Abstract
The cDNAs of two sorbitol transporters, common plantain (Plantago major) polyol transporter (PLT) 1 and 2 (PmPLT1 and PmPLT2), were isolated from a vascular bundle-specific cDNA library from common plantain, a dicot plant transporting Suc plus sorbitol in its phloem. Here, we describe the kinetic characterization of these sorbitol transporters by functional expression in Brewer's yeast (Saccharomyces cerevisiae) and in Xenopus sp. oocytes and for the first time the localization of plant PLTs in specific cell types of the vascular tissue. In the yeast system, both proteins were shown to be uncoupler sensitive and could be characterized as low-affinity and low-specificity polyol symporters. The Km value for the physiological substrate sorbitol is 12 mm for PmPLT1 and even higher for PmPLT2, which showed an almost linear increase in sorbitol transport rates up to 20 mm. These data were confirmed in the Xenopus sp. system, where PmPLT1 was analyzed in detail and characterized as a H+ symporter. Using peptide-specific polyclonal antisera against PmPLT1 or PmPLT2 and simultaneous labeling with the monoclonal antiserum 1A2 raised against the companion cell-specific PmSUC2 Suc transporter, both PLTs were localized to companion cells of the phloem in common plantain source leaves. These analyses revealed two different types of companion cells in the common plantain phloem: younger cells expressing PmSUC2 at higher levels and older cells expressing lower levels of PmSUC2 plus both PLT genes. The putative role of these low-affinity transporters in phloem loading is discussed.
The export of photoassimilates from higher plant source leaves occurs via the sieve element/companion cell complex (SE/CCC) of the phloem. In many plant species, such as in Arabidopsis, maize (Zea mays), sugar beet (Beta vulgaris), or tobacco (Nicotiana tabacum), assimilated CO2 is exported exclusively in the form of Suc. In numerous other plants, however, additional carbohydrates are used for this long distance transport. Examples are raffinose or stachyose in Cucurbitaceae (Kandler and Hopf, 1982; Keller and Pharr, 1996) or reduced monosaccharides, such as mannitol or sorbitol in Rosaceae, Plantaginaceae, or several other families (Barker, 1955; Webb and Burley, 1962; Zimmermann and Ziegler, 1975). The common properties of Suc and these additional compounds are that they are highly soluble, chemically inert, and not readily accessible for primary cellular metabolism. They can thus be stored and transported in high concentrations with no damage to the cells and without being degraded or modified.
cDNAs encoding Suc transporters involved in phloem loading have been cloned from several plants (Riesmeier et al., 1992; Riesmeier et al., 1993; Gahrtz et al., 1994; Sauer and Stolz, 1994; Bürkle et al., 1998; Aoki et al., 1999; Noiraud et al., 2000; Williams et al., 2000). In contrast, only a single cDNA of a mannitol transporter, AgMAT1 from celery (Apium graveolens; Noiraud et al., 2001) and two cDNAs for sorbitol transporters, PcSOT1 and PcSOT2 from sour cherry (Gao et al., 2003), has been cloned. So far, no transporters for raffinose or stachyose have been identified. This does not at all reflect the relative importance of Suc versus these other substances in phloem transport, because in many plants, phloem concentrations of oligosaccharides from the raffinose family or of polyols are comparable with or even higher than the concentrations of Suc. For example, Suc, raffinose, and stachyose concentrations in pumpkin (Cucurbita maxima) were found to be 180, 120, and 180 mm, and in peach (Prunus persica), the concentrations of Suc and sorbitol were shown to be 140 and 550 mm in the phloem sap. In common plantain, Suc and sorbitol concentrations are 800 and 300 mm, respectively (Lohaus and Fischer, 2002; G. Lohaus, unpublished data).
It is not really understood, why different plants use different compounds for long distance carbon allocation. For raffinose-transporting symplastic phloem loaders, such as cucurbits, it has been postulated that the difference in the Stoke's radii between Suc and raffinose (or stachyose) represents the actual “driving force” for long distance transport (Turgeon, 1996). The so-called “polymer trap model” is based on the assumption that Suc, the first precursor in raffinose biosynthesis, can traffic symplastically from mesophyll cells into the SE/CCC. The model is also based on the observation that galactinol, the second precursor in raffinose formation, is synthesized inside the companion cells (also called intermediary cells) of these plants (Bebee and Turgeon, 1992; Haritatos et al., 2000). The unproven conclusion is that Suc and monosaccharides can traffic into the companion cells, but raffinose cannot traffic back due to its higher Stoke's radius (trapping mechanism). According to this model, phloem transport of oligosaccharides from the raffinose family might be essential to drive phloem transport in symplastic loaders. The major drawback of this model is, however, that it has never been possible to show that plasmodesmata can discriminate between molecules, such as Suc or raffinose.
In contrast, there are several physiological reasons that might explain the long distance transport of polyols. Obviously, phloem transport of more reduced sugar alcohols may be advantageous for NADPH+-dependent reactions in sinks, such as the reduction of NO3-, which in many plants is performed in roots (Hansch et al., 2001). Mannitol has also been shown to act as antioxidant (Shen et al., 1997) and may have important functions in plant-pathogen interactions (Jennings et al., 1998). Moreover, it is known that polyols can serve as compatible solutes and the role of mannitol as an osmoprotectant in celery is well documented (Tarczynski et al., 1993; Everard et al., 1994; Stoop and Pharr, 1994a). Interestingly, mannitol synthesis is not up-regulated under stress (Everard et al., 1994), and increased mannitol concentrations in roots of stressed plants result primarily from reduced degradation of mannitol (Stoop and Pharr, 1994a, 1994b), which is constantly supplied by the phloem. Finally, phloem polyols were also shown to influence the phloem mobility of boron (B) by the formation of soluble mannitol-B-mannitol complexes in celery or of sorbitol-B-sorbitol complexes in peach (Penn et al., 1997). These complexes were identified in the phloem sap of these plants and are assumed to be the basis for the increased B efficiency of these plants (Hu et al., 1997). Genetically modified tobacco plants with enhanced sorbitol synthesis were shown to transport B in their phloem, whereas control plants did not (Bellaloui et al., 1999; Brown et al., 1999).
Despite these important functions of mannitol or sorbitol in higher plant physiology, little is known about the proteins involved in phloem loading of polyols—only the mannitol transporter AgMAT1 is thought to be involve in phloem loading (Noiraud et al., 2001)—and nothing is known about the identity of the cells catalyzing this step. Common plantain, a sorbitol-translocating plant (Wallart, 1981; Lohaus and Fischer, 2002), is highly resistant to drought, trampling, and other environmental stresses. Moreover, vascular tissue is easily purified from common plantain leaves and has been used to clone and characterize the Suc transporters PmSUC2 (Gahrtz et al., 1994; Stadler et al., 1995a) and PmSUC3 (Barth et al., 2003). Here, we describe the identification, characterization, and cellular localization of two phloemlocalized sorbitol transporters from plantain, an important step toward the understanding of potential physiological roles of sorbitol in higher plants.
RESULTS
Cloning of Two Polyol Transporter (PLT) cDNAs
The only characterized plant PLT putatively involved in phloem loading is the product of the AgMAT1 cDNA from celery (Noiraud et al., 2001), a Suc and mannitol translocating plant. Homologous cDNAs encoding sorbitol transporters were identified in fruits of sour cherry (Gao et al., 2003), and related genes have also been found in plants that neither transport polyols inside their phloem nor store polyols, such as in Arabidopsis (Munich Information Center for Protein Sequences nos. At2g16120, At2g20780, At2g16130, At3g18830, At2g18480, and At4g36670) and sugar beet (accession nos. U64902 and U64903). None of the encoded gene products has been characterized so far, but the presence of these genes suggests that they may have functions different from phloem loading and that mannitol or similar substrates may be transported in these plants under specific physiological conditions.
AgMAT1, PcSOT1, PcSOT2, and the uncharacterized proteins from Arabidopsis and sugar beet share a high degree of similarity (about 70% similarity on the protein level). Therefore, we hoped that a lowstringency screening (Sauer et al., 1990) of a vascular tissue-specific cDNA library (Gahrtz et al., 1994) from sorbitol-translocating common plantain (Wallart, 1981; Lohaus and Fischer, 2002) with an AgMAT1-derived probe might identify cDNAs potentially encoding transporters involved in the phloem loading of sorbitol.
