, pH 7.4, with or without the MOR-selective antagonist D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP; 300 n) to define MOR. For [3H]DAMGO saturation binding, membranes from whole brain (100 μg protein) were incubated for 60 min at 25 °C with increasing concentrations of [3H]DAMGO (0.09–12 n) in 50 m Tris base, pH 7.4. Membranes from spinal cord (100–200 μg protein) were incubated for 60 min at 25 °C with 12 n [3H]DAMGO in 50 m Tris base, pH 7.4. For all radioligand-binding assays, nonspecific binding was evaluated in the presence of 10 μ naloxone. Reactions were stopped by rapid filtration through a Brandel MLR-24 harvester (Brandel, Gaithersburg, MD), and bound radioligand was collected on GF/C filtermats (Whatman, Kent, UK) and rinsed three times with ice-cold 50 m Tris base, pH 7.4. Filters were dried, saturated with EcoLume scintillation cocktail (MP Biomedicals, Solon, OH) and radioactivity was counted using a Wallec 1450 MicroBeta counter (PerkinElmer, Waltham, MA).
Agonist-Stimulated [35S]GTPγS-Binding Assays
To measure binding of the non-hydrolyzable GTP analog guanosine-5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) to Gα proteins, membranes from whole brain (10 μg protein) or spinal cord (25–50 μg protein) were pre-incubated for 10 min at 25 °C with or without various concentrations of the opioid agonists DAMGO, methadone, morphine, or nalbuphine in [35S]GTPγS-binding buffer (50 m Tris base, pH 7.4, 5 m MgCl2, 100 m NaCl, 1 m EDTA, 2 m dithiothreitol, 100 μ GDP, and 0.4 U/ml adenosine deaminase). After pre-incubation, 0.1 n [35S]GTPγS was added and reactions were further incubated for 90 min at 25 °C. For saturation analysis of [35S]GTPγS binding, membranes from whole brain (10 μg protein) were pre-incubated for 10 min at 25 °C with or without 10 μ DAMGO in [35S]GTPγS-binding buffer, followed by incubation for 90 min at 25 °C with 0.1 n [35S]GTPγS, with or without various concentrations of unlabeled GTPγS (0.8–50 nM). For all [35S]GTPγS-binding assays, nonspecific binding was evaluated in the presence of 10 μ GTPγS. Binding reactions were stopped by rapid filtration, rinsed three times with ice-cold wash buffer (50 m Tris base, pH 7.4, 5 m MgCl2, and 100 m NaCl), and bound radioactivity was measured by liquid scintillation counting, as above.
Drugs
Morphine sulfate was from RTI (Research Triangle Park, NC). Methadone and nalbuphine were obtained through the Narcotic Drug and Opioid Peptide Basic Research Center at the University of Michigan (Ann Arbor, MI). For behavioral experiments, all drugs were diluted in sterile water. [3H]DPN, [3H]DAMGO and [35S]GTPγS were purchased from PerkinElmer. Adenosine deaminase was obtained from Calbiochem (San Diego, CA). DAMGO, CTAP, GDP, GTPγS, and all other chemicals were obtained from Sigma-Aldrich, unless otherwise noted.
Data Analysis
All data were analyzed using GraphPad Prism software, version 5.0. (GraphPad, San Diego, CA). Differences between genotypes were evaluated using Student's t-tests or one-way or two-way ANOVA with Bonferroni's post-tests, where appropriate. For all statistical tests, significance was set at p<0.05. In vivo potency (ED50) values were calculated by fitting the compiled data to an agonist vs normalized response curve (Hill slope=1), and values are expressed as the mean (95% CI). Where antinociception was near or below 50% MPE, ED50 values were extrapolated from the fitted data. Maximal radioligand-binding (Bmax) and binding affinity (K) values were derived by fitting each experiment to a one-site saturation-binding curve fit (Hill slope=1), while maximal [35S]GTPγS stimulation (Emax) and in vitro potency (EC50) values were calculated by fitting individual experiments to an agonist vs response curve fit (Hill slope=1); values are expressed as the mean±SEM.
RESULTS
Characterization of Transgenic Mice Lacking Gαo Protein
The full knockout, Gαo −/− mice did not often survive until weaning (∼21 days), whereas wild-type and Gαo +/− mice were obtained at frequencies predicted by Mendeleian genetics (Table 1) (χ2=11.07, df=1, p<0.001). Peri-natal lethality was also noted in the initial reports of two independently generated Gαo null mouse strains (Valenzuela et al, 1997; Jiang et al, 1998). These previous studies also reported several neurological abnormalities in Gαo −/− animals, including hyperactivity, tremor and turning behavior; however, no such gross behavioral abnormalities were noted for the Gαo +/− or Gαo −/− mice used in this study (Duan et al, 2007; unpublished observations). In adulthood (>8 weeks), body weight varied as a function of genotype (Table 1) (F(2,23)=14.54, p<0.001). Post hoc analysis revealed that those Gαo −/− mice that did survive weighed significantly less than their wild-type littermates, whereas Gαo +/− mice did not differ from wild-type controls.
Table 1. Physical Characteristics of Wild-Type and Gαo Transgenic Mice.
| Genotype | Body weight (g) |
Genotype frequency at weaning (%) |
|
|---|---|---|---|
| Expected | Observed (n=347) | ||
| Wild type | 28.6±1.3 (n=10) | 25.0 | 35.7 (n=124) |
| Gαo +/− | 27.6±0.8 (n=12) | 50.0 | 59.4 (n=206) |
| Gαo −/− | 18.2±1.4 (n=4)* | 25.0 | 4.9 (n=17) |
Asterisk indicates a statistical difference vs wild type by Bonferroni's post-test (p<0.001).
Supraspinal Antinociception in Gαo Transgenic Mice
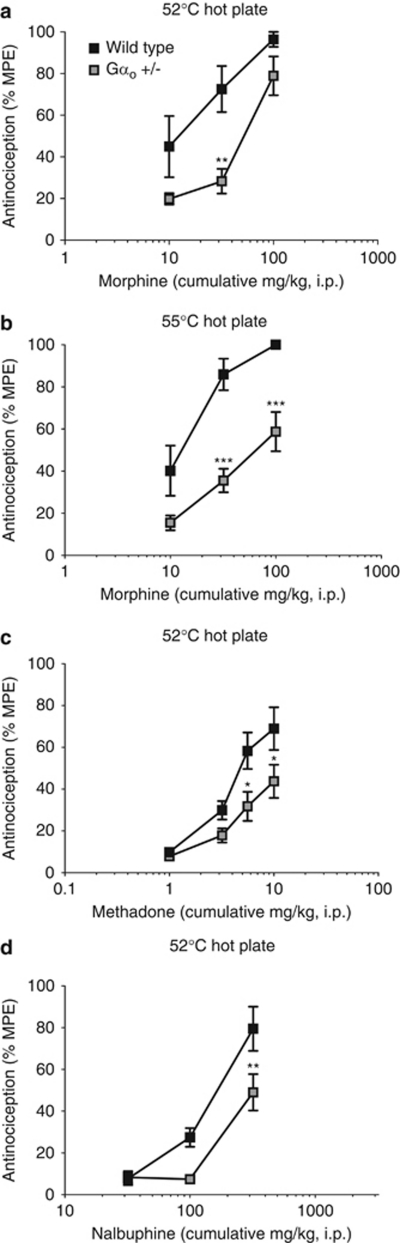
To determine whether Gαo is involved in opioid antinociception, Gαo +/− mice were evaluated for morphine antinociception in the hot plate test (Figure 1). In the 52 °C hot plate test, the baseline nociceptive threshold was not significantly different between wild-type (12.6±0.6 s; n=30) and Gαo +/− mice (12.2±0.5 s; n=32; t(60)=0.4885, p=0.627). Morphine produced a dose-dependent increase in antinociception that was significantly reduced (∼4-fold) in Gαo +/− mice when compared with wild-type controls, with ED50 values of 47.7 mg/kg (31.2–72.9) and 11.4 mg/kg (5.9–22.1), respectively (Figure 1a). Although there was no significant interaction, there were significant main effects of dose and genotype (dose: F(2,36)=19.88; p<0.001; genotype: F(1,36)=15.76, p<0.001).
