Abstract
Arsenic (As) accumulation in rice (Oryza sativa) may pose a significant health risk to consumers. Plants take up different As species using various pathways. Here, we investigated the contribution of the phosphate (Pi) transport pathway to As accumulation in rice grown hydroponically or under flooded soil conditions. In hydroponic experiments, a rice mutant defective in OsPHF1 (for phosphate transporter traffic facilitator1) lost much of the ability to take up Pi and arsenate and to transport them from roots to shoots, whereas transgenic rice overexpressing either the Pi transporter OsPht1;8 (OsPT8) or the transcription factor OsPHR2 (for phosphate starvation response2) had enhanced abilities of Pi and arsenate uptake and translocation. OsPT8 was found to have a high affinity for both Pi and arsenate, and its overexpression increased the maximum influx by 3- to 5-fold. In arsenate-treated plants, both arsenate and arsenite were detected in the xylem sap, with the proportion of the latter increasing with the exposure time. Under the flooded soil conditions, the phf1 mutant took up less Pi whereas the overexpression lines took up more Pi. But there were no similar effects on As accumulation and distribution. Rice grain contained predominantly dimethylarsinic acid and arsenite, with arsenate being a minor species. These results suggest that the Pi transport pathway contributed little to As uptake and transport to grain in rice plants grown in flooded soil. Transgenic approaches to enhance Pi acquisition from paddy soil through the overexpression of Pi transporters may not increase As accumulation in rice grain.
Inorganic arsenic (As) is a human carcinogen, with exposure coming mainly from drinking water and food (Tsuji et al., 2007; European Food Safety Authority, 2009). Recent studies have identified rice (Oryza sativa) as a major dietary source of inorganic As, which may pose a significant health risk (Kile et al., 2007; Mondal and Polya, 2008; Meharg et al., 2009). This is because paddy rice is rather efficient at As accumulation due to a combination of the anaerobic conditions prevailing in paddy soil, which leads to arsenite mobilization (Takahashi et al., 2004; Williams et al., 2007b; Xu et al., 2008), and the inadvertent uptake of arsenite through the rice silicic acid uptake pathway (Ma et al., 2008). This problem is further exacerbated by the widespread contamination of As in paddy fields as a result of irrigation with As-laden groundwater in south Asia (Meharg and Rahman, 2003; Dittmar et al., 2010), mining, and the past use of arsenical agrochemicals (Williams et al., 2007a; Zhu et al., 2008). As contamination of paddy soils not only compromises food safety but also can cause substantial losses in rice production (Panaullah et al., 2009). It is important, therefore, to understand the mechanisms of As uptake by rice.
As is a redox-sensitive metalloid, with arsenate [As(V)] and arsenite [As(III)] being commonly found in soil. The two inorganic As species are readily interconvertible depending on the environmental conditions (especially the redox potential and pH). Arsenate predominates under aerobic conditions, whereas anaerobic conditions in flooded paddy soil favor arsenite. Arsenite can also be methylated by soil microorganisms, producing various forms of methylated As (e.g. monomethylarsonous acid [MMA] and dimethylarsinic acid [DMA]; Cullen and Reimer, 1989). Plants are able to take up various As species through different mechanisms (for review, see Zhao et al., 2010b). Arsenate is a chemical analog of phosphate (Pi) and is taken up by Pi transporters. Evidence for this comes from physiological studies showing competitive inhibition of arsenate uptake by Pi (Abedin et al., 2002b) and isolation of arsenate-resistant mutants of Arabidopsis (Arabidopsis thaliana) defective in Pi transporters (Shin et al., 2004; González et al., 2005). In the rice genome, there are 13 sequences belonging to the Pht1 family encoding putative high-affinity Pi transporters (Paszkowski et al., 2002). Some of them have been characterized with regard to Pi uptake and transport (Ai et al., 2009). However, their roles in arsenate transport remain unknown. Furthermore, Pi uptake is highly regulated in plants (Raghothama, 1999; Chiou and Lin, 2011), and this regulatory system may also control arsenate uptake and translocation.
In contrast to arsenate, arsenite is present predominantly as a neutral molecule (arsenous acid) under the normal pH range that occurs in soil and in plant cells, and it permeates through the plasma membranes via the nodulin 26-like intrinsic protein (NIP) aquaporin channels (Bienert et al., 2008; Isayenkov and Maathuis, 2008; Ma et al., 2008). Arsenite influx into rice roots is mediated mainly through the silicic acid transporter NIP2;1 (Lsi1; Ma et al., 2008). Lsi1 also allows arsenite to leak to the external medium when the internal concentration is greater than the outside (Zhao et al., 2010a). In addition, another silicon (Si) transporter, Lsi2, mediates the efflux of both Si and arsenite from exodermal and endodermal cells toward the stele for xylem loading; Lsi2 plays a crucial role in As accumulation in rice shoots (Ma et al., 2008). Rice is a Si accumulator with both Lsi1 and Lsi2, being expressed strongly in rice roots (Ma et al., 2006, 2007); the Si uptake pathway thus constitutes the main route of arsenite uptake in rice. Compared with inorganic As, the methylated species MMA and DMA are taken up less efficiently by roots but are more readily transported via xylem and phloem (Abedin et al., 2002b; Raab et al., 2007; Li et al., 2009a; Carey et al., 2010; Ye et al., 2010). The rice aquaporin Lsi1 is also able to transport the undissociated form of MMA and DMA (Li et al., 2009a).
The dynamics of As speciation in paddy fields are complex, showing strong spatial and temporal variations (Stroud et al., 2011). While arsenite is the dominant species in the soil solution of flooded paddy soil (Takahashi et al., 2004; Xu et al., 2008; Li et al., 2009b), arsenate can also be present in considerable proportions (Stroud et al., 2011). Moreover, arsenite reaching the root surface may undergo oxidation to arsenate because of the oxygen released by rice roots. Rice roots (the mature zone) are typically coated with orange-colored iron plaque, which is formed from the oxidation of ferrous iron (Fe2+) and precipitation of ferric oxides/hydroxides (mainly ferrihydrite; Liu et al., 2006; Seyfferth et al., 2010). Iron plaque provides a strong sink for As, especially arsenate (Liu et al., 2006). The chemical reactions occurring in the rhizosphere of paddy rice add substantial complexity to the biogeochemical cycle of As. A particularly relevant question concerns the contribution of the Pi transport pathway to As accumulation in paddy rice. This is an important question, because strategies to enhance Pi acquisition in rice, an important goal for sustainable rice production, may inadvertently lead to excessive As accumulation if As is taken up as arsenate through the Pi pathway. Apart from root uptake and root-to-shoot translocation, it has been suggested that phloem transport of As to rice grain may also take the form of arsenate via the Pi pathway (Norton et al., 2010).
In this study, we first characterized Pi and arsenate uptake and root-to-shoot translocation in hydroponically grown rice using a mutant defective in Pi transport and two transgenic rice lines overexpressing a Pi transporter gene or a transcription factor regulating phosphorus (P) starvation responses. These rice lines and their wild types were then grown in soil under flooded conditions to evaluate the effects on P and As uptake, distribution, and As speciation in grain.
