Abstract
P2X1 receptors are ATP-gated ion channels expressed by smooth muscle and blood cells. Carboxyl-terminally His-FLAG-tagged human P2X1 receptors were stably expressed in HEK293 cells and co-purified with cytoskeletal proteins including actin. Disruption of the actin cytoskeleton with cytochalasin D inhibited P2X1 receptor currents with no effect on the time course of the response or surface expression of the receptor. Stabilization of the cytoskeleton with jasplakinolide had no effect on P2X1 receptor currents but decreased receptor mobility. P2X2 receptor currents were unaffected by cytochalasin, and P2X1/2 receptor chimeras were used to identify the molecular basis of actin sensitivity. These studies showed that the intracellular amino terminus accounts for the inhibitory effects of cytoskeletal disruption similar to that shown for lipid raft/cholesterol sensitivity. Stabilization of the cytoskeleton with jasplakinolide abolished the inhibitory effects of cholesterol depletion on P2X1 receptor currents, suggesting that lipid rafts may regulate the receptor through stabilization of the cytoskeleton. These studies show that the cytoskeleton plays an important role in P2X1 receptor regulation.
Keywords: Actin, ATP, Cytoskeleton, Lipid Raft, Protein Purification, Purinergic Receptor, Receptor Regulation
Introduction
P2X1 receptors for ATP are ligand-gated cation channels formed from the trimeric assembly of subunits with two transmembrane regions, intracellular amino and carboxyl termini and a large extracellular ligand binding loop (1, 2). They are expressed predominantly on smooth muscle and blood cells. In smooth muscle they contribute to sympathetic nerve-mediated contraction of arteries (3), vas deferens (4), and parasympathetic control of the urinary bladder (5). In the circulatory system P2X1 receptors have been shown to have a role in platelet activation (6) and the subtype selective antagonist NF449 has a protective role in thromboembolism models (7). The receptors are also expressed on mast cells (8), macrophages (9), neutrophils (where they are involved in migration) (10), and T cells where they redistribute to the immune synapse on activation (11). Regulation of P2X1 receptor responsiveness may therefore play an important role in the fine tuning of blood flow and immune responses.
P2X1 receptor channels show rapid desensitization during continued agonist application with the current decaying monoexponentially with a time constant of ∼250 ms (12, 13). In the whole cell recording mode P2X1 receptor currents do not fully recover from desensitization and decrease in amplitude on repeated application, a phenomenon referred to as run down (14). In the perforated patch recording configuration (that maintains the integrity of intracellular signaling pathways) reproducible P2X1 receptor currents are recorded when a 5-min washout period is given between agonist applications (13, 14). This suggests that intracellular factors that are dialyzed out of the cell during whole cell recording contribute to regulation of the receptor. Further evidence that P2X1 receptors can be regulated by intracellular factors comes from studies showing that P2X1 receptor currents are potentiated following activation of protein kinase C (15, 16). Interestingly, the potentiation of responses is not thought to result from direct phosphorylation of the receptor but of an interacting protein that has a regulatory role (15). Lipid rafts provide a mechanism for bringing together receptors and signaling molecules (17). P2X1 receptors are localized to lipid rafts in smooth muscle, platelets as well as in recombinant systems, and cholesterol depletion that disrupts the rafts inhibits P2X1 receptor responses (18–20). Taken together, these observations indicate that the P2X1 receptor can be regulated by intracellular signaling pathways and interacting proteins that may be localized together in lipid rafts.
Proteomic studies have identified interacting proteins for other P2X receptors with a focus on P2X2 and P2X7 receptors (21–25). In this study we have expressed an epitope-tagged P2X1 receptor in HEK293 cells, isolated the receptor, and identified associated proteins, including actin, by mass spectroscopy. In functional studies disruption of the actin cytoskeleton inhibited P2X1-, but not P2X2-receptor mediated responses, and chimeric P2X1/2 receptors showed that the intracellular amino terminus was involved. This was similar to that observed for the inhibition of the P2X1 receptor following cholesterol depletion, and we have now shown a link between the cytoskeleton and lipid rafts in receptor regulation.
MATERIALS AND METHODS
Protein Purification and Protein Complex Analysis
Human P2X1 receptors with a carboxyl-terminal His-FLAG tag were isolated utilizing an anti-FLAG® M2 affinity agarose gel column (Sigma). Starting material of 5- ×175-cm2 flasks of either native HEK293 or HEK293 stably expressing human P2X1 His-FLAG were lysed using buffer containing 150 mm NaCl, 40 mm Tris-HCl (pH 7.5), 8 mm Tris, 750 mm aminocaproic acid, 1% n-octyl glucoside, and protease inhibitor mixture (Sigma). Cell lysate was cleared by centrifugation (16,000 × g for 15 min) and supernatant rolled overnight with 2 ml of anti-FLAG® M2 affinity agarose gel. The agarose beads were placed in a column and flow-through collected. Beads were washed four times with 50 ml of TBS and a sample collected at each wash stage. Proteins were eluted with 1 ml aliquots of 0.1 mg/ml 3× FLAG peptide followed by two 1-ml aliquots of 0.1 m glycine (pH 3.5). Samples of fractions (10 μl) collected from each step were Western blotted with anti-P2X1 antibody (Alomone, Jerusalem, Israel) at 1:1000 dilution (as used previously) and positive fractions pooled and concentrated using Amicon Ultra 4 30,000 MWCO centrifugal filters (Millipore). To discover proteins with weak interactions to P2X1, some cells were treated prior to lysis with the cell-permeable cross-linking agent dithiobis(succinimidyl propionate) (DSP,2 100 μm in PBS) applied to the cells for 30 min and the cross-linking reaction stopped by addition of 1 m Tris. Purified His-FLAG-tagged human P2X1 receptor and associated proteins were run on a 10% SDS-polyacrylamide gel and stained using InstantBlue (Expedeon, Harston, UK). The stained bands were excised, trypsin-digested, and analyzed using mass spectrometry. LC-tandem MS analysis was carried out using either 4000 Q-Trap system or Thermo Orbitrap (Protein Nucleic Acid Chemistry Laboratory, University of Leicester, UK). Postrun tandem MS ion searches were carried out on Mascot (Matrix Science), and multiple protein analysis carried out with Scaffold2 (Proteome Software Inc.). Proteins were positively identified by a minimum of three peptides with >95% peptide identification probability giving an overall protein identification probability of >99%.