Figure 1.
Supraspinal antinociception produced by morphine, methadone, and nalbuphine in the hot plate test in Gαo transgenic mice. Antinociception was measured in wild-type and Gαo +/− mice 30 min following morphine in the (a) 52 °C or (b) 55 °C hot plate test, (c) 30 min following methadone in the 52 °C hot plate test, and (d) 15 min following nalbuphine in the 52 °C hot plate test. Data represent the mean±SEM for morphine at 52 °C (n=7) and 55 °C (n=9–10), for methadone (n=7–15), and for nalbuphine (n=8–10). Legend in panel (a) also describes panels (b) through (d). Asterisks indicate a statistical difference vs wild type by Bonferroni's post-test (*p<0.05, **p<0.01, ***p<0.001).
We hypothesized that increasing the efficacy requirements of the nociceptive system might further exaggerate this observed genotype difference; thus, the hot plate temperature was raised to 55 °C and Gαo transgenic mice were again evaluated for morphine supraspinal antinociception (Figure 1b). As expected, a decreased baseline nociceptive threshold was observed at the elevated hot plate temperature, and there were no significant differences between wild-type (7.5±0.8 s; n=9) and Gαo +/− mice at baseline (6.6±0.6 s; n=10; t(17)=0.8940, p=0.384). Morphine dose-dependently produced antinociception in both wild-type and Gαo +/− mice, but the ED50 was shifted ∼6-fold for Gαo +/− mice, with a value of 62.7 mg/kg (42.9–91.5) compared with 9.9 mg/kg (5.8–17.1) for wild-type littermates (Figure 1b). There were significant main effects of both dose (F(2,51)=25.37; p<0.001) and genotype (F(1,51)=41.98, p<0.001), although there was no significant interaction.
To determine whether Gαo has a role in the antinociception produced by opioid agonists other than morphine, Gαo transgenic mice were evaluated for either methadone or nalbuphine antinociception in the 52 °C hot plate test (Figures 1c and d). Like morphine, methadone produced a dose-dependent increase in antinociception (Figure 1c). The ED50 value for wild-type mice was 13.0 mg/kg (10.1–16.8), which was ∼2-fold higher than the extrapolated ED50 value for Gαo +/− mice of 5.8 mg/kg (4.4–7.6). There was no significant interaction; however, there were significant main effects of both dose (F(3,82)=33.12, p<0.001) and genotype (F(1,82)=19.87, p<0.001). The partial agonist nalbuphine also produced a dose-dependent stimulation of antinociception that was significantly reduced for Gαo +/− mice, compared with wild-type littermates (Figure 1d), with an ED50 value of 170.2 mg/kg (108.3–267.6) for wild-type mice. Extrapolation of the dose-response curve for Gαo +/− mice gave an ED50 value of 432.0 mg/kg (289.0–645.9), representing a ∼3-fold shift. There were significant main effects of both dose (F(2,48)=48.62, p<0.001) and genotype (F(1,48)=11.09; p=0.002), as well as a significant dose × genotype interaction (F(2,48)=3.377, p=0.043).
Spinal Antinociception in Gαo Transgenic Mice
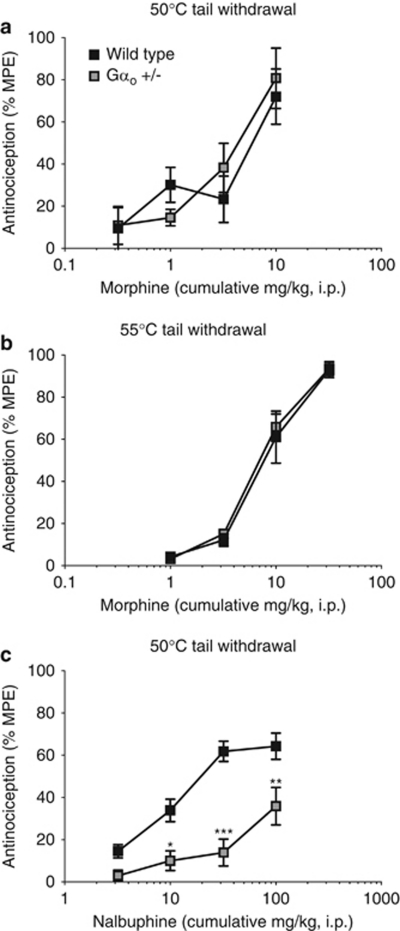
Gαo transgenic mice were also evaluated in the warm-water tail withdrawal test (Figure 2), the same antinociceptive measure that was utilized in the majority of antisense ODN studies (Raffa et al, 1994; Sanchez-Blazquez et al, 1995, 2001). In the 50 °C tail withdrawal test, the baseline tail flick latency was not significantly different between wild-type (3.2±0.4 s; n=13) and Gαo +/− mice (4.2±0.6 s; n=15; t(26)=1.399, p=0.174). Morphine produced a dose-dependent increase in antinociception in both wild-type and Gαo +/− mice, with ED50 values of 5.2 mg/kg (2.7–9.8) and 4.1 mg/kg (2.3–7.2), respectively (Figure 2a). There was a significant main effect of dose (F(3,44)=14.79, p<0.001), although the main effect of genotype (F(1,44)=0.1024, p=0.751) and the dose × genotype interaction were not significant.
Figure 2.
Ability of morphine and nalbuphine to induce spinal antinociception in the warm-water tail withdrawal test in Gαo transgenic mice. Antinociception was measured in wild-type and Gαo +/− mice 30 min following morphine in the (a) 50 °C or (b) 55 °C warm-water tail withdrawal test and (c) 15 min following nalbuphine in the 50 °C warm-water tail withdrawal test. Data represent the mean±SEM for morphine at 50 °C (n=6–7) and 55 °C (n=8) and for nalbuphine (n=7–8). Legend for panels (b) and (c) is the same as for panel (a). Asterisks indicate a statistical difference vs wild type by Bonferroni's post-test (*p<0.05, **p<0.01, ***p<0.001).