RESULTS
OsPHR2 Overexpression Enhanced Pi and Arsenate Uptake and Root-to-Shoot Translocation
Zhou et al. (2008) showed that overexpression of OsPHR2, a MYB-CC transcription factor and a homolog of Arabidopsis PHR1 (Rubio et al., 2001), up-regulates a number of Pi transporter genes in rice grown with sufficient Pi supply, resulting in excessive P accumulation in the shoots. In this study, we used an OsPHR2-overexpressing line (PHR2-Ov) to investigate the effect of PHR2 on Pi and arsenate uptake and root-to-shoot translocation. The ratio of root to shoot biomass of PHR2-Ov was slightly lower than that of Nipponbare, but the difference was not significant (Supplemental Fig. S1).
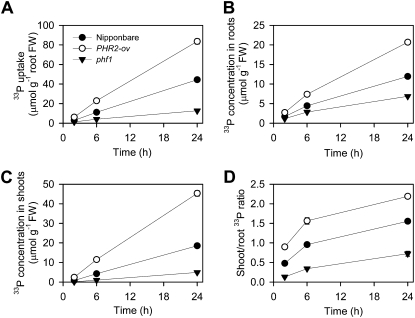
Over the 24-h time course, the uptake of 33P-labeled Pi by PHR2-Ov was approximately two times larger than that by the wild-type cv Nipponbare (Fig. 1A). PHR2-Ov had significantly higher concentrations of 33P in both roots and shoots than the wild type, with the effect on shoot 33P (2.7-fold increase) being greater than that on root 33P (1.7-fold increase; Fig. 1, B and C). The ratio of shoot to root 33P concentration increased with time and was 1.6 times higher in PHR2-Ov than in the wild type averaged across all time points (Fig. 1D). These results indicate that PHR2 overexpression in rice increases both the uptake and root-to-shoot translocation of Pi.
Figure 1.
Uptake and distribution of 33P-labeled Pi in Nipponbare, the PHR2 overexpression line, and the phf1 mutant of rice. A, Uptake per root fresh weight (FW). B, 33P concentrations in roots. C, 33P concentrations in shoots. D, Ratio of shoot to root 33P concentrations. Plants were supplied with 100 μm 33P-labeled Pi for 2, 6, and 24 h. Data are means ± se (n = 4); where error bars are not visible, se values are smaller than the symbol size.
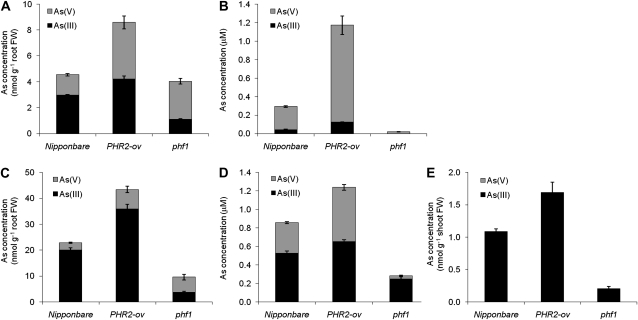
Unlike Pi, arsenate [As(V)] can be reduced rapidly to arsenite [As(III)] in plant roots (Zhao et al., 2009); therefore, it is necessary to analyze As speciation in order to determine the effect of PHR2 overexpression. When rice plants were exposed to 5 μm As(V) for 2 or 24 h in the presence of 100 μm Pi, root As(V) concentration of PHR2-Ov was 2.6 to 2.8 times higher than that of the wild type, compared with only a 1.4- to 1.8-fold difference in root As(III) concentration (Fig. 2, A and C). At 2 h, 65% and 49% of As in the roots of the wild type and PHR2-Ov, respectively, was in the form of As(III); the As(III) percentage increased to 88% and 83%, respectively, at 24 h. Xylem sap was collected at 2 and 24 h after As(V) exposure. At 2 h, As speciation in the xylem sap was dominated by As(V), accounting for 85% and 90% of the total As in the wild type and PHR2-Ov, respectively (Fig. 2B). Compared with the wild type, PHR2-Ov had 4.2 and 2.8 times more As(V) and As(III), respectively. By 24 h, As(III) had become the dominant As species in the xylem sap, accounting for 61% and 53% in the wild type and PHR2-Ov, respectively. At this time point, As(V) and As(III) concentrations in the xylem sap of PHR2-Ov were 76% and 24% larger than those of the wild type (Fig. 2D). At 2 h, shoot As was below the detection limit of the method used. At 24 h, only As(III) was detected in the shoots, with PHR2-Ov containing 56% higher As(III) than the wild type (Fig. 2E).
Figure 2.
As speciation in roots (A and C), xylem sap (B and D), and shoots (E) of Nipponbare, the PHR2-overexpressing line, and the phf1 mutant after 2 h (A and B) or 24 h (C–E) of exposure to 5 μm arsenate. Data are means ± se (n = 4). FW, Fresh weight.
OsPHF1 Mutation Greatly Decreased Pi and Arsenate Uptake and Root-to-Shoot Translocation
From an ethylmethane sulfonate-mutagenized population of the PHR2-Ov line, a number of arsenate-tolerant mutants with mutation in the OsPHF1 gene, which encodes a Pi transporter traffic facilitator, were isolated (P. Wu, unpublished data). In this study, we compared Pi and arsenate uptake and translocation between a rice phf1 mutant with a single nucleotide substitution and its wild type (PHR2-Ov). Compared with PHR2-Ov, phf1 had a significantly larger ratio of root to shoot biomass (Supplemental Fig. S1), a phenotype typically observed in P-deficient plants. Uptake of 33P-labeled Pi by phf1 was only 18% of the wild type (Fig. 1A), and 33P concentrations in roots and shoots were 38% and 8.5% of the wild type (Fig. 1, B and C). Root-to-shoot translocation of Pi was also affected markedly, with the shoot-to-root 33P ratio of the phf1 mutant being only 23% of the wild type (averaged across different time points; Fig. 1D).
Compared with PHR2-Ov, the rice phf1 mutant lost more than half of the arsenate uptake ability (Fig. 2). After exposure to 5 μm As(V) for 2 or 24 h, the roots of the phf1 mutant contained As(V) at levels that were 67% and 78%, respectively, of the wild type and contained As(III) at levels that were only 26% and 10% of the wild type (Fig. 2, A and C). A notable difference between the mutant and the wild type was in the percentage of As(III) in roots, only 27% to 39% in the mutant compared with 49% to 83% in the wild type, suggesting that arsenate reduction to arsenite was also impaired in the mutant. The reason for this effect is unclear. Analysis of As species in xylem sap showed even greater differences than those in the roots. At 2 h, As(V) in the xylem sap of phf1 was only 1.8% of the wild type and As(III) was not detectable (Fig. 2B), while at 24 h, As(V) and As(III) concentrations were 6% and 38% of the wild type (Fig. 2D). At 24 h, shoots contained As(III) only, with the level in phf1 being 12% of its wild type (Fig. 2E).