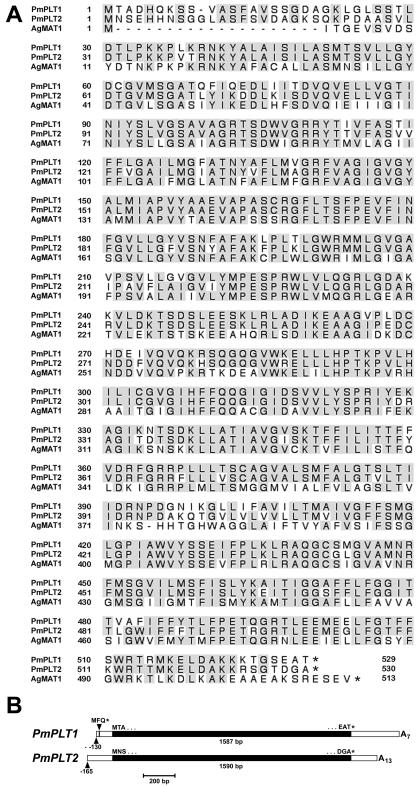
After the first screening, 10 to 15 positive signals were obtained per filter (7,000 plaque forming units), suggesting that between 0.05% and 0.1% of the clones in this library may encode PLT-like sequences. This was confirmed by sequencing the inserts of numerous randomly chosen positive λ-clones. All sequences could be assigned to two different, highly homologous cDNAs. The genes were named P. major PLT 1 and 2 (PmPLT1 and PmPLT2). The encoded proteins (Fig. 1A) are 529 amino acids (PmPLT1) and 530 amino acids (PmPLT2) long and share 83.0% identical amino acids. Moreover, they share 67.5% (PmPLT1) and 65.8% (PmPLT2) identity with AgMAT1. The number of identical amino acids shared with the cherry sorbitol transporters are similar.
Figure 1.
Comparison of PmPLT1, PmPLT2 (sorbitol transporters), and AgMAT1 (mannitol transporter) protein sequences and of the cDNA structures of PmPLT1 and PmPLT2. A, Amino acid sequences of PmPLT1, PmPLT2, and AgMAT1 were aligned with the program SeqVu (James Gardner, Garvan Institute of Medical Research, Sydney), and residues identical in all three sequences were highlighted. B, The structure of the longest PmPLT1 and PmPLT2 cDNAs are presented including the information from the 5′-RACE reactions. Arrowheads indicate the transcriptional start sites and the start (-111 bp) of the short ORF in the 5′-untranslated region of PmPLT1. The complete cDNA sequences were deposited in the EMBL data library. Accession numbers are AJ532589 (PmPLT1) and AJ532590 (PmPLT2).
Interestingly, the longest cDNA of PmPLT1 (1,987 bp) with 115-bp 5′-flanking sequence and 285-bp 3′-flanking sequence contained a short open reading frame (ORF) for the tripeptide Met-Phe-Gln starting 111 bp upstream from the predicted start-ATG of the cDNA. Such a 5′-ORF was absent from the 5′-flanking sequence of the longest PmPLT2 cDNA (1,891 bp with 145-bp 5′-flanking sequence and 156-bp 3′-flanking sequence). To test, whether this short 5′-ORF in PmPLT1 corresponds to the C terminus of an even longer ORF and whether a similar 5′-ORF is present in the complete 5′-untranslated sequence of PmPLT2, 5′-RACEs were performed with total RNA from common plantain vascular tissue. The PmPLT1-specific RACE-reactions showed that translation of the PmPLT1 gene starts at position -130 bp and that the 5′-ORF encodes only the tripeptide Met-Phe-Gln (Fig. 1B). The PmPLT2-specific 5′-RACE reactions showed that translation in the PmPLT2 gene starts at -165 and that the corresponding mRNA does not have a 5′-ORF (Fig. 1B).
Functional Expression in Yeast Depends on the 5′-Flanking Sequences
It had been claimed that acyclic polyols cannot be metabolized by Brewer's yeast (Saccharomyces cerevisiae; Canh et al., 1975). This was disproven later by the observation that bakers' yeast can induce expression of the sorbitol dehydrogenase gene, SDH1, when grown on sorbitol as the sole carbon source for at least 2 weeks (Sarthy et al., 1994). Such a delayed induction of sorbitol catabolism does not interfere with sorbitol transport tests performed with Glc-grown yeast cells, where endogenous genes for polyol uptake and metabolism stay repressed. Therefore, transport properties of PmPLT1 and PmPLT2 were analyzed by expressing of the longest cDNAs in the bakers' yeast strain SEY2102 (Emr et al., 1983) in sense and antisense orientation in the unique EcoRI-site of the NEV-E expression vector (Sauer and Stolz, 1994).
However, uptake analyses with 14C-sorbitol showed no detectable transport activity for any of the sense transformants (data not shown). For PmPLT1, this might be explained by the presence of the 5′-ORF described above. Therefore, truncated clones were generated by PCR lacking this 5′-ORF. But again, no transport activities could be observed after NEV-E-based expression of these cDNAs in yeast (data not shown). In a final attempt, cDNA constructs were generated for PmPLT1 and PmPLT2 with modified 5′-flanking sequences. This approach had been applied successfully before (Stadler et al., 1995b) and replaces the 5′-flanking sequence of a given cDNA by the sequence AAGCTTGTAAAAGAAATG. This sequence was taken from the 5′-flanking region of AtSTP1, the first higher plant transporter successfully expressed in yeast (Sauer et al., 1990). This sequence seems to be ideal for the bakers' yeast translation machinery and fits well to the consensus sequence (A/Y) A(A/Y) A(A/Y) AATG published for bakers' yeast (Hinnebusch and Liebman, 1991). With the modified PmPLT1 and PmPLT2 cDNAs, a third set of sense and antisense yeast lines was generated for and transport of 14C-sorbitol was analyzed.
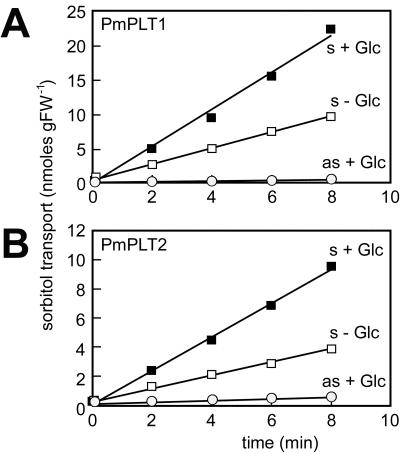
Obviously, the native 5′-flanking sequences of both cDNAs were the reason for the observed lack in expression with the first two sets of constructs. Sense yeast strains harboring PmPLT1 (strain MRYs1) or PmPLT2 (strain MRYs2) cDNA constructs with modified 5′-flanking sequences expressed the cDNAs and were able to incorporate 14C-sorbitol (Fig. 2). No transport activity was observed in control strains harboring the cDNAs in antisense direction (strains MRYas1 and MRYas2).
Figure 2.
PmPLT1 and PmPLT2 can be expressed in yeast cells and catalyze the uptake of 14C-sorbitol. Transport rates for 14C-sorbitol were determined with the transgenic yeast cells MRYs1 and MRYas1 (expressing PmPLT1 in sense or antisense orientation) or with MRYs2 and MRYas2 (expressing PmPLT2 in sense or antisense orientation). The concentration of 14C-labeled sorbitol was 0.1 mm in all experiments. Where indicated, d-Glc was added to a final concentration of 10 mm.
Transport Properties and Kinetic Parameters
All previously analyzed plant sugar transporters as well as the celery mannitol transporter were described as energy-dependent H+ symporters driven by the proton motive force (pmf) across the plasma membrane (Boorer et al., 1994, 1996; Williams et al., 2000; Noiraud et al., 2001). It was, therefore, expected that sorbitol transport by PmPLT1 and PmPLT2 might also be energy dependent. A first clue came from the observation that 14C-sorbitol transport in transgenic yeast was enhanced in the presence of Glc (Fig. 2). This has also been described for Suc transporters expressed in yeast cells (Riesmeier et al., 1992; Gahrtz et al., 1994; Sauer and Stolz, 1994), and the idea is that Glc metabolism provides energy for active transport of non-metabolizable substrates or activates the plasma membrane H+-ATPase.
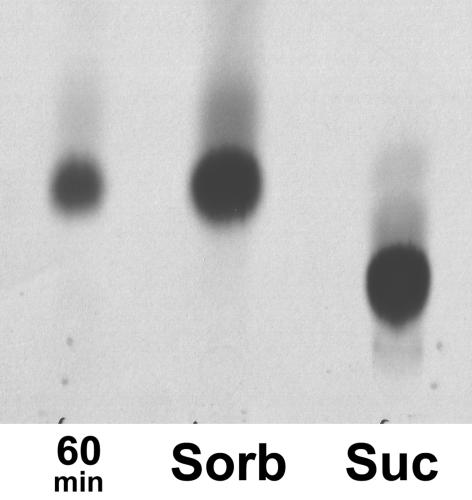
In addition, active sorbitol transport by PmPLT1 was shown more directly by comparing intracellular and extracellular sorbitol concentrations in MRYs1 cells after a 60-min incubation in 0.1 mm14C-sorbitol. During this time, the extracellular concentration of 14C-sorbitol was reduced to 0.075 mm, and from the incorporated amount of label, it was calculated that the cells should have an intracellular sorbitol concentration of 1.6 mm sorbitol, if sorbitol is not metabolized. This was confirmed by thin-layer chromatography of cellular extracts showing that the entire incorporated label was still 14C-sorbitol (Fig. 3). Thus, accumulation of sorbitol inside the yeast cells was more than 20-fold, which strongly supports an active transport mechanism by PmPLT1.