Given that morphine behaves as a full agonist in this test, which may preclude the identification of small differences between genotypes, it was hypothesized that increasing the efficacy requirement of the system by raising the water bath temperature to 55 °C (Figure 2b) should allow for the identification of such differences. Again, as predicted, a decreased baseline nociceptive threshold was observed at the elevated water temperature, and there were also no significant differences between wild-type (1.9±0.1 s; n=8) and Gαo +/− mice in this test (1.8±0.2 s; n=8; t(14)=0.6932, p=0.500). Against the 55 °C stimulus, morphine produced a dose-dependent increase in antinociception that was equivalent between wild-type and Gαo +/− mice, with ED50 values of 8.2 mg/kg (5.5–12.5) and 7.4 mg/kg (5.5–10.0), respectively (Figure 2b). There was no significant interaction or significant effect of genotype (genotype: F(1,56)=0.2371, p=0.628), but there was a significant main effect of dose (dose: F(3,56)=124.0, p<0.001).
As an alternative method of evaluating whether the efficacious antinociception produced by morphine was masking a mediatory role for Gαo, spinal antinociception was measured in the 50 °C warm-water tail withdrawal test in response to the low-efficacy agonist, nalbuphine (Figure 2c). Nalbuphine produced a dose-dependent stimulation of spinal antinociception that was significantly reduced (∼7-fold) in Gαo +/− mice when compared with wild-type littermates, with wild-type mice exhibiting an ED50 value of 24.2 mg/kg (17.5–33.4) (Figure 2a). Extrapolation of the nalbuphine dose-response for Gαo +/− mice revealed an ED50 value of 176.1 mg/kg (113.3–273.8). There were significant main effects of dose and genotype (dose: F(3,52)=20.35, p<0.001; genotype: F(1,52)=48.62, p<0.001), as well as a significant dose × genotype interaction (F(3,52)=3.552, p=0.021).
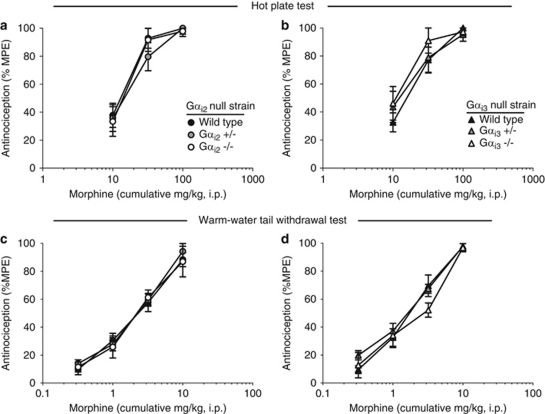
Antinociception in Gαi2 and Gαi3 Transgenic Mice
To confirm the importance of Gαo for opioid antinociception, transgenic mice lacking either Gαi2 (Gαi2 heterozygous null, Gαi2+/− Gαi2 homozygous null, Gαi2 −/−) or Gαi3 (Gαi3 heterozygous null, Gαi3+/− Gαi3 homozygous null, Gαi3 −/−), together with their respective wild-type littermates, were evaluated in the 52 °C hot plate and 50 °C warm-water tail withdrawal tests (Figure 3). Both the Gαi2 and the Gαi3 transgenic mouse strains were generated in parallel with Gαo transgenic mice, and inactivation of the appropriate Gα subunit has been previously confirmed by western blot analysis (Sowell et al, 1997; Duan et al, 2007). In the 52 °C hotplate test (Figures 3a and b), the baseline response latency was equivalent among all Gαi2 transgenic mouse genotypes (wild-type: 12.0±0.7 s, n=10; Gαi2 +/−: 11.6±0.7 s, n=9; Gαi2 −/−: 12.9±0.7 s, n=8; F(2,24)=0.8112, p=0.456). Morphine produced a dose-dependent increase in antinociception that was not different between wild-type, Gαi2 +/− and Gαi2 −/− mice, with ED50 values of 9.4 mg/kg (5.8–15.3), 12.0 mg/kg (6.9–20.8), and 10.6 mg/kg (6.0–18.8), respectively (Figure 3a). There was a significant main effect of dose (F(2,72)=59.03, p<0.001), but the main effect of genotype (F(2,72)=0.3274, p=0.722) and the dose × genotype interaction were not significant. Similarly, in Gαi3 transgenic mice, morphine produced a dose-dependent increase in antinociception that was equivalent between wild-type, Gαi3 +/− and Gαi3 −/− mice, with ED50 values of 13.2 mg/kg (8.5–20.4), 10.6 mg/kg (6.0–18.9) and 8.1 mg/kg (4.4–15.0), respectively (Figure 3b). There was a significant main effect of dose (F(2,69)=37.38, p<0.001), but not genotype (F(2,69)=0.7686, p=0.468), and no significant interaction. There were also no genotype-dependent differences observed in the baseline nociceptive threshold for these mice (wild-type: 13.3±1.4 s, n=9; Gαi3 +/−: 16.8±1.2 s, n=9; Gαi3 −/−: 16.2±1.4 s, n=8; F(2,23)=2.139, p=0.141).
Figure 3.
Morphine supraspinal and spinal antinociception in Gαi2 and Gαi3 transgenic mice. Antinociception produced 30 min following morphine was evaluated in the 52 °C hot plate test in (a) Gαi2 +/− and Gαi2 −/− mice and (b) Gαi3 +/− and Gαi3 −/− mice and in the 50 °C warm-water tail withdrawal test in (c) Gαi2 +/− and Gαi2 −/− mice and (d) Gαi3 +/− and Gαi3 −/− mice, together with their respective wild-type littermates. Data represent the mean±SEM for Gαi2 mice in the hot plate (n=8–10) and tail withdrawal tests (n=6–9) and for Gαi3 mice in the hot plate (n=8–9) and tail withdrawal tests (n=6–9). Legends for panels (c) and (d) are the same as for panels (a) and (b), respectively.
Gαi2 and Gαi3 transgenic mice were also evaluated for spinal antinociception in the 50 °C tail withdrawal test (Figures 3c and d). Baseline response latencies in this test were equivalent among all Gαi2 (wild-type: 5.0±0.4 s, n=9; Gαi2 +/−: 4.4±0.6 s, n=8; Gαi2 −/−: 3.9±0.5 s, n=6; F(2,20)=1.050, p=0.368) and Gαi3 transgenic mouse genotypes (wild-type: 5.6±0.4 s, n=8; Gαi3 +/−: 4.2±0.5 s, n=9; Gαi3 −/−: 4.4±0.8 s, n=6; F(2,20)=1.990, p=0.163). Morphine produced a dose-dependent increase in antinociception that was not different between wild-type, Gαi2+/− and Gαi2 −/− mice, with ED50 values of 2.2 mg/kg (1.6–2.9), 2.0 mg/kg (1.6–2.6), and 2.2 mg/kg (1.5–3.3), respectively (Figure 3c). There was no significant interaction or main effect of genotype (F(2,80)=0.2412, p=0.786), but there was a significant main effect of dose (F(3,80)=113.5, p<0.001). Similarly, in Gαi3 transgenic mice, morphine produced a dose-dependent increase in antinociception that was equivalent in wild-type, Gαi3 +/− and Gαi3 −/− mice, with ED50 values of 1.6 mg/kg (1.2–2.2), 1.4 mg/kg (1.2–1.7), and 2.0 mg/kg (1.4–3.1), respectively (Figure 3d). Although there was no significant interaction or main effect of genotype (F(2,80)=1.936, p=0.151), there was a significant main effect of dose (F(3,80)=171.1, p<0.001).