Cell Culture and Transfection of HEK293 Cells
Native HEK293 cells were maintained in minimal essential medium with Earle's salts (with GlutaMAXTM I; Invitrogen) supplemented with 10% fetal bovine serum and 1% nonessential amino acids (Invitrogen) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. A monolayer of native cells at 80–90% confluence in a 24-well culture dish was transiently transfected using 0.5 μg of DNA and 1 μl of Lipofectamine 2000 (Invitrogen) in 500 μl of serum-free Opti-MEM 1. After a 24-h incubation, cells were plated onto 13-mm no. 1 coverslips for electrophysiological experiments and left to grow in DMEM. Cells were subjected to experiments 24–48 h after transfection. Cells were transfected with wild-type human P2X1-5 or 7 receptors. P2X4 and P2X7 receptor DNAs were kindly given by Drs. F. Rassendren (CNRS Montpellier, France) and L.-H. Jiang (University of Leeds, UK). For the human P2X5 receptor, a 1353-bp DNA sequence was designed containing the full coding sequence for human P2X5, including exon 10 as described (26, 27) with 5′ HindIII and 3′ EcoRI restriction enzyme sites. The DNA fragment was codon optimized for Homo sapiens, checked for secondary structure, synthesized (Epoch Lifescience Inc., Missouri City, TX), and cloned into a pBluescript vector from which it was subsequently subcloned into pcDNA3. HEK293 cells were also co-transfected with human P2X1 and P2X5 DNAs. However, we only observed small P2X1/5-like currents (∼45 pA/pF) in 2 of 17 tested cells (three different transfections), and so the effects of actin disruption were not tested. Chimeric P2X1/2 receptors and EGFP-tagged P2X1 receptors were as described previously (13, 18).
Isolation of Rat Tail Artery Smooth Muscle Cells
Rat tail artery was dissected and smooth muscle cells isolated enzymatically as described previously (14). Cells were plated onto glass coverslips and recordings made within 1–6 h of isolation.
Electrophysiological Recordings and Solutions
Whole cell and permeabilized patch voltage clamp recordings were made using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Membrane currents were recorded at a holding potential of −70 mV (corrected for tip potential). Data were low pass-filtered at 1 kHz, digitized at a sampling interval of 200 μs, and acquired using a Digidata 1200 analog-to-digital converter with pClamp 9.2 acquisition software (Axon Instruments). Patch pipettes were filled with internal solution composed of 140 mm potassium gluconate, 10 mm EGTA, 10 mm HEPES, 5 mm NaCl (pH 7.3, adjusted with KOH) and had resistances in the range of 2–6 megohms. For perforated patch pipette recordings amphotericin B was added to the internal solution at a final concentration of 200 μg ml−1. The bath was perfused continuously with extracellular solution containing 150 mm NaCl, 2.5 mm KCl, 2.5 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, 10 mm glucose (pH 7.3, adjusted with NaOH). Agonists were applied with a U-tube perfusion system. For P2X1 receptors repeated applications of agonist (α,β-meATP) were separated by 5 min to allow recovery from receptor desensitization. P2X2–5 receptors were stimulated with 100 μm ATP; for P2X7 receptors BzATP (100 μm) was used, and the extracellular solution had reduced levels of divalent cations (0.3 mm CaCl2, 0 mm MgCl2). In the permeabilized patch configuration experiments drugs were added to the extracellular solution. For whole cell experiments we used cells pretreated in drug containing solution before recordings. Control untreated cells were recorded from every day, and comparisons between nontreated and treated cells were made between the cells from the same batch. All chemicals were purchased from Sigma.
Fluorescent Recovery after Photobleaching (FRAP) Measurements
Confocal fluorescence measurements were made on an Olympus inverted microscope with a confocal laser scanning module (Olympus FluoView1000). Cells were observed with a 60 × oil immersion objective lens (UPLSAPO 60XO, NA 1.35). The EGFP fluorescence was excited with a 488-nm argon laser at low power, and emission was detected and collected from 500 to 600 nm at a rate of 0.5 Hz. Each FRAP experiment started with collection of 30 base-line control images of the cell followed by 1-s photobleach of the region of interest with laser pulse at full power to photobleach most of region of interest fluorescence. Typically, 100 images were recorded at low laser power after bleaching. Average fluorescence intensities of bleached, nonbleached, and background regions were recorded for each time point. Fluorescence signals were background-subtracted, corrected for fading of fluorescence during the experiment and expressed as F/Fi ratios to normalize fluorescence levels (F) against starting fluorescence (Fi). Fluorescence intensities of the region of interest were obtained using FluoView software and plotted.
Cell Surface Biotinylation
HEK293 cells expressing P2X1 receptors were cultured in 6-well plates and treated with cytochalasin D (500 nm, 1 h). Surface proteins were biotinylated by the addition of 0.5 mg/ml sulfo-NHS-LC-biotin (sulfo-biotin; Pierce) diluted in PBS (30 min at 4 °C). Excess biotin was quenched with two 20-min (4 °C) washes with 100 mm glycine in PBS. Cells were lysed in 300 μl of buffer H (100 mm NaCl, 20 mm Tris-Cl (pH 7.4), 1% Triton X-100, and 10 μl/ml protease inhibitor mixture (P8340; Sigma)), incubated on ice for 20 min, and cleared by centrifugation (4 °C at 16,000 × g for 10 min). For isolation of biotinylated proteins, 30 μl of streptavidin-agarose beads (Sigma) were added to 200 μl of supernatant and mixed on a rolling shaker (4 °C, 3 h). The rest of the supernatant was kept to assess total protein for each sample. Beads were washed four times in buffer H and 30 μl of 2 × gel sample loading buffer added. Samples were separated on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose. Membranes were then processed with the primary anti-P2X1 receptor antibodies (1:1000; Alomone). Protein bands were visualized using an ECL Plus kit and Hyperfilm MP (Amersham Biosciences).
Data Analysis
Data were analyzed with CLAMPFIT (Axon Instruments) or ORIGIN 6.0 (Microcal Software, Northampton MA). The kinetics of FRAP has been analyzed by fitting the curve of fluorescence intensity with model curves with monoexponential rise and decay phases (13). The time constants of recovery (τ) was determined as time constant of the fast component and optimized by the gradient method to minimize the mean square error (28). Data in the text and graphs are shown as means ± S.E. of mean from n determinations as indicated and analyzed either by ANOVA followed by Dunnett's multiple comparisons test using the unpaired Student's t test as appropriate, and p < 0.05 was considered significant.