Overexpression of OsPT8 Markedly Increased Pi and Arsenate Uptake and Translocation
OsPT8 (OsPht1;8) is one of the 13 putative high-affinity Pi transporters in rice (Paszkowski et al., 2002). The OsPT8 gene is expressed in both root and shoot tissues of rice (Paszkowski et al., 2002; Jia et al., 2011). In this study, we constructed an overexpression vector of OsPT8 driven by a constitutive cauliflower mosaic virus 35S promoter and obtained two independent transgenic lines that were confirmed by quantitative reverse transcription (RT)-PCR and Southern-blot analysis (Supplemental Fig. S2). When grown in a normal nutrient solution (half-strength Kimura with 0.1 mm Pi), the two transgenic lines also contained significantly larger concentrations of P in roots and shoots than the wild type (cv Nipponbare; Supplemental Fig. S3). We then used one of the overexpression lines for more detailed characterization. Compared with the wild type, PT8-Ov had a significantly smaller ratio of root to shoot biomass (Supplemental Fig. S1).
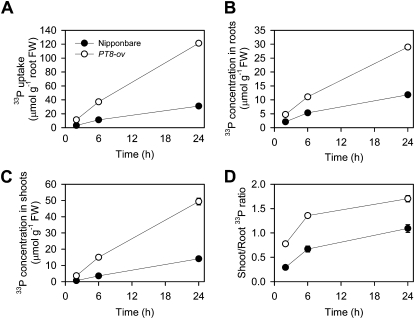
Averaged across the three time points between 2 and 24 h, the uptake of 33P-labeled Pi by PT8-Ov was 3.6 times larger than that of the wild type (Fig. 3A), 33P concentrations in roots and shoots were 2.3 and 4.6 times, respectively, larger than those in the wild type (Fig. 3, B and C), and the ratio of shoot to root 33P concentrations was 3.6 times larger (Fig. 3D).
Figure 3.
Uptake and distribution of 33P-labeled Pi in Nipponbare and the PT8 overexpression line of rice. A, Uptake per root fresh weight (FW). B, 33P concentrations in roots. C, 33P concentrations in shoots. D, Ratio of shoot to root 33P concentrations. Plants were supplied with 100 μm 33P-labeled Pi for 2, 6, and 24 h. Data are means ± se (n = 4); where error bars are not visible, se values are smaller than the symbol size.
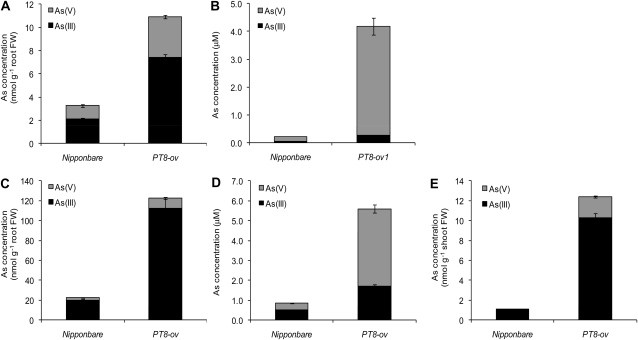
Arsenate speciation in roots, shoots, and xylem sap was determined after PT8-Ov and the wild type were exposed to 5 μm arsenate for 2 to 24 h (Fig. 4). OsPT8 overexpression increased the concentrations of As(V) and As(III) in roots by 2.0- and 2.5-fold, respectively, at 2 h and by 2.5- and 4.6-fold, respectively, at 24 h (Fig. 4, A and C). The percentage of As(III) was not significantly different between PT8-Ov (68% and 91% at 2 and 24 h, respectively) and the wild type (65% and 88% at 2 and 24 h, respectively). OsPT8 overexpression dramatically increased the As(V) concentration in the xylem sap, by 23- and 11-fold at 2 and 24 h, respectively (Fig. 4, B and D). The effect on As(III) concentration in the xylem sap was smaller but still highly significant, with 3.2- and 4.2-fold increases over the wild type at 2 and 24 h, respectively. As a result, OsPT8 overexpression increased the proportion of As(V) but decreased the proportion of As(III) in the xylem sap. At 24 h, shoot As concentration was increased by 10.4-fold compared with the wild type (Fig. 4E). Moreover, As(V), although undetectable in the wild-type shoots, accounted for 13% of the total As in the PT8-Ov shoots.
Figure 4.
As speciation in roots (A and C), xylem sap (B and D), and shoots (E) of Nipponbare and the PT8 overexpression line after 2 h (A and B) or 24 h (C–E) of exposure to 5 μm arsenate. Data are means ± se (n = 4). FW, Fresh weight.
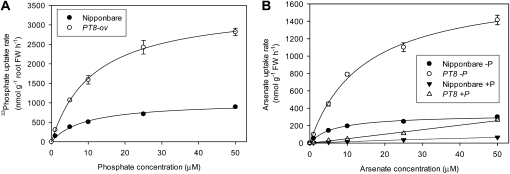
We further determined the Pi and arsenate uptake kinetics of PT8-Ov and its wild type. Within the concentration range (1–50 μm) in which high-affinity transporters are expected to operate, uptake of 33Pi and arsenate was greatly enhanced by OsPT8 overexpression (Fig. 5). The uptake kinetics can be described by a Michaelis-Menten equation, and the parameters obtained are shown in Table I. The maximum influx velocities (Vmax) of PT8-Ov for Pi and arsenate were 3.4 and 5.5 times larger than those of the wild type, respectively. The Km of Pi was slightly higher in PT8-Ov than in the wild type, and that of arsenate was about doubled. The Km values of Pi and arsenate were comparable, but Vmax for Pi was two to three times larger than that of arsenate. Arsenate uptake kinetics were also determined in the presence of 100 μm Pi in the solution. Arsenate influx was suppressed greatly by Pi in both PT8-Ov and the wild type, although it was still higher in the former than in the latter (Fig. 5B). In the presence of Pi, arsenate influx was linear over the range of arsenate concentrations tested, with the slope being about four times greater in PT8-Ov than in the wild type (Table I).
Figure 5.
Uptake kinetics of 33P-labeled Pi (A) and arsenate (B) in Nipponbare and the PT8 overexpression line of rice. Data are means ± se (n = 4). FW, Fresh weight.
Table I. Fitted parameters of Pi and arsenate uptake kinetics of Nipponbare and the PT8 overexpression line of rice.
| Rice Line | Vmax | Km | Linear Slope | r2adj |
| nmol g−1 root fresh wt h−1 | μm | |||
| Pi uptake kinetics | ||||
| Nipponbare | 1,020 ± 60 | 9.1 ± 1.6 | 0.987 | |
| PT8-Ov | 3,479 ± 54 | 11.4 ± 0.5 | 0.999 | |
| Arsenate uptake kinetics | ||||
| Nipponbare –P | 326 ± 13 | 6.5 ± 0.9 | 0.992 | |
| PT8-Ov –P | 1,796 ± 78 | 14.2 ± 1.6 | 0.996 | |
| Arsenate uptake kinetics | ||||
| Nipponbare +P | 5.2 ± 0.2 | 0.990 | ||
| PT8-Ov +P | 1.3 ± 0.04 | 0.992 | ||
Compared with the wild type, the expression of OsPT8 in both roots and shoots was increased in the PHR2-Ov line (Supplemental Fig. S2), suggesting that OsPT8 expression is under the regulation of PHR2. The expression of OsPT8 was also enhanced in the phf1 mutant, which can be attributed to P starvation in the mutant.