G Protein Expression in Gαo Transgenic Mouse Brain
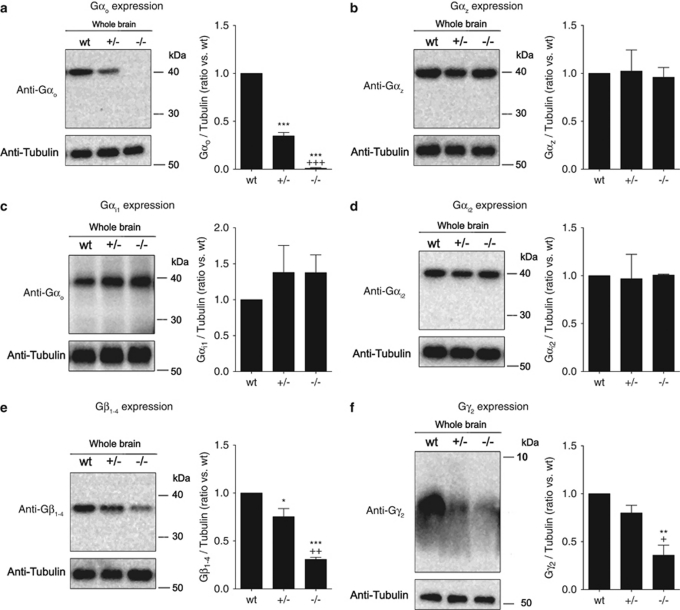
Western blot analysis of G protein expression in whole brain membrane samples confirmed the loss of Gαo protein in Gαo −/− mice (Figure 4a). Quantification of western blot images for Gαo revealed that, in comparison with wild-type controls, Gαo +/− mice express ∼60% less Gαo protein, which is close to the expected 50% reduction (Figure 4a). Across a panel of G protein subunits, including Gαi/o, Gβ, and Gγ proteins (Figure 4), the expression of Gαo (Figure 4a) (F(2,6)=527.9, p<0.001), Gβ1−4 (Figure 4e) (F(2,6)=46.53, p<0.001), and Gγ2 (Figure 4f) (F(2,6)=18.45, p=0.003) were significantly decreased as a function of genotype. In contrast, there were no compensatory changes noted for the expression of either Gαz (Figure 4b) (F(2,6)=0.0548, p=0.947), Gαi1 (Figure 4c) (F(2,6)=0.6938, p=0.536), or Gαi2 (Figure 4d) (F(2,6)=0.0189, p=0.981).
Figure 4.
G protein expression in whole brain homogenates from Gαo transgenic mice. Membranes from whole brain of wild-type (wt), Gαo +/− (+/−), and Gαo −/− (−/−) mice were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed for the expression of (a) Gαo, (b) Gαz, (c) Gαi1, (d) Gαi2, (e) Gβ1−4, or (f) Gγ2 using selective antibodies (see Materials and Methods section); membranes were also probed for tubulin as a loading control. G protein expression was quantified in Image J by normalizing G protein band intensity to tubulin band intensity, and data are plotted as a ratio of wt expression. Data represent the mean±SEM (n=3). Symbols indicate a statistical difference vs wt (*p<0.05, **p<0.01, ***p<0.001) or +/− (+p<0.05, ++p<0.01, +++p<0.001) by Bonferroni's post-test.
MOR Expression in Gαo Transgenic Mouse Brain and Spinal Cord
To evaluate whether the reduction in opioid antinociception observed in Gαo +/− mice could be explained by alterations at the receptor level, MOR expression was measured in membranes from whole brain or from spinal cord of Gαo transgenic mice (Table 2). Binding of a maximal concentration (4 nM) of the radiolabeled opioid antagonist [3H]DPN, representing the entire pool of MOR, delta- and kappa-opioid receptors, was unaffected by genotype in either whole brain (F(2,5)=0.3542, p=0.718) or spinal cord (t(4)=0.0097, p=0.993). To measure total MOR expression, maximal [3H]DPN binding was displaced using the MOR-selective antagonist CTAP (300 nM). Total MOR expression was also not different between genotypes (Table 2) in either whole brain (F(2,5)=0.6832, p=0.547) or spinal cord (t(4)=0.7611, p=0.489).
Table 2. Properties of Agonist and Antagonist Radioligand Binding in Membranes from Whole Brain or Spinal Cord of Wild-Type and Gαo Transgenic Mice.
| Tissue | Genotype |
[3H]DPN binding |
[3H]DAMGO binding |
||
|---|---|---|---|---|---|
| Total (fmol/mg protein) | MOR (fmol/mg protein)a | Bmax (fmol/mg protein) | KD (nM) | ||
| Whole brain | Wild type | 366±24 | 218±13b | 246±29 | 2.5±0.4 |
| Gαo +/− | 391±38 | 216±3 | 181±16 | 1.9±0.5 | |
| Gαo −/− | 407±38 | 233±12 | 121±17* | 3.5±1.3 | |
| Spinal cord | Wild type | 161±20 | 95±5 | 84±5c | ND |
| Gαo +/− | 161±14 | 101±6 | 93±6c | ND | |
Abbreviation: ND, not determined.
MOR expression was evaluated as the amount of bound [3H]DPN at a maximal concentration that was displaced by the MOR-selective antagonist CTAP (300 nM).
In wild-type whole brain, there is a trend for total MOR to be less than high-affinity MOR because binding was measured indirectly (see Materials and Methods).
In spinal cord, Bmax values were estimated using a single maximal concentration of [3H]DAMGO.
Data represent the mean±SEM (n=2–3 performed in at least duplicate). Asterisk indicates a statistical difference vs wild-type whole brain by Bonferroni's post-test (p<0.05).
In whole brain, maximal binding (Bmax) of [3H]DAMGO (Table 2), which, as an agonist, recognizes only high-affinity MOR, was significantly decreased in Gαo +/− and Gαo −/− mice when compared with wild-type controls (F(2,5)=10.44; p=0.016). There was no change across genotypes in the affinity (K) of [3H]DAMGO for high-affinity MOR sites (F(2,5)=1.398; p=0.330). In contrast, maximal (12 nM) [3H]DAMGO binding was unchanged in the spinal cord of Gαo +/− mice when compared with wild-type littermate controls (t(4)=1.186, p=0.301).