Figure 3.
Thin-layer chromatography of 14C-sorbitol accumulated in PmPLT1-expressing yeast cells. Cell extracts prepared with 80% (v/v) ethanol from yeast strain MRYs1 after a 60-min incubation in 14C-labeled sorbitol (60 min). 14C-Sorbitol (Sorb) and 14C-Suc (Suc) were used as standards.
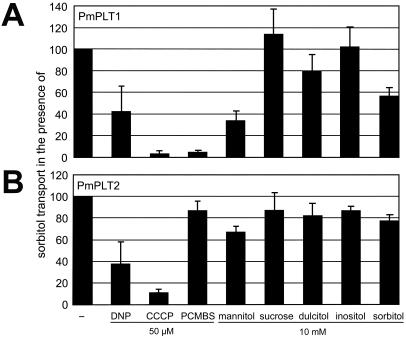
Finally, analyses of the sensitivities of PmPLT1 and PmPLT2 to uncouplers of proton gradients, such as carbonyl cyanide-m-chlorophenylhydrazone and dinitrophenol, confirmed that this active transport is driven by the proton motive force (Fig. 4).
Figure 4.
Transport properties of PmPLT1 and PmPLT2 in yeast cells. Transport of 14C-sorbitol (0.1 mm initial outside concentration) was analyzed in the presence of uncouplers (dinitrophenol or carbonyl cyanide-m-chlorophenylhydrazone) or in the presence of the SH-group inhibitor PCMBS. Inhibitors were added to a final concentration of 50 μm. Transport of 0.1 mm 14C-sorbitol was also analyzed in the presence of potential substrates added at a 100-fold excess (final concentrations 10 mm). Each bar results from at least three independent analyses (mean ± sd).
PmPLT1 is also sensitive to the SH-group inhibitor p-(chloromercuri) benzene sulfonic acid (PCMBS; Fig. 4A), whereas PmPLT2 is not (Fig. 4B). So far, PCMBS was known to inhibit the activity of plant Suc transporters with high specificity (Riesmeier et al., 1992; Sauer and Stolz, 1994) but not the activity of plant monosaccharide transporters (Ludwig et al., 2000). For mannitol transport in celery, it has been reported that the transporter is not (Salmon et al., 1995) or is only slightly (Noiraud et al., 2001) inhibited by PCMBS; the sorbitol transporters from cherry were not sensitive to PCMBS (Gao et al., 2003). The observed PCMBS sensitivity of PmPLT1 can be explained by comparing the Cys residues in PmPLT1 (six Cys residues) and PmPLT2 (five Cys residues). All five Cys residues found in PmPLT2 are conserved in PmPLT1. The sixth Cys is specific for PmPLT1 (Cys61 in Fig. 1) and is located in the predicted first extracellular loop between transmembrane helices 1 and 2. At this position, it seems to be accessible for the SH-group inhibitor. This Cys is not conserved in AgMAT1 (six Cys residues), PcSOT1 (five Cys residues), or PcSOT2 (six Cys residues) explaining the poor inhibition of these transporters by PCMBS (Noiraud et al., 2001).
Competition of 14C-sorbitol uptake with other compounds, such as unlabeled sorbitol, mannitol, dulcitol, inositol, or Suc (Fig. 4) revealed little effect of any of these compounds on 14C-sorbitol transport by PmPLT2, whereas a significant inhibition was observed for mannitol and unlabeled sorbitol on PmPLT1-driven 14C-sorbitol transport. On one hand, the observed lack of inhibition with PmPLT2 was in agreement with the high specificity described for the sorbitol transporters in cherry (Gao et al., 2003) and with earlier results from Salmon et al. (1995). These latter authors analyzed mannitol transport in plasma membrane vesicles prepared from celery phloem tissue and showed that a 20-fold excess of sorbitol had no inhibitory effect on 3H-mannitol transport. On the other hand, more recent analyses of recombinant AgMAT1 protein showed strong inhibition of mannitol transport by other sugar alcohols, such as sorbitol, xylitol, or dulcitol (Noiraud et al., 2001). Therefore, the mannitol transport capacity of PmPLT1- and PmPLT2-expressing yeast cells was tested directly using radiolabeled 14C-mannitol. Interestingly, both common plantain transporters catalyzed the transport of 14C-mannitol despite a poor inhibition of 14C-sorbitol transport by unlabeled mannitol in MRYs1. But again, the transport of 14C-mannitol (initial concentration 0.1 mm) was hardly inhibited by a 10-fold excess of unlabeled sorbitol (data not shown).
This result can only be explained with Km values that are significantly higher than the Km values published for AgMAT1 (Km = 0.3 mm) or PcSOT1 (Km = 0.6 mm) and PcSOT2 (Km = 0.3 mm). In this case, unlabeled polyol could be transported in addition to but not in competition with a second 14C-labeled polyol.
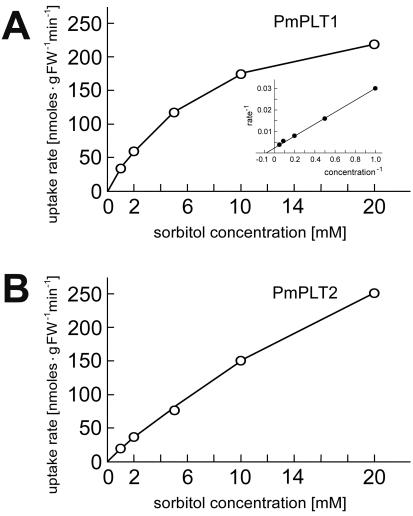
Analysis of the Km values confirmed this interpretation of the competition data. Figure 5 shows that the Km value for sorbitol of PmPLT1 is 12.3± 0.9 mm and even higher for PmPLT2 (the Km values for mannitol are about 5 and 30 mm for PmPLT1 and PmPLT2 in the yeast system; average of two analyses; data not shown). Thus, the Km values for both tested substrates are 1 to 2 orders of magnitude higher than the Km values of AgMAT1 (Noiraud et al., 2001) or the PcSOTs (Gao et al., 2003). These results characterize both common plantain PLTs as low-specificity and low-affinity sorbitol transporters.
Figure 5.
Analysis of substrate affinities of PmPLT1 and PmPLT2 for sorbitol in transgenic yeast cells. A, Michaelis-Menten plot for the sorbitol uptake by PmPLT1. Inset, Lineweaver-Burk plot of the same data set. B, Michaelis-Menten plot for the sorbitol uptake by PmPLT2. Uptake rates could not be saturated under the conditions analyzed. Each curve represents one out of three independent transport tests. From these analyses, the Km value for sorbitol of PmPLT1 was calculated to be 12.3 ± 0.9 mm. No Km value could be calculated for PmPLT2.
Functional Expression in Xenopus sp. Oocytes
The accumulation of sorbiol in PmPLT-expressing yeast cells, the sensitivity of polyol transport to uncouplers, and the increased transport rates in the presence of d-Glc provide indirect evidence for H+ polyol transport. For a direct analysis of the potential driving force, PmPLT1 transport was also analyzed in Xenopus sp. oocytes expressing injected PmPLT1 cRNA.
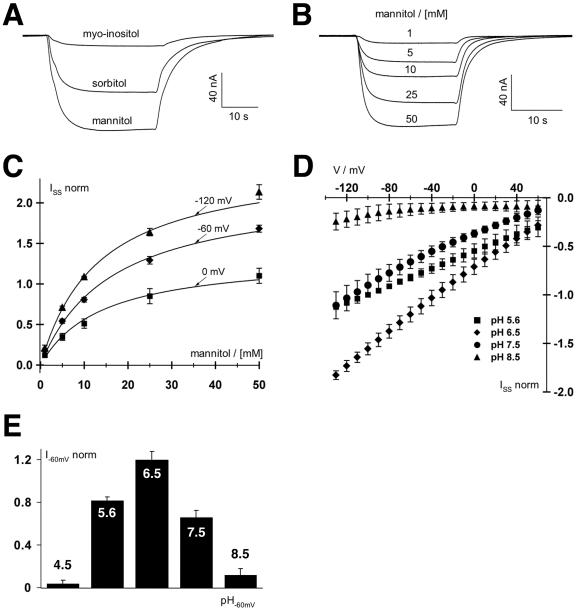
Inward currents were obtained in the presence of 30 mm sorbitol or mannitol, but little or no currents were observed with myoinositol (Fig. 6A) or Suc (data not shown) confirming the specificity of PmPLT1 for linear polyols. Due to the higher currents obtained with mannitol, this polyol was used for all further analyses. The mannitol-derived currents increased over a wide range of concentrations (Fig. 6B). Km values for mannitol (18.03 ± 2.38 mm at 0 mV, 16.94 ± 2.03 mm at -60 mV, and 15.15 ± 1.97 mm at -120 mV; mean ± sd; Fig. 6C) as well as analyses at different membrane potentials (Fig. 6D) revealed the dependence of this low-affinity transporter of the membrane potential. The slightly lower Km value (5 mm) that was determined for mannitol in the yeast system may reflect a higher membrane potential and other differences between the two expression systems.