Effects of PHR2 or PT8 Overexpression, or PHF1 Mutation, on Pi and As Uptake by Rice Grown in Flooded Soil
While the short-term hydroponic experiments described above were conducted to investigate the mechanisms of uptake and root-to-shoot translocation, a long-term experiment was carried out in which the transgenic lines, mutants, and their wild types were grown to maturity in soil under flooded conditions that are normal for paddy rice. The soil used contained a borderline level of available P (15 mg kg−1; by the Olsen extraction method), and the experiment included two levels of P, control (no P addition) and +100 mg P kg−1 soil. The phf1 mutant grew poorly in the control soil, resulting in no grain and a much reduced straw biomass compared with its wild type, PHR2-Ov (Supplemental Fig. S4). In the +P treatment, phf1 grew better but still produced significantly lower grain biomass than its wild type. There were no significant differences between the PT8-Ov and PHR2-Ov lines and their wild type (cv Nipponbare), except that PHR2-Ov produced a significantly smaller grain biomass than the wild type in the control treatment (Supplemental Fig. S4).
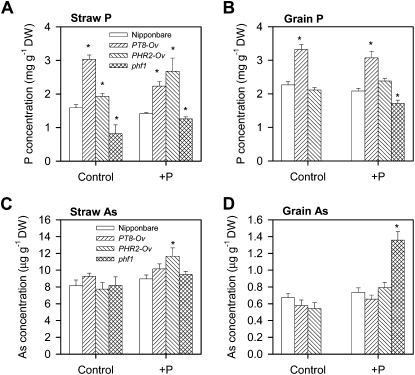
Overexpression of OsPT8 significantly increased total P concentrations in both grain and straw (by 46%–91%) but had no significant effect on straw or grain As concentration (Fig. 6). Overexpression of OsPHR2 significantly increased straw P concentration (by 21%–89%) but not grain P concentration. OsPHR2 overexpression increased straw As concentration significantly in the +P treatment (by 30%) but had no effect on straw As concentration in the control treatment or on grain As concentration in either treatment. The PHF1 mutation decreased both straw and grain P concentrations significantly (by 53%–58% in straw and by 28% in grain; note the absence of grain production by phf1 in the control treatment). In contrast, PHF1 mutation had no significant effect on straw or grain As concentration, except a significant increase in grain As concentration in the +P treatment, which was likely due to a much reduced grain biomass compared with the wild type. The total amounts of P and As accumulated in straw and grain are shown in Supplemental Figure S5. Again, there were more significant differences between the overexpressing lines or the mutant and their wild types in P accumulation than in As accumulation; for the latter, the only significant difference was between the phf1 mutant and its wild type, which was mainly due to poor growth of the mutant.
Figure 6.
Concentrations of P (A and B) and As (C and D) in straw (A and C) and grain (B and D) of different rice lines grown in the pot experiment under flooded soil conditions. * Significant difference (P < 0.05) from the wild type. DW, Dry weight.
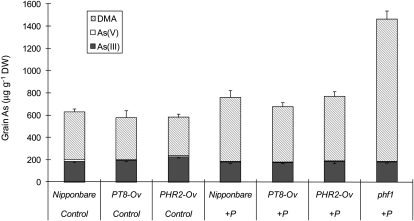
As speciation in rice grain was also analyzed. Unpolished rice grain contained mainly DMA (60%–87%) and As(III) (12%–38%), with As(V) being present only as a minor As species (0.6%–2.2%; Fig. 7). There were no significant differences between rice lines or between the control and + P treatments in As(III) concentration, with the exception of a significant increase in PHR2-Ov compared with the wild type in the control treatment. +P significantly decreased As(V) concentration in each rice line, but the overall effect on grain As was negligible because As(V) was a very minor component. +P also tended to increase DMA concentration, with the effect being significant for PHR2-Ov. Compared with PHR2-Ov, the phf1 mutant had significantly higher DMA in grain in the +P treatment, probably due to the much smaller grain biomass (see above).
Figure 7.
As speciation in rice grain from the soil experiment. DW, Dry weight.
DISCUSSION
Given the possibility of arsenite oxidation in the oxygenated rhizosphere of rice roots (Liu et al., 2006; Seyfferth et al., 2010) and the potential phloem transport of arsenate from the vegetative tissues to rice grain (Norton et al., 2010), it is important to investigate how the Pi transport pathway contributes to As accumulation in rice, a staple crop that makes a large contribution to the dietary intake of inorganic As (Kile et al., 2007; Mondal and Polya, 2008; Meharg et al., 2009).
Arsenate Uptake via the Pi Transport Pathway
Although it is well known that plants take up arsenate via the Pi transport pathway in general (Zhao et al., 2009), there has been little research on the role of individual Pi transporters and the regulatory components of Pi homeostasis in arsenate uptake and translocation in rice. Previous studies with Arabidopsis have shown that AtPht1;1 and AtPht1;4 are involved in Pi and arsenate uptake (Shin et al., 2004) and that AtPHF1 mutation resulted in increased arsenate tolerance (González et al., 2005). Mutation in AtPHF1 resulted in retention in the endoplasmic reticulum of the Pi transporter PHT1;1 (PT1) and possibly also other higher affinity Pi transporters (González et al., 2005). This study has provided direct evidence that arsenate is taken up by rice roots via Pi transporters and that the arsenate uptake is also subject to the control of the Pi regulatory system. Mutation in OsPHF1 resulted in decreased uptake of Pi and arsenate from nutrient solutions, whereas overexpression of OsPHR2 or OsPT8 produced the opposite effect (Figs. 1–4). PHF1 mutation may affect the trafficking of Pht1;1 (PT1), and possibly also some other Pi transporter proteins, from the endoplasmic reticulum to the plasma membrane (González et al., 2005). Interestingly, the expression of OsPT8 was enhanced in the mutant, probably due to a P-starvation response. The fact that the phf1 mutant was still able to take up some Pi and arsenate suggests that there exist Pi/arsenate transporters not under the control of PHF1. The transcription factor OsPHR2 has been shown to regulate the expression of multiple Pi transporters (Zhou et al., 2008). OsPT8 expression also appears to be under the regulation of OsPHR2 (Supplemental Fig. S2). The ability of OsPT8 to transport both Pi and arsenate was clearly demonstrated by the short-term uptake kinetics (Fig. 5; Table I). The Km values for Pi and arsenate of both the wild-type cultivar (Nipponbare) and PT8-Ov are within the range reported for the high-affinity transporters of Pi (typically 2–10 μm for Pi; Lee, 1982; Barber, 1984; Raghothama, 1999) and of arsenate (2–14 μm; Abedin et al., 2002b). Interestingly, Pi and arsenate had similar Km values in both the wild type and the PT8-overexpressing line, although the Vmax for Pi was two to three times larger than that of arsenate. Our results differ from previous studies with other plant species, which showed a higher affinity for Pi than for arsenate (Ullrich-Eberius et al., 1989; Meharg et al., 1994).