RESULTS
Purification of P2X1 Receptors and Identification of Associated Proteins
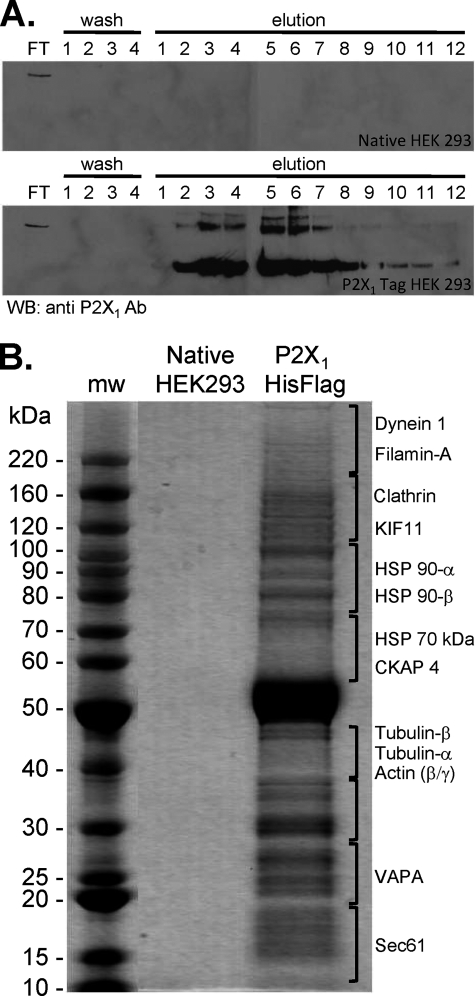
A His-FLAG epitope tag was added to the carboxyl terminus of the human P2X1 receptor to purify the receptor stably expressed in HEK293 cells. The tag had no effect on P2X1 receptor properties, similar to the lack of effect of addition of the much larger EGFP tag (13). An anti-FLAG affinity agarose gel column was used to isolate the P2X1 receptor and associated proteins. The P2X1 receptor was eluted with FLAG peptide and fractions containing the P2X1 receptor identified by Western blotting with an antibody directed against the receptor carboxyl terminus (Fig. 1). These fractions were pooled, concentrated, and run out on a gel, and proteins were visualized. In control studies where nontransfected HEK293 cells were used no P2X1 receptor or other proteins were detected in the elutions. Similarly when a general protein stain was used or when sections of the gel were excised digested and analyzed by mass spectrometry no proteins were detected. In contrast, when the P2X1 His-FLAG stable cell line was used and the eluate run on a gel a range of protein bands was detected. The strongest of these was ∼55 kDa; this band was excised from the gel subjected to tryptic digestion and analyzed by mass spectrometry and shown to correspond to the P2X1 receptor protein. A number of other protein bands were also detected following P2X1 receptor isolation, and these were subsequently identified and shown to contain a range of cytoskeletal proteins (supplemental Table 1) including actin and tubulin. P2X1-His-FLAG-expressing cells were also treated with the membrane-permeant cross-linking reagent DSP. This would be expected to isolate proteins that have a less stringent interaction with the receptor or are associated with P2X1 receptor accessory proteins within a signaling complex. Following cross-linking with DSP a number of additional cytoskeletal interacting proteins were identified following P2X1 receptor isolation (supplemental Table 1) including ezrin and annexins.
FIGURE 1.
Isolation of P2X1 receptors and associated proteins. A, anti-FLAG antibody-agarose beads were incubated with lysates of either native HEK293 cells or HEK293 cells stably expressing human P2X1 His-FLAG-tagged receptor. The agarose bead-protein complex was loaded onto a column and flow-through (FT) collected. Beads were washed four times and a sample of eluate kept (washes 1–4). 3× FLAG peptide (0.1 mg/ml) eluted 10 1-ml fractions (elutions 1–10) followed by two fractions eluted with 0.1 m glycine (pH 3.5) (elutions 11 and 12). Fractions were analyzed by Western blotting and probed with P2X1 antibody. No immunoreactivity was observed in HEK native cell lysate fractions, but strong immunoreactivity was observed in fractions E2–E8 from HEK293 cells expressing P2X1 receptor. B, fractions E2–E8 were pooled, concentrated, and run on SDS-PAGE after which the gel was stained for the presence of proteins. Cytoskeletal proteins are listed on the right as identified by mass spectrometery from HEK293 cells expressing P2X1 receptor lane gel slices. No proteins were identified from the HEK native receptor lane gel slices.
Effects of Cytoskeletal Disruption on P2X1 Receptor Currents
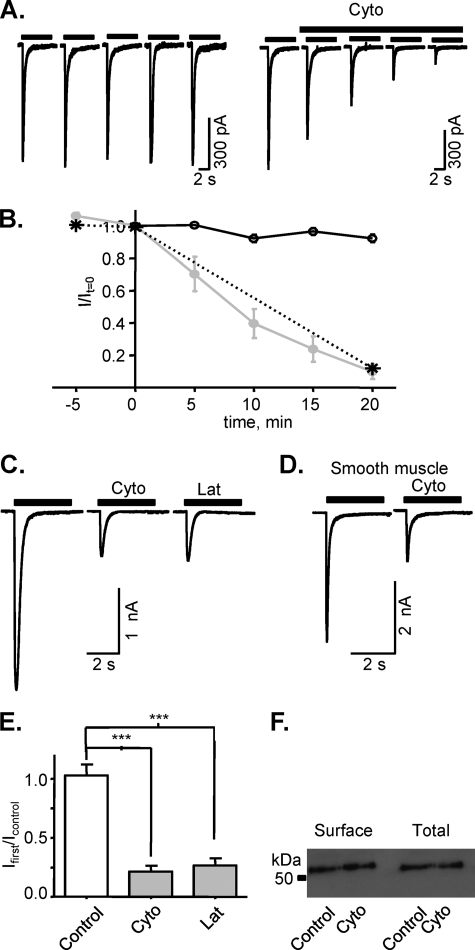
The protein purification studies suggested that P2X1 receptors interact with the actin cytoskeleton. To test whether this interaction is important for P2X1 receptor function cytochalasin D was used to disrupt the actin cytoskeleton by facilitating ATP-actin hydrolysis (29). In the permeabilized patch recording configuration (to maintain the integrity of intracellular signaling pathways) α,β-meATP (10 μm, an EC90 concentration) evoked reproducible inward currents (mean amplitude 740 ± 38 pA, current density 105 ± 6 pA/pF, n = 8) at recombinant human P2X1 receptors expressed in HEK293 cells. Cytochalasin D (5 μm, applied acutely in the bath) reduced the peak amplitude of currents; this effect was time-dependent and reached ∼80% inhibition by 20-min application (in control conditions average amplitude of current was 92.3 ± 2.4%, n = 5, and in the presence of cytochalasin it was 9.8 ± 4.6%, n = 6, of initial value, p < 0.0001; Fig. 2, A and B). This inhibitory effect was not dependent on repeated receptor activation because the extent of reduction in current amplitude was the same whether the P2X1 receptor was stimulated every 5 min during the 20-min application or was only stimulated at the end of the 20-min application period (Fig. 2, A and B). Cytochalasin treatment, however, had no effect on the time course of P2X1 receptor currents (decay time constant for P2X1 receptor mediated currents evoked by 10 μm α,β-meATP in control 220.6 ± 11.7 ms, n = 67 and 209.3 ± 28.4 ms, n = 12 after treatment with cytochalasin). Latrunculin-A (500 nm, 1-h treatment at room temperature; Fig. 2, C and D), which prevents actin monomers repolymerizing into filaments (mechanistically different from cytochalasin D) (30), also produced a similar reduction in peak amplitude (73.4 ± 6% decrease, n = 10, p = 0.0002).