Figure 6.
Biophysical analyses of PmPLT1 expressed in Xenopus sp. oocytes with the double-electrode voltage clamp technique. A, Polyol-induced inward H+ currents mediated by PmPLT1-injected oocytes. Increase in H+ currents in response to 30-s pulses of 30 mm mannitol, sorbitol, or myoinositol at pH 5.6 at a holding potential of -60 mV. Only mannitol and sorbitol but not myoinositol are substrates of PmPLT1. Relative currents were: Imannitol = 1, Isorbitol = 0.66 ± 0.11, and Imyoinositol = 0.12 ± 0.05 (data points were normalized to the currents at -60 mV in 30 mm mannitol; mean ± sd, n = 4). B, Whole-cell currents through PmPLT1 in response to a stepwise increase of mannitol concentration at pH 5.6 and a holding potential of -60 mV. C, Michaelis-Menten kinetics of PmPLT1 for mannitol at membrane potentials of 0, -60, and -120 mV. Data points represent the mean ± sd of five independent experiments normalized to the currents at -100 mV in 10 mm mannitol. Currents in the absence of mannitol were subtracted for leak correction. Continuous lines show the best nonlinear regression fits of the data points to the Michaelis-Menten equation. D and E, Mannitol-induced currents of oocytes injected with PmPLT1 cDNA are voltage and pH dependent. D, Whole-cell steady-state currents (ISS) in response to 10-mV voltage steps from 60 to -130 mV were recorded upon perfusion with 10 mm mannitol solutions at varying pH values from 4.5 to 8.5, as indicated. E, The pH optimum of PmPLT1 (determined at -120 mV) is 6.5. Data points represent mean ± sd, n = 4. Normalization to the currents at -100 mV in pH 5.6 and leak subtraction were performed as in C.
Figure 6, D and E, show that mannitol import by PmPLT1 is also proton dependent, and that PmPLT1 has a pH optimum at about pH 6.5. Addition of 10 mm Na+ did not alter the polyol-induced currents (data not shown).
Immunolocalization of the Sorbitol Transporters in Planta
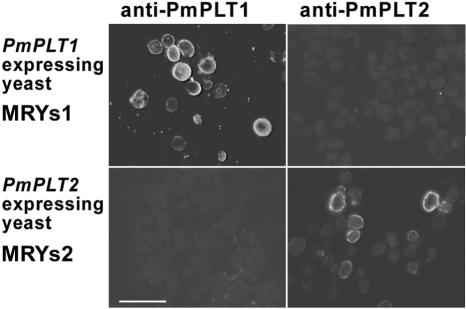
The large number of PmPLT1 and PmPLT2 cDNAs identified within the vascular tissue-specific library (0.05%-0.1% of all clones) suggests that both encoded proteins are strongly expressed in the phloem and involved in phloem loading of sorbitol. However, for a detailed understanding of their physiological function it is necessary to identify the precise cell type(s) where each of these genes is expressed. Therefore, antibodies were raised against peptides corresponding to the very N termini and the very C termini of the two transporters (amino acids 1-11 and 522-529 of PmPLT1 and amino acids 1-12 and 523-530 of PmPLT2). The specificity of the obtained antisera was tested on cross sections of the yeast strains MRYs1 (expresses PmPLT1) and MRYs2 (expresses PmPLT2). Figure 7 shows that the anti-PmPLT1 antiserum binds only to sections of yeast strain MRYs1 but not to sections of MRYs2 cells. Vice versa, anti-PmPLT2 antiserum binds only to sections of yeast strain MRYs2 but not to sections of MRYs1 cells. This shows the specificity of both antisera that were now used for immunolocalization of PmPLT1 and PmPLT2 in sections of common plantain source leaves.
Figure 7.
Specificity of anti-PmPLT1 and anti-PmPLT2 antisera. Sections of the PmPLT1-expressing yeast strain MRYs1 and the PmPLT2-expressing strain MRYs2 were fixed and embedded under the same conditions that were used for common plantain leaf material (Fig. 7). Sections were treated with anti-PmPLT1 or anti-PmPLT2 antiserum. Binding of antibody was detected under a fluorescence microscope by decoration with a fluorescent goat anti-rabbit IgG antiserum. Scale bar = 10 μm.
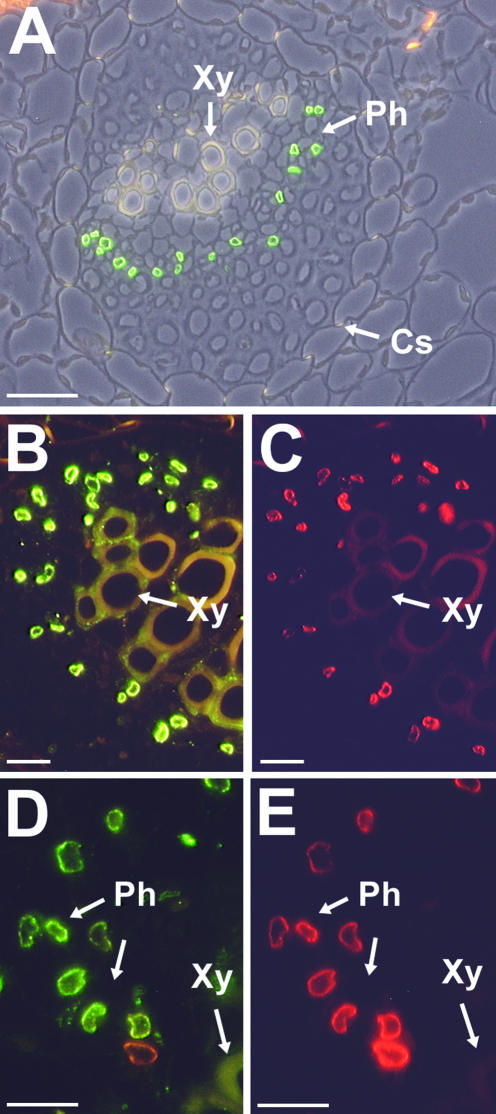
Figure 8A shows a cross section through a common plantain leaf treated with anti-PmPLT1 antiserum and decorated with Alexa Fluor 488-conjugated goat anti-rabbit IgG. Green fluorescence is clearly visible in individual cells of the phloem, suggesting that the labeled cells are part of the SE/CCC. Similar results were obtained with anti-PmPLT2 antiserum (data not shown). For a further characterization of the precise cell type, sections were double-stained with anti-PmPLT1 antiserum and with the monoclonal anti-PmSUC2 1A2 (Stolz et al., 1999). For detection of antibody binding, sections were decorated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (green fluorescence, localization of PmPLT1 or PmPLT2) and with Alexa Fluor 546-conjugated goat anti-mouse IgG (red fluorescence, localization of PmSUC2). PmSUC2 has previously been documented as a companion cell-specific Suc transporter by immunodetection (Stadler et al., 1995a) and can thus be used as an internal standard for the common plantain vascular system.
Figure 8.
Immunodetection of PmPLT1 and PmPLT2 proteins in sections from common plantain source leaves. A, The section was labeled with anti-PmPLT1 antiserum and with fluorescent goat anti-rabbit IgG antiserum. The resulting green fluorescence is found only in cells of the common plantain phloem. For this figure, a fluorescence image was superposed on a photo taken under white light. Similar results were obtained with sections treated with anti-PmPLT2 antiserum and with fluorescent goat anti-rabbit IgG antiserum (data not shown). B and C, The presented section was double labeled with anti-PmPLT1 antiserum (B) and with the monoclonal anti-PmSUC2 antiserum 1A2 (C). Binding of antibodies was visualized under a fluorescent microscope after simultaneous incubation with fluorescent goat anti-rabbit IgG antiserum (B; green fluorescence, PmPLT1 localization) and fluorescent goat anti-mouse IgG antiserum (C; red fluorescence, PmSUC2 localization in companion cells). D and E, The presented section was double labeled with anti-PmPLT2 antiserum in D and with the monoclonal anti-PmSUC2 antiserum 1A2 in E. Binding of antibodies was visualized under a fluorescent microscope after simultaneous incubation with fluorescent goat anti-rabbit IgG antiserum (D; green fluorescence, PmPLT2 localization) and fluorescent goat anti-mouse IgG antiserum (E; red fluorescence, PmSUC2 localization in companion cells). The weak orange signal in one of the companion cells in D results from the extremely strong PmSUC2 signal of this cell, which is seen in E. Xy, Xylem; Ph, phloem; Cs, Casparian stripes. Scale bars = 25 μm in A, 10 μm in B and C, and 10 μm in D and E.