Root-to-Shoot Translocation of Pi and As
In addition to the uptake by roots, Pi transporters are also involved in the root-to-shoot translocation of arsenate. As transported in the xylem sap consisted of both arsenate and arsenite, with their proportions changing over the time course of arsenate exposure; arsenate was initially the dominant species, but by 24 h the majority of As was arsenite in the wild-type cv Nipponbare. This time course reflects the reduction of arsenate to arsenite in rice roots (Figs. 2 and 4). Overexpression of OsPHR2 or, especially, OsPT8 increased the proportion of arsenate in the xylem sap, consistent with their role in increasing Pi and arsenate loading into the xylem vessels. A recent study showed that the transcription factor OsPHR2 acts to up-regulate OsPT2, which is localized to the vascular tissue of rice roots and is likely to mediate Pi (and arsenate) loading into the xylem (Liu et al., 2010a). In the OsPT8 overexpression line, the transporter is likely to be overexpressed in different cell types, including those in the vascular tissues (Jia et al., 2011). The concentration of arsenite in the xylem sap was also increased in the two overexpression lines and decreased in the phf1 mutant; these changes were caused by the altered arsenite pool in the root cells as a result of altered arsenate influx. Arsenate arriving in rice shoots was also reduced to arsenite, because in most cases, arsenite was the only As species detected in the shoots. Only in PT8-Ov did some arsenate in shoots remain unreduced, suggesting that the amount of arsenate delivered to the shoots exceeded the reduction capacity in this transgenic line (Fig. 4).
Although arsenate shares the Pi transport pathway, its reduction to arsenite causes a diversion from this pathway. Arsenite is transported via the silicic acid transporters in rice (the aquaporin Lsi1 and the efflux carrier Lsi2; Ma et al., 2008). Furthermore, arsenite has a high affinity for thiol compounds (Schmöger et al., 2000), and arsenite-phytochelatin complexes are the main storage form in roots (Raab et al., 2005; Liu et al., 2010b). Arsenite-phytochelatin complexes are transported into vacuoles via ATP-binding cassette transporter-type proteins (Song et al., 2010). A recent study showed that As is stored mainly in the vacuoles of pericycle and endodermal cells of rice roots, with a strong colocalization with sulfur (Moore et al., 2011). Complexation with thiol compounds and subsequent vacuolar sequestration constitute an important mechanism for arsenite detoxification in plants (Zhao et al., 2009), and this process may also explain the much lower root-to-shoot translocation of As than P (at 24 h, the shoot-to-root ratio of 33P is about 20–30 times higher than that of As).
Effect of the Overexpression of Pi Transporters on Pi Acquisition from the Soil
It is generally thought that Pi acquisition by plant roots growing in soil is limited by the diffusion of Pi to the root surface (Barber, 1984). Modeling studies showed that Pi uptake by soil-grown soybean (Glycine max) was most sensitive to changes in the root surface area and then to changes in soil Pi supply, but it was rather insensitive to changes in the parameters of Pi uptake kinetics (either changing Km or increasing Vmax; Silberbush and Barber, 1983). Similar results were obtained by Huguenin-Elie et al. (2009), who modeled Pi uptake by rice growing in either flooded or moist soil. This raises a question of whether the overexpression of Pi transporters, which increased Vmax (Table I), can be effective in increasing Pi uptake from the soil. Overexpression of AtPT1 in tobacco (Nicotiana tabacum) cultured cells was found to enhance Pi uptake from the solution and cell growth under Pi-limiting conditions (Mitsukawa et al., 1997). In contrast, Rae et al. (2004) reported that overexpression of the Pht1;1 gene in barley (Hordeum vulgare) did not improve Pi uptake from either nutrient solution or from soil at a range of P supply levels, possibly due to posttranscriptional regulation of the transporter activity. Due to the lack of an effect on the Pi uptake from nutrient solution, the study of Rae et al. (2004) could not adequately test the hypothesis of whether the expression of Pi transporters can increase Pi uptake from soil. In our study, Pi uptake from nutrient solution was increased markedly by overexpression of either OsPT8 or OsPHR2. Enhanced Pi uptake was also obtained in the soil pot experiment under two different levels of P supply (Fig. 6), indicating that an increased Vmax did lead to increased Pi acquisition from the soil. In the case of the PHR2-Ov line, increased root elongation and root hair proliferation under P-deficient conditions (Zhou et al., 2008), in addition to increased expression of Pi transporters, could also have contributed to the greater Pi acquisition from the soil. However, the root-shoot ratio of the PT8-Ov line was smaller than that of the wild type in hydroponic experiments; therefore, its superior ability to acquire Pi was not due to enhanced root growth. It is known that flooding soil increases Pi availability and decreases the diffusion limitation (Huguenin-Elie et al., 2009); both changes could have enhanced the effect of Vmax on Pi acquisition by rice from the flooded soil. The results from our study demonstrate that it is possible to increase Pi acquisition from soil by overexpressing Pi transporters in rice.