FIGURE 2.
Inhibition of P2X1 receptor-mediated currents by actin cytoskeleton disruption. Effects of actin cytoskeleton disruption by cytochalasin D (Cyto) or latrunculin A (Lat) on P2X1 receptor currents were tested for recombinant human P2X1 receptors expressed in HEK293 cells (A, C, E, and F) and native rat smooth muscle P2X1 receptors (D). Application of cytochalasin D (5 μm, gray symbols) in external solution reduced the amplitude of α,β-meATP-evoked currents (10 μm, applied every 5 min) in the permeabilized patch recording configuration (A and B). 20-min treatment with cytochalasin D before stimulating the P2X1 receptor is shown as a star. In the whole cell recording configuration P2X1 receptor currents in HEK293 cells or rat smooth muscle cells treated with cytochalasin D (500 nm,1 h) or latrunculin A (500 nm, 1 h) were reduced (C–E). Surface biotinylation showed that actin cytoskeleton disruption by cytochalasin D (500 nm,1 h) did not change either the total or surface level of P2X1 receptors expression in HEK293 cells (F). ***, p < 0.001.
The effects of cytoskeletal disruption were also tested on native P2X1 receptors from acutely dissociated rat tail artery smooth muscle cells. α,β-meATP (10 μm) evoked desensitizing currents from artery smooth muscle cells that were reduced by ∼65% following cytochalasin treatment (n = 10, Fig. 2C, 296.1 ± 34.1 pA/pF in control, 103.3 ± 24.9 pA/pF after treatment with cytochalasin D, p < 0.05). These results suggest that that the cytoskeleton plays an important regulatory role on P2X1 receptor function.
To determine whether cytochalasin treatment decreased the expression of P2X1 receptors membrane-impermeant sulfo-NHS LC biotin was used to label cell surface proteins selectively. These were then isolated with streptavidin beads, and Western blotting was used to quantify surface expression levels. The P2X1 receptor antibody detected a band of ∼55 kDa from HEK293 cells stably expressing the P2X1 receptor, consistent with the receptor running as a monomer under denaturing conditions (31). Cytochalasin treatment had no effect on the surface expression of P2X1 receptors (Fig. 2E), indicating that an effect on receptor trafficking or turnover is unlikely to account for the reduction in P2X1 receptor current amplitude in response to cytochalasin treatment.
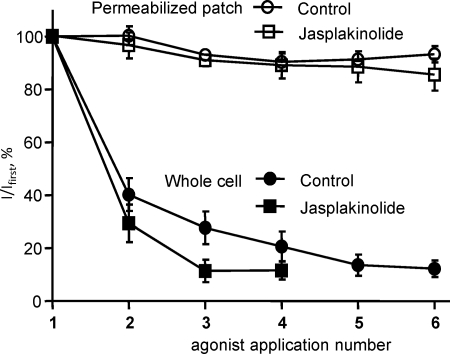
Stabilization of the Actin Cytoskeleton Has No Effect on P2X1 Receptor Currents
The actin cytoskeleton is dynamic and continually undergoing ATP-dependent repolymerization. The rundown of P2X1 receptor currents in the whole cell recording configuration, which results in dialysis of the cell, was partially rescued by the inclusion of 5 mm ATP in the patch electrode recording solution (but not by inclusion of GTP or PiP2, supplemental Figs. 1 and 2). This raised the possibility that disruption of ATP-dependent actin polymerization may underlie P2X1 receptor current rundown as has been shown for NMDA receptor channels (32). Stabilization of the actin cytoskeleton with jasplakinolide (30 nm, 1-h treatment at room temperature) had no effect on P2X1 receptor current rundown in whole cell recordings (Fig. 3), suggesting that rundown does not result from a disruption of the actin cytoskeleton. Jasplakinolide had no effect on the reproducibility of responses in the permeabilized patch configuration (Fig. 3). Jasplakinolide also reversed the effects of cytochalasin treatment (80 ± 4 and 51 ± 5% reduction in peak current amplitude for cytochalasin and cytochalasin + jasplakinolide, respectively, p < 0.05). These results show that it is not dynamic changes in actin polymerization, but an intact actin cytoskeleton that is required for normal P2X1 receptor function.
FIGURE 3.
Stabilization of the actin cytoskeleton by jasplakinolide does not prevent the rundown of P2X1 receptor-mediated currents in the whole cell recording configuration. In the permeabilized patch recording configuration, which supports intracellular signaling pathways, reproducible α,β-meATP (10 μm)-evoked currents were observed every 5 min both for control cells and cells treated with jasplakinolide (30 nm, 1 h) to stabilize the actin cytoskeleton. In the whole cell recording configuration P2X1 receptor currents decreased in amplitude following repeated application of agonist at 5-min intervals. This rundown was unaffected by jasplakinolide treatment.