Figure 8, B and C, clearly shows that PmSUC2 and PmPLT1 are localized within the very same cell type of the common plantain phloem. In contrast to the vascular bundle shown in Figure 8A, this section is from a larger bundle, where phloem is already seen on both sides of the xylem. The green fluorescence in Figure 8B results from the immunodetection of PmPLT1 and is identical to the PmSUC2-specific red fluorescence in Figure 8C. The identical result was obtained in double labeling-analyses with anti-PmPLT2 and anti-PmSUC2 antisera (Fig. 8, D and E). The green fluorescence in Figure 8D results from the immunodetection of PmPLT2 and is identical to the PmSUC2-specific red fluorescence in Figure 8E. These data show that the PLT genes PmPLT1 and PmPLT2 are expressed in the companion cells of the common plantain phloem.
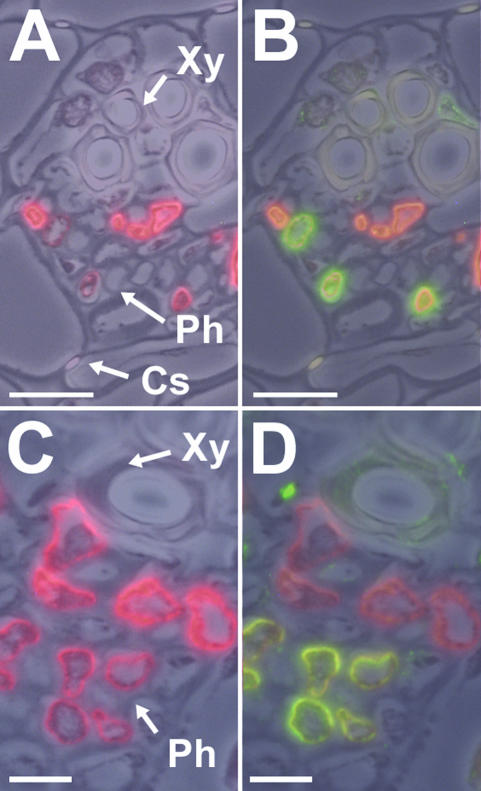
In our immunohistochemical analyses, we found that sometimes, especially in smaller and mediumsized common plantain vascular bundles, not all companion cells that were labeled with the anti-PmSUC2 antiserum were also labeled with the antisera raised against PmPLT1 or PmPLT2 (Fig. 9). Typically, companion cells that were labeled only by the monoclonal anti-PmSUC2 antibody seem to have higher levels of PmSUC2 protein than the other companion cells (i.e. stronger red fluorescence). Moreover, these “PmSUC2-only companion cells” are more frequently seen in the vicinity of the xylem, whereas the “PmSUC2-plus-PmPLT companion cells” are concentrated on the side of the phloem adjacent to the mesophyll (Fig. 9, B and D). One example for such a “PmSUC2-only” companion cell is also seen in the medium-sized vascular bundle shown in Figure 8D, where part of the strong PmSUC2-specific fluorescence is seen even with the filter for the green PmPLT2 signal.
Figure 9.
Immunodetection of PmPLT1 and PmPLT2 proteins in medium-sized veins of common plantain source leaves. A, Immuno-localization of PmSUC2 Suc transporter protein (red fluorescence) by immunodetection with the anti-PmSUC2 monoclonal antibody 1A2 in a small vein from a common plantain leaf. B, Additional labeling of the section shown in A with anti-PmPLT1 antiserum (green fluorescence). C, Immunolocalization of PmSUC2 Suc transporter protein (red fluorescence) by immunodetection with the anti-PmSUC2 monoclonal antibody 1A2 in a small vein from a common plantain leaf. D, Additional labeling of the section shown in C with anti-PmPLT2 antiserum (green fluorescence). Xy, Xylem; Ph, phloem; Cs, Casparian stripes. For the presented figures one (A and C) or two (B and D) fluorescence images were superposed on a photo taken under white light. Scale bars = 10 μm in A and B, and 5 μm in C and D.
DISCUSSION
This paper describes the molecular cloning and functional characterization of two higher plant sorbitol transporters and for the first time, to our knowledge, the immunohistochemical localization of the corresponding proteins. Moreover, the presented immunohistochemical data provide the first direct evidence for the existence of different types of companion cells in smaller veins during phloem development.
The model plant used for these analyses was common plantain, a dicot plant that allows simple isolation of pure vascular tissue (Gahrtz et al., 1994) and that is known to transport Suc and sorbitol in its phloem (Wallart, 1981; Lohaus and Fischer, 2002). The common plantain Suc transporters PmSUC1, PmSUC2, and PmSUC3 have been characterized (Gahrtz et al., 1994, 1996; Barth et al., 2003), and PmSUC2 was the first plant Suc transporter that has been localized on the cellular level (Stadler et al., 1995a). In the present paper, the analysis of the common plantain phloem is extended by the detailed characterization of two sorbitol transporters, PmPLT1 and PmPLT2. These two transporters differ in several functional properties, such as their Km, their sensitivity to PCMBS, their substrate specificity, or their response to Glc, from the previously described PLTs PcSOT1 and PcSOT2 from cherry (Gao et al., 2003).
PmPLT1 and PmPLT2 Are Low-Affinity H+ Symporters
Functional analyses of PmPLT1 and PmPLT2 in the yeast expression system clearly demonstrate that both transporters catalyze the transport of sorbitol, the polyol transported in the common plantain phloem (Fig. 2). Both transporters can also mediate the uptake of mannitol (data not shown; Fig. 3) and, therefore, it seems sensible to name these genes PLT1 and PLT2, although their physiological function is the transport of sorbitol. A low specificity for polyols was also described for the AgMAT1 mannitol transporter from celery (Noiraud et al., 2001), whereas a high specificity was found for the sour cherry sorbitol transporters (Gao et al., 2003).
In contrast to all previously described PLTs with Km values below 1 mm, PmPLT1 has a Km value for its physiological substrate sorbitol of 12 mm (Fig. 5), and the Km value for PmPLT2 seems to be even higher, because no saturation was observed over the concentration range analyzed (Fig. 5). Similar results were obtained for mannitol (yeast data not shown; Fig. 6). These substrate affinities are in the same order of magnitude as the Km values described for sorbitol transport in apple (Malus domestica) tissue (35-55 mm; Berüter, 1997) and in the same range like the Km value for phloem loading with sorbitol calculated from modeled carbon fluxes in a mature peach leaf (Moing et al., 1994). This not only shows that the common plantain sorbitol transporters have a more than 40- to 100-fold lower affinity than the proteins encoded by the previously cloned PLT cDNAs, but it also suggests different mechanism for the regulation of phloem loading with mannitol in celery and with sorbitol in common plantain. In celery, mannitol synthesis stays constant under stress, and the increase in root mannitol concentrations of stressed celery plants results from reduced mannitol catabolism (Everard et al., 1994; Stoop and Pharr, 1994a, 1994b). If AgMAT1 is responsible for phloem loading, maximal transport rates are reached already at 1 mm mannitol in the apoplast (Noiraud et al., 2001), which is ideal for a system where polyol synthesis is constant under stressed and unstressed conditions. In common plantain, however, a set of two transporters could respond with increased phloem loading rates to increasing apoplastic sorbitol concentrations between 0 and 100 mm. Therefore, it will be interesting to investigate whether in common plantain leaves the synthesis of sorbitol and its supply to the apoplastic space is increased under stress conditions.
Both common plantain transporters are highly sensitive to uncouplers of proton gradients (Fig. 4), both show increased transport rates, when Glc is added to activate the yeast plasma membrane ATPase in the recombinant yeast cells MRYs1 and MRYs2 (Fig. 2), and PmPLT1 was shown to accumulate sorbitol more than 20-fold inside the yeast strain MRYs1 (Fig. 3). A similar accumulation of sorbitol inside MRYs2 cells could be shown for PmPLT2 (data not shown). Taken together, these data suggest that both common plantain sorbitol transporters mediate an energy-dependent H+ sorbitol symport.
Direct proof for a H+ symport mechanism was obtained by expressing PmPLT1 in Xenopus sp. oocytes (Fig. 6). The observed inward currents were only obtained with mannitol and sorbitol, increased at higher substrate concentrations and with increasing membrane potentials, and did not respond to variations in the Na+ concentration. This demonstrates that the uptake of polyols depends on the electrical potential (ΔΨ) and on the protongradient (ΔpH) across the plasma membrane.