Effect of the Overexpression of Pi Transporters on As Acquisition from the Soil
While hydroponic experiments are useful for dissecting individual physiological processes, it is important to consider plants growing in soil, where complex chemical reactions, microbial processes, and plant-soil interactions occur, especially in the rhizosphere. This is particularly true for As, which is a redox-sensitive metalloid that can also be methylated by soil microorganisms. In this study, while overexpression of OsPT8 or OsPHR2 increased, and OsPHF1 mutation decreased, P acquisition by rice plants from flooded soil significantly, there was generally little effect on As accumulation (Figs. 6 and 7). These results strongly suggest that the Pi/arsenate transport pathway contributes little to As accumulation in rice grown under flooded soil conditions. This is, to our knowledge, a new and significant finding. It is likely that arsenite was the main form of As taken up by rice roots, via the silicic acid pathway (Ma et al., 2008). As speciation was not analyzed in the soil solutions of this study, but previous experiments using the same soil and similar experimental conditions showed that arsenite accounted for 70% to 95% of the soluble As (Xu et al., 2008; Li et al., 2009b). Although some oxidation of arsenite is possible in the rice rhizosphere, the oxidized arsenate may be preferentially retained by the iron plaque because of its greater affinity for iron oxides/hydroxides than arsenite. Indeed, Chen et al. (2005) showed that the formation of iron plaque decreased arsenate influx into rice roots but, surprisingly, increased arsenite influx. Rice roots may also take up some methylated As species from flooded soil, but this uptake is again mediated mainly through the silicic acid transporter Lsi1 (Li et al., 2009a). Furthermore, even if some arsenate is taken up from the relatively oxygenated rhizosphere of rice roots, the pentavalent As is efficiently reduced to arsenite in roots and shoots, so that arsenite became the dominant form transported in the xylem and phloem. While both arsenate and arsenite were present in the xylem sap from rice plants exposed to arsenate in hydroponic experiments (Figs. 2 and 4), the proportion of arsenate decreased with the exposure time. Analysis of xylem sap from rice plants grown in a flooded soil supplemented with arsenate revealed only arsenite and methylated As species (F.-J. Zhao and W.L. Ye, unpublished data). There are no previous reports of As speciation in the phloem sap of rice. A recent study of castor bean (Ricinus communis) showed that arsenite typically accounted for approximately 90% of the total As in the phloem sap (Ye et al., 2010). Analysis of As speciation in rice grain showed the dominance of arsenite and DMA, with arsenate being present at negligible concentrations (Fig. 7). Furthermore, there was a lack of an inhibitory effect from the addition of P fertilizer on As accumulation in soil-grown rice (Figs. 6 and 7; Abedin et al., 2002a). In fact, addition of P tended to increase DMA concentration in rice grain. This effect can be explained by Pi replacing the adsorbed arsenate/arsenite from the soil solid phase (e.g. Fe oxides/hydroxides), thus enhancing microbial methylation of inorganic As in the soil solution, leading to increased DMA accumulation in rice. Therefore, the use of P fertilizers would not suppress As accumulation in rice grown in flooded paddy soils. The significant increase in grain P concentration, but not in grain As concentration (Fig. 6), in the PT8-Ov line suggests that OsPT8 is involved in the phloem transport of Pi, but not As, to rice grain. A recent study also showed the involvement of OsPT8 in Pi transport from rachis to rice grain (Jia et al., 2011).
Taken together, our study shows that, while arsenate is taken up and loaded into the xylem via the Pi pathway in hydroponically grown rice, this pathway appears to contribute little to As uptake and transport to grain in rice plants grown in flooded soil. Our results imply that transgenic approaches to enhance Pi acquisition from paddy soil through the overexpression of Pi transporters may not result in increased As accumulation in rice grain. An exception would be when rice is grown under aerobic conditions either deliberately or due to drought, and in such a situation, a better understanding of Pi and arsenate transport mechanisms and their interactions could lead to improved management for both the essential nutrient P and the toxic element As.
MATERIALS AND METHODS
Plant Materials
Four rice (Oryza sativa) lines, cv Nipponbare, OsPHR2 overexpression line (PHR2-Ov), OsPT8 overexpression line (PT8-Ov), and OsPHF1 mutant (phf1), were used in this study. PHR2-Ov has been characterized previously (Zhou et al., 2008), and its wild type is Nipponbare. The generation of PT8-Ov is described below. Both PHR2-Ov and PT8-Ov used in the study were homozygous. phf1 is a mutant isolated from an ethylmethane sulfonate-mutagenized population of PHR2-Ov.
Generation of OsPT8 Overexpression Lines
For the cloning of OsPT8, 1,784 bp of cDNA, including the open reading frame of OsPT8, was amplified using Nipponbare cDNA as the template and primers 5′-CAGGGGGACTCGTCTTCTTC-3′ (forward) and 5′-GGGTTCATGATTCCC-3′ (reverse), which were designed based on a previously published sequence (GenBank accession no. AF536968; Paszkowski et al., 2002). The amplified product was first ligated into the pMD18-T vector, cleaved using XbaI and SalI, and ligated into a binary vector, modified pCAMBIA 1300 driven by the cauliflower mosaic virus 35S promoter. The construct was transformed into mature embryos from the seeds of Nipponbare via Agrobacterium tumefaciens (strain EHA105) as reported previously (Hiei et al., 1997). Overexpression of OsPT8 in two independent transgenic lines was confirmed by quantitative RT-PCR and Southern blotting.
For Southern-blot analysis, genomic DNA was extracted from leaves of wild-type and T1 transgenic plants of two independent lines (OsPT8-Ov1 and OsPT8-Ov2) using the SDS method. Eight micrograms of genomic DNA was digested with the enzyme HindIII overnight at 37°C. The digested DNA was separated on a 0.8% (w/v) agarose gel, transferred to a Hybond-N+ nylon membrane, and hybridized with the coding sequence of the hygromycin-resistant gene used as the hybridization probe following the procedure described previously (Zhou et al., 2008).
Total RNA was extracted from the shoot tissues of plants cultured in a Pi-sufficient nutrient solution using TRIzol reagent (Invitrogen). The first-strand cDNA was synthesized from 3 μg of RNA using SuperScript II reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed by using the SYBR PremixEx Taq (Perfect Real Time) Kit (TaKaRa Biomedicals) on the LightCycler480 machine (Roche Diagnostics). The amplification program for SYBR Green I was carried out at 94°C for 10 s, 58°C for 15 s, and 72°C for 15 s. Triplicate quantitative assays were performed on each cDNA sample. The relative level of expression was calculated using the equation 2−Δ(ΔCp). The relative quantification of each sample was determined by normalization to the amount of actin cDNA detected in the same sample. The primers used for quantitative RT-PCR are given in Supplemental Table S1.
Hydroponic Culture
Seeds were sterilized in 0.5% NaOCl for 10 min and rinsed three times with deionized water. Seeds were soaked in deionized water overnight and then germinated at 25°C for 2 d. Seedlings were transferred to a nylon net floating on a 0.5 mm CaCl2 solution in a glasshouse (12-h photoperiod with a light intensity of more than 350 μmol m−2 s−1, day/night temperatures of 28°C/25°C, and relative humidity of 70%). After 1 week, the solution was replaced with a modified half-strength Kimura nutrient solution with a low P concentration, containing 0.091 mm KNO3, 0.183 mm Ca(NO3)2, 0.274 mm MgSO4, 0.183 mm (NH4)2SO4, 10 μm KH2PO4, 1.0 μm MnCl2, 3 μm H3BO3, 0.1 μm (NH4)6Mo7O24, 0.4 μm ZnSO4, 0.2 μm CuSO4, 80 μm NaFe(III)-EDTA, and 2 mm MES (pH 5.5). Plants were grown in this nutrient solution for 1 week. Seedlings were then transferred to either 0.3-L pots (two plants per pot) or 1-L pots (three plants per pot) containing a half-strength Kimura solution but with Pi concentration increased to 100 μm. The pots were placed in a controlled-environment cabinet (photoperiod of 12 h, light intensity of 500 μmol m−2 s−1, day/night temperatures of 28°C/25°C, and relative humidity of 70%). Plants were grown for a further 3 d before being used in various experiments.