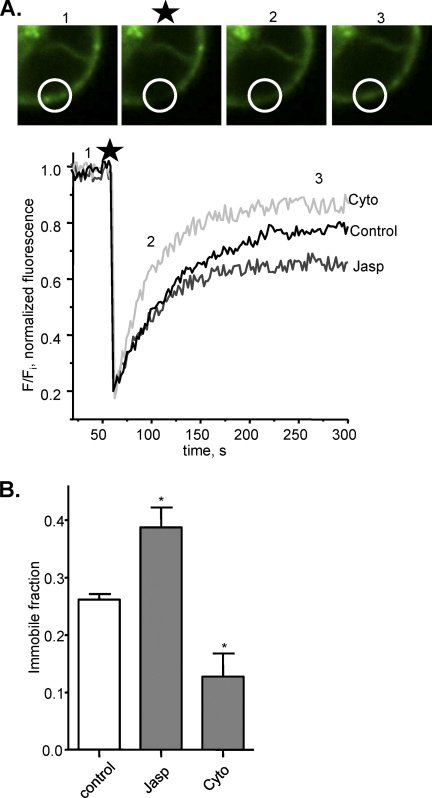
Cytoskeletal Regulation of P2X1 Receptor Mobility
FRAP studies on EGFP-tagged P2X1 receptors have shown that the receptors are mobile in the membrane (13). In the present study a brief laser pulse was used to photobleach an area of the cell surface, within 5 min P2X1-EGFP fluorescence had recovered in this region by ∼75% similar to that reported previously (13) (Fig. 4). This demonstrates that at least 75% of the receptors are mobile and moving within a 5-min time frame. The ∼25% fluorescence that does not recover most likely corresponds to bleached receptors that do not move out of the illuminated area and are referred to as the immobile fraction. Disruption of the cytoskeleton with cytochalasin D (500 nm, 1-h treatment at room temperature) increased the recovery in fluorescence and reduced the immobile fraction to 14.7 ± 4.4% (n = 9, p < 0.05) (Fig. 4). In contrast, stabilization of the cytoskeleton with jasplakinolide (30 nm, 1-h treatment at room temperature) increased the immobile fraction to 39.3 ± 2.8% (n = 20, p < 0.05) (Fig. 4). The change in the immobile fraction does not result from a general change in membrane fluidity and mobility of receptors as previous studies have shown that the immobile fraction of ionotropic nicotinic (33) or metabotropic P2Y receptors3 are not affected by actin disruption. These results demonstrate that the actin cytoskeleton regulates mobility of the P2X1 receptor in the membrane.
FIGURE 4.
Alterations in actin cytoskeleton status lead to changes in the fraction of immobile P2X1 receptors. A, representative snapshots and traces of FRAP for HEK293 cells expressing P2X1-EGFP receptors in control conditions (area bleached is shown by the circle, and numbers refer to the time of image, and star indicates the bleach event). The time course of FRAP is shown under control conditions and following treatment with either cytochalasin D (Cyto, 500 nm, 1 h) or jasplakinolide (Jasp, 30 nm, 1 h). B, summary data of the immobile fraction for P2X1 receptors after stabilization of actin cytoskeleton by jasplakinolide and after disruption of actin cytoskeleton by cytochalasin D (n = 9–20). *, p < 0.05.
Effects of Cytochalasin D at Other P2X Receptors
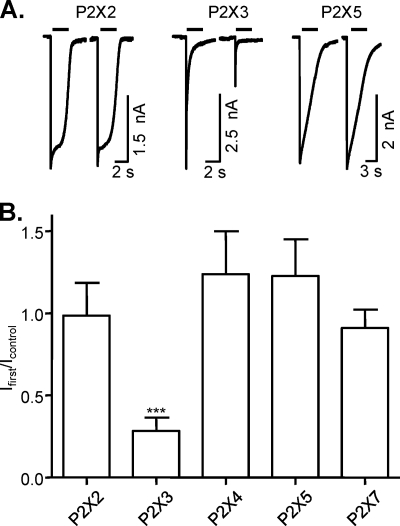
To gain an insight into the extent of cytoskeletal regulation of the P2X receptor family, the effects of cytochalasin D treatment (500 nm, 1-h treatment at room temperature) were determined on P2X2, 3, 4, 5, and 7 receptors transiently transfected into HEK293 cells. ATP-evoked currents at P2X3 receptors were reduced by ∼ 71.7 ± 8.2% (n = 17) following cytochalasin treatment (Fig. 5), but the time course of P2X3 receptor current desensitization was unaffected (data not shown). In contrast, homomeric P2X2, 4, 5, and 7 receptor currents were unaffected following cytoskeletal disruption. The lack of effect of cytochalasin on P2X7 receptor currents is consistent with previous studies on preassembled P2X7 receptors in acinar cells (34). These results demonstrate that regulation of P2X receptors by the cytoskeleton is subtype-specific.
FIGURE 5.
Differential sensitivity of P2X receptors to actin cytoskeleton disruption. The effect of actin cytoskeleton disruption by cytochalasin D was tested on currents mediated by P2X2, P2X3, P2X4, P2X5, and P2X7 receptors transiently transfected in HEK293 cells. Currents were evoked by application of ATP (100 μm) or BzATP (100 μm in 0.3 mm CaCl2, 0 mm MgCl2 containing solution) for the P2X7 receptor. A, representative traces of ATP-evoked currents of P2X receptors from control cells (left trace) and cells treated with cytochalasin D (right trace) (500 nm, 1 h). B, summary of the effects of actin cytoskeleton disruption for currents mediated by P2X2, P2X3, P2X4, P2X5, and P2X7 receptors (n = 8–14). ***, p < 0.001.
Chimeric P2X1/2 Receptors Identify the Intracellular Amino Terminus as Important for Actin Sensitivity
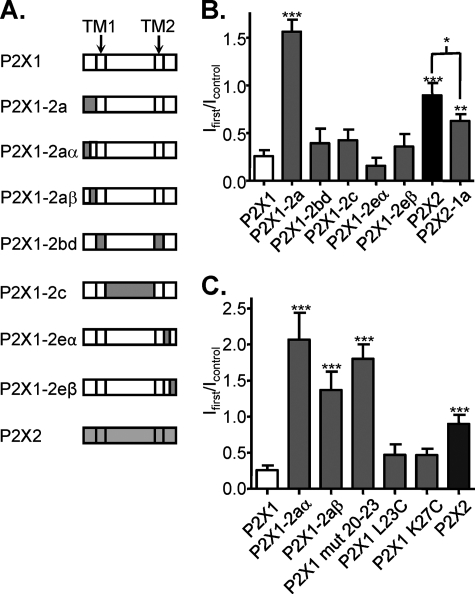
We have previously generated chimeric P2X1/2 receptors to address the molecular basis of regulation by lipid rafts, and these chimeras (18) have been used in this study to identify regions of the receptor important for cytoskeletal regulation. Chimeric P2X1/2 receptors where the two transmembrane regions (P2X1–2bd), the extracellular loop (P2X1–2c), or the intracellular carboxyl terminus (P2X1–2eα and P2X1–2eβ) of the P2X1 receptor were replaced with the corresponding region from the P2X2 receptor showed the same level of inhibition following cytochalasin treatment (500 nm, 1-h treatment at room temperature) as the homomeric P2X1 receptor (Fig. 6). In contrast swapping the amino terminus of the P2X1 receptor for that from the P2X2 receptors (P2X1/2a) produced a channel where cytochalasin treatment actually potentiated the responses to ∼156 ± 12% (n = 8) of control. The reciprocal chimera, replacing the amino terminus of the P2X1 receptor with the corresponding region from the P2X1 receptor (P2X2/1a), was inhibited by ∼32.6 ± 8.4% (n = 10) by cytochalasin. These results indicate that the intracellular amino terminus of the P2X1 receptor is important for regulation by the actin cytoskeleton.