Noiraud et al. (2001) and Gao et al. (2003) found a strong inhibition of polyol transport by d-Glc and d-Fru. This unexpected observation could not really be explained, and the authors speculated that the inhibition of AgMAT1 or of PcSOTs by d-Glc does not result from Glc uptake via the mannitol transporter and may thus rather be an artifact of the yeast system (Noiraud et al., 2001; Gao et al., 2003). The effect of d-Glc is totally different for the common plantain sorbitol transporters PmPLT1 and PmPLT2. Sorbitol transport (Fig. 2) and mannitol transport (data not shown) are significantly enhanced in the presence of d-Glc. This is identical to previous analyses of plant Suc transporters in the yeast system (e.g. Gahrtz et al., 1994; Barth et al., 2003).
PmPLT1 has a consensus sequences for N-glycosylation at Asn334 (Fig. 1). This sequence is located in the predicted extracellular loop between the transmembrane helices 7 and 8 and is not conserved in PmPLT2 or in any of the other cloned PLTs. During secretion, it might be exposed to the lumen of the endoplasmic reticulum and be glycosylated. However, western-blot analyses with plasma membrane extracts from the recombinant yeast strains MRYs1 and MRYs2 showed no difference between the apparent Mr of PmPLT1 and PmPLT2, suggesting that this site in PmPLT1 is not used for N-glycosylation (data not shown).
PmPLT1 Is Sensitive to PCMBS
An interesting observation is the inhibition of PmPLT1-driven but not of PmPLT2-driven sorbitol transport by PCMBS (Fig. 4). A comparison of the deduced protein sequences shows that there is one extra Cys residue in PmPLT1 that is exposed to the predicted extracellular side of the protein. The observed inhibition can only be explained by PCMBS binding to the unique Cys61 in the PmPLT1 protein. This finding is not only a confirmation for the predicted topology of these proteins but may also be used for future structure/function analyses.
Most importantly, however, this difference in PCMBS sensitivity shows that inhibition of phloem loading of sorbitol (and possibly also of other substrates) by PCMBS does not allow a prediction on the mechanism of loading. The PCMBS sensitivity or insensitivity of substrate import (including sorbitol) into leaf discs of several plant species has previously been used to predict an apoplastic or symplastic loading mechanism for the different plant species (Flora and Madore, 1996). However, our data clearly show that the PCMBS sensitivity of sorbitol transport can vary between different transporters of the very same species and the very same cell type.
PmPLT1 and PmPLT2 Are Phloem-Specific Transporters
Although both sorbitol transporters from common plantain are highly similar (83% sequence identity on the amino acid level), it was possible to raise antisera against N- and C-terminal peptides that specifically recognize only one of the two proteins (Fig. 7). These antisera were used for immunohistochemical analyses of common plantain leaf sections. Both transporters reacted exclusively with cells located in the phloem of the vascular tissue (Fig. 8A). Double labeling of leaf sections with anti-PmPLT1 antiserum and with the monoclonal anti-PmSUC2 antibody 1A2 (Stolz et al., 1999) revealed that these cells are the companion cells of the common plantain phloem (Fig. 8, B and C). Moreover, double labeling of leaf sections with anti-PmPLT2 antiserum and with the monoclonal anti-PmSUC2 antiserum 1A2 (Stolz et al., 1999) revealed an identical localization (Fig. 8, D and E). These analyses were possible because the PmSUC2 Suc transporter had previously been located in the companion cells of the common plantain vascular tissue (Stadler et al., 1995a).
The localization of both common plantain PLTs in the companion cells demonstrates that phloem loading of both compounds transported in common plantain, sorbitol and Suc, occurs primarily in the phloem companion cells of this plant (this paper; Stadler et al., 1995a). This is supported by the observation that expression of the PLT genes is source specific. In vascular bundles of sink leaves, neither PmSUC2 nor PmPLT proteins could be identified (data not shown). Also in Arabidopsis, phloem loading is catalyzed by a companion cell-specific transporter (AtSUC2; Truernit and Sauer, 1995; Stadler and Sauer, 1996). This is what one would expect according to the anatomical facts in minor veins, where the relatively large companion cells surround the small sieve elements in the center and mediate the contact to the leaf mesophyll. Moreover, the importance of companion cell-specific phloem loading is supported by the observation that a knock out-mutant in the Arabidopsis AtSUC2 gene (Gottwald et al., 2000) shows a severe phenotype and can hardly survive under normal growth conditions. Nevertheless, solanaceous plants, which represent the only group of plants that has been analyzed besides common plantain and Arabidopsis, seem to behave differently. All phloem Suc transporters that have been studied in different members of this family (potato, tomato, and tobacco) so far were found in the phloem sieve elements (Kühn et al., 1997; Barker et al., 2000; Weise et al., 2000). It will, therefore, be important to study the cellular localization e.g. of the celery mannitol and Suc transporters (Noiraud et al., 2000, 2001) for a better understanding of the physiological basis for these differences in the cell-specific expression of phloem loaders in different plant species.
Only recently, Barth et al. (2003) showed that common plantain has also a transport protein in its sieve elements. The Suc transporter PmSUC3 could be localized in common plantain sieve elements. Interestingly, this sieve element-specific transporter has a lower affinity to its substrate Suc than its companion cell-specific partner PmSUC2, and expression of PmSUC3 is also seen in the sink phloem (Barth et al., 2003). It was discussed that this might be a mechanism to regulate release and retrieval of Suc along the transport phloem and in apoplastically unloading sinks.
Smaller Veins Possess Different Types of Companion Cells
Most interestingly, not all companion cells seem to have the identical physiological function. As shown in Figure 9, certain companion cells seem to be specialized on the phloem loading of Suc, whereas others, with lower levels of PmSUC2 protein, seem to catalyze the simultaneous loading of Suc plus sorbitol. These different types of companion cells are only seen in smaller vascular bundles. Larger veins, as shown in Figure 8, or mature veins with bicollateral phloem (not shown) possess only or almost exclusively (Fig. 8, B-D) companion cells with both types of transporters. These data are likely to reflect different steps during phloem development. Obviously, the youngest SE/CCCs (next to the xylem) start with the expression of PmSUC2, and expression of both PmPLT genes is initiated only at later developmental stages. Moreover, this might be a mechanism to modulate the supply of Suc and/or sorbitol to different sink organs.
Differences between individual companion cells of the same vascular bundle have previously been described by Haritatos et al. (2000). In this paper, tobacco plants were analyzed expressing the GUS reporter gene under the control of the galactinol synthase promoter from melon (Cucumis melo). GUS histochemical staining was observed only in some of the companion cells and was absent from others, suggesting differences in the transcriptional activity for this transgene. Our data support this interpretation and represent the first direct proof for differences in the physiological functions of individual companion cells.
Similar Transporters Are Found in Other Plant Species That Do Not Translocate Polyols in Their Phloem
Database searches revealed a group of six sequences highly homologous to PmPLT1 and PmPLT2 also in the Arabidopsis genome (Munich Information Center for Protein Sequences nos. AtPLT1, At2g16120; AtPLT2, At2g16130; AtPLT3, At2g18480; AtPLT4, At2g20780; AtPLT5, At3g18830; and AtPLT6, At4g36670). Due to sequence identities of 50% to 66% (on the amino acid level) between these transporters and the so-far characterized PLTs (Noiraud et al., 2001; this paper), these genes are likely to encode PLTs. It will be interesting to study the physiological roles and the physiological substrates of these proteins in Arabidopsis, a plant that does not transport polyols in the phloem. Similarly, two homologous transporter genes (GenBank accession nos. U64902 and U64903) were found in sugar beet. The function of these transporters might be necessary during local polyol synthesis in specific cell types or under certain environmental conditions. The identification of the physiological roles of these genes but also the detailed physiological roles of phloem localized sorbitol transporters, such as PmPLT1 and PmPLT2, will have to be analyzed further in the future. This will include analyses in plants that do normally not translocate polyols, such as Arabidopsis, but also mutants of polyol transporting plant species.
MATERIALS AND METHODS
Strains
Common plantain (Plantago major) wild-type plants were grown in potting soil in the greenhouse under ambient conditions. For cloning in Escherichia coli, we used strain DH5a (Hanahan, 1983). Yeast (Saccharomyces cerevisiae) expression was performed with strain SEY2102 (Emr et al., 1983). For the preparation of genomic DNA, leaves of celery (Apium graveolens) were purchased on a local market.