G protein Activation in Gαo Transgenic Mouse Brain and Spinal Cord
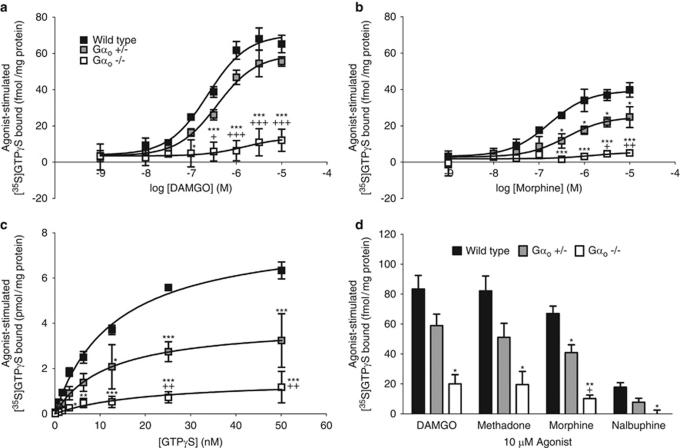
To examine the importance of Gαo for MOR function, the [35S]GTPγS-binding assay was utilized to evaluate the first component of MOR signaling, namely, G protein activation, in membranes from either whole brain or spinal cord of Gαo transgenic mice (Figure 5; Table 3). In whole brain, basal levels of [35S]GTPγS incorporation (Table 3) were significantly reduced in Gαo +/− and Gαo −/− mice, compared with wild-type littermates (F(2,5)=20.06, p=0.004), suggesting that Gαo is responsible for some, but not all, basal G protein activity. The MOR-selective agonist DAMGO produced a dose-dependent stimulation of [35S]GTPγS binding that was reduced in Gαo +/− and Gαo −/− mice when compared with wild-type controls (Figure 5a). There was a significant concentration × genotype interaction for this response (F(14,39)=6.700, p<0.001), including main effects of both concentration (F(7,39)=43.38, p<0.001) and genotype (F(2,39)=72.02, p<0.001). Maximal DAMGO-stimulated binding (Emax) (Table 3) was decreased in Gαo transgenic mice in a genotype-dependent manner (F(2,5)=64.69, p<0.001); this reduction in maximal stimulation was without a change in potency (EC50) between wild-type and Gαo +/− mice (t(2)=1.307, p=0.321). Morphine also produced a dose-dependent stimulation of [35S]GTPγS binding that was significantly decreased in Gαo +/− and Gαo −/− mice when compared with wild-type littermates (Figure 5b). There were significant main effects of both concentration and genotype, as well as a significant concentration × genotype interaction (concentration: F(7,39)=15.79, p<0.001; genotype: F(2,39)=51.45, p<0.001; concentration × genotype: F(14,39)=3.468, p=0.001). The Emax for morphine (Table 3) was reduced as a function of genotype (F(2,5)=16.24, p=0.007), and was accompanied by a nonsignificant trend toward a reduction in the EC50 value for Gαo +/− mice, compared with wild-type littermates (t(4)=2.407, p=0.074).
Figure 5.
Ability of opioid agonists to stimulate G protein activity in whole brain homogenates from Gαo transgenic mice. Agonist-stimulated [35S]GTPγS (0.1 nM) binding was measured in the presence of various concentrations of the opioid agonists (a) DAMGO or (b) morphine, (c) in the presence of 10 μM DAMGO plus increasing concentrations of unlabeled GTPγS, and (d) in the presence of 10 μM DAMGO, methadone, morphine, or nalbuphine in membrane homogenates from whole brain of wild-type, Gαo +/− and Gαo −/− mice. Nonspecific binding was evaluated in the presence of unlabeled GTPγS (10 μM). Data are plotted as agonist-stimulated [35S]GTPγS binding, defined as the increase in [35S]GTPγS incorporation in the presence of agonist over that of basal (measured in the absence of agonist), and represent the mean±SEM (n=2–3 performed in at least duplicate). Legend in (a) also applies to panels (b) and (c). Symbols indicate a statistical difference vs wild type (*p<0.05, **p<0.01, ***p<0.001) or Gαo +/− (+p<0.05, ++p<0.01, +++p<0.001) by Bonferroni's post-test.
Table 3. Properties of Agonist-Stimulated [35S]GTPγS Binding in Membranes from Whole Brain of Wild-Type and Gαo Transgenic Mice.
| Tissue | Genotype | Basal [35S]GTPγS binding |
Agonist-stimulated [35S]GTPγS binding |
DAMGO-stimulated [35S]GTPγS saturation binding |
||||
|---|---|---|---|---|---|---|---|---|
| (fmol/mg protein) |
DAMGO |
Morphine |
||||||
| Emax (fmol/mg protein) | EC50 (nM) | Emax (fmol/mg protein) | EC50 (nM | Bmax (pmol/mg protein) | K (nM) | |||
| Whole brain | Wild type | 62.2±2.5 | 71.3±3.2 | 287±91 | 39.4±4.5 | 165±14 | 8.31±1.01 | 14.2±4.0 |
| Gαo +/− | 49.1±5.8 | 59.4±4.0 | 382±23 | 25.6±3.9 | 350±76 | 4.26±1.31 | 16.5±6.7 | |
| Gαo −/− | 22.2±0.3**, + | 13.1±2.3***, +++ | NC | 5.2±1.0** | NC | 1.18±0.53* | 7.1±4.4 | |
| Spinal cord | Wild type | 59.0±10.9 | 46.4±5.4 | ND | 36.3±4.0 | ND | ND | ND |
| Gαo +/− | 49.0±11.1 | 35.0±6.7# | ND | 19.5±3.1## | ND | ND | ND | |
Abbreviations: NC, not calculated; ND, not determined.
Agonist-stimulated [35S]GTPγS binding is defined as the increase in [35S]GTPγS incorporation in the presence of agonist, over that of basal (measured in the absence of agonist). Data represent the mean±SEM (n=2–3 performed in duplicate). Symbols indicate a statistical difference vs wild type whole brain (*p<0.05, **p<0.01, ***p<0.001) or Gαo +/– whole brain (+p<0.05, +++p<0.001) by Bonferroni's post-test or vs wild-type spinal cord by Student's paired t-test (#p<0.05, ##p<0.01).
Given that DAMGO-stimulated [35S]GTPγS incorporation was significantly attenuated, saturation analysis of DAMGO-stimulated binding was performed (Figure 5c; Table 3) to measure the maximal number of G proteins (Bmax) activated by agonist-occupied MOR and the ability of agonist to induce formation of GTP-bound Gα (K) (Traynor and Nahorski, 1995; Selley et al, 1997). In membranes from whole brain, DAMGO-stimulated [35S]GTPγS incorporation was increased as a function of increasing concentration of GTPγS, but was significantly reduced in Gαo +/− and Gαo −/− mice when compared with wild-type controls (Figure 5c). There were significant main effects of both concentration and genotype, as well as a significant concentration × genotype interaction (concentration: F(7,44)=37.50, p<0.001; genotype: F(2,44)=79.22, p<0.001; concentration × genotype: F(14,44)=7.482, p<0.001). This reduction was manifested as a decrease in Bmax for GTPγS binding (F(2,5)=9.359, p=0.020), without an accompanying change in the K for GTPγS (Table 3) (F(2,5)=0.6608, p=0.556).