FIGURE 6.
Identification of the regions of the P2X1 receptor that contribute to actin sensitivity. A, schematic representation of chimeric P2X1/P2X2 receptors. P2X1/2aα and P2X1–2aβ chimeras constructs had residues 1–16 and 16–31 from the P2X1 receptor swapped with those from the P2X2 receptor, respectively. P2X1/2eα and P2X1/2eβ chimeras constructs had residues 354–366 and 367–459 from the P2X1 receptor swapped with those from the P2X2 receptor, respectively. B, summary of the effects of cytochalasin D (500 nm, 1 h) treatment on currents mediated by P2X1, P2X2, and P2X1/P2X2 chimeric receptors as fraction of the response to ATP in nontreated cells (n = 8–19). C, summary of the effects of cytochalasin D (500 nm, 1 h) treatment on currents mediated by P2X1/P2X2 chimeric receptors with modified N terminus (n = 6–19). *, p < 0.05; ***, p < 0.001.
Replacing either the first half of the amino terminus (P2X1/2aα) or the second half of the amino terminus (P2X1/2aβ) of the P2X1 receptor with the corresponding region of the P2X2 receptor removed the inhibitory effect of cytochalasin, and responses were actually potentiated (by 58%, n = 6, and 13%, n = 5, p < 0.001) (Fig. 6C). We have previously shown that the residues in the amino terminus (P2X1 L23C, K27C, and mut 20–23) just before the first transmembrane region are involved in regulation of the P2X1 receptor by cholesterol (18). We were interested in determining whether these mutations had an effect on the sensitivity to cytoskeletal disruption. ATP-evoked currents were potentiated following cytochalasin treatment for the mutant where the four amino acids (20–23) of the P2X1 receptor were replaced with those from the P2X2 receptor (P2X1 mut 20–23). The point mutations P2X1 L23C and K27C, which removed cholesterol sensitivity (18), were still inhibited, although to a smaller extent, by cytoskeletal disruption. These results indicate that there is considerable overlap in the role of the amino terminus in regulation of cholesterol and cytoskeletal sensitivity.
Stabilization of the Actin Cytoskeleton Abolishes Cholesterol Sensitivity of the P2X1 Receptor
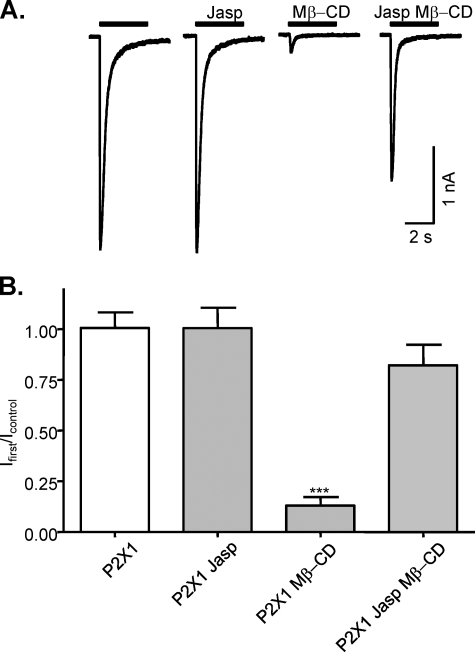
We have previously shown that cholesterol depletion with methyl-β-cyclodextrin (Mβ-CD) results in the inhibition of P2X1 receptor currents (18, 19). The role of the amino terminus in determining both cholesterol (18) and cytoskeletal sensitivity (this study) suggested that there may be some interplay between the cytoskeleton and cholesterol depletion in the regulation of P2X1 receptors (Fig. 7). Pretreatment with jasplakinolide to stabilize the actin cytoskeleton had no effect on P2X1 receptor currents (n = 9, p = 0.82) but removed the inhibitory effect of subsequent cholesterol disruption with Mβ-CD (n = 13, p < 0.001). These results suggest an interaction between the actin cytoskeleton and lipid rafts in the regulation of the P2X1 receptor.
FIGURE 7.
Stabilization of actin polymerization prevents disruption of lipid rafts that are essential for P2X1 receptors function. A, representative traces of P2X1 receptor-mediated currents evoked by application of α,β-meATP (10 mm) for nontreated cells and after incubation with jasplakinolide (Jasp, 30 nm, 1 h), Mβ-CD (10 mm, 1 h), or jasplakinolide (30 nm) together with Mβ-CD (10 mm, 1 h) followed by pretreatment with jasplakinolide (30 nm, 1 h). B, summary data of actin polymerization and disruption of lipid rafts on P2X1 receptor-mediated currents. P2X1 receptor currents were unaffected by treatment with jasplakinolide (30 nm, 1 h) which stabilizes polymerized actin cytoskeleton (n = 9). Mβ-CD (10 mm, 1 h) treatment reduced the peak amplitude of P2X1 receptor currents by >90% (n = 15). Treatment with jasplakinolide (30 nm, 1 h) together with Mβ-CD (10 mm, 1 h) followed by pretreatment with jasplakinolide (30 nm, 1 h) abolished the effect of Mβ-CD (n = 13). ***, p < 0.001.
DISCUSSION
To function effectively receptors are often found in microdomains with other signaling molecules or regulatory proteins. For example, the P2X1 receptor is expressed in cholesterol enriched lipid rafts that play an important role in regulation of the receptor (18, 19). The cytoskeleton plays a role in the organization and regulation of many receptors, including ligand gated ion channels (35). Previous studies have identified proteins interacting with P2X2 and P2X7 receptors (21–25) that have included the cytoskeletal proteins tubulin for P2X2 and β-actin, α-actinin as well as supervillin for the P2X7 receptor. We have now shown that the P2X1 receptor is also associated with a range of cytoskeletal proteins and that disruption of the actin cytoskeleton can regulate P2X receptor function in a subtype-specific manner.