Cloning of PmPLT1 and PmPLT2 cDNAs
A common plantain cDNA library in λ-gt10 that had previously been used for the cloning of the Suc transporter cDNAs PmSUC1 and PmSUC2 (Gahrtz et al., 1996) was screened with a radiolabeled probe derived from the AgMAT1 mannitol transporter gene of celery. To this end, genomic DNA was isolated from celery leaves, and a 1,700-bp AgMAT1 genomic fragment was isolated by PCR (AgMAT1-5′ primer, 5′-AAG TAT GCT TTT GCT TGT GCT C-3′; AgMAT1-3′primer, 5′-AGC CTG TTG GAA GAA ATG AAT AC-3′). The radiolabeled probe was used to screen 120,000 pfu of the λ-gt10 library at a density of 7,000 pfu plate-1. Phage-DNA was transferred to nitrocellulose filters, prehybridized, hybridized, and washed as described (Sauer et al., 1990). From more than 200 positive signals obtained after exposure of the filters to x-ray films (Kodak X-Omat AR, Eastman Kodak, Rochester, NY), 25 plaques were isolated and rescreened, and their EcoRI inserts were cloned into pGEM-T-easy (Promega, Mannheim, Germany) and sequenced. The obtained sequences turned out to result from two different common plantain mRNAs. For further analyses, the longest cDNAs for each mRNA were cloned into the EcoRI site of pUC19, yielding the plasmids pMR7 (PmPLT1) and pMR8 (PmPLT2). The corresponding genes were named PmPLT1 (insert from λ-phage 5B) and PmPLT2 (insert from λ-phage 9B).
Analysis of 5′-Flanking Sequences by 5′-RACE
The complete 5′-flanking regions of the PmPLT1 and PmPLT2 cDNAs were determined using the 5′/3′-RACE kit of Roche Diagnostics (Mannheim, Germany). The nested primers PmSBT1-SP1 (5′-GAG CTT TCC GGC ATC ACC GGA G-3′), PmSBT1-SP2 (5′-CTG GTG ATC AGC AGT CAT AGT TG-3′), PmPSBT1-SP3 (5′-GGT TTA ATT GAC TAG CTA GC-3′), PmSBT2-SP1 (5′-CGT CTG GCT TTT GAG ACT TAC-3′), PmSBT2-SP2 (5′-GCC ACC GGA GTT ATG GTG TTC AC-3′), and PmSBT2-SP3 (5′-GTG TGA GCC TAC TTG TGT GTT TGG C-3′) were used to generate full-length 5′ sequences from both cDNA clones after in vitro polyadenylation of their very 5′ ends. All treatments were performed according to the manufacturer's protocol.
Functional Expression of the cDNAs in Bakers' Yeast
For the functional expression in bakers' yeast, the EcoRI inserts from pMR7 and pMR8 were cloned into the unique EcoRI site of the yeast/E. coli shuttle vector NEV-E (Sauer and Stolz, 1994) in sense and antisense orientation. The four resulting plasmids were used to transform into yeast. In a second approach, the primers SBT1-5 (5′-AGT CTG CTT GAA TTC AAC TAT GAC TGC TGA TCA CCA GA-3′), SBT1-3 (5′-TCA GCA CAT AAG AAT TCT TAG GTA GCT TCA GAA CCA GT-3′), SBT2-5 (5′-ACA CTT GTT GAA TTC CCT AAC CAT CAT GAA TAG TGA AC-3′), and SBT2-3 (5′-CTC ACT ACT GAA TTC TTA GGC ACC ATC AGT ACC ACT CC-3′) were used to generate cDNAs lacking their 5′- and 3′-flanking sequences. Again, the four plasmids resulting from cloning of these cDNAs into NEV-E were used to transform yeast. In a third approach, cDNA clones were generated with a modified 5′-flanking sequence using the primers SBT1-5Eco (5′-CTC CGG AAT TCA AGC TTG TAA AAG AAA TGA CTG CTG ATC ACC AGA AGT CAA G-3′), SBT1-3, SBT2-5Eco (5′-CTC CGG AAT TCA AGC TTG TAA AAG AAA TGA ATA GTG AAC ACC ATA ACT CC-3′), and SBT2-3. Ligation into NEV-E gave the plasmids NEV::PLT1s (PmPLT1 in sense orientation), NEV::PLT1as (PmPLT1 in antisense orientation), NEV::PLT2s (PmPLT2 in sense orientation), and NEV::PLT2as (PmPLT2 in antisense orientation). These plasmids were used to transform the yeast strain SEY2102. The resulting strains were named MRYs1 (expressing PmPLT1 in sense orientation), MRYas1 (expressing PmPLT1 in antisense orientation), MRYs2 (expressing PmPLT2 in sense orientation), and MRYas2 (expressing PmPLT2 in antisense orientation).
Transport Measurements in Transgenic Yeast Cells
Uptake of radiolabeled substrates and analyses of inhibitor sensitivities and Km values were performed in 50 mm Na-PO4 buffer, pH 5.5, as described (Sauer et al., 1990).
Thin-Layer Chromatography
Yeast cells (A600 was 20) were incubated in 50 mm Na-PO4 buffer, pH 5.5, in the presence of 0.1 mm 14C-sorbitol for 60 min. At this point, 1 mL of yeast cells was harvested, washed extracted with 80% (v/v) ethanol, and subjected to thin-layer chromatography as described (Gahrtz et al., 1994). Radioactivity was determined by exposure to x-ray films (Kodak X-Omat AR, Eastman Kodak).
Immunohistochemical Techniques
The anti-PmPLT1 and anti-PmPLT2 antisera used in this paper were raised against mixtures of two protein-specific oligopeptides (PmPLT1, MTADHQKSSVA and KKTGSEAT; PmPLT2, MNSEHHNSGGLA and KRSGTDGA) that were used to immunize two rabbits and one guinea pig after coupling to a protein carrier (Pineda, Antikörper-Service, Berlin).
Common plantain tissue and yeast cells were prepared, fixed in methacrylate, sectioned, and transferred to adhesion microscope slides (Linaris, Wertheim-Bettingen, Germany) as previously described (Stadler and Sauer, 1996). Methacrylate was removed by incubation of the slides for 3 min in acetone. Sections were rehydrated by sequential incubation in ethanol of decreasing concentrations (100%, 95%, 80%, 60%, and 30% [v/v]) and blocked for 1 h (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 1% [w/v] skim milk powder). After overnight incubation with affinity-purified anti-PmSUC3 antiserum (diluted 1:10 in blocking buffer) and/or monoclonal anti-PmSUC2 antiserum (Stolz et al., 1999; diluted 1:2), sections were washed five times with blocking buffer. For detection of bound anti-PmPLT1 or anti-PmPLT2 antisera, sections were incubated for 1 h with a 1:300 dilution of Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, Leiden, Netherlands). For double stainings of sections with polyclonal anti-PmPLT1 or anti-PmPLT2 antisera and with monoclonal anti-PmSUC2 antiserum, Alexa Fluor 546 goat anti-mouse IgG (Molecular Probes) was used in addition (diluted 1:100). After five final washes with blocking buffer, the slides were rinsed with water and mounted in ProLong Antifade kit (Molecular Probes). Photographs were taken on a fluorescence microscope (Zeiss, Göttingen, Germany) with appropriate excitation light.
For antibody-peptide competition experiments, a conjugate of the specific peptide with ovalbumin was used. Before immunolocalization, the affinity-purified antiserum was incubated for 2 to 3 h at room temperature with 200 μg mL-1 conjugate or pure ovalbumin, respectively.
Heterologous Expression in Xenopus sp. Oocytes
For functional analysis, PmPLT1 cRNA was prepared using the mMESSAGE mMACHINE RNA Transcription Kit (Ambion, Austin, TX). Oocyte preparation and cRNA injection have been described elsewhere (Becker et al., 1996). In two-electrode voltage-clamp studies, oocytes were perfused with 30 mm K+ gluconate-containing solutions, based on Tris/MES buffers for pH values from 5.6 to 8.5 or citrate/Tris buffers for pH 4.5. The standard solution contained 10 mm MES/Tris, pH 5.6, 30 mm K+ gluconate, 1 mm CaCl2, and 1 mm MgCl2. In addition, 20 mm BaCl2 and 30 mm TEA-Cl were used to reduce cationic background conductances. Osmolarity was adjusted to 220 mOsmol using Suc. The content of polyols and the pH values are indicated in the figure legends. Steady-state currents (ISS) were recorded with single-pulse protocols to 500-ms test voltages from 60 to -130 mV from a holding potential (VH) of 0 mV.
Acknowledgments
We thank Anja Schillinger for excellent technical assistance and Angelika Wolf for growing the common plantain plants.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027136.