[35S]GTPγS binding stimulated by a maximal concentration of DAMGO, morphine, methadone, or nalbuphine was evaluated in whole brain homogenates from Gαo transgenic mice (Figure 5d). In wild-type mice, the opioid agonists tested elicited maximal [35S]GTPγS stimulation according to the rank order of efficacy DAMGO=methadone > morphine >> nalbuphine. When compared with wild-type controls, Gαo +/− and Gαo −/− mice exhibited a reduction in G protein stimulation across all opioid agonists tested, including: DAMGO (wild-type: 83.4±9.1 fmol/mg; Gαo +/−: 59.0±7.7 fmol/mg; Gαo −/−: 20.1±6.1 fmol/mg; F(2,5)=12.98, p=0.011), methadone (wild-type: 82.2±9.9 fmol/mg; Gαo +/−: 51.2±9.3 fmol/mg; Gαo −/−: 19.6±8.7 fmol/mg; F(2,5)=9.407, p=0.020), morphine (wild-type: 67.1±5.0 fmol/mg; Gαo +/−: 40.9±5.3 fmol/mg; Gαo −/−: 10.3±2.3 fmol/mg; F(2,5)=29.93, p=0.002), and nalbuphine (wild-type: 17.8±3.0 fmol/mg; Gααo +/−: 7.7±2.6 fmol/mg; Gαo −/−: 0.01±2.49 fmol/mg; F(2,5)=8.770, p=0.035).
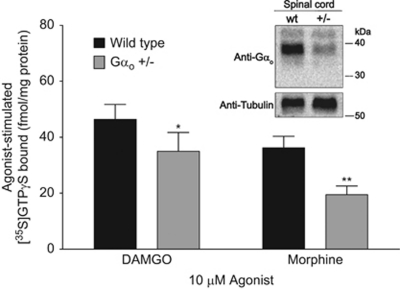
In spinal cord homogenates, basal levels of [35S]GTPγS incorporation (Table 3) were not different between Gαo +/− mice and their wild-type littermates (t(4)=0.6431, p=0.555), suggesting that Gαo is not as important for basal G protein activity in the spinal cord. DAMGO-stimulated binding (Figure 6; Table 3) was significantly reduced in Gαo +/− mice when compared with wild-type controls (t(2)=7.072, p=0.019). Similarly, morphine-stimulated [35S]GTPγS binding in spinal cord (Figure 6; Table 3) showed a decrease in Gαo +/− mice, as compared with wild-type littermates (t(2)=17.54, p=0.003). Western blot analysis of Gαo expression in spinal cord membranes confirmed that there was a significant reduction in Gαo protein levels in these samples, as compared with the loading control tubulin (Figure 6, inset).
Figure 6.
DAMGO- and morphine-stimulated G protein activity in spinal cord homogenates from Gαo transgenic mice. [35S]GTPγS (0.1 nM) incorporation stimulated by 10 μM DAMGO or morphine was evaluated in membrane homogenates from spinal cord of wild-type and Gαo +/− mice. Nonspecific binding was evaluated in the presence of unlabeled GTPγS (10 μM). Data are plotted as agonist-stimulated [35S]GTPγS binding, defined as the increase in [35S]GTPγS binding in the presence of agonist over that of basal (measured in the absence of agonist), and represent the mean±SEM (n=3 performed in quadruplicate). Asterisks indicate a statistical difference vs wild type by Student's paired t-test (*p<0.05, **p<0.01). Inset, representative western blot in spinal cord membranes showing reduced Gαo protein expression in Gαo +/− mice (+/−) when compared with wild-type (wt) controls; membranes were probed for tubulin as a loading control.
DISCUSSION
This study shows that reduction in the expression of the inhibitory Gα isoform, Gαo, attenuates MOR agonist-mediated antinociception in mice at both the supraspinal and the spinal level. However, whether a genotype-dependent difference was seen depended on the efficacy of the agonist and the strength of the noxious stimulus; a greater effect of the Gαo +/− genotype was manifested in the presence of the partial agonist nalbuphine or against a higher temperature stimulus. In contrast, there were no differences observed in the antinociceptive response to morphine in mice that were null for either Gαi2 or Gαi3, compared with their respective wild-type littermates, at either the supraspinal or the spinal level. Furthermore, the loss of Gαo protein in Gαo −/− mice resulted in a decrease in Gβ and Gγ expression, a reduction in the number of high-affinity MOR-binding sites, and consequently, attenuation of MOR agonist-stimulated [35S]GTPγS binding. Together, these results provide strong evidence that MOR coupling to Gαo is important for opioid antinociception.
MOR Agonist-Mediated Antinociception
In wild-type mice, there was no difference in the potency of morphine observed at the higher hot plate temperature of 55 °C when compared with 52 °C, and morphine remained fully effective at both temperatures. However, this effect of temperature was exaggerated in Gαo +/− mice such that a larger shift in the potency of morphine was realized at the higher hot plate temperature, and even at 100 mg/kg, full antinociception was not attained. This suggests a reduced efficiency of antinociceptive processing in the Gαo +/− mice, leading to a higher agonist efficacy requirement. In confirmation of this, methadone, which has higher efficacy than morphine (Adams et al, 1990; Peckham and Traynor, 2006; McPherson et al, 2010), showed a smaller genotype difference. These findings confirm a role for Gαo in opioid agonist-mediated supraspinal antinociception against a thermal stimulus, but also indicate that in the Gαo +/− mice, sufficient Gαo protein remains to give a robust response and/or that other Gαi/o proteins are involved in the response. However, this latter suggestion is less likely given the absence of a difference between Gαi2 or Gαi3 null mice and their wild-type littermates and the lack of compensatory changes in the expression of other Gαi/o proteins in Gαo null mice.
Surprisingly, in light of our findings using the hot plate test, but in agreement with previous ODN studies (Raffa et al, 1994; Sanchez-Blazquez et al, 1995, 2001; Standifer et al, 1996), we did not observe a genotype-dependent difference in the ability of systemic morphine to produce antinociception between wild-type and Gαo +/− mice using the tail withdrawal test. However, we did see a profound shift in the potency of the partial agonist nalbuphine, which has lower efficacy than morphine (Dykstra et al, 1997; Selley et al, 1998). This suggests, as with the hot plate test, that the relationship between the strength of the noxious stimulus and the efficacy of the ligand determines if a genotype difference is observed. These findings imply that blockade of spinal nociception, as measured in the tail withdrawal test, requires less agonist efficacy. As a result, even with a large reduction in Gαo protein, the system is still able to function efficiently.