The inhibition of P2X1 receptor currents following cytoskeletal disruption could result from a decrease in agonist sensitivity, a reduction in expression of the receptors, and/or an effect on the gating of the channel. The time course of P2X1 receptor current desensitization is a hallmark of the agonist sensitivity of the receptor (supplemental Fig. 3). Cytochalasin, however, had no effect on the time course of P2X1 receptor current desensitization. The lack of effect on the time course of desensitization of both native arterial smooth muscle and recombinant P2X1 receptors is consistent with a previous report on cytochalasin B-treated P2X1 receptors expressed in Xenopus oocytes (36). In another study it was reported that following 2–4 days of plating HEK293 cells stably expressing P2X1 receptors changed from a rounded to a flat phenotype. Associated with this was a considerable slowing in the time course of desensitization that reverted to the more rapid time course seen in rounded cells following disruption of the actin cytoskeleton (37). However, it is not clear whether this effect was also associated with a change back to the rounded phenotype and could be explained by improved agonist access to the cell. The finding in this study that actin disruption does not slow P2X1 receptor desensitization (predicted for a decrease in agonist potency) demonstrates that the reduction in current amplitude is unlikely to result from a change in agonist sensitivity. We have also shown that cytoskeletal disruption had no effect on the surface expression of P2X1 receptors (Fig. 2). Taken together, these data rule out a change in agonist sensitivity or expression levels as being responsible for the reduction in current amplitude. Therefore, an effect on the likelihood of the agonist-bound receptor opening, i.e. channel gating, is most likely to account for the reduction in P2X1 receptor responses following cytoskeletal disruption.
The inhibition of P2X1 receptor currents following actin depolymerization, with either cytochalasin or latrunculin, clearly demonstrates the importance of the cytoskeleton for receptor function and raised the possibility that ongoing dynamic polymerization of actin is required for receptor function. However, stabilization of the actin cytoskeleton with jasplakinolide had no effect on the reproducibility of P2X1 currents in the permeabilized patch configuration, demonstrating that dynamic changes in the cytoskeleton are not required for regulation of the P2X1 receptor, just an intact cytoskeleton. The current study shows that inclusion of ATP in the pipette solution can rescue P2X1 receptor current rundown. Given the contribution of the cytoskeleton to signaling microdomains, one possibility is that the cytoskeleton plays an important role in the maintenance of an ATP-dependent regulatory complex around the P2X1 receptor that can modulate channel gating.
Chimeras between actin-sensitive P2X1 and -insensitive P2X2 receptors identified the amino terminus of the P2X1 receptor to be essential for regulation by the cytoskeleton. The reciprocal amino-terminal chimera also conferred actin sensitivity on the P2X2 receptor (chimera P2X2–1a). A cluster of amino acids close to the first transmembrane region of the P2X1 receptor has previously been shown to be important for regulation by protein kinase C/phorbol esters and lipid rafts (18, 41, 42). Within this pre-TM1 region the mutant P2X1 mut20–23 removed sensitivity to both cytoskeletal disruption (this study) and cholesterol depletion (18). It has been shown recently that the P2X1 receptor migrates to the immune synapse following T cell activation (11). Interestingly, both filamentous actin and lipids congregate at the immune synapse (43), and our work suggests that the P2X1 could be trafficked to the immune synapse through its association with the actin cytoskeleton-rich lipid rafts.
P2X1 receptor currents were inhibited by both actin depolymerization and cholesterol depletion (17). However, pretreatment with jasplakinolide to stabilize the actin cytoskeleton abolished the inhibitory effect of cholesterol depletion. This suggested that either actin depolymerization was reducing lipid raft association or conversely that cholesterol depletion was interfering with the state of actin polymerization. We therefore determined whether cytoskeletal manipulations had an effect on the lipid raft association of the P2X1 receptor (supplemental Fig. 4). Cholesterol depletion with Mβ-CD resulted in redistribution of the P2X1 receptor within the density gradient consistent with movement out of the lipid rafts as we have shown previously (19). In contrast, disruption of the actin cytoskeleton with cytochalasin had no effect on the distribution/raft association of the P2X1 receptor (supplemental Fig, 4). These results demonstrate that the effects of actin disruption were not mediated directly by an effect on the lipid raft association of the P2X1 receptor. Stabilization of the actin cytoskeleton with jasplakinolide also had no effect on the P2X1 receptor association with lipid rafts on its own. Interestingly, jasplakinolide treatment did not prevent the redistribution of the P2X1 receptor within the density gradient in response to Mβ-CD (supplemental Fig. 4). These results show that even when the raft association of the P2X1 receptor was disrupted, stabilization of the actin cytoskeleton was sufficient to maintain efficient signaling through the P2X1 receptor. This suggests that cholesterol depletion may have a predominant effect on P2X1 receptor responsiveness through disrupting interactions with the actin cytoskeleton. This is consistent with studies where cholesterol depletion leads to actin depolymerization (38–40), which can be blocked by stabilization of the cytoskeleton with jasplakinolide (38).
In summary, we have shown that the actin cytoskeleton plays an important role in P2X1 receptor function. The intracellular amino terminus of the receptor is essential for this regulation and also contributes to protein kinase C-mediated potentiation of the receptor. Our results suggest that the close association of the P2X1 receptor with the actin cytoskeleton contributes to a localized signaling microenvironment important for regulation.
Supplementary Material
Acknowledgments
We thank Drs. Sharad Mistry and Andrew Bottrill at Protein Nucleic Acid Chemistry Laboratory, University of Leicester, for mass spectrometry; Dr. Catherine Vial for generation of the tagged receptor; Drs. Andrew Powell and Kelvin Agboh for initial studies on P2X1 receptor purification; Drs. Rebecca Allsopp and Hairuo Wen for generation of chimeras and mutants; and Dr. Sue Monkley for discussions on the actin cytoskeleton.
This work was supported by the Wellcome Trust.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4 and Table 1.
A. Bye, R. J. Evans, and M. P. Mahaut Smith, unpublished observations.
- DSP
- dithiobis(succinimidyl propionate)
- EGFP
- enhanced GFP
- FRAP
- fluorescent recovery after photobleaching
- Mβ-CD
- methyl-β-cyclodextrin
- pA/pF
- picoamperes per picofarad
- α,β-meATP
- α,β-methylene ATP.