Uptake Kinetics and Root-to-Shoot Translocation of 33Pi
To determine Pi uptake kinetics, 17-d old plants were transferred to 300-mL uptake solutions containing 0.5 mm CaCl2, 2 mm MES (pH 5.5), and various Pi concentrations (1, 5, 10, 25, and 50 μm) labeled with 10 kBq of 33P (H333PO4; supplied by Perkin-Elmer) per pot. Each Pi concentration was replicated in four pots. The uptake solutions were aerated vigorously and continuously during the experiment. After 30 min, roots were rinsed briefly with deionized water and then immersed in 300 mL of an ice-cold desorption solution (0.5 mm CaCl2, 100 μm KH2PO4 unlabeled with 33P, and 2 mm MES, pH 5.5) for 10 min to remove apoplastic 33P. Shoots and roots were separated. Roots were rinsed with deionized water three times and blotted dry with tissue paper, and the fresh weight was recorded. Roots were placed in a 20-mL scintillation vial, to which 1.5 mL of a tissue solubilizer (Soluene-350; Perkin-Elmer) was added. After 24 h, 10 mL of scintillation cocktail (Utima Gold; Perkin-Elmer) was added. 33P radioactivity was determined using a liquid scintillation counter (Tri-Carb 2100; Packard) and corrected for quenching with a preestablished quenching curve. A preliminary experiment showed little 33P in the shoots after 30 min of uptake. Pi uptake kinetics were fitted to the Michaelis-Menten equation using nonlinear regression of the software SigmaPlot (version 11.0).
To determine root-to-shoot translocation, 17-d-old plants were transferred to 1 L of fresh nutrient solution (half-strength Kimura with 100 μm KH2PO4) labeled with 10 kBq of 33P. Six plants (two per rice line) were placed in each pot. At 2, 6, and 24 h, roots were rinsed with deionized water and then immersed in 300 mL of an ice-cold desorption solution for 10 min as described above. At each time point, four replicate pots were harvested. Roots and shoots were separated, rinsed with deionized water, and blotted dry, and the fresh weights were recorded. The plant materials were digested with 2.5 mL of HNO3:HClO4 (85:15, v/v) at 175°C for 1.5 h. The digests were made up to 5 mL with deionized water, and 33P radioactivity was determined as described above.
Arsenate Uptake Kinetics
Arsenate uptake kinetics of Nipponbare and PT8-Ov were determined in the same way as for Pi uptake kinetics, with Pi being replaced with arsenate (1–50 μm). Arsenate uptake was determined both in the absence and presence of 100 μm Pi. As uptake in roots was determined by inductively coupled plasma mass spectrometry (ICP-MS).
Arsenate Uptake, Speciation, and Root-to-Shoot Translocation
Five-week-old plants were exposed to 5 μm arsenate in the half-strength Kimura nutrient solution with 100 μm Pi. After 2 and 24 h of arsenate exposure, stems were cut at 2 cm above the stem-root junction with a razor blade. Xylem exudates were collected by pipette for 30 min. Roots were then rinsed with deionized water and desorbed of apoplastic As with 1 L of an ice-cold desorption solution containing 1 mm K2HPO4, 0.5 mm CaCl2, and 2 mm MES (pH 5.5) for 10 min. Roots and shoots were frozen in liquid nitrogen for As speciation analysis. Four replicates were included for each time point.
Soil Pot Experiment
A pot experiment was conducted using an arable soil from the Rothamsted Experimental Farm in the United Kingdom (available Pi, 15 mg kg−1 by the Olsen P method; total As concentration, 11 mg kg−1, pH 6.4). Two kilograms of air-dried soil was placed in each pot. Two levels of Pi were included: control (no addition of P) and +P (100 mg kg−1 soil, added as KH2PO4). Arsenate (sodium arsenate) was added to each pot at 5 mg kg−1. Basal nutrients added to the soil included nitrogen (152 mg kg−1), potassium (126 mg kg−1), sulfur (30 mg kg−1), and magnesium (23 mg kg−1). Soil was then flooded with deionized water to maintain 2 to 3 cm of standing water on the soil surface. Ten-day-old rice seedlings of Nipponbare, PT8-Ov, PHR2-Ov, and phf1 were transplanted into the soil pots (one plant per pot). Each treatment was replicated in five pots. Plants were grown under flooded soil conditions until maturity inside a glasshouse.
Analysis of As Speciation Using HPLC-ICP-MS
Xylem sap was stored on ice during collection, diluted 3-fold with a Pi buffer solution (2 mm NaH2PO4 and 0.2 mm Na2-EDTA, pH 6.0), and analyzed immediately following collection. For the analysis of roots and shoots, samples were ground to fine powders in liquid N2 with a mortar and pestle. Aliquots (0.2–0.3 g) of the ground materials were extracted with 20 mL of Pi buffer solution for 1 h under sonication (Li et al., 2009a). For the determination of As speciation in rice grain, finely ground grain samples were extracted with 2 m trifluoroacetic acid according to Williams et al. (2005) and Xu et al. (2008). A reference material (NIST 1568a rice flour) was included for quality assurance. As species in the extracts and xylem sap were determined using anion-exchange HPLC-ICP-MS (Agilent LC1100 series and Agilent ICP-MS 7500ce; Agilent Technologies) as described previously (Li et al., 2009a).
Analysis of Total As and Total P Concentrations
Plant materials were ground to fine powders and digested with HNO3:HClO4 (85:15, v/v). Total As concentrations were determined by ICP-MS (Agilent 7500ce) and total P concentrations by ICP-atomic emission spectrometry (Optima 7300DV; Perkin-Elmer).
Data Analysis
Data were analyzed by ANOVA, followed by comparisons of means using the lsd test (P < 0.05).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Root-to-shoot ratio of four rice lines used in the arsenate uptake and translocation experiments.
Supplemental Figure S2. Quantitative RT-PCR of OsPT8 in the shoots of Nipponbare and OSPT8-Ov lines.
Supplemental Figure S3. P concentrations of roots and shoots of Nipponbare and OsPT8-Ov lines grown in a half-strength Kimura nutrient solution with 0.1 mm Pi
Supplemental Figure S4. Biomass of straw (A) and grain (B) of different rice lines grown in the soil pot experiment.
Supplemental Figure S5. The contents of P (A and B) and As (C and D) in straw (A and C) and grain (B and D) of different rice lines grown in the soil pot experiment.
Supplemental Table S1. Quantitative RT-PCR gene-specific primer sequences for OsPT8 and OsActin.