Previous studies have shown that ODN knockdown of Gα subunits inhibits antinociception in an agonist-specific manner, suggesting that different agonists may cause MOR to signal through different Gα proteins. For example, antinociception induced by the partial agonist buprenorphine in the warm-water tail withdrawal test was significantly reduced after administration of antisense ODNs targeting Gαi2, Gαi3, Gαo2, Gαz, or Gαq, whereas morphine antinociception was only attenuated in the presence of ODNs targeting Gαi2 or Gαz (Sanchez-Blazquez et al, 2001). However, in our study, morphine antinociception in the tail withdrawal test was not altered on loss of Gαo, Gαi2, or Gαi3. Our findings indicate this may be due to differences in relative agonist efficacy, which suggests that there is a Gαo protein reserve for full agonists such that even a significant knockdown of Gαo does not necessarily alter the ability of morphine to elicit antinociception, whereas a partial agonist, such as nalbuphine, is more susceptible. Indeed, Standifer et al (1996) reported a reduction in morphine antinociception in the radiant-heat tail flick assay in mice exhibiting >60% knockdown of Gαo. On the other hand, the reason(s) why knockdown of Gαi2 and other Gα subunits affected antinociception in a ligand-dependent manner in previous studies is not clear, but may be due to differences in the route of administration (central vs peripheral) or the approach used (ODN vs constitutive knockdown). For example, in our constitutive knockdown, although no compensatory changes in Gαi/o protein expression were observed, other developmental changes may have occurred to substitute for the loss of Gαo specifically.
MOR-Dependent G Protein Activation
Loss of Gαo, as determined by western blot, was accompanied by a reduction in both Gβ and Gγ subunits. Valenzuela et al (1997) observed a similar decrease in Gβ protein in ventricular membranes from a separately generated Gαo −/− mouse. This reduction in Gβγ is likely due to the instability of these subunits in the absence of sufficient concentrations of Gα protein (Hwang et al, 2005). A mechanism of regulated Gα and Gβy expression would prevent the accumulation of free Gβy dimers that are functionally competent in the absence of receptor agonist (Jiang et al, 1998). Reductions in free Gβ and Gγ levels were not observed in brains from mice lacking either Gαi2 or Gαi3 (data not shown), presumably due to the lower expression levels of these Gα proteins.
This decrease in Gαo and accompanying Gβ and Gγ subunits, in addition to reducing the antinociceptive response, also reduced the ability of MOR agonists to stimulate [35S]GTPγS incorporation in whole brain or spinal cord homogenates. Indeed, DAMGO- and morphine-stimulated binding of 0.1 nM [35S]GTPγS was abolished in whole brain homogenates from Gαo −/− mice, confirming the importance of Gαo for MOR signaling (Jiang et al, 1998, 2001). The reduction in Gαo and cognate Gβ and Gγ subunits also resulted in a decrease in high-affinity MOR-binding sites, but not total MOR sites, suggesting a reduction in heterotrimeric G protein coupling. However, high-affinity MOR binding was still present in the complete absence of Gαo, which could indicate that other Gαi/o subunits are taking the place of Gαo and providing a functional compensation, although there were no obvious increases in the levels of these isoforms. Indeed, analysis of DAMGO-stimulated [35S]GTPγS saturation binding revealed a Gα protein to high-affinity MOR ratio (Gα : MOR) of approximately 34 : 1 in wild-type mice, compared with 24 : 1 in Gαo +/− mice and 10 : 1 in Gαo −/− mice. These results suggest that, in the brain, Gα proteins other than Gαo are able to form complexes with MOR. Such complexes might also help to translocate MOR to the cell surface, as with delta-opioid receptor (DOR)/Gαi2 complexes that are preassembled in secretory vesicles before delivery to the plasma membrane (Zhao et al, 2011). However, G protein was not required for DOR translocation; if this was also true for MOR, it would explain the high level of low-affinity MOR present in the Gαo −/− mice.
In spinal cord homogenates, both total and high-affinity MOR numbers are considerably less than in whole brain of wild-type mice. Furthermore, there was no change in MOR expression observed in spinal cord tissue from Gαo +/− mice. This could be because of an overabundance of Gαo compared with MOR in the spinal cord. It is unlikely that other Gα subunits are making a bigger contribution in the spinal cord given that there is no difference in morphine antinociception in the tail withdrawal test between Gαi2 or Gαi3 null mice and their wild-type littermates. Similarly, differences between supraspinal and spinal antinociceptive circuitry have been demonstrated in a Gαz-deficient mouse (Hendry et al, 2000), although the mechanisms underlying these supraspinal vs spinal differences were not further characterized. Together, these findings suggest that MOR signaling in the spinal cord may be more efficient, such that full behavioral responses can be achieved at much lower MOR expression and/or on activation of a smaller fraction of the total pool of G proteins.
Concluding Remarks
The present results using Gαo +/− mice demonstrate that Gαo has an important role in opioid antinociception. Moreover, changes observed in opioid antinociception in Gαo +/− mice were paralleled by similar alterations in opioid-dependent signaling at the cellular level. This conclusion is further supported by the recent work of Kest et al (2009), who showed that Gαo expression modulates opioid dependence in mice by targeted knockdown of Gαo mRNA, which reduced the expression of withdrawal after chronic heroin or morphine. However, despite the strong evidence linking Gαo to opioid antinociception, these findings cannot be taken as absolute proof that MOR coupling to Gαo is required for morphine analgesia. Gαo is important for the signaling and activity of many neurotransmitter receptors in the central nervous system (reviewed in Jiang and Bajpayee, 2009). Thus, it is possible that non-opioid pathways are compromised in the Gαo +/− mice and contribute to the altered antinociceptive responses (Connor and Christie, 1999). These and other questions related to the consequences of regional knockdown of Gαo will be addressed in future studies. Nevertheless, the finding that in addition to antinociception, both high-affinity MOR expression and MOR agonist-stimulated G protein activity are reduced strongly supports the notion that the Gαo-MOR complex has a key role in opioid antinociception.
Acknowledgments
We acknowledge Jasmine Schimmel, Chelsea Smith, and Alexander Delgado for excellent technical assistance and/or animal husbandry. This work was supported by NIDA grant DA04087 (to JRT); JTL was supported by training grants DA007267 and GM007767.
The authors declare no conflict of interest.