References
- Aoki N, Hirose T, Takahashi S, Ono K, Ishimaru K, Ohsugi R (1999) Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.). Plant Cell Physiol 40: 1072-1078 [DOI] [PubMed] [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12: 1153-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SA (1955) Acyclic sugar alcohols. In K Peach, MV Tracey, eds, Modern Methods of Plant Analysis. Springer Verlag, Berlin, pp 158-192
- Barth I, Meyer S, Sauer N (2003) PmSUC3: kinetic characterization and cellular localization of a SUC3-type sucrose transporter from Plantago major. Plant Cell 15: 1375-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebee DU, Turgeon R (1992) Localization of galactinol and raffinose, and stachyose synthesis in Cucurbita pepo leaves. Planta 188: 354-361 [DOI] [PubMed] [Google Scholar]
- Becker D, Dreyer I, Hoth S, Reid JD, Busch H, Lehnen M, Palme K, Hedrich R (1996) Changes in voltage activation, Cs+ sensitivity, and ion permeability in H5 mutants of the plant K+ channel KAT1. Proc Natl Acad Sci USA 93: 8123-8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaloui N, Brown PH, Dandekar AM (1999) Manipulation of in vivo sorbitol production alters boron uptake and transport in tobacco. Plant Physiol 119: 735-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berüter J (1997) Characterization of the permeability of excised apple tissue for sorbitol. J Exp Bot 44: 519-528 [Google Scholar]
- Boorer KJ, Loo DDF, Frommer WB, Wright EM (1996) Transport mechanism of the cloned potato H+/sucrose cotransporter StSUT1. J Biol Chem 271: 25139-25144 [DOI] [PubMed] [Google Scholar]
- Boorer KJ, Loo DDF, Wright EM (1994) Steady-state and pre-steady-state kinetics of the H+/hexose cotransporter (STP1) from Arabidopsis thaliana expressed in Xenopus oocytes. J Biol Chem 269: 20417-20424 [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Hu H, Dandekar A (1999) Transgenically enhanced sorbitol synthesis facilitates phloem boron transport and increases tolerance of tobacco to boron deficiency. Plant Physiol 119: 17-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkle L, Hibberd JM, Quick WP, Kühn C, Hirner B, Frommer WB (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118: 59-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canh DS, Horak J, Kotyk A, Rihova L (1975) Transport of acyclic polyols in Saccharomyces cerevisiae. Folia Microbiol (Praha) 20: 320-325 [DOI] [PubMed] [Google Scholar]
- Emr SD, Scheckman R, Flessel MC, Thorner J (1983) An MFα1-SUC2 (σ-factor-invertase) gene fusion for study of protein localisation and gene expression in yeast. Proc Natl Acad Sci USA 80: 7080-7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora LL, Madore MA (1996) Significance of minor-vein anatomy to carbohydrate transport. Planta 198: 171-178 [Google Scholar]
- Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH (1994) Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol 106: 281-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahrtz M, Schmelzer E, Stolz J, Sauer N (1996) Expression of the PmSUC1 sucrose carrier gene from Plantago major L. is induced during seed development. Plant J 9: 93-100 [DOI] [PubMed] [Google Scholar]
- Gahrtz M, Stolz J, Sauer N (1994) A phloem specific sucrose-H+ symporter from Plantago major L. supports the model of apoplastic phloem loading. Plant J 6: 697-706 [DOI] [PubMed] [Google Scholar]
- Gao Z, Maurousset L, Lemoine R, Yoo SD, Van Nocker S, Loescher W (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol 131: 1566-1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979-13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D (1983) Studies on transformation of E. coli with plasmids. J Mol Biol 166: 557-580 [DOI] [PubMed] [Google Scholar]
- Hansch R, Fessel DG, Witt C, Hesberg C, Hoffmann G, Walch-Liu P, Engels C, Kruse J, Rennenberg H, Kaiser WM et al. (2001) Tobacco plants that lack expression of functional nitrate reductase in roots show changes in growth rates and metabolite accumulation. J Exp Bot 52: 1251-1258 [PubMed] [Google Scholar]
- Haritatos E, Ayre BG, Turgeon R (2000) Identification of phloem involved in assimilate loading in leaves by the activity of the galactinol synthase promoter. Plant Physiol 123: 929-937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG, Liebman SW (1991) Protein synthesis and translational control in Saccharomyces cerevisiae. In JR Broach, JR Pringle, EW Jones, The Molecular and Cellular Biology of the Yeast Saccharomyces. Volume I, Genome Dynamics, Protein Synthesis, and Energetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 627-735 [Google Scholar]
- Hu H, Penn SG, Lebrilla CB, Brown PH (1997) Isolation and characterization of soluble boron complexes in higher plants: the mechanism of phloem mobility of boron. Plant Physiol 113: 649-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings DB, Ehrenshaft M, Pharr DM, Williamson JD (1998) Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc Natl Acad Sci USA 95: 15129-15133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O, Hopf H (1982) Oligosaccharides based on sucrose (sucrosyl oligosaccharides). In FA Loewus, W Tanner, eds, Encyclopedia of Plant Physiology: Plant Carbohydrates I, Intracellular Carbohydrates New Series. Vol. 13a. Springer-Verlag, Berlin, pp 348-383 [Google Scholar]
- Keller F, Pharr DM (1996) Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosaccharides. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. Marcel Dekker, New York, pp 157-183
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298-1300 [DOI] [PubMed] [Google Scholar]
- Lohaus G, Fischer K (2002) Intracellular and intercellular transport of nitrogen and carbon. In C Foyer, G Noctor, eds, Advances in Photosynthesis. Kluwer Academic Publishers (in press)
- Ludwig A, Stolz J, Sauer N (2000) Plant sucrose-H+ symporters mediate the transport of vitamin H. Plant J 24: 503-509 [DOI] [PubMed] [Google Scholar]
- Moing A, Escobar-Gutiérrez A, Gaudillère JP (1994) Modeling carbon export out of mature peach leaves. Pant Physiol 106: 591-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiraud N, Delrot S, Lemoine R (2000) The sucrose transporter of celery: identification and expression during salt stress. Plant Physiol 122: 1447-1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiraud N, Maurousset L, Lemoine R (2001) Identification of a mannitol transporter, AgMaT1, in celery phloem. Plant Cell 13: 695-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn SG, Hu H, Brown PH, Lebrilla CB (1997) Direct analysis of sugar alcohol borate complexes in plant extracts by matrix-assisted laser desorption/ionization Fourier transform mass spectrometry. Anal Chem 69: 2471-2477 [DOI] [PubMed] [Google Scholar]
- Riesmeier JW, Hirner B, Frommer WB (1993) Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell 5: 1591-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11: 4705-4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S, Lemoine R, Jamai A, Bouché-Pillon S, Fromont C (1995) Study of sucrose and mannitol transport in plasma-membrane vesicles from phloem and non-phloem tissues of celery (Apium graveolens L.) petioles. Planta 197: 76-83 [Google Scholar]
- Sarthy AV, Schopp C, Idler KB (1994) Cloning and sequence determination of the gene encoding sorbitol dehydrogenase from Saccharomyces cerevisiae. Gene 140: 121-126 [DOI] [PubMed] [Google Scholar]
- Sauer N, Friedländer K, Gräml-Wicke U (1990) Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J 9: 3045-3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N, Stolz J (1994) SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana. Expression and characterization in baker's yeast and identification of the histidine tagged protein. Plant J 6: 67-77 [DOI] [PubMed] [Google Scholar]
- Shen B, Jensen RG, Bohnert HJ (1997) Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol 115: 527-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Brandner J, Schulz A, Gahrtz M, Sauer N (1995a) Phloem loading by the PmSUC2 sucrose carrier from Plantago major occurs into companion cells. Plant Cell 7: 1545-1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Sauer N (1996) The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Bot Acta 109: 299-306 [Google Scholar]
- Stadler R, Wolf K, Hilgarth C, Tanner W, Sauer N (1995b) Subcellular localization of the inducible Chlorella HUP1 monosaccharide-H+ symporter and cloning of a co-induced galactose-H+ symporter. Plant Physiol 107: 33-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz J, Ludwig A, Stadler R, Biesgen C, Hagemann K, Sauer N (1999) Structural analysis of a plant sucrose carrier using monoclonal antibodies and bacteriophage lambda surface display. FEBS Lett 453: 375-379 [DOI] [PubMed] [Google Scholar]
- Stoop JMH, Pharr DM (1994a) Mannitol metabolism in celery stressed by excess macronutrients. Plant Physiol 106: 503-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop JMH, Pharr DM (1994b) Growth substrate and nutrient salt environment alter mannitol to hexose partitioning in celery petioles. J Am Soc Hortic Sci 119: 237-242 [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259: 508-510 [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N (1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta 196: 564-570 [DOI] [PubMed] [Google Scholar]
- Turgeon R (1996) Phloem loading and plasmodesmata. Trends Plant Sci 1: 418-423 [Google Scholar]
- Wallart RAM (1981) Acyclic polyols as taxonomic characters. Proc K Ned Akad Wet Ser C 84: 77-87 [Google Scholar]
- Webb KL, Burley JWA (1962) Sorbitol translocation in apple. Science 137: 766. [DOI] [PubMed] [Google Scholar]
- Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity is localized in enucleate sieve elements of plants. Plant Cell 12: 1345-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants: a diversity of roles and complex regulation. Trends Plant Sci 5: 283-290 [DOI] [PubMed] [Google Scholar]
- Zimmermann MH, Ziegler H (1975) List of sugars and sugar alcohols in sieve-tube exudates. In MH Zimmermann, JA Milburn, eds, Encyclopedia of Plant Physiology. Springer Verlag, Berlin, pp 480-503