REFERENCES
- 1. Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. (1994) Nature 371, 516–519 [DOI] [PubMed] [Google Scholar]
- 2. Nicke A., Bäumert H. G., Rettinger J., Eichele A., Lambrecht G., Mutschler E., Schmalzing G. (1998) EMBO J. 17, 3016–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vial C., Evans R. J. (2002) Mol. Pharmacol. 62, 1438–1445 [DOI] [PubMed] [Google Scholar]
- 4. Mulryan K., Gitterman D. P., Lewis C. J., Vial C., Leckie B. J., Cobb A. L., Brown J. E., Conley E. C., Buell G., Pritchard C. A., Evans R. J. (2000) Nature 403, 86–89 [DOI] [PubMed] [Google Scholar]
- 5. Vial C., Evans R. J. (2000) Br. J. Pharmacol. 131, 1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gachet C. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 277–300 [DOI] [PubMed] [Google Scholar]
- 7. Hechler B., Magnenat S., Zighetti M. L., Kassack M. U., Ullmann H., Cazenave J. P., Evans R., Cattaneo M., Gachet C. (2005) J. Pharmacol. Exp. Ther. 314, 232–243 [DOI] [PubMed] [Google Scholar]
- 8. Wareham K., Vial C., Wykes R. C., Bradding P., Seward E. P. (2009) Br. J. Pharmacol. 157, 1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sim J. A., Park C. K., Oh S. B., Evans R. J., North R. A. (2007) Br. J. Pharmacol. 152, 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lecut C., Frederix K., Johnson D. M., Deroanne C., Thiry M., Faccinetto C., Marée R., Evans R. J., Volders P. G., Bours V., Oury C. (2009) J. Immunol. 183, 2801–2809 [DOI] [PubMed] [Google Scholar]
- 11. Woehrle T., Yip L., Elkhal A., Sumi Y., Chen Y., Yao Y., Insel P. A., Junger W. G. (2010) Blood 116, 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Surprenant A., North R. A. (2009) Annu. Rev. Physiol. 71, 333–359 [DOI] [PubMed] [Google Scholar]
- 13. Lalo U., Allsopp R. C., Mahaut-Smith M. P., Evans R. J. (2010) J. Neurochem. 113, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis C. J., Evans R. J. (2000) Br. J. Pharmacol. 131, 1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vial C., Tobin A. B., Evans R. J. (2004) Biochem. J. 382, 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ase A. R., Raouf R., Bélanger D., Hamel E., Séguéla P. (2005) J. Pharmacol. Exp. Ther. 315, 144–154 [DOI] [PubMed] [Google Scholar]
- 17. Allen J. A., Halverson-Tamboli R. A., Rasenick M. M. (2007) Nat. Rev. Neurosci. 8, 128–140 [DOI] [PubMed] [Google Scholar]
- 18. Allsopp R. C., Lalo U., Evans R. J. (2010) J. Biol. Chem. 285, 32770–32777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vial C., Evans R. J. (2005) J. Biol. Chem. 280, 30705–30711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vial C., Fung C. Y.., Goodall A. H., Mahaut-Smith M. P., Evans R. J. (2006) Biochem. Biophys. Res. Commun. 343, 415–419 [DOI] [PubMed] [Google Scholar]
- 21. Masin M., Kerschensteiner D., Dümke K., Rubio M. E., Soto F. (2006) J. Biol. Chem. 281, 4100–4108 [DOI] [PubMed] [Google Scholar]
- 22. Gendreau S., Schirmer J., Schmalzing G. (2003) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 786, 311–318 [DOI] [PubMed] [Google Scholar]
- 23. Chaumont S., Compan V., Toulme E., Richler E., Housley G. D., Rassendren F., Khakh B. S. (2008) Sci. Signal. 1, ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim M., Jiang L. H., Wilson H. L., North R. A., Surprenant A. (2001) EMBO J. 20, 6347–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu B. J., Saunders B. M., Jursik C., Wiley J. S. (2010) Blood 115, 1621–1631 [DOI] [PubMed] [Google Scholar]
- 26. Kotnis S., Bingham B., Vasilyev D. V., Miller S. W., Bai Y., Yeola S., Chanda P. K., Bowlby M. R., Kaftan E. J., Samad T. A., Whiteside G. T. (2010) Mol. Pharmacol. 77, 953–960 [DOI] [PubMed] [Google Scholar]
- 27. Bo X., Jiang L. H., Wilson H. L., Kim M., Burnstock G., Surprenant A., North R. A. (2003) Mol. Pharmacol. 63, 1407–1416 [DOI] [PubMed] [Google Scholar]
- 28. Pankratov Y. V., Krishtal O. A. (2003) Biophys. J. 85, 3375–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper J. A. (1987) J. Cell Biol. 105, 1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morton W. M., Ayscough K. R., McLaughlin P. J. (2000) Nat. Cell Biol. 2, 376–378 [DOI] [PubMed] [Google Scholar]
- 31. Ennion S., Hagan S., Evans R. J. (2000) J. Biol. Chem. 275, 29361–29367 [DOI] [PubMed] [Google Scholar]
- 32. Rosenmund C., Westbrook G. L. (1993) Neuron 10, 805–814 [DOI] [PubMed] [Google Scholar]
- 33. Fernandes C. C., Berg D. K., Gómez-Varela D. (2010) J. Neurosci. 30, 8841–8851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Q., Luo X., Zeng W., Muallem S. (2003) J. Biol. Chem. 278, 47554–47561 [DOI] [PubMed] [Google Scholar]
- 35. Sheng M., Pak D. T. (2000) Annu. Rev. Physiol. 62, 755–778 [DOI] [PubMed] [Google Scholar]
- 36. Werner P., Seward E. P., Buell G. N., North R. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 15485–15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parker K. E. (1998) J. Physiol. 510, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hering H., Lin C. C., Sheng M. (2003) J. Neurosci. 23, 3262–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brusés J. L., Chauvet N., Rutishauser U. (2001) J. Neurosci. 21, 504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwik J., Boyle S., Fooksman D., Margolis L., Sheetz M. P., Edidin M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13964–13969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ennion S. J., Evans R. J. (2002) Biochem. Biophys. Res. Commun. 291, 611–616 [DOI] [PubMed] [Google Scholar]
- 42. Wen H., Evans R. J. (2009) J. Neurochem. 108, 331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huppa J. B., Davis M. M. (2003) Nat. Rev. Immunol. 3, 973–983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.