References
- Abedin MJ, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J. (2002a) Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ Sci Technol 36: 962–968 [DOI] [PubMed] [Google Scholar]
- Abedin MJ, Feldmann J, Meharg AA. (2002b) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128: 1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai PH, Sun SB, Zhao JN, Fan XR, Xin WJ, Guo Q, Yu L, Shen QR, Wu P, Miller AJ, et al. (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809 [DOI] [PubMed] [Google Scholar]
- Barber SA. (1984) Soil Nutrient Bioavailability: A Mechanistic Approach. John Wiley & Sons, New York [Google Scholar]
- Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP. (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA. (2010) Grain unloading of arsenic species in rice. Plant Physiol 152: 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhu YG, Liu WJ, Meharg AA. (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165: 91–97 [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Lin SI. (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62: 185–206 [DOI] [PubMed] [Google Scholar]
- Cullen WR, Reimer KJ. (1989) Arsenic speciation in the environment. Chem Rev 89: 713–764 [Google Scholar]
- Dittmar J, Voegelin A, Maurer F, Roberts LC, Hug SJ, Saha GC, Ali MA, Badruzzaman ABM, Kretzschmar R. (2010) Arsenic in soil and irrigation water affects arsenic uptake by rice: complementary insights from field and pot studies. Environ Sci Technol 44: 8842–8848 [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (2009) Scientific opinion on arsenic in food. EFSA Journal 7, 1351: 1–199 [Google Scholar]
- González E, Solano R, Rubio V, Leyva A, Paz-Ares J. (2005) PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17: 3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Komari T, Kubo T. (1997) Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol 35: 205–218 [PubMed] [Google Scholar]
- Huguenin-Elie O, Kirk GJD, Frossard E. (2009) The effects of water regime on phosphorus responses of rainfed lowland rice cultivars. Ann Bot (Lond) 103: 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayenkov SV, Maathuis FJM. (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett 582: 1625–1628 [DOI] [PubMed] [Google Scholar]
- Jia HF, Ren HY, Gu M, Zhao JN, Sun SB, Zhang X, Chen J, Wu P, Xu G. (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156: 1164–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC. (2007) Dietary arsenic exposure in Bangladesh. Environ Health Perspect 115: 889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RB. (1982) Selectivity and kinetics of ion uptake by barley plants following nutrient deficiency. Ann Bot (Lond) 50: 429–449 [Google Scholar]
- Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ. (2009a) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150: 2071–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ. (2009b) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43: 3778–3783 [DOI] [PubMed] [Google Scholar]
- Liu F, Wang ZY, Ren HY, Shen CJ, Li Y, Ling HQ, Wu CY, Lian XM, Wu P. (2010a) OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J 62: 508–517 [DOI] [PubMed] [Google Scholar]
- Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J. (2010b) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol 152: 2211–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Zhu YG, Hu Y, Williams PN, Gault AG, Meharg AA, Charnock JM, Smith FA. (2006) Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ Sci Technol 40: 5730–5736 [DOI] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. (2006) A silicon transporter in rice. Nature 440: 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. (2007) An efflux transporter of silicon in rice. Nature 448: 209–212 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105: 9931–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharg AA, Naylor J, Macnair MR. (1994) Phosphorus nutrition of arsenate tolerant and nontolerant phenotypes of velvetgrass. J Environ Qual 23: 234–238 [Google Scholar]
- Meharg AA, Rahman MM. (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37: 229–234 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu YG, Feldmann J, et al. (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43: 1612–1617 [DOI] [PubMed] [Google Scholar]
- Mitsukawa N, Okumura S, Shirano Y, Sato S, Kato T, Harashima S, Shibata D. (1997) Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc Natl Acad Sci USA 94: 7098–7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Polya DA. (2008) Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: a probabilistic risk assessment. Appl Geochem 23: 2987–2998 [Google Scholar]
- Moore KL, Schröder M, Wu ZC, Martin BGH, Hawes CR, McGrath SP, Hawkesford MJ, Ma JF, Zhao FJ, Grovenor CRM. (2011) NanoSIMS analysis reveals contrasting patterns of arsenic and silicon localization in rice roots. Plant Physiol 156: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton GJ, Islam MR, Duan GL, Lei M, Zhu YG, Deacon CM, Moran AC, Islam S, Zhao FJ, Stroud JL, et al. (2010) Arsenic shoot-grain relationships in field grown rice cultivars. Environ Sci Technol 44: 1471–1477 [DOI] [PubMed] [Google Scholar]
- Panaullah GM, Alam T, Hossain MB, Loeppert RH, Lauren JG, Meisner CA, Ahmed ZU, Duxbury JM. (2009) Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 317: 31–39 [Google Scholar]
- Paszkowski U, Kroken S, Roux C, Briggs SP. (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 99: 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab A, Schat H, Meharg AA, Feldmann J. (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168: 551–558 [DOI] [PubMed] [Google Scholar]
- Raab A, Williams PN, Meharg A, Feldmann J. (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4: 197–203 [Google Scholar]
- Rae AL, Jarmey JM, Mudge SR, Smith FW. (2004) Over-expression of a high-affinity phosphate transporter in transgenic barley plants does not enhance phosphate uptake rates. Funct Plant Biol 31: 141–148 [DOI] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J. (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmöger MEV, Oven M, Grill E. (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfferth AL, Webb SM, Andrews JC, Fendorf S. (2010) Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L.) roots having variable Fe coatings. Environ Sci Technol 44: 8108–8113 [DOI] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ. (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39: 629–642 [DOI] [PubMed] [Google Scholar]
- Silberbush M, Barber SA. (1983) Sensitivity of simulated phosphorus uptake to parameters used by a mechanistic mathematical model. Plant Soil 74: 93–100 [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107: 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud JL, Norton GJ, Islam MR, Dasgupta T, White RP, Price AH, Meharg AA, McGrath SP, Zhao FJ. (2011) The dynamics of arsenic in four paddy fields in the Bengal delta. Environ Pollut 159: 947–953 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K. (2004) Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38: 1038–1044 [DOI] [PubMed] [Google Scholar]
- Tsuji JS, Yost LJ, Barraj LM, Scrafford CG, Mink PJ. (2007) Use of background inorganic arsenic exposures to provide perspective on risk assessment results. Regul Toxicol Pharmacol 48: 59–68 [DOI] [PubMed] [Google Scholar]
- Ullrich-Eberius CI, Sanz A, Novacky AJ. (1989) Evaluation of arsenate- and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba-G1. J Exp Bot 40: 119–128 [Google Scholar]
- Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA. (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39: 5531–5540 [DOI] [PubMed] [Google Scholar]
- Williams PN, Raab A, Feldmann J, Meharg AA. (2007a) Market basket survey shows elevated levels of As in South Central U.S. processed rice compared to California: consequences for human dietary exposure. Environ Sci Technol 41: 2178–2183 [DOI] [PubMed] [Google Scholar]
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. (2007b) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41: 6854–6859 [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Meharg AA, Zhao FJ. (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42: 5574–5579 [DOI] [PubMed] [Google Scholar]
- Ye WL, Wood BA, Stroud JL, Andralojc PJ, Raab A, McGrath SP, Feldmann J, Zhao FJ. (2010) Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol 154: 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF. (2010a) The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol 186: 392–399 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Ma JF, Meharg AA, McGrath SP. (2009) Arsenic uptake and metabolism in plants. New Phytol 181: 777–794 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP, Meharg AA. (2010b) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61: 535–559 [DOI] [PubMed] [Google Scholar]
- Zhou J, Jiao FC, Wu ZC, Li YY, Wang XM, He XW, Zhong WQ, Wu P. (2008) OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol 146: 1673–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YG, Sun GX, Lei M, Teng M, Liu YX, Chen NC, Wang LH, Carey AM, Deacon C, Raab A, et al. (2008) High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ Sci Technol 42: 5008–5013 [DOI] [PubMed] [Google Scholar]