References
- Adams JU, Paronis CA, Holtzman SG. Assessment of relative intrinsic activity of mu-opioid analgesics in vivo by using beta-funaltrexamine. J Pharmacol Exp Ther. 1990;255:1027–1032. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown DA, Sihra TS. Presynaptic signaling by heterotrimeric G-proteins. Handb Exp Pharmacol. 2008;174:207–260. doi: 10.1007/978-3-540-74805-2_8. [DOI] [PubMed] [Google Scholar]
- Carter BD, Medzihradsky F. Go mediates the coupling of the mu opioid receptor to adenylyl cyclase in cloned neural cells and brain. Proc Natl Acad Sci USA. 1993;90:4062–4066. doi: 10.1073/pnas.90.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Prather PL, Yu L, Law PY, Loh HH. Expression of the mu-opioid receptor in CHO cells: ability of mu-opioid ligands to promote alpha-azidoanilido[32P]GTP labeling of multiple G protein alpha subunits. J Neurochem. 1995;64:2534–2543. doi: 10.1046/j.1471-4159.1995.64062534.x. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Furman CA, Gilson TD, Traynor JR. Comparison of the relative efficacy and potency of mu-opioid agonists to activate Galpha(i/o) proteins containing a pertussis toxin-insensitive mutation. J Pharmacol Exp Ther. 2006a;317:858. doi: 10.1124/jpet.105.096818. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Linderman JJ, Traynor JR. Endogenous regulators of G protein signaling differentially modulate full and partial mu-opioid agonists at adenylyl cyclase as predicted by a collision coupling model. Mol Pharmacol. 2008;73:1538–1548. doi: 10.1124/mol.107.043547. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Traynor JR. Mediation of adenylyl cyclase sensitization by PTX-insensitive GalphaoA, Galphai1, Galphai2 or Galphai3. J Neurochem. 2006b;99:1494–1504. doi: 10.1111/j.1471-4159.2006.04176.x. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MD. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Duan SZ, Christe M, Milstone DS, Mortensen RM. Go but not Gi2 or Gi3 is required for muscarinic regulation of heart rate and heart rate variability in mice. Biochem Biophys Res Commun. 2007;357:139–143. doi: 10.1016/j.bbrc.2007.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA, Preston KL, Bigelow GE. Discriminative stimulus and subjective effects of opioids with mu and kappa activity: data from laboratory animals and human subjects. Psychopharmacology (Berl) 1997;130:14–27. doi: 10.1007/s002130050208. [DOI] [PubMed] [Google Scholar]
- Garzon J, de Antonio I, Sanchez-Blazquez P. In vivo modulation of G proteins and opioid receptor function by antisense oligodeoxynucleotides. Methods Enzymol. 2000;314:3–20. doi: 10.1016/s0076-6879(99)14091-6. [DOI] [PubMed] [Google Scholar]
- Garzon J, Martinez-Pena Y, Sanchez-Blazquez P. Gx/z is regulated by mu but not delta opioid receptors in the stimulation of the low Km GTPase activity in mouse periaqueductal grey matter. Eur J Neurosci. 1997;9:1194–1200. doi: 10.1111/j.1460-9568.1997.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Gierschik P, Milligan G, Pines M, Goldsmith P, Codina J, Klee W, et al. Use of specific antibodies to quantitate the guanine nucleotide-binding protein Go in brain. Proc Natl Acad Sci USA. 1986;83:2258–2262. doi: 10.1073/pnas.83.7.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry IA, Kelleher KL, Bartlett SE, Leck KJ, Reynolds AJ, Heydon K, et al. Hypertolerance to morphine in G(z alpha)-deficient mice. Brain Res. 2000;870:10–19. doi: 10.1016/s0006-8993(00)02387-8. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Rosenthal W, Trautwein W, Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. Nature. 1987;325:445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hwang JI, Choi S, Fraser ID, Chang MS, Simon MI. Silencing the expression of multiple Gbeta-subunits eliminates signaling mediated by all four families of G proteins. Proc Natl Acad Sci USA. 2005;102:9493–9498. doi: 10.1073/pnas.0503503102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Bajpayee NS. Molecular mechanisms of Go signaling. Neurosignals. 2009;17:23–41. doi: 10.1159/000186688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Gold MS, Boulay G, Spicher K, Peyton M, Brabet P, et al. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Smith SB, Schorscher-Petcu A, Austin JS, Ritchie J, Klein G, et al. Gnao1 (G alphaO protein) is a likely genetic contributor to variation in physical dependence on opioids in mice. Neuroscience. 2009;162:1255–1264. doi: 10.1016/j.neuroscience.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Offermanns S, Spicher K, Schultz G. mu and delta opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH-SY5Y cells. Neuron. 1993;10:233–242. doi: 10.1016/0896-6273(93)90314-h. [DOI] [PubMed] [Google Scholar]
- Lester PA, Traynor JR. Comparison of the in vitro efficacy of mu, delta, kappa and ORL1 receptor agonists and non-selective opioid agonists in dog brain membranes. Brain Res. 2006;1073–1074:290–296. doi: 10.1016/j.brainres.2005.12.066. [DOI] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, et al. mu-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moises HC, Rusin KI, Macdonald RL. mu-Opioid receptor-mediated reduction of neuronal calcium current occurs via a G(o)-type GTP-binding protein. J Neurosci. 1994;14:3842–3851. doi: 10.1523/JNEUROSCI.14-06-03842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AA, Seidman JG. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther. 2006;316:1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Martinez RP, Connelly CD. G-protein antisense oligodeoxyribonucleotides and mu-opioid supraspinal antinociception. Eur J Pharmacol. 1994;258:R5–R7. doi: 10.1016/0014-2999(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Garcia-Espana A, Garzon J. In vivo injection of antisense oligodeoxynucleotides to G alpha subunits and supraspinal analgesia evoked by mu and delta opioid agonists. J Pharmacol Exp Ther. 1995;275:1590–1596. [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Gomez-Serranillos P, Garzon J. Agonists determine the pattern of G-protein activation in mu-opioid receptor-mediated supraspinal analgesia. Brain Res Bull. 2001;54:229–235. doi: 10.1016/s0361-9230(00)00448-2. [DOI] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR. Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J Pharmacol Exp Ther. 1998;285:496–505. [PubMed] [Google Scholar]
- Selley DE, Sim LJ, Xiao R, Liu Q, Childers SR. Mu-opioid receptor-stimulated guanosine-5′-O-(gamma-thio)-triphosphate binding in rat thalamus and cultured cell lines: signal transduction mechanisms underlying agonist efficacy. Mol Pharmacol. 1997;51:87–96. doi: 10.1124/mol.51.1.87. [DOI] [PubMed] [Google Scholar]
- Sowell MO, Ye C, Ricupero DA, Hansen S, Quinn SJ, Vassilev PM, et al. Targeted inactivation of alphai2 or alphai3 disrupts activation of the cardiac muscarinic K+ channel, IK+Ach, in intact cells. Proc Natl Acad Sci USA. 1997;94:7921–7926. doi: 10.1073/pnas.94.15.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standifer KM, Rossi GC, Pasternak GW. Differential blockade of opioid analgesia by antisense oligodeoxynucleotides directed against various G protein alpha subunits. Mol Pharmacol. 1996;50:293–298. [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, et al. G alpha(o) is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proc Natl Acad Sci USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VC, Sadee W. Efficacy and tolerance of narcotic analgesics at the mu opioid receptor in differentiated human neuroblastoma cells. J Pharmacol Exp Ther. 1988;245:350–355. [PubMed] [Google Scholar]
- Zhao B, Wang HB, Lu YJ, Hu JW, Bao L, Zhang X. Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res. 2011;21:741–753. doi: 10.1038/cr.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]